Abstract
Objective: This study aimed to comprehensively evaluate perianal fistulas and their related complications using magnetic resonance imaging (MRI). Methods: We enrolled 115 eligible patients who underwent preoperative perianal MRI. Primary fistulas, internal and external openings, and related complications were evaluated using MRI. All fistulas were classified according to Park’s classification, Standard Practice Task Force classification, St. James’s grade, and the position of the internal opening. Results: In total, 169 primary fistulas were detected in 115 patients; 73 (63.5%) patients had a single primary tract and 42 (36.5%) patients had multiple primary tracts, and 198 internal and 129 external openings were identified. Based on Park’s classification, 150 (88.7%) primary fistulas were classified into the following types: intersphincteric (82, 54.7%), trans-sphincteric (58, 38.6%), suprasphincteric (8, 5.3%), extrasphincteric (1, 0.7%), and diffuse intersphincteric with trans-sphincteric (1, 0.7%) types. Based on St. James’s grade, 149 fistulas were classified into grade 1 (52, 34.9%), grade 2 (30, 20.1%), grade 3 (20, 13.4%), grade 4 (38, 25.5%), and grade 5 (9, 6.1%). We detected 92 (54.4%) simple and 77 (45.6%) complex perianal fistulas and 72 (42.6%) high and 97 (57.4%) low perianal fistulas. Furthermore, we detected 32 secondary tracts in 23 (20.0%) patients and 87 abscesses in 60 (52.2%) patients. Levator ani muscle involvement and extensive soft tissue edema were detected in 12 (10.4%) and 24 (20.9%) patients, respectively. Conclusion: MRI is a valuable and comprehensive tool that can not only be used to determine the general condition of perianal fistulas but also to classify them and identify related complications.
Keywords: Perianal fistula, classification, magnetic resonance imaging, complications
Introduction
A perianal fistula is an abnormal condition that develops between the anal canal and the perineal skin or in the perianal region [1]. Although its overall incidence is not high, it causes considerable morbidity in young and middle-aged men [1,2]. Patients with perianal fistula may be completely asymptomatic or present with local pain and secretions [3]. According to the cryptoglandular theory, perianal fistulas are developed by the occlusion of anal gland drainage into the lumen of the anal canal, spreading the infection to the fatty areolar tissue, which has little inherent resistance to its progression [4]. Surgery is the main treatment strategy for perianal fistulas and is based on the classification of the fistula and the degree of involvement of surrounding pelvic structures, particularly the detection of secondary fistula tracts and abscesses in the surrounding tissues. Surgery for perianal fistulas can minimize their recurrence rate and the risk of incontinence when eradicating the fistulas; however, a complex fistulizing process can greatly reduce treatment efficacy. Therefore, the precise and comprehensive preoperative evaluation of perianal fistulas is an important diagnostic strategy that can break this vicious circle and improve the success rate of surgery [5]. Studies have reported that magnetic resonance imaging (MRI) affects perianal fistula-related treatment outcomes and patient care [6,7].
MRI is the most accurate and widely accepted gold standard imaging technique to define the anatomy of the anal canal and perianal fistulas [8,9]. The use of an optimal MRI technique for the preoperative evaluation of perianal lesions can help accurately identify potential risk factors and assist physicians in selecting the best surgical approach. High-resolution MRI has been proven to provide superior results over the clinical examination, endoluminal ultrasound (EUS), and computed tomography (CT) for evaluating perianal fistulas, their classification, and associated complications [10,11]. MRI can accurately delineate the presence and course of the primary fistulous track and identify the presence and site of secondary extensions and accompanying abscesses [12-14].
An accurate and comprehensive evaluation of primary tracks, associated complications, and abscesses can improve surgical outcomes and minimize complications, such as fecal incontinence and recurrent lesions [15]. Therefore, herein, we evaluated a group of consecutive patients with perianal fistulas and identified the presence and course of a primary fistulous track as well as the classification and associated complications of fistulas, such as secondary fistula, abscess, levator ani muscle involvement, and edema. This study may provide objective support for optimizing treatment strategies and patient care based on the accurate and time-efficient analysis of perianal MRI examination results.
Materials and methods
Study population
In this study, we included patients with perianal fistulas who underwent preoperative MRI and surgery at the Xi’an Hospital of Traditional Chinese Medicine, Xi’an, China, from March 1, 2022, to August 15, 2022. This study was conducted following the Declaration of Helsinki (as revised in 2013) and was approved by the Clinical Research Ethical Committee of the Xi’an Hospital of Traditional Chinese Medicine (ref no: LLSCPJ2022061).
The inclusion criteria were as follows: (1) patients aged 11-80 years; (2) those with fistula symptoms and who were recommended for MRI by an anorectal physician; and (3) those with clinically diagnosed perianal fistula via physical or imaging examination of the pelvic region.
The exclusion criteria were as follows: (1) patients with fistulas due to Crohn’s disease (CD); (2) those who underwent perianal operation; (3) those with other perianal diseases (e.g., hemorrhoids, rectal prolapse, or perianal neoplasms); (4) those with poor-quality MRI images; and (5) those who could not tolerate MRI examination.
MRI equipment and image acquisition
Patients were placed in the supine position and examined using 1.5 tesla MRI scanners (Magnetom Avanto, Siemens Limited, Germany) equipped with a six-channel phased-array surface coil. None of the patients used oral special intestinal preparations or rectal contrast agents. After identifying the three planes, sagittal images, oblique coronal and axial images that were parallel and perpendicular to the long axis of the anal canal were obtained and used. The following images were taken: oblique axial T1-weighted turbo spin-echo (T1W TSE), sagittal fat-saturated (FS) T2-weighted TSE (T2W TSE), oblique coronal FS T2W TSE, oblique coronal T2W TSE, and oblique axial FS T2W TSE. The field of view (FOV) of the MRIs included the supralevator planes and levator ani muscles, which are the anatomical sites that can be affected during the clinical course of perianal fistulas.
The parameters of each sequence were as follows: oblique axial T1W TSE: time of repetition (TR)/time of echo (TE) = 3.79/1.53 ms, slice thickness = 1.4 mm, slice spacing = 0.0 mm, FOV = 260 mm, and matrix = 288 × 320; sagittal FS T2W TSE: TR/TE = 3500/83 ms, slice thickness = 2.0 mm, slice spacing = 0.0 mm, FOV = 280 mm, and matrix = 230 × 256; oblique coronal FS T2W TSE: TR/TE = 3500/83 ms, slice thickness = 2.0 mm, slice spacing = 0.0 mm, FOV = 280 mm, and matrix = 230 × 256; oblique coronal T2W TSE: TR/TE = 3500/83 ms, slice thickness = 2.0 mm, slice spacing = 0.0 mm, FOV = 280 mm, and matrix = 230 × 256; and oblique axial FS T2W TSE: TR/TE = 5000/83 ms, slice thickness = 2.0 mm, slice spacing = 0.0 mm, FOV = 280 mm, and matrix = 230 × 256.
MRI assessment
All MRI images were independently evaluated by at least two radiologists with > 10 years of experience in interpreting MRI results. In case of inconsistencies between their analysis, the final judgment was made by a radiologist with > 20 years of experience in interpreting MRI results.
Diagnosis of perianal fistulas using MRI was based on the shape (linear or oval structure surrounded by an irregular area), visualization of signal intensities (isointense to hyperintense on T2-weighted images), and extension of the fistula. When a perianal fistula was detected, its internal (the beginning of the fistula) and external openings were identified. The internal orifice is the opening through which the fistula drains into the lumen of the anal canal. The radial site of the internal opening was defined based on clock positions (12 o’clock anterior and 6 o’clock posterior). If the primary fistula terminated blindly in the subcutaneous fat tissue, it was named the sinus track. Because there may be more than one external opening other than the perianal region, the openings were defined as gluteal, perineal, labial, or retroscrotal openings.
Classification of the perianal fistulas
All perianal fistulas were classified according to Park’s classification, Standard Practice Task Force (SPTF) classification, St. James’s grade, and the position of the internal opening.
Park’s classification
According to Park’s classification, perianal fistulas were classified into intersphincteric, trans-sphincteric, suprasphincteric, and extrasphincteric types [16].
St. James’s grade
According to St. James’s grade, anal fistulas were divided into five grades (grades 1-5) based on MRI results [13]. This grading mainly considers the relationship between the fistula and sphincter and the presence of an abscess or a secondary fistula.
SPTF classification
The SPTF classification was developed by the American Society of Colon and Rectal Surgeons. According to the SPTF classification, perianal fistulas were classified into “simple” and “complex” types [17].
Classification according to the position of the internal opening
Perianal fistulas were classified into low and high types based on the position of the internal opening. The perianal fistulas with the internal opening located near the deep part of the upper external sphincter muscles were defined as high perianal fistulas, whereas those in lower regions of the external sphincter muscle were defined as low perianal fistulas.
Defining the secondary fistulas and abscesses
Secondary fistulas and abscesses were defined based on their anatomical location. Primary fistula-associated secondary tracks may extend into the ischioanal, gluteal, perineal, or supralevator regions. During image interpretation, according to the standards recommended by Singh et al. and Torkzad et al., fluid collection or extension of the fistula larger than 10 mm in diameter was determined as an abscess [18,19].
Statistical analysis
The primary outcomes of the study were comprehensive descriptions of the perianal fistula, its classification, and associated complications. SPSS 19.0 version statistical software was used and descriptive statistics were provided. Continuous variables were expressed as mean ± standard deviation (x̅ ± s) or median, and categorical variable were represented by percentage (%).
Results
General characteristics of the patients
A total of 115 patients met the inclusion criteria. Of these, 101 (87.8%) were males aged 37.8 ± 11.1 years (median = 39 years), and 14 (12.2%) were females aged 44.4 ± 14.5 years (median = 37 years). A total of 87 (75.7%) patients presented with the sensation of the anus bearing down and/or anal discomfort, and 27 (23.5%) patients had obvious perianal pain. Secretion outflow in the perianal region was observed in 77 (70.0%) patients, and skin itching was observed in 19 (16.5%) patients.
Evaluation of the primary fistulas and their internal and external openings
A total of 169 primary fistulas were detected in 115 patients. Seventy-three (63.5%) patients had single (Figure 1B), 34 (29.5%) patients had two, six (5.2%) patients had three (Figure 3E-H), one (0.9%) patient had four, and one (0.9%) patient had six primary tracts (Table 1). The inner diameter of the fistulas was 0.2-1.0 cm, and the length was 1.0-11.5 cm.
Figure 1.
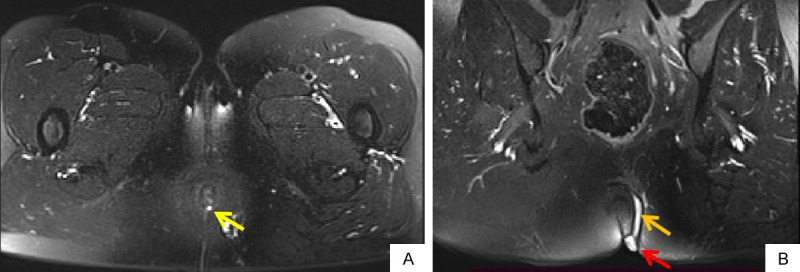
58-years old female patient with perianal fistula, intermittent secretion outflow for 1 year. Oblique axial FS T2WI (A) shows an internal opening at 6 o’clock (yellow arrow). Oblique coronal FS T2WI (B) shows fistula (orange arrow) and external opening (red arrow). FS T2WI: fat suppression T2 weighted imaging.
Figure 3.
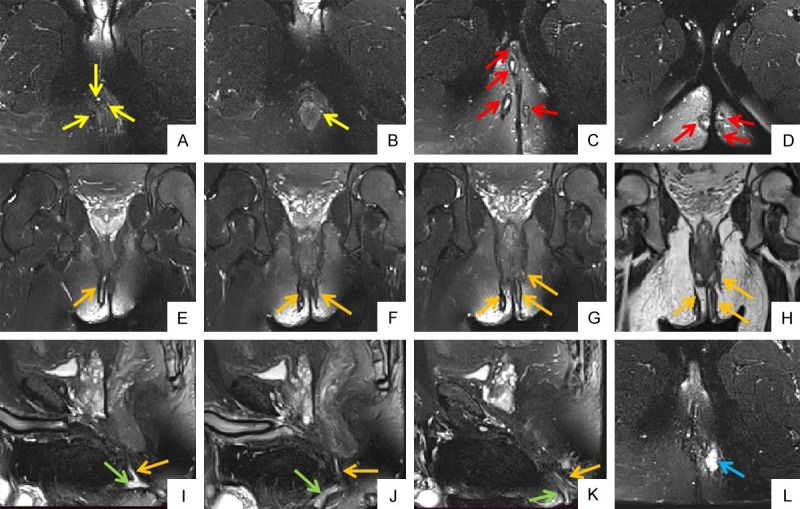
38-years old male patient with perianal fistula. Oblique axial FS T2WIs (A, B) show internal openings at 2 o’clock, 3 o’clock, 8 o’clock and 12 o’clock respectivly (yellow arrow). Oblique axial FS T2WIs (C, D) show multiple external openings on bilateral buttocks and right perineum (red arrow). Oblique coronal FS T2WIs (E-G) and oblique coronal T2WI (H) show bilateral intersphincteric and left transsphincteric anal fistula (orange arrow). Sagittal FS T2WIs (I-K) show primary fistula (orange arrow) and secondary fistula (green arrow). Oblique axial FS T2WI (L) shows associated abscess in left ischioanal space (blue arrow). FS T2WI: fat suppression T2 weighted imaging.
Table 1.
Evaluation of primary fistulas, internal and external openings of perianal fistulas
| N (%) | |
|---|---|
| Patients with primary fistula | 115 |
| With single primary fistula | 73 (63.5%) |
| With multiple primary fistulas | 42 (36.5%) |
| 2 primary fistulas | 34 (29.5%) |
| 3 primary fistulas | 6 (5.2%) |
| 4 primary fistulas | 1 (0.9%) |
| 6 primary fistulas | 1 (0.9%) |
| Internal opening | 198 |
| Form of internal openings | |
| independent internal openings | 29 (14.6%) |
| dependent internal openings | 169 (85.4%) |
| Site of internal openings | |
| 1-3 o’clock | 44 (22.2%) |
| 4-6 o’clock | 70 (35.4%) |
| 7-9 o’clock | 49 (24.7%) |
| 10-12 o’clock | 35 (17.7%) |
| Patients with external opening | 93 |
| With single external opening | 69 (74.2%) |
| With multiple external openings | 24 (25.8%) |
| 2 external openings | 16 (66.6%) |
| 3 external openings | 6 (25.0%) |
| 4 external openings | 1 (4.2%) |
| 6 external openings | 1 (4.2%) |
| External opening | 129 |
| Cutaneous distribution of external opening | |
| left buttock | 41 (31.8%) |
| right buttock | 40 (31.0%) |
| middle line of buttocks | 21 (16.3%) |
| left perineum | 10 (7.7%) |
| right perineum | 13 (10.1%) |
| middle line of perineum | 3 (2.3%) |
| middle-lower segment of the vagina | 1 (0.8%) |
Among the 115 patients, 198 internal openings (Figures 1A, 3A, 3B and 4A) were identified, 29 (14.6%) of which were independent internal openings. Regarding radial sites, among the 198 internal openings, 44 (22.2%) were at the 1-3 o’clock position, 70 (35.4%) at the 4-6 o’clock position, 49 (24.7%) at the 7-9 o’clock position, and 35 (17.7%) at the 10-12 o’clock position (Table 1). The distance between the inner opening and the anal verge was 0-6.6 cm.
Figure 4.
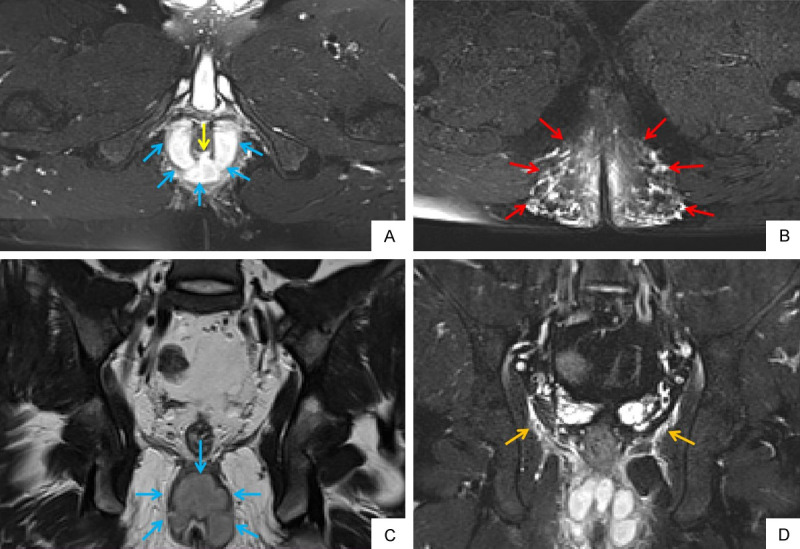
A 22-year old male patient with perianal fistula and abscess. Oblique axial FS T2WI (A) shows internal opening at 6 o’clock (yellow arrow). Oblique axial FS T2WI (A) and oblique coronal T2WI (C) show horseshoe abscess formation in intersphincter space (blue arrow). Oblique axial FS T2WI (B) shows extensive soft tissue edema in subcutaneous space of both buttocks (red arrow). Oblique coronal FS T2WI (D) shows inflammatory involvement of levator ani muscle (orange arrow). FS T2WI: fat suppression T2 weighted imaging.
A total of 50 (29.6%) primary fistulas in 22 (19.1%) patients showed a distal blind end. A total of 129 external openings were identified in 93 (80.9%) patients. Of these, 69 (74.2%) patients had one external opening (Figure 1B), and 24 (25.8%) had multiple external openings as follows: 16 (66.6%) patients, two; six (25.0%) patients, three; one (4.2%) patient, four; and one (4.2%) patient, six (Figure 3C and 3D). Among the 129 external openings, 41 (31.8%) had cutaneous openings in the left buttock, 40 (31.0%) in the right buttock, 21 (16.3%) in the middle line of both buttocks, 10 (7.7%) in the left perineum, 13 (10.1%) in the right perineum, 3 (2.3%) in the middle line of the perineum, and one (0.8%) in the middle-lower segment of the vagina (Table 1).
Various classifications of the perianal fistulas
Among the 169 primary fistulas, 150 (88.7%) were classified according to the Park’s classification; the intersphincteric (Figure 2D) and trans-sphincteric types (Figure 2A-C) were the most abundant (82; 54.7% and 58; 38.6%, respectively). The suprasphincteric type (Figure 2E) was relatively rare (8; 5.3%), and the extrasphincteric type was the least abundant (only one; 0.7%) (Figure 2F). The other fistula (0.7%) was of the diffuse intersphincteric with the trans-sphincteric type (Table 2). The fistulas that could not be classified using Park’s classification included 13 (68.4%), five (26.3%), and one (5.3%) submucosal, high deep intermuscular, and rectovaginal fistulas, respectively (Table 2).
Figure 2.
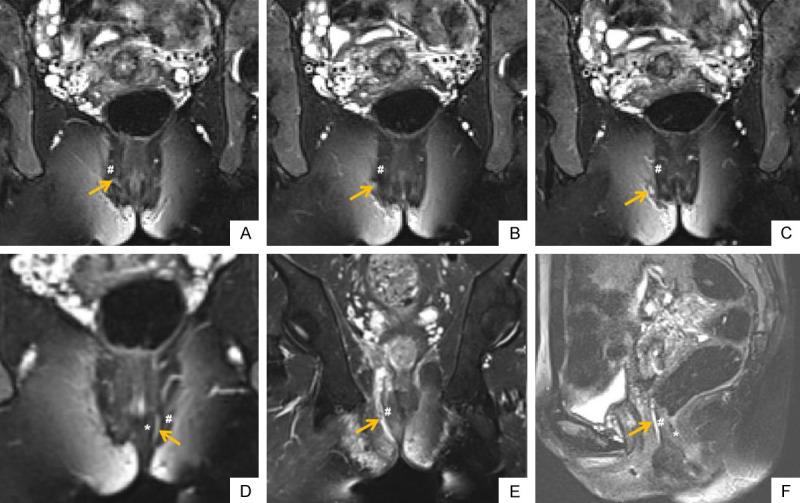
A cohort of perianal fistula cases, which was classified according to Park’s classification. Oblique coronal FS T2WIs (A-C) show the fistula passes through the external sphincter, which is a trans-sphincteric perianal fistula (orange arrow). Oblique coronal FS T2WI (D) shows a intersphincteric perianal fistula (orange arrow). Oblique coronal FS T2WI (E) shows a suprasphincteric perianal fistula (orange arrow). Sagittal FS T2WI (F) shows a extrasphincteric perianal fistula (orange arrow). * shows internal sphincter; # shows external sphincter. FS T2WI: fat suppression T2 weighted imaging.
Table 2.
Various classifications of perianal fistulas
| N (%) | |
|---|---|
| Primary fistulas | 169 |
| Park’s classification | 150 |
| intersphincteric | 82 (54.7%) |
| trans-sphincteric | 58 (38.6%) |
| suprasphincteric | 8 (5.3%) |
| eatrasphincteric | 1 (0.7%) |
| diffuse intersphincteric with trans-sphincteric | 1 (0.7%) |
| Other types except Park’s classification | 19 |
| submucosal | 13 (68.4%) |
| high deep intermuscular space | 5 (26.3%) |
| rectovaginal fistula | 1 (5.3%) |
| St. James’s grade | 149 |
| grade 1 | 52 (34.9%) |
| grade 2 | 30 (20.1%) |
| grade 3 | 20 (13.4%) |
| grade 4 | 38 (25.5%) |
| grade 5 | 9 (6.1%) |
| SPTF classification | 169 |
| simple | 92 (54.4%) |
| complex | 77 (45.6%) |
| Classification by position of internal opening | 169 |
| high site | 72 (42.6%) |
| low site | 97 (57.4%) |
Among the 169 primary fistulas, 149 were classified according to the St. James’s grade; 52 (34.9%), 30 (20.1%), 20 (13.4%), 38 (25.5%), and nine (6.1%) fistulas were of grades 1, 2, 3, 4, and 5, respectively (Table 2).
In total, 92 (54.4%) and 77 (45.6%) perianal fistulas were divided into simple and complex types, respectively, according to the SPTF classification (Table 2).
Further, 72 (42.6%) and 97 (57.4%) perianal fistulas were divided into high and low types, respectively, according to the position of the internal opening (Table 2).
Complications associated with the perianal fistulas
A total of 87 abscesses were detected in 60 (52.2%) patients (Figure 3L). Among them, 10 and 2 were horseshoe (Figure 4A, 4C) and Y-shaped abscesses, respectively. The maximum abscess diameter was 2.5 ± 1.7 cm. Abscess locations were as follows: 19 (21.8%) in the subcutaneous space of the buttocks, 15 (17.3%) in the intersphincteric space, 13 (14.9%) in the perianal region, seven (8.1%) in the high deep intermuscular space, seven (8.1%) in the subcutaneous space of the perineum, one (1.1%) in the perineal corpuscle, four (4.6%) in the levator ani region, five (5.7%) in the right ischioanal space, seven (8.1%) in the left ischioanal space, two (2.3%) in the right ischiorectal space, three (3.4%) in the submucosal space, two (2.3%) in the external sphincter region, and two (2.3%) in the internal sphincter region (Table 3).
Table 3.
Perianal fistula patients and associated conditions
| Patients(%) | N (%) | |
|---|---|---|
| Patients | 115 | |
| Male | 101 | |
| Female | 14 | |
| With or no abscesses | ||
| No abscesses | 55 (47.8%) | |
| With abscesses | 60 (52.2%) | 87 |
| subcutaneous space of buttocks | 19 (21.8%) | |
| intersphincteric | 15 (17.3%) | |
| Perianal | 13 (14.9%) | |
| high deep intermuscle space | 7 (8.1%) | |
| subcutaneous space of perineum | 7 (8.1%) | |
| perineal corpuscle | 1 (1.1%) | |
| levator ani region | 4 (4.6%) | |
| right ischioanal space | 5 (5.7%) | |
| left ischioanal space | 7 (8.1%) | |
| right ischiorectal space | 2 (2.3%) | |
| submucosal space | 3 (3.4%) | |
| external sphincter region | 2 (2.3%) | |
| internal sphincter region | 2 (2.3%) | |
| With or no secondary fistula | ||
| No secondary fistula | 92 (80.0%) | |
| With secondary fistula | 23 (20.0%) | 32 |
| 1 secondary fistula | 17 (73.9%) | 17 (53.1%) |
| 2 secondary fistulas | 4 (17.5%) | 8 (25.0%) |
| 3 secondary fistulas | 1 (4.3%) | 3 (9.4%) |
| 4 secondary fistulas | 1 (4.3%) | 4 (12.5%) |
| With or no levator ani muscle involvement | ||
| No levator ani muscle involvement | 103 (89.6%) | |
| With levator ani muscle involvement | 12 (10.4%) | |
| Involvement of bilateral levator ani muscle | 5 (41.7%) | |
| Involvement of right levator ani muscle | 6 (50.0%) | |
| Involvement of left levator ani muscle | 1 (8.3%) | |
| With or no extensive soft tissue edema | ||
| No extensive soft tissue edema | 91 (79.1%) | |
| With extensive soft tissue edema | 24 (20.9%) | |
| subcutaneous space of both buttocks | 8 (33.3%) | |
| subcutaneous space of left buttock | 1 (4.2%) | |
| subcutaneous space of right buttock | 2 (8.3%) | |
| subcutaneous space of both buttocks with subcutaneous space of perineum | 1 (4.2%) | |
| subcutaneous space of right buttock with subcutaneous space of perineum | 1 (4.2%) | |
| right ischioanal space with subcutaneous space of right buttock | 2 (8.3%) | |
| left ischioanal space with subcutaneous space of left buttock | 2 (8.3%) | |
| bilateral ischioanal space with bilateral subcutaneous space of buttocks | 2 (8.3%) | |
| subcutaneous space of perineum | 1 (4.2%) | |
| postanal space | 2 (8.3%) | |
| bilateral ischiorectal space | 1 (4.2%) | |
| bilateral subcutaneous space of buttocks with bilateral ischioanal space and right ischiorectal space | 1 (4.2%) |
A total of 32 secondary tracts were detected in 23 (20.0%) patients (25 primary tracts) (Figure 3I-K); 17 (73.9%) and six (26.1%) patients had one and multiple secondary tracts, respectively, of which four (17.5%) had two, one (4.3%) had three, and one (4.3%) had four secondary tracts (Table 3).
The involvement of the bilateral levator, right levator, and left levator ani muscles was detected in five (41.7%) (Figure 4D), six (50.0%), and one (8.3%) patient, respectively (Table 3).
Extensive soft tissue edema was observed in 24 (20.9%) patients. Edema distribution was as follows: the subcutaneous space of both buttocks in 8 (33.3%) patients (Figure 4B), subcutaneous space of the left buttock in one (4.2%) patient, subcutaneous space of the right buttock in two (8.3%) patients, subcutaneous space of both buttocks with the subcutaneous space of the perineum in one (4.2%) patient, subcutaneous space of the right buttock with the subcutaneous space of the perineum in one (4.2%) patient, right ischioanal space with the subcutaneous space of the right buttock in two (8.3%) patients, left ischioanal space with the subcutaneous space of the left buttock in two (8.3%) patients, bilateral ischioanal space with the bilateral subcutaneous space of the buttocks in two (8.3%) patients, subcutaneous space of the perineum in one (4.2%) patient, postanal space in one (4.2%) patient, bilateral ischiorectal space in two (8.3%) patients, and bilateral subcutaneous space of the buttocks with the bilateral ischioanal space and right ischiorectal space in one (4.2%) patient (Table 3).
Discussion
A total of 115 patients with 169 primary fistulas were identified in the present study, and an MRI evaluation of their perianal region revealed the presence and course of the fistulous track, classification, and associated complications. MRI was important in determining the fistulous track, internal and external openings, secondary tracks, abscess, Park’s and SPFT classifications, St. James’s grade, levator ani muscle involvement, and perianal soft tissue edema.
The etiology of the perianal fistula varies, and its etiological risk factors may include tuberculosis, pelvic infection, pelvic malignancy, delivery trauma, diverticulitis, and radiation therapy [14]. CD is an extremely common risk factor [20,21]. However, most cases of perianal fistula are considered idiopathic, and the most widely used theory for its pathogenesis is the cryptoglandular hypothesis. The anal glands distributed along the anal wall (mainly in the intersphincteric space) at the dentate line level are discharged into the anal canal through ducts opening into the crypts of Morgagni. Blockage of the drainage tube may lead to infections in the intersphincteric anal gland entering the intersphincteric space through the internal sphincter or penetrating the ischioanal space through the internal and external sphincters. This may subsequently lead to fistula tract or abscess formation in the corresponding location [5]. Patients with pelvic infection, pelvic tumors, leukopenia, and CD were excluded from our study at the time of enrollment. Therefore, all 115 patients were considered idiopathic. Therefore, perianal fistulas are possibly caused by anal gland infection and poor drainage.
Perianal fistula dominantly affects young and middle-aged men. In our study, 104 men and 14 women (ratio, 8.8:1.2) were included, and the median ages were 39 and 37, respectively. The above results are consistent with those of a previous study [22]. Some researchers believe that perianal fistulas tend to occur in young and middle-aged men because their anal glands are developing and cells are proliferating with exuberant secretions. Additionally, in men, the anal gland duct is curved [23], and siltation may easily occur because of excess secretions.
To reasonably manage the fistula and drain any abscess present, the course of the primary and secondary tracts and their relationship with the sphincter tissue should be determined via perianal fistula surgery [15]. The use of MRI for perianal fistulas was first reported in the early 1990s [24]. Previous study indicated that the surgeons’ awareness of the MRI results before fistula surgery could decrease the recurrence rate of anal fistulas [7]. The commonly used imaging techniques before introducing MRI to evaluate perianal fistulas included fistulography, EUS, and CT using rectal and intravenous contrast agents. However, some limitations exist in applying these technologies. Fistulography cannot show the anal sphincter and determine its relationship with the fistula [25]. Defining subtle fistulas and abscesses using CT is difficult because of its low soft tissue resolution [26]. Furthermore, the suprapapillary or secondary ducts cannot be detected using EUS owing to its limited FOV [10]. With technological advancements, MRI has proven to be crucial in evaluating perianal fistulas and is currently considered the gold standard method [27].
Most patients have a single primary fistula; however, some patients can have multiple fistulas. In our study, we found that 73 and 34 patients had single and multiple primary fistulas, respectively. Moreover, one patient had six primary fistulas, and the patient’s constitution and living habits were possibly the contributing factors for these fistulas. Once a perianal fistula is detected, the internal opening can be identified (the origin of the fistula can be located in the anus or lower rectum). The fistula drains into the lumen of the anal canal through its internal orifice. The internal orifice of most fistulas is located at the levels of the dentate line and posterior midline [28]. The anal region from the feet of the patient in the supine position is observed to determine the “anal clock”, which is a widely used term to describe the site and direction of the fistula tracts [12-14]. In our study, 70 (35.4%) internal openings were located at the 4-6 o’clock position, followed by 49 (29%) at the 7-9 o’clock position, implying that 64.4% of the internal openings were on the posterior wall of the anus-rectum. These results are consistent with those obtained from previous studies. Usually, primary fistula tracts have internal and external openings. The primary tract is called the sinus track when it blindly terminates in the subcutaneous fat tissue. In this study, 50 (29.6%) primary fistulas with blind ends were found. Meanwhile, more than one external cutaneous opening other than the perianal region, such as gluteal, perineal, labial, or retroscrotal openings, may exist. Only one such patient with six external cutaneous openings (two on the left buttock, two on the right buttock, and two on the right perineum) was identified.
Accurately identifying and classifying perianal fistulas based on MRI results are crucial to determine personalized treatment strategies and achieve treatment success. The course of the primary fistula tract is assessed according to its relationship with the internal and external anal sphincters. The four main types of fistulas using Park’s classification are determined based on their location: intersphincteric, trans-sphincteric, suprasphincteric, and extrasphincteric. We found that the intersphincteric (82; 54.7%) and trans-sphincteric types (58; 38.6%) are the most abundant. This result is consistent with that obtained from a previous study [28]. However, in our study, we found that 19 (11.2%) perianal fistulas were not suitable for Park’s classification. The most commonly found perianal fistula among the 19 was the submucosal perianal fistula possibly because the submucosa is relatively loose and inflammation tends to accumulate here. Additionally, five perianal fistulas were highly deep intermuscular fistulas, the infection of which spreads to the subpuborectal muscle, and the location was relatively deep. Despite its rarity, this type is highly likely to recur if drainage is incomplete; therefore, it must be separately presented and promptly reported to the clinician. A rectovaginal fistula, found in one patient in our study, was difficult to treat [29].
Compared with Park’s classification, St. James’s grade [13] includes relevant findings obtained from MRI and describes the characteristics of primary fistulous tract, secondary extensions, and associated abscesses. This classification uses reproducible anatomical markers that radiologists can easily understand and provide accurate information to surgeons. This classification system comprises five grades according to the anatomical structure obtained from MRI. In our study, among the 169 primary fistulas found, 149 tracts could be classified according to St. James’s grade. Furthermore, the quantitative relationships among the various types were as follows: grade 1 > grade 4 > grade 2 > grade 3 > grade 5. Grade 1 simply represents linear intersphincteric fistulas, which are the most abundant type of fistulas. This is consistent with the most intersphincteric fistula found using Park’s classification. Grade 5 was the least abundant, which includes the suprasphincteric and extrasphincteric types, similar to the findings obtained using the Park’s classification (both types account for 6% in total).
Additionally, we classified anal fistulas according to the SPTF classification [17], which is a classification system based on the complexity of perianal fistulas. An anal fistula identified as a complex fistula indicates an increase in the recurrence and fecal incontinence rate. Furthermore, we found that the proportion of complex perianal fistulas was 45.6%, which is higher than that found in other studies [30], possibly because our hospital is the northwest anal fistula center and the patients treated here are relatively serious.
Additionally, we classified perianal fistulas according to the location of the internal opening (high or low perianal fistulas). The difficulty of the operation increases with the higher position of the internal opening of the perianal fistula. In our study, 72 (42.6%) were high perianal fistulas, which is consistent with the results found in other studies [31].
If a fistulous tract exists, radiologists should not only describe the primary fistula but also evaluate its complications, such as secondary fistulas, abscesses, perianal soft tissue edema, and levator ani muscle involvement. The fistulas of the secondary tracks associated with a primary fistula extend into the ischioanal, gluteal, perineal, or supralevator regions. In this study, 23 patients (25 primary fistulas) had secondary fistulas, with 32 branches, of which six patients had multiple secondary fistulas. If the secondary fistula is not completely found and is not correctly handled, recurrence will easily occur. Therefore, to provide surgeons with accurate and comprehensive information, radiologists should carefully evaluate the perianal images and record them in detail.
Abscess and fistula in the perianal region represent the same disease process viewed at different times [28]. Any fluid collection or obvious widening of the fistula by > 10 mm in diameter is considered an abscess. In this study, 60 (52.5%) patients had 87 perianal abscesses; most of these were found in the subcutaneous space of the buttocks and intersphincter space. If the tissue in the sphincter space is loose and the abscess is easily blocked by the internal and external sphincters, the abscess is usually present in the intersphincter space. If the infection spreads to fatty areolar tissue, which has little inherent resistance to its progression, the abscess is usually present in the subcutaneous space of the buttocks. Horseshoe and Y-shaped abscesses wrap around the anal canal in U shape or Y shape respectively either posteriorly or anteriorly, respectively [12-14]. A total of 10 horseshoes and two Y-shaped abscesses were found in this study, which require careful surgery to ensure proper drainage.
The skeletal muscles above the puborectalis are fanned out to form the levator ani muscles, which separate the perineum from the pelvic cavity. Perianal inflammation spreads upward and can involve or even exceed the levator ani muscle and reach the ischiorectal fossa. The upward spread of inflammation or abscess, as indicated by the involvement of the levator ani muscles, should be clearly specified to the clinic. In this study, levator ani muscle involvement was observed in 12 of 115 patients. Meanwhile, the right levator ani muscle was relatively susceptible; further studies are warranted to identify the specific reasons for this.
Anal gland infection and poor drainage, which often cause surrounding soft tissue edema, are the most common causes of perianal fistula. We found 24 patients with extensive soft tissue edema in this study, and the edema was more likely to occur in the subcutaneous space of the buttocks. The wide range of the subcutaneous spaces and little inherent resistance of the fatty areolar tissue to edema progression are also possible causes of this finding.
This study has a few limitations. First, fistulas caused by inflammatory bowel diseases were excluded. This patient group will be covered by considering layered research in the near future. Second, the obtained results were not compared with surgical results, which will be a topic of focus in further studies.
In conclusion, MRI is a valuable, precise, and comprehensive tool that can not only show the primary fistula of perianal fistulas and internal and external openings but also be used for classification. Furthermore, it can clearly determine the associated complications, such as secondary fistulas, abscesses, levator ani muscle involvement, and perianal soft tissue edema.
Acknowledgements
The study was supported by the Health Commission Foundation of Shaanxi Province (No. 2022A02) and Xi’an Administration of Traditional Chinese Medicine Program (No. SZL202207).
Disclosure of conflict of interest
None.
References
- 1.Wlodarczyk M, Wlodarczyk J, Sobolewska-Wlodarczyk A, Trzcinski R, Dziki L, Fichna J. Current concepts in the pathogenesis of cryptoglandular perianal fistula. J Int Med Res. 2021;49:300060520986669. doi: 10.1177/0300060520986669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Jhaveri KS, Thipphavong S, Guo L, Harisinghani MG. MR imaging of perianal fistulas. Radiol Clin North Am. 2018;56:775–789. doi: 10.1016/j.rcl.2018.04.005. [DOI] [PubMed] [Google Scholar]
- 3.Iqbal N, Tozer PJ, Fletcher J, Lightner AL, Sackitey C, Corr A, Patel U, Ilangovan R, Lung P. Getting the most out of MRI in perianal fistula: update on surgical techniques and radiological features that define surgical options. Clin Radiol. 2021;76:784.e17–784.e25. doi: 10.1016/j.crad.2021.06.018. [DOI] [PubMed] [Google Scholar]
- 4.Halligan S, Stoker J. Imaging of fistula in ano. Radiology. 2006;239:18–33. doi: 10.1148/radiol.2391041043. [DOI] [PubMed] [Google Scholar]
- 5.Vo D, Phan C, Nguyen L, Le H, Nguyen T, Pham H. The role of magnetic resonance imaging in the preoperative evaluation of anal fistulas. Sci Rep. 2019;9:17947. doi: 10.1038/s41598-019-54441-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Beets-Tan RG, Beets GL, van der Hoop AG, Kessels AG, Vliegen RF, Baeten CG, van Engelshoven JM. Preoperative MR imaging of anal fistulas: does it really help the surgeon? Radiology. 2001;218:75–84. doi: 10.1148/radiology.218.1.r01dc0575. [DOI] [PubMed] [Google Scholar]
- 7.Buchanan G, Halligan S, Williams A, Cohen CR, Tarroni D, Phillips RK, Bartram CI. Effect of MRI on clinical outcome of recurrent fistula-in-ano. Lancet. 2002;360:1661–1662. doi: 10.1016/S0140-6736(02)11605-9. [DOI] [PubMed] [Google Scholar]
- 8.Liang C, Lu Y, Zhao B, Du Y, Wang C, Jiang W. Imaging of anal fistulas: comparison of computed tomographic fistulography and magnetic resonance imaging. Korean J Radiol. 2014;15:712–723. doi: 10.3348/kjr.2014.15.6.712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kumar N, Agarwal Y, Chawla AS, Jain R, Thukral BB. MRI of perianal fistulae: a pictorial kaleidoscope. Clin Radiol. 2015;70:1451–1461. doi: 10.1016/j.crad.2015.09.001. [DOI] [PubMed] [Google Scholar]
- 10.Amato A, Bottini C, De Nardi P, Giamundo P, Lauretta A, Realis Luc A, Piloni V. Evaluation and management of perianal abscess and anal fistula: SICCR position statement. Tech Coloproctol. 2020;24:127–143. doi: 10.1007/s10151-019-02144-1. [DOI] [PubMed] [Google Scholar]
- 11.Garg P, Singh P, Kaur B. Magnetic resonance imaging (MRI): operative findings correlation in 229 fistula-in-ano patients. World J Surg. 2017;41:1618–1624. doi: 10.1007/s00268-017-3886-x. [DOI] [PubMed] [Google Scholar]
- 12.Das GC, Chakrabartty DK. Best non-contrast magnetic resonance imaging sequence and role of intravenous contrast administration in evaluation of perianal fistula with surgical correlation. Abdom Radiol (NY) 2021;46:469–475. doi: 10.1007/s00261-020-02616-1. [DOI] [PubMed] [Google Scholar]
- 13.Morris J, Spencer JA, Ambrose NS. MR imaging classification of perianal fistulas and its implications for patient management. Radiographics. 2000;20:623–635. doi: 10.1148/radiographics.20.3.g00mc15623. discussion 635-627. [DOI] [PubMed] [Google Scholar]
- 14.de Miguel Criado J, del Salto LG, Rivas PF, del Hoyo LF, Velasco LG, de las Vacas MI, Marco Sanz AG, Paradela MM, Moreno EF. MR imaging evaluation of perianal fistulas: spectrum of imaging features. Radiographics. 2012;32:175–194. doi: 10.1148/rg.321115040. [DOI] [PubMed] [Google Scholar]
- 15.Konan A, Onur MR, Ozmen MN. The contribution of preoperative MRI to the surgical management of anal fistulas. Diagn Interv Radiol. 2018;24:321–327. doi: 10.5152/dir.2018.18340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Parks AG, Gordon PH, Hardcastle JD. A classification of fistula-in-ano. Br J Surg. 1976;63:1–12. doi: 10.1002/bjs.1800630102. [DOI] [PubMed] [Google Scholar]
- 17.Whiteford MH, Kilkenny J 3rd, Hyman N, Buie WD, Cohen J, Orsay C, Dunn G, Perry WB, Ellis CN, Rakinic J, Gregorcyk S, Shellito P, Nelson R, Tjandra JJ, Newstead G Standards Practice Task Force; American Society of Colon and Rectal Surgeons. Practice parameters for the treatment of perianal abscess and fistula-in-ano (revised) Dis Colon Rectum. 2005;48:1337–1342. doi: 10.1007/s10350-005-0055-3. [DOI] [PubMed] [Google Scholar]
- 18.Singh K, Singh N, Thukral C, Singh KP, Bhalla V. Magnetic resonance imaging (MRI) evaluation of perianal fistulae with surgical correlation. J Clin Diagn Res. 2014;8:RC01–4. doi: 10.7860/JCDR/2014/7328.4417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Torkzad MR, Ahlström H, Karlbom U. Comparison of different magnetic resonance imaging sequences for assessment of fistula-in-ano. World J Radiol. 2014;6:203–209. doi: 10.4329/wjr.v6.i5.203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Panes J, Garcia-Olmo D, Van Assche G, Colombel JF, Reinisch W, Baumgart DC, Dignass A, Nachury M, Ferrante M, Kazemi-Shirazi L, Grimaud JC, de la Portilla F, Goldin E, Richard MP, Diez MC, Tagarro I, Leselbaum A, Danese S ADMIRE CD Study Group Collaborators. Long-term efficacy and safety of stem cell therapy (Cx601) for complex perianal fistulas in patients with Crohn’s disease. Gastroenterology. 2018;154:1334–1342. e4. doi: 10.1053/j.gastro.2017.12.020. [DOI] [PubMed] [Google Scholar]
- 21.Adegbola SO, Dibley L, Sahnan K, Wade T, Verjee A, Sawyer R, Mannick S, McCluskey D, Bassett P, Yassin N, Warusavitarne J, Faiz O, Phillips R, Tozer PJ, Norton C, Hart AL. Development and initial psychometric validation of a patient-reported outcome measure for Crohn’s perianal fistula: the Crohn’s anal fistula quality of life (CAF-QoL) scale. Gut. 2021;70:1649–1656. doi: 10.1136/gutjnl-2019-320553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Balci S, Onur MR, Karaosmanoglu AD, Karcaaltincaba M, Akata D, Konan A, Ozmen MN. MRI evaluation of anal and perianal diseases. Diagn Interv Radiol. 2019;25:21–27. doi: 10.5152/dir.2018.17499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Erlichman DB, Kanmaniraja D, Kobi M, Chernyak V. MRI anatomy and pathology of the anal canal. J Magn Reson Imaging. 2019;50:1018–1032. doi: 10.1002/jmri.26776. [DOI] [PubMed] [Google Scholar]
- 24.Lunniss PJ, Armstrong P, Barker PG, Reznek RH, Phillips RK. Magnetic resonance imaging of anal fistulae. Lancet. 1992;340:394–396. doi: 10.1016/0140-6736(92)91472-k. [DOI] [PubMed] [Google Scholar]
- 25.Bhatt S, Jain BK, Singh VK. Multi detector computed tomography fistulography in patients of fistula-in-ano: an imaging collage. Pol J Radiol. 2017;82:516–523. doi: 10.12659/PJR.901523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Guniganti P, Lewis S, Rosen A, Connolly S, Raptis C, Mellnick V. Imaging of acute anorectal conditions with CT and MRI. Abdom Radiol (NY) 2017;42:403–422. doi: 10.1007/s00261-016-0982-6. [DOI] [PubMed] [Google Scholar]
- 27.Gecse KB, Bemelman W, Kamm MA, Stoker J, Khanna R, Ng SC, Panes J, van Assche G, Liu Z, Hart A, Levesque BG, D’Haens G World Gastroenterology Organization, International Organisation for Inflammatory Bowel Diseases IOIBD, European Society of Coloproctology and Robarts Clinical Trials; World Gastroenterology Organization International Organisation for Inflammatory Bowel Diseases IOIBD European Society of Coloproctology and Robarts Clinical Trials. A global consensus on the classification, diagnosis and multidisciplinary treatment of perianal fistulising Crohn’s disease. Gut. 2014;63:1381–1392. doi: 10.1136/gutjnl-2013-306709. [DOI] [PubMed] [Google Scholar]
- 28.Erden A. MRI of anal canal: normal anatomy, imaging protocol, and perianal fistulas: part 1. Abdom Radiol (NY) 2018;43:1334–1352. doi: 10.1007/s00261-017-1305-2. [DOI] [PubMed] [Google Scholar]
- 29.Cavusoglu M, Duran S, Sozmen Ciliz D, Tufan G, Hatipoglu Cetin HG, Ozsoy A, Sakman B. Added value of diffusion-weighted magnetic resonance imaging for the diagnosis of perianal fistula. Diagn Interv Imaging. 2017;98:401–408. doi: 10.1016/j.diii.2016.11.002. [DOI] [PubMed] [Google Scholar]
- 30.Clinical Guidelines Committee, Colorectal Surgeons Branch of Chinese Medical Doctor Association. Consensus of Chinese experts on the diagnosis and treatment of anal fistula (2020) Zhonghua Wei Chang Wai Ke Za Zhi. 2020;23:1123–1130. doi: 10.3760/cma.j.cn.441530-20200925-00537. [DOI] [PubMed] [Google Scholar]
- 31.Li J, Yang W, Huang Z, Mei Z, Yang D, Wu H, Wang Q. Clinical characteristics and risk factors for recurrence of anal fistula patients. Zhonghua Wei Chang Wai Ke Za Zhi. 2016;19:1370–1374. [PubMed] [Google Scholar]


