Abstract
Objective: To investigate the mechanism of Neferine in treating endometriosis fibrosis by TGF-β/ERK signaling pathway through a combination of network pharmacological analysis of Lotus embryos, in vivo animal experiments, and in vitro cell experiments. Methods: The active ingredients of the drug lotus embryos, the drug targets and the targets of endometriosis were determined from the TCMSP database, the Swiss Target Prediction database and GeneCard and Online Mendelian Inheritance in Man. The String database and Cytoscape 3.6.3 software were used to construct the network of common target protein interactions between drug and disease, as well as the target network. GO and KEGG enrichment analysis of the common targets was performed. We designed endometriosis mouse models with Neferine to investigate the therapeutic effect of Neferine on the fibrosis model of endometriosis and its mechanism of action. Different methods were used to evaluate the treated endometriotic lesion tissue and the untreated ectopic lesion tissue. The 12Z cells (human endometriosis immortalized cells) were cultured in vitro and treated with Neferine to detect cell viability and the effects of invasion and metastasis. Results: The results of GO function and KEGG enrichment analysis showed that the role pathways of lotus germ were TGF-β signaling pathway, ERK1/2 signaling pathway, IL-17 signaling pathway, TNF signaling pathway, AGE-RAGE signaling pathway, and PI3K-Akt signaling pathway. Neferine which is one of the effective active ingredients of lotus germ, significantly inhibited the expression of fibronectin, collagen I, connective tissue growth factor, and smooth muscle actin by activating the TGF-β/ERK pathway in vivo, which is required for the fibrosis process of endometriosis. Neferine also significantly inhibited the proliferation, invasion and metastasis ability of 12Z cells. Conclusion: Neferine inhibits the progression of endometriosis both in vitro and in vivo. Its mechanism of action may involve the regulation of the TGF-β/ERK signaling pathway, leading to the inhibition of fibrosis in endometriosis.
Keywords: Neferine, endometriosis, fibrosis, TGF-β/ERK signaling pathway
Introduction
Endometriosis (EMs) refers to the process of recurrent bleeding, injury, and repair of endometrial tissue outside the uterus with periodic changes in hormones, resulting in changes in the microenvironment, which leads to the activation of fibrosis-related factors, fibroplasia, and adhesions [1,2]. Excessive fibrosis can result in scarring, chronic pain, and changes in tissue function, leading to clinical symptoms such as dysmenorrhea, pelvic pain, and infertility.
In the course of the occurrence and development of EMs, more and more studies have proven that fibrosis is an inevitable pathological process of all EMs [3]. Vigano et al. realized in their study that ectopic endometrial matrix and glands only represent a small part of EMs lesions, while fibrosis in EMs lesions is a key part of the pathogenesis of the disease [4,5]. Donnez found in rectovaginal nodules, glandular epithelium without any surrounding stroma was observed deep within the fibrocystic tissue, and Muzii observed that in 40% of chocolate cysts of the ovary, neither endometrium epithelium nor the inner surface of the cyst could be recognized [6,7]. In addition, Somigliana also found that there was no endometrium in the pelvic adhesion lesions of heterotopia, but the fibrous lesions played a dominant role [7]. The main treatment method of western medicine for EMs is surgery or hormone therapy. While pseudopregnancy therapy and pseudomenopause therapy are commonly used to alleviate the conditions in endometriosis patients, they have a high relapse rate and come with many adverse effects. Additionally, these therapies are not conducive to pregnancy and may cause osteoporosis and menopausal syndromes, which limit their long-term use [3,4]. Therefore, finding a treatment scheme that has accurate and stable therapeutic effect, minimal side effects and is easily accepted by patients has become a focus and challenge of modern research. Despite an improved understanding of fibrosis in EMs, there is currently no safe and effective clinical treatment available for this serious condition. Therefore, the anti-fibrosis treatment of EMs is of great exploration value and clinical significance. In our study, we found, for the first time, that Neferine (Nef) can effectively inhibit the fibrosis progression of EMs and serve as a potential target therapeutic agent. Activity of the TGF-β1/MAPK/ERK pathway is transmitted into the nucleus and then regulates the expression of the collagen gene. ERK1/2 is mainly activated by phosphorylation of some factors such as hydrogen peroxide, ionizing radiation and growth factors. Activated p-ERK1/2 can promote the transcription and expression of some genes, participate in the regulation of cell growth, differentiation, proliferation, apoptosis and cell function. The ERK pathway plays an important role in promoting the fibrosis of multiple organs. Previous studies have also confirmed that inhibition of MAPKs is a potential target of anti-fibrosis therapy [8].
The role of fibrosis in the occurrence and development of EMs has received increasing attention, but the molecular mechanism underlying EMs fibrosis is still elusive.
Nef is a major dibenzylisoquinoline alkaloid extracted from lotus seeds. In recent years, Nef has been reported to have physiological activities such as anti-diabetes, anti-thrombosis, anti-oxidation, anti-inflammation, anti-forgetfulness, anti-fibrosis, and anti-hyperglycemia properties [8]. Although Nef has been studied for anti-fibrosis in the liver and pulmonary fibrosis in mice, there are currently no related reports on the treatment of EMs fibrosis. Therefore, this study aims to investigate the potential therapeutic effect of Nef on EMs fibrosis in mice by targeting the TGF-β/ERK pathway.
Materials and methods
Network pharmacology methods
The active ingredients of lotus embryos were obtained from the TCMSP (http://lsp.nwsuaf.edu.cn/tcmsp.php) database. The targets of the drugs were identified from the TCMSP database and the Swiss TargetPrediction database. The common targets between drugs and diseases were searched. The targets of the disease were identified from GeneCards and Online Mendelian Inheritance in Man databases. We used Cytoscape 3.8.0 to construct a drug-disease-targets Venn Diagram and the String database and Cytoscape 3.6.3 software to construct the network of common target protein interactions between drug and disease, as well as the target network. Finally, the GO and KEGG enrichment analyses of the common targets were performed using the DAVID database. The enrichment results were also displayed.
Experimental animals
In this experiment, C57BL/6 female mice aged 8 weeks and weighing approximately 18-20 g were selected. The mice were purchased from Beijing Vital River laboratory Animal Technology Co., Ltd. The use and treatment of the experimental animals were performed strictly in accordance with the animal ethics regulations of the Shanghai Hospital of Traditional Chinese Medicine.
Endometrial transplantation mouse model
A mouse model of EMs was established by intraperitoneal injection transplantation [9]. All mice were treated with 30 µg/kg estradiol for 3 days to unify the estrous cycle. The donor mice were dissected. The abdominal cavity was checked to locate the “Y” uterus, and the tissue was separated and ligated approximately 0.5 cm from the ovary. The donor mouse uterus was removed from the middle position. The adipose tissue was removed with forceps and opened longitudinally in a DMEM tissue culture medium containing serum, supplemented with 100 U/mL penicillin and 100 mg/mL streptomycin. Endometrial debris (approximately 3 mm in diameter; volume: 0.3 mL), including the myometrium, was collected using a 1.5 mL syringe. The recipient mice were injected using a 10 mL syringe needle. The entire process was performed under sterile conditions, and the injections in each mouse were completed within approximately 5 min.
Medication
After 21 days of modeling, the mice were randomly divided into 6 groups of 10 mice, including a Blank group (for normal saline), a Model group (for normal saline), a Neferine (B20598 Shanghai Source Biology Co., Ltd.) high dose group (30 mg/kg/d), a Neferine medium dose group (10 mg/kg/d), a Neferine low dose group (5 mg/kg/d), and a Dienogest group (2 mg/day, gavage, positive control).
Hematoxylin and eosin stain
To assess the morphology of eutopic and ectopic endometrium, H&E staining was performed on the removed tissues after sacrificing the mice. This procedure was also used to confirm the success of the mouse model of EMs. The eutopic and ectopic endometrium tissues were firstly washed in distilled water, then stained with hematoxylin dye for 10 min. After that, they were treated with 1% hydrochloric acid alcohol for differentiation with 0.6% ammonia. The tissue sections were placed in eosin dye solution for 3 min and successively dehydrated using 80%, 95%, and anhydrous ethanol.
Masson staining
In order to analyze the degree of endometrial fibrosis in different groups, Massonance staining was performed on the endometrial tissue. Endometrial tissue sections were washed in distilled water, mixed with ferroxyl solution at 1:1 ratio. The mixture was then added dropwise onto sections for 3 min, after which the sections were washed in distilled water, treated with 1% hydrochloride alcohol for 10 s, and then subjected to decolorization with 0.6% ammonia. Then, the sections were stained with Lichun red acid dye solution (C520005-0050 raw) for 10 min, phosphomolybdate acid solution for 3 min, and blue aniline solution, differentiated in glacial acetic acid solution for 1 min, and dehydrated with ethanol. At last, the sections were soaked in xylene solution, dried, and sealed with neutral gum.
Western-blotting
In order to analyze the differences in the expression levels of fibrosis-related proteins among different groups, Western blotting was performed on the endometrial tissue. Endometrial tissue was cut, homogenized and treated with RIPA lysate for 5 min. The tissue lysates were centrifuged and the supernatant was collected into 200 µL EP tubes. The protein samples were separated by 10% SDS-PAGE and then transferred onto a polyvinylidene difluoride (PVDF) membrane for 1 h at 300 mA. Membranes were blocked in 5% skim milk/TBST (Tris-buffered saline buffer containing 0.1% Tween-20) for 30 min and then treated with primary antibodies using TBST containing 3% skim milk. The antibodies were TGF-β (1:1,000), smooth muscle actin (α-SMA, 1:1,000), connective tissue growth factor (CTGF, 1:1,000), fibronectin (FN, 1:1,000), collagen I (COL-1, 1:1,000), ERK (1:1,000), and Phospho-p44/42 MAPK (Erk1/2; Thr202/Tyr204; 1:1,000). The washed membranes were probed with horseradish peroxidase-conjugated secondary antibody (1:1,000) in TBST containing 3% skimmed milk. Immune complexes were visualized using the ECL immunoblot system.
Immunohistochemistry
The expression of α-SMA was detected by anti-α-SMA. Briefly, after routine antigen retrieval, the fixed eutopic and ectopic endometrium tissues above were incubated with 3% H2O2 at room temperature for 10 min, washed with PBS, placed into 0.01 mmol/L citrate buffer (pH 6.0), and then subjected to microwave antigen retrieval for 20 min. Next, the eutopic and ectopic endometrium tissues were treated with primary anti-α-SMA antibody at 4°C overnight, and then probed with the HRP-conjugated goat anti-rabbit polyclonal secondary antibody at 37°C for 20 min. After that, the sections were subjected to staining using a DAB Horseradish Peroxidase Color Development Kit and counterstained with hematoxylin. Finally, the sections were mounted for further analysis. Images were captured using an inverted microscope at 40× and 100× magnifications (IX71; Olympus, Tokyo, Japan).
Immunofluorescence staining
The paraffin sections were dewaxed and dehydrated with alcohol at different concentrations for antigen repair. After washing thrice with 0.01 M PBST and blocking for 30 min, the sections were added with diluted primary antibodies and incubated overnight at 4°C in the dark. After 24 h, the sections were washed 5 times with PBS (5 min each time) and then incubated with Alexa Fluor 488-conjugated goat anti-mouse/rabbit IgG (H+L) secondary antibodies (Jackson) in a dark humidity chamber at 4°C for 2 h. After that, they were washed another three times with PBS. Finally, the sections were observed under a confocal microscope (TCS SP2; Leica Microsystems, Germany).
CCK8 proliferation assay
This experiment used 12Z cells (human endometrial immortalized cells) for in vitro experiments. The cell suspension (100 µL) was prepared in 96-well plates, which were pre-cultured in the incubator for 24 hours (at 37°C, 5% CO2). Then, 10 uL of low, medium, and high concentrations (15 µmol/L, 30 µmol/L, 60 µmol/L, respectively) were added to the culture plates and incubated for 12, 24 or 48 h. After that, 10 µL of CCK8 solution was added to each well for 2-h incubation, and the absorbance was measured by the microplate reader.
Wound healing assay
A marker pen was used to mark a line on the back of a 6-well plate, and about 5×105 cells were added to each well to allow adherence overnight. The next day, a scratch was made across the well using a pipette tip. Cells were washed three times with PBS and cultured in serum-free medium. Images were taken at 0, 6, 12, 24 hours to monitor cell migration and closure of the scratch.
Statistical methods
Statistical comparisons between two groups were processed by student t-test. One-way ANOVA followed by the Tukey test was used for data involving more than two groups. GraphPad Prism version 7.01 (GraphPad Software, San Diego, CA) statistical software was used to create analyses and graphics. A value of P<0.05 was considered statistically significant.
Results
Network pharmacology-based analysis
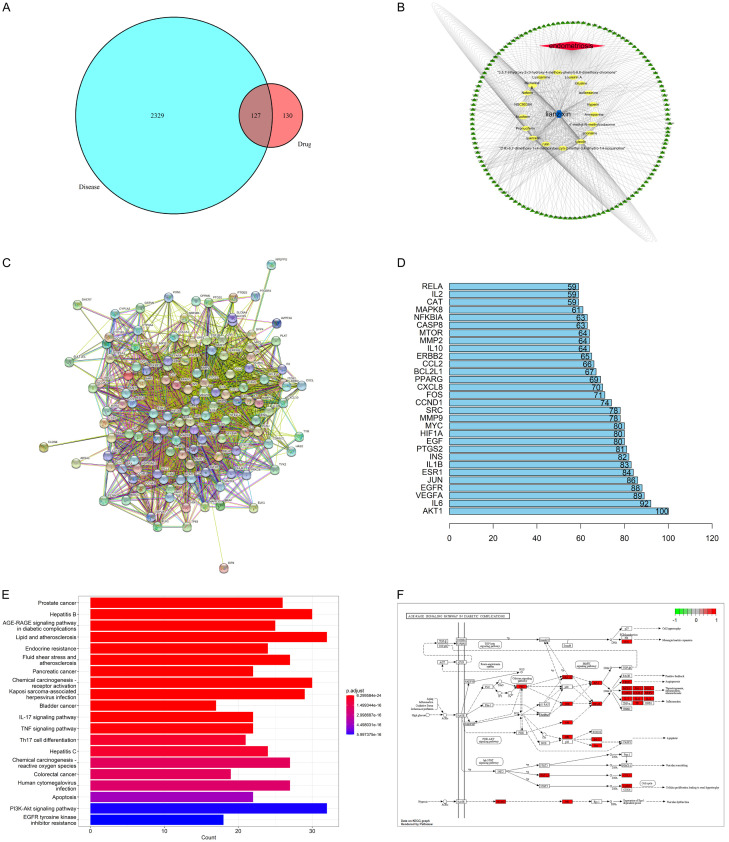
The TCMSP database was used to identify the active ingredient of lotus germ with DL greater than 0.18, resulting in the identification of 25 active components (Table 1), and 127 intersection targets were identified between lotus germ and EMs (Figure 1A). Eighteen active components with potential therapeutic effects on EMs were identified (Figure 1B). The following were the key action targets: AKT1, IL-6, VECFA, JUN, ESR1, IL1B, INS, MMP9, MAPK8, MMP2, IL-10, MTOR, IL-2, and others (Figure 1C and 1D). The following biological processes were primarily involved: RNA polymerase ll-specific, DNA-binding transcription factor binding, DNA-transcription factor binding, transcription coregulator binding, cytokine receptor-binding protein, serine/threonine/tyrosine kinase activity, cytokine activity, nuclear receptor activity, ligand-activated transcription factor activity, receptor-ligand activity, signaling receptor activator activity, transcription coactivator binding, heat shock protein binding, Hsp90 protein binding, a ubiquitin-like protein, ligase binding, nuclear receptor binding, heme binding, general transcription, initiation factor binding, tetrapyrrole binding, peptide binding, growth factor receptor binding, and others (Figure 1E). The lotus germ was predicted to primarily play a role in the treatment of EMs through the TGF-β signaling pathway, ERK1/2 signaling pathway, IL-17 signaling pathway, TNF signaling pathway, AGE-RAGE signaling pathway, and PI3K-Akt signaling pathway (Figure 1F).
Table 1.
Active Nelumbinis Plumula components
| Mol ID | Molecule Name | DL |
|---|---|---|
| MOL002419 | (R)-Norcoclaurine | 0.21 |
| MOL000415 | rutin | 0.68 |
| MOL004217 | Micheline B | 0.53 |
| MOL004368 | Hyperin | 0.77 |
| MOL005239 | Lysicamine | 0.41 |
| MOL000006 | luteolin | 0.25 |
| MOL000663 | lignoceric acid | 0.33 |
| MOL007206 | Armepavine | 0.29 |
| MOL007213 | Nuciferin | 0.4 |
| MOL008377 | Galuteolin | 0.79 |
| MOL009155 | 14-Methyl-24-methylene-dihydromangiferodiol | 0.8 |
| MOL009156 | 4’-methyl-N-methylcoclaurine | 0.26 |
| MOL009157 | NSC90384 | 0.8 |
| MOL009158 | Isolimocitrol-3-beta-D-glucoside | 0.81 |
| MOL009159 | 3,5,7-trihydroxy-2-(3-hydroxy-4-methoxy-phenyl)-6,8-dimethoxy-chromone | 0.44 |
| MOL009160 | Loureirin A | 0.19 |
| MOL009163 | Nelumboside | 0.62 |
| MOL009166 | anonaine | 0.47 |
| MOL009167 | (1R)-6,7-dimethoxy-1-(4-methoxybenzyl)-2-methyl-3,4-dihydro-1H-isoquinoline | 0.32 |
| MOL009168 | isoliensinine | 0.46 |
| MOL009169 | Liensinine | 0.46 |
| MOL009170 | lotusine | 0.28 |
| MOL009171 | Neferin | 0.42 |
| MOL009172 | Pronuciferin | 0.37 |
| MOL000098 | quercetin | 0.28 |
Figure 1.
A: Venn diagrams: Matching Targets of lotus germ and endometriosis; B: The compound-target network for lotus germ on endometriosis; C: The protein-protein interaction (PPI) network between lotus germ and critical targets of endometriosis (the purple nodes represent candidate active compounds; the green nodes represent potential protein targets; the edges represent the interactions between them; the nodes size are proportional to their degree); D: Hub gene: The genes from DEGs map Venn intersection to analyze the core genes of protein interactions; E: Analysis of the GO enrichment histogram for the treatment of endometriosis using lotus germ; F: KEGG pathway enrichment analysis showed that the top 20 significantly enriched pathways of lotus germ in the treatment of Endometriosis were connected with TGF-β signaling pathway, ERK1/2 signaling pathway, IL-17 signaling pathway, TNF signaling pathway, AGE-RAGE signaling pathway, and PI3K-Akt signaling pathway.
Establishment of the EMs mouse model
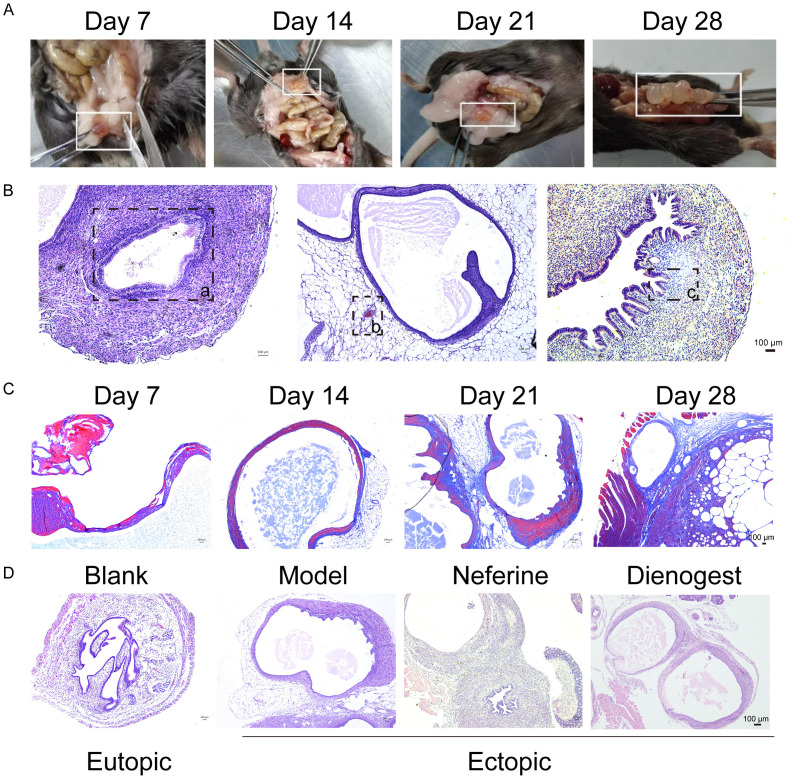
Ectopic lesions in the EMs mice were evaluated at different periods of modeling. On the 7th day of modeling, small ectopic lesions were observed in the abdominal cavity, showing bright red blood bubble or dark red sac. On the 14th day, the size of the ectopic lesion increased compared to that on the 7th day, and a single transparent or translucent vesicle was observed with a small number of tiny vessels. On the 21st day, there were one or more transparent vesicles, which were covered by connective tissue and had small vessels on the surface. On the 28th day of modeling, one or more transparent vesicles were still present. Clear fluid was detected in the capsule, and adhesions and blood vessels were observed around it (Figure 2A). Endometrial epithelial cells, glands, and stroma as well as angiogenesis and inflammatory cell infiltration, were observed in the capsule. The ectopic lesion exhibited a large and clear cyst cavity structure, appearing as multiple or single cysts, with an epithelial-cell layer, stromal cell layer, and muscle layer lining the cyst cavity wall. This indicated the successful establishment of the EMs mouse model (Figure 2B and 2D). Fibrotic staining was blue, whereas the rest was in light red. On day 7 of the model establishment, no obvious fibrosis staining was observed in the ectopic lesions, and only a few lesions were stained blue. Fibrotic staining and the cyst structure were clearly visible on day 14 after the model was established. On day 21, after the EMs model was established, the ectopic lesions showed significant fibrotic staining with a darker color and stable capsule structure. This indicated that the EMs mouse fibrosis model was successfully established, and the ectopic lesions were mature. The mouse model on day 28 showed substantial fibrotic staining, not considerably different from the mouse model on day 21 (Figure 2C).
Figure 2.
A: The growth of ectopic lesions at different time points; B: H&E staining was performed for the ectopic lesions: cystic structure (a), bleeding point (b), glandular structure (c); C: Masson staining results at different time points; D: H&E staining of ectopic lesions in each group. H&E: Hematoxylin and eosin.
Neferine suppresses extracellular matrix deposition to relieve EMs fibrosis through the TGF-β/ERK signaling pathway
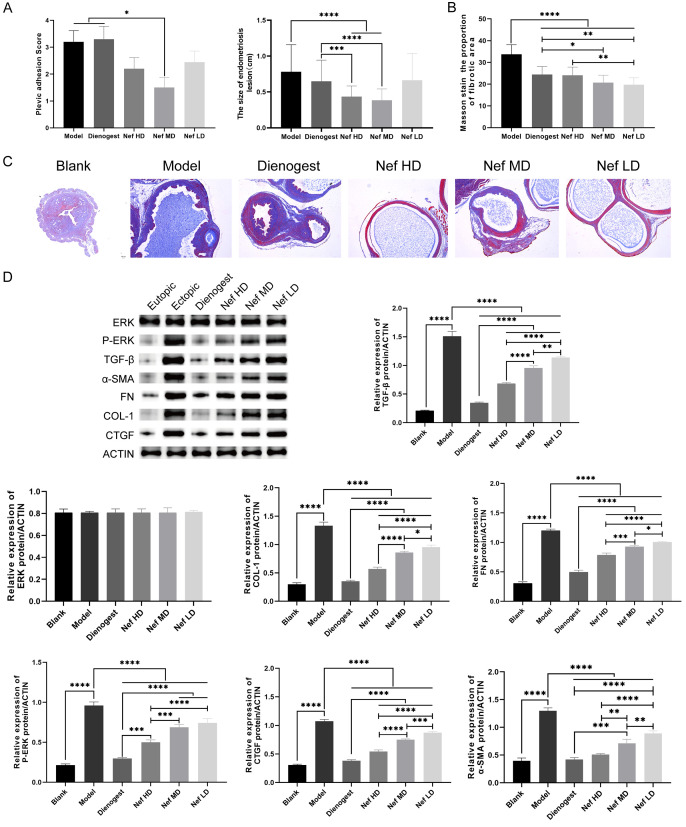
The degree of perifocal adhesion in each group was scored using the Haber adhesion scoring method. Compared with that in the model group, the adhesion score in the Nef medium dose group was significantly lower (P<0.05). There was no statistical difference in the degree of perifocal adhesion between the dienogest group and the Nef high- and low-dose group (P>0.05). The size of ectopic lesions in each group was determined. In this experiment, the greatest diameter of ectopic lesions was used as the reference. The results showed that compared with those in the model group, the ectopic lesions were significantly smaller in the Nef high-dose and Nef medium-dose groups (P<0.05). The difference in the sizes of ectopic lesions in the Nef low-dose group and dienogest group was not statistically significant (Figure 3A). The ratio of Masson-stained area in each group was calculated using the ImageJ software. The results showed that the staining area ratio of ectopic lesions in the dienogest group and the Nef low-, medium-, and high-dose groups decreased significantly when comparing with that in the model group (P<0.05; n=10, *P<0.05, **P<0.01, ***P<0.001; Figure 3B and 3C).
Figure 3.
A: Pelvic adhesion score and ectopic lesion size; B and C: The ratio of Masson fibrosis staining area; D: Western-blotting test the relative protein content of α-SMA, Col-1, CTGF, FN, TGF-β, ERK and phosphorylation of ERK1/2. The difference was statistically significant (P<0.05; n=10, *P<0.05, **P<0.01, ***P<0.001).
The results of Western blot showed that the protein levels of α-SMA, Col-1, CTGF, FN, TGF-β, and phosphorylation of ERK1/2 were significantly higher in the model group, dienogest group, and the 3 Nef groups than those in healthy mice (P<0.05). When comparing with the model group, the dienogest group and the 3 Nef groups showed lower protein levels of α-SMA, Col-1, CTGF, FN, TGF-β, and phosphorylation of ERK1/2, with the differences being statistically significant (P<0.05; *P<0.05, **P<0.01, ***P<0.001). However, the level of ERK did not statistically differ among the different groups (P>0.05; Figure 3D).
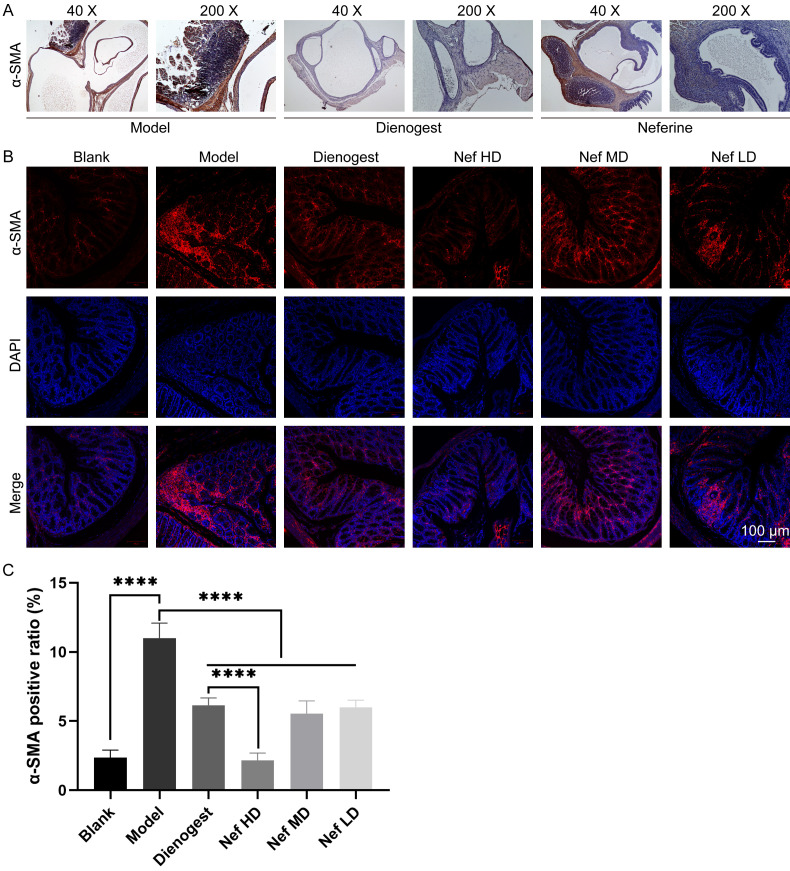
The immunohistochemistry results revealed that α-SMA expression was detected in the ectopic endometrium of mice. Specifically, the expression of α-SMA was found to be higher in the endometrial glands and stroma. The expression of α-SMA was low in the epithelial layer, stromal layer, and glandular epithelial layer, primarily in myometrial cells (Figure 4A).
Figure 4.
A: Immunohistochemistry analysis of α-SMA protein of uterus tissues obtained from different groups; B and C: Immunofluorescence staining results of α-SMA in each group. The difference was statistically significant (P<0.05; n=10, *P<0.05, **P<0.01, ***P<0.001).
The results of the immunofluorescence experiments showed that fluorescence intensity of α-SMA was higher in the ectopic endometrium of the model group than that in the treatment groups (Figure 4B and 4C). It is indicated that dienogest and Nef can inhibit α-SMA fluorescence, with high-dose Nef being the most effective (Figure 4B and 4C).
Nef inhibits the proliferation, invasion, and metastasis potential of 12Z cells
The results of the CCK8 cell proliferation assay showed that the survival rate of 12Z cells treated with 20 µmol/L Nef for 24 h was nearly 50%. The survival rate of 12Z cells treated with 40 µmol/L Nef for 12 h was nearly 50%. The survival rate of 12Z cells treated with 60 µmol/L Nef for more than 12 h was nearly 1%. This indicates that Nef can inhibit 12Z cell proliferation (Figure 5A). The results of the wound healing assay showed that Nef could inhibit the invasion and metastasis potential of 12Z cells (Figure 5B).
Figure 5.
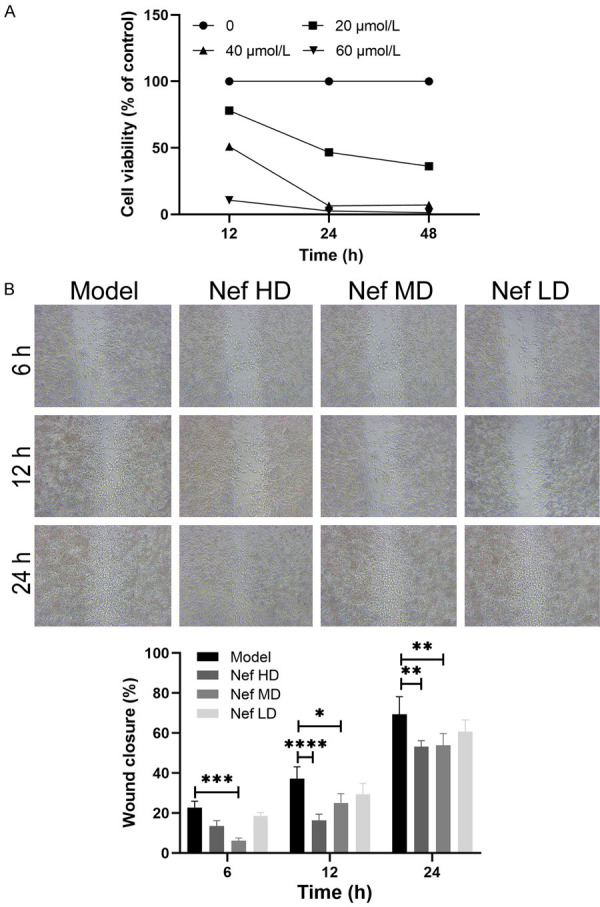
A: The results of CCK8 proliferation test of 12Z cells treated with Neferine; B: The wound healing assay results of 12Z cells treated with Neferine. The difference was statistically significant (*P<0.05; n=3, *P<0.05, **P<0.01, ***P<0.001).
Discussion
The TGF-β/ERK pathway is closely related to the expression of collagen genes, cell invasion, and epithelial-mesenchymal transformation during fibrosis. Various mediators contribute to the development of fibrosis, among which transforming growth factor-beta (TGF-β) is a crucial mediator that regulates the phenotype and function of fibroblasts during the induction of myofibroblast transformation. The lesion formation in EMs involves the activation of the TGF-β signaling pathway, which induces the transformation of fibroblasts to myofibroblasts or epithelial-mesenchymal transformation. This results in an increase in collagen expression and cell contractility, thereby leading to fibrosis. The development and progression of fibrosis are associated with persistent chronic inflammation, infections, ischemia, chemical or radioactive injuries, allergies, and other factors that cause chronic inflammation and repeated stimulation on organs, thus leading to fibrosis. The pathways related to fibrosis have been studied extensively. In addition to the TGF-β/Smad pathway, MAPK signaling is also recognized as an important contributor to fibrosis [10]. ERK1/2 is a part of the primary cascade of MAPK signaling. ERK1/2 activation is associated with TGF-β-induced epithelial-mesenchymal transformation and cell invasion. The inhibition of ERK1/2 signaling blocks epithelial-mesenchymal transformation in normal mouse mammary cells [11]. In addition, the ERK pathway is one of the primary signaling pathways of the TGF-β1 signal in the nucleus and regulates the expression of collagen genes [12]. ERK1/2 is stimulated by growth factors, hydrogen peroxide, and ionizing radiation. It is also activated after phosphorylation. Activated phosphorylation of ERK1/21/2 regulates gene transcription and expression, cell proliferation, and cell differentiation and participates in fibrosis in multiple organs.
TGF-β is also an inflammatory growth factor that regulates cell invasion, adhesion, vascular development, and other cell functions. The increase in TGF-β expression may further trigger retrograde menstruation and inflammation induced by endometrial cells, thus exacerbating EMs lesions. Evidence from recent studies have suggested that cell adhesion, invasion, and angiogenesis are inevitable pathological processes in endometrial lesions. Elevated TGF-β levels have been reported in the peritoneal fluid, serum, ectopic endometrium, and peritoneal tissue of patients with EMs [13]. The largest group of cells in the peritoneal cavity has also been reported to exhibit TGF-β overexpression, especially the TGF-β1 ligand, which is overexpressed in peritoneal fluid and participates in peritoneum-related pathology. In addition, the TGF-β1 protein level significantly increases the nerve fibers of peritoneal EMs lesions. These findings also suggest that TGF-β plays a significant role in the pathological course of EMs in women. The TGF-β subtypes associated with EMs are TGF-β1, TGF-β2, and TGF-β3, all three of which are expressed in endometrial tissue and localized to endometrial stromal cells, glandular cells and macrophages. They are regulated by the menstrual cycle and can be found in exfoliated endometrial tissue. Much of the evidence suggests that the TGF-β subtype plays an important signaling role in the human endometrium [13]. However, a review of different literature shows that the duration of higher TGF-β1 levels in EMs peritoneal fluid is not well understood [13], and it is unclear whether this is involved in the entire process of EMs lesion induction.
High estrogen expression is a unique feature of EMs, and the development of EMs lesions is closely regulated by estrogen. The MAPK/ERK signaling pathway has been implicated in ER activation. During MAPK/ERK signaling, ER binds into complexes with other molecules mediating this process, such as the PELP/MNAR-ER-Src complex and ER-Shc-IGFR complex. The two isoforms of the estrogen receptor, ERα and ERβ, exhibit different expression patterns in EMs tissue. The same was observed in the ectopic lesions of the EMs animal model by Chantalat et al. [14]. ERα expression was found to be higher in ovarian EMs lesion tissue compared to that in normal endometrium. However, contrasting results were also shown in other studies, indicating a complex mechanism of action of ERα in EMs progression [14]. This requires further investigation.
The results of this experiment showed that Nef could reduce the TGF-β protein expression levels. TGF-β is an important target in the pathogenesis of fibrosis. As an anti-fibrotic target, Nef could reduce the phosphorylation of ERK1/2 protein levels. It is speculated that the effect of Nef intervention on EMs fibrosis may be related to the TGF-β/ERK signaling pathway.
Extracellular matrix (ECM) proteins play an important role in EMs, as they do in other fibrosis diseases. Specifically, in the biological process of EMs-related fibrosis, excess ECM proteins, such as FN, COL-1, CTGF, and α-SMA, contribute to fibrosis-related factors.
Yuge et al. showed that in EMs, endometrial stromal cells (ESCs) strongly contract and promote scar formation. The enhancement of collagen contraction in EMs ectopic stromal cells (EcSCs) is related to myofibroblast differentiation and increased fibronectin expression [15]. COL-1 is an important component in EMs-related fibrosis [16,17] and may be a good target for EMs treatment. The high concentration of plasminogen activator inhibitor-1 in the intraperitoneal fluid of patients with EMs contributes to the development of peritoneal lesions [18]. Repeated damage to the site of the EMs lesion, followed by repair and healing, can eventually lead to fibrosis. This process can also cause damage to blood vessels, leading to platelet extravasation, aggregation, and activation. Richter [19] et al. observed that after treatment with activated platelets, the morphology of the EMs epithelial cell line changed from a round shape to a spindle shape, with an increase in the expression of both epithelial cell markers and stromal cell markers, as well as a significant increase in α-SMA expression. Myofibroblasts can be activated when α-SMA is newly expressed and incorporated into stress fiber-like bundles. Notably, TGF-β1 induced new α-SMA expression both in vitro and in vivo. Moreover, it could be cultured on rigid substrates such as the fibrotic scar, and activate different cells to differentiate into myofibroblasts [19]. Some scholars believe that the most critical function of mesothelial cells involved in EMs fibrosis is to undergo mesothelial to mesenchymal transition (MMT) and differentiate into fibroblasts [20]. Like epithelial cells, stromal cells can also undergo MMT upon induction by activated platelet-derived growth factor and TGF-β, enhancing invasion and migration potential, and calreticulin expression. Meanwhile, the expression of stromal markers is decreased, accompanied by increased cellular α-SMA expression, contractility, and collagen production. CTGF is known to promote angiogenesis, cell adhesion, and fibrosis and it also functions as a downstream mediator of TGF-β1, thereby involving in TGF-β1-induced fibrosis [21]. It is also implicated in fibrogenesis and the development of various organs. The results of this experiment showed that Nef reduced the expression of FN, COL-1, α-SMA and CTGF. Therefore, we speculate that Nef can potentially reduce the collagen contractility in EMs ectopic interstitial cells, inhibit the differentiation and activation of myofibroblasts, and alleviate the angiogenesis and ectopic cell adhesion in EMs ectopic lesions. Eventually, this could lead to a reduction in EMs fibrosis.
The increase in the cell migration capacity and invasiveness of EMs tissue via MAPK/ERK signaling is associated with TGF-β overexpression [22]. Cell migration is also supported by the downregulation of cytokines that maintain vascular integrity and the regulation of MMPs. Abnormalities in the MAPK pathway in EMs cells may have a genetic basis. The proliferation and enhanced activity of ectopic endometrial cells in patients with EMs is associated with the abnormal activation of extracellular signal-regulated kinases 1 and 2 (ERK1/2). The reduction of phosphorylated ERK protein expression can significantly reduce the proliferative potential of ectopic endometrial cells [23,24]. The results of this experiment showed that Nef was able to reduce the levels of phosphorylated ERK protein, which in turn lead to a decrease in the proliferation of ectopic endometrial cells. These findings suggest that Nef may hold a potential for the treatment of EMs. Cell experimental results showed that a certain concentration of methyllotus heart base could inhibit the proliferation, invasion, and migration of EMs cells. Epithelial-mesenchymal transformation is related to cell invasion and is one of the pathological mechanisms underlying EMs fibrosis. Whether Nef can reduce EM fibrosis by intervening in epithelial-mesenchymal transformation needs further investigation.
Nonetheless, due to limitations in time and funding, the TGF-β/ERK pathway was not manipulated to determine whether the effects of Nef were dependent on this pathway. Further experiments are needed to ensure the overall rigor of this research.
In conclusion, this study explored the mechanism of methylliensinine in the treatment of EMs fibrosis. The TGF-β/ERK signaling pathway is involved in multiple pathological processes of EMs fibrosis. Blocking TGF-β/ERK signaling pathway may slow down the fibrosis process of EMs by reducing the levels of fibrosis factors regulated by this pathway. This study also provides new treatment ideas and targeted drugs for EMs, and serves as a theoretical basis and reference for experimental research on EMs.
Disclosure of conflict of interest
None.
References
- 1.Nezhat C, Falik R, McKinney S, King LP. Pathophysiology and management of urinary tract endometriosis. Nat Rev Urol. 2017;14:359–372. doi: 10.1038/nrurol.2017.58. [DOI] [PubMed] [Google Scholar]
- 2.Greene AD, Lang SA, Kendziorski JA, Sroga-Rios JM, Herzog TJ, Burns KA. Endometriosis: where are we and where are we going? Reproduction. 2016;152:R63–78. doi: 10.1530/REP-16-0052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Viganò P, Ottolina J, Bartiromo L, Bonavina G, Schimberni M, Villanacci R, Candiani M. Cellular components contributing to fibrosis in endometriosis: a literature review. J Minim Invasive Gynecol. 2020;27:287–295. doi: 10.1016/j.jmig.2019.11.011. [DOI] [PubMed] [Google Scholar]
- 4.Guo SW. Cancer driver mutations in endometriosis: variations on the major theme of fibrogenesis. Reprod Med Biol. 2018;17:369–397. doi: 10.1002/rmb2.12221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Vigano P, Candiani M, Monno A, Giacomini E, Vercellini P, Somigliana E. Time to redefine endometriosis including its pro-fibrotic nature. Hum Reprod. 2018;33:347–352. doi: 10.1093/humrep/dex354. [DOI] [PubMed] [Google Scholar]
- 6.Donnez J, Nisolle M, Casanas-Roux F, Brion P, Da Costa Ferreira N. Stereometric evaluation of peritoneal endometriosis and endometriotic nodules of the rectovaginal septum. Hum Reprod. 1996;11:224–228. doi: 10.1093/oxfordjournals.humrep.a019024. [DOI] [PubMed] [Google Scholar]
- 7.Muzii L, Bianchi A, Bellati F, Cristi E, Pernice M, Zullo MA, Angioli R, Panici PB. Histologic analysis of endometriomas: what the surgeon needs to know. Fertil Steril. 2007;87:362–366. doi: 10.1016/j.fertnstert.2006.06.055. [DOI] [PubMed] [Google Scholar]
- 8.Somigliana E, Vigano P, Benaglia L, Busnelli A, Vercellini P, Fedele L. Adhesion prevention in endometriosis: a neglected critical challenge. J Minim Invasive Gynecol. 2012;19:415–421. doi: 10.1016/j.jmig.2012.03.004. [DOI] [PubMed] [Google Scholar]
- 9.Lake D, Corrêa SA, Müller J. Negative feedback regulation of the ERK1/2 MAPK pathway. Cell Mol Life Sci. 2016;73:4397–4413. doi: 10.1007/s00018-016-2297-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yuan M, Li D, An M, Li Q, Zhang L, Wang G. Rediscovering peritoneal macrophages in a murine endometriosis model. Hum Reprod. 2017;32:94–102. doi: 10.1093/humrep/dew274. [DOI] [PubMed] [Google Scholar]
- 11.Sritananuwat P, Sueangoen N, Thummarati P, Islam K, Suthiphongchai T. Blocking ERK1/2 signaling impairs TGF-β1 tumor promoting function but enhances its tumor suppressing role in intrahepatic cholangiocarcinoma cells. Cancer Cell Int. 2017;17:85. doi: 10.1186/s12935-017-0454-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Liu X, Song X, Lu J, Chen X, Liang E, Liu X, Zhang M, Zhang Y, Du Z, Zhao Y. Neferine inhibits proliferation and collagen synthesis induced by high glucose in cardiac fibroblasts and reduces cardiac fibrosis in diabetic mice. Oncotarget. 2016;7:61703–61715. doi: 10.18632/oncotarget.11225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chantalat E, Valera MC, Vaysse C, Noirrit E, Rusidze M, Weyl A, Vergriete K, Buscail E, Lluel P, Fontaine C, Arnal JF, Lenfant F. Estrogen receptors and endometriosis. Int J Mol Sci. 2020;21:2815. doi: 10.3390/ijms21082815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Young VJ, Ahmad SF, Duncan WC, Horne AW. The role of TGF-β in the pathophysiology of peritoneal endometriosis. Hum Reprod Update. 2017;23:548–559. doi: 10.1093/humupd/dmx016. [DOI] [PubMed] [Google Scholar]
- 15.Yuge A, Nasu K, Matsumoto H, Nishida M, Narahara H. Collagen gel contractility is enhanced in human endometriotic stromal cells: a possible mechanism underlying the pathogenesis of endometriosis-associated fibrosis. Hum Reprod. 2007;22:938–944. doi: 10.1093/humrep/del485. [DOI] [PubMed] [Google Scholar]
- 16.Matsuzaki S, Canis M, Darcha C, Dechelotte P, Pouly JL, Bruhat MA. Fibrogenesis in peritoneal endometriosis. A semi-quantitative analysis of type-I collagen. Gynecol Obstet Invest. 1999;47:197–199. doi: 10.1159/000010094. [DOI] [PubMed] [Google Scholar]
- 17.Mulayim N, Savlu A, Guzeloglu-Kayisli O, Kayisli UA, Arici A. Regulation of endometrial stromal cell matrix metalloproteinase activity and invasiveness by interleukin-8. Fertil Steril. 2004;81(Suppl 1):904–911. doi: 10.1016/j.fertnstert.2003.11.015. [DOI] [PubMed] [Google Scholar]
- 18.Bruse C, Bergqvist A, Carlström K, Fianu-Jonasson A, Lecander I, Astedt B. Fibrinolytic factors in endometriotic tissue, endometrium, peritoneal fluid, and plasma from women with endometriosis and in endometrium and peritoneal fluid from healthy women. Fertil Steril. 1998;70:821–826. doi: 10.1016/s0015-0282(98)00285-4. [DOI] [PubMed] [Google Scholar]
- 19.Richter K, Konzack A, Pihlajaniemi T, Heljasvaara R, Kietzmann T. Redox-fibrosis: impact of TGFβ1 on ROS generators, mediators and functional consequences. Redox Biol. 2015;6:344–352. doi: 10.1016/j.redox.2015.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Xu TH. Research progress on the mechanism of fibrosis in endometriosis. Chin J Difficult Complicated Cases. 2021;20:636–639. 644. [Google Scholar]
- 21.Li X, Liu H, Sun L, Zhou X, Yuan X, Chen Y, Liu F, Liu Y, Xiao L. MicroRNA-302c modulates peritoneal dialysis-associated fibrosis by targeting connective tissue growth factor. J Cell Mol Med. 2019;23:2372–2383. doi: 10.1111/jcmm.14029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rabajdová M, Špaková I, Klepcová Z, Smolko L, Abrahamovská M, Urdzík P, Mareková M. Zinc(II) niflumato complex effects on MMP activity and gene expression in human endometrial cell lines. Sci Rep. 2021;11:19086. doi: 10.1038/s41598-021-98512-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yotova IY, Quan P, Leditznig N, Beer U, Wenzl R, Tschugguel W. Abnormal activation of Ras/Raf/MAPK and RhoA/ROCKII signalling pathways in eutopic endometrial stromal cells of patients with endometriosis. Hum Reprod. 2011;26:885–897. doi: 10.1093/humrep/der010. [DOI] [PubMed] [Google Scholar]
- 24.Ngô C, Nicco C, Leconte M, Chéreau C, Arkwright S, Vacher-Lavenu MC, Weill B, Chapron C, Batteux F. Protein kinase inhibitors can control the progression of endometriosis in vitro and in vivo. J Pathol. 2010;222:148–157. doi: 10.1002/path.2756. [DOI] [PubMed] [Google Scholar]