Abstract
Objective: To observe the anti-aging effects of moxibustion on age-related alterations in middle-aged mice. Methods: Thirty, 9-month-old, male ICR mice were randomly divided into the moxibustion and control groups (N = 15). Mice in the moxibustion group were given mild moxibustion at the Guanyuan acupoint for 20 minutes every other day. After 30 treatments, neurobehavior tests, lifespan, gut microbiota composition and splenic gene expression were observed in the mice. Results: Moxibustion improved the locomotor activity as well as motor function, activated the SIRT1-PPARα signaling pathway, ameliorated age-related alterations in gut microbiota, and affected the expression of genes related to energy metabolism in spleen. Conclusion: Moxibustion ameliorated age-related alterations in neurobehavior and gut microbiota in middle-aged mice.
Keywords: Aging, moxibustion, behavior, gut microbiota, lifespan, transcriptome
Introduction
Aging is major challenge globally. According to the World Population Prospects 2022, it is estimated that by 2050, the percentage of people aged 65 years and above will increase from 10% in 2022 to 16%, and the average lifespan expectancy will further be prolonged to 77.2 years. Aging is closely associated with the development of various diseases, such as neurodegenerative diseases, metabolic diseases, cardiovascular diseases, cancer, etc., which leads to an increased mortality [1]. Therefore, the aim of anti-aging therapy is not only to improve life expectancy, but also to enhance the life quality in the elderly population.
Aging is accompanied by degenerative changes in the function of human tissues and organs, among which the brain is one of the most susceptible organs. Clinically, the main manifestation of brain aging is cognitive impairment, followed by motor dysfunction. Studies have found that the function of the hippocampus decreases with age, attributing to memory impairment [2]. In addition, the incidence of Parkinson’s disease (PD), which is characterized by movement disorders is also increased with age [3].
The pathogeny of aging is very complex. Previous studies have indicated that the main mechanisms involved are genomic instability, telomere shortening, epigenetic changes, abnormal nutrient sensing, mitochondrial defects, and dysregulated intercellular signaling [4]. Importantly, the gut microbiota has been recognized as a new anti-aging target. Numerous studies have confirmed age-related changes in the intestinal microecology. The diversity and composition of gut microbiota in the elderly differ discernibly from those in the young adults. The alterations involve a reduced diversity, a decreased abundance in beneficial bacteria as well as an increased abundance in harmful bacteria [5], which may be caused by the functional abnormity in the gastrointestinal tract, modifications in lifestyle and nutritional behavior, as well as deterioration of immune system function [5,6]. Moreover, research has highlighted that intestinal ecological imbalance may resulted in age-related diseases including Alzheimer’s disease (AD) and PD [7,8]. Therefore, preserving the health of intestinal flora is pivotal for the elderly people. Accordingly, probiotics have been demonstrated to improve health and prolong life by regulating the intestinal microbiome [9]. Significantly, more studies have focused on the connection between gut microbiota and nervous system function. Since the brain-gut-microbiome (BGM) axis involves the interaction between gut microbiome and brain, gut microbes will influence the function of the brain. In support with this, the transplantation of gut microbiota from a young mouse to an older mouse enhances the cognition of the latter [10].
Currently, the commonly accepted anti-aging measures include dietary intervention (DR), exercise, and drug therapy; however, most of them have limitations. Consequently, it is of great necessity to explore more safe and effective therapeutic approaches. Moxibustion therapy has been used in China for thousands of years as an indispensable external treatment in traditional Chinese medicine. Moxibustion therapy is widely accepted owing to its advantages of low toxicity and few side effects, a woundless process, and being easy to operate. Moxibustion practice involves burning moxa sticks which are made of Artemisia argyi and placing them above or on the skin of the acupoint or diseased area, to produce a warming effect. Moxibustion therapy has been applied to maintain health, prevent aging, and prolong lifespan since ancient times. Intriguingly, modern studies have also confirmed effect of moxibustion in maintaining health and delaying deterioration in older people [11]. In addition, animal experiments have demonstrated that the anti-aging mechanism of moxibustion includes the regulation of the inflammatory response [12], the enhancement of antioxidant capacity [13], the scavenging of free radicals [14], and the improvement of neurosecretion function [15], as well as telomerase activity [16]. However, few studies have explored the influence of moxibustion on lifespan, or explained the anti-aging effect of moxibustion in relation to gut microbiota. Herein, we explored the influence of moxibustion treatment at Guanyuan (RN4) acupoint on the neurobehavior and lifespan of middle-aged male mice, as well as evaluated the alteration in gut microbiota and splenic gene expression by RNA sequencing (RNA-seq). Our study will provide valuable insights into how moxibustion influences age-related alterations and help understand the mechanism of moxibustion on anti-aging.
Materials and methods
Animals and experimental design
Thirty, 9-month-old (weighing 44 ± 3 g) SPF male ICR (Institute of Cancer Research) mice were purchased from the Beijing Vital River Laboratory Animal Technology Co., Ltd. (Beijing, China). All mice were given unlimited food and water and were kept in a temperature-controlled environment (22 ± 2°C), with humidity of 55 ± 5% and 12 h light/dark cycle. After 7 days of adaptation, the mice were randomly divided into the moxibustion or the control groups (n = 15/group). Animal handling was performed according to the National Institutes of Health Guide for the Care and Use of Laboratory Animals and approved by the Animal Ethics Committee of Beijing University of Chinese Medicine (BUCM-4-2016022401-1004).
Moxibustion intervention
In this study, moxibustion treatment on RN4, which is in the lower abdomen and 10 mm below the navel of mice, was performed in the moxibustion group. The moxa sticks (0.5 cm × 20 cm) were homemade with 3-year-old pure moxa (Nanyang Hanyi Moxa Co., Ltd., China). Plastic fixators with a hole on the bottom and a groove at the rear to hold the baffle were utilized to fasten the mice.
Mice in the moxibustion group were put in the fixator with their lower abdomen exposed to the hole, and the hair near the RN4 site was shaved. The fixators were then put on an iron-wire shelf, and indirect moxibustion intervention was performed 1 cm above the RN4 for 20 min. Mice in the control group went through the same procedure, except no moxibustion treatment. Mice in the moxibustion group and control groups were manipulated in room 1 and room 2, respectively (Figure 1). The intervention was performed every other day for 30 applications. At day 60, behavioral tests were conducted on all mice to observe the changes in cognitive and motor function.
Figure 1.

Intervening measures. The red dots represent Guanyuan acupoint.
Passive avoidance test
The passive avoidance test, which was modified based on the previous study, was performed to examine the learning and memory capability of mice [17]. Basically, the testing apparatus comprise a bright and a dark compartment joint by a guillotine door. The test includes a training trial and a testing trial. The training trial was performed on the first day. Specifically, the mice were put in the apparatus and were allowed to become accustomed the environment for 3 minutes, during which no power was suppled. The mice were then taken out, and the dark room was electrified (0.5 mA). Next, an operator placed the mice in the bright compartment of the apparatus with the guillotine door open, such that the mice would get an electric shock to their feet when entering the dark compartment. The number of electric shocks received by the mice within 5 minutes as well as the initial latency of mice entering the darkroom were recorded. If the mice did not enter the darkroom within 5 minutes, the latency was recorded as 300 s. On the second day, the testing trial was performed and the latency as well as the number of times entering the darkroom compartment but without electric shock were recorded.
Open field test
The open field test (OFT), which evaluates the locomotor activity and exploration ability of the mice was carried out following the procedure reported previously [18]. Briefly, the mice were first put in an open field box (L × W × H: 54 cm × 50 cm × 37 cm) and allowed to habituate for 3 minutes. Then, the mice were placed in the center of the box, and the total traveling steps were recorded along with vertical locomotor activity within 5 minutes. After each test, the apparatus was cleaned with 75% alcohol.
Rota-Rod test
The Rota-Rod test was conducted to evaluate the motor function of the mice [19]. Briefly, in the test, mice were placed on a rota-rod with a rotation speed of 40 rpm and were tested every 10 minutes for 3 times. The test time is 5 minutes. The latency to falling was recorded, and if the mice stayed on the rod for more than 300 s, the latency was recorded as 300 s. The test with the longest latency was employed in the final results.
Sample collection
At the end of the experiments, mice were anesthetized through subperitoneal injection of 3% pentobarbital sodium and then sacrificed. The liver tissues were dissected for immunohistochemical staining, and the spleen specimens were collected for Digital Gene Expression Profiling analysis. Additionally, the fecal samples were also collected for gut microbiota analysis. The spleen and feces samples were snap-frozen and stored for further RNA extraction. The remaining mice were kept without any intervention until death and the lifespans were recorded.
Immunohistochemistry assay (IHC)
The liver tissue samples were fixed in 4% paraformaldehyde solution (24 h, 4°C), paraffin embedded, and sliced into 4 µm sections. Subsequently, the liver sections were deparaffinized, hydrated, and incubated with primary antibodies against SIRT1 (ab110304; Abcam, USA), PPARα (ab61182; Abcam, USA), PPARγ (ab45036; Abcam, USA) and P53 (ab32389; Abcam, USA) at 4°C overnight. After rinsing, the slides were incubated with a corresponding secondary antibody for 20 min. Diaminobenzidine (DAB) solution (DAKO, Denmark) was used to visualize protein expression, and the nucleus was counter stained with hematoxylin solution. The images were observed under the BX51 microscope (Olympus, USA), and the integral optical density (IOD) of images was quantified by Image-pro Plus software.
Digital gene expression profiling analysis
The Digital Gene Expression Profiling (DGE) analysis was carried out as reported previously [20]. Briefly, Trizol reagent (Invitrogen, Canada) was used for total RNA extraction from 6 samples, and the cDNA libraries were constructed using NEBNext@ UltraTM RNA Library Preparation Kit (Illumina, USA). Subsequently, the library was sequenced on the Illumina HiSeq2500 platform. The reads from each sample were strictly filtered to obtain clean reads of high-quality, which were then aligned to the reference sequence using TopHat2.
The expression level of genes was calculated by counting the sequencing reads mapped on each gene using FPKM method (fragments per kilo bases per million mapped reads) [21]. Htseq (version 0.6.1) software was used to analyze the gene expression level of each sample [22]. The differential expression analysis was performed using DESeq [23]. Benjamini-Hochberg procedure was used to adjust P values for reducing false-positive results [24]. The differentially expressed genes (DEGs) between the two groups were determined based on the criterion of an adjusted P-value < 0.05 and |log2(fold change)| > 1.
Gene Ontology (GO) enrichment analysis was carried out using GOseq software [25] to reveal the biological function of these DEGs by mapping these genes to the corresponding GO terms. Pathway analysis of the DEGs was conducted by the KOBAS software on Kyoto Encyclopedia of Genes and Genomes (KEGG) database, to systematically access gene function and genome information. KEGG pathway analysis identifies the significantly enriched pathways compared with the whole genome. GO terms and pathways with an adjusted P-value < 0.05 were deemed significantly enriched.
Gut microbiota analysis
Total genomic DNA from each fecal sample was extracted by E.Z.N.A.® stool DNA Kit (Omega Bio-tek, Norcross, GA, USA). The variable V3-V4 regions of 16S rRNA were amplified using the primers: 338F (5’-ACTCCTACGGGGAGGAGCAG-3’) and 806R (5’-GGACTACHVGGGTWTCTAAT-3’). The PCR products were purified using AxyPrep DNA Gel Extraction Kit (Axygen, CA, USA), which were then used to construct the library by using TruSeqTM DNA Sample Prepration Kit (Illumina, San Diego, USA). The PCR amplicon products were paired end sequenced (2 × 300 bp) via Illumina MiSeq platform (Illumina, San Diego, USA).
The sequencing reads were spliced and filtered to optimize the quality for subsequent analysis. The Operational Taxonomic Units (OTU) analysis annotates OTU for species taxonomy and computes the abundance of each OTU annotation result. By clustering the samples’ sequencing results, OTU analysis divided the sequences into many groups according to their similarities. The taxonomic analysis of OTU with 97% sequence similarity was conducted using Uparse software (version 7.0.1090) and RDP classifier Bayesian algorithm (version 2.11) with the confidence threshold set at 0.7.
Based on results from OTU analysis, further comparison of microbial differences was performed. The Chao1 and Shannon indices were calculated to detect the alpha diversity, which reflected the richness and diversity of microbial community. The Chao1 index was analyzed using Mann-Whitney U test, while the Shannon index was analyzed by unpaired Student’s t-test. We further used principal co-ordinates analysis (PCoA) and non-metric multidimensional scaling analysis (NMDS) to detect the beta diversity which explored the similarity in community composition between the two groups, according to the Bray-Curtis dissimilarity and ANOSIM analysis and displayed by R software (version 3.3.1). The linear discriminant analysis effect size (LEfSe) was applied to identify biomarkers in each group using Mann-Whitney U test. In LEfSe analysis, linear discriminant analysis (LDA) determines the effect of each species and the default threshold value was set at 3.0. The bacterial community and the relative abundance of OTUs in each sample were assessed from phylum to genera, and the differences in abundance between the two groups were displayed by R software, using Mann-Whitney U test.
Statistical analysis
The data were shown as the means ± standard deviation (SD) or medians and interquartile ranges (IQRs). Student’s unpaired t-test was applied to analyze the data with normal distribution; while the Mann-Whitney U test was used for other data analysis. SPSS 20.0 was used for statistical analysis, and a P-value < 0.05 indicated a statistical significance. Kaplan Meier method was used for survival analysis and the comparison between groups was done with Breslow test.
Results
Moxibustion improved locomotor activity and motor function
Before and after the experiment, no significant difference in body weight was found between the two groups (P > 0.05; Figure 2A). In terms of lifespan, although moxibustion intervention did not extend the survival time of the mice (P > 0.05; Figure 2B), the median survival was increased by 16.5%, indicating that moxibustion intervention might play a beneficial role in longevity. In the open field test, vertical exploration of mice in moxibustion group increased remarkably (P < 0.05; Figure 2D), although the difference in traveling distance between the two groups was not statistically significant (P > 0.05; Figure 2C), suggesting that moxibustion intervention enhanced exploration ability. Moreover, the latency in the moxibustion group was significantly longer compared to the control group in Rota-Rod test (P < 0.05; Figure 2E), suggesting a better motor function in moxibustion-treated mice, although the passive avoidance test showed similar results between these two groups (P > 0.05; Supplementary Table 1).
Figure 2.
Moxibustion improved locomotor activity and motor function of mice. A. Effects of moxibustion on Body Weight (n = 15/group). B. The survival rate displayed by month (n = 11/group). C, D. The total horizontal traveling distance and frequency of vertical exploration in OFT (n = 15/group). E. The latency time in Rota-Rod test (n = 15/group). Con: the control group. Mox: the moxibustion group. *P < 0.05.
Moxibustion activated SIRT1-related pathway
The Immunohistochemistry (IHC) assay revealed that the expression levels of SIRT1 and PPARα in the moxibustion group were significantly increased (P < 0.05; Figure 3A, 3B), but there was no significant difference in P53 (P > 0.05; Figure 3C), suggesting that moxibustion activated SIRT1-PPARα signalling pathway.
Figure 3.

Moxibustion promoted the protein expression of SIRT1 and PPARα in liver tissues (n = 4/group). A. The SIRT1 relative expression tested via IHC assay. B. The PPARα relative expression tested via IHC assay. C. The P53 relative expression tested via IHC assay. Con: the control group. Mox: the moxibustion group. *P < 0.05.
Moxibustion modulated gene expression in the spleen
Approximately 146 million raw reads were obtained from the DGE analysis, of which 96.45% were clean reads. Among them, 12 differentially expressed genes (DEGs) were recognized, including 7 up-regulated and 5 down-regulated unigenes (Figure 4A). All DEGs are listed in Supplementary Table 2.
Figure 4.
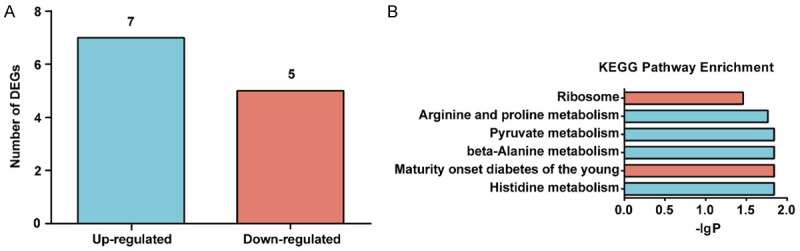
Moxibustion altered splenic gene expression based on RNA-Seq (n = 3/group). A. Number of DEGs. Cut-off value of |log2FC| > 1 and P-adj < 0.05. B. The KEGG enrichment pathway analysis of DEGs. The blue column represents up regulation, while the orange column represents down regulation.
GO enrichment analysis revealed that these 12 unigenes were assigned to 312 GO terms, but none of them were significantly enriched with P-values < 0.05. The top ranked GO terms were carnosine biosynthetic process, carnosine synthase activity, carnosine metabolic process, intracellular distribution of mitochondria, mitochondrial calcium ion transport, etc. (Supplementary Table 3).
KEGG analysis displayed that the DEGs were enriched in 6 signal transduction pathways (P-adj < 0.05, Figure 4B). Of these, the significantly up-regulated pathways were related to metabolism of histidine, β-Alanine, pyruvate, arginine, and prolin, while the significantly down-regulated pathways were maturity onset diabetes of the young and ribosome.
Moxibustion improved the diversity of the gut microbiota
First, the alpha diversity analysis was used to compare the gut microbiota diversity between the two groups at the OTU level. The Shannon index was calculated to characterize the community diversity, which demonstrated the evenness of individual distribution in the community. In addition, the Chao1 index was computed to identify the community richness. We found that the Shannon index (P < 0.05; Figure 5A) was higher in the moxibustion group than in the control group, suggesting a significantly higher community diversity after moxibustion. Nevertheless, the differences in the Chao1 index (P > 0.05; Figure 5B) between the two groups were not statistically significant, suggesting similar community richness.
Figure 5.
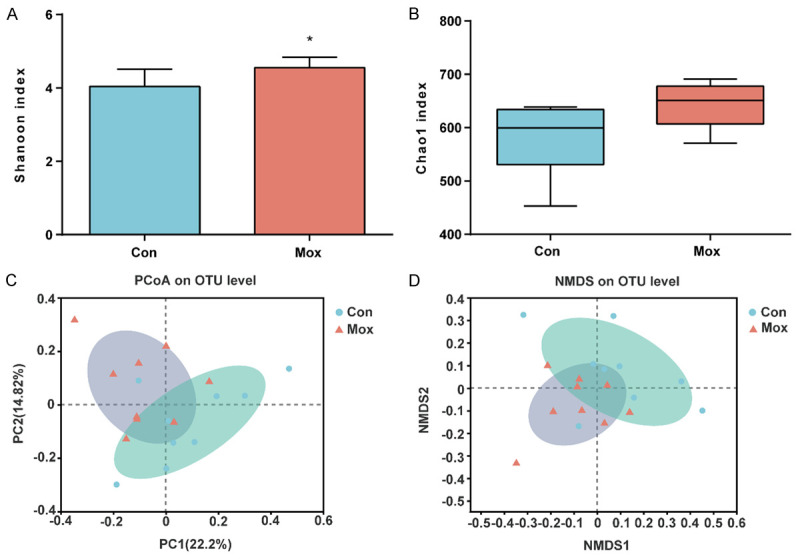
Moxibustion altered Alpha- and Beta-diversity indices of the gut microbiota (n = 9/group). A. Shannon index. B. Chao1 index. C. PCoA plot based on the Bray-Curtis. D. NMDS plot based on the Bray-Curtis. Con: the control group. Mox: the moxibustion group. *P < 0.05.
Furthermore, we performed the beta diversity analysis visualized by PCoA and NMDS to explore the similarity in community composition between the control and the moxibustion groups. As the greater distance in the figure represented greater difference in flora composition and structure, PCoA and NMDS results divided the samples from the control and moxibustion groups into two communities (P < 0.05; Figure 5C, 5D), suggesting that moxibustion treatment significantly affected the gut microbiota structure of mice.
Moxibustion altered the composition of the gut microbiota
To identify the characteristic microbiota at different taxonomic levels of each group, LEfSe was performed and displayed in a cladogram of the biomarkers (Figure 6A) and an LDA score histogram (Figure 6B) and found that the predominant taxa of the contral group were c_Actinobacteria, p_Actinobacteria, f_Bacteroidaceae, g_Bacteroides, etc., while the key microorganisms of the moxibustion group were o_Clostridiales, c_Clostridia, f_Lachnospiraceae, g_unclassified_f_Lachnospiraceae, f_Ruminococcaceae, g_Roseburia, etc.
Figure 6.
Moxibustion changed the composition of the gut microbiota by LEfSe analysis (n = 9/group). A. Taxonomic cladogram of differential abundance microbiota (phylum to genus). B. LDA scores of differential abundance microbiota. LDA score (log10) > 3 and P < 0.05. Con: the control group. Mox: the moxibustion group.
At the phylum level, as displayed in Figure 7A, Bacteroidetes, Firmicutes, Actinobacteria and Proteobacteria were the top phyla in both the control and moxibustion groups, among which Bacteroidetes and Firmicutes account for more than 90% of the proportion. The proportion of Bacteroidetes decreased while the proportion of Firmicutes increased in the moxibustion group compared to the control group, although the differences were not statistically significant (P > 0.05; Figure 7B). In addition, the Firmicutes (F)/Bacteroidetes (B) ratio in the moxibustion group was increased, although not significantly (P > 0.05). Furthermore, an increased proportion of Tenericutes while a decreased proportion of Actinobacteria and Cyanobacteria was found in the moxibustion group (P < 0.05; Figure 7B). Community abundance with significant differences from class to family levels was shown in Supplementary Figures 1, 2.
Figure 7.
Moxibustion changed the relative abundance of the gut microbiota at the phylum and genus levels (n = 9/group). A. The gut microbiota composition at the phylum level (top 10 phyla of abundance). B. Differences in dominant gut microbiota composition at the phylum level (top 10 phyla of abundance). C. The gut microbiota composition at the genus level (top 20 genera of abundance). D. Differences in dominant gut microbiota composition at the genus level (top 20 genera of abundance).
At the genus level, as displayed in Figure 7C, the major microbiota in both groups were norank_f_Bacteroidales_S24-7_group, norank_f_Erysipelotrichaceae, Lachnospiraceae_NK4A136_group, Lactobacillus, Bacteroides, Alistipes, Alloprevotella, unclassified_f_Lachnospiraceae, Turicibacter, etc. Notably, the moxibustion group exhibited an increased abundance in unclassified_f_Lachnospiraceae, norank_f_Lachnospiraceae, Roseburia, Lachnoclostridium, etc., and a significantly decreased abundance of Bacteroides (P < 0.05; Figure 7D), supporting the results from LEfSe analysis.
Discussion
The brain is one of most susceptible organs to aging. After middle age, the age-related decline of brain function is greatly accelerated, accompanied by the growing incidence of neurodegenerative disorders such as AD and PD. As brain aging leads to neurobehavioral changes, studies have shown that the cognitive function of mice declines from middle age [26]. Although moxibustion has been proven to improve the learning and memory ability of mice with Alzheimer’s disease [27], our study found that it might not have a significant impact on the memory of the middle-aged mice. It is also notable that, we used passive avoidance test to measure memory in this experiment. However, the accuracy of passive avoidance test is affected by many factors. The large individual differences in the avoidance response, the unfamiliar environment, the smells and sounds during the test, and the stress reaction caused by electric stimulation, all of which may affect the behavioral results. In future study, we hope to improve the methods of passive avoidance test, and use different behavioral tests to evaluate the learning and memory ability more comprehensively and objectively. Although our study showed that moxibustion intervention didn’t significantly improve the learning and memory ability of mice, it improved the locomotor activity and motor function of mice in the OFT and Rota-Rod test.
SIRT1 is recognized as a longevity gene that participates in regulating the aging process, thus affecting lifespan. Caloric restriction, as one of the main anti-aging measures, has been reported to prolong lifespan and alleviate age-related diseases, by enhancing the activity of SIRT1 [28]. Caloric restriction prolongs the median lifespan of SIRT1 (+/-) mice, but not SIRT1 (-/-) mice [29], suggesting that SIRT1 mediates the life prolonging effect of caloric restriction. SIRT1 regulates cell metabolism and aging by acting on a variety of downstream signaling molecules, such as PPARs, P53, FOXO1, and PGC1α. In particular, SIRT1 not only activates PPARs to regulate nutrient metabolism [30], but also regulates apoptosis by acting on P53 [31]. In this study, although no significant changes were found in the lifespan of the mice, the median lifespan was extended by 16.5%, and IHC showed that moxibustion intervention activated the SIRT1-PPARα signaling pathway, all of which demonstrated the function of moxibustion in modulating energy metabolism and increasing life expectancy. Similar studies also suggested that consuming resveratrol, a SIRT1 activator, from middle age reduced the signs of aging and improved age-related adverse conditions, although no statistical difference was found in lifespan [32]. Our study failed to display a significant effect of moxibustion intervention on prolonging the lifespan of mice, which may be due to the fact that moxibustion as a health care therapy requires a long course of treatment, while our experimental period (30 times) was insufficient to achieve the expected effect. Our small sample size might also limit the accuracy of the results.
Our splenic gene sequencing results identified 12 DEGs by moxibustion treatment, and the GO terms of these genes were mainly related to carnosine metabolism and mitochondrial function. In addition, KEGG analysis showed that 6 pathways were significantly enriched, among which the metabolisms of histidine, β-Alanine, and pyruvate were significantly up-regulated, suggesting that moxibustion affected nutrients metabolism. Furthermore, the enhancement of amino acid metabolism might correlate with the regulation of carnosine metabolism by moxibustion. L-Carnosine, a small molecular dipeptide of β-Alanine and histidine, enriched in muscle and brain tissues, is a strong antioxidant and is considered as one of the most effective anti-aging substances [33]. A carnosine containing diet has been proven to reduce the aging characteristics of SAMP1 mice and prolong the mean lifespan by 20% [34]. In consistence with these findings, one study reported that oral carnosine administration delayed brain aging and ameliorated memory deficits of AD mice [35].
Our IHC and spleen RNA-seq results suggested the effects of moxibustion on energy metabolism. Similarly, our previous study also found that moxibustion at the Guanyuan acupoint enhanced cognitive function of AD modeled mice through promotion of metabolism [36,37]. As energy metabolism mainly occurs in mitochondria, our unpublished data indicated that moxibustion intervention alleviated the oxidative stress of brain tissue and ameliorated behavioral performance through regulating the function of the mitochondrial respiratory chain.
In this study, we found that moxibustion intervention improved the autonomous activity and motor function of mice, prolonged the median survival, and boosted the energy metabolism, which could be explained by the enhancing effects on yang qi of moxibustion at RN4. Based on the theory of traditional Chinese medicine, supplementing yang qi is one of the key functions of moxibustion. Around 1146 CE, the Bianque Heart Book has proposed that moxibustion is the first choice of treatment for yang-deficiency syndrome. RN4, an acupoint of the Ren meridian, where three yin meridians of the foot converge, has the effect of reinforcing kidney-yang. Yang qi is interpreted in modern vocabulary as referring to the nature of “upward, exuberant and enhanced”. Therefore, a human body sufficient in yang qi exhibits a strong metabolism, full of spirit and energy. Traditional Chinese medicine also proposes that the main pathogenesis of aging is “kidney yang deficiency”. As people grow older, the yang qi will gradually be depleted. The earliest written record of Chinese medicine, Huangdi Neijing, states the relationship between yang qi and lifespan: “When Yang is strong, you will live long, and when Yang is weak, you will die”. Based on the theory of traditional Chinese medicine, moxibustion at RN4, as an important health care therapy, can supplement the yang qi of the human body, thus delaying the aging process. Additionally, modern research has confirmed that a radiation effect is one of the functional mechanisms of moxibustion. Previous research has illustrated that the burning moxa radiation spectrum ranges between 0.8 and 5.6 μm, while the peak is around 1.5 μm, within the band of medical infrared [38]. Radiation effects not only raise the temperature and promote the energy metabolism of local issues, but also induce some active substances to enter the blood circulation, strengthening the metabolism of the affected organs [38].
Accumulating evidence has indicated the critical role of gut microbiota in the aging process. Gut microbiota has been proposed as a possible determinant of human lifespan as evidenced by the fact that beneficial species of the gut microbiome in centenarians have been preserved compared to elderly [39]. Other studies also found similar gut microbiota between the healthy older adults and young adults, suggesting the crucial role of gut microbiota in healthy aging [40]. Through the brain-gut-microbiome axis (BGM), gut microbiota participates in the regulation of nervous function, via the mechanisms of metabolism, and the immune and endocrine systems, etc. [41]. The age-related changes in the nervous system were connected to the imbalance of fecal microbiota. Indeed, the gut microbiota related to inflammation were in imbalance in elderly adults with cognitive impairment [42]. In line with this finding, clinical trials reported that probiotics enhanced the memory of AD patients [43], which was also supported by using animal experiments in which probiotics improved age-related behavior of SAMP8 mice via the BGM axis [44].
In this study, the alpha diversity of gut microbiota in the moxibustion group increased remarkably compared to the control group. A high diversity of microbiota is vital to maintain functional affluence and adaptability and, therefore, systematic robustness against environmental challenges. Conversely, a low diversity is found to link to the onset of many diseases [45]. The diversity of gut microbiota in the elderly reduced significantly [46]. In our beta diversity analysis, we found a significant difference in gut microbiota structure between moxibustion-treated and control mice, indicating the effects of moxibustion on gut microbiota structure, consistent with previous research [12].
In the aspect of composition, the four major phyla of gut microbiota are Bacteroidetes, Firmicutes, Actinobacia and Proteobateria. Bacteroides and Firmicutes are the dominant bacteria, accounting for more than 90% of gut microbiota. The relative abundance of Firmicutes (F) and Bacteroides (B) is an important indicator of gut microbiota status [47]. However, the change of F/B ratio with age still remains debatable. Some studies showed that, after adulthood, the F/B ratio in gut decreases with age [48], whereas others reported that it increases with age [49]. We found a trend of increased F/B ratio after moxibustion treatment in our study, although not statistically significant. Additionally, the relative abundance of f_Bacteroidaceae and g_Bacteroides, which is considered to be potentially harmful conditioned pathogens, declined in the moxibustion group. It has been found an increased abundance of Bacteroides during aging [48], which is positively correlated with cognitive impairment [50]. Furthermore, at the phylum level, the relative abundance of Actinobacteria and Cyanobacteria in the moxibustion group was reduced, while the relative abundance of Tenericetes was increased. Previous studies have also confirmed these age-related changes [51,52].
In our LSEF analysis, we identified that o_Clostridiales, c_Clostridia, f_lachnospiraceae, g_unclassified_f_Lachnospiraceae, f_Ruminococcaceae g_Roseburia, etc. were the key microorganisms in the moxibustion group, among which f_Lachnospiraceae and f_Ruminococcaceae are from o_Clostridiales, while g_Roseburia is from f_Lachnospiraceae. Previous studies have suggested that the abundance of Lachnospiracea, Roseburia and Ruminocochaceae in the gut display age-related alterations. The abundance of these microorganisms was decreased in the elderly compared with that in the young adults [39]. Similar study also indicated that Roseburia and Ruminococcaeae are more abundant in the gut of centenarians compared with the younger elderly [53]. Since Lachnospiracea, Ruminocochaceae and Roseburia produce short chain fatty acid (SCFA), especially butyric acid [54-56], previous studies have also revealed a positive relationship between brain aging and the reduction in the abundance of butyrate, as well as butyrate-producing bacteria in the gut [57]. SCFAs, which regulate the metabolism of nutrients, are mainly produced by beneficial intestinal microorganisms after dietary fiber digestion [58]. SCFAs participate in maintaining the health of both gut and brain. Furthermore, Butyrate, a crucial member of SCFAs, has been shown to have anti-inflammatory and neuroprotective effects [59], as well as to improve the cognitive function of animals with neurodegenerative diseases [60]. Although we did not measure the levels of intestinal SCFAs in this study, our previous studies found that moxibustion at RN4 increased the levels of various SCFAs in aged rats [12]. Collectively, our results indicated that, moxibustion intervention ameliorated age-related changes in gut microbiota. It is assumed that by regulating gut microbiota, moxibustion plays a role in healthy aging and improving nervous function.
Conclusion
In summary, our study suggested that, in middle-aged mice, moxibustion intervention improved the neurobehavioral performance, reversed the age-related imbalance of gut microbiota, and promoted energy metabolism, resulting in an anti-aging effect. Future research will be carried out to extend the intervention course and explore the correlation between gut microecological and senescence characteristics.
Acknowledgements
This study was funded by the National Natural Science Foundation of China (No: 82174475; 82174507).
Disclosure of conflict of interest
None.
Supporting Information
References
- 1.Li S, Kim HE. Implications of sphingolipids on aging and age-related diseases. Front Aging. 2022;2:797320. doi: 10.3389/fragi.2021.797320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Leal SL, Yassa MA. Neurocognitive aging and the hippocampus across species. Trends Neurosci. 2015;38:800–812. doi: 10.1016/j.tins.2015.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rani L, Sahu MR, Mondal AC. Age-related mitochondrial dysfunction in Parkinson’s disease: new insights into the disease pathology. Neuroscience. 2022;499:152–169. doi: 10.1016/j.neuroscience.2022.07.007. [DOI] [PubMed] [Google Scholar]
- 4.López-Otín C, Blasco MA, Partridge L, Serrano M, Kroemer G. The hallmarks of aging. Cell. 2013;153:1194–1217. doi: 10.1016/j.cell.2013.05.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Buford TW. (Dis)Trust your gut: the gut microbiome in age-related inflammation, health, and disease. Microbiome. 2017;5:80. doi: 10.1186/s40168-017-0296-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Salazar N, Valdes-Varela L, Gonzalez S, Gueimonde M, de Los Reyes-Gavilan CG. Nutrition and the gut microbiome in the elderly. Gut Microbes. 2017;8:82–97. doi: 10.1080/19490976.2016.1256525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wanapaisan P, Chuansangeam M, Nopnipa S, Mathuranyanon R, Nonthabenjawan N, Ngamsombat C, Thientunyakit T, Muangpaisan W. Association between gut microbiota with mild cognitive impairment and Alzheimer’s disease in a Thai population. Neurodegener Dis. 2022;22:43–54. doi: 10.1159/000526947. [DOI] [PubMed] [Google Scholar]
- 8.Onaolapo AY, Ojo FO, Olofinnade AT, Falade J, Lawal IA, Onaolapo OJ. Microbiome-based therapies in Parkinson’s disease: can tuning the microbiota become a viable therapeutic strategy? CNS Neurol Disord Drug Targets. 2022 doi: 10.2174/1871527321666220903114559. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 9.Boyajian JL, Ghebretatios M, Schaly S, Islam P, Prakash S. Microbiome and human aging: probiotic and prebiotic potentials in longevity, skin health and cellular senescence. Nutrients. 2021;13:4550. doi: 10.3390/nu13124550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Boehme M, Guzzetta KE, Bastiaanssen TFS, van de Wouw M, Moloney GM, Gual-Grau A, Spichak S, Olavarría-Ramírez L, Fitzgerald P, Morillas E, Ritz NL, Jaggar M, Cowan CSM, Crispie F, Donoso F, Halitzki E, Neto MC, Sichetti M, Golubeva AV, Fitzgerald RS, Claesson MJ, Cotter PD, O’Leary OF, Dinan TG, Cryan JF. Microbiota from young mice counteracts selective age-associated behavioral deficits. Nat Aging. 2021;1:666–676. doi: 10.1038/s43587-021-00093-9. [DOI] [PubMed] [Google Scholar]
- 11.Xu K, Wei Y, Liu C, Zhao L, Geng B, Mai W, Zhang S, Liang L, Zeng X, Deng D, Liu P. Effect of moxibustion treatment on degree centrality in patients with mild cognitive impairment: a resting-state functional magnetic resonance imaging study. Front Hum Neurosci. 2022;16:889426. doi: 10.3389/fnhum.2022.889426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ouyang XL, Duan HR, Jin Q, Luo X, Han L, Zhao BX, Li JT, Chen YX, Lin Y, Liu YJ, Huang YP, Shuang S, Huang C, He R, Yao Q, Xue Y, Guo SQ, Zhao J. Moxibustion may delay the aging process of Wistar rats by regulating intestinal microbiota. Biomed Pharmacother. 2022;146:112147. doi: 10.1016/j.biopha.2021.112147. [DOI] [PubMed] [Google Scholar]
- 13.Yang X, Wang W, Zhang Y, Wang J, Huang F. Moxibustion improves ovary function by suppressing apoptosis events and upregulating antioxidant defenses in natural aging ovary. Life Sci. 2019;229:166–172. doi: 10.1016/j.lfs.2019.05.040. [DOI] [PubMed] [Google Scholar]
- 14.Yang C, Xu B, Sun Y. Effects of acupuncture and moxibustion on MDA, GSH-Px, and hyp in the skin of senile mice. Journal of Acupuncture and Tuina Science. 2011;9:142–144. [Google Scholar]
- 15.Yao C, Zhao C, Zhang S, Liu S. Effect of moxibustion on testosterone secretion and apoptosis of spermatogenic cells in aging rats. Evid Based Complement Alternat Med. 2019;2019:5186408. doi: 10.1155/2019/5186408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wu HG, Guo LQ, Chen HP, Sun GJ, Zhang W, Shi Y, Zhang QJ, Liu ZHR. Moxibustion on telomerase activity in aging rat. Journal of Acupuncture and Tuina Science. 2007;5:74–78. [Google Scholar]
- 17.Rodenas-Gonzalez F, Blanco-Gandia MC, Minarro J, Rodriguez-Arias M. Cognitive profile of male mice exposed to a ketogenic diet. Physiol Behav. 2022;254:113883. doi: 10.1016/j.physbeh.2022.113883. [DOI] [PubMed] [Google Scholar]
- 18.Liu L, Cai F, Lu Y, Xie Y, Li H, Long C. Comparative lipidomic and metabolomic analyses reveal the mystery of lacquer oil from toxicodendron vernicifluum for the treatment of “Yuezi” disease in Nujiang, China: from anti-inflammation and anti-postpartum depression perspective. Front Pharmacol. 2022;13:914951. doi: 10.3389/fphar.2022.914951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Du X, Xu Y, Chen S, Fang M. Inhibited CSF1R alleviates ischemia injury via inhibition of microglia M1 polarization and NLRP3 pathway. Neural Plast. 2020;2020:8825954. doi: 10.1155/2020/8825954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Liu N, Jiang Y, Xing M, Zhao B, Hou J, Lim M, Huang J, Luo X, Han L. Digital gene expression profiling analysis of aged mice under moxibustion treatment. Evid Based Complement Alternat Med. 2018;2018:4767328. doi: 10.1155/2018/4767328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mortazavi A, Williams BA, McCue K, Schaeffer L, Wold B. Mapping and quantifying mammalian transcriptomes by RNA-Seq. Nat Methods. 2008;5:621–628. doi: 10.1038/nmeth.1226. [DOI] [PubMed] [Google Scholar]
- 22.Anders S, Pyl PT, Huber W. HTSeq--a python framework to work with high-throughput sequencing data. Bioinformatics. 2015;31:166–169. doi: 10.1093/bioinformatics/btu638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Robinson MD, McCarthy DJ, Smyth GK. edgeR: a bioconductor package for differential expression analysis of digital gene expression data. Bioinformatics. 2010;26:139–140. doi: 10.1093/bioinformatics/btp616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Benjamini Y, Hochberg Y. Controlling the false discovery rate: a practical and powerful approach to multiple testing. Journal of the Royal Statistical Society: Series B (Methodological) 1995;57:289–300. [Google Scholar]
- 25.Young MD, Wakefield MJ, Smyth GK, Oshlack A. Gene ontology analysis for RNA-seq: accounting for selection bias. Genome Biol. 2010;11:R14. doi: 10.1186/gb-2010-11-2-r14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kakae M, Miyanohara J, Morishima M, Nagayasu K, Mori Y, Shirakawa H, Kaneko S. Pathophysiological role of TRPM2 in age-related cognitive impairment in mice. Neuroscience. 2019;408:204–213. doi: 10.1016/j.neuroscience.2019.04.012. [DOI] [PubMed] [Google Scholar]
- 27.Ha L, Yang B, Wang S, An Y, Wang H, Cui Y. Effect of moxibustion on behavioral changes and expression of APP and BACE1 in hippocampus of SAMP8 mice. Evid Based Complement Alternat Med. 2020;2020:3598930. doi: 10.1155/2020/3598930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rachakatla A, Kalashikam RR. Calorie restriction-regulated molecular pathways and its impact on various age groups: an overview. DNA Cell Biol. 2022;41:459–468. doi: 10.1089/dna.2021.0922. [DOI] [PubMed] [Google Scholar]
- 29.Mercken EM, Hu J, Krzysik-Walker S, Wei M, Li Y, McBurney MW, de Cabo R, Longo VD. SIRT1 but not its increased expression is essential for lifespan extension in caloric-restricted mice. Aging Cell. 2014;13:193–196. doi: 10.1111/acel.12151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Schug TT, Li X. Sirtuin 1 in lipid metabolism and obesity. Ann Med. 2011;43:198–211. doi: 10.3109/07853890.2010.547211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Liu J, Zhang C, Hu W, Feng Z. Tumor suppressor p53 and metabolism. J Mol Cell Biol. 2019;11:284–292. doi: 10.1093/jmcb/mjy070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Pearson KJ, Baur JA, Lewis KN, Peshkin L, Price NL, Labinskyy N, Swindell WR, Kamara D, Minor RK, Perez E, Jamieson HA, Zhang Y, Dunn SR, Sharma K, Pleshko N, Woollett LA, Csiszar A, Ikeno Y, Le Couteur D, Elliott PJ, Becker KG, Navas P, Ingram DK, Wolf NS, Ungvari Z, Sinclair DA, de Cabo R. Resveratrol delays age-related deterioration and mimics transcriptional aspects of dietary restriction without extending life span. Cell Metab. 2008;8:157–168. doi: 10.1016/j.cmet.2008.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Li X, Yang K, Gao S, Zhao J, Liu G, Chen Y, Lin H, Zhao W, Hu Z, Xu N. Carnosine stimulates macrophage-mediated clearance of senescent skin cells through activation of the AKT2 signaling pathway by CD36 and RAGE. Front Pharmacol. 2020;11:593832. doi: 10.3389/fphar.2020.593832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gallant S, Semyonova M, Yuneva M. Carnosine as a potential anti-senescence drug. Biochemistry (Mosc) 2000;65:866–868. [PubMed] [Google Scholar]
- 35.Hegazy MA, Abdelmonsif DA, Zeitoun TM, El-Sayed NS, Samy DM. Swimming exercise versus L-carnosine supplementation for Alzheimer’s dementia in rats: implication of circulating and hippocampal FNDC5/irisin. J Physiol Biochem. 2022;78:109–124. doi: 10.1007/s13105-021-00845-6. [DOI] [PubMed] [Google Scholar]
- 36.He R, Liu J, Huang C, Liu J, Cui H, Zhao B. A urinary metabolomics analysis based on UPLC-MS and effects of moxibustion in APP/PS1 mice. Curr Alzheimer Res. 2020;17:753–765. doi: 10.2174/1567205017666201109091759. [DOI] [PubMed] [Google Scholar]
- 37.Ha L, Yu M, Yan Z, Rui Z, Zhao B. Effects of moxibustion and moxa smoke on behavior changes and energy metabolism in APP/PS1 mice. Evid Based Complement Alternat Med. 2019;2019:9419567. doi: 10.1155/2019/9419567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Deng H, Shen X. The mechanism of moxibustion: ancient theory and modern research. Evid Based Complement Alternat Med. 2013;2013:379291. doi: 10.1155/2013/379291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Biagi E, Franceschi C, Rampelli S, Severgnini M, Ostan R, Turroni S, Consolandi C, Quercia S, Scurti M, Monti D, Capri M, Brigidi P, Candela M. Gut microbiota and extreme longevity. Curr Biol. 2016;26:1480–1485. doi: 10.1016/j.cub.2016.04.016. [DOI] [PubMed] [Google Scholar]
- 40.Bian G, Gloor GB, Gong A, Jia C, Zhang W, Hu J, Zhang H, Zhang Y, Zhou Z, Zhang J, Burton JP, Reid G, Xiao Y, Zeng Q, Yang K, Li J. The gut microbiota of healthy aged Chinese is similar to that of the healthy young. mSphere. 2017;2:e00327-17. doi: 10.1128/mSphere.00327-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Martin CR, Osadchiy V, Kalani A, Mayer EA. The brain-gut-microbiome axis. Cell Mol Gastroenterol Hepatol. 2018;6:133–148. doi: 10.1016/j.jcmgh.2018.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Cattaneo A, Cattane N, Galluzzi S, Provasi S, Lopizzo N, Festari C, Ferrari C, Guerra UP, Paghera B, Muscio C, Bianchetti A, Volta GD, Turla M, Cotelli MS, Gennuso M, Prelle A, Zanetti O, Lussignoli G, Mirabile D, Bellandi D, Gentile S, Belotti G, Villani D, Harach T, Bolmont T, Padovani A, Boccardi M, Frisoni GB INDIA-FBP Group. Association of brain amyloidosis with pro-inflammatory gut bacterial taxa and peripheral inflammation markers in cognitively impaired elderly. Neurobiol Aging. 2017;49:60–68. doi: 10.1016/j.neurobiolaging.2016.08.019. [DOI] [PubMed] [Google Scholar]
- 43.Liu C, Guo X, Chang X. Intestinal flora balance therapy based on probiotic support improves cognitive function and symptoms in patients with Alzheimer’s disease: a systematic review and meta-analysis. Biomed Res Int. 2022;2022:4806163. doi: 10.1155/2022/4806163. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 44.Yang X, Yu D, Xue L, Li H, Du J. Probiotics modulate the microbiota-gut-brain axis and improve memory deficits in aged SAMP8 mice. Acta Pharm Sin B. 2020;10:475–487. doi: 10.1016/j.apsb.2019.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Li Z, Zhou J, Liang H, Ye L, Lan L, Lu F, Wang Q, Lei T, Yang X, Cui P, Huang J. Differences in Alpha diversity of gut microbiota in neurological diseases. Front Neurosci. 2022;16:879318. doi: 10.3389/fnins.2022.879318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Vemuri R, Gundamaraju R, Shastri MD, Shukla SD, Kalpurath K, Ball M, Tristram S, Shankar EM, Ahuja K, Eri R. Gut microbial changes, interactions, and their implications on human lifecycle: an ageing perspective. Biomed Res Int. 2018;2018:4178607. doi: 10.1155/2018/4178607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Teker HT, Ceylani T. Intermittent fasting supports the balance of the gut microbiota composition. Int Microbiol. 2023;26:51–57. doi: 10.1007/s10123-022-00272-7. [DOI] [PubMed] [Google Scholar]
- 48.Mariat D, Firmesse O, Levenez F, Guimaraes V, Sokol H, Dore J, Corthier G, Furet JP. The firmicutes/bacteroidetes ratio of the human microbiota changes with age. BMC Microbiol. 2009;9:123. doi: 10.1186/1471-2180-9-123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Vaiserman A, Romanenko M, Piven L, Moseiko V, Lushchak O, Kryzhanovska N, Guryanov V, Koliada A. Differences in the gut Firmicutes to Bacteroidetes ratio across age groups in healthy Ukrainian population. BMC Microbiol. 2020;20:221. doi: 10.1186/s12866-020-01903-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Saji N, Murotani K, Hisada T, Tsuduki T, Sugimoto T, Kimura A, Niida S, Toba K, Sakurai T. The relationship between the gut microbiome and mild cognitive impairment in patients without dementia: a cross-sectional study conducted in Japan. Sci Rep. 2019;9:19227. doi: 10.1038/s41598-019-55851-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Odamaki T, Kato K, Sugahara H, Hashikura N, Takahashi S, Xiao JZ, Abe F, Osawa R. Age-related changes in gut microbiota composition from newborn to centenarian: a cross-sectional study. BMC Microbiol. 2016;16:90. doi: 10.1186/s12866-016-0708-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Fasina OB, Wang J, Mo J, Osada H, Ohno H, Pan W, Xiang L, Qi J. Gastrodin from gastrodia elata enhances cognitive function and neuroprotection of AD mice via the regulation of gut microbiota composition and inhibition of neuron inflammation. Front Pharmacol. 2022;13:814271. doi: 10.3389/fphar.2022.814271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Wang F, Yu T, Huang G, Cai D, Liang X, Su H, Zhu Z, Li D, Yang Y, Shen P, Mao R, Yu L, Zhao M, Li Q. Gut microbiota community and its assembly associated with age and diet in Chinese centenarians. J Microbiol Biotechnol. 2015;25:1195–1204. doi: 10.4014/jmb.1410.10014. [DOI] [PubMed] [Google Scholar]
- 54.Berger K, Burleigh S, Lindahl M, Bhattacharya A, Patil P, Stalbrand H, Nordberg Karlsson E, Hallenius F, Nyman M, Adlercreutz P. Xylooligosaccharides increase bifidobacteria and lachnospiraceae in mice on a high-fat diet, with a concomitant increase in short-chain fatty acids, especially butyric acid. J Agric Food Chem. 2021;69:3617–3625. doi: 10.1021/acs.jafc.0c06279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Song L, Sun Q, Zheng H, Zhang Y, Wang Y, Liu S, Duan L. Roseburia hominis alleviates neuroinflammation via short-chain fatty acids through histone deacetylase inhibition. Mol Nutr Food Res. 2022;66:e2200164. doi: 10.1002/mnfr.202200164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Ouyang Y, Liu D, Zhang L, Li X, Chen X, Zhao C. Green alga enteromorpha prolifera oligosaccharide ameliorates ageing and hyperglycemia through gut-brain axis in age-matched diabetic mice. Mol Nutr Food Res. 2022;66:e2100564. doi: 10.1002/mnfr.202100564. [DOI] [PubMed] [Google Scholar]
- 57.Cuervo-Zanatta D, Syeda T, Sanchez-Valle V, Irene-Fierro M, Torres-Aguilar P, Torres-Ramos MA, Shibayama-Salas M, Silva-Olivares A, Noriega LG, Torres N, Tovar AR, Ruminot I, Barros LF, Garcia-Mena J, Perez-Cruz C. Dietary fiber modulates the release of gut bacterial products preventing cognitive decline in an alzheimer’s mouse model. Cell Mol Neurobiol. 2023;43:1595–1618. doi: 10.1007/s10571-022-01268-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Luo P, Lednovich K, Xu K, Nnyamah C, Layden BT, Xu P. Central and peripheral regulations mediated by short-chain fatty acids on energy homeostasis. Transl Res. 2022;248:128–150. doi: 10.1016/j.trsl.2022.06.003. [DOI] [PubMed] [Google Scholar]
- 59.Strasser B, Ticinesi A. Intestinal microbiome in normal ageing, frailty and cognition decline. Curr Opin Clin Nutr Metab Care. 2023;26:8–16. doi: 10.1097/MCO.0000000000000878. [DOI] [PubMed] [Google Scholar]
- 60.Wang C, Zheng D, Weng F, Jin Y, He L. Sodium butyrate ameliorates the cognitive impairment of Alzheimer’s disease by regulating the metabolism of astrocytes. Psychopharmacology (Berl) 2022;239:215–227. doi: 10.1007/s00213-021-06025-0. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.





