Abstract
Objective: This study was designed to analyze risk factors for postoperative pulmonary infection (PPI) in patients with non-small cell lung cancer (NSCLC) based on regression models and to construct a corresponding nomogram prediction model. Methods: A total of 244 patients with NSCLC who received surgical treatment from June 2015 to January 2017 were retrospectively analyzed. According to the PPI, they were assigned to a pulmonary infection group (n=27) or non-pulmonary infection group (n=217). The independent risk factors for PPI in NSCLC patients were screened through least absolute shrinkage and selection operator (LASSO) and logistic regression analysis, and a corresponding nomogram prediction model was constructed. Results: A total of 244 NSCLC patients were included, including 27 with PPI (11.06%). According to LASSO regression-based screening, age, diabetes mellitus (DM), tumor node metastasis (TNM) staging, chemotherapy regimen, chemotherapy cycle, post-chemotherapy albumin (g/L), pre-chemotherapy KPS and operation time were all significant and found to be the influencing factors for PPI. The risk model constructed based on LASSO was 0.0035770333 + (0.0020227686* age) + (0.057554487* DM) + (0.016365428* TNM staging) + (0.048514458* chemotherapy regimen) + (0.00871801* chemotherapy cycle) + (-0.002096683* post-chemotherapy albumin (g/L) + (-0.00090206* pre-chemotherapy Karnofsky performance score (KPS)) + (0.000296876* operation time). The pulmonary infection group got significantly higher risk scores than the non-pulmonary infection group (P<0.0001). According to receiver operating characteristic (ROC) curve-based analysis, the area under the curve (AUC) of risk score in predicting pulmonary infection was 0.894. Based on 4 independent predictors, a risk-prediction nomogram model was constructed to predict pulmonary infection in NSCLC patients after surgery. The internal verification C-index was 0.900 (95% CI: 0.839-0.961), and the calibration curves were well fitted with the ideal ones. Conclusion: The prediction model based on a regression model for PPI in NSCLC patients demonstrates good prediction efficiency, which is conducive to early screening of high-risk patients and further improvement of treatment regimen.
Keywords: Regression model, non-small cell lung cancer, postoperative pulmonary infection, risk factor, nomogram prediction model
Introduction
Lung cancer (LC), one of the most common cancers, is the primary cause of cancer-associated deaths worldwide. Non-small cell lung cancer (NSCLC) accounts for approximate 80% of all LCs [1]. According to estimation, 2020 has seen 2.09 million new cases of LC. As the primary cause of cancer-related deaths, LC results in 25% of such deaths [2]. The incidence of NSCLC varies worldwide, with a higher incidence in developed countries and a lower one in developing countries [3]. However, due to the increase of tobacco use and exposure to environmental pollutants, NSCLC has a growing incidence in many countries, especially in low-income and middle-income countries [4]. NSCLC is primarily treated by the combination of surgery, radiotherapy, and chemotherapy [5]. Surgical intervention that is generally regarded as the most effective option for early NSCLC brings the risk of postoperative complications, including postoperative infection [6].
Postoperative infection is a common and possibly serious surgical complication, which greatly impacts patients’ rehabilitation and overall outcomes [7]. The risk of postoperative infection is the highest in the first few weeks after surgery, which is probably due to bacteria, viruses or fungi that enter the body through the surgical incision [8]. Symptoms of postoperative infection can range from mild (such as fever and redness at the surgical site) to severe (such as pneumonia or sepsis) [9]. It greatly compromises the recovery of patients, increases the risk of complications, and results in a longer hospitalization and higher medical expenses [10]. However, opinions on risk factors of postoperative pulmonary infection (PPI) vary extensively. For example, Wang et al. [11] found that surgical procedure, surgical duration, inspired fraction of oxygen in one-lung ventilation, and postoperative pain were risk factors for postoperative infection in lung cancer patients. Another study [12] showed that age 75 years or older, forced expiratory volume in 1 second as a percentage of forced vital capacity (FEV1%) less than 70%, advanced pathologic stage, and induction therapy were risk factors for lung infection in patients.
Thus, postoperative infection, as one common and serious complications of NSCLC surgery, greatly compromises the patients’ prognosis. Accordingly, it is necessary to better understand the factors for postoperative infections in NSCLC patients and strategies for preventing and treating these infections in order to improve the patients’ prognosis and reduce medical expense.
Materials and methods
Research objects
A total of 244 NSCLC patients who received surgical treatment from June 2015 to January 2017 were retrospectively analyzed. According to the PPI, they were assigned to a pulmonary infection group (n=27) and non-pulmonary infection group (n=217). This study was performed with permission from the Medical Ethics Committee of the First Affiliated Hospital of Nanchang University. A flowchart was constructed for this study (Figure 1).
Figure 1.
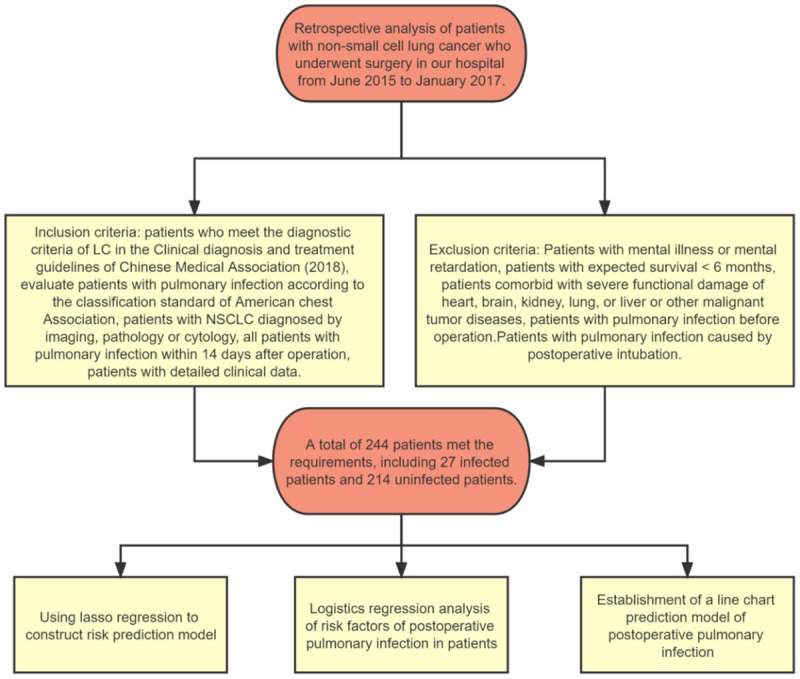
Flow chart of patient enrollment and analysis.
Inclusion and exclusion criteria
Inclusion criteria: patients diagnosed with NSCLC according to the diagnostic criteria in the Chinese Medical Association LC Clinical Diagnosis and Treatment Guidelines (2018) [13]; patients who were evaluated for pulmonary infection according to the American Thoracic Society classification standards, patients who were confirmed with imaging, pathological, or cytological examination; patients with detailed clinical information. Exclusion criteria: patients with mental illness or mental retardation, patients with expected survival <6 months, patients comorbid with severe functional damage of heart, brain, kidney, lung, or liver or other malignant tumor diseases, patients with pulmonary infection before operation; patients with pulmonary infection caused by postoperative intubation.
Clinical data collection
The data of patients were collected according to their follow-up and the hospital information system, including sex, age, body mass index (BMI), history of drinking, tumor type, diabetes mellitus (DM), hypertension, cardiovascular diseases (coronary heart disease, myocardial infarction, angina pectoris, heart failure and transient cerebral ischemia), pulmonary diseases (bronchitis, chronic obstructive pulmonary disease, bronchial asthma, tuberculosis), Tumor Node Metastasis (TNM) staging, chemotherapy regimen (cisplatin, carboplatin, nedaplatin, paclitaxel, gemcitabine, docetaxel, etoposide), chemotherapy cycle, pre-chemotherapy karnofsky performance score (KPS), post-chemotherapy white blood cell count (WBC), post-chemotherapy albumin content, and post-chemotherapy hemoglobin content, operation time, and intraoperative blood loss.
Diagnostic criteria of pulmonary infection
Diagnostic criteria covered (1) body temperature >38°C; (2) peripheral white blood cell counts >15×109 L-1; (3) clinical symptoms of pulmonary infection such as cough and expectoration; (4) moist rales in the lungs, and (5) obvious infection lesions in the lungs of the patients. When a lung cancer patient met any four of the above five diagnostic criteria within 14 days after operation, the patient was diagnosed with PPI.
Outcome measures
Primary outcome measures
A risk assessment model for PPI was established based on lasso regression. The main influencing factors of PPI were analyzed by logistics regression.
Secondary outcome measures
The predictive model was constructed by nomogram, and the value of lasso risk score in predicting PPI in patients was analyzed by receiver operator characteristic (ROC) curve.
Statistical analyses
This study used R language software (R Foundation for Statistical Computing, Vienna, Austria) for data screening and analysis, and established a model. The least absolute shrinkage and selection operator (LASSO) regression was conducted to screen predictors of non-zero coefficient, and logistic regression to screen the influencing factors. The nomogram was drawn by R (R3.5.3) software package and rms software package. The consistency index (C-index) was calculated by the latter, and its clinical value was verified through ROC curves. This study also adopted Graph Pad Prism 8.0 for data visualization. P<0.05 implied a significant difference.
Results
Comparison of clinical data
According to screening results of patients’ clinical data, the two groups were not greatly different in gender, BMI, history of drinking, hypertension, cardiovascular diseases, pulmonary diseases, tumor type, pre-chemotherapy pulmonary function, post-chemotherapy WBC, intraoperative blood loss or post-chemotherapy hemoglobin content (P>0.05, Table 1). Compared with the non-pulmonary infection group, the pulmonary infection group had a notably higher proportion of patients who were over 60 years old, suffered DM, were at phase III-IV in TNM staging, received single-drug chemotherapy regimen, experienced chemotherapy cycle ≥2, or had post-chemotherapy albumin content <30 g/L, pre-chemotherapy KPS <80 or operation time ≥180 min (P<0.05, Table 1).
Table 1.
Comparison of clinical data
| Factor | Pulmonary infection group (n=27) | Non-pulmonary infection group (n=217) | X2 value | P value |
|---|---|---|---|---|
| Gender | ||||
| Male | 13 | 123 | 0.708 | 0.399 |
| Female | 14 | 94 | ||
| Age | ||||
| ≥60 years old | 18 | 80 | 8.873 | 0.003 |
| <60 years old | 9 | 137 | ||
| BMI (kg/m2) | ||||
| ≥25 | 8 | 49 | 0.666 | 0.414 |
| <25 | 19 | 168 | ||
| History of drinking | ||||
| Yes | 6 | 56 | 0.162 | 0.686 |
| No | 21 | 161 | ||
| Diabetes mellitus | ||||
| Yes | 15 | 60 | 8.783 | 0.003 |
| No | 12 | 157 | ||
| Hypertension | ||||
| Yes | 10 | 90 | 0.195 | 0.658 |
| No | 17 | 127 | ||
| Cardiovascular disease | ||||
| Yes | 11 | 78 | 0.238 | 0.625 |
| No | 16 | 139 | ||
| Pulmonary diseases | ||||
| Yes | 6 | 62 | 0.481 | 0.488 |
| No | 21 | 155 | ||
| Tumor type | ||||
| Lung adenocarcinoma | 16 | 135 | 1.030 | 0.597 |
| Squamous cell carcinoma | 8 | 69 | ||
| Other | 3 | 13 | ||
| TNM staging | ||||
| Phase I-II | 12 | 152 | 7.142 | 0.008 |
| Phase III-IV | 15 | 65 | ||
| Chemotherapy regimen | ||||
| Single | 14 | 60 | 6.656 | 0.010 |
| Combined | 13 | 157 | ||
| Chemotherapy cycle | ||||
| ≥2 | 18 | 81 | 8.573 | 0.003 |
| <2 | 9 | 136 | ||
| Pre-chemotherapy pulmonary function (%) | ||||
| ≥70 | 17 | 140 | 0.025 | 0.873 |
| <70 | 10 | 77 | ||
| Post-chemotherapy white blood cell count (109/L) | ||||
| ≥4 | 12 | 106 | 0.186 | 0.666 |
| <4 | 15 | 111 | ||
| Post-chemotherapy albumin (g/L) | ||||
| ≥30 | 13 | 159 | 7.287 | 0.007 |
| <30 | 14 | 58 | ||
| Post-chemotherapy hemoglobin (g/L) | ||||
| ≥90 | 13 | 115 | 0.226 | 0.634 |
| <90 | 14 | 102 | ||
| Pre-chemotherapy KPS score | ||||
| ≥80 | 10 | 138 | 7.097 | 0.008 |
| <80 | 17 | 79 | ||
| operation time | ||||
| ≥180 min | 12 | 40 | 9.688 | 0.002 |
| <180 min | 15 | 177 | ||
| Intraoperative blood loss | ||||
| ≥200 mL | 10 | 72 | 0.160 | 0.689 |
| <200 mL | 17 | 145 |
Note: BMI: Body mass index; KPS: Karnofsky performance scale; TNM: Tumor Node Metastasis.
LASSO-based screening of predictors and construction of risk score
According to LASSO regression analysis on collected data, age, DM, TNM staging, chemotherapy regimen, chemotherapy cycle, post-chemotherapy albumin (g/L), pre-chemotherapy KPS and operation time were all significant (P<0.05), and 1se and min were all acceptable to seven indexes in lambda selection. Therefore, ambda.1se (0.02199) was selected for analysis (Figure 2). Based on lambda.1se, a risk score formula was constructed: 0.0035770333 + (0.0020227686* age) + (0.057554487* DM) + (0.016365428* TNM staging) + (0.048514458* chemotherapy regimen) + (0.00871801* chemotherapy cycle) + (-0.002096683* post-chemotherapy albumin (g/L) + (-0.00090206* pre-chemotherapy KPS)) + (0.000296876* operation time).
Figure 2.
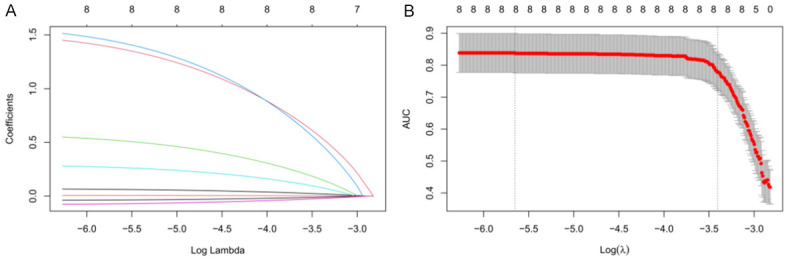
Lasso-based screening of predictors. A, B: The coefficient distribution of the least absolute shrinkage and selection operator (LASSO) repression analysis and the calculation of adjustment parameters (lambda) based on partial likelihood deviation of 10 times cross-validation.
Value of LASSO-derived risk model in predicting pulmonary infection in patients
Based on the risk score formula, the risk score of each sample was acquired. According to comparison results, the pulmonary infection group had significantly higher risk scores than the non-pulmonary infection group (P<0.0001, Figure 3A). According to ROC curve-based analysis, the area under the curve (AUC) of risk score in predicting pulmonary infection was 0.894, with a specificity of 78.57%, a sensitivity of 91.24%, Youden index of 69.81%, and cut-off value of <0.163. The results indicate a high predictive value of this model (Figure 3B).
Figure 3.
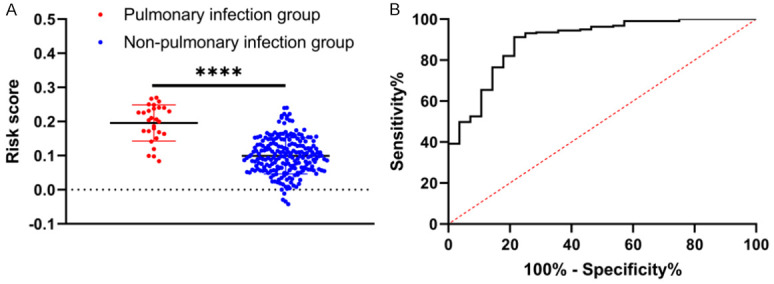
The level and predictive value of risk score in patients with pulmonary infection. A: The level of risk score in patients with pulmonary infection. B: ROC curve of risk score in forecasting pulmonary infection in patients. Note: ***, P<0.0001.
Multivariate logistic regression analysis
In order to determine whether the risk score can serve as one independent predictor of pulmonary infection, multivariate logistic regression was carried out on age, DM, TNM staging, chemotherapy regimen, chemotherapy cycle, post-chemotherapy albumin (g/L), pre-chemotherapy KPS, operation time and risk score. The data were assigned before regression (Table 2). Then the backward LR method was adopted to analyze all the data. The results showed that TNM stage, chemotherapy cycle, chemotherapy regimen, and risk score were independent factors for the prediction of pulmonary infection (P<0.05, Table 3).
Table 2.
Assignment of factors
| Factor | Assignment |
|---|---|
| Age (years) | ≥60=1, <60=0 |
| Diabetes mellitus | Yes =1, No =0 |
| TNM staging | Phase I-II =0, phase III-IV =1 |
| Chemotherapy regimen | Single =1, combined =0 |
| Chemotherapy cycle | ≥2=1, <2=0 |
| Post-chemotherapy albumin (g/L) | ≥30=0, <30=1 |
| Pre-chemotherapy KPS | ≥80=0, <80=1 |
| operation time | ≥180 min =1, <180 min =0 |
| Risk score | ≥0.163=1, <0.163=0 |
| Infection | Infected =1, uninfected =0 |
Note: TNM: Tumor Node Metastasis.
Table 3.
Multivariate logistic regression analysis
| Factor | β | SE | Wals | P value | OR value | 95% CI | |
|---|---|---|---|---|---|---|---|
|
| |||||||
| Lower limit | Upper limit | ||||||
| Age | 0.993 | 0.580 | 2.936 | 0.087 | 2.700 | 0.867 | 8.407 |
| Diabetes mellitus | 0.029 | 0.615 | 0.002 | 0.963 | 1.029 | 0.308 | 3.437 |
| TNM staging | 2.210 | 0.671 | 10.853 | 0.001 | 9.117 | 2.448 | 33.953 |
| Chemotherapy regimen | 1.364 | 0.590 | 5.357 | 0.021 | 3.913 | 1.232 | 12.427 |
| Chemotherapy cycle | 1.587 | 0.613 | 6.694 | 0.010 | 4.888 | 1.469 | 16.262 |
| Post-chemotherapy albumin | 0.766 | 0.598 | 1.641 | 0.200 | 2.150 | 0.666 | 6.938 |
| Pre-chemotherapy KPS | 0.573 | 0.591 | 0.938 | 0.333 | 1.773 | 0.556 | 5.651 |
| Operation time | -0.210 | 0.681 | 0.095 | 0.758 | 0.810 | 0.213 | 3.080 |
| Risk score | 3.997 | 0.681 | 34.431 | <0.001 | 54.434 | 14.324 | 206.866 |
Note: TNM: Tumor Node Metastasis.
Construction of a nomogram risk model
Based on 4 independent predictors, a nomogram model was constructed to predict the risk of pulmonary infection in NSCLC patients after surgery. The left endpoint of each score line was 0, and the right endpoint was 17.5, 6, 32.5, and 100 respectively, from the TNM, with a total score of 156 (Figure 4A). For instance, for a patient with TNM stage of III, a single chemotherapy regimen, a chemotherapy cycle of 1, and risk score of 0.171. The score was 10 + 6 + 0 + 100=116, and the corresponding probability of pulmonary infection was 63% according to the nomogram model. The Bootstrap method (after the original data was repeatedly sampled for 1,000 times) was adopted to verify the nomogram model internally. According to the results, the C-index of internal verification was 0.900 (95% CI: 0.839-0.961), and the calibration curves were well fitted with the ideal ones (Figure 4B).
Figure 4.
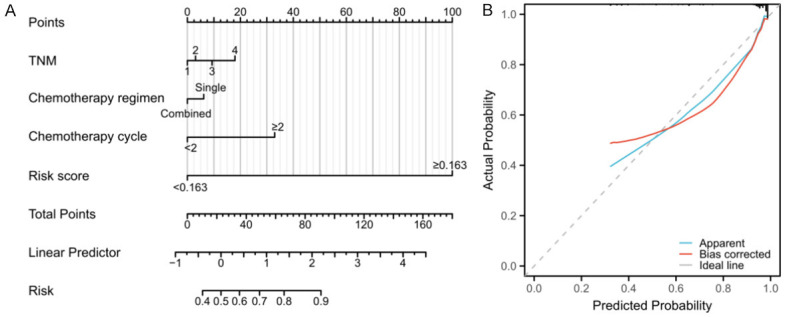
Nomogram risk model. Note: TNM: Tumor Node Metastasis.
Discussion
This study retrospectively analyzed the risk factors for PPI in NSCLC patients, and found that age, chemotherapy cycle, pre-chemotherapy KPS, and risk score were predictors of PPI in the patients. The study also successfully constructed a nomogram risk model for the evaluation of PPI.
Without obvious manifestations in the early stage, NSCLC has often entered the middle or advanced stage already at the time of diagnosis, and the patient has thus lost the optimal timing for surgical treatment, so that it can only be treated with chemotherapy, radiotherapy, and targeted therapy, and the 5-year survival rate is less than 15% [14]. Neoadjuvant chemotherapy combined with surgery is a crucial method for the therapy of NSCLC. According to prior research [15], neoadjuvant chemotherapy can strongly reduce the tumor volume and staging and facilitate operation. However, not all patients can acquire effective alleviation after surgical treatment, and the occurrence of postoperative complications is the key to the patients’ prognosis [16]. PPI is a frequent and serious complication after cancer surgery. Postoperative infection can be triggered by various factors, such as surgical incision, prolonged immobility, decreased immunity, and exposure to bacteria [17]. Reportedly, the occurrence of pulmonary infection is associated with the clinical characteristics, treatment factors, and immune dysfunction of patients [18]. However, there is a great heterogeneity in the prediction efficiency among multiple factors, and a consistent and effective prediction model for pulmonary infection in NSCLC patients has not yet been reached [19].
In this study, the factors affecting patients’ pulmonary infection were predicted through LASSO risk regression [20]. LASSO regression is highly useful in the cases where the number of predictions exceeds that of observations, because it helps to reduce the complexity of the model by eliminating irrelevant or redundant variables [21]. Additionally, with its ability to deal with the combination of continuous, binary and classified predictors, LASSO regression becomes a general tool for regression analysis [22]. Logistic regression, as a nonlinear probabilistic prediction model, can help study the association of classified observation results with some covariates, and is thus generally adopted to analyze the high-risk factors inducing diseases in clinical practice [23]. Compared to LASSO logistics regression for prediction of continuous results, most clinical indicators are continuous detection indicators, and user-defined binary classification may cause biased model results [24]. In this study, age, DM, TNM staging, chemotherapy regimen, chemotherapy cycle, post-chemotherapy albumin (g/L), pre-chemotherapy KPS, and operation time were found to be correlated with pulmonary infection. Subsequently, all eight factors were verified to be prognostic factors for pulmonary infection through LASSO regression analysis. The risk model is adopted to quantify the probability of specific events, which is crucial in efficacy prediction, disease diagnosis, and prognosis evaluation [25]. According to the model coefficient, a risk model of pulmonary infection was constructed. According to the calculation of a risk score formula, infected patients had a notably higher risk score than non-infected patients, and the ROC-AUC of risk score in predicting pulmonary infection of patients was 0.894, indicating a high clinical value of the risk model constructed by LASSO in predicting PPI of NSCLC patients.
Finally, this study determined the independent predictive value of risk score in PPI of NSCLC patients. According to Logistic regression analysis, TNM staging, chemotherapy cycle, chemotherapy regimen, and risk score were independent factors for pulmonary infection prediction. With the aggravation of the patient’s condition, the function of various tissues and organs deteriorates, which results in degenerative changes in lung structure and function, decreased respiratory muscle tension, and correspondingly weakened tolerance to surgery, making the patients more prone to pulmonary infection [26].
Additionally, the chemotherapy cycle was found to have a strong correlation with patients’ infection, which is mainly because with the increase of the number of chemotherapy cycles. Patients have weakened immunity, worse constitution and gradually reduced tolerance, thus facing a higher risk of infection [27]. It is well known that chemotherapy increases the risk for infection in cancer patients, including postoperative lung infection. The specific chemotherapy regimen adopted may also affect the risk of PPI. A prior study has found that patients receiving platinum-based regimens/neoadjuvant chemotherapy may face a higher risk of PPI than patients who received non-platinum-based regimens/no neoadjuvant chemotherapy [28]. In this study, risk score was found to be an independent predictor of PPI in NSCLC patients, indicating the possibility of using risk score as an independent predictor of PPI in NSCLC patients. At the end of the study, a nomogram was constructed based on the logistic regression results. Nomogram is a graph based on multivariate regression model, which integrates multiple prediction indexes and is drawn by several calibrated line segments. It can visualize complex data, making the prediction model more intuitive and readable, and facilitating individualized risk assessment of patients. In order to avoid over-fitting, C-index, calibration curve, ROC curve and decision curve can be adopted for model verification to make the results more reliable. In this study, the results showed that the C-index of internal verification was 0.900 (95% CI: 0.839-0.961), and the calibration curves were well fitted with the ideal ones, which verified a high prediction accuracy of the nomogram model in forecasting pulmonary infection.
This study has successfully constructed a risk model for pulmonary infection based on LASSO regression and logistics regression. However, it still has some limitations. First, the samples enrolled in this study are relatively few, so we are unable to divide the data into verification sets and training sets to verify the effectiveness of the model internally. Second, in such a single-center study, whether the model is universal or not needs more data support. Therefore, we hope to collect more data in future research to improve the research conclusions.
To sum up, the prediction model based on a regression model for PPI of NSCLC patients demonstrates good predictive efficiency, which is conducive to early screening of high-risk patients and further improvement of treatment regimen.
Disclosure of conflict of interest
None.
References
- 1.Mithoowani H, Febbraro M. Non-small-cell lung cancer in 2022: a review for general practitioners in oncology. Curr Oncol. 2022;29:1828–1839. doi: 10.3390/curroncol29030150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Alexander M, Kim SY, Cheng H. Update 2020: management of non-small cell lung cancer. Lung. 2020;198:897–907. doi: 10.1007/s00408-020-00407-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Xiang G, Gu L, Chen X, Wang F, Chen B, Zhao J, Lu Y, Chang F, Zhu Y. Economic evaluation of first-line camrelizumab for advanced non-small-cell lung cancer in China. Front Public Health. 2021;9:743558. doi: 10.3389/fpubh.2021.743558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Liu T, Guo W, Luo K, Li L, Dong J, Liu M, Shi X, Wang Z, Zhang J, Yin J, Qiu N, Lu M, Chen D, Jia X, Liu H, Gu Y, Xiong Y, Zheng G, Xu G, He Z, Zhang Z. Smoke-induced SAV1 gene promoter hypermethylation disrupts YAP negative feedback and promotes malignant progression of non-small cell lung cancer. Int J Biol Sci. 2022;18:4497–4512. doi: 10.7150/ijbs.73428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Duma N, Santana-Davila R, Molina JR. Non-small cell lung cancer: epidemiology, screening, diagnosis, and treatment. Mayo Clin Proc. 2019;94:1623–1640. doi: 10.1016/j.mayocp.2019.01.013. [DOI] [PubMed] [Google Scholar]
- 6.Le Pechoux C, Pourel N, Barlesi F, Lerouge D, Antoni D, Lamezec B, Nestle U, Boisselier P, Dansin E, Paumier A, Peignaux K, Thillays F, Zalcman G, Madelaine J, Pichon E, Larrouy A, Lavole A, Argo-Leignel D, Derollez M, Faivre-Finn C, Hatton MQ, Riesterer O, Bouvier-Morel E, Dunant A, Edwards JG, Thomas PA, Mercier O, Bardet A. Postoperative radiotherapy versus no postoperative radiotherapy in patients with completely resected non-small-cell lung cancer and proven mediastinal N2 involvement (Lung ART): an open-label, randomised, phase 3 trial. Lancet Oncol. 2022;23:104–114. doi: 10.1016/S1470-2045(21)00606-9. [DOI] [PubMed] [Google Scholar]
- 7.Isono T, Kagiyama N, Takano K, Hosoda C, Nishida T, Kawate E, Kobayashi Y, Ishiguro T, Takaku Y, Kurashima K, Yanagisawa T, Takayanagi N. Outcome and risk factor of immune-related adverse events and pneumonitis in patients with advanced or postoperative recurrent non-small cell lung cancer treated with immune checkpoint inhibitors. Thorac Cancer. 2021;12:153–164. doi: 10.1111/1759-7714.13736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chen Y, Wen F, Chen H, Zhao Y, Ding L, Lu W, Liu Y, Xue Y. Analysis of the pathogenic bacteria, drug resistance, and risk factors of postoperative infection in patients with non-small cell lung cancer. Ann Palliat Med. 2021;10:10005–10012. doi: 10.21037/apm-21-2364. [DOI] [PubMed] [Google Scholar]
- 9.Asakawa A, Horio H, Yamamichi T, Okui M, Harada M. Clinical features of HIV-infected patients with non-small-cell lung cancer after lung resection. Gen Thorac Cardiovasc Surg. 2020;68:38–42. doi: 10.1007/s11748-019-01149-9. [DOI] [PubMed] [Google Scholar]
- 10.Pastorino U, Tagliabue E. Non-human determinants of lung cancer outcome: a target for inhibition of pro-metastatic effect of surgery, triggered by postoperative bacterial pneumonia. J Thorac Oncol. 2019;14:2039–2041. doi: 10.1016/j.jtho.2019.09.084. [DOI] [PubMed] [Google Scholar]
- 11.Wang JY, Pang QY, Yang YJ, Feng YM, Xiang YY, An R, Liu HL. Development and validation of a nomogram for predicting postoperative pulmonary infection in patients undergoing lung surgery. J Cardiothorac Vasc Anesth. 2022;36:4393–4402. doi: 10.1053/j.jvca.2022.08.013. [DOI] [PubMed] [Google Scholar]
- 12.Shiono S, Yoshida J, Nishimura M, Hagiwara M, Hishida T, Nitadori J, Nagai K. Risk factors of postoperative respiratory infections in lung cancer surgery. J Thorac Oncol. 2007;2:34–38. doi: 10.1097/JTO.0b013e31802bafb6. [DOI] [PubMed] [Google Scholar]
- 13.Chinese Medical Association; Oncology Society of Chinese Medical Association; Chinese Medical Association Publishing House. Chinese Medical Association guidelines for clinical diagnosis and treatment of lung cancer (Edition 2018) Zhonghua Zhong Liu Za Zhi. 2018;40:935–964. doi: 10.3760/cma.j.issn.0253-3766.2018.12.012. [DOI] [PubMed] [Google Scholar]
- 14.Chaudhary S, Singh A, Kumar P, Kaushik M. Strategic targeting of non-small-cell lung cancer utilizing genetic material-based delivery platforms of nanotechnology. J Biochem Mol Toxicol. 2021;35:e22784. doi: 10.1002/jbt.22784. [DOI] [PubMed] [Google Scholar]
- 15.Svaton M. Targeted therapy of non-small cell lung cancer. Klin Onkol. 2021;34:48–52. doi: 10.48095/ccko2021S48. [DOI] [PubMed] [Google Scholar]
- 16.Tomita M, Ayabe T, Nakamura K. The advanced lung cancer inflammation index is an independent prognostic factor after surgical resection in patients with non-small-cell lung cancer. Interact Cardiovasc Thorac Surg. 2018;26:288–292. doi: 10.1093/icvts/ivx329. [DOI] [PubMed] [Google Scholar]
- 17.Uda K, Matsui H, Fushimi K, Yasunaga H. Preoperative short-term plus postoperative physical therapy versus postoperative physical therapy alone for patients undergoing lung cancer surgery: retrospective analysis of a nationwide inpatient database. Eur J Cardiothorac Surg. 2018;53:336–341. doi: 10.1093/ejcts/ezx301. [DOI] [PubMed] [Google Scholar]
- 18.Kim HJ, Cha SI, Kim CH, Lee J, Cho JY, Lee Y, Kim GJ, Lee DH. Risk factors of postoperative acute lung injury following lobectomy for nonsmall cell lung cancer. Medicine (Baltimore) 2019;98:e15078. doi: 10.1097/MD.0000000000015078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yao ZH, Liao WY, Ho CC, Chen KY, Shih JY, Chen JS, Lin ZZ, Lin CC, Chih-Hsin Yang J, Yu CJ. Real-world data on prognostic factors for overall survival in EGFR mutation-positive advanced non-small cell lung cancer patients treated with first-line gefitinib. Oncologist. 2017;22:1075–1083. doi: 10.1634/theoncologist.2016-0331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.McEligot AJ, Poynor V, Sharma R, Panangadan A. Logistic lasso regression for dietary intakes and breast cancer. Nutrients. 2020;12:2652. doi: 10.3390/nu12092652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lu J, Wang X, Sun K, Lan X. Chrom-lasso: a lasso regression-based model to detect functional interactions using Hi-C data. Brief Bioinform. 2021;22:bbab181. doi: 10.1093/bib/bbab181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Alhamzawi R, Ali HTM. The Bayesian adaptive lasso regression. Math Biosci. 2018;303:75–82. doi: 10.1016/j.mbs.2018.06.004. [DOI] [PubMed] [Google Scholar]
- 23.Stoltzfus JC. Logistic regression: a brief primer. Acad Emerg Med. 2011;18:1099–1104. doi: 10.1111/j.1553-2712.2011.01185.x. [DOI] [PubMed] [Google Scholar]
- 24.Li Y, Lu F, Yin Y. Applying logistic lasso regression for the diagnosis of atypical Crohn’s disease. Sci Rep. 2022;12:11340. doi: 10.1038/s41598-022-15609-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Li YM, Li ZL, Chen F, Liu Q, Peng Y, Chen M. A LASSO-derived risk model for long-term mortality in Chinese patients with acute coronary syndrome. J Transl Med. 2020;18:157. doi: 10.1186/s12967-020-02319-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zhao J, Li S, Xu H. Effect of psychological nursing on postoperative complications and negative emotion in patients with non-small cell lung cancer. Minerva Med. 2021;112:673–674. doi: 10.23736/S0026-4806.20.06579-9. [DOI] [PubMed] [Google Scholar]
- 27.Yang F, Li X, Chen KZ, Jiang GC, Wang J. Tolerability and toxicity of adjuvant cisplatin and gemcitabine for treating non-small cell lung cancer. Chin Med J (Engl) 2013;126:2087–2091. [PubMed] [Google Scholar]
- 28.Rades D, Hansen HC, Schild SE, Janssen S. A new diagnosis-specific survival score for patients to be irradiated for brain metastases from non-small cell lung cancer. Lung. 2019;197:321–326. doi: 10.1007/s00408-019-00223-6. [DOI] [PubMed] [Google Scholar]


