Abstract
Dystonia is a movement disorder in which excessive muscle contractions cause abnormal movement. It is classified according to its clinical manifestations (onset, distribution, temporal and associated features) and etiology (pathology and inheritance). Deep brain stimulation (DBS) is a surgical procedure used to treat medically intractable dystonia. In this study, we aim to share our experience with general anesthesia in systemic idiopathic dystonia that was not controlled by drugs, along with a literature review. A 21-year-old man with generalized idiopathic dystonia and developmental delay was scheduled to undergo deep brain stimulator implantation under general anesthesia. Intubation of the endotracheal tube and fixation of the stereotactic frame were performed in the intensive care unit (ICU) under sedation and neuromuscular blockade before arrival at the operating room. Total intravenous anesthesia was administered. After an uneventful surgery, the patient was discharged to the ICU with an endotracheal tube. As dystonia has a wide clinical spectrum and DBS requires special anesthetic considerations, anesthesiologists should adopt proper anesthetic depth and neuromuscular blockade according to each patient’s condition.
Keywords: Idiopathic generalized dystonia, deep brain stimulation, general anesthesia
Introduction
Dystonia refers to dyskinesia that causes abnormal posture or movement due to continuous or intermittent muscle contraction [1]. It is a common form of movement disorder [2] and is classified according to clinical features based on age and site of onset, temporal distribution, accompanying symptoms, as well as according to the mechanisms based on pathology and heredity. Many types of dystonia exist with various treatments, including counseling, physical therapy, oral medications, botulinum toxin injection, and surgical intervention [1]. Deep brain electrode insertion can be performed for abnormal movement disorders that do not respond to drugs [3]. It has the peculiarities of stereotactic frame installation, magnetic resonance imaging, and arousal surgery, and requires careful airway management and anesthesia maintenance. For safe anesthesia and airway management in patients with dystonia, anesthesiologists must select the appropriate anesthesia method for each patient after identifying the patient’s movement, posture, and comorbidities in the pre-anesthetic evaluation. In this study, we aim to share our experience with general anesthesia in systemic idiopathic dystonia that was not controlled by drugs, along with a literature review.
Case
A 21-year-old man (height, 160 cm; weight, 30 kg) underwent deep brain stimulator implantation for uncontrolled systemic idiopathic dystonia. The patient was first diagnosed with a developmental disorder at the age of 2 years and continued to receive drug treatment at the Department of Pediatrics and Psychiatry to manage autism spectrum disorder and cerebral palsy. However, at the age of 20 years, the dystonia worsened, and the symptoms of bending the back and neck backward, and losing strength of the whole body and falling forward were observed.
There were no abnormal findings in the preoperative blood test and electrocardiogram, and there were no specific findings except for mild scoliosis on a simple chest radiograph.
Since brain magnetic resonance imaging (MRI) failed despite sufficient sedation due to exacerbation of dystonia; on the morning of the surgery, after muscle relaxant administration in the intensive care unit, endotracheal intubation, head frame installation, brain MRI, and admission to the operating room were performed. Midazolam 3 mg, remifentanil 0.2 mcg/mL, atracurium 6 mcg/mL, and succinylcholine 400 mg were intravenously administered in the MRI room 1.5 h before moving the patient to the operating room. The patient entered the operating room with ambu bagging of 5 L/min of oxygen. After arriving at the operating room, non-invasive blood pressure monitoring, electrocardiogram, pulse oxygen saturation, waveform variable index, and esophageal temperature were initiated. The following parameter values were noted: blood pressure, 169/112 mmHg; heart rate, 97/min; and oxygen saturation, 99%. Glycopyrrolate 0.2 mg, lidocaine 40 mg, propofol at a target concentration of 1.0 µg/mL, and remifentanil at a target concentration of 1.0 ng/mL were intravenously administered to induce anesthesia. Trans of Four (TOF) was confirmed to be 0%, and no additional administration of neuromuscular blockers was performed. Mechanical ventilation was initiated, the end-tidal carbon dioxide partial pressure was maintained at approximately 31 mmHg, and a ductus arteriosus was inserted into the right radial artery for continuous blood pressure monitoring. Thirty minutes after anesthesia induction, the patient’s movement was detected before the operation began, and 20 mg of rocuronium was administered. Subsequently, TOF was 0%. To maintain anesthesia, propofol was injected intravenously at a target concentration of 2.0 to 2.5 µg/mL and remifentanil at a target concentration of 0.1 to 1.0 ng/mL. The operation was completed with a stable systolic blood pressure of < 130 mmHg. It was confirmed that the patient’s TOF was consistently 0%, and sugmmadex 120 mg was injected intravenously to ensure recovery from the neuromuscular blockade. Since spontaneous breathing was insufficient, the patient was kept in the intensive care unit while maintaining intubation (Figure 1). After monitoring the patient for more than 1 h in the intensive care unit, he recovered stable spontaneous breathing and was extubated. The next day, he was transferred to a general ward, and he was discharged a week later as his condition improved to the point where he was able to use a wheelchair. Currently, he is able to live a sedentary life, and his dystonia in the trunk and neck has improved.
Figure 1.
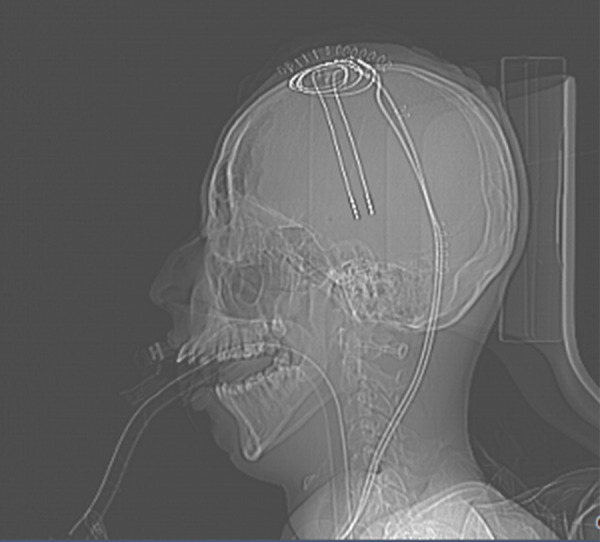
Deep brain stimulation electrodes. Lateral X-ray of electrodes (white, upper right) in the brain.
Discussion
Dystonia is generally defined as repetitive twisting movements or chronic or intermittent excessive muscle contractions [1]. In addition, it is classified according to the age of onset, site of involvement, temporal distribution, and clinical features of accompanying symptoms. There are many types of dystonia depending on the cause [4]. Various treatments are available, including counseling, physical therapy, oral medications, botulinum toxin injection, and surgical intervention. Deep brain stimulation (DBS) is a surgical intervention method for dystonia when the patient does not respond to conservative treatments, such as drug therapy [1,5]. Stimulation electrodes were implanted under general anesthesia and positioned based on the direct anatomy of the posteroventrobasal globus pallidus internus (GPi). GPi stimulation is an effective treatment for patients with dystonia with DYT1 mutations [5].
To prepare for a stereotactic procedure, such as deep brain stimulator implantation, it is necessary to check for a blood coagulation disorder, including medication history. The most common type of anesthesia used for patients undergoing deep brain stimulator implantation is monitored anesthetic care or local anesthesia with mild sedation [6]. This is because this procedure requires intraoperative neurophysiological monitoring of awake patients and precise localization of the target nuclei [7]. Therefore, it is necessary to ensure adequate sedation and analgesia during surgery and thorough monitoring to prevent excessive respiratory depression or the disappearance of symptoms [8].
However, general anesthesia may be planned for patients who cannot cooperate because of accompanying developmental disabilities, such as in the present case [9]. Therefore, it is necessary to perform a brain MRI under sedation after fixing the stereotactic frame before surgery; however, the frame makes mask ventilation and endotracheal intubation difficult. The patient had dystonic movements, including intermittent retrocollisis, and only mild scoliosis was observed on chest radiography. Although the frequency of difficult endotracheal intubation has been reported to be low in cervical torticollis, a representative of focal dystonia [10,11], anesthesiologists should consider the possibility of difficult endotracheal intubation in advance and conduct a thorough interview and physical examination. In particular, our patient had a history of failed brain MRIs despite sufficient sedation. Therefore, considering the possibility of respiratory depression, airway failure, and imaging failure, endotracheal intubation and head frame installation were performed after sedation and neuromuscular block in the intensive care unit, and a brain MRI was performed. Therefore, it is important to determine anesthesia and airway management methods in advance according to the patient’s condition.
Unlike awakening surgery, there are no restrictions on anesthetic agents, because microelectrode recordings are impossible during general anesthesia. Since anesthetics do not uniformly affect brain regions, there have been insufficient studies on how anesthetics affect microelectrode recordings [12,13]. We administered total intravenous anesthesia with propofol and remifentanil. Continuous blood pressure monitoring was performed through the ductus arteriosus because increased blood pressure could cause intracerebral hemorrhage as a complication. After anesthesia induction, additional intravenous rocuronium was administered based on TOF monitoring and patient movement. This patient was sedated, and neuromuscular blocking agents were administered before the patient entered the operating room; however, TOF monitoring was not performed in the intensive care unit and MRI room. Therefore, it was difficult to predict the appropriate dose of neuromuscular blocking agents when inducing general anesthesia in the operating room. Accordingly, the patient was extubated after injecting sugammadex at a sufficient dose, and spontaneous breathing was monitored for more than 1 h in the intensive care unit.
Tsaroucha et al. reported an anesthetic method for Inguinal hernia repair surgery for patients with focal dystonia [11]. The patient had cervical dystonia called spasmodic torticollis (ST), which is the most common primary focal dystonia [14]. Recently, DBS has been known to be an effective first-line treatment for ST [15,16], and patients underwent surgery while being treated with DBS. Bhoyar et al. also reported the experience of DBS anesthesia in patients with Meige’s syndrome [17]. Meige syndrome is a rare form of facial dystonia. However, in the case of refractory cases, pallidal DBS was reported to be effective [18,19]. In this patient, dexmedetomidine was used for anesthesia, and hemodynamic stability was maintained. Table 1 compares the generalized idiopathic dystonia anesthesia in the current study with the aforementioned anesthesia experiences in the literature. Dexmedetomidine, a relatively selective alpha2-adrenoceptor agonist, is suggested as an anesthetic for patients with dystonia by minimizing respiratory function deterioration and maintaining hemodynamic stability [20].
Table 1.
Comparison of other dystonia cases
| Include the literature | Case | Athanasia [11] | Kalpesh [17] |
|---|---|---|---|
| Disease | Generalized idiopathic dystonia | Idiopathic focal dystonia | Meige’s syndrome |
| Type of surgery | Deep brain stimulation insertion | Inguinal hernia repair | Deep brain stimulation insertion |
| Sedative agent | midazolam 3 mg propofol 1.0 mcg/mL by TIVA | propofol 2.5 mg/kg by TIVA | Dexmedetomidine 1 mcg/kg (60 mcg) continuous IV infusion @ 0.5 mcg/kg/h |
| Neuromuscular blockage agent | atracurium 6 mcg/cc, succinylcholine 400 mg, rocuronium 20 mg | rocuronium 70 mg | vecuronium 6 mg |
| Neuromuscular block reversal agent | sugammadex 120 mg | sugammadex 140 mg | neostigmine and glycopyrrolate |
| Complication | none | none | none |
TIVA: total intravenous anesthesia.
The use of local or general anesthesia during deep brain stimulator implantation is not contraindicated. With advances in medicine, the survival rate of patients with rare diseases, such as dystonia, will increase. As the indications for DBS increase, there is an increased risk of patients entering the operating room, or of operation in other departments for patients who already have undergone DBS. Therefore, standardized anesthesia protocols for patients undergoing DBS transplantation as well as orthopedic and gynecological surgeries, unrelated to neurosurgery, for patients of DBS transplantation are required.
Acknowledgements
We would like to thank Chungbuk National University Hospital for financial support. We would like to thank Editage (www.editage.co.kr) for the English language editing.
Disclosure of conflict of interest
None.
References
- 1.Jinnah HA. The dystonias. Continuum (Minneap Minn) 2019;25:976–1000. doi: 10.1212/CON.0000000000000747. [DOI] [PubMed] [Google Scholar]
- 2.Lee JY, Deogaonkar M, Rezai A. Deep brain stimulation of globus pallidus internus for dystonia. Parkinsonism Relat Disord. 2007;13:261–265. doi: 10.1016/j.parkreldis.2006.07.020. [DOI] [PubMed] [Google Scholar]
- 3.Toda H, Hamani C, Lozano A. Deep brain stimulation in the treatment of dyskinesia and dystonia. Neurosurg Focus. 2004;17:E2. doi: 10.3171/foc.2004.17.1.2. [DOI] [PubMed] [Google Scholar]
- 4.Albanese A, Bhatia K, Bressman SB, DeLong MR, Fahn S, Fung VS, Hallett M, Jankovic J, Jinnah HA, Klein C, Lang AE, Mink JW, Teller JK. Phenomenology and classification of dystonia: a consensus update. Mov Disord. 2013;28:863–873. doi: 10.1002/mds.25475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Coubes P, Vayssiere N, El Fertit H, Hemm S, Cif L, Kienlen J, Bonafe A, Frerebeau P. Deep brain stimulation for dystonia. Surgical technique. Stereotact Funct Neurosurg. 2002;78:183–191. doi: 10.1159/000068962. [DOI] [PubMed] [Google Scholar]
- 6.Venkatraghavan L, Manninen P, Mak P, Lukitto K, Hodaie M, Lozano A. Anesthesia for functional neurosurgery: review of complications. J Neurosurg Anesthesiol. 2006;18:64–67. doi: 10.1097/01.ana.0000181285.71597.e8. [DOI] [PubMed] [Google Scholar]
- 7.Halpern C, Hurtig H, Jaggi J, Grossman M, Won M, Baltuch G. Deep brain stimulation in neurologic disorders. Parkinsonism Relat Disord. 2007;13:1–16. doi: 10.1016/j.parkreldis.2006.03.001. [DOI] [PubMed] [Google Scholar]
- 8.Lagueny A, Burbaud P, Le Masson G, Bergouignan FX, Ferrer X, Julien J. Involvement of respiratory muscles in adult-onset dystonia: a clinical and electrophysiological study. Mov Disord. 1995;10:708–713. doi: 10.1002/mds.870100603. [DOI] [PubMed] [Google Scholar]
- 9.Panov F, Gologorsky Y, Connors G, Tagliati M, Miravite J, Alterman RL. Deep brain stimulation in DYT1 dystonia: a 10-year experience. Neurosurgery. 2013;73:86–93. doi: 10.1227/01.neu.0000429841.84083.c8. discussion 93. [DOI] [PubMed] [Google Scholar]
- 10.Mac TB, Girard F, McKenty S, Chouinard P, Boudreault D, Ruel M, Bouvier G. A difficult airway is not more prevalent in patients suffering from spasmodic torticollis: a case series. Can J Anaesth. 2004;51:250–253. doi: 10.1007/BF03019105. [DOI] [PubMed] [Google Scholar]
- 11.Tsaroucha A, Moschovou T, Melemeni A, Argyra E. Anesthetic management of a patient with spasmodic torticollis treated with a deep brain stimulator implant operated for inguinal hernia. J Med Cases. 2016;7:376–378. [Google Scholar]
- 12.Velly LJ, Rey MF, Bruder NJ, Gouvitsos FA, Witjas T, Regis JM, Peragut JC, Gouin FM. Differential dynamic of action on cortical and subcortical structures of anesthetic agents during induction of anesthesia. Anesthesiology. 2007;107:202–212. doi: 10.1097/01.anes.0000270734.99298.b4. [DOI] [PubMed] [Google Scholar]
- 13.Bos MJ, Buhre W, Temel Y, Joosten EA, Absalom AR, Janssen ML. Effect of anesthesia on microelectrode recordings during deep brain stimulation surgery: a narrative review. J Neurosurg Anesthesiol. 2021;33:300–307. doi: 10.1097/ANA.0000000000000673. [DOI] [PubMed] [Google Scholar]
- 14.Zabani I, Vaghadia H. Refractory dystonia during propofol anaesthesia in a patient with torticollis-dystonia disorder. Can J Anaesth. 1996;43:1062–1064. doi: 10.1007/BF03011910. [DOI] [PubMed] [Google Scholar]
- 15.Smith DL, DeMario MC. Spasmodic torticollis: a case report and review of therapies. J Am Board Fam Pract. 1996;9:435–441. [PubMed] [Google Scholar]
- 16.Venkatraghavan L, Luciano M, Manninen P. Review article: anesthetic management of patients undergoing deep brain stimulator insertion. Anesth Analg. 2010;110:1138–1145. doi: 10.1213/ANE.0b013e3181d2a782. [DOI] [PubMed] [Google Scholar]
- 17.Bhoyar KV, Gujjar P, Shinde S, Kotak N. Anesthetic management of deep brain stimulator implantation in Meige’s syndrome. J Anaesthesiol Clin Pharmacol. 2012;28:111–3. doi: 10.4103/0970-9185.92459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhan S, Sun F, Pan Y, Liu W, Huang P, Cao C, Zhang J, Li D, Sun B. Bilateral deep brain stimulation of the subthalamic nucleus in primary Meige syndrome. J Neurosurg. 2017;128:897–902. doi: 10.3171/2016.12.JNS16383. [DOI] [PubMed] [Google Scholar]
- 19.Reese R, Gruber D, Schoenecker T, Bäzner H, Blahak C, Capelle HH, Falk D, Herzog J, Pinsker MO, Schneider GH, Schrader C, Deuschl G, Mehdorn HM, Kupsch A, Volkmann J, Krauss JK. Long-term clinical outcome in meige syndrome treated with internal pallidum deep brain stimulation. Mov Disord. 2011;26:691–698. doi: 10.1002/mds.23549. [DOI] [PubMed] [Google Scholar]
- 20.Maurtua MA, Cata JP, Martirena M, Deogaonkar M, Rezai A, Sung W, Lotto M, Niezgoda J, Schubert A. Dexmedetomidine for deep brain stimulator placement in a child with primary generalized dystonia: case report and literature review. J Clin Anesth. 2009;21:213–216. doi: 10.1016/j.jclinane.2008.06.038. [DOI] [PubMed] [Google Scholar]


