Summary
Background
Integrase inhibitor (INSTI) with boosted darunavir (DRV/r), a regimen with a high-resistance barrier, avoiding NRTI toxicities, might be a switching option in children living with HIV (CLWHIV).
Methods
SMILE is a randomised non-inferiority trial evaluating safety and antiviral efficacy of once-daily INSTI + DRV/r vs. continuing on current standard-of-care (SOC) triple ART (2NRTI + boosted PI/NNRTI) in virologically-suppressed CLWHIV aged 6–18 years. The primary outcome is the proportion with confirmed HIV-RNA ≥50 copies/mL by week 48, estimated by Kaplan–Meier method. Non-inferiority margin was 10%. Registration number for SMILE are: ISRCTN11193709, NCT #: NCT02383108.
Findings
Between 10th June 2016 and 30th August 2019, 318 participants were enrolled from Africa 53%, Europe 24%, Thailand 15% and Latin America 8%, 158 INSTI + DRV/r [153 Dolutegravir (DTG); 5 Elvitegravir (EVG)], 160 SOC. Median (range) age was 14.7 years (7.6–18.0); CD4 count 782 cells/mm3 (227–1647); 61% female. Median follow-up was 64.3 weeks with no loss to follow-up. By 48 weeks, 8 INSTI + DRV/r vs. 12 SOC had confirmed HIV-RNA ≥50 copies/mL; difference (INSTI + DRV/r-SOC) −2.5% (95% CI: −7.6, 2.5%), showing non-inferiority. No major PI or INSTI resistance mutations were observed. There were no differences in safety between arms. By week 48, difference (INSTI + DRV/r-SOC) in mean CD4 count change from baseline was −48.3 cells/mm3 (95% CI: −93.4, −3.2; p = 0.036). Difference (INSTI + DRV/r-SOC) in mean HDL change from baseline was −4.1 mg/dL (95% CI: −6.7, −1.4; p = 0.003). Weight and Body Mass Index (BMI) increased more in INSTI + DRV/r than SOC [difference: 1.97 kg (95% CI: 1.1, 2.9; p < 0.001), 0.66 kg/m2 (95% CI: 0.3, 1.0; p < 0.001)].
Interpretation
In virologically-suppressed children, switching to INSTI + DRV/r was non-inferior virologically, with similar safety profile, to continuing SOC. Small but significant differences in CD4, HDL-cholesterol, weight and BMI were observed between INSTI + DRV/r vs. SOC although clinical relevance needs further investigation. SMILE data corroborate adult findings and provide evidence for this NRTI-sparing regimen for children and adolescents.
Funding
Fondazione Penta Onlus, Gilead, Janssen, INSERM/ANRS and UK MRC. ViiV-Healthcare provided Dolutegravir.
Keywords: HIV, Children, Adolescents, Randomised, Paediatrics, Dual therapy, Maintenance, Simplification, Dolutegravir, Darunavir, Virological suppressed
Research in context.
Evidence before this study
At the time SMILE started, standard antiretroviral treatment regimens in children and adolescents included 2 nucleoside/nucleotide reverse transcriptase inhibitors (NRTIs) with either a non-nucleoside reverse transcriptase inhibitor (NNRTI) or a boosted protease inhibitor (bPI). This implies in some cases, pill burden, sometimes BID regimens and related toxicities due to long-term exposure with the risk of cumulative side-effects. A dual therapy strategy combining bPI + INSTI could be a potential option to reduce NRTI-related drug toxicity.
We searched PubMed for clinical trials and cohort studies published up to October 7, 2022, that described randomized control trials or open-label studies using the search terms in title (dolutegravir OR Integrase inhibitor OR darunavir OR boosted protease inhibitor OR ritonavir) AND (dual therapy OR second-line ART OR maintenance OR simplification OR switching OR switch) AND (trial OR randomized OR randomised OR open label OR open-label). Only one randomized trial included dolutegravir with boosted darunavir in virologically suppressed adults, corresponding to the DUALIS study that showed non-inferiority of switching to DTG + DRV/r vs. continuing with triple therapy including DRV/r, in virologically-suppressed PLWHIV.
Added value of this study
To our knowledge, this was the first randomized trial of dual therapy with INSTI plus DRV/r in children and adolescents as maintenance therapy after prolonged viral suppression. The study showed that switching to an INSTI plus DRV/r is highly efficacious and non-inferior virologically in maintaining virological suppression at 48 weeks, with similar safety profile to continuing SOC and low risk of treatment -emergent resistance. SMILE data corroborate similar findings in adults and provide evidence for an effective NRTI-sparing regimen for children and adolescents as maintenance therapy.
Implications of all the available evidence
WHO guidelines suggest that innovative approaches including new drugs with minimal risk of cross-resistance such as INSTIs and second-generation NNRTIs and PIs need to be investigated in children as data are lacking. SMILE results contribute to filling this research gap by providing data on the potential use of the DTG + DRV/r combination in children. They also provide evidence of efficacy of a regimen that can be used when NRTI/NNRTIs are contraindicated or not tolerated. In addition, the use of a once daily potent dual combination can be useful to simplify complex regimen in children and adolescents.
Introduction
Triple-drug antiretroviral therapy (ART) has been the mainstay of treatment in adults living with HIV and is included among the current recommendations in adults, adolescents and children living with HIV.1, 2, 3 At the time this trial started, standard treatment regimens in children and adolescents included 2 nucleoside/nucleotide reverse transcriptase inhibitors (NRTIs) with either a non-nucleoside reverse transcriptase inhibitor (NNRTI) or a boosted protease inhibitor (bPI). Whilst treatment has dramatically improved disease outcomes, important limitations remain for children and young people such as pill burden, twice-daily dosing and side effects, which may be particularly challenging in CLWHIV because they start ART early and for life. Although new fixed dose combinations, such as tenofovir/lamivudine/dolutegravir (TLD) are increasingly available in low and middle income countries (LMIC), evaluation of alternative NRTI sparing regimen remains relevant. Children may be more vulnerable to adverse effects of drugs due to metabolic changes related to growth.
NRTIs may contribute to mitochondrial toxicity which is dose-related and cumulative. Therefore, it is desirable to reduce cumulative NRTI exposure in children. A dual therapy strategy combining bPI + integrase inhibitor (INSTI) could be a potential option to reduce NRTI-related drug toxicity. In addition, WHO recommendations suggest that third-line regimens in children should include new drugs with minimal risk of cross-resistance to previously used regimens, such as INSTIs, second-generation NNRTIs and PIs and that these innovative approaches need to be investigated in children as data are lacking.
The SMILE-PENTA-17 trial is the first randomised trial in children to assess outcomes following switching from a fully suppressive 3 drug regimen including 2 NRTIs to ritonavir boosted DRV (DRV/r) plus a once daily INSTI compared with continuing current therapy. Our hypothesis is that children with chronic HIV infection on ART with suppressed viral load will maintain similar levels of suppression with once daily INSTI + DRV/r compared to continued standard of care triple ART.
Methods
Study design and participants
SMILE is an open-label, multicentre, randomized, non-inferiority phase 2/3 trial which enrolled children living with HIV (CLWHIV) aged 6–18 years, who were virologically-suppressed (HIV-1 RNA <50 copies/mL) for at least 12 months and had been on a 3-drug bPI or NNRTI containing regimen for at least 24 weeks. Main exclusion criteria included pregnancy, significant liver disease, known biliary abnormalities, Hepatitis B or Hepatitis C co-infection, diagnosis of tuberculosis and on anti-tuberculosis treatment, evidence of resistance to DRV/r or INSTI and prior INSTI exposure for >2 weeks.
Participants were randomized 1:1 to either switch to INSTI + DRV/r (NRTI-sparing regimen) or to continue on current SOC triple ART regimen according to a computer-generated randomization list incorporated within the study database. Randomization was stratified by age (6 < 12 years, 12 < 18 years) and region (Africa vs. other).
Of note, under version 1.1 of the protocol, EVG was the study INSTI. Due to the company's plan to withdraw EVG from the market as a single agent, recruitment was temporarily suspended and restarted under a new version 2.0 using DTG with the caveat that children would only be enrolled if aged ≥12 years. For children randomised to INSTI + DRV/r, criteria to resume SOC included: HIV-RNA ≥50 copies/mL on two consecutives measures within 4 weeks; new or recurrent CDC stage C or severe stage B event and toxicity according to the toxicity grading for clinical events; pregnancy; three unconfirmed HIV-1 RNA ≥50 copies/mL during any 12-month period.
Procedures
The trial was approved by local/National Ethics Committees and relevant Competent Authorities. All participants/families provided informed consent as appropriate before any procedure was performed.
Participants were seen at screening, enrolment, 4, 12, 24, 36 and 48 weeks and then every 12–16 weeks until the last participant reached 48 weeks follow-up (31st July 2020).
At each visit, weight and height were measured, a pregnancy test (in all females of childbearing potential) was performed and HIV clinical progression, adverse events, notable events (pregnancies, suicidal ideation or behaviours, drug induced liver injury and COVID-19 contact) and adherence to treatment were assessed. HIV-1 RNA was measured at each study visit (week 4 test was optional); participants with HIV-1 RNA ≥50 copies/mL returned within 4 weeks for HIV-1 RNA confirmatory test. A plasma sample for resistance testing was requested for participants who had confirmed HIV-1 RNA ≥50 copies/mL up to week 48 censoring date [week 54 date (48 + 6 weeks)]., CD4 measurements, fasting lipids/glucose, were performed at baseline, 24 and 48 weeks; biochemistry and haematology tests were performed at baseline, weeks 4, 24 and 48 and optionally at weeks 12 and 36. Paediatric Quality of Life (PedsQL™) was evaluated at baseline, weeks 24 and 48 and acceptability questionnaires were completed at baseline and week 48.
Outcomes
The primary endpoint was virological failure by 48 weeks defined as HIV-1 RNA ≥50 copies/mL (confirmed within 4 weeks) at any time up to week 48. Main secondary endpoints included efficacy (HIV-1 RNA ≥50 copies/mL, ≥400 copies/mL at weeks 24 and 48; new resistance mutations defined by the IAS-USA list of mutations 20194; death, new or recurrent CDC stage C or severe stage B event (defined as severe lung disease including LIP, nephropathy, cardiomyopathy, failure to thrive in absence of remediable causes, recurrent bacterial pneumonia, severe or recurrent oral candidiasis); changes in CD4 absolute and percentage from baseline to weeks 24 and 48 and safety (grade 3 or 4 laboratory and clinical adverse events particularly lipodystrophy; any adverse event leading to discontinuation or modification of the treatment regimen; changes in ART; changes in blood lipids from baseline to weeks 24 and 48). Other secondary endpoints were height and weight, adherence as measured by questionnaire and visual analogue scale and acceptability and quality of life over 48 weeks as assessed by patient completed questionnaires.
All serious adverse events (SAE), notable events, CDC stage C and severe stage B events and clinical grade 3/4 events during the trial were reviewed by an independent Endpoint Review Committee, blinded to randomized allocation. The Independent Data Monitoring Committee reviewed interim data including recruitment progress, efficacy and safety data.
Sample size
Non-inferiority of INSTI + DRV/r was assessed by the difference between the INSTI + DRV/r arm and SOC arm in the estimated proportion of participants with confirmed HIV-RNA ≥50 copies/mL by week 48 after randomisation. Non-inferiority was to be inferred if the upper bound of the two-sided 95% confidence interval (CI) for the difference between the two arms (INSTI + DRV/r-SOC) was less than 10% (the non-inferiority margin). Assuming 90% of participants in both arms of SMILE maintained HIV-1 RNA <50 copies/mL through 48 weeks, a total of 300 participants (150 per arm) was determined to provide 80% power for the upper bound of the 95% CI for the difference in virological suppression to be less than 10%, allowing for 5% loss to follow-up.
Statistical analysis
For analysis of the primary endpoint, follow-up was to week 48 censoring date [week 54 date (48 + 6 weeks)]. For safety and occurrence of new or recurrent CDC stage C or severe stage B events or death analysis, the primary analysis included all follow-up to the latest of 31st July 2020 (date last enrolled participant reached 48 weeks follow-up) or week 48 censoring date.
Comparisons between randomised arms were intent-to-treat, adjusting for stratification factors. The primary outcome was assessed by time to virologic failure (confirmed HIV-1 RNA ≥50 copies/mL) using Kaplan Meier curves to estimate the proportion of participants failing in each arm at any time up to the week 48 censoring date. The cumulative failure function for each randomized arm was estimated as a weighted mean of the corresponding stratum-specific cumulative failure functions (estimated from a Cox model adjusting for stratification factors and randomized arm), with weights proportional to the number of participants in each stratum at baseline. The estimated probability of treatment failure by week 48 was compared between arms. 95% bias-corrected confidence intervals were estimated using bootstrap (sampled 1000 times and stratified by stratification factors). A per-protocol analysis of the primary endpoint was performed by censoring the follow-up of participants who discontinued any component of the allocated treatment for any reason, except for change due to change in national clinical guidelines in the SOC arm. Interruption (stop) of the regimen for >31 days for any reason was defined as treatment discontinuation and participants were censored at interruption date. In addition, the proportion of subjects with HIV-1 RNA ≥50 copies/mL at week 48 was determined using the FDA snapshot algorithm5 (Appendices 2 and 3 for details and flowchart description of the FDA snapshot analysis algorithm). All statistical tests were 2-sided and comparisons were adjusted for the stratification factors.
Changes in continuous variables from baseline to weeks 24 and 48 in each randomised group were calculated using normal regression of absolute values, adjusting for the baseline measurement and stratification factors. Average treatment differences through follow-up to week 48 were estimated by fitting linear mixed models with random effect for intercept and fixed effects for treatment assignment, post-randomisation study visit and adjustment covariates (baseline level of the outcome variable and stratification factors).
Differences between treatment groups in binary outcome variables were tested using Chi-squared tests or Fisher's exact tests, as appropriate and logistic regression models for adjusted analyses. Clinical and laboratory adverse events were classified by System Organ Class according to the Medical Dictionary for Regulatory Activities (MedDRA) version 23.0. The total number of adverse events and number of patients with at least one event were computed. Adverse event rates (per 100-person years) in each arm were calculated as the number of events/total person years at risk∗100. Adverse events were compared between arms by Cox proportional hazards regression for time to first event and Poisson regression (adjusted for clustering within participants) for total event rates.
Role of the funding source
Members of the Trial Management Group and Paediatric European Network for Treatment of AIDS Foundation were authors of the paper who were involved in study design, data collection, data analysis, and interpretation, or writing the report. Pharmaceutical Companies who provided dolutegravir and darunavir, reviewed the manuscript.
Results
Patients
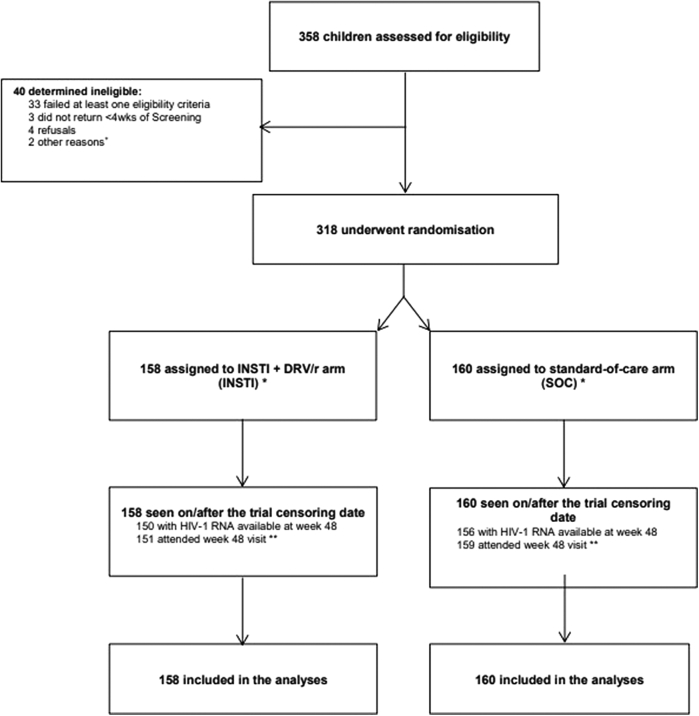
318 participants were randomized between 10th June 2016 and 30th August 2019 (158 INSTI + DRV/r; 160 SOC). All participants randomized met eligibility criteria for enrolment and were included in the final analysis: Uganda 110, South Africa 60, Ukraine 50, Thailand 47, Argentina 13, Mexico 12 and Europe 26 (See SMILE CONSORT diagram in Fig. 1).
Fig. 1.
SMILE total population CONSORT diagram. + One participant was ineligible due to interruption in recruitment due to EVG withdrawal off the market. One participant was ineligible due to viral resistance to Darunavir. ∗ 5 participants in each arm were randomised before temporary suspension due to EVG withdrawal from the market. ∗∗ During the COVID-19 pandemic, sites were advised they could conduct follow-up visits by phone if there was an additional risk to come in person. Eight participants missed the trial endpoint visit at week 48 (7 in INSTI + DRV/r; 1 in SOC); 5 out of the 8 participants came in 1–6 days late and missed week 48 max window (fell in week 60 window), 3 participants missed week 48 clinic visit/telephone follow-up.
Baseline characteristics were generally well-balanced between arms (Table 1). Most participants had been vertically infected (95%) with median (IQR) for age, weight and Body Mass Index (BMI) at enrolment of 14.7 (13.6, 16.3) years, 47.8 (43.5, 52.2) kg, and 19.2 (17.8, 21.1) kg/m2, respectively. More females (61%) were enrolled and 55%were of black-African ethnic origin. Median (IQR) CD4% was 36% (32, 40) and CD4 count was 782 cells/mm3 (631, 984). Most participants (66%) had CD4 counts between 500 and 1000 cells/mm3. The overall median (range) cumulative ART exposure at enrolment was 111, 2, 3, 4, 5, 6, 7, 8, 9, 10, 11, 12, 13, 14, 15, 16, 17 years; 26% had only been exposed to NRTI and PI; 54% had only been exposed to NRTI and NNRTI; 20% had been exposed to NRTI, NNRTI and PI (Appendix 1). Overall, 59% were receiving NNRTI- and 41% bPI-based regimens immediately prior to randomisation. In the INSTI + DRV/r arm, 5 (3%) participants started EVG and 153 (97%) DTG at enrolment. Median (IQR) follow-up overall was 64.3 (55.4, 72.0) weeks. None of the participants were lost to follow-up. All participants were seen on or after the trial censoring date of 31 July 2020. Overall visit attendance was very high (99% by week 48; 98% by end of trial).
Table 1.
Baseline characteristics and HIV related parameters.
| INSTI + DRV/r | SOC | Total | |
|---|---|---|---|
| Participants randomised | 158 | 160 | 318 |
| Sex [n] | 158 | 160 | 318 |
| Male | 66 (42%) | 58 (36%) | 124 (39%) |
| Female | 92 (58%) | 102 (64%) | 194 (61%) |
| Ethnic origin [n] | 158 | 160 | 318 |
| White | 32 (20%) | 38 (24%) | 70 (22%) |
| Black-African | 87 (55%) | 87 (54%) | 174 (55%) |
| Black-other | 0 (0%) | 1 (1%) | 1 (0%) |
| Asian | 29 (18%) | 22 (14%) | 51 (16%) |
| Mixed black-white | 2 (1%) | 1 (1%) | 3 (1%) |
| Othera | 8 (5%) | 11 (7%) | 19 (6%) |
| Route of HIV infection [n] | 158 | 160 | 318 |
| Mother to child | 146 (92%) | 155 (97%) | 301 (95%) |
| Unknown | 12 (8%) | 5 (3%) | 17 (5%) |
| Age (years) [n] | 158 | 160 | 318 |
| Median (IQR) | 14.7 (13.5, 16.4) | 14.6 (13.7, 16.3) | 14.7 (13.6, 16.3) |
| [Range] | [9.5–18.0] | [7.6–17.9] | [7.6–18.0] |
| ≥6–12 years | 2 (1%) | 2 (1%) | 4 (1%) |
| ≥12–18 years | 156 (99%) | 158 (99%) | 314 (99%) |
| Weight (kg) [n] | 158 | 160 | 318 |
| Median (IQR) | 47.3 (42.9, 51.8) | 48.0 (44.0, 53.0) | 47.8 (43.5, 52.2) |
| [Range] | [28.5–96.3] | [22.1–82.2] | [22.1–96.3] |
| Height (cm) [n] | 158 | 160 | 318 |
| Median (IQR) | 157 (152, 162) | 158 (153, 163) | 158 (152, 163) |
| [Range] | [134–189] | [120–182] | [120–189] |
| Body mass index (kg/m2) [n] | 158 | 160 | 318 |
| Median (IQR) | 19.1 (17.7, 21.1) | 19.2 (17.9, 21.1) | 19.2 (17.8, 21.1) |
| [Range] | [14.8–33.3] | [15.3–30.3] | [14.8–33.3] |
| Weight-for-age z-scoreb[n] | 158 | 160 | 318 |
| Median (IQR) | −0.4 (−1.2, 0.2) | −0.4 (−1.1, 0.2) | −0.4 (−1.2, 0.2) |
| [Range] | [−3.7 to 3.2] | [−4.1 to 2.0] | [−4.1 to 3.2] |
| <−3 | 4 (3%) | 3 (2%) | 7 (2%) |
| ≥−3 to < −2 | 12 (8%) | 10 (6%) | 22 (7%) |
| ≥−2 to <0 | 90 (57%) | 96 (60%) | 186 (58%) |
| ≥0 | 52 (33%) | 51 (32%) | 103 (32%) |
| Height-for-age z-scoreb[n] | 158 | 160 | 318 |
| Median (IQR) | −0.7 (−1.4, −0.1) | −0.6 (−1.4, 0.1) | −0.7 (−1.4, 0.0) |
| [Range] | [−3.5 to 2.2] | [−3.1 to 1.9] | [−3.5 to 2.2] |
| <−3 | 3 (2%) | 1 (1%) | 4 (1%) |
| ≥−3 to < −2 | 16 (10%) | 14 (9%) | 30 (9%) |
| ≥−2 to <0 | 103 (65%) | 99 (62%) | 202 (64%) |
| ≥0 | 36 (23%) | 46 (29%) | 82 (26%) |
| BMI-for-age z-scoreb[n] | 158 | 160 | 318 |
| Median (IQR) | −0.1 (−0.9, 0.6) | −0.2 (−0.8, 0.7) | −0.1 (−0.8, 0.6) |
| [Range] | [−3.5 to 2.8] | [−3.1 to 2.8] | [−3.5 to 2.8] |
| <−3 | 1 (1%) | 1 (1%) | 2 (1%) |
| ≥−3 to < −2 | 3 (2%) | 2 (1%) | 5 (2%) |
| ≥−2 to <0 | 77 (49%) | 85 (53%) | 162 (51%) |
| ≥0 | 77 (49%) | 72 (45%) | 149 (47%) |
| CD4% [n] | 156 | 159 | 315 |
| Median (IQR) | 36 (32, 40) | 36 (32, 41) | 36 (32, 40) |
| [Range] | [17–53] | [17–54] | [17–54] |
| <30% | 27 (17%) | 30 (19%) | 57 (18%) |
| ≥30–40% | 83 (53%) | 80 (50%) | 163 (52%) |
| ≥40% | 46 (29%) | 49 (31%) | 95 (30%) |
| Missing [n] | 2 | 1 | 3 |
| CD4 (cells/mm3) [n] | 156 | 159 | 315 |
| Median (IQR) | 775 (636, 967) | 803 (630, 985) | 782 (631, 984) |
| [Range] | [283–1642] | [227–1647] | [227–1647] |
| <350 | 3 (2%) | 2 (1%) | 5 (2%) |
| ≥350–500 | 15 (10%) | 12 (8%) | 27 (9%) |
| ≥500–1000 | 100 (64%) | 107 (67%) | 207 (66%) |
| ≥1000–1500 | 37 (24%) | 35 (22%) | 72 (23%) |
| ≥1500 | 1 (1%) | 3 (2%) | 4 (1%) |
| Missing [n] | 2 | 1 | 3 |
| Antiretroviral regimen prior to randomisation | |||
| NRTI backbone [n (%)] | |||
| ABC 3TC | 63 (39.9%) | 51 (31.9%) | 114 (35.8%) |
| ABC ZDV | 2 (1.3%) | 0 (0.0%) | 2 (0.6%) |
| ABC ddI | 0 (0.0%) | 1 (0.6%) | 1 (0.3%) |
| FTC TAF | 1 (0.6%) | 2 (1.3%) | 3 (0.9%) |
| FTC TDF | 28 (17.7%) | 28 (17.5%) | 56 (17.6%) |
| TDF 3TC | 16 (10.1%) | 21 (13.1%) | 37 (11.6%) |
| ZDV 3TC | 48 (30.4%) | 57 (35.6%) | 105 (33.0%) |
| Anchor drug [n (%)] | |||
| NNRTI | 92 (58.2%) | 97 (60.6%) | 189 (59.4%) |
| EFV | 71 (77.2%) | 68 (70.1%) | 139 (73.5%) |
| NVP | 21 (22.8%) | 28 (28.9%) | 49 (25.9%) |
| RLP | 0 (0.0%) | 1 (1.0%) | 1 (0.5%) |
| PI | 66 (41.8%) | 63 (39.4%) | 129 (40.6%) |
| ATV | 11 (16.7%) | 11 (17.5%) | 22 (17.1%) |
| DRV | 2 (3.0%) | 3 (4.8%) | 5 (3.9%) |
| LOP | 53 (80.3%) | 49 (77.8%) | 102 (79.1%) |
Other ethnic origins reported by sites include Mixed Asian & White, Hispano, Latino and Coloured.
British 1990 Reference data used for calculation of z-scores: STATA zanthro function. The British 1990 Reference data (0–23 years) was used for standardisation of BMI, weight and height because it covers the full age range of SMILE participants.
Efficacy
Non-inferiority of dual therapy (INSTI + DRV/r) compared with continuing on SOC triple ART regimen was demonstrated (non-inferiority margin 10%). By 48 weeks, 8/158 (estimated probability of failure 5.0%) participants had met the primary endpoint (confirmed HIV-1 RNA ≥50 copies/mL) in the INSTI + DRV/r arm compared to 12/160 (7.6%) participants in SOC arm; difference (INSTI + DRV/r - SOC) was −2.5% (95% CI: −7.6%, 2.5%) (Table 2). Results were similar in the per-protocol analysis (difference of −2.6% (95% CI: −7.7%, 2.2%; p = 0.316) (Appendix 2). Sensitivity and FDA snapshot analyses showed consistent supporting results (Appendices 2 and 3). At week 24, cross-sectional proportions of participants with HIV-1 RNA ≥400 copies/mL and ≥50 copies/mL were similar in both arms; at week 48, proportions were lower in the INSTI + DRV/r arm, but differences were not statistically significant (Table 2). By end of trial, there was only 1 new severe CDC stage B event (nephropathy) diagnosed in INSTI + DRV/r arm compared to none in SOC arm.
Table 2.
Efficacy endpoints comparing INSTI + DRV/r dual therapy vs. SOC.
| INSTI + DRV/r | SOC | INSTI + DRV/r vs. SOC | |
|---|---|---|---|
| Participants | 158 | 160 | |
| Participants with week 24 HIV-1 RNA data | 158 | 160 | |
| Participants with week 48 HIV-1 RNA data | 150 | 156 | |
| Primary endpoint: Virological failure (confirmed HIV-1 RNA ≥50 copies/mL) | Adjusted differencea (95% CI) p-value |
||
| Participants with confirmed HIV-1 RNA ≥50 copies/mL at any time up to week 48 | 8 | 12 | |
| Estimated probability of failure (95% CI) | 0.050 (0.019, 0.088)c | 0.076 (0.038, 0.120)c | −0.025 (−0.076, 0.025)c p = 0.335 |
| Secondary efficacy endpoints: Cross sectional viral outcomesd | Adjusted differencea (95% CI) p-value |
||
| Participants with HIV-1 RNA ≥50 copies/mL at week 24 | 7 | 7 | |
| Proportion ≥50 copies/mL (95% CI) | 0.044 (0.021, 0.090) | 0.044 (0.021, 0.089) | 0.001 (−0.045, 0.046) p = 0.985 |
| Participants with HIV-1 RNA ≥50 copies/mL at week 48 | 7 | 13 | |
| Proportion ≥50 copies/mL (95% CI) | 0.047 (0.022, 0.095) | 0.083 (0.049, 0.139) | −0.037 (−0.092, 0.018) p = 0.195 |
| Participants with HIV-1 RNA ≥400 copies/mL at week 24 | 2 | 4 | |
| Proportion ≥400 copies/mL (95% CI) | 0.013 (0.003, 0.050) | 0.025 (0.009, 0.065) | −0.012 (−0.042, 0.017) p = 0.428 |
| Participants with HIV-1 RNA ≥400 copies/mL at week 48 | 4 | 7 | |
| Proportion ≥400 copies/mL (95% CI) | 0.027 (0.010, 0.069) | 0.045 (0.021, 0.092) | −0.018 (−0.060, 0.023) p = 0.392 |
| Secondary efficacy endpoints: Drug resistance mutations | Adjusted difference %a (95% CI) p-value | ||
| Participants (%) with resistance test available by week 48 (% of those meeting primary endpoint by week 48) | 6 (75%) | 5 (42%)f | |
| Participants (%) with major IASe mutation | 1 (17%) | 2 (40%) | −23 (−76, 29) p = 0.711 |
| Participants with: | |||
| Major IASe NRTI mutation | 1 (17%) | 1 (20%) | |
| Major IASe NNRTI mutation | 1 (17%) | 2 (40%) | |
| Major IASe PI mutation | 0 (0%) | 0 (0%) | |
| Major IASe IN mutation | 0 (0%) | 0 (0%) | |
| Secondary efficacy endpoints: CD4 count and CD4% | Adjusted differenceb (95% CI) p-value | ||
| Mean change in CD4 count (cells/mm3) to week 24 from baseline (SE) | −31.2 (16.3) | 0.1 (16.3) | −31.5 (−76.5,13.5) p = 0.170 |
| Mean change in CD4 count (cells/mm3) to week 48 from baseline (SE) | −43.8 (16.5) | 5.2 (16.1) | −48.3 (−93.4, −3.2) p = 0.036 |
| CD4≤500 cells/mm3 at week 48 (n (%)) | 21 (14%) | 15 (10%) | 4 (−3, 12) p = 0.234 |
| Mean change in CD4% to week 24 from baseline (SE) | −1.6 (0.3) | −0.1 (0.3) | −1.5 (−2.3, −0.6) p = 0.001 |
| Mean change in CD4% to week 48 from baseline (SE) | −1.8 (0.4) | 0.2 (0.4) | −1.9 (−3.0, −0.9) p < 0.001 |
SE, standard error; CI, confidence interval; n, number; P, p-value.
Adjusted for stratification factors: Age (≥6 < 12 yrs; ≥12 < 18 yrs) and Region (African; Non-African).
Adjusted for stratification factors and baseline value.
Bias corrected 95% CI obtained by bootstrap resampling (1000 times).
Result nearest to scheduled visit week 24 or 48 is used when multiple results fall in the same nominal week window.
Resistance defined according to IAS 2019.
INSTI gene sequencing failed in 1 SOC participant.
Of the 20 participants reaching the primary endpoint, 11 samples (6 INSTI + DRV/r; 5 SOC) were successfully sequenced for resistance (reverse transcriptase, protease and INSTI genes; INSTI gene sequencing failed in 1 out of the 5 SOC samples). Overall, 3/11 participants with resistance results had major IAS resistance mutations (1/6 INSTI + DRV/r vs. 2/5 SOC; p = 0.711); 2 had major NRTI mutations (1 INSTI + DRV/r, 1 SOC) and 3 had major NNRTI mutations (1 INSTI + DRV/r, 2 SOC). There were no major PI or INSTI mutations (Table 2). Despite the efforts made to have the complete resistance data, 9/20 resistance test results were missing (2 in INSTI + DRV/r and 7 in SOC) either because there was no suitable stored sample (5/9) or because sample was below site limit for resistance testing (4/9). The 2 participants in the INSTI + DRV/r arm re-suppressed after ART change. In the SOC arm: 3 did not re-suppress and 4 re-suppressed without ART change.
Both CD4% and count were significantly lower in the INSTI + DRV/r arm compared to SOC over time. The difference in mean change in CD4% from baseline (INSTI + DRV/r-SOC) to week 24 was −1.5% (95% CI: −2.3, −0.6; p = 0.001) and to week 48 was −1.9% (95% CI: −3.0, −0.9; p < 0.001). The difference in mean change in CD4 count from baseline (INSTI + DRV/r-SOC) to week 24 was −31.5 cells/mm3 (95% CI: −76.5, 13.5; p = 0.170) and to week 48 was −48.3 cells/mm3 (95% CI: −93.4, −3.2; p = 0.036) (Table 2). There was no significant difference between arms in the proportion of participants with CD4 count ≤500 cells/mm3 at 48 weeks (14% in INSTI + DRV/r vs. 10% in SOC arm, p = 0.234). No significant differences between arms in terms of changes from baseline to week 24 or 48 were observed for CD8%, CD8 count and CD4%/CD8% ratio (Appendix 4).
Safety and tolerability
Overall, by end of trial, there were 9 serious adverse events (SAEs) reported in 8 participants (4 [4] INSTI + DRV/r vs. 5 [4] SOC; p = 0.986, comparing proportion of participants with ≥1 SAEs between arms) (Table 3). The majority of SAEs were hospitalisations (67%, 6/9). There was no difference between arms in time to first SAE (p = 0.993) or SAE rate (p = 0.763) (Table 3). In total, there were 38 Grade 3/4 clinical or laboratory events reported in 32 participants (13 [13] INSTI + DRV/r vs. 25 [19] SOC). Although not statistically significant, the proportion of participants with ≥1 Grade 3/4 events (p = 0.280) and the rate of Grade 3/4 events (p = 0.079) were somewhat higher in SOC than in INSTI + DRV/r (Table 3); this was driven by 9 expected increased bilirubin events in participants taking atazanavir in the SOC arm. There were 10 ART modifying events in 8 participants (4 [4] INSTI + DRV/r vs. 6 [4] SOC; p = 0.986), with no significant difference between arms in time to first ART modifying event (p = 0.959) or ART modifying event rate (p = 0.575) (Table 3).
Table 3.
Safety endpoints comparing INSTI + DRV/r dual therapy vs. SOC.
| INSTI + DRV/r | SOC | INSTI + DRV/r vs. SOC | |
|---|---|---|---|
| Participants | 158 | 160 | |
| SAFETY AND TOLERABILITY | |||
| Serious Adverse Events (SAEs) by MedDRA SOC Terms reported to end of trial | |||
| Number of events [participants]i | 4 [4] | 5 [4] | p = 0.986l |
| Gastrointestinal disorders | 1 [1] | 0 [0] | |
| Hepatobiliary disorders | 1 [1] | 0 [0] | |
| Infections and infestations | 1 [1] | 1 [1] | |
| Injury, poisoning and procedural complications | 0 [0] | 1 [1] | |
| Psychiatric disorders | 0 [0] | 3 [2] | |
| Renal and urinary disorders | 1 [1] | 0 [0] | |
| SAE rate (per 100-person years) (95% CI) | 1.9 (0.7, 5.0) | 2.3 (1.0, 5.6) | |
| Rate Ratiom(95% CI) | 0.81 (0.20, 3.22) | 1 (ref) | p = 0.763 |
| Hazard Ratiog(95% CI) | 1.01 (0.25, 4.02) | 1 (ref) | p = 0.993 |
| Grade 3/4 clinical and laboratory adverse events (AEs) by MedDRA SOC Terms reported to end of trial | |||
| Number of events [participants]i | 13 [13] | 25j[19] | p = 0.280l |
| Blood and lymphatic system disorders | 1 [1] | 3 [3] | |
| Gastrointestinal disorders | 1 [1] | 0 [0] | |
| Hepatobiliary disorders | 1 [1] | 6 [4] | |
| Infections and infestations | 1 [1] | 1 [1] | |
| Injury, poisoning and procedural complications | 0 [0] | 1 [1] | |
| Investigations | 6 [6] | 8 [7] | |
| Metabolism and nutrition disorders | 1 [1] | 0 [0] | |
| Nervous system disorders | 0 [0] | 1 [1] | |
| Pregnancy, puerperium and perinatal conditions | 0 [0] | 1 [1] | |
| Psychiatric disorders | 0 [0] | 3 [2] | |
| Renal and urinary disorders | 2 [2] | 1 [1] | |
| Grade 3/4 AE rate (per 100-person years) (95% CI) | 6.1 (3.5, 10.5) | 11.6 (7.9, 17.2) | |
| Rate Ratiom(95% CI) | 0.52 (0.26, 1.08) | 1 (ref) | p = 0.079 |
| Hazard Ratiog(95% CI) | 0.65 (0.32, 1.32) | 1 (ref) | p = 0.237 |
| ART Modifying Events by MedDRA SOC Terms reported to end of trial | |||
| Number of events [participants]i | 4 [4] | 6 [4] | p = 0.986l |
| Gastrointestinal disorders | 1 [1] | 0 [0] | |
| Hepatobiliary disorders | 1 [1] | 1 [1] | |
| Investigations | 0 [0] | 3 [2] | |
| Psychiatric disorders | 1 [1] | 2 [2] | |
| Psychiatric disorders + Metabolism and nutrition disorders + Psychiatric disorders | 1 [1] | 0 [0] | |
| ART modifying AE rate (per 100-person years) (95% CI) | 1.9 (0.7, 5.0) | 2.8 (1.3, 6.2) | |
| Rate Ratiom(95% CI) | 0.67 (0.17, 2.67) | 1 (ref) | p = 0.575 |
| Hazard Ratiog(95% CI) | 1.04 (0.26, 4.15) | 1 (ref) | p = 0.959 |
| Notable Events reported to end of trial | |||
| Number of events [participants]i | |||
| Pregnancy | 1 [1] | 2 [2] | p = 1.000l |
| Suicidal ideation | 0 [0] | 1 [1] | p = 1.000l |
| Drug induced liver injury | 2 [2] | 1 [1] | p = 0.621l |
| COVID-19 contact | 1 [1] | 0 [0] | p = 0.497l |
| ART REGIMEN | |||
| On initial regimen at end of trial (n (%)) | 145 (91.8%)a | 131 (81.9%)b | |
| Not on initial regimen at end of trial (n (%)) | 13 (8.2%) | 29 (18.1%) | |
| ART changes [Number of participants] | 19 [14] | 40 [32] | |
| Increase in viral loadc | 7 [7] | 0 [0] | |
| Combination of failure indicatorsd | 0 [0] | 1 [1] | |
| Simplification | 3 [3] | 9 [7] | |
| Drug availabilitye | 2 [2] | 0 [0] | |
| Adverse event | 3 [3] | 6 [3] | |
| Patient/carer decision | 0 [0] | 1 [1] | |
| Pregnancya | 1 [1] | 0 [0] | |
| Concomitant drug interaction | 0 [0] | 1 [1] | |
| Change in National clinical guidelines | 0 [0] | 21 [21] | |
| Adverse event AND Patient/carer decisiona | 1 [1] | 0 [0] | |
| Otherf | 2 [2] | 0 [0] | |
| Unknown | 0 [0] | 1 [1] | |
| Treatment interruption > 31 days [Number of participants] | 1 [1] | 1 [1] | |
| Time to first ART change by end of trialn[excluding changes for changes in national clinical guidelines] | |||
| Total number of ART changes [Number of participants] | 20 [14] | 20 [13] | |
| Hazard Ratiog (95% CI) | 1.12 (0.52, 2.38) | 1 (ref) | p = 0.777 |
| LIPIDS/GLUCOSE | Adjusted differenceh (95% CI) p-value | ||
| Mean change in fasting total cholesterol (mg/dL) to week 24 from baseline (SE) | 4.3 (2.0) | −2.2 (2.0) | 6.4 (0.9, 11.9) p = 0.022 |
| Mean change in fasting total cholesterol (mg/dL) to week 48 from baseline (SE) | −0.6 (2.2) | −1.0 (2.2) | 0.3 (−5.7, 6.4) p = 0.916 |
| Mean change in fasting LDL (mg/dL) to week 24 from baseline (SE) | 9.0 (1.7) | −1.0 (1.7) | 9.9 (5.2, 14.6) p < 0.001 |
| Mean change in fasting LDL (mg/dL) to week 48 from baseline (SE) | 2.8 (1.9) | −2.1 (1.9) | 4.7 (−0.7, 10.0) p = 0.088 |
| Mean change in fasting HDL (mg/dL) to week 24 from baseline (SE) | −5.6 (0.9) | −1.8 (0.9) | −3.9 (−6.3, −1.5) p = 0.002 |
| Mean change in fasting HDL (mg/dL) to week 48 from baseline (SE) | −6.4 (1.0) | −2.4 (0.9) | −4.1 (−6.7, −1.4) p = 0.003 |
| Mean change in fasting glucose (mg/dL) to week 24 from baseline (SE) | −2.5 (0.7) | −0.5 (0.8) | −1.9 (−3.9, 0.1) p = 0.063 |
| Mean change in fasting glucose (mg/dL) to week 48 from baseline (SE) | −1.2 (0.8) | 1.0 (0.8) | −2.3 (−4.3, −0.3) p = 0.026 |
| ANTHROPOMETRIC MEASURES | Adjusted differenceh (95% CI) p-value | ||
| Mean change in weight-for-age z-scorek to week 48 from baseline (SE) | 0.19 (0.0) | −0.02 (0.0) | 0.21 (0.1, 0.3) p < 0.001 |
| Mean change in weight (kg) to week 48 from baseline (SE) | 5.08 (0.3) | 3.11 (0.3) | 1.97 (1.1, 2.9) p < 0.001 |
| Mean change in height (cm) to week 48 from baseline (SE) | 3.10 (0.2) | 2.76 (0.2) | 0.36 (−0.2, 0.9) p = 0.230 |
| Mean change in BMI-for-age z-scorek to week 48 from baseline (SE) | 0.23 (0.0) | 0.01 (0.0) | 0.22 (0.1, 0.3) p < 0.001 |
| Mean change in BMI to week 48 from baseline (SE) | 1.25 (0.1) | 0.59 (0.1) | 0.66 (0.3, 1.0) p < 0.001 |
SE, standard error; CI, confidence interval; n, number; P, p-value; MedDRA SOC, MedDRA system organ class.
One participant in INSTI + DRV/r arm changed ART but later returned to initial regimen before end of trial–a positive pregnancy test at week 24 resulted in stop of DTG + DRV/r and re-start of triple ART therapy as per protocol; following 4 days on triple ART therapy, adverse event (vomiting) was reported and participant resumed dual therapy DTG + DRV/r by own decision.
Three participants in SOC arm changed ART but later returned to initial regimen before end of trial—changed ART for simplification, then returned to initial regimen due to adverse event; changed ART for Change in National clinical guidelines then returned to initial regimen due to adverse event; ART was stopped at week 72 for unknown reason followed by restart at week 84 due to patient/carer decision.
Increase in viral load defined as confirmed VL ≥50 copies/mL. Note that one participant had confirmed VL ≥50 copies/mL but did not change ART. This decision was made by the investigator based on examination of subsequent viral load data, in consultation with the participant, family and the SMILE chief investigator.
Combination of failure indicators defined as “Any combination of increase in VL/falling CD4/progression/resistance”.
EVG withdrawal from the market as a single entity.
ART changes for “other” reasons include “Transition to three component therapy”; “Persistent cough”.
Adjusted for stratification factors: Age (≥6 < 12 yrs; ≥12 < 18 yrs) and Region (African; Non-African).
Adjusted for stratification factors and baseline value.
Number of events [participants] shows total number of events reported in participants. When participants have multiple events, the total number of events reported are higher than number participants in brackets.
Atazanavir had probable/definitive causal relationship to 9 increased bilirubin events reported in SOC arm.
Based on British 1990 Reference data (0–23 years).
Chi-squared test or Fisher's exact test used, as appropriate, to compare number of participants with ≥1 events between arms.
Adjusted for stratification factors and event clustering within individuals.
With the exception of changes for change in national clinical guidelines, any other ART changes for any reason are included in the analysis of time to first ART change. Regimen interruptions for >31 days are considered as ART discontinuations and are also included in the analysis.
Overall, notable events were reported in 8 participants including 3 pregnancies (1 INSTI + DRV/r; 2 SOC), 1 suicidal ideation (0 INSTI + DRV/r; 1 SOC), 3 drug induced liver injuries (2 INSTI + DRV/r; 1 SOC), of which only 1 in INSTI + DRV/r group switched to SOC; 1 COVID-19 contact (1 INSTI + DRV/r; 0 SOC) (Table 3). The outcome of pregnancies included induced abortion in 2 participants (1 INSTI + DRV/r; 1 SOC) and 1 live birth in one participant in the SOC arm.
By end of trial, 145 (92%) participants in INSTI + DRV/r arm vs. 131 (82%) in SOC remained on their initial ART regimen (Table 3). The main treatment changes were for changes in national clinical guidelines (0 INSTI + DRV/r vs. 21 SOC), simplification (3 INSTI + DRV/r vs. 9 SOC), increase in viral load [confirmed HIV-1 RNA ≥50 copies/mL] (7 INSTI + DRV/r vs. 0 SOC; protocol-required change in INST + DRV/r arm but not SOC) and AE (6 SOC vs. 3 INSTI + DRV/r). There was no significant difference between arms in time to first ART change (p = 0.777), excluding changes for change in national clinical guidelines in the SOC arm (Table 3).
Fasting total cholesterol increased significantly from baseline to week 24 but not to week 48 in the INSTI + DRV/r arm compared to SOC arm (Table 3). Fasting LDL increased significantly from baseline to week 24 and to a lesser extent to week 48 in the INSTI + DRV/r arm compared to SOC arm (difference week 24: 9.9 mg/dL (95% CI: 5.2, 14.6; p < 0.001); week 48: 4.7 mg/dL (95% CI: −0.7, 10.0; p = 0.088)). Fasting HDL decreased significantly from baseline to week 24 and 48 in the INSTI + DRV/r arm compared to SOC arm (difference week 24: −3.9 mg/dL (95% CI: −6.3, −1.5; p = 0.002); week 48: −4.1 mg/dL (95% CI: −6.7, −1.4; p = 0.003)) (Table 3).
Fasting glucose decreased significantly from baseline to week 48 in the INSTI + DRV/r arm compared to SOC arm (Table 3).
Weight, weight-for-age, BMI and BMI-for-age had increased significantly more in INSTI + DRV/r arm than SOC at 48 weeks; differences in mean change from baseline (INSTI + DRV/r-SOC) were 1.97 kg (95% CI: 1.1, 2.9), 0.21 Z-score (95% CI: 0.1, 0.3), 0.66 kg/m2 (95% CI: 0.3, 1.0), 0.22 Z-score (95% CI: 0.1, 0.3), respectively (Table 3). Height at week 48 was similar between arms (Table 3). A very small number of participants were obese (BMI ≥30 kg/m2) at baseline (2/158 INSTI vs. 1/160 SOC) and at follow-up visits (week 24: 4/158 INSTI vs. 0/160 SOC; week 48: 4/145 INSTI vs. 3/153 SOC).
Adherence to treatment was very high throughout follow-up and was similar between arms based on both participant and parent/carer reports (Appendix 5 Tables 1 and 2). Participant and parent/carer reported mood and sleep-related symptoms were similar between arms (Appendix 5 Tables 3–6). At the end of study, 52 (64%) participants in INSTI + DRV/r arm reported that taking INSTI + DRV/r made things a lot easier for them compared to pre-study medication and 66 (81%) reported being happy to stay on INSTI + DRV/r (Appendix 5 Tables 7 and 8). There was no evidence of a difference in quality of life over follow-up between arms, based on participant and parent/carer reports (data not shown).
Discussion
In this first randomised trial comparing switching virologically-suppressed children to DRV/r in combination with once-daily INSTI vs. continuing a SOC regimen with 2 NRTIs + bPI/NNRTI, non-inferiority was shown at 48 weeks with no safety concerns. Maintenance of virological suppression was high over the study period with no emergence of PI or INSTI genotypic resistance among the small number of patients with virological failure. Of note, this is consistent with the results of the adult study DUALIS, which had a similar design to SMILE.9 Tolerability was deemed good with low and comparable rates of adverse events in both arms and most participants remained on their initial ART regimen to the end of trial. The occurrence of mood and sleep-related symptoms was infrequent with no significant differences between arms, which is reassuring and supports the wide safety experience on DTG in children and adolescents,6 and the large experience on DTG in adult trials.7,8
SMILE efficacy results are in keeping with adult studies. DUALIS, a randomized open-label study, showed non-inferiority of switching to DTG + DRV/r vs. continuing with triple therapy including DRV/r as maintenance, in virologically-suppressed adults living with HIV.9 Other observational studies have confirmed the effectiveness and safety of dual therapy with DTG + DRV/r in virologically-suppressed adults, even if heavily pre-treated.10, 11, 12 The combination DTG + DRV/r, both drugs with high genetic barrier to resistance, would offer an additional potential advantage in terms of resistance compared to other two-drug regimens studied in virologically-suppressed adult patients showing high rates of virological success, including 3TC plus a bPI13, 14, 15 or the combination of DTG with rilpivirine.16
Regarding CD4 outcomes, a small but significant decrease both in the absolute and percentage was observed at 48 weeks in the INSTI + DRV/r arm. Of note, the proportion of patients with CD4 >500 cells/mm3 at the end of the trial remained high with no statistically significant difference between arms. Nevertheless, this CD4 difference over time is of uncertain clinical significance and needs further investigation, for example whether this was a chance finding or whether there is a direct effect of INSTI on CD4 cell proliferation17 or whether it reflects redistribution of CD4 cells from blood to lymphoid tissues. In DUALIS, where all participants were on DRV/r+2NRTIs at baseline, no significant differences in CD4 count between arms were detected at 48 weeks with a median increase from baseline of 30 cells/mm3 in the dual therapy arm vs. 32 cells/mm3 in the SOC arm.9
An increase in LDL-cholesterol was observed from baseline to week 24, that was associated with a significant decrease in HDL-cholesterol and higher total cholesterol/HDL ratio in the INSTI + DRV/r arm compared to SOC. Due to the neutral effect of DTG on lipids in adults7,8 and adolescents,18 the observed changes in lipids are likely to be attributable to DRV/r in the study arm. In SMILE, the effect was small, and most patients continued to have cholesterol levels within the normal range. As differences were of a lesser extent at week 48, longer follow-up could have provided additional information on the persistence and clinical significance of this observation.
Other dual alternatives for simplification in suppressed patients with a more favourable lipid profile should be explored like DTG + 3TC which has already been tested in adults19,20 and is under investigation in children within the ongoing PENTA D3 trial.21
Weight and BMI increased significantly more in the INSTI + DRV/r arm than in the SOC arm. There is mounting evidence in adults that INSTIs, particularly DTG, maybe associated with more weight gain than other classes of antiretroviral drugs,22, 23, 24 although in the large ODYSSEY trial, enrolling predominantly African children and adolescents, weight gain was not greater in DTG vs. PI or NNRTI- based ART over 96 weeks of follow-up.6 Alternatively, withdrawing an NRTI (one third of patients were on a zidovudine/lamivudine (ZDV/3TC) containing regimen at baseline) or NNRTI such EFV which have potential effects on weight or fat redistribution might have also contributed to the differences in observed weight between arms. Ethnicity may influence INSTI-related weight gain. African adolescents may be prone to weight gain as suggested in a large retrospective observational study in which virologically suppressed African adolescents had an increase in BMI after switching to dolutegravir.25 Nevertheless, in SMILE, INSTI-related weight gain was not restricted to the black African participants. Small observational studies in Caucasian young adults with perinatal HIV infection in Europe did not find a link between weight gain and INSTI.26 Although SMILE is the first randomised trial observing this association in a large population of CLWHIV, excess weight gain in the INSTI + DRV/r group over 48 weeks was relatively modest with a very low proportion of children with a BMI ≥30 kg/m2 at the end of the trial.
In 2018, WHO published interim guidelines recommending dolutegravir (DTG)-containing regimens as the preferred first and second-line antiretroviral therapy (ART) regimens for people living with HIV.3 As more data on the efficacy, safety and PK on DTG across all age groups are available,5 it is increasingly being used in children and adolescents. Monitoring of weight and BMI gain should be considered when using DTG in CLWHIV.
SMILE results provide evidence for the use of the DTG + DRV/r combination in other contexts for example when NRTI/NNRTIs are contraindicated or not tolerated or to simplify some complex regimens in virologically suppressed children/adolescents. This strategy, not evaluated in children before and supported by a randomised clinical trial, remains relevant as it provides additional data for switching strategies in virologically suppressed CLWHIV and could be included in guidelines as an “alternative” switch option in the cases mentioned above. The dual therapy DTG + DRV/r can also be further evaluated in children failing therapy in the light of the positive results of the D2EFT trial in adults failing first line therapy.27
The lack of efficacy of DRV/r + INSTI in treating HBV limits its potential use in the context of HBV/HIV co-infection.
Limitations of the study include the open-label design, as well as the heterogeneity in baseline ART-regimens in the SOC arm that precludes a detailed analysis of the observed changes in CD4, cholesterol and weight gain over time. Also, nearly half of resistance testing was not available for those who failed. Of note the two children from the dual therapy arm with missing resistance test re-suppressed after treatment change, which is potentially reassuring.
Almost all patients were treated with DTG and over 12 years of age, and therefore, the results obtained in this study should not be extrapolated to other INSTI or to children less than 12 years old.
In conclusion in virologically-suppressed children, switching to INSTI + DRV/r was non-inferior virologically, and shows similar safety profile, to continuing SOC. There was no occurrence of major genotypic PI or INSTI mutations in failing participants, suggesting this regimen is robust. INSTI + DRV/r dual therapy as an NRTI sparing regimen could be a suitable alternative switch option in virologically-suppressed in children and adolescents to simplify complex regimens. Further research on this combination is needed, including its potential impact on weight, lipid and distribution of CD4 lymphocytes subsets. Further research on its use also in patients with virological failure should be considered.
Contributors
The study was designed by ACom, MKC, YS, DMG, ABab, CG and JTR. ACom, MKC, YS, ABab and JTR conceived and drafted the manuscript. MKC and ABab undertook the statistical analysis. Data were verified and interpreted by ACom, MKC, YS, TRC, ABam, YR, TN, DF, DMG, ABab and JTR.
All authors reviewed and approved the final manuscript. All authors had final responsibility for the decision to submit for publication.
Data sharing statement
The SMILE data are held at the Medical Research Council (MRC) Clinical Trials Unit at University College London, UK, which encourages optimal use of data by using a controlled access approach to data sharing, incorporating a transparent and robust system to review requests and provide secure data access consistent with the relevant ethics committee approvals. All requests for data are considered and can be initiated by contacting the corresponding author: alexandra.compagnucci@inserm.fr.
Declaration of interests
ACom, YS, YR and ACoe declare that their institution Inserm received a grant for the trial through PENTA Foundation “agreement PENTA-Inserm” started 14th September 2014 and declare support for scientific meetings from PENTA Foundation. AVi declares funding given from PENTA Foundation to her institution for the conduct of the study. ABab declares a grant from Medical Research Council, UKRI to his Institution. PA and ADK declare sponsored study materials and participant recruitment costs from PENTA foundation. PA, ADK and CG declare a research grant for part of the study from Gilead and funded drug supply for the study from ViiV Healthcare. NPR declares a financial support for the research paid to her institution from PENTA Foundation and from Merck. NPR declares that her institution receives the drugs for the study from PENTA Foundation. NPR declares a support for attending ID week in 2022 from AstraZeneca.
All other authors declare no competing interests.
Footnotes
Supplementary data related to this article can be found at https://doi.org/10.1016/j.eclinm.2023.102025.
Contributor Information
Alexandra Compagnucci, Email: alexandra.compagnucci@inserm.fr, alexandra.compagnucci@aphp.fr.
SMILE-PENTA17-ANRS 152 Trial Group:
A. Compagnucci, Y. Saidi, Y. Riault, A. Coelho, C. Kouakam, L. Picault, M. Ndiaye, L. Meyer, C. Cagnot, S. Circosta, L. Léger, S. Simanic, A. Arulananthan, D.M. Gibb, A. Babiker, M. Chan, D. Ford, F. Hudson, L. Harper, A. Bamford, A. Nolan, K. Widuch, S. Townsend, N. Van-Looy, L. Gao, E. Little, A. Turkova, S. Fabiane, J. Calvert, J. Blackstone, K. Scott, J. Inshaw, A. Nunn, A. Nardone, D. Bilardi, T.R. Cressey, S. Chalermpantmetagul, W. Khamduang, G. Jourdain, N. Ngo Giang Huong, D. Chinwong, C. Saenjum, R. Peongjakta, P. Sukrakanchana, L. Laomanit, A. Kaewbundit, J. Khamkon, K. Than-in-at, C. Meeboon, W. Sripaoraya, N. Krueduangkam, N. Kruenual, W. Khamjakkaew, S. Klinprung, C. Giaquinto, G. Morkunaite, D. Hirt, L. Prieto Tato, T. Niehues, D. Plonné, C. Morén, T. Noguera, M.A. Muñoz Fernández, R. Bologna, S. Arazi, A.G. Fedullo, M. Taicz, E. Vicentini, M. Moragas, A. Mangano, M. Dell’Orso, M. Gatto, V. Reliquet, A. Soria, E. Paredes, N. Chereau, M. Tching Sin, L. Flet, A. Rodallec, C. Hemon, N. Elenga, M.D. Terrine, N. Blaise, S. Augustin, P. Mespoulhe, H. Pouchain, N. Pavia-Ruz, R. Muñoz- Hernández, A. Neri-Macias, M.D. Jarillo-Quijada, C. Espinosa-Sotero, L. Marques, C. Teixeira, A. Fernandes, R. Nunes, H. Nascimento, J. Tuna, A. Padrao, I. Ferraz, A.C. Mendes, C. Correira, H. Pinheiro, A.C. Matos, A.C. Sampaio, A. Oliveira, A. Caldeira, M. Tavares, A. Reis Melo, C. Castro, C. Faria, C. Prucha, R. Ribeiro, F. Monteiro, M.F. Candeias, T. Silva Milhiero, E. Gomes Neves, A. Oliveira, R. Corte-Real, M. Morgado, D. Mendes, M. Cardão, A. Violari, N. Ramsagar, A. Liberty, M. Nyati, L. Maseko, M. Khunene, S. Mkhize, Z. Essack, N. Akoojee, U. Singh, Y. Fourie, S. Govender, A. Vadee, R. Lakha, J. Erasmus, A. Mamiane, T. Daniel, P. Bhana, N. Maduna, M. Cotton, M. Groenewald, G. Slade, J. Coetzee, L. Ganger, S. Weldon, M. Wessels, L. Hoorn, S. Pieterse, C. Makola, K. Smith, M. Isaacs, A. Cweya, S. Fry, S. Barnabas, M. Theunissen, N. Nduna, M. Smuts, P. Rojo Conejo, C. Epalza, L. Prieto Tato, M. Fernández, M.J. Mellado Peña, T. Sainz Costa, L. Escosa García, P. Gomez Salcedo, C. Fortuny Guasch, T. Noguera Julian, C. Estepa, M. Cubells, E. Sans, E. Bruno, L. Prieto, P. Mendez García, A. Murciano Cabeza, M. Coto, R. Torrent, M. Torres Arauz, M. Navarro Gómez, A. Mur, S. Guillén Martin, M. Moreno, J.T. Ramos Amador, I. Garcia, C. Kalhert, T. Wachinger, B. Wohlwend, S. Hafner, G. Dollenmaier, P. Paioni, R. Signorell, J. Boni, A. Duppenthaler, B. Mann, C. Saegesser, M. Barbani, C. Ngampiyaskul, P. Greetanukroh, P. Khannak, P. Tearsansern, W. Chamjamrat, N. Chanto, T. Thapwai, K. Thungkham, P. Puangmalai, C. Ruklao, P. Ounchanum, S. Khusuwan, S. Denjanta, Y. Thaweesombat, J. Thewsoongnoen, K. Kaewmamueng, P. Kamboua, S. Pongprapass, W. Srisuk, A. Kongponoi, J. Limplertjareanwanich, S. Kanjanavanit, C. Saewtrakool, P. Yingyong, D. Chutima, R. Junkaew, T. Chankun, U. Srirompotong, P. Sudsaard, K. Kongsuk, T. Petpranee, S. Srirojana, D. Donngernl, A. Kamkoonmongkol, N. Na Kalasin, P. Phunkhum, A.R. Kekitiinwa, P. Amuge, D. Bbuye, J. Nalubwama, S. Namanda, M. Nsibuka Kisekka, A. Kirabira, L. Lawrence, G. Agaba, G. Ahimbisibwe, A. Nalugo, F. Namuli, R. Kadhuba, R. Namuddu, I. Nabwire, L. Kiyimba, A. Baita, J. Tikabibamu, L. Nakandi, G.P. Kisitu, N. Nabukeera Barung, C.M. Kityo, V. Musiime, E. Kaudha, A. Nanduudu, E. Mujyambere, S.P. Labeja Ocitti, J. Ategeka, E. Nambi, R. Nazzinda, D. Rutebarika, R. Basiimwa, R. Mbabazi, P. Kyobutungi, M. Nabalamba, A. Nakalyango, J. Tumusiime, S. Nakabuye, J. Mwebaza, S. Oruk, J. Namusanje, A. Musiime, L. Mugarura, M. Ojok, J. Kitabalwa, C. Katemba, M. Nannungi, E. Bagirigomwa, D. Odoch, E. Rubanga, D. Mulima, E.L. Babu, D. Baliruno, C. Inyakuwal, E.D. Williams, A. Mulindwa, A. Uyungrwoth, I. Raus, O. Mostovenko, T. Stepchenkova, A. Volokha, N. Primak, J. Kenny, A. Callaghan, M. Ahmad, S. Vergnano, M. Ross, F. Manyi, D. Nayagam, S. Hawkins, C. Ball, E. Hamlyn, C. Gilmour, S. Gilmour-White, S. Doshi, E. Fuller, A. Adebayo, K. Tupper, E. Nsirim, S. Welch, J. Daglish, L. Thrasyvoulou, E. Irvine, K. Gandhi, Y. Vaughn-Gordon, and N. Sibanda
Appendix A. Supplementary data
References
- 1.PENTA guidelines for treatment of paediatric HIV-1 infection 2014: optimising health in preparation for adult life. PENTA steering committee. HIV Med. 2018;19:e1–e42. doi: 10.1111/hiv.12217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Guidelines for the use of antiretroviral agents in pediatric HIV infection. Developed by the HHS panel on antiretroviral therapy and medical management of HIV-infected children-A working group of the office of AIDS research advisory Council (OARAC) http://aidsinfo.nih.gov/guidelines Access december 2021.
- 3.Updated recommendations on HIV prevention, infant diagnosis, antiretroviral initiation and monitoring: march 2021. World Health Organization; Geneva: 2021. [PubMed] [Google Scholar]
- 4.Wensing A.M., Calvez V., Ceccherini-Silberstein F., et al. 2019 update of the drug resistance mutations in HIV-1. Top Antivir Med. 2019;27:111–121. [PMC free article] [PubMed] [Google Scholar]
- 5.Food Drug Administration Center for Drugs Evaluation Research . FDA; Maryland, USA: 2015. Human immunodeficiency virus-1 infection: developing antiretroviral drugs for treatment - guidance for industry; p. 9. [Google Scholar]
- 6.Turkova A., White E., Mujuru H.A., et al. Dolutegravir as first- or second-line treatment for HIV-1 infection in children. N Engl J Med. 2021;385:2531–2543. doi: 10.1056/NEJMoa2108793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Molina J.M., Clotet B., van Lunzen J., et al. FLAMINGO study team Once-daily dolutegravir versus darunavir plus ritonavir for treatment-naive adults with HIV-1 infection (FLAMINGO): 96-week results from a randomised, open-label, phase 3b study. Lancet HIV. 2015;4:e127–e136. doi: 10.1016/S2352-3018(15)00027-2. [DOI] [PubMed] [Google Scholar]
- 8.Cahn P., Sierra Madero J., Arribas J.R., et al. Dolutegravir plus lamivudine versus dolutegravir plus tenofovir disoproxil fumarate and emtricitabine in antiretroviral-naive adults with HIV-1 infection (GEMINI-1 and GEMINI-2): week 48 results from two multicentre, double-blind, randomised, non-inferiority, phase 3 trials. Lancet. 2019;393(10167):143–155. doi: 10.1016/S0140-6736(18)32462-0. [DOI] [PubMed] [Google Scholar]
- 9.Spinner C.D., Kümmerle T., Schneider J., et al. Efficacy and safety of switching to dolutegravir with boosted darunavir in virologically suppressed adults with HIV-1: a randomized, open-label, multicenter, phase 3, noninferiority trial: the DUALIS study. Open Forum Infect Dis. 2020;9 doi: 10.1093/ofid/ofaa356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Navarro J., Santos J.R., Silva A., et al. Effectiveness of once/day dolutegravir plus boosted darunavir as a switch strategy in heavily treated patients with human immunodeficiency virus. Pharmacotherapy. 2019;39:501–507. doi: 10.1002/phar.2227. [DOI] [PubMed] [Google Scholar]
- 11.Vizcarra P., Fontecha M., Monsalvo M., Vivancos M.J., Rojo A., Casado J.L. Efficacy and safety of dolutegravir plus boosted-darunavir dual therapy among highly treatment-experienced patients. Antivir Ther. 2019;24:467–471. doi: 10.3851/IMP3319. [DOI] [PubMed] [Google Scholar]
- 12.Hawkins K.L., Montague B.T., Rowan S.E., et al. Boosted darunavir and dolutegravir dual therapy among a cohort of highly treatment-experienced individuals. Antivir Ther. 2019;24:513–519. doi: 10.3851/IMP3330. [DOI] [PubMed] [Google Scholar]
- 13.Arribas J.R., Girard P.-M., Landman R., et al. Dual treatment with lopinavir-ritonavir plus lamivudine versus triple treatment with lopinavir-ritonavir plus lamivudine or emtricitabine and a second nucleos(t)ide reverse transcriptase inhibitor for maintenance of HIV-1 viral suppression (OLE): a random. Lancet Infect Dis. 2015;15:785–792. doi: 10.1016/S1473-3099(15)00096-1. [DOI] [PubMed] [Google Scholar]
- 14.Perez-Molina J.A., Rubio R., Rivero A., et al. Simplification to dual therapy (atazanavir/ritonavir + lamivudine) versus standard triple therapy (atazanavir/ritonavir + two nucleos[t]ides) in virologically stable patients on antiretroviral therapy: 96- week results from an open-label, non-inferiority. J Antimicrob Chemother. 2017;72:246–253. doi: 10.1093/jac/dkw379. [DOI] [PubMed] [Google Scholar]
- 15.Pulido F., Ribera E., Lagarde M., et al. Dual therapy with darunavir and ritonavir plus lamivudine vs triple therapy with darunavir and ritonavir plus tenofovir disoproxil fumarate and emtricitabine or abacavir and lamivudine for maintenance of human immunodeficiency virus type 1 viral suppression. Clin Infect Dis. 2017;316:191–210. doi: 10.1093/cid/cix734. [DOI] [PubMed] [Google Scholar]
- 16.Llibre J.M., Hung C.C., Brinson C., et al. Efficacy, safety, and tolerability of dolutegravir-rilpivirine for the maintenance of virological suppression in adults with HIV-1: phase 3, randomised, non-inferiority SWORD-1 and SWORD-2 studies. Lancet. 2018;391(10123):839–849. doi: 10.1016/S0140-6736(17)33095-7. [DOI] [PubMed] [Google Scholar]
- 17.Korencak M., Byrne M., Richter E., et al. Effect of HIV infection and antiretroviral therapy on immune cellular functions. JCI Insight. 2019;4(12) doi: 10.1172/jci.insight.126675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Giacomet V, Lazzarin S, Manzo A, et al. Body fat and lipid profile changes in HIV-infected youths switched to dolutegravir. Poster presented at CROI 2020. Abstract 827.
- 19.Borghetti A., Baldin G., Lombardi F., et al. Efficacy and tolerability of lamivudine plus dolutegravir as a switch strategy in a multicentre cohort of patients with suppressed HIV-1 replication. HIV Med. 2018;19:452–454. doi: 10.1111/hiv.12611. [DOI] [PubMed] [Google Scholar]
- 20.Calza L., Colangeli V., Borderi M., et al. Simplification to dual therapy containing lamivudine and raltegravir or dolutegravir in HIV-infected patients on virologically suppressive antiretroviral therapy. J Antimicrob Chemother. 2020;75:3327–3333. doi: 10.1093/jac/dkaa319. [DOI] [PubMed] [Google Scholar]
- 21.DTG/3TC fixed dose formulations for the maintenance of virological suppression in children with HIV infection aged 2 to <15 Years old (D3 (Penta 21)) ClinicalTrials.gov Identifier: NCT04337450.
- 22.Eckard A.R., McComsey G.A. Weight gain and integrase inhibitors. Curr Opin Infect Dis. 2020;33:10–19. doi: 10.1097/QCO.0000000000000616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Venter W.D.F., Moorhouse M., Sokhela S., et al. Dolutegravir plus two different prodrugs of tenofovir to treat HIV. N Engl J Med. 2019;381:803–815. doi: 10.1056/NEJMoa1902824. [DOI] [PubMed] [Google Scholar]
- 24.Bai R., Lv S., Wu H., Dai L. Effects of different integrase strand transfer inhibitors on body weight in patients with HIV/AIDS: a network meta-analysis. BMC Infect Dis. 2022;22(1):118. doi: 10.1186/s12879-022-07091-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Thivalapill N., Simelane T., Mthethwa N., et al. Transition to dolutegravir is associated with an increase in the rate of body Mass Index change in a cohort of virally suppressed adolescents. Clin Infect Dis. 2021;73(3):e580–e5862. doi: 10.1093/cid/ciaa1652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Taramasso L., Di Biagio A., Bovis F., et al. Switching to integrase inhibitors unlinked to weight increase in perinatally HIV-infected young adults and adolescents: a 10-year observational study. Microorganisms. 2020;8:864. doi: 10.3390/microorganisms8060864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Matthews G, Borok M, Eriobou N, et al. D2EFT: dolutegravir and darunavir evaluation in adults failing first-line HIV Therapy-CROI 2023 Oral Abstract Session-12. Abstract number 198.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.