Abstract
Purpose
To investigate the relationship between complement pathway activities and progression of geographic atrophy (GA) secondary to age-related macular degeneration in samples collected from patients enrolled in the Chroma and Spectri trials.
Design
Chroma and Spectri were phase III, double-masked, and sham-controlled, 96-week trials.
Participants
Aqueous humor (AH) samples collected at baseline and week 24 visits from 81 patients with bilateral GA across all 3 treatment groups (intravitreal lampalizumab 10 mg every 6 weeks, every 4 weeks, or corresponding sham procedures) were tested, along with patient-matched plasma samples collected at baseline.
Methods
Antibody capture assays using the Simoa platform were used to measure the levels of complement factor B, the Bb fragment of complement factor B, intact complement component 3 (C3), processed C3, intact complement C4, and processed C4. Complement factor D levels were measured using enzyme-linked immunosorbent assay.
Main Outcome Measures
Correlations of complement levels and activities (i.e., processed:intact ratio of complement component) in AH and plasma with baseline GA lesion size and growth rate.
Results
In baseline AH, there were strong correlations (Spearman’s rho ≥ 0.80) between intact complement proteins, between processed complement proteins, and between linked processed and intact complement proteins; weak correlations (rho ≤ 0.24) were found between complement pathway activities. There were no strong correlations between complement protein levels and activities measured in AH and plasma at baseline (rho ≤ 0.37). Baseline complement levels and activities in AH and plasma did not correlate with baseline GA lesion size or change from baseline in GA lesion area at week 48 (i.e., annualized growth rate). There were no strong correlations between changes in complement levels/activities in the AH from baseline to week 24 and annualized GA lesion growth rate. Genotype analysis did not reveal a meaningful correlation between complement-related, age-related macular degeneration risk single-nucleotide polymorphisms and complement levels and activities.
Conclusions
Complement levels or activities in AH and plasma did not correlate with GA lesion size or growth rate. This suggests that local complement activation as measured in AH does not appear to be related to GA lesion progression.
Financial Disclosure(s)
Proprietary or commercial disclosure may be found after the references.
Keywords: Complement pathway, Lampalizumab, Chroma and Spectri trials, Geographic atrophy, Lesion growth
Estimates suggest that geographic atrophy (GA) secondary to age-related macular degeneration (AMD) affects 0.44% of the global population.1 The cardinal early sign of GA is degeneration of retinal pigment epithelium, photoreceptors, and underlying choriocapillaris.2,3 Slowly enlarging parafoveal scotomas then cause irreversible visual dysfunction, and severe central vision loss ensues if the fovea becomes atrophic.4 Not surprisingly, GA exerts a significant burden on affected patients, their caregivers, and ophthalmic health care services because there are no approved therapies to halt disease progression or mitigate associated vision loss.3,5, 6, 7, 8, 9 An additional pressing challenge is that GA prevalence will increase in line with the aging population because GA disproportionately affects older individuals.1,10 Further, gaps in our knowledge regarding GA pathophysiology have hampered development of therapeutic agents in this area.5
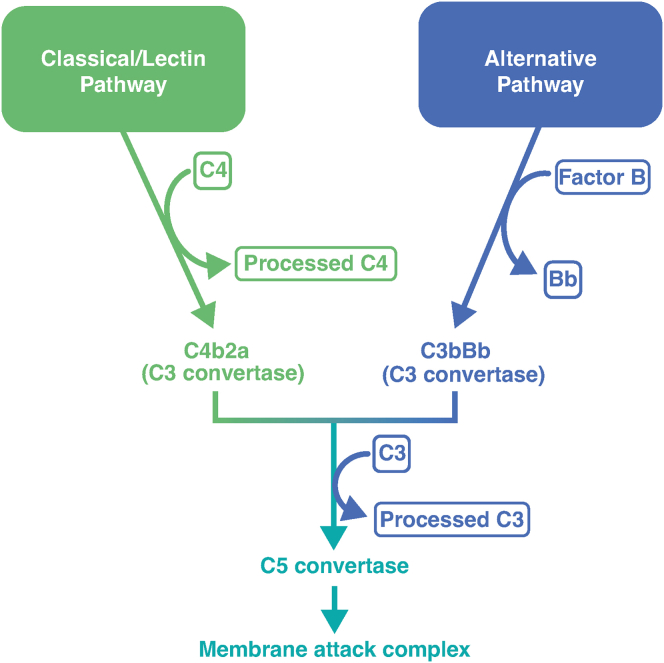
Genetic variation in the alternative complement cascade has been implicated in advanced AMD, including GA.2,3 The risk of developing advanced disease is associated with polymorphisms in genes encoding alternative complement pathway proteins, including complement factor H (CFH), complement factor I (CFI), and complement factor B (CFB).11, 12, 13 In addition, serum levels of complement proteins were found to be higher in patients with AMD vs. controls.13, 14, 15 One of these complement proteins, complement factor D (CFD), fulfills a key regulatory role in the alternative pathway.2 Complement factor D binds the C3b/CFB complex (C3bFB) and cleaves CFB into 2 fragments, Ba and Bb, to form the complement component 3 (C3) convertase,2 C3bBb, which in turn, catalyzes cleavage of additional C3.2
CFD is a rate-limiting enzyme of the alternative pathway and is present in the plasma at relatively low concentrations compared with other complement proteins16,17; hence, it was considered a therapeutic target in GA.16, 17, 18 However, the identically designed phase III Chroma and Spectri trials conducted in patients with bilateral GA and no previous active choroidal neovascularization showed that inhibiting CFD activity via intravitreal injection of lampalizumab, an antigen-binding fragment of a humanized monoclonal antibody, did not reduce GA lesion area enlargement over 48 weeks when compared with sham.5 A post hoc pharmacodynamic analysis of aqueous humor (AH) samples collected during the Chroma and Spectri trials reaffirmed that lampalizumab engages CFD and inhibits CFD-mediated cleavage of CFB18,19; however, the effects of lampalizumab intervention did not translate into a reduction in downstream complement pathway activation.20 Nevertheless, the Chroma and Spectri trials provide a unique opportunity to analyze the role of the complement pathway in GA progression.
We conducted a post hoc analysis of the Chroma and Spectri trials to assess the correlations of complement levels and activities in AH and plasma with baseline GA lesion size and growth rate. These additional insights into GA pathophysiology may support future drug research and development in this area.
Methods
Study Design
This was a post hoc analysis of complement activities in longitudinal baseline and week 24 AH samples collected from patients included in the 2 identically designed, phase III, and double-masked, sham-controlled Chroma (NCT02247479) and Spectri (NCT02247531) trials, which were conducted at 275 sites in 23 countries. The trial conduct, design and eligibility criteria, and baseline characteristics and outcomes have been published previously.5 In brief, patients were randomized in a 2:1:2:1 ratio to receive intravitreal lampalizumab 10 mg every 6 weeks, sham procedure every 6 weeks, intravitreal lampalizumab 10 mg every 4 weeks, or sham procedure every 4 weeks for 96 weeks. These trials adhered to the tenets of the Declaration of Helsinki and were conducted in accordance with the International Conference on Harmonisation E6 Guidelines for Good Clinical Practice and with applicable local, state, and federal laws. All sites received institutional review board or ethics committee approval before study initiation and all participants provided written informed consent.5
Assessment of GA Lesion Area and Growth Rate
GA lesion area was measured using blue-light fundus autofluorescence. Images were evaluated at the Doheny Image Reading Center and graded at a central reading center (GRADE Reading Center).5 For this post hoc analysis, we assessed GA lesion area at baseline, week 24, and week 48. In addition, we assessed mean change in GA lesion area from baseline to week 48, or annualized GA lesion growth rate. Annualized GA lesion growth rate was defined as the linear slope of a regression obtained on each study day for each patient, which were then multiplied by 48 weeks and 7 days. For assessment of the relationships between complement levels and activities and annualized GA lesion growth rate, only the pooled sham group was analyzed. Otherwise, for baseline comparisons between complement levels and activities and GA lesion size, patients across all treatment and sham groups were used for the analysis.
Assessment of Complement Levels and Activities in AH and Plasma Samples
Optional AH and plasma samples were collected from patients who consented to the procedure and sample acquisition. The AH sample was collected in the clinic by the treating physician before any treatment was administered. The collection consisted of an anterior chamber paracentesis whereby a drop of topical anesthetic was placed on the cornea and a 30-gauge needle was passed through the limbus into the anterior chamber and approximately 0.1 ml of aqueous fluid was removed. The plasma sample was collected in a 5 ml BD Vacutainer PPT (plasma preparation tube) containing K2-EDTA using standard venipuncture techniques. Upon collection, samples were gently inverted at least 8 to 10 times prior to centrifugation at a minimum of 1500 × g for 10 minutes at ambient temperature, and approximately 2.5 ml of the separated plasma was transferred to a new tube and aliquoted. All samples were subsequently frozen, shipped on dry ice, and stored at <−70°C at a central laboratory prior to analysis.
We used novel, well-characterized techniques to measure complement activities in AH, as described elsewhere.20 Briefly, assays used a Simoa (Quanterix) platform to measure levels of full-length CFB, the Bb fragment, full-length C3 (intact C3), the C3c, iC3b, and C3b fragments of C3 (processed C3); full-length complement C4 (intact C4); and the C4c and C4b fragments of C4 (processed C4). Further testing on all complement assays was performed to confirm assay compatibility in their existing configurations in plasma. Complement factor D concentrations were determined using an enzyme-linked immunosorbent assay, as previously described.21 Fig S1 (available at www.ophthalmologyscience.org) shows the components assessed in the study and their relationship with each other in the context of the complement pathway.
Complement levels and activities in AH and plasma at baseline were compared. To that end, patient-matched samples of AH and plasma collected at baseline from patients in the Chroma and Spectri trials were selected in a masked manner and analyzed using the 6 complement assays. These assays were qualified for use with AH as well as plasma, and hence, enabled analysis of complement activities between sample matrices.
For each assay, AH samples were measured in duplicate with a percent coefficient of variation of up to 30 for each analyte; samples with percent coefficient of variation greater than 30% (n = 2) were excluded from the analysis. Samples with analyte levels below the assay reportable range were assigned the value of the assay lower limit of quantification.
Assessment of Complement-Related AMD Risk Single-Nucleotide Polymorphisms and Complement Levels and Activities
In a subset of patients who provided consent, we performed whole-genome sequencing, as described previously,22 modified to exclude single-nucleotide polymorphisms (SNPs) with allele depth and allele balance test of P < 0.01. Previously identified complement-related AMD risk SNPs11 with minor allele frequencies greater than 0.1 were evaluated for correlations with complement levels and activities (defined as the ratio of processed:intact complement component) in AH and plasma at baseline. In some instances, the index SNP described in Fritsche et al (2016)11 did not pass quality control in our whole-genome screening. In these instances, a surrogate SNP in strong linkage disequilibrium (R2 > 0.8) was used instead of the originally identified AMD risk SNP. If no SNP was identified with R2 > 0.8, then this locus was not assessed in our whole genome sequencing.
Statistical Analysis
Mean and median complement levels and activities in AH20 and plasma at baseline were assessed across both treatment groups and sham group. Correlations of complement levels/activities in AH and plasma with GA lesion size and growth rate were calculated using Spearman’s rank correlation coefficient, a nonparametric measure to assess the strength of association. Due to the relatively small sample size, no adjustments were made for other factors in these analyses. Correlations between complement-related AMD–risk SNPs and complement levels/activities were also calculated using Spearman’s rank correlation coefficient. Statistical analyses were performed using R Statistical Software.23
Results
GA Area Metrics
Data on GA progression in the Chroma and Spectri trials have been published previously.5 In summary, across all treatment groups, GA lesions grew at a rate of approximately 2.0 mm2 per year.
Longitudinal baseline and follow-up week 24 AH samples from 97 patients were available for the analysis. Of these, 81 patients had valid patient-matched baseline plasma test results for all assays and were included in the current analysis. Baseline and demographic characteristics for this population are shown in Table 1.
Table 1.
Demographic and Baseline Characteristics of Patients Providing Aqueous Humor and Plasma Samples
| Sham (n = 26) | LQ6W (n = 26) | LQ4W (n = 29) | Overall (N = 81) | |
|---|---|---|---|---|
| Sex, no. (%) | ||||
| Female | 12 (46.2) | 16 (61.5) | 23 (79.3) | 51 (63.0) |
| Male | 14 (53.8) | 10 (38.5) | 6 (20.7) | 30 (37.0) |
| Age at parent reference date (years) | ||||
| Mean (SD) | 80.0 (8.39) | 79.3 (8.38) | 79.1 (7.72) | 79.5 (8.06) |
| Median [min, max] | 81.5 [60.0, 93.0] | 81.5 [61.0, 91.0] | 80.0 [63.0, 91.0] | 81.0 [60.0, 93.0] |
| Tobacco use, no. (%) | ||||
| Current | 3 (11.5) | 1 (3.8) | 3 (10.3) | 7 (8.6) |
| Never | 8 (30.8) | 15 (57.7) | 9 (31.0) | 32 (39.5) |
| Previous | 15 (57.7) | 10 (38.5) | 17 (58.6) | 42 (51.9) |
| Baseline study eye GA area by FAF (mm2) | ||||
| Mean (SD) | 6.15 (2.85) | 9.69 (5.04) | 8.47 (3.33) | 8.12 (4.06) |
| Median [min, max] | 5.35 [2.94, 13.9] | 8.63 [2.90, 17.7] | 7.55 [4.25, 17.3] | 7.54 [2.90, 17.7] |
| Baseline study eye GA lesion contiguity, no. (%) | ||||
| Multifocal | 22 (84.6) | 21 (80.8) | 24 (82.8) | 67 (82.7) |
| Not multifocal | 4 (15.4) | 5 (19.2) | 5 (17.2) | 14 (17.3) |
| Baseline study eye location of GA lesion, no. (%) | ||||
| Nonsubfoveal | 11 (42.3) | 12 (46.2) | 15 (51.7) | 38 (46.9) |
| Subfoveal | 15 (57.7) | 14 (53.8) | 14 (48.3) | 43 (53.1) |
| Baseline study eye GA lesion distance from fovea (μm) | ||||
| Mean (SD) | 170 (303) | 82.6 (116) | 132 (180) | 128 (213) |
| Median [min, max] | 0 [0, 1200] | 0 [0, 351] | 52.0 [0, 572] | 0 [0, 1200] |
| Baseline study eye hyperautofluorescence pattern, no. (%) | ||||
| Banded | 1 (3.8) | 4 (15.4) | 3 (10.3) | 8 (9.9) |
| Diffuse | 25 (96.2) | 22 (84.6) | 26 (89.7) | 73 (90.1) |
| Baseline study eye BCVA (ETDRS letters) | ||||
| Mean (SD) | 64.9 (10.7) | 64.0 (11.8) | 63.3 (9.24) | 64.0 (10.5) |
| Median [min, max] | 64.0 [44.0, 84.0] | 61.5 [50.0, 86.0] | 64.0 [50.0, 85.0] | 64.0 [44.0, 86.0] |
| Baseline study eye low-luminance deficit (ETDRS letters) | ||||
| Mean (SD) | 24.4 (12.4) | 31.4 (13.5) | 28.9 (14.8) | 28.2 (13.8) |
| Median [min, max] | 23.0 [2.00, 52.0] | 32.0 [4.00, 61.0] | 29.0 [6.00, 59.0] | 28.5 [2.00, 61.0] |
| Missing, no. (%) | 0 | 1 (3.8) | 2 (6.9) | 3 (3.7) |
| Baseline study eye presence of reticular pseudodrusen, no. (%) | ||||
| No | 16 (61.5) | 14 (53.8) | 18 (62.1) | 48 (59.3) |
| Questionable | 2 (7.7) | 4 (15.4) | 2 (6.9) | 8 (9.0) |
| Yes | 8 (30.8) | 8 (30.8) | 9 (31.0) | 25 (30.9) |
BCVA = best-corrected visual acuity; FAF = fundus autofluorescence imaging; GA = geographic atrophy; max = maximum; min = minimum; LQ4W, lampalizumab 10 mg every 4 weeks; LQ6W, lampalizumab 10 mg every 6 weeks; SD = standard deviation.
Correlation of Complement Protein Levels and Activities in AH and Plasma
Table S2 (available at www.ophthalmologyscience.org) shows the mean baseline plasma complement levels for patients with longitudinal AH and baseline plasma samples (N = 81) in all treatment groups and the sham group. Table S3 (available at www.ophthalmologyscience.org) shows that there were no strong correlations between levels and activities of complement proteins measured in AH and plasma at baseline (rho ≤ 0.37).
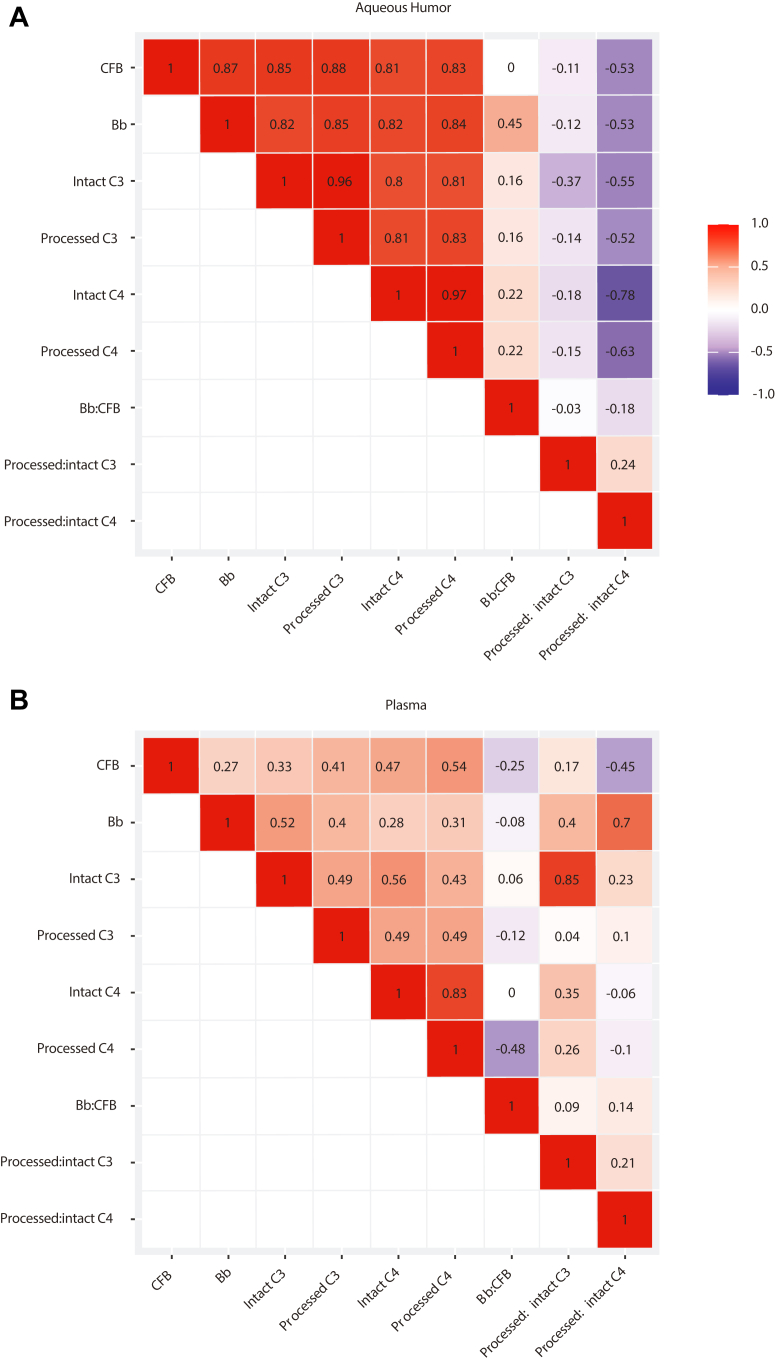
Figure 2 shows that in AH at baseline, there were strong correlations (rho ≥ 0.80) between intact complement proteins (e.g., between CFB and intact C3), between processed complement proteins (e.g., between Bb and processed C3), and between linked processed and intact complement proteins (e.g., between intact C3 and processed C3). Weak correlations (rho ≤ 0.24) were found between complement pathway activities (e.g., between Bb:CFB and processed:intact C3).
Figure 2.
Correlations between baseline complement protein levels and activities within (A) aqueous humor and (B) plasma (N = 81). C3 = complement component 3; C4 = complement component 4; CFB = complement factor B.
In contrast to the strong correlations observed in the AH, only weak to moderate correlations (rho = 0.27–0.56) between intact complement proteins, between processed complement proteins, and between linked processed and intact complement proteins were observed in plasma at baseline. The only exception was the strong correlation observed between intact C4 and processed C4 (rho = 0.83). Similar to AH, only weak correlations (rho ≤ 0.21) were observed between plasma complement pathway activities.
Correlation of Complement Levels and Activities with GA Lesion Size and Area
No meaningful correlations between the baseline complement levels/activities in either AH or plasma and baseline GA lesion size were found (Table 3). Complement activity was not prognostic of GA lesion area growth rate. In the sham group, no substantial correlations between baseline complement levels/activities in either AH or plasma and annualized GA lesion growth rate were found (Table 4).
Table 3.
Correlations Between Baseline Complement Levels and Activities and Baseline GA Lesion Size in Treatment and Sham Groups (N = 81)
| Complement Levels/Activities | Correlation (rho) |
|---|---|
| Aqueous humor | |
| Bb | 0.141 |
| CFB | 0.142 |
| Bb:CFB | 0.099 |
| Intact C3 | 0.109 |
| Processed C3 | 0.109 |
| Processed:intact C3 | −0.047 |
| Intact C4 | 0.119 |
| Processed C4 | 0.119 |
| Processed:intact C4 | −0.105 |
| Plasma | |
| Bb | −0.196 |
| CFB | −0.217 |
| Bb:CFB | 0.060 |
| Intact C3 | −0.021 |
| Processed C3 | 0.007 |
| Processed:intact C3 | 0.008 |
| Intact C4 | −0.151 |
| Processed C4 | −0.052 |
| Processed:intact C4 | 0.047 |
C3 = complement component 3; C4 = complement component 4; CFB = complement factor B; GA = geographic atrophy.
Table 4.
Correlations Between Baseline Complement Levels and Activities and Annualized GA Lesion Growth Rate in the Sham Group Only (N = 26)
| Complement Levels and Activities | Correlation (rho) |
|---|---|
| Aqueous humor | |
| Bb | 0.147 |
| CFB | 0.126 |
| Bb:CFB | 0.081 |
| Intact C3 | 0.041 |
| Processed C3 | 0.066 |
| Processed:intact C3 | 0.256 |
| Intact C4 | −0.012 |
| Processed C4 | −0.010 |
| Processed:intact C4 | 0.222 |
| Plasma | |
| Bb | −0.193 |
| CFB | −0.244 |
| Bb:CFB | 0.056 |
| Intact C3 | −0.226 |
| Processed C3 | 0.148 |
| Processed:intact C3 | 0.256 |
| Intact C4 | −0.348 |
| Processed C4 | −0.029 |
| Processed:intact C4 | 0.209 |
C3 = complement component 3; C4 = complement component 4; CFB = complement factor B; GA = geographic atrophy.
In sham-treated patients, changes in complement levels/activities in the AH from baseline to week 24 did not show a high level of correlation with annualized GA lesion growth rate (Table 5 and Fig S3 [available at www.ophthalmologyscience.org]). Similar results were obtained when patients from the lampalizumab treatment groups were included in the analyses (data not shown).
Table 5.
Correlation Between Change from Baseline to Week 24 in Aqueous Humor Complement Levels and Activities and Annualized Geographic Atrophy Lesion Growth Rate in the Sham Group Only (N = 26)
| Complement Levels and Activities | Correlation (rho) |
|---|---|
| Bb | 0.045 |
| CFB | −0.014 |
| Bb:CFB | 0.007 |
| Intact C3 | 0.285 |
| Processed C3 | 0.311 |
| Processed:intact C3 | 0.031 |
| Intact C4 | 0.296 |
| Processed C4 | 0.214 |
| Processed:intact C4 | −0.187 |
C3 = complement component 3; C4 = complement component 4; CFB = complement factor B.
Genetics and Complement Activities
Complement-related AMD–risk SNPs were identified in the following genes: CFI, CFH, C2/CFB, and C3. No meaningful correlations of complement-related AMD–risk SNPs with AH and plasma complement levels/activities were found (Table S6).
Discussion
In this post hoc analysis of the Chroma and Spectri trials, we found that neither baseline complement levels nor activities in AH or plasma were prognostic for GA lesion growth rate, nor did they reflect GA lesion size. Additionally, we did not observe a correlation between increases in GA lesion area and increases in complement protein levels or activities in AH, further highlighting a lack of relationship between complement activities and GA progression. This suggests that although there is strong genetic evidence and some limited biochemical evidence for complement activation contributing to the risk for developing advanced AMD,11,13 complement activities may not directly drive GA lesion growth in advanced disease. To our knowledge, this is the first study to investigate the role of ocular complement protein levels and activities specifically in GA progression.
Our analyses showed that complement levels and activities between AH and plasma were not well correlated in patients. This suggests that the complement levels and activity measured in AH are not simply a reflection of systemic complement activity, but instead may be a composite measurement reflecting also local complement production and/or activation within the eye. Consistent with this hypothesis, we found that complement activity is greater in AH than in plasma, in agreement with previous studies.24 Taken together, these results emphasize the potential importance of AH measurements for the purposes of studying ocular complement activities.
Recent studies have assessed the relationship between complement levels and AMD progression. A prospective study in 79 patients with AMD showed significantly increased complement levels in AH in patients with early, intermediate, and neovascular AMD vs. age-matched controls.25 In addition, a study of complement activation in 797 patients with AMD and 945 controls found that AMD stage was significantly associated with complement activation levels in serum (higher for intermediate AMD and central GA), and that complement activation was more pronounced in individuals with genetic polymorphisms associated with AMD risk.13 Altered levels of complement activation in serum samples were associated with the SNPs rs10922109 and rs570618 (CFH gene) and rs116503776 (CFB gene).13 However, 15 other selected AMD-associated variants in C3, CFB, CFH, CFI, or complement C9 were not significantly linked with complement activation.13 In our analyses, no meaningful correlations were observed between complement-related AMD–risk SNPs and complement levels or activities in either plasma or AH among patients with bilateral GA. However, this study was not powered to specifically assess these associations, thus the lack of association was possibly due to the much smaller patient sample size of 81 patients in our study compared with the 797 patients with AMD and 945 controls in the above study.13 It remains to be determined as to what characteristics might identify patients who are more likely to benefit from complement-inhibiting therapy.
The strengths of our study are the sensitivity and specificity of the assays,20 which showed strong correlations between linked, processed, and intact complement proteins, and a weak correlation between the different complement pathway activities. The GA lesion area and growth rate data from this post hoc analysis are representative of the data published for the full Chroma and Spectri cohorts, and in line with findings from the GA Progression study and Proxima A and B trials.5,7,26
Limitations of this analysis include the technical challenges associated with measuring retinal complement activities. We believe that the proximity of the anterior chamber to the retina makes it a more relevant compartment for monitoring retinal complement activities compared with measuring complement activities in peripheral blood; however, there remains a possibility that AH complement activities might not accurately reflect retinal complement activities. Along these lines, the vast majority of complement protein is produced in the liver and enters the circulation before reaching the eye,27 and complement levels have been reported as much higher in plasma than in AH.20,24 While some of this protein may have already been activated before entering the eye, previous research has found that activation of complement was increased in AH relative to systemic complement activation in patients with AMD.24 However, our assays cannot distinguish between locally and systemically activated complement in AH.
While previous genetic11 and histological analyses28, 29, 30 have strongly implicated the complement pathway in the progression toward advanced stages of AMD, the lack of complement associations with GA lesion size or growth found in this study would suggest that other biological processes may be contributing more significantly to GA lesion growth. Consistent with this idea, in patients with GA secondary to AMD, intravitreal administration of the complement C5 inhibitor, avacincaptad pegol (Zimura), reduced the mean rate of GA growth a modest 17.3% based on GA area, compared with sham treatment in the phase III GATHER2 study.31,32 Similar efficacy was observed in the phase III DERBY and OAKS studies using the complement component 3 inhibitor pegcetacoplan (APL-2; Apellis Pharmaceuticals) where GA lesion growth was reduced 16% to 22% compared with sham treatment.33, 34, 35 Follow-up evaluation of these treatments, along with further research, is needed to better elucidate the role of the complement system across the different stages of AMD progression.
Acknowledgments
Acknowledgments. The authors would like to acknowledge Teresa Davancaze for development of the CFD assay and sample testing support, Patrick Zoder for sample testing and project management support, Eric Wakshul for assay development and technical support, Janis Allen for assay development and project management support, Brian Yaspan and Amy Dressen for patient genotyping support, and Kelly Loyet for provision of assay protocols and reagents.
Data Sharing. Qualified researchers may request access to individual patient level data through the clinical study data request platform (https://vivli.org/). Further details on Roche’s criteria for eligible studies are available here (https://vivli.org/members/ourmembers/). For further details on Roche’s Global Policy on the Sharing of Clinical Information and how to request access to related clinical study documents, see here (https://www.roche.com/research_and_development/who_we_are_how_we_work/clinical_trials/our_commitment_to_data_sharing.htm).
Manuscript no. XOPS-D-22-00203.
Footnotes
Supplemental material available atwww.ophthalmologyscience.org
Disclosures:
All authors have completed and submitted the ICMJE disclosures form.
The authors declare the following financial interests/personal relationships which may be considered as potential competing interests: L.A.H.: Medical writing support – Envision Pharma Group; Funding – Genentech, Inc; Employee – Genentech; Stock and stock options – Roche; Equipment, materials, drugs, medical writing, gifts or other services, including financial and non-financial interests – Genentech, Inc.
M.C.C.: Medical writing support – Envision Pharma Group; Funding – Genentech, Inc; Employee – Genentech; Stock and stock options – Roche; Equipment, materials, drugs, medical writing, gifts or other services, including financial and non-financial interests – Genentech, Inc.
R.E.: Medical writing support – Envision Pharma Group; Funding – Genentech, Inc; Employee – Genentech; Stock and stock options – Roche; Equipment, materials, drugs, medical writing, gifts or other services, including financial and non-financial interests – Genentech, Inc.
V.S.: Medical writing support – Envision Pharma Group; Funding – Genentech, Inc; Employee – Genentech; Stock and stock options – Roche; Equipment, materials, drugs, medical writing, gifts or other services, including financial and non-financial interests – Genentech, Inc.
All authors report support from Genentech, Inc, a member of the Roche Group, for third-party writing assistance for this manuscript.
Genentech, Inc, a member of the Roche Group, provided financial support for the study and participated in the study design; conducting of the study; data collection, management, analysis, and interpretation; and preparation, review, and approval of the manuscript. Third-party writing assistance was provided by Nibedita Gupta, PhD, CMPP, of Envision Pharma Group and funded by Genentech, Inc, a member of the Roche Group.
HUMAN SUBJECTS: These trials adhered to the tenets of the Declaration of Helsinki and were conducted in accordance with the International Conference on Harmonization E6 Guidelines for Good Clinical Practice and with applicable local, state, and federal laws. All sites received institutional review board or ethics committee approval before study initiation and all participants provided written informed consent.
No animal subjects were used in this study.
Author Contributions:
Conception and design: Steffen, Chang
Data Collection: Edmonds
Analysis and Interpretation: Edmonds, Steffen, Honigberg, Chang
Obtained funding: N/A; Overall responsibility: Edmonds, Steffen, Honigberg, Chang
Meeting Presentation: Data were previously published in an abstract in Association for Research in Vision and Ophthalmology Conference (ARVO) 2020; however, due to the COVID-19 pandemic, data were not presented at the meeting.
Supplementary Data
Figure S1.
Schematic showing the 6 complement components measured in this study.
Figure S3.
Correlations between Annualized GA Growth Rate and complement levels and activities in Sham Groups.
References
- 1.Wong W.L., Su X., Li X., et al. Global prevalence of age-related macular degeneration and disease burden projection for 2020 and 2040: a systematic review and meta-analysis. Lancet Glob Health. 2014;2:e106–e116. doi: 10.1016/S2214-109X(13)70145-1. [DOI] [PubMed] [Google Scholar]
- 2.Boyer D.S., Schmidt-Erfurth U., van Lookeren Campagne M., et al. The pathophysiology of geographic atrophy secondary to age-related macular degeneration and the complement pathway as a therapeutic target. Retina. 2017;37:819–835. doi: 10.1097/IAE.0000000000001392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Holz F.G., Strauss E.C., Schmitz-Valckenberg S., van Lookeren Campagne M. Geographic atrophy: clinical features and potential therapeutic approaches. Ophthalmology. 2014;121:1079–1091. doi: 10.1016/j.ophtha.2013.11.023. [DOI] [PubMed] [Google Scholar]
- 4.Sunness J.S., Rubin G.S., Applegate C.A., et al. Visual function abnormalities and prognosis in eyes with age-related geographic atrophy of the macula and good visual acuity. Ophthalmology. 1997;104:1677–1691. doi: 10.1016/s0161-6420(97)30079-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Holz F.G., Sadda S.R., Busbee B., et al. Efficacy and safety of lampalizumab for geographic atrophy due to age-related macular degeneration: Chroma and Spectri phase 3 randomized clinical trials. JAMA Ophthalmol. 2018;136:666–677. doi: 10.1001/jamaophthalmol.2018.1544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chakravarthy U., Bailey C.C., Scanlon P.H., et al. Direct ophthalmic healthcare resource use among patients with geographic atrophy in a large cohort from the United Kingdom. Ophthalmol Retina. 2019;3:920–926. doi: 10.1016/j.oret.2019.06.012. [DOI] [PubMed] [Google Scholar]
- 7.Holekamp N., Wykoff C.C., Schmitz-Valckenberg S., et al. Natural history of geographic atrophy secondary to age-related macular degeneration: results from the prospective Proxima A and B clinical trials. Ophthalmology. 2020;127:769–783. doi: 10.1016/j.ophtha.2019.12.009. [DOI] [PubMed] [Google Scholar]
- 8.Künzel S.H., Möller P.T., Lindner M., et al. Determinants of quality of life in geographic atrophy secondary to age-related macular degeneration. Invest Ophthalmol Vis Sci. 2020;61:63. doi: 10.1167/iovs.61.5.63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Patel P.J., Ziemssen F., Ng E., et al. Burden of illness in geographic atrophy: a study of vision-related quality of life and health care resource use. Clin Ophthalmol. 2020;14:15–28. doi: 10.2147/OPTH.S226425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rudnicka A.R., Kapetanakis V.V., Jarrar Z., et al. Incidence of late-stage age-related macular degeneration in American Whites: systematic review and meta-analysis. Am J Ophthalmol. 2015;160:85–93. doi: 10.1016/j.ajo.2015.04.003. [DOI] [PubMed] [Google Scholar]
- 11.Fritsche L.G., Igl W., Bailey J.N., et al. A large genome-wide association study of age-related macular degeneration highlights contributions of rare and common variants. Nat Genet. 2016;48:134–143. doi: 10.1038/ng.3448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gorin M.B. Genetic insights into age-related macular degeneration: controversies addressing risk, causality, and therapeutics. Mol Aspects Med. 2012;33:467–486. doi: 10.1016/j.mam.2012.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Heesterbeek T.J., Lechanteur Y.T.E., Lorés-Motta L., et al. Complement activation levels are related to disease stage in AMD. Invest Ophthalmol Vis Sci. 2020;61:18. doi: 10.1167/iovs.61.3.18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Scholl H.P.N., Charbel Issa P., Walier M., et al. Systemic complement activation in age-related macular degeneration. PLoS One. 2008;3 doi: 10.1371/journal.pone.0002593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Stanton C.M., Yates J.R., den Hollander A.I., et al. Complement factor D in age-related macular degeneration. Invest Ophthalmol Vis Sci. 2011;52:8828–8834. doi: 10.1167/iovs.11-7933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Do D.V., Pieramici D.J., van Lookeren Campagne M., et al. A phase Ia dose-escalation study of the anti-factor D monoclonal antibody fragment FCFD4514S in patients with geographic atrophy. Retina. 2014;34:313–320. doi: 10.1097/IAE.0b013e3182979ddd. [DOI] [PubMed] [Google Scholar]
- 17.Volanakis J.E., Narayana S.V.L. Complement factor D, a novel serine protease. Protein Sci. 1996;5:553–564. doi: 10.1002/pro.5560050401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Katschke K.J., Jr., Wu P., Ganesan R., et al. Inhibiting alternative pathway complement activation by targeting the factor D exosite. J Biol Chem. 2012;287:12886–12892. doi: 10.1074/jbc.M112.345082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yaspan B.L., Williams D.F., Holz F.G., et al. Targeting factor D of the alternative complement pathway reduces geographic atrophy progression secondary to age-related macular degeneration. Sci Transl Med. 2017;9 doi: 10.1126/scitranslmed.aaf1443. [DOI] [PubMed] [Google Scholar]
- 20.Edmonds R., Steffen V., Honigberg L.A., Chang M.C. Alternative complement pathway inhibition by lampalizumab: analysis of data from Chroma and Spectri phase 3 clinical trials. Ophthalmol Sci. 2023 doi: 10.1016/j.xops.2023.100286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Le K.N., Gibiansky L., van Lookeren Campagne M., et al. Population pharmacokinetics and pharmacodynamics of lampalizumab administered intravitreally to patients with geographic atrophy. CPT Pharmacometrics Syst Pharmacol. 2015;4:595–604. doi: 10.1002/psp4.12031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dressen A., Abbas A.R., Cabanski C., et al. Analysis of protein-altering variants in telomerase genes and their association with MUC5B common variant status in patients with idiopathic pulmonary fibrosis: a candidate gene sequencing study. Lancet Respir Med. 2018;6:603–614. doi: 10.1016/S2213-2600(18)30135-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.R Core Team R: a language and environment for statistical computing. 2021. https://www.R-project.org/
- 24.Schick T., Steinhauer M., Aslanidis A., et al. Local complement activation in aqueous humor in patients with age-related macular degeneration. Eye (Lond) 2017;31:810–813. doi: 10.1038/eye.2016.328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Altay L., Sitnilska V., Schick T., et al. Early local activation of complement in aqueous humour of patients with age-related macular degeneration. Eye (Lond) 2019;33:1859–1864. doi: 10.1038/s41433-019-0501-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Schmitz-Valckenberg S., Sahel J.A., Danis R., et al. Natural history of geographic atrophy progression secondary to age-related macular degeneration (geographic atrophy progression study) Ophthalmology. 2016;123:361–368. doi: 10.1016/j.ophtha.2015.09.036. [DOI] [PubMed] [Google Scholar]
- 27.Lubbers R., van Essen M.F., van Kooten C., Trouw L.A. Production of complement components by cells of the immune system. Clin Exp Immunol. 2017;188:183–194. doi: 10.1111/cei.12952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Johnson L.V., Leitner W.P., Staples M.K., Anderson D.H. Complement activation and inflammatory processes in drusen formation and age related macular degeneration. Exp Eye Res. 2001;73:887–896. doi: 10.1006/exer.2001.1094. [DOI] [PubMed] [Google Scholar]
- 29.Johnson L.V., Ozaki S., Staples M.K., et al. A potential role for immune complex pathogenesis in drusen formation. Exp Eye Res. 2000;70:441–449. doi: 10.1006/exer.1999.0798. [DOI] [PubMed] [Google Scholar]
- 30.Mullins R.F., Russell S.R., Anderson D.H., Hageman G.S. Drusen associated with aging and age-related macular degeneration contain proteins common to extracellular deposits associated with atherosclerosis, elastosis, amyloidosis, and dense deposit disease. FASEB J. 2000;14:835–846. [PubMed] [Google Scholar]
- 31.Jaffe G.J., Westby K., Csaky K.G., et al. C5 inhibitor avacincaptad pegol for geographic atrophy due to age-related macular degeneration: a randomized pivotal phase 2/3 trial. Ophthalmology. 2021;128:576–586. doi: 10.1016/j.ophtha.2020.08.027. [DOI] [PubMed] [Google Scholar]
- 32.IVERIC Bio I Iveric Bio announces positive topline data from Zimura® GATHER2 phase 3 clinical trial in geographic atrophy. 2022. https://investors.ivericbio.com/news-releases/news-release-details/iveric-bio-announces-positive-topline-data-zimurar-gather2-phase Available at:
- 33.Liao D.S., Grossi F.V., El Mehdi D., et al. Complement C3 inhibitor pegcetacoplan for geographic atrophy secondary to age-related macular degeneration: a randomized phase 2 trial. Ophthalmology. 2020;127:186–195. doi: 10.1016/j.ophtha.2019.07.011. [DOI] [PubMed] [Google Scholar]
- 34.Apellis announces top-line results from phase 3 DERBY and OAKS studies in geographic atrophy (GA) and plans to submit NDA to FDA in the first half of 2022. https://investors.apellis.com/news-releases/news-release-details/apellis-announces-top-line-results-phase-3-derby-and-oaks Available at:
- 35.Apellis Pharmaceuticals I Apellis announces 24-month results showing increased effects over time with pegcetacoplan in phase 3 DERBY and OAKS studies in geographic atrophy (GA) 2022. https://investors.apellis.com/news-releases/news-release-details/apellis-announces-24-month-results-showing-increased-effects Available at:
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.