Abstract
A heterotrophic-specialist model was proposed previously to divide wastewater treatment plant (WWTP) heterotrophs into sub-guilds of consumers of readily or slowly degradable substrates (RDS or SDS, respectively). The substrate degradation rate model coupled to metabolic considerations predicted that RNA and polyhydroxyalkanoate (PHA) levels would be positively correlated in the activated sludge communities with high RNA and PHA occurring in RDS-consumers, and low RNA with no PHA accumulation occurring in SDS-consumers because their external substrates are always present. This prediction was verified in previous studies and in the current one. Thus, RNA and PHA levels were used as biomarkers of the RDS- and SDS-consumer sub-guilds for cell sorting using flow cytometry of samples from three WWTPs. Subsequently, 16S rRNA gene amplicon sequencing revealed that the sorted groups were highly similar over time and among WWTPs, and demonstrated a clear segregation by RNA levels. Predicted ecophysiological traits based on 16S rRNA phylogeny suggested that the high-RNA population showed RDS-consumer traits such as higher rrn copy numbers per genome. Using a mass-flow immigration model, it appeared that the high-RNA populations exhibited high immigration rates more frequently than low-RNA populations, but the differences in frequencies were less with increasing solids residence times.
Keywords: heterotrophic guilds, activated sludge, microbial community, deterministic factor, flow cytometry, fluorescence-activated cell sorting
1. Introduction
The central workforce of activated sludge wastewater treatment plants (WWTPs) is the microbial community. Identification of the microbial community members and understanding their microbial ecology are of high importance for the development of new processes and mathematical models, and for the prevention of operational failures. Several studies showed that, even though different activated sludge systems have diverse microbial community structures, they share a similar core of abundant populations [1,2]. However, the mechanisms underlying microbial community assembly are not fully understood. The microbial community is affected by various factors that can be classified as neutral or deterministic factors [3–5]. Neutral factors refer to random phenomena such as immigration with the wastewater influents, removal (wastage) of biomass, and fortuitous births and deaths causing drift in population abundances. All these factors do not confer selective advantage to given populations. Conversely, deterministic factors provide selective pressure on the populations and they include process configuration (e.g. conventional, enhanced biological phosphorus removal and nitrification–denitrification), operational parameters (e.g. solids retention time (SRT)) and influent substrate composition [6–13].
Particularly for heterotrophic bacteria, it appears that the substrate utilization is one of the major factors selecting the populations assembling in the activated sludge mixed liquor community, which may be the result of the specialization of populations in consuming specific types of organic substrates [6,14]. Aiming to link such observations with current activated sludge modelling frameworks, the heterotrophic-specialist model was proposed as the main mechanism structuring the populations of activated sludge heterotrophs in a series of substrate sub-guilds [14–16]. The sub-guild classification also amounts to a kinetic classification in the context of activated sludge models because the consumed organic compounds are considered as either slowly degradable substrates (SDS) or readily degradable substrates (RDS) [17].
In our previous study, we hypothesized that the heterotrophic-specialist model predicted a positive correlation between the cellular RNA level (high instantaneous growth rate indicator) and the accumulation of storage compounds (indicator of exceedance of carbon supply beyond growth rate capacity due to very fast substrate uptake) [15]. The hypothesis was based on simulations with the Activated Sludge Model No. 3 (ASM3) of substrate feast–famine cycles in conventional plug-flow reactors showing accumulation of storage compounds such as polyhydroxybutyrate only by RDS-consumers but not SDS-consumers [15]. The slow substrate uptake by SDS-consumers provides them access to their external substrate at all times, and they do not experience feast–famine. The feast–famine cycle experienced by RDS-consumers leads also to the accumulation of high cellular RNA levels to sustain high biomass formation rates during the feast phase [18]. The predicted positive correlation between RNA and polyhydroxyalkanoate (PHA) was verified at nine WWTPs in both winter and summer [15], supporting the classification of heterotrophs as RDS-consumer and SDS-consumer specialists. Thus, RNA and PHA can be used as biomarkers of the heterotrophic sub-guilds.
The current study addresses three questions arising from the previous study on the sub-guilds defined on the basis of RNA and PHA levels. First, does one find similar populations in the sub-guilds at different WWTPs? Second, what are the ecophysiological traits related to the sub-guilds? Third, are the sub-guild assemblies influenced differently by immigration?
One of the potential ecological traits that differs between the RDS- and SDS-consumers is the level of ribosomal RNA operon (rrn) copy number per genome. While rapid growth (i.e. biomass formation) requires more ribosomes than slow growth, the capacity to rapidly increase the ribosome content correlates to rrn copies in a genome [19–23]. Thus, rrn copy number plays an ecological role in substrate utilization and maximum reproductive rates [24–26]. A few studies have investigated the rrn copy number in WWTP microbial communities. A study of an anaerobic sludge digester reported that rrn copy number increased with increasing the organic loading rate [27]. In plug-flow reactors, concentrations of RDS are very high at the beginning of reactors (feast phase), which may be a selection pressure for high rrn species capable of rapid metabolic adaptation. Therefore, RDS-consumer sub-guild may have higher average rrn copy number than SDS-consumers, a hypothesis to be tested. The study aims also to identify other potential traits related to the sub-guilds.
To answer the questions raised above, three WWTPs were sampled on three different days over a 1-year period. The WWTPs were chosen because they exhibited the most differences in taxonomic composition among the plant we studied previously around Montreal (Quebec), Canada [28]. Activated sludge samples were sorted based on RNA and PHA levels using fluorescence-activated cell sorting (FACS), and the sorted samples were characterized by Illumina high-throughput 16S rRNA gene amplicon sequencing. Genomes predicted from 16S rRNA were used to determine possible differences in the distributions of rrn copy numbers and other potential ecophysiological traits between the sorted groups [29]. Finally, a mass-flow immigration model was used to calculate the fraction of immigration characterizing the biomass of each population.
2. Materials and methods
2.1. Activated sludge samples
Influent and activated sludge samples were obtained from Pincourt, Cowansville, and LaPrairie WWTPs in Québec, Canada on 3 days (table 1). Samples were fixed on site in 4% paraformaldehyde in phosphate-buffered saline solution for 4 h, followed by 1 h of 75% methanol fixation. After fixation, samples were stored in 50% ethanol at –80°C until analyses [15].
Table 1.
Characteristics of activated sludge WWTPs and influent of monthly average.
| plant characteristicsa | LaPrairie | Cowansville | Pincourt | ||||||
|---|---|---|---|---|---|---|---|---|---|
| process | conventional PFRb | oxidation ditch | EBPR (summer)/conventional PFR (winter)c | ||||||
| SRT (d) | 7 | 10 | 15 | ||||||
| HRT (h) | 15 | 18 | 8 | ||||||
| ratio of origin of COD (residential : industrial) | 45% : 55% | 90% : 10% | 90% : 10% | ||||||
| sampling date | 25 May 2016 | 25 May 2017 | 14 Aug 2017 | 25 May 2016 | 25 May 2017 | 14 Aug 2017 | 25 May 2016 | 25 May 2017 | 14 Aug 2017 |
| flow rate (m3 d−1) | 52 848 | 63 701 | 57 246 | 10 659 | 16 059 | 13 829 | 6855 | 12 506 | 6534 |
| influent | |||||||||
| COD (mg l−1) | 531 | 404 | 400 | 363 | 213 | 282 | 373 | 148 | 343 |
| BOD5 (mg l−1) | 252 | 135 | 125 | 89 | 56 | 38 | 91 | 35 | 62 |
| TSS (mgl−1) | 295 | 224 | 230 | 256 | 154 | 214 | 166 | 58 | 152 |
| (mg-N l−1) | 16.5 | 12.1 | 15.6 | N/A | N/A | N/A | N/A | N/A | N/A |
| P total (mg-P l−1) | 12.5 | 10.7 | 14.0 | 2.6 | 2.0 | 2.1 | 3.4 | 1.4 | 3.3 |
| effluent | |||||||||
| COD (mg l−1) | 26.0 | 33.2 | 45.5 | 15.3 | 13.9 | 8.5 | 37.0 | 10.0 | 31.0 |
| BOD5 (mg −1 | 4.0 | 4.8 | 7.7 | 2.9 | 9.9 | 3.2 | 5.2 | 9.1 | 19.0 |
| TSS (mg −1 | 6.1 | 7.6 | 23.4 | 2.9 | 4.1 | 6.0 | 5.4 | 10.0 | 5.0 |
| (mg-N l−1) | 0.4 | 1.1 | 0.7 | 0.2 | 5.0 | 0.1 | 17.0 | 6.1 | 6.0 |
| P total (mg-P l−1) | 1.90 | 2.86 | 9.43 | 0.11 | 0.28 | 0.17 | 0.50 | 0.35 | 0.49 |
| aeration tank | |||||||||
| dissolved O2 (mg l−1) | 3.4 | 3.8 | 2.4 | 1.0 | 0.4 | 0.7 | 3.3 | 2.8 | 2.1 |
| MLSS (mg l−1) | 2584 | 2514 | 1998 | 7382 | 10 366 | 11 593 | 3114 | N/A | 2802 |
| MLVSS (mg l−1) | 2012 | 1981 | 1521 | 4998 | 6599 | 6019 | 2641 | N/A | 2265 |
| MLVSS/MLSS (%) | 78 | 79 | 76 | 68 | 64 | 52 | 85 | N/A | 81 |
aSRT: solids retention time; HRT: hydraulic retention time; COD: chemical oxygen demand; BOD5: 5-day biochemical oxygen demand; TSS: total suspended solids; MLSS: mixed liquor suspended solids; MLVSS: mixed liquor volatile suspended solids.
bPFR: plug-flow reactor.
cEBPR: enhanced biological phosphorus removal; summer: approximate date 15 May–15 November; winter: 16 November–14 May.
2.2. Flow cytometry cell sorting
Cell sorting process followed the protocol developed previously [15] using BD FACSAria™ cell sorter (BD Biocsiences, San Jose, USA). Mixed liquor samples were stained to detect RNA, DNA and PHA by using respectively SYTO RNASelect (Molecular Probe®, Invitrogen, Carlsbad, CA, emission filter 530 ± 30 nm), 7-aminoactinomycin D (7-AAD; Molecular Probe®, Invitrogen, Carlsbad, CA, emission filter 650+ nm) and Nile Red (Sigma-Aldrich, St Louis, USA, emission filter 585 ± 26 nm). Stained cells were sorted using FACS by first selecting events with minimal values of side and forward scatter (gate P3; electronic supplementary material, figure S1a), and then activating the sorting using a 488 nm laser to acquire the signal of SYTO RNASelect (RNA stain) relative to 7-AAD (DNA stain) (electronic supplementary material, figure S1b). A subsample of cells selected by side and forward scatter (gate P3; electronic supplementary material, figure S1a) was collected without sorting to test the impact of the flow cytometry protocol on the taxonomic composition of the sample. Sub-gates were set to capture high RNA per DNA cells (high-RNA gate) and low RNA per DNA cells (low-RNA gate). In the sub-gates, Nile Red signal intensity was divided into high-PHA and low-PHA level gates. For each WWTP, seven samples were analysed: influent (INF), mixed liquor (ML), unsorted (Un) and four sorted sub-samples: high-RNA low-PHA (HL), high-RNA high-PHA (HH), low-RNA low-PHA (LL) and low-RNA high-PHA (LH). The total number of cells sorted is summarized in the electronic supplementary material, table S1.
2.3. DNA extraction, PCR amplification and amplicon sequencing
DNA extraction was performed using 10% Chelex® 100 Resin (Bio-Rad, Hercules, USA) and Proteinase K (Sigma-Aldrich, Oakville, Canada) on a heating block [15]. DNA was amplified using a nested PCR protocol to produce sufficient amplicon quantity for sequencing. First, 16S rRNA genes were amplified using universal primer pair 27F/1492R [30]. The amplicons were diluted and used as the template for the next PCR using 926F/1392R [31] with adaptors targeting the V6–V8 regions of 16S rRNA genes. Then, sample-specific barcodes were added to the amplicons by a final PCR. PCR conditions are listed in the electronic supplementary material, table S2. All reactions were carried out with primer concentration at 0.3 µM, Mg2+ 1.5 mM, dNTP 200 µM, Taq DNA polymerase 1.25 U/50 µl reaction (all reagents from New England Biolab). The amplicons were cleaned up using QIAquick PCR purification kit (Qiagen Inc., Toronto, Canada), then quantified using Quant-iT™ PicoGreen™ dsDNA Assay Kit (Life Technologies Inc., Burlington, ON, Canada). The amplicon libraries were pooled with equal mass quantity and sequenced on Illumina Miseq PE300 platform at McGill University and Génome Québec Innovation Centre (Montréal, QC, Canada).
2.4. Bioinformatics and data analysis
The raw de-multiplexed sequence data were paired, primer-stripped, quality-filtered, and chimera-removed using USEARCH algorithm [32]. Operational taxonomic units (OTUs) were picked at 97% similarity level using open-reference protocol with the UCLUST algorithm [32]. Taxonomy was assigned based on Greengenes reference database [33,34] and diversity was analysed using Quantitative Insights Into Microbial Ecology (Qiime) pipelines [35] and R ‘vegan’ packages [36]. The total quality-filtered sequence reads were between 36 409 and 75 001 per sample, with median of 50 676 reads per sample. For standardization, each sample was rarefied to 36 000 reads. OTUs were clustered at 97% similarity level (resulting in 6685 OTUs) then assigned with taxonomy. Beta-diversity variation between groups was computed using permutational multi-variate analysis of variance (PERMANOVA) test; the p-value was calculated with 999 permutations. A group of abundant genera were picked for top 10 genera from each sample. The comparison of OTU abundance between groups was tested using the Odds Ratio test to obtain the significantly enriched OTUs [15,37]. Closed reference OTUs were input in PICRUSt [29] to predict metagenomes using database IMG [38] and functional profiles using database KEGG [39]. The weighted average rrn copy number was calculated using the total sequence of closed reference OTUs and normalized OTUs. The comparison of functions between groups was tested using two-sided Welch's t-test. Raw sequences can be accessed from NCBI GenBank (BioProject ID: PRJNA496295).
2.5. Microbial immigration and growth rate model
The derivation of the microbial immigration and growth rate model is detailed in Guo et al. [5]. In summary, the immigration ratio of the ith population (mi) is the proportion of the mixed liquor biomass derived from the influent biomass (equation (2.1)):
| 2.1 |
where XBio,i,Inf and XBio,i,ML are the concentrations of the ith population in influent and mixed liquor, respectively, θx is SRT, θ is hydraulic retention time (HRT), fBio,Capt,i is the fraction of influent ith population captured by the mixed liquor flocs (assumed to be 100%) and bi is the decay rate.
The model also relates the immigration ratio to the specific growth rate of the ith population (µi) through equation (2.2):
| 2.2 |
The model defines the relationship between the DNA fractions of the ith population in the influent and the mixed liquor measured by 16S rRNA amplicon sequencing (f16S,Inf,i and f16S,ML,i, respectively), and reveals that the log–log plot of these fractions makes the populations with the same immigration ratio appear on a 1 : 1 line with the y-intercept (term between square brackets) function of mi (equation (2.3)):
| 2.3 |
where XTot,Inf and XTot,ML are the total concentrations of suspended solids in influent and mixed liquor, γDNA,Inf and γDNA,ML are the masses of DNA per gram of suspended solid in influent and mixed liquor.
From equation (2.3), the zero net growth rate line (µnet,i = µi − bi = 0, common reference line in the graphs) is obtained by equation (2.4):
| 2.4 |
3. Results
3.1. Microbial community composition and diversity
Similar to our previous study [15], the correlation trend between the cellular RNA and PHA levels was observed for all samples (electronic supplementary material, figure S1c). Samples of AS mixed liquor processed or not by flow cytometry and of influent were analysed using 16S rRNA gene amplicon sequencing. Proteobacteria was the most abundant phylum in all samples, followed by Bacteroidetes, Firmicutes, Planctomycetes and OP11 (electronic supplementary material, figure S2). To highlight differences at the genus level, the 10 most abundant genera in each sample were selected, which assembled a set of 63 genera observed in all samples (figure 1). The hierarchical clustering of samples based on this set of genera showed grouping into AS mixed liquor and influent samples, and the mixed liquor samples were generally further grouped by WWTP of origin (with a few exception of sorted AS mixed liquor samples from LaPrairie) (figure 1).
Figure 1.
Assemblage of the most abundant 10 genera from each sample. Influent (INF) and mixed liquor (ML) samples were obtained from LaPrairie (LP), Cowansville and Pincourt on three sampling dates: A (25 May 2016), B (25 May 2017) and C (14 August 2017). ML samples were processed by flow cytometer to get unsorted (Unsort) and sorted samples: high-RNA low-PHA (HL), high-RNA high-PHA (HH), low-RNA low-PHA (LL) and low-RNA high-PHA (LH). Hierarchical clustering used Euclidean distance and algorithm based on the selected abundant genera. OTU names are shown at genus or higher taxonomic level when genera were not identified. Colour key indicates relative abundance of genera in log scale.
The genera Flavobacterium and Acinetobacter, and unidentified genera of the families Oxalobacteraceae, Aeromonadaceae, Carnobacteriaceae and Comamonadaceae were highly prevalent in all influent and mixed liquor samples. A few taxa were only abundant at one plant such as the family Methylophilaceae, which was highly abundant only in the LaPrairie mixed liquor, and 4 to 30 times more abundant in the influent of LaPrairie than the ones of the other two plants. These observations are similar to abundant and specific genera of activated sludge WWTPs reported in a previous Danish study [1].
The simple flow cytometry processing (i.e. no sorting) of mixed liquor samples generally decreased the richness (number of OTUs) and first-order Hill number (exponential of Shannon index) compared to the unprocessed sample (electronic supplementary material, figure S3), which was due to the loss of rare taxa. In turn, the alpha diversities of sorted samples were typically lower than that of the unsorted sample after flow cytometry processing, supporting the expected selection of specific taxa in the sorted samples. For unexplained reasons, the only exceptions were for the low-RNA high-PHA samples of Pincourt mixed liquor that showed slightly higher diversity than the flow cytometry processed but unsorted samples (electronic supplementary material, figure S3).
The compositions of the influent microbial communities were relatively more similar among different WWTPs than the ones of the mixed liquor communities, and the communities of the two types of samples were significantly different from each other (PERMANOVA tests, p < 0.05) (electronic supplementary material, table S3; figure 2a). Furthermore, the community compositions of the influent and the mixed liquor samples within each WWTP varied less over time than between WWTPs (figure 1).
Figure 2.
Principal coordinate analysis using Bray–Curtis distance of microbial community samples from Cowansville, LaPrairie (LP) and Pincourt. (a) All sample types: influent (INF), mixed liquor (ML), unsorted (Un) and sorted sub-samples: high-RNA low-PHA (HL), high-RNA high-PHA (HH), low-RNA low-PHA (LL) and low-RNA high-PHA (LH). (b) Average Bray–Curtis distances to influent (INF) and mixed liquor (ML) samples of each plant. Significant group letters identified group of flow cytometry samples that were not different from each other (electronic supplementary material, table S3). (c) Principal coordinate analysis of unsorted and sorted groups. (d) PCoA2–PCoA3 plot of unsorted and sorted samples.
A PCoA plot of all Bray–Curtis distances between communities generally displayed the mixed liquor samples processed by FACS in a gradient between the unprocessed mixed liquor samples (left in figure 2a) and the influent samples (right in figure 2a). The position of the processed samples was evaluated by pooling all the distances of the processed sample communities to the communities in influent or unprocessed mixed liquor samples of the same WWTP obtained on the same day (figure 2b). This analysis revealed that all sorted samples were more similar to the mixed liquor than to the influent. The composition of the high-RNA (HH and HL) sorted samples was relatively more similar to the influent communities and the low-RNA (LL and LH) samples were relatively more similar to the unprocessed mixed liquor communities. The community composition from all the flow cytometry processed mixed liquor samples clustered based on their WWTPs of origin (figure 2c), while the cell sorting significantly (PERMANOVA p < 0.05; electronic supplementary material, table S3) differentiated the sample compositions based on the RNA level (figure 2d). Finally, the level of PHA did not significantly (PERMANOVA p > 0.05) differentiate the resulting sorted communities from the ones of the same RNA level (electronic supplementary material, table S3), suggesting that the RNA level is the primary cellular biomarker defining the sub-guilds.
3.2. Predicted functional characteristics
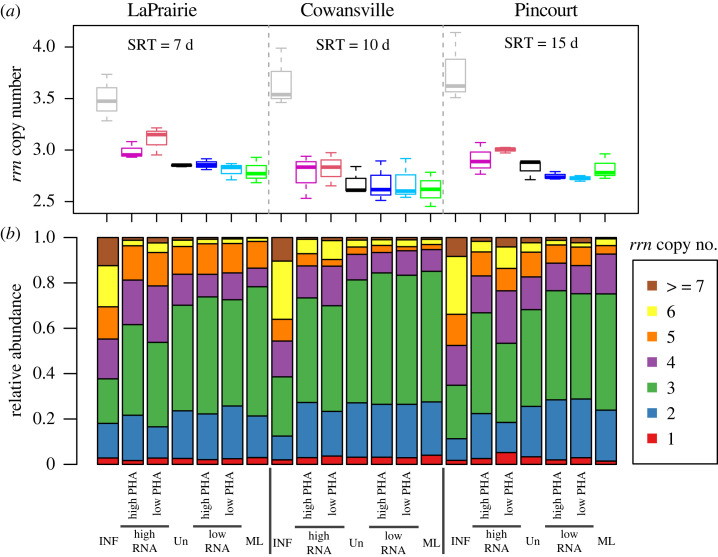
The possible functional characteristics of the high-RNA and low-RNA sub-guilds were assessed with the 16S rRNA gene amplicon sequence data. The data were used to infer the rrn copy number per genome using the IMG [38] and KEGG [39] databases, and the metagenome composition was predicted using PICRUSt [29]. The sorted high-RNA samples harboured higher rrn copies per genome than the low-RNA population (p < 0.05), mainly due to the increase of taxa with predictions of greater than four copies per genome (figure 3). Parallel to the similarity of their community compositions (figure 2), the unprocessed mixed liquor samples showed rrn copies per genome similar to the low-RNA sorted mixed liquor samples, and the influent bacteria displayed much higher rrn copies per genome than the ones in the mixed liquor but more similar to the high-RNA sub-guilds. The higher rrn copies per genome in the high-RNA sub-guild than in the low-RNA sub-guild appears to support the prediction that the high-RNA sub-guild consume RDS.
Figure 3.
(a) Average 16S rRNA operon number of influent (INF), mixed liquor (ML), unsorted (Un) and sorted samples. (b) Total relative abundance of OTUs of different rrn copy number. ANOVA showed that high-RNA group has significantly higher rrn copy number than low-RNA group (p < 0.05).
Predictions of ecophysiological traits of metagenomes by PICRUSt using 16S rRNA amplicon sequencing profiles showed significant differences at KEGG orthology (KO) level 2 and level 3. Membrane transporters, translation factors, and DNA replication and repair orthologs were predicted to be significantly more prevalent in high-RNA than in low-RNA (p < 0.05) (electronic supplementary material, figure S4a). Intriguingly, membrane transporters were also predicted to be more abundant in the influent than in the mixed liquor (p < 0.05) (electronic supplementary material, figure S4a), raising the question as to whether this is due to the higher similarity between the high-RNA sub-guild and the influent community (figure 2b). To clarify this question, closer examination of the predicted transporter systems showed that the trend is mainly due to sugar transporters linked to the phosphotransferase system (PTS) for the high-RNA samples, while it is due to ABC transporters and secretion systems for the influent samples (electronic supplementary material, figure S4b). Similarly, the prevalences of predicted genetic information processing functions are characteristics that set apart the high-RNA and influent samples. Therefore, sub-guild function was the main reason of high-RNA and low-RNA variation rather than influent community similarity.
Membrane transporters, translation factors, and DNA replication and repair could be associated with the consumption of RDS as the metabolism needs to be constantly adjusted through the feast–famine cycle that would be experienced in the activated sludge reactor. The low-RNA sub-guild appeared to house a significantly higher (p < 0.05) prevalence of metabolic functions than the high-RNA samples, including the metabolisms of amino acids (from protein hydrolysis), glycans (from polymers), cofactors and vitamins, and lipids (electronic supplementary material, figure S4), which are part of complex macromolecules and may represent SDS.
3.3. Immigration of high-RNA and low-RNA population from sewers
OTUs significantly enriched in the high-RNA or low-RNA sorted samples were identified by testing their abundances using the Odds Ratio test (p < 0.05, high-RNA versus low-RNA samples) [15]. The general enrichment in high-RNA and low-RNA groups could be compared by pooling the three WWTPs together. There were 52 genera significantly enriched in high-RNA samples and 74 genera enriched in low-RNA samples (electronic supplementary material, table S4). Some of the high-RNA OTUs were abundant in the influent samples, such as Acinetobacter, a genus in the family Aeromonadaceae, and a few genera within the order Clostridiales. Some of the abundant low-RNA OTUs belonged to the genera Flavobacterium and Rhodobacter, and unidentified genera within the families Chitinophagaceae, Cytophagaceae, Sphingomonadaceae and Methylophilaceae. These enrichments were observed to be common among all studied WWTPs, suggesting that the compositions of the heterotrophic sub-guilds were shared across WWTPs.
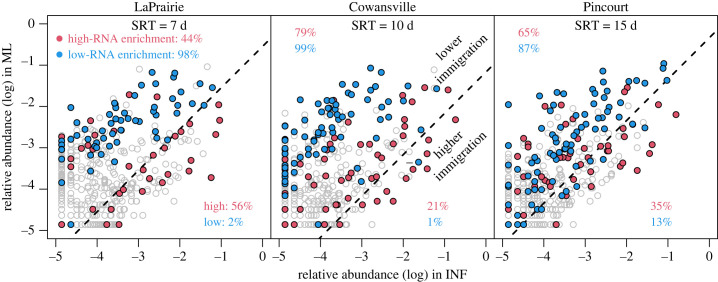
The fate of the high-RNA and low-RNA populations during their immigration from the sewer to the activate sludge WWTP was evaluated using a mass-flow immigration model [5]. The log–log plot of the relative abundances of OTUs in the influent and mixed liquor (figure 4) displays populations with the same immigration ratio (mi) on the same 1 : 1 line (equation (2.3)). In figure 4, a reference line was plotted corresponding to zero net growth rate (equation (2.4)), which is when the substrate consumption matches the needs of biomass decay. The immigration ratio decreases perpendicular to this line in the top-left direction and decreases in the bottom-right direction (figure 4). The low-RNA populations tended to have lower immigration ratios (2%, 1% and 13% of enrichments in the higher immigration zone, below the zero net growth rate dash line), while the high-RNA populations tended to have higher immigration ratios (56%, 21% and 35%, respectively) (figure 4). In addition to the feast–famine cycle selecting for specific metabolic characteristics, the immigration from the influent with populations with high rrn copies per genome may also have contributed to the relatively high rrn copies per genome in the high-RNA sub-guild [40]. The difference between high- and low-RNA populations was the most extreme in LaPrairie (lowest SRT) and least extreme in Pincourt (highest SRT). The impact of SRT on immigration (comparing WWTWs) showed that difference between high-RNA and low-RNA populations was lowest at highest SRT (in Pincourt), indicating that high SRT may be beneficial for low-RNA population immigration.
Figure 4.
Distribution of enriched OTUs in high-RNA and low-RNA groups on mixed liquor–influent log biplot. Dashed line represents zero net growth rate, below which indicates negative net growth rate and vice versa. Numbers indicate the percentage of OTU numbers with low (above dashed line) and high (below dashed line) immigration efficiency from influent.
4. Discussion
4.1. RNA level for heterotrophic sub-guild classification
The heterotrophic-specialist model describes that heterotrophs are either consuming RDS or SDS; based on metabolic considerations [18], it was hypothesized that RDS-consumers have high levels of RNA and PHA (storage compounds) in feast–famine feeding regimes or equivalently plug-flow reactors, while the levels of these macromolecules should be low or not occurring in SDS-consumers. This hypothesis provided a positive correlation prediction across taxa between RNA and PHA levels that was previously observed at nine WWTPs in both winter and summer [15]. This correlation was confirmed once more based on three additional observations obtained on different dates at a subset of three WWTPs from the initial nine. However, phylogenetic compositions of the samples sorted based on the levels of RNA and PHA revealed that the sub-guild classification primarily depended on the level of RNA but not PHA. This could be explained by the correlation between PHA and RNA, which leads to the capture of PHA signals during the RNA level segregation and weakens the variance for PHA level. It may also be because all samples were from the summer. Indeed, it was previously observed that PHA levels were generally lower during the summer than the winter [15]. Other studies also observed lower accumulation of PHA at higher temperature in activated sludge laboratory-scale reactors [41,42]. Consequently, PHA may have been at relatively low levels in our samples. Furthermore, possible false negative may come from cells with abnormally high neutral lipid content [43] or from extracellular lipid particles (i.e. SDS), which would have increased the noise and reduced the additional classification power of Nile Red once the RNA level sorting was achieved.
The differences in ecophysiological traits between high-RNA and low-RNA groups (electronic supplementary material, figure S4) showed links with substrate metabolism and cell growth that supported the heterotrophic-specialist model. RNA translation factors, DNA replication and repair (more abundant in high-RNA sorted samples) are functions linked to protein synthesis and cell growth, supporting the sub-guild functional definitions. Membrane transporter, PTS and pyruvate metabolism (which is the heart of heterotrophic central metabolism) were also significantly more abundant in high-RNA sorted samples, which are functions compatible with rapid metabolism and fast growth rates [44]. Furthermore, high-RNA sorting enriched for organisms with higher rrn copy numbers per genome (figure 3). All these observations are consistent with the hypothesis that the high-RNA sub-guild specialize in consuming RDS.
In the low-RNA group, lipid metabolism, amino acid (from protein hydrolysis) metabolism and glycan (polymers from cell walls or exopolymeric substances) metabolism and other large-molecule metabolisms were more prevalent. Lipids generally belong to SDS, and a large proportion of proteins exists in wastewater in forms of particulates, which may be consumed at slow rates [45]. While these functions were typically enriched in the mixed liquor compared to the influent samples, their higher prevalence in the low-RNA groups inferred that the low-RNA group is specialized in the degradation of SDS, consistent with the original prediction of the heterotrophic-specialist model.
4.2. Enrichment in high-RNA and low-RNA groups
The organisms generally enriched in high-RNA and low-RNA sorted samples (electronic supplementary material, table S4) can provide information to identify their ecological roles in activated sludge. High-RNA enrichments in the order Clostridiales and the family Desulfobacteraceae are probably high-rate immigrants from the influent. Conversely, OTUs in the family Moraxellaceae show diverse ecological roles in various environments. Within this family, Acinetobacter is a common genus in influent and mixed liquor, members of which were shown to consume RDS [46,47]. Previous studies hypothesized them to be RDS-consumers, and their relative abundances in the mixed liquor were found to exhibit diurnal dynamics similar to substrate loadings [48]. However, other studies suggested that their net growth rate in the activated sludge mixed liquor was negative due to their high abundance in influent [1]. In combination, literature observations and our data suggest that genera like Acinetobacter may result from both high immigration rate from influent and growth on RDS substrates; thus, they may have a profound impact on the expressed heterotrophic activities in activated sludge.
In low-RNA groups, Candidatus Microthrix in phylum Actinobacteria are filamentous bacteria reported to degrade long chain fatty acids and probably specialize in lipid consumption [49]. Genera in the order Saprospirales were reported in the microbial composition of filamentous protein-hydrolysing organism (PHO) in activated sludge [50]. It was determined that the Saprospiraceae (phylum Bacteroidetes) took the highest portion in the epiflora PHO, which were considered slow-growing. It was reported that the family Cytophagaceae are degraders of macromolecules such as polysaccharides or proteins [51]. Moreover, genera in family Flavobacteriaceae (genus Flavobacterium), family Rhodobacteraceae (genus Rhodobacter), family Xanthomonadaceae and phylum TM7 were reported as slow growers and large-molecule degraders [1,52–54]. The composition of enrichment in the low-RNA groups concurs with the functional sub-guild classification of SDS consumption in general.
The only noticeable exception to the SDS-consumer interpretation of the low-RNA sub-guild is the enrichment of a population of Methylophilaceae in samples obtained from LaPrairie. Methylophilaceae are methanol users, which would typically be considered RDS. In a previous study, this population was sorted into the high-RNA samples obtained from the same WWTP but in the winter rather than in the summer [15]. The discrepancy here may be due to the different sampling season, and further investigation is needed to resolve the matter.
4.3. Links between influent immigration and high-RNA populations
Sorting mixed liquor samples on the level of RNA revealed that similarity between the high-RNA populations and influent community was higher than between low-RNA sample populations and influent community (figure 2). This was true at all sampling sites and on all days despite variations in community compositions. A priori, this suggests that a larger fraction of the members of the high-RNA population originates from the influent in the process of immigration. However, the situation is more nuanced. Some OTUs in the high-RNA enrichments showed high immigration ratio, others exhibited low immigration (figure 4). Based on the heterotrophic-specialist model prediction, high-RNA group represents high growth rate (low immigration). Our results showed both high and low immigration OTUs coexist in the high-RNA group. Consequently, the high-RNA group is probably an assemblage of OTUs with specific ecophysiological characteristics at the same time as being influenced by relatively high influent immigration.
The resemblance of the high-RNA and influent samples with respect to microbial composition (figure 2), predicted metagenomic functional profile and rrn copy numbers (figure 3) suggest an ecological similarity. The high-RNA population was hypothesized to be RDS-consumers, which may select for organisms with a fast substrate uptake ecological strategy (i.e. r-strategy). The sewer is a reactor rich in substrates and nutrients, providing an environment for microbial growth [55], especially for the RDS-consumers because the low retention time in a sewer favours the fast degradation of RDS. These factors may contribute to the establishment of a fast-growing community of bacteria in influent wastewater (characterized by high rrn copy number) that successfully occupy a number of the RDS-degradation niches.
Although there are limitations of using RNA as biomarker to study microbial community growth and activity [56], our study provides a methodology of testing the ecological sub-guild of RDS- and SDS-consumer groups and the usability of biomarkers RNA and PHA at population level. The sub-guild sorting using RNA as biomarker did have limitations and could be improved by exploring more details of species/strains in mixed-culture community. Possible future directions may be combination of more biomarkers, or normalization methods.
This study demonstrates the complementarity of using generic biomarker (RNA level) and calculating the immigration ratios or the net growth rates with mass-flow immigration model to reveal novel ecological patterns in the assembly of the activated sludge community. To be conclusive, however, the patterns observed should be replicated at other WWTPs, and the impact of operational variables (e.g. SRT, dissolved oxygen, and concentration-specific substrate and inhibitors) should be assessed. These data will support the development of general ecological hypotheses and models for the assembly of heterotrophs in activated sludge, for example, improve activated sludge modelling by including the influent immigration impact, and splitting the lumped heterotrophic biomass into sub-groups. In turn, the resulting models will likely guide the optimization of WWTP designs and the implementation of solutions to operational problems such as bulking and foaming.
5. Conclusion
This study used the flow cytometry cell sorting method and biomarkers RNA and PHA to investigate the classification of heterotrophic populations in activated sludge. Results showed that RNA is sufficient to separate the community in reproducible groups of populations, and PHA does not provide additional sorting resolution. Implications from predicted functional profiles showed that the low-RNA populations may contain a higher prevalence of large-molecule metabolism-related functions. Determination of the immigration ratios by a mass-flow immigration modelling approach revealed that an important subset of the high-RNA population was characterized by high immigration, which was not the case for the low-RNA populations. This relationship, however, may be dependent on operational conditions such as the SRT at different WWTPs.
Acknowledgements
We thank all the staff at WWTPs Cowansville, Pincourt and LaPrairie that assisted us with sampling, and the Core Facilities of L'Institut de recherche en immunologie et en cancérologie (IRIC, Montreal) for their help with cell sorting. We are grateful for the technical advice on Illumina sequencing and bioinformatics analysis from Génome Québec Innovation Centre (Montréal).
Data accessibility
Raw sequences can be accessed from NCBI GenBank (BioProject ID: PRJNA496295).
The data are provided in the electronic supplementary material [57].
Authors' contributions
B.G.: conceptualization, data curation, formal analysis, funding acquisition, investigation, methodology, visualization and writing—original draft; D.F.: conceptualization, funding acquisition, project administration, resources, supervision, validation and writing—review and editing.
All authors gave final approval for publication and agreed to be held accountable for the work performed therein.
Conflict of interest declaration
The authors declare no competing interests.
Funding
This work received funding from Natural Sciences and Engineering Research Council of Canada Discovery grants (grant nos. RGPIN 341393-11 and RGPIN-2016-06498) and a Collaborative Research and Development grant (grant no. CRDPJ 417654-11) in collaboration with Air Liquide Canada and the Régie d'Assainissement des Eaux du Bassin LaPrairie. B.G. was funded by McGill University with the MEDA doctoral scholarship and Graduate Mobility Award.
References
- 1.Saunders AM, Albertsen M, Vollertsen J, Nielsen PH. 2016. The activated sludge ecosystem contains a core community of abundant organisms. ISME J. 10, 11-20. ( 10.1038/ismej.2015.117) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zhang T, Shao MF, Ye L. 2012. 454 pyrosequencing reveals bacterial diversity of activated sludge from 14 sewage treatment plants. ISME J. 6, 1137-1147. ( 10.1038/ismej.2011.188) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dottorini G, Michaelsen TY, Kucheryavskiy S, Andersen KS, Kristensen JM, Peces M, Wagner DS, Nierychlo M, Nielsen PH. 2021. Mass-immigration determines the assembly of activated sludge microbial communities. Proc. Natl Acad. Sci. USA 118, e2021589118. ( 10.1073/pnas.2021589118) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mei R, Liu WT. 2019. Quantifying the contribution of microbial immigration in engineered water systems. Microbiome 7, 144. ( 10.1186/s40168-019-0760-0) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Guo B, Liu C, Gibson C, Klai N, Lin X, Frigon D. 2022. Wastewater influent microbial immigration and contribution to resource consumption in activated sludge using taxon-specific mass-flow immigration model. bioRxiv. ( 10.1101/2022.08.15.504022) [DOI]
- 6.Frigon D, Guthrie RM, Bachman GT, Royer J, Bailey B, Raskin L. 2006. Long-term analysis of a full-scale activated sludge wastewater treatment system exhibiting seasonal biological foaming. Water Res. 40, 990-1008. ( 10.1016/j.watres.2005.12.015) [DOI] [PubMed] [Google Scholar]
- 7.Yi T, Lee EH, Kang S, Shin J, Cho KS. 2012. Structure and dynamics of microbial community in full-scale activated sludge reactors. J. Ind. Microbiol. Biot. 39, 19-25. ( 10.1007/s10295-011-0994-8) [DOI] [PubMed] [Google Scholar]
- 8.Mielczarek AT, Nguyen HTT, Nielsen JL, Nielsen PH. 2013. Population dynamics of bacteria involved in enhanced biological phosphorus removal in Danish wastewater treatment plants. Water Res. 47, 1529-1544. ( 10.1016/j.watres.2012.12.003) [DOI] [PubMed] [Google Scholar]
- 9.Flowers JJ, Cadkin TA, McMahon KD. 2013. Seasonal bacterial community dynamics in a full-scale enhanced biological phosphorus removal plant. Water Res. 47, 7019-7031. ( 10.1016/j.watres.2013.07.054) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ju F, Guo F, Ye L, Xia Y, Zhang T. 2014. Metagenomic analysis on seasonal microbial variations of activated sludge from a full-scale wastewater treatment plant over 4 years. Env. Microbiol. Rep. 6, 80-89. ( 10.1111/1758-2229.12110) [DOI] [PubMed] [Google Scholar]
- 11.Wells GF, Park HD, Eggleston B, Francis CA, Criddle CS. 2011. Fine-scale bacterial community dynamics and the taxa-time relationship within a full-scale activated sludge bioreactor. Water Res. 45, 5476-5488. ( 10.1016/j.watres.2011.08.006) [DOI] [PubMed] [Google Scholar]
- 12.Albuquerque MG, Carvalho G, Kragelund C, Silva AF, Barreto Crespo MT, Reis MA, Nielsen PH. 2013. Link between microbial composition and carbon substrate-uptake preferences in a PHA-storing community. ISME J. 7, 1-12. ( 10.1038/ismej.2012.74) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Shchegolkova NM, Krasnov GS, Belova AA, Dmitriev AA, Kharitonov SL, Klimina KM, Melnikova NV, Kudryavtseva AV. 2016. Microbial community structure of activated sludge in treatment plants with different wastewater compositions. Front. Microbiol. 7, 90. ( 10.3389/fmicb.2016.00090) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lu H, Chandran K, Stensel D. 2014. Microbial ecology of denitrification in biological wastewater treatment. Water Res. 64, 237-254. ( 10.1016/j.watres.2014.06.042) [DOI] [PubMed] [Google Scholar]
- 15.Guo B, Manchester M, Luby T, Frigon D. 2018. Composition of heterotrophic specialized sub-guilds defined by a positive RNA and polyhydroxyalkanoate correlation in activated sludge. Water Res. 144, 561-571. ( 10.1016/j.watres.2018.07.059) [DOI] [PubMed] [Google Scholar]
- 16.Frigon D, Arnaiz E, Oerther DB, Raskin L. 2002. Who eats what? Classifying microbial populations based on diurnal profiles of rRNA levels. Water Sci. Technol. 46, 1-9. [PubMed] [Google Scholar]
- 17.Henze M, Gujer W, Mino T, van Loosdrecht M. 2000. Activated sludge models ASM1, ASM2, ASM2d and ASM3. London, UK: IWA Publishing. [Google Scholar]
- 18.Frigon D, Muyzer G, van Loosdrecht M, Raskin L. 2006. rRNA and poly-beta-hydroxybutyrate dynamics in bioreactors subjected to feast and famine cycles. Appl. Environ. Microbiol. 72, 2322-2330. ( 10.1128/AEM.72.4.2322-2330.2006) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Klappenbach JA, Dunbar JM, Schmidt TM. 2000. rRNA operon copy number reflects ecological strategies of bacteria. Appl. Environ. Microbiol. 66, 1328-1333. ( 10.1128/AEM.66.4.1328-1333.2000) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lauro FM, et al. 2009. The genomic basis of trophic strategy in marine bacteria. Proc. Natl Acad. Sci. USA 106, 15 527-15 533. ( 10.1073/pnas.0903507106) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Roller BR, Stoddard SF, Schmidt TM. 2016. Exploiting rRNA operon copy number to investigate bacterial reproductive strategies. Nat. Microbiol. 1, 16160. ( 10.1038/nmicrobiol.2016.160) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Stevenson BS, Schmidt TM. 2004. Life history implications of rRNA gene copy number in Escherichia coli. Appl. Environ. Microbiol. 70, 6670-6677. ( 10.1128/AEM.70.11.6670-6677.2004) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Stoddard SF, Smith BJ, Hein R, Roller BR, Schmidt TM. 2015. rrnDB: improved tools for interpreting rRNA gene abundance in bacteria and archaea and a new foundation for future development. Nucleic Acids Res. 43, D593-D598. ( 10.1093/nar/gku1201) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dethlefsen L, Schmidt TM. 2007. Performance of the translational apparatus varies with the ecological strategies of bacteria. J. Bacteriol. 189, 3237-3245. ( 10.1128/JB.01686-06) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nemergut DR, et al. 2016. Decreases in average bacterial community rRNA operon copy number during succession. ISME J. 10, 1147-1156. ( 10.1038/ismej.2015.191) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Shrestha PM, Noll M, Liesack W. 2007. Phylogenetic identity, growth-response time and rRNA operon copy number of soil bacteria indicate different stages of community succession. Environ. Microbiol. 9, 2464-2474. ( 10.1111/j.1462-2920.2007.01364.x) [DOI] [PubMed] [Google Scholar]
- 27.Wu L, et al. 2017. Microbial functional trait of rRNA operon copy numbers increases with organic levels in anaerobic digesters. ISME J. 11, 2874-2878. ( 10.1038/ismej.2017.135) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Isazadeh S, Jauffur S, Frigon D. 2016. Bacterial community assembly in activated sludge: mapping beta diversity across environmental variables. MicrobiologyOpen 5, 1050-1060. ( 10.1002/mbo3.388) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Langille MG, et al. 2013. Predictive functional profiling of microbial communities using 16S rRNA marker gene sequences. Nat. Biotechnol. 31, 814-821. ( 10.1038/nbt.2676) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Weisburg WG, Barns SM, Pelletier DA, Lane DJ. 1991. 16S ribosomal DNA amplification for phylogenetic study. J. Bacteriol. 173, 697-703. ( 10.1128/jb.173.2.697-703.1991) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Engelbrektson A, Kunin V, Wrighton KC, Zvenigorodsky N, Chen F, Ochman H, Hugenholtz P. 2010. Experimental factors affecting PCR-based estimates of microbial species richness and evenness. ISME J. 4, 642-647. ( 10.1038/ismej.2009.153) [DOI] [PubMed] [Google Scholar]
- 32.Edgar RC. 2010. Search and clustering orders of magnitude faster than BLAST. Bioinformatics. 26, 2460-2461. ( 10.1093/bioinformatics/btq461) [DOI] [PubMed] [Google Scholar]
- 33.McDonald D, Price MN, Goodrich J, Nawrocki EP, DeSantis TZ, Probst A, Andersen GL, Knight R, Hugenholtz P. 2012. An improved Greengenes taxonomy with explicit ranks for ecological and evolutionary analyses of bacteria and archaea. ISME J. 6, 610-618. ( 10.1038/ismej.2011.139) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Werner JJ, Koren O, Hugenholtz P, DeSantis TZ, Walters WA, Caporaso JG, Angenent LT, Knight R, Ley RE. 2012. Impact of training sets on classification of high-throughput bacterial 16 s rRNA gene surveys. ISME J. 6, 94-103. ( 10.1038/ismej.2011.82) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Caporaso JG, et al. 2010. QIIME allows analysis of high-throughput community sequencing data. Nat. Methods 7, 335-336. ( 10.1038/nmeth.f.303) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Oksanen J, et al. 2017. Vegan: community ecology package. See https://CRAN.R-project.org/package=vegan.
- 37.Cornfield J. 1951. A method of estimating comparative rates from clinical data; applications to cancer of the lung, breast, and cervix. J. Natl Cancer Inst. 11, 1269-1275. [PubMed] [Google Scholar]
- 38.Markowitz VM, et al. 2012. IMG: the Integrated Microbial Genomes database and comparative analysis system. Nucleic Acids Res. 40, D115-D122. ( 10.1093/nar/gkr1044) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kanehisa M, Goto S, Sato Y, Furumichi M, Tanabe M. 2012. KEGG for integration and interpretation of large-scale molecular data sets. Nucleic Acids Res. 40, D109-D114. ( 10.1093/nar/gkr988) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Guo B, Liu C, Gibson C, Frigon D. 2019. Wastewater microbial community structure and functional traits change over short timescales. Sci. Total Environ. 662, 779-785. ( 10.1016/j.scitotenv.2019.01.207) [DOI] [PubMed] [Google Scholar]
- 41.Krishna C, Van Loosdrecht MCM. 1999. Effect of temperature on storage polymers and settleability of activated sludge. Water Res. 33, 2374-2382. ( 10.1016/S0043-1354(98)00445-X) [DOI] [Google Scholar]
- 42.Chinwetkitvanich S, Randall CW, Panswad T. 2004. Effects of phosphorus limitation and temperature on PHA production in activated sludge. Water Sci. Technol. 50, 135-143. [PubMed] [Google Scholar]
- 43.Kacmar J, Carlson R, Balogh SJ, Srienc F. 2006. Staining and quantification of poly-3-hydroxybutyrate in Saccharomyces cerevisiae and Cupriavidus necator cell populations using automated flow cytometry. Cytometry A 69, 27-35. ( 10.1002/cyto.a.20197) [DOI] [PubMed] [Google Scholar]
- 44.Deutscher J, Francke C, Postma PW. 2006. How phosphotransferase system-related protein phosphorylation regulates carbohydrate metabolism in bacteria. Microbiol. Mol. Biol. Rev. 70, 939-1031. ( 10.1128/MMBR.00024-06) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Huang MH, Li YM, Gu GW. 2010. Chemical composition of organic matters in domestic wastewater. Desalination 262, 36-42. ( 10.1016/j.desal.2010.05.037) [DOI] [Google Scholar]
- 46.Vandewalle JL, Goetz GW, Huse SM, Morrison HG, Sogin ML, Hoffmann RG, Yan K, McLellan SL. 2012. Acinetobacter, Aeromonas and Trichococcus populations dominate the microbial community within urban sewer infrastructure. Environ. Microbiol. 14, 2538-2552. ( 10.1111/j.1462-2920.2012.02757.x) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Seviour RJ, Mino T, Onuki M. 2003. The microbiology of biological phosphorus removal in activated sludge systems. FEMS Microbiol. Rev. 27, 99-127. ( 10.1016/S0168-6445(03)00021-4) [DOI] [PubMed] [Google Scholar]
- 48.Frigon D. 2005. Proposed mechanism explaining seasonal biological foaming in activated sludge systems: foam-causing bacteria specialize in consuming lipids. PhD thesis, University of Illinois at Urbana-Champaign, USA. [Google Scholar]
- 49.Nielsen PH, Roslev P, Dueholm TE, Nielsen JL. 2002. Microthrix parvicella, a specialized lipid consumer in anaerobic-aerobic activated sludge plants. Water Sci. Technol. 46, 73-80. [PubMed] [Google Scholar]
- 50.Xia Y, Kong Y, Nielsen PH. 2007. In situ detection of protein-hydrolysing microorganisms in activated sludge. FEMS Microbiol. Ecol. 60, 156-165. ( 10.1111/j.1574-6941.2007.00279.x) [DOI] [PubMed] [Google Scholar]
- 51.McBride MJ, Liu W, Lu X, Zhu Y, Zhang W. 2014. The family Cytophagaceae. In The prokaryotes: other major lineages of bacteria and the archaea (eds Rosenberg E, DeLong EF, Lory S, Stackebrandt E, Thompson F), pp. 577-593. Berlin, Germany: Springer. [Google Scholar]
- 52.McIlroy SJ, Starnawska A, Starnawski P, Saunders AM, Nierychlo M, Nielsen PH, Nielsen JL. 2016. Identification of active denitrifiers in full-scale nutrient removal wastewater treatment systems. Environ. Microbiol. 18, 50-64. ( 10.1111/1462-2920.12614) [DOI] [PubMed] [Google Scholar]
- 53.Ginige MP, Keller J, Blackall LL. 2005. Investigation of an acetate-fed denitrifying microbial community by stable isotope probing, full-cycle rRNA analysis, and fluorescent in situ hybridization-microautoradiography. Appl. Environ. Microbiol. 71, 8683-8691. ( 10.1128/AEM.71.12.8683-8691.2005) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Bernardet JF, Bowman JP. 2015. Flavobacterium. In Bergey's manual of systematics of archaea and bacteria. Wiley/Bergey's Manual Trust. ( 10.1002/9781118960608.gbm00312) [DOI] [Google Scholar]
- 55.McLellan SL, Roguet A. 2019. The unexpected habitat in sewer pipes for the propagation of microbial communities and their imprint on urban waters. Curr. Opin. Biotechnol. 57, 34-41. ( 10.1016/j.copbio.2018.12.010) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Blazewicz SJ, Barnard RL, Daly RA, Firestone MK. 2013. Evaluating rRNA as an indicator of microbial activity in environmental communities: limitations and uses. ISME J. 7, 2061-2068. ( 10.1038/ismej.2013.102) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Guo B, Frigon D. 2023. Cellular RNA levels define heterotrophic substrate-uptake rate sub-guilds in activated sludge microbial communities. Figshare. ( 10.6084/m9.figshare.c.6669414) [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Citations
- Guo B, Frigon D. 2023. Cellular RNA levels define heterotrophic substrate-uptake rate sub-guilds in activated sludge microbial communities. Figshare. ( 10.6084/m9.figshare.c.6669414) [DOI] [PMC free article] [PubMed]
Data Availability Statement
Raw sequences can be accessed from NCBI GenBank (BioProject ID: PRJNA496295).
The data are provided in the electronic supplementary material [57].