Abstract
Background
Adverse childhood experiences have been linked to increased multimorbidity, with physical and mental health consequences throughout life. Chronic pain is often associated with mood disorders, such as major depressive disorder (MDD); both have been linked to adverse childhood experiences. It is unclear how the effect of adverse childhood experiences on neural processing impacts on vulnerability to chronic pain, MDD, or both, and whether there are shared mechanisms. We aimed to assess evidence for central neural changes associated with adverse childhood experiences in subjects with chronic pain, MDD, or both using systematic review and meta-analysis.
Methods
Electronic databases were systematically searched for neuroimaging studies of adverse childhood experiences, with chronic pain, MDD, or both. Two independent reviewers screened title, abstracts, and full text, and assessed quality. After extraction of neuroimaging data, activation likelihood estimate meta-analysis was performed to identify significant brain regions associated with these comorbidities.
Results
Forty-nine of 2414 studies were eligible, of which 43 investigated adverse childhood experiences and MDD and six investigated adverse childhood experiences and chronic pain. None investigated adverse childhood experiences, chronic pain, and MDD together. Functional and structural brain abnormalities were identified in the superior frontal, lingual gyrus, hippocampus, insula, putamen, superior temporal, inferior temporal gyrus, and anterior cerebellum in patients with MDD exposed to adverse childhood experiences. In addition, brain function abnormalities were identified for patients with MDD or chronic pain and exposure to adverse childhood experiences in the cingulate gyrus, inferior parietal lobule, and precuneus in task-based functional MRI studies.
Conclusions
We found that adverse childhood experiences exposure can result in different functional and structural brain alterations in adults with MDD or chronic pain compared with those without adverse childhood experiences.
Systematic review protocol
PROSPERO CRD42021233989.
Keywords: adverse childhood experiences, chronic pain, depression, early life adversity, major depressive disorder, MRI, neuroimaging, neuropathic pain
Editor's key points.
-
•
The association between adverse childhood experiences and chronic pain or depression has been well established, but the neural substrates are poorly understood.
-
•
This systematic review with meta-analysis of the long-term effects of adverse childhood experiences on chronic pain and depression identifies limited literature examining the neural correlates of chronic pain and adverse childhood experiences, with no studies of adverse childhood experiences and comorbid chronic pain and depression.
-
•
The authors highlight the need for further research to improve understanding of the neural mechanisms underlying chronic pain, adverse childhood experiences, and depression.
Chronic pain is defined as pain that lasts longer than 3 months, or pain that persists beyond normal healing time.1 The prevalence of chronic pain in the UK is 35–53% of the population, increasing with older age, and often associated with comorbid conditions such as depression.2, 3, 4, 5 A recent systematic review found that over half of patients with fibromyalgia had co-existing major depressive disorder (MDD),6, 7, 8 although the nature of the link between pain and depression is not clear.7 At a global level, chronic pain conditions are the leading cause of years lived with disability,9 and, independent of pain, MDD has a prevalence of approximately 10%.10, 11, 12
Adverse childhood experiences (adverse childhood experiences) are defined as repeated aversive experiences that represent a deviation from the expected environment and require adaptation.13,14 Examples include physical, sexual, and emotional abuse, parental illness, criminality, violence, neglect, and poverty.13, 14, 15, 16, 17 Chronic pain has been found to be related to adverse childhood experiences18; for example exposure to adverse childhood experiences may be a risk factor for fibromyalgia in later life.19 As with chronic pain, adverse childhood experiences have been proposed as a risk factor for developing mood disorders later in life.20,21 Adverse childhood experiences are associated with elevated risk for multimorbidity with both mental and physical health problems. Although reasons for this are poorly understood, long-term immune system and neurobiological changes may play a role in altering vulnerability to chronic pain and MDD in adult life.22,23
The quality of parental care, nutrition, cognitive stimulation, and socioeconomic status during early child development have been shown to affect brain morphology and functionality throughout the life course.24 Neuroimaging is an important tool in advancing understanding of the neural correlates of chronic pain25 and depression, and allows exploration of the impact of adverse childhood experiences, with the potential to identify common mechanisms.24,26, 27, 28 There have been some neuroimaging studies of chronic pain and adverse childhood experiences identifying involvement of various brain regions, such as the insular cortex, basal ganglia, amygdala, hippocampal cortex, and prefrontal cortex16,17,26; however these studies had not examined the comorbidity of adverse childhood experiences and chronic pain. Adverse childhood experiences and MDD neuroimaging studies share several common features, with reported abnormalities of the hippocampus, amygdala, anterior insular, and superior temporal gyrus,29, 30, 31 but these studies had revealed the abnormalities without the comorbidity of adverse childhood experiences and MDD. A notable brain region strongly implicated as affected by adverse childhood experiences and depression in neuroimaging studies is the hippocampus; specifically, there is robust evidence for a smaller hippocampal volume.30,32, 33, 34, 35, 36, 37, 38 There is limited understanding of how comorbid chronic pain and MDD are affected by adverse childhood experiences.
In this systematic review and meta-analysis, we aimed to synthesise evidence from relevant neuroimaging studies to investigate the impact of adverse childhood experiences on the neural correlates of chronic pain, and depression, both alone and in combination.
Methods
Literature
After prospective registration on PROSPERO (https://www.crd.york.ac.uk/prospero/display_record.php?RecordID=233989; Registration number: CRD42021233989), a systematic literature search, data extraction, and meta-analysis were carried out. The search was conducted using the following electronic databases, MEDLINE (OVID), Embase (OVID), the Cochrane Central Registry of Controlled Trials (CENTRAL) (the Cochrane Library), PsycINFO (OVID), PubMed, Neurosynth, Sleuth, and Web of Science. An advanced search strategy was developed using a list of MeSH and keywords in combination or alone, such as chronic pain, neuropathic pain, MDD, depression, anxiety, neuroimaging, MRI, and early life adversity. Searches were customised for each database. The searches were restricted to English language, whereas no publication status, or date restrictions were imposed on the systematic search. Searches were imported into Rayyan for removal of duplicates, screening, and study selection (https://www.rayyan.ai/).
Eligibility criteria
Eligible studies were on humans, were randomised or non-randomised, or observational (cross-sectional or longitudinal, cohort). To be eligible for review, studies had to include participants with chronic pain, depression with a history of adverse childhood experiences, or both, and have quantitative data and neuroimaging measures (i.e. structural and functional MRI), positron emission tomography (PET) or single-photon emission computed tomography (SPECT). Participants/study population of selected papers were adults aged 16 yr or older. Chronic pain was defined as pain that had been present for at least 3 months, and adverse childhood experiences were defined as repeated aversive experiences that represent a deviation from the expected environment and require adaptation and had been identified by a questionnaire such as the Childhood Trauma Questionnaire (CTQ).39 Standard depression rating scales40, 41, 42 were converted to a summary measure using a recognised conversion table in order to allow comparisons.42 Studies were excluded if patients had acute pain, there was no diagnosis of depression, or depression severity was mild. Studies involving participants with brain injury, brain tumours, stroke, or neurodegenerative conditions were also excluded.
Study selection
To investigate the associations between chronic pain, mood disorder, and the effects of adverse childhood experiences on brain structure and function, neuroimaging studies identified through systematic searching were assessed for inclusion by two independent reviewers (GA, EL). Firstly, title and abstract screening was carried out, assessing against the predefined inclusion and exclusion criteria. Secondly, where it was not possible to determine eligibility from the title and abstract, full-text screening was performed. When discrepancies were raised, they were resolved through discussion, or, when they remained, by consultation with a third party (DS, LC). Cohen's kappa43 was computed to assess the agreement between two reviewers ‘include’, ‘maybe include’, or ‘exclude’ decisions during the full-text screening.
When the selection process was completed, data were extracted according to our predefined protocol. Data extracted from each study included publication details (e.g. authors, year of publication, country where the study was carried out) and demographic characteristics of the sample such as age and sex. Moreover, the clinical diagnosis was extracted for MDD and chronic pain along with type and scale of measurements for adverse childhood experiences, MDD, and chronic pain. The neuroimaging method used was also extracted along with the modality, the patient groups, use of contrast (if task-related functional MRI [fMRI]) and the resulting significant coordinates (x, y, z coordinates of the brain regions).
Quality assessment
There is no standardised tool for assessing the risk of bias in MRI (structural and functional), to the best of our knowledge. Therefore, we modified a version of the Newcastle–Ottawa scale,44,45 which is used for assessing the quality of non-randomised studies, including case-control and cohort studies, in meta-analyses. The risk of bias tool included four categories, study selection aspects (adequate case definition: MDD, chronic pain, and adverse childhood experiences, representative of the cases, selection of controls and definition of controls), comparability (age, sex, and other variables), exposure (ascertain of exposure and drop-out rate), and statistical interference (uncorrected P-value threshold and correction for multiple testing at each voxel).
Activation likelihood estimate meta-analysis method
A coordinate-based meta-analysis approach, activation likelihood estimate (ALE), of extracted neuroimaging results46, 47, 48 was used to analyse studies within BrainMap GingerALE 3.0.2 (http://www.brainmap.org/ale). Talairach space coordinates were converted into Montreal Neurological Institute (MNI) coordinates using the ‘icbm2tal’ transform.49 To take into account the spatial statistical uncertainty of the reported foci in the ALE method, foci were modelled as a 3D Gaussian probability distribution.48 Considering between-subject and between-template variance for each study, the full width half maximum (FWHM) of the Gaussian probability was computed.46 FWHM of the modelled probability was computed through combining the activation probabilities of the reported foci, for each analysed study.46 By combining the activation probabilities of the reported foci, modelled activation maps were computed for each analysed study. The union of these modelled activation maps as ALE scores were calculated within a grey matter on a voxel-by-voxel basis. ALE scores are tested against a null distribution of random spatial events to test for statistical significance.46
Cluster-level family-wise error (I) has greater sensitivity and specificity and is less prone to type one error with regard to the convergence in comparison with voxel-wise thresholding. In particular, the foci convergence is achieved over the random clustering of the foci-noise via testing against the null hypothesis of random spatial association between experiments.46 Thus, the I was used to guide the meta-analysis. Statistical significance was set at a corrected threshold of P<0.05 (using a threshold derived from the permutation of 1000 cluster-forming events assuming a voxel level of P<0.05). Computations involving the same group of subjects were pooled into one experiment to control for sample overlap.48,50 A random-effects analysis was used (GingerALE 3.0.2) with incorporation of variable uncertainty based on subject size,46 where the subject size is determined as the smallest number of participants in the contrast of the groups.51 We performed a meta-analysis across all fMRI and voxel-based morphology paradigms contrasting participants who experienced adverse childhood experiences with participants who did not experience adverse childhood experiences. In addition, where there was a sufficient number of relevant studies, we aimed to perform separate exploratory meta-analyses on MDD (or chronic pain) participants with adverse childhood experiences, MDD participants without adverse childhood experiences, separately for fMRI and VBM and fMRI MDD with adverse childhood experiences compared with healthy controls. An assessment of heterogeneity is frequently used in non-neuroimaging studies; however, there is not yet any accepted method to calculate this for coordinate-based meta-analyses. Therefore, we were unable to assess this formally.
Results
After removing duplicates, 228 studies on mood disorder and adverse childhood experiences and 15 studies on chronic pain and adverse childhood experiences were identified, from title and abstract screening. After title, abstract, and – where appropriate – full-text screening, 43 studies were identified as meeting inclusion criteria for mood disorders and adverse childhood experiences, with six studies for chronic pain and adverse childhood experiences.32, 33, 34, 35, 36, 37, 38,52, 53, 54, 55, 56, 57, 58, 59, 60, 61, 62, 63, 64, 65, 66, 67, 68, 69, 70, 71, 72, 73, 74, 75, 76, 77, 78, 79, 80, 81, 82, 83, 84, 85, 86, 87, 88, 89, 90, 91, 92, 93 No studies were identified of adverse childhood experiences with comorbid mood disorder and chronic pain. Cohen's kappa was computed with good agreement between the two reviewers: kappa=0.69 (95% confidence interval [CI], 0.57–0.82; P<0.0001) for full-text study selection. Of the 108 full-text studies screened, 43 met inclusion criteria: 26 were structural MRI studies, seven were resting-state fMRI studies, and 10 were task-based fMRI studies (Supplementary material). Most of these studies were cohort studies or case-control studies. Details of the included structural studies can be found in Supplementary Table S2, and task-based fMRI studies can be found in Supplementary Table S3. Summary tables of imaging findings for the structural and task-based fMRI can be found in Table 1, Table 2, respectively. Table 3 summarises identified resting state fMRI studies: it was not possible to carry out ALE meta-analysis because of inadequately reported coordinates in those studies. The risk of bias is summarised in the Supplementary material. The assessment of study quality showed no study at high risk, nine studies at an intermediate risk of bias, and 40 studies at low risk of bias.
Table 1.
Summary table of the main result for the structural MRI studies. adverse childhood experiences, adverse childhood experiences; BDI-II, Beck's Depression Inventory; CECA, Childhood Experience of Care and Abuse; CM, childhood maltreatment; CN, childhood neglect; CTQ, Childhood Trauma Questionnaire; CTQ-SF Childhood Trauma Questionnaire-Short Form; EA, emotional abuse; ELA, early life adversity; GMV, grey matter volume; HAD, Hospital Anxiety and Depression Scale; HAMD, Hamilton depression scale; HAMD-D, Hamilton Rating Scale for Depression; HDRS, Hamilton Depression Rating; HC, healthy control; IBS, irritable bowel syndrome; IDS-SR, Inventory of Depressive Symptomatology-Self report; LS, life stress; MADRS, Montgomery Åsberg Depression Rating Scale; MDD, major depressive disorder; PHQ, Patient Health Questionnaire; QIDS, Quick Inventory for Depression Symptomatology; SA, sexual abuse; SDS, Self-rating Depression Scale; ROD, recent onset of depression; SPD, somatoform pain disorder.
| Authors | Structural MRI |
|
|---|---|---|
| Contrast of interest | Main results | |
| Studies examining the relationship of MDD or chronic pain with adverse childhood experiences and without adverse childhood experiences | ||
| Chaney and colleagues53 | MDD+CM>MDD-CM | Compared with MDD without CM, MDD with CM: smaller hippocampal volume, larger dorsomedial prefrontal cortex, and orbitofrontal cortex. |
| Colle and colleagues55 | MDD+ELA>MDD-ELA | MDD without ELA, compared with MDD with ELA: smaller hippocampal volumes found in males only. |
| Gerritsen and colleagues35 | MDD+CM>MDD-CM | CM: no change in hippocampal volume. MDD: was associated with smaller hippocampal volume. MDD was related to smaller hippocampal volume, in participants with CM. |
| Monninger and colleagues67 | LS | Reduce cortical thickness in the right medial orbitofrontal cortex and increased depressive symptoms were associated with chronic LS during infancy. Reduce cortical thickness in the left medial orbitofrontal cortex was associated with chronic LS during childhood and negatively correlated with the left caudal ACC and the left parahippocampal surface area. CT of the right transverse temporal lobe and the left entorhinal cortex volume was inversely related to LS during adolescence. |
| Oshri and colleagues69 | adverse childhood experiences | Participants with higher adverse childhood experiences scores had smaller right amygdala volumes and smaller central-medial nuclei. Moreover, participants with higher adverse childhood experiences scores with increased anxiety, depressive symptoms and alcohol use showed smaller basolateral amygdala volume. |
| Peng and colleagues70 | MDD-CN>MDD+CN | Compared with patients without CN, patients with CN showed increased WM densities in bilateral sublobar extra-nucleus and right brainstem midbrain |
| Salokangas and colleagues72 | ROD+adverse childhood experiences >ROD-adverse childhood experiences | ROD was not found to be associated with changes in the amygdala–hippocampus complex in adulthood. ROD patients with experiences of physical abuse in early life showed that are mediating the reduction of frontal lobe. |
| Van Harmelen and colleagues80 | CEM>NO CEM | Compared with non-CEM participants, CEM participants: reduction in bilateral dorsal medial prefrontal cortex, independent of comorbid psychopathology |
| Vythilingam and colleagues81 | MDD+CA>MDD-CA | Compared with participants with MDD without CA and HC, participants with MDD with CA showed bilateral smaller hippocampal volumes. |
| Yang and colleagues85 | MDD+CM>MDD-CM | For MDD with CM compared with MDD without CM: smaller left inferior occipital gyrus and larger left cerebellum anterior lobe and left superior temporal gyrus. Interaction effect for the CM and MDD for the MDD with CM compared with MDD without CM showed increased left superior frontal gyrus and right middle frontal gyrus and smaller right inferior frontal gyrus. |
| Studies examining the different relationships of MDD, chronic pain, Healthy controls, and adverse childhood experiences | ||
| Chaney and colleagues53 | MDD+CM>HC | Compared with HC, MDD with CM had smaller left orbitofrontal cortex and left dorsomedial prefrontal cortex. |
| Peng, and colleagues70 | HC>MDD+CN | Compared with HC, MDD patients with CN showed decreased WM densities in bilateral inferior parietal lobules. |
| Yang and colleagues85 | HC+CM>HC-CM | The main effect of CM had been observed for HC with CM compared with HC without CM, smaller left posterior cingulate cortex and larger right inferior frontal gyrus. |
Table 2.
Summary table of the main result for the functional MRI studies. adverse childhood experiences, adverse childhood experiences; BDI-II, Beck's Depression Inventory; CECA, Childhood Experience of Care and Abuse; CM, childhood maltreatment; CTQ, Childhood Trauma Questionnaire; CTQ-SF, Childhood Trauma Questionnaire-Short Form; EA, emotional abuse; ELA, early life adversity; ELT, early life trauma; GMV, grey matter volume; HAD, Hospital Anxiety and Depression Scale; HAMD, Hamilton depression scale; HAMD-D, Hamilton Rating Scale for Depression; HC, healthy control; HDRS, Hamilton Depression Rating; IBS, irritable bowel syndrome; IDS-SR, Inventory of Depressive Symptomatology-Self report; IP, internalizing psychopathology; MADRS, Montgomery Åsberg Depression Rating Scale; MDD, major depressive disorder; MSPD, multisomatoform pain disorder; PA, physical abuse; PHQ, Patient Health Questionnaire; QIDS, Quick Inventory for Depression Symptomatology; SA, sexual abuse; SDS, Self-rating Depression Scale; SPD, somatoform pain disorder. ∗Structural MRI provides detailed images of the brain's anatomy, whereas task-based functional MRI provides information about brain activity and function during a specific task.
| Author | Task-based functional MRI∗ |
|||
|---|---|---|---|---|
| Task | Group of participants | Contrast of interest | Main results | |
| Grant and colleagues59 | Riksen flanker task of selective attention | MDD+ELA | Sad>neutral | In contrast to the sad > neutral faces, the right amygdala showed higher activation for the unipolar depressed patients with ELA. Increased activation of the amygdala was found, and a positive correlation with the PA. Other forms of abuse and neglect were also found to have a weaker relationship. The correlation was significant when the patient had experienced ELA, and it was not significant for depressed patients. |
| MDD+ELA | positive>neutral | For positive > neutral faces, sexual abuse showed a correlation with amygdala response. | ||
| Hentze and colleagues62 | Affective ToM task | MDD | Emotional focus | The contrast of emotional focus revealed a negative correlation between amygdala and MADRS scores (depression). |
| CTQ | Perspective taking | Perspective taking revealed a positive correlation between amygdala activation and CTQ total scores. | ||
| Miller and colleagues66 | Continuous performance task (CPT); Go/No-go | MDD+CM> MDD-CM | CM vs no CM | During working memory updating on CPT, patients with and without CM differed in the activation of the right dorsolateral prefrontal cortex. |
| Noll-Hussong and colleagues88 | Empathy-for-pain paradigm | MSPD+abuse > MSPD–abuse |
MSPD+abuse vs > MSPD–abuse |
Abused patients in contrast to those with no experience of abuse showed an activation in the left lateral and medial superior frontal gyrus. No-abused patients in contrast with the abuse group showed an activation in the left hippocampus. |
| Peters and colleagues71 | Face/shapes interaction | IP+ELA | Angry>Shapes | Cuneus showed increase activation in the angry > shapes contrast for IP+ELA. |
| IP+ELA | Fearful > Shapes | Increased activation in the anterior and posterior cingulate, superior parietal, precuneus, cuneus, superior frontal and inferior temporal was demonstrated in the contrast of fearful > shapes for IP+ELA. | ||
| Ringel and colleagues89 | Three sets of repeated 39 s rectal distension separated by a 39-s rest period | All abuse>All non-abuse | All abuse vs All non-abuse | Abused patients in contrast to non-abuse patients with IBS showed an activation in the left mid-cingulate cortex and left posterior cingulate cortex. |
| IBS+abuse > ALL | IBS with abuse vs all | IBS patients with abuse in contrast with all others showed an activation in the left mid-cingulate cortex, bilateral posterior cingulate cortex and a de-activation in bilateral supra genual cingulate cortex. | ||
| Skokauskas and colleagues74 | Emotional attention shifting task | MDD | Judgement of emotion minus judgement of geometry after emotional neutral stimuli | Decrease activation with the contrast judgement of emotion minus judgement of geometry after emotional neutral stimuli on patients with MDD on the fusiform gyrus |
| MDD+SA | Judgement of emotion minus judgement of geometry after emotional negative stimuli | Increased activation with the contrast of judgement of emotion minus judgement of geometry after emotional negative stimuli on patients with MDD and experiences of SA on the left inferior parietal lobe. | ||
| Grant and colleagues60 | Gender identification variant of the Eriksen flanker task of selective attention | MDD>HC | MDD>HC | Based on medial prefrontal cortex–amygdala connectivity within the MDD group, association between exposure to ELT with failure of inhibition was observed. Association between negative causal pathways from medial prefrontal cortex to amygdala with non-ELT MDD patients, even though a reduced dorsolateral prefrontal cortex input compared with HC. |
| Tozzi and colleagues77 | Attentional cognitive emotional task | HC>MDD | HC>MDD | HC in contrast to MDD patients revealed an activation in the right middle frontal gyrus, right hippocampus, right precuneus and left lingual gyrus. |
| Tozzi and colleagues78 | Valence recognition of emotional images. | MDD>HC | MDD>HC | MDD patients compared with HC during valence recognition of emotional images showed de-activation in the bilateral inferior frontal gyrus. |
Table 3.
Summary table of patient characteristics, and main result for resting state MRI studies. adverse childhood experiences, adverse childhood experiences; BDI-II, Beck's Depression Inventory; CECA, Childhood Experience of Care and Abuse; CM, childhood maltreatment; CN, childhood neglect; chronic pain, chronic pain; CTQ, Childhood Trauma Questionnaire; CTQ-SF, Childhood Trauma Questionnaire-Short Form; EA, emotional abuse; EAL, early adverse life; ELA, early life adversity; ELS, early life stress; GMV, grey matter volume; GUPI, Genitourinary Pain Index; HAD, Hospital Anxiety and Depression Scale; HAMD, Hamilton Depression Scale; HAMD-D, Hamilton Rating Scale for Depression; HC, healthy control; HDRS, Hamilton Depression Rating; IBS, irritable bowel syndrome; IDS-SR, Inventory of Depressive Symptomatology-Self report; MADRS, Montgomery Åsberg Depression Rating Scale; MDD, major depressive disorder; PHQ, Patient Health Questionnaire; QIDS, Quick Inventory for Depression Symptomatology; RSN, Resting State Network; SA, sexual abuse; SDS, Self-rating Depression Scale; SPD, somatoform pain disorder; UCPPS, urological chronic pelvic pain syndrome.
| Author | Country | Study population |
Type of study | Assessment of ELA | Assessment of chronic pain | Assessment of depression | Assessment of anxiety disorders | Resting-state functional MRI |
||
|---|---|---|---|---|---|---|---|---|---|---|
| n | Mean age | Sex | Main results | |||||||
| Cisler and colleagues54 | USA | HC=12 ELS-MDD=7 ELS+MDD=19 |
HC=25.92 (5.33) ELS-MDD=27.43 (7.39) ELS+MDD=31.28 (8.57) |
– | An examination of global connectivity and hub-like properties in women with MDD with and without ELS compared with HC. | CTQ | n.a. | HAMD | n.a. | Between resilient individuals there were hub-like properties and decreased global connectivity for the right ventrolateral prefrontal cortex and for the dorsal anterior cingulate decreased local network connectivity. Between susceptible individuals there were hub-like properties and decreased global connectivity for the left amygdala and for the dorsal anterior cingulate decrease hub-like properties and for the left ventrolateral prefrontal cortex decreased local connectivity. |
| Wang and colleagues82 | China | HC=20 MDD-CN=20 MDD+CN=18 |
HC=27.9 (4.4) MDD-CN=28.2 (8.7) MDD+CN=28.3 (6.2) |
HC=11F MDD-CN=8F MDD+CN=10F |
An investigation in MDD patients with and without CM of the whole-brain functional connectivity patterns. | CTQ | n.a. | HDRS Self-rating Depression Scale |
n.a. | Compared with HC, MDD group in bilateral ventral medial prefrontal cortex/ventral anterior cingulate cortex revealed decreased functional connectivity strength. Compared with the MDD group without CM, MDD with CM in brain regions within the prefrontal–limbic–thalamic–cerebellar circuitry showed widespread reduction of functional connectivity strength, whereas the reductions were correlated with childhood neglect measurements. |
| Wu and colleagues83 | China | HC=58 MDD=29 |
HC=27.9 (5.9) MDD=26.7 (6.0) |
HC=34F MDD=17F |
An examination of certain brain functional connectivity patterns and their relationship to certain affective temperaments. In addition, whether the FCs contribute to depressive symptoms. | CTQ | n.a. | Temperament Evaluation of Memphis (TEMPS) HDRS |
Hamilton Anxiety Rating Scale (HAM-A) | Compared with HC, in MDD patients the covariation between the partial least square's functional connectivity profile and the partial least squares affective–temperament profile was enhanced. The somatisation symptom dimension was associated with the affective temperament modulated functional connectivity profile in MDD patients when there was adjusted for age, sex, duration of illness, age on set and HARS scores. |
| Xu and colleagues84 | China | HC=17 MDD+CM=15 MDD-CM=14 |
HC=28.94 (5.92) MDD+CM=28.33 (5.81) MDD-CM=32.36 (6.23) |
HC=7F MDD+CM=6F MDD-CM=5F |
An investigation of brain functionality in MDD patients with CM experience via a resting-state fMRI. | CTQ | n.a. | HAMD-17 | n.a. | In the prefrontal cortex there was an increased amplitude of low-frequency fluctuation and altered function connection which was associated with MDD patients with CM compared with MDD without CM. MDD patients with CM from patients without CM were differentiated by the left frontal middle gyrus. |
| Yu and colleagues86 | USA | HC=39 MDD=189 |
HC=37.1 (14.7) MDD=37.3 (13.0) |
HC=25F MDD=123F |
An investigation in patients with MDD and healthy controls for the network connectivity differences within and between RSNs. | CTQ | n.a. | QIDS HAMD |
Mood and anxiety symptom questionnaire anxious arousal (MASQ) | Compared with HC, MDD patients were characterised by a network model with abnormalities in the decrease within-network connectivity in the FPN, the dorsal attention network, and the cingulo-opercular network, task-positive RSNs. The second abnormality is an increase of within-network connectivity in the DMN and salience network, intrinsic networks. The last abnormality is an increase of within-network connectivity in the sensorimotor network and visual network, sensory networks. The history of childhood trauma and current symptoms in MDD patients were associated with a multivariate pattern of different within- and between-network connectivities, which involves the cingulo-opercular network, FPN, dorsal attention network, subcortical regions, ventral attention network, auditory network, visual network, and sensorimotor network |
| Gupta and colleagues92 | USA | HC=58 IBS=110 |
– | HC=30F IBS=72F |
An investigation in IBS patients compare with HC on the integrity of resting state networks, emotional/pain networks and default mode network related to EALs and sex. | Early adverse life trauma (ETI) | n.a. | HAD | n.a. | A positive correlation between left frontal parietal ICN-striatum connectivity and Early Adverse Life was demonstrated primarily in male participants. Female participants had a positive correlation with the connectivity of right putamen and right frontal parietal ICN. IBS patients showed negative correlations with right frontal parietal ICN – precentral gyrus connectivity and positive correlation with left parietal ICN – right superior parietal lobe connectivity. |
| Gupta and colleagues93 | USA | HC=86 UCPPS=85 |
HC=37.9 (12.23) UCPPS=39.36 (12.8) |
HC=59F UCPPS=56F |
An investigation of the role of EAL's in the central processes of chronic pain. | Childhood Traumatic Early Adversity (CTES) | Baseline GUPI QoL score; Pain severity; Urinary Severity | n.a. | n.a. | Compared with HC, UCPPS showed lower centrality in the right anterior insular. Compared with males HC, males UCPPS showed lower centrality in the right anterior insular. Compared with females with UCPPS, males with UCPPS showed lower centrality in the left posterior cingulate, middle temporal gyrus, angular gyrus and superior temporal sulcus, although it had greater centrality in the anterior midcingulate cortex and precuneus. In females with UPPS an association was observed between higher reports of ELAs and greater centrality in the left precuneus and left anterior midcingulate cortex. |
Structural MRI experiments, voxel-based morphology were split based upon disease status:
-
1.
MDD participants exposed to adverse childhood experiences were compared with MDD participants not exposed to adverse childhood experiences (five experiments; 13 foci; 277 participants), which revealed eight significant clusters in the anterior, occipital, temporal, frontal lobe, and putamen and insula (further details in Table 4).
-
2.
MDD participants and healthy subjects exposed to adverse childhood experiences were compared with MDD participants and healthy subjects not exposed to adverse childhood experiences (11 experiments; 26 foci; 628 participants), which revealed one significant cluster in the occipital and anterior lobe (further details in Table 4).
-
3.
Chronic pain participants exposed to adverse childhood experiences compared with chronic pain participants not exposed to adverse childhood experiences: no studies were identified.
Table 4.
Three groups of fMRI experiments and two groups of voxel based morphology significant meta-analysis results using GingerALE. ALE, Activation likelihood estimation; BA, Brodmann area; CP, chronic pain; ELA, early life adversity; Hem, Hemispheres; MDD, major depressive disorder; MNI, Montreal Neurological Institute coordinates. ∗ Broadman Area not applicable.
| Cluster | Hem | Lobes | Brain regions | BA | MNI |
ALE | P | Z | ||
|---|---|---|---|---|---|---|---|---|---|---|
| x | y | z | ||||||||
| fMRI meta-analysis results | ||||||||||
| MDD with adverse childhood experiences (n=95) compared with HC (n=69)59,74,76 | 164 participants | |||||||||
| 1 | Left | Parietal | Superior parietal lobule | 7 | –30 | –42 | 48 | 0.0012 | 0.0180 | 2.09 |
| Left | Parietal | Inferior parietal lobule | 40 | –33 | –49 | 49 | 0.0086 | 0.0003 | 3.41 | |
| 2 | Right | Limbic | Parahippocampal gyrus | 30 | 21 | –48 | 4 | 0.0009 | 0.0210 | 2.03 |
| Right | Limbic | Parahippocampal gyrus | 30 | 12 | –49 | 5 | 0.0090 | 0.0002 | 3.62 | |
| 3 | Left | Frontal | Middle frontal gyrus | 9 | –42 | 14 | 22 | 0.0095 | 2.3E–05 | 4.08 |
| Left | Frontal | Inferior frontal gyrus | 9 | –54 | 23 | 22 | 0.0092 | 6.1E–05 | 3.84 | |
| Left | Frontal | Middle frontal gyrus | 9 | –45 | 29 | 31 | 0.0086 | 0.0003 | 3.41 | |
| 4 | Right | Frontal | Middle frontal gyrus | 9 | 35 | 35 | 31 | 0.0051 | 0.0031 | 2.73 |
| 5 | Left | Temporal | Middle temporal gyrus | 37 | –60 | –52 | –11 | 0.0092 | 6.1E–05 | 3.84 |
| Left | Temporal | Inferior temporal gyrus | 37 | –57 | –46 | –17 | 0.0089 | 0.0002 | 3.52 | |
| 6 | Right | Sublobar | Putamen/globus pallidus | ∗ | 20 | –4 | 10 | 0.0065 | 0.0014 | 2.99 |
| MDD with adverse childhood experiences (n=116) compared with MDD without adverse childhood experiences (n=154)59,66,71,74 | 270 participants | |||||||||
| 1 | Right | Occipital | Middle occipital gyrus | 18 | 29 | –90 | 24 | 0.0180 | 1.1E–08 | 5.6 |
| Right | Occipital | Superior occipital gyrus | 19 | 24 | –90 | 38 | 0.0170 | 8.1E–08 | 5.24 | |
| Right | Occipital | Cuneus | 18 | 22 | –81 | 28 | 0.0000 | 0.0830 | 1.38 | |
| Right | Parietal | Precuneus | 7 | 23 | –61 | 45 | 0.0005 | 0.0440 | 1.71 | |
| 2 | Left | Parietal | Superior parietal lobule | 40 | –36 | –54 | 51 | 0.0022 | 0.0160 | 2.15 |
| Left | Parietal | Inferior parietal lobule | 40 | –33 | –40 | 49 | 0.0000 | 0.0800 | 1.4 | |
| MDD or CP or HC: with adverse childhood experiences (n=139) > without adverse childhood experiences (n=217)66,71,74,88,89 | 356 participants | |||||||||
| 1 | Left | Limbic | Posterior cingulate gyrus | 31 | –12 | –34 | 42 | 0.0110 | 1.4E–05 | 4.18 |
| Parietal | Inferior parietal lobule | 40 | –36 | –54 | 51 | 0.0022 | 0.0200 | 2.05 | ||
| Left | Parietal | Precuneus | 7 | –20 | –46 | 48 | 0.0070 | 0.0018 | 2.92 | |
| Left | Parietal | Inferior parietal lobule | 40 | –45 | –34 | 49 | 0.0069 | 0.0018 | 2.91 | |
| Structural (VBM) meta-analysis results | ||||||||||
| MDD with adverse childhood experiences (n=154) compared with MDD without adverse childhood experiences (n=123)52,53,65,70,85 | 277 participants | |||||||||
| 1 | Left | Anterior | Cerebellum | ∗ | –2 | –58 | –6 | 0.0093 | 2.5E–05 | 4.06 |
| Left | Occipital | Lingual gyrus | 18 | –8 | –72 | 6 | 0.0090 | 3.2E–05 | 3.99 | |
| Left | Anterior | Cerebellum | ∗ | –6 | –50 | –20 | 0.0089 | 0.0001 | 3.81 | |
| Right | Anterior | Cerebellum | ∗ | 8 | –34 | –14 | 0.0078 | 0.0004 | 3.37 | |
| 2 | Right | Temporal | Hippocampus | ∗ | 32 | –37 | 5 | 0.0075 | 0.0004 | 3.36 |
| 3 | Right | Sub-lobar | Superior insula | ∗ | 27 | –13 | 20 | 0.0078 | 0.0004 | 3.37 |
| 4 | Left | Sub-lobar | Putamen | ∗ | –25 | –17 | 18 | 0.0078 | 0.0004 | 3.37 |
| 5 | Left | Temporal | Superior temporal gyrus | 41 | –51 | –33 | 14 | 0.0084 | 0.0001 | 3.71 |
| 6 | Right | Temporal | Inferior temporal gyrus | 20 | 46 | –32 | –21 | 0.0086 | 8.1E–05 | 3.77 |
| 7 | Left | Frontal | Superior frontal gyrus | 8 | –17 | 32 | 37 | 0.0090 | 6.5E–05 | 3.83 |
| 8 | Right | Frontal | Superior frontal gyrus | 6 | 26 | 11 | 51 | 0.0090 | 6.5E–05 | 3.83 |
| MDD and HC with adverse childhood experiences (n=315) >MDD and HC without adverse childhood experiences (n=313)52,53,64,65,70,80,85 | 628 participants | |||||||||
| 1 | Left | Occipital | Lingual gyrus | 18 | –8 | –72 | 6 | 0.0180 | 1.7E–08 | 5.52 |
| Left | Anterior | Cerebellum | ∗ | –2 | –58 | –6 | 0.0093 | 0.0001 | 3.74 | |
| Left | Anterior | Cerebellum | ∗ | –6 | –50 | –20 | 0.0089 | 0.0002 | 3.58 | |
Task-based fMRI experiments were split based upon disease status:
-
1.
MDD participants exposed to adverse childhood experiences compared with healthy subjects (five experiments; 18 foci; 164 participants) which revealed six significant clusters in the parietal, limbic, frontal, temporal lobe and putamen/globus pallidus (further details in Table 4).
-
2.
MDD participants exposed to adverse childhood experiences compared with MDD participants not exposed adverse childhood experiences (seven experiments; 28 foci; 270 participants), which revealed two significant clusters in the occipital and parietal lobes (further details in Table 4).
-
3.
MDD, or chronic pain participants or healthy subjects exposed to adverse childhood experiences compared with those not exposed to adverse childhood experiences (10 experiments; 37 foci; 296 participants), which revealed one significant cluster in the limbic and parietal lobe (further details in Table 4).
-
4.
Chronic pain participants exposed to adverse childhood experiences compared with chronic pain participants not exposed to adverse childhood experiences (two experiments; nine foci; 23 participants) were not analysed because of the small group size.
Discussion
Adverse childhood experiences involving stress, injury, or diseases can change the brain at different levels, including epigenetic,94 cell biological, and systems and network levels.95,96 Using a systematic review and meta-analysis, we have explored adverse childhood experiences as a potential modulator for chronic pain and depression in adulthood. Significant structural alterations for patients with depression and adverse childhood experiences in the hippocampus, insula, and putamen were identified, with functional alteration in the precuneus. Investigation of the effects of adverse childhood experiences on chronic pain or depression revealed significant functional changes in the posterior cingulate cortex (PCC) and precuneus (Fig 1). We have shown that adverse childhood experiences have been linked with alterations in the function of the brain in people with chronic pain. Epigenetic and epidemiological studies have shown a relationship between adverse childhood experiences and chronic pain in later life94,96, 97, 98, 99, 100; however, we have identified a gap in the neuroimaging literature studying the long-term impact of adverse childhood experiences on neural processing in people with chronic pain. There are neuroimaging studies examining psychosocial adversities (e.g. physical, sexual, emotional abuse), but there are no studies examining non-psychosocial adversities such as those arising from serious medical conditions (e.g. asthma, cancer, epilepsy in early life) which have often been overlooked.101
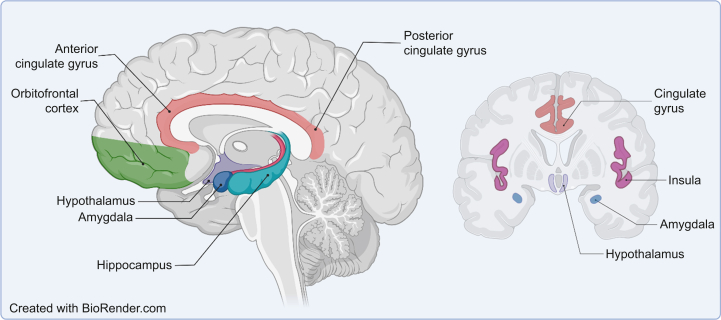
Fig 1.
Key brain regions that are commonly associated with pain perception, including the insula, anterior and posterior cingulate, hypothalamus, hippocampus, amygdala, orbitofrontal cortex, and thalamus.
The reward-motivation network (including the prefrontal cortex, nucleus accumbens, hippocampus, and ventral tegmentum) and the descending pain modulatory system (prefrontal cortex, anterior cingulate cortex [ACC], amygdala, hypothalamus) are implicated in vulnerability to painful conditions with evidence for structural, functional, and neurochemical alterations in the brain (Table 5).102 Other areas that might not affect risk of developing chronic pain, but are relevant to pain perception include changes in the insula, thalamus, orbitofrontal, primary and secondary somatosensory cortex, and dorsal ACC extending to mid-ACC.102,103 There is also an extensive literature around the involvement of the reward-motivation and salience networks in people with MDD.104,105 The neural correlates of emotional processing have been well studied in people who have experienced adverse childhood experiences, although reward processing has received less attention, but there is evidence that suggests a deficit in reward sensitivity.106,107
Table 5.
Listing specific brain areas and their possible relation to pain, chronic pain, MDD and adverse childhood experiences. ACC, anterior cingulate cortex; adverse childhood experiences, adverse childhood experiences; MDD, major depressive disorder; PCC, posterior cingulate cortex.
| Regions | Involvement of MDD, chronic pain, and adverse childhood experiences |
|---|---|
| Prefrontal cortex | Involvement in vulnerability to painful conditions (reward-motivation network and descending pain modulatory system).102 Involvement of the reward-motivation and salience networks in people with MDD.104,105 Deficit in reward sensitivity and reward anticipation in adverse childhood experiences.106,107 |
| ACC | Individuals who are vulnerable to painful conditions may have changes in the reward-motivation and descending pain modulatory systems in the brain.102 These changes may not necessarily affect the risk of developing chronic pain, but they do play a role in how pain is perceived.103,108,126 Activated by other aversive experiences, leading to negative emotions and anxiety.108, 109, 110 MDD may have alterations in the reward-motivation and salience networks.104,105 adverse childhood experiences have been linked to a deficit in reward sensitivity and reward anticipation.106,107 |
| Amygdala | Vulnerability to painful conditions may have changes in the descending pain modulatory system in the brain.102 They play a role in how pain is perceived.103 MDD have alterations in the reward-motivation and salience networks104,105 which can contribute to their vulnerability to painful conditions. |
| Hypothalamus/thalamus | Vulnerability to painful conditions (descending pain modulatory system),102 may not affect risk of developing chronic pain but is a relevant region to pain perception.103 Involvement with the MDD.104,105 |
| Nucleus accumbens | Individuals who are at risk of experiencing painful conditions may have changes in the reward-motivation network in the brain.102 MDD may have alterations in the reward-motivation and salience networks,104,105 which can make them more susceptible to painful conditions. adverse childhood experiences have been linked to a deficit in reward sensitivity.106,107 Anhedonia, chronic pain, and depression is linked to dopamine abnormalities affected by inputs from the hippocampus. Research also suggests that childhood adversity can lead to dysfunction of the basal ganglia during reward anticipation. |
| Ventral tegmentum | Vulnerability to painful conditions (reward-motivation network).102 Involvement of the reward-motivation and salience networks in people with MDD.104,105 Deficit in reward sensitivity and dysfunction during reward anticipation in humans exposed to adverse childhood experiences.106,107 |
| Hippocampus | Vulnerability to painful conditions (reward-motivation network), is a relevant region to pain perception.103 Involvement of the reward-motivation and salience networks in people with MDD.104,105 Children exposed to traumatic events have smaller hippocampus and weaker activity in that region during memory tasks.116 This is associated with difficulties in learning and memory in individuals with chronic pain.130, 131, 132 Changes in the structure of the hippocampus, which is often impacted by chronic pain, may contribute to the emergence of depression.133,134 |
| Insula | It is an important area in regard to the perception of pain.103,108 It is activated by other negative experiences as well, leading to negative emotions and anxiety.108, 109, 110 Blunted signal in aversive stimuli in MDD. Mediates changes in the default mode network and frontoparietal network facilitating responses to salient stimuli.108,115 Increased insula activation in children exposed to violence.116, 117, 118, 119, 120, 121 |
| Orbitofrontal cortex | It is still an area of the brain that plays a role in the perception of pain.103 MDD may have alterations in the reward-motivation and salience networks.104,105 |
| Primary/secondary somatosensory cortex | A significant area in terms of pain.103 |
| PCC | It is a significant area in terms of the sensation and perception of pain,103 and episodic memory retrieval.122 MDD have been found to have abnormal activity in this region, and healthy individuals who have experienced adverse childhood experiences. PCC activation may be more related to the emotional and memory-related aspects of stimuli, rather than the actual experience of pain.122 |
Our findings have demonstrated an impact of adverse childhood experiences on neuroanatomically plausible areas associated with mood and pain processing. Major cortical projections from the spinothalamic tracts (associated with pain) include the posterior insula, parietal operculum, and mid-cingulate cortex.108 Posterior insula and parietal operculum stimulation and focal seizures can trigger pain, lesions can cause pain deficits, and cortical insula damage has been correlated with neuropathic pain.108 Other regions, including anterior insula and anterior cingulate, influence the experience of pain but are also activated by other aversive experiences generating negative affect and anxiety.108, 109, 110 Lesions in the posterior insula111 and mid-cingulate cortex112 can result in patients continuing to recognise pain but with reduced or absent suffering; pain asymbolia.113 The anterior insula is thought to alter responsiveness to specific stimuli114 because of its involvement in the salience network,108 which mediates changes in the default mode network and frontoparietal network facilitating responses to salient stimuli.115 Notably, a systematic review on childhood adversity and neural development highlighted the importance of the salience network,116 with increased insula activation in children exposed to violence.117, 118, 119, 120, 121
A meta-analysis of functional imaging studies reported that activation of the PCC was associated with the experience of pain and episodic memory retrieval122 consistent with earlier reports.123, 124, 125 The anterior mid-cingulate cortex (aMCC) is also strongly associated with the experience of pain,108,126 and for some patients, small lesions of the aMCC relieve the distress of intractable pain.112 Relative to the aMCC though, a larger and more posterior part of the cingulate is also associated with pain.122 Furthermore, there is evidence for a rostral–caudal segregation of function, with the anterior PCC region more associated with pain and the posterior PCC region more associated with memory.122 PCC activation appears consistently associated with the emotional salience of stimuli and, despite evidence for segregation, there is a tendency for emotion and memory-related activations to overlap.122 Our meta-analysis study is consistent with these reports, such that patients with chronic pain and MDD were found to have reproducibly abnormal PCC activity. Furthermore, it is worth noting that healthy subjects with a history of adverse childhood experiences also have abnormal PCC activation. Consequently, abnormal activation of this region may be associated more with emotion and memory-related activations of the PCC rather than experiences of pain.
The hippocampus has an important role in the storage and retrieval of long-term explicit memories127 and in associative learning.128 It also has an important role in terminating the stress response via its regulatory role in the hypothalamic–pituitary–adrenal axis.129 Hippocampal volume and functional abnormalities have been reported inconsistently in deprivation-exposed children.116 Nevertheless, in children exposed to threat-related adversity, reduced hippocampal volumes have been reported, consistent with our findings including reduced activation during a memory task.116 Reduced hippocampal volume has been reported in patients with chronic pain: fibromyalgia,130 complex regional pain syndrome (CRPS), and chronic back pain (CBP),131 with evidence for learning and memory deficits in patients with chronic pain.132 Chronic pain and depression comorbidity is common, and it has been suggested that hippocampal abnormalities observed in patients with chronic pain may be related to the mood component.133 Anhedonia, chronic pain, and depression have all been correlated with blunted striatum activation which may be related to dopaminergic abnormalities modulated by hippocampal afferents.134 Studies on animals and humans have revealed reduced neurogenesis, neuroplasticity, neurotropic factors and increased hippocampal inflammation in both patients with depression and chronic pain.132
The reward system includes the caudate and putamen,135 with these regions responding to both the receipt and anticipation of reward.136 Consistent with anhedonia being a prominent clinical feature of depression, the brain reward circuitry appears abnormal in depression with functional dysregulations and brain structure changes,137 in accordance with our findings, such as blunted striatal responses to anticipated reward in maltreated children.106,107 Blunted reward-linked striatal activity has also been observed in patients with adverse postnatal experiences.138 Dysfunction of the basal ganglia during reward anticipation in humans exposed to childhood adversity may reflect abnormalities in dopaminergic circuits reported in animal studies.139,140
Limitations
Potential limitations of this study should be noted. First, an assessment of heterogeneity is often done in non-neuroimaging studies, but there is no methodology for assessing heterogeneity for ALE or other coordinate-based meta-analyses.141 The ALE method provides an estimation of the probability that an activity in a specific region may differ between groups of patients and not an estimate of the mean difference in the regional signal change.142 Thus, traditional measures of heterogeneity are not applicable.141,143,144 Second, the limited number of neuroimaging studies available in the literature investigating chronic pain with adverse childhood experiences limited the meta-analysis results. Third, adverse childhood experiences have been associated with other psychiatric disorders, such as post-traumatic stress disorder (PTSD) and anxiety, whereas our study focused only on patients with depression. Recall bias,145 unfortunately, cannot be eliminated because of the systematic error that occurs when the participants did not remember previous adverse childhood experiences accurately when answering the questionnaire. Prospective longitudinal cohort studies and pre-existing datasets that include information collected during childhood, which can be further linked to healthcare records in later life (e.g. Generation Scotland: STRADL-Pain,146, 147, 148 ALSPAC149), are approaches to address this limitation.
Conclusions
This review has investigated the neural correlates of adverse childhood experiences on chronic pain and depression. It is notable that there are few neuroimaging investigations into patients with chronic pain who have experienced adverse childhood experiences. Nevertheless, significant brain structure and function correlations with adverse childhood experiences were observed in people with major depressive disorder or chronic pain in comparison with healthy controls. In the former, correlations were found in the hippocampus, superior insula, putamen, and precuneus, and in the latter, significant correlations were found in the dorsal anterior cingulate and precuneus. Our results indicate the existence of brain structural and functional abnormalities associated with adverse childhood experiences – some of which may be characteristic for adverse childhood experiences and others more related to comorbid depression and chronic pain. Moreover, they are suggesting a shared neural correlates for comorbidity and possibly increasing the vulnerability to develop later in life depression, chronic pain, or both.
Authors’ contributions
Concept: GA, EL, LC, JDS
Study design: GA, EL, LC, JDS
Interpretation of data: GA, EL, LC, JDS
Drafting the manuscript: GA, EL, LC, JDS
Article screening and selection: GA, EL
Data extraction, analysis, and quality assessment of studies: GA
Read and approved the final version of the manuscript: all authors
Acknowledgements
We are grateful to Research Librarian, Scott McGregor at Library and Learning and Culture and Information, University of Dundee for his kind assistance with design of the database searches. LC and JDS are part of the UKRI Advanced Pain Discovery Platform.
Declarations of interest
LC is a member of the British Journal of Anaesthesia and BJA Open editorial boards. GA, EL, and JDS have no conflicts of interests to declare.
Funding
The study is part of E-PaiD PhD project, funded by TENOVUS Scotland Research PhD Studentship, ref T20-18.
Handling editor: Jonathan Hardman
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.bja.2023.03.008.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- 1.Treede R.D., Rief W., Barke A., et al. A classification of chronic pain for ICD-11. Pain. 2015;156:1003–1007. doi: 10.1097/j.pain.0000000000000160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Fayaz A., Croft P., Langford R., et al. Prevalence of chronic pain in the UK: a systematic review and meta-analysis of population studies. BMJ Open. 2016;6 doi: 10.1136/bmjopen-2015-010364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cassell A., Edwards D., Harshfield A., et al. The epidemiology of multimorbidity in primary care: a retrospective cohort study. Br J Gen Pract. 2018;68:e245–e251. doi: 10.3399/bjgp18X695465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Krishnan R.R.K., France R.D., Pelton S., et al. Chronic pain and depression. ii. Symptoms of anxiety in chronic low back pain patients and their relationship to subtypes of depression. Pain. 1985;22:289–294. doi: 10.1016/0304-3959(85)90029-6. [DOI] [PubMed] [Google Scholar]
- 5.McWilliams L.A., Cox B.J., Enns M.W. Mood and anxiety disorders associated with chronic pain: an examination in a nationally representative sample. Pain. 2003;106:127–133. doi: 10.1016/s0304-3959(03)00301-4. [DOI] [PubMed] [Google Scholar]
- 6.Kleykamp B.A., Ferguson M.C., McNicol E., et al. The prevalence of psychiatric and chronic pain comorbidities in fibromyalgia: an ACTION systematic review. Semin Arthritis Rheum. 2021;51:166–174. doi: 10.1016/j.semarthrit.2020.10.006. [DOI] [PubMed] [Google Scholar]
- 7.Fava M., Kendler K.S. Major depressive disorder. Neuron. 2000;28:335–341. doi: 10.1016/s0896-6273(00)00112-4. [DOI] [PubMed] [Google Scholar]
- 8.Sheng J., Liu S., Wang Y., et al. The link between depression and chronic pain: neural mechanisms in the brain. Neural Plast. 2017;2017 doi: 10.1155/2017/9724371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.James S.L., Abate D., Abate K.H., et al. Global, regional, and national incidence, prevalence, and years lived with disability for 354 diseases and injuries for 195 countries and territories, 1990–2017: a systematic analysis for the global burden of disease study 2017. Lancet. 2018;392:1789–1858. doi: 10.1016/S0140-6736(18)32279-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Avenevoli S., Swendsen J., He J.-P., et al. Major depression in the national comorbidity survey–adolescent supplement: prevalence, correlates, and treatment. J Am Acad Child Adolesc Psychiatry. 2015;54:37–44. doi: 10.1016/j.jaac.2014.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hasin D.S., Sarvet A.L., Meyers J.L., et al. Epidemiology of adult DSM-5 major depressive disorder and its specifiers in the United States. JAMA Psychiatry. 2018;75:336–346. doi: 10.1001/jamapsychiatry.2017.4602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Whiteford H.A., Degenhardt L., Rehm J., et al. Global burden of disease attributable to mental and substance use disorders: findings from the global burden of disease study 2010. Lancet. 2013;382:1575–1586. doi: 10.1016/S0140-6736(13)61611-6. [DOI] [PubMed] [Google Scholar]
- 13.Turecki G., Ota V.K., Belangero S.I., et al. Early life adversity, genomic plasticity, and psychopathology. Lancet Psychiatry. 2014;1:461–466. doi: 10.1016/S2215-0366(14)00022-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Merrick M.T., Ford D.C., Ports K.A., et al. Vital signs: estimated proportion of adult health problems attributable to adverse childhood experiences and implications for prevention—25 states, 2015–2017. MMWR Morb Mortal Wkly Rep. 2019;68:999. doi: 10.15585/mmwr.mm6844e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.McLaughlin K.A., Green J.G., Gruber M.J., et al. Childhood adversities and first onset of psychiatric disorders in a national sample of us adolescents. Arch Gen Psychiatry. 2012;69:1151–1160. doi: 10.1001/archgenpsychiatry.2011.2277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Krugers H.J., Arp J.M., Xiong H., et al. Early life adversity: lasting consequences for emotional learning. Neurobiol Stress. 2017;6:14–21. doi: 10.1016/j.ynstr.2016.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dube S.R., Felitti V.J., Dong M., et al. The impact of adverse childhood experiences on health problems: evidence from four birth cohorts dating back to 1900. Prev Med. 2003;37:268–277. doi: 10.1016/s0091-7435(03)00123-3. [DOI] [PubMed] [Google Scholar]
- 18.Burke N.N., Finn D.P., McGuire B.E., Roche M. Psychological stress in early life as a predisposing factor for the development of chronic pain: clinical and preclinical evidence and neurobiological mechanisms. J Neurosci Res. 2017;95:1257–1270. doi: 10.1002/jnr.23802. [DOI] [PubMed] [Google Scholar]
- 19.Low L.A., Schweinhardt P. Early life adversity as a risk factor for fibromyalgia in later life. Pain Res Manag. 2012;2012 doi: 10.1155/2012/140832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Elovainio M., Pulkki-Råback L., Jokela M., et al. Socioeconomic status and the development of depressive symptoms from childhood to adulthood: a longitudinal analysis across 27 years of follow-up in the young Finns study. Soc Sci Med. 2012;74:923–929. doi: 10.1016/j.socscimed.2011.12.017. [DOI] [PubMed] [Google Scholar]
- 21.Mathur A., Graham-Engeland J.E., Slavish D.C., et al. Recalled early life adversity and pain: the role of mood, sleep, optimism, and control. J Behav Med. 2018;41:504–515. doi: 10.1007/s10865-018-9917-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gill H., El-Halabi S., Majeed A., et al. The association between adverse childhood experiences and inflammation in patients with major depressive disorder: a systematic review. J Affect Disord. 2020;272:1–7. doi: 10.1016/j.jad.2020.03.145. [DOI] [PubMed] [Google Scholar]
- 23.Ehlert U. Enduring psychobiological effects of childhood adversity. Psychoneuroendocrinology. 2013;38:1850–1857. doi: 10.1016/j.psyneuen.2013.06.007. [DOI] [PubMed] [Google Scholar]
- 24.Edwards R.R., Dworkin R.H., Turk D.C., et al. Patient phenotyping in clinical trials of chronic pain treatments: IMMPACT recommendations. Pain. 2016;157:1851–1871. doi: 10.1097/j.pain.0000000000000602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tracey I. Neuroimaging mechanisms in pain: from discovery to translation. Pain. 2017;158:S115–S122. doi: 10.1097/j.pain.0000000000000863. [DOI] [PubMed] [Google Scholar]
- 26.Martucci K.T., Ng P., Mackey S. Neuroimaging chronic pain: what have we learned and where are we going? Future Neurol. 2014;9:615–626. doi: 10.2217/FNL.14.57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lee M., Tracey I. Imaging pain: a potent means for investigating pain mechanisms in patients. Br J Anaesth. 2013;111:64–72. doi: 10.1093/bja/aet174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nusslock R., Miller G.E. Early-life adversity and physical and emotional health across the lifespan: a neuroimmune network hypothesis. Biol Psychiatry. 2016;80:23–32. doi: 10.1016/j.biopsych.2015.05.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Herzog J.I., Schmahl C. Adverse childhood experiences and the consequences on neurobiological, psychosocial, and somatic conditions across the lifespan. Front Psychiatry. 2018;9:420. doi: 10.3389/fpsyt.2018.00420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rao U., Chen L.-A., Bidesi A.S., et al. Hippocampal changes associated with early-life adversity and vulnerability to depression. Biol Psychiatry. 2010;67:357–364. doi: 10.1016/j.biopsych.2009.10.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ploner M., Lee M.C., Wiech K., et al. Flexible cerebral connectivity patterns subserve contextual modulations of pain. Cereb Cortex. 2010;21:719–726. doi: 10.1093/cercor/bhq146. [DOI] [PubMed] [Google Scholar]
- 32.Carballedo A., Morris D., Zill P., et al. Brain-derived neurotrophic factor Val66Met polymorphism and early life adversity affect hippocampal volume. Am J Med Genet. 2013;162:183–190. doi: 10.1002/ajmg.b.32130. [DOI] [PubMed] [Google Scholar]
- 33.Frodl T., Reinhold E., Koutsouleris N., et al. Childhood stress, serotonin transporter gene and brain structures in major depression. Neuropsychopharmacology. 2010;35:1383–1390. doi: 10.1038/npp.2010.8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Frodl T., Reinhold E., Koutsouleris N., et al. Interaction of childhood stress with hippocampus and prefrontal cortex volume reduction in major depression. J Psychiatr Res. 2010;44:799–807. doi: 10.1016/j.jpsychires.2010.01.006. [DOI] [PubMed] [Google Scholar]
- 35.Gerritsen L., van Velzen L., Schmaal L., et al. Childhood maltreatment modifies the relationship of depression with hippocampal volume. Psychol Med. 2015;45:3517–3526. doi: 10.1017/S0033291715001415. [DOI] [PubMed] [Google Scholar]
- 36.Lenze S.N., Xiong C., Sheline Y.I. Childhood adversity predicts earlier onset of major depression but not reduced hippocampal volume. Psychiatry Res Neuroimaging. 2008;162:39–49. doi: 10.1016/j.pscychresns.2007.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Opel N., Redlich R., Zwanzger P., et al. Hippocampal atrophy in major depression: a function of childhood maltreatment rather than diagnosis? Neuropsychopharmacology. 2014;39:2723–2731. doi: 10.1038/npp.2014.145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Saleh A., Potter G.G., McQuoid D.R., et al. Effects of early life stress on depression, cognitive performance and brain morphology. Psychol Med. 2017;47:171–181. doi: 10.1017/S0033291716002403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bernstein D.P., Fink L., Handelsman L., Foote J. APA; Washington, DC: 1998. Childhood trauma questionnaire. Assessment of family violence: a Handbook for researchers and practitioners. [Google Scholar]
- 40.Williams J.B. A structured interview guide for the Hamilton Depression Rating Scale. Arch Gen Psychiatry. 1988;45:742–747. doi: 10.1001/archpsyc.1988.01800320058007. [DOI] [PubMed] [Google Scholar]
- 41.Rush A.J., Gullion C.M., Basco M.R., et al. The inventory of depressive symptomatology (IDS): psychometric properties. Psychol Med. 1996;26:477–486. doi: 10.1017/s0033291700035558. [DOI] [PubMed] [Google Scholar]
- 42.Rush A.J., Trivedi M.H., Ibrahim H.M., et al. The 16-item Quick Inventory of Depressive Symptomatology (QIDS), Clinician Rating (QIDS-C), and self-report (QIDS-SR): a psychometric evaluation in patients with chronic major depression. Biol Psychiatry. 2003;54:573–583. doi: 10.1016/s0006-3223(02)01866-8. [DOI] [PubMed] [Google Scholar]
- 43.Warrens M.J. Five ways to look at Cohen’s kappa. J Psychol. 2015;5:1. [Google Scholar]
- 44.Stang A. Critical evaluation of the Newcastle Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur J Epidemiol. 2010;25:603–605. doi: 10.1007/s10654-010-9491-z. [DOI] [PubMed] [Google Scholar]
- 45.Gentili C., Messerotti Benvenuti S., Lettieri G., et al. ROI and phobias: the effect of ROI approach on an ale meta-analysis of specific phobias. Hum Brain Mapp. 2019;40:1814–1828. doi: 10.1002/hbm.24492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Eickhoff S.B., Laird A.R., Grefkes C., et al. Coordinate-based activation likelihood estimation meta-analysis of neuroimaging data: a random-effects approach based on empirical estimates of spatial uncertainty. Hum Brain Mapp. 2009;30:2907–2926. doi: 10.1002/hbm.20718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Laird A.R., Fox P.M., Price C.J., et al. ALE meta-analysis: controlling the false discovery rate and performing statistical contrasts. Hum Brain Mapp. 2005;25:155–164. doi: 10.1002/hbm.20136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Turkeltaub P.E., Eden G.F., Jones K.M., Zeffiro T.A. Meta-analysis of the functional neuroanatomy of single-word reading: method and validation. NeuroImage. 2002;16:765–780. doi: 10.1006/nimg.2002.1131. [DOI] [PubMed] [Google Scholar]
- 49.Lancaster J.L., Tordesillas-Gutiérrez D., Martinez M., et al. Bias between MNI and Talairach coordinates analyzed using the ICBM-152 brain template. Hum Brain Mapp. 2007;28:1194–1205. doi: 10.1002/hbm.20345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Müller V.I., Cieslik E.C., Laird A.R., et al. Ten simple rules for neuroimaging meta-analysis. Neurosci Biobehav Rev. 2018;84:151–161. doi: 10.1016/j.neubiorev.2017.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Eickhoff S.B., Bzdok D., Laird A.R., et al. Activation likelihood estimation metaanalysis revisited. NeuroImage. 2012;59:2349–2361. doi: 10.1016/j.neuroimage.2011.09.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ahn S.J., Kyeong S., Suh S.H., et al. What is the impact of child abuse on gray matter abnormalities in individuals with major depressive disorder: a case control study. BMC Psychiatry. 2016;16:397. doi: 10.1186/s12888-016-1116-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Chaney A., Carballedo A., Amico F., et al. Effect of childhood maltreatment on brain structure in adult patients with major depressive disorder and healthy participants. JPN. 2014;39:50–59. doi: 10.1503/jpn.120208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Cisler J.M., James G.A., Tripathi S., et al. Differential functional connectivity within an emotion regulation neural network among individuals resilient and susceptible to the depressogenic effects of early life stress. Psychol Med. 2013;43:507–518. doi: 10.1017/S0033291712001390. [DOI] [PubMed] [Google Scholar]
- 55.Colle R., Segawa T., Chupin M., et al. Early life adversity is associated with a smaller hippocampus in male but not female depressed in-patients: a case-control study. BMC Psychiatry. 2017;17:71. doi: 10.1186/s12888-017-1233-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Frodl T., Skokauskas N., Frey E.M., et al. BDNF Val66Met genotype interacts with childhood adversity and influences the formation of hippocampal subfields. Hum Brain Mapp. 2014;35:5776–5783. doi: 10.1002/hbm.22584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Frodl T., Janowitz D., Schmaal L., et al. Childhood adversity impacts on brain subcortical structures relevant to depression. J Psychiatr Res. 2017;86:58–65. doi: 10.1016/j.jpsychires.2016.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Frost C.P., Meyerand M.E., Birn R.M., et al. Childhood emotional abuse moderates associations among corticomotor white matter structure and stress neuromodulators in women with and without depression. Front Neurosci. 2018;12:256. doi: 10.3389/fnins.2018.00256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Grant M.M., Cannistraci C., Hollon S.D., et al. Childhood trauma history differentiates amygdala response to sad faces within MDD. J Psychiatr Res. 2011;45:886–895. doi: 10.1016/j.jpsychires.2010.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Grant M.M., White D., Hadley J., et al. Early life trauma and directional brain connectivity within major depression. Hum Brain Mapp. 2014;35:4815–4826. doi: 10.1002/hbm.22514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Graziano R.C., Bruce S.E., Paul R.H., et al. The effects of bullying in depression on white matter integrity. Behav Brain Res. 2019;363:149–154. doi: 10.1016/j.bbr.2019.01.054. [DOI] [PubMed] [Google Scholar]
- 62.Hentze C., Walter H., Schramm E., et al. Functional correlates of childhood maltreatment and symptom severity during affective theory of mind tasks in chronic depression. Psychiatry Res Neuroimaging. 2016;250:1–11. doi: 10.1016/j.pscychresns.2016.02.004. [DOI] [PubMed] [Google Scholar]
- 63.Jaworska N., MacMaster F.P., Gaxiola I., et al. A preliminary study of the influence of age of onset and childhood trauma on cortical thickness in major depressive disorder. Biomed Res Int Biomed Res Int. 2014;2014 doi: 10.1155/2014/410472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Lu S., Xu R., Cao J., et al. The left dorsolateral prefrontal cortex volume is reduced in adults reporting childhood trauma independent of depression diagnosis. J Psychiatr Res. 2019;112:12–17. doi: 10.1016/j.jpsychires.2019.02.014. [DOI] [PubMed] [Google Scholar]
- 65.Lu X.W., Guo H., Sun J.R., et al. A shared effect of paroxetine treatment on gray matter volume in depressive patients with and without childhood maltreatment: a voxel-based morphometry study. CNS Neurosci Ther. 2018;24:1073–1083. doi: 10.1111/cns.13055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Miller S., McTeague L.M., Gyurak A., et al. Cognition-childhood maltreatment interactions in the prediction of antidepressant outcomes in major depressive disorder patients: results from the iSPOT-D trial. Depress Anxiety. 2015;32:594–604. doi: 10.1002/da.22368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Monninger M., Kraaijenvanger E.J., Pollok T.M., et al. The long-term impact of early life stress on orbitofrontal cortical thickness. Cereb Cortex. 2020;30:1307–1317. doi: 10.1093/cercor/bhz167. [DOI] [PubMed] [Google Scholar]
- 68.Ohashi K., Anderson C.M., Bolger E.A., et al. Childhood maltreatment is associated with alteration in global network fiber-tract architecture independent of history of depression and anxiety. Neuroimage. 2017;150:50–59. doi: 10.1016/j.neuroimage.2017.02.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Oshri A., Gray J.C., Owens M.M., et al. Adverse childhood experiences and amygdalar reduction: high-resolution segmentation reveals associations with subnuclei and psychiatric outcomes. Child Maltreat. 2019;24:400–410. doi: 10.1177/1077559519839491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Peng H., Ning Y., Zhang Y., et al. White-matter density abnormalities in depressive patients with and without childhood neglect: a voxel-based morphometry (VBM) analysis. Neurosci Lett. 2013;550:23–28. doi: 10.1016/j.neulet.2013.06.048. [DOI] [PubMed] [Google Scholar]
- 71.Peters A.T., Burkhouse K.L., Kinney K.L., Phan K.L. The roles of early-life adversity and rumination in neural response to emotional faces amongst anxious and depressed adults. Psychol Med. 2019;49:2267–2278. doi: 10.1017/S0033291718003203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Salokangas R.K.R., Hietala J., Armio R.L., et al. Effect of childhood physical abuse on social anxiety is mediated via reduced frontal lobe and amygdala-hippocampus complex volume in adult clinical high-risk subjects. Schizophr Res. 2020;24:101–109. doi: 10.1016/j.schres.2020.05.041. [DOI] [PubMed] [Google Scholar]
- 73.Sara P., Veronica A., Silvia B., et al. Impact of early and recent stress on white matter microstructure in major depressive disorder. J Affect Disord. 2018;225:289–297. doi: 10.1016/j.jad.2017.08.017. [DOI] [PubMed] [Google Scholar]
- 74.Skokauskas N., Carballedo A., Fagan A., Frodl T. The role of sexual abuse on functional neuroimaging markers associated with major depressive disorder. World J Biol Psychiatry. 2015;16:513–520. doi: 10.3109/15622975.2015.1048723. [DOI] [PubMed] [Google Scholar]
- 75.Tatham E.L., Ramasubbu R., Gaxiola-Valdez I., et al. White matter integrity in major depressive disorder: implications of childhood trauma, 5-HTTLPR and BDNF polymorphisms. Psychiatry Res Neuroimaging. 2016;253:15–25. doi: 10.1016/j.pscychresns.2016.04.014. [DOI] [PubMed] [Google Scholar]
- 76.Tozzi L., Carballedo A., Wetterling F., et al. Single-nucleotide polymorphism of the FKBP5 gene and childhood maltreatment as predictors of structural changes in brain areas involved in emotional processing in depression. Neuropsychopharmacology. 2016;41:487–497. doi: 10.1038/npp.2015.170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Tozzi L., Farrell C., Booij L., et al. Epigenetic changes of FKBP5 as a link connecting genetic and environmental risk factors with structural and functional brain changes in major depression. Neuropsychopharmacology. 2018;43:1138–1145. doi: 10.1038/npp.2017.290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Tozzi L., Garczarek L., Janowitz D., et al. Interactive impact of childhood maltreatment, depression, and age on cortical brain structure: mega-analytic findings from a large multi-site cohort. Psychol Med. 2020;50:1020–1031. doi: 10.1017/S003329171900093X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Ugwu I.D., Amico F., Carballedo A., et al. Childhood adversity, depression, age and gender effects on white matter microstructure: a DTI study. Brain Struct Funct. 2015;220:1997–2009. doi: 10.1007/s00429-014-0769-x. [DOI] [PubMed] [Google Scholar]
- 80.van Harmelen A.L., van Tol M.J., van der Wee N.J., et al. Reduced medial prefrontal cortex volume in adults reporting childhood emotional maltreatment. Biol Psychiatry. 2010;68:832–838. doi: 10.1016/j.biopsych.2010.06.011. [DOI] [PubMed] [Google Scholar]
- 81.Vythilingam M., Heim C., Newport J., et al. Childhood trauma associated with smaller hippocampal volume in women with major depression. Am J Psychiatry. 2002;159:2072–2080. doi: 10.1176/appi.ajp.159.12.2072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Wang L.F., Dai Z.J., Peng H.J., et al. Overlapping and segregated resting-state functional connectivity in patients with major depressive disorder with and without childhood neglect. Hum Brain Mapp. 2014;35:1154–1166. doi: 10.1002/hbm.22241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Wu H., Wu C., Wu F., et al. Covariation between childhood-trauma related resting-state functional connectivity and affective temperaments is impaired in individuals with major depressive disorder. Neuroscience. 2021;453:102–112. doi: 10.1016/j.neuroscience.2020.08.002. [DOI] [PubMed] [Google Scholar]
- 84.Xu Z.X., Zhang J., Wang D., et al. Altered brain function in drug-naive major depressive disorder patients with early-life maltreatment: a resting-state fMRI study. Front Psychiatry. 2019;10:255. doi: 10.3389/fpsyt.2019.00255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Yang S., Cheng Y., Mo Y., et al. Childhood maltreatment is associated with gray matter volume abnormalities in patients with first-episode depression. Psychiatry Res Neuroimaging. 2017;268:27–34. doi: 10.1016/j.pscychresns.2017.07.005. [DOI] [PubMed] [Google Scholar]
- 86.Yu M., Linn K.A., Shinohara R.T., et al. Childhood trauma history is linked to abnormal brain connectivity in major depression. PNAS. 2019;116:8582–8590. doi: 10.1073/pnas.1900801116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Yuan M.L., Rubin-Falcone H., Lin X.J., et al. Smaller left hippocampal subfield CA1 volume is associated with reported childhood physical and/or sexual abuse in major depression: a pilot study. J Affect Disord. 2020;272:348–354. doi: 10.1016/j.jad.2020.03.169. [DOI] [PubMed] [Google Scholar]
- 88.Noll-Hussong M., Otti A., Laeer L., et al. Aftermath of sexual abuse history on adult patients suffering from chronic functional pain syndromes: an fMRI pilot study. J Psychosom Res. 2010;68:483–487. doi: 10.1016/j.jpsychores.2010.01.020. [DOI] [PubMed] [Google Scholar]
- 89.Ringel Y., Drossman D.A., Leserman J.L., et al. Effect of abuse history on pain reports and brain responses to aversive visceral stimulation: an fMRI study. Gastroenterology. 2008;134:396–404. doi: 10.1053/j.gastro.2007.11.011. [DOI] [PubMed] [Google Scholar]
- 90.Meyer E., Morawa E., Nacak Y., et al. Insular cortical thickness in patients with somatoform pain disorder: are there associations with symptom severity and childhood trauma? Front Psychiatry. 2020;11 doi: 10.3389/fpsyt.2020.497100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Gupta A., Labus J., Kilpatrick L.A., et al. Interactions of early adversity with stress-related gene polymorphisms impact regional brain structure in females. Brain Struct Funct. 2016;221:1667–1679. doi: 10.1007/s00429-015-0996-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Gupta A., Kilpatrick L.A., Braun A., et al. Early adverse life events: influence on resting state connectivity in somatosensory, cognitive and pain regions in male and female patients with irritable bowel syndrome. Gastroenterology. 2013;144 S-150–S-151. [Google Scholar]
- 93.Gupta A., Bhatt R.R., Naliboff B.D., et al. Impact of early adverse life events and sex on functional brain networks in patients with urological chronic pelvic pain syndrome (UCPPS): a MAPP research network study. PLoS One. 2019;14 doi: 10.1371/journal.pone.0217610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.McGowan P.O., Sasaki A., D’alessio A.C., et al. Epigenetic regulation of the glucocorticoid receptor in human brain associates with childhood abuse. Nat Neurosci. 2009;12:342–348. doi: 10.1038/nn.2270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Gonzalez A. The impact of childhood maltreatment on biological systems: implications for clinical interventions. Paediatr Child Health. 2013;18:415–418. [PMC free article] [PubMed] [Google Scholar]
- 96.Lang J., McKie J., Smith H., et al. Adverse childhood experiences, epigenetics and telomere length variation in childhood and beyond: a systematic review of the literature. Eur Child Adolesc Psychiatry. 2020;29:1329–1338. doi: 10.1007/s00787-019-01329-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Nelson C.A., Bhutta Z.A., Harris N.B., et al. Adversity in childhood is linked to mental and physical health throughout life. BMJ. 2020;371 doi: 10.1136/bmj.m3048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Denk F., McMahon S.B. Chronic pain: emerging evidence for the involvement of epigenetics. Neuron. 2012;73:435–444. doi: 10.1016/j.neuron.2012.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Provençal N., Arloth J., Cattaneo A., et al. Glucocorticoid exposure during hippocampal neurogenesis primes future stress response by inducing changes in DNA methylation. PNAS. 2020;117:23280–23285. doi: 10.1073/pnas.1820842116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Relton C.L., Davey Smith G. Epigenetic epidemiology of common complex disease: prospects for prediction, prevention, and treatment. PLoS Med. 2010;7 doi: 10.1371/journal.pmed.1000356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.McLaughlin K.A., Sheridan M.A. Beyond cumulative risk: a dimensional approach to childhood adversity. Curr Dir Psychol Sci. 2016;25:239–245. doi: 10.1177/0963721416655883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Denk F., McMahon S.B., Tracey I. Pain vulnerability: a neurobiological perspective. Nat Neurosci. 2014;17:192–200. doi: 10.1038/nn.3628. [DOI] [PubMed] [Google Scholar]
- 103.Flynn F.G. Anatomy of the insula functional and clinical correlates. Aphasiol. 1999;13:55–78. [Google Scholar]
- 104.Johnston B.A., Tolomeo S., Gradin V., et al. Failure of hippocampal deactivation during loss events in treatment-resistant depression. Brain. 2015;138:2766–2776. doi: 10.1093/brain/awv177. [DOI] [PubMed] [Google Scholar]
- 105.Rupprechter S., Romaniuk L., Series P., et al. Blunted medial prefrontal corticolimbic reward-related effective connectivity and depression. Brain. 2020;143:1946–1956. doi: 10.1093/brain/awaa106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Teicher M.H., Samson J.A. Annual research review: enduring neurobiological effects of childhood abuse and neglect. J Child Psychol Psychiatry. 2016;57:241–266. doi: 10.1111/jcpp.12507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Kraaijenvanger E.J., Pollok T.M., Monninger M., et al. Impact of early life adversities on human brain functioning: a coordinate-based meta-analysis. Neurosci Biobehav Rev. 2020;113:62–76. doi: 10.1016/j.neubiorev.2020.03.008. [DOI] [PubMed] [Google Scholar]
- 108.Garcia-Larrea L., Peyron R. Pain matrices and neuropathic pain matrices: a review. Pain. 2013;154:S29–S43. doi: 10.1016/j.pain.2013.09.001. [DOI] [PubMed] [Google Scholar]
- 109.Shackman A.J., Salomons T.V., Slagter H.A., et al. The integration of negative affect, pain and cognitive control in the cingulate cortex. Nat Rev Neurosci. 2011;12:154–167. doi: 10.1038/nrn2994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Paulus M.P., Stein M.B. An insular view of anxiety. Biol Psychiatry. 2006;60:383–387. doi: 10.1016/j.biopsych.2006.03.042. [DOI] [PubMed] [Google Scholar]
- 111.Berthier M., Starkstein S., Leiguarda R. Asymbolia for pain: a sensory-limbic disconnection syndrome. Ann Neurol. 1988;24:41–49. doi: 10.1002/ana.410240109. [DOI] [PubMed] [Google Scholar]
- 112.Deng Z., Pan Y., Li D., et al. Effect of bilateral anterior cingulotomy on chronic neuropathic pain with severe depression. World Neurosurg. 2019;121:196–200. doi: 10.1016/j.wneu.2018.10.008. [DOI] [PubMed] [Google Scholar]
- 113.Brooks J., Tracey I. The insula: a multidimensional integration site for pain. Pain. 2007;128:1–2. doi: 10.1016/j.pain.2006.12.025. [DOI] [PubMed] [Google Scholar]
- 114.Uddin L.Q., Nomi J.S., Hébert-Seropian B., et al. Structure and function of the human insula. J Clin Neurophysiol. 2017;34:300–306. doi: 10.1097/WNP.0000000000000377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Uddin L.Q. Salience processing and insular cortical function and dysfunction. Nat Rev Neurosci. 2015;16:55–61. doi: 10.1038/nrn3857. [DOI] [PubMed] [Google Scholar]
- 116.McLaughlin K.A., Weissman D., Bitrán D. Childhood adversity and neural development: a systematic review. Annu Rev Psychol. 2019;1:277–312. doi: 10.1146/annurev-devpsych-121318-084950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Cisler J.M., Esbensen K., Sellnow K., et al. Differential roles of the salience network during prediction error encoding and facial emotion processing among female adolescent assault victims. Biol Psychiatry. 2019;4:371–380. doi: 10.1016/j.bpsc.2018.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Ganzel B.L., Kim P., Gilmore H., et al. Stress and the healthy adolescent brain: evidence for the neural embedding of life events. Dev Psychopathol. 2013;25:879–889. doi: 10.1017/S0954579413000242. [DOI] [PubMed] [Google Scholar]
- 119.Marusak H.A., Etkin A., Thomason M.E. Disrupted insula-based neural circuit organization and conflict interference in trauma-exposed youth. NeuroImage Clin. 2015;8:516–525. doi: 10.1016/j.nicl.2015.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.McCrory E., De Brito S.A., Viding E. The impact of childhood maltreatment: a review of neurobiological and genetic factors. Front Psychiatry. 2011;2:48. doi: 10.3389/fpsyt.2011.00048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.McLaughlin K.A., Sheridan M.A., Tibu F., et al. Causal effects of the early caregiving environment on development of stress response systems in children. PNAS. 2015;112:5637–5642. doi: 10.1073/pnas.1423363112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Nielsen F.Å., Balslev D., Hansen L.K. Mining the posterior cingulate: segregation between memory and pain components. Neuroimage. 2005;27:520–532. doi: 10.1016/j.neuroimage.2005.04.034. [DOI] [PubMed] [Google Scholar]
- 123.Maddock R.J., Garrett A.S., Buonocore M.H. Remembering familiar people: the posterior cingulate cortex and autobiographical memory retrieval. Neuroscience. 2001;104:667–676. doi: 10.1016/s0306-4522(01)00108-7. [DOI] [PubMed] [Google Scholar]
- 124.Maddock R.J., Garrett A.S., Buonocore M.H. Posterior cingulate cortex activation by emotional words: fMRI evidence from a valence decision task. Hum Brain Mapp. 2003;18:30–41. doi: 10.1002/hbm.10075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Maddock R.J. The retrosplenial cortex and emotion: new insights from functional neuroimaging of the human brain. Trends Neurosi. 1999;22:310–316. doi: 10.1016/s0166-2236(98)01374-5. [DOI] [PubMed] [Google Scholar]
- 126.Peyron R., Laurent B., Garcia-Larrea L. Functional imaging of brain responses to pain. a review and meta-analysis (2000) Neurophysiol Clin. 2000;30:263–288. doi: 10.1016/s0987-7053(00)00227-6. [DOI] [PubMed] [Google Scholar]
- 127.Eldridge L.L., Knowlton B.J., Furmanski C.S., et al. Remembering episodes: a selective role for the hippocampus during retrieval. Nat Neurosci. 2000;3:1149–1152. doi: 10.1038/80671. [DOI] [PubMed] [Google Scholar]
- 128.Davachi L. Item, context and relational episodic encoding in humans. Curr Opin Neurobiol. 2006;16:693–700. doi: 10.1016/j.conb.2006.10.012. [DOI] [PubMed] [Google Scholar]
- 129.Murthy S., Gould E. Early life stress in rodents: animal models of illness or resilience? Front Behav Neurosci. 2018;12:157. doi: 10.3389/fnbeh.2018.00157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.McCrae C.S., O’Shea A.M., Boissoneault J., et al. Fibromyalgia patients have reduced hippocampal volume compared with healthy controls. J Pain Res. 2015;8:47. doi: 10.2147/JPR.S71959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Grilli M. Chronic pain and adult hippocampal neurogenesis: translational implications from preclinical studies. J Pain Res. 2017;10:2281. doi: 10.2147/JPR.S146399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Mokhtari T., Tu Y., Hu L. Involvement of the hippocampus in chronic pain and depression. Brain Sci Adv. 2019;5:288–298. [Google Scholar]
- 133.Mutso A.A., Radzicki D., Baliki M.N., et al. Abnormalities in hippocampal functioning with persistent pain. J Neurosci. 2012;32:5747–5756. doi: 10.1523/JNEUROSCI.0587-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Leknes S., Tracey I. A common neurobiology for pain and pleasure. Nat Rev Neurosci. 2008;9:314–320. doi: 10.1038/nrn2333. [DOI] [PubMed] [Google Scholar]
- 135.Schultz W. Reward functions of the basal ganglia. J Neural Transm. 2016;123:679–693. doi: 10.1007/s00702-016-1510-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Lafer B., Renshaw P.F., Sachs G.S. Major depression and the basal ganglia. Psychiatr Clin N Am. 1997;20:885–896. doi: 10.1016/s0193-953x(05)70350-6. [DOI] [PubMed] [Google Scholar]
- 137.Sachs-Ericsson N.J., Hajcak G., Sheffler J.L., et al. Putamen volume differences among older adults: depression status, melancholia, and age. J Geriatr Psychiatry Neurol. 2018;31:39–49. doi: 10.1177/0891988717747049. [DOI] [PubMed] [Google Scholar]
- 138.Novick A.M., Levandowski M.L., Laumann L.E., et al. The effects of early life stress on reward processing. J Psychiatr Res. 2018;101:80–103. doi: 10.1016/j.jpsychires.2018.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Dillon D.G., Holmes A.J., Birk J.L., et al. Childhood adversity is associated with left basal ganglia dysfunction during reward anticipation in adulthood. Biol Psychiatry. 2009;66:206–213. doi: 10.1016/j.biopsych.2009.02.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Willner P. Chronic mild stress (CMS) revisited: consistency and behavioural neurobiological concordance in the effects of CMS. Neuropsychobiology. 2005;52:90–110. doi: 10.1159/000087097. [DOI] [PubMed] [Google Scholar]
- 141.Higgins J., Green S. [updated march 2011]. The Cochrane Collaboration; 2011. Cochrane Handbook for systematic reviews of interventions.www.cochrane-handbook.org Available from: [Google Scholar]
- 142.Zhang W.-N., Chang S.-H., Guo L.-Y., et al. The neural correlates of reward-related processing in major depressive disorder: a meta-analysis of functional magnetic resonance imaging studies. J Affect Disord. 2013;151:531–539. doi: 10.1016/j.jad.2013.06.039. [DOI] [PubMed] [Google Scholar]
- 143.Acar F., Seurinck R., Eickhoff S.B., Moerkerke B. Assessing robustness against potential publication bias in activation likelihood estimation (ALE) meta-analyses for fMR. PLoS One. 2018;13 doi: 10.1371/journal.pone.0208177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Costa C., Cristea I.A., Dal Bò E., et al. Brain activity during facial processing in autism spectrum disorder: an activation likelihood estimation (ALE) meta-analysis of neuroimaging studies. J Child Psychol Psychiatry. 2021;62:1412–1424. doi: 10.1111/jcpp.13412. [DOI] [PubMed] [Google Scholar]
- 145.Raphael K. Recall bias: a proposal for assessment and control. Int J Epidemiol. 1987;16:167–170. doi: 10.1093/ije/16.2.167. [DOI] [PubMed] [Google Scholar]
- 146.Habota T., Sandu A.-L., Waiter G.D., et al. Cohort profile for the STratifying Resilience and Depression Longitudinally (STRADL) study: a depression-focused investigation of Generation Scotland, using detailed clinical, cognitive, and neuroimaging assessments. Wellcome Open Res. 2021;4:185. doi: 10.12688/wellcomeopenres.15538.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Libby G., Smith A., McEwan N.F., et al. The walker project: a longitudinal study of 48 000 children born 1952–1966 (aged 36–50 years in 2002) and their families. Paediatr Perinat Epidemiol. 2004;18:302–312. doi: 10.1111/j.1365-3016.2004.00575.x. [DOI] [PubMed] [Google Scholar]
- 148.Smith B.H., Campbell A., Linksted P., et al. Cohort profile: generation Scotland: scottish Family Health Study (GS:SFHS). the study, its participants and their potential for genetic research on health and illness. Int J Epidemiol. 2013;42:689–700. doi: 10.1093/ije/dys084. [DOI] [PubMed] [Google Scholar]
- 149.Fraser A., Macdonald-Wallis C., Tilling K., et al. Cohort profile: the avon longitudinal study of parents and children: ALSPAC mothers cohort. Int J Epidemiol. 2013;42:97–110. doi: 10.1093/ije/dys066. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.