Abstract
Preserved physical function is key for successful liver transplantation (LT); however, prehabilitation strategies are underdeveloped. We created a smartphone application (app), EL-FIT (Exercise and Liver FITness), to facilitate exercise training in end-stage liver disease (ESLD). In this feasibility study, we tested EL-FIT app usage and the accuracy of physical activity data transfer and obtained feedback from initial users. A total of 28 participants used the EL-FIT app and wore a physical activity tracker for 38 ± 12 days (age, 60 ± 8 years; 57% males; Model for End-Stage Liver Disease–sodium, 19 ± 5). There was fidelity in data transfer from the tracker to the EL-FIT app. Participants were sedentary (1957 [interquartile range, 873–4643] steps/day) at baseline. Level of training assigned by the EL-FIT app agreed with that from a physical therapist in 89% of cases. Participants interacted with all app features (videos, perceived exertion, and gamification/motivational features). We rearranged training data to generate heart rate–validated steps as a marker of performance and showed that 35% of the participants had significant increases in their physical performance. Participants emphasized their interest in having choices to better engage in exercise, and they appreciated the sense of community the EL-FIT app generated. We showed that patients with ESLD are able to use and interact with the EL-FIT app. This novel smartphone app has the potential of becoming an invaluable tool for home-based prehabilitation in LT candidates.
Every year in the United States, 20% to 25% of liver transplantation (LT) candidates are removed from the waiting list because of death or clinical deterioration (ie, become “too sick to transplant”) and miss their life-saving procedure.(1,2) Physical decline leading to frailty has been increasingly recognized as a key driving factor for waitlist removal along with liver failure.(3,4) Frailty is present in 18% to 25% of patients with end-stage liver disease (ESLD), and it is associated with nearly twice the mortality of reference populations as well as increased hospital admission, waitlist mortality, post-transplant complications, and health care costs.(3,5–7) Frailty is also a progressive condition for patients on the waiting list as its prevalence doubles from the time of listing to the time of LT.(8,9)
Multiple clinical trials have shown that exercise interventions are feasible, safe, and effective in reversing the metrics of frailty among patients with ESLD and LT candidates.(10–12) An expert consensus endorsed by the American Society of Transplantation recommended exercise to combat frailty, proposing prehabilitation programs as a strategy to reduce waitlist removal and improve LT outcomes.(13) The concern of transplant providers is, however, the lack of availability of prehabilitation programs specific for LT candidates with or without frailty. This is attributed to the limited number of exercise professionals who are experienced with ESLD, unclear training levels and endpoints for this population, the lack of reimbursement (except as a part of hospital disposition once disability has ensued), and the burden imposed on patients and their caregivers (ie, transportation, training fees). As such, prehabilitation for LT candidates is still seldomly prescribed by LT providers.
EL-FIT, Exercise and Liver FITness, is a smartphone application (app) developed for patients with cirrhosis to physically train at home while paired to a personal activity tracker. It was developed by LT providers while considering the physical and cognitive limitations of ESLD. The EL-FIT app includes a stratification algorithm facilitating exercise prescriptions at proper training levels with educational and exercise/workout videos of corresponding intensity, and it can monitor daily steps, heart rate, and sleep time and displays the collected data in a user-friendly format. The EL-FIT app also includes motivational and gamification features to incentivize patients to become physically active, such as a leaderboard specific to each training level, earned badges for training accomplishments, and the possibility of participants congratulating each other via emojis. Finally, an exercise professional can send encouraging push notifications to participants and review achievements on a Web-based dashboard where detailed training databases can be downloaded. By facilitating remote monitoring and physical training, the EL-FIT app can become an invaluable tool to generalize home-based prehabilitation for LT candidates.
The primary aim of this study was to determine the feasibility of ESLD LT candidates using the EL-FIT app and interacting with its features. As secondary aims, we investigated the adequacy of the EL-FIT app training-level prescriptions and data transfer fidelity from the tracker to the EL-FIT app databases. Finally, we performed focus group exit interviews to better understand participant and caregiver perspectives and technology acceptability (qualitative feedback).
Patients and Methods
Patients with cirrhosis and ESLD, aged 40 to 70 years, Model for End-Stage Liver Disease (MELD) ≥13, and listed or undergoing evaluation for LT at the Thomas E. Starzl Transplantation Institute were invited to participate following a baseline evaluation by our LT physical therapist (P.M.B.) between July and December 2019. Having a smartphone or a tablet—either the patient or caregiver—was a requisite for participation, and we excluded those without home wireless internet or an unlimited data plan, recurrent or persistent overt hepatic encephalopathy (HE), or unavailability for a follow-up visit 4 to 8 weeks after enrollment. The session with our LT physical therapist allowed us to (1) have objective evidence on each participant’s physical performance; (2) know the EL-FIT app level of training each participant could be assigned to; and (3) systematically educate participants on generic physical activity recommendations (our current standard of care). The Liver Frailty Index (LFI) and 6-minute walk test (6MWT) were obtained during the physical therapy baseline session to investigate frailty, which was defined as values ≥4.5 and ≤250 m, respectively.(3,7) After signing the informed consent, the participants were given a Fitbit 3 and had EL-FIT and Fitbit apps downloaded to their smartphone/tablet. This was followed by a brief introduction on the components and functionality of the EL-FIT app and Fitbit by a research coordinator. Afterward, the participants and caregivers were asked to explore all features of the EL-FIT app up until their follow-up visit. A medical physical exam and EncephalApp Stroop test were performed at baseline to investigate overt and minimal HE. Clinical data were obtained by a review of records within the University of Pittsburgh Medical Center’s secure electronic record domain under the provisions of the University of Pittsburgh Institutional Review Board.
EL-FIT FIELD TESTING
Following completion of the beta version, the EL-FIT app was field-tested in 10 healthy volunteers to ensure proper functioning of all of its features, video display, and database construction. During this stage, we could confirm the correct functioning of all app features and a perfect matching of the collected step counts between the EL-FIT and Fitbit apps.
TECHNOLOGY DESCRIPTION
The EL-FIT app was developed for both iOS and Android smartphone/tablet platforms. Its features include an exercise prescription tool with 3 training levels and 5 simplified screens (Fig. 1).
FIG. 1.
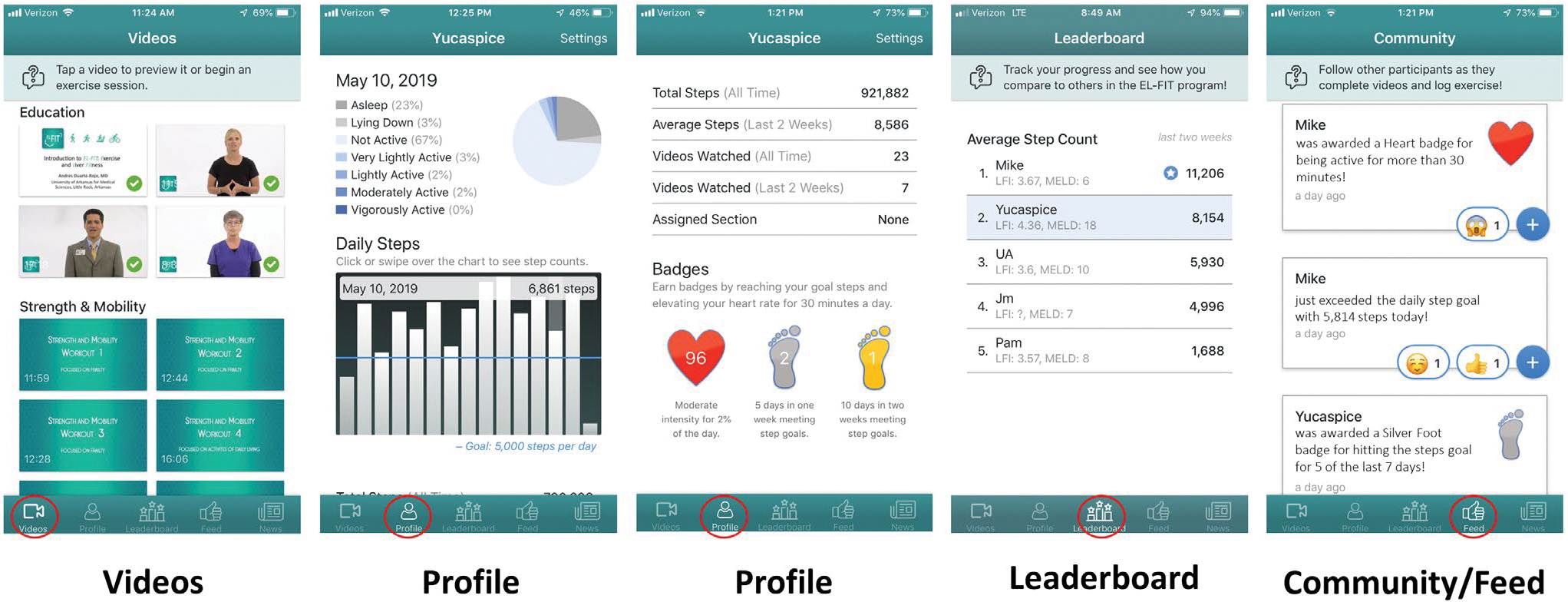
EL-FIT screenshots showing the main features of the app. Videos shows the Education and Strength & Mobility videos for patients who are frail. Profile displays the percentage of their day performing very light to vigorous activities and sleep time along with a bar chart with the total number of steps per day, statistics on the number of videos watched, and the number of badges received. Leaderboard demonstrates performance in average daily steps for 5 volunteers included in the Strength & Mobility training level; below each nickname, the corresponding LFI and MELD can be observed for each participant. Community/Feed is an automatic feed of achievements from each participant that allows EL-FIT members to congratulate and cheer for each other via emojis.
Training-Level Stratification Algorithm
During the sign-up process, the operator enters critical data pertaining to physical/liver function and exercise safety, which runs a stratification algorithm, assigns a training level, and unlocks corresponding exercise videos and a daily step goal (Fig. 2). The algorithm uses standard clinical data available to any clinician, and it does not include the LFI or 6MWT.
FIG. 2.
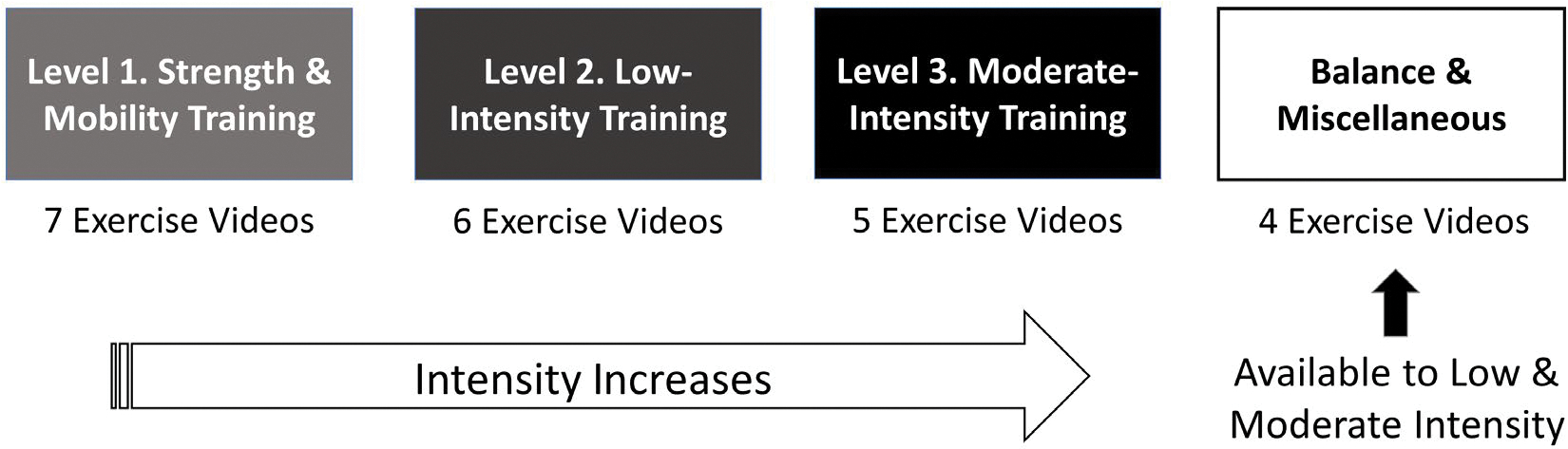
Exercise training levels allocated by stratification algorithm. The EL-FIT app considers the following 3 levels of training: (1) Strength & Mobility Training, which starts with exercises in a chair for patients who are frail and builds up physical challenges until patients can do basic standing exercises; (2) Low-Intensity Training, with various workouts that assume patients have gained full mobility; and (3) Moderate-Intensity Training, which includes more complex workouts for the physically robust with no limitations. The stratification algorithm considers liver and physical function data to allocate patients to 1 of these 3 training levels. Patients can graduate from 1 level and move on to the next level. A fourth section, Balance & Miscellaneous, includes workouts aiming to prevent falls, help with back pain, and a basic Tai Chi form favoring coordination. This fourth section is only made available to patients allocated to Low-Intensity Training or Moderate-Intensity Training.
Videos Screen
This screen allows subjects to play educational videos and train along the exercise videos. Examples of exercises included in the videos are shown in the Supporting Information. Exercise videos are presented in such a sequence that they are increasingly physically challenging and thus favoring fitness buildup, both within and between training-level sections. At the end of each exercise video, participants are queried to record the intensity of their workout using an emoji version of the Borg’s rate of perceived exertion (Supporting Fig. 1). The date and time each video is watched and the rate of perceived exertion is recorded in a database to monitor adherence.
Profile Screen
In this section, the participants can see their daily achievements in terms of walking, watched/executed videos, and badges. It also displays the percentage of their day that they are active or asleep, showing the intensity of their activities according to cadence, where sedentary is 0 to 19 steps/minute, very light is 20 to 39 steps/minute; light is 40 to 79 steps/minute, moderate is 80 to 130 steps/minute, and vigorous is >130 steps/minute.(14) The EL-FIT app gives 3 types of badges based on step goal accomplishments: a heart badge for those reaching a moderate intensity of >28 minutes/day; a silver foot for those reaching their step goals 5 days/week; and a gold foot, which is the equivalent of 2 consecutive silver foot badges.
Leaderboard Screen
Here participants can compare their achievements in average daily steps with that of other participants on the same training level while comparing their MELD and LFI.
Community/Feed Screen
The training accomplishments of the exercising community are automatically posted on the Community/Feed screen (eg, surpassing a step goal), and there is an option for EL-FIT members to congratulate each other via emojis. EL-FIT members are identified by a given nickname to protect privacy.
News Screen
All communications from a provider’s Web-based dashboard to EL-FIT members are posted on the News screen and can be directed either to all members or personalized to a single participant.
FOCUS GROUP
An exit interview in groups of up to 4 participants and their corresponding caregivers was set up for the follow-up visit. Participants were asked open-ended questions for them to express what benefits the novel technology brought to them in terms of physical activity and adherence to exercise recommendations and whether there were any novel or improved features they would like to see in the EL-FIT based on the barriers they experienced while using the app. These interviews were facilitated by an exercise physiologist and behaviorist (R.J.R.) who was unaware of each participant’s physical performance and extent of interaction with the EL-FIT app. Reassessments of the LFI and 6MWT were not pursued during this research visit as a change in frailty metrics was not part of our study aims.
DATA MANAGEMENT AND STATISTICS
All clinical information entered into the EL-FIT app by an investigator and the automatically generated usability or training information were stored in a cloud and downloaded following completion of the study for analysis. We used descriptive statistics (mean ± standard deviation or median [interquartile range], unless specified) after assessing the distribution of data with the Shapiro-Wilk test as well as the chi-square, analysis of variance, Wilcoxon, and Mann-Whitney U tests as needed. Appropriateness of the EL-FIT app exercise prescription (ie, training-level stratification algorithm) was investigated by determining the proportion of participants whose prescription matched that of the LT physical therapist (reference) using the McNemar test of symmetry. The LT physical therapist, who is acquainted with the EL-FIT app training levels and exercise videos, independently provided an exercise prescription, and to maintain concealment she was never made aware of the results from the EL-FIT stratification algorithm. Intraday steps, heart rate, and sleep data were obtained following the Fitbit application programming interface terms of service. Once downloaded from the EL-FIT dashboard, intraday data were transferred to Stata version 12 (StataCorp, College Station, TX) for analysis.
Results
A total of 28 participants consented and 25 completed the study after a mean of 38 ± 12 days. Of the participants, 2 did not stay in the clinic long enough to complete setting up the smartphone apps after the consent was signed (ie, no data recorded), and 1 participant contributed only 5 days of data before being called for LT. Table 1 shows the baseline characteristics of the participants. More than half of the participants were males and obese, with a predominance of nonalcoholic steatohepatitis/cryptogenic, and less than half used beta-blockers or reported prior falls. Although no participant had overt HE by the time of enrollment, almost all had prior HE or minimal HE, and most of them had prior varices (esophageal or gastric) and ascites needing frequent paracentesis; decompensated cirrhosis (Child-Turcotte-Pugh B/C class) accounted for two thirds of the cohort. Frailty was present in 15% of patients according to the LFI and in 27% according to the 6MWT. Using the LT physical therapist prescription as reference, the EL-FIT app correctly prescribed the level of training in 89% of cases with no significant difference between the 2 strategies (P = 0.22). Specifically, the physical therapist and EL-FIT app stratification algorithm prescribed the Strength & Mobility training level in 24 and 26 participants, respectively. The 4 participants allocated to Moderate-Intensity Training by the physical therapist had 1 concordant prescription, 2 to Strength & Mobility and 1 to Low-Intensity Training by the EL-FIT app. Reported history of falls and the degree of liver dysfunction is what drove the EL-FIT app stratification algorithm to prescribe lower levels of training. Training-level prescription was not associated with the presence of frailty (by LFI and 6MWT) for either the EL-FIT app or LT physical therapist (data not shown); however, there was concordance in the Strength & Mobility prescription being provided to all frail patients by either method. Daily step count at baseline (first week of assessment) was 1957 (873–4643) steps/day with 19 (76%) participants walking less than 5000 steps/day (ie, sedentary) and 14 (56%) walking less than 2500 steps/day (ie, basal activity).
TABLE 1.
Baseline Characteristics of Included Participants (n = 28)
| Variable | Descriptive Statistic |
|---|---|
|
| |
| Age, years | 60 ± 8 |
| Sex, male | 16 (57) |
| Body mass index, kg/m2 | 32 ± 6 |
| Obese | 17 (61) |
| Etiology | |
| NASH/cryptogenic | 16 (57) |
| Alcohol | 8 (28) |
| Viral | 2 (7) |
| Autoimmune | 1 (4) |
| Other | 1 (4) |
| Listed for LT | 20 (71) |
| β-blocker | 12 (44) |
| Falls | 9 (32) |
| Education, years | 13 ± 2 |
| Encephalapp | |
| Stroop off + on, seconds | 207 (185–247) |
| Minimal HE | 27 (96) |
| MO-Log | 24 (23–24) |
| Prior HE | 25 (89) |
| Varices/prior bleeding | 24 (85) / 3 (11) |
| Ascites or hydrothorax | 27 (96) |
| Refractory ascites | 25 (89) |
| Child-Turcotte-Pugh | 8 ± 2 |
| Class B/C | 19 (68) |
| MELD-Na | 19 ± 5 |
| Type 2 diabetes mellitus | 13 (48) |
| LFI | 3.8 ± 0.7 |
| Frail | 4 (15) |
| 6MWT, meters | 306 ± 102 |
| Frail | 8 (27) |
NOTE: Data are presented as mean ± standard deviation, number (percentage), or median (interquartile range).
Regarding the EL-FIT app usability, the participants showed ample interaction with all of the EL-FIT app features although with wide variability. As shown in Table 2, 77% of participants watched at least 1 of the videos, including exercising videos, and most of them completed at least 1 video section (each with 4 to 7 videos) while reporting on the intensity of the activity they had executed. The presence of frailty by the 6MWT did not influence the proportion of patients watching videos (83% versus 79% in nonfrail patients; P = 0.81) or the number of videos watched per patient (8 [5–11] versus 12 [3–45] in nonfrail patients; P = 0.48), although a trend was observed for patients defined by the LFI as frail watching less videos (33% versus 83% [P = 0.057] and 0 [0–9] versus 10 [5–37] [P = 0.11], respectively). A subanalysis restricted to exercise training videos showed similar results (data not shown). Interestingly, the EL-FIT app recorded more participants self-reporting their rate of perceived exertion than training videos watched (at least 3 participants more), which suggests that some were scrolling through videos without exercising. Regarding performance-based accomplishments, 85% of the participants exceeded their EL-FIT assigned daily step goal for an average of 12 days (representing 32% of monitored time), and between 35% and 58% gained badges for their training both in terms of walking cadence (heart badge) and walking steps (foot badges). Frailty by 6MWT was associated with participants reaching their step goals on fewer days and fewer badges earned (except for heart badges), whereas frailty by LFI did not affect any form of goal accomplishment (data not shown).
TABLE 2.
Usability of the EL-FIT App Features Among Patients With Advanced Liver Disease
| EL-FIT Goal Accomplishment | Participants, n = 26, n (%) | Repeat per Participant, Median (Range) |
|---|---|---|
|
| ||
| Videos | 20 (77) | 13 (3–109) |
| Educational videos | 18 (69) | 7 (3–14) |
| Training videos | 20 (77) | 9 (1–100) |
| Section completion | 19 (69) | 2 (1–5) |
| Educational videos | 17 (65) | 1 (1–1) |
| Training videos | 13 (50) | 1 (1–4) |
| RPE reporting | 23 (88) | 14 (1–70) |
| Daily walking goal accomplished | 22 (85) | 12 (2–73) |
| Heart badge | 11 (42) | 16 (2–71) |
| Silver foot badge | 15 (58) | 4 (1–12) |
| Gold foot badge | 9 (35) | 3 (1–6) |
The EL-FIT databases collected a total of 1.26 million minutes with a step count and 1.06 million minutes with a heart rate (corresponding to 84% of total). Agreement between the EL-FIT and Fitbit output was excellent in terms of minute-based step count (ρ = 1, P < 0.001) and heart rate (ρ = 0.99, P < 0.001), thus confirming the fidelity of data transmission. Baseline heart rate was 81 ± 10 bpm (range, 65–124), with no difference between participants using or not using a β-blocker (79 ± 9 versus 84 ± 11; P = 0.32). Aiming to improve the accuracy of step count reporting while adjusting for the time when a participant is not wearing the personal activity tracker, we then compared the raw output to a method defining a reliable reporting day as that with >1000 accumulated minutes (70% of total day duration), where each minute-based step count was accompanied by heart rate tracking (validated steps). We lost 1% of steps data (from 1.26 to 1.25 million minutes) when considering only days with >1000 accumulated minutes and 1% more of heart rate data (from 1.06 to 1.03 million minutes) when accounting only for the step counts accompanied by heart rate within the same minute. Table 3 shows the results for the average daily step count using raw data and validated steps. It can be seen that the validated steps method provides higher median values for the daily step count during the study period, which resulted from the systematic elimination of minutes with “0” steps as an output (95% of eliminated minute-based steps had a 0 [n = 220,136]), that is, when the tracker was not being worn by the participant. A similar analysis with cadence showed that there was a net gain of 6 minutes of activity per participant (Table 4), which corresponds to a relative gain of 12% given how sedentary participants behaved (approximately 96% of recorded time).
TABLE 3.
Comparison Between Raw and Heart Rate–Adjusted Step Data With > 1000 Accumulated Minutes (Validated Steps)
| EL-FIT Daily Steps | All | Week 1 | Week 2 | Week 3 | Week 4 | Week 5 | Week 6 | P Value |
|---|---|---|---|---|---|---|---|---|
|
| ||||||||
| Steps | 2087 (764–4567) | 1957 (873–4643) | 1891 (693–3990) | 2384 (940–4655) | 1660 (585–3750) | 1732 (610–4002) | 2343 (910–4696) | <0.001 |
| Validated steps | 2408 (964–5117) | 2417 (1023–5813) | 2043 (930–4328) | 2791 (1138–5555) | 2005 (656–3957) | 2143 (890–4039) | 2661 (1498–5538) | <0.001 |
| P | 0.003 | 0.19 | 0.28 | 0.17 | 0.28 | 0.15 | 0.32 | |
NOTE: Data are presented as median (interquartile range).
TABLE 4.
Intensity of Physical Activity According to Cadence
| Cadence Category (Steps per Minute), % | ||||||
|---|---|---|---|---|---|---|
|
|
||||||
| EL-FIT Daily Steps | Minutes Analyzed | 0–19 (Sedentary) | 20–39 (Very Light) | 40–79 (Light) | 80–130 (Moderate) | >130 (Vigorous) |
|
| ||||||
| All steps | 1,268,444 | 96.4 | 1.9 | 0.9 | 0.8 | 0 |
| Validated steps | 1,037,612 | 95.9 | 2.2 | 1 | 0.9 | 0 |
When we analyzed the change in daily average step by week, we found no specific pattern in the variability among participants (Table 3). Of note, for most participants (n = 9, 39%), the best physical performance occurred on week 1 of the study (at baseline). Defining change as an average increase or decrease in > 500 steps/day per week for 2 weeks of the 5 weeks analyzed past baseline, we found that physical activity decreased in 7 (30%) and remained unchanged in 8 (35%), whereas it increased in 8 (35%); in 3 participants, no more than 2 weeks of data were accumulated for analysis. The step counts per these groups can be found in Fig. 3. There were no differences in activity change when considering LT candidates versus non-candidates (increased activity in 31% and 43%, respectively; P = 0.51). Similarly, the presence of frailty by any of the 2 used tools was not associated with activity change (data not shown).
FIG. 3.
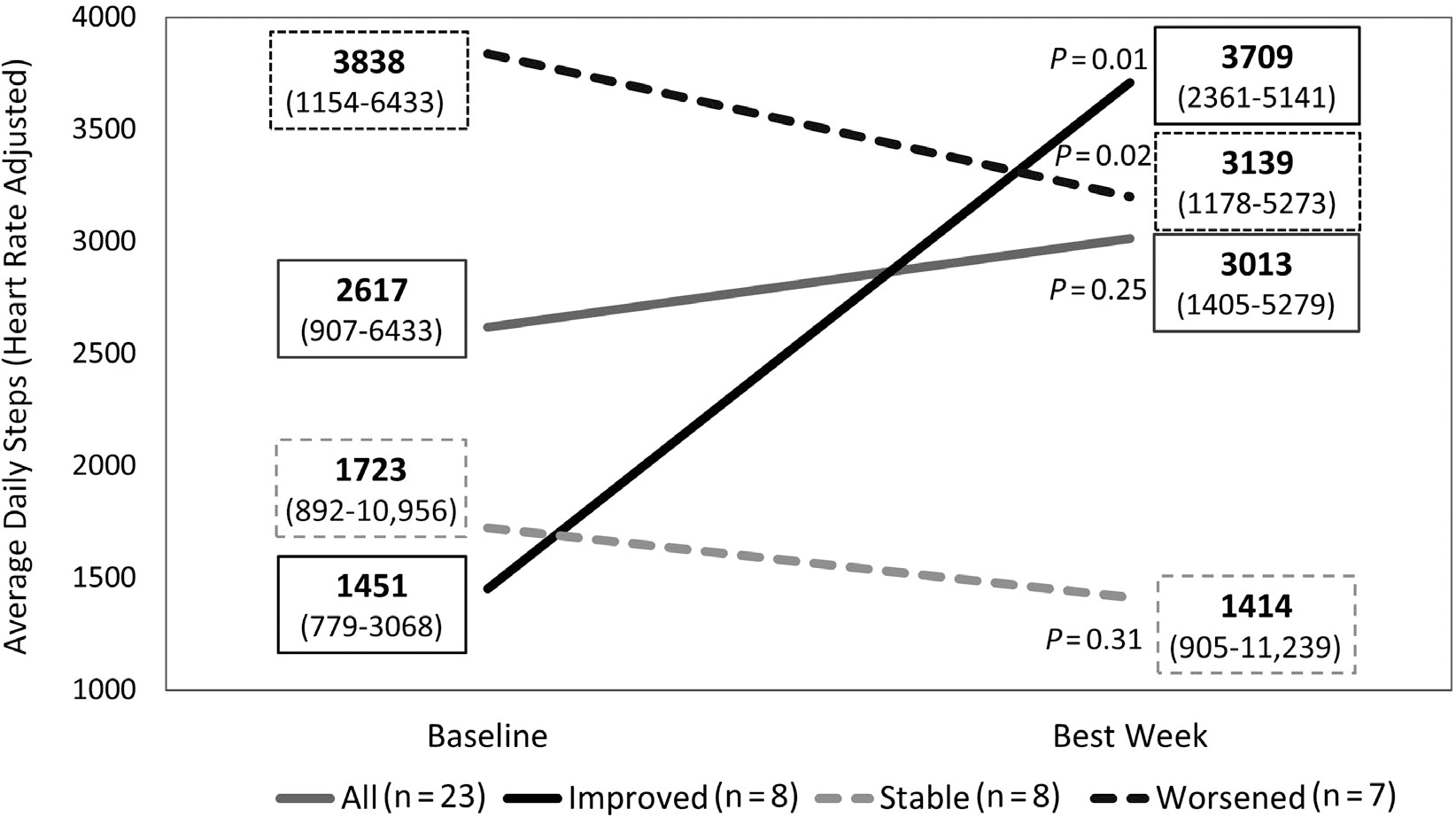
Patterns of daily step performance while using the EL-FIT app. Changes in daily step count from baseline (week 1) to the week of best performance for all patients and for groups of patients showing a pattern of improvement from baseline (increase in more than 500 steps/day per week for 2 of up to 5 follow-up weeks), deterioration (decrease in more than 500 steps/day per week for 2 of up to 5 follow-up weeks), or stability (none of the above). Numbers in boxes represent median and interquartile range.
Results from the focus group are shown in Table 5. Participants and their caregivers often expressed a preference for having greater choices among different types of exercise and having a platform composed of peers exercising under the same medical circumstances. Despite the short duration of the study, some participants reported benefits in lower body strength and improved daily function. We identified the potential refinements users wanted for the app, including improved technical support, enhanced social interaction functionality, and improved video availability and usability.
TABLE 5.
Results From Qualitative Part of Study
| Patients reported as positive |
| 1. Benefits of educational videos showing activities to increase physical function |
| 2. Ability to monitor steps, thus emphasizing the importance of physical mobility |
| 3. Ability to choose from walking and exercise videos, thus favoring engagement |
| 4. Gain in lower body strength and physical function, facilitating daily activities |
| 5. Having a platform promoting social support from others with similar medical circumstances |
| 6. Feedback, which was motivating and kept them focused |
| 7. Enhanced perception of care from the medical team |
| Areas for potential improvement |
| 1. Need for additional assistance using the technology |
| 2. Opportunities to enhance interaction, social support, and a sense of community |
| 3. More variety in the videos provided |
| 4. Guidance on what videos are more appropriate based on medical state (eg, swelling) |
| 5. Autonomy to select the appropriate exercise videos based on medical state |
Discussion
An acceptable level of physical function is crucial for patients with ESLD to become LT candidates and for candidates to successfully reach LT once added to the waiting list.(15) Despite the recognition of the deleterious effects of frailty in ESLD, we still lack a standardized treatment for this condition.(4) Exercise and increasing physical activity constitute, however, the foundation of interventions to improve frailty.(16) Significant barriers need to be overcome before exercise programs or prehabilitation are incorporated into hepatology practice and LT programs. Apart from difficulties in patients getting an appropriate exercise prescription (ie, lack of exercise professionals comfortable with treating ESLD), treatment endpoints and adherence monitoring strategies are yet to be defined, particularly for home-based prehabilitation. The EL-FIT app was created considering the challenges of home-based prehabilitation by experts in the field of exercise in cirrhosis while following a methodology to select workout routines that are both feasible and safe in ESLD. The EL-FIT app facilitates exercise prescriptions and demonstrates ESLD-specific workouts for patients to train, and when paired with a tracker, it records and processes daily steps, heart rate, and sleep time for patients and providers to monitor progress and accomplishments. As such, the EL-FIT app can aid with the identification of reachable training endpoints and the remote monitoring of physical status.
Our results attest to the feasibility of the EL-FIT app being used by patients who are severely decompensated, including those with prior overt and minimal HE. Although the prevalence of frailty in our cohort was lower than that reported in a multicenter LFI study,(17) it did not differ in terms of 6MWT,(7) and there were no consistent major differences in app interaction when comparing frail to nonfrail participants with the 2 methods. Participants (± their caregivers) were able to explore the features of the EL-FIT app, including educational videos, exercising with videos, reporting perceived exertion, accomplishing the walking goal set by the stratification algorithm, and earning badges for their accomplishments. The most frequent explanation we got from patients when asked why they did not click on the EL-FIT videos was that they were not asked to do so, which further speaks of memory issues in the setting of minimal HE, variability on technology acceptance, and the need to include EL-FIT app operation/goal refreshers following initial installation and training. Also, 3 participants were medically prohibited to continue interacting with EL-FIT past the first 1 to 2 weeks of the study. The stratification algorithm closely followed our LT physical therapist recommendations, except for 3 participants in whom prior falls or the degree of liver dysfunction made the app default to Strength & Mobility. This is an intended feature of the app to maximize safety (eg, Strength & Mobilty for potentially frail patients), which is key when considering that many centers might use the EL-FIT app without the support of an exercise professional. Nonetheless, whenever the app assigns a training level lower than appropriate for the participant, the latter can graduate from it and be moved to the next level. Badges are a gamification feature of the EL-FIT app that intends to motivate participants to exercise, and along with the daily walking goal, they are automatically displayed on the Feed screen, allowing participants to react to each other’s accomplishments, thus favoring socialization of exercise and a sense of community. The generation of a sense of community was actually one of the main features participants liked about the EL-FIT app, and this was also favored by the Leaderboard, where patients could compare their accomplishments to that of peers experiencing similar medical circumstances. The Leaderboard also displays the MELD and the LFI of each participant within each training category, thus favoring a fair competition. Notably, by tracking exercise videos and the rate of perceived exertion separately we were able to identify a subset of participants who did not watch the videos long enough to be credited, yet they reported some intensity of exercise. This evidence further supports the need for objective monitoring of patient’s training efforts over self-reporting, something our group has previously reported.(18) With the aid of a tracker, we hope to soon include algorithms for the identification of exercise bouts in the EL-FIT app based on objective training data, which would further expand reporting of adherence to exercise interventions as a key element in home-based prehabilitation.
Accurate reporting of step count from an activity tracker is crucial to detect response to treatment. Because the Fitbit 3 is waterproof and can be continuously worn (except for charging time), we were able to use these features to develop a new definition for a reliable step-tracking method that considers ≥70% (ie, >1000) minutes per day with a concomitantly recorded heart rate. Using this strict definition caused us to lose some data (16% of minute-based steps lacked concomitant heart rate and another 2% had <1000 minutes recorded), but it mainly eliminated from the database cells with a “0,” which are entered by default when the tracker is not being worn. Yet we retained >80% of total recorded time while showing higher step counts. This contrasts with existing literature defining reliable personal activity tracking at 10 hours per day.(19,20) As such, it is possible that with this new and more accurate definition we will be able to identify smaller step count differences following an exercise intervention. Cadence or the number of steps walked per minute, a recently proposed measure to track intensity of training, also improved when considering our validated steps method, with a relative increase in daily activity of 12%. This is of particular interest to patients with ESLD who function with very low energy expenditures and changes following an exercise intervention tend to be of low magnitude.(12,14,21)
When compared with the general population, our participants were sedentary and most performed only basal activities.(19–22) Such inactivity is not uncommon for patients with decompensated cirrhosis, HE, and refractory ascites.(12) We recently reported that a low level of functioning in steps/day can predict clinical admission to the hospital,(23) and thus a tracker could provide a novel method for monitoring patients on the waiting list at high risk for decompensation or death once their output is properly integrated into the electronic medical record.(24) When analyzed by week, many participants performed at their best on the first week of monitoring, what is likely a reflection of the “novelty effect,” a phenomenon in which participants briefly acquire interest in a new technology, device, or metric, making them react to such feedback (ie, their step counts).(25–27) This novelty effect likely aggravated the observed decline in daily steps for some participants, but despite it, we were still able to see an improvement of >2000 steps/day in approximately one third of them. This is remarkable given that no exercise intervention apart from the EL-FIT app and a tracker were provided to participants during the study period. We expect the integration of exercise coaches into the EL-FIT program to significantly increase exercise accomplishments in a larger proportion of participants, although the novelty effect and self-driven increase in daily steps will need to be considered in the planning and power calculation of future clinical trials.
Finally, our qualitative data yielded some valuable information on the benefits participants perceived from the EL-FIT app and the research team. Of particular interest was the perceived need of being able to choose an exercising strategy, the need for feedback (which was delivered exclusively via the EL-FIT app from peers or researchers), and the need for a sense of community promoting physical activity engagement. Our focus group also revealed some barriers that need to be overcome to make the EL-FIT app more user friendly and for it to become a generalized exercise tool in ESLD. We have created a Web site to generate awareness of the importance of physical function among patients with chronic liver diseases and to interact with patients and providers regarding EL-FIT availability and updates (www.el-fit.pitt.edu).
Some of the limitations of our study include selection bias, sample size, and the lack of a control group. Acceptance and usage of technology are still not universal, and the fact that study participants (or their caregivers) had to have a smartphone or a tablet inherently imposed a selection bias toward people with wider technology access and more favorable socioeconomic status. Although not a bias in the research setting, the need for a personal activity tracker would become one during clinical implementation. Currently, the EL-FIT app is compatible with any Fitbit and Apple Watch, but we have plans to expand the pool of compatible trackers. Future research efforts will need to account for such bias while investigating a technology platform allowing equitable access to the EL-FIT app. Given the aims of our study and based on our prior experience, we had estimated that 20 to 30 participants would suffice to report on ease of use and data transfer fidelity. Although a control group attending physical therapy only would have allowed us to better quantify the novelty effect and the impact of the EL-FIT app on physical activity, our design did not aim to answer such research questions; rather, upcoming studies are expected to investigate the efficacy of the EL-FIT app. The fact that all participants received a baseline exercise prescription and a follow-up phone call 1 month after by our LT physical therapist, per our standard of care, brings all of our participants to a leveled ground. It is likely that some degree of the Hawthorne effect is in play in the study, encouraging participants to become more physically active at least temporarily, although researchers did not promote physical activity (except for generic push notifications to all EL-FIT app users) or influenced the standard of care. Further studies are needed to investigate how frail patients specifically behave when exposed to the EL-FIT app and whether a training effect can be observed in such a “difficult-to-train” population.
In summary, our findings support patients with ESLD being able to use and interact with the EL-FIT app, including excellent agreement between the EL-FIT level of training prescription and that from our LT physical therapist. Knowledge gained from this study will help us improve the EL-FIT app usability in ESLD and the design of future clinical trials while overcoming some of the barriers to implementation and adherence. The smartphone app transferred tracker-derived training data with fidelity and rearranged it to make it more meaningful to patients and providers. Through a novel step count reporting method—validated steps—it can also facilitate the identification of training endpoints, detect potential changes to an intervention, and more accurately monitor patients remotely. As such, the EL-FIT app has the potential of becoming an invaluable tool for home-based prehabilitation in LT candidates.
Supplementary Material
Acknowledgments:
The authors thank Elliot B. Tapper, M.D., for his critical review of the educational videos and Nancy Howes, PT/MSc, and Deborah Josbeno, PT/Ph.D., for their critical review of all EL-FIT exercise videos. The authors also recognize the services rendered by exercise professionals Sheery Woods, Karen Johnson, Rodrigo Ruiz, and Amanda and Eric Martin as well as that of Kenneth Childres and Jean Romano from Creative Services at the University of Arkansas for Medical Sciences.
This study was funded by the American Association for the Study of Liver Diseases Innovation Fund and a Pilot and Feasibility Grant from the Pittsburgh Liver Research Center (National Institutes of Health/National Institute of Diabetes and Digestive and Kidney Diseases P30DK120531).
Abbreviations:
- 6MWT
6-minute walk test
- app
application
- EL-FIT
Exercise and Liver FITness
- ESLD
end-stage liver disease
- HE
hepatic encephalopathy
- LFI
Liver Frailty Index
- LT
liver transplantation
- MELD
Model for End-Stage Liver Disease
- MELD-Na
Model for End-Stage Liver Disease–sodium
- MO-log
modified orientation log
- NASH
nonalcoholic steatohepatitis
- RPE
rate of perceived exertion
Footnotes
Portions of this article were presented at the Digestive Disease Week Shark Tank Session 2019 in San Diego, CA, and at the American Transplant (Virtual) Congress 2020.
Renee J. Rogers consults for Naturally Slim. Amit D. Tevar advises for CSL Behring. Andrés Durante-Rojo advises for Axcella, Inc. John M. Jakicic advises for WW International, Inc., Spark360, and Naturally Slim.
REFERENCES
- 1).Kwong A, Kim WR, Lake JR, Smith JM, Schladt DP, Skeans MA, et al. OPTN/SRTR 2018 annual data report: liver. Am J Transplant 2020;20(suppl 1):193–299. [DOI] [PubMed] [Google Scholar]
- 2).Goldberg D, French B, Trotter J, Shetty K, Schiano T, Reddy KR, et al. Underreporting of liver transplant waitlist removals due to death or clinical deterioration: results at four major centers. Transplantation 2013;96:211–216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3).Lai JC, Covinsky KE, Dodge JL, Boscardin WJ, Segev DL, Roberts JP, et al. Development of a novel frailty index to predict mortality in patients with end-stage liver disease. Hepatology 2017;66:564–574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4).Duarte-Rojo A, Ruiz-Margain A, Montano-Loza AJ, Macias-Rodriguez RU, Ferrando A, Kim WR. Exercise and physical activity for patients with end-stage liver disease: Improving functional status and sarcopenia while on the transplant waiting list. Liver Transpl 2018;24:122–139. [DOI] [PubMed] [Google Scholar]
- 5).Dunn MA, Josbeno DA, Tevar AD, Rachakonda V, Ganesh SR, Schmotzer AR, et al. Frailty as tested by gait speed is an independent risk factor for cirrhosis complications that require hospitalization. Am J Gastroenterol 2016;111:1768–1775. [DOI] [PubMed] [Google Scholar]
- 6).Salim TI, Nestlerode LC, Lucatorto EL, Wasserman TL, Din HA, Landsittel DP, et al. Frailty as tested by gait speed is a risk factor for liver transplant respiratory complications. Am J Gastroenterol 2020;115:859–866. [DOI] [PubMed] [Google Scholar]
- 7).Yadav A, Chang YH, Carpenter S, Silva AC, Rakela J, Aqel BA, et al. Relationship between sarcopenia, six-minute walk distance and health-related quality of life in liver transplant candidates. Clin Transplant 2015;29:134–141. [DOI] [PubMed] [Google Scholar]
- 8).McCabe P, Wong RJ. More severe deficits in functional status associated with higher mortality among adults awaiting liver transplantation. Clin Transplant 2018;32:e13346. [DOI] [PubMed] [Google Scholar]
- 9).McCabe P, Gish RG, Cheung R, Wong RJ. More severe deficits in performance status at time of liver transplant is associated with significantly higher risk of death following liver transplantation. J Clin Gastroenterol 2019;53:e392–e399. [DOI] [PubMed] [Google Scholar]
- 10).Tapper EB, Martinez-Macias R, Duarte-Rojo A. Is exercise beneficial and safe in patients with cirrhosis and portal hypertension? Curr Hepatology Rep 2018;17:175–183. [Google Scholar]
- 11).Williams FR, Vallance A, Faulkner T, Towey J, Durman S, Kyte D, et al. Home-based exercise in patients awaiting liver transplantation: a feasibility study. Liver Transpl 2019;25:995–1006. [DOI] [PubMed] [Google Scholar]
- 12).Chen HW, Ferrando A, White MG, Dennis RA, Xie J, Pauly M, et al. Home-based physical activity and diet intervention to improve physical function in advanced liver disease: a randomized pilot trial. Dig Dis Sci 2020;65:3350–3359. [DOI] [PubMed] [Google Scholar]
- 13).Lai JC, Sonnenday CJ, Tapper EB, Duarte-Rojo A, Dunn MA, Bernal W, et al. Frailty in liver transplantation: an expert opinion statement from the American Society of Transplantation Liver and Intestinal Community of Practice. Am J Transplant 2019;19:1896–1906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14).Chen HW, Ferrando AA, Dunn MA, Kim WR, Duarte-Rojo A. Cadence from physical activity trackers for monitoring of home-based exercise intensity in advanced liver disease. Liver Transpl 2020;26:718–721. [DOI] [PubMed] [Google Scholar]
- 15).Lai JC, Dodge JL, Kappus MR, Dunn MA, Volk ML, Duarte-Rojo A, et al. Changes in frailty are associated with waitlist mortality in patients with cirrhosis: a multi-center cohort study. J Hepatol 2020;73:575–581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16).Tandon P, Ismond KP, Riess K, Duarte-Rojo A, Al-Judaibi B, Dunn MA, et al. Exercise in cirrhosis: translating evidence and experience to practice. J Hepatol 2018;69:1164–1177. [DOI] [PubMed] [Google Scholar]
- 17).Lai JC, Dodge JL, Kappus MR, Dunn MA, Volk ML, Duarte-Rojo A, et al. Changes in frailty are associated with waitlist mortality in patients with cirrhosis. J Hepatol 2020;73:575–581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18).Dunn MA, Josbeno DA, Schmotzer AR, Tevar AD, DiMartini AF, Landsittel DP, et al. The gap between clinically assessed physical performance and objective physical activity in liver transplant candidates. Liver Transpl 2016;22:1324–1332. [DOI] [PubMed] [Google Scholar]
- 19).Lee IM, Shiroma EJ, Kamada M, Bassett DR, Matthews CE, Buring JE. Association of step volume and intensity with all-cause mortality in older women. JAMA Intern Med 2019;179:1105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20).Spartano NL, Davis-Plourde KL, Himali JJ, Andersson C, Pase MP, Maillard P, et al. Association of accelerometer-measured light-intensity physical activity with brain volume: the Framingham heart study. JAMA Netw Open 2019;2:e192745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21).Serper M, Barankay I, Chadha S, Shults J, Jones LS, Olthoff KM, et al. A randomized, controlled, behavioral intervention to promote walking after abdominal organ transplantation: results from the LIFT study. Transpl Int 2020;33:632–643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22).Tudor-Locke C, Hatano Y, Pangrazi RP, Kang M. Revisiting, “how many steps are enough?”. Med Sci Sports Exerc 2008;40:S537–S543. [DOI] [PubMed] [Google Scholar]
- 23).Hassan MA, Dunn MA, Bloomer PM, Gougol AH, Serper M, Jorgensen D, et al. Cross-validation of performance-based frailty against daily step count from personal activity trackers—refining prehabilitation tools in liver transplant candidates. Am J Transplant 2020;20;516. [Google Scholar]
- 24).Pevnick JM, Fuller G, Duncan R, Spiegel BM. A large-scale initiative inviting patients to share personal fitness tracker data with their providers: initial results. PLoS One 2016;11:e0165908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25).Marttinen R, Daum D, Fredrick RN 3rd, Santiago J, Silverman S. Students’ perceptions of technology integration during the F.I.T. Unit. Res Q Exerc Sport 2019;90:206–216. [DOI] [PubMed] [Google Scholar]
- 26).Shin G, Feng Y, Jarrahi MH, Gafinowitz N. Beyond novelty effect: a mixed-methods exploration into the motivation for long-term activity tracker use. JAMIA Open 2019;2:62–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27).Calvo C, Hermida RC, Ayala DE, Lopez JE, Fernandez JR, Dominguez MJ, et al. The ‘ABPM effect’ gradually decreases but does not disappear in successive sessions of ambulatory monitoring. J Hypertens 2003;21:2265–2273. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


