Abstract
COVID-19 refers to viral respiratory infections and is the predisposing factor for the development of venous and arterial thrombotic events due to a pronounced inflammatory response, platelet activation, endothelial dysfunction, and stasis. Recent studies have confirmed a high incidence of thromboembolic events, especially in the group of patients with severe coronavirus pneumonia. There have been an increasing number of reports of peripheral arterial thrombosis as well. Most cases of arterial thrombosis are noted in critical ill patients in intensive care setting. However, an increase of adverse arterial events was also noted in cases of asymptomatic or mild forms of COVID-19. Herein, we report a case of patient with asymptomatic SARS-CoV-2 infection, who developed a threatening lower limb ischemia. Our own clinical observation suggests that COVID-19-associated arterial thrombosis can be successfully treated by embolectomy, administration of in-hospital parenteral anticoagulation, and continuation of antithrombotic therapy with a “vascular” dose of rivaroxaban after discharge.
Keywords: coronavirus disease 2019, SARS-CoV-2, thrombosis, acute limb ischemia
A novel β-coronavirus (severe acute respiratory syndrome coronavirus 2 [SARS-CoV-2]), which presumably occurred in the Chinese city of Wuhan (Hubei province) in December 2019, was the first to cause human viral pneumonia and the ongoing pandemic. 1 Unsurprisingly, a large number of publications have now appeared on the high incidence of cardiovascular events associated with SARS-CoV-2 infection.2,3 Many authors point out the prevalence of venous and arterial thrombotic complications in critically ill patients with severe course of coronavirus disease 2019.2,3 At the same time, during the second pandemic peak, reports of peripheral arterial thrombosis in cases of asymptomatic or mild forms of COVID-19 started to occur in literature. 4 However, acute limb ischemia often occurs in the absence of serious comorbid conditions and is accompanied by high rates of amputations and deaths.
Some specialists consider prescription of therapeutic doses of low-molecular-weight heparins as a basic treatment option in COVID-19-associated arterial thrombosis. Anti-inflammatory and other drug products, such as anticytokine drugs, angiotensin-converting enzyme inhibitors, and statins, also may be used. According to British experts, in case of drug therapeutic failure, preference should be given to open, rather than endovascular surgery. On the other hand, to date, there are no clear recommendations for outpatient antithrombotic therapy for patients who have had COVID-19-associated arterial thrombosis.
This report describes an unusual clinical case of treatment of a patient with acute arterial thrombosis of iliofemoral segment and threatening lower limb ischemia secondary to asymptomatic SARS-CoV-2 infection. Our case adds to the literature regarding arterial thrombosis in COVID-19. To the best of our knowledge, there have been no more than four previous clinical reports of similar acute limb ischemia associated with asymptomatic SARS-CoV-2 infection.
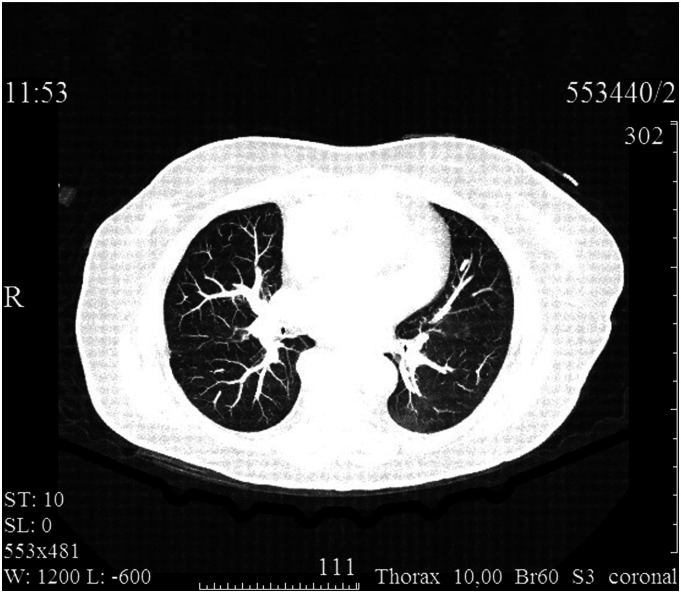
A 70-year-old female patient was admitted to a vascular center on an emergency basis due to sudden (occurred 3 hours ago) right lower limb ischemia. On admission, she complained of intense pain and loss of sensitivity in the foot and lower leg, limited mobility in the ankle and knee joints. No respiratory or other symptoms of viral infection (dry cough, dyspnea, fever, and myalgia) were observed. No history of intermittent claudication or other manifestations of chronic arterial insufficiency. The medical history included coronary artery disease, arterial hypertension, and diabetes mellitus, which required daily administration of acetylsalicylic acid 100 mg, medium-dose statins, β-blockers, angiotensin-converting enzyme inhibitors, and metformin. Physical examination revealed calf pain, pronounced hypothermia, and mottled skin, as well as absence of pulse in the right lower limb arteries. The clinical picture corresponded to stage IIb acute limb ischemia according to Rutherford. On the contralateral limb, normal (2+) pulse was detected in the femoral and popliteal arteries and weakened (1+) pulse was detected in the anterior and posterior tibial arteries. Duplex angioscanning confirmed thrombotic occlusion of the right iliofemoral arterial segment. Electrocardiography data showed normal sinus rhythm. The nasopharyngeal smear for SARS-CoV-2 by PCR was positive. (The material was collected on the day of admission, and the result was obtained the next day.) Computed tomography (CT) of thoracic organs revealed multiple subpleural and perivascular zones of ground glass opacity in the pulmonary tissue with signs of consolidation in the peripheral regions (Figure 1). Blood tests revealed abnormalities that are common in COVID-19: leukocytosis, neutrophilia, and lymphocytopenia, as well as increased D-dimer and fibrinogen levels (up to 1190 ng/mL and 5.59 g/L, respectively).
Figure 1.
Computed tomography imaging at admission: Bilateral multisegmental viral COVID-19 pneumonia.
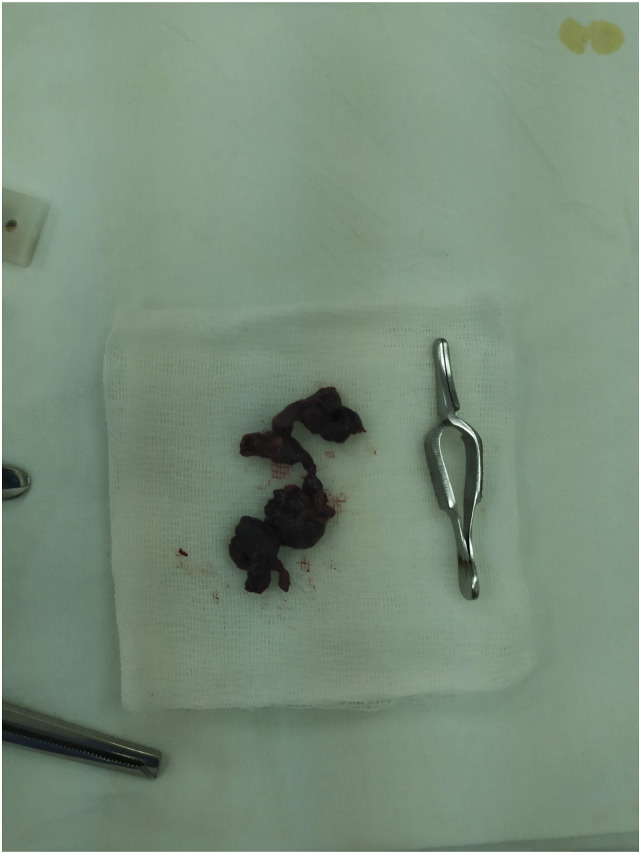
Given the rapid progression of threatening limb ischemia, the patient underwent emergency surgery. Short-term preoperative patient preparation included anesthesia (2% trimeperidine solution) and intravenous bolus administration of unfractionated heparin 5000 IU. For antibiotic prophylaxis, cefazolin 2.0 g was administered intravenously. In an operating theater, under spinal anesthesia (.5% bupivacaine solution), via longitudinal section in the upper third of the right thigh, the common, superficial, and deep femoral arteries (CFA, SFA, and DFA, respectively) were sequentially isolated: dense on palpation, with areas of pronounced atherocalcinosis, nonpulsatile. Above the bifurcation, the anterior wall of the CFA was dissected transversely; in the lumen, dark cherry red thrombotic masses of soft elastic texture were detected. The latter were removed from the distal and proximal arterial segments using a 5 F/80 cm Fogarty arterial embolectomy catheter (Figure 2). A pulsatile central blood flow and a satisfactory retrograde blood flow from the SFA and DFA were achieved. After placing a continuous suture on the arteriotomic opening using a USP 6/0 polypropylene suture, blood flow in the limb was restored. Fasciotomies were not performed. Thirty minutes after the completion of surgical revascularization, the capillary refill time in the foot was 2 seconds. The early postoperative period was favorable; the patient received parenteral anticoagulation therapy (dalteparin sodium); motor activity was restored within the first day after revascularization; and 2 days after the surgery, the patient was discharged for outpatient treatment.
Figure 2.
Removed thrombi from the lumen of the arterial iliofemoral segment.
Elderly age, coronary artery disease, arterial hypertension, and diabetes mellitus allowed classification of the patient as a high risk of major adverse cardiovascular events. In addition, acute right lower limb ischemia (stage IIb according to Rutherford), pronounced atherocalcinosis, and open revascularization of the iliofemoral arterial segment indicated a high risk of amputation. Considering the absence of history of bleeding and in order to reduce the risks of major adverse cardiovascular and limb events, antithrombotic therapy with a “vascular” 2.5 mg dose of rivaroxaban was initiated twice daily in addition to daily administration of acetylsalicylic acid 100 mg. After hospital discharge, the patient has been supervised by the operating surgeon for 3 months.
Recently, more data have been becoming available on thrombotic events in patients with asymptomatic or mild forms of SARS-CoV-2 infection. 4 The coronavirus is likely to be directly damaging to endothelial cells, thus inducing apoptosis and endotheliitis with activation of macrophages, granulocytes, platelets, and coagulation factors. 3 Thus, the necessary prerequisites are created for the mechanisms of local thrombus formation, which are likely to be dependent not only on the infectious process severity and risk factors associated with the patient’s hospitalization. Our observation confirms this assumption and supports the arguments in favor of the need to assess the risk of thrombotic complications, particularly in comorbid patients with mild or asymptomatic course of coronavirus infection.
An unknown component of antithrombotic strategy in patients with COVID-19-associated arterial thrombosis is the optimal duration of anticoagulation. Typically, all patients with arterial embolism or thrombosis in situ receive lifelong anticoagulant/antiplatelet therapy. However, the mechanisms of thrombus formation in SARS-CoV-2 infection differ from those in atherothrombosis in situ. Therefore, it remains to be seen whether to draw an analogy between COVID-19-associated arterial thrombosis and provoked deep vein thrombosis and use standard anticoagulation for 3 months, or prescribe lifelong antithrombotic therapy. The results of the ongoing COVER study may answer this and many other questions related to the aspects of management of vascular patients during the COVID-19 pandemic.
In conclusion, the presented clinical observation and literature data indicate a high risk of arterial thrombotic complications not only in severe course of SARS-CoV-2 infection but also in cases of asymptomatic or mild forms of COVID-19. Stage IIb acute arterial limb ischemia is associated with an extremely poor prognosis, and therefore, timely diagnosis and active surgical tactics along with parenteral therapeutic anticoagulation allow prevention of amputation and adverse cardiovascular events in the short run. The combination of rivaroxaban with aspirin is the only antithrombotic therapy regimen that has demonstrated acceptable safety and the ability to improve long-term results of surgical treatment of patients with peripheral arterial disease. However, to confirm the findings, randomized clinical studies are required in patients with COVID-19-associated arterial events.
Footnotes
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
References
- 1.Zhu N, Zhang D, Wang W, et al. A novel coronavirus from patients with pneumonia in China, 2019. N Engl J Med. 2020;382(8):727-733. DOI: 10.1056/NEJMoa2001017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Klok FA, Kruip MJHA, van der Meer NJM, et al. Confirmation of the high cumulative incidence of thrombotic complications in critically ill ICU patients with COVID-19: an updated analysis. Thromb Res. 2020;191:148-150. DOI: 10.1016/j.thromres.2020.04.041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kunutsor SK, Laukkanen JA. Incidence of venous and arterial thromboembolic complications in COVID-19: A systematic review and meta-analysis. Thromb Res. 2020;196:27-30. DOI: 10.1016/j.thromres.2020.08.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Veyre F, Poulain-Veyre C, Esparcieux A, et al. Femoral arterial thrombosis in a young adult after nonsevere COVID-19. Ann Vasc Surg. 2020;69:85-88. DOI: 10.1016/j.avsg.2020.07.013 [DOI] [PMC free article] [PubMed] [Google Scholar]