Key Points
Question
Is there a difference in second primary cancer risk after intensity-modulated radiotherapy (IMRT) vs 3-dimensional conformal radiation therapy (3DCRT) for prostate cancer in older adult men?
Findings
In a large-scale, population-based cohort from 2002 to 2015, the risk for developing a solid cancer was reduced with IMRT compared with 3DCRT, but only for patients treated during the earlier portion of the study period (2002 to 2005) and most notably for colon cancer.
Meaning
Treatment with IMRT for prostate cancer is not associated with an increased risk of second primary cancers, either solid or hematologic, and any inverse associations may be associated with calendar year of treatment.
Abstract
Importance
Compared with 3-dimensional conformal radiotherapy (3DCRT), intensity-modulated radiotherapy (IMRT) can spare nearby tissue but may result in increased scatter radiation to distant normal tissue, including red bone marrow. It is unclear whether second primary cancer risk varies by radiotherapy type.
Objective
To evaluate whether radiotherapy type (IMRT vs 3DCRT) is associated with second primary cancer risk among older men treated for prostate cancer.
Design, Setting, and Participants
In this retrospective cohort study of a linked database of Medicare claims and Surveillance, Epidemiology, and End Results (SEER) Program population-based cancer registries (2002-2015), male patients aged 66 to 84 diagnosed with a first primary nonmetastatic prostate cancer from 2002 to 2013, as reported to SEER, and who received radiotherapy (IMRT and/or 3DCRT without proton therapy) within the first year following prostate cancer were identified. The data were analyzed from January 2022 through June 2022.
Exposure
Receipt of IMRT and 3DCRT, based on Medicare claims.
Main Outcomes and Measures
The association between radiotherapy type and development of a subsequent hematologic cancer at least 2 years after prostate cancer diagnosis or a subsequent solid cancer at least 5 years after prostate cancer diagnosis. Hazard ratios (HRs) and 95% CIs were estimated using multivariable Cox proportional regression.
Results
The study included 65 235 2-year first primary prostate cancer survivors (median [range] age, 72 [66-82] years; 82.2% White patients) and 45 811 5-year survivors with similar demographic characteristics (median [range] age, 72 [66-79] years; 82.4% White patients). Among 2-year prostate cancer survivors (median [range] follow-up, 4.6 [0.003-12.0] years), 1107 second hematologic cancers were diagnosed (IMRT, 603; 3DCRT, 504). Radiotherapy type was not associated with second hematologic cancers overall or any specific types evaluated. Among 5-year survivors (median [range] follow-up, 3.1 [0.003-9.0] years), 2688 men were diagnosed with a second primary solid cancer (IMRT, 1306; 3DCRT, 1382). The overall HR for IMRT vs 3DCRT was 0.91 (95% CI, 0.83-0.99). This inverse association was restricted to the earlier calendar year period of prostate cancer diagnosis (HR2002-2005 = 0.85; 95% CI, 0.76-0.94; HR2006-2010 = 1.14; 95% CI, 0.96-1.36), with a similar pattern observed for colon cancer (HR2002-2005 = 0.66; 95% CI, 0.46-0.94; HR2006-2010 = 1.06; 95% CI, 0.59-1.88).
Conclusions and Relevance
The results of this large, population-based cohort study suggest that IMRT for prostate cancer is not associated with an increased risk of second primary cancers, either solid or hematologic, and any inverse associations may be associated with calendar year of treatment.
This cohort study evaluates whether radiotherapy type (intensity-modulated radiotherapy vs 3-dimensional conformal radiotherapy) was associated with second primary cancer risk among older men treated for prostate cancer.
Introduction
Intensity-modulated radiotherapy (IMRT) has replaced 3-dimensional conformal radiotherapy (3DCRT) as the clinical radiotherapy standard for patients with prostate cancer to enable dose escalation to the tumor and spare nearby tissue.1 However, IMRT’s increased scatter radiation to distant normal tissue, including red bone marrow, may confer greater risk of developing subsequent cancers, a serious late adverse effect of radiotherapy.2 An initial report among 5-year prostate cancer survivors diagnosed from 2002 to 2006 with follow-up through 2011 reported reduced risk for subsequent colon (n = 72) and rectal (n < 25) cancers following IMRT vs 3DCRT,3 but no association between radiotherapy type and other second solid cancers, or with hematologic cancers among 2-year survivors diagnosed from 2002 to 2009 with follow-up through 2011. In this study, we extend those analyses with follow-up through 2015 to address the limited follow-up in the previous report.
Methods
Within the linked Surveillance, Epidemiology and End Results (SEER) Program–Medicare database,4 we identified a retrospective cohort of male patients aged 66 to 84 diagnosed with first primary nonmetastatic prostate cancer from 2002 to 2013, as reported to 17 SEER registries (eFigure in Supplement 1). This study was exempt from ethics committee review by the National Institutes of Health Office of Human Subjects Research because it relied on deidentified existing data. This study followed the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) reporting guideline. The data were analyzed from January 2022 through June 2022.
Treatment data (IMRT, 3DCRT, proton therapy, brachytherapy, surgery, chemotherapy, hormone therapy) were ascertained from inpatient and outpatient Medicare claims. To ensure complete treatment data ascertainment within the first year following prostate cancer diagnosis, we required at least 1 year of Part A (in-patient hospital, skilled nursing facility, and hospice care), Part B (physician and outpatient services), and non–health maintenance organization Medicare coverage pre–prostate cancer diagnosis and post–prostate cancer diagnosis (or until death for less than 1 year survivors). We further restricted the study population to men with at least 1 inpatient or outpatient IMRT or 3DCRT Medicare claim less than 1 year after diagnosis. We excluded men with proton therapy claims before the start of analytic follow-up due to small numbers, precluding separate analyses.
Because latencies for radiation-related hematologic and solid cancers differ,5 analytic follow-up began 2 years or 5 years following prostate cancer diagnosis for hematologic and solid cancers, respectively, and continued until the earliest of second cancer diagnosis, age 85 years (due to potential underascertainment of cancer diagnoses in the older adult population),6 first Medicare claim for proton therapy (due to potential effect on second primary cancer risk), death, or December 2015 (last available data at the time of analysis). Hematologic cancer analyses further excluded individuals who received chemotherapy in the first year. Multivariable Cox proportional hazards regression models (person-year time-scale) estimated hazard ratios (HRs) and 95% CIs of second primary cancers after IMRT vs 3DCRT (SAS, version 9.4, SAS Institute Inc), adjusting for age at prostate cancer diagnosis, tumor grade, race, Charlson comorbidity score, and receipt of chemotherapy (for hematologic cancers, this only applied to chemotherapy received after 1 year), hormone therapy, brachytherapy, and surgery.
We conducted exploratory analyses stratified by receipt of brachytherapy within 1 year following prostate cancer diagnosis to evaluate potential differences in risk by treatment approach, and by calendar year of prostate cancer diagnosis to enable comparison with the previous report. We estimated the cumulative incidence of developing second solid cancers and hematologic cancers, accounting for competing risk of mortality (Stata, version 15.1; StataCorp).7 Statistical significance for all analyses was considered a 2-sided P < .05.
Results
Among 65 235 2-year prostate cancer survivors (Table), 63.4% received IMRT (±3DCRT). Recipients of both IMRT and 3DCRT were predominantly identified as White (IMRT, 84.9%; 3DCRT, 80.6%; total, 82.2%). The median (range) age at prostate cancer diagnosis of was 72 (66-82) years. Treatment with IMRT was more frequently used after 2006. More 3DCRT recipients had well or moderately differentiated prostate cancer diagnoses compared with IMRT (65.3% vs 36.9%). Similar patterns were observed among 45 811 5-year prostate cancer survivors (median [range] age, 72 [66-79] years; 82.4% White patients).
Table. Selected Characteristics Among Patients Treated With 3DCRT or IMRT for Prostate Cancer in the Linked SEER-Medicare Cohort.
| Characteristic | No. (%) | |||
|---|---|---|---|---|
| 2-y survivors, analysis cohort for subsequent hematologic cancers | 5-y survivors, analysis cohort for subsequent solid cancers | |||
| 3DCRT alonea | IMRTa | 3DCRT alonea | IMRTa | |
| Total | 23 875 (36.6) | 41 360 (63.4) | 19 242 (42.0) | 26 569 (58.0) |
| Age at diagnosis | ||||
| 66-69 | 7138 (29.9) | 10 818 (26.2) | 6174 (32.1) | 7545 (28.4) |
| 70-74 | 9394 (39.3) | 15 908 (38.5) | 7972 (41.4) | 11 030 (41.5) |
| ≥75 | 7343 (30.8) | 14 634 (35.4) | 5096 (26.5) | 7994 (30.1) |
| Raceb | ||||
| Black | 2344 (9.8) | 4979 (12.0) | 1911 (9.9) | 3201 (12.0) |
| Other | 1264 (5.3) | 3045 (7.4) | 1012 (5.3) | 1941 (7.3) |
| White | 20 267 (84.9) | 33 336 (80.6) | 16 319 (84.8) | 21 427 (80.6) |
| Calendar year of prostate cancer | ||||
| 2002-2005 | 15 151 (59.2) | 10 444 (25.3) | 12 851 (66.8) | 8724 (32.8) |
| 2006-2010 | 7159 (25.4) | 20 992 (50.8) | 6391 (33.2) | 17 845 (67.2) |
| 2011-2013 | 1565 (13.6) | 9924 (11.6) | NA | NA |
| Charlson comorbidity index in 12 mos before prostate cancer diagnosis | ||||
| 0 | 17 261 (72.3) | 27 773 (67.1) | 14 168 (73.6) | 18 592 (70.0) |
| 1 | 4649 (19.5) | 8800 (21.3) | 3679 (19.1) | 5445 (20.5) |
| ≥2 | 1965 (8.2) | 4787 (11.6) | 1395 (7.2) | 2532 (9.5) |
| Grade of prostate cancer | ||||
| Well or moderately differentiated | 15 599 (65.3) | 15 274 (36.9) | 13 087 (68.0) | 11 009 (41.4) |
| Poorly differentiated or undifferentiated | 8276 (34.7) | 26 086 (63.1) | 6155 (32.0) | 15 560 (58.6) |
| Received brachytherapyc | ||||
| No/unknown | 7793 (32.6) | 31 757 (76.8) | 5945 (30.9) | 19 343 (72.8) |
| Yes | 16 082 (67.4) | 9603 (23.2) | 13 297 (69.1) | 7226 (27.2) |
| Received hormonal therapyc | ||||
| No/unknown | 13 133 (55.0) | 18 636 (45.1) | 10 573 (54.9) | 12 244 (46.1) |
| Yes | 10 742 (45.0) | 22 724 (54.9) | 8669 (45.1) | 14 325 (53.9) |
| Received prostatectomyc | ||||
| No/unknown | 23 147 (97.0) | 39 815 (96.3) | 18 609 (96.7) | 25 585 (96.3) |
| Yes | 728 (3.0) | 1545 (3.7) | 633 (3.3) | 984 (3.7) |
| Received chemotherapyc | ||||
| No/unknown | 23 875 (100) | 41 360 (100) | 18 529 (96.3) | 25 405 (95.6) |
| Yes | 0 | 0 | 713 (3.7) | 1164 (4.4) |
| Vital status at end of follow-upd | ||||
| Alive | 16 469 (69.0) | 32 129 (77.7) | 14 455 (75.1) | 21 761 (81.9) |
| Developed second cancere | 2921 (12.2) | 3634 (8.8) | 1714 (8.9) | 1648 (6.2) |
| Deceased | 4485 (18.8) | 5597 (13.5) | 3073 (16.0) | 3160 (11.9) |
Abbreviations: 3DCRT, 3-dimensional conformal radiation therapy; IMRT, intensity modulated radiation therapy; SEER, Surveillance, Epidemiology, and End Results.
Radiation therapy type based on International Classification of Diseases, Ninth Revision (ICD-9), and Healthcare Common Procedure Coding System (HCPCS) codes on Medicare claims. IMRT, Current Procedural Terminology (CPT) codes 77295, (76370 and 77290), (77014 and 77290). 3DCRT, HCPCS and CPT codes: 0073T, 77301, 77338, 77385, 77386, 77418, G0174, G6015, G6016, G0178, G1074. This group also included patients who had no claims for IMRT or proton beam therapy but had claims for external beam radiation therapy not otherwise specified (HCPCS and CPT codes: 77401, 77402, 77403, 77404, 77405, 77406, 77407, 77408, 77409, 77410, 77411, 77412, 77413, 77414, 77415, 77416, G6003-G6014, 77422, 77423). The 2-year (hematologic) and 5-year (solid) restrictions correspond to the expected minimum latency periods for developing radiation-related cancers.5 Patients who received proton therapy (CPT codes: 77380, 77381, 77520, 77521, 77522, 77523, 77524, 77525) before the start of analytic follow-up were excluded.
Race was included as reported in the Medicare Master Beneficiary Summary File. The category of other and unknown includes patients classified in the original variable as “unknown,” “other,” “Asian,” “Hispanic,” and “North American Native.”
This was received during the first year following prostate cancer diagnosis.
Patients were followed up from 2 years or 5 years following prostate cancer diagnosis until the earliest of second cancer diagnosis, age 85 years, death, date of first Medicare claim for proton therapy, or December 31, 2015.
Includes all second cancers (hematologic, solid, and those that could not be classified as hematologic or solid).
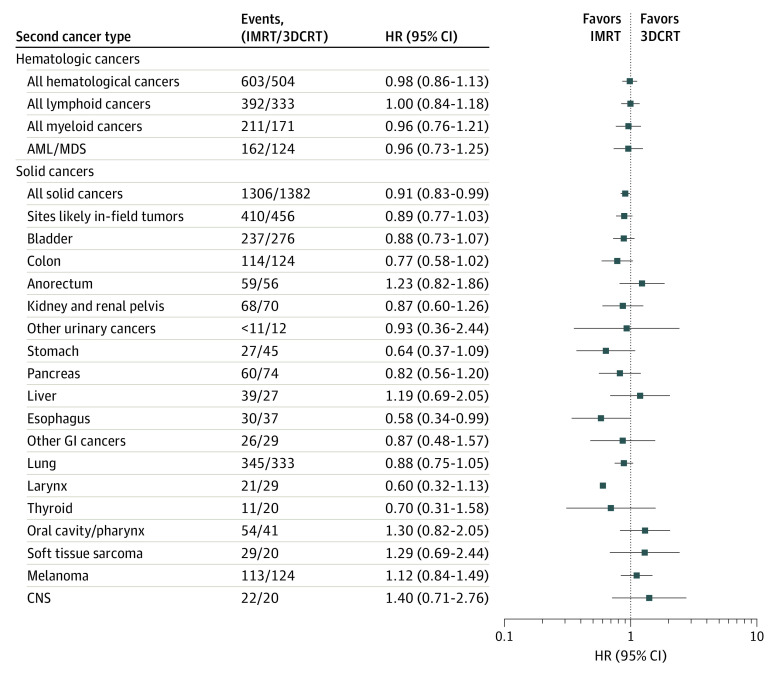
During a median (range) follow-up of 4.6 (0.003-12.0) years (ie, 6.6 years since first primary prostate cancer diagnosis), 1107 second hematologic cancers were diagnosed among 65 235 2-year prostate cancer survivors, including 504 after 3DCRT (10-year cumulative incidence, 2.3%; 95% CI, 2.1%-2.6%) and 603 after IMRT (10-year cumulative incidence, 2.4%; 95% CI, 2.2%-2.6%). Radiotherapy type was not associated with second hematologic cancers overall or any specific types including acute myeloid leukemia and myelodysplastic syndrome (Figure).
Figure. Association Between Radiotherapy Type and Second Primary Cancers Among Male Prostate Cancer Survivors in the Linked SEER-Medicare Cohort.
Models were adjusted for time-fixed variables of age at diagnosis, race, grade at prostate diagnosis, Charlson comorbidity index, and prostatectomy using the categories presented in the Table and time-dependent covariates of chemotherapy (for analyses of hematologic cancers this only includes chemotherapy received after the first year), brachytherapy, and hormone therapy. Treatment variables were ascertained from Medicare claims. Age, calendar year, and tumor characteristics were ascertained from SEER. Follow-up began at 2 years and 5 years (corresponding to the expected minimum latency periods for radiation-related cancers) following prostate cancer diagnosis, for hematologic cancers and solid cancers, respectively. Men were followed until the earliest of age 85 years, second cancer, Medicare claim for proton therapy, death or December 31, 2015 (last available data). Case counts of less than 11 were suppressed to protect patient confidentiality. Abbreviations: AML/MDS, acute myeloid leukemia/myelodysplastic syndrome; 3DCRT, 3-dimensional conformal radiation therapy; CNS, central nervous system; GI, gastrointestinal; HR, hazard ratio; IMRT, intensity-modulated radiation therapy; SEER, Surveillance, Epidemiology, and End Results.
Among 5-year survivors followed up for a median (range) of 3.1 (0.003-9.0) years (ie, 8.1 years since first primary prostate cancer diagnosis), 2688 men were diagnosed with a second primary solid cancer, including 1306 after IMRT (10-year cumulative incidence, 7.3%; 95% CI, 6.9%-7.7%) and 1382 after 3DCRT (10-year cumulative incidence, 7.5%; 95% CI, 7.1%-7.9%). The overall HR for IMRT vs 3DCRT was 0.91 (95% CI, 0.83-0.99) (Figure). Radiotherapy type was not statistically significantly associated with specific solid cancers or the combined group of proximate sites (bladder, colon, rectum/anus), although HRs were less than 1 for most gastrointestinal and genitourinary cancers.
Analyses stratified by calendar year suggested that the association of radiotherapy type with second primary cancer diagnoses was restricted to the earlier years of prostate cancer diagnosis for solid cancers overall (HR2002-2005 = 0.85; 95% CI, 0.76-0.94; HR2006-2010 = 1.14; 95% CI, 0.96-1.36) and for colon cancer specifically (HR2002-2005 = 0.66; 95% CI, 0.46-0.94; HR2006-2010 = 1.06; 95% CI, 0.59-1.88) but not other proximate sites (bladder and anorectal cancers; eTable in Supplement 1).
Results were consistent among men who did and did not receive brachytherapy in the first year following prostate cancer diagnosis (eTable in Supplement 1), in sensitivity analyses using attained age as the timescale, and in models with additional adjustment for median income within each patient’s zip code (a measure of socioeconomic status).
Discussion
Consistent with an earlier report within the linked SEER-Medicare database,3 risk for hematologic cancers, including acute myeloid leukemia and myelodysplastic syndrome, was not associated with IMRT vs 3DCRT in this retrospective cohort study. The present study’s results, based on larger case counts and a broader range of calendar years, suggest that the inverse association between IMRT vs 3DCRT and risk for all solid cancers as well as colon cancer does not persist in more recent calendar years. This may reflect changes in treatment volume or expansion of IMRT to broader populations (eg, more aggressive disease, increased comorbidities, community oncology centers) over time.
Strengths
To our knowledge, this cohort study includes the longest follow-up of any observational analysis of second primary cancers following prostate cancer IMRT, which is critical because of the known latency effects in developing radiation-related cancers.5 The large study population and range of prostate cancer diagnosis years allowed for calendar-year stratified analyses to further explore time-specific trends in selected second cancers.
Limitations
Key limitations of this cohort study include the lack of specific dosimetric data, including delivered dose, field size, and energy levels, which precluded quantification of the dose-response association between radiotherapy and second cancer risk and consideration of dose volume. Although stratification by receipt of prostatectomy could have served as a proxy for field size, the small proportion of men who received prostatectomy precluded such an analysis. We also lacked the sample size to evaluate proton therapy. Lastly, we lacked information on other potential second cancer risk factors, such as smoking status or genetic susceptibility. Adjustment for zip code–level median income did not appear to be associated with the present study’s results, but this metric might not have sufficiently accounted for socioeconomic status.
Conclusions
The present cohort study’s results suggest that IMRT for prostate cancer is not associated with an increased risk of second solid or hematologic cancers. The inverse association previously reported for colon cancer was only observed in this analysis among men treated from 2002 to 2005. Further studies are needed to continue monitoring outcomes after IMRT and to incorporate patients treated with proton therapy as its use becomes more widespread.8
eFigure 1. Selection of analytic cohorts
eTable 1. Hazard ratios for selected second solid cancer types, by receipt of brachytherapy in the first year following prostate cancer diagnosis and by calendar year of prostate cancer diagnosis.
Data Sharing Statement
References
- 1.Fischer-Valuck BW, Rao YJ, Michalski JM. Intensity-modulated radiotherapy for prostate cancer. Transl Androl Urol. 2018;7(3):297-307. doi: 10.21037/tau.2017.12.16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hall EJ, Wuu CS. Radiation-induced second cancers: the impact of 3D-CRT and IMRT. Int J Radiat Oncol Biol Phys. 2003;56(1):83-88. doi: 10.1016/S0360-3016(03)00073-7 [DOI] [PubMed] [Google Scholar]
- 3.Journy NM, Morton LM, Kleinerman RA, Bekelman JE, Berrington de Gonzalez A. Second primary cancers after intensity-modulated vs 3-dimensional conformal radiation therapy for prostate cancer. JAMA Oncol. 2016;2(10):1368-1370. doi: 10.1001/jamaoncol.2016.1368 [DOI] [PubMed] [Google Scholar]
- 4.Enewold L, Parsons H, Zhao L, et al. Updated overview of the SEER-Medicare data: enhanced content and applications. J Natl Cancer Inst Monogr. 2020;2020(55):3-13. doi: 10.1093/jncimonographs/lgz029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Berrington de Gonzalez A, Bouville A, Rajaraman P. Ionizing Radiation. In: Thun M, Linet MS, Cerhan JR, Haiman CA, Schottenfeld D, eds. Cancer Epidemiology and Prevention. Oxford University Press; 2018. [Google Scholar]
- 6.Jones D, Di Martino E, Bradley SH, et al. Factors affecting the decision to investigate older adults with potential cancer symptoms: a systematic review. Br J Gen Pract. 2021;72(714):e1-e10. doi: 10.3399/BJGP.2021.0257 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Coviello V, Boggess M. Cumulative incidence estimation in the presence of competing risks. Stata J. 2004;4:103-112. doi: 10.1177/1536867X0400400201 [DOI] [Google Scholar]
- 8.Royce TJ, Efstathiou JA. Proton therapy for prostate cancer: a review of the rationale, evidence, and current state. Urol Oncol. 2019;37(9):628-636. doi: 10.1016/j.urolonc.2018.11.012 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eFigure 1. Selection of analytic cohorts
eTable 1. Hazard ratios for selected second solid cancer types, by receipt of brachytherapy in the first year following prostate cancer diagnosis and by calendar year of prostate cancer diagnosis.
Data Sharing Statement