Abstract
Pott's spine, commonly known as spinal tuberculosis (TB), is an extrapulmonary form of TB caused by Mycobacterium TB. Pott's paraplegia occurs when the spine is involved. Spinal TB is usually caused by the hematogenous spread of infection from a central focus, which can be in the lungs or another location. Spinal TB is distinguished by intervertebral disc involvement caused by the same segmental arterial supply, which can result in severe morbidity even after years of approved therapy. Neurological impairments and spine deformities are caused by progressive damage to the anterior vertebral body. The clinical, radiographic, microbiological, and histological data are used to make the diagnosis of spinal TB. In Pott's spine, combination multidrug antitubercular therapy is the basis of treatment. The recent appearance of multidrug-resistant/extremely drug-resistant TB and the growth of human immunodeficiency virus infection have presented significant challenges in the battle against TB infection. Patients who come with significant kyphosis or neurological impairments are the only ones who require surgical care. Debridement, fusion stabilization, and correction of spinal deformity are the cornerstones of surgical treatment. Clinical results for the treatment of spinal TB are generally quite good with adequate and prompt care.
Keywords: Tuberculosis, Pott’s disease, Spinal tuberculosis, Kyphosis, Medical treatment of spinal tuberculosis, Surgical treatment of spinal tuberculosis, Drugs resistance
Core Tip: Pott's spine is a type of spinal tuberculosis (TB) characterized by hematogenous dissemination of mycobacterium from a primary lesion. It accounts for roughly half of all skeletal TB patients. The most frequent type of spinal TB is para-discal TB. Untreated infections can result in consequences such as a cold abscess, paraplegia, and deformity, all of which may necessitate surgical intervention. Rapid molecular approaches have aided in the detection of spinal TB and drug resistance, but it remains difficult due to sample collection issues and the paucibacillary nature of spinal TB. The presence of human immunodeficiency virus, which is endemic in some areas, increases the burden and complexity of care. Moreover, the emergence of multidrug-resistant TB and extremely drug-resistant TB has posed a big challenge in the management.
INTRODUCTION
Tuberculosis (TB) is a disease characterized by poverty, economic distress, vulnerability, stigma, and discrimination[1-3]. TB affects roughly one-quarter of the world's population. In 2021, 10.6 million people were infected with TB, equating to 134 cases per 100000 people. Human immunodeficiency virus (HIV)-positive individuals accounted for 6.7% of all TB cases. Geographically, the WHO areas of South-East Asia (45%), Africa (23%), and the Western Pacific (18%) had the highest percentages of TB cases in 2021, while the Eastern Mediterranean (8.1%), the Americas (2.9%), and Europe (2.2%) had the lowest percentages[4]. Despite being a disease that may be prevented and treated, TB is still not completely under control on a worldwide scale. This is caused by a variety of factors, such as rising HIV infection rates, drug misuse, an increase in the population of developing nations, and the migration of people to developed countries. Involvement of the skeletal system has been documented in 1%-2% of all TB patients and 10% of extrapulmonary TB cases[5,6]. More than half of all skeletal TB patients are caused by spinal TB, which commonly affects the productive age group and is costly to the family and the nation. It is more common in developing countries, where many people continue to live in poverty, have poor nutrition, are overcrowded, and lack proper hygiene. There is no difference in susceptibility to TB depending on gender. Because of longer life expectancies, diabetes, cancer therapy, HIV, and greater use of immunosuppressive drugs, spinal TB is becoming more common among the elderly[7,8].
The clinical presentations of spinal TB are often mild. Patients may show up with typical symptoms including weight loss, evening fever, lack of appetite, and back pain that doesn't go away with physiotherapy, or they may show up with no symptoms at all. The importance of taking a detailed history and completing a thorough examination of the spine and other joints cannot be overemphasized. A TB family history, interactions with a patient with active TB, habits, and socioeconomic situations might all be indicators of an early diagnosis. After the clinical examination, one should be able to recognize the pathology and any new issues that may require further testing and treatment.
SIGNS AND SYMPTOMS
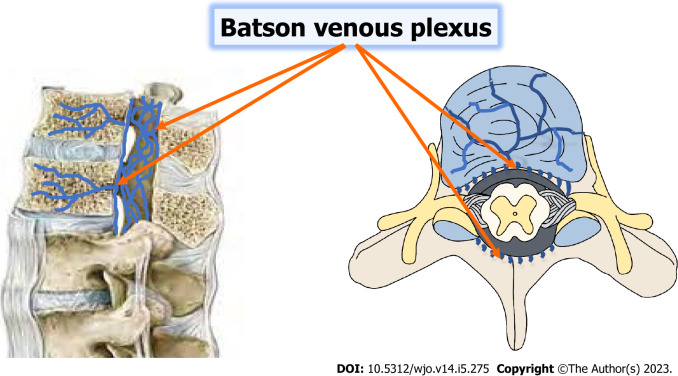
According to several retrospective studies, the lower thoracic and upper lumbar vertebrae account for 90% of individuals with spinal TB. Overall, the thoracolumbar junction is most frequently affected, followed by the lumbar, and cervical areas. The biomechanical change from an inflexible thoracic spine to a dynamic lumbar spine makes the thoracolumbar area susceptible to microtrauma, which may facilitate the seeding of TB bacteria. This region is commonly affected. TB bacteria often migrate from a primary location, such as the lungs or genitourinary system, to the vertebral body via a hematogenous pathway[9-13]. Each vertebra has a rich vascular plexus in the subchondral region, which makes it easier for TB bacteria to spread to the paradisiacal area. Two neighboring vertebrae are supplied by the same segmental artery, so often both are affected. A valveless system known as Batson's venous plexus connects the spine to the intra-abdominal and intra-thoracic chambers. Whereas TB bacilli propagating through the Batson's plexus generate core lesions in the vertebral body as well as involvement of non-contiguous vertebrae, dissemination through the anterior or posterior longitudinal ligaments leads in the involvement of numerous contiguous vertebrae[14,15]. The proportion of non-contiguous spinal involvement in skeletal TB was 63.6%, according to a published case series using FDG-PET SCAN to assess therapy response[16].
The clinical presentation is generally determined by a number of parameters, such as age, location of the lesions, duration, and so on. Immunological conditions, co-morbidities, and the emergence of issues such as a cold abscess, deformity, secondary infection, and neurological sequelae further complicate the neurological picture. The most common symptom is chronic low back pain, which is subtle at first, gradually worsening, dull and aching in nature, and typically non-radiating. There may be a link between constitutional symptoms such as malaise, lack of appetite, and weight loss. There may have been previously healed cases of pulmonary TB, cases in other places, or contact with a patient who had the disease[17,18]. Nighttime discomfort is a hallmark of this condition. If it's there, it wakes the sufferer. The absence of a protective muscle spasm, which indicates the spine's instability, is associated with the abrupt, excruciating pain. Night discomfort in the supine position is also connected to swelling and venous engorgement. Back pain in skeletal TB is particularly resistant to conservative treatment. When a root is squeezed owing to abscess development or a bone fragment, radicular pain might be a presenting symptom. Radicular discomfort directed to the belly might be mistaken for cholecystitis, pancreatitis, appendicitis, and renal disorders, leading to a delay in diagnosis and, in some cases, inappropriate examinations and operations[19]. There is muscular spasm on examination, which might manifest as prominent paraspinal muscles in the thoracolumbar spine and sciatic pain due to a unilateral spasm. Local sensitivity for the affected area might be evoked. Patients move with extreme caution while supporting the afflicted portion. The "Tripod Sign" with thoracolumbar spine involvement is one of the symptoms of skeletal TB[20,21].
Neurological deficit
Neurology is involved in 23% to 76% of cases of spinal TB. As the spinal cord terminates at L1 and the canal is somewhat large here, paraparesis is more common in the thoracic and cervical spines than below L1. Neurological symptoms might range from minor gait problems to total bladder and bowel incontinence. Patients in impoverished regions typically present after experiencing weakness. Patients first walk awkwardly and slowly as a result of their discomfort and weakness. Weakness in all four limbs is a sign of cervical cord compression. Whereas lumbar spine involvement is defined by lower motor neuron symptoms, thoracic cord compression is characterized by paralysis in both lower limbs, including or excluding bladder and bowel involvement[22]. When a disease is actively spreading or the body is healed slowly, paresis might develop. The active state is produced by either direct compression from an abscess, inflammatory tissue, sequestrum, or instability, or intrinsic causes such inflammation, meningitis, infective thrombi, or vascular damage brought on by endarteritis. Paraplegia is caused by the spinal cord expanding over the internal gibs, bony ridges, scarring, or disease recurrence in cured diseases.
With a spinal cord compression, motor function frequently deteriorates first, followed by sensory and autonomic impairments. Compression frequently starts anteriorly in an anterior lesion and progresses posteriorly. Spasticity may first develop without the patient being aware of it. During a clinical examination, it can be identified by brisk deep tendon reflexes and an extensor plantar response. The anterior columns gradually get implicated as a result of increased compression, which results in a loss of motor function. Further compression results in involvement of the lateral spinothalamic pathways, which results in a loss of pain, warmth, and rough touch. Complete loss of feeling happens when the posterior columns are involved, and by then, bladder and bowel irregularities have developed. When compression is applied for a long time, flaccidity and flexor spasms take the place of spasticity. Lesions at the conus medullaris or cauda equina may present mixed symptoms of upper motor neuron and lower motor neuron lesions with asymmetrical loss of feeling when bladder and bowel function is impaired early. It is possible to categorize neurological abnormalities in spinal TB using the Frankel and ASIA ratings, which were initially developed to identify neurological deficits in acute spinal injuries[23]. The most appropriate categorization of Pott paraplegia with spinal cord involvement is the modified Tuli classification[24]. Pott paraplegia was classified into five stages (Table 1). Despite the fact that the majority of neurologic abnormalities would fit into this category, neurological impairments in intraspinal granulomas, cauda equina syndromes, conus medullaris syndromes, or TB of other uncommon sites may not correlate to any of the phases described above.
Table 1.
Classification of paraplegia in tuberculosis of the spine[24]
|
Stage
|
Complaints
|
Motor
|
Sensory
|
Autonomic
|
| I | Nil | Plantar extensor/ankle clonus; ASIA motor score−100 | Nil | Nil |
| II | Able to walk with support | ASIA motor score; Tetra paresis (60–100); Paraparesis (80–100) | Lateral column; Involvement | Nil |
| III | Confined to bed; Can move limbs | ASIA motor score; Tetra paresis (0–30); Paraparesis (50–80) | Lateral column; Involvement | May be present |
| IV | No limb movement | ASIA motor score; Tetra paresis (0); Paraparesis (50) | Both lateral and posterior column involvement | May be present |
| V | Flexor spasms | Flaccid paralysis | Complete loss | Complete loss of bladder and bowel control |
Cold abscess
In at least half of cases with spinal TB, paravertebral abscesses are seen. As the name suggests, a cold abscess lacks signs of inflammation, including redness, dolor, and rubor, yet its existence indicates an active infection. Most cold abscesses can evoke a fluctuation, and they typically have a well-defined boundary with smooth, uniform borders. The skin above an abscess is typically swollen and glossy if it is large. Subcutaneous and dermal tissue are steadily destroyed by superficial abscesses, leading to a sinus that discharges. The abscess's pus is often light yellow or white. It contains caseous material with detritus and sequestered bone and has no unpleasant odor indicative of a pyogenic or fungal infection. When the underlying disease process is under control, tubercular sinuses gradually heal with a thin scar after a protracted period of time. Common symptoms of inflammation brought on by secondary infection might heal with an unsightly fibrotic scar that is anchored to the underlying tissue. According to culture, the presence of pyogenic organisms in the pus does not rule out TB. Paravertebral abscesses have a tendency to migrate along the path of least resistance after inflicting damage to the surrounding areolar tissue, including the muscular, subpleural, sub-peritoneal, perivascular, perineural, and fascial planes, and may manifest as a superficial abscess far from the initial focus[25-27].
Swellings in the axilla, subscapular region, or anterior or posterior triangles of the neck might be signs of retropharyngeal abscesses in the cervical spine. The usual pharyngo-vertebral crepitus, which is felt when the larynx is gently pressed from side to side against the vertebral column, is absent in cases with retropharyngeal abscesses. Together with stridor, hoarseness, and dysphagia, it can also present as a potentially lethal condition. An abscess in the arm or forearm might occur if the infection spreads through the brachial plexus. A cold abscess is identified as a fusiform paravertebral swelling in the thoracic area. It may occasionally adhere to the arcuate ligament or the diaphragm aperture. It can also travel via the intercostal arteries and cause a protrusion in the chest wall. A lumbar spine cold abscess is generally detected in the Petit's triangle or groin. It has the potential to migrate down the psoas muscle and cause a hip pseudo-flexion deformity. It can occasionally appear as a tumor in the Scarpa's triangle or a gluteal abscess and follow the femoral or gluteal arteries. If the obturator vessels are followed, the abscess will show up in the adductor region. The abscess may develop in the gluteal area, the posterior thigh, or, on rare occasions, even the popliteal fossa if the sciatic nerve is suspected. Understanding the distribution of the pus is crucial since it can take the examiner away from the source of the TB focus. Although involvement of the thoracic spine may diffuse into the lumbar region through the diaphragmatic orifice, the emphasis on the cervical spine may present as an abscess in the mediastinum or elbow. A lumbar spine abscess might appear in the thigh, calf, or even the ankle[28,29].
Spine deformity
Patients may appear to have deformities during either the active or healed stages. After a surgical debridement, deformities may appear right away, or it may take years for older patients whose skeletal architecture has been weakened by osteoporosis to develop. TB first affects the anterior column, and increasing deterioration causes kyphotic deformity and, eventually, instability. If therapy is started at this point, the lesion heals without significant deformity when the intervertebral disc is destroyed and the cancellous bones come into contact with one another. Further degradation occurs if therapy is delayed, resulting in increased deformity. The clinical presentation is determined by the number of vertebrae affected. Further collapse will result in vertebral body retropulsion, resulting in cord compression and neurological impairment. Regular follow-up is critical in the development of children until they reach adulthood. Children who have facet damage during an active illness are more likely to suffer severe deformities later in life. Severe abnormalities can impair quality of life, impede cardiac function, and cause cerebral deficits. Kyphosis is less problematic in the cervical and lumbar spines than it is in the thoracic and thoracolumbar spines because of natural lordosis[30,31].
Pediatric spinal TB
Despite the fact that there may not be any discernible trends today, children are more influenced than adults. Due to their juvenile skeletons, flexibility, and levels of activity, it is crucial to have a high level of suspicion when a child complains of back discomfort at the spine clinic. Children's vertebral bodies contain more cartilage, which is quickly damaged by active diseases and mechanical stress. A neurological sequela can result from asymmetric loading of the ring apophysis, which causes the formation of new deformities or the advancement of previously existing deformities. Even after being pronounced cured, children should continue to get frequent clinical and radiological checks until they reach skeletal maturity, since residual deformity might worsen due to a developing spine and physical activity. More than two of the four spine-at-risk indicators were present in one-third of the kids, which is an undesirable development. Children under the age of seven, people with more than three vertebral bodies sick, and people with conditions affecting the lower thoracic or thoracolumbar junction are more likely to progress[32-34]. There are four symptoms of "spine at risk" in children: (1) Retropulsion of the affected vertebra; (2) Facet subluxation; (3) Lateral translation of vertebrae; and (4) Toppling of one vertebra over the other.
Children who exhibit two or more of these symptoms may have posterior facet dislocation and necessitate surgical treatment, according to Rajasekaran[35,36]. Additionally, he proposed three categories for how the deformity progresses in children: Type 1 curves (curvature increases until growth stops or skeletal maturity is reached, at which point surgical intervention is necessary); type 2 curves (the deformity lessened as the child grew); and type 3 curves (there was minimal change in the deformity either during the active or healed phases of the disease).
Spinal TB in elderly
As people live longer, more and more older people are being identified as having spinal TB. Compromised health, co-morbidities, and medication interactions are specific issues that older people face. The elderly population has a threefold increased chance of experiencing drug reactions, a sixfold increased risk of mortality, and a twentyfold increased risk of receiving an incorrect diagnosis. Malignancy, diabetes, poor nutrition, immunosuppression, prolonged hospitalization, and other variables are risk factors for an increased incidence of spinal TB. When assessing an elderly person, look for signs of TB meningitis, disseminated TB, or other system involvement. Elderly low back discomfort is frequently disregarded in terms of degenerative conditions[37-39].
Atypical presentations
Intervertebral disc prolapses, isolated abscesses without skeletal involvement, and pure intraspinal granulomas are a few examples of unusual clinical presentations. Atypical radiographic appearances include circumferential vertebral involvement, solitary vertebral involvement, isolated meningeal, neural, or perineural involvement without any vertebral destruction, concentric vertebral collapse, isolated posterior arch involvement, and multifocal osseous lesions[40-43].
LABORATORY DIAGNOSIS
Spinal TB is diagnosed based on clinical and radiographic cues, as well as microbiological and histological markers. Diagnosis is challenging, even when there is a strong clinical suspicion of spinal TB. Clinical symptoms lack specificity, and testing for TB infection may yield conflicting results. Spinal TB is usually paucibacillary, and the sites of infection may be difficult to collect specimens adequate for molecular testing, histology, culture, or microscopy. Despite this, mycobacterium isolation from clinical samples is critical for both diagnostic confirmation and drug susceptibility testing. Diagnostic delays are prevalent due to the subtle clinical features of spinal TB[44,45]. It should be underlined that early identification of TB spine is crucial in minimizing deformity and neurological damage and improving patient outcomes from this illness. If discovered early, TB of the spine may typically be treated with antitubercular medication without the need for surgery. Despite technological developments, the diagnosis is still predicated on a strong clinical index of suspicion based on the history and examination results, which are complemented with biochemical and radiographic evidence[46,47].
Microscopy
Because of the high lipid content in their cell walls, mycobacteria are also known as acid fast bacilli (AFB). When exposed to acid alcohol, this binds to the fuchsin dye, preventing it from escaping from the cells. Early diagnosis is aided by the presence of AFB on microscopy, as well as a history of constitutional TB symptoms and evidence of pulmonary lesions on chest X-rays. Acid fast smears, in addition to supporting diagnosis, can aid in monitoring therapeutic response. The main limitation of smears is that they require a minimum of 10000 AFB per ml of material to be recognized, which is typically not the case with paucibacillary diseases like spinal TB[48,49]. Acid fast staining procedures include:
Carbol fuchsin stains: A fuchsin and phenol (carbolic acid) combination.
Ziehl–Neelsen (ZN, hot stain): Mycobacteria are stained red in this smear, whereas the backgrounds are bright blue. It is called "hot staining" because heat is used to help carbol fuchsin penetrate the bacilli. The smear is then decolored with 20% H2SO4 before being counterstained with methylene blue. Tubercular bacilli are acid fast because they resist decolorization by H2SO4 due to their high lipid content in the cell wall. Ziehl-Neelsen staining has the benefit of being a dependable, repeatable, and inexpensive process that may also be used to evaluate antitubercular treatment response. The limitations of this technology are that it has poor sensitivity and cannot distinguish between various species of Mycobacteria[17].
Kinyoun (cold stain): When compared to ZN staining, this procedure uses a higher quantity of phenol, removing the requirement for heat to penetrate carbol fuchsin. Mycobacteria look red on a pale blue background, similar to ZN staining.
Fluorochrome stains: rhodamine, a second fluorochrome, with or without auramine O. Compared to ZN staining, fluorochrome staining has the benefit of being able to scan a substantially broader region of the smear. This leads to greater sensitivity to detect bacteria as well as a reduction in the time necessary to scan the smear[50].
Culture
Culture methods identify substantially fewer tubercle bacilli (10-100/mL of specimen) than microscopy. Furthermore, the isolated bacilli can be utilized to identify the species as well as for drug susceptibility tests. As a result, the presence of TB bacilli in culture is regarded as the "gold standard" for TB diagnosis. The limitation of traditional culture procedures is the length of time required to detect noticeable growth, which can range from 4 to 8 wk. The various cultural mediums used to grow Mycobacterium TB are classified as follows:
Solid medias
Egg-based: Whole eggs or egg yolks, potato flour, salts, and glycerol that have been stiffened by inspissation are among the ingredients. They have a long shelf life and promote the growth of the majority of mycobacteria. The Lowenstein-Jensen (LJ) medium is the most often used egg-based medium.
Agar-based: Compared to egg-based media, this medium has a higher chemical definition. Colonies may therefore appear considerably sooner than in egg-based media (10-12 d).
Selective media: Due to the usage of antibacterial medications that prevent contaminating germs, this medium is more selective, allowing the development of Mycobacteria alone. It is therefore frequently used in conjunction with a non-selective egg or agar medium. Both the Gruft modification, which enhances LJ media with nalidixic acid and penicillin, and the Mitchison selective 7H11 medium, which enhances LJ media with carbenicillin, polymyxin B, trimethoprim, and amphotericin B, are examples of selective media.
Liquid medias
Mycobacteria growth indicator tube (MGIT): To help identify tubercle bacilli development in modified Middlebrook 7H9 culture, the MGIT includes a fluorescence-quenching-based oxygen sensor (silicon rubber impregnated with ruthenium pentahydrate).
BACTEC 460 TB system: A method that is semi-automated and uses palmitic acid that has been 14C-labeled as the carbon source for the medium. The apparatus detects 14CO2, which is produced as a result of this being digested in the presence of bacteria. Bacilli in a smear-positive specimen for TB often take 9 to 14 d to be found. The drawbacks of this approach are the inability to monitor colony shape, difficulties detecting mixed cultures, contamination overgrowth, expense, and radioactive disposal.
Automated continuous monitoring systems: Similar to the MGIT system, the BACTEC 9000 MB system uses a fluorescence quenching-based oxygen sensor to identify growth. The MB/BacT ALERT 3D system monitors the presence and generation of CO2 dissolved in growth media using a colorimetric CO2 sensor in each bottle and reflected light. When the bacilli grow, CO2 is produced. This gas diffuses to the sensor across the membrane, dissolves in water, and builds up as hydrogen ions. The amount of CO2 generated is proportional to the development of microorganisms in the medium, leading to the accumulation of hydrogen ions and a decrease in the sensor's pH. As a result, the color shifts from dark to bright green or yellow[51].
Serological tests
IgM levels are known to be a predictor of TB activity and have been proven to decrease during a three-month period after starting medication. IgG levels, on the other hand, show an increasing trend over the same time period and are therefore non-diagnostic but suggestive of chronic or cured disorders. Interferon (IFN) gamma, a cytokine produced by the body as part of its cell-mediated inflammatory response to tubercular antigen, is detected by the Interferon Gamma Release Assay, an ELISA test. One such test is QuantiFERON-TB Gold, which can detect IFN gamma in patients with spinal TB and vertebral body collapse with a sensitivity of 84% and specificity of 95%. While ELISA has improved the detection of these antibodies, it cannot tell the difference between current and latent infections or pulmonary and extrapulmonary TB[52,53].
Molecular testing methods
Nucleic ACID probes: Ribosomal RNA (rRNA), which is abundant in cells and culture, serves as a genetic target in this approach. Stable DNA-RNA complexes are produced when single-stranded radiolabeled (acridine ester) DNA probes hybridize with rRNA. An instrument that is proportional to the amount of probe present measures the light an unhybridized probe produces after it is deactivated. To assess positivity, a specified threshold is applied. It takes two hours to complete this technique[54].
In situ hybridization: This technique uses an oligonucleotide probe that has been fluorescein-labeled, and interpretation is done by direct fluorescence microscopy viewing. The phrase "fluorescence in situ hybridization" (FISH) is a popular one[54].
Nucleic acid amplification (NAA) methods: Nowadays, the PCR technique is often used in research and diagnostic applications. This technique works by amplifying certain DNA sequences into several copies that may be distinguished using gel electrophoresis separation. Synthetic oligonucleotide primers that are complementary to a certain DNA sequence are used to achieve amplification. The target DNA is amplified a million times as a result of this technique. The most often amplified target is the IS 6110 repetitive element, which is present in many copies in the majority of M. tuberculosis strains. The precision and expertise of the technician performing the assay determine the efficacy of PCR for TB. Using real-time PCR, the amount of M. tuberculosis in a clinical sample may be quantified while the detection time is sped up. In a closed system, the entire amplification and detection process takes place in a single reaction vessel. The risk of amplicon contamination in the lab is thereby diminished. There is no need for electrophoresis or post-amplification processing because this process is entirely automated.
Xpert MTB/rifampicin (RIF) test: An automated PCR test called the Xpert MTB/RIF Test (Cepheid, Sunnyvale, California) may detect rifampicin resistance and TB in less than two hours. To conduct the test, lysis reagent and tissue samples acquired during a biopsy are combined, and a mixture is then swiftly oscillated between the two. Two mL of the mixture are removed and put through the GeneXpert machine after being allowed to stand. After around 90 minutes, the results will be available[55,56]. Rapid results, a fully automated system, simultaneous detection of rifampicin resistance, the ability to detect very low amounts of TB bacilli, and the ability to differentiate between typical and atypical mycobacteria are all advantages of this system. The Xpert MTB/RIF test has the disadvantage of detecting non-viable pathogens, missing mono drug resistance, and having a single gene target.
Multiplex PCR: Instead of amplifying just one M. tuberculosis gene, the multiplex PCR aims to do so. Under these circumstances, it has been demonstrated that multiplex PCR, which amplifies both the IS6110 and MPB 64 genes identified in mycobacterium, has higher sensitivity and specificity. It has been established that both genes may be amplified from a single tissue sample to lower the expense of this inquiry[57,58].
Nanotechnology
Despite improvements in the field of TB diagnosis, there is still no point-of-care test for M. tuberculosis that is accurate in detecting children, extrapulmonary TB, or HIV-associated TB. This gap will be filled by nanotechnology, which will provide a point-of-care diagnosis that is rapid, effective, and affordable and uses particles with a size range of 1-100 nm. The possibility of using different physiological fluids, such as blood, sputum, or urine samples from patients, in nano-diagnostic methods to get reliable and quick results using affordable and portable tools, seems to be promising in the detection of infectious diseases like TB[59-61].
Among the nano-diagnostic techniques being explored for TB are:
Gold nanoparticle (NP)-based TB diagnostic techniques: For colorimetric detection of M. tuberculosis, gold NPs are combined with DNA probes. If complementary DNA is present, the nanoprobe solution remains pink at a wavelength of 526 nm, when complementary DNA is missing from the samples, the solution becomes purple.
Dipstick with gold NPs: Alkanethiol derivatives were used to coat colloidal gold NPs with the M. tuberculosis antigen; this coating produced a pronounced red color when mixed with serum containing antibodies.
Detection based on silica NPs: Indirect immunofluorescence microscopy has been created to detect M. tuberculosis using NPs linked with fluorescent dye.
Detection system based on quantum dots: In this approach, one probe accurately binds to the mycobacterium's 23S rRNA gene, and when the mycobacterium is treated with sulfuric acid and chromium quantum dots, a second probe accurately identifies the IS900 conserved sequence. As a consequence of hybridization with target gene sequences of mycobacterium DNA isolated from probable TB patient samples, a sandwich is produced. The conjugates of quantum dots and magnetic beads are then exposed to ultraviolet light, which causes red fluorescence that can be seen with the unaided eye.
Biosensors detection: Based on finding short nucleotide sequences of M. tuberculosis DNA, this is done. Sensors are classified as mass/piezoelectric, biological, electrical, or optical.
Erythrocyte sedimentation rate and C-reactive protein
In spinal TB, the erythrocyte sedimentation rate (ESR) is often greater than 20 mm/hour (60%-90% sensitivity) and decreases with treatment response. It is, however, not a particularly sensitive test. C-reactive protein (CRP) has a higher specificity (71% sensitivity) than ESR[62,63].
IMAGING MODALITIES
Plain radiographs
In the early stages of illness, plain radiographs may be normal. Before the lesions become radiographically visible, a 30% mineral loss must occur. Various publications have reported an average of 3.4 to 3.8 degrees of vertebral involvement over the years. Immunocompromised patients, diabetics, and people with hemoglobinopathies may have extensive spinal involvement. A "skipped lesion" occurs in 7% of patients when two non-contiguous vertebrae are implicated without the involvement of neighboring vertebral bodies and intervertebral discs; the mechanism is assumed to be infection transmission through the Batson's plexus of veins[64,65] (Figure 1). The thoracic spine is the most commonly involved area, followed by the lumbar region. The posterior arch is more commonly impacted than the vertebral body. There are four radiographic forms of spinal involvement: para-discal, anterior, central, neural arch, or appendiceal (pedicles, laminae, spinous processes, or transverse processes).
Figure 1.
The Batson venous plexus is a network of valveless veins that connects the deep pelvic veins and thoracic veins (which drain the inferior end of the urine bladder, breast, and prostate) to the internal vertebral venous plexuses in the human body.
Para-discal type
It is the most typical type of vertebral involvement, in which two contiguous vertebrae close to the disc space are involved at the same time. This points to a shared blood supply in this area. On radiographs, it appears as a decrease in intervertebral disc space associated with endplate irregularities in the adjacent vertebrae. Plain radiographs show tuberculous granulation tissue and abscess development in the paravertebral area as soft tissue shadows next to the spine. It is best apparent on a lateral radiograph in the cervical area as an enhanced prevertebral soft tissue shadow. Abscesses below the T4 spinal level have a distinctive fusiform shape (like a bird’s nest), but bigger abscesses may have a broad posterior mediastinal shadow. A globular-shaped shadow might form when an abscess is under strain. The psoas shadow in the lumbar region enlarges as an indication of an abscess tracking down the muscle. In cases of long-standing destruction of adjacent para-discal vertebral bodies, the spine may angle, and one or both bodies may show posterior convexity with wedge collapse. The most frequent spinal deformity is kyphotic deformity, which is caused by involvement of the thoracic vertebrae. Multiple involvement of neighboring vertebrae may result in a significant kyphotic deformity[66].
Central type
When the infection starts in the center of the vertebral body and spreads via Batson's venous plexus or the branches of the posterior vertebral artery, the pattern of involvement described above occurs. Later, with axial stress, the diseased vertebral body collapses due to trabecular bone loss. Because the loss of disc space and paravertebral shadow is minor in comparison to the para-discal type, it is sometimes mistaken for having neoplastic origins. However, with a longer follow-up, there may be a reduction in the nearby disc area[45].
Anterior type
When the disease process begins just below the anterior longitudinal ligament and periosteum, this pattern is observed. This results in erosions of the vertebral body's anterior aspect, which may be detected on lateral radiographs as uneven cortical borders. Multiple adjacent vertebrae may be involved if the infection spreads beneath the anterior or posterior longitudinal ligaments. Vertebral body collapse with loss of neighboring disc space is generally minor and appears later[41,42].
Appendiceal type
This involves either isolated or mixed involvement of the neural arch (pedicles and laminae), transverse processes, and spinous processes. When there are indirect symptoms of involvement, such as paravertebral shadows or erosive alterations with an undamaged disc, these lesions may be suspected radiographically. Routine radiography makes it difficult to see the involvement of the posterior spinal joints. Posterior spinal articulations may also be affected, resulting in lateral translation, an uncommon malformation. The fundamental drawback of radiography is that it is insensitive in the early stages of illness. The craniovertebral and cervicodorsal junctions are two spinal locations that are difficult to identify on X-ray. Plain X-rays make it difficult to assess spinal cord alterations, soft tissue involvement, and the exact location and extent of abscesses. So, the appearance of any of the radiographic signs may be a sign that the disease process has progressed to a fair degree[67,68].
COMPUTED TOMOGRAPHY
Computed tomography (CT) reveals findings significantly earlier than normal radiography because it shows more detail of bone irregularity, disruption, sclerosis, and disc collapse. Fragmentary, osteolytic, sclerotic, and subperiosteal bone disintegration patterns have all been documented. Aside from bony detail, paraspinal abscesses are assessed better than plain radiography. It's essential for detecting calcification within an abscess or bone pieces within epidural lesions. It is quite useful for providing direction for percutaneous diagnostic sampling, particularly in inaccessible locations. The main drawback is that magnetic resonance imaging (MRI)scores higher than CT when evaluating the effect of the disease on brain structures. In spinal TB, radiological evidence of healing lags behind clinical and laboratory results. Several months after the start of combination therapy, many patients may not exhibit any signs of improvement on X-rays or CT scans, which should not be construed as a sign that the treatment is failing. Nevertheless, if the pictures are repeated more than 6 months after the commencement of the treatment and do not demonstrate improvement, the possibility of an extra lesion or a condition that is therapeutically resistant should be taken into account[69,70].
MAGNETIC RESONANCE IMAGING
Because of its improved soft tissue contrast and capacity to spot and classify anomalies in the spinal cord, bone marrow, and intervertebral disc, MRI outperforms other imaging modalities. For the entire examination of the tuberculous spine, MRI is the modality of choice. The craniovertebral junction, cervicodorsal junction, neural arch components, vertebral appendages, sacroiliac joint region, sacrum, and coccyx are just a few tough places where it is very helpful in diagnosing disorders. Standard MRI procedures include fat-suppressed T1W, T2W, and short tau inversion recovery sequences in the axial, sagittal, and coronal planes, as well as contrast-enhanced T1W sequences after gadolinium contrast injection. An abnormal marrow signal intensity that appears hypointense on T1W sequences and hyperintense on T2W sequences, showing heterogeneous enhancement and a lack of cortical definition, is indicative of the vertebral body being involved. Contiguous vertebral body disease with disc degeneration is common. Loss of normal internuclear cleft with increased signal on T2W images, as well as post-contrast enhancement, are indications of disc involvement. Because mycobacterium lacks proteolytic enzymes, disc involvement occurs later than in pyogenic spondylitis. The “floating disc sign” may arise infrequently if there is severe spinal damage with disc sparing. In children, the disc is highly hydrated and more susceptible to infection.
The intercostal space, mediastinum, pleural cavity, or even the intercostal arteries themselves may get enclosed by the paraspinal collection as it just barely breaches the anterior longitudinal ligament in the thoracic region. When the psoas muscle is involved in the lumbar area, there is a loss of typical muscle shape, increased muscular size, and uniform signal intensity on T1W imaging. In T2W imaging, the psoas abscess appears as a high-signal fluid with dense peripheral post-contrast enhancement. Although posterior element involvement is less likely in TB than in a pyogenic infection, it is nonetheless more common. The disease's involvement manifests as an aberrant signal and in the homogeneous amplification of the afflicted spot. The posterior elements can be afflicted alone, although they are most typically encountered in conjunction with anterior element abnormalities. Composite lesions, or panvertebral lesions, are defined as involving both the posterior and anterior components. The epidural/subdural space or the spinal cord may be involved in addition to granulomatous lesions inside the spinal canal. Around 61% of afflicted vertebrae have epidural extension that may be seen on an MRI. When the spinal cord is squeezed from the front or the rear, compression myelopathy can happen[71-73].
MEDICAL MANAGEMENT
Multidrug antitubercular therapy
Unlike other infections, TB needs multidrug treatment for a variety of microbiological reasons, as mentioned below: Mycobacteria exist in four types in the human body: (1) Extracellular fast dividing; (2) Extracellular slowly dividing; (3) Intracellular intermittently dividing; and (4) Dormant bacilli. As a result, it needs the use of many medications that are effective against various bacterial types. Rifampicin destroys slowly growing bacteria, ethambutol kills intracellular bacteria inside macrophages, and streptomycin kills rapidly multiplying bacteria. In contrast, isoniazid, ethambutol, and streptomycin all kill rapidly multiplying bacteria[74].
Mycobacteria's modest growth rate is both a blessing and a curse. While the illness progresses slowly, medications that work on quick multipliers become ineffective, limiting therapy choices. As a result, it is necessary to use medications for a longer period of time.
The drug permeability is minimal due to the restricted permeability of mycobacterial cell walls and intra-macrophage bacilli. As a result, specialized medications that penetrate macrophages and thick bacterial cell walls are required for therapy[75].
Mycobacteria are well-known for rapidly acquiring antibiotic resistance to monotherapy. Isoniazid resistance mutations occur at a rate of 1 in 106, while rifampicin resistance mutations occur at a rate of 1 in 108. Resistance to both can be found in 1 in 1014 people. As a result, a minimum of two medications are administered in combination to prevent the development of resistance[76].
Most anti-tubercular medications, with the exception of thioacetazone, have a protracted period of action known as the "lag-effect." This quality made it possible to take medications on an intermittent schedule, which was essential for the success of directly observed therapy[77].
FIRST-LINE DRUGS
Despite decades of extensive study, only a few medications have been shown to be effective. Since the current anti-tubercular treatments consist of the five first-line drugs isoniazid (INH), RIF, ethambutol (EMB), pyrazinamide (PZA), and streptomycin, which are the most efficient and least toxic, it is advised to use these drugs with caution and a focus on compliance in order to prevent the emergence of resistance. Prolonged use of numerous medicines may increase the risk of a variety of side effects and problems. To achieve a safe and effective therapy, a detailed understanding of pharmacokinetics, medication interactions, and side effect profiles is required, as well as monthly monitoring.
INH
Isoniazid is an important drug in the treatment and prevention of TB. INH is a prodrug that, when activated by the catalase-peroxidase KatG, creates a variety of radicals. The bonding of the radicals with nicotinamide adenine dinucleotide (NAD) causes an INH-NAD adduct, which inhibits the enoyl-ACP reductase InhA of the fatty acid synthase type II (FASII) pathway, ultimately leading to cell death. INH is especially effective against rapidly developing mycobacteria. It can pass the blood-brain barrier and act on both intracellular and extracellular bacteria. Only dividing bacteria are killed when INH penetrates the bacterial cell; mycobacteria in the stationary phase are unaffected. INH is bacteriostatic for the first 1-4 days, thereafter, it becomes bactericidal, which coincides with its lack of acid fastness. Multiple genes in different pathways are involved in INH resistance. A mutation in the katG gene is the most prevalent source of resistance, followed by mutations in other genes such as inhA, ahpC, kasA, and ndh. Adults should take 5 mg/kg, while children should take 10 mg/kg. Peripheral neuropathy, lethargy, hepatitis, and, in rare cases, convulsions, insanity, and a lupus-like condition are among the side effects. Pyridoxine (10-25 mg/d) is also indicated to help reduce the risk of peripheral neuropathy. INH is a cytochrome P450 inhibitor that has been shown to raise the plasma concentrations of anticonvulsants, benzodiazepines, acetaminophen, and oral anticoagulants[78,79].
RIF
Rifampicin, a rifamycin derivative, inhibits messenger RNA elongation by binding to the β-subunit of the RNA polymerase. Both intracellularly and extracellularly, it is effective against bacteria that quickly proliferate and slowly metabolize. This impact on bacteria with irregular metabolism provides a "sterilizing effect". Adults should take 10 mg/kg, while children should take 15-20 mg/kg. High dosages can cause hepatotoxicity; hence, the maximum daily dose shouldn't exceed 600 mg. Additional adverse effects include hemolytic anemia, gastrointestinal distress, purpura, and orange-red urine stains. Rifampicin is a cytochrome P450 inducer, which necessitates dose changes for other medications that are processed in the liver, such as oral hypoglycemics, anticonvulsants, antifungals, protease inhibitors, non-nucleoside reverse transcriptase inhibitors, cardiac therapies, and so on. Rifampicin resistance is most frequently caused by a mutation in the 81-bp rpoB gene (codons 507–533), which codes for the β-subunit of RNA polymerase[80,81].
PZA
An amidase enzyme produced by the pncA gene transforms the prodrug PZA into its active form, pyrazinoic acid. PZA is thought to hinder membrane transport, trans-translation, and coenzyme A production, all of which are required for bacteria to thrive. The primary characteristic of PZA is its ability to combat non-replicating persisters in an inflammatory, acidic environment. The first two months of treatment, when acute inflammatory changes are still apparent, are when it works best. It has a great "sterilizing effect" and is critical in decreasing the length of chemotherapy. Adults should take 25 mg/kg, while children should take 35 mg/kg. The recognized side effects are hepatotoxicity, hyperuricemic arthralgia, exanthema, and pruritis. Cyclosporine and gout patients need dosage adjustments. PncA mutations in the 561 bp open reading frame or an 82 bp putative promoter region are the main causes of PZA resistance in M. tuberculosis[82,83].
EMB
Ethambutol is only effective against growing bacteria (bacteriostatic), where it prevents mycobacterial arabinogalactan biosynthesis by inhibiting the enzyme arabinosyltransferases. Other pathways include glycerol metabolism and RNA synthesis disruption. Adults should take 15 mg/kg, while youngsters should take 15-25 mg/kg. Dose-dependent retrobulbar neuritis is the most significant side effect. The central fibers are frequently compromised, leading to loss of visual acuity, scotomas, and the inability to discern between green and red colors. The effects are reversible if recognized early and the medicine is stopped. Abdominal discomfort, eosinophilia, peripheral neuritis, myocarditis, and hypersensitivity are some of the other side effects. The drug is not recommended for children due to the difficulties in evaluating visual acuity consistently. Dosage adjustments are necessary for patients with low creatinine clearance (30 mL/min). The embB gene, which codes for arabinosyltransferases, has codon 306, which is the most often occurring mutation for EMB resistance[84,85].
Streptomycin
Streptomycin is a Streptomyces griseus aminocyclitol glycoside. It is highly effective against actively proliferating bacilli found in cavities when administered intramuscularly. The ribosomal proteins S12 and 16S rRNA, which are encoded by the genes rpsL and rrs, respectively, are not translated as a result of their action. The fact that it is active at an alkaline pH is significant. Adults should take 15 mg/kg of streptomycin, whereas children should take 15-25 mg/kg. Ototoxicity, vestibulotoxicity, nephrotoxicity, rashes, eosinophilia, and fever are among the side effects. The most prevalent mutation giving streptomycin resistance is a lysine to arginine change in codon 43 of rpsL. Streptomycin can cross the placenta and cause fetal ototoxicity and nephrotoxicity; hence, it is not recommended for use during pregnancy. All other first-line medications are safe to use while pregnant. Children's pharmacokinetics differ from those of adults. Children metabolize medicines quicker than adults, and their blood concentrations are substantially lower, necessitating a greater body weight dosage[86,87].
The first-line anti-tubercular medications' dose, pharmacological activities, and side effects are summarized in Table 2.
Table 2.
The first-line anti-tubercular medications' dose, pharmacological activities, and side effects[85]
|
Anti-tuberculosis drugs
|
Dose (mg/kg)
|
Pharmacological activities
|
Side effects
|
| Isoniazid | 5 | -Inhibits the enoyl-ACP reductase InhA of the fatty acid synthase type II (FASII) pathway | -Peripheral neuropathy, lethargy, hepatitis, insanity, convulsions, and a lupus-like condition |
| Rifampicin | 10 | -Inhibits messenger RNA elongation by binding to the β-subunit of the RNA polymerase | -Hemolytic anemia, gastrointestinal distress, purpura, and orange-red urine stains |
| Ethambutol | 15 | -Inhibits enzyme arabinosyltransferases, glycerol metabolism, and RNA synthesis | -Abdominal discomfort, eosinophilia, peripheral neuritis, myocarditis, and hypersensitivity |
| Pyrazinamide | 25 | -Disrupts membrane energetics and inhibits membrane transport function | -Hepatotoxicity, pruritis, hyperuricemic arthralgia, and exanthema |
| Streptomycin | 15 | -Binds to the small 16S rRNA of the 30S ribosomal subunit irreversibly, interfering with the binding of formyl-methionyl-tRNA to the 30S subunit | -Ototoxicity, rashes, fever, nephrotoxicity, eosinophilia, and vestibulotoxicity |
SECOND-LINE DRUGS
The emergence of first-line antibiotic resistance has required the frequent use of second-line treatments. Second-line medications are less effective, have more toxicity, and are more expensive than first-line treatments. The most often used second-line medications include fluoroquinolones, injectable aminoglycosides, ethionamide, and cycloserine.
Fluoroquinolones
Fluoroquinolones (FQs) are antibiotics with broad spectrum action against M. tuberculosis in vitro and in vivo. They are bactericidal, with the mechanism of action being DNA synthesis inhibition. Fluoroquinolones that are routinely utilized include ciprofloxacin, ofloxacin, levofloxacin, and moxifloxacin. The suggested daily dose is 400-600 mg. Adverse effects such as headache, gastric discomfort, rashes, and dizziness are uncommon. A mutation in DNA gyrase, the biological target for FQs, is the most prevalent route for drug resistance[88,89].
Aminoglycosides
Amikacin (AMK), kanamycin (KAN), and capreomycin (CAP) are injectable aminoglycosides that are crucial to the treatment of multidrug-resistant (MDR) TB. They are made from Streptomyces and are bactericidal. AMK and KAN work by inhibiting protein translation, but capreomycin works by inhibiting mRNA-tRNA translocation by attaching to the 70S ribosome. All three drugs are administered in a single dose of 15 mg/kg per day. Due to the adverse effects, which include renal toxicity, ototoxicity, and electrolyte abnormalities, frequent monitoring of hearing and renal function is required. Resistance to AMK and KAN is associated with changes in the 16S rRNA, and cross-resistance between both drugs is frequent. Capreomycin resistance is linked to a TlyA mutation and is more likely to result in treatment failure and death[90,91].
Cycloserine
Cycloserine is a bacteriostatic drug derived from Streptomyces orchidaceus. It prevents the formation of mycobacterial cell walls. Moreover, cycloserine could successfully permeate bone and reach quantities that were equivalent to those in plasma, which supports its use in the treatment of osteoarticular TB[92]. The recommended daily dosage is 1 g, taken as a single dose. Those with psychiatric disorders and renal insufficiency should avoid using the medication since it might cause seizures, headaches, and psychosis. Because of its high stomach tolerance and low drug-drug interactions, it may be used to treat MDR and extremely drug-resistant (XDR) TB[93].
Ethionamide/prothionamide
Iso-nicotinic acid is used to make the bacteriostatics ethionamide and protionamide. The mechanism of action is similar to that of INH in that it inhibits InhA of mycolic acid production. The medication is administered in a single daily dosage of 1 g. Severe gastrointestinal intolerance, hepatitis, peripheral neuropathy, hypothyroidism, and depression are among the adverse effects. There is a lot of cross-resistance between the two medications[94,95].
Para-aminosalicylic acid
Para-aminosalicylic acid was one of the first anti-tubercular medications to be developed. However, due to quick resistance and gastrointestinal intolerance, it was replaced. Hypothyroidism, hepatic dysfunction, and hypersensitivity are some of the other side effects. A single or two split dosages of 12 g are advised[96].
NOVEL DRUGS
In view of rising treatment failure in M. tuberculosis, there is an unmet need for innovative medicines that act on novel targets and have higher effectiveness while requiring fewer drug interactions and having a lower toxicity profile.
Delamanid
Delamanid is a prodrug (dihydro-nitroimidazo-oxazole derivative) that inhibits the formation of mycobacterial cell wall components when activated by the enzyme deazaflavin-dependent nitroreductase (Rv3547). Headache, nausea, dizziness, and QT prolongation are all side effects. The safety profile is good, with the least amount of toxicity and no major drug interactions with other anti-TB drugs. Given the inconsistent findings of effectiveness trials, WHO recommends using the medicine only in a more protracted MDR regimen if no viable alternative can be discovered[97,98].
Bedaquiline
Bedaquiline is a new anti-tubercular drug that was released in 2012. It targets the mycobacterial ATP synthase and binds to one of its components to prevent it from functioning. Like other second-line medications, it is advised for use in MDR and XDR-TB patients. Its distinct mode of action makes it less likely than other anti-TB medications to produce cross-resistance. Among the side effects that have been seen are nausea, arthralgia, headaches, hemoptysis, chest pain, anorexia, rashes, and an increase in hepatic transaminases[99]. The most significant side effect is QT prolongation; hence, it should not be used with other QT-prolonging medications. Mutations in the rv0678 gene, which encodes the MmpL5 efflux pump repressor, have been linked to low-level bedaquline resistance and cross-resistance to clofazimine.
SURGICAL MANAGEMENT
Chemotherapy is the primary treatment for spinal TB. Long-term antitubercular chemotherapy is a crucial component of the treatment of spinal TB. Surgery for TB of the spine is a contentious subject, with no clear consensus on the indications for surgical therapy. To prevent and cure complications of spinal TB, such as cold abscess, deformity, and neurological deficiency, surgical intervention may be necessary. Excisional treatment and antitubercular therapy were shown to be equivalent in studies. In fact, excisional treatment alone causes anterior column deficiency, deformity, and delayed neurological damage[100].
According to Mak et al[100] the following circumstances warrant surgical intervention:
Progressive neurological deficit: Early surgical decompression contributes to the resolution of the deficiency, which lowers morbidity and improves quality of life.
Progressive spinal deformity: More kyphosis in the thoracic spine may be tolerated if the lumbar spine compensates, but an excessive loss of lordosis in the lumbar spine is not acceptable. Kyphosis of sixty degrees should be surgically corrected since it can cause paraplegia and cardiovascular impairment.
Failure of conservative treatment: Following 3-4 wk of chemotherapy with or without a brace or bed rest, any increase in discomfort or neurological deficit should be treated surgically as a therapeutic failure.
Uncertain diagnosis: Making a diagnosis or determining medication sensitivity might be challenging when there isn't a surface abscess or when percutaneous biopsy samples are either insufficient or impossible to acquire. In these circumstances, surgery should be done to collect enough tissue samples.
END POINT OF CHEMOTHERAPY
The major issue with spinal TB is that there are no clear standards for determining "healed status." Therefore, it is difficult to determine when to discontinue using anti-tubercular medications. In order to determine the end point of treatment for spinal TB, the "gold standard" in pulmonary TB—repeated tissue biopsy and culture conversion—is not used because, the paucibacillary nature of the TB spine makes it an invasive, time-consuming, and consequently impractical method with a very poor yield. Additionally, there is no established method for estimating the total body burden of M. tuberculosis or forecasting clinical results. Therefore, the trifecta of clinical improvement, laboratory markers, and radiographic evaluation continues to be used as corroborated indicators of healing status.
Clinical improvement
Pain: Although the majority of spinal TB patients who are recovering report lessening of their pain, this cannot be taken as an absolute criterion because there can be several different causes of back pain, and more importantly, healing can take place without a strong bony fusion, which can result in persistent pain from spinal instability despite the disease's recovery. In addition, there is currently no accurate metric for measuring the degree of pain.
Deformity: The same is true for spinal deformity, which in most individuals either worsens or remains as the illness progresses and collapses.
Neurology: Although the patient's neurology improves when the diseased soft tissue crushing the neural components is reduced, in a small number of cases, neurologic dysfunction may continue despite the illness healing due to chronic bony compression.
Laboratory improvement
ESR: In most situations, ESR is increased. It is used to track how well patients respond to therapy. Failure to normalize following therapy should raise concerns about primary drug resistance or alternate causation.
CRP: CRP levels have been shown to be up to 75% higher in patients with spinal TB. It is more targeted toward viral and inflammatory lesions. It takes around 2 wk to detect a change, whereas ESR takes about 4 wk and hence has more relevance in monitoring therapy response.
Imging improvement
MRI: There are numerous well-known criteria for determining spinal TB healing.
Even after the infection has been completely eradicated, an MRI may still reveal soft tissues and even sterile abscesses.
MRI often lags behind clinical recovery by up to 3 mo.
MRI may be too sensitive when utilized as a sole source of information to assess the degree of a current infection, inflammatory edema, or active disease pus.
Poor disease management is indicated by the development of new lesions while receiving treatment, the worsening of existing lesions, marrow edema, and new bone damage or abscesses, particularly when these symptoms are coupled with clinical deterioration[101,102].
PROGNOSIS
Younger age and earlier diagnosis have been reported to be favorable prognostic factors. The severity of the disease (number of damaged vertebrae) and the extent of spinal deterioration (instability, deformity, abnormalities) influence the clinical outcome. The existence of paraplegic symptoms at the time of the initial diagnosis is considered a bad prognosis. Immunodeficiency (HIV, drug addiction, and alcoholism), malnutrition, and poverty are additional risk factors[103,104].
CONCLUSION
Early identification and prompt treatment enhance the prognosis for spinal TB. Even in the absence of neurological symptoms and indications, individuals who appear to have persistent back pain must be viewed with a high degree of clinical suspicion. Medical treatment is generally effective. However, MDR/XDR TB is increasing and should be identified early by using molecular methods to diagnose spinal TB and drug resistance. Advanced instances with significant bone involvement, abscess development, or paraplegia require surgical intervention. Young individuals are susceptible to spinal TB; thus, measures should be taken for effective prevention. The only strategy to avoid spinal TB is to stop the spread of the disease.
Footnotes
Conflict-of-interest statement: The authors declare no conflicts of interest related to this article.
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Peer-review started: December 29, 2022
First decision: February 20, 2023
Article in press: April 12, 2023
Specialty type: Infectious diseases
Country/Territory of origin: Thailand
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Kuroki H, Japan; Paparoupa M, Germany S-Editor: Ma YJ L-Editor: A P-Editor: Yuan YY
Contributor Information
Wattana Leowattana, Department of Clinical Tropical Medicine, Faculty of Tropical Medicine, Mahidol University, Rachatawee 10400, Bangkok, Thailand. wattana.leo@mahidol.ac.th.
Pathomthep Leowattana, Department of Clinical Tropical Medicine, Faculty of Tropical Medicine, Mahidol University, Rachatawee 10400, Bangkok, Thailand.
Tawithep Leowattana, Department of Medicine, Faculty of Medicine, Srinakarinwirot University, Wattana 10110, Bangkok, Thailand.
References
- 1.Janse Van Rensburg A, Dube A, Curran R, Ambaw F, Murdoch J, Bachmann M, Petersen I, Fairall L. Comorbidities between tuberculosis and common mental disorders: a scoping review of epidemiological patterns and person-centred care interventions from low-to-middle income and BRICS countries. Infect Dis Poverty. 2020;9:4. doi: 10.1186/s40249-019-0619-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zaheen A, Bloom BR. Tuberculosis in 2020 - New Approaches to a Continuing Global Health Crisis. N Engl J Med. 2020;382:e26. doi: 10.1056/NEJMp2000325. [DOI] [PubMed] [Google Scholar]
- 3.García de Viedma D. Pathways and strategies followed in the genomic epidemiology of Mycobacterium tuberculosis. Infect Genet Evol. 2019;72:4–9. doi: 10.1016/j.meegid.2019.01.027. [DOI] [PubMed] [Google Scholar]
- 4.World Health Organization. TB disease burden. [Accessed on 12 December, 2022]. Available from: https://www.who.int/teams/global-tuberculosis-programme/tb-reports/global-tuberculosis-report-2022/tb-disease-burden/2-1-tb-incidence .
- 5.Rodriguez-Takeuchi SY, Renjifo ME, Medina FJ. Extrapulmonary Tuberculosis: Pathophysiology and Imaging Findings. Radiographics. 2019;39:2023–2037. doi: 10.1148/rg.2019190109. [DOI] [PubMed] [Google Scholar]
- 6.Moule MG, Cirillo JD. Mycobacterium tuberculosis Dissemination Plays a Critical Role in Pathogenesis. Front Cell Infect Microbiol. 2020;10:65. doi: 10.3389/fcimb.2020.00065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Louw QA, Tawa N, Van Niekerk SM, Conradie T, Coetzee M. Spinal tuberculosis: A systematic review of case studies and development of an evidence-based clinical guidance tool for early detection. J Eval Clin Pract. 2020;26:1370–1382. doi: 10.1111/jep.13309. [DOI] [PubMed] [Google Scholar]
- 8.Wang MS, Han C, Wang JL, Liu FL. The prevalence, diagnosis and surgical risk factors of spinal tuberculosis in children. Trop Med Int Health. 2020;25:834–838. doi: 10.1111/tmi.13411. [DOI] [PubMed] [Google Scholar]
- 9.Mulleman D, Mammou S, Griffoul I, Avimadje A, Goupille P, Valat JP. Characteristics of patients with spinal tuberculosis in a French teaching hospital. Joint Bone Spine. 2006;73:424–427. doi: 10.1016/j.jbspin.2005.10.013. [DOI] [PubMed] [Google Scholar]
- 10.Wang H, Li C, Wang J, Zhang Z, Zhou Y. Characteristics of patients with spinal tuberculosis: seven-year experience of a teaching hospital in Southwest China. Int Orthop. 2012;36:1429–1434. doi: 10.1007/s00264-012-1511-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Qu JT, Jiang YQ, Xu GH, Tang Y, Wang ZT, Ye XJ, Shi GH, Dong JW, Li J, Zhou JL, Hu Y. Clinical characteristics and neurologic recovery of patients with cervical spinal tuberculosis: should conservative treatment be preferred? A retrospective follow-up study of 115 cases. World Neurosurg. 2015;83:700–707. doi: 10.1016/j.wneu.2015.01.015. [DOI] [PubMed] [Google Scholar]
- 12.Wang P, Liao W, Cao G, Jiang Y, Rao J, Yang Y. Characteristics and Management of Spinal Tuberculosis in Tuberculosis Endemic Area of Guizhou Province: A Retrospective Study of 597 Patients in a Teaching Hospital. Biomed Res Int. 2020;2020:1468457. doi: 10.1155/2020/1468457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yang S, Yu Y, Ji Y, Luo DJ, Zhang ZY, Huang GP, He FY, Wu WJ, Mou XP. Multi-drug resistant spinal tuberculosis-epidemiological characteristics of in-patients: a multicentre retrospective study. Epidemiol Infect. 2020;148:e11. doi: 10.1017/S0950268820000011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zeng H, Liang Y, He J, Chen L, Su H, Liao S, Huang S, Qin H. Analysis of Clinical Characteristics of 556 Spinal Tuberculosis Patients in Two Tertiary Teaching Hospitals in Guangxi Province. Biomed Res Int. 2021;2021:1344496. doi: 10.1155/2021/1344496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kilborn T, Janse van Rensburg P, Candy S. Pediatric and adult spinal tuberculosis: imaging and pathophysiology. Neuroimaging Clin N Am. 2015;25:209–231. doi: 10.1016/j.nic.2015.01.002. [DOI] [PubMed] [Google Scholar]
- 16.Dureja S, Sen IB, Acharya S. Potential role of F18 FDG PET-CT as an imaging biomarker for the noninvasive evaluation in uncomplicated skeletal tuberculosis: a prospective clinical observational study. Eur Spine J. 2014;23:2449–2454. doi: 10.1007/s00586-014-3483-8. [DOI] [PubMed] [Google Scholar]
- 17.Procopie I, Popescu EL, Pleșea RM, Dorobanțu M, Mureșan RF, Lupașcu-Ursulescu CV, Pleșea IE, Anușca DN. Clinical-Morphological Aspects in Spinal Tuberculosis. Curr Health Sci J. 2018;44:250–260. doi: 10.12865/CHSJ.44.03.08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Liu Z, Wang J, Chen GZ, Li WW, Wu YQ, Xiao X, Zhang YL, Yang Y, Hu WK, Sun ZC, Wang XY. Clinical Characteristics of 1378 Inpatients with Spinal Tuberculosis in General Hospitals in South-Central China. Biomed Res Int. 2019;2019:9765253. doi: 10.1155/2019/9765253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Shi T, Zhang Z, Dai F, Zhou Q, He Q, Luo F, Hou T, Xu J. Retrospective Study of 967 Patients With Spinal Tuberculosis. Orthopedics. 2016;39:e838–e843. doi: 10.3928/01477447-20160509-03. [DOI] [PubMed] [Google Scholar]
- 20.Kumar K. Spinal tuberculosis, natural history of disease, classifications and principles of management with historical perspective. Eur J Orthop Surg Traumatol. 2016;26:551–558. doi: 10.1007/s00590-016-1811-x. [DOI] [PubMed] [Google Scholar]
- 21.Chandra SP, Singh A, Goyal N, Laythalling RK, Singh M, Kale SS, Sharma MS, Suri A, Singh P, Garg A, Sarkar C, Tripathi M, Sharma BS, Mahapatra AK. Analysis of changing paradigms of management in 179 patients with spinal tuberculosis over a 12-year period and proposal of a new management algorithm. World Neurosurg. 2013;80:190–203. doi: 10.1016/j.wneu.2012.12.019. [DOI] [PubMed] [Google Scholar]
- 22.Jain AK, Kumar J. Tuberculosis of spine: neurological deficit. Eur Spine J. 2013;22 Suppl 4:624–633. doi: 10.1007/s00586-012-2335-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Frankel HL, Hancock DO, Hyslop G, Melzak J, Michaelis LS, Ungar GH, Vernon JD, Walsh JJ. The value of postural reduction in the initial management of closed injuries of the spine with paraplegia and tetraplegia. I. Paraplegia. 1969;7:179–192. doi: 10.1038/sc.1969.30. [DOI] [PubMed] [Google Scholar]
- 24.Jain AK, Sinha S. Evaluation of systems of grading of neurological deficit in tuberculosis of spine. Spinal Cord. 2005;43:375–380. doi: 10.1038/sj.sc.3101718. [DOI] [PubMed] [Google Scholar]
- 25.Zhang Z, Hao Y, Wang X, Zheng Z, Zhao X, Wang C, Zhang X. Minimally invasive surgery for paravertebral or psoas abscess with spinal tuberculosis - a long-term retrospective study of 106 cases. BMC Musculoskelet Disord. 2020;21:353. doi: 10.1186/s12891-020-03344-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Divya A, Shukla DP, Bahubali VH, Bharath RD, Nandeesh BN, Kruthika P, Srinivas D, Siddaiah N. Consumption of spine by tuberculosis in the era of directly observed treatment, short-course and genomic diagnosis. Indian J Tuberc. 2021;68:73–79. doi: 10.1016/j.ijtb.2020.08.017. [DOI] [PubMed] [Google Scholar]
- 27.Gehlot PS, Chaturvedi S, Kashyap R, Singh V. Pott's Spine: Retrospective Analysis of MRI Scans of 70 Cases. J Clin Diagn Res. 2012;6:1534–1538. doi: 10.7860/JCDR/2012/4618.2552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Misra UK, Warrier S, Kalita J, Kumar S. MRI findings in Pott's spine and correlating clinical progress with radiological findings. Neuroradiology. 2020;62:825–832. doi: 10.1007/s00234-020-02402-2. [DOI] [PubMed] [Google Scholar]
- 29.Raut AA, Naphade PS, Ramakantan R. Imaging Spectrum of Extrathoracic Tuberculosis. Radiol Clin North Am. 2016;54:475–501. doi: 10.1016/j.rcl.2015.12.013. [DOI] [PubMed] [Google Scholar]
- 30.Yusof MI, Hassan E, Rahmat N, Yunus R. Spinal tuberculosis: the association between pedicle involvement and anterior column damage and kyphotic deformity. Spine (Phila Pa 1976) 2009;34:713–717. doi: 10.1097/BRS.0b013e31819b2159. [DOI] [PubMed] [Google Scholar]
- 31.Rajasekaran S. Kyphotic deformity in spinal tuberculosis and its management. Int Orthop. 2012;36:359–365. doi: 10.1007/s00264-011-1469-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rajasekaran S. Natural history of Pott's kyphosis. Eur Spine J. 2013;22 Suppl 4:634–640. doi: 10.1007/s00586-012-2336-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Jain AK, Sreenivasan R, Mukunth R, Dhammi IK. Tubercular spondylitis in children. Indian J Orthop. 2014;48:136–144. doi: 10.4103/0019-5413.128747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Pinto D, Dhawale A, Shah I, Rokade S, Shah A, Chaudhary K, Aroojis A, Mehta R, Nene A. Tuberculosis of the spine in children - does drug resistance affect surgical outcomes? Spine J. 2021;21:1973–1984. doi: 10.1016/j.spinee.2021.06.001. [DOI] [PubMed] [Google Scholar]
- 35.Rajasekaran S. The natural history of post-tubercular kyphosis in children. Radiological signs which predict late increase in deformity. J Bone Joint Surg Br. 2001;83:954–962. doi: 10.1302/0301-620x.83b7.12170. [DOI] [PubMed] [Google Scholar]
- 36.Rajasekaran S. Buckling collapse of the spine in childhood spinal tuberculosis. Clin Orthop Relat Res. 2007;460:86–92. doi: 10.1097/BLO.0b013e31806a9172. [DOI] [PubMed] [Google Scholar]
- 37.Rajagopalan S, Yoshikawa TT. Tuberculosis in the elderly. Z Gerontol Geriatr. 2000;33:374–380. doi: 10.1007/s003910070034. [DOI] [PubMed] [Google Scholar]
- 38.Yew WW, Yoshiyama T, Leung CC, Chan DP. Epidemiological, clinical and mechanistic perspectives of tuberculosis in older people. Respirology. 2018;23:567–575. doi: 10.1111/resp.13303. [DOI] [PubMed] [Google Scholar]
- 39.Teale C, Goldman JM, Pearson SB. The association of age with the presentation and outcome of tuberculosis: a five-year survey. Age Ageing. 1993;22:289–293. doi: 10.1093/ageing/22.4.289. [DOI] [PubMed] [Google Scholar]
- 40.Pu F, Feng J, Yang L, Zhang L, Xia P. Misdiagnosed and mismanaged atypical spinal tuberculosis: A case series report. Exp Ther Med. 2019;18:3723–3728. doi: 10.3892/etm.2019.8014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wang LN, Wang L, Liu LM, Song YM, Li Y, Liu H. Atypical spinal tuberculosis involved noncontiguous multiple segments: Case series report with literature review. Medicine (Baltimore) 2017;96:e6559. doi: 10.1097/MD.0000000000006559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Pande KC, Babhulkar SS. Atypical spinal tuberculosis. Clin Orthop Relat Res. 2002:67–74. doi: 10.1097/00003086-200205000-00010. [DOI] [PubMed] [Google Scholar]
- 43.Babhulkar SS, Tayade WB, Babhulkar SK. Atypical spinal tuberculosis. J Bone Joint Surg Br. 1984;66:239–242. doi: 10.1302/0301-620X.66B2.6707060. [DOI] [PubMed] [Google Scholar]
- 44.Cormican L, Hammal R, Messenger J, Milburn HJ. Current difficulties in the diagnosis and management of spinal tuberculosis. Postgrad Med J. 2006;82:46–51. doi: 10.1136/pgmj.2005.032862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sagane SS, Patil VS, Bartakke GD, Kale KY. Assessment of Clinical and Radiological Parameters in Spinal Tuberculosis: Comparison between Human Immunodeficiency Virus-Positive and Human Immunodeficiency Virus-Negative Patients. Asian Spine J. 2020;14:857–863. doi: 10.31616/asj.2019.0251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kanna RM, Babu N, Kannan M, Shetty AP, Rajasekaran S. Diagnostic accuracy of whole spine magnetic resonance imaging in spinal tuberculosis validated through tissue studies. Eur Spine J. 2019;28:3003–3010. doi: 10.1007/s00586-019-06031-z. [DOI] [PubMed] [Google Scholar]
- 47.Wang G, Dong W, Lan T, Fan J, Tang K, Li Y, Yan G, Jiang G, Ma Y, Shang Y, Qin S, Huang H. Diagnostic accuracy evaluation of the conventional and molecular tests for Spinal Tuberculosis in a cohort, head-to-head study. Emerg Microbes Infect. 2018;7:109. doi: 10.1038/s41426-018-0114-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Purohit M, Mustafa T. Laboratory Diagnosis of Extra-pulmonary Tuberculosis (EPTB) in Resource-constrained Setting: State of the Art, Challenges and the Need. J Clin Diagn Res. 2015;9:EE01–EE06. doi: 10.7860/JCDR/2015/12422.5792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Yao Y, Song W, Wang K, Ma B, Liu H, Zheng W, Tang Y, Zhou Y. Features of 921 Patients With Spinal Tuberculosis: A 16-Year Investigation of a General Hospital in Southwest China. Orthopedics. 2017;40:e1017–e1023. doi: 10.3928/01477447-20171012-03. [DOI] [PubMed] [Google Scholar]
- 50.Garg SK, Tiwari RP, Tiwari D, Singh R, Malhotra D, Ramnani VK, Prasad GB, Chandra R, Fraziano M, Colizzi V, Bisen PS. Diagnosis of tuberculosis: available technologies, limitations, and possibilities. J Clin Lab Anal. 2003;17:155–163. doi: 10.1002/jcla.10086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Otu J, Antonio M, Cheung YB, Donkor S, De Jong BC, Corrah T, Adegbola RA. Comparative evaluation of BACTEC MGIT 960 with BACTEC 9000 MB and LJ for isolation of mycobacteria in The Gambia. J Infect Dev Ctries. 2008;2:200–205. doi: 10.3855/jidc.263. [DOI] [PubMed] [Google Scholar]
- 52.Garg RK, Somvanshi DS. Spinal tuberculosis: a review. J Spinal Cord Med. 2011;34:440–454. doi: 10.1179/2045772311Y.0000000023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Jain AK, Jena SK, Singh MP, Dhammi IK, Ramachadran VG, Dev G. Evaluation of clinico-radiological, bacteriological, serological, molecular and histological diagnosis of osteoarticular tuberculosis. Indian J Orthop. 2008;42:173–177. doi: 10.4103/0019-5413.40253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Azadi D, Motallebirad T, Ghaffari K, Shojaei H. Mycobacteriosis and Tuberculosis: Laboratory Diagnosis. Open Microbiol J. 2018;12:41–58. doi: 10.2174/1874285801812010041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Held M, Laubscher M, Zar HJ, Dunn RN. GeneXpert polymerase chain reaction for spinal tuberculosis: an accurate and rapid diagnostic test. Bone Joint J. 2014;96-B:1366–1369. doi: 10.1302/0301-620X.96B10.34048. [DOI] [PubMed] [Google Scholar]
- 56.Solanki AM, Basu S, Biswas A, Roy S, Banta A. Sensitivity and Specificity of Gene Xpert in the Diagnosis of Spinal Tuberculosis: A Prospective Controlled Clinical Study. Global Spine J. 2020;10:553–558. doi: 10.1177/2192568219858310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Sharma K, Appannanavar SB, Modi M, Singh M, Sharma A, Varma S. Role of multiplex polymerase chain reaction using IS6110 and Protein b for the diagnosis of extra-pulmonary tuberculosis: North India. Indian J Pathol Microbiol. 2015;58:27–30. doi: 10.4103/0377-4929.151164. [DOI] [PubMed] [Google Scholar]
- 58.Sharma K, Sharma A, Sharma SK, Sen RK, Dhillon MS, Sharma M. Does multiplex polymerase chain reaction increase the diagnostic percentage in osteoarticular tuberculosis? A prospective evaluation of 80 cases. Int Orthop. 2012;36:255–259. doi: 10.1007/s00264-011-1241-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Dahiya B, K Mehta P. Utility of nanoparticle-based assays in the diagnosis of tuberculosis. Nanomedicine (Lond) 2021;16:1263–1268. doi: 10.2217/nnm-2021-0077. [DOI] [PubMed] [Google Scholar]
- 60.Sheffee NS, Rubio-Reyes P, Mirabal M, Calero R, Carrillo-Calvet H, Chen S, Chin KL, Shakimi NAS, Anis FZ, Suraiya S, Sarmiento ME, Norazmi MN, Acosta A, Rehm BHA. Engineered Mycobacterium tuberculosis antigen assembly into core-shell nanobeads for diagnosis of tuberculosis. Nanomedicine. 2021;34:102374. doi: 10.1016/j.nano.2021.102374. [DOI] [PubMed] [Google Scholar]
- 61.Cheon HJ, Lee SM, Kim SR, Shin HY, Seo YH, Cho YK, Lee SP, Kim MI. Colorimetric Detection of MPT64 Antibody Based on an Aptamer Adsorbed Magnetic Nanoparticles for Diagnosis of Tuberculosis. J Nanosci Nanotechnol. 2019;19:622–626. doi: 10.1166/jnn.2019.15905. [DOI] [PubMed] [Google Scholar]
- 62.Sudprasert W, Piyapromdee U, Lewsirirat S. Neurological Recovery Determined by C-Reactive Protein, Erythrocyte Sedimentation Rate and Two Different Posterior Decompressive Surgical Procedures: A Retrospective Clinical Study of Patients with Spinal Tuberculosis. J Med Assoc Thai. 2015;98:993–1000. [PubMed] [Google Scholar]
- 63.Li W, Liu Z, Xiao X, Xu Z, Sun Z, Zhang Z, Wang X. Early surgical intervention for active thoracic spinal tuberculosis patients with paraparesis and paraplegia. BMC Musculoskelet Disord. 2021;22:213. doi: 10.1186/s12891-021-04078-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Ansari S, Amanullah MF, Ahmad K, Rauniyar RK. Pott's Spine: Diagnostic Imaging Modalities and Technology Advancements. N Am J Med Sci. 2013;5:404–411. doi: 10.4103/1947-2714.115775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Ärztin CB. Human anatomy study guide. (Access 26 Dec 2022). Available from: https://www.kenhub.com/en/library/education/human-anatomy-study-guide .
- 66.Jain AK, Rajasekaran S, Jaggi KR, Myneedu VP. Tuberculosis of the Spine. J Bone Joint Surg Am. 2020;102:617–628. doi: 10.2106/JBJS.19.00001. [DOI] [PubMed] [Google Scholar]
- 67.Moon MS. Tuberculosis of spine: current views in diagnosis and management. Asian Spine J. 2014;8:97–111. doi: 10.4184/asj.2014.8.1.97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Rasouli MR, Mirkoohi M, Vaccaro AR, Yarandi KK, Rahimi-Movaghar V. Spinal tuberculosis: diagnosis and management. Asian Spine J. 2012;6:294–308. doi: 10.4184/asj.2012.6.4.294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Li J, Huang X, Chen F, Dai F, Zhou Q, Luo F, Xu J, Zhang Z. Computed Tomography-Guided Catheterization Drainage to Cure Spinal Tuberculosis With Individualized Chemotherapy. Orthopedics. 2017;40:e443–e449. doi: 10.3928/01477447-20170117-02. [DOI] [PubMed] [Google Scholar]
- 70.Gupta P, Prakash M, Sharma N, Kanojia R, Khandelwal N. Computed tomography detection of clinically unsuspected skeletal tuberculosis. Clin Imaging. 2015;39:1056–1060. doi: 10.1016/j.clinimag.2015.07.033. [DOI] [PubMed] [Google Scholar]
- 71.Gouliamos AD, Kehagias DT, Lahanis S, Athanassopoulou AA, Moulopoulou ES, Kalovidouris AA, Trakadas SJ, Vlahos LJ. MR imaging of tuberculous vertebral osteomyelitis: pictorial review. Eur Radiol. 2001;11:575–579. doi: 10.1007/s003300000631. [DOI] [PubMed] [Google Scholar]
- 72.Kumar Y, Gupta N, Chhabra A, Fukuda T, Soni N, Hayashi D. Magnetic resonance imaging of bacterial and tuberculous spondylodiscitis with associated complications and non-infectious spinal pathology mimicking infections: a pictorial review. BMC Musculoskelet Disord. 2017;18:244. doi: 10.1186/s12891-017-1608-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Guo H, Lan S, He Y, Tiheiran M, Liu W. Differentiating brucella spondylitis from tuberculous spondylitis by the conventional MRI and MR T2 mapping: a prospective study. Eur J Med Res. 2021;26:125. doi: 10.1186/s40001-021-00598-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Tiberi S, du Plessis N, Walzl G, Vjecha MJ, Rao M, Ntoumi F, Mfinanga S, Kapata N, Mwaba P, McHugh TD, Ippolito G, Migliori GB, Maeurer MJ, Zumla A. Tuberculosis: progress and advances in development of new drugs, treatment regimens, and host-directed therapies. Lancet Infect Dis. 2018;18:e183–e198. doi: 10.1016/S1473-3099(18)30110-5. [DOI] [PubMed] [Google Scholar]
- 75.Lambert PA. Cellular impermeability and uptake of biocides and antibiotics in Gram-positive bacteria and mycobacteria. J Appl Microbiol. 2002;92 Suppl:46S–54S. [PubMed] [Google Scholar]
- 76.Goossens SN, Sampson SL, Van Rie A. Mechanisms of Drug-Induced Tolerance in Mycobacterium tuberculosis. Clin Microbiol Rev. 2020;34 doi: 10.1128/CMR.00141-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Pasipanodya JG, Gumbo T. A meta-analysis of self-administered vs directly observed therapy effect on microbiologic failure, relapse, and acquired drug resistance in tuberculosis patients. Clin Infect Dis. 2013;57:21–31. doi: 10.1093/cid/cit167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Unissa AN, Subbian S, Hanna LE, Selvakumar N. Overview on mechanisms of isoniazid action and resistance in Mycobacterium tuberculosis. Infect Genet Evol. 2016;45:474–492. doi: 10.1016/j.meegid.2016.09.004. [DOI] [PubMed] [Google Scholar]
- 79.Vilchèze C, Jacobs WR Jr. The Isoniazid Paradigm of Killing, Resistance, and Persistence in Mycobacterium tuberculosis. J Mol Biol. 2019;431:3450–3461. doi: 10.1016/j.jmb.2019.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Zaw MT, Emran NA, Lin Z. Mutations inside rifampicin-resistance determining region of rpoB gene associated with rifampicin-resistance in Mycobacterium tuberculosis. J Infect Public Health. 2018;11:605–610. doi: 10.1016/j.jiph.2018.04.005. [DOI] [PubMed] [Google Scholar]
- 81.Nusrath Unissa A, Hanna LE. Molecular mechanisms of action, resistance, detection to the first-line anti tuberculosis drugs: Rifampicin and pyrazinamide in the post whole genome sequencing era. Tuberculosis (Edinb) 2017;105:96–107. doi: 10.1016/j.tube.2017.04.008. [DOI] [PubMed] [Google Scholar]
- 82.Stehr M, Elamin AA, Singh M. Pyrazinamide: the importance of uncovering the mechanisms of action in mycobacteria. Expert Rev Anti Infect Ther. 2015;13:593–603. doi: 10.1586/14787210.2015.1021784. [DOI] [PubMed] [Google Scholar]
- 83.Njire M, Tan Y, Mugweru J, Wang C, Guo J, Yew W, Tan S, Zhang T. Pyrazinamide resistance in Mycobacterium tuberculosis: Review and update. Adv Med Sci. 2016;61:63–71. doi: 10.1016/j.advms.2015.09.007. [DOI] [PubMed] [Google Scholar]
- 84.Pawar A, Jha P, Konwar C, Chaudhry U, Chopra M, Saluja D. Ethambutol targets the glutamate racemase of Mycobacterium tuberculosis-an enzyme involved in peptidoglycan biosynthesis. Appl Microbiol Biotechnol. 2019;103:843–851. doi: 10.1007/s00253-018-9518-z. [DOI] [PubMed] [Google Scholar]
- 85.Singh R, Dwivedi SP, Gaharwar US, Meena R, Rajamani P, Prasad T. Recent updates on drug resistance in Mycobacterium tuberculosis. J Appl Microbiol. 2020;128:1547–1567. doi: 10.1111/jam.14478. [DOI] [PubMed] [Google Scholar]
- 86.Sharma P, Kumar B, Gupta Y, Singhal N, Katoch VM, Venkatesan K, Bisht D. Proteomic analysis of streptomycin resistant and sensitive clinical isolates of Mycobacterium tuberculosis. Proteome Sci. 2010;8:59. doi: 10.1186/1477-5956-8-59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Ruiz P, Rodríguez-Cano F, Zerolo FJ, Casal M. Investigation of the in vitro activity of streptomycin against Mycobacterium tuberculosis. Microb Drug Resist. 2002;8:147–149. doi: 10.1089/107662902760190707. [DOI] [PubMed] [Google Scholar]
- 88.Miotto P, Cirillo DM, Migliori GB. Drug resistance in Mycobacterium tuberculosis: molecular mechanisms challenging fluoroquinolones and pyrazinamide effectiveness. Chest. 2015;147:1135–1143. doi: 10.1378/chest.14-1286. [DOI] [PubMed] [Google Scholar]
- 89.Ginsburg AS, Grosset JH, Bishai WR. Fluoroquinolones, tuberculosis, and resistance. Lancet Infect Dis. 2003;3:432–442. doi: 10.1016/s1473-3099(03)00671-6. [DOI] [PubMed] [Google Scholar]
- 90.Vianna JF, S Bezerra K, I N Oliveira J, Albuquerque EL, Fulco UL. Binding energies of the drugs capreomycin and streptomycin in complex with tuberculosis bacterial ribosome subunits. Phys Chem Chem Phys. 2019;21:19192–19200. doi: 10.1039/c9cp03631h. [DOI] [PubMed] [Google Scholar]
- 91.Quenard F, Fournier PE, Drancourt M, Brouqui P. Role of second-line injectable antituberculosis drugs in the treatment of MDR/XDR tuberculosis. Int J Antimicrob Agents. 2017;50:252–254. doi: 10.1016/j.ijantimicag.2017.01.042. [DOI] [PubMed] [Google Scholar]
- 92.Zhang T, Yu X, Wen S, Xue Y, Xiao H, Ren R, Wang F, Dong L, Qin S, Huang H. Bone Penetration of Cycloserine in Osteoarticular Tuberculosis Patients of China. Antimicrob Agents Chemother. 2022;66:e0222421. doi: 10.1128/aac.02224-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Alghamdi WA, Alsultan A, Al-Shaer MH, An G, Ahmed S, Alkabab Y, Banu S, Barbakadze K, Houpt E, Kipiani M, Mikiashvili L, Schmidt S, Heysell SK, Kempker RR, Cegielski JP, Peloquin CA. Cycloserine Population Pharmacokinetics and Pharmacodynamics in Patients with Tuberculosis. Antimicrob Agents Chemother. 2019;63 doi: 10.1128/AAC.00055-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Yun HY, Chang MJ, Jung H, Chang V, Wang Q, Strydom N, Yoon YR, Savic RM. Prothionamide Dose Optimization Using Population Pharmacokinetics for Multidrug-Resistant Tuberculosis Patients. Antimicrob Agents Chemother. 2022;66:e0189321. doi: 10.1128/aac.01893-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Thee S, Garcia-Prats AJ, Donald PR, Hesseling AC, Schaaf HS. A review of the use of ethionamide and prothionamide in childhood tuberculosis. Tuberculosis (Edinb) 2016;97:126–136. doi: 10.1016/j.tube.2015.09.007. [DOI] [PubMed] [Google Scholar]
- 96.Abulfathi AA, Donald PR, Adams K, Svensson EM, Diacon AH, Reuter H. The pharmacokinetics of para-aminosalicylic acid and its relationship to efficacy and intolerance. Br J Clin Pharmacol. 2020;86:2123–2132. doi: 10.1111/bcp.14395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.von Groote-Bidlingmaier F, Patientia R, Sanchez E, Balanag V Jr, Ticona E, Segura P, Cadena E, Yu C, Cirule A, Lizarbe V, Davidaviciene E, Domente L, Variava E, Caoili J, Danilovits M, Bielskiene V, Staples S, Hittel N, Petersen C, Wells C, Hafkin J, Geiter LJ, Gupta R. Efficacy and safety of delamanid in combination with an optimised background regimen for treatment of multidrug-resistant tuberculosis: a multicentre, randomised, double-blind, placebo-controlled, parallel group phase 3 trial. Lancet Respir Med. 2019;7:249–259. doi: 10.1016/S2213-2600(18)30426-0. [DOI] [PubMed] [Google Scholar]
- 98.Blair HA, Scott LJ. Delamanid: a review of its use in patients with multidrug-resistant tuberculosis. Drugs. 2015;75:91–100. doi: 10.1007/s40265-014-0331-4. [DOI] [PubMed] [Google Scholar]
- 99.Li Y, Sun F, Zhang W. Bedaquiline and delamanid in the treatment of multidrug-resistant tuberculosis: Promising but challenging. Drug Dev Res. 2019;80:98–105. doi: 10.1002/ddr.21498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Mak KC, Cheung KM. Surgical treatment of acute TB spondylitis: indications and outcomes. Eur Spine J. 2013;22 Suppl 4:603–611. doi: 10.1007/s00586-012-2455-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Singh R, Magu NK, Rohilla RK. Clinicoradiologic Profile of Involvement and Healing in Tuberculosis of the Spine. Ann Med Health Sci Res. 2016;6:311–327. doi: 10.4103/amhsr.amhsr_188_15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Maurya VK, Sharma P, Ravikumar R, Debnath J, Sharma V, Srikumar S, Bhatia M. Tubercular spondylitis: A review of MRI findings in 80 cases. Med J Armed Forces India. 2018;74:11–17. doi: 10.1016/j.mjafi.2016.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Sharma A, Chhabra HS, Chabra T, Mahajan R, Batra S, Sangondimath G. Demographics of tuberculosis of spine and factors affecting neurological improvement in patients suffering from tuberculosis of spine: a retrospective analysis of 312 cases. Spinal Cord. 2017;55:59–63. doi: 10.1038/sc.2016.85. [DOI] [PubMed] [Google Scholar]
- 104.Trecarichi EM, Di Meco E, Mazzotta V, Fantoni M. Tuberculous spondylodiscitis: epidemiology, clinical features, treatment, and outcome. Eur Rev Med Pharmacol Sci. 2012;16 Suppl 2:58–72. [PubMed] [Google Scholar]