ABSTRACT
Introduction:
The endocannabinoid system (ECS), discovered in the 1990s, is a system involved with maintaining cellular homeostasis by down-regulating the damaging inflammatory responses and upregulating regenerative processes. Cannabidiol (CBD), tetrahydrocannabivarin (THCV), and cannabidivarin (CBDV) are all phytocannabinoids found in varying quantities in hemp extract. These three cannabinoids have novel therapeutic effects on hair regrowth through the ECS. The method of action is different from and synergistic with current hair regrowth therapies. The three cannabinoids are fat-soluble and poorly absorbed past the epidermis, but topical application easily reaches hair follicles where they act as partial or full CB1 antagonist and agonist of transient receptor potential vanilloid-1 (TRPV1) and vanilloid receptor-4 (TRPV4). All these ECS receptors relate to hair follicle function. Blocking the CB1 receptor on the hair follicle has been shown to result in hair shaft elongation; in addition, the hair follicle cycle (anagen, catagen, and telogen phases) is controlled by TRPV1. The effects of CBD on hair growth are dose dependent and higher doses may result in premature entry into the catagen phase through a different receptor known as TRPV4. CBD has also been shown to increase Wnt signaling, which causes dermal progenitor cells to differentiate into new hair follicles and maintains anagen phase of the hair cycle.
Objective:
This study was conducted on subjects with androgenetic alopecia (AGA), as follow-up to a prior published study using hemp extract high in CBD without CBDV or THCV. That study showed an average 93.5% increase in hair numbers after 6 months of use. This subsequent study is being done to determine if daily topical application of a hemp-oil high in CBD, THCV, and CBDV concentrations would result in improved hair regrowth in the area of the scalp most affected by AGA.
Materials and Methods:
A case series study was done of 31 (15 men and 16 women, 27 Caucasian, 2 Asian, and 1 mixed race) subjects with AGA. They used a once-daily topical hemp extract formulation, averaging about 33 mg/day for 6 months. A hair count of the greatest area of alopecia was carried out before treatment was started and again after 6 months of treatment. To facilitate consistent hair count analysis, a permanent tattoo was placed at the point for maximum hair loss on the scalp. The subjects were also asked to qualitatively rate their psychosocial perception of “scalp coverage” improvement after the study was completed. The qualitative scale included “very unhappy,” “unhappy,” “neutral,” “happy,” and “very happy.” The subjects were photographed in a standard manner before and after the study. The photographs were compared for improvements in “scalp coverage” by an independent physician. The qualitative scale included “none,” “mild,” “moderate,” and “extensive” improvement of scalp coverage.
Results:
The results revealed that all subjects had some regrowth. This ranged from 31.25% (from 16 to 21 hairs) to 2000% (from 1 to 21 hairs). The average increase was statistically significant 246% (15.07 hairs/cm2 increase) in men and 127% (16.06 hairs/cm2) in women. There were no reported adverse effects. All subjects rated their psychosocial perception of the effects of the hair loss, as “happy” or “very happy.” Independent review of the photographs revealed evidence of “mild” to “extensive” scalp coverage improvements for all of the subjects.
Conclusion:
Although the exact mechanism of therapeutic effects is not known, THCV and CBDV are most likely functioning as full CB1 receptor neutral antagonists and CBD is most likely functioning as a partial CB1 receptor antagonist and potentially through Wnt messaging. All three cannabinoids were functioning as TRPV1 agonists. The addition of menthol through the peppermint extract is probably acting through promoting a rapid onset of anagen phase. This topical hemp formulation was superior to oral finasteride, 5% minoxidil once daily foam and CBD topical extract alone. Since this hemp extract works through novel mechanisms entirely different from both finasteride and minoxidil, it can be used in conjunction with these current drugs and would be expected to have synergistic effects. However, safety and efficacy of this combination would be to be evaluated.
Keywords: Androgenetic alopecia, cannabidiol, cannabidivarin, endocannabinoid system, tetrahydrocannabivarin
INTRODUCTION
Androgenetic alopecia (AGA) is a common condition that occurs in both men and women and increases in prevalence with age. It is by far the most common cause of baldness. It generally starts in the third and fourth decades of life and significantly increases in prevalence in women after menopause.[2,3] Is it estimated that 50% of Caucasian men and 19% of Caucasian women are affected by the age of 50 years, and there is a lower prevalence and severity of the condition in Asian and African men.[4] AGA may adversely impact a person both psychologically and socially, especially in women.[5,6] The condition is characterized by follicular miniaturization in a specific pattern due to the effects of systemic androgens and genetic factors.[7]
In the male pattern phenotype, the hairline regresses at the bitemporal regions and at the vertex, and in the female pattern, there is diffuse thinning with preservation of the frontal hairline; however, the pathogenesis is the same.[5] AGA develops due to a disturbance in the cyclic transformation of hair follicles from active hair shaft growth and pigment production (anagen) to apoptosis-driven (cell death) hair follicle involution (catagen).[2]
The pathology of hair loss, especially female AGA, is complex with many gaps in the understanding of pathophysiology. In normal hair growth, the hair follicles cycle through distinct phases.[8] Growth, known as anagen phase, can last up to 6 years. Ninety percent of the follicles are usually in this phase. In this phase, the hair shaft extends and thickens due to matrix cell activity. This is followed for the catagen or resting phase. Only about 1% of follicles are usually in this phase. In this phase, the follicle regresses. This is followed by a period of quiescence known as the telogen phase. About 9% of follicles are usually in this phase. The last phase is call the exogen phase with the release of the hair shaft. The proportion of follicles in anagen phase declines with age.[9,10]
The signals that cause the transition between the various phases of the hair follicle are not well understood. There is complex signaling cycles between the root sheath and the dermal papillae, involving a number of protein families including the fibroblast growth factors, bone morphogenic proteins, sonic hedgehog, and Wnt signaling, are involved.[10]
In males, androgens are usually involved, and this has led to the term of AGA. It is known that in many men dihydrotestosterone (DHT), synthesized from testosterone by 5α reductases, is a major player.[11] The intracellular signaling cascade after androgen receptor binding by DHT is poorly understood. In women, there is less evidence regarding the involvement of androgens. The higher prevalence of AGA in postmenopausal women has led to the association with hormones.[12]
Current treatment
Only two medications, topical minoxidil, and oral finasteride are the Food and Drug Administration (FDA) approved for the treatment of AGA. Then can be used alone or in combination for synergistic effects.[13] There have been no new medications FDA approved in over 15 years. Finasteride, a 5α reductase inhibitor, blocks the conversion of testosterone to DHT[14] has proven useful in the treatment of AGA.[3] However, finasteride is associated with a number of side effects, including sexual dysfunction that in some cases persists after ceasing therapy.[15] Despite a small number of studies showing efficacy, the use of finasteride in women is controversial, and only off-label, uncontrolled studies and anecdotal evidence have reported positive results.[16] Finasteride treatment in premenopausal women is also problematic, requiring concomitant contraceptive treatment due to the teratogenic nature of the compound.[16]
Topical minoxidil is the only known treatment that is effective in both men and women; however, the mechanism of action is unknown[17] Minoxidil is generally well tolerated but is associated with a number of side effects, including an initial increase in hair shedding and exacerbation of hair loss after withdrawal from treatment.[18]
Unfortunately, these medications offer limited results.[19,20,21] Recently, the combination of topical minoxidil and topical finasteride has shown more promising results.[13,22] Surgical hair transplantation is the only current successful permanent option. Several other medical options, including antiandrogens such as spironolactone, oral contraceptives, cyproterone, flutamide, dutasteride, prostaglandin analogs, and ketoconazole are reported to be beneficial. However, these treatments can be associated with significant adverse effects and are expensive.[21] Laser and light therapies have also become popular despite the lack of documented profound benefit.[23]
A Phase III clinical trial of men with AGA was conducted in 2021. The study used an investigational new topical drug called SM04554. SM04554 works by modulating the Wnt pathway that is postulated to initiate and maintain the anagen phase of the hair cycle.[24] Wnt signaling also causes dermal progenitor cells to differentiate into new hair follicles. It is interesting to note that cannabidiol (CBD) has also been shown to increase Wnt signaling.[25] To date, however, there is little basic science or clinical research on CBD and Wnt signaling.
Recently, with the increasing acceptance of cannabis sativa-based therapies, hemp extract has come under consideration as a possible, effective, safe, inexpensive nonprescription, topical AGA therapy. A 2021 case study[26] of CBD-rich hemp extract revealed 93.5% average increase in hair regrowth. Hemp extract works through the endocannabinoid system (ECS) in the body and has novel effects on hair follicle elongation and hair matrix keratinocytes activated through ECS receptors in the hair follicle cells.[26] As such, the therapeutic effects of hemp extract would complement the physiologic effects of minoxidil, finasteride, and antiandrogen therapies.
Tetrahydrocannabivarin (THCV) and cannabidivarin (CBDV) are full CB1 antagonists, compared to CBD which is a partial CB1 antagonist.[27,28] Therefore, the therapeutic effects from CB1 blockade should be more marked with the addition of THCV and CBDV.[27,29,30] Until recently, hemp extract with significant percentages of THCV and/or CBDV has been unavailable commercially.
A 2014 study investigated hair growth in mice using 3% peppermint oil compared to 3% minoxidil and jojoba oil. The results showed that peppermint oil (40% menthol) “showed the most prominent hair growth effects; a significant increase in dermal thickness, follicle number, and follicle depth.” The researchers suggested that peppermint oil induces a rapid anagen stage.[1]
Endocannabinoid system and hair follicles
The ECS was discovered in the 1990s. In essence, it is a system involved with maintaining cellular homeostasis in response to excess oxidative stress. It downregulates the damaging inflammatory response, and it upregulates regenerative processes. It is comprised of at several receptors, including cannabinoid receptor 1 and 2 (CB1 and CB2), transient receptor potential vanilloid-1 (TRPV1), and transient receptor potential vanilloid-4 (TRPV4). It has at least two messenger molecules known as the endocannabinoids, anandamide, and 2-arachidonylglycerol.[31] One of the many systems that the ECS is involved with is thermoregulation within the skin. There are a substantial number of CB1 and CB2 receptors on various cell types within the skin.[32] CB1 receptors are well expressed in hair follicle cells.[33]
The hair follicle cycle (anagen, catagen, and telogen phases) is controlled by the TRPV1.[34] TRPV1 receptors are found on hair matrix keratinocytes. Mouse studies have shown that activation TRPV1 receptors promote hair follicle regression (catagen) and hair matrix keratinocyte apoptosis (cell death) and retardation of hair shaft elongation.[34] Endocannabinoids, and cannabis-derived phytocannabinoids, such as tetrahydrocannabinol (THC) and CBD, message TRPV1 receptors. It is postulated that CBD has therapeutic effects through TRPV1 receptors by such excessive activation of the receptor that then become desensitized.[35]
THC is a CB1 receptor agonist, and it has been shown to dose-dependently inhibit hair shaft elongation, decrease proliferation of hair matrix keratinocytes, and induce intraepithelial apoptosis and premature hair follicle regression (catagen). These effects from THC were inhibited by a selective CB1 antagonist.[33,34]
The available research suggests that THC and other CB1 agonists can be used to manage unwanted hair growth, and likewise, CB1 antagonists, such as CBD, THCV, and CBDV, can be used to promote hair growth.[33] CBD is a CB1 partial antagonist that probably produces its effects through negative allosteric modulation of the CB1 receptor.[28,36] Whereas, THCV and CBDV are full neutral CB1 receptor antagonists.[28]
A recent study of human hair follicle cultured cells revealed that the use of lower doses of CBD resulted in hair shaft elongation, probably through CB1 antagonism.[37] However, much higher doses resulted in premature entry into the catagen phase, probably through a different receptor, the TRPV4. Therefore, the dosing of the topical CBD needs to be evaluated to obtain positive hair growth.
Cannabidiol
Over the past decade, CBD has been extensively researched for a myriad of therapeutic benefits.[38] CBD does not cause euphoria or addiction. It is safe, with a wide therapeutic window and few adverse effects. The topical application of CBD has not been associated with any significant adverse effects.[32,34] CBD in an oral form has been FDA approved for the treatment of recalcitrant epilepsy. CBD in sublingual, oral, inhaled, and topical versions are relatively inexpensive and widely available as nutraceuticals. It is estimated that 14% of the U.S. population has tried CBD products.[39]
CBD is fat-soluble and poorly absorbed past the epidermis, but topical application of CBD easily reaches hair follicles where it is a CB1 antagonist and TRPV1 and TRPV4 agonist.[37]
Varins
THCV and CBDV, known together as the “varins,” have not been as extensively researched because of the dearth of available hemp extract containing any significant amounts of these two cannabinoids. There has been considerable research of ingested varins derived from marijuana (high-THC Cannabis sativa) for the treatment of epilepsy,[40] obesity,[41] and diabetes mellitus.[41]
MATERIALS AND METHODS
The study is a case series of adults presenting to a “Hair and Scalp” center in Clearwater, Florida. Adult subjects, who were not currently using minoxidil or finasteride, were offered the opportunity to receive the hemp-based formulation free of charge. Thirty-one subjects, 15 males and 16 females, representing a variety of racial backgrounds (27 Caucasian, 2 Asian, and 2 Mixed-race), presented AGA with Norwood-Hamilton scale classification score of 3V or higher. The predefined endpoints were hair counts obtained in a defined, representative area of scalp hair loss, and investigator clinical assessment of hair growth.
Ages ranged from 31 to 65 for the females and from 39 to 64 for the males. The subjects gave their written informed consent for this 6-month trial. The study adhered to the Helsinki guidelines and was institutionally approved. None of the subjects were currently using minoxidil or finasteride. No other hair loss treatments were used during the 6 months of the research.
The subjects were given a one-ounce dispenser and advised to apply a thin layer once each morning to the areas of baldness. The formulation was made of a whole plant extract (CBD 60.00%, CBDV 12.63%, THCV 3.71%, delta 9 THC 0.18%, cannabigerol 0.86%, and cannabinol 0.05%). This hemp extract was independently analyzed by ACS Laboratory, Sun City Center, Florida. Each one ounce of the formulation contained active ingredients of 1 g of this hemp extract, 5 g of menthol, 600 g of peppermint oil-infused into a vehicle of 5 g of ethanol, 600 g of Emu oil and 14.9 g of Hexafluoroacetone (HFA) 134A (1,11,2-tetrafluoroethane) propellant, and 900 ml of dimethicone. The one-ounce foam spray or tincture lasted approximately 1 month on average. This is an average daily dose of 33 mg of topically applied hemp extract. The subjects were advised that they could use blow dryers, conditioners, and shampoos. The formulation was replaced as needed throughout the 6-month trial.
A hair count of the greatest area of alopecia was carried out before treatment was started and again after 6 months of treatment. To facilitate consistent hair count analysis a permanent black tattoo dot was placed at the point of maximum hair loss on the scalp. The nonvellus hairs within the 1 cm2 around the tattoo were pulled through the opening of a one-centimeter mold with a surgical skin hook and a hair count taken using a Bodelin ProScope with ×50 magnification.
RESULTS
For all males, the baseline hair count was 6.13/cm2 and at 6 months, it was 21.20/cm2 (one-tailed paired t-test P < 0.00001). This represented an average increase of 246% or 15.50% additional hairs in the one square centimeter mold. For all females, the baseline hair count was 12.69/cm2 and at 6 months, it was 28.75/cm2 (one-tailed paired t-test P < 0.00001). This represented an average increase of 127% or 15.50% additional hairs in the 1 cm2 mold. For all adults, the baseline hair count was 9.50/cm2 and it increased after 6 months to 25.00 (one-tailed paired t-test P < 0.00001). This represented an average increase of 164% or 15.50% additional hairs in the one square centimeter mold. All subjects had some increase in hair count. The increase ranged from 31.25% in a female (16–21 hairs/cm2) to 2000% in a male (1–21 hairs/cm2). In general, the increased hair counts were associated with a cosmetically pleasing result.
All subjects rated their psychosocial perception of the effects of the hair loss, as “happy” (17 out of 31, 55%), or “very happy” (14 out of 31, 45%).
An Independent physician review of the photographs revealed evidence of “mild” to “extensive” scalp coverage improvements for all of the subjects.
The following photographs are representative images of before and after treatment in a male and a female from the study.
Figures 1-4 show a male (top) and female (bottom) patient's results, starting from baseline (Day 1, left) to completion of the study (Day 180, right).
Figure 1.
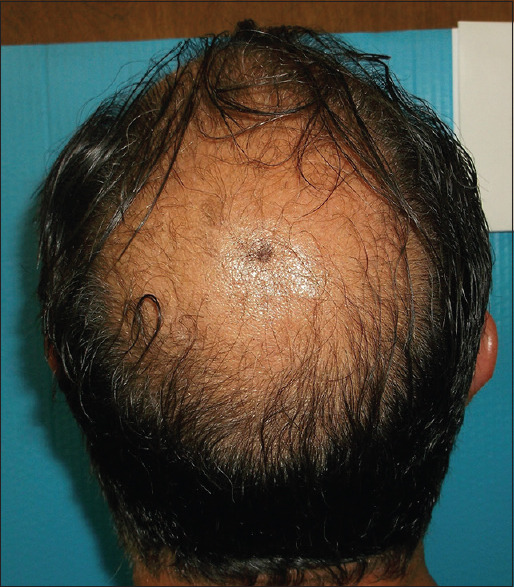
Male– Day 0–2 hairs/cm2
Figure 4.
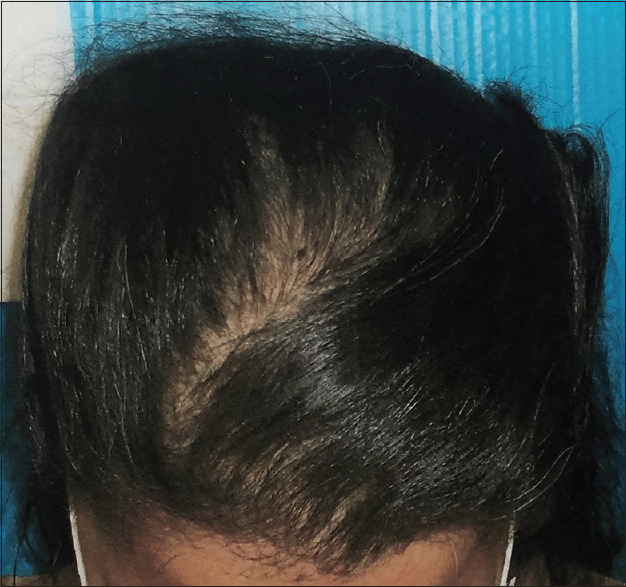
Female– Day 180-37 hairs/cm2
Figure 2.
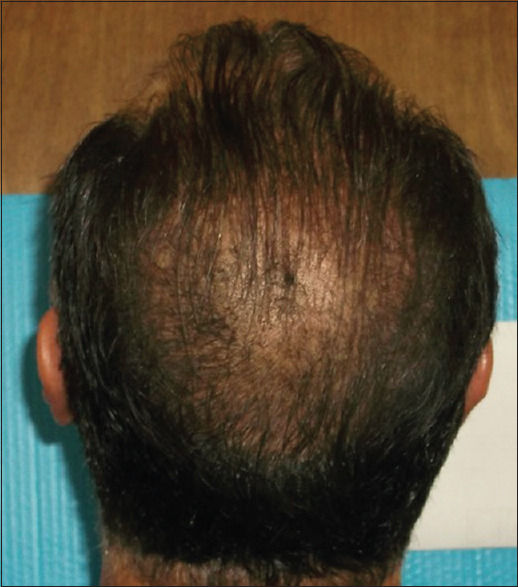
Male– Day 180-19 hairs/cm2
Figure 3.
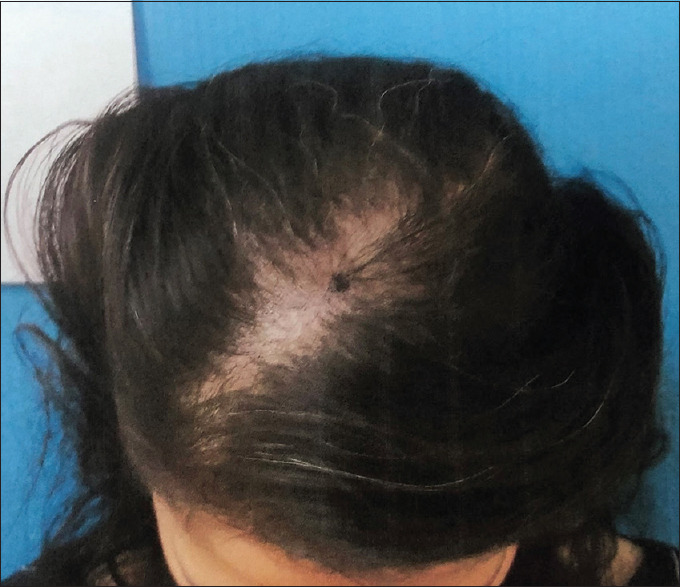
Female– Day 0–16 hairs/cm2
DISCUSSION
This case study suggests that topical hemp extract high in THCV, CBDV, CBD, menthol, and peppermint oil is associated with significant hair regrowth in both men and women with AGA. This topical was superior to high-CBD hemp extract alone.[26] In general, men did better than women. On average, there was a 164% (P < 0.00001) increase in nonvellus hair after 6 months of once-daily use. All subjects had some regrowth and cosmetic benefits.
Although the exact mechanism of therapeutic effects is not entirely clear, CBD is most likely functioning as a CB1 receptor antagonist, through negative allosteric effects, and potentially also through Wnt messaging. THCV and CBDV are acting as full CB1 neutral antagonists and through TRPV1 agonism. The menthol and peppermint (40% menthol) are probably acting by promoting the rapid onset of anagen phase.[1]
The safety of topically applied hemp extract has been previously well documented. Once again, there is no reported significant adverse effects for 6-month application of this hemp extract topical.[24,25]
As was discussed above, topical hemp formulation has superior results to finasteride and 5% minoxidil once daily foam. Since this hemp extract works through novel mechanisms entirely different from finasteride and minoxidil, it can be used in conjunction with these current drugs and would be expected to have synergistic effects. However, safety of this combination would need to be evaluated.
Financial support and sponsorship
This study was supported by CBDLuxe, Golden, CO for supplying the tested topical hemp extract.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Oh JY, Park MA, Kim YC. Peppermint oil promotes hair growth without toxic signs. Toxicol Res. 2014;30:297–304. doi: 10.5487/TR.2014.30.4.297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cranwell W, Sinclair R. Male Androgenetic Alopecia. South Dartmouth. 2016. [Last accessed on 2016 Feb 29]. Available from: https://www.ncbi.nlm.nih.gov/books/NBK278957/
- 3.Mella JM, Perret MC, Manzotti M, Catalano HN, Guyatt G. Efficacy and safety of finasteride therapy for androgenetic alopecia: A systematic review. Arch Dermatol. 2010;146:1141–50. doi: 10.1001/archdermatol.2010.256. [DOI] [PubMed] [Google Scholar]
- 4.Krupa Shankar D, Chakravarthi M, Shilpakar R. Male androgenetic alopecia: Population-based study in 1,005 subjects. Int J Trichology. 2009;1:131–3. doi: 10.4103/0974-7753.58556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Levy LL, Emer JJ. Female pattern alopecia: Current perspectives. Int J Womens Health. 2013;5:541–56. doi: 10.2147/IJWH.S49337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cash TF. The psychological effects of androgenetic alopecia in men. J Am Acad Dermatol. 1992;26:926–31. doi: 10.1016/0190-9622(92)70134-2. [DOI] [PubMed] [Google Scholar]
- 7.Salman KE, Altunay IK, Kucukunal NA, Cerman AA. Frequency, severity and related factors of androgenetic alopecia in dermatology outpatient clinic: Hospital-based cross-sectional study in Turkey*. An Bras Dermatol. 2017;92:35–40. doi: 10.1590/abd1806-4841.20175241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Stenn KS. The molecular and structural biology of hair: Introduction. Ann N Y Acad Sci. 1991;642:xi–xiii. doi: 10.1111/j.1749-6632.1991.tb24375.x. [DOI] [PubMed] [Google Scholar]
- 9.Whiting DA. Male pattern hair loss: Current understanding. Int J Dermatol. 1998;37:561–6. doi: 10.1046/j.1365-4362.1998.00542.x. [DOI] [PubMed] [Google Scholar]
- 10.Courtois M, Loussouarn G, Hourseau C, Grollier JF. Ageing and hair cycles. Br J Dermatol. 1995;132:86–93. doi: 10.1111/j.1365-2133.1995.tb08630.x. [DOI] [PubMed] [Google Scholar]
- 11.Kaufman KD. Androgens and alopecia. Mol Cell Endocrinol. 2002;198:89–95. doi: 10.1016/s0303-7207(02)00372-6. [DOI] [PubMed] [Google Scholar]
- 12.Messenger AG. Hair through the female life cycle. Br J Dermatol. 2011;165(Suppl 3):2–6. doi: 10.1111/j.1365-2133.2011.10628.x. [DOI] [PubMed] [Google Scholar]
- 13.Chen L, Zhang J, Wang L, Wang H, Chen B. The efficacy and safety of finasteride combined with topical minoxidil for androgenetic alopecia: A systematic review and meta-analysis. Aesthetic Plast Surg. 2020;44:962–70. doi: 10.1007/s00266-020-01621-5. [DOI] [PubMed] [Google Scholar]
- 14.Drake L, Hordinsky M, Fiedler V, Swinehart J, Unger WP, Cotterill PC, et al. The effects of finasteride on scalp skin and serum androgen levels in men with androgenetic alopecia. J Am Acad Dermatol. 1999;41:550–4. [PubMed] [Google Scholar]
- 15.Irwig MS, Kolukula S. Persistent sexual side effects of finasteride for male pattern hair loss. J Sex Med. 2011;8:1747–53. doi: 10.1111/j.1743-6109.2011.02255.x. [DOI] [PubMed] [Google Scholar]
- 16.Stout SM, Stumpf JL. Finasteride treatment of hair loss in women. Ann Pharmacother. 2010;44:1090–7. doi: 10.1345/aph.1M591. [DOI] [PubMed] [Google Scholar]
- 17.Messenger AG, Rundegren J. Minoxidil: Mechanisms of action on hair growth. Br J Dermatol. 2004;150:186–94. doi: 10.1111/j.1365-2133.2004.05785.x. [DOI] [PubMed] [Google Scholar]
- 18.Kulick MI. Topical minoxidil: Its use in treatment of male pattern baldness. Ann Plast Surg. 1988;21:273–5. [PubMed] [Google Scholar]
- 19.Adil A, Godwin M. The effectiveness of treatments for androgenetic alopecia: A systematic review and meta-analysis. J Am Acad Dermatol. 2017;77:136–41.e5. doi: 10.1016/j.jaad.2017.02.054. [DOI] [PubMed] [Google Scholar]
- 20.Gupta AK, Charrette A. Topical minoxidil: Systematic review and meta-analysis of its efficacy in androgenetic alopecia. Skinmed. 2015;13:185–9. [PubMed] [Google Scholar]
- 21.York K, Meah N, Bhoyrul B, Sinclair R. A review of the treatment of male pattern hair loss. Expert Opin Pharmacother. 2020;21:603–12. doi: 10.1080/14656566.2020.1721463. [DOI] [PubMed] [Google Scholar]
- 22.Suchonwanit P, Srisuwanwattana P, Chalermroj N, Khunkhet S. A randomized, double-blind controlled study of the efficacy and safety of topical solution of 0.25% finasteride admixed with 3% minoxidil vs. 3% minoxidil solution in the treatment of male androgenetic alopecia. J Eur Acad Dermatol Venereol. 2018;32:2257–63. doi: 10.1111/jdv.15171. [DOI] [PubMed] [Google Scholar]
- 23.Holmäng S, Lele SM, Johansson SL. Squamous cell carcinoma of the renal pelvis and ureter: Incidence, symptoms, treatment and outcome. J Urol. 2007;178:51–6. doi: 10.1016/j.juro.2007.03.033. [DOI] [PubMed] [Google Scholar]
- 24. NCT03742518. A Study Evaluating the Efficacy and Safety of SM04554 Topical Solution in Male Subjects With Androgenetic Alopecia. c2018. [Updated 2021 Dec 28; cited 2022 Jan 12]. Available from: https://clinicaltrials.gov/ct2/show/NCT03742518.
- 25.Vallee A, Lecarpentier Y, Guillevin R, Vallee JN. Effects of cannabidiol interactions with Wnt/beta-catenin pathway and PPARgamma on oxidative stress and neuroinflammation in Alzheimer's disease. Acta Biochim Biophys Sin (Shanghai) 2017;49:853–66. doi: 10.1093/abbs/gmx073. [DOI] [PubMed] [Google Scholar]
- 26.Smith G, Satino J. Hair Regrowth with Cannabidiol (CBD)-rich hemp extract. [Last accessed on 2021 Aug 15];Original Rep. 2021 4:53–9. doi: 10.26828/cannabis/2021.01.003. Available from: https://publications.sciences.ucf. edu/cannabis/index.php/Cannabis/article/view/78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Laprairie RB, Bagher AM, Kelly ME, Denovan-Wright EM. Cannabidiol is a negative allosteric modulator of the cannabinoid CB1 receptor. Br J Pharmacol. 2015;172:4790–805. doi: 10.1111/bph.13250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pertwee RG. Inverse agonism and neutral antagonism at cannabinoid CB1 receptors. Life Sci. 2005;76:1307–24. doi: 10.1016/j.lfs.2004.10.025. [DOI] [PubMed] [Google Scholar]
- 29.McPartland JM, Duncan M, Di Marzo V, Pertwee RG. Are cannabidiol and Δ9-tetrahydrocannabivarin negative modulators of the endocannabinoid system.A systematic review? Br J Pharmacol. 2015;172:737–53. doi: 10.1111/bph.12944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bolognini D. Pharmacological Properties of the Phytocannabinoids Δ9-Tetrahydrocannabivarin and Cannabidiol. Doctoral Thesis. 2010. [Last accessed on 2021 Aug 15]. Available from: http://hdl.handle.net/10277/269.
- 31.Smith GL. Medical Cannabis: Basic Science and Clinical Applications. Beverly Farms (MA): Aylesbury Press; 2016. p. 225. [Google Scholar]
- 32.Tóth KF, Ádám D, Bíró T, Oláh A. Cannabinoid signaling in the skin: Therapeutic potential of the “C (ut) annabinoid” system. Molecules. 2019;24:918. doi: 10.3390/molecules24050918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Telek A, Bíró T, Bodó E, Tóth BI, Borbíró I, Kunos G, et al. Inhibition of human hair follicle growth by endo- and exocannabinoid. FASEB J. 2007;21:3534–41. doi: 10.1096/fj.06-7689com. [DOI] [PubMed] [Google Scholar]
- 34.Bíró T, Tóth BI, Haskó G, Paus R, Pacher P. The endocannabinoid system of the skin in health and disease: novel perspectives and therapeutic opportunities. Trends Pharmacol Sci. 2009;30:411–20. doi: 10.1016/j.tips.2009.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Muller C, Morales P, Reggio PH. Cannabinoid ligands targeting TRP channels. Front Mol Neurosci. 2018;11:487. doi: 10.3389/fnmol.2018.00487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Chung H, Fierro A, Pessoa-Mahana CD. Cannabidiol binding and negative allosteric modulation at the cannabinoid type 1 receptor in the presence of delta-9-tetrahydrocannabinol: An In Silico study. PLoS One. 2019;14:e0220025. doi: 10.1371/journal.pone.0220025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Adil A, Godwin M. The effectiveness of treatments for androgenetic alopecia: A systematic review and meta-analysis. J Am Acad Dermatol. 2017;77:136–41.e5. doi: 10.1016/j.jaad.2017.02.054. [DOI] [PubMed] [Google Scholar]
- 38.Azer VB, Blackledge J, Charles AM, Chen O, Kernan J, Nadeau P, et al. Collective View of CBD. Cowen Outperform. 2019 [Google Scholar]
- 39.Corroon J, Phillips JA. A cross-sectional study of cannabidiol users. Cannabis Cannabinoid Res. 2018;3:152–61. doi: 10.1089/can.2018.0006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Gaston TE, Friedman D. Pharmacology of cannabinoids in the treatment of epilepsy. Epilepsy Behav. 2017;70:313–8. doi: 10.1016/j.yebeh.2016.11.016. [DOI] [PubMed] [Google Scholar]
- 41.Abioye A, Ayodele O, Marinkovic A, Patidar R, Akinwekomi A, Sanyaolu A. Δ9-tetrahydrocannabivarin (THCV): A commentary on potential therapeutic benefit for the management of obesity and diabetes. J Cannabis Res. 2020;2:6. doi: 10.1186/s42238-020-0016-7. [DOI] [PMC free article] [PubMed] [Google Scholar]


