Abstract
Immuno-oncology has revolutionized cancer treatment and has opened up new opportunities for developing vaccination methods. DNA-based cancer vaccines have emerged as a promising approach to activating the bodily immune system against cancer. Plasmid DNA immunizations have shown a favorable safety profile and there occurs induction of generalized as well as tailored immune responses in preclinical and early-phase clinical experiments. However, these vaccines have notable limitations in immunogenicity and heterogeneity and these require refinements. DNA vaccine technology has been focusing on improving vaccine efficacy and delivery, with parallel developments in nanoparticle-based delivery systems and gene-editing technologies such as CRISPR/Cas9. This approach has showcased great promise in enhancing and tailoring the immune response to vaccination. Strategies to enhance the efficacy of DNA vaccines include the selection of appropriate antigens, optimizing insertion in a plasmid, and studying combinations of vaccines with conventional strategies and targeted therapies. Combination therapies have attenuated immunosuppressive activities in the tumor microenvironment and enhanced the capability of immune cells. This review provides an overview of the current framework of DNA vaccines in oncology and focuses on novel strategies, including established combination therapies and those still under development.The challenges that oncologists, scientists, and researchers need to overcome to establish DNA vaccines as an avant-garde approach to defeating cancer, are also emphasized. The clinical implications of the immunotherapeutic approaches and the need for predictive biomarkers have also been reviewed upon. We have also tried to extend the role of Neutrophil extracellular traps (NETs) to the DNA vaccines. The clinical implications of the immunotherapeutic approaches have also been reviewed upon. Ultimately, refining and optimizing DNA vaccines will enable harnessing the immune system's natural ability to recognize and eliminate cancer cells, leading the world towards a revolution in cancer cure.
Keywords: DNA-based vaccines, Cancer vaccines, Combination therapy, Adjuvants, Immunotherapy, Immune response, Vaccine development
Introduction
Cancer is a term that brings the most trepidation in the whole thesaurus of medicine [1]. Accurate and early diagnosis of cancer patterns and their stages becomes essential as the treatment regimens are distinct. The wonted pharmacotherapy for cancer comprises surgery, radiotherapy, and systemic therapy, alone or in a combined form. However, cytotoxic cancer therapy proves to be unendurable for the patients and provides no lifelong protection as well. We have entered an era where this six-lettered word is accountable for nearly one in six deaths [2]. The global epidemiological fact of the year 2020 features the figure of cancer deaths to be around 9.9 million. Among this, 5.5 million affected were males, surpassing women by 11%. The prevalence of cancer has increased, reaching a peak of about 1.93 million in 2020 [3]. What’s interesting to note is that the age-adjusted rates demonstrate a decrease in cancer mortality despite notable increases in cancer incidence, new cases of cancer and cancer deaths, since the turn of the century [4]. To make this decrement in cancer mortality significant, we need certain quantum leaps and DNA vaccines can be the one, with right efforts.
To comprehend the mechanism of DNA vaccine, we first need to grasp the relation between the immune system and cancer. Immunosuppressive Tumor Microenvironment (TME) [5] and Malfunctional antitumor T-cells define this relation. TME constitutes composite networks of cells, typically including cancer cells, a wide variety of immune cells, stromal cells, blood vessels, extracellular matrix, and regulatory proteins. Cancer cells survive by evading the immune attacks directed within the TME, making the environment immunosuppressive through various mechanisms. T-cell mediated reactions against tumor cells involve ensuing steps: (a) Arrest of neoantigens released from dead tumor cells by antigen-presenting cells (APCs); (b) Presentation of captured antigens to T-cells; (c) T-cell reaction against tumor antigens (TAs); (d) Advancement of effector T-cells towards tumor locus; (e) Tumor infiltration by T-cells; (f) Recognition and binding of effector T-cells to the TAs; (g) Destruction of cancer cells by antigen-specific T-cells. Dysfunction of cell-mediated immunity was recognized with the discovery of Cytotoxic T-lymphocyte protein 4 (CTLA-4) and programmed cell death protein 1 (PD-1) in the 1990s [6]. Both of these serve as negative immune regulators, also called immune checkpoints. Normally, T-cell activation against tumor antigens requires two signals—recognition of the antigen and co-stimulatory signals from APCs. CTLA-4 and CD-28 are structurally homologous T-cell receptors and share the co-stimulators, B7-1 and B7-2. On their complex formation with the shared ligands, CD-28 stimulates immune response as opposed to CTLA-4, which inhibits it. In a healthy host, an intricate balance is maintained between the activation and inhibition of immune responses. Tumors, however, shift the equilibrium towards the annihilation of immune response by promoting CTLA-4 and PD-1 [7, 8]. Due to complexity of TME and the prowess of tumor cells in dodging immunity, bodily protection mechanisms fall flat and this is where immune therapy shows up.
Inter-tumoral and Intra-tumoral heterogeneity are the major drawbacks of the increasing anticlimactic responses of cytotoxic chemotherapeutic regimens. In 2013, the journal Science, declared immunotherapy for treating cancer as the “Breakthrough of the Year” [9]. Subsequently, Immunotherapy has reinvigorated the field of onco-therapy. There have been various classifications of Immunotherapy including Adoptive cell transfers (ACT) [10], Immune checkpoint inhibitors (ICI) [11], Cancer vaccines, Antibody therapies, and Cytokine therapies. ACT involves the infusion of T-cells that are genetically modified to respond against tumor cells. Chimeric antigen receptor (CAR) [5] T-cells and T-cell receptor-modified T-cells are two of the subtypes of ACT. ICIs being a recent inclusion in monoclonal antibodies, targets the negative immune regulators. Oncolytic virus vaccines are the subtype of cancer vaccines where the virus exists naturally or is genetically manipulated to target cancer cells, without affecting the normal cells [9]. Cancer vaccines target mutational antigens and TAAs [12]. They can be categorized into (a) Cell-based; (b) Peptide-based; (c) Viral and Bacterial based; (d) Gene-based vaccines. Interleukin-2 and Interferon-alpha serve as classic therapeutic cytokines in cancer therapy. Neoantigens being a recent breakthrough are based on individual genetic mutations [13, 14].
Being the epicenter of this review, DNA vaccines showcase the astounding technological advancements in genetic vaccines. These are a type of Nucleic acid vaccines (NAV) which have been a recent approach [15]. These constitute genetic information against tumor antigens [16] and pass it on to the host, which helps elicit adequate immune responses [17]. DNA vaccines can be administered through various routes, better ones being electroporation, sonoporation, DNA tattooing, and gene gun [18]. Once the desired gene sequence enters the nucleus, it gets tagged with major histocompatibility complex-I (MHC-I) and major histocompatibility complex-II (MHC-II) molecules which activate cluster of differentiation (CD) cells, CD4 and CD8 along with B-cells [19]. DNA vaccines offer better antigen specificity and safety, causing fewer side effects in comparison with other nontargeted therapies [20]. Nevertheless, DNA vaccines have expressed poor immunogenicity, being its major fallback [18]. The ultimate idea of this review aims to explore and peruse every facet of DNA-based vaccination, thus paving the way for better furtherance in onco-therapeutics.
DNA-based vaccine constructs based on the strategies to enhance immunogenicity
Chimeric DNA vaccines
A few reasons that are responsible for the failure of approval of DNA vaccines for cancer are the selection of target antigens, formulation processes of vaccines, and surpassing the issue of tolerance. To overpower these limitations, the concept of chimeric DNA vaccines to oppose cancer antigens was introduced. Utilizing xenogeneic elements like proteins or peptides, which are extracted from another species and are notably homologous to the self-ortholog [21]. As these sequences are similar and not the same, they could help circumvent the immune tolerance and elicit a potential immunogenic response [22]. The fine difference between this non-self antigen and the indigenous proteins is accountable for the stimulation of T- and B-cell responses against the xenoantigens [23]. These responses may cross-react with the native antigens or self antigens. ONCEPT stands to be the first xenogeneic DNA vaccine to be approved for canine melanoma, as previously mentioned. The efficacy of the chimeric vaccines could be magnified by combination with immune modulators. Mesothelin(MSLN) has been identified as a tumor antigen in various malignancies like ovarian and pancreatic tumors. An antigen-specific connective tissue growth factor lined with mesothelin (CTGF/MSLN) was combined with epigallocatechin-3-gallate (EGCG), an immunomodulator [24]. This combination enhanced the antigen-specific anti-tumor effects of the vaccine through the maturation of dendritic cells.
Apart from the xenogeneic approach to elicit reaction against cancer antigens, there occurs a few other strategies. One of them is, the simultaneous co-injection of vaccine with the peptides that induce CD4 T-cells. These peptides are exemplified by the use of long peptides or the Pan HLA-DR epitope (PADRE) peptides, which stimulate the CD4 cells as well as the dendritic cells [25].
Nonetheless, few studies have observed the response of self-antibodies to be robust with the autologous compared to the xenogeneic vaccination. The apparent reason is the specificity for the homologous antigen of antibodies, triggered by the DNA vaccines [22]. Ergo the chimeric DNA vaccines have a significant drawback of low-affinity antibody response. For surpassing this limitation of low-affinity antibody reaction, hybrid vaccines can also be fabricated where the chimeric elements include the xenogeneic as well as autologous antigen domains. One such example is introduction of plasmid that encodes for chimeric neu-HER2 antigen for induction of immune response opposing the ErbB2 + tumors [26].
MUC1-based DNA vaccines
Mucin 1 (MUC1) exists as a transmembrane protein in humans and embodies the three parts: (a) The extracellular domain of the N-terminal that consists of a variable number of 20 amino acid tandem repeat (VNTR) units (b) The transmembrane domain (c) Intracellular C-terminal region [27]. The fundamental portion of this peptide carries five O-linked glycosylation sites on threonine or serine residues of MUC1 VNTR. This protein is mainly expressed in breasts, ovaries, lungs, kidneys, pancreas, and colon. In cells with normal physiologic function, these proteins are highly glycosylated. Conversely, in the tumorigenic cells, it is either hypo- or aberrantly glycosylated, and this makes it a potential target of interest [28].
MUC-1 encoded recombinant eukaryotic vector expression was introduced through the intramuscular route in the animal models and this resulted in stimulation of T- and B- cells [29, 30]. The three notable mechanisms through which these vaccines work are: (a) The MUC-1 peptide is released into the extracellular space and is exposed to the APCs. These APCs then aid the endocytosis of the peptide and present the fragments of MUC-1 to the MHC-II molecules. This presentation ultimately leads to the induction of CD4 T cells to get differentiated into Th1 and Th2 cells (b) The MUC-1 protein is secreted into the extracellular region and directly binds to the receptors of B-cell to induce the production of antibodies (c) Mucin peptide is degraded to peptides that contain 8–12 amino acids and this elicits differentiation of CD8 T-cells to cytotoxic T-cells through MHC-I pathway. For the anti-tumor vaccine response to be effective, the candidate receiving the vaccine should be able to produce both cellular as well as humoral immunity. The vaccines with just the MUC1 peptide fail to demonstrate adequate efficacy and hence, different T- and B- cell epitopes and adjuvants are used [31]. A chimeric MUC-1 DNA vaccine had been designed that encoded the mucin gene, the gene which had a deletion of the transmembrane and C-terminal, and this was fused with the human heat shock protein (HSP70) gene. This vaccine demonstrated requisite efficacy in exerting anti-tumor effects in mice models [32].
Neoantigen and personalized DNA vaccines
Although TAAs comprise the major part of DNA vaccines, immunization through non-mutated antigens has failed to showcase significant results when compared to the standard protocols. The reason behind these inadequate results is the concept of immune tolerance, as these antigens are normally present in the tissues and prevent strong immune responses [33, 34]. However, the development of neoantigens has turned the tables and proffered us the ability to target tumors more specifically. Neoantigens are consequences of the alterations in the tumor-specific genes and this generates newer epitopes. This therapy targets multiple cancer antigens, which occur to be unique in every patient. Each patient embodies a unique set of tumor antigens and is subject to clonal variation. With the intersubject variability, there occurs intratumoral heterogeneity which consists of a higher amount of branched mutations. These mutations increase the number of subclones within a tumor and renders the weak neoantigen-specific retaliation of T-cells [35]. Along with the benefit of tailored targeting in tumors, neoantigens have shown minimal adverse effects [36, 37]. Cold tumors are converted to hot ones, as proinflammatory cytokines tend to accumulate, and exposure to T-cells increases [38]. This process is also accompanied by the enhanced regulation of PD-L1 in the TME. Due to these benefits, tumors become more susceptible to immune checkpoint blockers (ICBs) [39].
Neoantigens are identified as ‘non-self’ antigens by the host immune system and this renders them incapable of evading the immune tolerance. The process for the generation of these clever neoantigens first involves the extraction of the genetic information from the tumor biopsy, followed by exon sequencing. The mutations that led to the development of the tumorigenic sequence are identified by comparing the tumor-acquired sequence to the normal genetic map. The antigens that are cognized by the MHC-I or MHC-II, are selected on the basis of certain antigen-prediction algorithms [40]. However, not all antigens identified through this process are immunogenic and hence establishing an optimum protocol is needed [18, 41]. With this, another drawback of neoantigens is their manufacturing time. The approximate time needed for development is 5 months which seems inconvenient in treating cancer, where time is of value [42]. Overpowering these limitations, DNA vaccines, in contrast to RNA and peptide-based vaccines, stimulate a significantly potent CD8 response against the desired neoantigens and hence are more trusted upon. A good majority of these vaccines are in ongoing trials, evaluating efficacy in solid tumors and combinational therapies. In a nutshell, neoantigens offer a good quantum of personalization and tailored treatment allowing cancer patients to achieve better remission.
Polyepitope-based DNA vaccines
Alterations in somatic DNA form the tumor neoantigens, which render the changes in protein sequences and induce adaptive immune reactions. Our capability of identifying neoantigens is constantly improving with the developments in the technologies of gene sequencing. The sequencing of cancer cells coupled with the epitope prediction algorithms are utilized for recognizing and ranking neoantigens to integrate them into personalized cancer vaccines [34, 43]. The primacy of DNA vaccines is associated with the fact that a good number of sequences could be delivered in a single construct. The basic concept of polyepitope DNA vaccines starts with CTLs. MHC-I molecules present antigens, which are recognized as short peptides (generally comprising of 8–10 amino acids) by the CTLs. These short peptides or fragments are designated the term, T cytotoxic cell determinants (DTcs) [44]. Polyepitope DNA vaccines are sequenced with several DTcs in a minigene construct to induce CTL responses against an extensive target repertoire. In another set of words, a comprehensive response by CTL could be elicited by the simultaneous delivery of atypical and immunodominant epitopes, as a polyepitope DNA vaccine. A single epitope vaccine has been reported to encode greater than 20 antigens [44].
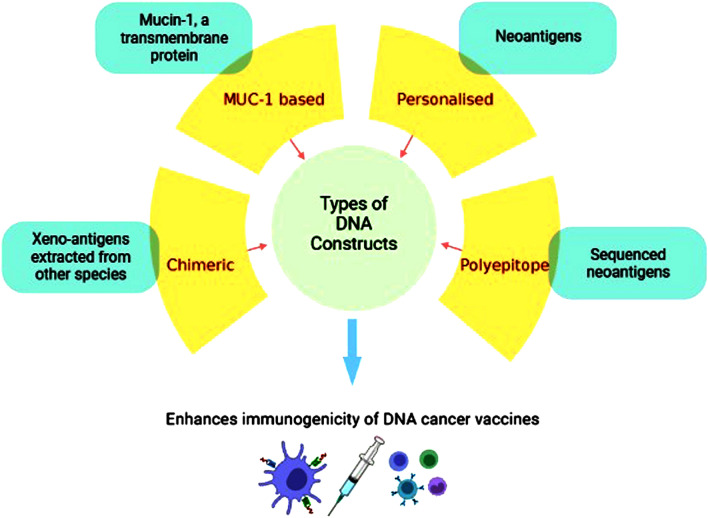
In comparison to the synthetic long peptide (SLP), DC, and RNA, the production of polyepitope vaccines in the format of DNA plasmid, is observed to have relatively easy manufacturing, a desirable safety profile, and molecular flexibility [37, 45–49]. One of the studies reported that polyepitope constructs encoding 20–25 epitopes, with or without spacers, fused with the mutant form of ubiquitin, can be efficiently processed [50]. The model DNA vaccines based on this approach were successfully able to showcase remarkable results in vivo. Preclinical trials evaluating the polyepitope-based DNA vaccines demonstrated induction of anti-tumor response. Moreover, clinical trials observed the stimulation of neoantigen-specific T-cell reactions. Though CD8 + T-cells are the prime elements in inducing anti-tumor responses when the medium occurs to be vaccination, CD4 + T-cells have been found to elicit broader and strengthened immune response immune response [51–54]. Hence, T-helper (Th) peptides are co-administered with the DNA vaccines to enhance the stimulation of Th-cells and ultimately the CTL responses. One such example of Th peptide is pan DR epitope (PADRE), which indeed increased the anti-tumor effects of the immune system [18, 55]. A number of clinical trials are in the ongoing phase to evaluate the efficacy of polyepitope DNA vaccines for breast, cervical, ovarian, and pancreatic cancers [36, 56–60].The illustration depicting the Types of DNA constructs has been depicted in Fig. 1.
Fig. 1.
Established constructs to enhance immunogenicity of DNA-based cancer vaccines. There have been established four types of DNA constructs to increase the immunogenic potential of DNA-based cancer vaccines. These include a Chimeric DNA vaccines utilizing the xeno-antigens b MUC-1 based DNA vaccines consisting of the mucin peptides c Personalized DNA vaccines that encode neoantigens specific to each patient d Polyepitope DNA vaccines that embody the sequences of multiple neoantigens or epitopes
Obstacles encountered and challenge to be fulfilled for the approval of DNA-based vaccines for cancer
DNA-based cancer vaccine has well-nigh approached its purpose. It has a comprehensible design and possesses long-term protective potential. It is cost-effective in terms of production and safer compared to live-attenuated vaccines. Moreover, it retains stability at room temperature and has a good solubility profile [61]. Nevertheless, these assets have been outranked by a few but considerable drawbacks that withheld the approval of DNA-based vaccines in humans, against cancer. The potential drawback of DNA vaccines is the failure to induce requisite immunogenicity and the responsible factors stand out to be the lack of optimal DNA transfection and immunostimulation. The DNA transfection capacity of these vaccines gives non-uniform results due to the complexity of cellular and nuclear membranes in each individual [62]. The plasmids are required to cross the phospholipid-rich cell membrane through pinocytosis or endocytosis. The plasmids are also required to escape the degradation process carried out by lysosomes, endosomes, and nucleases. These challenges could be subdued by improving the delivery of plasmids by physical and chemical means. Starting with the physical methods, plasmid insertion through a needle, be it intradermal (ID), intramuscular (IM), transdermal or mucosal, has demonstrated poor transfection [63]. Insertion through needle causes DNA to get concentrated in the intracellular spaces, instead of getting into the cells itself where they can get transcribed into mRNA. Ergo the use of ID or IM electroporation has replaced the needle delivery system. Electroporation involves application of electric current through needles, following and adjacent to the site of DNA insertion (insertion through needle and syringe) [64, 65].
Apart from electroporation, several other physical techniques like Particle-Mediated Epidermal Delivery (PMED) and Needle-Free Injection System (NFIS) are also considered [66, 67]. Advancing towards the chemical methods, these include the biological pharmaceuticals to enhance the transfection capacity [63]. Liposomes, one of the biopharmaceuticals, are spherical molecules embodying the cholesterol and phospholipid moieties into a lipid bilayer to allow fusion with lipid enriched cell membranes [68]. This fusion renders easy insertion of DNA into the cells. Liposomes play a dual role by contributing in the enhancement of transfection efficiency as well as acting as an adjuvant. The delivery systems could be improvised by inculcating the biodegradable polymeric micro-particles and recent technologies of nano-particles. These amphiphilic particles should be of the size 0.5–10 µm [63]. Along with the physical and chemical delivery techniques, another strategy that has been useful in increasing the immunogenicity is the use of vaccine cocktails that embody DNA along with the plasmids that encode adjuvanting immunomodulatory elements. Cytokines and dinucleotide motifs are the exemplifications of these molecular adjuvants. 5'—C—phosphate—G—3' (CpG) motif is a dinucleotide motif and is ideally matched with the DNA vaccine to incorporate in into the backbone of the vaccine.It has three types namely, A, B, and C [69]. The A type triggers cellular immune response wheras the B type induces humoral immunity. CpG-C has the capability of stimulating both cellular and humoral immune responses. Interleukin (IL)-2, is a major cytokine used as an adjuvant and it potentially induces the lymphocytes [70]. This adjuvant has been approved for use in mitigating the metastatic melanoma and renal cell carcinoma [71]. IL-15, similar to IL-2, stimulates the proliferation of NK- and T-cells.Granulocyte–macrophage colony-stimulating factor (GM-CSF), another adjuvant cytokine, engages the APCs to the vaccination site and promotes maturation of dendritic cells [72]. The utilization of polymeric carriers exemplified by, polylactic acid (PLA), polylactic-co-glycolic acid (PLGA), and chitosan, have exhibited propitious results as adjuvants and delivery assistants in the form of nano-particles [73].
The Toll-like receptor (TLR) ligands have also been a recent adjuvant of interest [74]. These adjuvant-encoded plasmids have the benefit of expressing proteins for the same span as that of the antigens and allow stimulation of immunity for longer periods of time [75]. Secondly, with concern towards safety issues, these vaccines have a propensity to induce systemic inflammatory reactions by precipitating excessive cytokine release that can lead to consistent fever. To elucidate this, we need to evince the inflammatory immune pathways that get affected by the introduction of DNA vaccines and employ methods that prevent cytokine storm (as seen with the adjuvant-encoded plasmids) [63, 76]. Plasmid DNA vectors contain the bacterial region elements like the origin of replication and makers of selection. These functional elements are futile once the cell culture has been halted and tend to cause negative effects on efficacy and stability of vaccines. Accompanying these detrimental effects, safety outcomes get compromised due to the possibility of horizontal transmission of antibiotic resistance markers to the enteric bacterial populations of host [77]. The strategy devised against the transmission of resistance markers, is the development of small bacterial RNA-based antibiotic free selection markers, also called as non-coding markers. Moreover, these markers are smaller in size with less than 200 base pairs, thus decreasing the vector size and increasing the transfection efficiency (smaller vectors are resistant to the shear forces encountered during delivery) [78, 79]. Further addressing the safety issues, there occurs the potential of shedding of vaccines to the environment and it’s spread through predatory animals. DNA vaccines also carry the risk of integrating into the host’s genome, which ultimately disrupts the expression of host genome. Thus to monitor such issues, guidelines and regulatory frameworks are established and these vary with the countries. The requirements for the regulation are based on the species being tested. The DNA vaccinated animals need to be labeled with Genetically Modified Organism (GMO) to help impede the widespread use of DNA vaccines [80]. The DNA constructs must be thoroughly checked for their integration into the host chromosome and the transparency associated with health risk assessments and environmental harm should be maintained. Also, it is toilsome to parallel the cytology and biomolecular mechanisms of cancer cells and this eventually complicates the recognition and suppression of tumor antigens [16]. Additionally, tumor neoantigens have a tendency to mutate which renders the antigen-targeted vaccines inefficacious [81].
Also, the compound immunosuppressive environs of tumor cells retard the efficacy of these vaccines. We need to outrun these immune-evading abilities of cancer antigens and enhance their presentation by APCs to increasingly deploy antigen-specific T-cells [82]. The most apparent reason for the inability to develop vaccines against cancer is the differences encountered in the anatomy of animal models and humans. On the brighter side, in august of 2021, ZyCoV-D, the world’s first DNA-based preventive vaccine was approved by the Drug Controller General of India (DCGI), fo r Covid-19 infection by the virus Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) [83, 84]. DNA-based immunization has received approval for use in animals to mitigate several veterinary diseases, one of which is Oncept. It is the United States Food and Drug Administration (USFDA)-approved xenogeneic human tyrosinase plasmid DNA vaccine, used to mitigate malignant melanoma in dogs. It targets a self-antigen termed tyrosinase which is upregulated in several malignant melanomas, in dogs as well as humans [85]. Furthermore, a novel technology utilizes CRISPR/Cas9 based gene-editing technology, in vaccine development. This technology consists of a series of short repetitions, which are interspaced with short sequences in the genome of E.coli. It has showcased promising efficiency, simplicity, specificity, cost-efficiency and flexibility, and this leads us towards an optimistic future of genetically engineered vaccines [86]. With this progressive pace, it is likely that all these obstacles will be resolved and DNA vaccines will soon accomplish in treating cancers. The above discussed obstacles are depicted in Fig. 2.
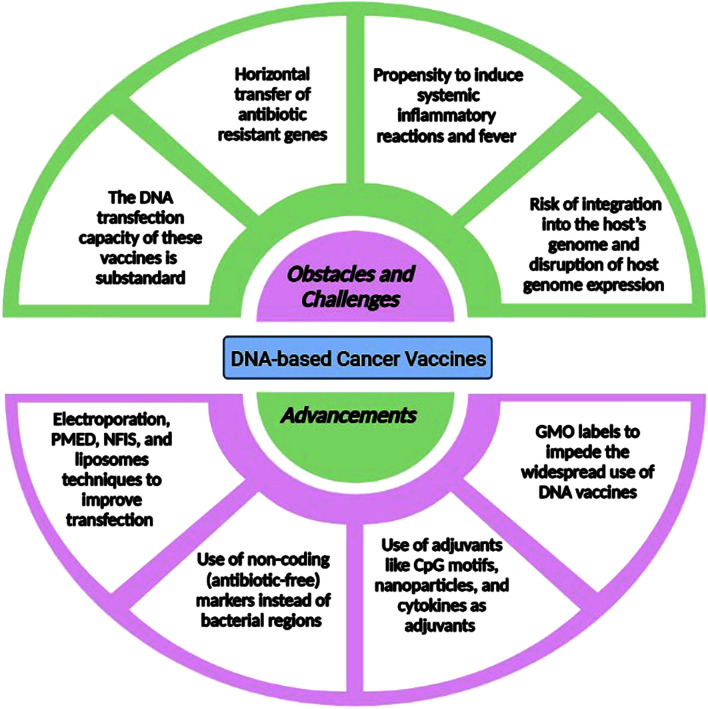
Fig. 2.
Obstacles and challenges encountered and advancements needed for approval of DNA vaccines. The approval of DNA vaccines faces several obstacles and challenges, including issues with stability, low immunogenicity, and the potential for integration into the host genome. Additionally, the delivery of DNA vaccines requires advanced technology to efficiently deliver the plasmid DNA into the target cells. Despite these challenges, significant advancements have been made in recent years, including the development of new delivery systems, adjuvants, and strategies to enhance the immunogenicity of DNA vaccines. Further research is needed to address these challenges and advance the development and approval of DNA vaccines
Immune mechanisms and features of DNA-based cancer vaccines
As discussed previously, the Immunosuppressive TME and the malfunction of T-cells are the flagbearers in the evasion of the tumor cells from body’s immune system. TME along with stromal cells, blood vessels, extracellular matrix, and regulatory proteins, also embody the immune cells. Of these immune cells, myeloid-derived stem cells (MDSCs) and regulatory T-cells (Tregs) are the prominent ones. MDSCs are the tumor-infitrating macrophages that have the immunosuppressive phenotype and are involved in anti-inflammatory processes [19, 87]. Tregs express the CD4 and CD25 along with the transcription factor- forkhead box protein 3 gene (FoxP3), all of which are the regulators of adaptive immunity [88]. Tregs, through the release of suppressive cytokines like IL-35 and Transforming growth factor-β (TGF-β), are capable of suppressing the immune reactions against tumors [89]. Also, the cancer cells downregulate the expressive abilities of MHC molecules ( by promoting non-classical MHC-I molecules such as HLA-E and HLA-G) and target antigens [76]. Tumor expands it’s tolerance towards the draining lymph nodes and enhances the activity of Tregs. Tumors negatively affect the maturation and differentiation of the APCs by the release of soluble factors like vascular endothelial growth factor (VEGF), TGF- β, and IL-10 [90]. In brief, immunosuppressive MDSCs, Tregs, VEGF, IL-10, coupled with the dysfunction of the immunostimulatory T-cells, are responsible for the tumor’s ability to escape the immune forces and ultimately disrupt hematopoiesis.
Ergo the conceptualization behind cancer immunotherapy is to induce adequate retaliation of immunity against cancer cells. For specific cancer antigens, respective and persistent immune responses are needed and this is where the postulation of Nucleic acid-based vaccines developed [91]. Nucleic acid vaccines, either DNA or messenger RNA (mRNA)-based, first need to be embodied into the cytoplasm and subsequently into the nucleus to eventuate gene expression [17]. The constitution of plasmid DNA and the promptness of immunity, are the two main features of DNA vaccines. To begin with, vectors to insert the DNA could be viral vectors, liposomes, naked plasmid DNA and gene- or particle gun mediated direct DNA delivery [92]. All these approaches carry the genetic particulars of a tumor-expressed antigen and guide the immunity to elicit a response against that specific antigen. TAs are antigenic proteins that are overexpressed in tumor tissues, ergo they can act as tumor markers. With the advancements in gene sequencing and profiling, TAs are identified and incorporated as key elements in cancer vaccines. Classification of TAs renders the two classes, Tumor-specific antigens (TSAs) and Tumor-associated antigens (TAAs) [16]. TSAs otherwise known as Mutational antigens or Neoantigens are the results of mutations in self-antigens. These are stringently confined to the tumors and are not expressed in normal cells. P53, Ras, and Bcr-Abl are the commonly encountered TSAs [65]. TAAs on the contrary, do not undergo mutations. They are the self-antigens that are upregulated in tumor cells in comparison to the normal ones. Silent gene products like Cancer/testis antigens, prostate-specific antigen (PSA), prostatic acid phosphatase (PAP), Tyrosinase, and human epidermal growth factor receptor 2 (HER2)/neu are included in TAAs [93, 94]. With the introduction of plasmid DNA, the antigen gets determined and the immunity can distinguish against which substance should it respond. Embarking upon the immune mechanisms elicited, the antigens after being expressed get affixed with the APCs and are then presented to the T-cells.
As depicted in Fig. 3, the genetic information encoded by DNA plasmid of the vaccine, gets processed either by aid of intrinsic adjuvant properties or through expression of TAs. The benefit of these vaccines is that both human leukocyte antigens (HLAs), HLA-1 and HLA-2 are employed as the APCs, through endogenous and exogenous pathways, respectively. This exhibits the coveted antigens to CD4+ and CD8+ T-cells, the beneficiary being the induction of cellular as well as the humoral immune responses [95]. Before the recognition of cancer cell antigens, the CTLs (CD8+ T-cells) are elucidated with the aid of Type 1 conventional CD103+ migrating DCs ( a type of APC) through: (a) Co-stimulatory molecules (like CD80/86 and CD28/152) (b) Adhesion of cancer antigen to MHC-I (c) Pro-inflammatory cytokines like Tumor antigen-tailored CTLs identify the epitopes of tumor antigens, following the formation of complex with T-cell receptors (TCR) [96]. The TCR signaling renders tumor cell death through three main pathways: (a) TNF-related apoptosis-inducing ligand (TRAIL) (b) release of perforin and serine protease via degranulation (c) enhanced regulation of cluster of differentiation ligand (CD95L) [97]. The activated CD8+ T-cells have been observed to release IFN-ϒ and TNF-α and showcase reduction in tumor cells [98]. IFN-ϒ, TNF-α, and IL-2, on the other hand, are produced by the CD4+ T-cells and demonstrated improvements in patient endurance [99]. Innate immune responses get activated on their exposure to intrinsic adjuvant properties like double-stranded DNA (dsDNA) and guanine residues (CpG) of the vaccine [100].
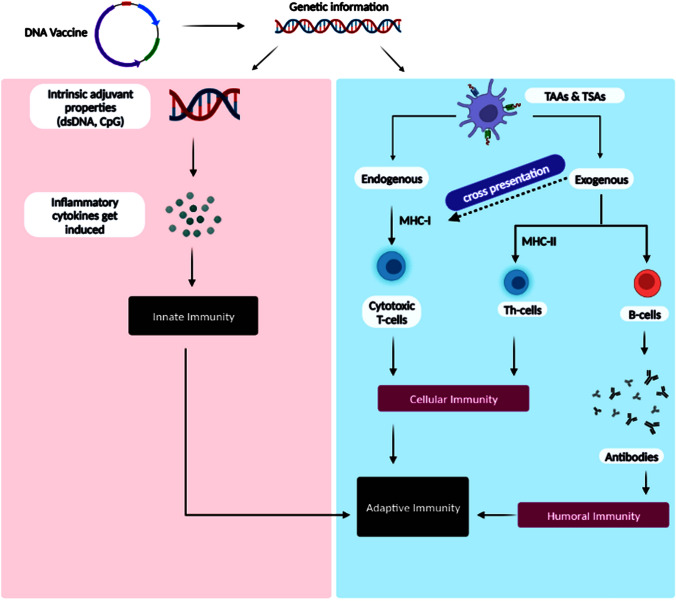
Fig. 3.
Illustration demonstrating the mechanism of action of the DNA-based cancer vaccines in stimulating the adaptive and innate immunities against cancer cells. The vaccine encodes tumor-specific antigens, which are taken up by dendritic cells and presented to T cells. This results in the activation of both innate and adaptive immune responses against cancer cells. Innate immune cells, such as natural killer cells, release cytokines and chemokines that recruit additional immune cells to the site of the tumor. The activated T cells then migrate to the tumor site and release cytotoxic molecules that kill cancer cells. This mechanism leads to a systemic anti-tumor immune response, reducing the risk of cancer recurrence
The innate immune reactions, consisting of NK cells, neutrophils, and macrophages, recognize the cancer antigens through the Fc receptors of tumor-bound antibodies (the antibodies that have identified the epitopes on tumor cell surfaces) and phagocytose the cancer cells [101]. These vaccines can be delivered systemically or topically. Systemic routes involve oral, pulmonary, and intravenous whereas topical routes involve intramuscular, subcutaneous, transdermal, and intradermal. The mechanism of action is reported to vary with the routes of administration. Systemic routes tend to activate more APCs of secondary lymphoid organs compared to topical ones [76]. In intramuscular delivery route, myocytes get transfected. Myocytes not being a professional APC, forms complex with MHC-I to stimulate CD8+ lymphocytes, but fails to induce the regulatory T-cells. However, due to inflammation and the release of cytokines caused by vaccination, professional APCs like dendritic cells get recruited. These then phagocytose the transfected somatic cells followed by processing and presentation of exogenous antigens through complexes with MHC class I and II [102]. On the other hand, Gene-gun aided intradermal administration of plasmid DNA transfects the immature Langerhans cells which then present endogenous antigens to the CD8+ T-cells through MHC-I molecules. APCs can also be transfected directly, to trigger CD8+ T-cells through MHC-I presentation [93]. All these findings elaborate on the profound mechanisms worked out by DNA immunizations in abolishing malignancies.
Advantages and disadvantages
Concerning good science-and-society policy-making, DNA-based cancer vaccines are more of a cautionary tale. It is important to weigh out all the pros and cons of these vaccines considering individual patients. To start with, DNA vaccines extend plenteous benefits among all the immunization technologies. These vaccines are free of any kind of infectious agents, as seen with the vaccines consisting of dead bacteria or their live-attenuated forms, and thus are innocuous. These vaccines are keenly priced, contemplating the mass production and storage costs [102]. These are stable at ambient temperature reducing the transportation values [93]. They corroborate the appropriate processing and presentation of the required gene targets, the contributing factor being the in-vivo presentation of the antigen and in-house post-translational modifications of antigen as seen with natural infections [65]. Recombinant DNA technologies ease the rapid modifications in antigens [94]. As mentioned previously, they activate adaptive as well as humoral immunity.
They provide safety against various vector-related issues due to the stimulation of innate immunity [92]. Bumping out these advantages, DNA vaccines hold a few limitations that have impeded their approval to treat cancers in humans. One of the cardinal disadvantages of DNA vaccines is its substandard immunogenicity. For the moderate provocation of the immune system, larger quantities of approximately 5–10 mg are needed [103]. The immune responses stimulated by these vaccines are limited to protein-based antigens and are not useful for sugar-coated bacteria. The body may develop tolerance against the antigens introduced through these vaccines. Lastly, there always remains a menace of autoimmunity and the integration of the plasmid DNA into the genome of the host [94].
Description of vaccines under development or trials
Immunotherapy is consistently being explored to defeat cancers and improve the prospects of onco-therapy. Few studies [16] are under clinical trials to evaluate DNA-based cancer vaccines for their efficiency to mitigate the cancers of Breast, Prostate, Ovarian, Lung, Brain, and Cervix and the same have been enlisted in Table 1. Looking towards a broader view of DNA vaccines, these vaccines are being maximally evaluated in the fields of Breast and Prostate cancers. With more than 2 million cases in the year 2020, Breast cancer has been incessantly marking its position as the most frequently occurring cancer in women [104]. With this consideration, DNA vaccines are being scrutinized for about eighteen classes of histologically differing breast cancers, common ones being HER2 positive, HER2 negative, and Triple Negative Breast cancers (TNBCs). To begin with, HER2-positive breast cancers embody tumor cells that test positive for Human epidermal growth factor receptor 2—a proliferative gene.
Table 1.
Ongoing clinical trials focusing on the development of the DNA vaccines against cancer
| Sr No | NCT | Study type and allocation | Title | Status | Phase | Total no. of patients | Regimen | Condition | Outcomes | References |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | NCT05455658 | Interventional | STEMVAC in patients with early stage triple negative breast cancer | Recruiting | II | 33 | STEMVAC in combination with sargramostim | Triple negative breast cancer |
1 Outcome: cellular immune response 2 Outcome: incidence of AEs, RFS |
[105, 106] |
| 2 | NCT02157051 | Interventional, non-randomizsed | Vaccine therapy in treating patients with HER2-negative stage III-IV breast cancer | Active, not recruiting | I | 42 | STEMVAC in combination with sargramostim | Triple negative breast cancer |
1 Outcome: incidence of toxicity, immunologic efficacy 2 Outcome: Memory Th1 dominant immune response, modulation of Tregs, Modulation of MDSC, STEMVAC specific Type 1 immune response |
[107, 108] |
| 3 | NCT02780401 | Interventional | Vaccine therapy in preventing cancer recurrence in patients with non-metastatic, node positive, HER2 negative breast cancer that is in remission (WOKVAC) | Active, not recruiting | I | 24 | WOKVAC in combination with Sargramostim | Non-metastatic, node positive, HER2-negative breast cancer |
1 Outcome: incidence of toxicity 2 Outcome: Assessment of IgG antibodies and T-helper Th1:Th2 ratio, modulation of Tregs, Modulation of MDSC, Assessment of the immunogenicity of WOKVAC |
[105, 109, 110] |
| 4 | NCT05242965 | Interventional, randomized | A Multiple antigen vaccine (STEMVAC) for the treatment of patients With stage IV non-squamous non-small cell lung cancer | Recruiting | II | 40 | STEMVAC combined with sargramostim | Non-small cell lung cancer |
1 Outcome: Change from baseline percentage of CD8 + TIL in patients, incidence of AEs 2 Outcome; ORR, OS, PFS, Magnitude of the immune response, Vaccine induced T-cells traffic to tumor |
[16, 111] |
| 5 | NCT03988283 | Interventional | Neoepitope-based personalized DNA vaccine approach in pediatric patients with recurrent brain tumors | Not yet recruiting | I | 10 | Personalized neoantigen DNA vaccine | Recurrent brain tumors |
1 Outcome: safety and tolerability 2 Outcome: median PFS and median OS |
[112, 113] |
| 6 | NCT04015700 | Interventional | Neoantigen-based personalized DNA vaccine in patients with newly diagnosed, unmethylated glioblastoma | Active, not recruiting | I | 9 | Personalized neoantigen DNA vaccine Plasmid encoded IL-12 | Unmethylated glioblastoma |
1 Outcome: safety and tolerability 2 Outcome: immunogenicity, PFS, OS |
[114, 115] |
| 7 | NCT03600350 | Interventional | pTVG-HP and Nivolumab in patients with non-metastatic PSA-recurrent prostate cancer | Active, not recruiting | II | 19 |
DNA vaccine encoding prostatic acid phosphatase when combined with Nivolumab Sargramostim |
Non-metastatic, PSA-recurrent prostate cancer |
1 Outcome: patients with acceptable toxicities boundaries, PSA-CR rate 2 Outcome: PSA doubling time, PSA response rate, median radiographic PFS, metastasis free-survival rate |
[116, 117] |
| 8 | NCT04090528 | Interventional, randomized | pTVG-HP DNA vaccine with or without pTVG-AR DNA vaccine and pembrolizumab in patients with castration-resistant, metastatic prostate cancer | Recruiting | II | 60 |
pTVG-HP combined with pTVG-AR Pembrolizumab |
Castration-resistant, metastatic prostate cancer |
1 Outcome: PFS 2 Outcome: ORR, OS, safety and tolerability, median radiographic PFS |
[105, 118] |
| 9 | NCT03199040 | Interventional, randomized | Neoantigen DNA vaccine alone vs. neoantigen DNA vaccine plus Durvalumab in triple negative breast cancer patients following standard of care therapy | Active, not recruiting | I | 18 | Neoantigen DNA vaccine in combination Durvalumab | Triple negative breast cancer |
1 Outcome: safety 2 Outcome: immune response |
[50, 119] |
| 10 | NCT04131413 | Interventional, non-randomized | HPV DNA vaccine via electroporation for HPV16 positive cervical neoplasia | Recruiting | I | 48 | pNGVL4aCRTE6E7L2 | HPV16 positive cervical neoplasia | 1 Outcome: no. of patients experiencing DLTs | [120, 121] |
| 11 | NCT04989946 | Interventional, randomized | Androgen deprivation, with or without pTVG-AR, and with or without nivolumab, in patients with newly diagnosed, high-risk prostate cancer | Recruiting | I/II | 39 | pTVG-AR combined with Degarelix, Nivolumab | Prostate cancer |
1 Outcome: pCR, MRD, incidence of AEs, toxicity rates 2 Outcome: PFS at 1 year, RCB |
[122, 123] |
| 12 | NCT04329065 | Interventional | Concurrent WOKVAC vaccination, chemotherapy, and HER2-targeted monoclonal antibody therapy before surgery for the treatment of patients with breast cancer | Recruiting | II | 16 | WOKVAC and Paclitaxel and Trastuzumab Pertuzumab | Breast cancer |
1 Outcome: Enumeration of the number of T-bet + , CD4 + , and CD8 + T-cells in TIL 2 Outcome: incidence of AEs, Induction of type 1 helper cell (Th1) immunity against HER2, IGF-1R, and IGFBP2 |
[124, 125] |
| 13 | NCT03439085 | Interventional | DNA Plasmid-encoding Interleukin-12/HPV DNA plasmids therapeutic vaccine INO-3112 and Durvalumab in treating patients with recurrent or metastatic human papillomavirus associated cancers | Active, not recruiting | II | 77 | MEDI0457 with Durvalumab | Recurrent/metastatic human papilloma virus associated cancers |
1 Outcome: ORR 2 Outcome: ORR, PFS, OS, DCR |
[126, 127] |
| 14 | NCT03603808 | Interventional | VGX-3100 and electroporation in treating patients with hiv-positive high-grade anal lesions | Recruiting | II | 80 | VGX-3100 | HIV-associated anal neoplasia |
1 Outcome: ORR 2 Outcome: safety and tolerability, ORR, CRR, viral clearance anal swab |
[128, 129] |
| 15 | NCT04251117 | Interventional | GNOS-PV02 personalized neoantigen vaccine, INO-9012 and pembrolizumab in subjects with advanced HCC | Recruiting | I/II | 36 |
Personalized neoantigen DNA vaccine (GNOS-PV02) and plasmid encoded IL-12 (INO-9012) with Pembrolizumab (MK-3475) |
Advanced hepatocellular carcinoma |
1 Outcome; AEs, immunogenicity 2 Outcome: ORR, DCR, DOR, PFS, OS |
[130, 131] |
pNGVL3-hICD and sargramostim in advanced stage HER2 positive breast cancer and ovarian cancer
This vaccine encodes the intracellular domain of the HER-2/neu proto-oncogene and elicits the cellular and humoral immune responses against HER2-upregulated tumor cells [132]. A single-arm phase-I clinical trial enrolling 66 adult females with HER2-positive BC or Ovarian Germ cell and Epithelial cancers to investigate the efficacy of pNGVL3-hICD, a DNA plasmid-based vaccine in combination with Sargramostim-a recombinant granulocyte–macrophage colony-stimulating factor (rGM-CSF) [16, 132]. It is an open-label study where patients are receiving pNGVL3-hICD with Sargramostim as an adjuvant, intradermally once a month for 3 months in the absence of disease progression or unacceptable toxicity. Three arms are divided on the basis of doses, that is, 10 mcg, 100 mcg and 500mcg of plasma. The patients had completed appropriate treatment for their primary disease and were off cytotoxic chemotherapy and corticosteroids for at least 1 month before enrollment. The primary objective of the study is to determine the safety of intradermal administration of 3 doses of pNGVL3-hICD admixed with a fixed dose of Sargramostim. The outline of the trial aims to study the side effects and identify the best dose of pNGVL3-hICD. The results procured till now portray the intermediate dose of 100 mcg to be immunogenic, where immunogenicity lasted for 60 weeks. The estimated completion date of this trial is December 1, 2024.
STEMVAC with GM-CSF in HER2-negative advanced stage breast cancer
STEMVAC is a multi-antigen vaccine that uses genetic engineering to manipulate DNA and instructs the cell to produce target antigens [133]. This phase-I Non-randomized clinical trial aims to study the side effects and the most efficacious dose of CD105/Yb-1/SOX2/CDH3/MDM2-polyepitope plasmid DNA vaccine with 100 mcg of recombinant human granulocyte–macrophage colony-stimulating factor as an adjuvant (NCT02157051) [107]. 42 adult patients were divided inro 3 dose arms of 150mcg, 300 mcg, and 600 mcg of STEMVAC. The patients who have had completed standard of care and recovered with mild to no residual toxicity from recent therapy had been enrolled and assigned one of the three arms. Patients also received 2 additional booster doses of STEMVAC vaccines at 3 and 6 months after the third vaccine in the absence of unacceptable toxicity or disease progression. Primary endpoints investigated safety and immunogenicity, with secondary outcomes being persistence of immune response following vaccination, and the induction of MDSCs and Tregs. The results obtained till date portray the dose of 300 mcg to be highly persistent [134]. After completion of study treatment, patients will be followed up twice yearly for up to 5 years. The completion date has been estimated to be February 10, 2027.
WOKVAC with sargramostim in non-metastatic, node positive, and HER2 negative breast cancer
WOKVAC is DNA plasmid-based vaccine that encodes three proteins namely insulin-like growth factor binding protein 2 (IGFBP2), HER2, and insulin-like growth factor receptor-1 (IGF-1R) [107]. These proteins are overexpressed in pre-invasive and high-risk breast lesions and are associated with progression to invasive breast cancer. A phase I trial that evaluates the side effects and an appropriate dose of pUMVC3-IGFBP2-HER2-IGF1R with Sargramostim in preventing cancer recurrence in patients with non-metastatic, node-positive, human epidermal growth factor receptor 2 negative breast cancer (NCT02780401) [134]. In total, 24 adult patients were enrolled who were in remission and had no evidence of disease. It is a single-arm open-label study where the first includes patients receiving WOKVAC with sargramostim ID on day 1 and the courses being repeated every 28 days for up to 3 courses in the absence of disease progression or unacceptable toxicity. Patients with axillary lymph node dissection (ALND) will be receiving a vaccine in the contralateral arm and for the ones with bilateral ALND, vaccine will be administered in the thigh. After completion of study treatment, patients will be followed up at 1, 6 months, and annually for up to 5 years. The completion date of the trial is estimated to be March 31, 2025.
WOKVAC in combination with chemotherapeutic and HER-2 targeted immunotherapeutic agents in breast cancer
A single-group, open-label, phase II study investigates the efficacy of the combination of WOKVAC with paclitaxel, pertuzumab and trastuzumab (NCT04329065). Patients will be receiving trastuzumab and pertuzumab on day 1 and paclitaxel infusion on days 1, 8, and 15. WOKVAC will be administered on day 13 of a 21-day cycle. The immuno-chemotherapeutic combination with vaccine will be primarily assessed on the basis of the number of tumor-infiltrating lymphocytes (TILs) [92]. The trial results will be giving us an insight on the combination of chemotherapy, monoclonal antibodies, and the DNA vaccine. The completion date of this trial has been predicted to be June 30, 2027 [124].
STEMVAC with Sargramostatin in triple negative breast cancer
A phase 2 trial scrutinizes STEMVAC T-helper (Th1) Polyepitope Plasmid-based Vaccine admixed with Sargramostim for its ability to treat patients with stage IB-III triple-negative breast cancer (NCT05455658) [135]. With the total of 33 adult patients, STEMVAC vaccine with GM-CSF was administered intradermally every month for 3 months in the absence of disease progression or unacceptable toxicity. Patients will then be receiving STEMVAC vaccine with sargramostatin ID booster injections 3 months after the 3rd vaccination and 6 months after the 1st booster vaccination. The primary outcomes to be evaluated include the stimulation of specific Th1 immune response and secondary points investigate on safety parameters. After completion of study treatment, patients will be followed up at 28 days, and then annually for 5 years. The estimated completion date is mentioned as April 30, 2024.
pTVG-HP in combination with pTVG-AR with pembrolizumab for prostate cancer—trial combining two DNA vaccines
These vaccines are the plasmid DNAs where pTVG-HP encodes for the human prostatic acid phosphatase and pTVG-AR encodes for the ligand-binding domain of androgen receptors [105]. One of the two experimental arms of a randomized, open-label and multi-center study, describes the administration of two DNA vaccines, with pTVG-HP given on days 1 and 8 for cycles 1,2,5 and 6 (NCT04090528). Alternatively, pTVG-AR, to be administered on days 1 and 8 for cycles 3, 4, 7 and 8. The vaccines will be given in combination with the monoclonal antibody, Pembrolizumab, on day 1. The trial aims at evaluating the progression-free survival (PFS) as a primary outcome with several secondary outcomes. The completion date has been estimated to in December of 2025 [105].
Mammoglobin-A DNA vaccine in combination with neoadjuvant chemotherapy of endocrine therapy for treating Breast Cancer
Mammoglobin-A occurs as an exceptional target for breast tumors, with it being a member of the secretoglobulin superfamily that involves dimeric proteins being mainly expressed in mucosal tissues [136, 137]. It is having a near-universal high expression coupled with good specificity, which makes it an extraordinary target for breast cancer prevention or treatment. A non-randomized, open-label study has been established to assess the efficacy of mammoglobin-A DNA vaccine in patients with ER+ , HER2- breast cancer (NCT02204098). One of the arms involves the administration of this vaccine in combination with the neoadjuvant chemotherapy and other arm is set to administer vaccine, combined with endocrine therapy. The outcomes to be measured include safety parameters, immune response, objective response rate (ORR), PFS and overall survival (OS). The estimated completion date of the trial is August 31, 2028 [138].
In a nutshell, DNA vaccines are currently undergoing testing for a wide range of cancer types, including commonly occurring cancers of breast, prostate, ovarian and cervical, coupled with determining its efficacy in relatively rare tumors of lung, brain and anus. These trials are being conducted to evaluate the effectiveness of DNA vaccines as both preventative and therapeutic treatments. The breadth of these trials suggests that DNA vaccines have the potential to become a prominent form of onco-therapy in the future, offering a promising new approach to cancer treatment.
Completed clinical trials demonstrating the efficacy of DNA vaccines
Numerous trials have completed their goal of evaluating DNA vaccines in various types of cancers including melanoma, multiple myeloma, cervical cancers, breast cancers, ovarian cancers, prostate cancers, bladder cancers, and lung cancers. Some of the crucial ones have been introduced in Table 2, and few of these have been described. An open-label, non-randomized, phase 2 study evaluated the safety, tolerability, and immunogenicity of the INO-3112, a DNA vaccine, in women with cervical cancer [139, 140]. Here, VGX-3100 embodies HPV-16 and HPV-18 plasmids and INO-9012 consists of DNA that encodes interleukin-12 (IL-12). Eighteen female participants were enrolled who had a biopsy-proven, stage IB-IVB invasive cervical cancer, which were inoperable and associated with human papilloma virus (HPV) 16 or 18, and the females who have already been administered two types of therapy. These two therapies had divided patients into two cohorts. Cohort I included patients who have had standard chemotherapy with a curative intent whereas, Cohort II included patients who had recurrent HPV-associated cervical cancer following the salvage therapy. The study highlights that the vaccine induced a strong antibody response, with the stimulation of CD8+ T-cells with cytotoxic T-cell (CTL) phenotype. Also, it produced antigen-specific CTLs. The adverse events were mild to moderate [141]. Grade 3 adverse events reported were viral gastroenteritis, tension headache, and wrist headache. However, these grade 3 adverse events were not related to the treatment. With the laboratory parameters, only two of them had moderate hypoglycemia not requiring any treatment. This data portrays the capability of this vaccine to drive strong response to the administered antigens and the vaccine could be deduced to have a near curative future.
Table 2.
Completed clinical trials that assessed the profile of DNA vaccines
| Sr No | NCT | Study type and allocation | Title | Phase | Total no. of patients | Regimen | Outcome | Condition | Remarks | References |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | NCT00849121 | Interventional, randomized | Two-Arm study of a DNA vaccine encoding prostatic acid phosphatase (PAP) in patients with non-metastatic castrate-resistant prostate cancer | I | 17 |
pTVG-HP with rhGM-CSF Every 3 Months Post Week 12 pTVG-HP with rhGM-CSF Variable Dosing Post Week 12 |
Non-metastatic castrate-resistant prostate cancer | Excellent PAP-specific CTL response was observed. Also, the PSA doubling time did rise during the course of treatment. After receiving the therapy for year, 12/17 patients were free of metastasis | [18, 143] | |
| 2 | NCT02172911 | Interventional, non-randpmized | A study of INO-3112 DNA vaccine with electroporation in participants with cervical cancer | I/II | 10 |
Curative therapy with INO-3112 Salvage therapy with INO-3112 |
Antibody responses against HPV oncoproteins were detected in up to 60% of patients | HPV-16 and/or 18-positive cervical carcinoma | TRAEs were all Grade 1, primarily injection site related. Adjuvant MEDI0457 (INO-3112) is feasible after chemoradiation for locally advanced and/or recurrent cervical cancer. More than half of the patients developed detectable immune responses to HPV antigens after treatment | [58, 144] |
| 3 | NCT01341652 | Interventional, randomized | Phase II PAP plus GM-CSF Versus GM-CSF alone for non-metastatic prostate cancer | II | 99 |
pTVG-HP vaccine with GM-CSF GM-CSF |
Two-year MFS was not different between study arms (41.8% vaccine v 42.3%. PAP-specific T cells were detected in both cohorts, including multifunctional PAP-specific T-helper 1–biased T cells | Non-metastatic prostate cancer | Except for a subset with quickly progressing disease, pTVG-HP therapy did not show a general increase in 2-year MFS in patients with castration-sensitive prostate cancer | [139, 145] |
| 4 | NCT01440816 | Interventional, non-randomized | IL-12 gene and in Vivo electroporation-mediated plasmid DNA vaccine therapy in patients with merkel cell cancer (MCC) | II | 15 |
One cycle of tavo with dose of 0.5 mg/ml followed by electroporation and proceeded to definitive treatment Four cycles of tavo with dose of 0.5 mg/ml followed by electroporation, with 12 weeks between each cycle and continuing till 12 months |
The ORR was 25% (3/12) in cohort B, with 2 patients experiencing durable clinical benefit (16 and 55 + months, respectively). Two cohort A patients were recurrence-free at 44 + and 75 + months, respectively | Merkel cell carcinoma | The therapy was safe and feasible and no toxicities were observed. Several individuals experienced systemic immune responses and clinically significant benefits as a result of the sustained local production of the IL12 protein and local inflammation | [146, 147] |
| 5 | NCT00199849 | Interventional, non-randomized | NY-ESO-1 plasmid DNA (pPJV7611) cancer vaccine | I | 18 |
4 µg administered as 4 × 1 µg PMEDs 8 µg administered as 8 × 1 µg PMEDs 8 µg as a cluster dosage of 4 doses (6 6 8 8) as 2 × 1 µg PMEDs per day |
Prostate cancer Bladder cancer Non-small cell lung cancer Esophageal cancer Sarcoma |
Most patients demonstrated an overall rise in antibody titer and NY-ESO-1-specific CD4 + and CD8 + T cells. Besides that, not all patients experienced a positive delayed-type hypersensitivity (DTH) reaction to the protein NY-ESO-1 | [148, 149] | |
| 6 | NCT00398073 | Interventional, randomized | Vaccine therapy in treating patients with stage IIB, stage IIC, stage III, or stage IV melanoma | I | 35 |
Insertion of mouse gp100 DNA through PMED Insertion of mouse gp100 DNA through intramuscular injection |
Melanoma | The trial demonstrated that the evoked immune responses reduce tumor development and increase survival | [102, 150] |
The NCT01341652 phase 2 study assessed the effectiveness of pTVG-HP in conjunction with rhGM-CSF [142]. Ninety-nine patients, with non-metastatic, castration-sensitive prostate cancer were enrolled. The patients were randomized to get administered with either pTVG-HP intradermally, combined with 200 mcg of GM-CSF or 200 mcg alone. Two-year Metastasis-free interval was the primary endpoint to be evaluated. The secondary outcomes to be investigated included modulations in prostate-specific antigen doubling time, median time to radiographic disease progression, PSA, PFS, and observed toxicities. The treatment with pTVG-HP did not showcase a significant increase in 2-year metastasis-free survival (MFS). However, a Prespecified 18F-NaF PET/CT imaging revealed significant effects on micro-metastatic bone disease. In brief, this study did not showcase satisfying results and needs further analysis [139]. The ongoing trials are investigating the combination of pTVG-HP with the PD-1 inhibitors.
An interesting investigation is being conducted by an open-label, non-randomized, phase 2 study, on the effectiveness of ImmunoPulse IL-12 in Merkel cell carcinoma [151]. This vaccine is the combination of tavokinogene telseplasmid, a plasmid encoding IL-12 and in vivo electroporation-mediated plasmid DNA vaccine. Fifteen participants were enrolled in the study, to evaluate the primary and the secondary endpoints. Twelve of the participants exhibited a two-fold increase in the expression of IL-12 in the tumoral tissue. Adverse effects were seen more with the administration of four cycles compared to a single cycle of vaccine and the only significant adverse effect witnessed was injection site inflammation. In a nutshell, the completed clinical trials are relatively less and they fail to portray satisfactory results. However, pTVG-HP has apparently shown encouraging results and could be a pioneer in the near future. Additively, a good number of trials are in the ongoing phase and they could lead as to a therapeutic direction soon.
Union of DNA vaccines with other therapies
DNA vaccines, solitarily, are incapable of overcoming the tumor’s immune escape strategies including the selection of tumor cells that efficiently lack the immunogenic antigens as well as the sufficient enrollment of the immune suppressing cells in the TME [152]. Nevertheless, they could be potentiated by amalgamating them with the strategies that silence the recruitment of immune-suppressive cells coupled with adequate induction of the immune response against TAs in the TME [18]. Literature evidences regarding the combination of DNA vaccine with, the conventional approaches embodying chemotherapy, radiotherapy, and surgical interventions, could prove well. Figure 4 illustrates the various combinatorial strategies pf the DNA-based cancer vaccines and how the combinations affect the tumor cells. Some of the preclinical trials depicting the combination of DNA vaccines with other therapies are listed in Table 3 and the completed clinical trials have been enlisted in Table 4.
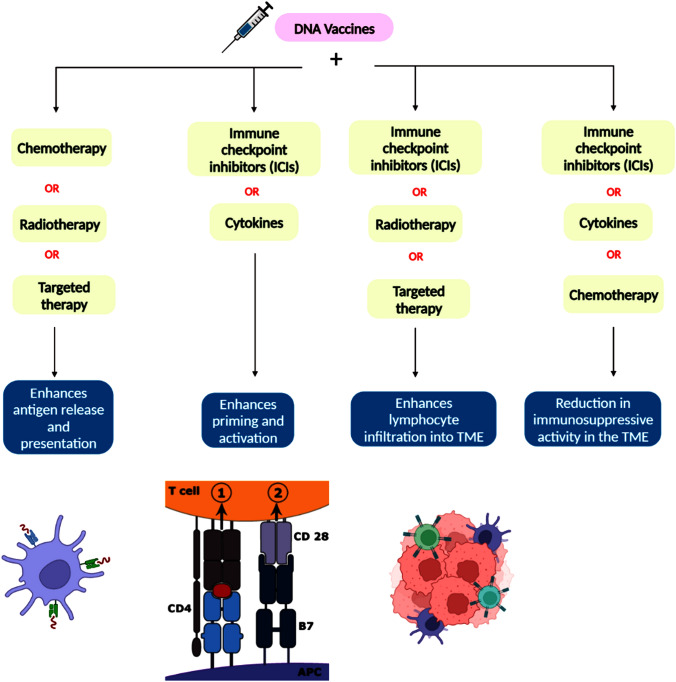
Fig. 4.
Illustration demonstrating the combinational strategies of DNA-based cancer vaccines with conventional therapies, targeted therapies, immune therapies and cytokines in opposing the capabilities of tumor cells. The vaccine targets specific tumor antigens, while conventional therapies, such as chemotherapy, radiation therapy, and surgery, eliminate bulk tumor cells. Targeted therapies, such as monoclonal antibodies and small molecule inhibitors, interfere with specific molecules involved in tumor growth and progression. Immune checkpoint inhibitors release the brakes on the immune system, allowing for greater T cell activation against cancer cells. Finally, cytokines, such as interferons and interleukins, stimulate immune cells and increase their cytotoxicity against tumor cells. The combination of these strategies has the potential to enhance the efficacy of cancer treatment and improve patient outcomes
Table 3.
Preclinical Studies of DNA-Based Cancer Vaccines in Murine Models as a part of new vaccine discovery and development
| Sr No | Murine model and cancer-type | Treatment regimen | Critical findings | References |
|---|---|---|---|---|
| 1 |
C57BL/6 mice Cervical cancer (TC-1 cells) |
DNA Vaccine: HPV plasmid encoding E6 and E7 antigens Combination therapy: pVAX1-ISG15 encoding an optimized mouse adjuvant ISG15 |
Strong immune response was observed with an increase in IFN-γ secretion | [153] |
| 2 |
BALB/c mice Murine breast cancer (D2F2 cells) |
DNA Vaccine: pVAX-E2A, encoding Her2/neu antigen Combination therapy: pVAX-CCL4, encoding CCL4, chemoattractant for immune effector cells |
Combination therapy improved tumor protection. Also, CCL4 produced a Th1 anti-Her2/neu response | [154] |
| 3 |
BALB/c mice Murine breast cancer (4 T1 cells) |
DNA Vaccine: CpVR-FAP, encoding fibroblast associated protein (FAP) Combination therapy: Cyclophosphamide, chemotherapy agent |
Combination therapy elevated median survival time of mice | [155] |
| 4 |
BALB/c mice Colon cancer (CT26/HER2 cells) |
DNA Vaccine: pVAX1-HER2, coding HER2 antigen Combination therapy: Gemcitabine, chemotherapy agent; anti-Gr1 antibody; anti-PD-L1 Ab |
Combination of vaccine + anti-PD-L1 Ab failed to delay tumor growth. The addition of anti-Gr1 or gemcitabine delayed tumor growth | [156] |
| 5 |
DBA/2 mice Mastocytoma (P815 cells) |
DNA Vaccine: Optimized pVAX2-P1A vaccine, encoding P815A Combination therapy: Anti-CTLA4, anti-PD1 |
The survival rate reached to 90%. IL-12 production increased and metastasis formation decreased | [157] |
| 6 |
HHDII-DR1 mice Sarcoma |
DNA Vaccine: SSX2-optimized vaccine, encoding modified cancer testis antigen Combination therapy: Ab anti-PD-1/L1 |
Increase of PD-L1 expression on tumor cells. CTL from immunized mice expressed more PD-1, increasing the antitumor efficacy of the combination with ICB | [158] |
| 7 |
C57BL/6 mice Melanoma (B16F10 cells) |
DNA Vaccine: pVAX1-MUCI, encoding mucin I glycoprotein Combination therapy: pVAX1-Flt3L, encoding Fms-like tyrosinase 3-ligand |
Specific CTL and antibodies were observed and tumor growth suppression was seen | [159] |
| 8 |
BALB/c mice Colorectal cancer (CT- 26/NIS cells) |
DNA Vaccine: pcDNA-hNIS, expressing human sodium/io- dide symporter (hNIS) | Elevation was seen in the IgG2a/IgG1 ratio, and the number of INF-g-secreting cells and IFN-g production increased. Th1 response was demonstrated and tumor growth slowed down | [160] |
| 9 |
BALB/c mice Murine breast cancer (4 T1 cells) |
DNA Vaccine: pVAX1-mCr-1, encoding mouse Cripto-1 oncofetal protein | A protective immune response was observed against cancer stem cells and lung metastasis seemed to be reduced | [161] |
| 10 |
BALB/c mice Murine breast cancer (4 T1 cells) |
DNA Vaccine: CpVR-FAP, encoding FAP | An increase in IL-2 production and a delay of tumor growth was observed. Specific CTL response against FAP was seen | [162] |
| 11 |
BALB/c mice Kidney cancer (RenCa cells) |
DNA Vaccine: pVAX1-G250-F2A-CTLA4, containing the co-expression gene G250-CTLA4, linked by Furin-2A (F2A) | Humoral and cellular-specific immune response against CTLA4 and G250 was observed. The rate of tumor growth reduced and there was an increase in INFγ and IL-4 (Th1/2 response) | [163] |
Table 4.
Completed clinical trials combining DNA Vaccines with other therapies
| Sr. No | NCT no | Study type and allocation | Title | Phase | Total no. of patients | Regimen | Outcome | Condition | Remarks | References |
|---|---|---|---|---|---|---|---|---|---|---|
| DNA Vaccines in combination with chemotherapy/ICIs | ||||||||||
| 1 | NCT03532217 | Interventional | Neoantigen DNA vaccine in combination with Nivolumab/Ipilimumab and PROSTVAC in metastatic hormone-sensitive prostate cancer | I | 19 | PROSTVAC/Ipilimumab/Nivolumab/Neoantigen DNA vaccine | Treatment was well-tolerated with only 2 (2.4%) grade 3 TRAEs of colitis, and no grade 4 + TRAEs | Metastatic hormone-sensitive prostate cancer | Increases in activation/co-stimulatory/co-inhibitory seen after treatment with Prostvac/ICB, suggest immune priming | [164, 165] |
| 2 | NCT01673217 | Interventional | Decitabine, vaccine therapy, and pegylated liposomal doxorubicin hydrochloride in treating patients with recurrent ovarian epithelial cancer, fallopian tube cancer, or peritoneal cancer | I | 18 | Decitabine intravenously (IV) over 3 h on day 1, pegylated liposomal doxorubicin hydrochloride IV on day 8, and NY-ESO-1 peptide vaccine emulsified in incomplete Freund's adjuvant and sargramostim subcutaneously on day 15 | – |
Recurrent fallopian tube cancer, Recurrent ovarian epithelial cancer, Recurrent primary peritoneal cavity cancer |
– | [166] |
| 3 | NCT00113984 | Interventional | Vaccine and antibody treatment of prostate cancer | I | 30 |
Biological: PROSTVAC-V/TRICOM Biological: PROSTVAC-F/TRICOM Drug: MDX-010 Drug: Sargramostim |
3 of 30 patients had grade 1 reactions and 26 had grade 2 reactions. 21 patients had grade 2 or greater immune-related adverse events | Prostatic neoplasm | The use of a vaccine targeting PSA that also enhances co-stimulation of the immune system did not seem to exacerbate the immune-related adverse events associated with ipilimumab | [167, 168] |
| DNA vaccines in combination with targeted therapy | ||||||||||
| 4 | NCT02529930 | Interventional, non-randomized | An exploratory safety and immunogenicity study of HPV16 + immunotherapy VB10.16 in Women With HSIL; CIN 2/3) | I/II | 34 | VB10.16 Immunotherapy (DNA vaccine) | AEs were mainly mild to moderate and related to the injection site. No SAEs or DLTs were reported | High Grade Cervical Intraepithelial Neoplasia | Strongly encouraging phase 1/2a safety, tolerability, and immunogenicity results for a therapeutic HPV16 DNA plasmid vaccine VB10 was reported | [169, 170] |
| 5 | NCT00179309 | Interventional, randomized | Docetaxel alone or in combination with vaccine to treat breast cancer | II | 48 |
Arm 1: PANVAC + docetaxel Arm 2: docetaxel alone |
PFS is 6.6 vs. 3.8 months in A vs. B. toxicity levels were same in both the arms | Breast cancer | This study demonstrated that the combination of PANVAC with DOC in metastatic breast cancer may provide a clinical benefit compared to DOC alone | [171, 172] |
Combining DNA vaccines with chemotherapy
The recent years have shed light on how chemotherapy plays a two-way role in mitigation of tumor. Induction of TA release along with the increased activity of T-cells in the TME and removal of immunity suppressing cells have been witnessed in several chemotherapeutic drugs like paclitaxel, cyclophosphamide, and gemcitabine [173–175]. Among several preclinical studies, one of the study evaluated the combination of DNA vaccines with cyclophosphamide and the results showcased decrease in the VEGF and IL-10 coupled with increase in the survival ratio of mice [176]. As apparent in the table representing the ongoing clinical trials, the combination strategies involving DNA vaccines and relevant chemotherapeutic agents may be crucially involved in tumor therapies in the succeeding years. Toll-like receptor 4 (TLR4) simulation mediated re-establishment of sensitivity to the checkpoint blockade has been encountered in chemotherapeutic treatments [177].
Combining DNA vaccines with targeted therapies
Targeted therapies which amplify priming of T-cells and stimulate the release of TAs, could be combined with the DNA vaccines. Though not tested in combination with DNA vaccines, decrease in the volume of tumor was noted when sunitinib, a tyrosine kinase inhibitor (TKI) was combined with a carcinoembryonic antigen (CEA)-encoded viral vaccine [178]. However, future studies might evaluate the FDA-approved TKIs like axitinib, cabozantinib, and pazopanib in combination with the DNA vaccines.
Combining DNA vaccines with immune checkpoint inhibitors
The co-stimulatory molecules that mediate the signaling mechanisms of antigen presentation are key elements in the regulation of T-cells [179]. The tumors have the ability to mimic this costimulatory molecule and inhibit the activation of immune cells against TAs, thus escaping immunity [180]. To be specific, the tumor cells express ligands that get attached to the inhibitory receptors like PD-1, CTLA-4, T-cell immunoglobulin and mucin-domain containing-3 (TIM-3), and lymphocyte activation gene-3 (LAG-3), present on the T-cells [181]. One way to override such escape of tumor cells is to block these inhibitory receptors on T-cells and prevent their interaction with tumor-released ligands. The inhibition of CTLA-4 that had been assessed in preclinical models showcased a delay in the growth of the tumor [182]. Tumor rejection was witnessed when the same CTLA-4 blockade was investigated in melanoma patients [183]. Enhanced activation of T-cells, along with the inhibition of IL-10 and TGF-β, could be held responsible for these antitumor effects. Additive to this benefit, the immune memory has been reported to be favorable with the CTLA-4 inhibitors [184]. Also, the anti-PD-1 antibodies have received FDA-approval for a variety of cancers, as these antibodies exhibited significantly efficient results [185]. The tumors with the enhanced burden of tumor possess increased neoantigens. These high amounts of neoantigens could be recognized well by the antitumor mechanisms and ultimately making such cancers susceptible to the therapies involving ICIs [186]. Ergo the combination of vaccines with ICIs could be proven beneficial in future.
Combining DNA vaccines with cytokines
The efficiency of the vaccine on the T-cells can be enhanced by the aid of immunostimulatory cytokines. They could be injected in form of proteins or could be encoded through target-encoded vaccines. GM-CSF, IL-12, and IL-2 are the most frequently involved in the clinical studies [187]. FDA has approved IL-2, as it forms more of effector and regulatory T-cells by differentiating the immature T-cells [188]. Also, it had shown efficacious results in mitigating metastatic renal cell cancer and metastatic melanoma. GM-CSFs, like Sargramostim, which has been already introduced in the ongoing clinical trials, stimulates the maturation of dendritic cells and induces activation and proliferation of T-cells as well [189]. The combination of IL-12, another potent cytokine, enhances the efficacy of vaccines, as it gets involves in activation and recruitment of T-cells [190]. The amalgamation of DNA vaccine with a plasmid that encodes IL-12 has been tested against cervical cancer in preclinical mouse models and this has showcased decrement in the MDSCs, which conveys a therapeutic future of this combination [191]. IL-12 is also being assessed for its combination with neoantigen DNA vaccine and pembrolizumab, in a clinical trial, against advanced hepatocellular carcinoma (HCC) [130]. Along with these three cytokines, other cytokines like IL-7, IL-15 and Interferon- γ (INF-γ) and other adjuvants like TLR activators, could also be utilized to form combination with DNA vaccines [192].
Other approaches that can be made use of in combination with DNA vaccines include the endocrine and radiation therapies. The radiation therapies, when assessed for combination with vaccines in preclinical studies, effects like a decrease in tumor volume, increased damage to the cancer cells and enhanced release of TAs [193]. In hormone-involved cancers such as prostate and breast cancers in which endocrinal therapy is the mainstay strategy, letrozole has showcased the decrement in regulatory T-cells [194]. Moreover, increments in the number of T-cells and regeneration of the thymus cells have been witnessed with the therapies exerting androgen deprivation in prostate cancers [152]. One of the ongoing trials is assessing the capability of the Mammoglobin-A DNA vaccine in combination with letrozole, exemestane, goserelin, and tamoxifen [138].
The vaccine targets specific tumor antigens, while conventional therapies, such as chemotherapy, radiation therapy, and surgery, eliminate bulk tumor cells. Targeted therapies, such as monoclonal antibodies and small molecule inhibitors, interfere with specific molecules involved in tumor growth and progression. Immune checkpoint inhibitors release the brakes on the immune system, allowing for greater T-cell activation against cancer cells. Finally, cytokines, such as interferons and interleukins, stimulate immune cells and increase their cytotoxicity against tumor cells. The combination of these strategies has the potential to enhance the efficacy of cancer treatment and improve patient outcomes.
Clinical implications of cancer immunotherapy
The Immunotherapeutic strategies against cancer has nearly acquired the stature of the conventional therapy in the field of oncology. High-grade tumors are being mitigated with the amalgamations of standard conventional chemotherapy with immunotherapy, taking into consideration, the higher chances of achieving longer remission periods and improved patient compliance. It abolishes the growth of tumor cells by utilizing and strengthening the body’s immune system against the tumors. The present clinical scenario includes immunotherapies such as monoclonal antibodies (mAbs) (Therapeutic, Bispecific, Immune checkpoint), adoptive cell therapy (CAR-T, TCR-T, TILs, CAR-NK), small molecule drugs (PD-1/PD-L1 inhibitors, IDO1 inhibitors), oncolytic viruses, and lastly the essence of this literature, the vaccines [195]. The mAb therapy has been proved to be a promising approach in clinical cases [196]. Nonetheless, due to their characteristic of immunogenicity, mAbs elicit serious adverse reactions and hence require strict monitoring, where recently developed conjugation therapies of mAbs and Fc-engineered antibodies come to rescue [197]. Small molecule inhibitors, with relatively better profile than mAbs, stand out to be a better immunotherapeutic alternative for solid tumors [198]. Proteolysis targeting chimeras (PROTAC), newer forms of small molecule inhibitor, are being tested in clinical trials [199, 200]. Advancing towards adoptive cell therapies, CAR-T/TCR-T are being less utilized due to safety-concerns and off-target outcomes [201–203]. However, these agents have been successfully used in treating hematological tumors and in combination with the small molecule inhibitors for solid tumors [204, 205]. With the oncolytic viruses, there occurs confrontation of targeted delivery of virus, as the virus needs to get past the systemic immune forces of the body. Nano-particle aided delivery of virus is being developed to overcome this issue [206]. Lastly, preventive and therapeutic vaccines against cancer are being developed, with the fabrication of Gardasil and Cervarix being a major achievement [207]. However, the vaccines, especially the DNA vaccines need to be invested upon, both monetarily and intelligently. In comparison to the monotherapy, combinational approach of immunotherapy has been more promising in clinical world and FDA has approved a handful of them. The sphere of immunotherapy will be constantly expanding and evolving, explicitly with the advancements in oncology, immunology, biotechnology, and bioinformatics.
Way forward
Numerous virus-like particles (VLP) and cell-based vaccines have been fabricated against cancers with the FDA-approval with DNA vaccines facing considerable barriers which we have been introduced to in the previous parts of this paper [95]. Nevertheless, these newer times have been advancing in combining vaccination strategies with conventional ones and this has led to a resurgence in the clinical trials evaluating oncologic vaccines [208]. This amalgamation has, on a greater part diminished the unmanageable toxic events of cytotoxic chemotherapeutic agents. It counterbalances the immunosuppressive TME and presents greater synergistic antitumor effects as well [19]. Even so, we need to establish future studies to characterize the role of combination chemoimmunotherapy and maximize the therapeutic activity to an even greater extent by heedfully selecting the agents, including the novel immune adjuvants, and revitalizing the time of administration. Moreover, there’s a need to evaluate the combinations in early-staged cancers besides the advanced ones. Though the DNA vaccines can be administered intradermally or intramuscularly, device-aided administration—electroporation, is frequently used. Moreover, the vaccines that express a single tumor antigen, have shown increased efficacy and immunogenicity when extraporated. Ergo clinical trials are increasingly incorporating the technique of extraporation to achieve optimal efficacy outcomes. Also, the backbone comprising of DNA and vector, could be upgraded by making use of minicircle DNA (mcDNA). mcDNA is a creation that embodies just the required eukaryotic expressions and eliminates the unnecessary prokaryotic sequences, thus preventing inflammatory reactions [209]. Immunotherapy’s variable response necessitates the establishment of predictive biomarkers, which would also help in monitoring the associated side effects. To establish these biomarkers, we need to make progress in genomic and proteomic tools along with advancements in bioinformatics [18]. Finally, the role of Neutrophil Extracellular Traps (NETs) is being explored in the DC-based cancer vaccines. These vaccines initially tend to induce the recruitment of neutrophils against tumors, however longer exposures to neutrophil-produced NETs demonstrated exhaustion of T-cell immune responses, DC cell death and ultimately the exertion of tumor-protective effects. As DNA-based cancer vaccines stimulate the dendritic cells (APCs) through complex involvement of molecular pattern recognition receptors, the same role of NETs could be applied to the DNA-based cancer vaccines [210, 211]. To summarize, we need to explore more on the immunoregulatory pathways and these advances will pave the pathways for the making of potential combinatorial cancer vaccines and strengthen the wide spectrum of therapeutic modalities.
In the future, DNA vaccines are anticipated to play a pivotal role in the management of stroke and myocardial infarction (MI) by effectively reducing thrombo-inflammation through their impact on neutrophil extracellular traps (NETs) formation. Thrombo-inflammation refers to the intricate interplay between inflammation and the formation of blood clots, which can contribute to the development and progression of stroke and MI. NETs are web-like structures composed of chromatin (DNA and histones) decorated with antimicrobial peptides and enzymes. Although NETs are primarily intended to capture and kill pathogens, excessive NET formation can lead to harmful effects, including the promotion of thrombosis and inflammation in stroke and MI [212, 213]. DNA vaccines could be designed to target pathogen associated molecular patterns (PAMPs) responsible for the development of stroke and MI. By inducing an immune response against these PAMPs, DNA vaccines might potentially mitigate the excessive NET formation triggered by activated neutrophils. This probable mechanism has the potential to reduce thrombotic events and dampen the inflammatory response in the affected tissues. Therefore, it is important to acknowledge that the role of DNA vaccines in managing thrombo-inflammation, particularly in stroke and MI, would be an area of ongoing research and development. However, to prove these probable mechanisms, preclinical studies are warranted to demonstrate promising probable effects of DNA vaccine in this context.
Conclusion
Genes, being the groundwork of a human body, the idea of fabricating a gene-based vaccine to fight off cancer is undoubtedly one of the greatest pioneers in oncology. DNA vaccines have been devised as one of the new generation biotechnologies, that is progressively being modified and employed in cancer therapeutics. When compared to the conventional vaccines, these genetic vaccines have been proved safe, as they carry the genetic information for antigen. Also, they are relatively stable and are economic in production. Recent advancements in the design and delivery of these vaccines, combined with the identification of optimal tumor antigens and combination with other immunotherapies, have shown great potential in preclinical and clinical studies. Despite these challenges, the continued development and testing of DNA-based cancer vaccines across a wide range of cancer types highlights their potential to play a significant role in the future of cancer therapy. With ongoing research and advancements, DNA vaccines may offer new and effective ways to combat this devastating disease.
Acknowledgements
The authors are grateful to Prof. Gaurang B. Shah, Department of Pharmacology, L. M. College of Pharmacy, Ahmedabad, Gujarat, India for kind support and guidance in manuscript preparation. The authors also extend their appreciation to L. M. College of Pharmacy, Ahmedabad, India for providing continuous library resources support throughout literature survey and data collection.
Abbreviations
- ACT
Adoptive cell transfers
- APC
Antigen presenting cell
- CAR
Chimeric antigen receptor
- CD
Cluster of differentiation
- CD95L
Cluster of differentiation ligand
- CEA
Carcinoembryonic antigen
- CpG
5'—C—phosphate—G—3' (guanine residues)
- CTGF
Connective tissue growth factor
- CTL
Cytotoxic T-lymphocyte
- CTLA-4
Cytotoxic T-lymphocyte protein 4
- DCGI
Drug Controller General of India
- DNA
Deoxyribonucleic acid
- dsDNA
Double stranded DNA
- DTcs
T cytotoxic cell determinants
- EGCG
Epigallocatechin-3-gallate
- FOXP3
Forkhead box protein 3 gene
- GM-CSF
Granulocyte–macrophage colony-stimulating factor
- GMO
Genetically Modified Organism
- HCC
Hepatocellular carcinoma
- HER2
Human epidermal growth factor receptor 2
- HLA
Human leukocyte antigens
- HPV
Human papilloma virus
- HSP70
Heat-shock protein 70
- ICB
Immune checkpoint blockers
- ICI
Immune checkpoint inhibitors
- ID
Intradermal
- IDO
Indoleamine 2,3-dioxygenase
- IGF-1R
Insulin-like growth factor binding protein 2
- IGFBP2
Insulin-like growth factor binding protein 2
- IL
Interleukin
- IM
Intramuscular
- LAG-3
Lymphocyte activation gene-3
- mAbs
Monoclonal antibodies
- mcDNA
Minicircle DNA
- MDSCs
Myeloid-derived suppressor cells
- MFS
Metastasis free survival
- MHC
Major histocompatibility complex
- mRNA
Messenger RNA
- MSLN
Mesothelin
- MUC1
Mucin1
- NAV
Nucleic acid vaccine
- NETs
Neutrophil extracellular traps
- NFIS
Needle-Free Injection System
- ORR
Objective response rate
- OS
Overall survival
- PADRE
Pan HLA-DR epitope
- PAP
Prostatic acid phosphatase
- PD-1
Programmed cell death protein 1
- PFS
Progression-free survival
- PLA
Polylactic acid
- PLGA
Polylactic-co-glycolic acid
- PMED
Particle-Mediated Epidermal Delivery
- PROTAC
Proteolysis targeting chimeras
- PSA
Prostate-specific antigen
- rGM-CSF
Recombinant GM-CSF
- RNA
Ribonucleic acid
- SARS-CoV-2
Severe acute respiratory syndrome coronavirus 2
- SLP
Synthetic long peptide
- TA
Tumor antigen
- TAA
Tumor-associated antigen
- TCR-T
T-cell receptor-engineered T-cell
- TGF-β
Transforming growth factor-β
- Th1 cell
T-helper cell
- TILs
Tumor-infiltrating lymphocytes
- TIM-3
T-cell immunoglobulin and mucin-domain containing-3
- TKI
Tyrosine kinase inhibitor
- TLR
Toll-like receptor
- TME
Tumor microenvironment
- TRAIL
TNF-related apoptosis-inducing ligand
- Tregs
T-regulatory cells
- TSA
Tumor-specific antigen
- USFDA
United States Food and Drug Administration
- VEGF
Vascular endothelial growth factor
- VLP
Virus-like particles
- VNTR
Variable number of tandem repeat
Author contributions
AP, YS, NK, HP, AS: manuscript first draft preparation and subsequent editing, literature and data survey, Figures and diagram conception and designing. PP: manuscript draft review and editing; Referencing. MRC: topic conception, design of content and skeleton, reviewed manuscript first draft review and editing; figures and tables conception, designing and editing.
Funding
The authors declare that no funds, grants, or other support have been received during or for the preparation of this manuscript.
Data availability
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
Declarations
Conflict of interest
The authors declare no conflict of interest to report.
Ethical approval
Not applicable.
Consent to participate
Not applicable.
Consent to publish
Not applicable.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Aanshi Pandya, Yesha Shah, Nirjari Kothari, Humzah Postwala, Aayushi Shah have contributed equally to this work.
References
- 1.Robbins SL, Cotran RS. Pathologic basis of disease: Saunders; 1979.
- 2.Jeschke J. Editorial: Advances in cancer detection and diagnosis: from liquid biopsies and molecular biomarkers to opportunistic intratumoral bacteria. Curr Opin Oncol. 2023 doi: 10.1097/CCO.0000000000000930. [DOI] [PubMed] [Google Scholar]
- 3.Xi Y, Xu P. Global colorectal cancer burden in 2020 and projections to 2040. Transl Oncol. 2021;14(10):101174. doi: 10.1016/j.tranon.2021.101174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Xia C, Dong X, Li H, Cao M, Sun D, He S, et al. Cancer statistics in China and United States, 2022: profiles, trends, and determinants. Chin Med J. 2022;135(05):584–590. doi: 10.1097/CM9.0000000000002108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lokman NA, Price ZK, Hawkins EK, Macpherson AM, Oehler MK, Ricciardelli C. 4-Methylumbelliferone inhibits cancer stem cell activation and overcomes chemoresistance in ovarian cancer. Cancers. 2019 doi: 10.3390/cancers11081187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dieu-Nosjean M-C, Caux C. The biology of PD1 and CTLA-4 as immunotherapeutic targets and the issue of biomarkers. Med Sci. 2019;35(12):957–965. doi: 10.1051/medsci/2019192. [DOI] [PubMed] [Google Scholar]
- 7.Chen DS, Mellman I. Elements of cancer immunity and the cancer-immune set point. Nature. 2017;541(7637):321–330. doi: 10.1038/nature21349. [DOI] [PubMed] [Google Scholar]
- 8.Yu WD, Sun G, Li J, Xu J, Wang X. Mechanisms and therapeutic potentials of cancer immunotherapy in combination with radiotherapy and/or chemotherapy. Cancer Lett. 2019;452:66–70. doi: 10.1016/j.canlet.2019.02.048. [DOI] [PubMed] [Google Scholar]
- 9.Abbott M, Ustoyev Y. Cancer and the immune system: the history and background of immunotherapy. Semin Oncol Nurs. 2019;35(5):1509. doi: 10.1016/j.soncn.2019.08.002. [DOI] [PubMed] [Google Scholar]
- 10.Shell SS, Prestwich EG, Baek S-H, Shah RR, Sassetti CM, Dedon PC, et al. DNA methylation impacts gene expression and ensures hypoxic survival of Mycobacterium tuberculosis. PLoS Pathog. 2013;9(7):e1003419. doi: 10.1371/journal.ppat.1003419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ottaviano M, De Placido S, Ascierto PA. Recent success and limitations of immune checkpoint inhibitors for cancer: a lesson from melanoma. Virchows Arch. 2019;474(4):421–432. doi: 10.1007/s00428-019-02538-4. [DOI] [PubMed] [Google Scholar]
- 12.Wojtukiewicz MZ, Rek MM, Karpowicz K, Górska M, Polityńska B, Wojtukiewicz AM, et al. Inhibitors of immune checkpoints—PD-1, PD-L1, CTLA-4—new opportunities for cancer patients and a new challenge for internists and general practitioners. Cancer Metastasis Rev. 2021;40(3):949–982. doi: 10.1007/s10555-021-09976-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Takahashi Y, Demachi-Okamura A, Oya Y, Nakada T, Sakakura N, Kuroda H, et al. Research advance in tumor specific antigens. AME Med J. 2020 doi: 10.21037/amj-20-121. [DOI] [Google Scholar]
- 14.Snyder A, Makarov V, Merghoub T, Yuan J, Zaretsky JM, Desrichard A, et al. Genetic basis for clinical response to CTLA-4 blockade in melanoma. N Engl J Med. 2014;371(23):2189–2199. doi: 10.1056/NEJMoa1406498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bianchi M, Meng C, Ivashkiv LB. Inhibition of IL-2-induced Jak-STAT signaling by glucocorticoids. Proc Natl Acad Sci USA. 2000;97(17):9573–9578. doi: 10.1073/pnas.160099797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Girard N, Popat S, Shoshkova S, Fear S, Gracia JP. 1033P IMreal Cohort 2: Second interim analysis of efficacy and safety data in patients (pts) with locally advanced or metastatic non-small cell lung cancer (NSCLC) receiving atezolizumab (atezo) under real-world conditions. Ann Oncol. 2022;33:S1027–S1028. doi: 10.1016/j.annonc.2022.07.1159. [DOI] [Google Scholar]
- 17.Jahanafrooz Z, Baradaran B, Mosafer J, Hashemzaei M, Rezaei T, Mokhtarzadeh A, et al. Comparison of DNA and mRNA vaccines against cancer. Drug Discovery Today. 2020;25(3):552–560. doi: 10.1016/j.drudis.2019.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lopes A, Vandermeulen G, Préat V. Cancer DNA vaccines: current preclinical and clinical developments and future perspectives. J Exp Clin Cancer Res. 2019;38(1):146. doi: 10.1186/s13046-019-1154-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fioretti D, Iurescia S, Fazio VM, Rinaldi M. DNA vaccines: developing new strategies against cancer. J Biomed Biotechnol. 2010;2010:1743. doi: 10.1155/2010/174378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Amara S, Tiriveedhi V. The five immune forces impacting DNA-based cancer immunotherapeutic strategy. Int J Mol Sci. 2017;18(3):650. doi: 10.3390/ijms18030650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Strioga MM, Darinskas A, Pasukoniene V, Mlynska A, Ostapenko V, Schijns V. Xenogeneic therapeutic cancer vaccines as breakers of immune tolerance for clinical application: to use or not to use? Vaccine. 2014;32(32):4015–4024. doi: 10.1016/j.vaccine.2014.05.006. [DOI] [PubMed] [Google Scholar]
- 22.Riccardo F, Bolli E, Macagno M, Arigoni M, Cavallo F, Quaglino E. Chimeric DNA vaccines: an effective way to overcome immune tolerance. In: Savelyeva N, Ottensmeier C, editors. Cancer vaccines. Cham: Springer International Publishing; 2017. pp. 99–122. [DOI] [PubMed] [Google Scholar]
- 23.Lee S-H, Danishmalik SN, Sin J-I. DNA vaccines, electroporation and their applications in cancer treatment. Hum Vaccines Immunother. 2015;11(8):1889–1900. doi: 10.1080/21645515.2015.1035502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chen Y-L, Chang M-C, Chiang Y-C, Lin H-W, Sun N-Y, Chen C-A, et al. Immuno-modulators enhance antigen-specific immunity and anti-tumor effects of mesothelin-specific chimeric DNA vaccine through promoting DC maturation. Cancer Lett. 2018;425:152–163. doi: 10.1016/j.canlet.2018.03.032. [DOI] [PubMed] [Google Scholar]
- 25.Safavi A, Kefayat A, Abiri A, Mahdevar E, Behnia AH, Ghahremani F. In silico analysis of transmembrane protein 31 (TMEM31) antigen to design novel multiepitope peptide and DNA cancer vaccines against melanoma. Mol Immunol. 2019;112:93–102. doi: 10.1016/j.molimm.2019.04.030. [DOI] [PubMed] [Google Scholar]
- 26.Quaglino E, Riccardo F, Macagno M, Bandini S, Cojoca R, Ercole E, et al. Chimeric DNA vaccines against ErbB2+ carcinomas: from mice to humans. Cancers. 2011;3(3):3225–3241. doi: 10.3390/cancers3033225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hossain MK, Wall KA. Immunological evaluation of recent MUC1 glycopeptide cancer vaccines. Vaccines. 2016;4(3):25. doi: 10.3390/vaccines4030025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cheever MA, Allison JP, Ferris AS, Finn OJ, Hastings BM, Hecht TT, et al. The prioritization of cancer antigens: A National Cancer Institute Pilot project for the acceleration of translational research. Clin Cancer Res. 2009;15(17):5323–5337. doi: 10.1158/1078-0432.CCR-09-0737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gao T, Cen Q, Lei H. A review on development of MUC1-based cancer vaccine. Biomed Pharmacother. 2020;132:110888. doi: 10.1016/j.biopha.2020.110888. [DOI] [PubMed] [Google Scholar]
- 30.Rajčáni J, Moško T, Režuchová I. Current developments in viral DNA vaccines: shall they solve the unsolved? Rev Med Virol. 2005;15(5):303–325. doi: 10.1002/rmv.467. [DOI] [PubMed] [Google Scholar]
- 31.Gaidzik N, Westerlind U, Kunz H. The development of synthetic antitumour vaccines from mucin glycopeptide antigens. Chem Soc Rev. 2013;42(10):4421–4442. doi: 10.1039/C3CS35470A. [DOI] [PubMed] [Google Scholar]
- 32.Choi D-H, Woo JK, Choi Y, Seo H-S, Kim C-W. A novel chimeric DNA vaccine: enhancement of preventive and therapeutic efficacy of DNA vaccine by fusion of Mucin 1 to a heat shock protein 70 gene. Mol Med Rep. 2011;4(5):885–890. doi: 10.3892/mmr.2011.525. [DOI] [PubMed] [Google Scholar]
- 33.Brennick CA, George MM, Corwin WL, Srivastava PK, Ebrahimi-Nik H. Neoepitopes as cancer immunotherapy targets: key challenges and opportunities. Immunotherapy. 2017;9(4):361–371. doi: 10.2217/imt-2016-0146. [DOI] [PubMed] [Google Scholar]
- 34.Li L, Goedegebuure SP, Gillanders WE. Preclinical and clinical development of neoantigen vaccines. Ann Oncol. 2017;28(12):xii11–xii17. doi: 10.1093/annonc/mdx681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Shemesh CS, Hsu JC, Hosseini I, Shen B-Q, Rotte A, Twomey P, et al. Personalized cancer vaccines: clinical landscape, challenges, and opportunities. Mol. 2021;29(2):555–570. doi: 10.1016/j.ymthe.2020.09.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Aurisicchio L, Pallocca M, Ciliberto G, Palombo F. The perfect personalized cancer therapy: cancer vaccines against neoantigens. J Exper Clin Cancer Res. 2018;37(1):86. doi: 10.1186/s13046-018-0751-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Keskin DB, Anandappa AJ, Sun J, Tirosh I, Mathewson ND, Li S, et al. Neoantigen vaccine generates intratumoral T cell responses in phase Ib glioblastoma trial. Nature. 2019;565(7738):234–239. doi: 10.1038/s41586-018-0792-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Duan Q, Zhang H, Zheng J, Zhang L. Turning cold into hot: firing up the tumor microenvironment. Trends Cancer. 2020;6(7):605–618. doi: 10.1016/j.trecan.2020.02.022. [DOI] [PubMed] [Google Scholar]
- 39.Sahin U, Türeci Ö. Personalized vaccines for cancer immunotherapy. Science (New York, NY) 2018;359(6382):1355–1360. doi: 10.1126/science.aar7112. [DOI] [PubMed] [Google Scholar]
- 40.Charneau J, Suzuki T, Shimomura M, Fujinami N, Mishima Y, Hiranuka K, et al. Development of antigen-prediction algorithm for personalized neoantigen vaccine using human leukocyte antigen transgenic mouse. Cancer Sci. 2022;113(4):1113–1124. doi: 10.1111/cas.15291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lee CH, Yelensky R, Jooss K, Chan TA. Update on tumor neoantigens and their utility: why it is good to be different. Trends Immunol. 2018;39(7):536–548. doi: 10.1016/j.it.2018.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hellmann MD, Snyder A. Making it personal: neoantigen vaccines in metastatic melanoma. Immunity. 2017;47(2):221–223. doi: 10.1016/j.immuni.2017.08.001. [DOI] [PubMed] [Google Scholar]
- 43.Li L, Goedegebuure P, Mardis ER, Ellis MJC, Zhang X, Herndon JM, et al. Cancer genome sequencing and its implications for personalized cancer vaccines. Cancers. 2011;3(4):4191–4211. doi: 10.3390/cancers3044191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Durántez M, López-Vázquez AB, de Cerio AL, Huarte E, Casares N, Prieto J, et al. Induction of multiepitopic and long-lasting immune responses against tumour antigens by immunization with peptides, DNA and recombinant adenoviruses expressing minigenes. Scand J Immunol. 2009;69(2):80–89. doi: 10.1111/j.1365-3083.2008.02202.x. [DOI] [PubMed] [Google Scholar]
- 45.Carreno BM, Magrini V, Becker-Hapak M, Kaabinejadian S, Hundal J, Petti AA, et al. Cancer immunotherapy. A dendritic cell vaccine increases the breadth and diversity of melanoma neoantigen-specific T cells. Science (New York, NY) 2015;348(6236):803–808. doi: 10.1126/science.aaa3828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Rizvi NA, Hellmann MD, Snyder A, Kvistborg P, Makarov V, Havel JJ, et al. Cancer immunology. Mutational landscape determines sensitivity to PD-1 blockade in non-small cell lung cancer. Science (New York, NY). 2015;348(6230):124–128. doi: 10.1126/science.aaa1348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ott PA, Hu Z, Keskin DB, Shukla SA, Sun J, Bozym DJ, et al. An immunogenic personal neoantigen vaccine for patients with melanoma. Nature. 2017;547(7662):217–221. doi: 10.1038/nature22991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Sahin U, Derhovanessian E, Miller M, Kloke BP, Simon P, Löwer M, et al. Personalized RNA mutanome vaccines mobilize poly-specific therapeutic immunity against cancer. Nature. 2017;547(7662):222–226. doi: 10.1038/nature23003. [DOI] [PubMed] [Google Scholar]
- 49.Hilf N, Kuttruff-Coqui S, Frenzel K, Bukur V, Stevanović S, Gouttefangeas C, et al. Actively personalized vaccination trial for newly diagnosed glioblastoma. Nature. 2019;565(7738):240–245. doi: 10.1038/s41586-018-0810-y. [DOI] [PubMed] [Google Scholar]
- 50.Li L, Zhang X, Wang X, Kim SW, Herndon JM, Becker-Hapak MK, et al. Optimized polyepitope neoantigen DNA vaccines elicit neoantigen-specific immune responses in preclinical models and in clinical translation. Genome Med. 2021;13(1):56. doi: 10.1186/s13073-021-00872-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Galaine J, Borg C, Godet Y, Adotévi O. Interest of tumor-specific CD4 T helper 1 cells for therapeutic anticancer vaccine. Vaccines (Basel) 2015;3(3):490–502. doi: 10.3390/vaccines3030490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Protti MP, De Monte L, Di Lullo G. Tumor antigen-specific CD4+ T cells in cancer immunity: from antigen identification to tumor prognosis and development of therapeutic strategies. Tissue Antigens. 2014;83(4):237–246. doi: 10.1111/tan.12329. [DOI] [PubMed] [Google Scholar]
- 53.Efremova M, Finotello F, Rieder D, Trajanoski Z. Neoantigens generated by individual mutations and their role in cancer immunity and immunotherapy. Front Immunol. 2017;8:1679. doi: 10.3389/fimmu.2017.01679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Cho HI, Celis E. Design of immunogenic and effective multi-epitope DNA vaccines for melanoma. Cancer Immunol Immunother. 2012;61(3):343–351. doi: 10.1007/s00262-011-1110-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Park JY, Jin D-H, Lee C-M, Jang MJ, Lee SY, Shin HS, et al. CD4+ TH1 cells generated by Ii-PADRE DNA at prime phase are important to induce effectors and memory CD8+ T cells. J Immunother. 2010;33(5):510. doi: 10.1097/CJI.0b013e3181d75cef. [DOI] [PubMed] [Google Scholar]
- 56.Xiuli Z, Goedegebuure SP, Nancy BM, Tammi V, Michael DM, Feng G, et al. Neoantigen DNA vaccines are safe, feasible, and capable of inducing neoantigen-specific immune responses in patients with triple negative breast cancer. medRxiv. 2021 doi: 10.1101/2021.11.19.21266466. [DOI] [Google Scholar]
- 57.Disis M, Liu Y, Stanton S, Gwin W, Coveler A, Liao J, et al. 546 A phase I dose escalation study of STEMVAC, a multi-antigen, multi-epitope Th1 selective plasmid-based vaccine, targeting stem cell associated proteins in patients with advanced breast cancer. J Immunother Cancer. J Immunother Cancer. 2022 doi: 10.1136/jitc-2022-SITC2022.0546. [DOI] [Google Scholar]
- 58.Hasan Y, Spiotto MT, Furtado LV, Tergas AI, Lee NK, Brooks RA, et al. A phase 1/2A trial of synthetic DNA vaccine immunotherapy targeting HPV-16 and -18 after chemoradiation for cervical cancer. J Clin Oncol. 2018;36(15_Suppl):5525. doi: 10.1200/JCO.2018.36.15_suppl.5525. [DOI] [Google Scholar]
- 59.Gwin WR, Childs J, Higgins D, Shakalia H, Liao JB, Disis ML. Phase II study of neoadjuvant IGFBP-2 vaccination with neoadjuvant carboplatin and paclitaxel in advanced ovarian cancer. J Clin Oncol. 2018 doi: 10.1200/JCO.2018.36.15_suppl.TPS5613. [DOI] [Google Scholar]
- 60.Guo J, Tang L, Li K, Ma Q, Luo S, Cheng R, et al. Application of nanotechnology in therapeutic cancer vaccines. Adv Nanobiomed Res. 2023 doi: 10.1002/anbr.202200122. [DOI] [Google Scholar]
- 61.Paston SJ, Brentville VA, Symonds P, Durrant LG. Cancer vaccines, adjuvants, and delivery systems. Front Immuno. 2021 doi: 10.3389/fimmu.2021.627932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.McNamara MA, Nair SK, Holl EK. RNA-based vaccines in cancer immunotherapy. J Immunol Res. 2015 doi: 10.1155/2015/794528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Suschak JJ, Williams JA, Schmaljohn CS. Advancements in DNA vaccine vectors, non-mechanical delivery methods, and molecular adjuvants to increase immunogenicity. Hum Vaccines Immunother. 2017;13(12):2837–2848. doi: 10.1080/21645515.2017.1330236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Ledesma-Feliciano C, Chapman R, Hooper JW, Elma K, Zehrung D, Brennan MB, et al. Improved DNA vaccine delivery with needle-free injection systems. Vaccines. 2023;11(2):280. doi: 10.3390/vaccines11020280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Li L, Petrovsky N. Molecular mechanisms for enhanced DNA vaccine immunogenicity. Expert Rev Vaccines. 2016;15(3):313–329. doi: 10.1586/14760584.2016.1124762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Jorritsma SHT, Gowans EJ, Grubor-Bauk B, Wijesundara DK. Delivery methods to increase cellular uptake and immunogenicity of DNA vaccines. Vaccine. 2016;34(46):5488–5494. doi: 10.1016/j.vaccine.2016.09.062. [DOI] [PubMed] [Google Scholar]
- 67.Papania MJ, Zehrung D, Jarrahian C. Technologies to improve immunization: Plotkin's Vaccines. Amsterdam: Elsevier; 2018. pp. 1320–1353.e17. [Google Scholar]
- 68.Tretiakova DS, Vodovozova EL. Liposomes as adjuvants and vaccine delivery systems. Biochem Mosc Suppl A. 2022 doi: 10.1134/S1990747822020076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Shirota H, Tross D, Klinman DM. CpG oligonucleotides as cancer vaccine adjuvants. Vaccines (Basel) 2015;3(2):390–407. doi: 10.3390/vaccines3020390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Jiang T, Zhou C, Ren S. Role of IL-2 in cancer immunotherapy. Oncoimmunology. 2016;5(6):e1163462. doi: 10.1080/2162402x.2016.1163462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Liu C, Xie Y, Sun B, Geng F, Zhang F, Guo Q, et al. MUC1- and SURVIVIN-BASED DNA vaccine combining immunoadjuvants CpG and interleukin-2 in a bicistronic expression plasmid generates specific immune responses and antitumour effects in a murine colorectal carcinoma model. Scand J Immunol. 2018;87(2):63–72. doi: 10.1111/sji.12633. [DOI] [PubMed] [Google Scholar]
- 72.Haddad D, Ramprakash J, Sedegah M, Charoenvit Y, Baumgartner R, Kumar S, et al. Plasmid vaccine expressing granulocyte-macrophage colony-stimulating factor attracts infiltrates including immature dendritic cells into injected muscles. J Immunol (Baltimore, Md: 1950) 2000;165(7):3772–3781. doi: 10.4049/jimmunol.165.7.3772. [DOI] [PubMed] [Google Scholar]
- 73.Uddin MN, Allon A, Roni MA, Kouzi S. Overview and future potential of fast dissolving buccal films as drug delivery system for vaccines. J Pharm Pharm Sci. 2019;22(1):388–406. doi: 10.18433/jpps30528. [DOI] [PubMed] [Google Scholar]
- 74.Wan C, Yi L, Yang Z, Yang J, Shao H, Zhang C, et al. The Toll-like receptor adaptor molecule TRIF enhances DNA vaccination against classical swine fever. Vet Immunol Immunopathol. 2010;137(1–2):47–53. doi: 10.1016/j.vetimm.2010.04.008. [DOI] [PubMed] [Google Scholar]
- 75.Manam S, Ledwith BJ, Barnum AB, Troilo PJ, Pauley CJ, Harper LB, et al. Plasmid DNA vaccines: tissue distribution and effects of DNA sequence, adjuvants and delivery method on integration into host DNA. Intervirology. 2000;43(4–6):273–281. doi: 10.1159/000053994. [DOI] [PubMed] [Google Scholar]
- 76.Hobernik D, Bros M. DNA vaccines-how far from clinical use? Int J Mol Sci. 2018 doi: 10.3390/ijms19113605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Sørensen SJ, Bailey M, Hansen LH, Kroer N, Wuertz S. Studying plasmid horizontal transfer in situ: a critical review. Nat Rev Microbiol. 2005;3(9):700–710. doi: 10.1038/nrmicro1232. [DOI] [PubMed] [Google Scholar]
- 78.Yin W, Xiang P, Li Q. Investigations of the effect of DNA size in transient transfection assay using dual luciferase system. Anal Biochem. 2005;346(2):289–294. doi: 10.1016/j.ab.2005.08.029. [DOI] [PubMed] [Google Scholar]
- 79.Hornstein BD, Roman D, Arévalo-Soliz LM, Engevik MA, Zechiedrich L. Effects of circular DNA length on transfection efficiency by electroporation into HeLa Cells. PLoS ONE. 2016;11(12):e0167537. doi: 10.1371/journal.pone.0167537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Myhr AI. DNA vaccines: regulatory considerations and safety aspects. Curr Issues Mol Biol. 2017;22(1):79–88. doi: 10.21775/cimb.022.079. [DOI] [PubMed] [Google Scholar]
- 81.Guo Y, Lei K, Tang L. Neoantigen vaccine delivery for personalized anticancer immunotherapy. Front Immunol. 2018 doi: 10.3389/fimmu.2018.01499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Shimizu K, Iyoda T, Okada M, Yamasaki S, Fujii SI. Immune suppression and reversal of the suppressive tumor microenvironment. Int Immunol. 2018;30(10):445–454. doi: 10.1093/intimm/dxy042. [DOI] [PubMed] [Google Scholar]
- 83.Dey A, Chozhavel Rajanathan TM, Chandra H, Pericherla HPR, Kumar S, Choonia HS, et al. Immunogenic potential of DNA vaccine candidate, ZyCoV-D against SARS-CoV-2 in animal models. Vaccine. 2021;39(30):4108–4116. doi: 10.1016/j.vaccine.2021.05.098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Samal KC, Sahoo J, Yadav N, Pradhan P. ZyCoV-D: World's first needle-free DNA vaccine’s emergency approval in India. Biot Res Today. 2021;3(8):714–716. [Google Scholar]
- 85.Zuleger CL, Kang C, Ranheim EA, Kurzman ID, Macklin MD, Newton MA, et al. Pilot study of safety and feasibility of DNA microseeding for treatment of spontaneous canine melanoma. Vet Med Sci. 2017;3(3):134–145. doi: 10.1002/vms3.65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Teng M, Yao Y, Nair V, Luo J. Latest advances of virology research using CRISPR/Cas9-based gene-editing technology and its application to vaccine development. Viruses. 2021;13(5):779. doi: 10.3390/v13050779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Sica A, Bronte V. Altered macrophage differentiation and immune dysfunction in tumor development. J Clin Investig. 2007;117(5):1155–1166. doi: 10.1172/JCI31422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Zhang S, Wu M, Wang F. Immune regulation by CD8+ Treg cells: novel possibilities for anticancer immunotherapy. Cell Mol Immunol. 2018;15(9):805–807. doi: 10.1038/cmi.2018.170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Collison LW, Workman CJ, Kuo TT, Boyd K, Wang Y, Vignali KM, et al. The inhibitory cytokine IL-35 contributes to regulatory T-cell function. Nature. 2007;450(7169):566–569. doi: 10.1038/nature06306. [DOI] [PubMed] [Google Scholar]
- 90.Hiam-Galvez KJ, Allen BM, Spitzer MH. Systemic immunity in cancer. Nat Rev Cancer. 2021;21(6):345–359. doi: 10.1038/s41568-021-00347-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Bepari A, Sakre N, Rahman I, Niazi SK, Dervesh AM. The assessment of drug utilization study of anticancer drugs using who prescribing indicators in a government tertiary care hospital of the Hyderabad-Karnataka Region of India. Open Access Maced J Med Sci. 2019;7(7):1203. doi: 10.3889/oamjms.2019.249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Gordy JT, Luo K, Zhang H, Biragyn A, Markham RB. Fusion of the dendritic cell-targeting chemokine MIP3α to melanoma antigen Gp100 in a therapeutic DNA vaccine significantly enhances immunogenicity and survival in a mouse melanoma model. J Immunother Cancer. 2016;4:96. doi: 10.1186/s40425-016-0189-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Khan KH. DNA vaccines: roles against diseases. Germs. 2013;3(1):26–35. doi: 10.11599/germs.2013.1034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Stachyra A, Góra-Sochacka A, Sirko A. DNA vaccines against influenza. Acta Biochim Pol. 2014;61(3):515–522. doi: 10.18388/abp.2014_1873. [DOI] [PubMed] [Google Scholar]
- 95.Liu J, Fu M, Wang M, Wan D, Wei Y, Wei X. Cancer vaccines as promising immuno-therapeutics: platforms and current progress. J Hematol Oncol. 2022;15(1):28. doi: 10.1186/s13045-022-01247-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Katopodi T, Petanidis S, Charalampidis C, Chatziprodromidou I, Eskitzis P, Tsavlis D, et al. Tumor-infiltrating dendritic cells: decisive roles in cancer immunosurveillance, immunoediting, and tumor T cell tolerance. Cells. 2022;11(20):3183. doi: 10.3390/cells11203183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Gupta M, Wahi A, Sharma P, Nagpal R, Raina N, Kaurav M, et al. Recent advances in cancer vaccines: challenges, achievements, and futuristic prospects. Vaccines. 2022;10(12):2011. doi: 10.3390/vaccines10122011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Bhat P, Leggatt G, Waterhouse N, Frazer IH. Interferon-γ derived from cytotoxic lymphocytes directly enhances their motility and cytotoxicity. Cell Death Dis. 2017;8(6):e2836. doi: 10.1038/cddis.2017.67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Chen C, Gao F-H. Th17 Cells Paradoxical Roles in Melanoma and Potential Application in Immunotherapy. Front Immunol. 2019 doi: 10.3389/fimmu.2019.00187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Dustin D, Gu G, Fuqua SAW. ESR1 mutations in breast cancer. Cancer. 2019;125(21):3714–3728. doi: 10.1002/cncr.32345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Souza-Fonseca-Guimaraes F, Cursons J, Huntington ND. The emergence of natural killer cells as a major target in cancer immunotherapy. Trends Immunol. 2019;40(2):142–158. doi: 10.1016/j.it.2018.12.003. [DOI] [PubMed] [Google Scholar]
- 102.Rezaei T, Davoudian E, Khalili S, Amini M, Hejazi M, de la Guardia M, et al. Strategies in DNA vaccine for melanoma cancer. Pigment Cell Melanoma Res. 2021;34(5):869–891. doi: 10.1111/pcmr.12933. [DOI] [PubMed] [Google Scholar]
- 103.Anderson RJ, Schneider J. Plasmid DNA and viral vector-based vaccines for the treatment of cancer. Vaccine. 2007;25(Suppl 2):B24–34. doi: 10.1016/j.vaccine.2007.05.030. [DOI] [PubMed] [Google Scholar]
- 104.Łukasiewicz S, Czeczelewski M, Forma A, Baj J, Sitarz R, Stanisławek A. Breast cancer-epidemiology, risk factors, classification, prognostic markers, and current treatment strategies-an updated review. Cancers. 2021 doi: 10.3390/cancers13174287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Kyriakopoulos CE, Eickhoff JC, Ferrari AC, Schweizer MT, Wargowski E, Olson BM, et al. Multicenter phase I trial of a DNA vaccine encoding the androgen receptor ligand-binding domain (pTVG-AR, MVI-118) in patients with metastatic prostate cancer. Clin Cancer Res. 2020;26(19):5162–5171. doi: 10.1158/1078-0432.Ccr-20-0945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.STEMVAC in patients with early stage triple negative breast cancer https://clinicaltrials.gov/ct2/show/NCT05455658?term=NCT05455658&draw=2&rank=1.
- 107.Higgins D, Childs J, Salazar LG, Disis ML. Abstract OT1–01–01: A phase I trial of the safety and immunogenicity of a multiple antigen vaccine (STEMVAC) in HER2 negative advanced stage breast cancer patients. Cancer Res. 2016 doi: 10.1158/1538-7445.SABCS15-OT1-01-01. [DOI] [Google Scholar]
- 108.Vaccine therapy in treating patients with HER2-negative stage III-IV breast cancer https://clinicaltrials.gov/ct2/show/NCT02157051?term=NCT02157051&draw=2&rank=1.
- 109.Pavlenko M, Roos AK, Lundqvist A, Palmborg A, Miller AM, Ozenci V, et al. A phase I trial of DNA vaccination with a plasmid expressing prostate-specific antigen in patients with hormone-refractory prostate cancer. Br J Cancer. 2004;91(4):688–694. doi: 10.1038/sj.bjc.6602019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.vaccine therapy in preventing cancer recurrence in patients with non-metastatic, node positive, HER2 negative breast cancer that is in remission (WOKVAC) https://clinicaltrials.gov/ct2/show/NCT02780401?term=NCT02780401&draw=2&rank=1.
- 111.A multiple antigen vaccine (STEMVAC) for the treatment of patients with stage IV non-squamous non-small cell lung cancer https://clinicaltrials.gov/ct2/show/NCT05242965?term=NCT05242965&draw=2&rank=1.
- 112.Niemi JV, Sokolov AV, Schiöth HB. Neoantigen vaccines; clinical trials, classes, indications, adjuvants and combinatorial treatments. Cancers. 2022;14(20):5163. doi: 10.3390/cancers14205163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Neoepitope-based personalized DNA vaccine approach in pediatric patients with recurrent brain tumors https://clinicaltrials.gov/ct2/show/NCT03988283?term=NCT03988283&draw=2&rank=1
- 114.Wang C, Yu M, Zhang W. Neoantigen discovery and applications in glioblastoma: An immunotherapy perspective. Cancer Lett. 2022;550:215945. doi: 10.1016/j.canlet.2022.215945. [DOI] [PubMed] [Google Scholar]
- 115.Neoantigen-based personalized DNA vaccine in patients with newly diagnosed, unmethylated glioblastoma https://clinicaltrials.gov/ct2/show/NCT04015700?term=NCT04015700&draw=2&rank=1
- 116.Emamekhoo H, Kyriakopoulos C, Liu G, McNeel DG. Phase II trial of a DNA vaccine encoding prostatic acid phosphatase (pTVG-HP) and nivolumab (Nivo) in patients (pts) with nonmetastatic, PSA-recurrent prostate cancer (PCa) J Clin Oncol. 2020 doi: 10.1200/JCO.2020.38.6_suppl.TPS273. [DOI] [Google Scholar]
- 117.pTVG-HP and Nivolumab in patients with non-metastatic PSA-recurrent prostate cancer https://clinicaltrials.gov/ct2/show/NCT03600350?term=NCT03600350&draw=2&rank=1.
- 118.pTVG-HP DNA vaccine with or without pTVG-AR DNA vaccine and pembrolizumab in patients with castration-resistant, metastatic prostate cancer https://clinicaltrials.gov/ct2/show/NCT04090528?term=NCT04090528&draw=1&rank=1.
- 119.Neoantigen DNA vaccine alone vs. Neoantigen DNA vaccine plus durvalumab in triple negative breast cancer patients following standard of care therapy https://clinicaltrials.gov/ct2/show/NCT03199040?term=NCT03199040&draw=2&rank=1.
- 120.Huber B, Wang JW, Roden RBS, Kirnbauer R. RG1-VLP and other L2-based, broad-spectrum HPV vaccine candidates. J Clin Med. 2021;10(5):1044. doi: 10.3390/jcm10051044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.HPV DNA vaccine via electroporation for HPV16 positive cervical neoplasia https://clinicaltrials.gov/ct2/show/NCT04131413?term=NCT04131413&draw=2&rank=1.
- 122.Pozas J, Álvarez Rodríguez S, Fernández VA, Burgos J, Santoni M, Manneh Kopp R, et al. Androgen receptor signaling inhibition in advanced castration resistance prostate cancer: what is expected for the near future? Cancers. 2022;14(24):6071. doi: 10.3390/cancers14246071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Androgen deprivation, with or without pTVG-AR, and with or without Nivolumab, in patients with newly diagnosed, high-risk prostate cancer https://clinicaltrials.gov/ct2/show/NCT04989946?term=NCT04989946&draw=2&rank=1.
- 124.Corti C, Giachetti PPMB, Eggermont AMM, Delaloge S, Curigliano G. Therapeutic vaccines for breast cancer: has the time finally come? Eur J Cancer. 2022;160:150–174. doi: 10.1016/j.ejca.2021.10.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Concurrent WOKVAC vaccination, chemotherapy, and HER2-targeted monoclonal antibody therapy before surgery for the treatment of patients with breast cancer https://clinicaltrials.gov/ct2/show/NCT04329065?term=NCT04329065&draw=2&rank=1.
- 126.Rogers JE, Leung M, Johnson B. Metastatic or locally recurrent anal squamous cell carcinoma (SCAC): current clinical trial landscape and novel approaches. Cancer Manag Res. 2022;14:2065–2077. doi: 10.2147/cmar.S331429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.DNA Plasmid-encoding Interleukin-12/HPV DNA plasmids therapeutic vaccine INO-3112 and durvalumab in treating patients with recurrent or metastatic human papillomavirus associated cancers https://clinicaltrials.gov/ct2/show/NCT03439085?term=NCT03439085&draw=2&rank=1.
- 128.Yan F, Cowell LG, Tomkies A, Day AT. Therapeutic vaccination for HPV-mediated cancers. Curr Otorhinolaryngol Rep. 2023;11(1):44–61. doi: 10.1007/s40136-023-00443-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.VGX-3100 and electroporation in treating patients With HIV-positive high-grade anal lesions https://clinicaltrials.gov/ct2/show/NCT03603808?term=NCT03603808&draw=2&rank=1.
- 130.Yarchoan M, Gane E, Marron T, Rochestie S, Cooch N, Peters J, et al. 453 Personalized DNA neoantigen vaccine (GNOS-PV02) in combination with plasmid IL-12 and pembrolizumab for the treatment of patients with advanced hepatocellular carcinoma. J Immunother Cancer. 2021 doi: 10.1136/jitc-2021-SITC2021.453. [DOI] [Google Scholar]
- 131.GNOS-PV02 personalized neoantigen vaccine, INO-9012 and Pembrolizumab in Subjects With Advanced HCC https://clinicaltrials.gov/ct2/show/NCT04251117?term=NCT04251117&draw=2&rank=1.
- 132.Disis ML, Dang Y, Coveler AL, Higgins D, Childs J, Salazar LG. Final report and long-term outcomes: Phase I trial of a HER2 intracellular plasmid-based vaccine in HER2+ advanced stage breast cancer. J Clin Oncol. 2021;39(15_Suppl):2619. doi: 10.1200/JCO.2021.39.15_suppl.2619. [DOI] [Google Scholar]
- 133.Dang Y, Cecil D, Corulli L, Rodmaker E, Disis M. Optimizing immunization schedules for therapeutic vaccination. J Immunother Cancer. 2022 doi: 10.1136/jitc-2022-SITC2022.1176. [DOI] [Google Scholar]
- 134.Pallerla S, Abdul AU, Comeau J, Jois S. Cancer vaccines, treatment of the future: with emphasis on HER2-positive breast cancer. Int J Mol Sci. 2021;22(2):779. doi: 10.3390/ijms22020779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Hosseini M, Seyedpour S, Khodaei B, Loghman A-H, Seyedpour N, Yazdi M-H, et al. Cancer vaccines for triple-negative breast cancer: a systematic review. Vaccines. 2023;11(1):146. doi: 10.3390/vaccines11010146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Zafrakas M, Petschke B, Donner A, Fritzsche F, Kristiansen G, Knüchel R, et al. Expression analysis of mammaglobin A (SCGB2A2) and lipophilin B (SCGB1D2) in more than 300 human tumors and matching normal tissues reveals their co-expression in gynecologic malignancies. BMC Cancer. 2006;6:88. doi: 10.1186/1471-2407-6-88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Li L, Goedegebuure SP, Fleming TP, Gillanders WE. Developing a clinical development paradigm for translation of a mammaglobin-A DNA vaccine. Immunotherapy. 2015;7(7):709–711. doi: 10.2217/imt.15.40. [DOI] [PubMed] [Google Scholar]
- 138.Kim SW, Goedegebuure P, Gillanders WE. Mammaglobin-A is a target for breast cancer vaccination. Oncoimmunology. 2016;5(2):e1069940. doi: 10.1080/2162402x.2015.1069940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.McNeel DG, Eickhoff JC, Johnson LE, Roth AR, Perk TG, Fong L, et al. Phase II trial of a DNA vaccine encoding prostatic acid phosphatase (pTVG-HP [MVI-816]) in patients with progressive, nonmetastatic, castration-sensitive prostate cancer. J Clin Oncol. 2019;37(36):3507–3517. doi: 10.1200/jco.19.01701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Yang A, Farmer E, Wu TC, Hung CF. Perspectives for therapeutic HPV vaccine development. J Biomed Sci. 2016;23(1):75. doi: 10.1186/s12929-016-0293-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Bagarazzi ML, Yan J, Morrow MP, Shen X, Parker RL, Lee JC, et al. Immunotherapy against HPV16/18 generates potent TH1 and cytotoxic cellular immune responses. Sci Transl Med. 2012 doi: 10.1126/scitranslmed.3004414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Liu G, Fong L, Antonarakis ES, Eickhoff JC, Wargowski EG, Johnson LE, et al. Randomized phase II trial of a DNA vaccine encoding prostatic acid phosphatase (pTVG-HP) versus GM-CSF adjuvant in patients with PSA-recurrent prostate cancer. J Clin Oncol. 2019;37(15_Suppl):5037. doi: 10.1200/JCO.2019.37.15_suppl.5037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Two-Arm study of a DNA vaccine encoding prostatic acid phosphatase (PAP) in patients with non-metastatic castrate-resistant prostate cancer https://clinicaltrials.gov/ct2/show/study/NCT00849121?term=NCT00849121&draw=2&rank=1.
- 144.A study of INO-3112 DNA vaccine with electroporation in participants with cervical cancer https://clinicaltrials.gov/ct2/show/study/NCT02172911?term=NCT02172911&draw=2&rank=1.
- 145.Phase II PAP Plus GM-CSF versus GM-CSF alone for non-metastatic prostate cancer https://clinicaltrials.gov/ct2/show/study/NCT01341652?term=NCT01341652&draw=2&rank=1.
- 146.Bhatia S, Longino NV, Miller NJ, Kulikauskas R, Iyer JG, Ibrani D, et al. Intratumoral delivery of plasmid IL12 Via electroporation leads to regression of injected and noninjected tumors in merkel cell carcinoma. Clin Cancer Res. 2020;26(3):598–607. doi: 10.1158/1078-0432.CCR-19-0972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.IL-12 gene and in vivo electroporation-mediated plasmid DNA vaccine therapy in patients with merkel cell cancer (MCC) https://clinicaltrials.gov/ct2/show/record/NCT01440816?term=NCT01440816&draw=2&rank=1.
- 148.NY-ESO-1 Plasmid DNA (pPJV7611) Cancer vaccine https://clinicaltrials.gov/ct2/show/NCT00199849?term=NCT00199849&draw=2&rank=1.
- 149.Huang T, Liu L, Lv Z, Zhao K, Yi Q, Zhang J. Recent advances in DNA vaccines against lung cancer: a mini review. Vaccines. 2022;10(10):1586. doi: 10.3390/vaccines10101586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Vaccine therapy in treating patients with stage IIB, stage IIC, stage III, or stage IV melanoma https://clinicaltrials.gov/ct2/show/NCT00398073?term=NCT00398073&draw=2&rank=1.
- 151.Zhu Z, Liu W, Gotlieb V. The rapidly evolving therapies for advanced melanoma—Towards immunotherapy, molecular targeted therapy, and beyond. Crit Rev Oncol Hematol. 2016;99:91–99. doi: 10.1016/j.critrevonc.2015.12.002. [DOI] [PubMed] [Google Scholar]
- 152.Gatti-Mays ME, Redman JM, Collins JM, Bilusic M. Cancer vaccines: Enhanced immunogenic modulation through therapeutic combinations. Hum Vaccines Immunother. 2017;13(11):2561–2574. doi: 10.1080/21645515.2017.1364322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Villarreal DO, Wise MC, Siefert RJ, Yan J, Wood LM, Weiner DB. Ubiquitin-like molecule ISG15 acts as an immune adjuvant to enhance antigen-specific CD8 T-cell tumor immunity. Mol Ther. 2015;23(10):1653–1662. doi: 10.1038/mt.2015.120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Nguyen-Hoai T, Pham-Duc M, Gries M, Dörken B, Pezzutto A, Westermann J. CCL4 as an adjuvant for DNA vaccination in a Her2/neu mouse tumor model. Cancer Gene Ther. 2016;23(6):162–167. doi: 10.1038/cgt.2016.9. [DOI] [PubMed] [Google Scholar]
- 155.Xia Q, Geng F, Zhang FF, Liu CL, Xu P, Lu ZZ, et al. Cyclophosphamide enhances anti-tumor effects of a fibroblast activation protein α-based DNA vaccine in tumor-bearing mice with murine breast carcinoma. Immunopharmacol Immunotoxicol. 2017;39(1):37–44. doi: 10.1080/08923973.2016.1269337. [DOI] [PubMed] [Google Scholar]
- 156.Danishmalik SN, Sin JI. Therapeutic tumor control of HER2 DNA vaccines is achieved by an alteration of tumor cells and tumor microenvironment by Gemcitabine and Anti-Gr-1 Ab treatment in a HER2-expressing tumor model. DNA Cell Biol. 2017;36(9):801–811. doi: 10.1089/dna.2017.3810. [DOI] [PubMed] [Google Scholar]
- 157.Lopes A, Vanvarenberg K, Kos Š, Lucas S, Colau D, Van den Eynde B, et al. Combination of immune checkpoint blockade with DNA cancer vaccine induces potent antitumor immunity against P815 mastocytoma. Sci Rep. 2018;8(1):15732. doi: 10.1038/s41598-018-33933-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Rekoske BT, Smith HA, Olson BM, Maricque BB, McNeel DG. PD-1 or PD-L1 blockade restores antitumor efficacy following SSX2 epitope-modified DNA vaccine immunization. Cancer Immunol Res. 2015;3(8):946–955. doi: 10.1158/2326-6066.Cir-14-0206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Gao FS, Zhan YT, Wang XD, Zhang C. Enhancement of anti-tumor effect of plasmid DNA-carrying MUC1 by the adjuvanticity of FLT3L in mouse model. Immunopharmacol Immunotoxicol. 2018;40(4):353–357. doi: 10.1080/08923973.2018.1498099. [DOI] [PubMed] [Google Scholar]
- 160.Son HY, Apostolopoulos V, Chung JK, Kim CW, Park JU. Protective efficacy of a plasmid DNA vaccine against transgene-specific tumors by Th1 cellular immune responses after intradermal injection. Cell Immunol. 2018;329:17–26. doi: 10.1016/j.cellimm.2018.04.003. [DOI] [PubMed] [Google Scholar]
- 161.Witt K, Ligtenberg MA, Conti L, Lanzardo S, Ruiu R, Wallmann T, et al. Cripto-1 plasmid DNA vaccination targets metastasis and cancer stem cells in murine mammary carcinoma. Cancer Immunol Res. 2018;6(11):1417–1425. doi: 10.1158/2326-6066.CIR-17-0572. [DOI] [PubMed] [Google Scholar]
- 162.Xia Q, Zhang FF, Geng F, Liu CL, Xu P, Lu ZZ, et al. Anti-tumor effects of DNA vaccine targeting human fibroblast activation protein α by producing specific immune responses and altering tumor microenvironment in the 4T1 murine breast cancer model. Cancer Immunol Immunother. 2016;65(5):613–624. doi: 10.1007/s00262-016-1827-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Zhao Y, Wei Z, Yang H, Li X, Wang Q, Wang L, et al. Enhance the anti-renca carcinoma effect of a DNA vaccine targeting G250 gene by co-expression with cytotoxic T-lymphocyte associated antigen-4(CTLA-4) Biomed Pharmacother. 2017;90:147–152. doi: 10.1016/j.biopha.2017.03.015. [DOI] [PubMed] [Google Scholar]
- 164.Neoantigen DNA vaccine in combination with Nivolumab/Ipilimumab and PROSTVAC in metastatic hormone-sensitive prostate cancer https://clinicaltrials.gov/ct2/show/NCT03532217?term=dna+vaccine%2C+chemotherapy+combination&recrs=e&cond=Cancer&draw=2&rank=1
- 165.Shah K, Ganapathy A, Borkowski A, Shah N, Bansal D, Beck R, et al. A pilot trial of neoantigen DNA vaccine in combination with nivolumab/ipilimumab and prostvac in metastatic hormone-sensitive prostate cancer (mHSPC) J Clin Oncol: Off J Am Soc Clin Oncol. 2022;40(16_Suppl):5068. doi: 10.1200/JCO.2022.40.16_suppl.5068. [DOI] [Google Scholar]
- 166.Decitabine, vaccine therapy, and pegylated liposomal doxorubicin hydrochloride in treating patients with recurrent ovarian epithelial cancer, Fallopian tube cancer, or peritoneal cancer https://clinicaltrials.gov/ct2/show/NCT01673217?term=dna+vaccine%2C+chemotherapy+combination&recrs=e&cond=Cancer&draw=1&rank=2.
- 167.Vaccine and antibody treatment of prostate cancer https://clinicaltrials.gov/ct2/show/NCT00113984?term=DNA+vaccine+combined+with+cytokines&cond=Cancer&draw=2&rank=3.
- 168.Madan RA, Mohebtash M, Arlen PM, Vergati M, Rauckhorst M, Steinberg SM, et al. Ipilimumab and a poxviral vaccine targeting prostate-specific antigen in metastatic castration-resistant prostate cancer: a phase 1 dose-escalation trial. Lancet Oncol. 2012;13(5):501–508. doi: 10.1016/S1470-2045(12)70006-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.An exploratory safety and immunogenicity study of HPV16+ Immunotherapy VB10.16 in Women With HSIL; CIN 2/3) https://clinicaltrials.gov/ct2/show/NCT02529930?term=DNA+vaccines%2C+targeted+therapy&recrs=e&cond=Cancer&phase=123&draw=2&rank=1.
- 170.Hillemanns P, Petry KU, Woelber L, Böhmer G, Stubsrud E, Skjørestad I, et al. Abstract CT209: Safety, efficacy and immunogenicity of VB10.16, a therapeutic DNA vaccine targeting human papillomavirus (HPV) 16 E6 and E7 proteins for high grade cervical intraepithelial neoplasia (CIN 2/3): 6-month data from an exploratory open-label phase I/2a trial. Cancer Res. 2019 doi: 10.1158/1538-7445.AM2019-CT209. [DOI] [Google Scholar]
- 171.Docetaxel Alone or in Combination With Vaccine to Treat Breast Cancer https://clinicaltrials.gov/ct2/show/NCT00179309?term=DNA+vaccines%2C+targeted+therapy&recrs=e&cond=Cancer&phase=123&draw=2&rank=3.
- 172.Heery C, Ibrahim N, Madan RA, Mohebtash M, Arlen P, McMahon S, et al. LBA14—A phase 2 randomized trial of docetaxel alone or in combination with therapeutic cancer vaccine, CEA-, MUC-1-Tricom. Ann Oncol. 2012 doi: 10.1016/S0923-7534(20)34312-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Sevko A, Kremer V, Falk C, Umansky L, Shurin MR, Shurin GV, et al. Application of paclitaxel in low non-cytotoxic doses supports vaccination with melanoma antigens in normal mice. J Immunotoxicol. 2012;9(3):275–281. doi: 10.3109/1547691X.2012.655343. [DOI] [PubMed] [Google Scholar]
- 174.Swan D, Gurney M, Krawczyk J, Ryan AE, O'Dwyer M. Beyond DNA damage: exploring the immunomodulatory effects of cyclophosphamide in multiple myeloma. HemaSphere. 2020;4(2):e350. doi: 10.1097/hs9.0000000000000350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Sasso MS, Lollo G, Pitorre M, Solito S, Pinton L, Valpione S, et al. Low dose gemcitabine-loaded lipid nanocapsules target monocytic myeloid-derived suppressor cells and potentiate cancer immunotherapy. Biomaterials. 2016;96:47–62. doi: 10.1016/j.biomaterials.2016.04.010. [DOI] [PubMed] [Google Scholar]
- 176.Xia Q, Zhang F-F, Geng F, Liu C-L, Wang Y-Q, Xu P, et al. Improvement of anti-tumor immunity of fibroblast activation protein α based vaccines by combination with cyclophosphamide in a murine model of breast cancer. Cell Immunol. 2016;310:89–98. doi: 10.1016/j.cellimm.2016.08.006. [DOI] [PubMed] [Google Scholar]
- 177.Zeng Q, Jewell CM. Directing toll-like receptor signaling in macrophages to enhance tumor immunotherapy. Curr Opin Biotechnol. 2019;60:138–145. doi: 10.1016/j.copbio.2019.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Mougel A, Terme M, Tanchot C. Therapeutic cancer vaccine and combinations with antiangiogenic therapies and immune checkpoint blockade. Front Immunol. 2019 doi: 10.3389/fimmu.2019.00467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Azuma M. Co-signal Molecules in T-Cell Activation. In: Azuma M, Yagita H, editors. Co-signal molecules in T Cell activation: immune regulation in health and disease. Singapore: Springer Singapore; 2019. pp. 3–23. [Google Scholar]
- 180.Garrido F, Aptsiauri N. Cancer immune escape: MHC expression in primary tumours versus metastases. Immunology. 2019;158(4):255–266. doi: 10.1111/imm.13114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Andrews LP, Yano H, Vignali DAA. Inhibitory receptors and ligands beyond PD-1, PD-L1 and CTLA-4: breakthroughs or backups. Nat Immunol. 2019;20(11):1425–1434. doi: 10.1038/s41590-019-0512-0. [DOI] [PubMed] [Google Scholar]
- 182.Rupp T, Genest L, Babin D, Legrand C, Hunault M, Froget G, et al. Anti-CTLA-4 and anti-PD-1 immunotherapies repress tumor progression in preclinical breast and colon model with independent regulatory T cells response. Transl Oncol. 2022;20:101405. doi: 10.1016/j.tranon.2022.101405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Lisi L, Lacal PM, Martire M, Navarra P, Graziani G. Clinical experience with CTLA-4 blockade for cancer immunotherapy: from the monospecific monoclonal antibody ipilimumab to probodies and bispecific molecules targeting the tumor microenvironment. Pharmacol Res. 2022;175:105997. doi: 10.1016/j.phrs.2021.105997. [DOI] [PubMed] [Google Scholar]
- 184.Zappasodi R, Serganova I, Cohen IJ, Maeda M, Shindo M, Senbabaoglu Y, et al. CTLA-4 blockade drives loss of Treg stability in glycolysis-low tumours. Nature. 2021;591(7851):652–658. doi: 10.1038/s41586-021-03326-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Lurain K, Ramaswami R, Yarchoan R, Uldrick TS. Anti-PD-1 and Anti-PD-L1 monoclonal antibodies in people living with HIV and cancer. Curr HIV/AIDS Rep. 2020;17(5):547–556. doi: 10.1007/s11904-020-00525-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Gjoerup O, Brown CA, Ross JS, Huang RSP, Schrock A, Creeden J, et al. Identification and utilization of biomarkers to predict response to immune checkpoint inhibitors. AAPS J. 2020;22(6):132. doi: 10.1208/s12248-020-00514-4. [DOI] [PubMed] [Google Scholar]
- 187.Veatch JR, Singhi N, Srivastava S, Szeto JL, Jesernig B, Stull SM, et al. A therapeutic cancer vaccine delivers antigens and adjuvants to lymphoid tissues using genetically modified T cells. J Clin Investig. 2021 doi: 10.1172/JCI144195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Buchbinder EI, Dutcher JP, Daniels GA, Curti BD, Patel SP, Holtan SG, et al. Therapy with high-dose Interleukin-2 (HD IL-2) in metastatic melanoma and renal cell carcinoma following PD1 or PDL1 inhibition. J Immunother Cancer. 2019;7(1):49. doi: 10.1186/s40425-019-0522-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Lazarus HM, Ragsdale CE, Gale RP, Lyman GH. Sargramostim (rhu GM-CSF) as cancer therapy (systematic review) and an immunomodulator. A drug before its time? Front Immunol. 2021 doi: 10.3389/fimmu.2021.706186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Cheng EM, Tsarovsky NW, Sondel PM, Rakhmilevich AL. Interleukin-12 as an in situ cancer vaccine component: a review. Cancer Immunol Immunother. 2022;71(9):2057–2065. doi: 10.1007/s00262-022-03144-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Diniz MO, Sales NS, Silva JR, Ferreira LC. Protection against HPV-16-associated tumors requires the activation of CD8+ effector memory T cells and the control of myeloid-derived suppressor cells. Mol Cancer Ther. 2016;15(8):1920–1930. doi: 10.1158/1535-7163.Mct-15-0742. [DOI] [PubMed] [Google Scholar]
- 192.Dajon M, Iribarren K, Cremer I. Dual roles of TLR7 in the lung cancer microenvironment. Oncoimmunology. 2015;4(3):e991615. doi: 10.4161/2162402X.2014.991615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Jagodinsky JC, Harari PM, Morris ZS. The promise of combining radiation therapy with immunotherapy. Int J Radiat Oncol Biol Phys. 2020;108(1):6–16. doi: 10.1016/j.ijrobp.2020.04.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Mohammadi E, Tabatabaei M, Habibi-Anbouhi M, Tafazzoli-Shadpour M. Chemical inhibitor anticancer drugs regulate mechanical properties and cytoskeletal structure of non-invasive and invasive breast cancer cell lines: study of effects of Letrozole, Exemestane, and Everolimus. Biochem Biophys Res Commun. 2021;565:14–20. doi: 10.1016/j.bbrc.2021.05.083. [DOI] [PubMed] [Google Scholar]
- 195.Liu C, Yang M, Zhang D, Chen M, Zhu D. Clinical cancer immunotherapy: Current progress and prospects. Front Immunol. 2022;13:961805. doi: 10.3389/fimmu.2022.961805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Teige I, Mårtensson L, Frendéus BL. Targeting the Antibody Checkpoints to Enhance Cancer Immunotherapy-Focus on FcγRIIB. Front Immunol. 2019;10:481. doi: 10.3389/fimmu.2019.00481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Brandsma AM, Jacobino SR, Meyer S, ten Broeke T, Leusen JH. Fc receptor inside-out signaling and possible impact on antibody therapy. Immunol Rev. 2015;268(1):74–87. doi: 10.1111/imr.12332. [DOI] [PubMed] [Google Scholar]
- 198.Liu C, Zhou F, Yan Z, Shen L, Zhang X, He F, et al. Discovery of a novel, potent and selective small-molecule inhibitor of PD-1/PD-L1 interaction with robust in vivo anti-tumour efficacy. Br J Pharmacol. 2021;178(13):2651–2670. doi: 10.1111/bph.15457. [DOI] [PubMed] [Google Scholar]
- 199.Hu M, Zhou W, Wang Y, Yao D, Ye T, Yao Y, et al. Discovery of the first potent proteolysis targeting chimera (PROTAC) degrader of indoleamine 2,3-dioxygenase 1. Acta pharmaceutica Sinica B. 2020;10(10):1943–1953. doi: 10.1016/j.apsb.2020.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Hu B, Zhou Y, Sun D, Yang Y, Liu Y, Li X, et al. PROTACs: new method to degrade transcription regulating proteins. Eur J Med Chem. 2020;207:11269. doi: 10.1016/j.ejmech.2020.112698. [DOI] [PubMed] [Google Scholar]
- 201.Naran K, Nundalall T, Chetty S, Barth S. Principles of immunotherapy: implications for treatment strategies in cancer and infectious diseases. Front Microbiol. 2018;9:3158. doi: 10.3389/fmicb.2018.03158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Morgan RA, Chinnasamy N, Abate-Daga D, Gros A, Robbins PF, Zheng Z, et al. Cancer regression and neurological toxicity following anti-MAGE-A3 TCR gene therapy. J Immunother. 2013;36(2):133–151. doi: 10.1097/CJI.0b013e3182829903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Darvishi M, Tosan F, Nakhaei P, Manjili DA, Kharkouei SA, Alizadeh A, et al. Recent progress in cancer immunotherapy: overview of current status and challenges. Pathol Res Pract. 2023;241:15424. doi: 10.1016/j.prp.2022.154241. [DOI] [PubMed] [Google Scholar]
- 204.Chung H, Jung H, Noh JY. Emerging approaches for solid tumor treatment using CAR-T cell therapy. Int J Mol Sci. 2021 doi: 10.3390/ijms222212126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Abbasi S, Totmaj MA, Abbasi M, Hajazimian S, Goleij P, Behroozi J, et al. Chimeric antigen receptor T (CAR-T) cells: Novel cell therapy for hematological malignancies. Cancer Med. 2023;12(7):7844–7858. doi: 10.1002/cam4.5551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Roy DG, Bell JC. Cell carriers for oncolytic viruses: current challenges and future directions. Oncolytic Virother. 2013;2:47–56. doi: 10.2147/ov.S36623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Roy V, Jung W, Linde C, Coates E, Ledgerwood J, Costner P, et al. Differences in HPV-specific antibody Fc-effector functions following Gardasil® and Cervarix® vaccination. npj Vaccines. 2023;8(1):39. doi: 10.1038/s41541-023-00628-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Yang B, Jeang J, Yang A, Wu TC, Hung CF. DNA vaccine for cancer immunotherapy. Hum Vaccin Immunother. 2014;10(11):3153–3164. doi: 10.4161/21645515.2014.980686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Eusébio D, Neves AR, Costa D, Biswas S, Alves G, Cui Z, et al. Methods to improve the immunogenicity of plasmid DNA vaccines. Drug Discov Today. 2021;26(11):2575–2592. doi: 10.1016/j.drudis.2021.06.008. [DOI] [PubMed] [Google Scholar]
- 210.Chan L, Wood GA, Wootton SK, Bridle BW, Karimi K. Neutrophils in dendritic cell-based cancer vaccination: the potential roles of neutrophil extracellular trap formation. Int J Mol Sci. 2023;24(2):896. doi: 10.3390/ijms24020896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Demkow U. Neutrophil extracellular traps (NETs) in cancer invasion, evasion and metastasis. Cancers. 2021;13(17):4495. doi: 10.3390/cancers13174495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Dhanesha N, Patel RB, Doddapattar P, Ghatge M, Flora GD, Jain M, et al. PKM2 promotes neutrophil activation and cerebral thromboinflammation: therapeutic implications for ischemic stroke. Blood. 2022;139(8):1234–1245. doi: 10.1182/blood.2021012322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Chorawala MR, Prakash P, Doddapattar P, Jain M, Dhanesha N, Chauhan AK. Deletion of extra domain a of fibronectin reduces acute myocardial ischaemia/reperfusion injury in hyperlipidaemic mice by limiting thrombo-inflammation. Thromb Haemost. 2018;118(8):1450–1460. doi: 10.1055/s-0038-1661353. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.