Abstract
C2-Symmetrical scaffolds are privileged ligands in metal catalysis and are also widely used in organocatalysis. Among these, 2,5-disubstituted pyrrolidines hold a paramount importance, especially since they also find application in medicinal chemistry. This review highlights the stereoselective syntheses of these C2-symmetrical nitrogen heterocycles. It includes synthetic strategies based on the use of the chiral pool as well as the more recent sequences designed following major achievements in asymmetric catalysis.
Keywords: pyrrolidine, nitrogen heterocycle, natural product, C2 symmetry, asymmetric synthesis, organometallic catalysis, organocatalysis, biocatalysis
1. Introduction
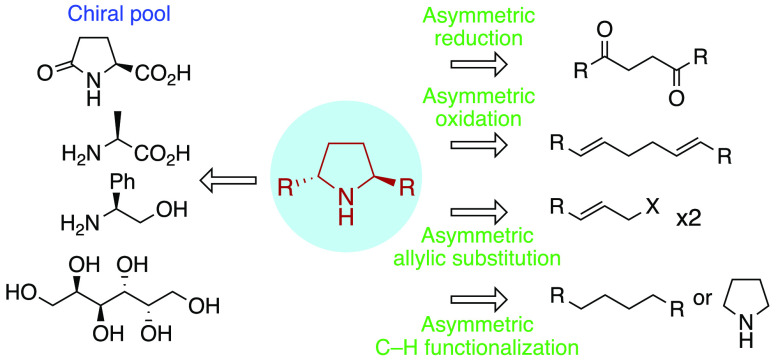
Pyrrolidines are ubiquitous in natural products and bioactive compounds as well as privileged motifs in stereoselective synthesis. In this respect, a recent study showed that the pyrrolidine ring is ranking in the fifth position among the most predominant nitrogen heterocycles for U.S. FDA approved pharmaceuticals.1 In addition, the 2021 Nobel Prize in chemistry was awarded for the advent of organocatalysis, where pyrrolidine-based catalysts hold a key importance, notably in the field of aminocatalysis.2 Among pyrrolidines, 2,5-disubstituted ones are particularly important (Figure 1). Indeed, C2-symmetrical pyrrolidines 1–3 have been broadly used as chiral auxiliaries for a variety of transformations.3−7 Pyrrolidine 2 was also among the very first aminocatalysts enabling high enantioselectivities8 as well as a key motif in the design of important chiral phosphosphoramidite ligands such as 5.9 Moreover, chiral Lewis base 4 is an efficient organocatalyst for the chemoselective acylation of polyhydroxylated substrates.10,11 On the other hand, 2,5-disubstituted pyrrolidines are frequently encountered in natural products, notably in the venoms of amphibians12 and ants13 such as (−)-pyrrolidine 197B (6) and gephyrotoxin 7. Last but not least, this motif is also relevant for the pharmaceutical industry, as exemplified by Ombistavir 8, an antiviral drug recently approved for the treatment of hepatitis C virus.14
Figure 1.
Important 2,5-disubstituted pyrrolidines.
Initially, enantioenriched 2,5-disubstituted pyrrolidines were obtained by resolution of racemates with chiral acids.4,5 Because of their importance, many research groups then designed stereoselective strategies to access them. In this review, we will first discuss early asymmetric syntheses starting from the chiral pool and then focus on more recent syntheses involving an enantioselective step or relying on asymmetric catalysis.
2. Asymmetric Strategies from the Chiral Pool
2.1. Syntheses Starting from Amino Acids
To the best of our knowledge, the first stereoselective strategy toward the synthesis of enantiopure 2,5-disubstituted pyrrolidines was developed by Rapoport in the context of the total synthesis of naturally occurring alkaloids (Scheme 1).15 They started from l-pyroglutamic acid 9 that was transformed into thiolactam 10 after some functional group manipulation. S-Alkylation of 10 with triflate 11, followed by the addition of triphenylphosphine and a tertiary amine base, led to vinylogous carbamate 12 which was selectively transformed into cis-pyrrolidine 13 by hydrogenolysis and a decarboxylation mediated by palladium on charcoal and ammonium formate. Then intermediate 13 could be elaborated to enantiopure cis-2,5-dialkylpyrrolidines 15via two different synthetic sequences, each of them requiring several steps.
Scheme 1. Synthesis of 15 from Pyroglutamic Acid by Rapoport and Co-workers.
Pyroglutamic acid was also the starting point for other synthetic strategies leading to 2,5-disubstituted pyrrolidines. For example, Somfai reported the synthesis of Katsuki’s pyrrolidine 3 according to the following route (Scheme 2).16 Pyroglutamic acid 9 was transformed into hemiaminal 18. Lewis acid activation of 18 and trans-selective addition of cyanide led to 19 that was then reduced to the corresponding alcohol 20 with partial epimerization. Removal of the silicon protecting group, double Williamson etherification with methyl iodide, and final deprotection of the nitrogen afforded 3 in good overall yield. Importantly, after etherification, the cis-isomer could be easily removed by chromatography, so that the desired trans-product was obtained diastereoisomerically pure.
Scheme 2. Synthesis of 3 from Pyroglutamic Acid by Somfai and Ahman.
The group of Onomura reported the synthesis of both cis- and trans-2,5-disubstituted pyrrolidines starting from the pyroglutamic acid-derived hemiaminal 20 (Scheme 3).17 Under Lewis acidic conditions, the in situ-formed iminium can be trapped by an electron rich aromatic, such as mesitylene. The selectivity of the addition could be controlled by varying the protecting group on the nitrogen. Carbamates favor the formation of cis-pyrrolidines such as 21 while a benzamide gives the trans-pyrrolidine 22 as the major isomer. Saponification of the methyl ester of 22 followed by electrochemical decarboxylative methoxylation led to hemiaminal 23 that was further reacted under the previously developed conditions to selectively afford C2-symmetrical pyrrolidine 24.
Scheme 3. Synthesis of 24 from Pyroglutamic Acid by Onomura and Co-workers.
To illustrate the high diversity provided by this method, hemiaminals 25 were also the starting point for Trost and co-workers to access a variety of trans-2,5-disubstituted pyrrolidines required for the synthesis of chiral phosphine ligand 28(18) or sterically constrained binuclear zinc catalyst 29 (Scheme 4).19 To this purpose, they developed a copper-mediated addition of Grignard reagents to 25 that proceeds with good to excellent diastereoselectivities to afford valuable pyrrolidines 26. Then a double Grignard addition to the ester moiety of 26 followed by the removal of the Boc-protecting group yielded prolinol 27. A similar strategy was later employed by Pihko and co-workers to synthesize a range of prolinol-based organocatalysts such as 30 required for an enantioselective Mukaiyama-Michael addition.20
Scheme 4. Synthesis of 26 and 27 from Pyroglutamic Acid by Trost and Co-workers.
The importance of C2-symmetric pyrrolidines 1 and 3 as chiral auxiliaries3 has been a considerable driving force for the development of stereoselective strategies to access them. In this respect, the group of Schlessinger developed the first stereoselective synthesis of each enantiomer of trans-2,5-dimethylpyrrolidines 1 starting from readily available d- or l-alanine (Scheme 5).21 The synthesis begins with the reduction of d-alanine 31 to the corresponding aminoalcohol and the selective protection of the amine with benzyl chloroformate. Then tosylation of the alcohol followed by a Finkelstein reaction with potassium iodide gave 32 which was engaged in a copper-mediated reaction with allyl magnesium bromide to afford 33. Compound 33 was cyclized by an intramolecular aminomercuration followed by a reduction with sodium borohydride according to a procedure developed by Harding and co-workers.22 The 5-exo-trig cyclization proceeds with high stereocontrol through a chairlike transition state. The desired trans-pyrrolidine 1 was finally obtained after the removal of the Cbz group. This route was further optimized by the group of Welch, who developed a more robust cuprate addition to reproducibly access intermediate 33 on a decagram scale.23
Scheme 5. Synthesis of 1 from d-Alanine by Schlessinger and Co-workers.
2.2. Syntheses Starting from Phenylglycinol
Early on, Husson and co-workers developed a general route to access trans-2,5-disubstituted pyrrolidines starting from (R)-phenylglycinol 35 (Scheme 6).24 Condensation with formaldehyde, followed by the addition of potassium cyanide, gave aminonitrile 36, which was then reacted with bromoaldehyde 37 to afford intermediate 38. Alkylation of the anion of 38 with heptyl bromide led to 39 as an inconsequential mixture of diastereoisomers since its reduction with Li/NH3 occurs with high stereocontrol (dr >20:1) to afford 40. Then the addition of butyl magnesium bromide to 40 gave predominantly the trans-2,5-dialkylpyrrolidine 41 from which the chiral auxiliary could be removed by hydrogenolysis to access stereopure pyrrolidine 42. This strategy was further applied to the synthesis of several trans-2,5-dialkylpyrrolidines,25 and conveniently, an improved protocol for the synthesis of intermediate 38 was also developed by the same group.26
Scheme 6. Synthesis of trans-2,5-Dialkylpyrrolidines from (R)-Phenylglycinol by Husson and Co-workers.
Inspired by the work of Husson et al., Higashiyama and co-workers demonstrated the versatility of the method by extending its scope to the synthesis of trans-2,5-bis(aryl) pyrrolidines (Scheme 7).27 The key steps are two diastereoselective additions of Grignard reagents, first to chiral imines and then to 1,3-oxazolidines. The synthesis starts with the condensation of (R)-phenylglycinol 35 with aromatic aldehydes to afford imines 43. These imines were then reacted with Grignard reagent 44 prepared from bromopropionaldehyde dimethyl acetal. The additions proceed on the Re face with complete diastereoselectivity to afford chiral (R)-benzylamines 45, which were then converted into 46 with a catalytic amount of methanolic hydrochloric acid. Oxazolidinones 46 were further reacted with other aryl Grignard reagents to give the stereopure adducts 47. The addition of the Grignard reagent to the in situ-formed iminium is believed to be directed by the alkoxy group of the chiral auxiliary, thus leading to a trans-(R,R)-disubstituted pyrrolidine. Removal of the chiral auxiliary, however, limits the overall efficiency of the method as it requires a two-step and moderately yielding procedure involving substitution of the hydroxyl group by a phenyl sulfide followed by reductive fragmentation mediated by lithium di-tert-butylbiphenylide (LiDBB). Of note, this route afforded so far only symmetrical disubstituted pyrrolidines 48, but it offers the opportunity to access related nonsymmetrical pyrrolidines as shown later by the group of Katritsky.
Scheme 7. Synthesis of trans-2,5-Diarylpyrrolidines from (R)-Phenylglycinol by Higashiyama and Co-workers.
Again starting from phenylglycinol as initial source of chirality, the group of Katritzky developed a similar strategy (Scheme 8).28 The condensation between benzotriazole BtH, (S)-phenylglycinol, and succindialdehyde equivalent 49 under acidic conditions directly led to oxazolopyrrolidine 50 in 80% yield on a 100 mmol scale. Intermediate 50 could be activated by BF3·Et2O and reacted with a range of nucleophilic partners such as allylsilanes, silyl enol ethers, and triethyl phosphite to selectively afford addition products 51 as single isomers. Then the addition of a Grignard reagent to 51 led to the corresponding pyrrolidines 52 with good to excellent trans-diastereoselectivities. However, the direct addition of an excess of Grignard reagents to 50 proved much less selective, as varying mixtures of cis- and trans-substituted pyrrolidines were obtained, with the cis-products being formed predominantly. Conveniently, the two isomers could be easily separated after chromatography.
Scheme 8. Synthesis of 2,5-Disubstituted Pyrrolidines from (R)-Phenylglycinol by Katritzky and Co-workers.
2.3. Syntheses Starting from Carbohydrates
Misiti and Marzi developed a concise stereoselective synthesis of Katsuki’s chiral auxiliary 56 starting from 53, which can be accessed from d-mannitol (Scheme 9).29 Selective benzylation of the primary alcohols followed by tosylation of the remaining secondary alcohols led to C2-symmetrical compound 54. Then a double nucleophilic substitution with benzylamine followed by a chemoselective N-debenzylation afforded optically pure (R)-56.
Scheme 9. Synthesis of 56 from d-Mannitol by Misiti and Marzi.
d-Mannitol was also used by the group of Kibayashi for the enantiodivergent synthesis of various naturally occurring trans-2,5-dialkylpyrrolidines since it can be readily transformed into bisepoxides (S,S)- and (R,R)-57 in two and four steps, respectively (Scheme 10).30 (R,R)-57 was reacted with two equivalents of an organocuprate to give the corresponding C2-symmetrical 1,4-diol 58; however, the formation of the targeted pyrrolidines requires several additional steps. After conversion to the cyclic sulfate 59 in two steps, nucleophilic displacement of the sulfate by an azide, followed by the activation of the remaining alcohol as a mesylate, led to 60. Reduction of the azide to an amine directly triggered a 5-exo-tet cyclization to afford enantiopure trans-2,5-dialkylpyrrolidine 61.
Scheme 10. Synthesis of 61 from d-Mannitol by Kibayashi and Co-workers.
Sasaki et al. reported a stereocontrolled synthesis of 2,5-disubstituted pyrrolidines by reacting two chiral starting materials, a glycerol-derived bistriflate 63 and an aminosulfone 62 (Scheme 11).31 Multiple deprotonation of 62 by an excess of n-BuLi followed by the addition of 63 led to the corresponding pyrrolidine via two sequential SN2 displacements, including a stereospecific cyclization. Removal of the sulfone under reductive conditions afforded the corresponding pyrrolidine 64. Since each enantiomer of 63 is readily available, both cis- and trans-64 could be accessed via this route.
Scheme 11. Synthesis of cis- and trans-64 by Sasaki and Co-workers.
2.4. Miscellaneous
Davis et al. developed a conceptually different route to access 2,5-disubstituted pyrrolidines.32 Their strategy relies on a diastereoselective Mannich reaction and then an iodocyclization to forge the pyrrolidine ring in a stereoselective manner (Scheme 12). First, the addition of the enolate of methyl acetate to chiral sulfinimine 65 gave Mannich product 66. Oxidation of the sulfinyl moiety led to sulfonamides, and the ester group was then reduced with DIBAL-H to afford aldehydes 67, which were reacted with phosphonium ylides to give 68 as mixtures of olefin isomers. In the presence of iodine and base, the Z-isomers could undergo a diastereoselective iodocyclization through a chairlike transition state, while the E-isomers did not react under these conditions because of the unfavorable A1,3 strain. Removal of the iodide with Bu3SnH followed by reductive cleavage of the sulfonamide protecting group with Na/NH3 afforded trans-2,5-substituted pyrrolidines, such as 2. This strategy is appropriate for the preparation of specific targets, as demonstrated by the synthesis of (−)-pyrrolidine 197B,12,30 an alkaloid present in poison frogs of the family Dendrobates histrionicus and in the venom of fire ants of the genus Solenopsis and Monomorium latinode.
Scheme 12. Synthesis of trans-2,5-Disubstituted Pyrrolidines by Davis and Co-workers.
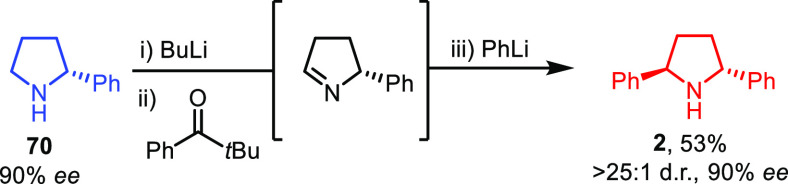
By comparison, more powerful and versatile is the method developed by Seidel and co-workers relying on a one-pot C–H functionalization of unprotected cyclic amines that proceeds selectively α to the nitrogen.33 Their operationally simple procedure involves an intermolecular hydride transfer to generate an imine, which is then reacted in situ with an organolithium reagent. Interestingly, their method was tested on enantioenriched pyrrolidine 70 to directly access 2 as the single trans-isomer without any erosion of the enantiopurity (Scheme 13).
Scheme 13. Synthesis of 2 by Seidel and Co-workers.
3. Enantioselective and Catalytic Asymmetric Strategies
3.1. Reductive Strategies from 1,4-Ketones
3.1.1. Enantioselective Reduction to 1,4-Diols
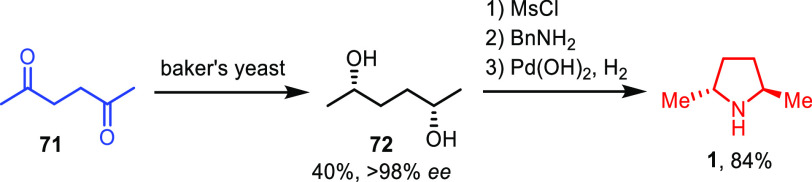
Masamune et al. developed a very concise synthesis of chiral auxiliary 1 starting from 2,5-hexanedione 71 that was reduced to diol 72 with excellent stereoselectivities using baker’s yeast (Scheme 14).34 Indeed, a high enantioselectivity was obtained because of the Horeau amplification effect,35,36 and only traces of the corresponding meso-diol were detected. Moreover, a single recrystallization gave stereopure diol 72 on a multigram scale. The diol was then activated as a bismethanesulfonate and reacted with benzyl amine to form the pyrrolidine ring via two consecutive nucleophilic displacements. Removal of the benzyl protecting group afforded stereopure 1 in good overall yield.
Scheme 14. Catalytic Enantioselective Synthesis of 1 by Masamune and Co-workers.
The strategy of Masamune and co-workers is versatile as it can be applied to the preparation of related C2-symmetrical pyrrolidines, in particular, pyrrolidines substituted with bulkier aromatic rings. Along this line, the group of Chong reported the asymmetric reduction of diketone 73 with (−)-diisopinocampheylchloroborane (Ipc2BCl) to access diol 74 with excellent stereoselectivities (Scheme 15).37 The diol was again activated as a bismesylate and reacted with allylamine to form a pyrrolidine ring. The allyl group was finally removed by a rhodium(I)-catalyzed olefin isomerization followed by hydrolysis to afford enantiopure free pyrrolidine 2.
Scheme 15. Synthesis of 2 by Chong and Co-workers.
The group of Steel later developed a catalytic protocol to access diol 74(38) relying on the Masui version of the Corey–Bakshi–Shibata reaction (Scheme 16).39 Then they successfully repeated the synthetic sequence developed by the group of Chong to obtain pyrrolidine 2. Interestingly, they could also apply this protocol for the asymmetric reduction of the homologated diketone to access the corresponding C2-symmetrical piperidine. This strategy was further applied by the group of Pihko to the synthesis of symmetrical and nonsymmetrical 2,5-diarylpyrrolidine organocatalysts.40,41 Later, the group of Yamada reported a complementary catalytic system based on a chiral β-ketoiminato cobalt(II) complex for the reduction of several diketones (Scheme 16) to access the corresponding C2-symmetrical azetidines, pyrrolidines, and piperidines.42
Scheme 16. Catalytic Enantioselective Synthesis of 2 by the Groups of Steel and Yamada.
3.1.2. Biocatalyzed Transaminations and Reductive Aminations
Turner et al. developed multienzymatic cascade processes involving a transaminase and a reductive aminase that led to enantiomerically pure 2,5-disubstituted pyrrolidines 77 starting from nonsymmetrical 1,4-diketones 75 (Scheme 17).43 Both R- and S-selective transaminases were identified for the first step, fully converting 1,4-diketones into enantiopure dihydropyrroles 76. Then a R-selective reductive aminase enabled stereoselective reduction of the obtained dihydropyrroles to the corresponding pyrrolidines in the same pot. Interestingly, this enzyme operates more or less independently of the existing stereogenic center, thus giving access to both cis-(2S,5R)- and trans-(2R,5R)-pyrrolidines 77 depending on the selected enzyme combination.
Scheme 17. Biocatalytic Synthesis of Pyrrolidines by the Group of Turner.
3.2. Direct C–H Functionalization of Pyrrolidines
3.2.1. Enantioselective Lithiation
The group of Beak pioneered a versatile strategy to access enantioenriched pyrrolidines through the asymmetric lithiation of N-Boc-pyrrolidine 78 followed by a reaction with an electrophilic partner (Scheme 18).44 Indeed, the complexation of s-BuLi by (−)-sparteine enables an enantioselective deprotonation of 78 and the corresponding lithiated species 79 is configurationally stable at low temperature. It can then be reacted with a range of electrophiles to afford enantioenriched pyrrolidines 80 with good selectivities. By performing two consecutive asymmetric lithiation/methylation sequences, they could convert 78 into enantiopure 82 in 50% overall yield on a gram scale.45
Scheme 18. Synthesis of 82 by Enantioselective Deprotonation by Beak and Co-workers.
Based on the work of Beak, Campos and co-workers later developed an enantioselective palladium-catalyzed arylation of N-Boc-pyrrolidine (Scheme 19).46 To preserve the stereochemical integrity of the lithiated species 79 at higher temperatures, they performed a transmetalation with ZnCl2 at low temperature, and the corresponding organozinc reagent proved to be configurationally stable at room temperature. Pleasingly, this organozinc reagent could also undergo stereoretentive transmetalations with organopalladium species generated from the oxidative addition to a range of arylbromides to afford the corresponding cross-coupling products consistently with 92% ee. Having developed an enantioselective direct arylation of N-Boc-pyrrolidine, the authors also performed two sequential arylations to access the protected chiral auxiliary 84 in an analogous fashion to Beak and co-workers.
Scheme 19. Synthesis of 84 by Enantioselective Deprotonation by Campos and Co-workers.
The sequential bisarylation of readily available N-Boc-pyrrolidine developed by Campos and co-workers is highly efficient and modular as it allows the rapid synthesis of a range of symmetrical and unsymmetrical 2,5-diarylpyrrolidines 86 with consistently high selectivities, independently of the nature of the aryl substituents. This straightforward strategy was exploited by the group of Trost (Scheme 20) to access a variety of phosphoramidite ligands incorporating a chiral pyrrolidine unit for the development of enantioselective trimethylenemethane [3 + 2] cycloadditions.47 Along this line, they synthesized several 2,5-diarylpyrrolidines to finally obtain chiral ligand 87, which proved to be very efficient for the targeted cycloadditions. This methodology was further applied by the group of Denmark to synthesize chiral bishydrazone ligands 88 for palladium-catalyzed atroposelective cross-coupling reactions with aryldimethylsilanolates.48 Overall, this double arylation strategy is powerful to synthesize stereopure 2,5-diarylpyrrolidines; however, the fact that only one enantiomer can be accessed as (+)-sparteine is not available from nature stands as a limitation especially since the use of chiral sparteine surrogates has proven to be still less efficient so far.
Scheme 20. Synthesis of trans-2,5-Diarylpyrrolidines by Trost and Co-workers.
3.2.2. Catalytic Asymmetric C–H Insertion of Carbenes
As highlighted with previously described enantioselective lithiations, the direct difunctionalization of a pyrrolidine moiety is a powerful strategy to access 2,5-disubstituted analogs. Davies et al. developed a catalytic version for this strategy enabled by two consecutive rhodium(II)-catalyzed C–H insertions (Scheme 21).49 The use of Rh1 as a catalyst and donor–acceptor diazo precursors 89 led to C2-symmetrical pyrrolidines 90 with high enantio- and diastereocontrol.
Scheme 21. Synthesis of 90 by Stereoselective C–H Insertions by Davies and Co-workers.
3.3. Catalytic Enantioselective Allylic Substitution
Given the importance of chiral ligand 87,47,50,51 an elegant catalytic strategy was developed by the group of Feringa to access both enantiomers of pyrrolidine 94 precursor of 87 (Scheme 22).52 To this purpose, they performed a double iridium-catalyzed branched-selective allylic substitution with carbonate 91 and ammonia as the nucleophilic partner. The use of chiral iridacycle Ir1 as a catalyst led to high yields and selectivities.53 Importantly, contrary to the strategy involving the enantioselective deprotonation with sparteine, both enantiomers of Ir1 are readily available, so each enantiomer of 92 can be accessed. Stoichiometric protonation of the amine followed by a ring-closing metathesis with a Hoveyda-Grubbs second generation catalyst enabled the formation of dihydropyrrole 93 that was then hydrogenated with diimide to afford the desired pyrrolidine 94.
Scheme 22. Synthesis of 94 by Enantioselective Allylic Substitutions by Feringa and Co-workers.
3.4. Catalytic Enantioselective Dihydroxylation
Corey and Sprott specifically targeted pyrrolidine 99 as a valuable C2-symmetrical unit for the design of novel cationic Lewis acid catalysts such as 100 (Scheme 23).54 Their synthesis started from 1,4-diene 95, easily obtained by a reductive dimerization of cinnamyl bromide, which was engaged in a double Sharpless dihydroxylation to afford 96 as a single stereoisomer. The tetraol 96 was then converted into the biscyclic carbonate 97. Hydrogenolysis with Raney nickel and mesylation of the resulting diol led to 98 that was reacted with benzylamine. Finally, the removal of the benzyl group afforded pyrrolidine 99. This strategy should theoretically give access to various 2,5-dibenzylpyrrolidines, but, as a limitation, the diversity is strictly linked to the choice of the starting material.
Scheme 23. Synthesis of 99 by Enantioselective Bisdihydroxylation by Corey and Sprott.
3.5. Catalytic Stereoselective C(sp3)–H Amination
Recently, Saget, Darses, Dauban, and co-workers envisaged the synthesis of 2,5-disubstituted pyrrolidines through a de novo strategy from simple hydrocarbons involving two consecutive C(sp3)–H amination reactions (Scheme 24).55 A rhodium(II)-catalyzed asymmetric nitrene C–H insertion with chiral rhodium catalyst Rh2 and sulfonimidamide S*-NH2 enabled the first intermolecular C–H amination on benzylic positions of substrates 101.56 The amination proceeds with high regioselectivities on the most electron-rich benzylic position of 1,4-diarylbutane substrates and diastereoselectivities from 9:1 to >20:1 were consistently obtained for 102. Then a second intramolecular amination via a 1,5-HAT inspired by recent studies on hypoiodite mediated Hofmann–Löffler–Freytag reactions57 led to the desired symmetrical and nonsymmetrical pyrrolidines 103 with moderate to good trans-diastereoselectivities. Interestingly, the cyclization was not restricted to benzylic positions, as shown with pyrrolidine 103e. Of note, this sequence was also applied to alkanes, therefore making these broadly available compounds useful building blocks for the synthesis of heterocycles. Conveniently, stereopure pyrrolidines could be isolated after chromatography and the chiral auxiliary could be removed under reductive conditions to afford the corresponding free pyrrolidines.
Scheme 24. Synthesis of trans-2,5-Disubstituted Pyrrolidines 103 by Two Consecutive C(sp3)–H Aminations by Saget, Darses, Dauban, and Co-workers.
4. Conclusion
In conclusion, the asymmetric synthesis of enantioenriched 2,5-disubstituted pyrrolidines was the purpose of several studies over the past few decades. Many disconnections have been successfully achieved, involving each of the five bonds present in the pyrrolidine ring. Interestingly, with the advent of catalytic C–H functionalization reactions, new streamlined strategies have been uncovered from readily available pyrrolidines thereby offering the opportunity to access new pseudo C2-symmetrical scaffolds of potential application as ligands in catalysis. On the other hand, application of C–H functionalization reactions leads to design synthetic schemes for the preparation of substituted pyrrolidines from alkanes. Finally, we notice that strategies involving radical intermediates are still to be reported. But according to the recent achievements in photoredox catalysis and electrochemistry, new methods involving radical intermediates are expected to appear in the near future.58
Acknowledgments
We wish to thank the French National Research Agency (program no. ANR-19-CE07-0043-02; fellowship to J.G.), the COMUE Université Paris-Saclay (program IDEX Paris/Saclay CDE-2018-002093; fellowship to Y.L.), and the ICSN for their support.
Data Availability Statement
The data underlying this study are available in the published article.
Author Contributions
All authors have given approval to the final version of the manuscript. CRediT: Yanis Lazib data curation (equal), methodology (equal), writing-original draft (equal); Junio Guimaraes Naves data curation (equal), methodology (equal), writing-original draft (equal); Agnès Labande project administration (lead), writing-review & editing (supporting); Philippe Dauban conceptualization (lead), funding acquisition (lead), project administration (lead), writing-review & editing (lead); Tanguy Saget conceptualization (lead), writing-review & editing (lead).
The authors declare no competing financial interest.
References
- Vitaku E.; Smith D. T.; Njardarson J. T. Analysis of the Structural Diversity, Substitution Patterns, and Frequency of Nitrogen Heterocycles among U.S. FDA Approved Pharmaceuticals: Miniperspective. J. Med. Chem. 2014, 57 (24), 10257–10274. 10.1021/jm501100b. [DOI] [PubMed] [Google Scholar]
- Melchiorre P.; Marigo M.; Carlone A.; Bartoli G. Asymmetric Aminocatalysis—Gold Rush in Organic Chemistry. Angew. Chem., Int. Ed. 2008, 47 (33), 6138–6171. 10.1002/anie.200705523. [DOI] [PubMed] [Google Scholar]
- Whitesell J. K. C2 Symmetry and Asymmetric Induction. Chem. Rev. 1989, 89 (7), 1581–1590. 10.1021/cr00097a012. [DOI] [Google Scholar]
- Whitesell J. K.; Felman S. W. Asymmetric Induction. 2. Enantioselective Alkylation of Cyclohexanone via a Chiral Enamine. J. Org. Chem. 1977, 42 (9), 1663–1664. 10.1021/jo00429a047. [DOI] [Google Scholar]
- Kawanami Y.; Ito Y.; Kitagawa T.; Taniguchi Y.; Katsuki T.; Yamaguchi M. Asymmetric Alkylation of Carboxyamides by Using Trans-2,5-Disubstituted Pyrrolidines as Chiral Auxiliaries. Tetrahedron Lett. 1984, 25 (8), 857–860. 10.1016/S0040-4039(01)80046-0. [DOI] [Google Scholar]
- Stafford J. A.; Heathcock C. H. Daphniphyllum Alkaloids. Part 8. Asymmetric Total Synthesis of (−)-Secodaphniphylline. J. Org. Chem. 1990, 55 (20), 5433–5434. 10.1021/jo00307a006. [DOI] [Google Scholar]
- Kim B. H.; Lee H. B.; Hwang J. K.; Kim Y. G. Asymmetric Induction in the Conjugate Addition of Thioacetic Acid to Methacrylamides with Chiral Auxiliaries. Tetrahedron Asymmetry 2005, 16 (6), 1215–1220. 10.1016/j.tetasy.2005.01.037. [DOI] [Google Scholar]
- Halland N.; Braunton A.; Bachmann S.; Marigo M.; Jo̷rgensen K. A. Direct Organocatalytic Asymmetric α-Chlorination of Aldehydes. J. Am. Chem. Soc. 2004, 126 (15), 4790–4791. 10.1021/ja049231m. [DOI] [PubMed] [Google Scholar]
- Trost B. M.; Stambuli J. P.; Silverman S. M.; Schwörer U. Palladium-Catalyzed Asymmetric [3 + 2] Trimethylenemethane Cycloaddition Reactions. J. Am. Chem. Soc. 2006, 128 (41), 13328–13329. 10.1021/ja0640750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawabata T.; Muramatsu W.; Nishio T.; Shibata T.; Schedel H. A Catalytic One-Step Process for the Chemo- and Regioselective Acylation of Monosaccharides. J. Am. Chem. Soc. 2007, 129 (42), 12890–12895. 10.1021/ja074882e. [DOI] [PubMed] [Google Scholar]
- Hashimoto H.; Ueda Y.; Fujimura K.; Takasu K.; Kawabata T. Approach Toward Reversal of Chemoselectivity in Catalytic Silylation of Pyranosides. Eur. J. Org. Chem. 2022, 2022 (37), e202200949. 10.1002/ejoc.202200949. [DOI] [Google Scholar]
- Daly J. W. Thirty Years of Discovering Arthropod Alkaloids in Amphibian Skin. J. Nat. Prod. 1998, 61 (1), 162–172. 10.1021/np970460e. [DOI] [PubMed] [Google Scholar]
- Attygalle A. B.; Morgan E. D. Chemicals from the Glands of Ants. Chem. Soc. Rev. 1984, 13 (3), 245. 10.1039/cs9841300245. [DOI] [Google Scholar]
- Feld J. J.; Kowdley K. V.; Coakley E.; Sigal S.; Nelson D. R.; Crawford D.; Weiland O.; Aguilar H.; Xiong J.; Pilot-Matias T.; DaSilva-Tillmann B.; Larsen L.; Podsadecki T.; Bernstein B. Treatment of HCV with ABT-450/r–Ombitasvir and Dasabuvir with Ribavirin. N. Engl. J. Med. 2014, 370 (17), 1594–1603. 10.1056/NEJMoa1315722. [DOI] [PubMed] [Google Scholar]
- Shiosaki K.; Rapoport H. α-Amino Acids as Chiral Educts for Asymmetric Products. Chirospecific Syntheses of the 5-Butyl-2-Heptylpyrrolidines from Glutamic Acid. J. Org. Chem. 1985, 50 (8), 1229–1239. 10.1021/jo00208a016. [DOI] [Google Scholar]
- Åhman J.; Somfai P. Carbon-Carbon Bond Formation via N-Tosyliminium Ions. Tetrahedron 1992, 48 (43), 9537–9544. 10.1016/S0040-4020(01)88321-6. [DOI] [Google Scholar]
- Onomura O.; Kirira P. G.; Tanaka T.; Tsukada S.; Matsumura Y.; Demizu Y. Diastereoselective Arylation of L-Proline Derivatives at the 5-Position. Tetrahedron 2008, 64 (32), 7498–7503. 10.1016/j.tet.2008.06.004. [DOI] [Google Scholar]
- Trost B. M.; Thaisrivongs D. A.; Donckele E. J. Palladium-Catalyzed Enantioselective Allylic Alkylations through C-H Activation. Angew. Chem., Int. Ed. 2013, 52 (5), 1523–1526. 10.1002/anie.201207870. [DOI] [PubMed] [Google Scholar]
- Trost B. M.; Miege F. Development of ProPhenol Ligands for the Diastereo- and Enantioselective Synthesis of β-Hydroxy-α-Amino Esters. J. Am. Chem. Soc. 2014, 136 (8), 3016–3019. 10.1021/ja4129394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Claraz A.; Sahoo G.; Berta D.; Madarász Á.; Pápai I.; Pihko P. M. A Catalyst Designed for the Enantioselective Construction of Methyl- and Alkyl-Substituted Tertiary Stereocenters. Angew. Chem., Int. Ed. 2016, 55 (2), 669–673. 10.1002/anie.201509302. [DOI] [PubMed] [Google Scholar]
- Schlessinger R. H.; Iwanowicz E. J. The Synthesis of Either (+) or (−) Trans-2,5-Dimethylpyrrolidine. Tetrahedron Lett. 1987, 28 (19), 2083–2086. 10.1016/S0040-4039(00)96049-0. [DOI] [Google Scholar]
- Harding K. E.; Burks S. R. Synthesis of Trans-2,5-Dimethylpyrrolidine by Intramolecular Amidomercuration. J. Org. Chem. 1981, 46 (19), 3920–3922. 10.1021/jo00332a036. [DOI] [Google Scholar]
- Yamazaki T.; Gimi R.; Welch J. T. An Optimized Synthesis of (2S,5S)-2,5-Dimethylpyrrolidine. Synlett 1991, 1991 (8), 573–574. 10.1055/s-1991-20802. [DOI] [Google Scholar]
- Huang P. Q.; Arseniyadis S.; Husson H.-P. Asymmetric Synthesis X: A Chiral Pyrrolidine Synthon for a New Approach to the Synthesis of Alkaloids. Tetrahedron Lett. 1987, 28 (5), 547–550. 10.1016/S0040-4039(00)95778-2. [DOI] [Google Scholar]
- Arseniyadis S.; Huang P. Q.; Piveteau D.; Husson H.-P. Asymmetric Synthesis: XII: Stereocontrolled Electrophilic-Nucleophilic α,α’-Substitution of the Pyrrolidine Ring. Tetrahedron 1988, 44 (9), 2457–2470. 10.1016/S0040-4020(01)81697-5. [DOI] [Google Scholar]
- Royer J.; Husson H.-P. Asymmetric Synthesis XI: A Short Synthesis of the Chiral Pyrrolidine Synthon, 2-Cyano-5-Oxazolopyrrolidine. Tetrahedron Lett. 1987, 28 (49), 6175–6178. 10.1016/S0040-4039(00)61839-7. [DOI] [Google Scholar]
- Higashiyama K.; Inoue H.; Takahashi H. Diastereoselective Addition of Chiral Imines and 1,3-Oxazolidines with Grignard Reagents; Asymmetric Synthesis of (R)-2-Aryl- and (R,R)-2,5-Bis(Aryl)Pyrrolidines. Tetrahedron 1994, 50 (4), 1083–1092. 10.1016/S0040-4020(01)80819-X. [DOI] [Google Scholar]
- Katritzky A. R.; Cui X.-L.; Yang B.; Steel P. J. Asymmetric Syntheses of 2-Substituted and 2,5-Disubstituted Pyrrolidines from (3S,5R,7aR)-5-(Benzotriazol-1-Yl)-3-Phenyl[2,1-b]Oxazolopyrrolidine. J. Org. Chem. 1999, 64 (6), 1979–1985. 10.1021/jo9821426. [DOI] [PubMed] [Google Scholar]
- Marzi M.; Misiti D. Asymmetric Synthesis of Trans-(2R,5R)-Bis(Benzyloxymethyl)Pyrrolidine. Tetrahedron Lett. 1989, 30 (44), 6075–6076. 10.1016/S0040-4039(01)93858-4. [DOI] [Google Scholar]
- Machinaga N.; Kibayashi C. Enantioselective Total Synthesis of (+)- and (−)-Pyrrolidine 197B, a New Class of Alkaloid from the Dendrobatid Poison Frog: Assignment of the Absolute Configuration. J. Org. Chem. 1991, 56 (4), 1386–1393. 10.1021/jo00004a011. [DOI] [Google Scholar]
- Wang Q.; Sasaki N. A.; Riche C.; Potier P. A Versatile Method for the Facile Synthesis of Enantiopure Trans- and Cis -2,5-Disubstituted Pyrrolidines. J. Org. Chem. 1999, 64 (23), 8602–8607. 10.1021/jo991052d. [DOI] [Google Scholar]
- Davis F. A.; Song M.; Augustine A. Asymmetric Synthesis of Trans-2,5-Disubstituted Pyrrolidines from Enantiopure Homoallylic Amines. Synthesis of Pyrrolidine (−)-197B. J. Org. Chem. 2006, 71 (7), 2779–2786. 10.1021/jo052566h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen W.; Ma L.; Paul A.; Seidel D. Direct α-C–H Bond Functionalization of Unprotected Cyclic Amines. Nat. Chem. 2018, 10 (2), 165–169. 10.1038/nchem.2871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Short R. P.; Kennedy R. M.; Masamune S. An Improved Synthesis of (−)-(2R,5R)-2,5-Dimethylpyrrolidine. J. Org. Chem. 1989, 54 (7), 1755–1756. 10.1021/jo00268a049. [DOI] [Google Scholar]
- Vigneron J. P.; Dhaenens M.; Horeau A. Nouvelle Methode Pour Porter Au Maximum La Purete Optique d’un Produit Partiellement Dedouble sans l’aide d’aucune Substance Chirale. Tetrahedron 1973, 29 (7), 1055–1059. 10.1016/0040-4020(73)80060-2. [DOI] [Google Scholar]
- Polavarapu P. L. Optical Purity, Enantiomeric Excess and the Horeau Effect. Org. Biomol. Chem. 2020, 18 (35), 6801–6806. 10.1039/D0OB01497D. [DOI] [PubMed] [Google Scholar]
- Michael Chong J.; Clarke I. S.; Koch I.; Olbach P. C.; Taylor N. J. Asymmetric Synthesis of Trans-2,5-Diphenylpyrrolidine: A C2-Symmetric Chiral Amine. Tetrahedron Asymmetry 1995, 6 (2), 409–418. 10.1016/0957-4166(95)00026-L. [DOI] [Google Scholar]
- Aldous D. J.; Dutton W. M.; Steel P. G. A Simple Enantioselective Preparation of (2S,5S)-2,5-Diphenylpyrrolidine and Related Diaryl Amines. Tetrahedron Asymmetry 2000, 11 (12), 2455–2462. 10.1016/S0957-4166(00)00208-1. [DOI] [Google Scholar]
- Masui M.; Shioiri T. A Practical Method for Asymmetric Borane Reduction of Prochiral Ketones Using Chiral Amino Alcohols and Trimethyl Borate. Synlett 1997, 1997 (03), 273–274. 10.1055/s-1997-779. [DOI] [Google Scholar]
- Kemppainen E. K.; Sahoo G.; Piisola A.; Hamza A.; Kótai B.; Pápai I.; Pihko P. M. Mukaiyama–Michael Reactions with Trans-2,5-Diarylpyrrolidine Catalysts: Enantioselectivity Arises from Attractive Noncovalent Interactions, Not from Steric Hindrance.. Chem. – Eur. J. 2014, 20 (20), 5983–5993. 10.1002/chem.201304240. [DOI] [PubMed] [Google Scholar]
- Kortet S.; Claraz A.; Pihko P. M. Catalytic Enantioselective Total Synthesis of (+)–Lycoperdic Acid. Org. Lett. 2020, 22 (8), 3010–3013. 10.1021/acs.orglett.0c00772. [DOI] [PubMed] [Google Scholar]
- Sato M.; Gunji Y.; Ikeno T.; Yamada T. Efficient Preparation of Optically Pure C2-Symmetrical Cyclic Amines for Chiral Auxiliary. Synthesis 2004, 2004 (9), 1434–1438. 10.1055/s-2004-822366. [DOI] [Google Scholar]
- Costa B. Z.; Galman J. L.; Slabu I.; France S. P.; Marsaioli A. J.; Turner N. J. Synthesis of 2,5-Disubstituted Pyrrolidine Alkaloids via A One-Pot Cascade Using Transaminase and Reductive Aminase Biocatalysts. ChemCatChem. 2018, 10 (20), 4733–4738. 10.1002/cctc.201801166. [DOI] [Google Scholar]
- Kerrick S. T.; Beak P. Asymmetric Deprotonations: Enantioselective Syntheses of 2-Substituted Tert-(Butoxycarbonyl)Pyrrolidines. J. Am. Chem. Soc. 1991, 113 (25), 9708–9710. 10.1021/ja00025a066. [DOI] [Google Scholar]
- Beak P.; Kerrick S. T.; Wu S.; Chu J. Complex Induced Proximity Effects: Enantioselective Syntheses Based on Asymmetric Deprotonations of N-Boc-Pyrrolidines. J. Am. Chem. Soc. 1994, 116 (8), 3231–3239. 10.1021/ja00087a008. [DOI] [Google Scholar]
- Campos K. R.; Klapars A.; Waldman J. H.; Dormer P. G.; Chen C. Enantioselective, Palladium-Catalyzed α-Arylation of N -Boc-Pyrrolidine. J. Am. Chem. Soc. 2006, 128 (11), 3538–3539. 10.1021/ja0605265. [DOI] [PubMed] [Google Scholar]
- Trost B. M.; Silverman S. M.; Stambuli J. P. Development of an Asymmetric Trimethylenemethane Cycloaddition Reaction: Application in the Enantioselective Synthesis of Highly Substituted Carbocycles. J. Am. Chem. Soc. 2011, 133 (48), 19483–19497. 10.1021/ja207550t. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denmark S. E.; Chang W.-T. T.; Houk K. N.; Liu P. Development of Chiral Bis-Hydrazone Ligands for the Enantioselective Cross-Coupling Reactions of Aryldimethylsilanolates. J. Org. Chem. 2015, 80 (1), 313–366. 10.1021/jo502388r. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies H. M. L.; Hansen T.; Hopper D. W.; Panaro S. A. Highly Regio-, Diastereo-, and Enantioselective C–H Insertions of Methyl Aryldiazoacetates into Cyclic N-Boc-Protected Amines. Asymmetric Synthesis of Novel C2-Symmetric Amines and Threo-Methylphenidate. J. Am. Chem. Soc. 1999, 121 (27), 6509–6510. 10.1021/ja9910715. [DOI] [Google Scholar]
- Trost B. M.; Silverman S. M.; Stambuli J. P. Palladium-Catalyzed Asymmetric [3 + 2] Cycloaddition of Trimethylenemethane with Imines. J. Am. Chem. Soc. 2007, 129 (41), 12398–12399. 10.1021/ja0753389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trost B. M.; Mata G. Enantioselective Palladium-Catalyzed [3 + 2] Cycloaddition of Trimethylenemethane and Fluorinated Ketones. Angew. Chem., Int. Ed. 2018, 57 (38), 12333–12337. 10.1002/anie.201807308. [DOI] [PubMed] [Google Scholar]
- Teichert J.; Feringa B. Catalytic Asymmetric Synthesis of 2,5-Naphthylpyrrolidine. Synthesis 2010, 2010 (07), 1200–1204. 10.1055/s-0029-1218646. [DOI] [Google Scholar]
- Hartwig J. F.; Stanley L. M. Mechanistically Driven Development of Iridium Catalysts for Asymmetric Allylic Substitution. Acc. Chem. Res. 2010, 43 (12), 1461–1475. 10.1021/ar100047x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sprott K. T.; Corey E. J. A New Cationic, Chiral Catalyst for Highly Enantioselective Diels–Alder Reactions. Org. Lett. 2003, 5 (14), 2465–2467. 10.1021/ol034706k. [DOI] [PubMed] [Google Scholar]
- Lazib Y.; Retailleau P.; Saget T.; Darses B.; Dauban P. Asymmetric Synthesis of Enantiopure Pyrrolidines by C(Sp3)–H Amination of Hydrocarbons. Angew. Chem., Int. Ed. 2021, 60 (40), 21708–21712. 10.1002/anie.202107898. [DOI] [PubMed] [Google Scholar]
- Liang C.; Collet F.; Robert-Peillard F.; Müller P.; Dodd R. H.; Dauban P. Toward a Synthetically Useful Stereoselective C–H Amination of Hydrocarbons. J. Am. Chem. Soc. 2008, 130 (1), 343–350. 10.1021/ja076519d. [DOI] [PubMed] [Google Scholar]
- Stateman L. M.; Nakafuku K. M.; Nagib D. A. Remote C–H Functionalization via Selective Hydrogen Atom Transfer. Synthesis 2018, 50 (8), 1569–1586. 10.1055/s-0036-1591930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaw M. H.; Shurtleff V. W.; Terrett J. A.; Cuthbertson J. D.; MacMillan D. W. C. Native Functionality in Triple Catalytic Cross-Coupling: Sp3 C–H Bonds as Latent Nucleophiles. Science 2016, 352 (6291), 1304–1308. 10.1126/science.aaf6635. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data underlying this study are available in the published article.