Abstract
Air pollution is a complex mixture of gases and particulate matter, with adsorbed organic and inorganic contaminants, to which exposure is life-long. Epidemiological studies increasingly associate air pollution with multiple neurodevelopmental disorders and neurodegenerative diseases, findings supported by experimental animal models. This breadth of neurotoxicity across these central nervous system diseases and disorders likely reflects shared vulnerability of their inflammatory and oxidative stress–based mechanisms and a corresponding ability to produce brain metal dyshomeostasis. Future research to define the responsible contaminants of air pollution underlying this neurotoxicity is critical to understanding mechanisms of these diseases and disorders and protecting public health.
Keywords: air pollution, ultrafine particles, PM2.5, neurodevelopmental disorders, neurodegenerative disease, brain metal dyshomeostasis
1. ELUSIVE ETIOLOGIES OF NEURODEVELOPMENTAL DISORDERS AND NEURODEGENERATIVE DISEASES: DOES AIR POLLUTION PLAY A ROLE?
Extensive efforts have been undertaken to delineate the underlying etiologies of neurodevelopmental disorders (NDDs) such as autism spectrum disorder (ASD), schizophrenia (SCZ), and attention deficit hyperactivity disorder (ADHD) and neurodegenerative diseases (NDGDs) such as Alzheimer’s disease (AD), Parkinson’s disease (PD), and multiple sclerosis (MS). Both NDDs and NDGDs are increasing in prevalence. Efforts to link these NDDs and NDGDs to genetic etiologies alone have proven to be of limited explanatory capacity, especially when considered in relation to increasing prevalence.
Albeit still at a relatively early stage, a sizeable body of epidemiological research has now associated these NDDs and NDGDs with exposures to air pollution (AP). To date, the development of animal model–based research has been more limited, particularly for studies based on human-relevant exposure conditions, likely because of the paucity of facilities for ambient whole-body inhalation exposures. Nevertheless, considered collectively, the epidemiological and animal model studies already provide strong support for a role of AP exposures in both NDDs and NDGDs and as such have marked significance for clinical and public health. The findings also support revised consideration of environmental regulations for such exposures.
This review highlights the epidemiological evidence from meta-analyses examining associations of AP with NDDs and NDGDs as well as evidence of human brain pathology. Importantly, animal model results are noted, summarized, and reviewed. This is followed by a discussion relating the breadth of central nervous system diseases and disorders associated with AP to the shared features of NDDs and NDGDs and to brain metal dyshomeostasis, and of why such disorders could increase even as air quality improves. Lastly, some specific considerations for future research are presented.
2. AIR POLLUTION: A COMPLEX MIXTURE OF PARTICLES AND GASES AND ADSORBED CONTAMINANTS
AP is actually a complex mixture that includes primary pollutants emitted directly from a source and secondary pollutants consequently formed in the atmosphere from chemical reactions. Specifically, AP is largely a product of vehicle exhaust and industrial emissions, and it includes particles with adhering volatile organic and inorganic contaminants [including metals and trace elements (1)], which are adsorbed onto a carbon base, and atmospheric gases (CO2, CO, NO, ozone, SO2). As such, environmental ambient AP is a dynamic complex mixture, the components and chemistry of which continuously change over time (even very brief time periods) in response to conditions that include weather, season, geography, and local industry and traffic conditions, among others (2). To focus the scope of this review, discussion is restricted primarily to particulate matter (PM) toxicity.
AP health effects are related to PM size, traditionally designated as coarse (<10 μm, or PM10), fine (<2.5 μm, or PM2.5), or ultrafine particles (UFPs) (<100 nm or 0.1 μm; nanoparticles). With a greater surface area per mass ratio for contamination by organic and inorganic contaminants, UFPs are considered the most reactive component of AP (3). Specific contaminants of UFPs are highly dependent upon chemical interactions with airborne gases, as well as with geographical area and associated industries and traffic conditions. Current regulation of PM AP exposures in the United States, which is overseen by the Environmental Protection Agency, is restricted to levels of PM10 and PM2.5, while UFPs, despite their greater potential for contamination, are not currently regulated, based on both the contention that their toxicity is insufficiently established and the current lack of adequate monitoring data. Gases in AP, specifically CO, SO2, and NO2, are also regulated in the United States, as is one other contaminant, the metal lead (Pb). The current annual primary standard for PM2.5 in the United States is 12 μg/m3, while the updated annual average World Health Organization guidelines are 5 μg/m3.
As this complexity indicates, outcomes across human studies of AP can be difficult to compare, even for comparisons based on, for example, only exposures to PM2.5, the adsorbed contaminant profiles can differ, meaning that the populations were exposed to a different chemical mixture. An understanding of AP health effects will require speciation of the chemical composition of the exposures to determine which contaminant(s) underlies observed neurotoxicity. Such information could be used to implement new regulations for controlling these contaminants in AP.
3. ROUTES OF AIR POLLUTION TO THE BRAIN: A LIFELONG EXPOSURE
The potential toxicity of AP to the brain is augmented by its multiple routes of exposure across life. In utero, AP PM inhaled by the mother can directly affect placenta and thereby influence fetal brain development (4). Animal models further support such a mechanism. For example, nose-only diesel exhaust exposure inhalation reduced placental efficiency and decreased placental blood flow and fetal vessel volume (5), while concentrated inhaled PM2.5 exposure for 15 days of gestation in rats reduced placental mass, size, and surface area (6), suggesting that a placental inflammatory reaction had taken place (7). A recent study of occupational exposures to nanoparticles based on a job exposure matrix found UFP exposures to be associated with placental hypoplasia (8). A review of human perfused placenta noted that placental transport was dependent on characteristics that included size and surface modifications of PM (9).
Adsorbed contaminants may also cross the placenta. Trace elements essential to fetal brain development can cross from maternal into fetal circulation. Both iron (Fe) and sulfur (S), for example, move actively into fetal circulation. The fetal need for Fe actually increases across pregnancy (10), with Fe traversing fetal endothelium to reach fetal circulation (11). Other nanoparticle-based metal contaminants shown to be capable of placental transfer and accumulation in fetal tissue include Pb, cadmium, mercury, copper (Cu), and vanadium (12, 13). In the case of Fe, regulatory mechanisms for its homeostasis within the brain are not established until later in development (14), thus leaving the fetal brain potentially vulnerable to early trace metal accumulation and consequent brain metal dyshomeostasis.
Following birth, both direct and indirect exposure routes to the brain are operative. While differences in deposition within lung regions occur in response to particle size, UFPs show efficient translocation into the blood circulation (15), from which they can then travel into the brain.
Of additional concern are extrapulmonary routes of exposure, consisting of nasal olfactory uptake and brain distribution via olfactory and trigeminal nerves (16). These nanoparticles/contaminants actually bypass the blood-brain barrier and thus are not necessarily indexed by peripheral markers (17). A study comparing levels of metals in postmortem brain tissue and ventricular fluid in individuals with AD and nondemented elderly controls reported that brain metal concentrations were elevated in individuals with AD, whereas levels in ventricular fluid were not indicative of brain metal concentrations in either group (18), with the exception of Pb. Metals and trace elements shown to be taken up into the brain via this pathway to date (19) include manganese (Mn), cadmium, Pb, Fe, uranium, nickel, thallium, cobalt, chromium, zinc (Zn), mercury, and aluminum (Al). Notably, increases in and imbalance of such metals, particularly Fe, have been implicated in multiple NGDGs, including AD and PD (20). Furthermore, brain trace metal imbalances have been implicated in multiple other NDGDs as well (21). Whether translocation occurs via the vagal nerve to the brainstem has yet to be ascertained.
The lack of clear mechanisms for removal of metal contaminants from the brain further exacerbates concern regarding the nasal olfactory route. Fe was reported to have an extremely slow turnover in rodent brain (half-life of approximately 9 months), with estimates on the order of decades when extrapolated to humans (22). Further, a recent study reported that Fe deposition in the brain may lead to dysfunction of the glymphatic system, which is considered an important fluid clearance system of the brain (23).
4. EPIDEMIOLOGICAL EVIDENCE FOR A ROLE OF AIR POLLUTION IN NEURODEVELOPMENTAL DISORDERS AND NEURODEGENERATIVE DISEASES
Given the scope of the epidemiological literature, the review of population exposure effects here focuses primarily on the most recent meta-analyses of studies that have utilized PM2.5 as the relevant exposure. These studies have primarily focused on exposures during pregnancy and/or early development, or on aging populations. Human studies without specific exposure metrics were not included.
4.1. Epidemiological Studies and Meta-Analyses of Neurodevelopmental Disorders
Epidemiological studies of the role of AP in NDDs have been reported in populations from multiple different countries based on different metrics of exposure, such as PM levels or surrogates of traffic such as NOx parameters. As noted above, given the breadth of sites from which such studies emerge, the specific contaminants and their levels within AP will also necessarily differ. Nevertheless, a substantive body of positive associations have emerged as indicated in recent meta-analyses.
Studies of ASD, the most extensively examined NDD in relation to AP exposures, have reported both positive and null associations across studies. However, recent meta-analyses suggest a positive overall significant link (24–26). The most recent meta-analysis (which included 28 studies that corresponded to a total of 758,997 newborns, of which 47,190 were diagnosed with ASD) found that increases of 5 μg/m3 of PM2.5 increased risk of ASD regardless of the model used, whether AP exposures were examined during preconception (+17% risk), pregnancy (+5–16%, dependent upon model), or the postnatal period (+11–16%), suggesting a broad period of vulnerability, although the most consistent effects were found with exposures during pregnancy (26). These findings were not influenced by parental age or newborn sex and suggest that pregnancy and the postnatal period are the most at-risk periods.
Studies of AP and ADHD have also included reports of both positive and null associations. A recent meta-analysis of studies published through December 2018 (27) noted that 9 of 12 studies found positive associations between PM exposures and ADHD, although a high risk of bias was noted. Since then, a prospective study in Taiwan (708,515 families) reported that prenatal AP exposure was associated with childhood hyperactivity disorder in children diagnosed before 8 years of age (28); a study in Korea reported that across a total of 7,200 ADHD- related hospital admissions over 2 years, short term exposures to PM10, NO2 (considered a marker of traffic), and SO2 were considered positive risk factors, particularly in those 15–19 years of age compared to 10–14 years of age (29). In a large Canadian prospective study of 37,000 births with 1,217 ADHD diagnoses, incidence of ADHD was increased in relation to levels of PM2.5 (30).
Positive associations between SCZ and AP have also been suggested. A meta-analysis focused on hospital admissions for psychiatric care (SCZ, mental disorder, mental and behavioral disorder, panic attack, depression) reported positive associations across 19 studies for multiple metrics of AP exposure, with the strongest associations being with PM10 (13 of 16 studies) and PM2.5 (8 of the 12 studies) exposures (31). A retrospective cohort study in London of 13,887 individuals found associations between multiple metrics of AP exposures and mental health service use among individuals 15 years of age or older with first presentation of psychotic disorders (32). Recent studies from China specifically related to SCZ reported an increased relapse risk or risk of hospitalization for SCZ in relation to PM2.5/PM10 exposures in a cohort of 6,220 hospital readmissions (33). A Danish study that included 230,884 individuals, with 2,189 diagnosed with SCZ, found associations between NO2 and development of SCZ, particularly in men exposed between birth and 10 years of age (34).
4.2. Epidemiological Studies and Meta-Analyses of Neurodegenerative Diseases
AP has also been associated with NDGDs, including AD, PD, and MS (35). Studies of AD/dementia associations with AP include several recent meta-analyses. An analysis that included 9 studies with a population size exceeding ten million 30–85-year-olds across the United States, the United Kingdom, Canada, Spain, Italy, Sweden, and Taiwan found that each 10 μg/m3 in PM2.5 was positively associated with increased AD risk (odds ratio of 1.95) (36). Another meta-analysis of 4 studies (from Canada, Taiwan, the United Kingdom, and the United States) selected based on a longitudinal cohort study design, with no overlap in study population, a population aged 50 years or older, accessibility of detailed descriptions of PM2.5 exposure assessment, and clear definitions of dementia that included more than 12 million people, found that a 10-μg/m3 increase in PM2.5 was positively associated with dementia (odds ratio of 3.26) as well as with AD (odds ratio of 4.82) (37). Earlier analyses have also provided support for an association of AP with AD/dementia (38, 39).
A meta-analysis of the association of AP with PD that included 9 studies based on PM2.5 and 6 studies based on PM10 in which sample sizes ranged from 126 to 9,817,806 (median 53,219), the number of PD cases ranged from 72 to 119,425 (median 509), and exposure durations ranged from 2 to 22 years (median 7) (40) also found marginally significant increases in risk ratios for increases of 10 μg/m3 in PM2.5 (risk ratio of 1.8) but not for PM10. Similarly, a meta-analysis based on 13 studies likewise reported a small increase in relative risk of 1.06 in populations with the number of PD cases ranging from 104 to 9.8 million (41), while a significant increase was reported in another meta-analysis based on seven studies prior to 2017, with an odds ratio of 1.34 (38). A subsequent retrospective cohort study in Korea of 338 individuals with newly diagnosed PD found that comparisons of highest versus lowest quartile of NO2 exposure increased the hazard ratio for PD (1.41), but no statistically significant associations were found with either PM2.5 or PM10 (42). In contrast, a prospective cohort study in China with 47,516 participants found a significant increase in PD risk (hazard ratio of 1.5) per each interquartile range increase in PM2.5 (43). In addition, a positive nonlinear PM2.5–PD association (measured for first hospitalization) was shown to plateau at 11 μg/m3 in a study in New York state that included 264,075 cases of AD and 114,514 cases of PD (44). As noted in that study, average annual PM2.5 concentrations averaged 8.1 μg/m3, a value below current national US standards. In the largest study to date, that is, a longitudinal cohort study in the contiguous United States of a population of more than 63 million individuals of age 65 or older with over 1 million cases of PD, each 5-μg/m3 increase in annual PM2.5 concentrations was associated with a significant hazard ratio of 1.13 (45).
Inconsistent associations have been reported for MS, and the most recent meta-analysis included 10 studies, based on criteria of being either a case-control, cross-sectional, or cohort study with definite PM diameter, that examined MS relapse and incidence (46). Risk ratio across all studies, where population sizes ranged from 52 to 9,72,756, revealed a significant correlation between PM2.5 and PM10 with MS incidence and relapse. In addition, a meta-analysis based on the three included studies of PM2.5 revealed a significant association. Another recent meta-analysis of six studies with MS case numbers ranging from 424 to 9,247 found an association of PM10 but not PM2.5 with MS (47). However, in a study in Israel of 287 MS patients, PM2.5 was associated with MS relapses in nonsmoking subjects (48), while in a study of 1,246 hospitalizations in France, PM2.5 concentration at 3 weeks prior to hospitalization was significantly associated with risk of hospitalization for MS relapse (49). In a study of 1,435 MS patients in Padua, Italy (50), MS prevalence was strongly correlated with the annual average PM2.5 concentration.
5. PATHOLOGICAL EVIDENCE FOR A ROLE OF AIR POLLUTION IN NEURODEVELOPMENTAL DISORDERS AND NEURODEGENERATIVE DISEASES
5.1. Brain Pathology and Developmental Air Pollution Exposures
Studies have also begun to evaluate pathological changes in the brain in children in response to developmental AP exposures, primarily via magnetic resonance imaging (MRI), with results that not only further confirm effects of AP on the developing brain but also provide evidence of changes consistent with known brain changes in NDDs. For example, a study of 332 US African-American or Dominican youth from 6 to 14 years of age reported associations of PM2.5 with thinning of dorsal parietal cortices, thickening of postero-inferior and mesial wall cortices, reduced white matter volume, disorganization of white matter in internal capsule and frontal lobe, and increased N-acetyl-l-aspartate concentrations in frontal lobe. Interestingly, the authors note the collective impacts in particular on the cortico-striato-thalamo-cortical circuitry connecting frontal, temporal, and parietal cortices with basal ganglia nuclei and thalamus, circuitry implicated to date in multiple NDDs, including SCZ (51) and ADHD (52), with observed effects greater in boys (53).
A study that included four different sites in the United States and China found AP to be related to the progression of hippocampal atrophy after a first episode of SCZ (54). An extensive assessment in 3,133 preadolescents in Rotterdam (55) reported that AP exposures during pregnancy were associated with increased volumes of cerebellum, putamen, pallidum, and amygdala and with reduced volumes of corpus callosum and hippocampus; AP exposures during childhood likewise resulted in reductions in volumes of corpus callosum and hippocampus. Reductions in corpus callosum size in response to PM2.5, particularly during pregnancy, have been reported in other studies in children as well (56). A recent examination of 10,343 9–10-year-old children observed hemisphere-specific differences in brain structure in response to PM2.5, with, for example, reductions in volume of the left but not right hemisphere nucleus accumbens (57), even at PM2.5 exposure concentrations (1.72–15.9 μg/m3) that are generally lower than the US Environmental Protection Agency regulations (12 μg/m3). PM2.5 exposures during the first year of life were associated with reduced gray matter volumes in the left pre- and postcentral gyri and cerebellum and inferior parietal lobe in 135 12-year-olds (58). A study examining elemental carbon and NO2 as a marker of vehicle exhaust reported alterations in functional connectivity in 263 8–12-year-old children (59), specifically weaker functional connectivity of the default mode network between medial frontal cortex and angular gyrus but stronger functional connectivity between the medial frontal cortex seed region of the default mode network and frontal operculum. Opposite effects were found when functional connectivity was assessed by children’s age or motor response speed, suggesting that AP slows brain maturation.
Another study assessed the role of Cu contamination in PM2.5 as a potential source of adverse effects. Cu, while required for brain development, is toxic in excess (60). Cu in PM2.5 was associated with increased gray matter and lower white matter concentrations in basal ganglia, without an effect on tissue volume. Functional MRI analyses revealed a reciprocal reduction of functional connectivity between the caudate nucleus and operculum of frontal lobe, consistent with anatomic findings. Correspondingly, these effects of Cu were associated with poorer motor performance in children, effects that remained significant following adjustment for several other elements, including carbon, Pb, Fe, and antimony.
5.2. Brain Pathology in Adults
Studies of brain pathology likewise provide further support for a role of AP in NDGDs. Several studies indicate vulnerability of white matter. In a study of 1,302 normal, aging US women without dementia, an increase of 3.22 μg/m3 in PM2.5 was associated with significantly lower white matter volume in total brain and in temporal lobe and corpus callosum (61), findings consistent with a prior report by these authors (62) of reduced white matter but not gray matter volume in women without dementia, with findings independent of geographical region, demographics, socioeconomic status, lifestyle, or cardiovascular risk factors. Further, in a study of 8,600 participants from the United Kingdom averaging 55.6 years of age, PM2.5 exposure was negatively associated with white matter volume, an effect that was actually exacerbated by vigorous physical activity (63).
Other studies report associations of AP with gray matter volume changes as well. In the study of 8,600 participants from the United Kingdom discussed above, PM2.5 exposure was negatively associated with both gray matter and white matter volume. Another study from the United Kingdom of 18,278 individuals averaging 62 years of age found an inverse association of left hippocampal volume with PM2.5 (64). A study of 212 participants from Barcelona revealed higher gray matter volume in cerebellum and white matter volume in the splenium of corpus callosum, the superior longitudinal fasciculus, and cingulum cingulate gyrus (65). In a study of 957 participants of an average age of 67 years from four cities in the Republic of Korea (66), PM10 exposures were negatively associated with thickness of the frontal and temporal lobes, as well as with reductions in volume of the pallidum, hippocampus, amygdala, and nucleus accumbens, while PM2.5 exposures were associated with reduced temporal cortex thickness, but with increases in the occipital and cingulate cortices, and with reductions in nucleus accumbens volume, with generally stronger impacts in men. In a sample of 1,364 women aged 71–89 years, PM2.5 was associated with reductions in cortical gray matter volume, particularly in the bilateral superior, middle, and medial frontal gyri (67).
In a study of 615 participants with an age range of 55–85 years, AP was associated with language and short-term memory deficits and with atrophy of the fronto-parietal network, which is associated with these cognitive processes in both sexes, specifically in the right hemisphere. In a compelling longitudinal study (68) of 998 women aged 73–87 years who were given brain scans separated by approximately 4 years, PM2.5 was associated with greater reductions in both immediate recall and new learning, such that each increment of 2.81 μg/m3 of PM2.5 accelerated the annual decline rate in immediate free recall new learning by 14.8–19.3%. Importantly, long-term PM2.5 exposures increased the AD pattern similarity scores, an MRI-derived neuroanatomical risk measure for AD.
Brain infarcts have also been associated with AP. In a study of 1,400,503 participants aged 18 years or older from 174 cities in China, each standard deviation increase in AP was associated with a 16–42% increase in the risk of brain infarct (69). Similarly, covert brain infarcts were reported in a population of 943 individuals with a mean age of 68 (70).
6. ANIMAL MODELS EXAMINING THE ROLE OF AIR POLLUTION ON THE BRAIN
In the discussion of animal models in this section, studies employing inhalation exposures are highlighted, as methods such as intranasal or intratracheal instillation do not fully capture important chemical modifications in inhalation exposures. While gases in AP, for example, ozone and SO2, have also received attention, this literature is currently far less expansive and consequently is not included here. The studies summarized below are selected highlights, but a more extensive review of inhalation studies in animal models is provided in Supplemental Tables 1 and 2.
6.1. Developmental Exposure Models
Studies of inhalation exposures during the periods encompassing brain development in experimental animals, while limited to date, support the vulnerability of the developing brain to AP and provide biological plausibility for the epidemiological studies (see Supplemental Table 1). Studies from our laboratory have examined the impact of concentrated ambient fine-particle/UFP exposures during gestation (6 h per day from gestational day 0.5 to 16.5) (71, 72), as well as concentrated ambient UFP exposures during early postnatal development in the mouse (73–77), which mirrors human third-trimester brain development (78). Observed effects were dependent upon the developmental period of exposure, offspring sex, and brain region. Gestational exposure increased corpus callosum size and produced premature and excessive myelination in both offspring sexes at postnatal day (PND) 14, consistent with a shift toward a more activated microglia state and a more mature oligodendrocyte phenotype in corpus callosum (71). These exposures also slightly (approximately 10%) but significantly reduced mean hippocampal area but did not alter frontal cortical thickness. All effects occurred in the absence of any maternal or offspring overt toxicity (72). Ventriculomegaly persisted in females but not in males in adulthood, while increased corpus callosum area and myelination persisted in both sexes. Notably, increases in levels of Fe, a primary driver of myelination (79), were found in the female but not male corpus callosum, and these levels correlated significantly with increases in myelin density, while increased Fe concentrations were found in cerebellum of both offspring sexes (80).
Postnatal exposures of mice to concentrated ambient UFP were carried out on PND 4–7 and 10–13 for 4 h per day, a period of very high rates of both neurogenesis and gliogenesis (81) and, as expected given differences in ongoing brain development at the time of exposure (75–77), produced different neuropathological consequences. Male-specific and persistent ventriculomegaly was seen even at PND 270. Male-specific reductions in corpus callosum size and myelination were seen at PND 14 but resolved by PND 270. Effects in corpus callosum were most pronounced in the truncus and medial external capsule, regions that were under development at the time of exposures. Striatal myelination was not affected, consistent with its development after the exposure window used in these studies. Male-specific and persistent excitatory-inhibitory glutamate imbalance was observed in frontal cortex, and 340% increases in microglial activation were seen in male corpus callosum at PND 270. Behavioral features included male-biased impulsivity, cognitive inflexibility, and reduced social novelty preference (73, 82). In another study, postnatal exposures of mice to inhaled PM2.5, albeit at very high exposure concentrations, likewise resulted in reductions in corpus callosum size, although sex differences were not considered in the study (83).
Aerosolized resuspended nanoparticulate matter exposure (340 μg/m3) in rats from development to adulthood reduced neurogenesis in dentate gyrus and produced glial activation, hippocampal Fe deposits, and microbleeds, coupled with behavioral deficits in impulsivity and impaired short-term memory (84). MRI examination in male rat offspring exposed during gestation and lactation to PM2.5 in a traffic tunnel at concentrations of approximately 200 μg/m3 revealed decreased structural integrity of gray matter in anterior cingulate as well as hippocampus, in addition to cognitive and social behavioral deficits (85). Direct exposures to ambient AP during gestation and early postnatal development produced sex-dependent differences that included increased hippocampal neurogenesis in males and increased granular cell layer width and reduced lateral ventricle volume in females (86). No systemic toxicity was found in any of these studies. Neurons derived from offspring cerebral cortex at 24 h showed an increase in undifferentiated neurons but no reduction in total numbers of neurons following prenatal exposure (87) of mice to reaerosolized urban nanoparticles (350 μg/m3) for 5 h, 3 times per week for 10 weeks, including gestation.
Another series of studies examining developmental exposures of mice to 250 μg/m3 diesel exhaust PM from embryonic day 0 through weaning reported alterations in levels of transcription factors relevant to neurogenesis in whole brain at PND 3 in male offspring and reductions in neurogenesis in the subgranular zone of hippocampus at PND 60 in both sexes (88). Earlier studies by this group reported increases in IL-6 in placenta and the fetal brain, as well as downregulated expression of reelin (RELN), critical to neuronal migration (89). Studies of diesel exhaust at lower concentrations have reported more limited but male-specific neurotoxicity, consisting of increased inflammation in corpus callosum and cortex, but no changes were found in behavioral function (90). Deficits in elevated plus maze and water maze performance and altered levels of hippocampal glutamatergic receptors have also been reported after exposures of mice to higher levels (350–400 μg/m3) of diesel exhaust (91).
6.2. Adult Exposure Models
An even more extensive literature has emerged examining AP exposures in adult animal models (see Supplemental Table 2). Inhalation exposures of adult male mice to resuspended nanoparticulate matter increased microglial activation and produced white matter injury and axonal degradation in corpus callosum (92). A 14-month exposure to ambient PM2.5 in a tunnel in northern California of TgF344-AD rats that express human AD risk genes or normal, wild-type littermates increased amyloid deposition, hyperphosphorylated tau levels, and neuronal loss while also producing cognitive deficits, with effects being age, genotype, and sex dependent (93). A 4-week exposure of male mice to ambient AP (28 ± 8 μg/m3) in a highly polluted area of Buenos Aires produced mitochondrial dysfunction and increased oxidant production (94). Interestingly, chronic exposure of male mice to ambient PM2.5 with an average concentration of 16.32 μg/m3 (95) suggested a preferential vulnerability of cortex relative to striatum, with increased numbers of neurons and microglia; such effects partially reversed following cessation of exposure. Exposures of APP/PS1 mice, another genetic model of AD, to average PM2.5 levels of 25.8 μg/m3 increased hippocampal Aβ plaque load as well as microgliosis and astrogliosis (96). An interesting study of exposure of male mice to ambient PM2.5 for 3, 6, 9, or 12 months at concentrations ranging from 17 to 57 μg/m3, while showing cognitive deficits only at the 9-month time point, did reveal increases in both astroglial and microglial activation across exposure periods (97).
A 9-month exposure of C57Bl6J male mice to inhaled concentrated ambient PM2.5 (concentration of 65.7 ± 34.2 μg/m3) increased protein levels of the beta-site amyloid precursor protein cleaving enzyme (BACE), amyloid precursor protein (APP) processing, and Aβ1–40 levels, while decreasing APP levels, but it did not influence tau and produced no significant inflammation. While the findings suggest an impact of PM2.5 on Aβ pathways, increases in BACE and Aβ1–40 levels would not necessarily increase APP processing, as noted by the authors, so further studies are warranted to better understand the pattern of outcomes.
Two studies have examined specific components of AP in relation to AD-like neuropathology. Male C57Bl6J mice were exposed to 0, 2.5, or 5.0 mg/m3 NO2 for 5 h per day for 4 weeks in a whole-body inhalation chamber (98). The high NO2 concentration increased phosphorylated tau protein expression in both cortex and hippocampus, reduced glutamate receptor expression, and produced thinner postsynaptic densities, consistent with impaired synaptic function.
Diesel exhaust adult models have also been developed. A 14-week diesel exhaust exposure produced a male-biased increase in neuroinflammation and oxidative stress (99), while in another study, exposures of rats to either 200 or 1,000 μg/m3 produced both microglial and astrocytic activation (100). Diesel engine exhaust exposure for 6 h per day, 5 days per week for either 3 or 13 weeks (101) in female 5X familial Alzheimer’s disease mice increased cortical Aβ plaque formation and Aβ42 following the 3-week but not 13-week exposure, a finding the authors attributed to a ceiling effect. Adult rats (sex not specified) exposed to ambient PM2.5 for 24 h per day, 7 days per week for 12 weeks (average of 44 μg/m3), but not 6 weeks (average of 27 μg/m3), showed increased middle cerebral artery narrowing and expression of multiple proinflammatory cytokines.
Experimental animal studies have also begun to address neuronal atrophy, impaired neurogenesis, and impaired synaptic function. In one such study (102), young (3 months old) or aged (18 months old) female C57Bl6J mice were exposed to resuspended traffic-related nanoparticles for 5 h per day, 3 days per week for a period of 10 weeks (342 ± 49 μg/m3) which resulted in the young but not old mice showing selective increases in neurite atrophy and reduced myelination in hippocampal CA1 regions. The absence of effect in aged females was attributed to a possible ceiling effect, and the selective effect on CA1 was considered to be of interest given its vulnerability in AD (103). Effects of acute PM2.5 diesel exhaust exposure (a single 6-h exposure) in adult mice at a concentration of 250–300 μg/m3 found a male-specific reduction in neurogenesis in the hippocampal subgranular zone, subventricular zone, and olfactory bulb (104), effects that could be mitigated by blocking microglial activation.
7. HOW COULD AIR POLLUTION INFLUENCE RISK FOR SUCH A WIDE ARRAY OF NEURODEVELOPMENTAL DISORDERS AND NEURODEGENERATIVE DISEASES? SHARED FEATURES AND MECHANISMS
As the above discussion indicates, an accumulating body of epidemiological, pathological, and experimental evidence links AP exposures to multiple NDDs and NDGDs. One question raised by this collective evidence is how AP could influence so many different NDDs and NDGDs. While research typically focuses on the differences between various NDDs and NDGDs, it is actually the case that they share numerous mechanisms of injury and pathological characteristics (shared outcomes), which may be corresponding targets of AP (Figure 1).
Figure 1.
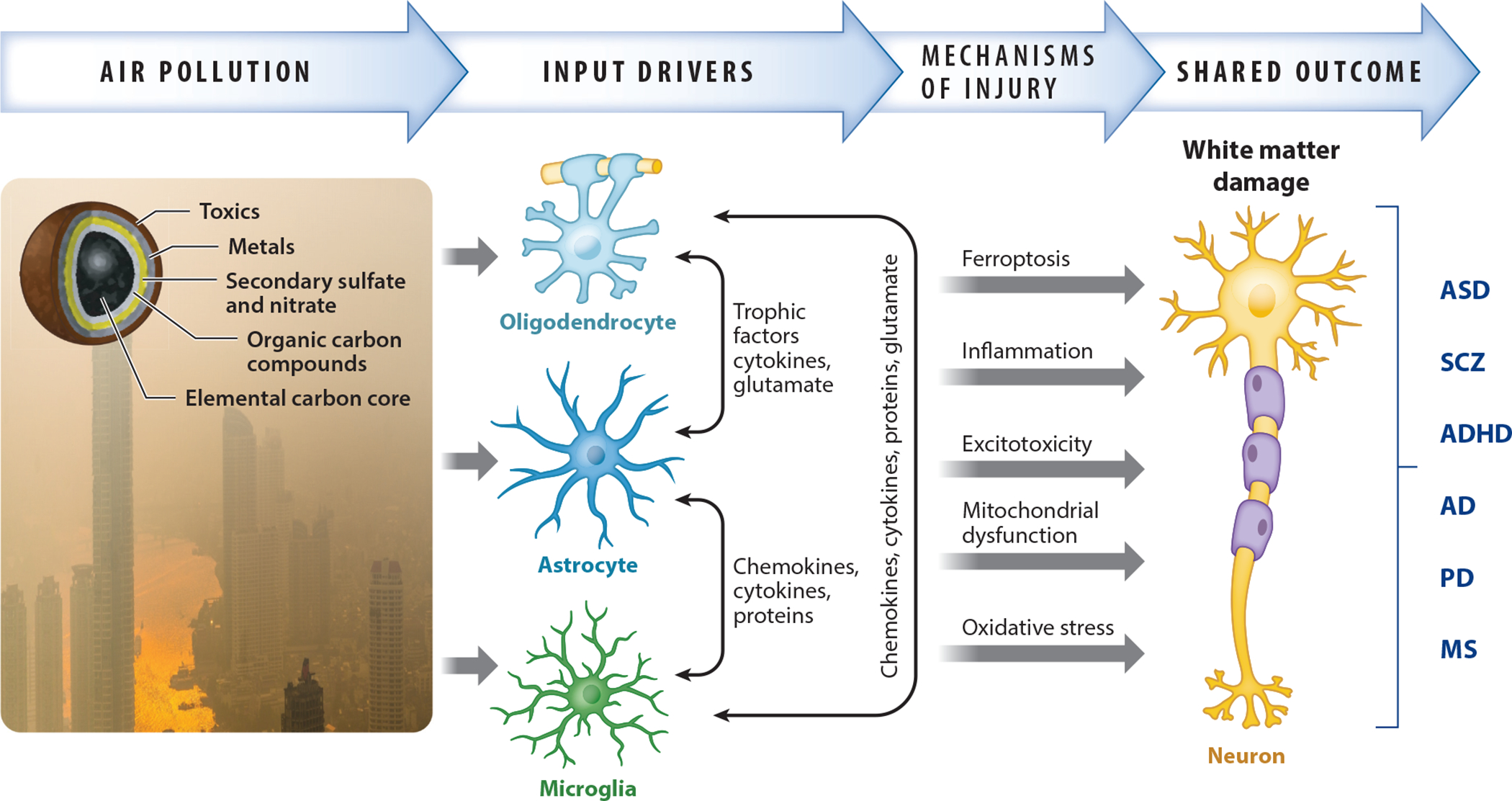
Schematic example of how mechanisms of neurotoxicity produced by central nervous system exposures to air pollution, a complex mixture of gases and particulate matter with adsorbed organic and inorganic contaminants, can lead to a pathological feature such as demyelination that is shared across NDDs and NDGDs. Via its effects on multiple cell types in the brain and their interactions, air pollution leads to various mechanisms of injury, which are also shared across NDDs and NDGDs, that underlie such outcomes. Photo by Peggy Anke on Unsplash. Abbreviations: AD, Alzheimer’s disease; ADHD, attention deficit hyperactivity disorder; ASD, autism spectrum disorder; MS, multiple sclerosis; NDD, neurodevelopmental disorder; NDGD, neurodegenerative disease; PD, Parkinson’s disease; SCZ, schizophrenia.
Inflammation and microglial activation are considered etiological factors in both NDDs and NDGDs (105, 106), thus constituting shared mechanisms of injury in these disorders. Similarly, AP is an inflammatory stimulus, as has been repeatedly documented for cardiopulmonary systems (107) and more recently seen in studies of AP (108) and associated experimental animal models (109). Thus, AP-induced inflammation may serve as a mechanism that sets off other upstream effects or converges on shared downstream outcomes, such as white matter damage, that underlie both NDDs and NDGDs.
Another hypothesized mechanism of injury attributed to both NDDs and NDGDs is mitochondrial dysfunction (110), and studies of AP’s effects on the brain are also demonstrating its adverse impact on mitochondrial function (94, 111); indeed, UFPs have been found in mitochondria of the brain and heart biopsies of Mexico City inhabitants who were chronically exposed to AP (112). Whether such effects reflect direct effects on mitochondria or are a response to AP-related inflammation is not yet known.
Alterations in glutamatergic function are also prominent in NDDs and NDGDs (113–115). Alterations in brain glutamatergic function have been reported in both developing and adult exposure models of AP (77, 116–118). Glutamate has multiple roles in the brain, including regulation of induction of synapses and their interconnection with astrocytes, cell migration, synaptic spatial organization of the cerebellum, cell differentiation, and apoptosis. Excess glutamate is an excitotoxin that can result in lipid peroxidation as well as neuronal dysfunction, white matter damage, and degeneration.
As exemplified in Figure 1, multiple mechanisms of injury known to be triggered by AP could converge on white matter damage, an outcome shared across NDDs and NDGDs (119, 120). White matter alterations also appear to be a prominent target of AP. Of particular note, in a study of 186 8–12-year-old children who underwent brain MRI, increased PM2.5 during the third trimester of pregnancy was associated with both a reduction in the corpus callosum volume and a higher hyperactivity score, although statistical significance did not survive correction for multiple comparison (56). Nevertheless, such findings are remarkably similar to those reported in animal models following early postnatal (human third trimester brain development equivalent) exposures to concentrated ambient UFPs that likewise showed reductions in corpus callosum size accompanied by hypomyelination (75). To date, alterations in white matter, particularly reductions in volume and other features, seem to be the most frequently reported change in human MRI studies, in both children and adults (53, 55, 56, 61–63).
Another example of a shared outcome is ventriculomegaly (enlarged lateral ventricles), which are reported in studies of both NDDs and NDGDs (121, 122). While assessment of lateral ventricle size in animal studies of AP exposure has been quite limited to date, it has been reported in response to UFP exposures during the early postnatal period (equivalent to human third trimester brain development), which in the case of developmental exposure was male biased and appeared to persist over the lifetime (75). Whether adult AP exposures either produce or further increase lateral ventricle size remains to be determined.
8. AIR POLLUTION AND BRAIN METAL DYSHOMEOSTASIS
As noted above, PM is a source of adsorbed contaminants, including metals and trace elements, that directly enter the brain via nasal olfactory uptake, thereby bypassing the blood-brain barrier. Levels of Fe, Al, and silicon are among the highest in AP, with lesser levels of S, Zn, Pb, and Cu. Correspondingly, our studies of inhaled ambient UFPs during the postnatal period revealed pronounced changes in brain metal content, with marked increases in Fe, S, Cu, and calcium (Ca) and to a lesser extent Al, at the expense of Mn and Zn (123).
Of particular note with respect to exposures to inhaled metals is the well-documented increase in brain Fe in many major NGDGs. Fe is known to accumulate in the brain with normal aging, but excess levels occur in NDGDs such as AD, PD, and MS (124, 125) and can underlie ferroptosis, a mechanism of cell death produced by increased Fe and associated oxidative stress and lipid peroxidation (126). Such increases are often hypothesized to reflect alterations in the brain, changes in peripheral Fe metabolism, aging-related pathophysiological changes in barriers such as the blood-brain barrier, or changes in the gastrointestinal tract as sources of increased brain Fe (21).
However, the possibility that life-long exposures to metals via AP underlie these increases and lead to brain metal dyshomeostasis does not appear to have been considered. It would be particularly interesting to consider the nasal olfactory uptake of AP contaminants via olfactory and trigeminal nerves in relation to Braak staging for AD and PD (127–129). It has been proposed that PD originates at two sites, that is, the olfactory bulb and medulla oblongata (brainstem), via vagal nerve interconnections; AD staging has highlighted the entorhinal region located between hippocampus and the transentorhinal region as an initiation point. These are regions that receive olfactory nerve input (127) that would then contain AP contaminants that move into allocortex (olfactory bulb, entorhinal cortex, and hippocampus). In addition, potential vagal nerve retrograde transport into the brainstem from PM circulating in the bloodstream may be occurring, although the latter has yet to be examined.
Correspondingly, studies have demonstrated inflammation in olfactory epithelium in response to AP (130), as well as human olfactory-brain uptake of inhaled UFPs (131). For example, olfactory bulbs of mice exposed intranasally to 14 nm of carbon black particles for 4 weeks exhibited elevated messenger RNA levels for multiple markers of cytokine activity and inflammation; such inflammation can influence various cognitive behaviors (132). Repeated intranasal instillation of lipopolysaccharide, an inflammatory model, produced olfactory bulb atrophy and olfactory sensory neuron degeneration (133).
In addition to increased Fe, dyshomeostasis of other metals is also seen in AD (124), including reductions in Cu (despite concurrent increases in Cu in serum/plasma) (134) and disruptions in Zn concentrations (135). Ca, also a contaminant of PM, can impair lung (136) and cardiac function (137). While the potential impact of elevated Ca from AP on brain function has yet to be considered, it is notable that postnatal exposure of mice to concentrated ambient UFPs markedly increased brain Ca levels in addition to Fe and Cu (138). The toxicity of excess Ca in the brain in relation to AD has long been known (139).
While metal/elemental disturbances have likewise been reported with NDDs (140), much of the focus of this literature has been on serum metal levels, which may not reflect brain metal levels. For example, alterations in trace metals levels in serum or in hair or nails are reported and sometimes correlated with features or severity of ASD (141); the observed metal profiles can differ by sex (142) and geographic location. Alterations in brain metal levels in ASD have been reported (143, 144) as have correlations of trace metal levels with neuroinflammatory markers (145). Similar findings are reported in SCZ, particularly for Zn and Cu (146, 147), as well as in ADHD (148). Interestingly, however, a recent meta-analysis of studies in children with ADHD found an inconsistent association of systemic Fe with ADHD, whereas a significant association was found between reduced brain thalamic Fe and ADHD (149).
9. THE DECLINE IN AIR POLLUTION DOES NOT INCLUDE UFPS
Another important question is how increasing prevalence of NDDs and NDGDs could be associated with AP exposures when AP levels are being regulated and have declined. It has been assumed that regulation of PM10 and PM2.5 levels would also reduce levels of UFPs, considered the most toxic component of AP. However, it now appears that PM2.5 and UFP levels are actually not correlated (150), likely due to a lower PM2.5 level reducing the scavenging of UFPs. Thus, regulating PM2.5 concentrations does little to reduce UFPs (150). This finding raises significant questions as to the adequacy of current regulations and may also explain why, even with cleaner air (by mass), AP could continue to contribute risks for NDDs and NDGDs.
10. FUTURE RESEARCH NEEDS FOR ADVANCING THE UNDERSTANDING OF THE ATTRIBUTABLE RISK OF AIR POLLUTION ON NEURODEVELOPMENTAL DISORDERS AND NEURODEGENERATIVE DISEASES
A critical need to further our understanding of the contribution of AP to NDDs and NDGDs is the development/implementation of in vivo animal models of inhalation exposures. Nonphysiological bolus-type instillation exposures are quite disparate from ambient exposures in terms of chemistry, dose-rate, and absence of deposition in the respiratory tract. It is important to note that UFPs form protein coronas on biological substrates, the nature of which can influence their targets and ultimate impact. Furthermore, the protein corona is not static; its composition evolves as UFPs travel, such as when crossing the blood-brain barrier, meaning that the initial composition is not predictive of fate and effects after it crosses the blood-brain barrier (151).
An additional need is assessment of the role of specific contaminants in producing the associated neurotoxicity, a need underscored by the reports of metal dyshomeostasis in both groups of diseases and disorders. As noted above, metal and trace element contaminants are one such class of potential toxicants. Organic contaminants are another possibility, as demonstrated by correlations of polycyclic aromatic hydrocarbons with reductions in white matter in human studies (152). In addition, AP contamination can include endotoxins (153), which is notable as lipopolysaccharide is an inflammatory stimulus used to model NDDs and causes delayed myelination, neuronal loss, and cerebellar hypoplasia following neonatal administration (154). While this review has focused on PM toxicity, gases such as SO2 and H2S are also a component of AP. For example, an increased odds ratios for ASD was found for several gaseous components of AP (155), with effect estimates of a 59%, 37%, and 340% risk increase per 10-ppb increase in ozone, CO, and NO2 levels (95% confidence interval 3.31–5.85), respectively, and 17% per 1-ppb increase in SO2 level. SO2 exposures during pregnancy and in the first 12 months postnatally were also associated with poor subclinical neurodevelopment, primarily deficiencies in fine motor behavior following adjustment for relevant covariates (156). Further assessments of the potential for these contaminants to phenocopy NDDs and NDGDs provide information that is not only highly relevant to mechanisms of NDDs and NDGDs but, when coupled with concentration response functions, also relevant to potential regulatory actions. The inclusion of both sexes in such studies is also requisite to understanding mechanisms that underlie the sex differences seen in NDDs and NDGDs.
As of yet, the current understanding of the impact of AP exposure timing and duration is limited. With respect to brain development, studies of exposures during specific trimesters are important but to date have been mostly pursued in epidemiological studies. In the case of NDGDs, several additional questions need consideration. For example, can developmental exposures, perhaps via fetal programming, ultimately set conditions of vulnerability to NDGDs, that is, consistent with a fetal basis of adult disease hypothesis? Or do NDGDs arise from adult exposure, and, in either case, must these exposures be sustained or episodic?
Supplementary Material
ACKNOWLEDGMENTS
The authors were supported by National Institutes of Health grants R01 ES032260, R35 ES031689-01A1, and P30 ES001247.
Footnotes
DISCLOSURE STATEMENT
The authors are not aware of any affiliations, memberships, funding, or financial holdings that might be perceived as affecting the objectivity of this review.
LITERATURE CITED
- 1.Friedlander SK. 1973. Chemical element balances and identification of air pollution sources. Environ. Sci. Technol. 7:235–40 [DOI] [PubMed] [Google Scholar]
- 2.Yu HL, Lin YC, Kuo YM. 2015. A time series analysis of multiple ambient pollutants to investigate the underlying air pollution dynamics and interactions. Chemosphere 134:571–80 [DOI] [PubMed] [Google Scholar]
- 3.Lin CC, Chen SJ, Huang KL, Hwang WI, Chang-Chien GP, Lin WY. 2005. Characteristics of metals in nano/ultrafine/fine/coarse particles collected beside a heavily trafficked road. Environ. Sci. Technol. 39:8113–22 [DOI] [PubMed] [Google Scholar]
- 4.Saenen ND, Vrijens K, Janssen BG, Madhloum N, Peusens M, et al. 2016. Placental nitrosative stress and exposure to ambient air pollution during gestation: a population study. Am. J. Epidemiol. 184:442–49 [DOI] [PubMed] [Google Scholar]
- 5.Valentino SA, Tarrade A, Aioun J, Mourier E, Richard C, et al. 2016. Maternal exposure to diluted diesel engine exhaust alters placental function and induces intergenerational effects in rabbits. Part. Fibre Toxicol. 13:39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Soto SF, Melo JO, Marchesi GD, Lopes KL, Veras MM, et al. 2017. Exposure to fine particulate matter in the air alters placental structure and the renin-angiotensin system. PLOS ONE 12:e0183314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.de Melo JO, Soto SF, Katayama IA, Wenceslau CF, Pires AG, et al. 2015. Inhalation of fine particulate matter during pregnancy increased IL-4 cytokine levels in the fetal portion of the placenta. Toxicol. Lett. 232:475–80 [DOI] [PubMed] [Google Scholar]
- 8.Pasquiou A, Pelluard F, Manangama G, Brochard P, Audignon S, et al. 2021. Occupational exposure to ultrafine particles and placental histopathological lesions: a retrospective study about 130 cases. Int. J. Environ. Res. Public Health 18:12719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Aengenheister L, Favaro RR, Morales-Prieto DM, Furer LA, Gruber M, et al. 2021. Research on nanoparticles in human perfused placenta: state of the art and perspectives. Placenta 104:199–207 [DOI] [PubMed] [Google Scholar]
- 10.Rao R, Georgieff MK. 2002. Perinatal aspects of iron metabolism. Acta Paediatr. Suppl. 91:124–29 [DOI] [PubMed] [Google Scholar]
- 11.Gambling L, Danzeisen R, Fosset C, Andersen HS, Dunford S, et al. 2003. Iron and copper interactions in development and the effect on pregnancy outcome. J. Nutr. 133:1554S–56S [DOI] [PubMed] [Google Scholar]
- 12.McArdle HJ, Andersen HS, Jones H, Gambling L. 2008. Copper and iron transport across the placenta: regulation and interactions. J. Neuroendocrinol. 20:427–31 [DOI] [PubMed] [Google Scholar]
- 13.Goyer RA. 1990. Transplacental transport of lead. Environ. Health Perspect. 89:101–5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Siddappa AJ, Rao RB, Wobken JD, Leibold EA, Connor JR, Georgieff MK. 2002. Developmental changes in the expression of iron regulatory proteins and iron transport proteins in the perinatal rat brain. J. Neurosci. Res. 68:761–75 [DOI] [PubMed] [Google Scholar]
- 15.Oberdorster G, Sharp Z, Atudorei V, Elder A, Gelein R, et al. 2002. Extrapulmonary translocation of ultrafine carbon particles following whole-body inhalation exposure of rats. J. Toxicol. Environ. Health A 65:1531–43 [DOI] [PubMed] [Google Scholar]
- 16.Oberdorster G, Sharp Z, Atudorei V, Elder A, Gelein R, et al. 2004. Translocation of inhaled ultrafine particles to the brain. Inhal. Toxicol. 16:437–45 [DOI] [PubMed] [Google Scholar]
- 17.Ibanez C, Suhard D, Tessier C, Delissen O, Lestaevel P, et al. 2014. Intranasal exposure to uranium results in direct transfer to the brain along olfactory nerve bundles. Neuropathol. Appl. Neurobiol. 40:477–88 [DOI] [PubMed] [Google Scholar]
- 18.Szabo ST, Harry GJ, Hayden KM, Szabo DT, Birnbaum L. 2016. Comparison of metal levels between postmortem brain and ventricular fluid in Alzheimer’s disease and nondemented elderly controls. Toxicol. Sci. 150:292–300 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tjalve H, Henriksson J. 1999. Uptake of metals in the brain via olfactory pathways. Neurotoxicology 20:181–95 [PubMed] [Google Scholar]
- 20.Barnham KJ, Bush AI. 2014. Biological metals and metal-targeting compounds in major neurodegenerative diseases. Chem. Soc. Rev. 43:6727–49 [DOI] [PubMed] [Google Scholar]
- 21.Jellinger KA. 2013. The relevance of metals in the pathophysiology of neurodegeneration, pathological considerations. Int. Rev. Neurobiol. 110:1–47 [DOI] [PubMed] [Google Scholar]
- 22.Dallman PR, Spirito RA. 1977. Brain iron in the rat: Extremely slow turnover in normal rats may explain long-lasting effects of early iron deficiency. J. Nutr. 107:1075–81 [DOI] [PubMed] [Google Scholar]
- 23.Zhou W, Shen B, Shen WQ, Chen H, Zheng YF, Fei JJ. 2020. Dysfunction of the glymphatic system might be related to iron deposition in the normal aging brain. Front. Aging Neurosci. 12:559603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chun H, Leung C, Wen SW, McDonald J, Shin HH. 2020. Maternal exposure to air pollution and risk of autism in children: a systematic review and meta-analysis. Environ. Pollut. 256:113307. [DOI] [PubMed] [Google Scholar]
- 25.Imbriani G, Panico A, Grassi T, Idolo A, Serio F, et al. 2021. Early-life exposure to environmental air pollution and autism spectrum disorder: a review of available evidence. Int. J. Environ. Res. Public Health 18:1204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Dutheil F, Comptour A, Morlon R, Mermillod M, Pereira B, et al. 2021. Autism spectrum disorder and air pollution: a systematic review and meta-analysis. Environ. Pollut. 278:116856. [DOI] [PubMed] [Google Scholar]
- 27.Donzelli G, Llopis-Gonzalez A, Llopis-Morales A, Cioni L, Morales-Suárez-Varela M. 2019. Particulate matter exposure and attention-deficit/hyperactivity disorder in children: a systematic review of epidemiological studies. Int. J. Environ. Res. Public Health 17:67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Shih P, Huang CC, Pan SC, Chiang TL, Guo YL. 2020. Hyperactivity disorder in children related to traffic-based air pollution during pregnancy. Environ. Res. 188:109588. [DOI] [PubMed] [Google Scholar]
- 29.Park J, Sohn JH, Cho SJ, Seo HY, Hwang IU, et al. 2020. Association between short-term air pollution exposure and attention-deficit/hyperactivity disorder-related hospital admissions among adolescents: a nationwide time-series study. Environ. Pollut. 266:115369. [DOI] [PubMed] [Google Scholar]
- 30.Yuchi W, Brauer M, Czekajlo A, Davies HW, Davis Z, et al. 2022. Neighborhood environmental exposures and incidence of attention deficit/hyperactivity disorder: a population-based cohort study. Environ. Int. 161:107120. [DOI] [PubMed] [Google Scholar]
- 31.Bernardini F, Trezzi R, Quartesan R, Attademo L. 2020. Air pollutants and daily hospital admissions for psychiatric care: a review. Psychiatr. Serv. 71:1270–76 [DOI] [PubMed] [Google Scholar]
- 32.Newbury JB, Stewart R, Fisher HL, Beevers S, Dajnak D, et al. 2021. Association between air pollution exposure and mental health service use among individuals with first presentations of psychotic and mood disorders: retrospective cohort study. Br. J. Psychiatry 219:678–85 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ji Y, Liu B, Song J, Pan R, Cheng J, et al. 2021. Particulate matter pollution associated with schizophrenia hospital re-admissions: a time-series study in a coastal Chinese city. Environ. Sci. Pollut. Res. Int. 28:58355–63 [DOI] [PubMed] [Google Scholar]
- 34.Antonsen S, Mok PLH, Webb RT, Mortensen PB, McGrath JJ, et al. 2020. Exposure to air pollution during childhood and risk of developing schizophrenia: a national cohort study. Lancet Planet. Health 4:e64–73 [DOI] [PubMed] [Google Scholar]
- 35.Cristaldi A, Fiore M, Oliveri Conti G, Pulvirenti E, Favara C, et al. 2021. Possible association between PM2.5 and neurodegenerative diseases: a systematic review. Environ. Res. 208:112581. [DOI] [PubMed] [Google Scholar]
- 36.Fu P, Yung KKL. 2020. Air pollution and Alzheimer’s disease: a systematic review and meta-analysis. J. Alzheimer’s Dis. 77:701–14 [DOI] [PubMed] [Google Scholar]
- 37.Tsai TL, Lin YT, Hwang BF, Nakayama SF, Tsai CH, et al. 2019. Fine particulate matter is a potential determinant of Alzheimer’s disease: a systemic review and meta-analysis. Environ. Res. 177:108638. [DOI] [PubMed] [Google Scholar]
- 38.Fu P, Guo X, Cheung FMH, Yung KKL. 2019. The association between PM2.5 exposure and neurological disorders: a systematic review and meta-analysis. Sci. Total Environ. 655:1240–48 [DOI] [PubMed] [Google Scholar]
- 39.Power MC, Adar SD, Yanosky JD, Weuve J. 2016. Exposure to air pollution as a potential contributor to cognitive function, cognitive decline, brain imaging, and dementia: a systematic review of epidemiologic research. Neurotoxicology 56:235–53 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Han C, Lu Y, Cheng H, Wang C, Chan P. 2020. The impact of long-term exposure to ambient air pollution and second-hand smoke on the onset of Parkinson disease: a review and meta-analysis. Public Health 179:100–10 [DOI] [PubMed] [Google Scholar]
- 41.Kasdagli MI, Katsouyanni K, Dimakopoulou K, Samoli E. 2019. Air pollution and Parkinson’s disease: a systematic review and meta-analysis up to 2018. Int. J. Hyg. Environ. Health 222:402–9 [DOI] [PubMed] [Google Scholar]
- 42.Jo S, Kim YJ, Park KW, Hwang YS, Lee SH, et al. 2021. Association of NO2 and other air pollution exposures with the risk of Parkinson disease. JAMA Neurol. 78:800–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Yu Z, Wei F, Zhang X, Wu M, Lin H, et al. 2021. Air pollution, surrounding green, road proximity and Parkinson’s disease: a prospective cohort study. Environ. Res. 197:111170. [DOI] [PubMed] [Google Scholar]
- 44.Nunez Y, Boehme AK, Weisskopf MG, Re DB, Navas-Acien A, et al. 2021. Fine particle exposure and clinical aggravation in neurodegenerative diseases in New York state. Environ. Health Perspect. 129:27003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Shi L, Wu X, Danesh Yazdi M, Braun D, Abu Awad Y, et al. 2020. Long-term effects of PM2.5 on neurological disorders in the American Medicare population: a longitudinal cohort study. Lancet Planet. Health 4:e557–65 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lotfi F, Mansourian M, Mirmoayyeb O, Najdaghi S, Shaygannejad V, Esmaeil N. 2022. Association of exposure to particulate matters and multiple sclerosis: a systematic review and meta-analysis. Neuroimmunomodulation 29:21–27 [DOI] [PubMed] [Google Scholar]
- 47.Tang C, Li QR, Mao YM, Xia YR, Guo HS, et al. 2021. Association between ambient air pollution and multiple sclerosis: a systemic review and meta-analysis. Environ. Sci. Pollut. Res. Int. 28:58142–53 [DOI] [PubMed] [Google Scholar]
- 48.Elgabsi M, Novack L, Yarza S, Elgabsi M, Shtein A, Ifergane G. 2021. An impact of air pollution on moderate to severe relapses among multiple sclerosis patients. Mult. Scler. Relat. Disord. 53:103043. [DOI] [PubMed] [Google Scholar]
- 49.Januel E, Dessimond B, Colette A, Annesi-Maesano I, Stankoff B. 2021. Fine particulate matter related to multiple sclerosis relapse in young patients. Front. Neurol. 12:651084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Tateo F, Grassivaro F, Ermani M, Puthenparampil M, Gallo P. 2019. PM2.5 levels strongly associate with multiple sclerosis prevalence in the province of Padua, Veneto region, North-East Italy. Mult. Scler. 25:1719–27 [DOI] [PubMed] [Google Scholar]
- 51.Avram M, Brandl F, Bäuml J, Sorg C. 2018. Cortico-thalamic hypo- and hyperconnectivity extend consistently to basal ganglia in schizophrenia. Neuropsychopharmacology 43:2239–48 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Zhu Y, Jiang X, Ji W. 2018. The mechanism of cortico-striato-thalamo-cortical neurocircuitry in response inhibition and emotional responding in attention deficit hyperactivity disorder with comorbid disruptive behavior disorder. Neurosci. Bull. 34:566–72 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Peterson BS, Bansal R, Sawardekar S, Nati C, Elgabalawy ER, et al. 2022. Prenatal exposure to air pollution is associated with altered brain structure, function, and metabolism in childhood. J. Child Psychol. Psychiatry. In press [DOI] [PubMed] [Google Scholar]
- 54.Worthington MA, Petkova E, Freudenreich O, Cather C, Holt D, et al. 2020. Air pollution and hippocampal atrophy in first episode schizophrenia. Schizophr. Res. 218:63–69 [DOI] [PubMed] [Google Scholar]
- 55.Lubczynska MJ, Muetzel RL, El Marroun H, Hoek G, Kooter IM, et al. 2021. Air pollution exposure during pregnancy and childhood and brain morphology in preadolescents. Environ. Res. 198:110446. [DOI] [PubMed] [Google Scholar]
- 56.Mortamais M, Pujol J, Martinez-Vilavella G, Fenoll R, Reynes C, et al. 2019. Effects of prenatal exposure to particulate matter air pollution on corpus callosum and behavioral problems in children. Environ. Res. 178:108734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Cserbik D, Chen JC, McConnell R, Berhane K, Sowell ER, et al. 2020. Fine particulate matter exposure during childhood relates to hemispheric-specific differences in brain structure. Environ. Int. 143:105933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Beckwith T, Cecil K, Altaye M, Severs R, Wolfe C, et al. 2020. Reduced gray matter volume and cortical thickness associated with traffic-related air pollution in a longitudinally studied pediatric cohort. PLOS ONE 15:e0228092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Pujol J, Martinez-Vilavella G, Macia D, Fenoll R, Alvarez-Pedrerol M, et al. 2016. Traffic pollution exposure is associated with altered brain connectivity in school children. NeuroImage 129:175–84 [DOI] [PubMed] [Google Scholar]
- 60.Pujol J, Fenoll R, Macia D, Martinez-Vilavella G, Alvarez-Pedrerol M, et al. 2016. Airborne copper exposure in school environments associated with poorer motor performance and altered basal ganglia. Brain Behav. 6:e00467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Chen C, Hayden KM, Kaufman JD, Espeland MA, Whitsel EA, et al. 2021. Adherence to a MIND-like dietary pattern, long-term exposure to fine particulate matter air pollution, and MRI-based measures of brain volume: the Women’s Health Initiative Memory Study-MRI. Environ. Health Perspect. 129:127008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Chen JC, Wang X, Wellenius GA, Serre ML, Driscoll I, et al. 2015. Ambient air pollution and neurotoxicity on brain structure: evidence from Women’s Health Initiative Memory Study. Ann. Neurol. 78:466–76 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Furlong MA, Alexander GE, Klimentidis YC, Raichlen DA. 2021. Association of air pollution and physical activity with brain volumes. Neurology 98:e416–26 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Hedges DW, Erickson LD, Kunzelman J, Brown BL, Gale SD. 2019. Association between exposure to air pollution and hippocampal volume in adults in the UK Biobank. Neurotoxicology 74:108–20 [DOI] [PubMed] [Google Scholar]
- 65.Falcón C, Gascon M, Molinuevo JL, Operto G, Cirach M, et al. 2021. Brain correlates of urban environmental exposures in cognitively unimpaired individuals at increased risk for Alzheimer’s disease: a study on Barcelona’s population. Alzheimer’s Dement. 13:e12205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Cho J, Noh Y, Kim SY, Sohn J, Noh J, et al. 2020. Long-term ambient air pollution exposures and brain imaging markers in Korean adults: the Environmental Pollution-Induced Neurological Effects (EPINEF) study. Environ. Health Perspect. 128:117006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Casanova R, Wang X, Reyes J, Akita Y, Serre ML, et al. 2016. A voxel-based morphometry study reveals local brain structural alterations associated with ambient fine particles in older women. Front. Hum. Neurosci. 10:495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Younan D, Petkus AJ, Widaman KF, Wang X, Casanova R, et al. 2020. Particulate matter and episodic memory decline mediated by early neuroanatomic biomarkers of Alzheimer’s disease. Brain 143:289–302 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Wu J, Ning Y, Gao Y, Shan R, Wang B, et al. 2021. Association between ambient air pollution and MRI-defined brain infarcts in health examinations in China. Int. J. Environ. Res. Public Health 18:4325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Wilker EH, Preis SR, Beiser AS, Wolf PA, Au R, et al. 2015. Long-term exposure to fine particulate matter, residential proximity to major roads and measures of brain structure. Stroke 46:1161–66 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Klocke C, Allen JL, Sobolewski M, Blum JL, Zelikoff JT, Cory-Slechta DA. 2017. Exposure to fine and ultrafine particulate matter during gestation alters postnatal oligodendrocyte maturation, proliferation capacity, and myelination. Neurotoxicology 65:196–206 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Klocke C, Allen JL, Sobolewski M, Mayer-Proschel M, Blum JL, et al. 2017. Neuropathological consequences of gestational exposure to concentrated ambient fine and ultrafine particles in the mouse. Toxicol. Sci. 156:492–508 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Allen JL, Conrad K, Oberdorster G, Johnston CJ, Sleezer B, Cory-Slechta DA. 2013. Developmental exposure to concentrated ambient particles and preference for immediate reward in mice. Environ. Health Perspect. 121:32–38 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Allen JL, Klocke C, Morris-Schaffer K, Conrad K, Sobolewski M, Cory-Slechta DA. 2017. Cognitive effects of air pollution exposures and potential mechanistic underpinnings. Curr. Environ. Health Rep. 4:180–91 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Allen JL, Liu X, Pelkowski S, Palmer B, Conrad K, et al. 2014. Early postnatal exposure to ultrafine particulate matter air pollution: persistent ventriculomegaly, neurochemical disruption, and glial activation preferentially in male mice. Environ. Health Perspect. 122:939–45 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Allen JL, Liu X, Weston D, Prince L, Oberdorster G, et al. 2014. Developmental exposure to concentrated ambient ultrafine particulate matter air pollution in mice results in persistent and sex-dependent behavioral neurotoxicity and glial activation. Toxicol. Sci. 140:160–78 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Allen JL, Oberdorster G, Morris-Schaffer K, Wong C, Klocke C, et al. 2017. Developmental neurotoxicity of inhaled ambient ultrafine particle air pollution: parallels with neuropathological and behavioral features of autism and other neurodevelopmental disorders. Neurotoxicology 59:140–54 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Clancy B, Finlay BL, Darlington RB, Anand KJ. 2007. Extrapolating brain development from experimental species to humans. Neurotoxicology 28:931–37 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Badaracco ME, Siri MV, Pasquini JM. 2010. Oligodendrogenesis: the role of iron. Biofactors 36:98–102 [DOI] [PubMed] [Google Scholar]
- 80.Klocke C, Sherina V, Graham UM, Gunderson J, Allen JL, et al. 2018. Enhanced cerebellar myelination with concomitant iron elevation and ultrastructural irregularities following prenatal exposure to ambient particulate matter in the mouse. Inhal. Toxicol. 30:381–96 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Bandeira F, Lent R, Herculano-Houzel S. 2009. Changing numbers of neuronal and non-neuronal cells underlie postnatal brain growth in the rat. PNAS 106:14108–13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Sobolewski M, Anderson T, Conrad K, Marvin E, Klocke C, et al. 2018. Developmental exposures to ultrafine particle air pollution reduces early testosterone levels and adult male social novelty preference: risk for children’s sex-biased neurobehavioral disorders. NeuroToxicology 68:203–11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Di Domenico M, Benevenuto SGM, Tomasini PP, Yariwake VY, de Oliveira Alves N, et al. 2020. Concentrated ambient fine particulate matter (PM2.5) exposure induce brain damage in pre and postnatal exposed mice. Neurotoxicology 79:127–41 [DOI] [PubMed] [Google Scholar]
- 84.Woodward NC, Haghani A, Johnson RG, Hsu TM, Saffari A, et al. 2018. Prenatal and early life exposure to air pollution induced hippocampal vascular leakage and impaired neurogenesis in association with behavioral deficits. Transl. Psychiatry 8:261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Nephew BC, Nemeth A, Hudda N, Beamer G, Mann P, et al. 2020. Traffic-related particulate matter affects behavior, inflammation, and neural integrity in a developmental rodent model. Environ. Res. 183:109242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Patten KT, González EA, Valenzuela A, Berg E, Wallis C, et al. 2020. Effects of early life exposure to traffic-related air pollution on brain development in juvenile Sprague-Dawley rats. Transl. Psychiatry 10:166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Davis DA, Bortolato M, Godar SC, Sander TK, Iwata N, et al. 2013. Prenatal exposure to urban air nanoparticles in mice causes altered neuronal differentiation and depression-like responses. PLOS ONE 8:e64128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Cole TB, Chang YC, Dao K, Daza R, Hevner R, Costa LG. 2020. Developmental exposure to diesel exhaust upregulates transcription factor expression, decreases hippocampal neurogenesis, and alters cortical lamina organization: relevance to neurodevelopmental disorders. J. Neurodev. Disord. 12:41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Chang YC, Daza R, Hevner R, Costa LG, Cole TB. 2019. Prenatal and early life diesel exhaust exposure disrupts cortical lamina organization: evidence for a reelin-related pathogenic pathway induced by interleukin-6. Brain Behav. Immun. 78:105–15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Morris-Schaffer K, Merrill AK, Wong C, Jew K, Sobolewski M, Cory-Slechta DA. 2019. Limited developmental neurotoxicity from neonatal inhalation exposure to diesel exhaust particles in C57BL/6 mice. Part. Fibre Toxicol. 16:1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Ehsanifar M, Jafari AJ, Nikzad H, Zavareh MS, Atlasi MA, et al. 2019. Prenatal exposure to diesel exhaust particles causes anxiety, spatial memory disorders with alters expression of hippocampal proinflammatory cytokines and NMDA receptor subunits in adult male mice offspring. Ecotoxicol. Environ. Saf. 176:34–41 [DOI] [PubMed] [Google Scholar]
- 92.Connor M, Lamorie-Foote K, Liu Q, Shkirkova K, Baertsch H, et al. 2021. Nanoparticulate matter exposure results in white matter damage and an inflammatory microglial response in an experimental murine model. PLOS ONE 16:e0253766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Patten KT, Valenzuela AE, Wallis C, Berg EL, Silverman JL, et al. 2021. The effects of chronic exposure to ambient traffic-related air pollution on Alzheimer’s disease phenotypes in wildtype and genetically predisposed male and female rats. Environ. Health Perspect. 129:57005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Calabro V, Garces M, Caceres L, Magnani ND, Marchini T, et al. 2021. Urban air pollution induces alterations in redox metabolism and mitochondrial dysfunction in mice brain cortex. Arch. Biochem. Biophys. 704:108875. [DOI] [PubMed] [Google Scholar]
- 95.Bernardi RB, Zanchi ACT, Damaceno-Rodrigues NR, Veras MM, Saldiva PHN, et al. 2021. The impact of chronic exposure to air pollution over oxidative stress parameters and brain histology. Environ. Sci. Pollut. Res. Int. 28:47407–17 [DOI] [PubMed] [Google Scholar]
- 96.Sahu B, Mackos AR, Floden AM, Wold LE, Combs CK. 2021. Particulate matter exposure exacerbates amyloid-β plaque deposition and gliosis in APP/PS1 mice. J. Alzheimer’s Dis. 80:761–74 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Shou Y, Zhu X, Zhu D, Yin H, Shi Y, et al. 2020. Ambient PM2.5 chronic exposure leads to cognitive decline in mice: from pulmonary to neuronal inflammation. Toxicol. Lett. 331:208–17 [DOI] [PubMed] [Google Scholar]
- 98.Yan W, Ku T, Yue H, Li G, Sang N. 2016. NO2 inhalation causes tauopathy by disturbing the insulin signaling pathway. Chemosphere 165:248–56 [DOI] [PubMed] [Google Scholar]
- 99.Ehsanifar M, Montazeri Z, Taheri MA, Rafati M, Behjati M, Karimian M. 2021. Hippocampal inflammation and oxidative stress following exposure to diesel exhaust nanoparticles in male and female mice. Neurochem. Int. 145:104989. [DOI] [PubMed] [Google Scholar]
- 100.Chen Z, Chen F, Fang Z, Zhao H, Zhan C, et al. 2021. Glial activation and inflammation in the NTS in a rat model after exposure to diesel exhaust particles. Environ. Toxicol. Pharmacol. 83:103584. [DOI] [PubMed] [Google Scholar]
- 101.Hullmann M, Albrecht C, van Berlo D, Gerlofs-Nijland ME, Wahle T, et al. 2017. Diesel engine exhaust accelerates plaque formation in a mouse model of Alzheimer’s disease. Part. Fibre Toxicol. 14:35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Woodward NC, Pakbin P, Saffari A, Shirmohammadi F, Haghani A, et al. 2017. Traffic-related air pollution impact on mouse brain accelerates myelin and neuritic aging changes with specificity for CA1 neurons. Neurobiol. Aging 53:48–58 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Padurariu M, Ciobica A, Mavroudis I, Fotiou D, Baloyannis S. 2012. Hippocampal neuronal loss in the CA1 and CA3 areas of Alzheimer’s disease patients. Psychiatr. Danub. 24:152–58 [PubMed] [Google Scholar]
- 104.Coburn JL, Cole TB, Dao KT, Costa LG. 2018. Acute exposure to diesel exhaust impairs adult neurogenesis in mice: prominence in males and protective effect of pioglitazone. Arch. Toxicol. 92:1815–29 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Santos S, Ferreira H, Martins J, Gonçalves J, Castelo-Branco M. 2022. Male sex bias in early and late onset neurodevelopmental disorders: shared aspects and differences in autism spectrum disorder, attention deficit/hyperactivity disorder, and schizophrenia. Neurosci. Biobehav. Rev. 135:104577. [DOI] [PubMed] [Google Scholar]
- 106.Ransohoff RM. 2016. How neuroinflammation contributes to neurodegeneration. Science 353:777–83 [DOI] [PubMed] [Google Scholar]
- 107.Arias-Pérez RD, Taborda NA, Gómez DM, Narvaez JF, Porras J, Hernandez JC. 2020. Inflammatory effects of particulate matter air pollution. Environ. Sci. Pollut. Res. Int. 27:42390–404 [DOI] [PubMed] [Google Scholar]
- 108.Hahad O, Lelieveld J, Birklein F, Lieb K, Daiber A, Münzel T. 2020. Ambient air pollution increases the risk of cerebrovascular and neuropsychiatric disorders through induction of inflammation and oxidative stress. Int. J. Mol. Sci. 21:4306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Costa LG, Cole TB, Dao K, Chang YC, Coburn J, Garrick JM. 2020. Effects of air pollution on the nervous system and its possible role in neurodevelopmental and neurodegenerative disorders. Pharmacol. Ther. 210:107523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Li H, Uittenbogaard M, Hao L, Chiaramello A. 2021. Clinical insights into mitochondrial neurodevelopmental and neurodegenerative disorders: their biosignatures from mass spectrometry-based metabolomics. Metabolites 11:233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Daiber A, Kuntic M, Hahad O, Delogu LG, Rohrbach S, et al. 2020. Effects of air pollution particles (ultrafine and fine particulate matter) on mitochondrial function and oxidative stress: implications for cardiovascular and neurodegenerative diseases. Arch. Biochem. Biophys. 696:108662. [DOI] [PubMed] [Google Scholar]
- 112.González-Maciel A, Reynoso-Robles R, Torres-Jardón R, Mukherjee PS, Calderón-Garcidueñas L. 2017. Combustion-derived nanoparticles in key brain target cells and organelles in young urbanites: culprit hidden in plain sight in Alzheimer’s disease development. J. Alzheimer’s Dis. 59:189–208 [DOI] [PubMed] [Google Scholar]
- 113.Moretto E, Murru L, Martano G, Sassone J, Passafaro M. 2018. Glutamatergic synapses in neurodevelopmental disorders. Prog. Neuropsychopharmacol. Biol. Psychiatry 84:328–42 [DOI] [PubMed] [Google Scholar]
- 114.Hu W, MacDonald ML, Elswick DE, Sweet RA. 2015. The glutamate hypothesis of schizophrenia: evidence from human brain tissue studies. Ann. N. Y. Acad. Sci. 1338:38–57 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Onaolapo AY, Onaolapo OJ. 2021. Peripheral and central glutamate dyshomeostasis in neurodegenerative disorders. Curr. Neuropharmacol. 19:1069–89 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Allen JL, Liu X, Weston D, Prince L, Oberdörster G, et al. 2014. Developmental exposure to concentrated ambient ultrafine particulate matter air pollution in mice results in persistent and sex-dependent behavioral neurotoxicity and glial activation. Toxicol. Sci. 140:160–78 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Davis DA, Akopian G, Walsh JP, Sioutas C, Morgan TE, Finch CE. 2013. Urban air pollutants reduce synaptic function of CA1 neurons via an NMDA/NO˙ pathway in vitro. J. Neurochem. 127:509–19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Haghani A, Johnson R, Safi N, Zhang H, Thorwald M, et al. 2020. Toxicity of urban air pollution particulate matter in developing and adult mouse brain: comparison of total and filter-eluted nanoparticles. Environ. Int. 136:105510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Cainelli E, Arrigoni F, Vedovelli L. 2020. White matter injury and neurodevelopmental disabilities: a cross-disease (dis)connection. Prog. Neurobiol. 193:101845. [DOI] [PubMed] [Google Scholar]
- 120.Bankston AN, Mandler MD, Feng Y. 2013. Oligodendroglia and neurotrophic factors in neurodegeneration. Neurosci. Bull. 29:216–28 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Thorup E, Jensen LN, Bak GS, Ekelund CK, Greisen G, et al. 2019. Neurodevelopmental disorder in children believed to have isolated mild ventriculomegaly prenatally. Ultrasound Obstet. Gynecol. 54:182–89 [DOI] [PubMed] [Google Scholar]
- 122.Marques F, Sousa JC, Brito MA, Pahnke J, Santos C, et al. 2017. The choroid plexus in health and in disease: dialogues into and out of the brain. Neurobiol. Dis. 107:32–40 [DOI] [PubMed] [Google Scholar]
- 123.Cory-Slechta DA, Sobolewski M, Oberdörster G. 2020. Air pollution-related brain metal dyshomeostasis as a potential risk factor for neurodevelopmental disorders and neurodegenerative diseases. Atmosphere 11:1098 [Google Scholar]
- 124.Mezzaroba L, Alfieri DF, Colado Simão AN, Vissoci Reiche EM. 2019. The role of zinc, copper, manganese and iron in neurodegenerative diseases. Neurotoxicology 74:230–41 [DOI] [PubMed] [Google Scholar]
- 125.Thirupathi A, Chang YZ. 2019. Brain iron metabolism and CNS diseases. In Advances in Experimental Medicine and Biology, Vol. 1173, ed. Chang YZ. Singapore: Springer. 10.1007/978-981-13-9589-5_1 [DOI] [PubMed] [Google Scholar]
- 126.Masaldan S, Bush AI, Devos D, Rolland AS, Moreau C. 2019. Striking while the iron is hot: iron metabolism and ferroptosis in neurodegeneration. Free Radic Biol. Med. 133:221–33 [DOI] [PubMed] [Google Scholar]
- 127.Braak H, Braak E. 1995. Staging of Alzheimer’s disease-related neurofibrillary changes. Neurobiol. Aging 16:271–78 [DOI] [PubMed] [Google Scholar]
- 128.Braak H, Del Tredici K, Rub U, de Vos RA, Jansen Steur EN, Braak E. 2003. Staging of brain pathology related to sporadic Parkinson’s disease. Neurobiol. Aging 24:197–211 [DOI] [PubMed] [Google Scholar]
- 129.Hawkes CH, Del Tredici K, Braak H. 2007. Parkinson’s disease: a dual-hit hypothesis. Neuropathol. Appl. Neurobiol. 33:599–614 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Ajmani GS, Suh HH, Pinto JM. 2016. Effects of ambient air pollution exposure on olfaction: a review. Environ. Health Perspect. 124:1683–93 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Nakane H 2012. Translocation of particles deposited in the respiratory system: a systematic review and statistical analysis. Environ Health Prev. Med. 17:263–74 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Hasegawa Y, Namkung H, Smith A, Sakamoto S, Zhu X, et al. 2021. Causal impact of local inflammation in the nasal cavity on higher brain function and cognition. Neurosci. Res. 172:110–15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Hasegawa-Ishii S, Shimada A, Imamura F. 2019. Neuroplastic changes in the olfactory bulb associated with nasal inflammation in mice. J. Allergy Clin. Immunol. 143:978–89.e3 [DOI] [PubMed] [Google Scholar]
- 134.Squitti R, Ventriglia M, Simonelli I, Bonvicini C, Costa A, et al. 2021. Copper imbalance in Alzheimer’s disease: meta-analysis of serum, plasma, and brain specimens, and replication study evaluating ATP7B gene variants. Biomolecules 11:960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Isaev NK, Stelmashook EV, Genrikhs EE. 2020. Role of zinc and copper ions in the pathogenetic mechanisms of traumatic brain injury and Alzheimer’s disease. Rev. Neurosci. 31:233–43 [DOI] [PubMed] [Google Scholar]
- 136.Baccarelli AA, Zheng Y, Zhang X, Chang D, Liu L, et al. 2014. Air pollution exposure and lung function in highly exposed subjects in Beijing, China: a repeated-measure study. Part. Fibre Toxicol. 11:51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Cakmak S, Dales R, Kauri LM, Mahmud M, Van Ryswyk K, et al. 2014. Metal composition of fine particulate air pollution and acute changes in cardiorespiratory physiology. Environ. Pollut. 189:208–14 [DOI] [PubMed] [Google Scholar]
- 138.Cory-Slechta DA, Sobolewski M, Marvin E, Conrad K, Merrill A, et al. 2019. The impact of inhaled ambient ultrafine particulate matter on developing brain: potential importance of elemental contaminants. Toxicol. Pathol. 47:976–92 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Green KN, LaFerla FM. 2008. Linking calcium to Aβ and Alzheimer’s disease. Neuron 59:190–94 [DOI] [PubMed] [Google Scholar]
- 140.Scassellati C, Bonvicini C, Benussi L, Ghidoni R, Squitti R. 2020. Neurodevelopmental disorders: metallomics studies for the identification of potential biomarkers associated to diagnosis and treatment. J. Trace Elem. Med. Biol. 60:126499. [DOI] [PubMed] [Google Scholar]
- 141.Lakshmi Priya MD, Geetha A. 2011. Level of trace elements (copper, zinc, magnesium and selenium) and toxic elements (lead and mercury) in the hair and nail of children with autism. Biol. Trace Elem. Res. 142:148–58 [DOI] [PubMed] [Google Scholar]
- 142.Skalny AV, Simashkova NV, Skalnaya AA, Klyushnik TP, Bjørklund G, et al. 2017. Assessment of gender and age effects on serum and hair trace element levels in children with autism spectrum disorder. Metab. Brain Dis. 32:1675–84 [DOI] [PubMed] [Google Scholar]
- 143.Mold M, Umar D, King A, Exley C. 2018. Aluminium in brain tissue in autism. J. Trace Elem. Med. Biol. 46:76–82 [DOI] [PubMed] [Google Scholar]
- 144.Exley C, Clarkson E. 2020. Aluminium in human brain tissue from donors without neurodegenerative disease: a comparison with Alzheimer’s disease, multiple sclerosis and autism. Sci. Rep. 10:7770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Skalny AV, Simashkova NV, Skalnaya AA, Klyushnik TP, Zhegalova IV, et al. 2018. Trace element levels are associated with neuroinflammatory markers in children with autistic spectrum disorder. J. Trace Elem. Med. Biol. 50:622–28 [DOI] [PubMed] [Google Scholar]
- 146.Cai L, Chen T, Yang J, Zhou K, Yan X, et al. 2015. Serum trace element differences between Schizophrenia patients and controls in the Han Chinese population. Sci. Rep. 5:15013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Joe P, Petrilli M, Malaspina D, Weissman J. 2018. Zinc in schizophrenia: a meta-analysis. Gen. Hosp. Psychiatry 53:19–24 [DOI] [PubMed] [Google Scholar]
- 148.Scassellati C, Bonvicini C, Faraone SV, Gennarelli M. 2012. Biomarkers and attention-deficit/hyperactivity disorder: a systematic review and meta-analyses. J. Am. Acad. Child Adolesc. Psychiatry 51:1003–19.e20 [DOI] [PubMed] [Google Scholar]
- 149.Degremont A, Jain R, Philippou E, Latunde-Dada GO. 2021. Brain iron concentrations in the pathophysiology of children with attention deficit/hyperactivity disorder: a systematic review. Nutr. Rev. 79:615–26 [DOI] [PubMed] [Google Scholar]
- 150.de Jesus AL, Rahman MM, Mazaheri M, Thompson H, Knibbs LD, et al. 2019. Ultrafine particles and PM2.5 in the air of cities around the world: Are they representative of each other? Environ. Int. 129:118–35 [DOI] [PubMed] [Google Scholar]
- 151.Cox A, Andreozzi P, Dal Magro R, Fiordaliso F, Corbelli A, et al. 2018. Evolution of nanoparticle protein corona across the blood–brain barrier. ACS Nano 12:7292–300 [DOI] [PubMed] [Google Scholar]
- 152.Peterson BS, Rauh VA, Bansal R, Hao X, Toth Z, et al. 2015. Effects of prenatal exposure to air pollutants (polycyclic aromatic hydrocarbons) on the development of brain white matter, cognition, and behavior in later childhood. JAMA Psychiatry 72:531–40 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Barraza F, Jorquera H, Heyer J, Palma W, Edwards AM, et al. 2016. Short-term dynamics of indoor and outdoor endotoxin exposure: case of Santiago, Chile, 2012. Environ. Int. 92–93:97–105 [DOI] [PubMed] [Google Scholar]
- 154.Cardoso FL, Herz J, Fernandes A, Rocha J, Sepodes B, et al. 2015. Systemic inflammation in early neonatal mice induces transient and lasting neurodegenerative effects. J. Neuroinflamm. 12:82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Jung CR, Lin YT, Hwang BF. 2013. Air pollution and newly diagnostic autism spectrum disorders: a population-based cohort study in Taiwan. PLOS ONE 8:e75510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Lin CC, Yang SK, Lin KC, Ho WC, Hsieh WS, et al. 2014. Multilevel analysis of air pollution and early childhood neurobehavioral development. Int. J. Environ. Res. Public Health 11:6827–41 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


