Abstract

The ability of modern agriculture to meet future food demand imposed by accelerating growth of the world’s population is a major challenge, and fertilizers play a key role by replacing nutrients in agricultural soil. Given the need for fertilizers, their cost in nonrenewable resources and energy, and the consequences of the greenhouse gas emissions required to make them, people have begun to explore ways to make fertilizer manufacturing and use more sustainable. Using data from the CAS Content Collection, this review examines and analyzes the academic and patent literature on sustainable fertilizers from 2001 to 2021. The breakdown of journal and patent literature publication over time on this topic, country or region of publications, the substances included in published research, among other things allow us to understand the general progress in the field as well as the classes of materials and concepts driving innovation. We hope that this bibliometric analysis and literary review will assist researchers in relevant industries to discover and implement ways to supplement conventional fertilizers and nutrient sources while improving the efficiency and sustainability of waste management and ammonia production.
Keywords: Sustainable fertilizers, nutrient recovery from waste and wastewater, green ammonia, biorefineries, struvite
Introduction
The global human population exceeded 8 billion on November 15, 2022. According to the World Population Prospects 2022, it will grow to 8.5 billion in 2030, reaching 9.7 billion in 2050.1 Feeding this population will require a sustainable agricultural system which provides food economically while using water, energy, and nutrients efficiently, causing a minimum of harm to the environment, and using as few nonrenewable resources as possible.2
Fertilizers replace nutrients that are depleted from the soil when plants are grown. The most prevalent nutrients for fertilizers are nitrogen, phosphorus, and potassium; calcium, magnesium, sulfur, boron, copper, manganese, molybdenum, and zinc are added less often or in lower amounts as micronutrients.3−6 Nitrogen can be supplied alone as anhydrous ammonia, urea, urea-ammonium nitrate, etc., or in combination with phosphorus.. Phosphorus is commonly supplied as phosphates, including monoammonium phosphate or more complex phosphates such as struvite or the calcium phosphate hydroxyapatite (Ca5(OH)(PO4)3). Potassium, when needed alone, is used as potassium sulfate or potassium chloride. The forms in which the nutrients are supplied and when they are provided determine how effectively the nutrients are used by the crops.
A variety of different sources of plant nutrients are available for use in the form of organic fertilizers (manure, alfalfa meal, blood meal, fish meal, wood ashes, and waste from water or sewage treatment)7 or synthetic fertilizers. In 2016, it was estimated that nearly half of the world population was supported using synthetic fertilizers in the previous year, indicating that both synthetic and nonsynthetic fertilizers are necessary to adequately feed the world’s population.8 For example, in 2020, 147 million tons of ammonia, 219 million tons of phosphate, and 44 million tons of potash were industrially produced or mined.9−11 The demand for ammonia, phosphate, and potash in 2020 were also predicted to be 119 million tons, 46 million tons, and 37 million tons, respectively.12 Currently no shortages in produced supplies and recoverable reserves are predicted. However, industrial production and mining have significant energy, transport, and environmental costs and are subject to geopolitical and economic conditions. These factors influence changes in waste management and fertilizer manufacture to make fertilizers more sustainable.
Risks and Costs of Fertilizer Production
Fertilizer manufacture and transport require energy and resources and contribute significantly to global CO2 emissions.13 The sustainability of fertilizer manufacture in part depends on reducing its energy and environmental costs.
Nitrogen in synthetic fertilizers is primarily derived from the Haber-Bosch process, in which nitrogen from the air is reduced with hydrogen to yield ammonia in the presence of an iron-based catalyst at high temperature.14−17 The heat and pressure needed for the Haber-Bosch process and the hydrogen used in its manufacture use 1.8% of total world energy generation and is responsible for 1.8% of global CO2 emissions.18−20 The phosphorus in fertilizers comes from mined phosphate rock. Phosphate deposits are limited (with supplies estimated to last 40–400 years)21 and concentrated mainly in six countries (Morocco, Western Sahara, Iraq, China, Algeria, and Syria).22,23 According to the United States Geological Service: “No substitutes exist for potassium as an essential plant nutrient and as an essential nutritional requirement for animals and humans.” Potassium supplies are nominally sufficient, most of the potassium used in fertilizers comes from Belarus, Canada, and Russia, making its supply potentially dependent on international relations and sanctions.24,25
Defined as “fertilizers derived from animal products and plant residues containing sufficient nitrogen”,26 organic fertilizers have a different set of concerns. In particular, the bulk of organic fertilizers makes it impractical and costly to transport them over significant distances, requiring them to be made locally and limiting the scalability of manufacture. Fertilizers obtained from water and sewage treatment wastes require testing for and management of pharmaceutical and heavy metal contamination and inactivation or removal of pathogens.27,28
Possible Pathways to Sustainable Fertilizers
Given the need for fertilizer, its costs in nonrenewable resources and energy, and the consequences of the CO2 emissions required to make it, people have begun to explore ways to make fertilizer manufacture and use more sustainable. The US EPA defined sustainability as “[the ability to] create and maintain the conditions under which humans and nature can exist in productive harmony to support present and future generations”.29 An alternative way to look at sustainable fertilizers and soil amendments are those which reduce use of energy and resource intensive ingredients; reuse and recycle waste materials; and recover nutrients from wastes and wastewaters. These ideas constitute guiding principles that can be used for sustainable agricultural production of fertilizers, as well as management of wastes. Here recovery is used for processes which directly generate useful waste, while recycling processes convert wastes to useful products.30 Reducing the resource, energy, and environmental cost of fertilizer manufacturing could improve fertilizer sustainability. Phosphorus and potassium deposits are concentrated geographically and their mining and manufacture requires limited resources. Manufacture of nitrogen fertilizers can be made more sustainable by replacing the use of natural gas with renewable energy for hydrogen generation and developing more efficient catalysts to reduce the temperatures and pressures needed to generate ammonia.31 Methods to generate ammonia electrochemically or photochemically would facilitate the use of renewable and non-CO2-generating energy to make nitrogen fertilizers. More sustainable nutrient recovery processes for fertilizers and more sustainable formulations of fertilizers, such as the use of nanomaterials, are also being studied. For example, a “greener” fertilizer production that uses less harsh polar organic acids, such as citric acid, in addition to mechanical size reduction of phosphate rock in wet slurries to produce a sustained-release nano- formulation was recently patented.32 The described nanoformulation provided performance in corn equivalent to commercial fertilizers but with a 50% reduction in the amount used. The development and use of nanofertilizers is another potential strategy that can improve the efficiency of nutrient use.33 Nanomaterials are applied as biofertilizers, major element fertilizers, and nutrient delivery systems that can encapsulate fertilizers and protect them from leaching.34 A combination of biotechnology and nanotechnology has developed smart fertilizers, fertilizers with controlled nutrient release through degradable delivery systems that lessen the negative impact on the environment.35 Bioformulation fertilizers may contain micro- or nanoencapsulated microorganisms beneficial for plant nutrient fixation and mobilization.36 Use of these types of fertilizers is a promising step toward sustainable agriculture and provides new mechanisms of action and nanoenabled formulations of agrochemicals for more efficient use of resources.37
Increasing the effectiveness of fertilizer application makes them more sustainable by potentially increasing nutrient bioavailability, reducing labor costs or ingredient amounts required, or reducing wasted material or pollution. Fertilizer effectiveness depends strongly on the timing, method, and form of application,6 while efficient ingredient use can be improved through formulation. For example, controlled- or sustained-release fertilizers provide plant nutrients in a formulation that delays or extends their availability. They may be formulated via reduction in size of particles, the addition of coatings, or by altering fertilizer chemical sources for properties such as solubility.38−41 Controlled-release formulation promotes sustainability via the reduction of required chemicals, lowering the amount of fertilizer application, and reducing nutrient loss through soil runoff or volatilization. Ostara Nutrient Recovery Technologies, Inc. have developed “intermediate-release” fertilizer compositions that may include struvite (considered to be the slow-release portion), and schertelite (an intermediate-release portion) and may also include fast-release sources like monoammonium phosphate, diammonium phosphate, and/or superphosphates.42 Inclusion of recovered nutrients like struvite in combination with alternative mineral phosphorus sources that improve timing of release, also demonstrates that recovered nutrients can be repurposed in formulations with better use efficiency.
The use of additives that reduce microbial degradation of fertilizer would also reduce fertilizer waste. Nitrogen is metabolized by soil bacteria to nitrate, useless for plant nutrition, while urea (an alternative to ammonia and ammonium nitrate) is processed by urease enzymes to CO2 and gaseous ammonia, which unable to function as a nutrient.43 Inhibiting the conversion of nitrogen to nitrate and of urea to ammonia gas via the addition of urease and nitrification inhibitors to fertilizer formulations thus reduces the amount of excess nitrogen needed in fertilizers.44 The addition of living microorganisms to fertilizers which colonize the soil or plants (biofertilizers) can increase the bioavailability and supply of nutrients to crops, also providing a means to reduce the amount of fertilizer applied and increase the efficiency of nutrient use.45,46
Reduction in nutrient use would also reduce the amount of fertilizer contamination in surface water, improving water quality and decreasing eutrophication.47−53 Application of biostimulants such as Ficosagro (microbial complex with seaweed extracts) and Cystium-k (pure extract of the seaweed Macrocystis Pyrifera) to the soil and plants respectively can increase crop productivity by 6 to 15% (depending on the type of crop) while significantly reducing the use of mineral fertilizers. This leads to a reduction in the nitrogen and phosphorus load that leaches from the crops into the seas and oceans.54
Other sources of organic fertilizers may be applicable as well. The use of waste as fertilizer or fertilizer components can both improve fertilizer sustainability and decrease pollution. The waste from sewage and wastewater treatment can be used as fertilizer directly, but wastewater processing to reduce the levels of nitrogen and phosphorus is often necessary. If the phosphorus and nitrogen from wastewater processing could be effectively converted to phosphates and nitrogen useful for fertilizer, then eutrophication from wastewater pollution would be reduced while making more sustainable fertilizers. For example, struvite is a product of water treatment that may be used as a fertilizer.55 Improvements in the processing methods for sewage treatment could also make those wastes more useful fertilizers by reducing the content of heavy metals, pathogens, and undesired contaminants, reducing the amount of synthetic fertilizer needed.56,57
The development of biorefineries may also be able to improve agricultural sustainability by using manure and agricultural wastes as sustainable fertilizers for valuable products while also generating components for sustainable fertilizers.58,59 Biorefineries are designed to use biomass, including crops raised for such, as refinery feedstock to replace nonrenewable sources for plastics, fuels, or other important materials. To sustain the crop yields necessary to replace other feedstocks, large amounts of fertilizer are needed. Growing crops and raising livestock on one farm allows livestock manure to be used as fertilizer. The need to transport manure (which is bulky and expensive to transport significant distances) is elided and applications of synthetic fertilizer can be reduced. The waste from the biorefined crops and crop wastes can be used as food for livestock or as additional fertilizer; in some cases, it may also be used for biogas or as fuel to provide energy for the farm. Biorefineries thus may improve the sustainability of fertilizer production and the circularity of the economy through reuse and recycling of wastes for new products including nutrients for fertilizers.
Academic and patent literature from 2001 to 2021 on sustainable fertilizers were retrieved from the CAS Content Collection in an effort to understand the general progress of the field as well as the classes of materials and concepts driving innovation. Data from 2022 was incomplete and so was excluded from figures, though 2022 publications and patents were examined as references for parts of this report. Publication volumes over time, country or region, by research topic, and by the substances included in published research were analyzed to find trends and insights. First, the use of biogenerated and organic wastes in production of organic or organomineral fertilizers and soil amendments, with particular focus on trending innovations in the production and use of biochar, is examined. Trends in processes for recovery of useful nutrients from wastewater, the integration of wastes for nutrient recovery, and recycling processes in biorefineries are reviewed. Finally, trends in catalytic nitrogen fixation to reduce fossil fuel consumption and carbon dioxide emissions of fertilizer production are discussed. A bibliometric analysis of journal and patent publications provides insight into how fertilizers may be produced and formulated more sustainably and how wastes may be managed more effectively to provide sustainable organic and inorganic nutrients for agriculture and industry.
General Publication Trends on Sustainable Fertilizers: Reuse, Recycling, and Recovery
To get a better overall scope of current publication trends on fertilizer recycling, recovery, and sustainable fertilizer topics, a more general search query was used (See SI Methods: Search Query 1). This query retrieved more than 120,000 patents and 125,000 journal publications over the 2001–2021 period and was used to generate Figures 1–3, 21, S1–S4, and S8, as well as Table 1. Patents saw an almost exponential increase until 2017, they then rapidly decreased (Figures 1 and S1), while journal publications continue increasing and have yet to reach a plateau (Figure S1). Further analysis demonstrated that the patent publication trend of all countries (Figures 1 and S1) is highly influenced by the patent publications of China (Figure S2). This steep publication peak of patents is not seen in the patent publication trends for the rest of the combined countries when excluding China (Figure S3). Instead, journal publications were the focus compared to patents for the rest of the world. China’s journal publications, on the other hand, lag compared to its patent publications (Figure S2) from 2001 to 2021.
Figure 1.
Journal and patent publication numbers for the years 2001–2021 on the topic of fertilizers, sustainability, recycling, and recovery.
Figure 3.
Total number of journals published by Top 15 countries for the period of 2001–2021 on the topic of fertilizers, sustainability, recycling, and recovery.
Figure 21.
Publication numbers on biorefinery-associated concepts from 2001 to 2021 on the topic of fertilizers, sustainability, recycling, and recovery.
Table 1. Substances Found Associated with the Topic of Fertilizers, Sustainability, Recycling, and Recovery.
| Substance Name | REG Number | No. of Publications |
|---|---|---|
| Nitrogen | 7727–37–9 | 43,716 |
| Phosphorus | 7723–14–0 | 32,837 |
| Potassium | 7440–09–7 | 25,387 |
| Nitrate | 14797–55–8 | 9,612 |
| Ammonium | 14798–03–9 | 8,293 |
| Ammonia NH3 | 7664–41–7 | 5,686 |
| Phosphate (PO4)3– | 14265–44–2 | 3,349 |
| Phosphorus pentoxide P2O5 | 1314–56–3 | 2,869 |
| Ammonium nitrate NH4NO3 | 6484–52–2 | 2,644 |
| Potassium oxide K2O | 12136–45–7 | 2,298 |
| Ammonium sulfate (NH4)2SO4 | 7783–20–2 | 2,242 |
| Potassium chloride KCl | 7447–40–7 | 2,205 |
| Potassium sulfate K2SO4 | 7778–80–5 | 1,534 |
| Diammonium hydrogen phosphate (NH4)2HPO4 | 7783–28–0 | 1,101 |
| Potassium nitrate KNO3 | 7757–79–1 | 1,005 |
| Phosphoric acid H3PO4 | 7664–38–2 | 944 |
| Calcium dihydrogen phosphate CaH2PO4 | 7758–23–8 | 840 |
| Ammonium dihydrogen phosphate (NH4)H2PO4 | 7722–76–1 | 808 |
| Monopotassium phosphate KPO4 | 7778–77–0 | 750 |
| Struvite (NH4)Mg(PO4)·6H2O | 15490–91–2 | 583 |
| Ammonium chloride NH4Cl | 12125–02–9 | 546 |
| Calcium hydrogen phosphate CaHPO4 | 7757–93–9 | 424 |
| Potassium carbonate KCO3 | 584–08–7 | 406 |
| Hydroxyapatite Ca5(OH)(PO4)3 | 1306–06–5 | 298 |
| Tricalcium phosphate Ca3(PO4)2 | 7758–87–4 | 285 |
| Ammonium bicarbonate NH4HCO3 | 1066–33–7 | 275 |
| Calcium ammonium nitrate Ca(NH4)x(NO3)x | 15245–12–2 | 271 |
| Ammonium acetate NH4CH3CO2 | 631–61–8 | 232 |
| Potassium hydroxide KOH | 1310–58–3 | 225 |
| Urea ammonium nitrate CH6N4O4 | 15978–77–5 | 210 |
| Dipotassium phosphate K2HPO4 | 7758–11–4 | 205 |
| Aluminum phosphate AlPO4 | 7784–30–7 | 154 |
| Magnesium ammonium phosphate MgNH4PO4 | 7785–21–9 | 137 |
| Ferric phosphate FePO4 | 10045–86–0 | 118 |
| Monosodium phosphate NaH2PO4 | 7558–80–7 | 114 |
| Fluorapatite Ca5F(PO4)3 | 1306–05–4 | 113 |
| Ammonium hydroxide NH4OH | 1336–21–6 | 106 |
| Phosphoric acid, ammonium salt NH4H2PO4 | 10124–31–9 | 97 |
| Potassium silicate | 1312–76–1 | 95 |
| Calcium magnesium phosphate CaMgPO4 | 25618–23–9 | 87 |
| Disodium phosphate Na2HPO4 | 7558–79–4 | 67 |
| Tripotassium phosphate K3PO4 | 7778–53–2 | 66 |
| Ammonium carbonate NH4CO3 | 506–87–6 | 66 |
| Potassium superoxide KO2 | 12030–88–5 | 60 |
| Pyrophosphoric acid H4P2O7 | 2466–09–3 | 56 |
| Potassium iodide KI | 7681–11–0 | 53 |
| Phosphoric acid, ammonium magnesium salt (1:1:1), hexahydrate NH4MgPO4·6H2O | 13478–16–5 | 52 |
| Iron phosphate | 10402–24–1 | 51 |
Still, China is the world leader in patent and journal publications related to sustainable fertilizers (Figures 2 and3) throughout the 2001–2021 period. This could be due to controlled-, sustained- or slow-release fertilizer publications in China, which have risen steeply and reached a peak of about ten times more than other countries between the years 2014–2016 (Figure S4).60−62 Claims of sustainability as it relates to these publications are likely due to the reduction of needed applications and loss of nutrients through runoff, leaching and volatilization. Patent trends in China may have been influenced by China’s agricultural policies from 2017 and the 14th Five-Year National Agricultural Green Development Plan published in 2021 which emphasize the promotion of green and more sustainable agricultural practices and crop production modes utilizing agricultural wastes and manures (as detailed in a USDA foreign Agricultural Service Global Agricultural Information Network report).63,64 However, in a related analysis, it was noted that agricultural support practices (direct and indirect subsidies) that encourage improved agricultural productivity, may encourage fertilizer use methods and farming practices which may not be well-aligned with policies on environmental sustainability.65 Thus, the overall pattern of peak and decline in sustainable fertilizer patent filings in China may be due to conflicting incentives in the Chinese agricultural or patent systems.
Figure 2.

Total number of patents published by Top 15 countries for the period of 2001–2021 on the topic of fertilizers, sustainability, recycling, and recovery.
An analysis of the top patent publishing countries (Figure 2) revealed that China was followed in descending order by Japan, Republic of Korea, the United States, the Russian Federation, and Germany. In the case of journal publications (Figure 3), India is in second place followed by the United States, Brazil, Japan, and Germany.
Using Wastes and Wastewater as Nutrient Sources
Since the late twentieth century, nations have implemented treatments to remove nitrogen and phosphorus from bio-organic wastes to reduce the eutrophication of surface waters. Biological and chemical processes are the most common methods to remove nitrogen and phosphorus from wastewater. While recently, waste biosolids have been incinerated, reducing the volume of waste but still producing ash.66 Land application of manure and biosolids has been one avenue to derive soil nutrients and manage waste. However, overapplication of manure or treated biosolids to land can result in nutrient buildup and runoff that also results in eutrophication of surface waters.67 Odors, pathogens, heavy metals, or other micropollutants such as drugs and hormones are also a source of concern for land application or release of effluents into waterways.68,69 To address these problems, alternative systems and processes are being developed to extract fertilizer nutrients from waste or wastewater.
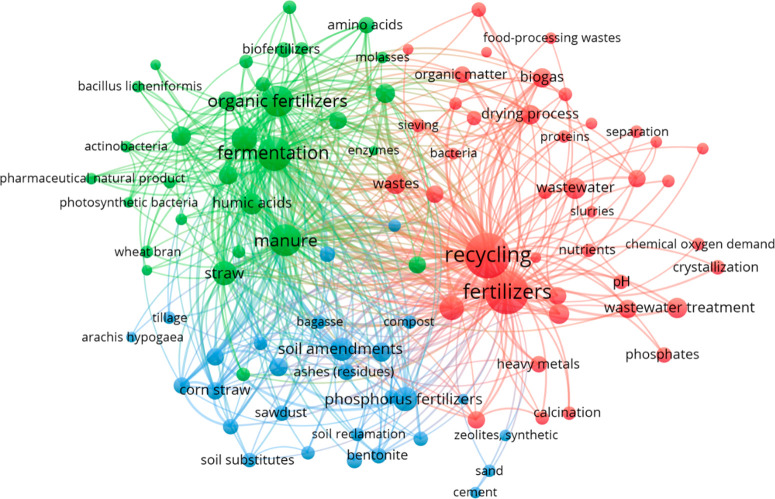
To find more sources and processes for recovery or recycling of the main fertilizer nutrients N, P, and K from wastes and wastewater, a second search query was applied (see SI Methods: Search Query 2) to filter the previously obtained data set. This smaller data set was then used to identify trends related to recycling nutrients for fertilizers from these sources such as types of wastes and wastewaters used in fertilizers and soil amendments, processes for recovery of nutrients therefrom, and substances and their functions related thereto. Data obtained on these topics were used to generate Figures 4–20, S5–S7, and Table 2. The recent research landscape in this area can be visualized in many ways; we begin by presenting the most commonly co-occurring concepts found to be important within each respective study in a clustered network diagram generated by VOSviewer. In these diagrams, the nearer the concepts are in the diagram the more often they are found together in documents, colors also represent groups of more closely associated concepts, and the larger the node circumference the more times this concept appeared in publications.70,71 The generated maps show three main clusters (red, blue, green), which indicate that organic nutrient sources, organic waste-derived products, and related processes such as wastewater treatment and fermentation are core topics in both journal (Figure 4) and patent (Figure 5) publications.
Figure 4.
Top co-occurring concepts in journals on fertilizers, sustainability, recycling, and recovery topics with a focus on wastes and wastewaters.
Figure 20.
Number of publications on physical treatment concepts for struvite production from 2001 to 2021.
Table 2. Key Substances in Nutrient Recovery from Wastes and Wastewaters Research.
| Substance Class | Substance | REG No. | Publications | Feature/Areas of Interest | Example Publications |
|---|---|---|---|---|---|
| Elements | Carbon | 7440–44–0 | 13,787 | Sorbent | (95, 177, 195,219, 220) |
| Graphite | 7782–42–5 | 58 | Coagulator | (221−224) | |
| Sorbent | |||||
| Electrode material for electrochemical wastewater treatment | |||||
| Graphene | 1034343–98–0 | 56 | Sorbent | (225, 226) | |
| Electrode material for electrochemical wastewater treatment | |||||
| Oxides/Hydroxides | Silica (SiO2) | 7631–86–9 | 1,214 | Sorbent Flocculant | (227−230) |
| Calcium oxide | 1305–78–8 | 917 | Sorbent | (231−234) | |
| Precipitating agent | |||||
| Magnesium oxide | 1309–48–4 | 882 | Struvite precipitating agent | (52, 235, 236) | |
| Sorbent | |||||
| Alumina | 1344–28–1 | 688 | Sorbent | (232, 237−239) | |
| Filtration membrane | |||||
| Iron oxide (Fe2O3) | 1309–37–1 | 650 | Sorbent | (196, 240−243) | |
| Zinc oxide | 1314–13–2 | 340 | Sorbent | (244−246) | |
| Precipitating agent | |||||
| Titania | 13463–67–7 | 323 | Flocculant | (247, 248) | |
| Sorbent | |||||
| Iron oxide | 1332–37–2 | 171 | Sorbent | (249, 250) | |
| Manganese oxide (MnO2) | 1313–13–9 | 137 | Sorbent | (251−253) | |
| Manganese oxide (MnO) | 1344–43–0 | 113 | Sorbent | (205,254,255) | |
| Copper oxide (CuO) | 1317–38–0 | 102 | Sorbent | (256−258) | |
| Manganese oxide | 11129–60–5 | 80 | Sorbent | (259) | |
| Iron oxide (Fe3O4) | 1317–61–9 | 53 | Magnetic Sorbent | (260−264) | |
| Calcium hydroxide | 1305–62–0 | 226 | Precipitating agent | (265−267) | |
| Biosorbent | |||||
| Pretreatment agent | |||||
| Coagulant | |||||
| Magnesium hydroxide | 1309–42–8 | 82 | Precipitating agent | (268−270) | |
| Coagulant | |||||
| Aluminum hydroxide | 21645–51–2 | 73 | Sorbent | (271−273) | |
| Metal salts | Calcium carbonate (CaCO3) | 471–34–1 | 1,820 | Precipitating agent | (175, 274, 275) |
| Sorbent | |||||
| Calcium chloride | 10043–52–4 | 862 | Hydroxyapatite precipitating agent | (276, 277) | |
| Magnesium chloride | 7786–30–3 | 279 | Forward osmosis membrane component | (269, 278−281) | |
| Struvite precipitating agent | |||||
| Iron chloride (FeCl3) | 7705–08–0 | 187 | Flocculant | (269, 282−284) | |
| Magnesium carbonate | 546–93–0 | 79 | Struvite precipitating agent | (52, 285) | |
| Zinc chloride | 7646–85–7 | 76 | Absorbent | (286) | |
| Magnesium nitrate | 10377–60–3 | 102 | Hydroxyapatite precipitating agent | (287) | |
| Minerals, clays | Gypsum (CaSO4·2H2O) | 13397–24–5 | 1,182 | Soil additive | (288−290) |
| Nutrient solubility | |||||
| Improver | |||||
| Phosphorus loss inhibitor | |||||
| Dolomite CaMg(CO3)2 | 16389–88–1 | 444 | Hydroxyapatite precipitating agent | (291−294) | |
| Sorbent | |||||
| Calcite | 13397–26–7 | 388 | Precipitating agent | (295−299) | |
| Sorbent | |||||
| Kaolinite (Al2(OH)4(Si2O5) | 1318–74–7 | 237 | Sorbent | (300, 301) | |
| Vermiculite | 1318–00–9 | 206 | Sorbent | (302−304) | |
| Ion exchanger | |||||
| Montmorillonite | 1318–93–0 | 158 | Sorbent Ion exchanger | (305−307) | |
| Pyrite | 1309–36–0 | 136 | Sorbent | (308−310) | |
| Hematite Fe2O3 | 1317–60–8 | 127 | Sorbent | (311−313) | |
| Nutrient delivery enhancer | |||||
| Goethite | 1310–14–1 | 124 | Sorbent | (314−316) | |
| Clinoptilolite | 12173–10–3 | 111 | Sorbent | (317−320) | |
| Ion-exchanger | |||||
| Betaine | 107–43–7 | 82 | Sorbent | (244, 321) | |
| Magnetite Fe3O4 | 1309–38–2 | 76 | Sorbent | (322−324) | |
| Muscovite | 1318–94–1 | 73 | Sorbent | (325) | |
| Ferrihydrite (Fe5(OH)9O3) | 39473–89–7 | 71 | Sorbent | (324, 326, 327) | |
| Palygorskite | 12174–11–7 | 68 | Sorbent | (328−330) | |
| Magnesite | 13717–00–5 | 65 | Struvite precipitating agent | (331−335) | |
| Sorbent | |||||
| Aragonite | 14791–73–2 | 61 | Sorbent | (336, 337) | |
| Gibbsite | 14762–49–3 | 60 | Sorbent | (338−340) | |
| Biotite | 1302–27–8 | 51 | Precipitating agent | (341, 342) | |
| Polymers | Starch | 9005–25–8 | 1,597 | Sorbent | (343−345) |
| Bioadditive | |||||
| Cellulose | 9004–34–6 | 999 | Sorbent | (194, 346, 347) | |
| Filtration membrane | |||||
| Hemicellulose | 9034–32–6 | 456 | Sorbent | (348, 349) | |
| Ion-exchange membrane | |||||
| Chitosan | 9012–76–4 | 282 | Sorbent | (53, 297, 350−354) | |
| Flocculant | |||||
| Encapsulant |
Figure 5.
Top co-occurring concepts in patents on fertilizers, sustainability, recycling, and recovery topics with a focus on wastes and wastewaters.
A closer examination of the paired topics closely associated with “recycling” in journals reveals that “nutrients”, “phosphates”, “nitrates”, “nitrites”, “phosphorus fertilizers”, “nitrogen fertilizers”, “soil amendments”, and “manure” are also associated most closely with “wastewater treatment sludge”, “wastewater”, and “wastewater treatment”. Recycling also frequently co-occurs with “fertilizer experiment” and “economics” in the journal set (Figure S5).
In patents (Figure S6), co-occurring concepts with the term “recycling” reveal the application of processes such as “aerobic fermentation”, “anaerobic fermentation”, “wastewater treatment”, and “composting” using wastes such as “agricultural wastes”, “straw”, “food-processing wastes”, “municipal wastes”, “bagasse”, “sludges”, “ashes”, “manure”, “wastewater”, “fly ash”, “sawdust”, and “biomass”. Associated products such as “phosphorus fertilizers”, “potassium fertilizers”, “organic fertilizers”, “biofertilizers”, “compost”, “soil amendments”, “charcoal” (in this context meaning biochar), and “cement” also appear. These concepts reflect patent application interest in the recycling of waste for recovery of nutrients for use as fertilizers or other products.
The concepts in Figure 5 highlight top co-occurring terms from author designated “sustainable” fertilizers or soil amendments in patents. In this context sustainable can mean having components derived from renewable sources such as organic wastes and byproducts or wastewaters, but also include formulations providing the feature of controlled-, slow- or sustained-release to extend the availability of fertilizer nutrients, or prevent the loss thereof, through addition of renewable resource components or other additives. For example, since 2015 at least 1281 patents containing such materials have the feature of controlled-, slow- or sustained-release. Slow-release fertilizers using recycled waste materials such as bagasse biochar in a nanogel-coated formulation of ammonium salts or urea improves both nitrogen release and water use efficiency.72 Agricultural waste biomass, food wastes, and vermicompost can be carbonized to biochar and formulated into slow-release fertilizer as a porous carrier for inorganic nutrients73 This fertilizer was produced with lower energy cost, lower carbon dioxide emissions, and formulated as granular pellets for easier transport. Thus, recycled organic wastes may provide support for improved delivery efficiency of inorganic nutrients in formulations as well.
To get an idea of what types of wastes as formulation components and nutrient sources are being used most in patents published over the last two decades, the CAS Content Collection data (See SI Methods: Search Query 2) was used to obtain the sums of patents for several categories (Figure 6). Results showed that patents were mostly focused on the use of agricultural and food wastes, followed by manure, sludges, wastewater, and ashes, with biomass, algae, and urine sources being of less focus.
Figure 6.

Patent trends of waste types used as nutrient sources from 2001 to 2021.
Examining patenting trends on products derived from said broad categories of wastes demonstrates a strong focus on phosphate recovery from manure, wastewaters, or urine. Plant-based agricultural wastes and food processing wastes were more often but not entirely associated with composts, soil amendments, as feedstock for biochar and biofuels, or used in multicomponent fertilizer formulations. Returning to the idea of a circular economic approach, chemical fertilizer nutrients can be derived from wastes, which are represented broadly by phosphates, nitrates, nutrients, trace elements, and superphosphates in Figure 7. However, patent literature on deriving agricultural uses from wastes over the last two decades has been dominated by the production of soil amendments. The generation of biogas for power is the next most discussed topic in patents. Biochar, often derived from biomass feedstocks such as agricultural wastes or algae, is also discussed in patents. Biomass, whether grown for fuel or as a process waste byproduct can contribute to the circular bioeconomy through its use in biochar, soil amendments, compost, biofuels, and as absorbents of nutrients.
Figure 7.

Patents including products derived from wastes and wastewaters from 2001 to 2021.
As previously mentioned, phosphates, nitrates and trace elements were associated with waste recycling, wastewater recycling, and sustainable fertilizers. Table 1 illustrates the forms of nitrogen, phosphorus, and potassium associated with these publications. Elemental nutrients are most likely to have been measured in soils, plants, or fertilizer products, while salt and mineral forms are either manufactured chemical forms of fertilizer or were derived from wastes or wastewaters. Struvite, a nutrient often generated from precipitation from waste slurries and wastewaters, is discussed in more detail later in this report.
While terms referring to potassium or potassium fertilizers appeared infrequently in association with wastes or wastewater, sustainable sources for potassium have been studied.74−76 Historically, potassium has been derived from potash mining, brines, wood ash and other ashes, potassium-silicate minerals, and even from kelp, though generally mining of potash has been economically more competitive as an industrial enterprise.74 Availability and affordability of mined potash globally has become an issue, particularly for poorer nations in the Southern Hemisphere.74 The earlier use of ashes and kelp as potassium sources indicates that improved technologies and methods for nutrient recovery may make these sources useful again. Kelp is farmed for other products such as alginic acid and carrageenan, used in foods, and is recently being proposed as a low-cost means for carbon sequestration and mitigation of ocean acidification.75 The promise of another profitable potassium product from kelp might cause further interest in its farming.
A variety of methods have been used to recover potassium struvite (MgKPO4·6H2O) from biowaste, such as pig slurry after nitrification-denitrification;76 by combined partial nitration-anammox from municipal wastewater;77 by selective recovery of two struvite forms, magnesium ammonium phosphate (MgNH4PO4 ·6H2O) and potassium struvite; by CO2-assisted extraction from poultry litter;78 extraction of potassium struvite from pumpkin wastes;79 from sugar cane vinasse by an integrated electrodialysis and precipitation process;80 and in multiple potassium mineral forms found in biochar produced from sugar palm fiber, coconut fiber, durian shell, and palm oil fruit.81 The variety of methods and sources for the recovery of potassium struvite from wastes or wastewater are consistent with interest in its sustainable recovery.
Sewage sludge generated by wastewater treatment plants is a source of both biogas energy and nutrients such as phosphorus, nitrogen and potassium.82−84 The nutrient content depends on sludge type (biochemically treated activated sludge, anaerobically digested sludge, lime treated sludge) and processing methods.85 Sewage sludge can also be a primary feedstock for producing biochar.86,87 Biochar produced via sewage sludge pyrolysis is rich in P, N, and K-based nutrients which makes it a potential fertilizer if heavy metal, drug, pathogen, and other contaminants can be managed in an economically feasible and safe manner.84,88−91 Charcoal and ashes derived from municipal sewage sludge contain a lot of phosphorus-based nutrients.92 Hydrochars prepared by hydrothermal carbonization of wastewater sludge are not only important adsorbents of nutrients, but also have potential as controlled-release fertilizers and methane production enhancers.93−95
Common techniques for nutrient recovery from sewage sludge and other bioorganic wastes are anaerobic digestion, composting, and vermicomposting. Anaerobic digestion (AD) is a natural biological process in which microorganisms break down organic materials in closed spaces where there is no air (or oxygen). AD is the preferred treatment method for organic fractions of agricultural, industrial, and municipal solid waste.96 AD of organic matter proceeds via hydrolysis (polymer decomposition), acidogenesis (volatile fatty acid production), acetogenesis (acetic acid production), and methanogenesis (methane production).97 The process produces two main products: digestate, from which nutrients may be extracted, and biogas.98 Nutrient recovery from anaerobically digested swine wastewater in the form of crystallized struvite was achieved by employing a sequencing batch reactor and a continuous-flow reactor,99 while calcium phosphate and struvite were accumulated during anaerobic digestion of sludge from biological phosphate removal treatment.100
Compost and vermicompost are waste-derived products found in the set of patents associated with sustainable fertilizer productions (Figure 7). Composting is the aerobic, thermophilic, microorganism-mediated bioconversion of organic matter into humic substances called compost.101 The process of composting is as follows: Organic waste (Protein + Cellulose + Lignin) + O2→ Compost + CO2+ H2O + Heat.
Usually, composting proceeds through three phases utilizing different microorganisms.102 Initial decomposition is carried out by moderate-temperature mesophilic microorganisms for a couple of days; then the mesophiles are replaced with thermophilic microorganisms and heating continues for anywhere from a few days to several months. Finally, the cooling and maturation phase proceeds for several months yielding compost. A typical percentage of N-, P-, and K-based nutrients in compost is 1–2%, 0.7%, and 1.2%, respectively.103 A strong research trend is the immobilization of microorganisms in compost to increase the content of nitrogen, phosphorus and potassium nutrients.104
Vermicomposting uses worms and bacteria in combination to convert solid organic wastes coming from different sources such as food, plants, animals, pharmaceuticals, and sewage into organic fertilizers.105−107 Vermicompost duration usually takes approximately 28–125 days. The resulting vermicompost constitutes N, P, K, and Mg amounts, on average, of about 2.8%, 0.85%, 2.3%, and 0.38%, respectively, though nutrient content strongly depends on vermicompost feedstock and treatments.108 For example, vermicompost prepared from coconut husk mixed with either pig slurry or poultry manure allowed the recovery of microbial biomass carbon and nutrients. The highest N and K recovery was observed for 20% feedstock substitution with pig slurry, while poultry manure substitution recorded higher P recovery.109
Reuse and recycling of solid wastes for plant nutrition products mainly involves biotransformation to usable soil amendments or use of incineration, pyrolysis, and gasification. These last three methods and gasification coupled with ash melting are widely used for energy and nutrient recovery from municipal solid wastes.110 Incineration in this case is the combustion of waste to produce heat and electricity, where the remaining products are nutrient-rich ashes that can be used as a part of fertilizer feedstock. Sewage sludge ashes are particularly rich in phosphorus111 and ashes from biomass rich in potassium, calcium and magnesium are also useful for fertilizing purposes.112 Similarly, pyrolysis is the heating of waste under a limited supply of oxygen; it is an important method for the processing of livestock waste.113 Biomass pyrolysis produces bio-oil and biochar. A small amount of biochar can be also prepared via gasification, a form of pyrolysis applied at higher temperatures to mainly produce gases.114 For example, gasification of organic waste in supercritical water at 600 °C yielded both energy and biochar containing nitrogen nutrients such as ammonium salts.115
Biochar quality can be improved by the choice of waste feedstock (biomass crops, agricultural residues, agroforestry, and sewage sludges116) and pyrolysis temperatures. For instance, biochar produced from sewage sludges at 450 °C contains all possible forms of phosphorus nutrients and a variety of N-based nutrients.117 Similarly, in another study, varying the pyrolysis temperature of sewage sludge used as feedstock affected the availability of nutrients in the resulting biochar. Mercl et al. observed that the pyrolysis of anaerobically stabilized sewage sludge at 320 °C resulted in an increment in pH and a significant drop in the content of available Ca, Mg, K, and S. However, this lower, less energetically demanding temperature showed the highest content of available P.118
Due to the high number of patent publications on biochar (Figure 7), the overall publication trends on this concept in relation to wastes and wastewaters was generated (Figure 8). This showed an overall increase in the patent and journals from 2014 to 2021. It also shows a sharp rise in journal publications in 2020 and 2021. This is due to the flexibility of biochar applications and the continued research into other possible uses. Apart from discussing the processes to make biochar, many of the documents describe its use as a soil amendment, as an adsorbent or carrier for nutrients or agrochemicals, or as a sorbent for pollutants.
Figure 8.

Patents and journals from 2001 to 2021 including the CAS term for biochar on fertilizers, sustainability, recycling, and recovery topics with a focus on wastes and wastewaters. The years 2001, 2002, and 2008 had values of zero.
Biochar characteristics can be manipulated through composition of feedstock, pyrolysis temperatures, or other chemical additives whereby it can be used as an adsorbent for ammonium, nitrate, and phosphate from wastewaters. The adsorbed nutrients can then be released via a desorption process, in acid/base solutions, by ion exchange, or by biodegradation to release nutrients.119 Nitrogen-doped biochar can also be used for pollutant removal from wastewaters.120 Nitrogen-doped biochar can be made through the pyrolysis of nitrogen-rich biomass obtained by either using sources high in nitrogen or by supplementation of biomass with external nitrogen sources. Kasera et al. list several nitrogen-rich sources of biomass including food wastes (bean dregs, watermelon rind, banana peels), Torula yeast (Candida utilis), municipal sewage sludge, human hair, iris, water hyacinth, tea, cat tails (Typha angustifolia), green algae, spirulina, shrimp shells, and chitosan. Biomass low in nitrogen (such as agar, corn straw, bagasse, cotton stalks, bamboo chips, bagasse, cellulose, anaerobic digested fiber) can be treated with external nitrogen sources such as ammonia gas, ammonium hydroxide, urea, ammonium chloride, or melamine. The use of novel treatments such as surface oxygen modification and post-treatment to add nitrogen sources to the biochar, as well as the temperature and time of pyrolysis, is important for determining the effectiveness of the biochar as a slow-release fertilizer.
Potassium-doped biochar can be made using sewage sludge as feedstock by treatment with potassium acetate prior to pyrolysis, resulting in a novel PK fertilizer.90 A recent study by Kassem et al. demonstrated the use of cellulose and montmorillonite-modified biochar in nanocomposite film coatings on superphosphate to produce slow-release phosphorus granules that also assist with water retention in soil.121 The water-retaining cellulosic granule coating material was prepared by pyrolysis of lignocellulosic biomass with montmorillonite. The use of cellulosic materials as coating modifiers and in biochar production provides new uses for these materials in making sustainable fertilizer formulations.
Engineering and modification of biochar provides many possible ways to use bioderived waste materials. In another study, biochar derived from corn silage, cow manure, and pig slurry fermentation waste as feedstock was used as a sorbent; modified with ferric iron and calcium by chemisorption, the biochar recovered phosphorus from sludge wastewater.122 The dewatered fermentation wastes are pyrolyzed to make biochar using waste heat from biogas combustion and pyrolysis gas. The authors compare the costs of the calcium-biochar sorbent vs ferric-biochar sorbent, struvite, or sludge water at an application rate of 1 kg CaP/ha. Biochar sorbents are less expensive than struvite or direct sludge water applications and the phosphorus from the biochar is more bioavailable to plants. From these studies, we can see the promise of biochar for recycling waste feedstocks into useful adsorbent carriers of nutrients, as alternative crystallizing agents for struvite that could replace more expensive chemical precipitants, or as sorbents for the removal of pollutants from wastewaters.123
Hydrothermal carbonization of anaerobically digested biomass is a way to make another type of biochar, referred to as “hydrochar”. In a study by Jamal Alhnidi et al., the hydrothermal carbonization of biogas digestate of cattle manure was simulated using a model nutrient solution of glucose (as the organic carbon source) and known amounts of potassium dihydrogen phosphate, ammonium chloride, potassium chloride, sodium nitrate, and sodium nitrite.124 Modeling was used to determine how carbon, nitrogen, and phosphorus are incorporated into or lost to process waters and gases during the formation of biochar. The gases measured included CO2, CO, methane, N2, nitrous oxide, and ammonia. Ammonia and nitrate were found in both the hydrochar and the process water; the relative amounts of ammonia and nitrates depended on the feedstock composition and the conditions for anaerobic digestion of the cattle manure. The carbon from the manure ended up in the biochar, while phosphorus was mainly lost to the wastewater. Thus, processes for anaerobic digestion of biomass feedstock to produce biogas can be integrated with use of the digestate for nutrient recovery as biochar and phosphorus recovery from wastewater. Still, these processes must be carefully managed to be efficient sources for fertilizer.
For a further comparison, publication trends for “charcoal”, “ashes”, “wastewater treatment sludge”, and “sewage sludge fertilizers” were determined (Figure 9). An increase in the number of publications concerning the recovery of nutrients from wastewater treatment sludge and biochar (charcoal) was observed. On the contrary the term “sewage sludge fertilizers”, indexed in documents focused more on sewage sludge formulated into a fertilizer product, remained low. Still, biochar publications, many incorporating use of sewage sludge as feed stock, increased. The low number of indexed “sewage sludge fertilizer” publications could be due to the use of sewage sludge being constrained by regulations to limit the transfer of organic contaminants, heavy metals, and pathogens to agricultural products and other soils.125,126 Regulations require the monitoring of biosolids derived from them, set limits on specific contaminants of concern, and determine when derived products can be applied to soil used for fruits, vegetables, and grazing animals. These rules increase the costs of biosolids in fertilizer, reducing the commercial interest in them.
Figure 9.
Publications including the CAS terms for ash, biochar (charcoal), wastewater treatment sludge, and sewage sludge fertilizers from 2001 to 2021 on fertilizers, sustainability, recycling, and recovery topics with a focus on wastes and wastewaters.
The publications related to ashes (including sludge incinerator ash, fly ash, and bone ash) increased substantially from 2015 to 2017, but decreased in the latter half of the decade. Smol et al. discussed the potential for the use of sewage sludge ash (SSA) in Poland as a source of phosphorus for agriculture, where other sources for phosphorus are limited or not economically viable.127 Roughly 20,000 t of SSA were generated in Poland in 2014; both EU and Polish laws encourage its use instead of its disposal. The largest current sources of SSA are in or near large cities, requiring transport of SSA to agricultural areas. However, economies of scale are most favorable near the larger sources of sewage sludge. A second problem is the level of contaminants in SSA. Previous evaluations of SSA indicate that it may be too high in cadmium and lead to be used on agricultural fields under the relevant EU regulations, with measurements of Cd and Pb levels of 3–25 ppm and 20–750 ppm, respectively.127 It is also possible that the levels of heavy metals and other contaminants in SSA obtained from smaller more rural areas may be lower than that obtained from more urban or industrial areas, thus making their use in local agricultural fields more attractive. For smaller sources of SSA, mobile facilities may be useful or necessary for further use. If the levels of contaminants are consistent with those measured previously, SSA in Poland (or the sludge from which it is generated) may require further treatment, increasing the costs for its use. The report generated by Smol et al. thus provides a more local analysis and knowledge gaps for the potential use of SSA as a phosphorus source.
Phosphorus precipitates and phosphoric acid can also be recovered from different wastewater sources using different techniques and conditions.128−130 Phosphorus, nitrogen and even potassium sources can also be recovered from more refined waste streams. For example, separation of urine at the source can reduce contamination of the wastewater, reducing downstream costs of nutrient recovery scaled up.131−133 Though many promising technologies for recovery of phosphorus and other nutrients have not been practiced on large scale and few economic analyses of these technologies have been tested, newer regulations governing wastes and wastewaters may encourage the use of recovered phosphorus, nitrogen, potassium, and trace elements in fertilizers.125,126
Growth in journal publications on recovering and recycling nutrients from waste and wastewater (Figures 9 and 10) certainly reflects interest in this growing field. Small and large businesses have begun incorporating recovered nutrients into fertilizers134 and this trend is expected to continue to rise along with patent publication. While no single technology or set of technologies will work in every circumstance, having a variety of nutrient recovery methods improves the likelihood that methods amenable to local needs and resources exist and are useful for a specific circumstance.135−137
Figure 10.

Number of publications on wastewater treatment for nutrient recovery throughout 2001–2021.
Wastewater Treatment Processes for Nutrient Recovery
Wastewater treatment processes that recover acceptable amounts of nutrients with minimal environmental impact are a key challenge. Environmental impact of nutrient recovery technologies can be estimated by life cycle assessment (LCA), which allows us to compare potential environmental impacts of fertilizers obtained from recovered nutrients to that of conventional fertilizers.137−141 This modeling helps to determine costs and benefits of sustainable wastewater treatment systems with integrated nutrient recovery (struvite and biosolids), water purification for irrigation, energy production (biogas), and useful chemical production.142 Still, development of newer, more energy-efficient, cost-effective, modular, transportable, or multiple-use integrated systems could improve the recovery of nutrients and other products. For example, emerging technologies such as ion-exchange electrolysis and reverse osmosis have already proved themselves useful for recovering nutrients from wastewater.143
Innovative technologies for developing sustainable fertilizers and nutrient delivery systems include the production of struvite and other fertilizer alternatives.144−150 Integrated commercial processes of phosphorus recovery as struvite (such as the AirPrex, PEARL, AshDec, and RecoPhos processes) are commonly employed at wastewater treatment plants.151 The AirPrex process for struvite production subjects wastewater sludge to CO2 stripping by aeration followed by addition of Mg salts in a reactor to form struvite.152−154 Ostara’s PEARL is widely used for phosphorus recovery from municipal and industrial wastewater and occurs via the controlled precipitation of crystalline struvite.155,156 The AshDec process uses anaerobic stabilization of wastewater sludge followed by incineration to produce phosphate-rich ashes free of contamination with heavy metals such as Pb, As, and Cd.157,158 The RecoPhos process is a thermochemical process for the generation of white phosphorus or phosphoric acid from sewage sludge ashes. Ash phosphates are formed and reduced to phosphorus in a thin film on the surface of coke particles; evaporation of the phosphorus then allows it to be isolated without further reactions.159−161 The innovative reactor used in the RecoPhos method allows the reduction of ash phosphates in the thin film on the surface of coke particles. The reduced P is evaporated from the film without reacting with other elements.
To compare multiple wastewater treatment processes associated with recycling or recovery of nutrients, we divided them into three categories: biological, chemical, and physical (Figure 10). Based on 2001–2021 publications, biological processes predominate over chemical and physical methods. The number of publications describing biological methods has increased by 40% over this period. Apparent growth was observed until 2012, after that the number of publications stabilized. The number of chemical publications increased by 20% by 2012, after which it remained practically unchanged until 2021. Publications on physical methods follow the same trend as chemical publications, although their growth is slightly higher: a 27% increase from 2001 to 2012. Figure S7 shows that patents make up a significant part of the publications on chemical and physical methods. About half of all publications on physical methods and chemical methods (53% and 49.5%, respectively) are patents, while the contribution of patents on biological methods is only 37%.
Phosphorus removal from wastewater by struvite precipitation was a common focus of publications addressing wastewater nutrient recovery. It can be used either alone or in complex fertilizer formulations with other waste-derived products, microbial inoculants, or conventional inorganic fertilizers.194−198 Struvite precipitation from wastewater has the potential to generate 17.3 kg of struvite/million liters per day of sewage thereby reducing carbon dioxide emissions by 53% and reducing imports of chemical fertilizers by 0.38 Mt per year.162 Publications using struvite have increased substantially, with the hexahydrate [(NH4)Mg(PO4)·6H2O] being the dominant form studied (Figure S8). Although it has potential utility as a PK fertilizer, little has been published on potassium struvite [MgK(PO4)·6H2O].194−198
Since precipitation of struvite from different types of wastewaters is a prominent technology for the recovery of phosphorus, we compared the publications associated with wastewater treatment for struvite production and overall publications on nutrient recovery from wastewater. The results show that biological, chemical, and physical methods are all used to recover struvite from wastewater (Figure 11B). Moreover, chemical methods are reported in 36% of publications, three times more often than in overall wastewater treatment publications (Figure 11A,B). Figure 11B also demonstrates that, though chemical processes play a significant role in struvite production, biological methods (46%) are in the lead. For a discussion on specific processes associated with biological, chemical, or physical methods, see the following sections.
Figure 11.
(A) Ratio of biological, chemical, and physical methods used in all publications associated with wastewater treatment from 2001 to 2021. (B) Ratio of publications on biological, chemical, and physical methods associated with wastewater treatment for struvite production from 2001 to 2021.
Biological Processes
Biological wastewater treatment systems commonly use ammonification and nitrification to remove nitrogenous substances from sewage. Ammonification converts amino acids, proteins, and other nitrogen sources to ammonia or ammonium salts, while nitrification converts ammonia or ammonium salts to nitrates. Biological processes for nitrogen removal can be co-opted and integrated into systems for reuse to produce products, energy, or for ecological services. Biological phosphorus removal can be accomplished using phosphorus-accumulating organisms, such as activated-sludge bacteria in an anaerobic/aerobic system, membranes with microbial biofilms, or algae in an aerobic lagoon system. It incorporates phosphorus into biomass for bacterial or algal growth. An example of microalgae incorporation for nitrogen and phosphorus recovery is discussed by Rajendran et al., where they evaluated several different systems for nutrient recovery from wastewater, examining energy consumption, cost, and efficiency of recovery and demonstrating that microalgal recovery could save on costs as compared to other systems.162 Estimates of the variation in levels of N and P in different types of Indian wastewaters are also compiled from several references therein, with levels ranging from 20 to 85 mg/L total nitrogen and 4–15 mg/L total phosphorus in municipal wastewater to 1000–1200 mg/L total nitrogen and 500–1500 mg/L total phosphorus in distillery spent wash or piggery wastewater.162
A newer bioprocess involves using an algal-bacterial symbiosis system (ABSS).163 ABSS uses algae and bacteria cooperatively; oxygen production in photosynthesis by algae drives the bacterial process of ammonia oxidation, nitrite oxidation and phosphorus bioaccumulation. The byproducts of bacteria feed the algae (CO2, polyphosphate, and nitrate) using cooperative nutrient exchange between organisms. These systems have mainly been used in the treatment of swine, domestic, and industrial wastewaters, but they may also be useful for treating aquaculture wastewaters (tail waters).163 The efficiency of the removal/recovery of nutrients and pollutants from tail waters via ABSS can be affected by environmental and other factors of the system including light, pH, dissolved oxygen, carbon sources (to support the bacteria), salinity, algae, bacteria, proportions of algae and bacteria, types of bioreactors (suspended biomass or immobilized in biofilm or by cell entrapment), and the overall processes used.
Figure 12 shows the annual number of publications on wastewater treatment using biological methods. The number of academic publications increased by 37.5% over this period. It doubled from 2001 to 2012, then remained almost unchanged and increased again in 2021. The number of patents increased by 23% from 2001 to 2017, with a steep decrease between 2018 and 2021. The results indicate a significant academic interest in biological wastewater treatment, consistent with the observed increase in journal publications on bioelectrochemical wastewater treatment systems, microbial nutrient recovery cells, and microbial electrodialysis cells.164
Figure 12.

Number of publications on biological wastewater treatment for nutrient recovery throughout 2001–2021.
Specific biological processes were characterized using CAS concepts (Figure 13). Due to each concept being associated with one specific publication, we can extract the total number of publications where this concept/process is mentioned. Among them the concepts associated, “Wastewater treatment sludge” is the most popular with 3575 publications, while 750 documents refer to “Secondary wastewater treatment sludge”, and 625 documents refer to “Municipal wastewater treatment sludge”. “Anaerobic wastewater treatment” is found in 800 publications. “Wastewater denitrification” occurs in 400 publications and “Dephosphorization wastewater treatment” is the least common concept.
Figure 13.
Number of publications on biological wastewater treatment concepts from 2001 to 2021.
Due to the association of struvite production and biological processes (Figure 11B), a deeper look into biological wastewater treatment concepts connected to struvite production was conducted (Figure 14). In the case of struvite production, “Anaerobic wastewater treatment” has a higher occurrence (78%) when compared to biological wastewater treatment in general (20%). It confirms the efficiency of struvite precipitation from the liquid phase of anaerobic digestates. “Dephosphorization wastewater treatment” and “Wastewater denitrification” were common concepts as well, occurring in 20% and 17.5% of documents, consistent with recovering phosphorus and nitrogen from wastewater in the form of struvite.
Figure 14.
Number of publications on biological wastewater treatment concepts from 2001 to 2021 related to struvite production.
Chemical Processes
Precipitation, crystallization, and ion-exchange are broadly applied for chemical wastewater treatment. Chemical precipitation, used after anaerobic treatment, is the most common chemical technology for phosphate recovery from municipal wastewater,57 while the formation of struvite has been commercialized as a treatment process for phosphorus and ammonia recovery from wastewater sludge dewatering.165 Ca2+ and Mg2+ ions are often used as phosphate precipitators to form Ca5(OH)(PO4)3 (hydroxyapatite) and NH4MgPO4·6H2O (struvite).166,167 Aluminum salts such as alum (aluminum sulfate or polyaluminum chloride), iron salts, and lime (calcium hydroxide) are also used to chemically precipitate phosphate, specifically during the primary sedimentation phase of wastewater treatment.168−170 Phosphate precipitates are then removed in the separation unit using sedimentation and flotation tanks. Magnesium-based precipitation combined with other precipitating agents or sorbents such as fly ash may also be used to recover nutrients in the form of struvite. Chemical precipitation combined with adsorption can also be applied to recover phosphate from sewage sludge using zinc–aluminum layered double hydroxides as adsorbents.171
One of the important parameters for chemical precipitation is pH, due to pH values affecting nutrient concentrations and the solubility of precipitates.172,173 A recent thermodynamic modeling study by Pindine and collaborators demonstrated that phosphorus–containing precipitates could be profitable when recovered from wastewater with high nitrogen-to-phosphorus ratios under optimal conditions.129 Temperature, pH, and MgCl2 addition schemes were modeled extensively in this study to support their findings. The crystallization of calcium phosphate carried out using CaCO3 particles as seeds also proved to be a useful method for phosphorus recovery from wastewater.174 It was found that the applied current, the CaCO3 particle size, and the feed rate can affect calcium phosphate precipitation.175 As an alternative seeding agent microalgae-derived biochar enriched with magnesium has also been used for seeding struvite crystallization.123
In ion-exchange membrane electrodialysis (ED), extraction of nutrients from wastewater occurs via application of ion-exchange membranes.176 It has been reported that over 95–98% of the phosphate and nitrate nutrients in wastewater were recovered using an ion-exchange membrane bioreactor.177 Rudong et al. demonstrated that selective electrodialysis using three consecutive ion-exchange membranes enhanced nitrate and phosphate recovery from secondary wastewater sludge.178 Other studies showed that simultaneous anionic and cationic selective ED could recover NH4+, K+, Ca2+, Mg2+, and PO43– from swine wastewater,179 and that ED showed high efficiency in recovery/removal of ammonium from anaerobic swine digestate.180 Integrating ED with a membrane bioreactor has resulted in >97% recovery of ammonium salts and >76% of phosphate from urine wastewater.181 Integrating bipolar electrodialysis membranes and membrane capacitive deionization techniques permit the simultaneous removal of phosphorus (89%) and nitrogen (77%).182 A solar energy-powered decoupled ED system with a separate anode and cathode, as well as an additional cation exchange membrane in the anode unit, was able to collect phosphates at a higher concentration and enhance the recovered struvite.183 All these studies demonstrate that ED has a very high potential for extraction of nutrients from waste.
In general, the number of publications on chemical wastewater treatment gradually increased from 2001 to 2007 (Figure 15). A fluctuation in journal publications occurred from 2002 to 2006, while an increase in patents in 2003 to 2007 was observed. Despite a noticeable decline in total publications from 2008 to 2011, the number of patents exceeded the number of journals during this period and continued as such until 2017. This indicates a strong commercial interest in struvite chemical precipitation. Novel technologies of struvite harvesting from wastewater were commercialized at that time. Among them AirPrex which was introduced to North American markets in 2014. Six MagPrex (AirPrex-based) orthophosphate removal/recovery systems are currently operational or under construction in the United States.184 In contrast, journal publications started to increase again in 2018 and surpassed the number of patents in the second half of the decade.
Figure 15.
Number of publications on chemical wastewater treatment for nutrient recovery throughout 2001–2021.
Comparing concepts used for chemical nutrient recovery from wastewater (Figure 16) and struvite production from wastewater (Figure 17), one can conclude that “Precipitation wastewater treatment” is a major concept in both cases, making up >90% of all the concepts. When not specifying struvite production (Figure 16), it is followed by “Wastewater treatment coagulation” with 210 publications, “Electrochemical wastewater treatment” with 180 publications, and “Oxidative wastewater treatment” with “Ion-exchange wastewater treatment” with 175 publications each. In documents for chemical methods for struvite production (Figure 17), “Crystallization wastewater treatment” occurred more frequently than in general chemical wastewater treatment methods. Both concepts, “Precipitation wastewater treatment” and “Crystallization wastewater treatment”, are directly related to struvite recovery processes. Interestingly, “Catalytic wastewater treatment” found in general chemical treatments was not associated with struvite production.
Figure 16.
Number of publications on Chemical wastewater treatment concepts from 2001 to 2021.
Figure 17.
Number of publications on Chemical treatment concepts for struvite production from 2001 to 2021.
Physical Processes
Physical processes for nutrient recovery from wastewaters mostly include forward osmosis, adsorption, and membrane filtration.
Forward osmosis (FO) can be applied to enhance nutrient recovery from wastewater.185−187 This technique uses an osmotic pressure gradient as a driving force and semipermeable membranes to separate dissolved solutes from water. The use of selective osmotic membranes improves phosphate and ammonium nutrient recovery.186,188 A hybrid system, containing both a FO apparatus and a microbial electrolysis cell, allowed the recovery of >99% of nitrogen as ammonium and >79% of phosphorus as struvite.189 Of the available phosphate in digested swine wastewater, 99% of it was recovered via FO with struvite precipitation.190 The combination of low-pressure reverse osmosis and nanofiltration is another promising technique for phosphorus and nitrogen recovery from anaerobic digestates.191 For example, an anaerobic osmotic membrane bioreactor in combination with membrane distillation has been shown to improve nutrient recovery from wastewater.192
Over the years, natural adsorbents such as zeolites, clays, biopolymers, and biochar have been investigated for nutrient recovery. Adsorption and removal of phosphate from wastewater can be accomplished using adsorbents such as synthetic metal hydroxides/oxides, carbonate minerals, clay minerals, zeolites, mesoporous silica, synthetic polymers, and biopolymers.193,194 Activated carbons and many types of biochar that are modified or doped to alter their adsorption capacities have been described.95,195,196 For example, biochar-mediated adsorption was able to recover 96% of ammonium and phosphorus from swine wastewater.197
The ability of engineered sorbents, including those derived from biowaste sources, to remove phosphates and ammonium from a variety of waste streams has improved significantly. Simultaneous recovery of ammonium and phosphate from urban sewage sludge using Na-, K-zeolites, and MgO has been achieved via formation of bobierrite Mg3(PO4)2 or struvite (MgNH4PO)4.198 In another study, a hybrid adsorption membrane ultrafiltration process was applied to the recovery of (N–P–K)-nutrients from potassium-rich sludge using reactive sorbents; Na-zeolites were used for NH4+ and K+ recovery, Ca-zeolites were applied to improve the removal of P via formation of Ca-phosphates (CaHPO4), and MgO facilitated the formation of Mg/NH4/PO4 minerals (struvite and magnesium phosphates).199 Natural zeolites such as K-clinoptilolite impregnated with metal oxides can be used to prepare hybrid reactive sorbents for ammonium and phosphate recovery from urban wastewater.200 The combination of biochar and clinoptilolite also resulted in improved ammonium, potassium, and dissolved organic content removal efficiencies compared to biochar alone.201
To recover nutrients from wastewater, oxides and hydroxides of divalent and trivalent metals (Ca2+, Fe3+, Al3+, Mg2+, La3+, and Mn3+) have been widely investigated because of their porosity and high surface area and hence high adsorption capacity.202,203 As previously shown, Mg and Mg modified adsorbents are a common theme in adsorption publications. Some other examples of this being: ammonia stripping and phosphate precipitation from wastewater in the form of struvite,204 and simultaneous removal of ammonia-nitrogen and phosphate as crystallized struvite from simulated swine wastewater using Mg-modified zeolites.205
Phosphorus removal from eutrophic waters using various functional nanomaterials such as carbon-based materials, zeolites, mesoporous silica, metal–organic frameworks, metal oxides and hydroxides, and biomass-derived materials have also been reviewed.206 Efficient phosphate recovery from eutrophic lakes (90%) was achieved using a hybrid adsorbent comprising of diatomite modified with dispersed magnesium oxide nanoflakes.207 Selective recovery and enrichment of phosphate from wastewater containing competing ions have been achieved by using adsorption combined with capacitive deionization.208 Effective sorption of phosphate, nitrite, and nitrate using chitosan hydrogel beads has also been demonstrated.209
Lastly, there has been an increased interest in the use of membranes for nutrient recovery from anaerobically digested slurries. The combination of microfiltration, ultrafiltration, and nanofiltration membranes have demonstrated >94% nitrogen recovery from digestate.210 The nanofiltration membrane NF270 permitted fractionation and recovery of ammonium (30–36%) and phosphate (83–95%) from dairy manure digestate across the 3 < pH < 11 range.211 However, membrane fouling is a challenge. Irreversible membrane fouling was attributed to the adsorption of substances related to humic acids and tyrosine to membranes.212,213 Effects of different filtration modes on membrane fouling have been examined while considering various parameters, including nutrient removal and sludge dewaterability.214 The addition of an inorganic coagulant (alum) reduced membrane fouling while improving phosphorus removal.215 Another improvement is precoagulation using poly aluminum chloride (PACl) or iron chloride as a coagulant, which improved membrane flux from 0.8 to 27.6 mL/m2/s.216 PACl-coagulation combined with sponge-membrane filtration can also effectively remove humic substances, polysaccharides, and other organic matter preventing membrane fouling and improving nutrient removal.217 Two-dimensional (2D) material-based membranes have also shown great promise in wastewater treatment. When laminated graphene oxide (GO)-cellulose nanocrystal hybrid membranes were fabricated and used, they allowed a high passage of desirable nutrients such as NO3– and H2PO4–.218
In the case of physical methods, patents contribute significantly to the total number of publications during the 2001–2021 period (Figure 18). Patent publications are important in physical wastewater treatment because physical processes such as dewatering, settling, filtration, absorption, and flocculation are broadly used in the preliminary treatment of wastewater. More than twice as many patents as journals were published in 2012. Another increase in patents is shown in 2017–2018, though a significant decrease occurred in the period 2019–2021. During this period, journals increased substantially, showing a reignited academic interest in the applications of physical methods.
Figure 18.
Number of publications on physical wastewater treatment for nutrient recovery throughout 2001–2021.
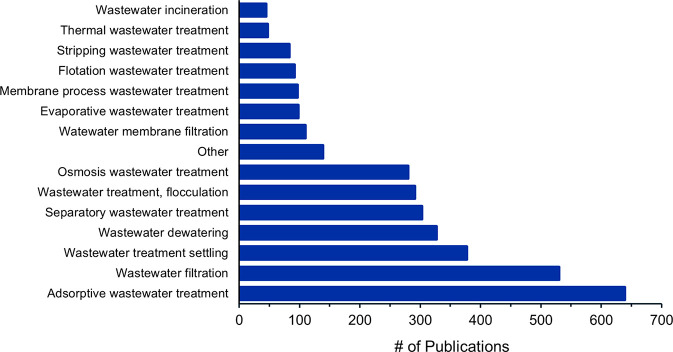
When looking at the top physical wastewater treatment concepts, “Adsorptive wastewater treatment” was the most prevalent (Figures 19 and 20). This concept is found in 91% publications on physical methods in general (Figure 19) and it is found in 82.5% of the publications on struvite production (Figure 20). “Wastewater filtration” contributes greatly to overall physical wastewater treatment (77%). “Osmosis wastewater treatment” was significantly less common despite the emergence of forward osmosis as a nutrient recovery technique, appearing in fewer than 300 documents (41%). For struvite production, only “Wastewater filtration” and “Wastewater treatment settling” are comparable with “Adsorptive wastewater treatment” accounting for 41% and 36%, respectively.
Figure 19.
Number of publications on physical wastewater treatment concepts from 2001 to 2021.
Substances Employed for Nutrient Recovery from Waste and Wastewater
Using Search Query 2 (see SI Methods) results, the main substances used to recover nutrients from waste/wastewater were identified, summarized, and divided by classes in Table 2. Several of them have already been mentioned in the previous process sections.
The class of elements is mostly represented by carbon (13,787 publications). Carbon stands for all types of activated carbons, including biochar. It is the most popular adsorbent known for its efficiency and low cost; often used for nutrient recovery from wastewater. Oxides are mostly represented by silica (1,214 publications), calcia (917 publications), and magnesia (882 publications). They can be employed not only as sorbents but also as flocculants (SiO2) and precipitating agents (CaO, MgO). Most frequently cited hydroxides including calcium, magnesium, and aluminum hydroxides are well-known as precipitating agents.
Metal salts are another class of broadly employed substances for nutrient recovery. Calcium carbonate (1,820 publications) is the most frequently reported compound and is used as a precipitating agent and sorbent. Other calcium salts along with magnesium, iron, and zinc salts are frequently used as precipitating agents and flocculants. A variety of natural minerals were also reported. For example, gypsum is a popular additive (1,182 publications) applied as a nutrient delivery improver and a nutrient loss inhibitor. Dolomite, calcite, magnesite, and biotite are precipitating agents. Vermiculite, montmorillonite, and clinoptilolite are ion-exchangers and sorbents.
Biopolymers also greatly contribute to nutrient recycling and recovery. Starch-based sorbents are the most frequently reported (1,597 publications). Cellulose (999 publications) is employed as an important component of filtration membranes. Hemicellulose (456 publications) is used as sorbent and in ion-exchange membranes. Chitosan (222 publications) is used as an encapsulant, sorbent, and/or flocculant.
Integrated Processes and Multipurpose Systems Utilizing Wastes: Biorefineries and More
As previously mentioned, recovery of nutrients using combinations of physical, chemical, and biological processes provides an alternative approach to sustainable fertilizer production. Biorefineries, farms which combine the production of biofuels, chemicals, and food with the use of the byproducts for energy and fertilizers, are one way to reduce the environmental impact of farming crops and livestock production.355
Locally produced biobased wastes that are not utilized for other purposes on-site must be transported for disposal or other processing. This adds to the cost of farming, food production, and other industrial processes that utilize biological sources. Integrating systems on-site for recycling and recovery of products from these wastes instead of transporting them for disposal improves the circularity of the economy and thus sustainability. In biorefineries, the recovery/recycling of nutrients from wastewater, the nonfuel fraction of algal biomass, and other biowaste makes a closed loop system. Such a locally adaptable, multipurpose system (an automated “zero waste” system) was recently patented in which the system was designed to be modular, mobile, and transportable.356 Waste inputs are converted to fertilizers, biogas/biofuels, chemicals, and/or clean water depending on which and how many modules are combined. Using separate process modules for separation and extraction, blend-heat, hydrolysis and acidification, first in - first out anaerobic digestion, aerobic-boost-blend, and formulation of the products to control nutrient release allows the system to respond effectively to local needs.
Biorefineries utilize different types of renewable biomass as a feedstock to produce a variety of products.59,357,358 Processes using algae biomass as a feedstock to produce biofuels, fertilizer nutrients, or other valuable byproducts have recently been patented.359 Animal-derived wastewaters and anaerobically digested manures have also been used to supply nutrients to biorefineries such as one using lipid-rich pig wastes to grow a lipid-accumulating algal biomass which can be used for biodiesel production.360 Anaerobic digestion of microalgal biomass can yield methane byproduct (biogas), while N and P absorbed into algal biomass digestate can subsequently be processed into N and P containing biochar for use as fertilizer.59,357,358
A macroalgal biorefinery with offshore seaweed cultivation can produce bioethanol, liquid fertilizers, and protein-rich ingredients for fish feed.113,361,362 On the other hand, struvite and other fertilizers can be produced from agricultural crop biorefineries under anaerobic digestion.363,364 Ammonium sulfate fertilizer can also be produced in an agro-biorefinery.365 It was also shown that several valuable products can be recovered from biorefinery-processed cellulosic primary sludge: methane (anaerobic digestion), short-chain fatty acids (acidogenic fermentation), and phosphorus as struvite (precipitation/crystallization).366
While biofuels may compete with food crops for fertilizer needs, integrating processes for biofuel production and nutrient recovery may reduce their fertilizer demand and thus make them more sustainable; for example, lipid-rich pig wastes were fed to a lipid-accumulating algal mass for potential use in biodiesel production.367 Biorefineries can use biobased wastes as feedstock for generating biogas, making biochar, or other products by combining processes, thus enhancing a circular approach to waste management.368 Considering the increased demand for renewable fertilizers, nutrients recovery from waste should be a key point of a biorefinery operation. Moreover, nutrient recovery not only provides a valuable marketable biorefinery product, but also prevents eutrophication through recovery of nutrients that otherwise might be released to the environment.58Figure 21 shows CAS concepts occurring in patents and journals published 2001–2021 and associated with biorefineries (see SI Methods: Broad Search). “Biomass combustion” is found mainly in journals and associated with the academic study of NOx emissions from N-fertilized soils.369−371 “Biomass refinery” is also prevalent in journals and connects to studies on fertilizer nutrient extraction from anaerobic digestates.363,364 At the same time, “biomass refining” occurs more frequently in patents (71%) and describes biochar-fertilizer production372 and other nutrients recovery from waste.373 The “Biomass gasification” contribution in patents is almost 40% and includes patents on chemical fertilizer production via manure gasification,374 as well as carbon-based organic fertilizer production through the combined gasification and carbonization of organic solid waste.375 “Biomass pyrolysis” is found mainly in journals but it is not exactly rare in patents (27%). Liquid fertilizers produced by biomass pyrolysis376 and N-fertilizers produced via urea-formaldehyde resin and biomass copyrolysis have also been patented.377
Green Ammonia Synthesis
Ammonia has had a significant global impact since the Haber-Bosch (H–B) process was discovered for its synthesis from hydrogen and nitrogen at the beginning of the 20th century. Today, ammonia plays a key role as an essential feedstock of inorganic fertilizers, supporting food production for approximately half of the world’s population.378 Ammonia synthesis is among the largest carbon dioxide-emitting industrial processes and is therefore a prime opportunity for the application of renewable energy.
Sustainable ammonia or green ammonia is ammonia synthesized using renewable energy, nitrogen, and water. The road to sustainable ammonia has been described as a transition of generations of technology.379 Brown ammonia is the current form of industrially produced ammonia via steam reforming and the H–B process. Blue ammonia, or low-carbon ammonia, is the result of brown ammonia with carbon capture and storage technology applied during manufacturing processes. Green ammonia is defined as zero-carbon ammonia, made using sustainable electricity, water, and air.31,380,381 Green ammonia synthesis has been proposed to first take place by substituting steam reforming of methane with green hydrogen generated from green energy-powered electrolysis of water.331,332 Application of the green hydrogen into a H–B process powered by renewable energy would generate green ammonia and zero carbon.382 An additional stage of green ammonia is the direct catalyzed synthesis of ammonia from water and nitrogen powered by renewable energy.
Direct reduction of nitrogen with water and electricity would be ideal for distributed ammonia generation, meaning that ammonia could be directly generated at the farm without the need of transportation or large-scale industrial equipment.383 To date, catalysts have only been shown to be capable of low rates of ammonia production and only in laboratory scale synthesis. New electrocatalysts, electrolytes, and systems must be developed that can produce ammonia in preference to hydrogen and achieve competitive production rates. However, this field has progressed quickly over the last 10–20 years and with continuous rigorous experimentation, a viable system may not be far off.
Miller and co-workers have discussed the economic feasibility of the direct electrochemical reduction of nitrogen (E-NR) versus the green ammonia synthesized by electrolysis of water in combination with the H–B process.383 Through their analysis, they found that the direct E-NR was less expensive than the alternative with electrolysis of water and H–B. The assumptions in their analysis of the direct electrochemical nitrogen reduction apply a 95% faradaic efficiency and 0.60 V overpotential for an energy efficiency of 62.2%. Interestingly a recent report by MacFarlane and co-workers demonstrated a nearly 100% faradaic efficiency for the electrochemical reduction of nitrogen at lithium. When a social cost of carbon emissions was applied to the current brown ammonia synthesis, the direct electrochemical reduction of ammonia becomes the most economically feasible route.
As an expert-curated resource, the CAS content is utilized here for the quantitative analysis of publications against variables including time, country/region, research area, and substance details. Query 3 (see SI Methods: Query 3) was used to retrieve documents that are specific to reports discussing catalytic nitrogen reduction. A total of 7,584 documents were used for the analysis described below. We have used this resource to investigate the recent progress toward nitrogen reduction reaction (NRR) with an emphasis on electrocatalysts and photocatalysts. The recent research landscape in this area can be visualized in many ways; we begin by presenting the most commonly co-occurring concepts found to be important within each respective study in a clustered network diagram generated by VOSviewer (Figure 22).
Figure 22.
Top 50 co-occurring concepts in green ammonia production literature from 2001–2021.
From 2001 to 2021, electrochemical reduction and related concepts were among the most frequently occurring concepts we have found associated with green ammonia and NRR, with “photocatalysts” occurring less frequently. The concept “photocatalysts” has significant overlap with several nanomaterial-related concepts. The top three nanomaterial-related concepts co-occurring with “photocatalysts” are “nanoparticles”, “nanosheets”, and “nanocomposites.” We also see that the “hydrogen evolution reaction” concept is indexed at a significant rate. This finding underscores that studies of NRR catalysts in the presence of water must consider the competing water reduction reaction to optimize ammonia production. Finally, the inclusion of a cluster of surface-oriented concepts such as “surface structure”, “surface area”, and “pore size distribution” shows the relevance of surface phenomena in catalyst design.
There has been a significant growth, from 2001–2021, in the publication volume of research toward the electrocatalytic or photocatalytic reduction of dinitrogen (Figure 23). In 2001, of publications investigating catalytic NRR, less than 1% were discussing photocatalytic or electrocatalytic nitrogen reduction. In 2021, publications discussing photocatalytic or electrocatalytic NRR grew to more than 25% of all catalytic NRR publications. Green ammonia synthesis was discussed primarily in journal publications with the number of patent documents reaching 20% of the total publication volume in 2020. The increased availability of green electricity likely leads to a preference for electrochemical methods for ammonia production.
Figure 23.
Publication trends and distinct substances used for catalysts by year in green ammonia production research from 2001 to 2021.
To analyze which fields of catalytic research may have been contributors to the growth in publications, we investigated the catalysts that were discussed within these manuscripts over time (Figure 24). The number of distinct substances with a role in catalytic green ammonia synthesis has grown continuously from 2001 to 2021, indicating that the research area of photocatalytic or electrocatalytic is continuing to expand. The most recent four or five years have shown dramatic growth in substances, with less than 100 distinct substances in 2017 to nearly 500 distinct substances in 2021. Specific subsets of substance classes, such as inorganic materials (e.g., metal oxides), organic/inorganic small molecules, elements, and coordination compounds make up many of the new catalysts used in green ammonia synthesis. The use of inorganic materials may more easily translate to large-scale production, while small molecules may more easily allow for mechanistic studies and optimization of catalysts.
Figure 24.

Publication trends and distinct substances used for catalysts by year in green ammonia synthesis research from 2001 to 2021.
A challenge faced in the development of green ammonia synthesis via NRR is the critical evaluation of catalysts. We analyzed the documents related to green ammonia synthesis to determine the prevalence of keywords important to catalyst evaluation. Of the 2,256 documents analyzed, 1,167 (52%) documents were found to have discussed reaction “rate”, “yield”, or “mechanism.” More than 40% of 1,500 documents with a focus on electrocatalysis discussed faradaic efficiencies. Recent reports have discussed the need for a set of standards for experimental reporting that would support the idea that ammonia formed during catalysis originated from N2.384 NRR experiments may be subject to contamination from exogenous ammonia or reducible forms of nitrogen that can affect the reporting of yields and catalyst efficiencies; therefore, control experiments are needed within the field.385 Experiments using 15N2 are important to confirm the source of nitrogen in ammonia. We analyzed the documents related to green ammonia synthesis to determine the prevalence of experiments utilizing 15N2 for experiments. Approximately 4.1% of the 2,256 documents were found to have discussed 15N or isotopically labeled reagents. This finding agrees with the assessment by NRR experts that there may be an insufficient use of control experiments.
Analysis of data from the CAS Content Collection indicates that interest in sustainable fertilizer production has grown, in part to reduce natural resource depletion and harmful greenhouse gas emissions. Sustainable fertilizer production encompasses “greener” processes for fertilizer production, such as green ammonia synthesis and the recovery of fertilizer nutrients from nitrogen- and phosphorus-containing wastes, and the development of more sustainable nutrient forms, such as nanomaterials, and of microbial and chemical additives and formulations to improve the efficiency of nutrient use. Journal publications discussing sustainable fertilizers have focused on soil, its properties, and their dependence on fertilizer use, while patent publications have focused on wastes, their processing methods, and their uses, such as in soil amendments. The recovery of phosphorus from wastes, wastewaters, wastewater treatment sludges, and incinerator ashes has also been an important research topic.
New regulations governing wastes and wastewaters encourage the use of recovered phosphorus, nitrogen, potassium, and trace elements in fertilizers, and the implementation of the methods on large scale will be required. One way to achieve reductions in resource use and emissions is using crop and livestock byproducts as fertilizers in biorefineries, reducing synthetic fertilizer use and transportation and energy costs while providing additional economic value. Publications in green ammonia production have focused on catalytic methods for reduction of nitrogen to ammonia and the optimization of processes and technology to improve yields and reduce costs.
Sustainable fertilizer production is likely to rely on a combination of improvements in the sustainable industrial production of ammonia and the use and processing of wastes for fertilizer use. Regulatory pressures on fertilizer use are likely to increase because of resource demands and eutrophication, while economic pressures are likely to demand more profitable integrated and multipurpose recovery technologies, suggesting that patent publications for sustainable fertilizer technologies, the use of recovered nutrients, and greener ammonia production will continue to rise. Implementing these technologies on scale may improve the health of soils and crops while providing sufficient food for growing populations and reducing the waste and greenhouse gas emissions of food production.
Acknowledgments
The authors sincerely appreciate the CAS Data, Analytics & Insights team for their assistance in data extraction. The authors also appreciate Dharmini Patel for project coordination, along with Peter Jap and Cristina Tomeo for insightful discussion. The authors are also grateful to Manuel Guzman, Gilles Georges, Michael Dennis, Dawn Riedel, Dawn George, and Hong Xie for executive sponsorship.
Supporting Information Available
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/acs.jafc.3c00454.
Journal and patent publication numbers for the years 2001–2021 on the topic of fertilizers, sustainability, recycling, and recovery; journal and patent publication trend from China from 2001–2021 on the topic of fertilizers, sustainability, recycling, and recovery; journal and patent publication trend all other countries excluding China from 2001–2021 on the topic of fertilizers, sustainability, recycling, and recovery; publication trends of countries on the topic of controlled release fertilizers; top co-occurring concepts in patents on fertilizers, sustainability, recycling, and recovery topics with a focus on wastes and wastewaters- focus on topics co-occurring with “recycling”; top co-occurring concepts in patents on fertilizers, sustainability, recycling, and recovery topics with a focus on wastes and wastewaters- focus on topics co-occurring with “recycling”; patents vs journals on biological, chemical, and physical methods of nutrient recovery from wastewater over 2001–2021; trend of main struvite forms found in patents and journal combined 2001–2021 for broader set of documents on sustainable fertilizers; interactive VosViewer map files (PDF)
The authors declare no competing financial interest.
Special Issue
Published as part of the Journal of Agricultural and Food Chemistryvirtual special issue “The Future of Agriculture and Food: Sustainable Approaches to Achieve Zero Hunger”.
Supplementary Material
References
- United Nations Department of Economic and Social Affairs Population Division . World Population Prospects 2022: Summary of Results; United Nations Department of Economic and Social Affairs, 2022. https://www.un.org/development/desa/pd/sites/www.un.org.development.desa.pd/files/wpp2022_summary_of_results.pdf.
- Urso J. H.; Gilbertson L. M. Atom Conversion Efficiency: A New Sustainability Metric Applied to Nitrogen and Phosphorus Use in Agriculture. ACS Sustainable Chem. Eng. 2018, 6 (4), 4453–4463. 10.1021/acssuschemeng.7b03600. [DOI] [Google Scholar]
- The International Plant Nutrition Institute . Nitrogen; The Fertilizer Institute, 2020. https://www.tfi.org/sites/default/files/tfi-nitrogen.pdf (accessed 09-13-2022).
- The International Plant Nutrition Institute . Phosphorus. The Fertilizer Institute, 2020. https://www.tfi.org/sites/default/files/tfi-phosphorus.pdf (accessed 09-13-2022).
- The International Plant Nutrition Institute . Potassium. The Fertilizer Institute, 2020. https://www.tfi.org/sites/default/files/tfi-potassium.pdf (accessed 09-13-2022).
- Culman S.; Fulford A.; Camberato J.; Steinke K.. Tri-State Fertilizer Recommendations for Corn, Soybean, Wheat, and Alfalfa; The Ohio State University, 2020. https://extensionpubs.osu.edu/tri-state-fertilizer-recommendations-for-corn-soybean-wheat-and-alfalfa/.
- McLaurin W.; Reeves W.; Gaskin J. W.; Harris G. H.; Kissel D. E.; Boyhan G. E.. How to Convert an Inorganic Fertilizer Recommendation to an Organic One; University of Georgia, 2014. https://extension.uga.edu/publications/detail.html?number=C853&title=How%20to%20Convert%20an%20Inorganic%20Fertilizer%20Recommendation%20to%20an%20Organic%20One (accessed 09-13-2022).
- Ritchie H.How many people does synthetic fertilizer feed?; Global Change Data Lab, 2017. https://ourworldindata.org/how-many-people-does-synthetic-fertilizer-feed (accessed 2022 September 13th).
- Apodaca L. E.Nitrogen (Fixed)-ammonia; U.S. Geological Survey, Mineral Commodity Summaries, 2022. https://pubs.usgs.gov/periodicals/mcs2022/mcs2022-nitrogen.pdf.
- Jasinski S. M.Phosphate Rock; U.S. Geological Survey, Mineral Commodity Summaries, 2022. https://pubs.usgs.gov/periodicals/mcs2022/mcs2022-phosphate.pdf.
- Jasinski S. M.Potash; U.S. Geological Survey, Mineral Commodity Summaries, 2022. https://pubs.usgs.gov/periodicals/mcs2022/mcs2022-potash.pdf.
- Food and Agriculture Organization Of the United Nations . World fertilizer trends and outlook to 2020; Food and Agriculture Organization Of the United Nations: Rome, 2017. https://www.fao.org/3/i6895e/i6895e.pdf.
- Walling E.; Vaneeckhaute C. Greenhouse gas emissions from inorganic and organic fertilizer production and use: A review of emission factors and their variability. J. Environ. Manage. 2020, 276, 111211. 10.1016/j.jenvman.2020.111211. [DOI] [PubMed] [Google Scholar]
- Galloway J. N.; Cowling E. B. Reactive Nitrogen and The World: 200 Years of Change. AMBIO: A Journal of the Human Environment 2002, 31 (2), 64–71. 10.1579/0044-7447-31.2.64. [DOI] [PubMed] [Google Scholar]
- Galloway J. N.; Townsend A. R.; Erisman J. W.; Bekunda M.; Cai Z.; Freney J. R.; Martinelli L. A.; Seitzinger S. P.; Sutton M. A. Transformation of the Nitrogen Cycle: Recent Trends, Questions, and Potential Solutions. Science 2008, 320 (5878), 889–892. 10.1126/science.1136674. [DOI] [PubMed] [Google Scholar]
- Sellars S.; Nunes V.. Synthetic Nitrogen Fertilizer in the U.S.; Department of Agricultural and Consumer Economics, University of Illinois at Urbana-Champaign, 2017. https://farmdocdaily.illinois.edu/2021/02/synthetic-nitrogen-fertilizer-in-the-us.html.
- Mikkelsen R.The Facts: Nitrogen Fertilizer; The Mosaic Company, 2022. https://www.cropnutrition.com/resource-library/the-facts-nitrogen-fertilizer (accessed 09-13-2022).
- International Energy Agency . Ammonia Technology Roadmap; IEA, 2021. https://iea.blob.core.windows.net/assets/6ee41bb9-8e81-4b64-8701-2acc064ff6e4/AmmoniaTechnologyRoadmap.pdf.
- Matassa S.; Batstone D. J.; Hülsen T.; Schnoor J.; Verstraete W. Can Direct Conversion of Used Nitrogen to New Feed and Protein Help Feed the World?. Environ. Sci. Technol. 2015, 49 (9), 5247–5254. 10.1021/es505432w. [DOI] [PubMed] [Google Scholar]
- The Royal Society . Ammonia: zero-carbon fertiliser, fuel and energy store Policy Briefing; The Royal Society, 2020. https://royalsociety.org/-/media/policy/projects/green-ammonia/green-ammonia-policy-briefing.pdf.
- de Boer M. A.; Wolzak L.; Slootweg J. C.. Phosphorus: Reserves, Production, and Applications. In Phosphorus Recovery and Recycling; Ohtake H., Tsuneda S., Eds.; Springer Singapore, 2019; pp 75–100. [Google Scholar]
- Cordell D.; Drangert J.-O.; White S. The story of phosphorus: Global food security and food for thought. Global Environmental Change 2009, 19 (2), 292–305. 10.1016/j.gloenvcha.2008.10.009. [DOI] [Google Scholar]
- Vaccari D. A. Phosphorus: a looming crisis. Sci. Am. 2009, 300 (6), 54–59. 10.1038/scientificamerican0609-54. [DOI] [PubMed] [Google Scholar]
- Schalk O.Prairies ramp up potash production amid Russia sanctions blowback; Canadian Dimension, 2022. https://canadiandimension.com/articles/view/prairies-ramp-up-potash-production-amid-russia-sanctions-blowback (accessed 09-13-2022).
- Chaudhury D. R.US carves out mechanism for insulating Russian fertilizer exports from sanctions; Economic Times, 2022. https://economictimes.indiatimes.com/news/international/world-news/us-carves-out-mechanism-for-insulating-russian-fertilizer-exports-from-sanctions/articleshow/90577961.cms (accessed 09-28-2022).
- United Nations . Glossary of Environment Statistics, Studies in Methods; 1997. https://stats.oecd.org/#:~:text=Definition%3A,plant%20residues%20containing%20sufficient%20nitrogen.
- Fijalkowski K.; Rorat A.; Grobelak A.; Kacprzak M. J. The presence of contaminations in sewage sludge - The current situation. J. Environ. Manage. 2017, 203, 1126–1136. 10.1016/j.jenvman.2017.05.068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kowalska A.; Grobelak A.; Almås Å. R.; Singh B. R. Effect of Biowastes on Soil Remediation, Plant Productivity and Soil Organic Carbon Sequestration: A Review. Energies 2020, 13 (21), 5813. 10.3390/en13215813. [DOI] [Google Scholar]
- United States Environmental Protection Agency . Learn About Sustainability; U.S. EPA, 2021. https://www.epa.gov/sustainability/learn-about-sustainability (accessed 09-13-2022).
- The Chemical Compliance Coach B.V . What is the Difference Between Recovey and Recycling?; The Chemical Compliance Coach, 2021. https://thechemicalcompliancecoach.com/what-is-the-difference-between-recovery-and-recycling/ (accessed 09-13-2022).
- Smart K. Review of Recent Progress in Green Ammonia Synthesis: Decarbonisation of fertiliser and fuels via green synthesis. Johnson Matthey Technol. Rev. 2022, 66 (3), 230–244. 10.1595/205651322X16334238659301. [DOI] [Google Scholar]
- Peiris K. T. H. P. A. L.; Amarasinghe M. T. U.; Munaweera M. T. I. S.; Karunaratne N. L. V. V.; Kottegoda N. S.. Method of making a nano-fertilizer composition for sustained release of macronutrients. WO2022180504, 2022.
- Raliya R.; Saharan V.; Dimkpa C.; Biswas P. Nanofertilizer for Precision and Sustainable Agriculture: Current State and Future Perspectives. J. Agric. Food. Chem. 2018, 66 (26), 6487–6503. 10.1021/acs.jafc.7b02178. [DOI] [PubMed] [Google Scholar]
- An C.; Sun C.; Li N.; Huang B.; Jiang J.; Shen Y.; Wang C.; Zhao X.; Cui B.; Wang C.; et al. Nanomaterials and nanotechnology for the delivery of agrochemicals: strategies towards sustainable agriculture. J. Nanobiotechnol. 2022, 20 (1), 11. 10.1186/s12951-021-01214-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Venugopalan V. K.; Nath R.; M. A S. C. Smart fertilizers - a way ahead for sustainable agriculture. J. Plant Nutr. 2022, 45 (13), 2068–2076. 10.1080/01904167.2022.2046065. [DOI] [Google Scholar]
- Mendes R.; Kruijt M.; de Bruijn I.; Dekkers E.; van der Voort M.; Schneider J. H. M.; Piceno Y. M.; DeSantis T. Z.; Andersen G. L.; Bakker P. A. H. M.; et al. Deciphering the Rhizosphere Microbiome for Disease-Suppressive Bacteria. Science 2011, 332 (6033), 1097–1100. 10.1126/science.1203980. [DOI] [PubMed] [Google Scholar]
- Schwab F. Opportunities and Limitations of Nanoagrochemicals. Helv. Chim. Acta 2023, 106, e202200136 10.1002/hlca.202200136. [DOI] [Google Scholar]
- Fertahi S.; Ilsouk M.; Zeroual Y.; Oukarroum A.; Barakat A. Recent trends in organic coating based on biopolymers and biomass for controlled and slow release fertilizers. J. Controlled Release 2021, 330, 341–361. 10.1016/j.jconrel.2020.12.026. [DOI] [PubMed] [Google Scholar]
- Liu C.; Ma H.; Gao Y.; Yao W.. Method for preparing ammonium zeolite with analcime powder and its use as slow-release nitrogen fertilizer or slow-release fertilizer carrier. CN108017415, 2018.
- Zhou X.; Zhou M.. Mixing device for coated controlled release fertilizer. CN109111301, 2019.
- Xu X.Slow and controlled release fertilizer. CN109776241, 2019.
- Britton A.; Verigin M.; Leatherwood R.; Sathyanarayana R. P. M.. Intermediate-Release Fertilizers and Methods for Making Same. WO/2023/065025, 2023.
- Modolo L. V.; da-Silva C. J.; Brandão D. S.; Chaves I. S. A minireview on what we have learned about urease inhibitors of agricultural interest since mid-2000s. J. Adv. Res. 2018, 13, 29–37. 10.1016/j.jare.2018.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ren Q.Preparation method of water retaining slow release fertilizer. CN108976003, 2018.
- Mahanty T.; Bhattacharjee S.; Goswami M.; Bhattacharyya P.; Das B.; Ghosh A.; Tribedi P. Biofertilizers: a potential approach for sustainable agriculture development. Environ. Sci. Pollut. Res. 2017, 24 (4), 3315–3335. 10.1007/s11356-016-8104-0. [DOI] [PubMed] [Google Scholar]
- Wang Q.; Chen G.; Li M.; Zhang S.. Slow release microbial fertilizer and preparation method thereof. CN108191553, 2018.
- Garcia-Fraile P.; Menendez E.; Rivas R. Role of bacterial biofertilizers in agriculture and forestry. AIMS Bioeng. 2015, 2 (3), 183–205. 10.3934/bioeng.2015.3.183. [DOI] [Google Scholar]
- Sharma S. B.; Sayyed R. Z.; Trivedi M. H.; Gobi T. A. Phosphate solubilizing microbes: sustainable approach for managing phosphorus deficiency in agricultural soils. SpringerPlus 2013, 2 (1), 587. 10.1186/2193-1801-2-587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thonar C.; Lekfeldt J. D. S.; Cozzolino V.; Kundel D.; Kulhánek M.; Mosimann C.; Neumann G.; Piccolo A.; Rex M.; Symanczik S.; et al. Potential of three microbial bio-effectors to promote maize growth and nutrient acquisition from alternative phosphorous fertilizers in contrasting soils. Chem. Biol. Technol. Agric. 2017, 4 (1), 7. 10.1186/s40538-017-0088-6. [DOI] [Google Scholar]
- Pang F.; Tao A.; Ayra-Pardo C.; Wang T.; Yu Z.; Huang S. Plant organ- and growth stage-diversity of endophytic bacteria with potential as biofertilisers isolated from wheat (Triticum aestivum L.). BMC Plant Biol. 2022, 22 (1), 276. 10.1186/s12870-022-03615-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adnan M.; Fahad S.; Saleem M. H.; Ali B.; Mussart M.; Ullah R.; Amanullah Jr.; Arif M.; Ahmad M.; Shah W. A.; et al. Comparative efficacy of phosphorous supplements with phosphate solubilizing bacteria for optimizing wheat yield in calcareous soils. Sci. Rep. 2022, 12 (1), 11997. 10.1038/s41598-022-16035-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quintana M.; Colmenarejo M. F.; Barrera J.; García G.; García E.; Bustos A. Use of a Byproduct of Magnesium Oxide Production To Precipitate Phosphorus and Nitrogen as Struvite from Wastewater Treatment Liquors. J. Agric. Food. Chem. 2004, 52 (2), 294–299. 10.1021/jf0303870. [DOI] [PubMed] [Google Scholar]
- Kumar I. A.; Viswanathan N. Development of multivalent metal ions imprinted chitosan biocomposites for phosphate sorption. Int. J. Biol. Macromol. 2017, 104, 1539–1547. 10.1016/j.ijbiomac.2017.02.100. [DOI] [PubMed] [Google Scholar]
- United Nations Department of Economic and Social Affairs . Nutrialgae - Novel sustainable algae-based fertilizers; UN Department of Economic and Social Affairs, 2021. https://sdgs.un.org/partnerships/nutrialgae-novel-sustainable-algae-based-fertilizers.
- Krishnamoorthy N.; Dey B.; Unpaprom Y.; Ramaraj R.; Maniam G. P.; Govindan N.; Jayaraman S.; Arunachalam T.; Paramasivan B. Engineering principles and process designs for phosphorus recovery as struvite: A comprehensive review. J. Environ. Chem. Eng. 2021, 9 (5), 105579. 10.1016/j.jece.2021.105579. [DOI] [Google Scholar]
- Carrillo V.; Fuentes B.; Gómez G.; Vidal G. Characterization and recovery of phosphorus from wastewater by combined technologies. Rev. Environ. Sci. Bio/Technol. 2020, 19 (2), 389–418. 10.1007/s11157-020-09533-1. [DOI] [Google Scholar]
- Ye Y.; Ngo H. H.; Guo W.; Liu Y.; Li J.; Liu Y.; Zhang X.; Jia H. Insight into chemical phosphate recovery from municipal wastewater. Sci. Total Environ. 2017, 576, 159–171. 10.1016/j.scitotenv.2016.10.078. [DOI] [PubMed] [Google Scholar]
- Carey D. E.; Yang Y.; McNamara P. J.; Mayer B. K. Recovery of agricultural nutrients from biorefineries. Bioresour. Technol. 2016, 215, 186–198. 10.1016/j.biortech.2016.02.093. [DOI] [PubMed] [Google Scholar]
- Naik S. N.; Goud V. V.; Rout P. K.; Dalai A. K. Production of first and second generation biofuels: A comprehensive review. Renewable Sustainable Energy Rev. 2010, 14 (2), 578–597. 10.1016/j.rser.2009.10.003. [DOI] [Google Scholar]
- Yang C.; Zeng Q.. Coated controlled-release fertilizer production apparatus. CN204529696, 2015.
- Huang J.Controlled-release functional fertilizer for whole growing period, and preparation method thereof. CN103524200, 2014.
- Hou Z.; Niu Y.; Shi X.; Li B.; Song J.; Liu S.; Wang Y.; Peng C.; Tian Z.; Lu F.. et al. Humic acid-containing controlled release blended fertilizer specially used for corn. CN104829396, 2015.
- Staff F. C.Plan for Green and Sustainable Ag Development; CH2021–0130; USDA, 2021. https://apps.fas.usda.gov/newgainapi/api/Report/DownloadReportByFileName?fileName=Plan%20for%20Green%20and%20Sustainable%20Ag%20Development_Beijing_China%20-%20People%27s%20Republic%20of_10-21-2021.
- Anderson L.China’s Annual Agricultural Policy Goals The 2017 No. 1 Document of the CCCPC and the State Council; CH17006; USDA, 2017. https://apps.fas.usda.gov/newgainapi/api/Report/DownloadReportByFileName?fileName=China%27s%202017%20Agricultural%20Policy%20Goals_Beijing_China%20-%20Peoples%20Republic%20of_2-15-2017.
- Wu Y.; Wang E.; Miao C. Fertilizer Use in China: The Role of Agricultural Support Policies. Sustainability 2019, 11 (16), 4391. 10.3390/su11164391. [DOI] [Google Scholar]
- United States Environmental Protection Agency . Biosolids Technology Fact Sheet; United States Environmental Protection Agency, 2018. https://www.epa.gov/sites/default/files/2018-11/documents/use-incineration-biosolids-management-factsheet.pdf (accessed 09-13-2022).
- Good A. G.; Beatty P. H. Fertilizing Nature: A Tragedy of Excess in the Commons. PLoS Biol. 2011, 9 (8), e1001124 10.1371/journal.pbio.1001124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pepper I. L.; Brooks J. P.; Gerba C. P.. Pathogens in Biosolids. In Advances in Agronomy, Vol. 90; Academic Press, 2006; pp 1–41. [Google Scholar]
- Kinney C. A.; Furlong E. T.; Zaugg S. D.; Burkhardt M. R.; Werner S. L.; Cahill J. D.; Jorgensen G. R. Survey of Organic Wastewater Contaminants in Biosolids Destined for Land Application. Environ. Sci. Technol. 2006, 40 (23), 7207–7215. 10.1021/es0603406. [DOI] [PubMed] [Google Scholar]
- van Eck N. J.; Waltman L.. VOSviewer Manual; Universiteit Leiden, 2023. https://www.vosviewer.com/documentation/Manual_VOSviewer_1.6.19.pdf.
- van Eck N. J.; Waltman L.. Visualizing Bibliometric Networks. In Measuring Scholarly Impact: Methods and Practice, Ding Y., Rousseau R., Wolfram D., Eds.; Springer International Publishing, 2014; pp 285–320. [Google Scholar]
- Hu C.; Yuan Z.; Huang W.; Cai Y.; Huang C.; Deng Z.; Chen R.. Nano gel-sugarcane leaf/bagasse composite coated slow-release nitrogen fertilizer and its preparation method. CN106748577, 2017.
- Park D. G.Method for manufacturing slow-release fertilizer using biochar produced from biomass. KR2020081907, 2020.
- Ciceri D.; Manning D. A. C.; Allanore A. Historical and technical developments of potassium resources. Sci. Total Environ. 2015, 502, 590–601. 10.1016/j.scitotenv.2014.09.013. [DOI] [PubMed] [Google Scholar]
- Smith H.Can Farming Seaweed Put the Brakes on Climate Change?; Sierra Magazine, 2021. https://www.sierraclub.org/sierra/2021-2-summer/stress-test/can-farming-seaweed-put-brakes-climate-change (accessed 12-12-2022).
- Company E.; Farrés M.; Colprim J.; Magrí A. Exploring the recovery of potassium-rich struvite after a nitrification-denitrification process in pig slurry treatment. Sci. Total Environ. 2022, 847, 157574. 10.1016/j.scitotenv.2022.157574. [DOI] [PubMed] [Google Scholar]
- Johansson S.; Ruscalleda M.; Saerens B.; Colprim J. Potassium recovery from centrate: taking advantage of autotrophic nitrogen removal for multi-nutrient recovery. J. Chem. Technol. Biotechnol. 2019, 94 (3), 819–828. 10.1002/jctb.5828. [DOI] [Google Scholar]
- Shashvatt U.; Benoit J.; Aris H.; Blaney L. CO2-assisted phosphorus extraction from poultry litter and selective recovery of struvite and potassium struvite. Water Res. 2018, 143, 19–27. 10.1016/j.watres.2018.06.035. [DOI] [PubMed] [Google Scholar]
- Atalay S.; Sargin I.; Arslan G. Crystallization of struvite-K from pumpkin wastes. J. Sci. Food Agric. 2022, 102 (2), 523–530. 10.1002/jsfa.11380. [DOI] [PubMed] [Google Scholar]
- Silva A. F. R.; Lebron Y. A. R.; Brasil Y. L.; Lange L. C.; Amaral M. C. S. Effect of electrolyte solution recycling on the potassium recovery from vinasse by integrated electrodialysis and K-struvite precipitation processes. Chem. Eng. J. 2022, 450, 137975. 10.1016/j.cej.2022.137975. [DOI] [Google Scholar]
- Prakongkep N.; Gilkes R. J.; Wiriyakitnateekul W. Forms and solubility of plant nutrient elements in tropical plant waste biochars. J. Plant Nutr. Soil Sci. 2015, 178 (5), 732–740. 10.1002/jpln.201500001. [DOI] [Google Scholar]
- Rosso D.; Lothman S. E.; Jeung M. K.; Pitt P.; Gellner W. J.; Stone A. L.; Howard D. Oxygen transfer and uptake, nutrient removal, and energy footprint of parallel full-scale IFAS and activated sludge processes. Water Res. 2011, 45 (18), 5987–5996. 10.1016/j.watres.2011.08.060. [DOI] [PubMed] [Google Scholar]
- Wei Y.; Zhu H.; Wang Y.; Li J.; Zhang P.; Hu J.; Liu J. Nutrients release and phosphorus distribution during oligochaetes predation on activated sludge. Biochem. Eng. J. 2009, 43 (3), 239–245. 10.1016/j.bej.2008.10.004. [DOI] [Google Scholar]
- Yuan H.; Lu T.; Wang Y.; Chen Y.; Lei T. Sewage sludge biochar: Nutrient composition and its effect on the leaching of soil nutrients. Geoderma 2016, 267, 17–23. 10.1016/j.geoderma.2015.12.020. [DOI] [Google Scholar]
- Ashekuzzaman S. M.; Forrestal P.; Richards K.; Fenton O. Dairy industry derived wastewater treatment sludge: Generation, type and characterization of nutrients and metals for agricultural reuse. J. Cleaner Prod. 2019, 230, 1266–1275. 10.1016/j.jclepro.2019.05.025. [DOI] [Google Scholar]
- Yin X.; Xi M.; Li Y.; Kong F.; Jiang Z. Improvements in physicochemical and nutrient properties of sewage sludge biochar by the co-pyrolysis with organic additives. Sci. Total Environ. 2021, 779, 146565. 10.1016/j.scitotenv.2021.146565. [DOI] [PubMed] [Google Scholar]
- Hossain M. K.; Strezov V.; Yin Chan K.; Nelson P. F. Agronomic properties of wastewater sludge biochar and bioavailability of metals in production of cherry tomato (Lycopersicon esculentum). Chemosphere 2010, 78 (9), 1167–1171. 10.1016/j.chemosphere.2010.01.009. [DOI] [PubMed] [Google Scholar]
- Liu H.; Basar I. A.; Nzihou A.; Eskicioglu C. Hydrochar derived from municipal sludge through hydrothermal processing: A critical review on its formation, characterization, and valorization. Water Res. 2021, 199, 117186. 10.1016/j.watres.2021.117186. [DOI] [PubMed] [Google Scholar]
- Kumar Awasthi M.; Wang M.; Pandey A.; Chen H.; Kumar Awasthi S.; Wang Q.; Ren X.; Hussain Lahori A.; Li D.-s.; Li R.; et al. Heterogeneity of zeolite combined with biochar properties as a function of sewage sludge composting and production of nutrient-rich compost. Waste Manage. 2017, 68, 760–773. 10.1016/j.wasman.2017.06.008. [DOI] [PubMed] [Google Scholar]
- Buss W.; Bogush A.; Ignatyev K.; Mašek O. Unlocking the Fertilizer Potential of Waste-Derived Biochar. ACS Sustainable Chem. Eng. 2020, 8 (32), 12295–12303. 10.1021/acssuschemeng.0c04336. [DOI] [Google Scholar]
- Zhang H.; Qian H.; Yu Q.; Feng Z.; He J.; Yu H.; Hao X.. Method for preparing low-lead plant-derived organic slow-release nitrogen fertilizer by extrusion texturization and enzymolysis. CN109485499, 2019.
- Liu H.; Hu G.; Basar I. A.; Li J.; Lyczko N.; Nzihou A.; Eskicioglu C. Phosphorus recovery from municipal sludge-derived ash and hydrochar through wet-chemical technology: A review towards sustainable waste management. Chem. Eng. J. 2021, 417, 129300. 10.1016/j.cej.2021.129300. [DOI] [Google Scholar]
- Ma J.; Luo H.; Li Y.; Liu Z.; Li D.; Gai C.; Jiao W. Pyrolysis kinetics and thermodynamic parameters of the hydrochars derived from co-hydrothermal carbonization of sawdust and sewage sludge using thermogravimetric analysis. Bioresour. Technol. 2019, 282, 133–141. 10.1016/j.biortech.2019.03.007. [DOI] [PubMed] [Google Scholar]
- Chu Q.; Xue L.; Singh B. P.; Yu S.; Müller K.; Wang H.; Feng Y.; Pan G.; Zheng X.; Yang L. Sewage sludge-derived hydrochar that inhibits ammonia volatilization, improves soil nitrogen retention and rice nitrogen utilization. Chemosphere 2020, 245, 125558. 10.1016/j.chemosphere.2019.125558. [DOI] [PubMed] [Google Scholar]
- Khoshbouy R.; Takahashi F.; Yoshikawa K. Preparation of high surface area sludge-based activated hydrochar via hydrothermal carbonization and application in the removal of basic dye. Environ. Res. 2019, 175, 457–467. 10.1016/j.envres.2019.04.002. [DOI] [PubMed] [Google Scholar]
- Logan M.; Visvanathan C. Management strategies for anaerobic digestate of organic fraction of municipal solid waste: Current status and future prospects. Waste Manage. Res. 2019, 37, 27–39. 10.1177/0734242X18816793. [DOI] [PubMed] [Google Scholar]
- Khalid A.; Arshad M.; Anjum M.; Mahmood T.; Dawson L. The anaerobic digestion of solid organic waste. Waste Manage. 2011, 31 (8), 1737–1744. 10.1016/j.wasman.2011.03.021. [DOI] [PubMed] [Google Scholar]
- Ma H.; Guo Y.; Qin Y.; Li Y.-Y. Nutrient recovery technologies integrated with energy recovery by waste biomass anaerobic digestion. Bioresour. Technol. 2018, 269, 520–531. 10.1016/j.biortech.2018.08.114. [DOI] [PubMed] [Google Scholar]
- Song Y.-H.; Qiu G.-L.; Yuan P.; Cui X.-Y.; Peng J.-F.; Zeng P.; Duan L.; Xiang L.-C.; Qian F. Nutrients removal and recovery from anaerobically digested swine wastewater by struvite crystallization without chemical additions. J. Hazard. Mater. 2011, 190 (1), 140–149. 10.1016/j.jhazmat.2011.03.015. [DOI] [PubMed] [Google Scholar]
- Marti N.; Bouzas A.; Seco A.; Ferrer J. Struvite precipitation assessment in anaerobic digestion processes. Chem. Eng. J. 2008, 141 (1), 67–74. 10.1016/j.cej.2007.10.023. [DOI] [Google Scholar]
- Füleky G.; Benedek S.. Composting to Recycle Biowaste. In Sociology, Organic Farming, Climate Change and Soil Science, Lichtfouse E., Ed.; Springer: Netherlands, 2010; pp 319–346. [Google Scholar]
- Cao R.; Wang J.; Ben W.; Qiang Z. The profile of antibiotic resistance genes in pig manure composting shaped by composting stage: Mesophilic-thermophilic and cooling-maturation stages. Chemosphere 2020, 250, 126181. 10.1016/j.chemosphere.2020.126181. [DOI] [PubMed] [Google Scholar]
- Raut M. P.; Prince William S. P. M.; Bhattacharyya J. K.; Chakrabarti T.; Devotta S. Microbial dynamics and enzyme activities during rapid composting of municipal solid waste – A compost maturity analysis perspective. Bioresour. Technol. 2008, 99 (14), 6512–6519. 10.1016/j.biortech.2007.11.030. [DOI] [PubMed] [Google Scholar]
- Sánchez Ó. J.; Ospina D. A.; Montoya S. Compost supplementation with nutrients and microorganisms in composting process. Waste Manage. 2017, 69, 136–153. 10.1016/j.wasman.2017.08.012. [DOI] [PubMed] [Google Scholar]
- Liu M. D.; Cong N.; Zhang W.; Wang Y. J. Vermicomposting of Chinese Medicine Residue with Earthworms. Adv. Mater. Res. (Durnten-Zurich, Switz.) 2012, 518–523, 3577–3584. 10.4028/www.scientific.net/AMR.518-523.3577. [DOI] [Google Scholar]
- Srivastava V.; Vaish B.; Singh R. P.; Singh P. An insight to municipal solid waste management of Varanasi city, India, and appraisal of vermicomposting as its efficient management approach. Environ. Monit. Assess. 2020, 192 (3), 191. 10.1007/s10661-020-8135-3. [DOI] [PubMed] [Google Scholar]
- Hill G. B.; Baldwin S. A. Vermicomposting toilets, an alternative to latrine style microbial composting toilets, prove far superior in mass reduction, pathogen destruction, compost quality, and operational cost. Waste Manage. 2012, 32 (10), 1811–1820. 10.1016/j.wasman.2012.04.023. [DOI] [PubMed] [Google Scholar]
- Hanc A.; Chadimova Z. Nutrient recovery from apple pomace waste by vermicomposting technology. Bioresour. Technol. 2014, 168, 240–244. 10.1016/j.biortech.2014.02.031. [DOI] [PubMed] [Google Scholar]
- Swarnam T. P.; Velmurugan A.; Pandey S. K.; Dam Roy S. Enhancing nutrient recovery and compost maturity of coconut husk by vermicomposting technology. Bioresour. Technol. 2016, 207, 76–84. 10.1016/j.biortech.2016.01.046. [DOI] [PubMed] [Google Scholar]
- Dong J.; Tang Y.; Nzihou A.; Chi Y. Key factors influencing the environmental performance of pyrolysis, gasification and incineration Waste-to-Energy technologies. Energy Convers. Manage. 2019, 196, 497–512. 10.1016/j.enconman.2019.06.016. [DOI] [Google Scholar]
- Parés Viader R.; Jensen P. E.; Ottosen L. M.; Thomsen T. P.; Ahrenfeldt J.; Hauggaard-Nielsen H. Comparison of phosphorus recovery from incineration and gasification sewage sludge ash. Water Sci. Technol. 2017, 75 (5), 1251–1260. 10.2166/wst.2016.620. [DOI] [PubMed] [Google Scholar]
- Wyciszkiewicz M.; Saeid A.; Malinowski P.; Chojnacka K. Valorization of Phosphorus Secondary Raw Materials by Acidithiobacillus ferrooxidans. Molecules 2017, 22 (3), 473. 10.3390/molecules22030473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cantrell K.; Ro K.; Mahajan D.; Anjom M.; Hunt P. G. Role of Thermochemical Conversion in Livestock Waste-to-Energy Treatments: Obstacles and Opportunities. Ind. Eng. Chem. Res. 2007, 46 (26), 8918–8927. 10.1021/ie0616895. [DOI] [Google Scholar]
- Brewer C. E.; Schmidt-Rohr K.; Satrio J. A.; Brown R. C. Characterization of biochar from fast pyrolysis and gasification systems. Environ. Prog. Sustainable Energy 2009, 28 (3), 386–396. 10.1002/ep.10378. [DOI] [Google Scholar]
- Lu Y.; Savage P. E. Supercritical water gasification of lipid-extracted hydrochar to recover energy and nutrients. J. Supercrit. Fluids 2015, 99, 88–94. 10.1016/j.supflu.2015.01.019. [DOI] [Google Scholar]
- Masebinu S. O.; Akinlabi E. T.; Muzenda E.; Aboyade A. O. A review of biochar properties and their roles in mitigating challenges with anaerobic digestion. Renewable Sustainable Energy Rev. 2019, 103, 291–307. 10.1016/j.rser.2018.12.048. [DOI] [Google Scholar]
- Bridle T. R.; Pritchard D. Energy and nutrient recovery from sewage sludge via pyrolysis. Water Sci. Technol. 2004, 50 (9), 169–175. 10.2166/wst.2004.0562. [DOI] [PubMed] [Google Scholar]
- Mercl F.; Kosnar Z.; Pierdona L.; ulloa-Murillo L. M.; Szakova J.; Tlustos P. Changes in availability of Ca, K, Mg, P and S in sewage sludge as affected by pyrolysis temperature. Plant, Soil Environ. 2020, 66 (4), 143–148. 10.17221/605/2019-PSE. [DOI] [Google Scholar]
- Marcińczyk M.; Ok Y. S.; Oleszczuk P. From waste to fertilizer: Nutrient recovery from wastewater by pristine and engineered biochars. Chemosphere 2022, 306, 135310. 10.1016/j.chemosphere.2022.135310. [DOI] [PubMed] [Google Scholar]
- Kasera N.; Kolar P.; Hall S. G. Nitrogen-doped biochars as adsorbents for mitigation of heavy metals and organics from water: a review. Biochar 2022, 4 (1), 17. 10.1007/s42773-022-00145-2. [DOI] [Google Scholar]
- Kassem I.; Ablouh E.-H.; El Bouchtaoui F.-Z.; Hannache H.; Ghalfi H.; Sehaqui H.; El Achaby M. Cellulose Nanofibers/Engineered Biochar Hybrid Materials as Biodegradable Coating for Slow-Release Phosphate Fertilizers. ACS Sustainable Chem. Eng. 2022, 10 (46), 15250–15262. 10.1021/acssuschemeng.2c04953. [DOI] [Google Scholar]
- Maroušek J.; Kolář L.; Strunecký O.; Kopecký M.; Bartoš P.; Maroušková A.; Cudlínová E.; Konvalina P.; Šoch M.; Moudrý J.; et al. Modified biochars present an economic challenge to phosphate management in wastewater treatment plants. J. Cleaner Prod. 2020, 272, 123015. 10.1016/j.jclepro.2020.123015. [DOI] [Google Scholar]
- Nageshwari K.; Chang S. X.; Balasubramanian P. Integrated electrocoagulation-flotation of microalgae to produce Mg-laden microalgal biochar for seeding struvite crystallization. Sci. Rep. 2022, 12 (1), 11463. 10.1038/s41598-022-15527-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alhnidi M.-J.; Wüst D.; Funke A.; Hang L.; Kruse A. Fate of Nitrogen, Phosphate, and Potassium during Hydrothermal Carbonization and the Potential for Nutrient Recovery. ACS Sustainable Chem. Eng. 2020, 8 (41), 15507–15516. 10.1021/acssuschemeng.0c04229. [DOI] [Google Scholar]
- United States Environmental Protection Agency . Biosolids Laws and Regulations; U.S. EPA, 2022. https://www.epa.gov/biosolids/biosolids-laws-and-regulations (accessed 09-01-2022).
- European Commission . Sewage sludge; European Commission, 2022. https://environment.ec.europa.eu/topics/waste-and-recycling/sewage-sludge_en (accessed 09-01-2022).
- Smol M.; Kulczycka J.; Kowalski Z. Sewage sludge ash (SSA) from large and small incineration plants as a potential source of phosphorus – Polish case study. J. Environ. Manage. 2016, 184, 617–628. 10.1016/j.jenvman.2016.10.035. [DOI] [PubMed] [Google Scholar]
- Li M.; Zhang H.; Sun H.; Mohammed A.; Liu Y.; Lu Q. Effect of phosphate and ammonium concentrations, total suspended solids and alkalinity on lignin-induced struvite precipitation. Sci. Rep. 2022, 12 (1), 2901. 10.1038/s41598-022-06930-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pindine G. P.; Trembly J. P.; Daramola D. A. Equilibrium-Based Temperature-Dependent Economic Analysis of the Recovery of Phosphorus from Different Wastewater Streams via Chemical Precipitation. ACS ES&T Water 2021, 1 (11), 2318–2326. 10.1021/acsestwater.1c00166. [DOI] [Google Scholar]
- Yetilmezsoy K.; Ilhan F.; Kocak E.; Akbin H. M. Feasibility of struvite recovery process for fertilizer industry: A study of financial and economic analysis. J. Cleaner Prod. 2017, 152, 88–102. 10.1016/j.jclepro.2017.03.106. [DOI] [Google Scholar]
- Ledezma P.; Kuntke P.; Buisman C. J. N.; Keller J.; Freguia S. Source-separated urine opens golden opportunities for microbial electrochemical technologies. Trends Biotechnol. 2015, 33 (4), 214–220. 10.1016/j.tibtech.2015.01.007. [DOI] [PubMed] [Google Scholar]
- Maurer M.; Pronk W.; Larsen T. A. Treatment processes for source-separated urine. Water Res. 2006, 40 (17), 3151–3166. 10.1016/j.watres.2006.07.012. [DOI] [PubMed] [Google Scholar]
- Biswas J. K.; Rana S.; Meers E.. Bioregenerative Nutrient Recovery from Human Urine: Closing the Loop in Turning Waste into Wealth In Biorefinery of Inorganics: Recovering Mineral Nutrients from Biomass and Organic Waste, 1st ed.; Meers E., Velthof G., Michels E., Rietra R., Eds.; John Wiley & Sons Ltd., 2020; pp 161–176. [Google Scholar]
- Schipper W.Success Factors for Implementing Phosphorus Recycling Technologies. In Phosphorus Recovery and Recycling, Ohtake H., Tsuneda S., Eds.; Springer: Singapore, 2019; pp 101–130. [Google Scholar]
- Chrispim M. C.; Scholz M.; Nolasco M. A. Phosphorus recovery from municipal wastewater treatment: Critical review of challenges and opportunities for developing countries. J. Environ. Manage. 2019, 248, 109268. 10.1016/j.jenvman.2019.109268. [DOI] [PubMed] [Google Scholar]
- Günther S.; Grunert M.; Müller S. Overview of recent advances in phosphorus recovery for fertilizer production. Eng. Life Sci. 2018, 18 (7), 434–439. 10.1002/elsc.201700171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mehta C. M.; Khunjar W. O.; Nguyen V.; Tait S.; Batstone D. J. Technologies to Recover Nutrients from Waste Streams: A Critical Review. Crit. Rev. Environ. Sci. Technol. 2015, 45 (4), 385–427. 10.1080/10643389.2013.866621. [DOI] [Google Scholar]
- Ahmed M.; Ahmad S.; Fayyaz-ul-Hassan; Qadir G.; Hayat R.; Shaheen F. A.; Raza M. A. Innovative Processes and Technologies for Nutrient Recovery from Wastes: A Comprehensive Review. Sustainability 2019, 11 (18), 4938. 10.3390/su11184938. [DOI] [Google Scholar]
- Pradel M.; Aissani L. Environmental impacts of phosphorus recovery from a “product” Life Cycle Assessment perspective: Allocating burdens of wastewater treatment in the production of sludge-based phosphate fertilizers. Sci. Total Environ. 2019, 656, 55–69. 10.1016/j.scitotenv.2018.11.356. [DOI] [PubMed] [Google Scholar]
- Hukari S.; Hermann L.; Nättorp A. From wastewater to fertilisers — Technical overview and critical review of European legislation governing phosphorus recycling. Sci. Total Environ. 2016, 542, 1127–1135. 10.1016/j.scitotenv.2015.09.064. [DOI] [PubMed] [Google Scholar]
- Sondh S.; Upadhyay D. S.; Patel S.; Patel R. N. A strategic review on Municipal Solid Waste (living solid waste) management system focusing on policies, selection criteria and techniques for waste-to-value. J. Cleaner Prod. 2022, 356, 131908. 10.1016/j.jclepro.2022.131908. [DOI] [Google Scholar]
- Wang X.; Daigger G.; Lee D.-J.; Liu J.; Ren N.-Q.; Qu J.; Liu G.; Butler D. Evolving wastewater infrastructure paradigm to enhance harmony with nature. Sci. Adv. 2018, 4 (8), eaaq0210 10.1126/sciadv.aaq0210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zulfiqar M.; Omar A. A.; Chowdhury S. Removal of Phosphate and Fluoride from Industrial Wastewater – A Short Review. Appl. Mech. Mater. 2014, 625, 805–808. 10.4028/www.scientific.net/AMM.625.805. [DOI] [Google Scholar]
- Bradford-Hartke Z.; Lane J.; Lant P.; Leslie G. Environmental Benefits and Burdens of Phosphorus Recovery from Municipal Wastewater. Environ. Sci. Technol. 2015, 49 (14), 8611–8622. 10.1021/es505102v. [DOI] [PubMed] [Google Scholar]
- Ishii S. K. L.; Boyer T. H. Life cycle comparison of centralized wastewater treatment and urine source separation with struvite precipitation: Focus on urine nutrient management. Water Res. 2015, 79, 88–103. 10.1016/j.watres.2015.04.010. [DOI] [PubMed] [Google Scholar]
- Lamastra L.; Suciu N. A.; Trevisan M. Sewage sludge for sustainable agriculture: contaminants’ contents and potential use as fertilizer. Chem. Biol. Technol. Agric. 2018, 5 (1), 10. 10.1186/s40538-018-0122-3. [DOI] [Google Scholar]
- Le Corre K. S.; Valsami-Jones E.; Hobbs P.; Parsons S. A. Phosphorus Recovery from Wastewater by Struvite Crystallization: A Review. Crit. Rev. Environ. Sci. Technol. 2009, 39 (6), 433–477. 10.1080/10643380701640573. [DOI] [Google Scholar]
- Zhang Y.; Desmidt E.; Van Looveren A.; Pinoy L.; Meesschaert B.; Van der Bruggen B. Phosphate Separation and Recovery from Wastewater by Novel Electrodialysis. Environ. Sci. Technol. 2013, 47 (11), 5888–5895. 10.1021/es4004476. [DOI] [PubMed] [Google Scholar]
- El Diwani G.; El Rafie S.; El Ibiari N. N.; El-Aila H. I. Recovery of ammonia nitrogen from industrial wastewater treatment as struvite slow releasing fertilizer. Desalination 2007, 214 (1), 200–214. 10.1016/j.desal.2006.08.019. [DOI] [Google Scholar]
- Medeiros D. L.; Queiroz L. M.; Cohim E.; Almeida-Neto J. A. d.; Kiperstok A. Human urine fertiliser in the Brazilian semi-arid: Environmental assessment and water-energy-nutrient nexus. Sci. Total Environ. 2020, 713, 136145. 10.1016/j.scitotenv.2019.136145. [DOI] [PubMed] [Google Scholar]
- Rufí-Salís M.; Brunnhofer N.; Petit-Boix A.; Gabarrell X.; Guisasola A.; Villalba G. Can wastewater feed cities? Determining the feasibility and environmental burdens of struvite recovery and reuse for urban regions. Sci. Total Environ. 2020, 737, 139783. 10.1016/j.scitotenv.2020.139783. [DOI] [PubMed] [Google Scholar]
- Wollmann I.; Gauro A.; Müller T.; Möller K. Phosphorus bioavailability of sewage sludge-based recycled fertilizers. J. Plant Nutr. Soil Sci. 2018, 181 (2), 158–166. 10.1002/jpln.201700111. [DOI] [Google Scholar]
- Wollmann I.; Möller K. Phosphorus bioavailability of sewage sludge-based recycled fertilizers in an organically managed field experiment. J. Plant Nutr. Soil Sci. 2018, 181 (5), 760–767. 10.1002/jpln.201700346. [DOI] [Google Scholar]
- Ewert W.; Wagenbach A. The project Wastewater Treatment Plant Lingen “Plus-Energy-WWTP with Phosphorus Recovery”. Hamb. Ber. Siedlungswasserwirtsch. 2012, 81, 17–32. [Google Scholar]
- Britton A.; Abrary F. P. Recovery of phosphate as struvite from wastewater streams. Proc. - Int. Fert. Soc. 2015, 765, 765–761. [Google Scholar]
- Maizel A. C.; Remucal C. K. The effect of advanced secondary municipal wastewater treatment on the molecular composition of dissolved organic matter. Water Res. 2017, 122, 42–52. 10.1016/j.watres.2017.05.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raniro H. R.; Bettoni Teles A. P.; Adam C.; Pavinato P. S. Phosphorus solubility and dynamics in a tropical soil under sources derived from wastewater and sewage sludge. J. Environ. Manage. 2022, 302, 113984. 10.1016/j.jenvman.2021.113984. [DOI] [PubMed] [Google Scholar]
- Petzet S.; Cornel P. Phosphorus recovery from wastewater. Issues Environ. Sci. Technol. 2013, 37, 110–143. 10.1039/9781849737883-00110. [DOI] [Google Scholar]
- Weigand H.; Bertau M.; Huebner W. P-fertilizer production from sewage sludge ash: Achievements and obstacles for the resource-efficient use of a waste stream. Muell Abfall 2012, 44 (5), 248–253. [Google Scholar]
- Weigand H.; Bertau M.; Hübner W.; Bohndick F.; Bruckert A. RecoPhos: Full-scale fertilizer production from sewage sludge ash. Waste Manage. 2013, 33 (3), 540–544. 10.1016/j.wasman.2012.07.009. [DOI] [PubMed] [Google Scholar]
- Schönberg A.; Raupenstrauch H. Beitrag zur Beschreibung der induktiven Erwärmung einer Graphitschüttung. BHM Berg- und Hüttenmännische Monatshefte 2016, 161 (7), 309–314. 10.1007/s00501-016-0503-5. [DOI] [Google Scholar]
- Gowd S. C.; Kumar D.; Lin R.; Rajendran K. Nutrient recovery from wastewater in India: A perspective from mass and energy balance for a sustainable circular economy. Bioresour. Technol. Rep. 2022, 18, 101079. 10.1016/j.biteb.2022.101079. [DOI] [Google Scholar]
- Sun X.; Li X.; Tang S.; Lin K.; Zhao T.; Chen X. A review on algal-bacterial symbiosis system for aquaculture tail water treatment. Sci. Total Environ. 2022, 847, 157620. 10.1016/j.scitotenv.2022.157620. [DOI] [PubMed] [Google Scholar]
- Meena R. A. A.; Yukesh Kannah R.; Sindhu J.; Ragavi J.; Kumar G.; Gunasekaran M.; Rajesh Banu J. Trends and resource recovery in biological wastewater treatment system. Bioresour. Technol. Rep. 2019, 7, 100235. 10.1016/j.biteb.2019.100235. [DOI] [Google Scholar]
- Cullen N.; Baur R.; Schauer P. Three years of operation of North America’s first nutrient recovery facility. Water Sci. Technol. 2013, 68 (4), 763–768. 10.2166/wst.2013.260. [DOI] [PubMed] [Google Scholar]
- Chen X.; Kong H.; Wu D.; Wang X.; Lin Y. Phosphate removal and recovery through crystallization of hydroxyapatite using Xonotlite as seed crystal. J. Environ. Sci. (Beijing, China) 2009, 21 (5), 575–580. 10.1016/S1001-0742(08)62310-4. [DOI] [PubMed] [Google Scholar]
- Desmidt E.; Ghyselbrecht K.; Zhang Y.; Pinoy L.; Van der Bruggen B.; Verstraete W.; Rabaey K.; Meesschaert B. Global Phosphorus Scarcity and Full-Scale P-Recovery Techniques: A Review. Crit. Rev. Environ. Sci. Technol. 2015, 45 (4), 336–384. 10.1080/10643389.2013.866531. [DOI] [Google Scholar]
- Filali-Meknassi Y.; Auriol M.; Tyagi R. D.; Comeau Y.; Surampalli R. Y. Phosphorus Co-Precipitation in the Biological Treatment of Slaughterhouse Wastewater in a Sequencing Batch Reactor. Pract. Period. Hazard., Toxic, Radioact. Waste Manage. 2005, 9 (3), 179–192. 10.1061/(ASCE)1090-025X(2005)9:3(179). [DOI] [Google Scholar]
- Moharami S.; Jalali M. Use of modified clays for removal of phosphorus from aqueous solutions. Environ. Monit. Assess. 2015, 187 (10), 639. 10.1007/s10661-015-4854-2. [DOI] [PubMed] [Google Scholar]
- Qiu L.-p.; Ma J. Parallel study on phosphorus removal by chemical precipitation with FeCl3 and AlCl3 in biological aerated filters. Xiandai Huagong 2007, 27 (Suppl. 1), 159–162. [Google Scholar]
- Cheng X.; Huang X.; Wang X.; Zhao B.; Chen A.; Sun D. Phosphate adsorption from sewage sludge filtrate using zinc–aluminum layered double hydroxides. J. Hazard. Mater. 2009, 169 (1), 958–964. 10.1016/j.jhazmat.2009.04.052. [DOI] [PubMed] [Google Scholar]
- Bi W.; Li Y.; Hu Y. Recovery of phosphorus and nitrogen from alkaline hydrolysis supernatant of excess sludge by magnesium ammonium phosphate. Bioresour. Technol. 2014, 166, 1–8. 10.1016/j.biortech.2014.04.092. [DOI] [PubMed] [Google Scholar]
- Huang H.; Liu J.; Ding L. Recovery of phosphate and ammonia nitrogen from the anaerobic digestion supernatant of activated sludge by chemical precipitation. J. Cleaner Prod. 2015, 102, 437–446. 10.1016/j.jclepro.2015.04.117. [DOI] [Google Scholar]
- Song Y.; Weidler P. G.; Berg U.; Nüesch R.; Donnert D. Calcite-seeded crystallization of calcium phosphate for phosphorus recovery. Chemosphere 2006, 63 (2), 236–243. 10.1016/j.chemosphere.2005.08.021. [DOI] [PubMed] [Google Scholar]
- Lei Y.; Narsing S.; Saakes M.; van der Weijden R. D.; Buisman C. J. N. Calcium Carbonate Packed Electrochemical Precipitation Column: New Concept of Phosphate Removal and Recovery. Environ. Sci. Technol. 2019, 53 (18), 10774–10780. 10.1021/acs.est.9b03795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ward A. J.; Arola K.; Thompson Brewster E.; Mehta C. M.; Batstone D. J. Nutrient recovery from wastewater through pilot scale electrodialysis. Water Res. 2018, 135, 57–65. 10.1016/j.watres.2018.02.021. [DOI] [PubMed] [Google Scholar]
- Johir M. A. H.; George J.; Vigneswaran S.; Kandasamy J.; Grasmick A. Removal and recovery of nutrients by ion exchange from high rate membrane bio-reactor (MBR) effluent. Desalination 2011, 275 (1), 197–202. 10.1016/j.desal.2011.02.054. [DOI] [Google Scholar]
- Liu R.; Wang Y.; Wu G.; Luo J.; Wang S. Development of a selective electrodialysis for nutrient recovery and desalination during secondary effluent treatment. Chem. Eng. J. 2017, 322, 224–233. 10.1016/j.cej.2017.03.149. [DOI] [Google Scholar]
- Ye Z.-L.; Ghyselbrecht K.; Monballiu A.; Pinoy L.; Meesschaert B. Fractionating various nutrient ions for resource recovery from swine wastewater using simultaneous anionic and cationic selective-electrodialysis. Water Res. 2019, 160, 424–434. 10.1016/j.watres.2019.05.085. [DOI] [PubMed] [Google Scholar]
- Wei C.-Y.; Pan S.-Y.; Lin Y.-I.; Cao T. N.-D. Anaerobic swine digestate valorization via energy-efficient electrodialysis for nutrient recovery and water reclamation. Water Res. 2022, 224, 119066. 10.1016/j.watres.2022.119066. [DOI] [PubMed] [Google Scholar]
- Wang Y.-K.; Geng Y.-K.; Pan X.-R.; Sheng G.-P. In situ utilization of generated electricity for nutrient recovery in urine treatment using a selective electrodialysis membrane bioreactor. Chem. Eng. Sci. 2017, 171, 451–458. 10.1016/j.ces.2017.06.002. [DOI] [Google Scholar]
- Gao F.; Wang L.; Wang J.; Zhang H.; Lin S. Nutrient recovery from treated wastewater by a hybrid electrochemical sequence integrating bipolar membrane electrodialysis and membrane capacitive deionization. Environ. Sci.: Water Res. Technol. 2020, 6 (2), 383–391. 10.1039/C9EW00981G. [DOI] [Google Scholar]
- Pan Y.; Zhu T.; He Z. Minimizing effects of chloride and calcium towards enhanced nutrient recovery from sidestream centrate in a decoupled electrodialysis driven by solar energy. J. Cleaner Prod. 2020, 263, 121419. 10.1016/j.jclepro.2020.121419. [DOI] [Google Scholar]
- MagPrex Offers a USA-Based Nutrient-Recovery Technology; Treatment Plant Opertator Magazine, 2020. https://www.tpomag.com/g/plant-proficiencies/2020/11/magprex-offers-a-usa-based-nutrient-recovery-technology (accessed 12-08-2022).
- Jafarinejad S. Forward osmosis membrane technology for nutrient removal/recovery from wastewater: Recent advances, proposed designs, and future directions. Chemosphere 2021, 263, 128116. 10.1016/j.chemosphere.2020.128116. [DOI] [PubMed] [Google Scholar]
- Xie M.; Shon H. K.; Gray S. R.; Elimelech M. Membrane-based processes for wastewater nutrient recovery: Technology, challenges, and future direction. Water Res. 2016, 89, 210–221. 10.1016/j.watres.2015.11.045. [DOI] [PubMed] [Google Scholar]
- Shi L.; Simplicio W. S.; Wu G.; Hu Z.; Hu H.; Zhan X. Nutrient Recovery from Digestate of Anaerobic Digestion of Livestock Manure: a Review. Curr. Pollut. Rep. 2018, 4 (2), 74–83. 10.1007/s40726-018-0082-z. [DOI] [Google Scholar]
- Hou D.; Lu L.; Sun D.; Ge Z.; Huang X.; Cath T. Y.; Ren Z. J. Microbial electrochemical nutrient recovery in anaerobic osmotic membrane bioreactors. Water Res. 2017, 114, 181–188. 10.1016/j.watres.2017.02.034. [DOI] [PubMed] [Google Scholar]
- Zou S.; Qin M.; Moreau Y.; He Z. Nutrient-energy-water recovery from synthetic sidestream centrate using a microbial electrolysis cell - forward osmosis hybrid system. J. Cleaner Prod. 2017, 154, 16–25. 10.1016/j.jclepro.2017.03.199. [DOI] [Google Scholar]
- Wu Z.; Zou S.; Zhang B.; Wang L.; He Z. Forward osmosis promoted in-situ formation of struvite with simultaneous water recovery from digested swine wastewater. Chem. Eng. J. 2018, 342, 274–280. 10.1016/j.cej.2018.02.082. [DOI] [Google Scholar]
- Adam G.; Mottet A.; Lemaigre S.; Tsachidou B.; Trouvé E.; Delfosse P. Fractionation of anaerobic digestates by dynamic nanofiltration and reverse osmosis: An industrial pilot case evaluation for nutrient recovery. J. Environ. Chem. Eng. 2018, 6 (5), 6723–6732. 10.1016/j.jece.2018.10.033. [DOI] [Google Scholar]
- Cong Nguyen N.; Cong Duong H.; Chen S.-S.; Thi Nguyen H.; Hao Ngo H.; Guo W.; Quang Le H.; Cong Duong C.; Thuy Trang L.; Hoang Le A.; et al. Water and nutrient recovery by a novel moving sponge – Anaerobic osmotic membrane bioreactor – Membrane distillation (AnOMBR-MD) closed-loop system. Bioresour. Technol. 2020, 312, 123573. 10.1016/j.biortech.2020.123573. [DOI] [PubMed] [Google Scholar]
- Bacelo H.; Pintor A. M. A.; Santos S. C. R.; Boaventura R. A. R.; Botelho C. M. S. Performance and prospects of different adsorbents for phosphorus uptake and recovery from water. Chem. Eng. J. 2020, 381, 122566. 10.1016/j.cej.2019.122566. [DOI] [Google Scholar]
- Anirudhan T. S.; Senan P. Adsorption of phosphate ions from water using a novel cellulose-based adsorbent. Chem. Ecol. 2011, 27 (2), 147–164. 10.1080/02757540.2010.547487. [DOI] [Google Scholar]
- Khalil A. M. E.; Eljamal O.; Amen T. W. M.; Sugihara Y.; Matsunaga N. Optimized nano-scale zero-valent iron supported on treated activated carbon for enhanced nitrate and phosphate removal from water. Chem. Eng. J. 2017, 309, 349–365. 10.1016/j.cej.2016.10.080. [DOI] [Google Scholar]
- Mehrabi N.; Soleimani M.; Yeganeh M. M.; Sharififard H. Parameter optimization for nitrate removal from water using activated carbon and composite of activated carbon and Fe2O3 nanoparticles. RSC Adv. 2015, 5 (64), 51470–51482. 10.1039/C5RA03920G. [DOI] [Google Scholar]
- Yu J.; Hu H.; Wu X.; Zhou T.; Liu Y.; Ruan R.; Zheng H. Coupling of biochar-mediated absorption and algal-bacterial system to enhance nutrients recovery from swine wastewater. Sci. Total Environ. 2020, 701, 134935. 10.1016/j.scitotenv.2019.134935. [DOI] [PubMed] [Google Scholar]
- Hermassi M.; Dosta J.; Valderrama C.; Licon E.; Moreno N.; Querol X.; Batis N. H.; Cortina J. L. Simultaneous ammonium and phosphate recovery and stabilization from urban sewage sludge anaerobic digestates using reactive sorbents. Sci. Total Environ. 2018, 630, 781–789. 10.1016/j.scitotenv.2018.02.243. [DOI] [PubMed] [Google Scholar]
- Hermassi M.; Valderrama C.; Gibert O.; Moreno N.; Querol X.; Batis N. H.; Cortina J. L. Recovery of nutrients (N-P-K) from potassium-rich sludge anaerobic digestion side-streams by integration of a hybrid sorption-membrane ultrafiltration process: Use of powder reactive sorbents as nutrient carriers. Sci. Total Environ. 2017, 599–600, 422–430. 10.1016/j.scitotenv.2017.04.140. [DOI] [PubMed] [Google Scholar]
- Guaya D.; Valderrama C.; Farran A.; Sauras T.; Cortina J. L. Valorisation of N and P from waste water by using natural reactive hybrid sorbents: Nutrients (N,P,K) release evaluation in amended soils by dynamic experiments. Sci. Total Environ. 2018, 612, 728–738. 10.1016/j.scitotenv.2017.08.248. [DOI] [PubMed] [Google Scholar]
- Kocatürk-Schumacher N. P.; Zwart K.; Bruun S.; Brussaard L.; Jensen L. S. Does the combination of biochar and clinoptilolite enhance nutrient recovery from the liquid fraction of biogas digestate?. Environ. Technol. 2017, 38 (10), 1313–1323. 10.1080/09593330.2016.1226959. [DOI] [PubMed] [Google Scholar]
- Saliu T. D.; Oladoja N. A. Assessing the suitability of solid aggregates for nutrient recovery from aqua systems. Journal of Water Process Engineering 2020, 33, 101000. 10.1016/j.jwpe.2019.101000. [DOI] [Google Scholar]
- Suresh Kumar P.; Korving L.; Keesman K. J.; van Loosdrecht M. C. M.; Witkamp G.-J. Effect of pore size distribution and particle size of porous metal oxides on phosphate adsorption capacity and kinetics. Chem. Eng. J. 2019, 358, 160–169. 10.1016/j.cej.2018.09.202. [DOI] [Google Scholar]
- Wu H.; Vaneeckhaute C. Nutrient recovery from wastewater: A review on the integrated Physicochemical technologies of ammonia stripping, adsorption and struvite precipitation. Chem. Eng. J. 2022, 433, 133664. 10.1016/j.cej.2021.133664. [DOI] [Google Scholar]
- Huang H.; Xiao D.; Pang R.; Han C.; Ding L. Simultaneous removal of nutrients from simulated swine wastewater by adsorption of modified zeolite combined with struvite crystallization. Chem. Eng. J. 2014, 256, 431–438. 10.1016/j.cej.2014.07.023. [DOI] [Google Scholar]
- Othman A.; Dumitrescu E.; Andreescu D.; Andreescu S. Nanoporous Sorbents for the Removal and Recovery of Phosphorus from Eutrophic Waters: Sustainability Challenges and Solutions. ACS Sustainable Chem. Eng. 2018, 6 (10), 12542–12561. 10.1021/acssuschemeng.8b01809. [DOI] [Google Scholar]
- Xie F.; Wu F.; Liu G.; Mu Y.; Feng C.; Wang H.; Giesy J. P. Removal of Phosphate from Eutrophic Lakes through Adsorption by in Situ Formation of Magnesium Hydroxide from Diatomite. Environ. Sci. Technol. 2014, 48 (1), 582–590. 10.1021/es4037379. [DOI] [PubMed] [Google Scholar]
- Gao F.; Li X.; Shi W.; Wang Z. Highly Selective Recovery of Phosphorus from Wastewater via Capacitive Deionization Enabled by Ferrocene-polyaniline-Functionalized Carbon Nanotube Electrodes. ACS Appl. Mater. Interfaces 2022, 14 (28), 31962–31972. 10.1021/acsami.2c06248. [DOI] [PubMed] [Google Scholar]
- Józwiak T.; Filipkowska U.; Szymczyk P.; Mielcarek A. Sorption of nutrients (orthophosphate, nitrate III and V) in an equimolar mixture of P-PO4, N-NO2 and N-NO3 using chitosan. Arabian J. Chem. 2019, 12 (8), 4104–4117. 10.1016/j.arabjc.2016.04.008. [DOI] [Google Scholar]
- Fernandes F.; Silkina A.; Fuentes-Grünewald C.; Wood E. E.; Ndovela V. L. S.; Oatley-Radcliffe D. L.; Lovitt R. W.; Llewellyn C. A. Valorising nutrient-rich digestate: Dilution, settlement and membrane filtration processing for optimization as a waste-based media for microalgal cultivation. Waste Manage. 2020, 118, 197–208. 10.1016/j.wasman.2020.08.037. [DOI] [PubMed] [Google Scholar]
- Gerardo M. L.; Aljohani N. H. M.; Oatley-Radcliffe D. L.; Lovitt R. W. Moving towards sustainable resources: Recovery and fractionation of nutrients from dairy manure digestate using membranes. Water Res. 2015, 80, 80–89. 10.1016/j.watres.2015.05.016. [DOI] [PubMed] [Google Scholar]
- Shi L.; Xie S.; Hu Z.; Wu G.; Morrison L.; Croot P.; Hu H.; Zhan X. Nutrient recovery from pig manure digestate using electrodialysis reversal: Membrane fouling and feasibility of long-term operation. J. Membr. Sci. 2019, 573, 560–569. 10.1016/j.memsci.2018.12.037. [DOI] [Google Scholar]
- Geng Z.; Hall E. R.; Bérubé P. R. Membrane fouling mechanisms of a membrane enhanced biological phosphorus removal process. J. Membr. Sci. 2007, 296 (1), 93–101. 10.1016/j.memsci.2007.03.019. [DOI] [Google Scholar]
- Maqbool T.; Khan S. J.; Lee C.-H. Effects of filtration modes on membrane fouling behavior and treatment in submerged membrane bioreactor. Bioresour. Technol. 2014, 172, 391–395. 10.1016/j.biortech.2014.09.064. [DOI] [PubMed] [Google Scholar]
- Song K.-G.; Kim Y.; Ahn K.-H. Effect of coagulant addition on membrane fouling and nutrient removal in a submerged membrane bioreactor. Desalination 2008, 221 (1), 467–474. 10.1016/j.desal.2007.01.107. [DOI] [Google Scholar]
- Luo H.; Lyu T.; Muhmood A.; Xue Y.; Wu H.; Meers E.; Dong R.; Wu S. Effect of flocculation pre-treatment on membrane nutrient recovery of digested chicken slurry: Mitigating suspended solids and retaining nutrients. Chem. Eng. J. 2018, 352, 855–862. 10.1016/j.cej.2018.07.097. [DOI] [Google Scholar]
- Deng L.; Ngo H.-H.; Guo W.; Zhang H. Pre-coagulation coupled with sponge-membrane filtration for organic matter removal and membrane fouling control during drinking water treatment. Water Res. 2019, 157, 155–166. 10.1016/j.watres.2019.03.052. [DOI] [PubMed] [Google Scholar]
- Gao H.; Wang Y.; Afolabi M. A.; Xiao D.; Chen Y. Incorporation of Cellulose Nanocrystals into Graphene Oxide Membranes for Efficient Antibiotic Removal at High Nutrient Recovery. ACS Appl. Mater. Interfaces 2021, 13 (12), 14102–14111. 10.1021/acsami.0c20652. [DOI] [PubMed] [Google Scholar]
- Alagha O.; Manzar M. S.; Zubair M.; Anil I.; Mu’azu N. D.; Qureshi A. Magnetic Mg-Fe/LDH Intercalated Activated Carbon Composites for Nitrate and Phosphate Removal from Wastewater: Insight into Behavior and Mechanisms. Nanomaterials 2020, 10 (7), 1361. 10.3390/nano10071361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alagha O.; Manzar M. S.; Zubair M.; Anil I.; Mu’azu N. D.; Qureshi A. Comparative Adsorptive Removal of Phosphate and Nitrate from Wastewater Using Biochar-MgAl LDH Nanocomposites: Coexisting Anions Effect and Mechanistic Studies. Nanomaterials 2020, 10 (2), 336. 10.3390/nano10020336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tian Y.; He W.; Zhu X.; Yang W.; Ren N.; Logan B. E. Improved Electrocoagulation Reactor for Rapid Removal of Phosphate from Wastewater. ACS Sustainable Chem. Eng. 2017, 5 (1), 67–71. 10.1021/acssuschemeng.6b01613. [DOI] [Google Scholar]
- Perera M. K.; Englehardt J. D.; Cohn J. L.; Dauer E. A.; Shukla D. Electrohydromodulation for phosphate recovery from wastewater. Sep. Purif. Technol. 2020, 247, 116909. 10.1016/j.seppur.2020.116909. [DOI] [Google Scholar]
- Zhang L.; Gao Y.; Li M.; Liu J. Expanded graphite loaded with lanthanum oxide used as a novel adsorbent for phosphate removal from water: performance and mechanism study. Environ. Technol. 2015, 36 (8), 1016–1025. 10.1080/09593330.2014.971884. [DOI] [PubMed] [Google Scholar]
- Monetti J.; Ledezma P.; Virdis B.; Freguia S. Nutrient Recovery by Bio-Electroconcentration is Limited by Wastewater Conductivity. ACS Omega 2019, 4 (1), 2152–2159. 10.1021/acsomega.8b02737. [DOI] [Google Scholar]
- Lai Y.-T.; Liu W.-T.; Chen L.-J.; Chang M.-C.; Lee C.-Y.; Tai N.-H. Electro-assisted selective uptake/release of phosphate using a graphene oxide/MgMn-layered double hydroxide composite. J. Mater. Chem. A 2019, 7 (8), 3962–3970. 10.1039/C8TA10518A. [DOI] [Google Scholar]
- Tran D. N. H.; Kabiri S.; Wang L.; Losic D. Engineered graphene–nanoparticle aerogel composites for efficient removal of phosphate from water. J. Mater. Chem. A 2015, 3 (13), 6844–6852. 10.1039/C4TA06308B. [DOI] [Google Scholar]
- Hamoudi S.; Saad R.; Belkacemi K. Adsorptive Removal of Phosphate and Nitrate Anions from Aqueous Solutions Using Ammonium-Functionalized Mesoporous Silica. Ind. Eng. Chem. Res. 2007, 46 (25), 8806–8812. 10.1021/ie070195k. [DOI] [Google Scholar]
- Hamoudi S.; Belkacemi K. Adsorption of nitrate and phosphate ions from aqueous solutions using organically-functionalized silica materials: Kinetic modeling. Fuel 2013, 110, 107–113. 10.1016/j.fuel.2012.09.066. [DOI] [Google Scholar]
- Yamashita T.; Aketo T.; Minowa N.; Sugimoto K.; Yokoyama H.; Ogino A.; Tanaka Y. Simultaneous removal of colour, phosphorus and disinfection from treated wastewater using an agent synthesized from amorphous silica and hydrated lime. Environ. Technol. 2013, 34 (8), 1017–1025. 10.1080/09593330.2012.733417. [DOI] [PubMed] [Google Scholar]
- Huang Y.; Wang C.; Zheng X.; Pan J.; Yan Y.. Preparation of lanthanum nitrate modified mesoporous silica nanostructural composite adsorbing material for selective adsorption of phosphate ions in wastewater. CN104667882, 2015.
- Hermassi M.; Valderrama C.; Moreno N.; Font O.; Querol X.; Batis N. H.; Cortina J. L. Fly ash as reactive sorbent for phosphate removal from treated waste water as a potential slow release fertilizer. J. Environ. Chem. Eng. 2017, 5 (1), 160–169. 10.1016/j.jece.2016.11.027. [DOI] [Google Scholar]
- Ahmad S. Z. N.; Hamdan R.; Al-Gheethi A.; Alkhadher S.; Othman N. Removal of phosphate from wastewater by steel slag with high calcium oxide column filter system; efficiencies and mechanisms study. J. Chem. Technol. Biotechnol. 2020, 95 (12), 3232–3240. 10.1002/jctb.6501. [DOI] [Google Scholar]
- Oladoja N. A.; Aboluwoye C. O.; Ololade I. A.; Adebayo O. L.; Olaseni S. E.; Adelagun R. O. A. Intercalation of Gastropod Shell Derived Calcium Oxide in Clay and Application in Phosphate Removal from Aqua Medium. Ind. Eng. Chem. Res. 2012, 51 (45), 14637–14645. 10.1021/ie301520v. [DOI] [Google Scholar]
- Liu W.; Zheng Z.; Sun F.; Miao M.; Cui M.-H.; Liu H.; Zhang H.; Zhang C.; Hu Z.; Liu H. Valorization of citric acid production wastewater as alternative carbon source for biological nutrients removal: A pilot-scale case study. J. Cleaner Prod. 2020, 258, 120576. 10.1016/j.jclepro.2020.120576. [DOI] [Google Scholar]
- Zhu D.; Chen Y.; Yang H.; Wang S.; Wang X.; Zhang S.; Chen H. Synthesis and characterization of magnesium oxide nanoparticle-containing biochar composites for efficient phosphorus removal from aqueous solution. Chemosphere 2020, 247, 125847. 10.1016/j.chemosphere.2020.125847. [DOI] [PubMed] [Google Scholar]
- Stolzenburg P.; Capdevielle A.; Teychené S.; Biscans B. Struvite precipitation with MgO as a precursor: Application to wastewater treatment. Chem. Eng. Sci. 2015, 133, 9–15. 10.1016/j.ces.2015.03.008. [DOI] [Google Scholar]
- Abbasi M.; Mirfendereski M.; Nikbakht M.; Golshenas M.; Mohammadi T. Performance study of mullite and mullite–alumina ceramic MF membranes for oily wastewaters treatment. Desalination 2010, 259 (1), 169–178. 10.1016/j.desal.2010.04.013. [DOI] [Google Scholar]
- Chen Y.; Su Y.; Zheng X.; Chen H.; Yang H. Alumina nanoparticles-induced effects on wastewater nitrogen and phosphorus removal after short-term and long-term exposure. Water Res. 2012, 46 (14), 4379–4386. 10.1016/j.watres.2012.05.042. [DOI] [PubMed] [Google Scholar]
- Tan X.; Yang Y.; Liu Y.; Li X.; Fan X.; Zhou Z.; Liu C.; Yin W. Enhanced simultaneous organics and nutrients removal in tidal flow constructed wetland using activated alumina as substrate treating domestic wastewater. Bioresour. Technol. 2019, 280, 441–446. 10.1016/j.biortech.2019.02.036. [DOI] [PubMed] [Google Scholar]
- Fang J.-M.; Wang Z.-Q.; Gong W.-Q.; Yang H.-G.; Meng Y.-W.; Zhang X.-Z. Ceramic Filter Balls Loaded with α -Fe2O3 and Their Application to NH3-N Wastewater Treatment. Chin. J. Chem. 2008, 26 (3), 459–462. 10.1002/cjoc.200890086. [DOI] [Google Scholar]
- Shan S.; Wang W.; Liu D.; Zhao Z.; Shi W.; Cui F. Remarkable phosphate removal and recovery from wastewater by magnetically recyclable La2O2CO3/γ-Fe2O3 nanocomposites. J. Hazard. Mater. 2020, 397, 122597. 10.1016/j.jhazmat.2020.122597. [DOI] [PubMed] [Google Scholar]
- Jahangiri-rad M.; Jamshidi A.; Rafiee M.; Nabizadeh R. Adsorption performance of packed bed column for nitrate removal using PAN-oxime-nano Fe2O3. J. Environ. Health Sci. Eng. 2014, 12 (1), 90. 10.1186/2052-336X-12-90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang H.; Liu K.; Ni Y. Synthesis of mesoporous α-Fe2O3 using cellulose nanocrystals as template and its use for the removal of phosphate from wastewater. J. Taiwan Inst. Chem. Eng. 2017, 71, 474–479. 10.1016/j.jtice.2016.12.008. [DOI] [Google Scholar]
- Nakarmi A.; Bourdo S. E.; Ruhl L.; Kanel S.; Nadagouda M.; Kumar Alla P.; Pavel I.; Viswanathan T. Benign zinc oxide betaine-modified biochar nanocomposites for phosphate removal from aqueous solutions. J. Environ. Manage. 2020, 272, 111048. 10.1016/j.jenvman.2020.111048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rathnayake S.; Unrine J. M.; Judy J.; Miller A.-F.; Rao W.; Bertsch P. M. Multitechnique Investigation of the pH Dependence of Phosphate Induced Transformations of ZnO Nanoparticles. Environ. Sci. Technol. 2014, 48 (9), 4757–4764. 10.1021/es404544w. [DOI] [PubMed] [Google Scholar]
- Cheng Y.-F.; Zhang Z.-Z.; Li G.-F.; Zhu B.-Q.; Zhang Q.; Liu Y.-Y.; Zhu W.-Q.; Fan N.-S.; Jin R.-C. Effects of ZnO nanoparticles on high-rate denitrifying granular sludge and the role of phosphate in toxicity attenuation. Environ. Pollut. 2019, 251, 166–174. 10.1016/j.envpol.2019.04.138. [DOI] [PubMed] [Google Scholar]
- Cheng P.; Chen D.; Liu W.; Cobb K.; Zhou N.; Liu Y.; Liu H.; Wang Q.; Chen P.; Zhou C.; et al. Auto-flocculation microalgae species Tribonema sp. and Synechocystis sp. with T-IPL pretreatment to improve swine wastewater nutrient removal. Sci. Total Environ. 2020, 725, 138263. 10.1016/j.scitotenv.2020.138263. [DOI] [PubMed] [Google Scholar]
- Berhanuddin M. S.; Aris A.; Chelliapan S.; Abdul M. Z.; Hasaan H. A. Nutrients removal by titanium dioxide-zeolite (TDZ) composite. Int. J. Curr. Res. Rev. 2021, 13 (13), 2–7. 10.31782/IJCRR.2021.131314. [DOI] [Google Scholar]
- Chen Z.; Chen Y.; Zheng X.; Wang X.; Wang Y.; Chen J. Comparison of complete nitritation–denitrification and partial nitritation–anammox for iron oxide wastewater treatment. J. Cleaner Prod. 2021, 294, 126281. 10.1016/j.jclepro.2021.126281. [DOI] [Google Scholar]
- Xu P.; Zeng G. M.; Huang D. L.; Feng C. L.; Hu S.; Zhao M. H.; Lai C.; Wei Z.; Huang C.; Xie G. X.; et al. Use of iron oxide nanomaterials in wastewater treatment: A review. Sci. Total Environ. 2012, 424, 1–10. 10.1016/j.scitotenv.2012.02.023. [DOI] [PubMed] [Google Scholar]
- Xu J.-J.; Cheng Y.-F.; Xu L.-Z.-J.; Zhu X.-L.; Zhu W.-Q.; Jin R.-C. The performance and microbial community in response to MnO2 nanoparticles in anammox granular sludge. Chemosphere 2019, 233, 625–632. 10.1016/j.chemosphere.2019.06.006. [DOI] [PubMed] [Google Scholar]
- Ge X.; Song X.; Ma Y.; Zhou H.; Wang G.; Zhang H.; Zhang Y.; Zhao H.; Wong P. K. Fabrication of hierarchical iron-containing MnO2 hollow microspheres assembled by thickness-tunable nanosheets for efficient phosphate removal. J. Mater. Chem. A 2016, 4 (38), 14814–14826. 10.1039/C6TA05386F. [DOI] [Google Scholar]
- Hu J.; Huang C.; Fan Z.; Xu G.; Lyu S.; He S.; Liang Y. Removal of Phosphate From Wastewater Using Manganese Dioxide. Shifa Yejin 2020, 39 (1), 68–71. 10.13355/j.cnki.sfyj.2020.01.014. [DOI] [Google Scholar]
- Nagy A.Removal of phosphate ions and ammonium ions from water. DE19505884, 1996.
- Xie H.; Yang Y.; Liu J.; Kang Y.; Zhang J.; Hu Z.; Liang S. Enhanced triclosan and nutrient removal performance in vertical up-flow constructed wetlands with manganese oxides. Water Res. 2018, 143, 457–466. 10.1016/j.watres.2018.05.061. [DOI] [PubMed] [Google Scholar]
- Mahdavi S.; Akhzari D. The removal of phosphate from aqueous solutions using two nano-structures: copper oxide and carbon tubes. Clean Technol. Environ. Policy 2016, 18 (3), 817–827. 10.1007/s10098-015-1058-y. [DOI] [Google Scholar]
- Acelas N. Y.; Martin B. D.; López D.; Jefferson B. Selective removal of phosphate from wastewater using hydrated metal oxides dispersed within anionic exchange media. Chemosphere 2015, 119, 1353–1360. 10.1016/j.chemosphere.2014.02.024. [DOI] [PubMed] [Google Scholar]
- Gupta M. K.; Tandon P. K.; Pandey V.; Afroz M.; Malviya T. Montmorillonite based copper oxide nanoparticles for the efficient remediation of phosphate and anti-bacterial activity against gram-negative bacteria. Sep. Sci. Technol. 2023, 58, 1–14. 10.1080/01496395.2022.2121724. [DOI] [Google Scholar]
- Pan B.; Han F.; Nie G.; Wu B.; He K.; Lu L. New Strategy To Enhance Phosphate Removal from Water by Hydrous Manganese Oxide. Environ. Sci. Technol. 2014, 48 (9), 5101–5107. 10.1021/es5004044. [DOI] [PubMed] [Google Scholar]
- Wu B.; Fang L.; Fortner J. D.; Guan X.; Lo I. M. C. Highly efficient and selective phosphate removal from wastewater by magnetically recoverable La(OH)3/Fe3O4 nanocomposites. Water Res. 2017, 126, 179–188. 10.1016/j.watres.2017.09.034. [DOI] [PubMed] [Google Scholar]
- Drenkova-Tuhtan A.; Schneider M.; Franzreb M.; Meyer C.; Gellermann C.; Sextl G.; Mandel K.; Steinmetz H. Pilot-scale removal and recovery of dissolved phosphate from secondary wastewater effluents with reusable ZnFeZr adsorbent @ Fe3O4/SiO2 particles with magnetic harvesting. Water Res. 2017, 109, 77–87. 10.1016/j.watres.2016.11.039. [DOI] [PubMed] [Google Scholar]
- Li N.; Tian Y.; Zhao J.; Zhan W.; Du J.; Kong L.; Zhang J.; Zuo W. Ultrafast selective capture of phosphorus from sewage by 3D Fe3O4@ZnO via weak magnetic field enhanced adsorption. Chem. Eng. J. 2018, 341, 289–297. 10.1016/j.cej.2018.02.029. [DOI] [Google Scholar]
- Wang W.; Zhang H.; Zhang L.; Wan H.; Zheng S.; Xu Z. Adsorptive removal of phosphate by magnetic Fe3O4@C@ZrO2. Colloids Surf., A 2015, 469, 100–106. 10.1016/j.colsurfa.2015.01.002. [DOI] [Google Scholar]
- Hao H.; Wang Y.; Shi B. NaLa(CO3)2 hybridized with Fe3O4 for efficient phosphate removal: Synthesis and adsorption mechanistic study. Water Res. 2019, 155, 1–11. 10.1016/j.watres.2019.01.049. [DOI] [PubMed] [Google Scholar]
- Hosni K.; Ben Moussa S.; Chachi A.; Ben Amor M. The removal of PO43– by calcium hydroxide from synthetic wastewater: optimization of the operating conditions. Desalination 2008, 223 (1), 337–343. 10.1016/j.desal.2007.01.213. [DOI] [Google Scholar]
- Yoshikawa E.; Sasaki A.; Endo M. Removal of boron from wastewater by the hydroxyapatite formation reaction using acceleration effect of ammonia. J. Hazard. Mater. 2012, 237–238, 277–282. 10.1016/j.jhazmat.2012.08.045. [DOI] [PubMed] [Google Scholar]
- Markou G.; Mitrogiannis D.; Muylaert K.; Çelekli A.; Bozkurt H. Biosorption and retention of orthophosphate onto Ca(OH)2-pretreated biomass of Phragmites sp. Journal of Environmental Sciences 2016, 45, 49–59. 10.1016/j.jes.2015.12.009. [DOI] [PubMed] [Google Scholar]
- Wu Q.; Bishop P. L.; Keener T. C.; Stallard J.; Stile L. Sludge digestion enhancement and nutrient removal from anaerobic supernatant by Mg(OH)2 application. Water Sci. Technol. 2001, 44 (1), 161–166. 10.2166/wst.2001.0039. [DOI] [PubMed] [Google Scholar]
- Wu Q.; Bishop P. L.; Keener T. C. A Strategy for Controlling Deposition of Struvite in Municipal Wastewater Treatment Plants. Water Environ. Res. 2005, 77 (2), 199–207. 10.2175/106143005X41771. [DOI] [PubMed] [Google Scholar]
- Yu R.; Ren H.; Wang Y.; Ding L.; Geng J.; Xu K.; Zhang Y. A kinetic study of struvite precipitation recycling technology with NaOH/Mg(OH)2 addition. Bioresour. Technol. 2013, 143, 519–524. 10.1016/j.biortech.2013.06.042. [DOI] [PubMed] [Google Scholar]
- Kawasaki N.; Ogata F.; Tominaga H. Selective adsorption behavior of phosphate onto aluminum hydroxide gel. J. Hazard. Mater. 2010, 181 (1), 574–579. 10.1016/j.jhazmat.2010.05.051. [DOI] [PubMed] [Google Scholar]
- Georgantas D. A.; Grigoropoulou H. P. Orthophosphate and metaphosphate ion removal from aqueous solution using alum and aluminum hydroxide. J. Colloid Interface Sci. 2007, 315 (1), 70–79. 10.1016/j.jcis.2007.06.058. [DOI] [PubMed] [Google Scholar]
- Xu R.; Zhang M.; Mortimer R. J. G.; Pan G. Enhanced Phosphorus Locking by Novel Lanthanum/Aluminum–Hydroxide Composite: Implications for Eutrophication Control. Environ. Sci. Technol. 2017, 51 (6), 3418–3425. 10.1021/acs.est.6b05623. [DOI] [PubMed] [Google Scholar]
- Gonzalez-Martinez A.; Leyva-Díaz J. C.; Rodriguez-Sanchez A.; Muñoz-Palazon B.; Rivadeneyra A.; Poyatos J. M.; Rivadeneyra M. A.; Martinez-Toledo M. V. Isolation and metagenomic characterization of bacteria associated with calcium carbonate and struvite precipitation in a pure moving bed biofilm reactor-membrane bioreactor. Biofouling 2015, 31 (4), 333–348. 10.1080/08927014.2015.1040006. [DOI] [PubMed] [Google Scholar]
- Zhao Z.; Song X.; Zhang Y.; Zhao Y.; Wang B.; Wang Y. Effects of iron and calcium carbonate on contaminant removal efficiencies and microbial communities in integrated wastewater treatment systems. Chemosphere 2017, 189, 10–20. 10.1016/j.chemosphere.2017.09.020. [DOI] [PubMed] [Google Scholar]
- Hermassi M.; Valderrama C.; Dosta J.; Cortina J. L.; Batis N. H. Evaluation of hydroxyapatite crystallization in a batch reactor for the valorization of alkaline phosphate concentrates from wastewater treatment plants using calcium chloride. Chem. Eng. J. 2015, 267, 142–152. 10.1016/j.cej.2014.12.079. [DOI] [Google Scholar]
- Zhao X. H.; Zhao Y. Q.; Kearney P. Transformation of beneficially reused aluminium sludge to potential P and Al resource after employing as P-trapping material for wastewater treatment in constructed wetland. Chem. Eng. J. 2011, 174 (1), 206–212. 10.1016/j.cej.2011.09.001. [DOI] [Google Scholar]
- Devia Y. P.; Imai T.; Higuchi T.; Kanno A.; Yamamoto K.; Sekine M.; Van Le T. Potential of magnesium chloride for nutrient rejection in forward osmosis. J. Water Resour. Prot. 2015, 7 (9), 730–740. 10.4236/jwarp.2015.79060. [DOI] [Google Scholar]
- Qiu G.; Ting Y.-P. Direct phosphorus recovery from municipal wastewater via osmotic membrane bioreactor (OMBR) for wastewater treatment. Bioresour. Technol. 2014, 170, 221–229. 10.1016/j.biortech.2014.07.103. [DOI] [PubMed] [Google Scholar]
- Aguado D.; Barat R.; Bouzas A.; Seco A.; Ferrer J. P-recovery in a pilot-scale struvite crystallisation reactor for source separated urine systems using seawater and magnesium chloride as magnesium sources. Sci. Total Environ. 2019, 672, 88–96. 10.1016/j.scitotenv.2019.03.485. [DOI] [PubMed] [Google Scholar]
- Zeng L.; Li X. Nutrient removal from anaerobically digested cattle manure by struvite precipitation. J. Environ. Eng. Sci. 2006, 5 (4), 285–294. 10.1139/s05-027. [DOI] [Google Scholar]
- Feng C.-h.; Zhou Y.-x.; Jiang J.-y.; Hu X. Influence of FeCl3 on phosphorus removal in SBBR system. Anquan Yu Huanjing Xuebao 2005, 5 (3), 23–25. [Google Scholar]
- Li S.; Zeng W.; Jia Z.; Wu G.; Xu H.; Peng Y. Phosphorus species transformation and recovery without apatite in FeCl3-assisted sewage sludge hydrothermal treatment. Chem. Eng. J. 2020, 399, 125735. 10.1016/j.cej.2020.125735. [DOI] [Google Scholar]
- Agbovi H. K.; Wilson L. D. Flocculation Optimization of Orthophosphate with FeCl3 and Alginate Using the Box–Behnken Response Surface Methodology. Ind. Eng. Chem. Res. 2017, 56 (12), 3145–3155. 10.1021/acs.iecr.6b04765. [DOI] [Google Scholar]
- Kirinovic E.; Leichtfuss A. R.; Navizaga C.; Zhang H.; Schuttlefield Christus J. D.; Baltrusaitis J. Spectroscopic and Microscopic Identification of the Reaction Products and Intermediates during the Struvite (MgNH4PO4·6H2O) Formation from Magnesium Oxide (MgO) and Magnesium Carbonate (MgCO3) Microparticles. ACS Sustainable Chem. Eng. 2017, 5 (2), 1567–1577. 10.1021/acssuschemeng.6b02327. [DOI] [Google Scholar]
- Bagreev A.; Locke D. C.; Bandosz T. J. H2S Adsorption/Oxidation on Adsorbents Obtained from Pyrolysis of Sewage-Sludge-Derived Fertilizer Using Zinc Chloride Activation. Ind. Eng. Chem. Res. 2001, 40 (16), 3502–3510. 10.1021/ie010165w. [DOI] [Google Scholar]
- Cheng K. Y.; Kaksonen A. H.; Douglas G. B. Sequential in situ hydrotalcite precipitation and biological denitrification for the treatment of high-nitrate industrial effluent. Bioresour. Technol. 2014, 172, 373–381. 10.1016/j.biortech.2014.09.050. [DOI] [PubMed] [Google Scholar]
- Matula J.; Pechova M. Influence of gypsum treatment on extractability of nutrients from soils. Plant, Soil Environ. 2005, 51 (8), 368–375. 10.17221/3612-PSE. [DOI] [Google Scholar]
- Elrashidi M. A.; West L. T.; Seybold C. A.; Benham E. C.; Schoeneberger P. J.; Ferguson R. Effects of gypsum addition on solubility of nutrients in soil amended with peat. Soil Sci. 2010, 175 (4), 162–172. 10.1097/SS.0b013e3181dd51d0. [DOI] [Google Scholar]
- Uusitalo R.; Ylivainio K.; Hyväluoma J.; Rasa K.; Kaseva J.; Nylund P.; Pietola L.; Turtola E. The effects of gypsum on the transfer of phosphorus and other nutrients through clay soil monoliths. Agric. Food Sci. 2012, 21 (3), 260–278. 10.23986/afsci.4855. [DOI] [Google Scholar]
- Kameda T.; Yoshioka T.; Okuwaki A. Development of new synthetic process for hydrotalcite using seawater and calcined dolomite and its application to wastewater treatment. Kagaku Kogyo 2001, 52 (10), 810–814. [Google Scholar]
- Johansson Westholm L. Substrates for phosphorus removal—Potential benefits for on-site wastewater treatment?. Water Res. 2006, 40 (1), 23–36. 10.1016/j.watres.2005.11.006. [DOI] [PubMed] [Google Scholar]
- Huang H.; Zhang D.; Guo G.; Jiang Y.; Wang M.; Zhang P.; Li J. Dolomite application for the removal of nutrients from synthetic swine wastewater by a novel combined electrochemical process. Chem. Eng. J. 2018, 335, 665–675. 10.1016/j.cej.2017.11.013. [DOI] [Google Scholar]
- Yang Y.; He Z.; Yang X.; Fan J.; Stoffella P.; Brittain C. Dolomite Phosphate Rock–Based Slow-Release Fertilizer for Agriculture and Landscapes. Commun. Soil Sci. Plant Anal. 2012, 43 (9), 1344–1362. 10.1080/00103624.2012.666308. [DOI] [Google Scholar]
- Larsdotter K.; Jansen J. l. C.; Dalhammar G. Biologically Mediated Phosphorus Precipitation In Wastewater Treatment With Microalgae. Environ. Technol. 2007, 28 (9), 953–960. 10.1080/09593332808618855. [DOI] [PubMed] [Google Scholar]
- Liu Y.; Sheng X.; Dong Y.; Ma Y. Removal of high-concentration phosphate by calcite: Effect of sulfate and pH. Desalination 2012, 289, 66–71. 10.1016/j.desal.2012.01.011. [DOI] [Google Scholar]
- Pap S.; Kirk C.; Bremner B.; Turk Sekulic M.; Shearer L.; Gibb S. W.; Taggart M. A. Low-cost chitosan-calcite adsorbent development for potential phosphate removal and recovery from wastewater effluent. Water Res. 2020, 173, 115573. 10.1016/j.watres.2020.115573. [DOI] [PubMed] [Google Scholar]
- Arias C. A.; Brix H.; Johansen N.-H. Phosphorus removal from municipal wastewater in an experimental two-stage vertical flow constructed wetland system equipped with a calcite filter. Water Sci. Technol. 2003, 48 (5), 51–58. 10.2166/wst.2003.0279. [DOI] [PubMed] [Google Scholar]
- Karageorgiou K.; Paschalis M.; Anastassakis G. N. Removal of phosphate species from solution by adsorption onto calcite used as natural adsorbent. J. Hazard. Mater. 2007, 139 (3), 447–452. 10.1016/j.jhazmat.2006.02.038. [DOI] [PubMed] [Google Scholar]
- Zhai Y.-t. Study on adsorption of phosphate in water by modified kaolinite. Anhui Nongye Kexue 2010, 38 (28), 15784–15785. [Google Scholar]
- Borges R.; Brunatto S. F.; Leitão A. A.; De Carvalho G. S. G.; Wypych F. Solid-state mechanochemical activation of clay minerals and soluble phosphate mixtures to obtain slow-release fertilizers. Clay Minerals 2015, 50 (2), 153–162. 10.1180/claymin.2015.050.2.01. [DOI] [Google Scholar]
- Hu G.-s.; Li Z.-y. Application of natural vermiculite in wastewater treatment. Beijing Gongshang Daxue Xuebao, Ziran Kexueban 2006, 24 (3), 13–16. [Google Scholar]
- Chen T.; Luo W.; Zhang F.-j.; Yu L.; Zhang G.-l. Application of modified vermiculite as fillings to biological fluidized bed in low C/N wastewater treatment. Jilin Daxue Xuebao, Diqiu Kexueban 2010, 40 (2), 394–398. [Google Scholar]
- Huang W.-Y.; Li D.; Liu Z.-Q.; Tao Q.; Zhu Y.; Yang J.; Zhang Y.-M. Kinetics, isotherm, thermodynamic, and adsorption mechanism studies of La(OH)3-modified exfoliated vermiculites as highly efficient phosphate adsorbents. Chem. Eng. J. 2014, 236, 191–201. 10.1016/j.cej.2013.09.077. [DOI] [Google Scholar]
- Song J.; Srivastava V.; Kohout T.; Sillanpää M.; Sainio T. Montmorillonite-anchored magnetite nanocomposite for recovery of ammonium from stormwater and its reuse in adsorption of Sc3+. Nanotechnol. Environ. Eng. 2021, 6 (3), 55. 10.1007/s41204-021-00151-y. [DOI] [Google Scholar]
- Jang J.; Lee D. S. Effective phosphorus removal using chitosan/Ca-organically modified montmorillonite beads in batch and fixed-bed column studies. J. Hazard. Mater. 2019, 375, 9–18. 10.1016/j.jhazmat.2019.04.070. [DOI] [PubMed] [Google Scholar]
- Tian S.; Jiang P.; Ning P.; Su Y. Enhanced adsorption removal of phosphate from water by mixed lanthanum/aluminum pillared montmorillonite. Chem. Eng. J. 2009, 151 (1), 141–148. 10.1016/j.cej.2009.02.006. [DOI] [Google Scholar]
- Si Z.; Song X.; Wang Y.; Cao X.; Wang Y.; Zhao Y.; Ge X. Natural pyrite improves nitrate removal in constructed wetlands and makes wetland a sink for phosphorus in cold climates. J. Cleaner Prod. 2021, 280, 124304. 10.1016/j.jclepro.2020.124304. [DOI] [Google Scholar]
- Di Capua F.; Mascolo M. C.; Pirozzi F.; Esposito G. Simultaneous denitrification, phosphorus recovery and low sulfate production in a recirculated pyrite-packed biofilter (RPPB). Chemosphere 2020, 255, 126977. 10.1016/j.chemosphere.2020.126977. [DOI] [PubMed] [Google Scholar]
- Chen T. H.; Wang J. Z.; Wang J.; Xie J. J.; Zhu C. Z.; Zhan X. M. Phosphorus removal from aqueous solutions containing low concentration of phosphate using pyrite calcinate sorbent. Int. J. Environ. Sci. Technol. 2015, 12 (3), 885–892. 10.1007/s13762-013-0450-6. [DOI] [Google Scholar]
- Claudio C.; di Iorio E.; Liu Q.; Jiang Z.; Barron V. Iron Oxide nanoparticles in soils: environmental and agronomic importance. J. Nanosci. Nanotechnol. 2017, 17 (7), 4449–4460. 10.1166/jnn.2017.14197. [DOI] [PubMed] [Google Scholar]
- Zhou D.-M.; Jin S.-Y.; Wang Y.-J.; Wang P.; Weng N.-Y.; Wang Y. Assessing the Impact of Iron-based Nanoparticles on pH, Dissolved Organic Carbon, and Nutrient Availability in Soils. Soil Sediment Contam. 2012, 21 (1), 101–114. 10.1080/15320383.2012.636778. [DOI] [Google Scholar]
- Huang B.; Miao A.-J.; Xiao L.; Yang L.-Y. Influence of nitrogen limitation on the bioaccumulation kinetics of hematite nanoparticles in the freshwater alga Euglena intermedia. Environ. Sci.: Nano 2017, 4 (9), 1840–1850. 10.1039/C7EN00477J. [DOI] [Google Scholar]
- Nowack B.; Stone A. T. Competitive adsorption of phosphate and phosphonates onto goethite. Water Res. 2006, 40 (11), 2201–2209. 10.1016/j.watres.2006.03.018. [DOI] [PubMed] [Google Scholar]
- Chen T.-C.; Shih Y.-J.; Chang C.-C.; Huang Y.-H. Novel adsorbent of removal phosphate from TFT LCD wastewater. J. Taiwan Inst. Chem. Eng. 2013, 44 (1), 61–66. 10.1016/j.jtice.2012.09.008. [DOI] [Google Scholar]
- Ioannou Z.; Dimirkou A.; Ioannou A. Phosphate Adsorption from Aqueous Solutions onto Goethite, Bentonite, and Bentonite–Goethite System. Water, Air, Soil Pollut. 2013, 224 (3), 1374. 10.1007/s11270-012-1374-3. [DOI] [Google Scholar]
- Huo H.; Lin H.; Dong Y.; Cheng H.; Wang H.; Cao L. Ammonia-nitrogen and phosphates sorption from simulated reclaimed waters by modified clinoptilolite. J. Hazard. Mater. 2012, 229–230, 292–297. 10.1016/j.jhazmat.2012.06.001. [DOI] [PubMed] [Google Scholar]
- Guaya D.; Hermassi M.; Valderrama C.; Farran A.; Cortina J. L. Recovery of ammonium and phosphate from treated urban wastewater by using potassium clinoptilolite impregnated hydrated metal oxides as N-P-K fertilizer. J. Environ. Chem. Eng. 2016, 4 (3), 3519–3526. 10.1016/j.jece.2016.07.031. [DOI] [Google Scholar]
- Bektas N.; Soysal D. Kinetics of phosphate removal using surfactant modified clinoptilolite. Fresenius Environ. Bull. 2004, 13 (4), 366–369. [Google Scholar]
- Williams A. T.; Zitomer D. H.; Mayer B. K. Ion exchange-precipitation for nutrient recovery from dilute wastewater. Environ. Sci.: Water Res. Technol. 2015, 1 (6), 832–838. 10.1039/C5EW00142K. [DOI] [Google Scholar]
- Yang H.; Liang C.; Luo F. Effects of adding betaine on biological nitrogen and phosphorus removal from simulated pickled vegetables wastewater. Water Sci. Technol. 2018, 77 (10), 2537–2544. 10.2166/wst.2018.214. [DOI] [PubMed] [Google Scholar]
- Lin X.; Xie Y.; Lu H.; Xin Y.; Altaf R.; Zhu S.; Liu D. Facile preparation of dual La-Zr modified magnetite adsorbents for efficient and selective phosphorus recovery. Chem. Eng. J. 2021, 413, 127530. 10.1016/j.cej.2020.127530. [DOI] [Google Scholar]
- Lin Y.-F.; Chen H.-W.; Chen Y.-C.; Chiou C.-S. Application of magnetite modified with polyacrylamide to adsorb phosphate in aqueous solution. J. Taiwan Inst. Chem. Eng. 2013, 44 (1), 45–51. 10.1016/j.jtice.2012.09.005. [DOI] [Google Scholar]
- Fu H.; Yang Y.; Zhu R.; Liu J.; Usman M.; Chen Q.; He H. Superior adsorption of phosphate by ferrihydrite-coated and lanthanum-decorated magnetite. J. Colloid Interface Sci. 2018, 530, 704–713. 10.1016/j.jcis.2018.07.025. [DOI] [PubMed] [Google Scholar]
- Abukhadra M. R.; Mostafa M. Effective decontamination of phosphate and ammonium utilizing novel muscovite/phillipsite composite; equilibrium investigation and realistic application. Sci. Total Environ. 2019, 667, 101–111. 10.1016/j.scitotenv.2019.02.362. [DOI] [PubMed] [Google Scholar]
- Ruby C.; Naille S.; Ona-Nguema G.; Morin G.; Mallet M.; Guerbois D.; Barthelemy K.; Etique M.; Zegeye A.; Zhang Y.; et al. Use of Ferrihydrite-Coated Pozzolana and Biogenic Green Rust to Purify Waste Water Containing Phosphate and Nitrate. Curr. Inorg. Chem. 2016, 6 (2), 100–118. 10.2174/1877944106999160603125459. [DOI] [Google Scholar]
- Wang X.; Hu Y.; Tang Y.; Yang P.; Feng X.; Xu W.; Zhu M. Phosphate and phytate adsorption and precipitation on ferrihydrite surfaces. Environ. Sci.: Nano 2017, 4 (11), 2193–2204. 10.1039/C7EN00705A. [DOI] [Google Scholar]
- Gan F.; Zhou J.; Wang H.; Du C.; Chen X. Removal of phosphate from aqueous solution by thermally treated natural palygorskite. Water Res. 2009, 43 (11), 2907–2915. 10.1016/j.watres.2009.03.051. [DOI] [PubMed] [Google Scholar]
- Gan F.; Zhou J.; Wang H.; Du C.; Zhang W.; Chen X. Phosphate adsorption on granular palygorskite- batch and column studies. Water Environ. Res. 2011, 83 (2), 147–153. 10.2175/106143010X12780288628372. [DOI] [PubMed] [Google Scholar]
- Ye H.; Chen F.; Sheng Y.; Sheng G.; Fu J. Adsorption of phosphate from aqueous solution onto modified palygorskites. Sep. Purif. Technol. 2006, 50 (3), 283–290. 10.1016/j.seppur.2005.12.004. [DOI] [Google Scholar]
- Gunay A.; Karadag D.; Tosun I.; Ozturk M. Use of magnesit as a magnesium source for ammonium removal from leachate. J. Hazard. Mater. 2008, 156 (1), 619–623. 10.1016/j.jhazmat.2007.12.067. [DOI] [PubMed] [Google Scholar]
- Liang H.; Guo P.; Yang Y.; Wang W.; Sun Z. Environmental application of engineering magnesite slag for phosphate adsorption from wastewater. Environ. Sci. Pollut. Res. 2022, 29 (39), 59502–59512. 10.1007/s11356-022-20029-z. [DOI] [PubMed] [Google Scholar]
- Mavhungu A.; Mbaya R.; Masindi V.; Foteinis S.; Muedi K. L.; Kortidis I.; Chatzisymeon E. Wastewater treatment valorisation by simultaneously removing and recovering phosphate and ammonia from municipal effluents using a mechano-thermo activated magnesite technology. J. Environ. Manage. 2019, 250, 109493. 10.1016/j.jenvman.2019.109493. [DOI] [PubMed] [Google Scholar]
- Huang H.; Xu C.; Zhang W. Removal of nutrients from piggery wastewater using struvite precipitation and pyrogenation technology. Bioresour. Technol. 2011, 102 (3), 2523–2528. 10.1016/j.biortech.2010.11.054. [DOI] [PubMed] [Google Scholar]
- Yu R.; Liu F.; Ren H.; Wu J.; Zhang X. Formation of magnesium hydrosilicate nanomaterials and its applications for phosphate/ammonium removal. Environ. Technol. 2018, 39 (17), 2162–2167. 10.1080/09593330.2017.1351495. [DOI] [PubMed] [Google Scholar]
- Wang F.; Peng L.; Xu N.; Yao Z.; Li D.; Cheng X. Enhanced phosphate removal from solution using Al-doped aragonite nanoparticles. Colloids Surf., A 2021, 630, 127638. 10.1016/j.colsurfa.2021.127638. [DOI] [Google Scholar]
- Xu N.; Wang Y.; Xu X.; Liu C.; Qian J.; Feng G. Mechanisms and Applications of the Synthesized Fusiform Aragonite for the Removal of High Concentration of Phosphate. Water, Air, Soil Pollut. 2016, 227 (2), 64. 10.1007/s11270-016-2757-7. [DOI] [Google Scholar]
- Wang S.-L.; Cheng C.-Y.; Tzou Y.-M.; Liaw R.-B.; Chang T.-W.; Chen J.-H. Phosphate removal from water using lithium intercalated gibbsite. J. Hazard. Mater. 2007, 147 (1), 205–212. 10.1016/j.jhazmat.2006.12.067. [DOI] [PubMed] [Google Scholar]
- Hong Z.-n.; Li J.-y.; Jiang J.; Li Z.-l.; Xu R.-k. Competition between bacteria and phosphate for adsorption sites on gibbsite: An in-situ ATR-FTIR spectroscopic and macroscopic study. Colloids Surf., B 2016, 148, 496–502. 10.1016/j.colsurfb.2016.09.026. [DOI] [PubMed] [Google Scholar]
- Heckman K.; Welty-Bernard A.; Vazquez-Ortega A.; Schwartz E.; Chorover J.; Rasmussen C. The influence of goethite and gibbsite on soluble nutrient dynamics and microbial community composition. Biogeochemistry 2013, 112 (1), 179–195. 10.1007/s10533-012-9715-2. [DOI] [Google Scholar]
- Zhang L.; Kim D.; Kim Y.; Wan J.; Jun Y.-S. Effects of phosphate on biotite dissolution and secondary precipitation under conditions relevant to engineered subsurface processes. Phys. Chem. Chem. Phys. 2017, 19 (44), 29895–29904. 10.1039/C7CP05158A. [DOI] [PubMed] [Google Scholar]
- Sarikhani M. R.; Khoshru B.; Oustan S. Efficiency of Some Bacterial Strains in Potassium Release from Mica and Phosphate Solubilization under In Vitro Conditions. Geomicrobiol. J. 2016, 33 (9), 832–838. 10.1080/01490451.2015.1117548. [DOI] [Google Scholar]
- Usharani K.; Lakshmanaperumalsamy P. Bio-treatment of phosphate from synthetic wastewater using Pseudomonas sp. YLW-7. J. Appl. Sci. Environ. Manage. 2010, 14 (2), 75–80. 10.4314/jasem.v14i2.57867. [DOI] [Google Scholar]
- Yang S.; Zhao Y.; Chen R.; Feng C.; Zhang Z.; Lei Z.; Yang Y. A novel tablet porous material developed as adsorbent for phosphate removal and recycling. J. Colloid Interface Sci. 2013, 396, 197–204. 10.1016/j.jcis.2012.12.077. [DOI] [PubMed] [Google Scholar]
- Chauhan K.; Kaur J.; Singh P.; Sharma P.; Sharma P.; Chauhan G. S. An Efficient and Regenerable Quaternary Starch for Removal of Nitrate from Aqueous Solutions. Ind. Eng. Chem. Res. 2016, 55 (9), 2507–2519. 10.1021/acs.iecr.5b03923. [DOI] [Google Scholar]
- Cui G.; Liu M.; Chen Y.; Zhang W.; Zhao J. Synthesis of a ferric hydroxide-coated cellulose nanofiber hybrid for effective removal of phosphate from wastewater. Carbohydr. Polym. 2016, 154, 40–47. 10.1016/j.carbpol.2016.08.025. [DOI] [PubMed] [Google Scholar]
- Yildiz E. Phosphate removal from water by fly ash using crossflow microfiltration. Sep. Purif. Technol. 2004, 35 (3), 241–252. 10.1016/S1383-5866(03)00145-X. [DOI] [Google Scholar]
- Zhao F.; Yang W.; Zeng Z.; Li H.; Yang X.; He Z.; Gu B.; Rafiq M. T.; Peng H. Nutrient removal efficiency and biomass production of different bioenergy plants in hypereutrophic water. Biomass Bioenergy 2012, 42, 212–218. 10.1016/j.biombioe.2012.04.003. [DOI] [Google Scholar]
- Orlando U. S.; Baes A. U.; Nishijima W.; Okada M. Preparation of agricultural residue anion exchangers and its nitrate maximum adsorption capacity. Chemosphere 2002, 48 (10), 1041–1046. 10.1016/S0045-6535(02)00147-9. [DOI] [PubMed] [Google Scholar]
- Agbovi H. K.; Wilson L. D. Design of amphoteric chitosan flocculants for phosphate and turbidity removal in wastewater. Carbohydr. Polym. 2018, 189, 360–370. 10.1016/j.carbpol.2018.02.024. [DOI] [PubMed] [Google Scholar]
- Fierro S.; del Pilar Sánchez-Saavedra M.; Copalcúa C. Nitrate and phosphate removal by chitosan immobilized Scenedesmus. Bioresour. Technol. 2008, 99 (5), 1274–1279. 10.1016/j.biortech.2007.02.043. [DOI] [PubMed] [Google Scholar]
- Fagundes T.; Bernardi E. L.; Rodrigues C. A. Phosphate Adsorption On Chitosan-FeIII-Crosslinking: Batch And Column Studies. J. Liq. Chromatogr. Relat. Technol. 2001, 24 (8), 1189–1198. 10.1081/JLC-100103441. [DOI] [Google Scholar]
- Leduc J.-F.; Leduc R.; Cabana H. Phosphate Adsorption onto Chitosan-Based Hydrogel Microspheres. Adsorpt. Sci. Technol. 2014, 32 (7), 557–569. 10.1260/0263-6174.32.7.557. [DOI] [Google Scholar]
- Karthikeyan P.; Meenakshi S. Fabrication of hybrid chitosan encapsulated magnetic-kaolin beads for adsorption of phosphate and nitrate ions from aqueous solutions. Int. J. Biol. Macromol. 2021, 168, 750–759. 10.1016/j.ijbiomac.2020.11.132. [DOI] [PubMed] [Google Scholar]
- Parajuli R.; Dalgaard T.; Birkved M. Can farmers mitigate environmental impacts through combined production of food, fuel and feed? A consequential life cycle assessment of integrated mixed crop-livestock system with a green biorefinery. Sci. Total Environ. 2018, 619–620, 127–143. 10.1016/j.scitotenv.2017.11.082. [DOI] [PubMed] [Google Scholar]
- Vogel W. S.; Hines T.. Automated zero waste systems and methods. WO2020077131, 2020.
- Anex R. P.; Lynd L. R.; Laser M. S.; Heggenstaller A. H.; Liebman M. Potential for Enhanced Nutrient Cycling through Coupling of Agricultural and Bioenergy Systems. Crop Sci. 2007, 47 (4), 1327–1335. 10.2135/cropsci2006.06.0406. [DOI] [Google Scholar]
- Octave S.; Thomas D. Biorefinery: Toward an industrial metabolism. Biochimie 2009, 91 (6), 659–664. 10.1016/j.biochi.2009.03.015. [DOI] [PubMed] [Google Scholar]
- Krivov A. D.Systems and methods of producing compositions from the nutrients recovered from waste streams using algal biomass. US20210207069, 2021.
- Olguín E. J.; Castillo O. S.; Mendoza A.; Tapia K.; González-Portela R. E.; Hernández-Landa V. J. Dual purpose system that treats anaerobic effluents from pig waste and produce Neochloris oleoabundans as lipid rich biomass. New Biotechnol. 2015, 32 (3), 387–395. 10.1016/j.nbt.2014.12.004. [DOI] [PubMed] [Google Scholar]
- Seghetta M.; Hou X.; Bastianoni S.; Bjerre A.-B.; Thomsen M. Life cycle assessment of macroalgal biorefinery for the production of ethanol, proteins and fertilizers – A step towards a regenerative bioeconomy. J. Cleaner Prod. 2016, 137, 1158–1169. 10.1016/j.jclepro.2016.07.195. [DOI] [Google Scholar]
- Javed F.; Aslam M.; Rashid N.; Shamair Z.; Khan A. L.; Yasin M.; Fazal T.; Hafeez A.; Rehman F.; Rehman M. S. U.; et al. Microalgae-based biofuels, resource recovery and wastewater treatment: A pathway towards sustainable biorefinery. Fuel 2019, 255, 115826. 10.1016/j.fuel.2019.115826. [DOI] [Google Scholar]
- Szymańska M.; Szara E.; Was A.; Sosulski T.; van Pruissen G. W. P.; Cornelissen R. L. Struvite—An Innovative Fertilizer from Anaerobic Digestate Produced in a Bio-Refinery. Energies 2019, 12 (2), 296. 10.3390/en12020296. [DOI] [Google Scholar]
- Barampouti E. M.; Mai S.; Malamis D.; Moustakas K.; Loizidou M. Exploring technological alternatives of nutrient recovery from digestate as a secondary resource. Renewable Sustainable Energy Rev. 2020, 134, 110379. 10.1016/j.rser.2020.110379. [DOI] [Google Scholar]
- Szymańska M.; Sosulski T.; Szara E.; Was A.; Sulewski P.; van Pruissen G. W. P.; Cornelissen R. L. Ammonium Sulphate from a Bio-Refinery System as a Fertilizer—Agronomic and Economic Effectiveness on the Farm Scale. Energies 2019, 12 (24), 4721. 10.3390/en12244721. [DOI] [Google Scholar]
- Crutchik D.; Frison N.; Eusebi A. L.; Fatone F. Biorefinery of cellulosic primary sludge towards targeted Short Chain Fatty Acids, phosphorus and methane recovery. Water Res. 2018, 136, 112–119. 10.1016/j.watres.2018.02.047. [DOI] [PubMed] [Google Scholar]
- Barbera E.; Bertucco A.; Kumar S. Nutrients recovery and recycling in algae processing for biofuels production. Renewable Sustainable Energy Rev. 2018, 90, 28–42. 10.1016/j.rser.2018.03.004. [DOI] [Google Scholar]
- Chojnacka K.; Moustakas K.; Witek-Krowiak A. Bio-based fertilizers: A practical approach towards circular economy. Bioresour. Technol. 2020, 295, 122223. 10.1016/j.biortech.2019.122223. [DOI] [PubMed] [Google Scholar]
- Jaeglé L.; Steinberger L.; Martin R. V.; Chance K. Global partitioning of NOx sources using satellite observations: Relative roles of fossil fuel combustion, biomass burning and soil emissions. Faraday Discuss. 2005, 130 (0), 407–423. 10.1039/b502128f. [DOI] [PubMed] [Google Scholar]
- Davidson E. A. The contribution of manure and fertilizer nitrogen to atmospheric nitrous oxide since 1860. Nat. Geosci. 2009, 2 (9), 659–662. 10.1038/ngeo608. [DOI] [Google Scholar]
- Harter J.; Krause H.-M.; Schuettler S.; Ruser R.; Fromme M.; Scholten T.; Kappler A.; Behrens S. Linking N2O emissions from biochar-amended soil to the structure and function of the N-cycling microbial community. ISME Journal 2014, 8 (3), 660–674. 10.1038/ismej.2013.160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun X.; Xuan X.; Chen S.; Li J.; Yang Z.; Zhao Y.; Wang M.. Cavitation and heating coupled device for preparing biochar fertilizer from waste biomass capable of improving specific surface area of the biochar. CN112723335, 2021.
- Krivov A. D.Systems and methods of producing compositions from the nutrients recovered from waste streams. WO2019133885, 2019.
- Sun W.Manure gasification production method of chemical fertilizer. CN113893906, 2022.
- Zhang X.; Lv F.; Han Z.. Organic solid waste recycling treatment and utilization method. CN113430012, 2021.
- Chen D.; Cen K.; Zhang Y.; Zhou J.; Ma H.. Method for preparing activated carbon and liquid fertilizer by pyrolysis of biomass based on combined action of biomass vinegar liquid and biomass oil. CN113233456, 2021.
- Zuo Z.; Luo S.; Li X.; Chen Y.; Gao L.; Peng B.; Ren D.; Guo J.. Urea-formaldehyde resin and biomass co-pyrolysis process system having high yield of biomass thermal desorption hydrogen desorption and method. CN113088307, 2021.
- Smil V.Enriching the Earth: Fritz Haber, Carl Bosch, and the Transformation of World Food Production; MIT Press, 2004. [Google Scholar]
- MacFarlane D. R.; Cherepanov P. V.; Choi J.; Suryanto B. H. R.; Hodgetts R. Y.; Bakker J. M.; Ferrero Vallana F. M.; Simonov A. N. A Roadmap to the Ammonia Economy. Joule 2020, 4 (6), 1186–1205. 10.1016/j.joule.2020.04.004. [DOI] [Google Scholar]
- Mauro da Silva Neiro S.; de Faria É. V.; Murata V. V. MILP Continuous-Time Production Scheduling Approaches for the Phosphate Fertilizer Industry. Ind. Eng. Chem. Res. 2022, 61 (11), 4031–4045. 10.1021/acs.iecr.1c04829. [DOI] [Google Scholar]
- Whalen J. M.; Matlin S. A.; Holme T. A.; Stewart J. J.; Mahaffy P. G. A Systems Approach to Chemistry Is Required to Achieve Sustainable Transformation of Matter: The Case of Ammonia and Reactive Nitrogen. ACS Sustainable Chem. Eng. 2022, 10, 12933. 10.1021/acssuschemeng.2c03159. [DOI] [Google Scholar]
- Heid O.; Beasley P.; Hughes T.. Load balancing of intermittent renewable energy for an electricity grid. WO2015192877, 2015.
- Jewess M.; Crabtree R. H. Electrocatalytic Nitrogen Fixation for Distributed Fertilizer Production?. ACS Sustainable Chem. Eng. 2016, 4 (11), 5855–5858. 10.1021/acssuschemeng.6b01473. [DOI] [Google Scholar]
- Greenlee L. F.; Renner J. N.; Foster S. L. The Use of Controls for Consistent and Accurate Measurements of Electrocatalytic Ammonia Synthesis from Dinitrogen. ACS Catal. 2018, 8 (9), 7820–7827. 10.1021/acscatal.8b02120. [DOI] [Google Scholar]
- Zhou F.; Azofra L. M.; Ali M.; Kar M.; Simonov A. N.; McDonnell-Worth C.; Sun C.; Zhang X.; MacFarlane D. R. Electro-synthesis of ammonia from nitrogen at ambient temperature and pressure in ionic liquids. Energy Environ. Sci. 2017, 10 (12), 2516–2520. 10.1039/C7EE02716H. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.