Abstract
Background
Millions of COVID-19 survivors experience a wide range of long-term symptoms after acute infection, giving rise to serious public health concerns. To date, few risk factors for post-COVID-19 conditions have been determined. This study evaluated the role of pre-infection sleep quality/duration and insomnia severity in the incidence of long-term symptoms after COVID-19.
Material and methods
This prospective study involved two assessments (April 2020 and 2022). At the baseline (April 2020), sleep quality/duration and insomnia symptoms in participants without current/prior SARS-CoV-2 infection were measured using the Pittsburgh Sleep Quality Index (PSQI) and the Insomnia Severity Index (ISI). At the follow-up (April 2022), we asked a group of COVID-19 survivors to retrospectively evaluate the presence of twenty-one symptoms (psychiatric, neurological, cognitive, bodily, and respiratory) that have been experienced one month (n = 713, infection in April 2020–February 2022) and three months after COVID-19 (n = 333, infection in April 2020–December 2021). In April 2022, participants also reported how many weeks passed to fully recover from COVID-19. Zero-inflated negative binomial models were used to estimate the effect of previous sleep on the number of long-term symptoms. Binomial logistic regressions were performed to evaluate the association between sleep variables, the incidence of each post-COVID-19 symptom, and the odds of recovery four/twelve weeks after infection.
Results
Analyses highlighted a significant effect of pre-infection sleep on the number of symptoms one/three months after COVID-19. Previous higher PSQI and ISI scores, and shorter sleep duration significantly increased the risk of almost every long-term symptom at one/three months from COVID-19. Baseline sleep problems were also associated with longer recovery times to return to the pre-infection daily functioning level after COVID-19.
Conclusions
This study suggested a prospective dose-dependent association of pre-infection sleep quality/quantity and insomnia severity with the manifestation of post-COVID-19 symptoms. Further research is warranted to determine whether preventively promoting sleep health may mitigate the COVID-19 sequelae, with substantial public health and societal implications.
Keywords: Insomnia, Post-COVID-19 symptoms, Prospective association, Public health, Sleep and immunity, Long COVID
1. Introduction
Estimates from the World Health Organization indicate that over 750 million Coronavirus Disease 2019 (COVID-19) cases have been confirmed globally (World Health Organization, 2023). While most individuals experience mild symptoms and recover quickly, recent meta-analyses suggested that 43–45% of COVID-19 survivors report signs and symptoms that continue or develop in the long run (Chen et al., 2022, O’Mahoney et al., 2023). This condition is commonly termed “long COVID” and encompasses over two-hundred long-term clinical manifestations (Davis et al., 2021). General symptoms include fatigue, body aches, fever, ageusia, and anosmia. Other symptoms are related to lung disease (i.e., cough, dyspnea) or involve neurological and cognitive dysfunctions (headache, brain fog, attention/concentration disorders, memory loss) and cardiovascular or gastrointestinal disorders. Moreover, a broad spectrum of psychiatric and psychological manifestations after acute infection were also identified, such as sleep disorders, depression, anxiety, post-traumatic stress disorder (PTSD), obsessive–compulsive disorders (OCD) and psychosis (Badenoch et al., 2021). This disabling condition pervasively impacts the daily life of COVID-19 survivors, also affecting their ability to resume a regular working routine (Davis et al., 2021, O’ Mahony et al., 2022).
Identifying potential antecedents of long-term symptoms represents a first-order medical challenge due to the burden on the international healthcare systems and the societal and economic costs (The Lancet, 2021). However, due to the multisystemic and heterogeneous nature of long COVID, its etiology remains poorly understood, and current evidence propose chronic inflammation and immune dysregulation as possible causes of long-term clinical manifestations after acute illness (Crook et al., 2021, Mazza et al., 2021, Mazza et al., 2020, Phetsouphanh et al., 2022).
Sleep plays a crucial role in human immunity (Besedovsky et al., 2012, Bryant et al., 2004), and poor sleep quality and inadequate sleep duration are associated with increased susceptibility to virus infections (Cohen et al., 2009, Prather et al., 2015). Moreover, a growing body of evidence linked sleep disturbances and short sleep duration with increased risk for inflammatory diseases due to the relationship between sleep problems and low sleep amount with sustained production of pro-inflammatory cytokines and other circulating markers of inflammation (Garbarino et al., 2021, Irwin et al., 2016).
Based on these assumptions, pre-infection sleep disturbances could play a role in predisposing people to experience long-term symptoms after COVID-19. However, to the best of our knowledge, no prospective studies have addressed this research question. In this study, sleep outcomes of validated questionnaires from a nationwide survey held during the first Italian lockdown (April 2020) were used as predictors of long COVID symptoms, which were retrospectively reported in April 2022 by a group of COVID-19 survivors infected in the previous two years. We hypothesized that sleep disturbances and shorter sleep duration could be prospectively associated with the occurrence of a wide range of long-term symptoms after one and three months from COVID-19 while accounting for established risk factors (age, gender, body mass index – BMI, COVID-19 severity; Chen et al., 2022, Huang et al., 2022, Sudre et al., 2021, Zeng et al., 2022). Finally, we investigated whether sleep issues before infection were related to longer recovery times to return to the pre-infection daily functioning level.
2. Material and methods
2.1. Participants and procedure
A total of 13,989 participants were surveyed during the first lockdown period in April 2020 via a web-based set of questionnaires (for a detailed description of the data collection procedure, see Salfi et al., 2021b). Subsequently, a total of 2,013 respondents were longitudinally evaluated in December 2020 (Salfi et al., 2021a). Finally, the overall sample surveyed in April 2020 was re-invited to take part in another longitudinal assessment in April 2022. A total of 2,759 Italians participated in the last data collection, while a total of 1,062 respondents participated in all three survey waves (Salfi et al., 2022). Baseline characteristics of participants followed-up in April 2022 (n = 2,759) and those who did not participate in the follow-up assessment (n = 11,230) are reported in Table S1 in the Supplementary Material.
Each assessment comprised an evaluation of sleep quality/duration and insomnia severity using the Pittsburgh Sleep Quality Index (PSQI; Curcio et al., 2013), and the Insomnia Severity Index (ISI; Castronovo et al., 2016).
The PSQI is a reliable questionnaire to evaluate sleep quality (Curcio et al., 2013). It consists of nineteen questions, from which a total score (range, 0–21) is calculated. A higher score indicates poorer sleep quality. The ISI is a validated screening instrument to assess the severity of insomnia symptoms (Castronovo et al., 2016). It comprised seven items and a higher total score (range, 0–28) point to more severe insomnia.
Moreover, in each survey wave we collected demographic and other information (for more details, see Salfi et al., 2021a, Salfi et al., 2021b, Salfi et al., 2022). In April 2022, we asked respondents if they have ever tested positive for COVID-19. If so, participants were asked to answer a set of ad hoc questions about their infection and symptomatology. We collected information about:
-
I.
the month of detected swab positivity;
-
II.
the COVID-19 severity in the acute stage of illness, using a multiple choice question with four alternative answers (i.e., no marked symptoms: absence of any symptom except for smell/taste dysfunctions; mild disease: e.g., cough, fever, muscle pains, etc., without pneumonia; moderate disease: non-severe pneumonia and having received different medications without the need of extra oxygen treatment; severe disease: severe pneumonia requiring extra-oxygen therapy and intravenous lines attached);
-
III.
the presence of long-term symptoms one and three months after the first infection according to Italian National Institute of Health guidelines for long COVID identification (over-tiredness, muscle weakness, breathlessness/dyspnea, concentration/attention difficulty, headache, asthenia, anxiety, diffuse body pain, sleep problems, memory problems, brain fog, deterioration of perceived health status, persistent cough, smell/taste dysfunctions, depression, appetite reduction, fever, PTSD, cardiovascular problems, OCD, psychosis);
-
IV.
the recovery time (in weeks) to return to the pre-infection daily functioning level.
From the overall April 2022 sample, a total of 973 participants (35.00%, mean age ± standard deviation, 33.40 ± 11.40 years; range, 18–81 years; 170 males) reported SARS-CoV-2 infection and provided the above-listed information. The temporal distributions of COVID-19 cases across the pandemic period in our sample and among the over 18-year Italian population are depicted in Fig. 1 .
Fig. 1.
Temporal distribution of COVID-19 cases among the over 18-year Italian population (weekly; dark blue line) and in the overall study sample (n = 973; monthly; light blue bars). Notes: Weekly national trend of COVID-19 cases was derived from the Italian National Institute of Health website (Istituto Superiore di Sanità, 2022).
The flow chart of participants analyzed in the study is reported in Fig. 2 .
Fig. 2.
Flow chart of study participants. Notes: Grey boxes indicate missing data or individuals excluded due to the study objectives. Blue boxes indicate analyzed samples. COVID-19 information was collected at follow-up in April 2022.
To estimate the role of sleep quality/quantity and insomnia severity in predicting long-term symptoms one month after COVID-19 (see 2.2.1 and 2.2.2 sections) and the odds of recovery in more than four weeks (see 2.2.3 section), the analyses were performed excluding 261 individuals. Specifically, we excluded people already infected during the first survey wave (due to possible COVID-19 effects on baseline sleep assessment) and participants who were infected in March/April 2022 (due to the insufficient time elapsed between COVID-19 and the retrospective evaluation in April 2022). Therefore, the analyses were performed on 713 people (33.38 ± 11.40 years; 18–81 years; 122 males) who reported the swab positivity from April 2020 to February 2022.
To evaluate the role of sleep quality/quantity and insomnia severity in predicting long-term symptoms three months after the infection (see 2.2.1 and 2.2.2 sections) and the risk for recovery in more than twelve weeks (see 2.2.3 section), the analyses were performed on the subgroup who indicated a swab positivity from April 2020 to December 2021 (n = 333; 33.09 ± 11.80 years; 18–70 years; 60 males). As shown in Fig. 2, this sample is entirely part of the group in which post-COVID-19 symptoms at one month from infection were analyzed. Specifically, the subgroup was selected by excluding other 380 subjects because they were infected less than three months before the retrospective assessment in April 2022.
The project was approved by the Institutional Review Board of the University of L’Aquila (protocol no. 43066). Respondents provided electronic consent to participate in the study in each survey wave.
2.2. Statistical analysis
We planned three analysis families to evaluate the predictive effect of the sleep variables [PSQI score, ISI score, and total sleep time (TST, extracted from item 4 of PSQI)] on (i) the number of long-term symptoms, (ii) the odds of each long-term symptom, and (iii) the odds of recovering the pre-infection daily functioning level in longer times (after 4 or 12 weeks). All analyses described in the following sections were adjusted for demographic confounding variables [age, gender, and BMI (weight/height2)]. All tests were two-tailed, and a p-value < 0.05 was considered significant.
A set of control analyses were also performed to evaluate the consistency of our results (see 2.2.4 section).
2.2.1. Analysis on the number of long-term symptoms after COVID-19
The numbers of symptoms at one/three months from COVID-19 were entered as dependent variables (range, 0–21), and the sleep variables (PSQI score, ISI score, TST) as separate predictors. Due to the nature of the dependent variables (count data), linear regressions are unsuitable (Coxe et al., 2009, Gardner et al., 1995). Therefore, we evaluated the possibility of performing Poisson regressions, which are typically used to model count data (Coxe et al., 2009, Gardner et al., 1995). However, preliminary analyses highlighted a violation of the equidispersion assumption (χ 2/df ranging from 3.94 to 5.48, greater than the acceptable value of 1.20, Payne et al., 2018). In this case, a Negative Binomial (NB) regression approach should be used (Gardner et al., 1995, Hilbe, 2011, Land et al., 2016). NB models provide estimates [exp(B)] that indicate the rate change of the count variable for a one-unit change in the predictor.
Due to the high prevalence of zero values in count variables (27.9% and 52.6% in one-month and three-month symptoms, respectively), data were modeled with Zero-Inflated NB regression (ZINB; Cameron and Trivedi, 2013, Green, 2021, Moghimbeigi et al., 2008). The ZINB approach combines two separate models: a ZI model and a NB model. The ZI portion is used to model the probability of a zero count, while the negative binomial portion is used to model the count itself. The ZINB model is useful in analyzing count data that has an excess of zero values because it accounts for this excess in the data and provides more accurate estimates of the underlying distribution. Therefore, the ZINB models allowed us to manage both the excess of zero observations and the overdispersion in the data.
Six ZINB models (M) were tested, evaluating the effect of PSQI, ISI, and TST in predicting the number of symptoms at one month (M1, M2, M3, respectively) and three months from infection (M4, M5, M6, respectively). The models were fitted using “countereg” R package (Zeileis et al., 2008). In the results section, we focused on the NB portion of ZINB models for addressing the role of sleep variables in predicting the number of long-term symptoms. A comparison of relative goodness-of-fit parameters (Akaike information criterion and the Bayesian information criterion) supported the use of ZINB regressions instead of NB (see Table S2 in the Supplementary Material). Moreover, a visual inspection of Tukey’s rootograms (Tukey, 1977) using the “countereg” R package (Kleiber and Zeileis, 2016) confirmed ZINB as the best-fit models.
2.2.2. Analysis on each long-term symptom after COVID-19
Binomial logistic regressions were performed to evaluate the association between sleep variables and the future occurrence of each long-term symptom one and three months after COVID-19. Analyses were performed using “glm” function of “stats” R package (R Core Team, 2022) In detail, each sleep variable was entered as a predictor (PSQI score, ISI score, TST), and each long-term symptom as a dichotomous dependent variable (yes, no). The criterion of a minimum of 5 outcome events per predictor variable (EPV) was adopted (Vittinghoff and McCulloch, 2007) to identify the analyzable long-term symptoms after infection. Consequently, some symptoms were excluded from the analysis on one-month (psychosis and OCD) and three-month symptoms (PTSD, fever, persistent cough, OCD, appetite reduction, cardiovascular problems, and psychosis) due to insufficient events. Considering the relaxed EPV criterion adopted to maximize the number of analyzable long-term symptoms, sensitivity analyses were performed replicating the binomial logistic models using the Firth's bias reduction method (Heinze and Puhr, 2010). This approach allows the parameter estimations to be more efficient and robust to small sample sizes and rare events by penalizing the likelihood function. These control analyses were applied using “logistf” R package (Heinze et al., 2022).
2.2.3. Analysis on recovery time after COVID-19
Binomial logistic regressions were performed to test the association between sleep characteristics (quality, duration, insomnia severity) and the recovery time to return to the pre-infection daily functioning level. The self-reported recovery time was entered as dichotomous dependent variable (≤4 weeks vs. > 4 weeks for the one-month symptoms sample; ≤ 12 weeks vs. > 12 weeks for the three-month symptoms sample), while sleep variables (PSQI score, ISI score, TST) were used as predictors.
2.2.4. Supplemental analyses
First, all analyses were further adjusted for the COVID-19 severity, considering the established relationship between acute illness severity and future long COVID symptoms (Chen et al., 2022, Huang et al., 2022, Taquet et al., 2021, Zeng et al., 2022).
Second, models were adjusted for education level.
Third, models were fitted including the number of months between April 2020 and the time of the infection as covariate. These analyses aimed at verifying if the predictive effect of sleep variables is affected by the time distance between the actual infection and the baseline sleep assessment. At the same time, the same analyses allowed us to evaluate if the relationship between sleep variables in April 2020 and post-COVID-19 symptoms/recovery time was impacted by the time elapsed between the infection and the retrospective assessment in April 2022 (as detailed in the “Supplemental statistical analysis” section in the Supplementary Material).
Fourth, we replicated the main results by performing a set of separate analyses using a different baseline assessment of sleep variables (December 2020; see “Supplemental results” section in the Supplementary Material). In these analyses, we included only people infected from December 2020 (after the questionnaire compilation).
Fifth, control models were performed estimating the relationship between sleep variables and the number of symptoms occurred only one month after the infection, but not three months later (see Table S6 in the Supplementary Material). These analyses aimed at confirming the predictive role of sleep features on post-COVID-19 symptoms that may unlikely be interpreted as functional consequences or comorbidities of poor sleep per se, due to their transient occurrence after COVID-19.
Sixth, sensitivity analyses were performed evaluating the relationship between sleep variables and the number of symptoms at one/three months from infection while controlling for the sleep variables collected in April 2022 (see Table S7 in the Supplementary Material). These models were fitted to exclude confounding effects due to the possibility that people who suffer from more sleep disturbances at follow-up may also be predisposed to retrospectively report more post-COVID-19 problems.
3. Results
3.1. Sample characteristics and COVID-19-related information
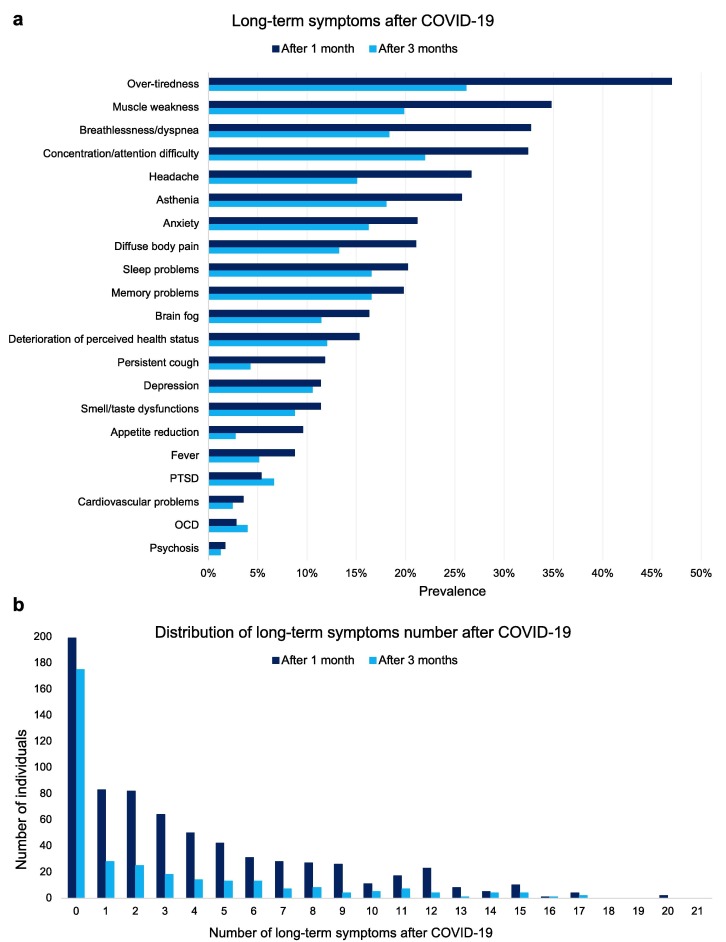
The composition of the analyzed samples is shown in Table 1 . Most participants were young, females, fell within the healthy weight range, and had at least a bachelor’s degree. The majority of respondents reported a mild COVID-19 severity in the acute stage and recovered the pre-infection daily functioning level in less than a month. Descriptive statistics of questionnaire scores assessing sleep quality, severity of insomnia symptoms, and sleep duration are also reported. The prevalence of each long-term symptom one month and three months after COVID-19 among the analyzed samples is reported in Fig. 3 a. The distribution of the number of symptoms at one and three months from the infection is shown in Fig. 3b.
Table 1.
Demographic characteristics, information about COVID-19, and descriptive statistics of questionnaire scores evaluating sleep quality, insomnia severity, and sleep duration among participants who were tested positive for SARS-CoV-2 from April 2020 to February 2022, and from April 2020 to December 2021.
| Variable | COVID-19 in April 2020–February 2022 (n = 713) |
COVID-19 in April 2020–December 2021 (n = 333) |
|---|---|---|
| N (%) or *mean (SD) | ||
| Age | ||
| Younger (18 to 30 years) | 390 (54.7) | 194 (58.3) |
| Middle-aged (31 to 50 years) | 250 (35.1) | 100 (30.0) |
| Older (>50 years) | 73 (10.2) | 39 (11.7) |
| Gender | ||
| Male | 122 (17.1) | 60 (18.0) |
| Female | 591 (82.9) | 273 (82.0) |
| BMI | ||
| Underweight range (<18.5) | 48 (6.7) | 23 (6.9) |
| Healthy weight range (18.5 to < 25) | 462 (64.8) | 205 (61.6) |
| Overweight range (25 to < 30) | 146 (20.5) | 69 (20.7) |
| Obesity (≥35) | 57 (8.0) | 36 (10.8) |
| Education | ||
| Middle/High school | 216 (30.3) | 106 (31.8) |
| Bachelor’s degree | 171(24.0) | 108 (32.4) |
| Master’s degree | 229 (32.1) | 78 (23.4) |
| Postgraduate | 97 (13.6) | 41(12.3) |
| COVID-19 severity | ||
| No marked symptoms | 183 (25.7) | 84 (25.2) |
| Mild disease | 490 (68.7) | 220 (66.1) |
| Moderate disease | 33 (4.6) | 22 (6.6) |
| Severe disease | 7 (1.0) | 7 (2.1) |
| Time to recover the pre-infection daily functioning level | ||
| ≤ 4 weeks | 479 (67.2) | 210 (63.1) |
| > 4 weeks | 234 (32.8) | 123 (35.9) |
| ≤ 12 weeks | 270 (81.1) | |
| > 12 weeks | 63 (18.9) | |
| PSQI score | *6.950 ± 3.575 | *6.885 ± 3.694 |
| ISI score | *8.279 ± 5.419 | *8.171 ± 5.330 |
| Sleep duration (hour) | *7.205 ± 1.358 | *7.215 ± 1.422 |
Notes: Demographic characteristics and questionnaire scores refer to the first assessment (April 2020), while information about COVID-19 was retrospectively collected in April 2022. Numbers preceded by an asterisk (*) are mean values ± standard deviation. BMI ranges were established according to the American Centers for Disease Control and Prevention (2023) guidelines.
Abbreviations: BMI, Body mass index; ISI, Insomnia Severity Index; PSQI, Pittsburgh Sleep Quality Index; SD, Standard deviation.
Fig. 3.
Prevalence of long-term symptoms (a) and distribution of symptoms number (b) one month and three months after COVID-19 in the analyzed samples. Notes: The “After 1 month” group consists of 713 individuals (dark blue bars), while the “After 3 months” group comprises 333 subjects (light blue bars). Abbreviations: OCD, obsessive–compulsive disorder; PTSD, post-traumatic stress disorder.
3.2. Association between pre-infection sleep and the number of long-term symptoms after COVID-19
Results from the NB portion of the ZINB models (Table 2 ) showed that lower sleep quality, more severe insomnia symptoms, and shorter sleep duration significantly predicted a higher number of long-term symptoms at one month and three months from COVID-19. A one-unit increase in PSQI and ISI scores, and a one-hour reduction of sleep duration predicted a 7.0% (95% CI: 4.6%–9.4%), 4.9% (95% CI: 3.3%–6.4%), and 12.5% (95% CI: 6.3%–19.0%) increase in the number of symptoms one month after infection, and an increase of 9.1% (95% CI: 4.7%–13.6%), 5.4% (95% CI: 2.7%–8.2%), and 17.2% (95% CI: 5.7%–30.0%) in the number of symptoms three months after COVID-19, respectively. A graphical representation of the relationships between sleep variables and the number of long-term symptoms is provided in Fig. 4 .
Table 2.
Results from the negative binomial portion of the ZINB models [exp(B), 95% confidence intervals, p-value] estimating the effect of sleep variables (PSQI score, ISI score, TST) and confounding factors (age, gender, BMI) in April 2020 on the number of long-term symptoms one month (M1, M2, M3) and three months after COVID-19 (M4, M5, M6).
| exp(B) |
95% CI |
p |
exp(B) |
95% CI |
p |
exp(B) |
95% CI |
p |
|
|---|---|---|---|---|---|---|---|---|---|
| M1: sleep quality | M2: insomnia severity | M3: sleep duration | |||||||
| Intercept | 2.325 | 1.461–3.699 | < 0.001 | 2.315 | 1.465–3.658 | < 0.001 | 9.182 | 4.494–18.761 | < 0.001 |
| Gender* | 0.912 | 0.724–1.149 | 0.433 | 0.878 | 0.701–1.101 | 0.260 | 0.819 | 0.649–1.034 | 0.093 |
| Age | 0.990 | 0.983–0.998 | 0.009 | 0.992 | 0.984–0.999 | 0.030 | 0.987 | 0.979–0.995 | 0.001 |
| BMI | 1.023 | 1.006–1.041 | 0.008 | 1.024 | 1.006–1.041 | 0.008 | 1.026 | 1.008–1.045 | 0.004 |
| PSQI score | 1.070 | 1.046–1.094 | < 0.001 | ||||||
| ISI score | 1.049 | 1.033–1.064 | < 0.001 | ||||||
| TST (hour) | 0.889 | 0.840–0.941 | < 0.001 | ||||||
| M4: sleep quality | M5: insomnia severity | M6: sleep duration | |||||||
| Intercept | 1.312 | 0.551–3.124 | 0.540 | 1.706 | 0.776–3.752 | 0.184 | 8.668 | 2.478–30.317 | 0.001 |
| Gender* | 0.925 | 0.592–1.446 | 0.733 | 0.999 | 0.644–1.550 | 0.997 | 0.816 | 0.522–1.278 | 0.375 |
| Age | 1.002 | 0.987–1.018 | 0.772 | 1.002 | 0.988–1.017 | 0.746 | 0.996 | 0.980–1.012 | 0.628 |
| BMI | 1.021 | 0.990–1.054 | 0.184 | 1.017 | 0.986–1.048 | 0.284 | 1.027 | 0.995–1.059 | 0.097 |
| PSQI score | 1.091 | 1.047–1.136 | < 0.001 | ||||||
| ISI score | 1.054 | 1.027–1.082 | < 0.001 | ||||||
| TST (hour) | 0.853 | 0.769–0.946 | 0.003 | ||||||
Notes: *Female was used as reference for “Gender” factor; significant values are in bold.
Abbreviations: BMI, Body mass index; CI, Confidence interval; ISI, Insomnia Severity Index; M, Model; PSQI, Pittsburgh Sleep Quality Index; TST, Total sleep time.
Fig. 4.
Relationships between sleep variables (PSQI score, ISI score, TST) in April 2020 and the number of long-term symptoms one month (M1, M2, M3) and three months after COVID-19 (M4, M5, M6). Notes: Light blue area represents 95% confidence intervals. Each model was adjusted for age, gender, and body mass index. Abbreviations: ISI, Insomnia Severity Index; M, Model; PSQI, Pittsburgh Sleep Quality Index; TST, Total sleep time.
Sensitivity analyses adjusting M1–6 for the COVID-19 severity, education level, or the time distance between swab positivity and the baseline assessment (April 2020; see Table S3 in the Supplementary Material) confirmed all the significant effects of sleep variables. Results were also confirmed using sleep outcomes collected in December 2020 as predictors of one-month symptoms (see Table S4 and Figure S1 in the Supplementary Material).
Furthermore, supplementary analyses showed that the occurred variations in sleep quality, insomnia severity, and sleep duration between April and December 2020 significantly predicted the number of long-term symptoms one month after infection (see Table S5 and Fig. S2 in the Supplementary Material).
Sensitivity analyses confirmed the role of sleep variables in predicting transient symptoms that were reported to occur only one month after the infection, but not three months later (see Table S6 in the Supplementary Material).
Finally, the relationship between sleep variables and the number of long-term symptoms one/three months after infection was confirmed while controlling for the sleep variables collected in April 2022 (see Table S7 in the Supplementary Material).
3.3. Association between pre-infection sleep and each long-term symptom after COVID-19
Results of binomial logistic regressions using sleep variables collected in April 2020 as predictors indicated that higher PSQI scores significantly increased the odds of each analyzed long-term symptom at one (Fig. 5 a) and three months from COVID-19 (Fig. 5b), except for smell/taste dysfunctions, cardiovascular problems experienced at one month from infection, and breathlessness/dyspnea three months after COVID-19. More severe insomnia symptoms were significantly associated with higher odds of all analyzed long-term symptoms, excluding smell/taste dysfunctions (Fig. 5c,d). Finally, a one-hour decrease in sleep duration was associated with higher odds of all one-month long-term symptoms, except for asthenia, memory problems, smell/taste dysfunctions, appetite reduction, and cardiovascular problems (Fig. 5e). Furthermore, reduced sleep duration was associated with increased risk for all symptoms at 3 months from infection, excluding over-tiredness, concentration/attention difficulty, anxiety, depression, and smell/taste dysfunctions (Fig. 5f).
Fig. 5.
Results of logistic regressions (odd ratios and 95% confidence intervals) evaluating the predictive effect of sleep variables (PSQI score, ISI score, TST) in April 2020 on the odds of each long-term symptom one month (a, c, e) and three months after COVID-19 (b, d, f). Notes: Long-term symptoms were ordered according to the prevalence data (top: most frequent) and represent the dependent variables. White dot indicates significance level at p < 0.05, grey dot at p < 0.01, and black dot at p < 0.001. “×” symbol indicates no statistically significant effect. Grey area indicates insufficient (<5) outcome events per predictor. Each model was adjusted for age, gender, and body mass index. Abbreviations: CI, Confidence Interval; ISI, Insomnia Severity Index; OCD, obsessive–compulsive disorder; OR, odd ratio; PSQI, Pittsburgh Sleep Quality Index; PTSD, post-traumatic stress disorder; TST, Total sleep time.
Control analyses using Firth's bias-reduced logistic regressions produced almost identical results to those obtained using the reported binomial logistic regressions, rejecting possible bias due to the low number of events per predictor for some long-term symptoms. Similarly, adjusting for self-reported COVID-19 severity, education level, or the time distance between SARS-CoV-2 infection and the baseline assessment (April 2020) confirmed the overall pattern of results.
Finally, logistic models using PSQI score, ISI score, and TST of December 2020 as predictors of symptoms at one month after COVID-19 partially confirmed the above-described results (see Fig. S3 in the Supplementary Material).
3.4. Association between pre-infection sleep and recovery time after COVID-19
Binomial logistic regression showed that poor sleep quality, more severe insomnia symptoms, and shorter sleep duration in April 2020 predicted longer recovery time to return to the pre-infection daily functioning level after COVID-19 (Fig. 6 ). A one-unit increase in PSQI and ISI scores, and a one-hour reduction of sleep duration were prospectively associated with higher odds of recovery in more than four weeks by 13.1% (95% CI: 7.9%–18.6%), 9.3% (95% CI: 6.1%–12.7%), and 16.8% (95% CI: 4.0%–31.2%), and after twelve weeks by 21.3% (95% CI: 11.9%–31.5%), 12.0% (95% CI: 6.2%–18.1%), and 25.3% (95% CI: 3.1%–52.4.4%), respectively. Adjusting for COVID-19 severity, education level, and the time distance between April 2020 assessment and the virus infection confirmed the above results. Finally, logistic regressions using sleep variables collected in December 2020 as predictors confirmed the significant association between recovery after 4 weeks with PSQI and ISI scores (see “Supplemental results” section in the Supplementary Material).
Fig. 6.
Results of logistic regressions (odd ratios and 95% confidence intervals) evaluating the predictive effect of sleep variables (PSQI score, ISI score, TST) in April 2020 on the odds of returning to the pre-infection daily functioning level after 4 and 12 weeks. Notes: Self-reported recovery after 4 and 12 weeks was evaluated on 713 and 333 subjects, respectively. White dot indicates significance level at p < 0.05, grey dot at p < 0.01, and black dot at p < 0.001. Each model was adjusted for age, gender, and body mass index. Abbreviations: CI, Confidence Interval; ISI, Insomnia Severity Index; OR, Odd ratio; PSQI, Pittsburgh Sleep Quality Index; TST, Total sleep time.
4. Discussion
Since the beginning of the pandemic, millions of people worldwide have reported signs and symptoms that continue or develop after COVID-19, affecting their ability to resume normal life and giving rise to serious public health concerns (The Lancet, 2021).
To the best of our knowledge, this study is the first to find a prospective association between pre-infection sleep disturbances and the occurrence of long-term symptoms after COVID-19. We highlighted significant dose-dependent relationships between previous sleep quality, insomnia severity, and sleep duration and the number of symptoms experienced at one and three months from the reported positive swab for SARS-CoV-2. Moreover, our study showed that lower sleep quality, more severe insomnia, and shorter sleep time lead to higher odds of experiencing a broad spectrum of clinical manifestations one and three months after COVID-19. Finally, we found that previous sleep problems are related to longer recovery times for returning to the self-perceived pre-infection daily functioning.
Our results were consistent with a recent study on a sample of female nurses that found an association between short sleep duration collected in 2017 and the occurrence of post-COVID-19 conditions (Siwen Wang et al., 2023).
Immune dysregulation and inflammatory mechanisms may explain the association between sleep and subsequent post-COVID-19 manifestations. Consistent literature showed that sleep disturbances are associated with heightened systemic inflammation, as evidenced by increased levels of pro-inflammatory cytokines (Cho et al., 2015, Ferrie et al., 2013, Irwin et al., 2016, Mills et al., 2007, Nowakowski et al., 2018), C-reactive protein (Cho et al., 2015, Ferrie et al., 2013, Ghilotti et al., 2021, Irwin et al., 2016, Liukkonen et al., 2007), and other markers of inflammation (Irwin et al., 2006, Nowakowski et al., 2018). This evidence has been proposed to explain the documented higher risk for inflammatory diseases in people with sleep disturbances (Garbarino et al., 2021, Irwin et al., 2016). On the other hand, findings from animal (Frere et al., 2022, Rutkai et al., 2022) and human models (Crook et al., 2021, Mazza et al., 2021, Mazza et al., 2020, Phetsouphanh et al., 2022) suggested that a possible mechanism behind the long COVID symptoms may involve an abnormal and persistent pro-inflammatory response weeks/months after infection. For example, Phetsouphanh and collaborators (2022) compared immune profiles of long COVID individuals with patients without long COVID, identifying a combination of inflammatory mediators eight months after infection as the correlate of persistent post-COVID-19 symptoms.
Another interpretation may involve the role of sleep loss as a driver of cellular stress and the consequent neuronal damage (Coulson et al., 2022) in the cognitive and neuropsychiatric manifestations of long COVID (Schilling et al., 2022).
Finally, pre-pandemic evidence suggested that insufficient sleep could impair the vaccination efficacy (Prather et al., 2021, Spiegel et al., 2002, Zimmermann and Curtis, 2019), and the importance of sleep health has also been advocated for the COVID-19 vaccination campaign (Benedict and Cedernaes, 2021, Rayatdoost et al., 2022). Since previous COVID-19 vaccination seems to reduce the risk of long COVID (Notarte et al., 2022), the link between sleep and post-COVID-19 symptoms may be mediated by the impact of sleep deficiency on the vaccination effectiveness. However, providing a clear understanding of the link between sleep and subsequent long-term symptoms after SARS-CoV-2 infection transcends the objectives and the capacity of the present investigation. Future studies should address this question to identify the potential underlying biobehavioral mechanisms of the sleep-long COVID relationship.
In a recent study, Wang and collaborators (2022) showed that pre-infection psychological distress was prospectively associated with the incidence of various post-COVID-19 conditions. Consistently, other studies demonstrated that a history of psychiatric disorders represented an independent predictor of post-acute sequelae of COVID-19 (Hirschtick et al., 2021, Tenforde et al., 2020) with personal resilience to stress positively associated with less severe post-COVID syndrome (Bahmer et al., 2022). The similarity with our findings is unsurprising considering the close relationship between sleep disturbances and short sleep duration with psychological conditions like anxiety (Alvaro et al., 2013, Cox and Olatunji, 2020), depression (Alvaro et al., 2013, Fang et al., 2019, Zhai et al., 2015), and stress disorders (Gardani et al., 2022, Zhang et al., 2019). Considering the established co-occurrence of sleep and psychological problems, psychological distress early in the pandemic may contribute to explaining the prospective association between previous sleep and long-term post-infection symptoms.
On the other hand, a growing body of evidence supports a causal role of sleep problems in the occurrence of mental health disorders (Baglioni et al., 2011, Hertenstein et al., 2019, Pigeon et al., 2017, Zhai et al., 2015), and treating sleep disturbances seems to improve the subsequent mental health outcomes (Freeman et al., 2017, Ho et al., 2016, Scott et al., 2021). In this view, we could speculate that pre-infection sleep disturbances may get involved in the predictive role of psychological distress in the incidence of post-COVID-19 symptoms.
In line with Wang and colleagues’ study (2022), smell/taste dysfunction represented the only long-term symptom not prospectively associated with all previous sleep outcomes. However, as argued by those authors (Wang et al., 2022), the variability in anosmia incidence in long COVID may be explained by genetic or binding activity differences of the cell entry receptor for SARS-CoV-2 (the angiotensin-converting enzyme 2 receptor; Butowt and von Bartheld, 2021) and may be independent from inflammatory mechanisms potentially involved in the effects of distress and sleep. Further investigations should address this research question.
Besides suggesting sleep problems and short sleep duration as risk factors for post-COVID-19 manifestations, models reported in the 3.2 section indicated that higher BMI and lower age were associated with more symptoms one month after infection. As regards the effect of BMI, these results were consistent with meta-analytic evidence identifying obesity as a risk factor for long COVID manifestations (Huang et al., 2022). As far as the age effect is concerned, our findings are consistent with some studies (e.g., Seeble et al., 2022), although the current literature presents inconsistencies: some investigations showed a positive association between age and post-COVID symptoms (Sudre et al., 2021, Thompson et al., 2022), one found a higher risk in the middle age group (Boscolo-Rizzo et al., 2021), while meta-analytic evidence did not support any association (Huang et al., 2022). Moreover, in the present study the negative associations were not replicated analyzing symptoms three months after COVID-19. The reduced sample size combined with the limited variability of data and the small dimension of the effects may explain the inconsistency in the results.
4.1. Strengths and limitations
The present study has several strengths. The predictive effect of sleep variables was obtained by adjusting for important confounding factors (age, gender, and BMI) that were associated by previous studies with a higher risk for long COVID symptoms (Huang et al., 2022, Sudre et al., 2021, Zeng et al., 2022). The main findings were also confirmed after controlling for the COVID-19 severity, which is an established predictor of long COVID conditions (Chen et al., 2022, Huang et al., 2022, Taquet et al., 2021, Zeng et al., 2022), but also a documented outcome of previous sleep problems (Huang et al., 2020, Jones et al., 2022). Furthermore, the results were replicated in the same sample by using a different baseline assessment in December 2020. Finally, our findings were confirmed after controlling for the time interval separating the infection from the baseline/follow-up evaluation, reducing the risk for a possible recall bias due to the time elapsed between COVID-19 and the retrospective assessment. At the same time, these sensitivity analyses suggested a predictive role of sleep features even after several months from COVID-19. Nevertheless, it is worth noting that also the changes in sleep quality/quantity over time could affect the post-COVID-19 sequelae. In fact, variations in sleep variables between April and December 2020 were associated with the subsequent number of symptoms one month after infection (see Table S5 and Fig. S2 in the Supplementary Material).
Several limitations should also be acknowledged. Notwithstanding our results rely on a longitudinal data collection, the long-term symptoms after COVID-19 were retrospectively reported by participants during the last follow-up assessment (April 2022) and sleep features were evaluated using (validated) self-report instruments. These characteristics could increase the risk for recall biases (due to the time elapsed between the event and its evaluation) and misclassifications (e.g., participants who are more health-aware are more likely to report both sleep problems and long COVID symptoms). Future longitudinal studies should confirm our results using objective sleep-assessment instruments (actigraphy, polysomnography), a clinical evaluation of sleep disturbances, and a timely assessment of long COVID symptoms.
Moreover, the absence of a control group without COVID-19 did not make it possible to evaluate if the relationship between previous sleep disturbances and some of the examined symptoms (e.g., over-tiredness, memory problems, psychiatric conditions) may be partially independent from the infection. However, logistic analyses highlighted a significant association between pre-COVID-19 sleep variables and symptoms that are not typically related to sleep problems and are specific of the long COVID syndrome (e.g., breathlessness/dyspnea, brain fog, diffuse body pain, persistent cough, appetite reduction). Sensitivity analyses (see Table S6 in the Supplementary Material) supported our interpretation, showing that pre-COVID-19 sleep features also predict symptoms that were reported to occur only one month after the infection, but not three months later. Due to their transient incidence after COVID-19, these relapsing symptoms may be unlikely interpreted as functional consequences or comorbidities of habitual poor sleep per se. Similarly, the association between poor sleep in April 2020 and longer recovery times to return to the pre-infection daily functioning level after infection corroborates the role of previous sleep in the long-term sequelae of COVID-19.
We also acknowledge that the lack of evaluation of potential pre-existing health conditions and behaviors did not allow our analyses to account for other risk factors for post-COVID-19 conditions (e.g., type 2 diabetes, asthma, smoking status, physical activity). Finally, our samples consisted of non-hospitalized adults and comprised a higher portion of female subjects, limiting the generalization of the results.
5. Conclusions
In conclusion, this study suggests that pre-existing sleep disturbances and inadequate sleep duration are associated with subsequent risk of long-term symptoms after COVID-19. Our findings could have large-scale implications considering the sleep-loss epidemic in our society (Chattu et al., 2018, Wang et al., 2023b) and the considerable rates of insomnia disorder and occasionally experienced insomnia symptoms among the adult population (10% and 20%, respectively; Morin and Jarrin, 2022) Furthermore, the present results may be even more relevant during a historical period that pervasively impacted the worldwide population’s sleep. Indeed, meta-analytic studies showed that half of the people experienced subthreshold and clinically significant insomnia symptoms in the first two pandemic years (AlRasheed et al., 2022), and sleep disturbances affected four out of ten people worldwide (Jahrami et al., 2022).
Raising public awareness about healthy sleep habits may represent an effective preventive approach to mitigate the COVID-19 repercussions in the long run, with substantial indirect effects at societal level. Further research is warranted to determine whether intervention aimed at promoting sleep quality/quantity could improve the long-term consequences of COVID-19.
Data availability statement
The data underlying this article will be shared on reasonable request to the corresponding author.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
CRediT authorship contribution statement
Federico Salfi: Conceptualization, Methodology, Investigation, Data curation, Formal analysis, Visualization, Writing – original draft, Writing – review & editing. Giulia Amicucci: Investigation. Domenico Corigliano: Investigation. Lorenzo Viselli: Investigation. Aurora D'Atri: Investigation, Writing – review & editing. Daniela Tempesta: Writing – review & editing. Michele Ferrara: Conceptualization, Methodology, Supervision, Writing – review & editing.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgment
We are grateful to all the Italians who participated in our study.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.bbi.2023.06.010.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
Data availability
Data will be made available on request.
References
- AlRasheed, M.M., Fekih-Romdhane, F., Jahrami, H., Pires, G.N., Saif, Z., Alenezi, A.F., Humood, A., Chen, W., Dai, H., Bragazzi, N., Pandi-Perumal, S.R., BaHammam, A.S., Vitiello, M. V., COMITY investigators, 2022. The prevalence and severity of insomnia symptoms during COVID-19: A global systematic review and individual participant data meta-analysis. Sleep Med 100, 7–23. 10.1016/j.sleep.2022.06.020. [DOI] [PMC free article] [PubMed]
- Alvaro P.K., Roberts R.M., Harris J.K. A Systematic Review Assessing Bidirectionality between Sleep Disturbances, Anxiety, and Depression. Sleep. 2013;36:1059–1068. doi: 10.5665/SLEEP.2810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Badenoch J.B., Rengasamy E.R., Watson C., Jansen K., Chakraborty S., Sundaram R.D., Hafeez D., Burchill E., Saini A., Thomas L., Cross B., Hunt C.K., Conti I., Ralovska S., Hussain Z., Butler M., Pollak T.A., Koychev I., Michael B.D., Holling H., Nicholson T.R., Rogers J.P., Rooney A.G. Persistent neuropsychiatric symptoms after COVID-19: a systematic review and meta-analysis. Brain Commun. 2021;4 doi: 10.1093/braincomms/fcab297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baglioni C., Battagliese G., Feige B., Spiegelhalder K., Nissen C., Voderholzer U., Lombardo C., Riemann D. Insomnia as a predictor of depression: A meta-analytic evaluation of longitudinal epidemiological studies. J. Affect. Disord. 2011;135:10–19. doi: 10.1016/j.jad.2011.01.011. [DOI] [PubMed] [Google Scholar]
- Bahmer T., Borzikowsky C., Lieb W., Horn A., Krist L., Fricke J., Scheibenbogen C., Rabe K.F., Maetzler W., Maetzler C., Laudien M., Frank D., Ballhausen S., Hermes A., Muljukov O., Haeusler K.G., Mokhtari N.E.E., Witzenrath M., Vehreschild J.J., Krefting D., Pape D., Montellano F.A., Kohls M., Morbach C., Störk S., Reese J.P., Keil T., Heuschmann P., Krawczak M., Schreiber S. Severity, predictors and clinical correlates of Post-COVID syndrome (PCS) in Germany: A prospective, multi-centre, population-based cohort study. EClinicalMedicine. 2022;51 doi: 10.1016/J.ECLINM.2022.101549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benedict C., Cedernaes J. Could a good night’s sleep improve COVID-19 vaccine efficacy? Lancet Respir. Med. 2021;9:447–448. doi: 10.1016/s2213-2600(21)00126-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Besedovsky L., Lange T., Born J. Sleep and immune function. Pflugers Archiv. 2012;463:121. doi: 10.1007/s00424-011-1044-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boscolo-Rizzo P., Guida F., Polesel J., Marcuzzo A.V., Capriotti V., D’Alessandro A., Zanelli E., Marzolino R., Lazzarin C., Antonucci P., Sacchet E., Tofanelli M., Borsetto D., Gardenal N., Pengo M., Tirelli G. Sequelae in adults at 12 months after mild-to-moderate coronavirus disease 2019 (COVID-19) Int. Forum Allergy Rhinol. 2021;11:1685. doi: 10.1002/ALR.22832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bryant P.A., Trinder J., Curtis N. Sick and tired: does sleep have a vital role in the immune system? Nat. Rev. Immunol. 2004;4:457–467. doi: 10.1038/nri1369. [DOI] [PubMed] [Google Scholar]
- Butowt R., von Bartheld C.S. Anosmia in COVID-19: Underlying Mechanisms and Assessment of an Olfactory Route to Brain Infection. Neuroscientist. 2021;27:582–603. doi: 10.1177/1073858420956905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cameron, C.A., Trivedi, P., 2013. Regression analysis of count data, 2nd ed. Cambridge University Press. 10.1017/cbo9781139013567.
- Castronovo V., Galbiati A., Marelli S., Brombin C., Cugnata F., Giarolli L., Anelli M.M., Rinaldi F., Ferini-Strambi L. Validation study of the Italian version of the Insomnia Severity Index (ISI) Neurol. Sci. 2016;37:1517–1524. doi: 10.1007/s10072-016-2620-z. [DOI] [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention Overweight & Obesity [WWW Document] 2023. https://www.cdc.gov/obesity/basics/adult-defining.html accessed 1.25.23.
- Chattu V.K., Manzar M.D., Kumary S., Burman D., Spence D.W., Pandi-Perumal S.R. The Global Problem of Insufficient Sleep and Its Serious Public Health Implications. Healthcare. 2018;7 doi: 10.3390/healthcare7010001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen C., Haupert S.R., Zimmermann L., Shi X., Fritsche L.G., Mukherjee B. Global Prevalence of Post-Coronavirus Disease 2019 (COVID-19) Condition or Long COVID: A Meta-Analysis and Systematic Review. J Infect Dis. 2022;226:1593–1607. doi: 10.1093/infdis/jiac136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho H.J., Seeman T.E., Kiefe C.I., Lauderdale D.S., Irwin M.R. Sleep disturbance and longitudinal risk of inflammation: Moderating influences of social integration and social isolation in the Coronary Artery Risk Development in Young Adults (CARDIA) study. Brain Behav. Immun. 2015;46:319–326. doi: 10.1016/j.bbi.2015.02.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen S., Doyle W.J., Alper C.M., Janicki-Deverts D., Turner R.B. Sleep habits and susceptibility to the common cold. Arch. Intern. Med. 2009;169:62–67. doi: 10.1001/archinternmed.2008.505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coulson R.L., Mourrain P., Wang G.X. Sleep deficiency as a driver of cellular stress and damage in neurological disorders. Sleep Med. Rev. 2022;63 doi: 10.1016/j.smrv.2022.101616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox R.C., Olatunji B.O. Sleep in the anxiety-related disorders: A meta-analysis of subjective and objective research. Sleep Med. Rev. 2020;51 doi: 10.1016/j.smrv.2020.101282. [DOI] [PubMed] [Google Scholar]
- Coxe S., West S.G., Aiken L.S. The analysis of count data: a gentle introduction to poisson regression and its alternatives. J. Pers. Assess. 2009;91:121–136. doi: 10.1080/00223890802634175. [DOI] [PubMed] [Google Scholar]
- Crook H., Raza S., Nowell J., Young M., Edison P. Long covid—mechanisms, risk factors, and management. BMJ. 2021;374 doi: 10.1136/bmj.n1648. [DOI] [PubMed] [Google Scholar]
- Curcio G., Tempesta D., Scarlata S., Marzano C., Moroni F., Rossini P.M., Ferrara M., de Gennaro L. Validity of the Italian Version of the Pittsburgh Sleep Quality Index (PSQI) Neurol. Sci. 2013;34:511–519. doi: 10.1007/s10072-012-1085-y. [DOI] [PubMed] [Google Scholar]
- Davis, H.E., Assaf, G.S., McCorkell, L., Wei, H., Low, R.J., Re’em, Y., Redfield, S., Austin, J.P., Akrami, A., 2021. Characterizing long COVID in an international cohort: 7 months of symptoms and their impact. EClinicalMedicine 38, 101019. 10.1016/j.eclinm.2021.101019. [DOI] [PMC free article] [PubMed]
- Fang H., Tu S., Sheng J., Shao A. Depression in sleep disturbance: A review on a bidirectional relationship, mechanisms and treatment. J. Cell Mol. Med. 2019;23:2324–2332. doi: 10.1111/JCMM.14170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrie J.E., Kivimäki M., Akbaraly T.N., Singh-Manoux A., Miller M.A., Gimeno D., Kumari M., Davey Smith G., Shipley M.J. Associations Between Change in Sleep Duration and Inflammation: Findings on C-reactive Protein and Interleukin 6 in the Whitehall II Study. Am. J. Epidemiol. 2013;178:956–961. doi: 10.1093/aje/kwt072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freeman D., Sheaves B., Goodwin G.M., Yu L.-M., Nickless A., Harrison P.J., Emsley R., Luik A.I., Foster R.G., Wadekar V., Hinds C., Gumley A., Jones R., Lightman S., Jones S., Bentall R., Kinderman P., Rowse G., Brugha T., Blagrove M., Gregory A.M., Fleming L., Walklet E., Glazebrook C., Davies E.B., Hollis C., Haddock G., John B., Coulson M., Fowler D., Pugh K., Cape J., Moseley P., Brown G., Hughes C., Obonsawin M., Coker S., Watkins E., Schwannauer M., MacMahon K., Siriwardena A.N., Espie C.A. The effects of improving sleep on mental health (OASIS): a randomised controlled trial with mediation analysis. Lancet Psychiatry. 2017;4:749–758. doi: 10.1016/s2215-0366(17)30328-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frere J.J., Serafini R.A., Pryce K.D., Zazhytska M., Oishi K., Golynker I., Panis M., Zimering J., Horiuchi S., Hoagland D.A., Møller R., Ruiz A., Kodra A., Overdevest J.B., Canoll P.D., Borczuk A.C., Chandar V., Bram Y., Schwartz R., Lomvardas S., Zachariou V., TenOever B.R. SARS-CoV-2 infection in hamsters and humans results in lasting and unique systemic perturbations after recovery. Sci. Transl. Med. 2022;14:3059. doi: 10.1126/scitranslmed.abq3059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garbarino S., Lanteri P., Bragazzi N.L., Magnavita N., Scoditti E. Role of sleep deprivation in immune-related disease risk and outcomes. Commun Biol. 2021;4:1–17. doi: 10.1038/s42003-021-02825-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gardani M., Bradford D.R.R., Russell K., Allan S., Beattie L., Ellis J.G., Akram U. A systematic review and meta-analysis of poor sleep, insomnia symptoms and stress in undergraduate students. Sleep Med. Rev. 2022;61 doi: 10.1016/j.smrv.2021.101565. [DOI] [PubMed] [Google Scholar]
- Gardner W., Mulvey E.P., Shaw E.C. Regression Analyses of Counts and Rates: Poisson, Overdispersed Poisson, and Negative Binomial Models. Psychol. Bull. 1995;118:392–404. doi: 10.1037/0033-2909.118.3.392. [DOI] [PubMed] [Google Scholar]
- Ghilotti F., Bellocco R., Trolle Lagerros Y., Thorson A., Theorell-Haglöw J., Åkerstedt T., Lindberg E. Relationship between sleep characteristics and markers of inflammation in Swedish women from the general population. J. Sleep Res. 2021;30:e13093. doi: 10.1111/jsr.13093. [DOI] [PubMed] [Google Scholar]
- Green J.A. Too many zeros and/or highly skewed? A tutorial on modelling health behaviour as count data with Poisson and negative binomial regression. Health Psychol. Behav. Med. 2021;9:436–455. doi: 10.1080/21642850.2021.1920416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heinze G., Puhr R. Bias-reduced and separation-proof conditional logistic regression with small or sparse data sets. Stat. Med. 2010;29:770–777. doi: 10.1002/sim.3794. [DOI] [PubMed] [Google Scholar]
- Heinze G., Ploner M., Dunkler D., Southworth H., Jiricka L. Package ‘logistf’ [WWW Document] Version. 2022;1(24):1. https://cran.r-project.org/web/packages/logistf/logistf.pdf accessed 1.18.23. [Google Scholar]
- Hertenstein E., Feige B., Gmeiner T., Kienzler C., Spiegelhalder K., Johann A., Jansson-Fröjmark M., Palagini L., Rücker G., Riemann D., Baglioni C. Insomnia as a predictor of mental disorders: A systematic review and meta-analysis. Sleep Med. Rev. 2019;43:96–105. doi: 10.1016/j.smrv.2018.10.006. [DOI] [PubMed] [Google Scholar]
- Hilbe, J.M., 2011. Negative Binomial Regression, 2nd ed. Cambridge University Press. Doi: 10.1017/cbo9780511973420.
- Hirschtick J.L., Titus A.R., Slocum E., Power L.E., Hirschtick R.E., Elliott M.R., McKane P., Fleischer N.L. Population-Based Estimates of Post-acute Sequelae of Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) Infection (PASC) Prevalence and Characteristics. Clin. Infect. Dis. 2021;73:2055–2064. doi: 10.1093/CID/CIAB408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ho F.Y.Y., Chan C.S., Tang K.N.S. Cognitive-behavioral therapy for sleep disturbances in treating posttraumatic stress disorder symptoms: A meta-analysis of randomized controlled trials. Clin. Psychol. Rev. 2016;43:90–102. doi: 10.1016/j.cpr.2015.09.005. [DOI] [PubMed] [Google Scholar]
- Huang Q., Jia M., Sun Y., Jiang B., Cui D., Feng L., Yang W. One-Year Temporal Changes in Long COVID Prevalence and Characteristics: A Systematic Review and Meta-Analysis. Value Health. 2022 doi: 10.1016/j.jval.2022.11.011. [DOI] [PubMed] [Google Scholar]
- Huang B., Niu Y., Zhao W., Bao P., Li D. Reduced sleep in the week prior to diagnosis of COVID-19 is associated with the severity of COVID-19. Nat Sci Sleep. 2020;12:999–1007. doi: 10.2147/nss.s263488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Irwin M.R., Wang M., Campomayor C.O., Collado-Hidalgo A., Cole S. Sleep Deprivation and Activation of Morning Levels of Cellular and Genomic Markers of Inflammation. Arch. Intern. Med. 2006;166:1756–1762. doi: 10.1001/archinte.166.16.1756. [DOI] [PubMed] [Google Scholar]
- Irwin M.R., Olmstead R., Carroll J.E. Sleep Disturbance, Sleep Duration, and Inflammation: A Systematic Review and Meta-Analysis of Cohort Studies and Experimental Sleep Deprivation. Biol. Psychiatry. 2016;80:40–52. doi: 10.1016/j.biopsych.2015.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Istituto Superiore di Sanità COVID-19 integrated surveillance: key national data [WWW Document] 2022. https://www.epicentro.iss.it/coronavirus/sars-cov-2-sorveglianza-dati accessed 6.29.22.
- Jahrami, H., Alhaj, O.A., Humood, A.M., Alenezi, A.F., Fekih-Romdhane, F., AlRasheed, M.M., Saif, Z.Q., Bragazzi, N.L., Pandi-Perumal, S.R., BaHammam, A.S., Vitiello, M. v., 2022. Sleep disturbances during the COVID-19 pandemic: A systematic review, meta-analysis, and meta-regression. Sleep Med Rev 62, 101591. 10.1016/j.smrv.2022.101591. [DOI] [PMC free article] [PubMed]
- Jones S.E., Maisha F.I., Strausz S., Cade B., Tervi A., Helaakoski V., Broberg M., Lammi V., Lane J.M., Redline S., Saxena R., Ollila H.M. Public health impact of poor sleep on COVID-19, influenza and upper respiratory infections. Sleep Med. 2022;100:S135. doi: 10.1016/j.sleep.2022.05.369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleiber C., Zeileis A. Visualizing Count Data Regressions Using Rootograms. Am. Stat. 2016;70:296–303. doi: 10.1080/00031305.2016.1173590. [DOI] [Google Scholar]
- Lancet T. Understanding long COVID: a modern medical challenge. Lancet. 2021;398:725. doi: 10.1016/s0140-6736(21)01900-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Land K.C., McCall P.L., Nagin D.S. A Comparison of Poisson, Negative Binomial, and Semiparametric Mixed Poisson Regression Models. Sociol. Methods Res. 2016;24:387–442. doi: 10.1177/0049124196024004001. [DOI] [Google Scholar]
- Liukkonen T., Räsänen P., Ruokonen A., Laitinen J., Jokelainen J., Leinonen M., Meyer-Rochow V.B., Timonen M. C-reactive protein levels and sleep disturbances: Observations based on the Northern Finland 1966 birth cohort study. Psychosom. Med. 2007;69:756–761. doi: 10.1097/psy.0b013e318157cb96. [DOI] [PubMed] [Google Scholar]
- Mazza M.G., de Lorenzo R., Conte C., Poletti S., Vai B., Bollettini I., Melloni E.M.T., Furlan R., Ciceri F., Rovere-Querini P., Benedetti F. Anxiety and depression in COVID-19 survivors: Role of inflammatory and clinical predictors. Brain Behav. Immun. 2020;89:594–600. doi: 10.1016/j.bbi.2020.07.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazza M.G., Palladini M., de Lorenzo R., Magnaghi C., Poletti S., Furlan R., Ciceri F., Rovere-Querini P., Benedetti F. Persistent psychopathology and neurocognitive impairment in COVID-19 survivors: Effect of inflammatory biomarkers at three-month follow-up. Brain Behav. Immun. 2021;94:138–147. doi: 10.1016/j.bbi.2021.02.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mills P.J., von Känel R., Norman D., Natarajan L., Ziegler M.G., Dimsdale J.E. Inflammation and Sleep in Healthy Individuals. Sleep. 2007;30:729–735. doi: 10.1093/sleep/30.6.729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moghimbeigi A., Eshraghian M.R., Mohammad K., Mcardle B. Multilevel zero-inflated negative binomial regression modeling for over-dispersed count data with extra zeros. J. Appl. Stat. 2008;35:1193–1202. doi: 10.1080/02664760802273203. [DOI] [Google Scholar]
- Morin C.M., Jarrin D.C. Epidemiology of Insomnia: Prevalence, Course, Risk Factors, and Public Health Burden. Sleep Med. Clin. 2022;17:173–191. doi: 10.1016/j.jsmc.2022.03.003. [DOI] [PubMed] [Google Scholar]
- Notarte K.I., Catahay J.A., Velasco J.V., Pastrana A., Ver A.T., Pangilinan F.C., Peligro P.J., Casimiro M., Guerrero J.J., Gellaco M.M.L., Lippi G., Henry B.M., Fernández-de-las-Peñas C. Impact of COVID-19 vaccination on the risk of developing long-COVID and on existing long-COVID symptoms: A systematic review. EClinicalMedicine. 2022;53 doi: 10.1016/j.eclinm.2022.101624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nowakowski S., Matthews K.A., Von Känel R., Hall M.H., Thurston R.C. Sleep characteristics and inflammatory biomarkers among midlife women. Sleep. 2018;41:zsy049. doi: 10.1093/sleep/zsy049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’ Mahony, L., Buwalda, T., Blair, M., Forde, B., Lunjani, N., Ambikan, A., Neogi, U., Barrett, P., Geary, E., O’Connor, N., Dineen, J., Clarke, G., Kelleher, E., Horgan, M., Jackson, A., Sadlier, C., 2022. Impact of Long COVID on health and quality of life. HRB Open Res 5, 31. 10.12688/HRBOPENRES.13516.1. [DOI] [PMC free article] [PubMed]
- O’Mahoney L.L., Routen A., Gillies C., Ekezie W., Welford A., Zhang A., Karamchandani U., Simms-Williams N., Cassambai S., Ardavani A., Wilkinson T.J., Hawthorne G., Curtis F., Kingsnorth A.P., Almaqhawi A., Ward T., Ayoubkhani D., Banerjee A., Calvert M., Shafran R., Stephenson T., Sterne J., Ward H., Evans R.A., Zaccardi F., Wright S., Khunti K. The prevalence and long-term health effects of Long Covid among hospitalised and non-hospitalised populations: A systematic review and meta-analysis. EClinicalMedicine. 2023;55 doi: 10.1016/j.eclinm.2022.101762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Payne E.H., Gebregziabher M., Hardin J.W., Ramakrishnan V., Egede L.E. An empirical approach to determine a threshold for assessing overdispersion in Poisson and negative binomial models for count data. Commun. Stat. Simul. Comput. 2018;47:1722. doi: 10.1080/03610918.2017.1323223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phetsouphanh, C., Darley, D.R., Wilson, D.B., Howe, A., Munier, C.M.L., Patel, S.K., Juno, J.A., Burrell, L.M., Kent, S.J., Dore, G.J., Kelleher, A.D., Matthews, G. v., 2022. Immunological dysfunction persists for 8 months following initial mild-to-moderate SARS-CoV-2 infection. Nat Immunol 23, 210–216. 10.1038/s41590-021-01113-x. [DOI] [PubMed]
- Pigeon W.R., Bishop T.M., Krueger K.M. Insomnia as a Precipitating Factor in New Onset Mental Illness: a Systematic Review of Recent Findings. Curr. Psychiatry Rep. 2017;19 doi: 10.1007/s11920-017-0802-x. [DOI] [PubMed] [Google Scholar]
- Prather A.A., Janicki-Deverts D., Hall M.H., Cohen S. Behaviorally Assessed Sleep and Susceptibility to the Common Cold. Sleep. 2015;38:1353–1359. doi: 10.5665/sleep.4968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prather A.A., Pressman S.D., Miller G.E., Cohen S. Temporal Links Between Self-Reported Sleep and Antibody Responses to the Influenza Vaccine. Int. J. Behav. Med. 2021;28:151–158. doi: 10.1007/s12529-020-09879-4. [DOI] [PubMed] [Google Scholar]
- R Core Team, 2022. R: A language and environment for statistical computing.
- Rayatdoost E., Rahmanian M., Sanie M.S., Rahmanian J., Matin S., Kalani N., Kenarkoohi A., Falahi S., Abdoli A. Sufficient Sleep, Time of Vaccination, and Vaccine Efficacy: A Systematic Review of the Current Evidence and a Proposal for COVID-19 Vaccination. Yale J. Biol. Med. 2022;95:221–235. [PMC free article] [PubMed] [Google Scholar]
- Rutkai I., Mayer M.G., Hellmers L.M., Ning B., Huang Z., Monjure C.J., Coyne C., Silvestri R., Golden N., Hensley K., Chandler K., Lehmicke G., Bix G.J., Maness N.J., Russell-Lodrigue K., Hu T.Y., Roy C.J., Blair R.V., Bohm R., Doyle-Meyers L.A., Rappaport J., Fischer T. Neuropathology and virus in brain of SARS-CoV-2 infected non-human primates. Nat. Commun. 2022;13:1745. doi: 10.1038/s41467-022-29440-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salfi F., D’Atri A., Tempesta D., Ferrara M. Sleeping under the waves: A longitudinal study across the contagion peaks of the COVID-19 pandemic in Italy. J. Sleep Res. 2021;30:e13313. doi: 10.1111/jsr.13313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salfi F., Lauriola M., D’Atri A., Amicucci G., Viselli L., Tempesta D., Ferrara M. Demographic, psychological, chronobiological, and work-related predictors of sleep disturbances during the COVID-19 lockdown in Italy. Sci. Rep. 2021;11:11416. doi: 10.1038/s41598-021-90993-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salfi F., Amicucci G., Corigliano D., Viselli L., D’Atri A., Tempesta D., Gorgoni M., Scarpelli S., Alfonsi V., Ferrara M. Two years after lockdown: Longitudinal trajectories of sleep disturbances and mental health over the COVID-19 pandemic, and the effects of age, gender and chronotype. J Sleep Res e13767. 2022 doi: 10.1111/JSR.13767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schilling C., Meyer-Lindenberg A., Schweiger J.I. Cognitive disorders and sleep disturbances in long COVID. Nervenarzt. 2022;93:779–787. doi: 10.1007/S00115-022-01297-Z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott A.J., Webb T.L., Martyn-St James M., Rowse G., Weich S. Improving sleep quality leads to better mental health: A meta-analysis of randomised controlled trials. Sleep Med. Rev. 2021;60 doi: 10.1016/j.smrv.2021.101556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seeble J., Waterboer T., Hippchen T., Simon J., Kirchner M., Lim A., Muller B., Merle U. Persistent Symptoms in Adult Patients 1 Year After Coronavirus Disease 2019 (COVID-19): A Prospective Cohort Study. Clin. Infect. Dis. 2022;74:1191–1198. doi: 10.1093/CID/CIAB611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spiegel K., Sheridan J.F., Van Cauter E. Effect of Sleep Deprivation on Response to Immunizaton. J. Am. Med. Assoc. 2002;288:1471–1472. doi: 10.1001/jama.288.12.1469. [DOI] [PubMed] [Google Scholar]
- Sudre C.H., Murray B., Varsavsky T., Graham M.S., Penfold R.S., Bowyer R.C., Pujol J.C., Klaser K., Antonelli M., Canas L.S., Molteni E., Modat M., Jorge Cardoso M., May A., Ganesh S., Davies R., Nguyen L.H., Drew D.A., Astley C.M., Joshi A.D., Merino J., Tsereteli N., Fall T., Gomez M.F., Duncan E.L., Menni C., Williams F.M.K., Franks P.W., Chan A.T., Wolf J., Ourselin S., Spector T., Steves C.J. Attributes and predictors of long COVID. Nat. Med. 2021;27:626–631. doi: 10.1038/s41591-021-01292-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taquet M., Geddes J.R., Husain M., Luciano S., Harrison P.J. 6-month neurological and psychiatric outcomes in 236 379 survivors of COVID-19: a retrospective cohort study using electronic health records. Lancet Psychiatry. 2021;8:416–427. doi: 10.1016/s2215-0366(21)00084-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tenforde, M.W., Kim, S.S., Lindsell, C.J., Billig Rose, E., Shapiro, N.I., Files, D.C., Gibbs, K.W., Erickson, H.L., Steingrub, J.S., Smithline, H.A., Gong, M.N., Aboodi, M.S., Exline, M.C., Henning, D.J., Wilson, J.G., Khan, A., Qadir, N., Brown, S.M., Peltan, I.D., Rice, T.W., Hager, D.N., Ginde, A.A., Stubblefield, W.B., Patel, M.M., Self, W.H., Feldstein, L.R., Hart, K.W., McClellan, R., Dorough, L., Dzuris, N., Griggs, E.P., Kassem, A.M., Marcet, P.L., Ogokeh, C.E., Sciarratta, C.N., Siddula, A., Smith, E.R., Wu, M.J., 2020. Symptom Duration and Risk Factors for Delayed Return to Usual Health Among Outpatients with COVID-19 in a Multistate Health Care Systems Network - United States, March-June 2020. MMWR Morb Mortal Wkly Rep 69, 993–998. 10.15585/MMWR.MM6930E1. [DOI] [PMC free article] [PubMed]
- Thompson E.J., Williams D.M., Walker A.J., Mitchell R.E., Niedzwiedz C.L., Yang T.C., Huggins C.F., Kwong A.S.F., Silverwood R.J., Di Gessa G., Bowyer R.C.E., Northstone K., Hou B., Green M.J., Dodgeon B., Doores K.J., Duncan E.L., Williams F.M.K., Walker A.J., MacKenna B., Inglesby P., Rentsch C.T., Curtis H.J., Morton C.E., Morley J., Mehrkar A., Bacon S., Hickman G., Bates C., Croker R., Evans D., Ward T., Cockburn J., Davy S., Bhaskaran K., Schultze A., Williamson E.J., Hulme W.J., McDonald H.I., Tomlinson L., Mathur R., Eggo R.M., Wing K., Wong A.Y.S., Forbes H., Tazare J., Parry J., Hester F., Harper S., Douglas I.J., Evans S.J.W., Smeeth L., Goldacre B., Steptoe A., Porteous D.J., McEachan R.R.C., Tomlinson L., Goldacre B., Patalay P., Ploubidis G.B., Katikireddi S.V., Tilling K., Rentsch C.T., Timpson N.J., Chaturvedi N., Steves C.J. Long COVID burden and risk factors in 10 UK longitudinal studies and electronic health records. Nat. Commun. 2022;13:1–11. doi: 10.1038/s41467-022-30836-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tukey J.W. Addison-Wesley Publishing Company; Reading, MA: 1977. Exploratory data analysis. [Google Scholar]
- Vittinghoff E., McCulloch C.E. Relaxing the Rule of Ten Events per Variable in Logistic and Cox Regression. Am. J. Epidemiol. 2007;165:710–718. doi: 10.1093/aje/kwk052. [DOI] [PubMed] [Google Scholar]
- Wang S., Quan L., Chavarro J.E., Slopen N., Kubzansky L.D., Koenen K.C., Kang J.H., Weisskopf M.G., Branch-Elliman W., Roberts A.L. Associations of Depression, Anxiety, Worry, Perceived Stress, and Loneliness Prior to Infection With Risk of Post–COVID-19 Conditions. JAMA Psychiat. 2022;79:1081–1091. doi: 10.1001/jamapsychiatry.2022.2640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang S., Li Y., Yue Y., Yuan C., Jae S., Kang H., Chavarro J.E., Bhupathiraju S.N., Roberts A.L. Adherence to Healthy Lifestyle Prior to Infection and Risk of Post–COVID-19 Condition. JAMA Intern. Med. 2023 doi: 10.1001/jamainternmed.2022.6555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang S., Rossheim M.E., Nandy R.R. Trends in prevalence of short sleep duration and trouble sleeping among US adults, 2005–2018. Sleep. 2023;46 doi: 10.1093/sleep/zsac231. [DOI] [PubMed] [Google Scholar]
- World Health Organization WHO Coronavirus (COVID-19) Dashboard [WWW Document] 2023. https://covid19.who.int accessed 1.29.23.
- Zeileis A., Kleiber C., Jackman S. Regression Models for Count Data in R. J. Stat. Softw. 2008;27:1–25. doi: 10.18637/jss.v027.i08. [DOI] [Google Scholar]
- Zeng, N., Zhao, Y.M., Yan, W., Li, C., Lu, Q.D., Liu, L., Ni, S.Y., Mei, H., Yuan, K., Shi, L., Li, P., Fan, T.T., Yuan, J.L., Vitiello, M. v., Kosten, T., Kondratiuk, A.L., Sun, H.Q., Tang, X.D., Liu, M.Y., Lalvani, A., Shi, J., Bao, Y.P., Lu, L., 2022. A systematic review and meta-analysis of long term physical and mental sequelae of COVID-19 pandemic: call for research priority and action. Mol. Psychiatry 28, 423–433. 10.1038/s41380-022-01614-7. [DOI] [PMC free article] [PubMed]
- Zhai L., Zhang H., Zhang D. Sleep duration and depression among adults: A meta-analysis of prospective studies. Depress. Anxiety. 2015;32:664–670. doi: 10.1002/da.22386. [DOI] [PubMed] [Google Scholar]
- Zhang Y., Ren R., Sanford L.D., Yang L., Zhou J., Zhang J., Wing Y.K., Shi J., Lu L., Tang X. Sleep in posttraumatic stress disorder: A systematic review and meta-analysis of polysomnographic findings. Sleep Med. Rev. 2019;48 doi: 10.1016/j.smrv.2019.08.004. [DOI] [PubMed] [Google Scholar]
- Zimmermann P., Curtis N. Factors That Influence the Immune Response to Vaccination. Clin. Microbiol. Rev. 2019;32 doi: 10.1128/cmr.00084-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data will be made available on request.