Abstract
Cardiovascular manifestations in human monkeypox virus (MPXV) infection has gained increasing recognition as significant complications with both social and clinical implications. Myocarditis, viral pericarditis, heart failure, and arrhythmias can occur, leading to adverse effects on individuals' health and quality of life. Understanding the detailed pathophysiology of these cardiovascular manifestations is essential for improved diagnosis and management. The social implications of these cardiovascular complications are multifaceted, ranging from public health concerns and the impact on individuals' quality of life to psychological distress and social stigma. Clinically, diagnosing and managing these complications present challenges, requiring a multidisciplinary approach and specialized care. The burden on healthcare resources necessitates preparedness and resource allocation to effectively address these complications. We delve into the pathophysiological mechanisms involved, including viral-induced cardiac damage, immune response, and inflammatory processes. Additionally, we explore the types of cardiovascular manifestations and their clinical presentations. Addressing cardiovascular manifestations' social and clinical implications in MPXV infection requires a comprehensive approach involving healthcare professionals, public health authorities, and communities. By prioritizing research, enhancing diagnosis and treatment strategies, and promoting preventive measures, we can mitigate the impact of these complications, improve patient care, and protect public health.
Keywords: Monkeypox, myocarditis, pericarditis, endothelial dysfunction
Introduction
Human monkeypox, caused by the Monkeypox virus (MPXV), is a rare and emerging zoonotic disease that belongs to the Orthopoxvirus genus1. Initially identified in 1970, this viral infection bears striking similarities to smallpox, both in terms of its clinical presentation and genetic makeup2. Over the years, several outbreaks have occurred, predominantly in the central and western regions of Africa3. While the respiratory and cutaneous manifestations of human monkeypox have been extensively studied, relatively less attention has been given to its impact on the cardiovascular system4.
Understanding the cardiovascular manifestations of human monkeypox is crucial as it may help improve diagnostic accuracy, guide clinical management, and enhance patient outcomes. This comprehensive review aims to summarize the current knowledge and provide an updated overview of the cardiovascular manifestations associated with human monkeypox virus infection.
Cardiovascular involvement in viral infections has been well-documented, with numerous viruses capable of causing myocarditis, pericarditis, and other cardiac complications5 , 6. In the case of monkeypox, the virus primarily targets the skin and mucous membranes, leading to characteristic skin lesions7. However, recent research has suggested that the virus can also invade endothelial cells and disseminate throughout the body, potentially affecting multiple organ systems, including the cardiovascular system8.
The cardiac manifestations of human monkeypox virus infection can vary widely, ranging from mild transient abnormalities to severe complications, such as myocarditis, congestive heart failure, and arrhythmias4. Patients may present with symptoms such as chest pain, shortness of breath, palpitations, and edema, necessitating a thorough cardiovascular evaluation9 , 10. Furthermore, the virus's impact on the vascular system, including endothelial dysfunction and thrombotic events, has also been observed, highlighting the need for comprehensive assessment and management11.
This review will delve into the pathogenesis of cardiovascular involvement in human monkeypox virus infection, exploring the mechanisms by which the virus interacts with the cardiovascular system. Additionally, it will discuss the clinical presentation, diagnostic approaches, and treatment strategies for cardiovascular complications associated with human monkeypox. Special emphasis will be placed on recent advances in research, including the use of imaging modalities, biomarkers, and antiviral therapies, to enhance early detection and targeted management of these cardiovascular manifestations.
By providing an updated understanding of the cardiovascular manifestations of human monkeypox virus infection, this review aims to contribute to the growing body of knowledge in this field. Ultimately, this knowledge can aid healthcare professionals in promptly identifying and effectively managing cardiovascular complications in patients with human monkeypox, thereby improving patient outcomes and reducing morbidity and mortality.
Methods
A comprehensive literature search was conducted using electronic databases, including PubMed, MEDLINE, Embase, and Scopus. The search terms used were combinations of keywords such as "human monkeypox," "Monkeypox virus," "cardiovascular manifestations," "myocarditis," "pericarditis," "congestive heart failure," "arrhythmias," "endothelial dysfunction," "thrombotic events," "diagnosis," and "treatment." Relevant articles published in English from the earliest available date to the date of the search were included.
Two independent reviewers (J.M. and H.A.) screened the titles and abstracts of the retrieved articles to identify potentially relevant studies. Full-text articles were obtained for further evaluation. Studies that specifically investigated cardiovascular manifestations associated with human monkeypox virus infection were included. Case reports, case series, observational studies, clinical trials, and reviews were considered for inclusion.
Types of cardiovascular manifestations
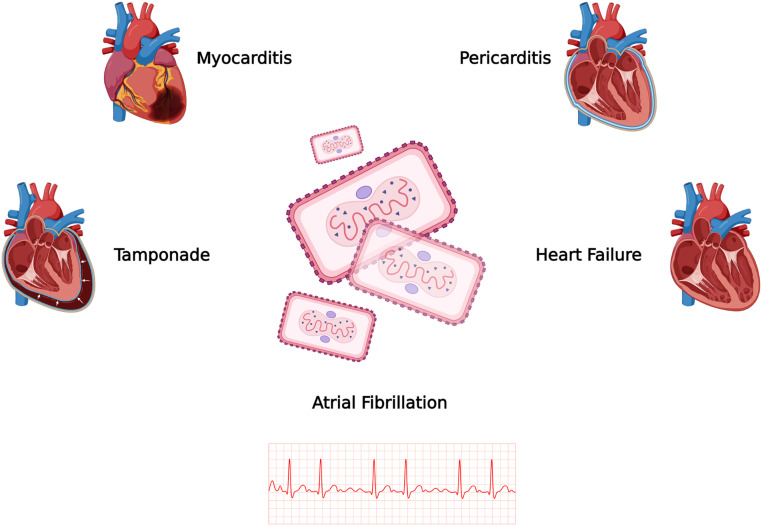
Cardiovascular manifestations associated with MPXV infection can vary in type and severity. Figure 1 and Table 1 demonstrate documented cardiovascular manifestations of MPXV. These manifestations can affect different components of the cardiovascular system and may include the following:
Figure 1.
Major cardiovascular manifestations reported in the literature
Table 1.
Study characteristics
| Author (year) [ref] | Country | Cardiac abnormality | MPXV diagnosis | Features |
|---|---|---|---|---|
| Shaik (2022)10 | USA | Pericarditis | PCR | Elevated inflammatory markers, widespread ST-elevation, mild pericardial effusion |
| Rodriguez (2022)20 | USA | Myocarditis | PCR | Elevated Troponin I and NT-Pro BNP, T-wave changes |
| Miller (2022)53 | USA | Atrial fibrillation | PCR | Tachycardia |
| Pinho (2022)54 | Portugal | Myocarditis | PCR | Elevated CRP, TroponinI, NT-Pro BNP, myocardial edema on CMR |
| Tan (2022)55 | Canada | Pericarditis | PCR | Elevated Ck and Troponin I, mild pericardial effusion |
Myocarditis
Myocarditis refers to inflammation of the myocardium, the muscular tissue of the heart12. MPXV infection can lead to myocarditis, which can range from mild inflammation to severe damage to the heart muscle13. Myocarditis can result in symptoms such as chest pain, fatigue, shortness of breath, and palpitations12. The pathogenesis is thought to involve a combination of direct viral damage to myocardial cells and an immune-mediated response to the infection13 , 14. The virus can directly invade and replicate within the myocardium, leading to cellular damage and death1. The immune response, triggered by the presence of viral antigens, can result in an inflammatory response characterized by the infiltration of immune cells, release of pro-inflammatory cytokines, and activation of cytotoxic T cells15. The interplay between direct viral damage and the immune response can contribute to the development of myocardial inflammation and subsequent cardiac dysfunction16. The clinical presentation of viral myocarditis can vary widely, ranging from mild flu-like symptoms to severe heart failure and life-threatening arrhythmias17. Common symptoms include chest pain, shortness of breath, palpitations, fatigue, and exercise intolerance. Some individuals may also experience fever, muscle aches, and gastrointestinal symptoms18. In severe cases, myocarditis can lead to acute heart failure, cardiogenic shock, or sudden cardiac death19. The diagnosis of viral myocarditis involves a combination of clinical evaluation, laboratory tests, imaging studies, and cardiac biopsy10. Diagnostic tests may include blood tests to detect viral genetic material or antibodies, electrocardiography (ECG) to assess heart rhythm and electrical activity, echocardiography to evaluate heart structure and function, and cardiac MRI or nuclear imaging to assess inflammation and myocardial damage10. Treatment of viral myocarditis aims to manage symptoms, support cardiac function, and reduce inflammation20. In mild cases, supportive measures such as rest, fluids, and over-the-counter pain relievers may be sufficient. In more severe cases, hospitalization, monitoring, and advanced interventions may be necessary. Treatment may involve medications such as anti-inflammatory drugs, immunosuppressive agents, and heart failure medications21. In cases of severe heart failure, advanced interventions like mechanical circulatory support or heart transplantation may be considered22. The prognosis of viral myocarditis varies depending on the severity of the condition, the specific virus involved, and individual patient factors23. Some individuals may experience a complete recovery with no long-term complications, while others may develop chronic heart failure or arrhythmias24. Early diagnosis, appropriate management, and close follow-up are crucial in optimizing outcomes and preventing long-term complications.
Pericarditis
Pericarditis is the inflammation of the pericardium, the thin sac that surrounds the heart25. MPXV infection can cause pericarditis, resulting in chest pain that is typically sharp and worsens with deep breathing or lying flat26. Other symptoms may include a low-grade fever, cough, and a characteristic friction rub heard on auscultation of the chest. Pericarditis can also lead to the accumulation of fluid around the heart (pericardial effusion), which can further compromise cardiac function27. The pathogenesis of viral pericarditis involves both direct viral invasion and immune-mediated mechanisms.
In some cases, the virus can directly invade the pericardium, leading to local inflammation28. In other cases, the immune response triggered by the viral infection can cause an inflammatory reaction in the pericardium29. This immune response involves the release of pro-inflammatory cytokines and the infiltration of immune cells into the pericardium, resulting in pericardial inflammation30. The clinical presentation of viral pericarditis can vary depending on the severity and underlying viral infection. Common symptoms include:
(a) Chest pain: Pericarditis typically presents with sharp, stabbing chest pain that worsens with deep breathing or lying flat. The pain may radiate to the neck, shoulders, or back. (b) Pericardial friction rub: A characteristic finding in pericarditis is the pericardial friction rub, which is a scratching or grating sound heard during the auscultation of the chest. (c) Fever: Viral pericarditis may be associated with fever, especially during the acute phase of the viral infection. (d) Other symptoms: Some individuals may experience symptoms such as fatigue, shortness of breath, cough, or abdominal discomfort31. The diagnosis of viral pericarditis involves a combination of clinical evaluation, laboratory tests, imaging studies, and the exclusion of other causes. Diagnostic tests may include (a) Physical examination: A thorough physical examination may reveal characteristic findings, such as a pericardial friction rub. (b) Electrocardiogram (ECG): ECG findings, such as widespread ST-segment elevation or PR-segment depression, can be suggestive of pericarditis. (c) Echocardiography: Echocardiography helps assess pericardial effusion, which is the accumulation of fluid in the pericardial space. (d) Blood tests: Blood tests may be performed to evaluate markers of inflammation, such as C-reactive protein (CRP) and erythrocyte sedimentation rate (ESR)32. Viral serology or polymerase chain reaction (PCR) tests can help identify the specific viral pathogen33. The treatment of viral pericarditis aims to alleviate symptoms, manage inflammation, and prevent complications34. The approach may include (a) Nonsteroidal anti-inflammatory drugs (NSAIDs): NSAIDs, such as ibuprofen or indomethacin, are often prescribed to reduce pain and inflammation. (b) Colchicine: Colchicine may be used in addition to NSAIDs to reduce inflammation and prevent recurrences. (c) Corticosteroids: In severe or refractory cases, corticosteroids may be considered to suppress the immune response and reduce inflammation. (d) Supportive care: Rest, adequate hydration, and symptomatic relief measures may be recommended to support the healing process35. The specific treatment approach will depend on the severity of symptoms, the presence of complications, and individual patient factors. The prognosis of viral pericarditis is generally favorable, with most cases resolving within a few weeks to months36. However, in some cases, complications such as cardiac tamponade (compression of the heart due to fluid accumulation) or chronic constrictive pericarditis (thickening and scarring of the pericardium) may develop37. Close monitoring, appropriate treatment, and follow-up care are essential to ensure a positive outcome.
Congestive Heart Failure
Congestive heart failure (CHF) can occur as a result of myocardial damage caused by MPXV infection38. Symptoms of CHF include fatigue, shortness of breath, swelling in the legs and ankles (edema), and difficulty performing physical activities39. Severe cases of CHF can be life-threatening and require immediate medical attention. The management of heart failure in monkeypox virus infection involves a comprehensive approach aimed at improving cardiac function, relieving symptoms, and preventing further complications40. Treatment strategies may include: (a) Medications: Heart failure medications, such as diuretics (to reduce fluid retention), angiotensin-converting enzyme (ACE) inhibitors, beta-blockers, and aldosterone antagonists, may be prescribed to improve cardiac function, reduce symptoms, and manage fluid balance. (b) Supportive measures: Lifestyle modifications, such as dietary changes (reducing salt intake) and regular physical activity, may be recommended to support heart health. (c) Monitoring: Regular follow-up visits and monitoring of cardiac function through echocardiography and other diagnostic tests may be necessary to assess response to treatment and adjust medications accordingly. (d) Advanced interventions: In severe cases of heart failure that are unresponsive to medical management, advanced interventions like implantable devices (such as pacemakers or defibrillators) or even heart transplantation may be considered41.
Arrhythmias
MPXV infection can disrupt the normal electrical conduction system of the heart, leading to arrhythmias4. MPXV can directly affect the electrical conduction system of the heart, disrupting the normal rhythm and timing of cardiac contractions. The virus can interfere with the generation and propagation of electrical signals, leading to abnormal heart rhythms. Certain factors can increase the likelihood of developing arrhythmias during monkeypox virus infection. These include the severity and extent of myocardial involvement, pre-existing heart conditions, and individual patient characteristics9. The presence of arrhythmias in monkeypox virus infection may manifest with various symptoms, including (a) Palpitations (b) Dizziness or lightheadedness (c) Syncope (d) Chest discomfort. It is important to note that arrhythmias may not always cause symptoms and can be incidentally detected during medical evaluation42. The management of arrhythmias in monkeypox virus infection involves various approaches depending on the type and severity of the arrhythmia. Treatment options may include: (a) Antiarrhythmic medications may be prescribed to regulate and stabilize the heart rhythm (b) Electrical cardioversion (c) Implantable devices (d) Treatment of underlying infection43. The specific treatment approach will depend on the type and severity of the arrhythmia, as well as the overall clinical condition of the individual.
Vascular Complications
MPXV infection can lead to endothelial dysfunction, which refers to impaired function of the endothelial cells lining the blood vessels44. Endothelial dysfunction can result in vasoconstriction, increased vascular resistance, and compromised blood flow to various organs, including the heart45. Additionally, endothelial dysfunction can contribute to the formation of blood clots (thrombosis) within the blood vessels, leading to potentially serious complications such as myocardial infarction, stroke, or peripheral vascular occlusion46.
Pathophysiology of cardiovascular manifestations
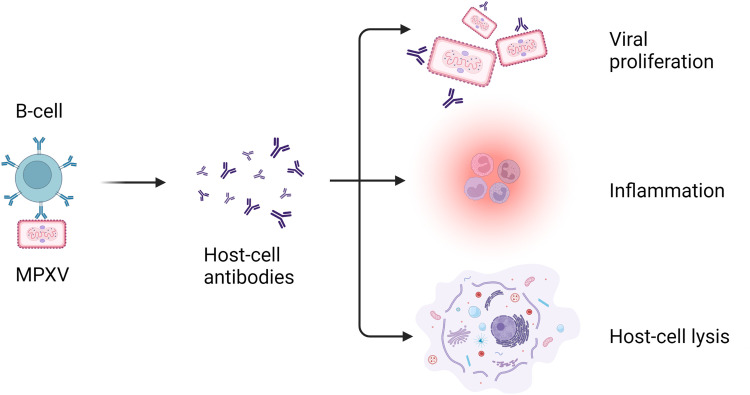
The pathophysiology of cardiovascular manifestations associated with MPXV infection is not yet fully understood. However, several mechanisms have been proposed based on studies and observations of viral infections in general and some specific findings related to MPXV. Figure 2 illustrates the pathophysiology of MPXV in causing cardiovascular manifestations. The following provides a detailed overview of the potential pathophysiological processes involved:
Figure 2.
Pathophysiology of monkeypox virus in immune cell response
Direct Viral Invasion
MPXV primarily targets the skin and mucous membranes, leading to characteristic skin lesions7. However, studies suggest that the virus can also invade endothelial cells, which line the blood vessels and play a crucial role in vascular function47. The direct invasion of endothelial cells by MPXV can disrupt their normal physiological functions, leading to endothelial dysfunction and subsequent cardiovascular manifestations45.
Inflammatory Response
Following the viral invasion, the immune system mounts an inflammatory response to control and eliminate the virus48. The release of pro-inflammatory cytokines, such as interleukin-6 (IL-6) and tumor necrosis factor-alpha (TNF-α), can induce endothelial cell activation and increase vascular permeability49. This inflammatory response can contribute to endothelial dysfunction and the development of cardiovascular complications44.
Indirect Effects
In addition to direct viral effects, the systemic response to MPXV infection can have indirect effects on the cardiovascular system. Fever, dehydration, electrolyte imbalances, and increased metabolic demands during the acute phase of the infection can strain the cardiovascular system and contribute to the development of cardiovascular manifestations.
Social and clinical implications
Public Health Concern
The emergence of cardiovascular manifestations in human monkeypox virus infection raises public health concerns due to the potential for severe outcomes and complications. Increased awareness, surveillance, and timely reporting of cases are crucial to monitor and control the spread of the virus50. Cardiovascular manifestations can significantly impact the quality of life of affected individuals. Symptoms such as chest pain, fatigue, shortness of breath, and limitations in physical activity can disrupt daily routines, hinder work productivity, and affect overall well-being.
Psychological Impact
The diagnosis of cardiovascular complications in the context of monkeypox virus infection can lead to emotional distress and anxiety for patients and their families51. Coping with the uncertainty of the disease and managing the potential long-term consequences can have psychological implications that require support and counseling. Individuals with monkeypox virus infection, particularly those experiencing cardiovascular manifestations, may face social stigma and discrimination due to fears of contagion or misconceptions about the disease. Education and awareness campaigns are essential to combat stigma and promote understanding within communities52.
Diagnostic Challenges
Cardiovascular manifestations in monkeypox virus infection may present with nonspecific symptoms, making diagnosis challenging. Distinguishing between viral myocarditis, pericarditis, and other cardiac conditions requires a thorough clinical evaluation, appropriate diagnostic tests, and expertise in infectious diseases and cardiology. The treatment and management of cardiovascular complications associated with monkeypox virus infection require a multidisciplinary approach involving infectious disease specialists, cardiologists, and other healthcare providers. Coordinating care, optimizing medications, and monitoring the progression of the disease can be complex, especially in severe cases requiring intensive care interventions.
Healthcare Resource Burden
The presence of cardiovascular manifestations in monkeypox virus infection can strain healthcare resources, including hospital facilities, diagnostic tests, medications, and specialized care. Adequate preparedness, capacity building, and resource allocation are necessary to effectively manage cases and ensure optimal patient outcomes. Individuals who experience cardiovascular complications during monkeypox virus infection may require long-term monitoring and follow-up. This includes regular cardiac evaluations, assessment of cardiac function, and adjustments to treatment plans based on the patient's clinical course and response to therapy.
Future directions
Future directions in the study of cardiovascular manifestations in human monkeypox virus (MPXV) infection are crucial for advancing our understanding, improving diagnosis and treatment, and effectively managing the associated complications.
Pathophysiological Mechanisms
Further research is needed to unravel the precise pathophysiological mechanisms underlying cardiovascular manifestations in MPXV infection. Studying the viral-host interactions, immune response, and genetic factors can provide insights into the mechanisms of viral-induced myocarditis, pericarditis, heart failure, and arrhythmias.
Biomarkers and Diagnostic Tools
The identification and validation of specific biomarkers can enhance the early detection, diagnosis, and monitoring of cardiovascular complications in MPXV infection. Developing novel diagnostic tools, including imaging modalities, serological tests, and molecular techniques, can improve accuracy and speed in diagnosing and monitoring cardiac involvement.
Risk Stratification
Identifying risk factors and developing risk stratification models can help predict the likelihood of developing cardiovascular complications in MPXV infection. Factors such as viral load, viral strain virulence, host genetics, and immune response profiles can aid in risk assessment and individualized patient management.
Treatment Strategies
Exploring targeted therapies, immunomodulatory agents, and antiviral interventions specific to cardiovascular complications in MPXV infection is crucial. Investigating the efficacy of existing antiviral drugs and immune-based therapies in mitigating cardiac damage and improving outcomes can pave the way for tailored treatment approaches.
Long-Term Outcomes and Follow-up
Long-term studies are needed to assess the impact of cardiovascular manifestations on the overall prognosis, quality of life, and long-term outcomes of individuals affected by MPXV infection. Understanding the natural history of cardiovascular complications and their sequelae can guide appropriate follow-up strategies and inform long-term management plans.
Prevention and Control Strategies
Developing effective prevention and control strategies for MPXV infection can indirectly reduce the incidence and severity of cardiovascular manifestations. This includes promoting public health measures, such as vaccination campaigns, surveillance systems, and early detection protocols, to limit the spread of the virus and minimize the risk of cardiac involvement.
Global Collaboration and Data Sharing
Encouraging collaboration among researchers, healthcare professionals, and public health agencies at a global scale is essential for sharing knowledge, resources, and data. Collaborative efforts can accelerate research, facilitate the development of standardized protocols, and enhance our understanding of the cardiovascular implications of MPXV infection.
Animal Models
Establishing reliable animal models that mimic the cardiovascular manifestations observed in human MPXV infection can aid in studying disease progression, testing potential therapies, and exploring novel interventions. Animal models can provide valuable insights into the pathogenesis and potential treatment options for cardiovascular complications.
Conclusion
In conclusion, cardiovascular manifestations in human monkeypox virus (MPXV) infection pose significant social and clinical implications. Myocarditis, viral pericarditis, heart failure, and arrhythmias can occur as complications of MPXV infection, leading to detrimental effects on individuals' health and well-being. The pathophysiology involves direct viral damage, immune response, and inflammatory processes that affect the myocardium and disrupt normal cardiac function. Addressing cardiovascular manifestations' social and clinical implications in MPXV infection necessitates a comprehensive approach involving healthcare professionals, public health authorities, and communities. By prioritizing research, enhancing diagnosis and treatment strategies, and promoting preventive measures, we can mitigate the impact of these complications, improve patient care, and protect public health.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Funding
The authors received no specific funding for this manuscript
References
- 1.Adnan N, Haq ZU, Malik A, Mehmood A, Ishaq U, Faraz M, Malik J, Mehmoodi A. Human monkeypox virus: An updated review. Medicine (Baltimore) 2022 Sep 2;101(35):e30406. doi: 10.1097/MD.0000000000030406. PMID: 36107544; PMCID: PMC9439836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.McCollum AM, Damon IK. Human monkeypox. Clin Infect Dis. 2014 Jan;58(2):260–267. doi: 10.1093/cid/cit703. Epub 2013 Oct 24Erratum in: Clin Infect Dis. 2014 Jun;58(12):1792. PMID: 24158414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gong Q, Wang C, Chuai X, Chiu S. Monkeypox virus: a re-emergent threat to humans. Virol Sin. 2022 Aug;37(4):477–482. doi: 10.1016/j.virs.2022.07.006. Epub 2022 Jul 9. PMID: 35820590; PMCID: PMC9437600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.El-Qushayri AE, Tawfik AG, Mahmoud-Elsayed H. Cardiovascular manifestations of monkeypox virus outbreak: An overview of the reported cases. Heart Lung. 2023;59:67–72. doi: 10.1016/j.hrtlng.2023.01.012. May-JunEpub 2023 Feb 3. PMID: 36739643; PMCID: PMC9896978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rahim A, Hameed A, Ishaq U, Malik J, Zaidi SMJ, Khurshid H, Malik A, Satti DI, Naz H. Cardiovascular sequelae of dengue fever: a systematic review. Expert Rev Cardiovasc Ther. 2022 Jun;20(6):465–479. doi: 10.1080/14779072.2022.2082945. Epub 2022 Jun 2. PMID: 35612830. [DOI] [PubMed] [Google Scholar]
- 6.Magadum A, Kishore R. Cardiovascular Manifestations of COVID-19 Infection. Cells. 2020 Nov 19;9(11):2508. doi: 10.3390/cells9112508. PMID: 33228225; PMCID: PMC7699571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wang X, Lun W. Skin Manifestation of Human Monkeypox. J Clin Med. 2023 Jan 24;12(3):914. doi: 10.3390/jcm12030914. PMID: 36769562; PMCID: PMC9918194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Aldhaeefi M, Rungkitwattanakul D, Unonu J, Franklin CJ, Lyons J, Hager K, Daftary MN. The 2022 human monkeypox outbreak: Clinical review and management guidance. Am J Health Syst Pharm. 2023 Jan 5;80(2):44–52. doi: 10.1093/ajhp/zxac300. PMID: 36259674. [DOI] [PubMed] [Google Scholar]
- 9.Jaiswal V, Sultana Q, Lahori S, Mukherjee D, Agrawal V, Doshi N, Shrestha AB, Huang H, Nasir YM, Naz S. Monkeypox-Induced Myocarditis: A Systematic Review. Curr Probl Cardiol. 2023 May;48(5) doi: 10.1016/j.cpcardiol.2023.101611. Epub 2023 Jan 28. PMID: 36716982; PMCID: PMC9883211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Shaik TA, Voloshyna D, Nasr TH, Makki A, Kosuru SH, Khan MH, Ghobriel NG, Sandhu QI, Saleem F. Monkeypox-Associated Pericarditis: A Maiden Case. Cureus. 2022 Sep 26;14(9):e29638. doi: 10.7759/cureus.29638. PMID: 36320991; PMCID: PMC9606631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Marietta M, Coluccio V, Luppi M. Monkeypox outbreak: after COVID-19, another challenge for the hemostatic system? Intern Emerg Med. 2022 Nov;17(8):2179–2183. doi: 10.1007/s11739-022-03112-8. Epub 2022 Oct 4. PMID: 36194336; PMCID: PMC9529604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cooper LT., Jr. Myocarditis. N Engl J Med. 2009 Apr 9;360(15):1526–1538. doi: 10.1056/NEJMra0800028. PMID: 19357408; PMCID: PMC5814110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dumont M, Guilhou T, Gerin M, Frémont-Goudot G, Nivose PL, Koubbi A, Joly V, Bouadma L, Yazdanpanah Y, André MH, de La Porte des Vaux C. Myocarditis in monkeypox-infected patients: a case series. Clin Microbiol Infect. 2023 Mar;29(3):390.e5–390.e7. doi: 10.1016/j.cmi.2022.12.001. Epub 2022 Dec 9. PMID: 36509373; PMCID: PMC9735378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Khan AH. The postcardiac injury syndromes. Clin Cardiol. 1992 Feb;15(2):67–72. doi: 10.1002/clc.4960150203. PMID: 1737407. [DOI] [PubMed] [Google Scholar]
- 15.Chen L, Deng H, Cui H, Fang J, Zuo Z, Deng J, Li Y, Wang X, Zhao L. Inflammatory responses and inflammation-associated diseases in organs. Oncotarget. 2017 Dec 14;9(6):7204–7218. doi: 10.18632/oncotarget.23208. PMID: 29467962; PMCID: PMC5805548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bartekova M, Radosinska J, Jelemensky M, Dhalla NS. Role of cytokines and inflammation in heart function during health and disease. Heart Fail Rev. 2018 Sep;23(5):733–758. doi: 10.1007/s10741-018-9716-x. PMID: 29862462. [DOI] [PubMed] [Google Scholar]
- 17.Elamm C, Fairweather D, Cooper LT. Pathogenesis and diagnosis of myocarditis. Heart. 2012 Jun;98(11):835–840. doi: 10.1136/heartjnl-2012-301686. Epub 2012 Mar 22. PMID: 22442199. [DOI] [PubMed] [Google Scholar]
- 18.Kindermann I, Barth C, Mahfoud F, Ukena C, Lenski M, Yilmaz A, Klingel K, Kandolf R, Sechtem U, Cooper LT, Böhm M. Update on myocarditis. J Am Coll Cardiol. 2012 Feb 28;59(9):779–792. doi: 10.1016/j.jacc.2011.09.074. PMID: 22361396. [DOI] [PubMed] [Google Scholar]
- 19.Kang M, Chippa V, An J. StatPearls Publishing; Treasure Island (FL): 2023 Jan. Viral Myocarditis.https://www.ncbi.nlm.nih.gov/books/NBK459259/ [Updated 2022 Sep 6]StatPearls [Internet]-Available from. [PubMed] [Google Scholar]
- 20.Rodriguez-Nava G, Kadlecik P, Filardo TD, Ain DL, Cooper JD, McCormick DW, Webber BJ, O'Laughlin K, Petersen BW, Narasimhan S, Sahni HK. Myocarditis Attributable to Monkeypox Virus Infection in 2 Patients, United States, 2022. Emerg Infect Dis. 2022 Dec;28(12):2508–2512. doi: 10.3201/eid2812.221276. Epub 2022 Sep 30. PMID: 36179413; PMCID: PMC9707588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Frustaci A, Chimenti C. Immunosuppressive therapy in myocarditis. Circ J. 2015;79(1):4–7. doi: 10.1253/circj.CJ-14-1192. Epub 2014 Dec 2. PMID: 25452202. [DOI] [PubMed] [Google Scholar]
- 22.Patel PM, Saxena A, Wood CT, O'Malley TJ, Maynes EJ, Entwistle JWC, Massey HT, Pirlamarla PR, Alvarez RJ, Cooper LT, Rame JE, Tchantchaleishvili V. Outcomes of Mechanical Circulatory Support for Giant Cell Myocarditis: A Systematic Review. J Clin Med. 2020 Dec 1;9(12):3905. doi: 10.3390/jcm9123905. PMID: 33271929; PMCID: PMC7761005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lampejo T, Durkin SM, Bhatt N, Guttmann O. Acute myocarditis: aetiology, diagnosis and management. Clin Med (Lond) 2021 Sep;21(5):e505–e510. doi: 10.7861/clinmed.2021-0121. PMID: 34507935; PMCID: PMC8439515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mlejnek D, Krejčí J. Myokarditidy a zánětlivé kardiomyopatie [Myocarditis and inflammatory cardiomyopathy] Vnitr Lek. 2017;63(7-8):507–512. FallCzech. PMID: 28933176. [PubMed] [Google Scholar]
- 25.Dababneh E, Siddique MS. StatPearls Publishing; Treasure Island (FL): 2023 Feb 12. Pericarditis. StatPearls [Internet]2023 Jan–PMID: 28613734. [PubMed] [Google Scholar]
- 26.Paiardi S, Pellegrino M, Cannata F, Bocciolone M, Voza A. Transitory effusive-constrictive pericarditis. Am J Emerg Med. 2018 Mar;36(3):524.e1. doi: 10.1016/j.ajem.2017.11.047. -524.e6Epub 2017 Nov 21PMID: 29169889. [DOI] [PubMed] [Google Scholar]
- 27.Hoit BD. Pericardial disease and pericardial tamponade. Crit Care Med. 2007 Aug;35(8):S355–S364. doi: 10.1097/01.CCM.0000271159.84639.2B. SupplPMID: 17667460. [DOI] [PubMed] [Google Scholar]
- 28.Cantarini L, Imazio M, Brizi MG, Lucherini OM, Brucato A, Cimaz R, Galeazzi M. Role of autoimmunity and autoinflammation in the pathogenesis of idiopathic recurrent pericarditis. Clin Rev Allergy Immunol. 2013 Feb;44(1):6–13. doi: 10.1007/s12016-010-8219-x. PMID: 21170606. [DOI] [PubMed] [Google Scholar]
- 29.Cantarini L, Lopalco G, Selmi C, Napodano S, De Rosa G, Caso F, Costa L, Iannone F, Rigante D. Autoimmunity and autoinflammation as the yin and yang of idiopathic recurrent acute pericarditis. Autoimmun Rev. 2015 Feb;14(2):90–97. doi: 10.1016/j.autrev.2014.10.005. Epub 2014 Oct 12. PMID: 25308531. [DOI] [PubMed] [Google Scholar]
- 30.Lopalco G, Rigante D, Cantarini L, Imazio M, Lopalco A, Emmi G, Venerito V, Fornaro M, Frediani B, Nivuori M, Brucato A, Iannone F. The autoinflammatory side of recurrent pericarditis: Enlightening the pathogenesis for a more rational treatment. Trends Cardiovasc Med. 2021 Jul;31(5):265–274. doi: 10.1016/j.tcm.2020.04.006. Epub 2020 May 3. PMID: 32376492. [DOI] [PubMed] [Google Scholar]
- 31.Awan A, Tiruneh F, Wessly P, Khan A, Iftikhar H, Barned S, Larbi D. Acute Pericarditis: Descriptive Study and Etiology Determination in a Predominantly African American Population. Cureus. 2017 Jul 6;9(7):e1431. doi: 10.7759/cureus.1431. PMID: 28924519; PMCID: PMC5587407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Imazio M, Spodick DH, Brucato A, Trinchero R, Markel G, Adler Y. Diagnostic issues in the clinical management of pericarditis. Int J Clin Pract. 2010 Sep;64(10):1384–1392. doi: 10.1111/j.1742-1241.2009.02178.x. Epub 2010 May 10. PMID: 20487049. [DOI] [PubMed] [Google Scholar]
- 33.Kogan E, Berezovskiy Y, Blagova O, Kukleva A, Semyonova L, Gretsov E, Ergeshov A. Morphologically, immunohistochemically and PCR proven lymphocytic viral peri-, endo-, myocarditis in patients with fatal COVID-19. Diagn Pathol. 2022 Feb 17;17(1):31. doi: 10.1186/s13000-022-01207-6. PMID: 35177093; PMCID: PMC8851780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chiabrando JG, Bonaventura A, Vecchié A, Wohlford GF, Mauro AG, Jordan JH, Grizzard JD, Montecucco F, Berrocal DH, Brucato A, Imazio M, Abbate A. Management of Acute and Recurrent Pericarditis: JACC State-of-the-Art Review. J Am Coll Cardiol. 2020 Jan 7;75(1):76–92. doi: 10.1016/j.jacc.2019.11.021. PMID: 31918837. [DOI] [PubMed] [Google Scholar]
- 35.Aikat S, Ghaffari S. A review of pericardial diseases: clinical, ECG and hemodynamic features and management. Cleve Clin J Med. 2000 Dec;67(12):903–914. doi: 10.3949/ccjm.67.12.903. PMID: 11127986. [DOI] [PubMed] [Google Scholar]
- 36.Imazio M, Brucato A, Barbieri A, Ferroni F, Maestroni S, Ligabue G, Chinaglia A, Cumetti D, Della Casa G, Bonomi F, Mantovani F, Di Corato P, Lugli R, Faletti R, Leuzzi S, Bonamini R, Modena MG, Belli R. Good prognosis for pericarditis with and without myocardial involvement: results from a multicenter, prospective cohort study. Circulation. 2013 Jul 2;128(1):42–49. doi: 10.1161/CIRCULATIONAHA.113.001531. Epub 2013 May 24. PMID: 23709669. [DOI] [PubMed] [Google Scholar]
- 37.Alerhand S, Adrian RJ, Long B, Avila J. Pericardial tamponade: A comprehensive emergency medicine and echocardiography review. Am J Emerg Med. 2022 Aug;58:159–174. doi: 10.1016/j.ajem.2022.05.001. Epub 2022 May 6. PMID: 35696801. [DOI] [PubMed] [Google Scholar]
- 38.Dayyab FM, Daiyab HM, Farahat RA. Precautions and recommendations towards possible cardiac manifestations of monkeypox vaccination. Int J Surg. 2022 Sep;105 doi: 10.1016/j.ijsu.2022.106898. Epub 2022 Sep 8. PMID: 36089260; PMCID: PMC9533822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Malik A, Brito D, Vaqar S, Chhabra L, Doerr C. StatPearls Publishing; Treasure Island (FL): 2022 Nov 7. Congestive Heart Failure (Nursing) StatPearls [Internet]2023 Jan–. PMID: 34662011. [PubMed] [Google Scholar]
- 40.Kearney MT, Cotton JM, Richardson PJ, Shah AM. Viral myocarditis and dilated cardiomyopathy: mechanisms, manifestations, and management. Postgrad Med J. 2001 Jan;77(903):4–10. doi: 10.1136/pmj.77.903.4. PMID: 11123385; PMCID: PMC1741887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.McDonagh TA, Metra M, Adamo M, Gardner RS, Baumbach A, Böhm M, Burri H, Butler J, Čelutkienė J, Chioncel O, Cleland JGF, Coats AJS, Crespo-Leiro MG, Farmakis D, Gilard M, Heymans S, Hoes AW, Jaarsma T, Jankowska EA, Lainscak M, Lam CSP, Lyon AR, McMurray JJV, Mebazaa A, Mindham R, Muneretto C, Francesco Piepoli M, Price S, Rosano GMC, Ruschitzka F, Kathrine Skibelund A, ESC Scientific Document Group 2021 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure. Eur Heart J. 2021 Sep 21;42(36):3599–3726. doi: 10.1093/eurheartj/ehab368. Erratum in: Eur Heart J. 2021 Oct 14;: PMID: 34447992. [DOI] [PubMed] [Google Scholar]
- 42.Dilaveris PE, Kennedy HL. Silent atrial fibrillation: epidemiology, diagnosis, and clinical impact. Clin Cardiol. 2017 Jun;40(6):413–418. doi: 10.1002/clc.22667. Epub 2017 Mar 8. PMID: 28273368; PMCID: PMC6490532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Li J, Gao M, Zhang M, Liu D, Li Z, Du J, Hou Y. Treatment of atrial fibrillation: a comprehensive review and practice guide. Cardiovasc J Afr. 2020;31(3):153–158. doi: 10.5830/CVJA-2019-064. May/Jun 23Epub 2020 Mar 18. PMID: 32186324; PMCID: PMC8762786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Fosse JH, Haraldsen G, Falk K, Edelmann R. Endothelial Cells in Emerging Viral Infections. Front Cardiovasc Med. 2021 Feb 24;8 doi: 10.3389/fcvm.2021.619690. PMID: 33718448; PMCID: PMC7943456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hadi HA, Carr CS, Al Suwaidi J. Endothelial dysfunction: cardiovascular risk factors, therapy, and outcome. Vasc Health Risk Manag. 2005;1(3):183–198. PMID: 17319104; PMCID: PMC1993955. [PMC free article] [PubMed] [Google Scholar]
- 46.Poredos P, Jezovnik MK. Endothelial Dysfunction and Venous Thrombosis. Angiology. 2018 Aug;69(7):564–567. doi: 10.1177/0003319717732238. Epub 2017 Sep 27. PMID: 28954526. [DOI] [PubMed] [Google Scholar]
- 47.Lum FM, Torres-Ruesta A, Tay MZ, Lin RTP, Lye DC, Rénia L, Ng LFP. Monkeypox: disease epidemiology, host immunity and clinical interventions. Nat Rev Immunol. 2022 Oct;22(10):597–613. doi: 10.1038/s41577-022-00775-4. Epub 2022 Sep 5. PMID: 36064780; PMCID: PMC9443635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Mueller SN, Rouse BT. Immune responses to viruses. Clinical Immunology. 2008:421–431. doi: 10.1016/B978-0-323-04404-2.10027-2. Epub 2009 May 15. PMCID: PMC7151814. [DOI] [Google Scholar]
- 49.Velazquez-Salinas L, Verdugo-Rodriguez A, Rodriguez LL, Borca MV. The Role of Interleukin 6 During Viral Infections. Front Microbiol. 2019 May 10;10:1057. doi: 10.3389/fmicb.2019.01057. PMID: 31134045; PMCID: PMC6524401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Hasan S, Saeed S. Monkeypox Disease: An Emerging Public Health Concern in the Shadow of COVID-19 Pandemic: An Update. Trop Med Infect Dis. 2022 Oct 3;7(10):283. doi: 10.3390/tropicalmed7100283. PMID: 36288024; PMCID: PMC9607171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Esch T, Stefano GB, Fricchione GL, Benson H. Stress in cardiovascular diseases. Med Sci Monit. 2002 May;8(5):RA93–RA101. PMID: 12011786. [PubMed] [Google Scholar]
- 52.Henderson C, Robinson E, Evans-Lacko S, Thornicroft G. Relationships between anti-stigma programme awareness, disclosure comfort and intended help-seeking regarding a mental health problem. Br J Psychiatry. 2017 Nov;211(5):316–322. doi: 10.1192/bjp.bp.116.195867. Epub 2017 Sep 21. PMID: 28935661; PMCID: PMC5663972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Miller MJ, Cash-Goldwasser S, Marx GE, Schrodt CA, Kimball A, Padgett K, Noe RS, McCormick DW, Wong JM, Labuda SM, Borah BF, Zulu I, Asif A, Kaur G, McNicholl JM, Kourtis A, Tadros A, Reagan-Steiner S, Ritter JM, Yu Y, Yu P, Clinton R, Parker C, Click ES, Salzer JS, McCollum AM, Petersen B, Minhaj FS, Brown E, Fischer MP, Atmar RL, DiNardo AR, Xu Y, Brown C, Goodman JC, Holloman A, Gallardo J, Siatecka H, Huffman G, Powell J, Alapat P, Sarkar P, Hanania NA, Bruck O, Brass SD, Mehta A, Dretler AW, Feldpausch A, Pavlick J, Spencer H, Ghinai I, Black SR, Hernandez-Guarin LN, Won SY, Shankaran S, Simms AT, Alarcón J, O'Shea JG, Brooks JT, McQuiston J, Honein MA, O'Connor SM, Chatham-Stephens K, O'Laughlin K, Rao AK, Raizes E, Gold JAW, SB; Morris. CDC Severe Monkeypox Investigations Team. Severe Monkeypox in Hospitalized Patients - United States, August 10-October 10, 2022. MMWR Morb Mortal Wkly Rep. 2022 Nov 4;71(44):1412–1417. doi: 10.15585/mmwr.mm7144e1. PMID: 36327164; PMCID: PMC9639440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Pinho AI, Braga M, Vasconcelos M, Oliveira C, Santos LD, Guimarães AR, Martins A, Chen-Xu J, Silva S, Macedo F. Acute Myocarditis: A New Manifestation of Monkeypox Infection? JACC Case Rep. 2022 Nov 2;4(21):1424–1428. doi: 10.1016/j.jaccas.2022.08.033. Epub 2022 Sep 2. PMID: 36249878; PMCID: PMC9528887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Tan DHS, Jaeranny S, Li M, Sukhdeo SS, Monge JC, Callejas MF, Hasso M, Fattouh R, Lalonde SD, Lam J, Mishra S. Atypical Clinical Presentation of Monkeypox Complicated by Myopericarditis. Open Forum Infect Dis. 2022 Aug 3;9(8):ofac394. doi: 10.1093/ofid/ofac394. PMID: 36043183; PMCID: PMC9416060. [DOI] [PMC free article] [PubMed] [Google Scholar]