Abstract
Background
Although exercise is recommended as part of the cystic fibrosis (CF) therapeutic routine, adherence to exercise is still limited. Digital health technologies can provide easy‐to‐access health information and may help improve healthcare and outcomes in individuals with long‐term conditions. However, its effects for delivering and monitoring exercise programs in CF have not yet been synthesized.
Objectives
To evaluate the benefits and harms of digital health technologies for delivering and monitoring exercise programs, increasing adherence to exercise regimens, and improving key clinical outcomes in people with CF.
Search methods
We used standard, extensive Cochrane search methods. The latest search date was 21 November 2022.
Selection criteria
We included randomized controlled trials (RCTs) or quasi‐RCTs of digital health technologies for delivering or monitoring exercise programs in CF.
Data collection and analysis
We used standard Cochrane methods. Our primary outcomes were 1. physical activity, 2. self‐management behavior, and 3. pulmonary exacerbations. Our secondary outcomes were 4. usability of technologies, 5. quality of life, 6. lung function, 7. muscle strength, 8. exercise capacity, 9. physiologic parameters, and 10. adverse events. We used GRADE to assess certainty of evidence.
Main results
We identified four parallel RCTs (three single‐center and one multicenter with 231 participants aged six years or older). The RCTs evaluated different modes of digital health technologies with distinct purposes, combined with diverse interventions.
We identified important methodologic concerns in the RCTs, including insufficient information on the randomization process, blinding of outcome assessors, balance of non‐protocol interventions across groups, and whether the analyses performed corrected for bias due to missing outcome data. Non‐reporting of results may also be a concern, especially because some planned outcome results were reported incompletely. Furthermore, each trial had a small number of participants, resulting in imprecise effects. These limitations on the risk of bias, and on the precision of effect estimates resulted in overall low‐ to very low‐certainty evidence. We undertook four comparisons and present the findings for our primary outcomes below. There is no information on the effectiveness of other modes of digital health technologies for monitoring physical activity or delivering exercise programs in people with CF, on adverse events related to the use of digital health technologies either for delivering or monitoring exercise programs in CF, and on their long‐term effects (more than one year).
Digital health technologies for monitoring physical activity
Wearable fitness tracker plus personalized exercise prescription compared to personalized exercise prescription alone
One trial (40 adults with CF) evaluated this outcome, but did not report data for any of our primary outcomes.
Wearable fitness tracker plus text message for personalized feedback and goal setting compared to wearable fitness tracker alone
The evidence is very uncertain about the effects of a wearable fitness tracker plus text message for personalized feedback and goal setting, compared to wearable technology alone on physical activity measured by step count at six‐month follow‐up (mean difference [MD] 675.00 steps, 95% confidence interval [CI] −2406.37 to 3756.37; 1 trial, 32 participants). The same study measured pulmonary exacerbation rates and reported finding no difference between groups.
Web‐based application to record, monitor, and set goals on physical activity plus usual care compared to usual care alone
Using a web‐based application to record, monitor, and set goals on physical activity plus usual care may result in little to no difference on time spent in moderate‐to‐vigorous physical activity measured via accelerometry compared to usual care alone at six‐month follow‐up (MD −4 minutes/day, 95% CI −37 to 29; 1 trial, 63 participants). Low certainty‐evidence from the same trial suggests that the intervention may result in little to no difference on pulmonary exacerbations during 12 months of follow‐up (median 1 respiratory hospitalization, interquartile range [IQR] 0 to 3) versus control (median 1 respiratory hospitalization, IQR 0 to 2; P = 0.6).
Digital health technologies for delivering exercise programs
Web‐based versus face‐to‐face exercise delivery
The evidence is very uncertain about the effects of web‐based compared to face‐to‐face exercise delivery on adherence to physical activity as assessed by the number of participants who completed all exercise sessions after three months of intervention (risk ratio 0.92, 95% CI 0.69 to 1.23; 1 trial, 51 participants).
Authors' conclusions
The evidence is very uncertain about the effects of an exercise program plus the use of a wearable fitness tracker integrated with a social media platform compared with exercise prescription alone and on the effects of receiving a wearable fitness tracker plus text message for personalized feedback and goal setting, compared to a wearable fitness tracker alone. Low‐certainty evidence suggests that using a web‐based application to record, monitor, and set goals on physical activity plus usual care may result in little to no difference in time spent in moderate‐to‐vigorous physical activity, total time spent in activity, pulmonary exacerbations, quality of life, lung function, and exercise capacity compared to usual care alone. Regarding the use of digital health technologies for delivering exercise programs in CF, the evidence is very uncertain about the effects of using a wearable fitness tracker plus personalized exercise prescription compared to personalized exercise prescription alone.
Further high‐quality RCTs, with blinded outcome assessors, reporting the effects of digital health technologies on clinically important outcome measures, such as physical activity participation and intensity, self‐management behavior, and the occurrence of pulmonary exacerbations in the long term are needed. The results of six ongoing RCTs identified through our searches may help clarify the effects of different modes of digital health technologies for delivering and monitoring exercise programs in people with CF.
Keywords: Adult, Humans, Cystic Fibrosis, Cystic Fibrosis/therapy, Digital Technology, Exercise, Exercise Therapy, Multicenter Studies as Topic, Muscle Strength, Quality of Life
Plain language summary
Digital interventions for delivering exercise in people with Cystic Fibrosis
Review question
What are the benefits and risks of using digital interventions for delivering or monitoring, or both, physical activity in people with cystic fibrosis (CF)?
Key messages
Taken together, the results of these trials suggest the following.
– Using a web‐based application to record, monitor, and set goals on physical activity plus usual care may result in little to no difference on physical activity and pulmonary exacerbations (flare up of disease) compared to usual care alone.
– The evidence is very uncertain about the effects of an exercise program plus the use of a wearable fitness tracker integrated with a social media platform plus exercise prescription, compared with exercise prescription alone, and on the effects of receiving a wearable fitness tracker plus text message for personalized feedback and goal setting, compared to a wearable fitness tracker alone.
– We are very uncertain about the effects of web‐based compared to face‐to‐face exercise delivery.
What is digital health technology?
When we talk about digital interventions, we mean using technology to allow communication and the sending of information between an individual and a healthcare provider to help manage a person's condition remotely. This can be done by mobile phone, tablet computer applications, or other types of technologies.
What is CF?
CF is an inherited disease that causes problems with the lungs, digestive system, and other organs. People with CF have thick and sticky mucus that blocks airways, leads to lung damage, and makes infections more likely. Most people with CF have respiratory symptoms such as coughing with more mucus and shortness of breath.
How digital health technologies can help people with CF?
A sedentary lifestyle may contribute to the progression of physical and functional impairment in people with CF, so exercise is recommended as part of the CF treatment plan. However, not everyone sticks to the exercise plan as they should. We wanted to see if digital technology can help people with CF to exercise and if it can keep track of the exercise they do.
What did we want to find out?
How can digital health technologies help deliver and monitor exercise programs in adults and children with CF?
What did we do?
To answer this question, we searched medical databases for all relevant trials on the topic. We collected and analyzed all the data currently available from the trials.
What did we find?
We found four trials involving 231 participants (aged six years or older). The trials lasted from three months to one year. These trials used different types of digital interventions. While one trial used a digital intervention to deliver an exercise program, the other three used a digital intervention to monitor people's physical activity.
Main results
One trial used a web‐based application to record, monitor, and set goals on physical activity plus usual care and investigators found there may be little to no difference on the amount of physical activity undertaken or on the number of pulmonary exacerbations that people experienced compared to usual care alone.
We are not confident in the results of the trial looking at whether a wearable fitness tracker with an exercise prescription can provide important benefits to people with CF compared to using only exercise prescription. We also cannot be sure whether using a wearable fitness tracker plus text message for personalized feedback and goal setting is effective compared to a wearable fitness tracker alone.
Furthermore, we are very uncertain on the effects of web‐based compared to face‐to‐face exercise delivery.
No trials formally evaluated whether digital interventions could lead to harms and we found little or no data on other important outcomes such as participation in physical activity, self‐management behavior (the ability to manage our actions), and the occurrence of pulmonary exacerbations.
There is no information on the effectiveness of other types of digital health technologies for monitoring physical activity or delivering exercise programs in people with CF, and on their long‐term effects (more than one year).
What are the limitations of the evidence?
We included only four trials, each with a small number of participants. The authors of these trials reported little information on how they were conducted. This decreased our confidence in their results. There may be bias when the people assessing a person's test results know which treatment the person received. In the included trials, it was not clear if the investigators tried to prevent the outcome assessors from knowing each person's treatment. Some trials also did not report in full the results that they planned in their protocol and this may also introduce bias. Therefore, we are uncertain about the effects of digital interventions for monitoring and delivering exercise in people with CF, and further better‐quality trials are needed to clarify its effects. Overall, we had only low to very low confidence in the results.
How up to date is this evidence?
The evidence is current to 21 November 2022.
Summary of findings
Summary of findings 1. Exercise prescription plus the use of a wearable fitness tracker versus exercise prescription alone.
| Exercise prescription plus the use of a wearable fitness tracker versus exercise prescription alone | ||||||
|
Patient or population: adults with CF Settings: home Intervention: wearable fitness tracker integrated with a social media platform with exercise prescription Comparison: exercise prescription alone | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of participants (trials) | Certainty of the evidence (GRADE) | Comments | |
| Exercise prescription alone | Wearable fitness tracker with exercise prescription | |||||
| Adherence to exercise training (long term) | This outcome result was not reported. | |||||
| Self‐management behavior (long term) | This outcome result was not reported. | |||||
| Time to subsequent pulmonary exacerbation (medium term) | This outcome result was not reported. | |||||
| Usability of digital health technology (medium term) | This outcome result was not reported. | |||||
| QoL: CFQ‐R scores (long term) | This outcome result was not reported at this time point. | |||||
|
Lung function – FEV1 % predicted (medium term) Follow‐up: 1 year |
The mean (SD) FEV1 was 67 (27) % predicted. | The mean FEV1 in the intervention group was 1.00 % predicted higher (15.46 lower to 17.46 higher). | — | 40 (1 RCT) | ⊕⊝⊝⊝ Very lowa,b | Bishay 2018 |
|
Exercise capacity – submaximal GXT (long term) |
This outcome result was not reported at this time point. | |||||
| *The basis for the assumed risk is provided in footnotes. The corresponding risk (and its 95% CI) is based on the assumed risk in the intervention group. CF: cystic fibrosis; CFQ‐R: Cystic Fibrosis Questionnaire – Revised; CI: confidence interval; FEV1: forced expiratory volume in 1 second; GXT: graded exercise test; MD: mean difference; QoL: quality of life; RCT: randomized controlled trial; SD: standard deviation. | ||||||
| GRADE Working Group grades of evidence
High certainty: we are very confident that the true effect lies close to that of the estimate of the effect. Moderate certainty: we are moderately confident in the effect estimate; the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low certainty: our confidence in the effect estimate is limited; the true effect may be substantially different from the estimate of the effect. Very low certainty: we have very little confidence in the effect estimate; the true effect is likely to be substantially different from the estimate of effect. | ||||||
a Downgraded one level due to serious imprecision (small number of participants). b Downgraded two levels due to very serious methodologic limitations. This is because the included trial did not provide sufficient information regarding the randomization process, blinding of outcome assessors, balance of non‐protocol interventions across groups, or whether the analyses were performed correcting for bias due to missing outcome data. This resulted in some concerns on bias arising from the randomization process, and high risk of bias due to deviations from intended interventions, missing outcome data, and measurement of the outcome, as this outcome could be influenced by the lack of blinding.
Summary of findings 2. Wearable fitness tracker plus text message for personalized feedback and goal setting compared to wearable fitness tracker alone.
| Wearable fitness tracker plus text message for personalized feedback and goal setting compared to wearable fitness tracker alone | ||||||
|
Patient or population: adults with CF Settings: outpatients Intervention: wearable fitness tracker plus text message personalized feedback and goal setting Comparison: wearable fitness tracker alone | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of participants (trials) | Certainty of the evidence (GRADE) | Comments | |
| Wearable fitness tracker | Wearable fitness tracker plus text message | |||||
| Adherence to exercise training (long term) | This outcome result was not reported. | |||||
| Self‐management behavior (long term) | This outcome result was not reported. | |||||
| Time to subsequent pulmonary exacerbation (medium term) | This outcome result was not reported. | |||||
| Usability of digital health technology (medium term) | This outcome result was not reported. | |||||
| QoL (long term) | This outcome was not reported at this time point. | |||||
|
Lung function – FEV1 % predicted (medium term) Follow‐up: 6 months |
The mean FEV1 was 2.83 (SD 1.29) % predicted. | The mean FEV1 in the intervention group was 0.34 % predicted lower (1.16 lower to 0.48 higher). | — | 32 (1 RCT) | ⊕⊝⊝⊝ Very lowa,b | Curran 2022 |
|
Exercise capacity (long term) |
This outcome was not reported at this time point. | |||||
| *The basis for the assumed risk is provided in footnotes. The corresponding risk (and its 95% CI) is based on the assumed risk in the intervention group. CF: cystic fibrosis; CFQ‐R: Cystic Fibrosis Questionnaire – Revised; CI: confidence interval; FEV1: forced expiratory volume in 1 second; QoL: quality of life; RCT: randomized controlled trial; SD: standard deviation. | ||||||
| GRADE Working Group grades of evidence
High certainty: we are very confident that the true effect lies close to that of the estimate of the effect. Moderate certainty: we are moderately confident in the effect estimate; the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low certainty: our confidence in the effect estimate is limited; the true effect may be substantially different from the estimate of the effect. Very low certainty: we have very little confidence in the effect estimate; the true effect is likely to be substantially different from the estimate of effect. | ||||||
a Downgraded two levels due to very serious imprecision (small number of participants). b Downgraded one level due to serious methodologic limitations. Due to the nature of intervention, participants could not be blinded to treatment allocation. Also, outcome assessors were not blinded to treatment allocation. This led to a high risk of bias due to deviations from the intended interventions and in the measurement of this result.
Summary of findings 3. Web‐based application to record, monitor and set goals on physical activity plus usual care compared to usual care alone.
| Web‐based application to record, monitor and set goals on physical activity plus usual care compared to usual care alone | ||||||
|
Patient or population: adolescents and adults with CF Settings: discharged from hospital Intervention: web‐based application to record, monitor and set goals on physical activity plus usual care Comparison: usual care alone | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of participants (trials) | Certainty of the evidence (GRADE) | Comments | |
| Usual care | Web‐based application | |||||
| Adherence to exercise training (long term) | This outcome result was not reported. | |||||
| Self‐management behavior (long term) | This outcome result was not reported. | |||||
|
Time to subsequent pulmonary exacerbation (medium term) Follow‐up: 12 months |
See comments | — | Not reported | ⊕⊕⊝⊝ Lowa, b | 1 RCT evaluated this outcome (Cox 2022). During 12 months of follow‐up, the authors reported there were no differences between groups on the time to first hospital admission due to respiratory causes. No further information was provided. | |
| Usability of digital health technology (medium term) | This outcome result was not reported. | |||||
| QoL (long term) | This outcome result was not reported at this time point. | |||||
|
Lung function – FEV % predicted (medium term) Follow‐up: 6 months |
The mean FEV1 was −1.2 (SDc 10.32) % predicted. | The mean FEV1 in the intervention group was 0.3 % predicted lower (5.2 lower to 4.6 higher). | — | 63 (1 RCT) | ⊕⊕⊝⊝ Lowa,b | Cox 2022 |
| Exercise capacity (long term) | This outcome result was not reported at this time point. | |||||
| *The basis for the assumed risk is provided in footnotes. The corresponding risk (and its 95% CI) is based on the assumed risk in the intervention group. CF: cystic fibrosis; CFQ‐R: Cystic Fibrosis Questionnaire – Revised; CI: confidence interval; FEV1: forced expiratory volume in 1 second; GXT: graded exercise test; MD: mean difference; QoL: quality of life; RCT: randomized controlled trial; SD: standard deviation. | ||||||
| GRADE Working Group grades of evidence
High certainty: we are very confident that the true effect lies close to that of the estimate of the effect. Moderate certainty: we are moderately confident in the effect estimate; the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low certainty: our confidence in the effect estimate is limited; the true effect may be substantially different from the estimate of the effect. Very low certainty: we have very little confidence in the effect estimate; the true effect is likely to be substantially different from the estimate of effect. | ||||||
a Downgraded one level due to serious imprecision (small number of participants). b Downgraded one level due to serious methodologic limitations. Due to the nature of intervention in the included trial, participants could not be blinded to treatment allocation. Also, there was a substantial number of losses in the trial (41%). This led to some concerns on the risk of bias due to deviations from intended interventions and high risk of bias due to missing outcome data. c SD was estimated using 95% CI provided in the primary study using Review Manager calculator.
Summary of findings 4. Web‐based versus face‐to‐face exercise delivery.
| Web‐based versus face‐to‐face exercise delivery | ||||||
|
Patient or population: children and adults with CF Settings: home Intervention: exercise program delivery via internet Comparison: exercise program delivery face‐to‐face | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of participants (trials) | Certainty of the evidence (GRADE) | Comments | |
| Face‐to‐face | Internet delivery | |||||
| Adherence to exercise training (long term) | This outcome result was not reported at this time point. | |||||
| Self‐management behavior (long term) | This outcome result was not reported. | |||||
| Time to subsequent pulmonary exacerbation (medium term) | This outcome result was not reported. | |||||
|
Usability of digital health technology (medium term) Follow‐up: 3 months |
See comments. | 1 RCT evaluated this outcome (Carr 2018). Usability data were collected via qualitative interviews and reported narratively. The authors reported that most people found Skype convenient and easy to use, and that it reduced travel, family demands, and impacted on privacy. There were a few technical issues reported, including loss of internet access, information technology skills, and pedagogic difficulties with the technology. No further data available for analysis. | ||||
|
QoL: CFQ‐R scores (long term) |
This outcome result was not reported at this time point. | |||||
|
Lung function (FEV1 % predicted) (medium term) Follow‐up: 3 months |
See comments | ⊕⊝⊝⊝ Very lowa,b | 1 RCT assessed this outcome (Carr 2018). The authors reported that after 3 months of intervention they found no difference between the groups for FEV1, but there were no analyzable data provided. | |||
| Exercise capacity | This outcome result was not reported. | |||||
| *The basis for the assumed risk is provided in footnotes. The corresponding risk (and its 95% CI) is based on the assumed risk in the intervention group. CF: cystic fibrosis; CFQ‐R: Cystic Fibrosis Questionnaire – Revised; CI: confidence interval; FEV1: forced expiratory volume in 1 second; QoL: quality of life; RCT: randomized controlled trial. | ||||||
| GRADE Working Group grades of evidence
High certainty: we are very confident that the true effect lies close to that of the estimate of the effect. Moderate certainty: we are moderately confident in the effect estimate; the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low certainty: our confidence in the effect estimate is limited; the true effect may be substantially different from the estimate of the effect. Very low certainty: we have very little confidence in the effect estimate; the true effect is likely to be substantially different from the estimate of effect. | ||||||
a Downgraded two levels due to risk of bias within the single included trial for this outcome. This is because the included trial did not provide sufficient information regarding the randomization process, blinding of outcome assessors, balance of non‐protocol interventions over the study groups, or whether the analyses were performed correcting for bias due to missing outcome data. This resulted in some concerns on bias arising from the randomization process, and high risk of bias due to deviations from intended interventions, missing outcome data, and measurement of the outcome, as this outcome could be influenced by the lack of blinding. b Downgraded one level due to imprecision caused by a small number of participants.
Background
Description of the condition
Cystic fibrosis (CF) is an autosomal recessive, life‐limiting disorder that affects approximately 100,000 people worldwide, with 7.97 per 100,000 births in the USA and 7.37 per 100,000 in the EU (Bell 2020). CF is caused by mutations in a gene on chromosome 7 that encodes for a protein called CF transmembrane conductance regulator (CFTR) (Lima 2014). The altered CFTR function is thought to result in decreased chloride secretion and increased sodium absorption, leading to water reabsorption across the epithelia (CF Foundation 2020). The water reabsorption promotes secretion dehydration and abnormal mucus clearance, resulting in the accumulation of thick sticky secretions in the lungs, pancreas, and other organs (Dyce 2015).
CF affects multiple systems, including reproductive organs, pancreas, liver, and intestines, resulting in an impairment in nutritional status (Naehrig 2017). Despite that, most morbidity and mortality stems from the respiratory effects. The accumulation of thick sticky secretions in the lungs favors infections and inflammation (Gautam 2015), thereby causing a progressive decline in lung function (Dasenbrook 2012). A cycle of recurrent infection, chronic inflammation, and progressive lung damage results in lung disease. The progress of the lung disease in combination with other factors such as malnutrition (due to exocrine and endocrine pancreatic insufficiency) and intrinsic muscle abnormalities contribute to ventilatory limitation during exercise in CF (Gruet 2017). Additionally, a sedentary lifestyle contributes to the progression of physical and functional impairment (Schneiderman 2014). The progressive respiratory disease ultimately results in respiratory failure, which is the primary cause of death in people with CF (CF Foundation 2020).
Of note, the life expectancy of people with CF has substantially improved (Keogh 2018). The median life expectancy of children with CF born in 1990 was estimated to be 40 years, double that of in the 1970s (Elborn 1991). Currently, the median survival is reported as 40.6 years in the USA (Stephenson 2017), 45.1 years in the UK (CF Trust 2016), and 50.9 years in Canada (Stephenson 2017); it has been predicted that the mean survival age of those born in 2000 may be over 50 years (Dodge 2007). This increase in life expectancy is possibly due to early diagnosis, advances in the treatment, and multiprofessional management in specialized centers (Dasenbrook 2012).
Description of the intervention
Despite the recent development of effective CFTR modulator therapies that show promise to improve pulmonary function and life expectancy in people with CF (Heijerman 2019; Middleton 2019), the management of CF will still involve a multidisciplinary team and a global approach. Exercise and physical activity are effective ways for improving overall health, and can provide important benefits beyond medications (Khoury 2019). While physical activity is considered to be any bodily movement that requires energy expenditure (Bull 2020), exercise is any type of physical activity that is planned, structured, repetitive, and has the purpose of improving or maintaining one or more components of physical fitness (WHO 2020b). Habitual physical activity, for example, is associated with improved pulmonary function (Schneiderman 2014), better exercise capacity, and decreased frequency of hospitalization (Cox 2016). Exercise leads to improved aerobic and anaerobic performance (Klijn 2004), better mucociliary clearance (Dwyer 2011), improved psychological health (Gupta 2019), and better quality of life (QoL) (Klijn 2004). Nevertheless, adherence to exercise remains problematic (Bernard 2008). An emerging body of literature suggests that digital interventions may be useful for providing supervised exercise therapy and facilitating adherence for people with several physical conditions (Chen 2018).
Digital technology refers to a wide variety of technologies, equipment, and applications that process information in the form of numeric codes, which can be processed by several devices such as smartphones, computers, and robots (Shah 2019). Recognizing the great potential for the accelerated technologic progress that we are living through to be harnessed to solve healthcare systems challenges, the World Health Organization (WHO) has created a global strategy on digital health (i.e. the use of digital technologies for improving health) (WHO 2020a). Instead of being a specific intervention, digital health technology is rather a means of enhancing care delivery and education (Velardo 2017). The WHO divides digital health technologies into four domains: live videoconferencing between a person and provider using telecommunication technology; store‐and‐forward transmission of patient data using an electronic communication system, such as an email or electronic medical record; remote patient monitoring, using electronic communication technologies to collect personal health data in one location and transmit to a provider in other location; and mobile health, using mobile communication devices, such as smartphones or tablets to deliver messages, through general packet radio service, third‐ and fourth‐generation mobile communications, global positioning systems, or Bluetooth technology (WHO 2011).
How the intervention might work
Although exercise training has several benefits for people with CF (Ward 2019), and physical activity promotion is part of the regular management of CF (Bradley 2015), visiting exercise specialist centers, especially for people living in remote areas, may be costly, time‐consuming, and represent a significant cross‐infection risk to people with CF. These challenges contribute to the low adherence to exercise in CF (Blakey 2018). Since digital health technologies have become more user‐friendly, providing care to people with chronic diseases such as CF has become increasingly more viable. Digital interventions such as smart devices, wearable biosensors, and live videoconferencing may be useful for providing supervised exercise training. These digital health technologies have the potential to connect the individual with the healthcare professional (Williams 2014), and can create the opportunity to support physical activity more flexibly, eliminate travel time, monitor physical activity and physiologic parameters, assess adherence, and reduce the potential risk of cross‐infectivity (Chen 2018).
One Cochrane Review found significant improvements in QoL and levels of activity in people with chronic obstructive pulmonary disease treated using computer and mobile technology compared to face‐to‐face or written instructions (McCabe 2017). Whether digital health technologies could lead to similar results in CF care is unknown. However, digital health technologies in CF could be used for exercise training prescription as well as for supporting physical activity participation by providing enhanced monitoring. The opportunity for improved monitoring may be useful to enhance self‐efficacy for self‐management (Cummings 2011), and to identify pulmonary exacerbations at an earlier stage allowing for earlier intervention (Lechtzin 2017). Self‐management could also have a positive impact on health behaviors. Improved self‐management in response to digital health technologies could help support exercise training, may encourage an individual's engagement (Sobnath 2017), and reduce the burden on healthcare systems. In this perspective, this intervention may also offer a more cost‐effective vehicle for practice and research. Furthermore, digital health technologies may be of value for a number of different purposes in CF care, also with potential for helping home monitoring (Calthorpe 2020; Moor 2022). For individuals who are geographically or socially isolated, or who find travel difficult due to their disease severity or comorbidities, digital health technologies may have the potential to connect the person with the healthcare professional (Wood 2017), and facilitate self‐management and adherence to treatment (Williams 2014). Perceived benefits to people with CF may include overcoming barriers such as the availability of transport, the flexibility of a schedule, a reduced number of outpatient department visits, and the reassurance of feeling constantly monitored by healthcare professionals (Fairbrother 2013).
Why it is important to do this review
Although exercise is recommended as part of the CF therapeutic routine, adherence to exercise is still limited. Digital health technologies can provide easy‐to‐access health information and may help improve healthcare and outcomes in individuals with long‐term conditions (Whitehead 2016). However, there have been small or heterogeneous studies whose results have not yet been synthesized. Recently, the use of some patient‐facing platforms has gained traction in CF (van Beurden 2021). In addition, the COVID‐19 pandemic has accelerated the dissemination of health interventions delivered via digital health technologies, and these interventions have become even more important for these vulnerable individuals who have been shielding during the pandemic. Furthermore, the risks of implementing these technologies among people with CF need to be addressed. Establishing this evidence base will help inform the clinical use of available effective resources and guide further research in this field.
Objectives
To evaluate the benefits and harms of digital health technologies for delivering and monitoring exercise programs, increasing adherence to exercise regimens, and improving key clinical outcomes in people with CF.
Methods
Criteria for considering studies for this review
Types of studies
We included randomized controlled trials (RCTs) or quasi‐RCTs (including cross‐over RCTs) reported in full text, published as an abstract only, and any unpublished trial identified. Cluster RCTs were not eligible for this review.
Types of participants
Individuals with CF of all ages and degrees of disease severity, diagnosed based on clinical criteria and sweat testing or genotype analysis. We did not employ any restrictions based on exacerbation status.
Types of interventions
The review included trials that compare the use of digital health technologies for two purposes, which we planned to report separately.
Digital health technologies for delivering exercise programs in CF
Digital health technologies for physical activity monitoring in CF
We included trials comparing interventions based on any type of digital health technology. Comparisons could include any digital health technology (such as smartphones and computer applications) used alone or in combination versus any type of comparator (such as a different type of digital health technology intervention (i.e. comparisons of two active methods of digital support using different frequency of digital monitoring or different modes of delivery), usual care, or delivering exercise programs in‐person or monitoring physical activity), as long as the effect of the digital health technology could be exclusively assessed (e.g. digital health technology plus exercise prescription versus exercise prescription alone or digital health technology for monitoring physical activity plus exercise orientation plus usual care versus exercise orientation plus usual care).
We defined exercise as a planned regimen of physical activity or exercise training, either alone or in combination, of defined types (e.g. resistance, endurance, flexibility, or neuromotor exercise), duration (e.g. minutes or hours), frequency (e.g. number of training sessions per week), intensity (e.g. light, moderate, or vigorous), and volume (e.g. metabolic equivalent of task (MET)/minute/week) and with the possibility of progression of the exercise regimen delivered via digital health technologies. We excluded trials if the interventions did not have a duration of at least two weeks.
Types of outcome measures
To assess the effects of digital health technologies for delivering exercise programs and for monitoring physical activity, we planned to analyze the following outcome measures.
Primary outcomes
-
Physical activity (measured objectively with devices such as pedometers, accelerometers, or activity monitors or subjectively using self‐report and validated questionnaires [e.g. International Physical Activity Questionnaire (IPAQ)])
Participation in physical activity (defined as number of steps, time spent in physical activity [e.g. minutes per day or week], energy expenditure [e.g. kilocalories or joules per day or week])
Adherence to exercise training (defined as the amount of completed exercise divided by the amount of prescribed exercise)
Intensity of physical activity (e.g. MET)
-
Self‐management behavior
Ability of the individual to fit treatment requirements for CF into their everyday activities (e.g. monitoring symptoms, monitoring of energy expenditure, communicating about illness or aspects of care)
Measures of self‐efficacy, coping, problem‐solving, or independence
-
Pulmonary exacerbations
Time to subsequent exacerbation
Number of pulmonary exacerbations (per participant per month, if available)
Secondary outcomes
Usability of digital health technologies (to participants and staff; measured using usability scales or questionnaires)
QoL (measured using validated instruments or participant reports, with generic or disease‐specific instruments, or both)
-
Lung function
Forced expiratory volume in one second (FEV1) reported as liters or % predicted
Forced vital capacity (FVC) reported as liters or % predicted
Lung Clearance Index (LCI)
Total lung capacity (TLC)
Functional residual capacity (FRC)
Forced expiratory flow between 25% and 75% of expiratory volume (FEF25–75)
-
Muscle strength
Isokinetic muscle force tests
Non‐isokinetic muscle force tests (e.g. handgrip strength)
-
Exercise capacity
Cardiopulmonary exercise testing (CPET) (e.g. Wingate anaerobic test [WaNT] and incremental maximal testing protocols)
Other tests of exercise capacity (e.g. six‐ and 12‐minute walk tests; shuttle tests; sit‐to‐stand test; three‐minute step test)
Physiologic parameters (e.g. oxygen saturation, heart rate, systemic blood pressure)
-
Adverse events related to the intervention
Serious adverse events (any untoward event related to the intervention that is life‐threatening, requiring hospitalization, or resulting in persistent or significant disability or death)
All other adverse events (an unfavorable medical occurrence, which may include abnormal signs, symptoms, or disease, temporarily associated with participation in the study (e.g. hemoptysis, exercise‐induced bronchospasm, and pneumothorax))
Timing of outcome assessment
We assessed each outcome at all time points reported in primary papers and planned to pool intervention periods into short‐term, intermediate‐term, and long‐term data, as defined below.
Short‐term: up to (but not including) three months after the start of the intervention
Intermediate‐term: from three months to one year after the start of the intervention
Long‐term: more than one year after the start of the intervention
Search methods for identification of studies
We searched for all relevant published and unpublished studies without restrictions on language, year, or publication status.
Electronic searches
The Cochrane Cystic Fibrosis and Genetic Disorders Group's Information Specialist conducted a search of the Group's Cystic Fibrosis Trials Register for relevant trials using the following terms: (physiotherapies & exercising:kw) AND (telehealth:kw).
The Cystic Fibrosis Trials Register is compiled from electronic searches of the Cochrane Central Register of Controlled Trials (CENTRAL) (updated each new issue of the Cochrane Library), weekly searches of MEDLINE, a search of Embase to 1995 and the prospective handsearching of two journals – Pediatric Pulmonology and the Journal of Cystic Fibrosis. Unpublished work is identified by searching the abstract books of three major cystic fibrosis conferences: the International Cystic Fibrosis Conference; the European Cystic Fibrosis Conference and the North American Cystic Fibrosis Conference. For full details of all searching activities for the register, see the relevant section of the Cochrane Cystic Fibrosis and Genetic Disorders Group's website.
Date of the latest search: 21 November 2022.
We also undertook separate searches of the following databases, registers, and trial registries:
CINAHL (EBSCO) (Cumulative Index to Nursing and Allied Health Literature; 1982 to 21 November 2022);
PEDro (Physiotherapy Evidence Database pedro.org.au/; searched 21 November 2022);
US National Institutes of Health Ongoing Trials Register ClinicalTrials.gov (www.clinicaltrials.gov; searched 21 November 2022);
The WHO International Clinical Trials Registry Platform (trialsearch.who.int/Default.aspx; searched 21 November 2022).
Details of the search strategies are presented in Appendix 1.
Searching other resources
We checked the bibliographies of included trials and any relevant systematic reviews identified for further references to relevant trials. We contacted experts and organizations in the field to obtain additional information on relevant trials. We also searched for errata or retractions from included trials and would have reported within the review the date if this had been done. However, we found no errata or retractions.
Data collection and analysis
Selection of studies
At least two out of three authors (ACP, AR, and MGN) independently screened the titles and abstracts of all the potential trials identified from the search for inclusion in the review. When disagreement arose on the suitability of a trial, we consulted another review author (SRP). Since each trial, rather than each report, was the unit of interest in the review, we excluded duplicates and collated multiple reports of the same trial. We present details of excluded trials with the reason for exclusion in the Characteristics of excluded studies table (Lefebvre 2022). A PRISMA flowchart is shown in Figure 1.
1.
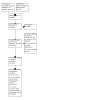
PRISMA flow diagram.
Data extraction and management
At least two out of three authors (ACP, AR, and MGN) independently extracted data using a standard data acquisition form that was piloted on at least one trial in the review to record the following details: study design (parallel or cross‐over or multi‐arm; single‐center or multicenter, participants and trial characteristics – age, gender, the severity of condition, diagnostic criteria – for baseline equality between groups, details on the number of participants screened for eligibility, randomized, analyzed, excluded, lost to follow‐up and dropped out, use of stratification, use of intention‐to‐treat (ITT) analysis); the setting; the detailed intervention; duration of studies; and outcome measures (continuous and dichotomous) and time points reported; funding for the trial, and notable conflicts of interest of trial authors. Although we used RoB 2 tool, we also extracted data on relevant information presented in the first version of the risk of bias tool (RoB 1) for further discussion, if needed, including: method of randomization and allocation concealment, blinding of personnel and outcome assessors, incomplete outcome data, and selective reporting. We resolved disagreements by consensus or if necessary, by consulting another review author (VFT) (Li 2022). One review author (ACP) entered the data into Review Manager Web and a second review author checked entries (HS) (RevMan Web 2022). We contacted the authors of two included trials to request additional data for other outcomes; however, to date we have not received any response (Bishay 2018; Carr 2018).
Assessment of risk of bias in included studies
At least two out of three authors (ACP, AR, and MGN) independently assessed the risk of bias for each outcome result using the RoB 2 outlined in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2022a). We assessed both the effect of assignment to the intervention and the effect of adhering to the intervention for each of the main outcome results and time points specified for inclusion in the summary of findings tables.
We assessed risk of bias according to the following domains.
Bias arising from the randomization process
Bias due to deviations from intended interventions
Bias due to missing outcome data
Bias in measurement of the outcome
Bias in selection of the reported result
We judged each potential source of bias as 'low', 'some concerns', or 'high' risk of bias, based on answers to the signaling questions. In 29 November 2022, we accessed the RoB 2 tool and used the Excel tool to record and manage RoB 2 assessments (riskofbias.info), and make the RoB 2 consensus decisions for the signaling questions. We resolved any disagreements by discussion or by involving another review author (ANA). We planned to assess cross‐over trials using the revised RoB 2 tool with additional considerations for cross‐over trials, and including the assessment of bias arising from period and carryover effects. As we did not find any cross‐over studies, we did not use this tool.
We summarized the risk of bias judgments for each outcome across all domains, with a justification for the judgments in the risk of bias table. We considered the overall risk of bias for each outcome assessed to be the least favorable assessment across the domains of bias (Higgins 2022a). We used the overall RoB 2 judgments for the specified outcomes for GRADE assessments.
In future versions of the review, if we are faced with missing evidence, we will apply the ROB‐ME tool to the appropriate outcomes (Page 2022).
Measures of treatment effect
For continuous outcomes (adherence, pulmonary function, QoL, usability, healthcare utilization, number of pulmonary exacerbations, participation in physical activity – number of steps, time spent in physical activity, energy expenditure, intensity of physical activity, muscle strength, exercise capacity, self‐management behavior), we reported the mean difference (MD) with 95% confidence intervals (CIs) as the measure of treatment effect.
If more than one trial had measured the same outcome using different tools or units of measurement, we would have calculated the pooled standardized mean difference (SMD) and 95% CI. For time‐to‐event outcomes (time to subsequent exacerbation), we planned to use hazard ratios (HRs) with 95% CIs. However, this outcome result was not reported in a way that allowed this analysis in the included trials.
For dichotomous outcomes (adherence and adverse events), we presented results as risk ratios (RR) with 95% CIs (Deeks 2022). If we had found skewed data, we planned to perform transformations of the original outcome data, where possible. If transformation was not possible, we planned to narratively describe any skewed data as medians, interquartile ranges (IQRs), and range (Higgins 2022b).
We considered using change‐from‐baseline or postintervention value scores, according to the data availability; however, we planned to summarize these types of results separately.
Unit of analysis issues
Where trials randomly allocated individual participants to a digital health technology or to a control intervention, we considered the participant as the unit of analysis (Higgins 2022c). For RCTs with a cross‐over design, we planned to use the results from paired analyses (Elbourne 2002). The reporting of data from cross‐over trials is generally variable with limited data published that are required for a paired analysis (Higgins 2022c). If these data were not available, we planned to use data from the first period of the trial and treat it as a parallel trial (Higgins 2022c). However, we did not find any eligible cross‐over RCTs to include in this review.
Dealing with missing data
Where there were missing or unavailable data, we contacted the authors of the included studies to request additional information (Bishay 2018; Carr 2018); however, we have not received responses to date. Although the trial authors did not respond, we still included the trials and reported all available information, and attempted to clarify the reason access to the missing data was not possible.
Where possible, we planned to perform an ITT analysis, considering all randomized participants in the treatment arm to which they were originally assigned. However, there was insufficient information provided. We assessed the extent to which trial investigators had employed an ITT analysis and, where possible, reported the numbers of participants who dropped out of each arm of the trial (Higgins 2022c). For outcomes with continuous data that were missing standard deviations (SDs), we planned to either calculate these from other available data such as standard errors (SEs), or impute them, on the basis of SDs for the same outcome using the same scale, or from other similar trials, if possible (Higgins 2022b). However, this was not needed, as there were no missing SDs in the included trials.
Assessment of heterogeneity
For trials with similar interventions and participants, assessing similar outcomes, we planned to pool the data in meta‐analyses and depict them in forest plots. We also planned to assess the level of heterogeneity using visual inspection of forest plots, Chi2 (P < 0.1), and the I² statistic, as defined in the Cochrane Handbook for Systematic Reviews of Interventions (Deeks 2022), as below.
0% to 40%: might not be important
30% to 60%: may represent moderate heterogeneitya
50% to 90%: may represent substantial heterogeneitya
75% to 100%: considerable heterogeneitya
aThe importance of the observed value of I2 statistic depends on the magnitude and direction of effects, and also the strength of evidence for heterogeneity (e.g. P value from the Chi2 test, or a CI for the I2 statistic; uncertainty in the value of the I2 statistic is substantial when the number of studies is small).
We also planned to perform prespecified subgroup analyses; however, we included few trials in this Cochrane Review and could not carry out any meta‐analyses.
Assessment of reporting biases
We planned to assess reporting biases by drawing a funnel plot (trial effect versus trial size), assuming we included a sufficient number of trials (more than 10 for each outcome) in the review. To test for asymmetry, we planned to perform a regression‐based method as suggested by Page (Page 2022).
As we did not have this number of trials, we did not perform a funnel plot analysis. As we were unable to assess publication biases via asymmetrical funnel plots, to minimize publication bias, we used multiple search strategies, search trial registries, and attempted to contact investigators for identifying unpublished data. We also assessed outcome reporting bias. To reduce this type of bias and to ensure all variables are reported, we attempted to identify the relevant trial protocols; if these were not available, we compared the methods section to the results section of each included trial.
Data synthesis
We had planned to group trials according to similarity of intervention, populations, and the outcomes measured. We assessed clinical homogeneity between studies by comparing trial characteristics and participant demographics. As the trials were clinically heterogeneous, considering the similarity of intervention, populations, and the outcomes measured, conducted a narrative synthesis as it would not be appropriate in these cases to combine results in a meta‐analysis (McKenzie 2022). We conducted separate narrative synthesis to examine effects of:
interventions for monitoring physical activity; and
interventions for delivering exercise programs.
Where trials examined the effects of multiple interventions, we planned to include participants from each arm in separate meta‐analyses. However, none of the included studies had evaluated the effects of multiple interventions.
Subgroup analysis and investigation of heterogeneity
We planned to carry out the following subgroup analyses on the primary outcomes to investigate their influence on the size of the treatment effect (if appropriate data were available):
age: pediatric (up to 18 years old) versus adult (over 18 years);
duration of intervention: up to 12 weeks versus more than 12 weeks;
type of intervention; and
disease severity based on lung function (FEV1% predicted, over 90%, 50% to 89%, below 50%).
However, these analyses were not possible because we did not have sufficient data.
Sensitivity analysis
We summarized each type of intervention (digital health technology for exercise prescription and digital health technology for physical activity monitoring) separately. To answer the question on whether digital health technology is effective for exercise prescription, we performed a primary analysis including all eligible studies using digital health technology for exercise training prescription in people with CF. We included trials on digital health technology for physical activity monitoring in a separate primary analysis.
We planned to perform the following sensitivity analyses for the primary outcomes:
by repeating each of the analyses after excluding trials with high risk of bias from the overall analyses;
by examining the effect of cross‐over trials on the results;
by comparing the results derived from a random‐effects model versus those obtained from a fixed‐effect model; and
by exploring the impact of including trials with incomplete data.
However, few trials were included in this review and these analyses could not be performed (Deeks 2022).
Summary of findings and assessment of the certainty of the evidence
We present a summary of findings table for each comparison in the review with the assessments of the certainty of evidence using the GRADE approach (Schünemann 2022). Where there were no data for individual outcomes, we identified this for that row in the table, by stating "data not reported". To inform our GRADE decision on downgrading for risk of bias, we used the overall risk of bias judgment derived from the RoB 2 Excel tool. We planned to rate the certainty of evidence for the following outcomes for each individual comparison, since we consider these are patient‐important outcomes:
adherence to exercise training (number of completed exercise sessions divided by the number of prescribed exercise sessions) (long‐term);
self‐management behavior (long‐term);
time to subsequent pulmonary exacerbation (medium‐term);
usability of digital health technologies to people with CF (medium‐term);
QoL (long‐term);
lung function (FEV1 % predicted) (medium‐term); and
exercise capacity (long‐term).
Results
Description of studies
Results of the search
Our searches retrieved 307 records. After assessing the abstracts for inclusion criteria, we assessed the full text of 19 trials (45 records). Of these, we excluded six trials (12 records) with justifications, three trials (three records) are awaiting classification, and six trials (10 records) are ongoing. We included the remaining four RCTs (20 records) in this systematic review (Figure 1). See Characteristics of included studies; Characteristics of excluded studies; Characteristics of ongoing studies; and Characteristics of studies awaiting classification tables for additional details on the trials.
Included studies
Setting
Three trials were single‐center, parallel RCTs (Bishay 2018; Carr 2018; Curran 2022), and one was a multicenter parallel RCT (Cox 2022). The trials had different durations, ranging from six months (Curran 2022), through nine months (Carr 2018) and 12 months (Cox 2022) to two years (Bishay 2018). One trial was conducted in the USA (Bishay 2018), one in the UK (Carr 2018), one in Ireland (Curran 2022), and one in Australia (Cox 2022). Three trials stated they recruited outpatients from a single center; two recruited only adults (Bishay 2018; Curran 2022), while the UK trial recruited both children and adults (Carr 2018). The final trial recruited adolescents and adults discharged from hospital at eight CF centers in Australia (Cox 2022).
Participants
The RCTs included 231 participants of both sexes (Bishay 2018; Carr 2018; Cox 2022; Curran 2022). The smallest trial recruited 33 adults with CF (Curran 2022) and the largest included 107 adolescents and adults with CF (Cox 2022). The mean age of participants in one trial was 35 (SD 14) years; however, this trial has only been reported as conference abstracts and there was no further detailed information on the characteristics of participants (Bishay 2018). The age range of participants in the second trial was 6.1 years to 51.5 years (Carr 2018). The third trial had a mean age of 26.7 (SD 7.8) years in the intervention group and 24.5 (SD 5.4) years in the control group (Curran 2022), and the last trial had a mean age of 21 (SD 6) years (Cox 2022).
There were similar numbers of males and females in two trials – there were 55% males and 45% females in one trial (Bishay 2018), and 43.9% males and 56.1% females in a second trial (Cox 2022). There were more females than males in the remaining trials; 32.5% males and 67.5% females (Carr 2018), and 39.4% males and 60.6% females (Curran 2022).
Bishay 2018 randomized participants by age and FEV1, and the authors reported that this resulted in two groups with similar demographic characteristics; the mean FEV1 at baseline was 70% predicted (SD 30%) (Bishay 2018). Carr 2018 reported that the median FEV1 % predicted at baseline was 76% predicted (range 28% to 106%). In the third trial, FEV1 was 69% predicted in the intervention group and 76% predicted in the control group (Curran 2022). In the final trial, FEV1 was 63% predicted in the intervention group and 72% predicted in the control group (Cox 2022).
Interventions
The included trials evaluated different types of digital health technologies with distinct purposes. While three trials assessed the use of a digital health technology for monitoring physical activity (Bishay 2018; Cox 2022; Curran 2022), one trial assessed its effects in delivering exercise programs (Carr 2018).
Bishay 2018 compared the effects of a personalized exercise prescription provided by a physical therapist plus the use of a wearable fitness tracker integrated with a social media platform to exercise prescription alone. In this trial, participants received a Fitbit and were monitored over the course of one year. However, as it was published only as an abstract, there was limited information on details of intervention, such as the frequency and duration of meetings with the physical therapist. Curran 2022 evaluated the effects of wearable technology (Fitbit Charge 2) linked to an online monitoring system (Fitabase), text message personalized feedback, and goal setting compared to the use of the same wearable technology linked to an online monitoring system, but without step count goals, and no text messages in adults with CF. This trial tested participants at baseline and 12 weeks with follow‐up at 24 weeks. Cox 2022 investigated the effects of a 12‐week intervention with a web‐based application (ActivOnline) to record and monitor physical activity, and set goals on physical activity plus usual care compared to usual care alone. Participants were followed up for six months for all outcomes, except for healthcare utilization (hospital admissions and hospital days), which assessed at 12 months following completion of the intervention period.
The trial that assessed the effects of a digital health technology for delivering exercise programs randomized participants to receive Tai Chi sessions (Carr 2018). Participants had no prior experience practicing Tai Chi and those who were taking part in any other interventional study or had participated in the pilot study were excluded. The trial delivered eight sessions over three months via Skype (the internet‐delivered group) or face‐to‐face either at home or other suitable venues, including hospital if convenient to the participant. The sessions employed a sequence of eight movements focused on developing key Tai Chi principles and selected for their specific effect on the respiratory system and assumed overall benefit in CF. Each session began with postural and breath awareness, and the end of each session included self‐massage. In this trial, participants were offered a DVD with three separate sections, in order to appeal to different ages and abilities: adults; children and people who may be incapacitated or in hospital experiencing an exacerbation; and those who needed to sit rather than stand whilst exercising (Carr 2018). There was a printed instruction booklet (including photographs), and stickers, diaries, and t‐shirts were offered, and participants were encouraged to practice the exercises for five to 10 minutes up to five times a week. The bespoke DVD and the booklet were given to each participant irrespective of group allocation. Forty participants completed all eight lessons (Carr 2018).
Outcomes
Only one trial reported results on adherence to exercise training (Carr 2018). Two trials reported the results for physical activity participation (Cox 2022; Curran 2022). One trial reported physical activity objectively measured via accelerometry (time spent in moderate‐to‐vigorous physical activity) and via a self‐reported measurement (Habitual Activity Estimation Scale [HAES]) (Cox 2022). The second trial reported this outcome by measuring Fitbit step count data and by the self‐reported measurement IPAQ (Curran 2022). Two trials reported results for pulmonary exacerbations, including the number of exacerbations (Curran 2022), and time to first hospital admission by medical record review (Cox 2022). No trials reported details on our other primary outcome, namely self‐management behavior (Bishay 2018; Carr 2018; Cox 2022; Curran 2022).
In terms of our secondary outcomes, one trial reported narrative data on usability of digital health technologies (Carr 2018), and all trials reported on QoL using the Cystic Fibrosis Questionnaire – Revised (CFQ‐R; Quittner 2009) (Bishay 2018; Carr 2018; Cox 2022; Curran 2022). All trials reported on lung function using FEV1 (Bishay 2018; Carr 2018; Cox 2022; Curran 2022), but only two trials reported on FVC (Carr 2018; Cox 2022), although Curran 2022 assessed but did not report results for FVC. Only one trial reported on exercise capacity using graded exercise test (GXT) (Bishay 2018), one trial using a cardiopulmonary exercise test (Curran 2022), and one trial using modified shuttle test (Cox 2022). One trial reported results on grip strength measured with hand dynamometry (Curran 2022). No trials reported on adverse effects of the intervention (Bishay 2018; Carr 2018; Cox 2022; Curran 2022).
Excluded studies
We excluded six trials (12 records) from this systematic review (Anifanti 2022; Bingham 2010; Happ 2013; Hebestreit 2022; Kenis‐Coskun 2022; NCT03522480).
In three trials, the intervention was ineligible as none used a digital health technology for delivering or monitoring exercise programs (Bingham 2010; Happ 2013; NCT03522480). One cross‐over RCT compared the use of a digital game with feedback to control software using featureless graphics to assess engagement with forced exhalation maneuvers that approximate those in airway clearance techniques (Bingham 2010); a second trial allocated participants to receive a respiratory therapy device to help them keep engaged in their respiratory therapy routine (NCT03522480); while the third used only telephone calls for reinforcement and for assessing progress and barriers (Happ 2013).
We excluded three trials because the effect of the digital health technology could not be exclusively evaluated among the interventions used (Anifanti 2022; Hebestreit 2022; Kenis‐Coskun 2022). In one trial, while the exercise group participated in a wearable activity tracker‐based exercise program over the course of one year, the control group was asked to participate in recreational physical activities, but refrain from any structured exercise intervention (Anifanti 2022). Thus, the intervention group received an exercise prescription plus a digital health technology for monitoring physical activity and the control group received no exercise prescription and no digital health technology, making it impossible to exclusively assess the effect of the digital health technology (Anifanti 2022). Similarly, in a second trial, the intervention group was multimodal and included several interventions beyond the digital health technology (Hebestreit 2022). The intervention group received a different exercise prescription (including the addition of at least three hours of vigorous physical activity) plus activity counseling plus motivation using a three‐axial pedometer worn on a daily basis and a web‐based activity log; while the control group received orientation to keep their physical activity level constant over the 12‐month study period, were not informed about their fitness level assessed with the exercise tests, did not receive any interpretation of the test results, were not given an evaluation of their answers to the activity questionnaires, and did not receive their individual pedometry results. Therefore, the effect of the digital health technology could not be exclusively evaluated (Hebestreit 2022). In the last excluded trial, the intervention group received telerehabilitation through a Zoom application plus an exercise program based on high‐intensity interval training and postural strengthening. After contacting the study authors, we were informed that the control group did not receive any interventions other than routine therapies. Participants in the control group were encouraged to perform their routine pulmonary rehabilitation such as airway clearance and exercises, however nothing was instructed for the purpose of the study. Therefore, the effect of the digital health technology could not be exclusively evaluated in this trial (Kenis‐Coskun 2022).
Studies awaiting classification
Three studies are awaiting classification (Burnett 2021; Kilic 2021; Mermis 2021). See Characteristics of studies awaiting classification table.
Ongoing studies
Six RCTs (10 reports) are still ongoing (ACTRN12620001237976; ISRCTN92573472; Lang 2019; NCT04249999; NCT04742049; Powers 2016). We will update this systematic review when the results of these trials are available. See Characteristics of ongoing studies table.
Setting
Three trials are enrolling participants in an outpatient setting (ACTRN12620001237976; NCT04742049; Powers 2016). The other three RCTs are performing the interventions at the participants' homes (ISRCTN92573472; Lang 2019; NCT04249999).
Participants
Two RCTs are recruiting only adults with CF (ACTRN12620001237976; ISRCTN92573472), two trials are recruiting only children (Lang 2019; NCT04742049), and the two remaining trials are including both children and adults (NCT04249999; Powers 2016). Target sample sizes range from 24 participants (ACTRN12620001237976) to 119 participants (Lang 2019). Limited data are available at present on the characteristics of the participants.
Interventions
Three trials will deliver an online exercise training protocol (ACTRN12620001237976; NCT04249999; NCT04742049). In the remaining three trials, participants will receive a wearable tracker to objectively track daily steps (ISRCTN92573472; Lang 2019; Powers 2016). Additionally, in one trial participants will also be given access to the 'Do More, B'More, Live Fit' webpage, which includes spotlighted exercises, instructional exercise photos, and videos (Powers 2016).
Outcomes
Considering our outcomes of interest, five ongoing trials will assess participation in physical activity (ISRCTN92573472; Lang 2019; NCT04249999; NCT04742049; Powers 2016). Four trials plan to assess lung function and QoL (ISRCTN92573472; Lang 2019; NCT04249999; Powers 2016), and four trials will assess exercise capacity (ISRCTN92573472; Lang 2019; NCT04742049; Powers 2016). Two trials plan to measure adherence to exercise (ACTRN12620001237976; Lang 2019); one trial to measure disease exacerbation (Lang 2019); one trial muscle strength (ISRCTN92573472); and one trial will measure physiologic parameters such as heart rate, blood pressure, and oxygen saturation (ISRCTN92573472). None of the identified ongoing trials state they will evaluate self‐management behavior and usability.
Risk of bias in included studies
We evaluated the risk of bias for each study result using RoB 2 and presented a summary (Figure 2). The details and rationale for each judgment per outcome, and the consensus decisions for the signaling questions of the risk of bias assessments are available at figshare.com/articles/dataset/ROB2_Consensus_form_1_xlsm/22122968. We were interested in both the effect of assignment to intervention and the effect of adhering to the intervention.
2.

Robvis plot to summarize of risk of bias judgments.
Due to the nature of the interventions, blinding of participants and personnel was probably not feasible in several studies. For some outcomes, assessors were also not blinded. Of note, we judged the absence of information regarding participants' possible deviations from the intended interventions to be of concern in studies where participants were not blinded. Additionally, to judge the measurement of the outcome domain, we considered whether the outcomes were objective or otherwise could have been influenced by the lack of blinding of outcome assessors. For some of these measurements (e.g. lung function, exercise capacity), good test quality depends on the efforts of both the participant and the technicians (Graham 2019; Schermer 2003; Seyedmehdi 2013). As adequate training, with appropriate feedback, and a motivated technologist are of particular importance to elicit maximum performance from the participant in spirometry and exercise capacity tests (Andreacci 2002; Ruppel 2012), we judged that the behavior of an unblinded outcome assessor biased toward the benefit of one intervention arm over the other could affect those results. Therefore, for these outcome results, we judged the lack of blinding to lead to a high risk of bias in the measurement of the outcome domain. For self‐reported outcomes (self‐reported physical activity and QoL), we judged that the lack of blinding of participants could also influence the outcome measurement.
We considered the overall risk of bias for each outcome assessed to be the least favorable assessment across the domains of bias (Higgins 2022a). As we judged all outcome results to have a high risk of bias in at least one domain, we judged all trials to have an overall high risk of bias for both the effects. Of note, non‐reporting of results was also a concern, especially because some planned outcome results were reported incompletely and because each comparison had only one study included. These results are specified below. In future updates of this review, we plan to use ROB‐ME to assess the risk of bias due to missing data, especially to try to evaluate to what extent these missing data influence the effect estimate in the syntheses including more studies.
1. Digital health technology for monitoring physical activity
1.1. Wearable fitness tracker plus personalized exercise prescription compared to personalized exercise prescription alone
Only Bishay 2018 evaluated this comparison. This study was only reported in conference abstracts and little information is available. We used the abstract reports (Bishay 2018) and the trial protocol (NCT02700243) to evaluate the risk of bias in this trial. Although no information was provided on blinding, due to the nature of the intervention, for all outcome results of this comparison participants were probably not blind to treatment allocation.
Quality of life
Investigators evaluated this outcome using the CFQ‐R after one year of the intervention. However, they did not provide sufficient information regarding the randomization process, blinding of outcome assessors, balance of non‐protocol interventions across groups, or whether the analyses were performed correcting for bias due to missing outcome data. There were no serious concerns raised due to selective reporting for the outcomes presented in our analysis.
Lung function
Bishay 2018 evaluated this outcome by measuring FEV1 % predicted. As for QoL, we judged there to be some concerns on the randomization process and high risk of bias due to deviations from intended interventions, missing outcome data, and measurement of the outcome, mainly because scarce information was available on the report.
Exercise capacity
Exercise capacity was evaluated using GXT (Bishay 2018). The same concerns raised for the other results of this comparison were also raised for this outcome result and these concerns lead to an overall high risk of bias for this outcome result.
As there was very little information not only regarding the inclusion all randomized participants in the analysis and the analysis of participants in the intervention groups to which they were randomized, but also on the occurrence of non‐protocol interventions, implementation of the intervention, and non‐adherence to the assigned intervention, we judged both the effect of assignment to the intervention and the effect of adhering to the intervention to have an overall high risk of bias for all outcomes in this comparison.
1.2. Wearable fitness tracker plus text message for personalized feedback and goal setting compared to wearable fitness tracker alone
Only one trial evaluated this comparison (Curran 2022). This trial was reported in multiple abstracts, a trial protocol (NCT03672058), and published in full (Curran 2022). We used the information from all reports to evaluate the risk of bias for this trial. For all outcome results of this comparison, participants could not be blinded to treatment allocation due to the nature of intervention. The authors reported that outcome assessors were also not blinded to treatment allocation.
Physical activity
Investigators measured physical activity using step count and IPAQ after three months of intervention and at six‐month follow‐up. The authors did not provide sufficient information on whether there were deviations from the intended intervention that arose because of the trial context or on the analysis used to assess the effect of assignment to intervention and the effect of adhering to intervention. This led to a high risk of bias due to possible deviations from intended interventions. As outcome assessors were not blinded in this trial, we judged that the assessment of IPAQ could have been influenced by knowledge of the intervention received. This led to a high risk of bias in the measurement of this result. However, this issue is not likely to have occurred for step counts, and we judged there to be a low risk of bias in the measurement of this specific result. There were no serious concerns for the other domains.
Pulmonary exacerbations
Curran 2022 reported exacerbation rates of participants. We judged there was high risk of bias due to possible deviations from intended interventions and in the measurement of the outcome result, since investigators provided little information on possible non‐protocol interventions and statistical analysis, and the outcome assessors were not blinded (this result could have been influenced by the lack of blinding).
Quality of life
Curran 2022 evaluated QoL using the CFQ‐R. Due to the lack of blinding of participants, caregivers, and outcomes assessors, and because this assessment can be influenced by the lack of blinding, we judged the QoL results at high risk of bias due to possible deviations from intended interventions and in the measurement of the outcome result.
Lung function
Curran 2022 evaluated lung function using FEV1 (liters/second) and FVC. The same concerns were raised for these outcome results as above because we judged that these results could have been influenced by the lack of blinding.
Muscle strength
Curran 2022 evaluated this outcome using hand dynamometry. We judged these results to be at high risk of bias due to possible deviations from intended interventions and in the measurement of the outcome result.
Exercise capacity
Investigators measured this outcome using a cardiopulmonary exercise test (highest amount of oxygen consumed at peak exercise [VO2peak]) (Curran 2022). We judged this outcome result to be potentially influenced by the lack of blinding of participants and outcome assessors, leading to a high risk of bias both for deviations from intended interventions and measurement of the outcome result.
As very little information was reported on the occurrence of non‐protocol interventions, implementation of the intervention, and non‐adherence to the assigned intervention, both the effect of assignment to the intervention and the effect of adhering to the intervention were at an overall high risk of bias for all outcomes in this comparison.
1.3. Web‐based application to record, monitor, and set goals on physical activity plus usual care compared to usual care alone
One trial evaluated this comparison (Cox 2022). This trial was reported as both in two abstracts and a full‐text paper (Cox 2022); its protocol was registered prospectively (ACTRN12617001009303). We used the information from all reports to evaluate the risk of bias for this trial. Due to the nature of intervention, participants could not be blinded to treatment allocation. The authors did not report on the occurrence of non‐protocol interventions, implementation of the intervention, and non‐adherence to the assigned intervention. However, as the authors reported both ITT and per‐protocol analyses using linear mixed models, accounting for recruitment site, and the baseline value of each outcome variable as a covariate, we judged this trial to have only some concerns on the risk of bias due to deviations from intended interventions for all outcome results both for the effect of assignment to the intervention and for the effect of adhering to the intervention. All assessments were performed by a blinded outcome assessor, except those assessed by the participants themselves. There was a substantial number of dropouts (according to the study flow diagram), with only 63/107 participants completing the primary outcome assessment (time spent in moderate‐to‐vigorous physical activity) after 12 weeks, and 55/107 completing the other outcomes. Thus, all outcome results were at high risk of bias for missing outcome data.
Physical activity
Cox 2022 measured physical activity via accelerometry (time spent in moderate‐to‐vigorous physical activity) and self‐reported using the HAES. As outcome assessors were blinded in this trial, we judged that for the time spent in moderate‐to‐vigorous physical activity, assessment of the outcome could not have been influenced by knowledge of intervention received but for HAES it may have been since it is a self‐reported measure and the participants were probably not blinded. This led to a high risk of bias in the measurement of the result for HAES but not for the time spent in moderate‐to‐vigorous physical activity, which we judged at low risk of bias. There were no serious concerns for the other domains, except for missing outcome data, which we judged at high risk of bias.
Pulmonary exacerbations
Cox 2022 evaluated pulmonary exacerbations by measuring the time to first hospital admission according to medical record review. Since participants were not blinded and there was a substantial number of losses to follow‐up, we judged there to be some concerns on the risk of bias due to deviations from the intended interventions and high risk of bias due to missing outcome data.
Quality of life
Investigators evaluated QoL using the CFQ‐R. We judged there to be some concerns on the risk of bias due to deviations from the intended interventions and high risk of bias due to missing outcome data.
Lung function
Cox 2022 evaluated lung function using FEV1 and FVC; the same concerns were raised for this outcome as above, as assessors were blinded to allocation.
Exercise capacity
Cox 2022 measured exercise capacity using the modified shuttle test. We had some concerns on the risk of bias due to deviations from the intended interventions (participants were not blinded) and judged there to be a high risk of bias due to missing outcome data (substantial number of losses to follow‐up). Less than half of all participants (47%) completed this assessment at baseline, and only 25% completed the assessment postintervention. This was primarily due to participants declining to undertake the test or completing their evaluation remotely.
2. Digital health technology for delivering exercise programs
2.1. Web‐based versus face‐to‐face exercise delivery
Only one trial evaluated this comparison and reported their results both as abstracts and full‐text articles (Carr 2018). The trial protocol was prospectively registered (NCT02054377). We considered all reports while evaluating the risk of bias in this trial. The results were focused on feasibility and on qualitative data from the RCT and there was little information on the methods and quantitative results. We contacted the primary authors to request additional information; however, no response has been received to date, and our assessments were based on the content of the published articles and abstracts. Of note, Carr 2018 did not present several numerical results that were planned in their protocol. Due to the nature of intervention, participants could not be blinded to treatment allocation. There was a substantial number of dropouts with 40/51 participants completing the three‐month intervention and being analyzed.
Physical activity
Carr 2018 evaluated adherence to exercise training by the number of participants who completed all exercise sessions after three months of intervention. The authors did not provide sufficient information regarding the randomization process, blinding of outcome assessors, balance of non‐protocol interventions across groups, or whether they corrected the analyses for bias due to missing outcome data. This resulted in some concerns on the randomization process and a high risk of bias due to deviations from the intended interventions, missing outcome data, and measurement of the outcome result.
Quality of life
Carr 2018 measured QoL using the CFQ‐R. We had some concerns on the randomization process and also judged there to be a high risk of bias for missing outcome data due to the lack of blinding of participants, caregivers, and outcomes assessors, which could have influenced this assessment. We judged this outcome at high risk of bias due to possible deviations from intended interventions and in the measurement of the outcome result.
Lung function
The authors evaluated lung function using FEV1 and FVC. The same concerns were raised for the results of this outcome as above, because we judged that these results could have been influenced by the lack of blinding.
Physiologic parameters
The authors measured participants' oxygen saturation. We judged that the lack of blinding could have very minimal to no influence on this assessment, and this resulted in some concerns on the randomization process and measurement of the outcome result and a high risk of bias due to deviations from the intended interventions, as there was no information on possible deviations from interventions and high risk of bias due to missing outcome data.
As there was very little information on the occurrence of non‐protocol interventions, implementation of the intervention, and non‐adherence to the assigned intervention, both the effect of assignment to the intervention and the effect of adhering to the intervention were judged at overall high risk of bias for all outcomes in this comparison.
Effects of interventions
See: Table 1; Table 2; Table 3; Table 4
We have graded the certainty of the evidence for those outcomes included in the summary of findings tables. For the definitions of these gradings, see Table 1; Table 2; Table 3; and Table 4.
1. Digital health technology for monitoring physical activity
1.1. Wearable fitness tracker plus personalized exercise prescription compared to personalized exercise prescription alone
Only one trial (40 adults with CF) assessed the effects of using a wearable fitness tracker integrated with a social media platform plus exercise prescription compared to exercise prescription alone for 12 months and presented change from baseline values for all reported outcomes (Bishay 2018; Table 1).
Primary outcomes
Physical activity
The trial did not report this outcome.
Self‐management behavior
The trial did not report this outcome.
Pulmonary exacerbations
The trial did not report this outcome.
Secondary outcomes
Usability of digital health technologies
The trial did not report this outcome.
Quality of life
The evidence is very uncertain about the effects of using a wearable fitness tracker integrated with a social media platform plus exercise prescription on QoL, compared to exercise prescription alone. The trial narratively reported no difference between groups in the mean QoL scores assessed using the CFQ‐R after one year of the intervention (Bishay 2018). The results of this trial were reported as a conference abstract and there was no further information available for analysis. We assessed the certainty of evidence as very low, due to serious imprecision and very serious risk of bias.
Lung function
The trial found that the provision of a wearable fitness tracker in addition to exercise prescription may make little or no difference to the change from baseline in lung function assessed by FEV1 % predicted compared to an exercise prescription alone at one year (MD 1.00 % predicted, 95% CI −15.46 to 17.46; 1 trial, 40 participants; very low‐certainty evidence; Analysis 1.1) (Bishay 2018). We downgraded the certainty of evidence due to serious imprecision and very serious risk of bias.
1.1. Analysis.

Comparison 1: Personalized exercise prescription plus wearable fitness tracker integrated with a social media platform compared to exercise prescription alone, Outcome 1: Lung function measured by the FEV1 (% predicted)
Other variables of lung function were not reported.
Muscle strength
The trial did not report this outcome.
Exercise capacity
Investigators measured exercise capacity using a GXT (performed on a bicycle which gives an accurate estimate of aerobic fitness level). We found that using a wearable fitness tracker with an exercise prescription may make little or no difference compared to an exercise prescription alone after one year of the intervention (MD −0.40, 95% CI −1.36 to 0.56; 1 trial, 40 participants; very low‐certainty evidence; Analysis 1.2). We downgraded the certainty of the evidence one level for serious imprecision and two levels for very serious risk of bias.
1.2. Analysis.

Comparison 1: Personalized exercise prescription plus wearable fitness tracker integrated with a social media platform compared to exercise prescription alone, Outcome 2: Exercise capacity measured by a submaximal graded exercise test
Physiologic parameters
Investigators reported no data on physiologic parameters.
Adverse events related to the intervention
The trial did not report this outcome.
1.2. Wearable fitness tracker plus text message for personalized feedback and goal setting compared to wearable fitness tracker alone
One trial (33 adults with CF) compared the effects of using a wearable fitness tracker integrated with an online monitoring system plus text message for personalized feedback and goal setting compared to wearable technology alone for three months (12 weeks) with a follow‐up at six months (24 weeks) (Curran 2022; Table 2). The trial presented change from baseline values for all outcomes reported.
Primary outcomes
Physical activity
We are very uncertain about the effect of using a wearable fitness tracker plus text message for personalized feedback and goal setting on physical activity compared to wearable technology alone. Receiving text messages with personalized feedback and goal setting may result in little to no difference on step count compared to not receiving them, both after three months of intervention (MD 1564.00 steps, 95% CI −1288.07 to 4416.07; 1 trial, 32 participants; very low‐certainty evidence; Analysis 2.1) and at six‐month follow‐up (MD 675.00 steps, 95% CI −2406.37 to 3756.37; 1 trial, 32 participants; very low‐certainty evidence; Analysis 2.1).
2.1. Analysis.

Comparison 2: Wearable fitness tracker (WFT) plus text message personalized feedback (TMPF) and goal setting (GS) compared to WFT alone, Outcome 1: Physical activity measured by step count
Receiving a wearable fitness tracker plus text message for personalized feedback and goal setting may result in little to no difference in the time spent walking compared to receiving a wearable fitness tracker alone both after three months (MD 578.54, 95% CI −264.22 to 1421.30; 1 trial, 32 participants; very low‐certainty evidence; Analysis 2.2) and at six‐month follow‐up (MD 181.50, 95% CI −805.21 to 1168.21; 1 trial, 32 participants; very low‐certainty evidence; Analysis 2.2). Similarly, receiving a wearable fitness tracker plus text message for personalized feedback and goal setting may result in little to no difference in the time spent in moderate physical activity compared to receiving a wearable fitness tracker alone both at three‐month (MD 262.50, 95% CI −494.75 to 1019.76; 1 trial, 32 participants; very low‐certainty evidence; Analysis 2.3) and six‐month follow‐up (MD −9.38, 95% CI −726.34 707.58; 1 trial, 32 participants; very low‐certainty evidence; Analysis 2.3).
2.2. Analysis.

Comparison 2: Wearable fitness tracker (WFT) plus text message personalized feedback (TMPF) and goal setting (GS) compared to WFT alone, Outcome 2: Physical activity measured by the IPAQ (walk)
2.3. Analysis.

Comparison 2: Wearable fitness tracker (WFT) plus text message personalized feedback (TMPF) and goal setting (GS) compared to WFT alone, Outcome 3: Physical activity measured by the IPAQ (moderate)
In addition, there may be no difference in receiving a wearable fitness tracker plus text message for personalized feedback and goal setting on the time spent in vigorous physical activity compared to receiving a wearable fitness tracker alone both at three‐month (MD −400.00, 95% CI −1906.72 to 1106.72; 1 trial, 32 participants; very low‐certainty evidence; Analysis 2.4) and six‐month follow‐up (MD −383.75, 95% CI −1705.78 to 938.28; 1 trial, 32 participants; very low‐certainty evidence; Analysis 2.4).
2.4. Analysis.

Comparison 2: Wearable fitness tracker (WFT) plus text message personalized feedback (TMPF) and goal setting (GS) compared to WFT alone, Outcome 4: Physical activity measured by the IPAQ (vigorous)
We downgraded the certainty of the evidence twice due to very serious imprecision and once due to serious risk of bias (Curran 2022).
Self‐management behavior
The trial did not report this outcome.
Pulmonary exacerbations
The authors reported there was no effect on exacerbation rates. The time point for this assessment was not provided, but the authors reported they would assess this outcome both at three and six months in trial protocol. There were no further data reported. This is very low‐certainty evidence; we downgraded twice due to very serious imprecision and once due to serious risk of bias (Curran 2022).
Secondary outcomes
Usability of digital health technologies
The trial did not report this outcome.
Quality of life
Using a wearable fitness tracker plus text message for personalized feedback and goal setting may result in little to no difference in QoL measured using the CFQ‐R (Health domain) compared to using a wearable fitness tracker alone, both after three months of intervention (MD 4.86, 95% CI −8.13 to 17.85; 1 trial, 32 participants; very low‐certainty evidence; Analysis 2.5) and at six‐month follow‐up (MD 1.74, 95% CI −8.83 to 12.31; 1 trial, 32 participants; very low‐certainty evidence; Analysis 2.5). The results for all other domains (Physical, Vitality, Emotion, Eat, Treatment, Health, Social, Body, Role, Weight, Respiratory, and Digestive) of the CFQ‐R were also not different between groups after three and six months. The certainty of evidence was very low due to very serious imprecision and serious risk of bias.
2.5. Analysis.

Comparison 2: Wearable fitness tracker (WFT) plus text message personalized feedback (TMPF) and goal setting (GS) compared to WFT alone, Outcome 5: Quality of life measured by the CFQ‐R (Health domain)
Lung function
Using a wearable fitness tracker plus text message for personalized feedback and goal setting may result in little to no difference on lung function measured by FEV1 compared to using a wearable fitness tracker alone, both after three months of intervention (MD −0.40 L/s, 95% CI −1.20 to 0.40; 1 trial, 32 participants; very low‐certainty evidence; Analysis 2.6) and at six‐month follow‐up (MD −0.34 L/s, 95% CI −1.16 to 0.48; 1 trial, 32 participants; very low‐certainty evidence; Analysis 2.6), but the evidence is very uncertain. According to the trial protocol, the authors planned to evaluate FVC, but these results were not reported. We downgraded the certainty of evidence due to very serious imprecision and serious risk of bias.
2.6. Analysis.

Comparison 2: Wearable fitness tracker (WFT) plus text message personalized feedback (TMPF) and goal setting (GS) compared to WFT alone, Outcome 6: Lung function measured by the FEV1 (L/s)
Muscle strength
We are very uncertain about the effects of using a wearable fitness tracker plus text message for personalized feedback and goal setting on muscle strength measured using hand dynamometry compared to using wearable fitness tracker alone, both after three months of intervention (MD −4.14, 95% CI −11.81 to 3.53; 1 trial, 32 participants; very low‐certainty evidence; Analysis 2.7), and at six‐month follow‐up (MD −8.88, 95% CI −17.01 to −0.75; 1 trial, 32 participants; very low‐certainty evidence; Analysis 2.7). We downgraded the certainty of evidence due to very serious imprecision and serious risk of bias.
2.7. Analysis.

Comparison 2: Wearable fitness tracker (WFT) plus text message personalized feedback (TMPF) and goal setting (GS) compared to WFT alone, Outcome 7: Muscle strength measured by hand dynamometry
Exercise capacity
Using a wearable fitness tracker plus text message for personalized feedback and goal setting may result in little to no difference on exercise capacity measured a cardiopulmonary exercise test (VO2peak) compared to using a wearable fitness tracker alone, both after three months of intervention (MD −2.97, 95% CI −9.18 to 3.24; 1 trial, 32 participants; very low‐certainty evidence; Analysis 2.8) and at six‐month follow‐up (MD −4.45, 95% CI −9.67 to 0.77; 1 trial, 32 participants; very low‐certainty evidence; Analysis 2.8). We downgraded the certainty of evidence due to very serious imprecision and serious risk of bias.
2.8. Analysis.

Comparison 2: Wearable fitness tracker (WFT) plus text message personalized feedback (TMPF) and goal setting (GS) compared to WFT alone, Outcome 8: Exercise capacity measured by a cardiopulmonary exercise test (VO2peak)
Physiologic parameters
Investigators did not report data on physiologic parameters.
Adverse events related to the intervention
The trial did not report this outcome.
1.3. Web‐based application to record, monitor, and set goals on physical activity plus usual care compared to usual care alone
One trial (107 adolescents and adults) investigated the effects of a 12‐week intervention with a web‐based application (ActivOnline) to record, monitor, and set goals on physical activity plus usual care compared to usual care alone during 12‐month follow‐up (Cox 2022; Table 3). Data were adjusted for baseline values and were reported as change from baseline; we reported the summary statistics directly from the paper.
Primary outcomes
Physical activity
Cox 2022 reported physical activity objectively measured via accelerometry (time spent in moderate‐to‐vigorous physical activity) and via a self‐reported measure (HAES).
Using a web‐based application to record, monitor, and set goals on physical activity plus usual care may result in little to no difference on time spent in moderate‐to‐vigorous physical activity, compared to usual care alone, both after three months of intervention (MD −14 minutes/day, 95% CI −45 to 16; 1 trial, 63 participants), and at six‐month follow‐up (MD −4 minutes/day, 95% CI −37 to 29; 1 trial, 63 participants).
Using a web‐based application to record, monitor, and set goals on physical activity plus usual care may result in little to no difference on total time spent in activity during weekdays, compared to usual care alone, both after three months of intervention (MD −0.8, 95% CI −2.6 to 0.9; 1 trial, 63 participants), and at six‐month follow‐up (MD −1.3, 95% CI −3.1 to 0.5; 1 trial, 63 participants).
Similarly, using a web‐based application to record, monitor, and set goals on physical activity plus usual care may result in little to no difference on time spent in activity during weekends, compared to usual care alone, both after three months of intervention (MD −0.2, 95% CI −2.3 to 2.0; 1 trial, 63 participants), and at six‐month follow‐up (MD −0.3, 95% CI −2.4 to 1.8; 1 trial, 63 participants).
The certainty of evidence was low due to serious imprecision and serious risk of bias.
Self‐management behavior
The trial did not report this outcome.
Pulmonary exacerbations
The trial reported results for pulmonary exacerbations, including the time to first hospital admission, by medical record review (Cox 2022). Using a web‐based application to record, monitor, and set goals on physical activity plus usual care may result in little to no difference on pulmonary exacerbations. During 12 months of follow‐up, investigators reported that 19 participants in the intervention group and 25 in the control group had at least one all‐cause hospital admission (RR 0.8, 95% CI 0.51 to 1.27). Additionally, 18 participants in the intervention group and 24 in the control group had at least one respiratory admission. We are unable to analyze these data in the review as the investigators did not report the number of participants assessed for this outcome.
The authors also reported there were no differences between groups on the median number of all‐cause hospitalizations per participant (intervention group 1 [IQR 0 to 3] hospitalizations versus control group 1 [IQR 0 to 2] hospitalizations; P = 1.0) or respiratory hospitalizations (intervention group 1 [IQR 0 to 3] hospitalizations versus control group 1 [IQR 0 to 2] hospitalizations; P = 0.6); nor for time to first admission (all‐cause or respiratory).
We assessed the certainty of evidence as low due to serious imprecision and serious risk of bias.
Secondary outcomes
Usability of digital health technologies
The trial did not report this outcome.
Quality of life
Using a web‐based application to record, monitor, and set goals on physical activity plus usual care may result in little to no difference to QoL, as measured using the CFQ‐R (Physical domain), both after three months (MD 0.9, 95% CI −11.4 to 13.2; 1 trial, 63 participants), and after six months (MD −10.1, 95% CI −22.7 to 2.5; 1 trial, 63 participants). It may also result in little to no difference in the CFQ‐R (Vitality domain), both after three months (MD −5.3, 95% CI −18.3 to 7.7; 1 trial, 63 participants), and after six months (MD −8.3, 95% CI −21.3 to 4.5; 1 trial, 63 participants); in the CFQ‐R (Treatment domain), both after three months (MD −2.3, 95% CI −11.3 to 6.7; 1 trial, 63 participants), and after six months (MD −7.3, 95% CI −16.5 to 1.9; 1 trial, 63 participants), and in the CFQ‐R (Respiratory domain), both after three months (MD −2.6, 95% CI −13.3 to 8.1; 1 trial, 63 participants), and after six months (MD −4.8, 95% CI −15.8 to 6.3; 1 trial, 63 participants).
We assessed the certainty of evidence as low due to serious imprecision and serious risk of bias.
Lung function
Using a web‐based application to record, monitor, and set goals on physical activity plus usual care may result in little to no difference on lung function, as measured using the FEV1 (liters), both after three months (MD 0.1 L, 95% CI −0.3 to 0.1; 1 trial, 63 participants), and after six months (MD 0.1 L, 95% CI −0.1 to 0.3; 1 trial, 63 participants). It may also result in little to no difference in the FEV1 (% predicted), both after three months (MD 0.3 % predicted, 95% CI −3.7 to 6.2; 1 trial, 63 participants), and after six months (MD −0.3 % predicted, 95% CI −5.2 to 4.6; 1 trial, 63 participants).
There were similar results for FVC (L) after three months (MD 0.2 L, 95% CI −0.2 to 0.6; 1 trial, 63 participants), and after six months (MD −0.1 L, 95% CI −0.5 to 0.3; 1 trial, 63 participants); and in FVC (% predicted), both after three months (MD 1.2 % predicted, 95% CI −4.3 to 6.7; 1 trial, 63 participants), and after six months (MD 1.1 % predicted, 95% CI −4.4 to 6.6; 1 trial, 63 participants).
We assessed the certainty of evidence as low due to serious imprecision and serious risk of bias.
Muscle strength
The trial did not report this outcome.
Exercise capacity
The trial assessed this outcome using the modified shuttle test (Cox 2022). Using a web‐based application to record, monitor, and set goals on physical activity plus usual care may result in little to no difference on exercise capacity after three months (MD −104.00, 95% CI −384.85 to 176.85; 1 trial, 26 participants).
We assessed the certainty of evidence as low due to serious imprecision and serious risk of bias.
Physiologic parameters
No data on physiologic parameters were reported for this comparison.
Adverse events related to the intervention
The trial did not report this outcome.
2. Digital health technology for delivering exercise programs
2.1. Web‐based versus face‐to‐face exercise delivery
One trial (51 children and adults) compared the effects of delivering an exercise program via the internet to delivering the program face‐to‐face for three months (Carr 2018; Table 4). The trial presented data for the change from baseline in the outcomes reported.
Primary outcomes
Physical activity
The evidence is very uncertain about the effects of web‐based compared to face‐to‐face exercise delivery on adherence to physical activity as assessed by the number of participants who completed all exercise sessions after three months of intervention (RR 0.92, 95% CI 0.69 to 1.23; 1 trial, 51 participants; very low‐certainty evidence; Analysis 3.1). We downgraded the evidence twice due to risk of bias and once due to imprecision.
3.1. Analysis.

Comparison 3: Web‐based versus face‐to‐face exercise delivery, Outcome 1: Adherence measured by the number of participants who completed all exercise sessions
Self‐management behavior
The trial did not report this outcome.
Pulmonary exacerbations
The trial did not report this outcome.
Secondary outcomes
Usability of digital health technologies
Carr 2018 reported usability qualitatively using interviews. The authors reported that most people found Skype convenient and easy to use, and that it reduced travel, family demands, and impacted on privacy. There were a few technical issues reported, including loss of internet access, information technology skills, and pedagogic difficulties with the technology. There were no further data available.
Quality of life
The evidence is very uncertain about the effects of web‐based compared to face‐to‐face exercise delivery on QoL measured using the CFQ‐R after three months of intervention. The authors reported there was no difference in change from baseline between groups in QoL, but the scores for each group before and after the delivery of the intervention were not provided. We graded the certainty of the evidence as very low after downgrading twice due to an overall high risk of bias and once due to imprecision (Carr 2018).
Lung function
The evidence is very uncertain about the effects of web‐based compared to face‐to‐face exercise delivery on lung function measured by the change from baseline in FEV1 and FVC after three months of the intervention. The authors reported that they found no difference between the groups for either measure, but provided no analyzable data. This evidence is very low‐certainty; we downgraded twice due to risk of bias and once due to imprecision.
Muscle strength
The trial did not report this outcome.
Exercise capacity
The trial did not report this outcome.
Physiologic parameters
The trial assessed oxygen saturation. The evidence is very uncertain about the effects on oxygen saturation of web‐based compared to face‐to‐face exercise delivery. The authors reported no differences between groups after three months of intervention. There was no additional information provided for further analysis. We graded the certainty of the evidence as very low after downgrading twice due to an overall high risk of bias and once due to imprecision (Carr 2018).
Adverse events related to the intervention
The trial did not report this outcome.
Discussion
Summary of main results
In this systematic review, we summarized the available evidence on the effects of digital health technology interventions for delivering and monitoring exercise programs in people with CF. We found only four RCTs with a small number of participants, which we judged to have an overall high risk of bias (Bishay 2018; Carr 2018; Cox 2022; Curran 2022). Furthermore, the trials used each digital health technology in combination with different interventions and for different purposes. Thus, we could not pool data in meta‐analyses. No trial reported on one of our primary outcomes, namely self‐management behavior, and none evaluated the outcomes in the long term (more than one year after the start of intervention). No trial reported a formal evaluation of adverse events, which makes conclusions difficult not only regarding the true efficacy, but also on the limitations of digital health technologies.
1. Digital health technologies for monitoring physical activity
Three trials assessed the effects of a digital health technology for monitoring physical activity in adults with CF (Bishay 2018; Cox 2022; Curran 2022). The results of one RCT suggested that there may be little to no difference between an exercise program plus the use of a wearable fitness tracker integrated with a social media platform compared with exercise prescription alone on QoL, lung function, and exercise capacity after one year of intervention, but the evidence is very uncertain (Bishay 2018). Similarly, results of one study, after three months of intervention and at six‐month follow‐up, suggested the evidence is very uncertain about the effects of receiving a wearable fitness tracker plus text message personalized feedback and goal setting on the step count, time spent walking, time spent in moderate physical activity, time spent in vigorous physical activity, exacerbation rates, QoL, lung function, muscle strength, and exercise capacity compared to not receiving them (Curran 2022). The results of the third study suggest that using a web‐based application to record, monitor, and set goals on physical activity plus usual care may result in little to no difference on time spent in moderate‐to‐vigorous physical activity (minutes/day), on total time spent in activity, pulmonary exacerbations, QoL, lung function, and exercise capacity compared to usual care alone, both after three months of intervention and at six‐month follow‐up (Cox 2022).
2. Digital health technologies for delivering exercise programs
One trial assessed the use of a digital health technology for delivering exercise programs in children and adults with CF (Carr 2018). This RCT addressed one of our primary outcomes, namely adherence to exercise, reporting no differences between groups after three months of intervention. Other reported outcomes included usability, QoL, lung function, and oxygen saturation; however, as the publications of this trial focused on feasibility and qualitative data from interviews, there were no analyzable data for the outcomes QoL, lung function, and oxygen saturation. For these outcomes, the authors only reported that no differences were found between the participants who were prescribed the exercise program via Skype and those to whom it was delivered on a face‐to‐face basis, but the evidence is very uncertain (Carr 2018). Regarding usability, the results from interviews conducted in this trial suggest that most people found Skype convenient and easy to use, but it had a few technical issues, including loss of internet access (access to internet was required for the intervention delivered via Skype), information technology skills, and pedagogic difficulties with the technology (Carr 2018). Of note, these results were not specified by age group, and it would be useful to know if different age groups experience the same issues with usability of digital health technology. Furthermore, future trials should include well‐reported standardized or validated scales for the assessment of outcomes such as usability. The trial did not report on our primary outcomes of self‐management behavior and pulmonary exacerbations, or the secondary outcomes of muscle strength, exercise capacity, and adverse events.
Overall completeness and applicability of evidence
Current evidence on the effects of digital health technologies for delivering and monitoring exercise programs in people with CF is scarce. Furthermore, the trials included in this review only had a small number of participants and assessed different types of digital health technologies, which were used for different purposes. Therefore, we could not perform meta‐analyses and the precision of effect estimates was jeopardized.
Due to several methodologic limitations and the insufficient reporting of outcome results in the included trials, several questions persist, and the results of this review are, therefore, of low to very‐low certainty. As the included trials only evaluated the use of a web‐based intervention for delivering an exercise program, a wearable tracker for monitoring physical activity, a web‐based application to record, monitor, and set goals on physical activity, and the use of text message personalized feedback and goal setting, the generalizability of our results is mostly limited to these digital health technologies. The results of six ongoing RCTs identified through our searches may clarify the effects of different types of digital health technologies for delivering and monitoring exercise programs in people with CF.
The review does not take into account the potential lack of technology and possible digital poverty that may exist or even a geographical lack of good internet access.
Quality of the evidence
The review included four RCTs with 231 participants. There were important methodologic concerns in these RCTs, including insufficient information on the randomization process, blinding of outcome assessors, balance of non‐protocol interventions across groups, and on whether the analyses were performed correcting for bias due to missing outcome data. Furthermore, these trials had a small number of participants and in most cases, data were insufficiently reported or reported in ways that did not allow extraction or further analysis on the precision of effect estimates. These limitations on risk of bias and precision of effect estimates resulted in an overall judgment of low to very low certainty on the body of available evidence, and no robust conclusions could be drawn.
Potential biases in the review process
We performed this review according to our prespecified protocol, which was planned in accordance with the recommendations of the Cochrane Handbook for Systematic Reviews of Interventions (Pereira Nunes Pinto 2021). We made efforts to identify and assess all relevant RCTs, regardless of language or publication status, by performing sensitive searches in the most important databases, clinical trial registers, and handsearching. We also contacted trial authors to request additional information (Bishay 2018; Carr 2018); however, no response has been received, and we assessed available evidence based on the content of the published articles and abstracts relating to the two trials. Notwithstanding, it has not been possible to determine whether these RCTs have assessed other outcomes and, therefore, the body of the available evidence may be larger than the evidence presented in our systematic review. As we found RCTs that were not sufficiently homogeneous in terms of interventions and comparisons, only a narrative synthesis was possible. Nevertheless, this scientifically rigorous systematic review exposes gaps in the literature and provides critical data that may help support further trials.
Agreements and disagreements with other studies or reviews
We are unaware of any published systematic reviews that have evaluated the effects of digital health technologies for monitoring or delivering exercise programs in people with CF. Exercise programs are already part of the regular management of CF (Bradley 2015), and one previous Cochrane Review found evidence that physical activity interventions for six months and longer likely improve exercise capacity when compared to no training, but showed little or no effect on lung function and health‐related QoL in people with CF, with rare adverse events (Radtke 2022). Radtke 2022 did not include studies using digital health technologies for delivering or monitoring exercise programs and our findings should be interpreted in addition to their findings, while prescribing exercise programs as part of the outpatient care in people with CF.
Authors' conclusions
Implications for practice.
The evidence is very uncertain about the effects of an exercise program plus the use of a wearable fitness tracker integrated with a social media platform compared with exercise prescription alone and on the effects of receiving a wearable fitness tracker plus text message personalized feedback and goal setting, compared to a wearable fitness tracker alone.
Low‐certainty evidence suggest that using a web‐based application to record, monitor, and set goals on physical activity plus usual care may result in little to no difference in the time spent in moderate‐to‐vigorous physical activity, total time spent in activity, pulmonary exacerbations, quality of life (QoL), lung function, and exercise capacity, compared to usual care alone. Regarding the use of digital health technologies for delivering exercise programs in cystic fibrosis (CF), the evidence is very uncertain about the effects of using a wearable fitness tracker plus personalized exercise prescription compared to personalized exercise prescription alone.
There is no information on the effectiveness of other modes of digital health technologies for monitoring physical activity or delivering exercise programs in people with CF, on adverse events related to the use of digital health technologies either for delivering or monitoring exercise programs in CF, and on their long‐term effects (more than one year).
Implications for research.
As this review included only four small randomized controlled trials (RCTs) using digital health technologies for different purposes (with an overall high risk of bias), no robust conclusions can be drawn. Further high‐quality RCTs evaluating the effects of digital health technologies are needed; outcome assessors should be blinded and trials should report on clinically important outcome measures such as physical activity participation and intensity, self‐management behavior, and the occurrence of pulmonary exacerbations in the long term. If these trials include individuals of different age groups, the stratification of randomization process by age and the reporting of results separately may help understand important information on which group(s) would benefit from digital health technology interventions. Furthermore, the inclusion of standardized or validated scales for the assessment of outcomes such as usability, and the clearer report of results as stated in the protocols registers is necessary. The results of six ongoing RCTs identified through our searches may clarify the effects of different modes of digital health technologies for delivering and monitoring exercise programs in people with CF.
History
Protocol first published: Issue 5, 2021
Risk of bias
Risk of bias for analysis 2.3 Physical activity measured by the IPAQ (moderate).
| Study | Bias | |||||||||||
| Randomisation process | Deviations from intended interventions | Missing outcome data | Measurement of the outcome | Selection of the reported results | Overall | |||||||
| Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | |
| Subgroup 2.3.1 At 3 months | ||||||||||||
| Curran 2022 | Low risk of bias | Details on randomization process were provided and seem adequate: "To reduce the possibility of selection bias, a researcher independent of the recruitment process (MC) completed the first random allocation using a sealed opaque envelope. Following this a minimization randomization procedure was conducted based on lung function, where FEV1 > 80% predicted was classified as having mild lung disease, 50–79% predicted classified as moderate lung disease, 30–49% as severe lung disease and < 30% indicated very severe disease." The authors report no important differences between groups at baseline. "There were no significant differences in any baseline characteristics between the groups, apart from self‐reported shortness of breath (SOB) whereby the INT group had significantly higher SOB than the AC group." | High risk of bias | Although no information is provided on participants, carers and people delivering interventions blinding, due to the nature of intervention, participants, carers and people delivering interventions could not be blind to treatment allocation. No information is provided on whether there were important non‐protocol interventions balanced across intervention groups No information is provided on whether there were failures in implementing the intervention that could have affected the outcome No information is provided on whether there was non‐adherence to the assigned intervention regimen that could have affected participants' outcomes No specific information was provided on whether an appropriate analysis was used to estimate the effect of adhering to the intervention No information was provided on whether there was a potential for a substantial impact (on the result) of the failure to analyze participants in the group they were randomized | Low risk of bias | The authors report one participant withdrew from the study for personal reasons. | Low risk of bias | The measurement instrument is adequate for evaluating this outcome The same measurement methods have been used in both study arms. The authors report that outcome assessors could not be blind. "Assessors were not blinded as the intervention involved the CF physiotherapists for the delivery of weekly text messages, who also assisted with repeating objective outcome measures" Assessment of the outcome could have been influenced by knowledge of intervention received. However, there was no information on whether this was likely to occur. | Low risk of bias | This result was analyzed in accordance with a pre‐specified analysis plan that was finalized before unblinded outcome data were available for analysis. There were no multiple eligible outcome measurements available for this outcome. There were no multiple eligible analyses available for this outcome. | High risk of bias | We considered this trial to have an overall risk of bias (considering the least favorable assessment across the domains of bias ‐ bias due to deviations from intended interventions and in the measurement of the outcome) |
| Subgroup 2.3.2 At 6 months | ||||||||||||
| Curran 2022 | Low risk of bias | Details on randomization process were provided and seem adequate: "To reduce the possibility of selection bias, a researcher independent of the recruitment process (MC) completed the first random allocation using a sealed opaque envelope. Following this a minimization randomization procedure was conducted based on lung function, where FEV1 > 80% predicted was classified as having mild lung disease, 50–79% predicted classified as moderate lung disease, 30–49% as severe lung disease and < 30% indicated very severe disease." The authors report no important differences between groups at baseline. "There were no significant differences in any baseline characteristics between the groups, apart from self‐reported shortness of breath (SOB) whereby the INT group had significantly higher SOB than the AC group." | High risk of bias | Although no information is provided on participants, carers and people delivering interventions blinding, due to the nature of intervention, participants, carers and people delivering interventions could not be blind to treatment allocation. No information is provided on whether there were important non‐protocol interventions balanced across intervention groups No information is provided on whether there were failures in implementing the intervention that could have affected the outcome No information is provided on whether there was non‐adherence to the assigned intervention regimen that could have affected participants' outcomes No specific information was provided on whether an appropriate analysis was used to estimate the effect of adhering to the intervention No information was provided on whether there was a potential for a substantial impact (on the result) of the failure to analyze participants in the group they were randomized | Low risk of bias | The authors report one participant withdrew from the study for personal reasons. | Low risk of bias | The measurement instrument is adequate for evaluating this outcome The same measurement methods have been used in both study arms. The authors report that outcome assessors could not be blind. "Assessors were not blinded as the intervention involved the CF physiotherapists for the delivery of weekly text messages, who also assisted with repeating objective outcome measures" Assessment of the outcome could have been influenced by knowledge of intervention received. However, there was no information on whether this was likely to occur. | Low risk of bias | This result was analyzed in accordance with a pre‐specified analysis plan that was finalized before unblinded outcome data were available for analysis. There were no multiple eligible outcome measurements available for this outcome. There were no multiple eligible analyses available for this outcome. | High risk of bias | We considered this trial to have an overall risk of bias (considering the least favorable assessment across the domains of bias ‐ bias due to deviations from intended interventions and in the measurement of the outcome) |
Risk of bias for analysis 2.4 Physical activity measured by the IPAQ (vigorous).
| Study | Bias | |||||||||||
| Randomisation process | Deviations from intended interventions | Missing outcome data | Measurement of the outcome | Selection of the reported results | Overall | |||||||
| Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | |
| Subgroup 2.4.1 At 3 months | ||||||||||||
| Curran 2022 | Low risk of bias | Details on randomization process were provided and seem adequate: "To reduce the possibility of selection bias, a researcher independent of the recruitment process (MC) completed the first random allocation using a sealed opaque envelope. Following this a minimization randomization procedure was conducted based on lung function, where FEV1 > 80% predicted was classified as having mild lung disease, 50–79% predicted classified as moderate lung disease, 30–49% as severe lung disease and < 30% indicated very severe disease." The authors report no important differences between groups at baseline. "There were no significant differences in any baseline characteristics between the groups, apart from self‐reported shortness of breath (SOB) whereby the INT group had significantly higher SOB than the AC group." | High risk of bias | Although no information is provided on participants, carers and people delivering interventions blinding, due to the nature of intervention, participants, carers and people delivering interventions could not be blind to treatment allocation. No information is provided on whether there were important non‐protocol interventions balanced across intervention groups No information is provided on whether there were failures in implementing the intervention that could have affected the outcome No information is provided on whether there was non‐adherence to the assigned intervention regimen that could have affected participants’ outcomes No specific information was provided on whether an appropriate analysis was used to estimate the effect of adhering to the intervention No information was provided on whether there was a potential for a substantial impact (on the result) of the failure to analyze participants in the group they were randomized | Low risk of bias | The authors report one participant withdrew from the study for personal reasons. | High risk of bias | The measurement instrument is adequate for evaluating this outcome The same measurement methods have been used in both study arms. The authors report that outcome assessors could not be blind. "Assessors were not blinded as the intervention involved the CF physiotherapists for the delivery of weekly text messages, who also assisted with repeating objective outcome measures" Assessment of the outcome could have been influenced by knowledge of intervention received. However, there was no information on whether this was likely to occur. | Low risk of bias | This result was analyzed in accordance with a prespecified analysis plan that was finalized before unblinded outcome data were available for analysis. There were no multiple eligible outcome measurements available for this outcome. There were no multiple eligible analyses available for this outcome. | High risk of bias | We considered this trial to have an overall risk of bias (considering the least favorable assessment across the domains of bias ‐ bias due to deviations from intended interventions and in the measurement of the outcome) |
| Subgroup 2.4.2 At 6 months | ||||||||||||
| Curran 2022 | Low risk of bias | Details on randomization process were provided and seem adequate: "To reduce the possibility of selection bias, a researcher independent of the recruitment process (MC) completed the first random allocation using a sealed opaque envelope. Following this a minimization randomization procedure was conducted based on lung function, where FEV1 > 80% predicted was classified as having mild lung disease, 50–79% predicted classified as moderate lung disease, 30–49% as severe lung disease and < 30% indicated very severe disease." The authors report no important differences between groups at baseline. "There were no significant differences in any baseline characteristics between the groups, apart from self‐reported shortness of breath (SOB) whereby the INT group had significantly higher SOB than the AC group." | High risk of bias | Although no information is provided on participants, carers and people delivering interventions blinding, due to the nature of intervention, participants, carers and people delivering interventions could not be blind to treatment allocation. No information is provided on whether there were important non‐protocol interventions balanced across intervention groups No information is provided on whether there were failures in implementing the intervention that could have affected the outcome No information is provided on whether there was non‐adherence to the assigned intervention regimen that could have affected participants’ outcomes No specific information was provided on whether an appropriate analysis was used to estimate the effect of adhering to the intervention No information was provided on whether there was a potential for a substantial impact (on the result) of the failure to analyze participants in the group they were randomized | Low risk of bias | The authors report one participant withdrew from the study for personal reasons. | High risk of bias | The measurement instrument is adequate for evaluating this outcome The same measurement methods have been used in both study arms. The authors report that outcome assessors could not be blind. "Assessors were not blinded as the intervention involved the CF physiotherapists for the delivery of weekly text messages, who also assisted with repeating objective outcome measures" Assessment of the outcome could have been influenced by knowledge of intervention received. However, there was no information on whether this was likely to occur. | Low risk of bias | This result was analyzed in accordance with a prespecified analysis plan that was finalized before unblinded outcome data were available for analysis. There were no multiple eligible outcome measurements available for this outcome. There were no multiple eligible analyses available for this outcome. | High risk of bias | We considered this trial to have an overall risk of bias (considering the least favorable assessment across the domains of bias ‐ bias due to deviations from intended interventions and in the measurement of the outcome) |
Risk of bias for analysis 2.5 Quality of life measured by the CFQ‐R (Health domain).
| Study | Bias | |||||||||||
| Randomisation process | Deviations from intended interventions | Missing outcome data | Measurement of the outcome | Selection of the reported results | Overall | |||||||
| Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | |
| Subgroup 2.5.1 At 3 months | ||||||||||||
| Curran 2022 | Low risk of bias | Details on randomization process were provided and seem adequate: "To reduce the possibility of selection bias, a researcher independent of the recruitment process (MC) completed the first random allocation using a sealed opaque envelope. Following this a minimization randomization procedure was conducted based on lung function, where FEV1 > 80% predicted was classified as having mild lung disease, 50–79% predicted classified as moderate lung disease, 30–49% as severe lung disease and < 30% indicated very severe disease." The authors report no important differences between groups at baseline. "There were no significant differences in any baseline characteristics between the groups, apart from self‐reported shortness of breath (SOB) whereby the INT group had significantly higher SOB than the AC group." | High risk of bias | Although no information is provided on participants, carers and people delivering interventions blinding, due to the nature of intervention, participants, carers and people delivering interventions could not be blind to treatment allocation. No information is provided on whether there were important non‐protocol interventions balanced across intervention groups No information is provided on whether there were failures in implementing the intervention that could have affected the outcome No information is provided on whether there was non‐adherence to the assigned intervention regimen that could have affected participants’ outcomes No specific information was provided on whether an appropriate analysis was used to estimate the effect of adhering to the intervention No information was provided on whether there was a potential for a substantial impact (on the result) of the failure to analyze participants in the group they were randomized | Low risk of bias | The authors report one patient withdrew from the study for personal reasons. | High risk of bias | The measurement instrument is adequate for evaluating this outcome The same measurement methods have been used in both study arms. The authors report that outcome assessors could not be blind. "Assessors were not blinded as the intervention involved the CF physiotherapists for the delivery of weekly text messages, who also assisted with repeating objective outcome measures" Assessment of the outcome could have been influenced by knowledge of intervention received. However, there was no information on whether this was likely to occur. | Low risk of bias | This result was analyzed in accordance with a pre‐specified analysis plan that was finalized before unblinded outcome data were available for analysis. There were no multiple eligible outcome measurements available for this outcome. There were no multiple eligible analyses available for this outcome. | High risk of bias | We considered this trial to have an overall risk of bias (considering the least favorable assessment across the domains of bias ‐ bias due to deviations from intended interventions and in the measurement of the outcome) |
| Subgroup 2.5.2 At 6 months | ||||||||||||
| Curran 2022 | Low risk of bias | Details on randomization process were provided and seem adequate: "To reduce the possibility of selection bias, a researcher independent of the recruitment process (MC) completed the first random allocation using a sealed opaque envelope. Following this a minimization randomization procedure was conducted based on lung function, where FEV1 > 80% predicted was classified as having mild lung disease, 50–79% predicted classified as moderate lung disease, 30–49% as severe lung disease and < 30% indicated very severe disease." The authors report no important differences between groups at baseline. "There were no significant differences in any baseline characteristics between the groups, apart from self‐reported shortness of breath (SOB) whereby the INT group had significantly higher SOB than the AC group." | High risk of bias | Although no information is provided on participants, carers and people delivering interventions blinding, due to the nature of intervention, participants, carers and people delivering interventions could not be blind to treatment allocation. No information is provided on whether there were important non‐protocol interventions balanced across intervention groups No information is provided on whether there were failures in implementing the intervention that could have affected the outcome No information is provided on whether there was non‐adherence to the assigned intervention regimen that could have affected participants’ outcomes No specific information was provided on whether an appropriate analysis was used to estimate the effect of adhering to the intervention No information was provided on whether there was a potential for a substantial impact (on the result) of the failure to analyze participants in the group they were randomized | Low risk of bias | The authors report one patient withdrew from the study for personal reasons. | High risk of bias | The measurement instrument is adequate for evaluating this outcome The same measurement methods have been used in both study arms. The authors report that outcome assessors could not be blind. "Assessors were not blinded as the intervention involved the CF physiotherapists for the delivery of weekly text messages, who also assisted with repeating objective outcome measures" Assessment of the outcome could have been influenced by knowledge of intervention received. However, there was no information on whether this was likely to occur. | Low risk of bias | This result was analyzed in accordance with a pre‐specified analysis plan that was finalized before unblinded outcome data were available for analysis. There were no multiple eligible outcome measurements available for this outcome. There were no multiple eligible analyses available for this outcome. | High risk of bias | We considered this trial to have an overall risk of bias (considering the least favorable assessment across the domains of bias ‐ bias due to deviations from intended interventions and in the measurement of the outcome) |
Risk of bias for analysis 2.6 Lung function measured by the FEV1 (L/s).
| Study | Bias | |||||||||||
| Randomisation process | Deviations from intended interventions | Missing outcome data | Measurement of the outcome | Selection of the reported results | Overall | |||||||
| Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | |
| Subgroup 2.6.1 At 3 months | ||||||||||||
| Curran 2022 | Low risk of bias | Details on randomization process were provided and seem adequate: "To reduce the possibility of selection bias, a researcher independent of the recruitment process (MC) completed the first random allocation using a sealed opaque envelope. Following this a minimization randomization procedure was conducted based on lung function, where FEV1 > 80% predicted was classified as having mild lung disease, 50–79% predicted classified as moderate lung disease, 30–49% as severe lung disease and < 30% indicated very severe disease." The authors report no important differences between groups at baseline. "There were no significant differences in any baseline characteristics between the groups, apart from self‐reported shortness of breath (SOB) whereby the INT group had significantly higher SOB than the AC group." | High risk of bias | Although no information is provided on participants, carers and people delivering interventions blinding, due to the nature of intervention, participants, carers and people delivering interventions could not be blind to treatment allocation. No information is provided on whether there were important non‐protocol interventions balanced across intervention groups No information is provided on whether there were failures in implementing the intervention that could have affected the outcome No information is provided on whether there was non‐adherence to the assigned intervention regimen that could have affected participants’ outcomes No specific information was provided on whether an appropriate analysis was used to estimate the effect of adhering to the intervention No information was provided on whether there was a potential for a substantial impact (on the result) of the failure to analyze participants in the group they were randomized | Low risk of bias | The authors report one participant withdrew from the study for personal reasons. | High risk of bias | The measurement instrument is adequate for evaluating this outcome The same measurement methods have been used in both study arms. The authors report that outcome assessors could not be blind. "Assessors were not blinded as the intervention involved the CF physiotherapists for the delivery of weekly text messages, who also assisted with repeating objective outcome measures" Assessment of the outcome could have been influenced by knowledge of intervention received. However, there was no information on whether this was likely to occur. | Low risk of bias | This result was analyzed in accordance with a pre‐specified analysis plan that was finalized before unblinded outcome data were available for analysis. There were no multiple eligible outcome measurements available for this outcome. There were no multiple eligible analyses available for this outcome. | High risk of bias | We considered this trial to have an overall risk of bias (considering the least favorable assessment across the domains of bias ‐ bias due to deviations from intended interventions and in the measurement of the outcome) |
| Subgroup 2.6.2 At 6 months | ||||||||||||
| Curran 2022 | Low risk of bias | Details on randomization process were provided and seem adequate: "To reduce the possibility of selection bias, a researcher independent of the recruitment process (MC) completed the first random allocation using a sealed opaque envelope. Following this a minimization randomization procedure was conducted based on lung function, where FEV1 > 80% predicted was classified as having mild lung disease, 50–79% predicted classified as moderate lung disease, 30–49% as severe lung disease and < 30% indicated very severe disease." The authors report no important differences between groups at baseline. "There were no significant differences in any baseline characteristics between the groups, apart from self‐reported shortness of breath (SOB) whereby the INT group had significantly higher SOB than the AC group." | High risk of bias | Although no information is provided on participants, carers and people delivering interventions blinding, due to the nature of intervention, participants, carers and people delivering interventions could not be blind to treatment allocation. No information is provided on whether there were important non‐protocol interventions balanced across intervention groups No information is provided on whether there were failures in implementing the intervention that could have affected the outcome No information is provided on whether there was non‐adherence to the assigned intervention regimen that could have affected participants’ outcomes No specific information was provided on whether an appropriate analysis was used to estimate the effect of adhering to the intervention No information was provided on whether there was a potential for a substantial impact (on the result) of the failure to analyze participants in the group they were randomized | Low risk of bias | The authors report one participant withdrew from the study for personal reasons. | High risk of bias | The measurement instrument is adequate for evaluating this outcome The same measurement methods have been used in both study arms. The authors report that outcome assessors could not be blind. "Assessors were not blinded as the intervention involved the CF physiotherapists for the delivery of weekly text messages, who also assisted with repeating objective outcome measures" Assessment of the outcome could have been influenced by knowledge of intervention received. However, there was no information on whether this was likely to occur. | Low risk of bias | This result was analyzed in accordance with a pre‐specified analysis plan that was finalized before unblinded outcome data were available for analysis. There were no multiple eligible outcome measurements available for this outcome. There were no multiple eligible analyses available for this outcome. | High risk of bias | We considered this trial to have an overall risk of bias (considering the least favorable assessment across the domains of bias ‐ bias due to deviations from intended interventions and in the measurement of the outcome) |
Risk of bias for analysis 2.7 Muscle strength measured by hand dynamometry.
| Study | Bias | |||||||||||
| Randomisation process | Deviations from intended interventions | Missing outcome data | Measurement of the outcome | Selection of the reported results | Overall | |||||||
| Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | |
| Subgroup 2.7.1 At 3 months | ||||||||||||
| Curran 2022 | Low risk of bias | Details on randomization process were provided and seem adequate: "To reduce the possibility of selection bias, a researcher independent of the recruitment process (MC) completed the first random allocation using a sealed opaque envelope. Following this a minimization randomization procedure was conducted based on lung function, where FEV1 > 80% predicted was classified as having mild lung disease, 50–79% predicted classified as moderate lung disease, 30–49% as severe lung disease and < 30% indicated very severe disease." The authors report no important differences between groups at baseline. "There were no significant differences in any baseline characteristics between the groups, apart from self‐reported shortness of breath (SOB) whereby the INT group had significantly higher SOB than the AC group." | High risk of bias | Although no information is provided on participants, carers and people delivering interventions blinding, due to the nature of intervention, participants, carers and people delivering interventions could not be blind to treatment allocation. No information is provided on whether there were important non‐protocol interventions balanced across intervention groups No information is provided on whether there were failures in implementing the intervention that could have affected the outcome No information is provided on whether there was non‐adherence to the assigned intervention regimen that could have affected participants’ outcomes No specific information was provided on whether an appropriate analysis was used to estimate the effect of adhering to the intervention No information was provided on whether there was a potential for a substantial impact (on the result) of the failure to analyze participants in the group they were randomized | Low risk of bias | The authors report one participant withdrew from the study for personal reasons. | High risk of bias | The measurement instrument is adequate for evaluating this outcome The same measurement methods have been used in both study arms. The authors report that outcome assessors could not be blind. "Assessors were not blinded as the intervention involved the CF physiotherapists for the delivery of weekly text messages, who also assisted with repeating objective outcome measures" Assessment of the outcome could have been influenced by knowledge of intervention received. However, there was no information on whether this was likely to occur. | Low risk of bias | This result was analyzed in accordance with a pre‐specified analysis plan that was finalized before unblinded outcome data were available for analysis. There were no multiple eligible outcome measurements available for this outcome. There were no multiple eligible analyses available for this outcome. | High risk of bias | We considered this trial to have an overall risk of bias (considering the least favorable assessment across the domains of bias ‐ bias due to deviations from intended interventions and in the measurement of the outcome) |
| Subgroup 2.7.2 At 6 months | ||||||||||||
| Curran 2022 | Low risk of bias | Details on randomization process were provided and seem adequate: "To reduce the possibility of selection bias, a researcher independent of the recruitment process (MC) completed the first random allocation using a sealed opaque envelope. Following this a minimization randomization procedure was conducted based on lung function, where FEV1 > 80% predicted was classified as having mild lung disease, 50–79% predicted classified as moderate lung disease, 30–49% as severe lung disease and < 30% indicated very severe disease." The authors report no important differences between groups at baseline. "There were no significant differences in any baseline characteristics between the groups, apart from self‐reported shortness of breath (SOB) whereby the INT group had significantly higher SOB than the AC group." | High risk of bias | Although no information is provided on participants, carers and people delivering interventions blinding, due to the nature of intervention, participants, carers and people delivering interventions could not be blind to treatment allocation. No information is provided on whether there were important non‐protocol interventions balanced across intervention groups No information is provided on whether there were failures in implementing the intervention that could have affected the outcome No information is provided on whether there was non‐adherence to the assigned intervention regimen that could have affected participants’ outcomes No specific information was provided on whether an appropriate analysis was used to estimate the effect of adhering to the intervention No information was provided on whether there was a potential for a substantial impact (on the result) of the failure to analyze participants in the group they were randomized | Low risk of bias | The authors report one participant withdrew from the study for personal reasons. | High risk of bias | The measurement instrument is adequate for evaluating this outcome The same measurement methods have been used in both study arms. The authors report that outcome assessors could not be blind. "Assessors were not blinded as the intervention involved the CF physiotherapists for the delivery of weekly text messages, who also assisted with repeating objective outcome measures" Assessment of the outcome could have been influenced by knowledge of intervention received. However, there was no information on whether this was likely to occur. | Low risk of bias | This result was analyzed in accordance with a pre‐specified analysis plan that was finalized before unblinded outcome data were available for analysis. There were no multiple eligible outcome measurements available for this outcome. There were no multiple eligible analyses available for this outcome. | High risk of bias | We considered this trial to have an overall risk of bias (considering the least favorable assessment across the domains of bias ‐ bias due to deviations from intended interventions and in the measurement of the outcome) |
Risk of bias for analysis 2.8 Exercise capacity measured by a cardiopulmonary exercise test (VO2peak).
| Study | Bias | |||||||||||
| Randomisation process | Deviations from intended interventions | Missing outcome data | Measurement of the outcome | Selection of the reported results | Overall | |||||||
| Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | |
| Subgroup 2.8.1 At 3 months | ||||||||||||
| Curran 2022 | Low risk of bias | Details on randomization process were provided and seem adequate: "To reduce the possibility of selection bias, a researcher independent of the recruitment process (MC) completed the first random allocation using a sealed opaque envelope. Following this a minimization randomization procedure was conducted based on lung function, where FEV1 > 80% predicted was classified as having mild lung disease, 50–79% predicted classified as moderate lung disease, 30–49% as severe lung disease and < 30% indicated very severe disease." The authors report no important differences between groups at baseline. "There were no significant differences in any baseline characteristics between the groups, apart from self‐reported shortness of breath (SOB) whereby the INT group had significantly higher SOB than the AC group." | High risk of bias | Although no information is provided on participants, carers and people delivering interventions blinding, due to the nature of intervention, participants, carers and people delivering interventions could not be blind to treatment allocation. No information is provided on whether there were important non‐protocol interventions balanced across intervention groups No information is provided on whether there were failures in implementing the intervention that could have affected the outcome No information is provided on whether there was non‐adherence to the assigned intervention regimen that could have affected participants’ outcomes No specific information was provided on whether an appropriate analysis was used to estimate the effect of adhering to the intervention No information was provided on whether there was a potential for a substantial impact (on the result) of the failure to analyze participants in the group they were randomized | Low risk of bias | The authors report one participant withdrew from the study for personal reasons. | High risk of bias | The measurement instrument is adequate for evaluating this outcome The same measurement methods have been used in both study arms. The authors report that outcome assessors could not be blind. "Assessors were not blinded as the intervention involved the CF physiotherapists for the delivery of weekly text messages, who also assisted with repeating objective outcome measures" Assessment of the outcome could have been influenced by knowledge of intervention received. However, there was no information on whether this was likely to occur. | Low risk of bias | This result was analyzed in accordance with a pre‐specified analysis plan that was finalized before unblinded outcome data were available for analysis. There were no multiple eligible outcome measurements available for this outcome. There were no multiple eligible analyses available for this outcome. | High risk of bias | We considered this trial to have an overall risk of bias (considering the least favorable assessment across the domains of bias ‐ bias due to deviations from intended interventions and in the measurement of the outcome) |
| Subgroup 2.8.2 At 6 months | ||||||||||||
| Curran 2022 | Low risk of bias | Details on randomization process were provided and seem adequate: "To reduce the possibility of selection bias, a researcher independent of the recruitment process (MC) completed the first random allocation using a sealed opaque envelope. Following this a minimization randomization procedure was conducted based on lung function, where FEV1 > 80% predicted was classified as having mild lung disease, 50–79% predicted classified as moderate lung disease, 30–49% as severe lung disease and < 30% indicated very severe disease." The authors report no important differences between groups at baseline. "There were no significant differences in any baseline characteristics between the groups, apart from self‐reported shortness of breath (SOB) whereby the INT group had significantly higher SOB than the AC group." | High risk of bias | Although no information is provided on participants, carers and people delivering interventions blinding, due to the nature of intervention, participants, carers and people delivering interventions could not be blind to treatment allocation. No information is provided on whether there were important non‐protocol interventions balanced across intervention groups No information is provided on whether there were failures in implementing the intervention that could have affected the outcome No information is provided on whether there was non‐adherence to the assigned intervention regimen that could have affected participants’ outcomes No specific information was provided on whether an appropriate analysis was used to estimate the effect of adhering to the intervention No information was provided on whether there was a potential for a substantial impact (on the result) of the failure to analyze participants in the group they were randomized | Low risk of bias | The authors report one participant withdrew from the study for personal reasons. | High risk of bias | The measurement instrument is adequate for evaluating this outcome The same measurement methods have been used in both study arms. The authors report that outcome assessors could not be blind. "Assessors were not blinded as the intervention involved the CF physiotherapists for the delivery of weekly text messages, who also assisted with repeating objective outcome measures" Assessment of the outcome could have been influenced by knowledge of intervention received. However, there was no information on whether this was likely to occur. | Low risk of bias | This result was analyzed in accordance with a pre‐specified analysis plan that was finalized before unblinded outcome data were available for analysis. There were no multiple eligible outcome measurements available for this outcome. There were no multiple eligible analyses available for this outcome. | High risk of bias | We considered this trial to have an overall risk of bias (considering the least favorable assessment across the domains of bias ‐ bias due to deviations from intended interventions and in the measurement of the outcome) |
Acknowledgements
We sincerely thank Mrs Nikki Jahnke, the Managing Editor, and the Cochrane Cystic Fibrosis and Genetic Disorders Editorial Group for her assistance in the development of the protocol for this review and the review.
We would like to thank the following people for commenting on the draft of the protocol for this review: Narelle Cox, Zoe Saynor, Amy MacDougall, Kerry Dwan, Sophie Lewis, and Natalie Hall. We would additionally like to thank the following people who commented on the draft of the full review: Narelle Cox, Zoe Saynor, Sanja Stanojevic, Kerry Dwan, Zoe Ellliot, and Natalie Hall.
This project was supported by the National Institute for Health and Care Research (NIHR), via Cochrane Infrastructure funding to the Cochrane Cystic Fibrosis and Genetic Disorders Group. The views and opinions expressed herein are those of the review authors and do not necessarily reflect those of the Systematic Reviews Programme, NIHR, Nation Health Service, or the Department of Health.
Appendices
Appendix 1. Electronic search strategies
| Database | Search strategy | Date to be searched |
| Physiotherapy Evidence Database (PEDro) (www.pedro.org.au/) |
SEARCH 1: [Advanced Search Form]
Abstract and title: "cystic fibrosis"
Method: clinical trial SEARCH 2: [Advanced Search Form] Abstract and title: mucoviscidosis Method: clinical trial |
From inception to 21 November 2022 |
| CINAHL database (EBSCO Health) (health.ebsco.com/products/the-cinahl-database) |
S1 MH randomized controlled trials S2 MH double‐blind studies S3 MH single‐blind studies S4 MH random assignment S5 MH pretest‐posttest design S6 TI (randomised OR randomized) S7 AB (random*) S8 TI (trial) S9 MH (sample size) AND AB (assigned OR allocated OR control) S10 MH (placebos) S11 PT (randomized controlled trial) S12 AB (control W5 group) S13 MH (crossover design) OR MH (comparative studies) S14 MH animals+ S15 MH (animal studies) S16 TI (animal model*) S17 S14 OR S15 OR S16 S18 MH (human) S19 S17 NOT S18 S20 S1 OR S2 OR S3 OR S4 OR S5 OR S6 OR S7 OR S8 OR S9 OR S10 OR S11 OR S12 OR S13 S21 S20 NOT S19 S22 MH "Cystic Fibrosis" S23 TX mucoviscidosis S24 S22 OR S23 S25 MH ("Computer‐Assisted Instruction" OR "Therapy, Computer Assisted" OR "Instant Messaging" OR "Electronic Mail" OR "Wireless Communications" OR "Signal Processing, Computer‐Assisted" OR "Computers, Handheld" OR "Computer Systems" OR Internet+ OR "Web Browsers" OR Videoconferencing OR Teleconferencing OR Software OR "Patient Portals" OR "Online Systems" OR "Cellular Phone" OR Smartphone OR "Mobile Applications" OR "Reminder Systems" OR "Text Messaging" OR "Communications Media" OR "Social Media" OR "Medical Informatics" OR Telemedicine OR telehealth+ OR "Computers, Portable+") S26 TI ((email* or e‐mail* or electronic mail* or messag* or SMS or MMS or texting or call* or phone* or cellphone* or cell‐phone* or smart watch* or smartwatch* or smartphone* or smart‐phone* or tablet* or pda or "personal digital assistant#" or reminder* or alert* or virtual* or portal or "social media" or "social networking" or facebook or twitter or instagram or youtube or whatsapp or skyp* or zoom or meet or video* or app* or television or radio or internet* or wireless* or bluetooth* or electronic* or digital* or tech* or online* or on‐line* or computer* or laptop# or device* or iphone* or ipod* or ipad* or android* or blackberr* or "palm pilot*" or wearable* or "Palm OS" or "Palm Pre classic" or nokia or symbian or INQ or HTC or sidekick or samsung or huawei or sony or LG or siemens or software or web* or remote* or distan*) N3 (deliver* or generat* or based or provid* or facilitat* or support* or therap* or treat* or intervention* or program* or feedback or monitor* or care* or consult*)) OR AB ((email* or e‐mail* or "electronic mail*" or messag* or SMS or MMS or texting or call* or phone* or cellphone* or cell‐phone* or "smart watch*" or smartwatch* or smartphone* or smart‐phone* or tablet* or pda or "personal digital assistant#" or reminder* or alert* or virtual* or portal or "social media" or "social networking" or facebook or twitter or instagram or youtube or whatsapp or skyp* or zoom or meet or video* or app* or television or radio or internet* or wireless* or bluetooth* or electronic* or digital* or tech* or online* or on‐line* or computer* or laptop# or device* or iphone* or ipod* or ipad* or android* or blackberr* or "palm pilot*" or wearable* or "Palm OS" or "Palm Pre classic" or nokia or symbian or INQ or HTC or sidekick or samsung or huawei or sony or LG or siemens or software or web* or remote* or distan*) N3 (deliver* or generat* or based or provid* or facilitat* or support* or therap* or treat* or intervention* or program* or feedback or monitor* or care* or consult*)) S27 TI (e‐health or ehealth or "e health" or "electronic health" or tele‐health* or telecare* or tele‐care* or telemanagement or tele‐management or teleconsultation or tele‐consultation or mhealth or m‐health or "m health" or "mobile health" or "virtual health" or "digital health" or technological aid* or telecommunication*) OR AB (e‐health or ehealth or "e health" or "electronic health" or tele‐health* or telecare* or tele‐care* or telemanagement or tele‐management or teleconsultation or tele‐consultation or mhealth or m‐health or "m health" or "mobile health" or "virtual health" or "digital health" or "technological aid*" or telecommunication*) S28 S25 or S26 or S27 S29 MH ("Exercise+" OR "Physical Fitness+" OR "Physical Endurance+" OR "Exertion" OR "Exercise Intensity" OR "Sports" OR "Physical Therapy" OR "Therapeutic Exercise" OR "Resistance Training" OR "Aerobic Exercises") S30 TI (treadmill* or run* or walk* or jog* or sprint* or row* or swim* or bicycl* or cycl* or danc* or yoga or pilates or "tai chi" or "tai ji" or qigong or "qi gong" or weight‐bearing or stretch* or exercis* or sport* or fitness* or gym*) OR AB (treadmill* or run* or walk* or jog* or sprint* or row* or swim* or bicycl* or cycl* or danc* or yoga or pilates or "tai chi" or "tai ji" or qigong or "qi gong" or "weight bearing" or stretch* or exercis* or sport* or fitness* or gym*) S31 TI (physical* N5 (fit* or activ* or movement* or train* or condition* or program* or therap*)) OR AB (physical* N5 (fit* or activ* or movement* or train* or condition* or program* or therap*)) S32 TI ((weight* or strength* or enduranc* or circuit*or aerobic* or resistance) N5 (program* or train* or session*)) OR AB ((weight* or strength* or enduranc* or circuit* or aerobic* or resistance) N5 (program* or train* or session*))) S33 S29 or S30 or S31 or S32 S34 S21 and S24 and S28 and S33 NOTE: Lines S1‐S21 are based on the Cochrane CINAHL Plus filter for identifying randomized trials. Available from:training.cochrane.org/handbook/version-6/chapter-4-tech-suppl(Box 3.f, page 65‐7). We omitted lines S6 and S15 from the original strategy because cluster RCTs were not eligible for this review. |
From inception to 21 November 2022 |
| WHO International Clinical Trials Registry Platform (ICTRP) (www.who.int/trialsearch) |
[Advanced Search Form]
In the Condition: "cystic fibrosis" OR mucoviscidosis In the Title: exercis* OR "physical* activ*" OR "Physical* Therap*" OR "physical* fit*" OR "physical* movement*" OR "physical* train*" OR "physical* condition*" OR "physical* program*" OR "physical* enduranc*" OR Exertion OR sport* OR treadmill* OR run* OR walk* OR jog* OR sprint* OR row* OR swim* OR bicycl* OR cycl* OR danc* OR yoga OR pilates OR "tai chi" OR "tai ji" OR qigong OR "qi gong" OR weight‐bearing OR stretch* OR fitness* OR gym* OR "weight* program*" OR "weight* train*" OR "weight* session*" OR "strength* program*" OR "strength* train*" OR "strength* session*" OR "enduranc* program*" OR "enduranc* train*" OR "enduranc* session*" OR "circuit* program*" OR "circuit* train*" OR "circuit* session*" OR "aerobic* program*" OR "aerobic* train*" OR "aerobic* session*" OR "resistance program*" OR "resistance train*" OR "resistance session*" |
From inception to 21 November 2022 |
| ClinicalTrials.gov (www.clinicaltrials.gov) |
SEARCH 1: [Advanced Search Form] Condition: "cystic fibrosis" OR mucoviscidosis Other terms: exercis* OR "physical* activ*" OR "Physical* Therap*" OR "physical* fit*" OR "physical* movement*" OR "physical* train*" OR "physical* condition*" OR "physical* program*" OR "physical* enduranc*" OR Exertion OR sport* OR treadmill* OR run* OR walk* Study type: Interventional Studies (Clinical Trials) SEARCH 2: [Advanced Search Form] Condition: "cystic fibrosis" OR mucoviscidosis Other terms: jog* OR sprint* OR row* OR swim* OR bicycl* OR cycl* OR danc* OR yoga OR pilates OR "tai chi" OR "tai ji" OR qigong OR "qi gong" OR weight‐bearing OR stretch* OR fitness* OR gym* Study type: Interventional Studies (Clinical Trials) SEARCH 3: [Advanced Search Form] Condition: "cystic fibrosis" OR mucoviscidosis Other terms: "weight* program*" OR "weight* train*" OR "weight* session*" OR "strength* program*" OR "strength* train*" OR "strength* session*" OR "enduranc* program*" OR "enduranc* train*" OR "enduranc* session*" Study type: Interventional Studies (Clinical Trials) SEARCH 4: [Advanced Search Form] Condition: "cystic fibrosis" OR mucoviscidosis Other terms: "circuit* program*" OR "circuit* train*" OR "circuit* session*" OR "aerobic* program*" OR "aerobic* train*" OR "aerobic* session*" OR "resistance program*" OR "resistance train*" OR "resistance session*" Study type: Interventional Studies (Clinical Trials) |
From inception to 21 November 2022 |
Data and analyses
Comparison 1. Personalized exercise prescription plus wearable fitness tracker integrated with a social media platform compared to exercise prescription alone.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1.1 Lung function measured by the FEV1 (% predicted) | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 1.1.1 At 1 year | 1 | 40 | Mean Difference (IV, Fixed, 95% CI) | 1.00 [‐15.46, 17.46] |
| 1.2 Exercise capacity measured by a submaximal graded exercise test | 1 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 1.2.1 At 1 year | 1 | 40 | Mean Difference (IV, Random, 95% CI) | ‐0.40 [‐1.36, 0.56] |
Comparison 2. Wearable fitness tracker (WFT) plus text message personalized feedback (TMPF) and goal setting (GS) compared to WFT alone.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 2.1 Physical activity measured by step count | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 2.1.1 At 3 months | 1 | 32 | Mean Difference (IV, Fixed, 95% CI) | 1564.00 [‐1288.07, 4416.07] |
| 2.1.2 At 6 months | 1 | 32 | Mean Difference (IV, Fixed, 95% CI) | 675.00 [‐2406.37, 3756.37] |
| 2.2 Physical activity measured by the IPAQ (walk) | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 2.2.1 At 3 months | 1 | 32 | Mean Difference (IV, Fixed, 95% CI) | 578.54 [‐264.22, 1421.30] |
| 2.2.2 At 6 months | 1 | 32 | Mean Difference (IV, Fixed, 95% CI) | 181.50 [‐805.21, 1168.21] |
| 2.3 Physical activity measured by the IPAQ (moderate) | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 2.3.1 At 3 months | 1 | 32 | Mean Difference (IV, Fixed, 95% CI) | 262.50 [‐494.76, 1019.76] |
| 2.3.2 At 6 months | 1 | 32 | Mean Difference (IV, Fixed, 95% CI) | ‐9.38 [‐726.34, 707.58] |
| 2.4 Physical activity measured by the IPAQ (vigorous) | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 2.4.1 At 3 months | 1 | 32 | Mean Difference (IV, Fixed, 95% CI) | ‐400.00 [‐1906.72, 1106.72] |
| 2.4.2 At 6 months | 1 | 32 | Mean Difference (IV, Fixed, 95% CI) | ‐383.75 [‐1705.78, 938.28] |
| 2.5 Quality of life measured by the CFQ‐R (Health domain) | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 2.5.1 At 3 months | 1 | 32 | Mean Difference (IV, Fixed, 95% CI) | 4.86 [‐8.13, 17.85] |
| 2.5.2 At 6 months | 1 | 32 | Mean Difference (IV, Fixed, 95% CI) | 1.74 [‐8.83, 12.31] |
| 2.6 Lung function measured by the FEV1 (L/s) | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 2.6.1 At 3 months | 1 | 32 | Mean Difference (IV, Fixed, 95% CI) | ‐0.40 [‐1.20, 0.40] |
| 2.6.2 At 6 months | 1 | 32 | Mean Difference (IV, Fixed, 95% CI) | ‐0.34 [‐1.16, 0.48] |
| 2.7 Muscle strength measured by hand dynamometry | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 2.7.1 At 3 months | 1 | 32 | Mean Difference (IV, Fixed, 95% CI) | ‐4.14 [‐11.81, 3.53] |
| 2.7.2 At 6 months | 1 | 32 | Mean Difference (IV, Fixed, 95% CI) | ‐8.88 [‐17.01, ‐0.75] |
| 2.8 Exercise capacity measured by a cardiopulmonary exercise test (VO2peak) | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 2.8.1 At 3 months | 1 | 26 | Mean Difference (IV, Fixed, 95% CI) | ‐2.97 [‐9.18, 3.24] |
| 2.8.2 At 6 months | 1 | 32 | Mean Difference (IV, Fixed, 95% CI) | ‐4.45 [‐9.67, 0.77] |
Comparison 3. Web‐based versus face‐to‐face exercise delivery.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 3.1 Adherence measured by the number of participants who completed all exercise sessions | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 3.1.1 At 3 months | 1 | 51 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.92 [0.69, 1.23] |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Bishay 2018.
| Study characteristics | |
| Methods | Single‐center parallel RCT |
| Participants | 40 adults with CF Age, mean: 35 (SD 14) years Sex: male 55%, female 45% Baseline graded exercise test, mean: fitness tracker + exercise prescription group: 6.1 (SD 1.8); exercise prescription alone group: 5.7 (SD 1.6) Baseline mean FEV1: 70 (SD 30) % predicted |
| Interventions |
Fitness tracker + exercise prescription (19 participants) Exercise prescription alone (21 participants) Exercise prescription was personalized and provided by a physical therapist |
| Outcomes |
Outcomes
Outcome measures not included in this review
Follow‐up: 12 months |
| Funding sources | Not described |
| Conflict of interest | Not described |
| Notes | Study reported as an abstract. No trial protocol available. Contact: lbishay@usc.edu; lara.bishay@childrens.harvard.edu |
Carr 2018.
| Study characteristics | |
| Methods | Single‐center parallel RCT |
| Participants | 51 children and adults with CF, aged ≥ 6 years without prior experience of practicing Tai Chi. People were excluded if they were taking part in any other interventional study or if they had participated in the pilot study. Age, median: 22.8 (range 6.1–51.5) years Sex: 32.5% males; 67.5% females Baseline FEV1, median: 76 (range 28–106) % predicted Baseline FVC, median: 92 (range 46–118) % predicted |
| Interventions |
Tai Chi lessons via Skype (the internet‐delivered group) (27 participants) Lessons in a face‐to‐face format (24 participants) Number of sessions: 8 Duration of intervention: 3 months Interventions: a sequence of 8 movements focused on developing key Tai Chi principles. Each session began with postural and breath awareness, and at the end of each session self‐massage was included. A DVD with sections, a booklet, stickers, diaries, and t‐shirts were offered. Participants were encouraged to practice the exercises for 5–10 minutes up to 5 times a week. 40 participants completed all 8 lessons. |
| Outcomes |
Outcomes
Outcomes not included in this review
Follow‐up: 3 months |
| Funding sources | Research grants from 2 charitable bodies (the Tracie Lawlor Trust for Cystic Fibrosis [the major grant funder] and the Cystic Fibrosis Trust). The authors reported neither of them contributed to trial design or their publication. |
| Conflict of interest | SB Carr reported receiving personal fees and non‐financial support for service on an advisory board, and accommodation and travel expenses to the advisory board and scientific committees from Vertex Pharmaceuticals, advisory board fees from Chiesi Pharmaceuticals and lecture fees from Teva Pharmaceuticals, outside the submitted work. P Ronan had no conflicts of interest to declare. A Lorenc had nothing to disclose. A Mian had nothing to disclose. SL Madge had nothing to disclose. N Robinson had no conflicts of interest to declare. |
| Notes | Trial protocol: NCT02054377 Contact: s.carr@rbht.nhs.uk |
Cox 2022.
| Study characteristics | |
| Methods | Multicenter parallel RCT |
| Participants | Target sample size: 75 Inclusion criteria: confirmed diagnosis of CF, hospital inpatient admission (including hospital in the home) for intravenous antibiotic therapy for a respiratory cause, able to provide informed consent, able to access the internet via computer or mobile device, males and females aged 12–35 years Exclusion criteria: presence of severe comorbidity limiting mobilization or physical activity participation, previous lung transplantation, pregnancy |
| Interventions |
Intervention group: participants used the ActivOnline program, via the internet and received usual care. Record their daily physical activity and exercise using a secure portal. When logging onto ActivOnline they were prompted to set goals, record their physical activity or exercise using a pedometer or other device of their choice (e.g. Fitbit or mobile telephone), and regularly entered data about that. If there was no activity logged for 3 days, the ActivOnline program issued a standardized alert message and emailed the participant. Participants sent questions at any time and clinicians were able to remind participants to review their goals each week. Usual care group: provided with details for an online information source regarding physical activity participation and physical activity targets for children and young adults (www.nhs.uk/Livewell/fitness/Pages/physical-activity-guidelines-for-young-people.aspx), as well as activity and exercise guidance, as indicated, as part of their routine clinical care on hospital discharge. |
| Outcomes |
Primary outcome
Secondary outcomes
Outcomes not included in this review
|
| Funding sources | UK CF Trust |
| Conflict of interest | Authors declared they had no competing interests. |
| Notes | Contact: narelle.cox@ monash.edu |
Curran 2022.
| Study characteristics | |
| Methods | Single‐center parallel RCT |
| Participants | Target sample size: 50 Inclusion criteria: confirmed diagnosis of CF (based on CF‐causing mutations or sweat chloride concentration during 2 tests > 60 mmol/L, or both); clinically stable (determined by those who are not experiencing a pulmonary exacerbation [defined as acute or subacute worsening of respiratory symptoms which warranted change in treatment such as new oral or intravenous antibiotics]) and attending University Hospital Limerick; access to a smartphone/tablet to access and ability to upload to Fitbit application; capacity and willingness to give explicit informed consent; aged ≥ 18 years. Exclusion criteria: FEV1 < 25% predicted (these individuals would typically require supplemental oxygen for exercise and at this point may require transplant assessment; furthermore, they are more likely to experience exacerbations and would not be suitable for a study over 24 weeks); on the waiting list for lung transplantation and those who have undergone lung transplantation; having had an exacerbation in the 4 weeks prior to study (participants could undergo testing once they finish their antibiotics and were deemed clinically stable by the respiratory consultant); dependent on supplemental oxygen for exercise; pregnancy; any cardiac, neurologic, or musculoskeletal impairment that may have impacted on the ability to participate in the activity intervention; participation in another clinical trial up to 4 weeks prior to the first baseline visit |
| Interventions |
Intervention group: wearable technology, text message feedback and goal setting. Participants provided with wearable technology (Fitbit Charge 2), educated on how to use it, and this was linked to an online monitoring system (Fitabase). Participants encouraged to enable Bluetooth and upload data regularly. Fitabase enables the physical therapists to access step count data remotely. Participants in both groups received a 'reminder' message on their mobile phone if data had not been synchronized via the Fitbit App in previous 7 days. This ensures data were continuously collected over study duration. Every week, participants were sent a 1‐way personalized text message by their CF physical therapist for 12 weeks overall. Control group: participants provided with a Fitbit Charge 2 and educated on how to use it. This was linked to Fitabase for data collection purposes. However, no feedback provided to participants on their physical activity levels throughout study period. |
| Outcomes |
Primary outcome
Secondary outcomes used
Outcomes not included in this review
|
| Funding sources | The Seed Funding Programme 2018 by the Health Research Institute, University of Limerick and Truck Run 4 Katie, a local charitable organization. |
| Conflict of interest | No competing interests disclosed Contact: maire.curran@ul.ie |
| Notes | |
BMI: body mass index; BREQ‐2: Behavioural Regulation In Exercise Questionnaire – 2; CAMM: Child and Adolescent Mindfulness Measure; CES‐D: Center for Epidemiological Studies – Depression; CF: cystic fibrosis; CFQ‐R: Cystic Fibrosis Questionnaire‐Revised; FEV1: forced expiratory volume in 1 second; FFMS: Five Facets Mindfulness Questionnaire; FVC: forced vital capacity; GAD‐7: General anxiety disorder; HADS: Hospital Anxiety and Depression Scale; MST: modified shuttle test; PHQ‐9: 9‐item Patient Heath Questionnaire; PSQI: Pittsburgh Sleep Quality Index; RCT: randomized controlled trial; SD: standard deviation.
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Anifanti 2022 | The effect of the digital health technology could not be exclusively evaluated. |
| Bingham 2010 | Did not use a digital health technology for delivering or monitoring exercise programs. |
| Happ 2013 | Did not use a digital health technology for delivering or monitoring exercise programs. |
| Hebestreit 2022 | The effect of the digital health technology could not be exclusively evaluated. |
| Kenis‐Coskun 2022 | The effect of the digital health technology could not be exclusively evaluated. |
| NCT03522480 | Did not use a digital health technology for delivering or monitoring exercise programs. |
Characteristics of studies awaiting classification [ordered by study ID]
Burnett 2021.
| Methods | No access to the text |
| Participants | No access to the text |
| Interventions | No access to the text |
| Outcomes | No access to the text |
| Notes | No access to the text. Contacted authors by e‐mail to check if details on study match the eligibility criteria for this review. No responses have been received. Contact: dburnett@kumc.edu |
Kilic 2021.
| Methods | Not clear. |
| Participants | 30 children with CF with mean age 11.16 (SD 3.02) years |
| Interventions |
Intervention: telerehabilitation with posture exercises, squat, crunch, oblique crunch, and lunge Control: brochure of the same exercises 1 session lasted 30–45 minutes |
| Outcomes |
|
| Notes | Not clear if a randomization process was performed or tried. Authors were contacted to check. No responses have been received. Contact: www.researchgate.net/profile/Kuebra‐Kilic‐7 |
Mermis 2021.
| Methods | Single‐center parallel RCT (1:1 randomization) Duration: 12 weeks |
| Participants | Adults with CF on highly effective modulator therapies ≥ 1 month Target enrolment: 40 participants; 9 participants were enrolled over a 4‐week period, but subsequent recruitment was halted due to COVID‐19. Completed by 8/9 participants (1 participant randomized to exercise discontinued for transplantation) |
| Interventions |
Intervention: usual care plus progressive increase in aerobic exercise plus fitness tracker Control: usual care Participants started with ≥ 60 minutes of weekly exercise during week 1, with a gradual increase to a target of 180 minutes of aerobic exercise by week 9; two‐thirds of total exercise at moderate aerobic and one‐third at vigorous aerobic exercise level. |
| Outcomes | Outcomes of interest to review
Outcomes not included in this review
|
| Notes | Contacted authors by e‐mail to check if details on interventions match the eligibility criteria for this review. Received no response. Contact: jmermis@kumc.edu |
CF: cystic fibrosis; FEV1: forced expiratory volume in 1 second; HbA1c: hemoglobin A1c; RCT: randomized controlled trial; SD: standard deviation; VO2: volume of oxygen.
Characteristics of ongoing studies [ordered by study ID]
ACTRN12620001237976.
| Study name | Virtual models for delivery of exercise training in cystic fibrosis (CF): an evaluation of patient engagement and feasibility |
| Methods | Single‐center parallel RCT |
| Participants | Target sample size: 24 Inclusion criteria: attending the adult CF center at The Prince Charles Hospital with a diagnosis of gene confirmed CF; lung function FEV1 30–70% predicted; age ≥ 18 years; males and females Exclusion criteria: severe pulmonary disease (FEV1 < 30% predicted); mild pulmonary disease (FEV1 > 70% predicted); use of domiciliary oxygen therapy; unable to perform exercise as determined by the medical or physical therapy team (or both); death is deemed imminent or the patient is actively being palliated; individuals or next of kin of individuals on their behalf do not consent to participate |
| Interventions |
Virtual care group: 2 models of interactive online exercise classes will deliver and promote exercise in adults with CF. The level of intensity will be monitored by modified Borg Score; however, all participants will be encouraged to self‐pace during the session based on their own exercise tolerance. Sessions will be 40 minutes in duration including warm‐up and cool‐down for both exercise models and program will be 8 weeks with sessions occurring twice‐weekly with a 3rd independent exercise session encouraged. Exercises prescribed to all participants will be standardized; however, progressions will be provided for participants for them to choose based on their exercise capacity (e.g. sit to stand OR squats OR jump squats). The models of interactive exercise will be: 1. a virtual exercise class that occurs in real time with 6 participants and 1 physical therapist via telehealth; 2. online exercise class where the participants will be provided with videos for exercise sessions that they will complete independently, unsupervised, in their own time. Control group: gold standard pulmonary rehab using a virtual model. This will involve telehealth via the Cisco Jabber platform, which will allow participants to interact with the physical therapist conducting the exercise class. The program will occur twice‐weekly with a 3rd independent exercise session encouraged. Progressions, modifications, and feedback on technique will be provided to all participants. |
| Outcomes |
Primary outcome
Outcomes not included in this review
|
| Starting date | 31 December 2020 |
| Contact information | kelly.burgess@health.qld.gov.au |
| Notes | Funding: The Physiotherapy Department, The Prince Charles Hospital, Queensland, Australia |
ISRCTN92573472.
| Study name | An evaluation of the efficacy of a 12‐week partially supervised, self‐regulated exercise intervention on physiological and psychometric indices in patients with cystic fibrosis: a randomized‐controlled trial |
| Methods | Single‐center parallel RCT |
| Participants | Target sample size: 30 Inclusion criteria: established diagnosis of CF (positive sweat chloride or genetic identification test); residing in the Republic of Ireland; lung function scores ≥ 50% predicted; must not have undergone lung transplantation; age ≥ 18 years; males and females Exclusion criteria: undergone lung transplantation; culturing MRSA, NTM, or Burkholderia cepacia |
| Interventions |
Exercise group: participants will receive a Fitbit device to objectively track daily steps and active minutes, to wear for the 12‐week intervention period. Participants in the exercise group will also receive an exercise manual (hardcopy) and access to an online exercise diary. Control group: participants will continue with usual care. |
| Outcomes |
Primary outcomes
Secondary outcomes
Outcomes not included in this review
|
| Starting date | 9 September 2019 |
| Contact information | nicola.hurley5@mail.dcu.ie |
| Notes | Funding: Cystic Fibrosis Ireland and The Mater Foundation Protocol was retrospectively registered |
Lang 2019.
| Study name | CyFiT telerehabilitation: technology based physiotherapy for peer driven participation in therapy, and quality of life |
| Methods | Single‐center parallel RCT |
| Participants | Target sample size: 110 Inclusion criteria: medically diagnosed with CF; have access to the internet in their local area (e.g. at home, a local health center, or the home of a family‐selected relative or friend), through a device that enables videoconferencing (e.g. personal computer, tablet, or phones); age 8–18 years; males and females Exclusion criteria: behavioral or intellectual difficulties that would prevent full participation in face‐to‐face physical therapy assessment, or physical therapy intervention via telehealth; acute or chronic medical comorbidity that requires more complex or frequent medical input (or both); involved in another study that precludes enrolment in any other study |
| Interventions |
CyFiT Telerehab group: children will be provided with a consumer‐based activity tracker to wear every day, including sleeping. Data collected by the activity tracker will be aggregated to a secure database where summaries are presented via online dashboards available for patients and treating clinicians. Data will be retrieved from activity trackers daily; however, frequency can be increased to every 30 seconds if required, so clinicians can have access to near‐real‐time physiologic data during real‐time videoconference consultations. Additional physical therapy follow‐up will be protocol‐triggered as per standard practice. Physical therapy consultations will be delivered via real‐time videoconferencing into the home with the same rationale as standard care. First 6 months: children will only be participating in 1‐to‐1 OPS via telehealth in the intervention group as clinically indicated. Treating physical therapists will have access to health‐related information via supplier web portal. 6–12 months: in addition to 1‐to‐1 OPS via telehealth, physical therapists can refer children in the CyFiT OPS group to an online group‐based exercise class. Group sessions will be delivered using the eHAB systems. Algorithm‐driven data analysis using health‐related information collected in Phase 1 will be used to generate potential risk score and visualized for clinicians for physical therapists. Usual OPS group: children allocated to the usual OPS group may receive OPS as face‐to‐face, telephone, telehealth (or a combination of these) follow‐up determined at the time by the treating physical therapist. A home exercise program will be performed independently and recorded via a self‐reported paper‐based exercise diary. Each review may involve any or a combination of the child's home exercise program, airway clearance techniques, use and maintenance of therapy equipment, and support for adherence. If telehealth is included as an intervention mode, this will be delivered through the standard Queensland Health Telehealth Network, which enables physical therapists to connect via real‐time videoconferencing. Physical therapists may choose to utilize Queensland Health Telehealth to replace some face‐to‐face reviews to better suit participant schedules or to provide video feedback. |
| Outcomes |
Primary outcome
Secondary outcomes
Outcomes not included in this review
|
| Starting date | 1 August 2017 |
| Contact information | lei.lang@uqconnect.edu.au |
| Notes | Funding: Health Practitioner Research Scheme 2017 Funding Round – Department of Health Allied Health Professions' Office of Queensland (Queensland Health) and Lady Cilento Children's Hospital |
NCT04249999.
| Study name | A randomised controlled trial of a novel web‐based intervention to promote physical activity participation in people with cystic fibrosis |
| Methods | Single‐center parallel RCT |
| Participants | Target sample size: 94 Inclusion criteria: confirmed diagnosis of CF; able to provide informed consent/assent; able to access the internet via computer or mobile device; aged 12–35 years (inclusive) Exclusion criteria: presence of severe comorbidity limiting mobilization or physical activity participation (e.g. orthopedic, cardiac, or neurologic condition); previous lung transplantation; pregnancy; unable to provide informed consent/assent |
| Interventions |
Intervention group: access to online physical activity platform (www.activonline.com.au) in addition to usual care Control group: no access to online physical activity platform; continue with usual care |
| Outcomes |
Primary outcomes
Secondary outcomes
Outcomes not included in this review
|
| Starting date | 7 May 2020 |
| Contact information | o.w.tomlinson@exeter.ac.uk |
| Notes |
NCT04742049.
| Study name | The effects of telerehabilitation on peripheral muscle function, physical activity level and sleep quality in pediatric cystic fibrosis patients having social isolation due to pandemic |
| Methods | Single‐center parallel RCT |
| Participants | Target sample size: 30 Inclusion criteria: diagnosis of CF and stable disease; volunteering to participate in the study; having a social isolation due to COVID‐19 pandemic; FEV1 > 40% predicted; age 8–14 years Exclusion criteria: acute pulmonary exacerbation at time of study or within the last month (or both); diagnosed with COVID‐19 before or during study; being physically or perceptually competent to exercise; allergic bronchopulmonary aspergillosis being treated with systemic steroid therapy; unable to complete the exercise training; FEV1 < 40% predicted |
| Interventions |
Intervention group: online exercise training protocol with each training protocol planned as 30 minutes for 3 days a week for 6 weeks with a physical therapist. Exercise protocol will start with the warm‐up exercises and finish with cool‐down exercises. Control group: an exercise document (brochure) including the same exercise protocol as for the intervention group will be sent to participants and they will be called by the physical therapist once a week for follow‐up. |
| Outcomes |
Primary outcome
Secondary outcomes
Outcomes not included in this review
|
| Starting date | 28 December 2020 |
| Contact information | fztktas@gmail.com |
| Notes |
Powers 2016.
| Study name | Do More, B'More, Live Fit: an outpatient fitness‐training pilot program designed to optimize the habit of exercise in adolescents and young adults with cystic fibrosis |
| Methods | Single‐center parallel RCT |
| Participants | Target sample size: 45 Inclusion criteria: diagnosis of CF and being cared for at Johns Hopkins; must have smart phone or computer (or both) with USB access to set‐up Fitbit Flex; age 12–21 years Exclusion criteria: FEV1 < 40% predicted; individuals already participating in vigorous physical activity as assessed by the study team such as participating in year‐round organized sports or aerobic exercise > 30 minutes > 5 times/week may or may not be included in this study at the discretion of the lead investigator and study team. |
| Interventions |
Intervention group: 30 participants will receive exercise prescriptions based upon their individual assessment. Endurance‐style exercise prescriptions may include walking, rope skipping or stair climbing to more complex Tabata‐style workouts. 2 additional 30‐minute physical therapy appointments are scheduled about 4–6 weeks and 8–10 weeks from enrollment. These appointments will vary based on initial assessment and previous exercise prescription success, but will include strength training for major muscle groups or flexibility exercises with yoga (or both) and reinforcement of previously learned techniques. Physical therapy will add additional individualized recommendations. Participants are enrolled to receive motivational messages starting 14 days after enrollment via preferred contact method (SMS, telephone call, email, or a combination of these) every 3–4 days over the 6‐month study. Participants are given access to Do More, B'More, Live Fit webpage which includes spotlighted exercises, instructional exercise photos, and videos. Participant Fitbit daily step goal is set based on a collaborative review between the participant and physical therapist and participants receive individualized exercise prescriptions based on their assessment. Throughout the study, these participants will receive customized encouragement and personalized fitness recommendations for physical activity at routine visits, baseline and follow‐up assessments at 3‐ and 6‐month clinic visits. At the 3‐month and 6‐month visits, study team members meet again with the participant for an additional 30–45 minutes to reinforce exercise through exercise prescriptions and individualized encouragement, export Fitbit data and review any missing data concerning for equipment failure or user error, and address any specific exercise concerns. Fitbit daily step goals may be adjusted based on collaborative review between the participant and physical therapist. Control group: at the baseline fitness assessment, the Fitbit daily step goal will be set at the manufacturer standard 10,000 steps. Throughout the study, these 30 participants will receive generic, non‐personalized encouragement and recommendations (if requested by the participant) for physical activity at routine clinic visits, baseline, and follow‐up assessments at 3‐ and 6‐month clinic visits. At the 3‐month and 6‐month visits, exercise is reinforced with generic encouragement, export Fitbit data, and review any missing data concerning for equipment failure or user error. |
| Outcomes |
Primary outcomes
Secondary outcomes
Outcomes not included in this review
|
| Starting date | 17 June 2015 |
| Contact information | Johns Hopkins University Principal Investigator: Peter Mogayzel, MD Contact: www.researchgate.net/profile/Peter‐Mogayzel |
| Notes | Trial protocol retrospectively registered |
BREQ‐2: Behavioural Regulation In Exercise Questionnaire – 2; CAPE‐PAC: Children's Assessment of Participation and Enjoyment and Preferences for Activities of Children; CES‐D: Center for Epidemiological Studies – Depression; CF: cystic fibrosis; CFQ‐R: Cystic Fibrosis Questionnaire – Revised; ECG: electrocardiogram; FEF25–75: forced expiratory flow between 25% and 75% of expiratory volume; FEV1: forced expiratory volume in 1 second; FVC: forced vital capacity; HADS: Hospital Anxiety and Depression Scale; HAES: Habitual Activity Estimation Scale; IPAQ: International Physical Activity Questionnaire; MRSA: methicillin‐resistant Staphylococcus aureus; MST: modified shuttle test; MVPA: moderate–vigorous physical activity; NTM: non‐tuberculous mycobacteria; OPS: outpatient physical therapy service; PSQI: Pittsburgh Sleep Quality Index; RCT: randomized controlled trial.
Differences between protocol and review
In this initial review version, instead of two review authors (ACP and AR) independently selecting studies, extracting data, and assessing the risk of bias of included studies as was planned, at least two out of three authors (ACP, AR, and MGN) independently undertook these tasks.
Also, in future updates of this review, we plan to use ROB‐ME to assess the risk of bias due to missing data, especially to try to evaluate to what extent the missing data influence the effect estimate in the syntheses.
Contributions of authors
| Task | Author(s) responsible |
| Protocol stage: draft the protocol | ACP, SRP, AR, ANA, HS, VFT |
| Review stage: select which trials to include (2 + 1 arbiter) | ACP, AR, MGN + SRP (arbiter) |
| Review stage: extract data from trials (2 people) | ACP, AR, MGN + VFT (for disagreements) |
| Review stage: assessment of risk of bias | ACP, AR, MGN + ANA (for disagreements) |
| Review stage: enter data into Review Manager Web | ACP + HS (to review it) |
| Review stage: carry out the analysis | ACP, ANA, HS |
| Review stage: interpret the analysis | ACP, SRP, AR, MGN, ANA, HS, VFT |
| Review stage: draft the final review | ACP, SRP, AR, MGN, ANA, HS, VFT |
| Review stage: draft the final review | ACP, SRP, AR, MGN, ANA, HS, VFT |
| Update stage: update the review | ACP, SRP, AR, MGN, ANA, HS, VFT |
Sources of support
Internal sources
-
Sources of Support, Other
No sources of support supplied
External sources
-
National Institute for Health & Care Research, UK
This systematic review was supported by the National Institute for Health & Care Research, via Cochrane Infrastructure funding to the Cochrane Cystic Fibrosis and Genetic Disorders Group.
Declarations of interest
ACP: none.
SRP: none.
AR: none.
MGN: none.
ANA: none.
HS: none.
VFT: none.
New
References
References to studies included in this review
Bishay 2018 {published data only}
- Bishay LC, Gould A, Prushingskaya O, Hill K, Greenberg J, Ng J, et al. Baseline submaximal exercise tolerance as an outcome measure in a cystic fibrosis exercise study. American Journal of Respiratory and Critical Care Medicine 2017;195(Abstract Issue. ATS Conference):C72. [ABSTRACT NO.: A6259] [CFGD REGISTER: MH60c] [Google Scholar]
- Bishay LC, Gould A, Prushinskaya OV, Hill K, Greenberg J, Sawicki GS, et al. Acceptability of a fitness tracker for patients with CF: a qualitative analysis within a randomized trial. Pediatric Pulmonology 2017;52(Suppl 47):490. [ABSTRACT NO.: 707] [CFGD REGISTER: MH60a] [Google Scholar]
- Bishay LC, Nelson E, Williams K, Greenberg J, Gould A, Bailey I, et al. Effect of a wearable fitness tracker on exercise tolerance for adults with cystic fibrosis: a pilot randomized clinical trial. Pediatric Pulmonology 2018;53(Suppl 2):345. [ABSTRACT NO.: 516] [CFGD REGISTER: MH60b] [Google Scholar]
- NCT02700243. Increase tolerance for exercise and raise activity through connectedness trial. clinicaltrials.gov/show/NCT02700243 (first received 7 March 2016). [CFGD REGISTER: MH60d]
Carr 2018 {published data only}
- Carr SB, Ronan P, Lorenc A, Mian A, Madge SL, Robinson N. Children and Adults Tai Chi study (CF-CATS2): a randomised controlled feasibility study comparing internet-delivered with face-to-face Tai Chi lessons in cystic fibrosis. ERJ Open Research 2018;4(4):1-9. [CFGD REGISTER: PE243f] [DOI: 10.1183/23120541.00042-2018] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carr SB, Ronan P, Robinson N, Agent P, Mian A, Madge S. A randomised controlled trial of tai chi in cystic fibrosis. Pediatric Pulmonology 2016;51(Suppl 45):376. [ABSTRACT NO.: 480] [CFGD REGISTER: PE243c] [Google Scholar]
- Lorenc A, Ronan P, Mian A, Madge S, Carr SB, Agent P, et al. Cystic Fibrosis – Children and Adults Tai Chi study (CF CATS2): can Tai Chi improve symptoms and quality of life for people with cystic fibrosis? Second phase study protocol. Chinese Journal of Integrative Medicine 2015:1-6. [CFGD REGISTER: PE243a] [DOI: 10.1007/s11655-015-2150-1] [DOI] [PubMed] [Google Scholar]
- Madge S, Ronan P, Robinson N, Agent P, Mian A, Carr SB. Assessing the benefits of tai chi in people with CF and the feasibility of delivering Tai Chi over the internet (Skype®). Pediatric Pulmonology 2016;51(Suppl 45):370. [ABSTRACT NO.: 465] [CFGD REGISTER: PE243b] [Google Scholar]
- NCT02054377. Cystic fibrosis – children and adults Tai Chi study. clinicaltrials.gov/show/NCT02054377 (first received 4 February 2014). [CFGD REGISTER: PE243h]
- Robinson N, Ronan P, Mian A, Madge S, Lorenc A, Agent P, et al. The potential of Tai Chi for long term health: exercise preferences for people with cystic fibrosis. BMC Complementary and Alternative Medicine 2017;17(Suppl 1):333. [ABSTRACT NO.: P324] [CFGD REGISTER: PE243d] [Google Scholar]
- Ronan P, Mian A, Carr SB, Madge SL, Lorenc A, Robinson N. Learning to breathe with Tai Chi online – qualitative data from a randomized controlled feasibility study of patients with cystic fibrosis. European Journal of Integrative Medicine 2020;40:101229. [CFGD REGISTER: PE243g] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ronan P, Robinson N, Carr S, Mian A, Lorenc A, Agent P, et al. Benefits for people with cystic fibrosis learning Tai Chi: comparison of self-reported to objective data in a non-acute/community population. BMC Complementary and Alternative Medicine 2017;17(Suppl 1):333. [ABSTRACT NO.: P325] [CFGD REGISTER: PE243e] [Google Scholar]
Cox 2022 {published data only}
- ACTRN12617001009303. Action: PACT. Be Active. Online. A trial to promote physical activity in young people with cystic fibrosis. www.anzctr.org.au/Trial/Registration/TrialReview.aspx?ACTRN=12617001009303 (first received 13 July 2017). [CFGD REGISTER: PE287b]
- Cox N, Eldridge B, Rawlings S, Dreger J, Corda J, Hauser J, et al. Web-based physical activity promotion in CF: a clinical trial. Respirology (Carlton, Vic.) 2022;27(Suppl 1):76-7. [Google Scholar]
- Cox NS, Eldridge B, Rawlings S, Dreger J, Corda J, Hauser J, et al. A web-based intervention to promote physical activity in adolescents and young adults with cystic fibrosis: protocol for a randomized controlled trial. BMC Pulmonary Medicine 2019;19(1):253. [CFGD REGISTER: PE287a] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox NS, Eldridge B, Rawlings S, Dreger J, Corda J, Hauser J, et al, Youth Activity Unlimited – A Strategic Research Centre of the UK Cystic Fibrosis Trust. Web-based physical activity promotion in young people with CF: a randomised controlled trial. Thorax 2022;0:1-8. [DOI: 10.1136/thoraxjnl-2022-218702] [DOI] [PubMed] [Google Scholar]
Curran 2022 {published data only}
- Curran M, Tierney AC, Collins L, Kennedy L, McDonnell C, Jurascheck AJ, et al. Steps Ahead: optimising physical activity in adults with cystic fibrosis: a pilot randomised trial using wearable technology, goal setting and text message feedback. Irish Journal of Medical Science 2022;191:S157-8. [CFGD REGISTER: PE309d] [DOI] [PubMed] [Google Scholar]
- Curran M, Tierney AC, Collins L, Kennedy L, McDonnell C, Jurascheck AJ, et al. Steps Ahead: optimising physical activity in adults with cystic fibrosis: a pilot randomised trial using wearable technology, goal setting and text message feedback. Journal of Cystic Fibrosis 2022 Nov 16 [Epub ahead of print]. [CFGD REGISTER: PE309e] [DOI: 10.1016/j.jcf.2022.11.002] [DOI] [PubMed]
- Curran M, Tierney AC, Collins L, Kennedy L, McDonnell C, Jurascheck AJ, et al. Steps Ahead: optimising physical activity in adults with cystic fibrosis: study protocol for a pilot randomised trial using wearable technology, goal setting and text message feedback. HRB Open Research 2020;3:21. [CFGD REGISTER: PE309b] [DOI] [PMC free article] [PubMed] [Google Scholar]
- NCT03672058. Steps Ahead: optimising physical activity and health in adults with cystic fibrosis. clinicaltrials.gov/show/NCT03672058 (first received 14 September 2018). [CFGD REGISTER: PE309a]
- Tierney A, Curran M, Collins L, Kennedy L, McDonnell C, Jurascheck A, et al. Steps Ahead: optimising physical activity in adults with cystic fibrosis – a pilot randomised trial using wearable technology, goal setting and text message feedback. Journal of Cystic Fibrosis 2022;21(Suppl):S131. [CFGD REGISTER: PE309c] [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Anifanti 2022 {published data only}
- Anifanti M, Giannakoulakos S, Hatziagorou E, Kampouras A, Tsanakas J, Deligiannis A, et al. Effects of a long-term wearable activity tracker-based exercise intervention on cardiac morphology and function of patients with cystic fibrosis. Sensors 2022;22:1-13. [DOI: 10.3390/s22134884] [DOI] [PMC free article] [PubMed] [Google Scholar]
Bingham 2010 {published data only}
- Bingham PM, Bates JH, Thompson-Figueroa J, Lahiri T. A breath biofeedback computer game for children with cystic fibrosis. Clinical Pediatrics 2010;49(4):337-42. [CFGD REGISTER: PE228a] [DOI] [PubMed] [Google Scholar]
- Bingham PM, Lahiri T, Ashikaga T. Pilot trial of spirometer games for airway clearance practice in cystic fibrosis. Respiratory Care 2012;57(8):1278-84. [CFGD REGISTER: PE228b] [DOI] [PubMed] [Google Scholar]
Happ 2013 {published data only}
- Happ MB, Hoffman LA, Higgins LW, Divirgilio D, Orenstein DM. Parent and child perceptions of a self-regulated, home-based exercise program for children with cystic fibrosis. Nursing Research 2013;62(5):305-14. [CFGD REGISTER: PE307b] [DOI] [PMC free article] [PubMed] [Google Scholar]
- NCT00609050. Exercise training study for patients with cystic fibrosis. clinicaltrials.gov/ct2/show/NCT00609050 (first received 6 February 2008). [CFGD REGISTER: PE307a]
Hebestreit 2022 {published data only}
- Hebestreit H, Kriemler S, Schindler C, Stein L, Karila C, Urquhart DS, et al. Effects of a partially supervised conditioning program in cystic fibrosis: an international multi-centre, randomised controlled trial (ACTIVATE-CF). Journal of Cystic Fibrosis 2021;20(Suppl):S8. [CFGD REGISTER: PE244e] [Google Scholar]
- Hebestreit H, Kriemler S, Schindler C, Stein L, Karila C, Urquhart DS, et al. Effects of a partially supervised conditioning program in cystic fibrosis: an international multicenter, randomized controlled trial (ACTIVATE-CF). American Journal of Respiratory and Critical Care Medicine 2022;205(3):330-9. [CFGD REGISTER: PE244d] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hebestreit H, Lands LC, Alarie N, Schaeff J, Karila C, Orenstein DM, et al. Effects of a partially supervised conditioning programme in cystic fibrosis: an international multi-centre randomised controlled trial (ACTIVATE-CF): study protocol. BMC Pulmonary Medicine 2018;18(1):31. [DOI: 10.1186/s12890-018-0596-6] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hebestreit H. How to counsel patients regarding exercise and habitual physical activity? Pediatric Pulmonology 2016;51(Suppl 45):188-9. [CFGD REGISTER: PE244a] [Google Scholar]
- NCT01744561. Effects of a partially supervised conditioning program in CF (ACTIVATE-CF). clinicaltrials.gov/ct2/show/NCT01744561 (first received 6 December 2012). [CFGD REGISTER: PE244c]
Kenis‐Coskun 2022 {published data only}
- Kenis-Coskun Ö, Aksoy AN, Kumaş EN, Yılmaz A, Güven E, Ayaz HH, et al. The effect of telerehabilitation on quality of life, anxiety, and depression in children with cystic fibrosis and caregivers: a single-blind randomized trial. Pediatric Pulmonology 2022;57(5):1262-71. [DOI: 10.1002/ppul.25860] [DOI] [PubMed] [Google Scholar]
NCT03522480 {published data only}
- NCT03522480. The effectiveness of the Jamboxx Respiratory Therapy Device: study 2. clinicaltrials.gov/show/NCT03522480 (first received 11 May 2018). [CFGD REGISTER: PE301]
References to studies awaiting assessment
Burnett 2021 {published data only}
- Burnett D, Barry A, Bustos A, Mermis J. Tele-rehab for improving healthcare access and clinical outcomes in cystic fibrosis. Journal of Allied Health 2021;51:74. [Google Scholar]
Kilic 2021 {published data only}
- Kilic K, Vardar-Yagli N, Ademhan-Tural D, Sunman B, Ozsezen B, Dogru D, et al. Effects of telerehabilitation on peripheral muscle function in pediatric cystic fibrosis patients during COVID-19 pandemic. European Respiratory Journal 2021;58(Suppl 65):PA1818. [DOI: 10.1183/13993003.congress-2021.PA1818] [DOI] [Google Scholar]
Mermis 2021 {published data only}
- Mermis J, Barry A, Bustos A, Burnett D. Feasibility of telehealth-based aerobic exercise training program for adults with cystic fibrosis. Journal of Cystic Fibrosis 2021;20(S2):S126. [ABSTRACT NO.: 260] [Google Scholar]
References to ongoing studies
ACTRN12620001237976 {published data only}
- ACTRN12620001237976. Virtual models for delivery of exercise training in cystic fibrosis (CF): an evaluation of patient engagement and feasibility. trialsearch.who.int/Trial2.aspx?TrialID=ACTRN12620001237976 (first received 18 November 2020). [CFGD REGISTER: PE336]
ISRCTN92573472 {published data only}
- ISRCTN92573472. The evaluation of a 12-week partially supervised, self-regulated exercise intervention in patients with cystic fibrosis. trialsearch.who.int/Trial2.aspx?TrialID=ISRCTN92573472 (first received 24 February 2020). [CFGD REGISTER: PE323]
Lang 2019 {published data only}
- ACTRN12617001035314. CyFiT Telerehabilitation: technology based physiotherapy for peer driven participation in therapy, and quality of life. trialsearch.who.int/Trial2.aspx?TrialID=ACTRN12617001035314 (first received 17 July 2017). [CFGD REGISTER: PE305a]
- Lang RL, Wilson C, Stockton K, Russell T, Johnston LM. CyFiT telehealth: protocol for a randomised controlled trial of an online outpatient physiotherapy service for children with cystic fibrosis. BMC Pulmonary Medicine 2019;19(1):21. [CFGD REGISTER: PE305b] [DOI] [PMC free article] [PubMed] [Google Scholar]
NCT04249999 {published data only}
- NCT04249999. ActivOnline: physical Activity in Cystic Fibrosis Trial UK. clinicaltrials.gov/show/NCT04249999 (first received 31 January 2020). [CFGD REGISTER: PE303]
NCT04742049 {published data only}
- NCT04742049. The effects of telerehabilitation on muscle function, physical activity and sleep in cystic fibrosis during pandemic. clinicaltrials.gov/show/NCT04742049 (first received 5 February 2021). [CFGD REGISTER: PE325]
Powers 2016 {published data only}
- NCT03109912. Do More, B'More, Live Fit: an outpatient fitness-training pilot program designed to optimize the habit of exercise in adolescents and young adults with cystic fibrosis. clinicaltrials.gov/show/nct03109912 (first received 12 April 2017). [CFGD REGISTER: PE246a]
- Powers KE, Herzog T, Loosen H, Berg K, Weir B, Riekert KA, et al. Step It Up: higher step count is significantly correlated with better exercise capacity in individuals with cystic fibrosis. American Journal of Respiratory and Critical Care Medicine 2017;195:A6135. [CFGD REGISTER: PE246d] [Google Scholar]
- Powers KE, Herzog TL, Loosen H, Berg K, Weir B, Riekert KA, et al. Self-reported physical activity does not correlate with ventilation inhomogeneity. Pediatric Pulmonology 2016;51(Suppl 45):370. [CFGD REGISTER: PE246c] [Google Scholar]
- Powers KE, Loosen H, Berg K, Herzog TL, Weir B, Riekert KA, et al. Ventilation inhomogeneity and lung function are associated with exercise capacity. Pediatric Pulmonology 2016;51(Suppl 45):369-70. [CFGD REGISTER: PE246b] [Google Scholar]
Additional references
Andreacci 2002
- Andreacci JL, Lemura LM, Cohen SL, Urbansky EA, Chelland SA, Duvillard SP. The effects of frequency of encouragement on performance during maximal exercise testing. Journal of Sports Sciences 2002;20(4):345-52. [DOI] [PubMed] [Google Scholar]
Bell 2020
- Bell SC, Mall MA, Gutierrez H, Macek M, Madge S, Davies JC, et al. The future of cystic fibrosis care: a global perspective. Lancet Respiratory Medicine 2020;8(1):65-124. [DOI: 10.1016/S2213-2600(19)30337-6] [DOI] [PMC free article] [PubMed] [Google Scholar]
Bernard 2008
- Bernard RS, Cohen LL, Moffett K. A token economy for exercise adherence in pediatric cystic fibrosis: a single-subject analysis. Journal of Pediatric Psychology 2008;34(4):354-65. [DOI: 10.1093/jpepsy/jsn101] [DOI] [PubMed] [Google Scholar]
Blakey 2018
- Blakey JD, Bender BG, Dima AL, Weinman J, Safioti G, Costello RW. Digital technologies and adherence in respiratory diseases: the road ahead. European Respiratory Journal 2018;52(5):1801147. [DOI: 10.1183/13993003.01147-2018] [DOI] [PMC free article] [PubMed] [Google Scholar]
Bradley 2015
- Bradley J, O'Neill B, Kent L, Hulzebos EH, Arets B, Hebestreit H, et al. Physical activity assessment in cystic fibrosis: a position statement. Journal of Cystic Fibrosis 2015;14(6):e25-32. [DOI] [PubMed] [Google Scholar]
Bull 2020
- Bull FC, Al-Ansari SS, Biddle S, Borodulin K, Buman MP, Cardon G, et al. World Health Organization 2020 guidelines on physical activity and sedentary behaviour. British Journal of Sports Medicine 2020;54(24):1451-62. [DOI] [PMC free article] [PubMed] [Google Scholar]
Calthorpe 2020
- Calthorpe RJ, Smith S, Gathercole K, Smyth AR. Using digital technology for home monitoring, adherence and self-management in cystic fibrosis: a state-of-the-art review. Thorax 2020;75(1):72-7. [DOI: 10.1136/thoraxjnl-2019-213233] [DOI] [PubMed] [Google Scholar]
CF Foundation 2020
- Cystic Fibrosis Foundation. About cystic fibrosis. www.cff.org/What-is-CF/About-Cystic-Fibrosis (accessed 10 June 2020).
CF Trust 2016
- UK Cystic Fibrosis Trust. UK Cystic Fibrosis Registry 2015 annual data report. www.cysticfibrosis.org.uk/~/media/documents/the-work-we-do/uk-cf-registry/full-registry-report-2015.ashx?la=en (accessed 23 June 2020).
Chen 2018
- Chen JJ, Cooper DM, Haddad F, Sladkey A, Nussbaum E, Radom-Aizik S. Tele-exercise as a promising tool to promote exercise in children with cystic fibrosis. Front Public Health 2018;6:269. [DOI: 10.3389/fpubh.2018.00269] [DOI] [PMC free article] [PubMed] [Google Scholar]
Cox 2016
- Cox NS, Alison JA, Button BM, Wilson JW, Morton JM, Holland AE. Physical activity participation by adults with cystic fibrosis: an observational study. Respirology 2016;21(3):511-8. [DOI] [PubMed] [Google Scholar]
Cummings 2011
- Cummings E, Hauser J, Cameron-Tucker H, Fitzpatrick P, Jessup M, Walters EH, et al. Enhancing self-efficacy for self-management in people with cystic fibrosis. Studies in Health Technology and Informatics 2011;169(1):33-7. [PubMed] [Google Scholar]
Dasenbrook 2012
- Dasenbrook EC, Konstan MW. Inhaled hypertonic saline in infants and young children with cystic fibrosis. JAMA 2012;307(21):2316-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Deeks 2022
- Deeks JJ, Higgins JP, Altman DG, editor(s). Chapter 10: Analysing data and undertaking meta-analyses. In: Higgins JP, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, Welch VA, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 6.3 (updated February 2022). Cochrane, 2022. Available from training.cochrane.org/handbook.
Dodge 2007
- Dodge JA, Lewis PA, Stanton M, Wilsher J. Cystic fibrosis mortality and survival in the UK: 1947–2003. European Respiratory Journal 2007;29(3):522-6. [DOI] [PubMed] [Google Scholar]
Dwyer 2011
- Dwyer TJ, Alison JA, McKeough ZJ, Daviskas E, Bye PT. Effects of exercise on respiratory flow and sputum properties in patients with cystic fibrosis. Chest 2011;139(4):870-7. [DOI] [PubMed] [Google Scholar]
Dyce 2015
- Dyce P. The diagnosis and management of cystic fibrosis-related diabetes. Journal of Diabetes Nursing 2015;19:92-6. [Google Scholar]
Elborn 1991
- Elborn S, Shale DJ, Britton JR. Cystic fibrosis: current survival and population estimates to the year 2000. Thorax 1991;46:881-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
Elbourne 2002
- Elbourne DR, Altman DG, Higgins JP, Curtin F, Worthington HV, Vail A. Meta-analyses involving cross-over trials: methodological issues. International Journal of Epidemiology 2002;31(1):140-9. [DOI] [PubMed] [Google Scholar]
Fairbrother 2013
- Fairbrother P, Pinnock H, Hanley J, McCloughan L, Sheikh A, Pagliari C, et al. Exploring telemonitoring and self-management by patients with chronic obstructive pulmonary disease: a qualitative study embedded in a randomized controlled trial. Patient Education and Counselling 2013;93(3):403-10. [DOI: 10.1016/j.pec.2013.04.003] [DOI] [PubMed] [Google Scholar]
Gautam 2015
- Gautam V, Shafiq N, Singh M, Ray P, Singhal L, Jaiswal NP, et al. Clinical and in vitro evidence for the antimicrobial therapy in Burkholderia cepacia complex infections. Expert Review of Anti-infective Therapy 2015;13(5):629-63. [DOI] [PubMed] [Google Scholar]
Graham 2019
- Graham BL, Steenbruggen I, Miller MR, Barjaktarevic IZ, Cooper BG, Hall GL, et al. Standardization of spirometry 2019 update. An Official American Thoracic Society and European Respiratory Society Technical statement. American Journal of Respiratory and Critical Care Medicine 2019;200(8):e70-88. [DOI] [PMC free article] [PubMed] [Google Scholar]
Gruet 2017
- Gruet M, Troosters T, Verges S. Peripheral muscle abnormalities in cystic fibrosis: etiology, clinical implications and response to therapeutic interventions. Journal of Cystic Fibrosis 2017;16(5):538-52. [DOI: 10.1016/j.jcf.2017.02.007] [DOI] [PubMed] [Google Scholar]
Gupta 2019
- Gupta S, Mukherjee A, Lodha R, Kabra M, Deepak KK, Khadgawat R, et al. Effects of exercise intervention program on bone mineral accretion in children and adolescents with cystic fibrosis: a randomized controlled trial. Indian Journal of Pediatrics 2019;86(11):987-94. [DOI: 10.1007/s12098-019-03019-x] [DOI] [PubMed] [Google Scholar]
Heijerman 2019
- Heijerman HG, McKone EF, Downey DG, Braeckel E, Rowe SM, Tullis E, et al. Efficacy and safety of the elexacaftor plus tezacaftor plus ivacaftor combination regimen in people with cystic fibrosis homozygous for the F508del mutation: a double-blind, randomised, phase 3 trial. Lancet 2019;394(10212):1940-8. [DOI: 10.1016/S0140-6736(19)32597-8] [DOI] [PMC free article] [PubMed] [Google Scholar]
Higgins 2022a
- Higgins JP, Savović J, Page MJ, Elbers RG, Sterne JA. Chapter 8: Assessing risk of bias in a randomized trial. In: Higgins JP, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, Welch VA, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 6.3 (updated February 2022). Cochrane, 2022. Available from training.cochrane.org/handbook.
Higgins 2022b
- Higgins JP, Li T, Deeks JJ, editor(s). Chapter 6: Choosing eIect measures and computing estimates of eIect. In: Higgins JP, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, Welch VA, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 6.1 (updated February 2022). Cochrane, 2022. Available from training.cochrane.org/handbook/archive/v6.1.
Higgins 2022c
- Higgins JP, Eldridge S, Li T, editor(s). Chapter 23: Including variants on randomized trials. In: Higgins JP, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, Welch VA, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 6.3 (updated February 2022). Cochrane, 2022. Available from training.cochrane.org/handbook.
Keogh 2018
- Keogh RH, Szczesniak R, Taylor-Robinson D, Bilton D. Up-to-date and projected estimates of survival for people with cystic fibrosis using baseline characteristics: a longitudinal study using UK patient registry data. Journal of Cystic Fibrosis 2018;17:218-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
Khoury 2019
- Khoury SR, Evans NS, Ratchford EV. Exercise as medicine. Vascular Medicine 2019;24(4):371-4. [DOI: 10.1177/1358863X19850316] [DOI] [PubMed] [Google Scholar]
Klijn 2004
- Klijn PH, Oudshoorn A, Ent CK, Net J, Kimpen JL, Helders PJ. Effects of anaerobic training in children with cystic fibrosis: a randomized controlled study. Chest 2004;125(4):1299-305. [DOI] [PubMed] [Google Scholar]
Lechtzin 2017
- Lechtzin N, Mayer-Hamblett N, West NE, Allgood S, Wilhelm E, Khan U, et al. Home monitoring of patients with cystic fibrosis to identify and treat acute pulmonary exacerbations. eICE study results. American Journal of Respiratory and Critical Care Medicine 2017;196:1144-51. [DOI] [PMC free article] [PubMed] [Google Scholar]
Lefebvre 2022
- Lefebvre C, Glanville J, Briscoe S, Littlewood A, Marshall C, Metzendorf M, et al. Chapter 4: Searching for and selecting studies. In: Higgins JP, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, Welch VA, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 6.3 (updated February 2022). Cochrane, 2022. Available from training.cochrane.org/handbook/archive/v6.1.
Li 2022
- Li T, Higgins JP, Deeks JJ, editor(s). Chapter 5: Collecting data. In: Higgins JP, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, Welch VA, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 6.3 (updated February 2022). Cochrane, 2022. Available from training.cochrane.org/handbook/archive/v6.1.
Lima 2014
- Lima TR, Guimarães FS, Ferreira AS, Penafortes JT, Almeida VP, Lopes AJ. Correlation between posture, balance control, and peripheral muscle function in adults with cystic fibrosis. Physiotherapy Theory and Practice 2014;30(2):79-84. [DOI] [PubMed] [Google Scholar]
McCabe 2017
- McCabe C, McCann M, Brady AM. Computer and mobile technology interventions for self-management in chronic obstructive pulmonary disease. Cochrane Database of Systematic Reviews 2017, Issue 5. Art. No: CD011425. [DOI: 10.1002/14651858.CD011425] [DOI] [PMC free article] [PubMed] [Google Scholar]
McKenzie 2022
- McKenzie JE, Brennan SE. Chapter 12: Synthesizing and presenting findings using other methods. In: Higgins JP, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, Welch VA, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 6.3 (updated February 2022). The Cochrane Collaboration, 2022. Available from training.cochrane.org/handbook.
Middleton 2019
- Middleton PG, Mall MA, Dřevínek P, Lands LC, McKone EF, Polineni D, et al. Elexacaftor-tezacaftor-ivacaftor for cystic fibrosis with a single Phe508del allele. New England Journal of Medicine 2019;381(19):1809-19. [DOI: 10.1056/NEJMoa1908639] [DOI] [PMC free article] [PubMed] [Google Scholar]
Moor 2022
- Moor CC. Home monitoring for cystic fibrosis: the future is now. Journal of Cystic Fibrosis 2022;21(1):15-7. [DOI: 10.1016/j.jcf.2021.12.005] [DOI] [PubMed] [Google Scholar]
Naehrig 2017
- Naehrig S, Chao CM, Naehrlich L. Cystic Fibrosis. Deutsches Arzteblatt International 2017;21(33-4):564-74. [DOI: 10.3238/arztebl.2017.0564] [DOI] [PMC free article] [PubMed]
Page 2022
- Page MJ, Higgins JP, Sterne JA. Chapter 13: Assessing risk of bias due to missing results in a synthesis. In: Higgins JP, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, Welch VA, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 6.3 (updated February 2022). Cochrane, 2022. Available from training.cochrane.org/handbook.
Quittner 2009
- Quittner AL, Modi AC, Wainwright C, Otto K, Kirihara J, Montgomery AB. Determination of the minimal clinically important difference scores for the Cystic Fibrosis Questionnaire-Revised respiratory symptom scale in two populations of patients with cystic fibrosis and chronic Pseudomonas aeruginosa airway infection. Chest 2009;135(6):1610-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Radtke 2022
- Radtke T, Nevitt SJ, Hebestreit H, Kriemler S. Physical exercise training for cystic fibrosis. Cochrane Database of Systematic Reviews 2022, Issue 8. Art. No: CD002768. [DOI: 10.1002/14651858.CD002768.pub5] [DOI] [PMC free article] [PubMed] [Google Scholar]
RevMan Web 2022 [Computer program]
- Review Manager Web (RevMan Web). Version 4.12.0. The Cochrane Collaboration, 2022. Available at revman.cochrane.org.
Ruppel 2012
- Ruppel GL, Enright PL. Pulmonary function testing. Respiratory Care 2012;57(1):165-75. [DOI] [PubMed] [Google Scholar]
Schermer 2003
- Schermer TR, Jacobs JE, Chavannes NH, Hartman J, Folgering HT, Bottema BJ, et al. Validity of spirometric testing in a general practice population of patients with chronic obstructive pulmonary disease (COPD). Thorax 2003;58(10):861-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Schneiderman 2014
- Schneiderman JE, Wilkes DL, Atenafu EG, Nguyen T, Wells GD, Alarie N, et al. Longitudinal relationship between physical activity and lung health in patients with cystic fibrosis. European Respiratory Journal 2014;43:817-23. [DOI] [PubMed] [Google Scholar]
Schünemann 2022
- Schünemann HJ, Higgins JP, Vist GE, Glasziou P, Akl EA, Skoetz N, et al. Chapter 14: Completing 'Summary of findings' tables and grading the certainty of the evidence. In: Higgins JP, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, Welch VA, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 6.3 (updated February 2022). Cochrane, 2022. Available from training.cochrane.org/handbook.
Seyedmehdi 2013
- Seyedmehdi SM, Attarchi M, Yazdanparast T, Lakeh MM. Quality of spirometry tests and pulmonary function changes among industrial company workers in Iran: a two-year before-and-after study following an intensive training intervention. Primary Care Respiratory Journal 2013;22(1):86-91. [DOI] [PMC free article] [PubMed] [Google Scholar]
Shah 2019
- Shah SG, Nogueras D, Woerden H, Kiparoglou V. Effectiveness of digital technology interventions to reduce loneliness in adults: a protocol for a systematic review and meta-analysis. BMJ Open 2019;9:e032455. [DOI] [PMC free article] [PubMed] [Google Scholar]
Sobnath 2017
- Sobnath DD, Philip N, Kayyali R, Nabhani-Gebara S, Pierscionek B, Vaes AW, et al. Features of a mobile support app for patients with chronic obstructive pulmonary disease: literature review and current applications. JMIR mHealth and uHealth 2017;5(2):e17. [DOI] [PMC free article] [PubMed] [Google Scholar]
Stephenson 2017
- Stephenson AL, Sykes J, Stanojevic S, Quon BS, Marshall BC, Petren K, et al. Survival comparison of patients with cystic fibrosis in Canada and the United States: a population-based cohort study. Annals of Internal Medicine 2017;166(8):537-46. [DOI: 10.7326/M16-0858] [DOI] [PMC free article] [PubMed] [Google Scholar]
van Beurden 2021
- Beurden S, Williams C, Enderby B, Metcalf B, Cockcroft E, Smith J, et al. Feasibility study of a novel digital intervention promoting physical activity in young people with cystic fibrosis. fundingawards.nihr.ac.uk/award/NIHR201999 (accessed prior to 17 May 2023).
Velardo 2017
- Velardo C, Shah SA, Gibson O, Clifford G, Heneghan C, Rutter H, et al. Digital health system for personalized COPD long-term management. BMC Medical Informatics and Decision Making 2017;17:19. [DOI] [PMC free article] [PubMed] [Google Scholar]
Ward 2019
- Ward N, Stiller K, Holland AE. Exercise as a therapeutic intervention for people with cystic fibrosis. Expert Reviews in Respiratory Medicine 2019;13(5):449-58. [DOI: 10.1080/17476348.2019] [DOI] [PubMed] [Google Scholar]
Whitehead 2016
- Whitehead L, Seaton P. The effectiveness of self-management mobile phone and tablet apps in long-term condition management: a systematic review. Journal of Medical Internet Research 2016;18(5):e97. [DOI] [PMC free article] [PubMed] [Google Scholar]
WHO 2011
- World Health Organization. m-Health: new horizons for health through mobile technologies: second global survey on e-health. apps.who.int/iris/handle/10665/44607 (accessed prior to 17 May 2023). [ISBN: 978 92 4 156425 0]
WHO 2020a
- World Health Organization. Draft global strategy on digital health 2020–2024. www.who.int/docs/default-source/documents/gs4dhdaa2a9f352b0445bafbc79ca799dce4d.pdf?sfvrsn=f112ede5_38 (accessed prior to 5 October 2020).
WHO 2020b
- World Health Organization. WHO guidelines on physical activity and sedentary behaviour. www.who.int/publications/i/item/9789240015128 (accessed prior to 17 May 2023). [ISBN: 978-92-4-001512-8 (electronic version)]
Williams 2014
- Williams V, Price J, Hardinge M, Tarassenko L, Farmer A. Using a mobile health application to support self-management in COPD: a qualitative study. British Journal of General Practice 2014;64(624):e392-400. [DOI: 10.3399/bjgp14X680473] [DOI] [PMC free article] [PubMed] [Google Scholar]
Wood 2017
- Wood J, Mulrennan S, Hill K, Cecins N, Morey S, Jenkins S. Telehealth clinics increase access to care for adults with cystic fibrosis living in rural and remote Western Australia. Journal of Telemedicine and Telecare 2017;23(7):673-9. [DOI: 10.1177/1357633X16660646] [DOI] [PubMed] [Google Scholar]
References to other published versions of this review
Pereira Nunes Pinto 2021
- Pereira Nunes Pinto AC, Piva SR, Rocha A, Atallah ÁN, Saconato H, Trevisani VF. Digital technology for delivering and monitoring exercise programs for people with cystic fibrosis. Cochrane Database of Systematic Reviews 2021, Issue 5. Art. No: CD014605. [DOI: 10.1002/14651858.CD014605] [DOI] [PMC free article] [PubMed] [Google Scholar]


