Abstract
Simple Summary
Liver cancer is a prevalent gastrointestinal carcinoma and is closely linked to chronic inflammation, including both hepatic and extrahepatic inflammations. However, the genetic association between inflammatory traits and liver cancer has not been systematically investigated. In this study, we aimed to explore the potential causal associations between immune-mediated diseases, circulating inflammatory biomarkers and cytokines, and liver cancer using Mendelian randomization (MR) analysis. To our best knowledge, this is the most comprehensive MR study on this topic to date, involving more than 200 inflammatory traits. This is an important contribution to the field, as it provides insights into the potential causal inflammatory factors of liver cancer.
Abstract
Liver cancer is closely linked to chronic inflammation. While observational studies have reported positive associations between extrahepatic immune-mediated diseases and systemic inflammatory biomarkers and liver cancer, the genetic association between these inflammatory traits and liver cancer remains elusive and merits further investigation. We conducted a two-sample Mendelian randomization (MR) analysis, using inflammatory traits as exposures and liver cancer as the outcome. The genetic summary data of both exposures and outcome were retrieved from previous genome-wide association studies (GWAS). Four MR methods, including inverse-variance-weighted (IVW), MR-Egger regression, weighted-median, and weighted-mode methods, were employed to examine the genetic association between inflammatory traits and liver cancer. Nine extrahepatic immune-mediated diseases, seven circulating inflammatory biomarkers, and 187 inflammatory cytokines were analyzed in this study. The IVW method suggested that none of the nine immune-mediated diseases were associated with the risk of liver cancer, with odds ratios of 1.08 (95% CI 0.87–1.35) for asthma, 0.98 (95% CI 0.91–1.06) for rheumatoid arthritis, 1.01 (95% CI 0.96–1.07) for type 1 diabetes, 1.01 (95% CI 0.98–1.03) for psoriasis, 0.98 (95% CI 0.89–1.08) for Crohn’s disease, 1.02 (95% CI 0.91–1.13) for ulcerative colitis, 0.91 (95% CI 0.74–1.11) for celiac disease, 0.93 (95% CI 0.84–1.05) for multiple sclerosis, and 1.05 (95% CI 0.97–1.13) for systemic lupus erythematosus. Similarly, no significant association was found between circulating inflammatory biomarkers and cytokines and liver cancer after correcting for multiple testing. The findings were consistent across all four MR methods used in this study. Our findings do not support a genetic association between extrahepatic inflammatory traits and liver cancer. However, larger-scale GWAS summary data and more genetic instruments are needed to confirm these findings.
Keywords: liver cancer, immune-mediated disease, inflammatory biomarkers, inflammatory cytokines
1. Introduction
Liver cancer is a common digestive system malignancy, with approximately 906,000 new cases and 830,000 deaths reported worldwide in 2020 [1]. It has been determined that liver cancer is derived from sustained hepatic inflammation caused by a suite of factors including viral hepatitis, alcohol consumption, and/or fatty liver disease [2]. Moreover, mounting evidence has suggested that extrahepatic chronic inflammations also increase the risk of liver cancer. For instance, previous epidemiological studies have reported a positive association between periodontitis and the risk of liver cancer [3,4]. A population-based cohort study showed that psoriasis, psoriatic arthritis, and rheumatoid arthritis were associated with an increased risk of liver cirrhosis [5]. Based on the UK Biobank cohort, He et al. reported that inflammatory bowel disease and its subtypes Crohn’s disease and ulcerative colitis are significantly associated with an elevated risk of liver cancer [6]. On the other hand, in addition to liver enzymes, numerous blood inflammatory biomarkers have been found to be associated with liver cancer risk. For example, Zhu et al. found that serum levels of C-reactive protein (CRP) are associated with the risk of liver cancer in a dose–response manner [7]. A similar positive association was observed between levels of IL6 and liver cancer [8]. These findings suggested that chronic inflammation is closely involved in hepatic tumorigenesis.
Despite mounting evidence from previous observational studies, it is hard to conclude that chronic immune-mediated diseases and inflammatory biomarkers are causal with the onset of liver cancer because of potential unmeasured confounders or reverse causality in observational studies. The inherent pitfalls of observational studies to some extent impede a full understanding of the association between chronic extrahepatic inflammation and liver cancer, which merits further investigations from other perspectives. Mendelian randomization (MR) analysis that leverages genetic information can serve as a valuable complement to observational studies [9] and has been widely used to explore the causal associations between exposures and diseases [10,11,12,13].
To date, many MR analyses have been performed to assess the association between inflammatory biomarkers and diseases [11,14,15,16]. However, only a few MR analyses have been conducted to assess the association between inflammation and liver cancer [7,17]. Moreover, these MR studies considered only a limited number of inflammatory biomarkers. Given the close relationship between chronic inflammations and liver cancer, it is necessary to systematically examine their impact on liver cancer. To this end, in the current study, we applied two-sample MR methods to assess the genetic associations of ten extrahepatic immune-mediated diseases, seven circulating inflammatory biomarkers (e.g., CRP and leukocyte count), and 228 blood inflammatory cytokines with the risk of liver cancer. Our findings not only provide important complementary information to previous epidemiological studies but also offer novel insights into the pathogenesis of liver cancer.
2. Methods
2.1. Study Design
We conducted a two-sample MR analysis, where the extrahepatic immune-mediated diseases were considered primary exposures, and circulating inflammatory biomarkers and cytokines were secondary exposures. The outcome of interest was liver cancer. For this analysis, we utilized GWAS summary data of the exposures and the outcome from study populations with the same ethnic background (i.e., Europeans), but without any overlap in individuals, to ensure their independence.
2.2. GWAS of Exposures
We examined ten immune-mediated diseases in this study, namely asthma [18], rheumatoid arthritis [19], type 1 diabetes [20], psoriasis [21], Crohn’s disease [22], ulcerative colitis [22], celiac disease [23], multiple sclerosis [24], systemic lupus erythematosus [25], and periodontitis [26]. We also retrieved GWAS summary data of seven circulating inflammatory biomarkers, including CRP [27], leukocyte count, eosinophil count, basophil count, neutrophil count, lymphocyte count, and monocyte count [28]. The details of the selected GWAS for the exposures are presented in Supplementary Tables S1 and S2. All selected GWASs were conducted on individuals of European ancestry, with large sample sizes, and quality control procedures were implemented. Further information on these GWASs can be found in the respective studies.
For circulating inflammatory cytokines, we retrieved the GWAS summary data from a plasma proteome GWAS study that involved 4907 aptamers in 35,559 Icelanders [29]. All plasma samples were measured with the SomaScan version 4 assay (SomaLogic), which contains 5284 aptamers providing a measurement of the relative binding of the plasma sample to each of the aptamers in relative fluorescence units. The genotype information was derived from Illumina SNP chips, long-range phased, and imputed based on the sequenced dataset. After quality control processes, 27.2 million imputed variants with minor allele frequency (MAF) > 0.01% and imputation information > 0.9 were analyzed in GWAS. Each of the 4907 aptamers that were tested underwent rank-inverse normalization, with adjustment for age, sex, and sample age for both the deCODE Health study and the remaining studies. The resulting residuals were then standardized again using rank-inverse normalization and used as phenotypes for genome-wide association testing via a linear mixed model implemented in BOLT-LMM. Our study retrieved GWAS summary data of 228 inflammatory cytokines, including 37 chemokines, 82 interleukins, 44 growth factors, 22 interferons, 37 tumor necrosis factors (TNF), and 6 other types.
2.3. GWAS of Outcome
The summary genetic statistics of liver cancer were retrieved from the FinnGen research project (https://r7.finngen.fi/, accessed on 20 January 2023; Version R7). FinnGen is a public–private partnership project combining genotype data from Finnish biobanks and digital health record data from Finnish health registries. The GWAS for liver cancer, defined as malignant neoplasm of the liver and intrahepatic bile ducts in the FinnGen study, included 518 cases and 238,678 controls without any type of cancer. More information about the GWAS in the FinnGen study can be found on their website (https://finngen.gitbook.io/documentation/, accessed on 20 January 2023). Briefly, DNA samples were genotyped with Illumina (Illumina Inc., San Diego, CA, USA) and Affymetrix arrays (Thermo Fisher Scientific, Santa Clara, CA, USA). In sample-wise quality control steps, individuals with ambiguous gender, high genotype missingness (>5%), excess heterozygosity, and non-Finnish ancestry were excluded. In variant-wise quality control steps, variants with high missingness (>2%), low HWE p-value (<1 × 10−6), and low minor allele count (<3) were excluded. Age, sex, 10 principal components, and FinnGen 1 or 2 chip or legacy genotyping batch were used as covariates in the GWAS, which was implemented using Regenie software (V2.2.4).
2.4. Mendelian Randomization Analysis
2.4.1. Selection of Instrumental Variables
We used a multistep process to select the genetic instrumental variables (IVs). First, we extracted SNPs that were associated with the exposures at the conventional genome-wide association study (GWAS) threshold (p < 5 × 10−8). Next, we clumped the SNPs based on linkage disequilibrium (LD) estimates from the European samples in the 1000 Genomes project, using an LD threshold of R2 < 0.01 and a window size of 10,000 kb. We then extracted the corresponding beta coefficients and standard errors of the selected SNPs from the GWAS of liver cancer. For SNPs that were not present in the GWAS of liver cancer, we retrieved data on an SNP proxy with an LD estimate of R2 > 0.8 with the requested SNP. Finally, we corrected or excluded ambiguous SNPs with inconsistent alleles and palindromic SNPs with ambiguous strands. To ensure the reliability of IVs, we calculated the F-statistics to assess the strength of the relationship between IVs and phenotype using the following equation [30]:
where R2 is the proportion of phenotype that can be explained by the genetic information, k is the number of instruments used in the model, and n is the sample size. An F-statistic > 10 indicates the suitability of the IVs, namely, meeting the first assumption of MR analysis [31].
2.4.2. Statistical Analysis and Sensitivity Analysis
We conducted a two-sample MR analysis using the following steps to investigate the potential causal relationship between immune-mediated diseases and circulating inflammatory biomarkers and liver cancer [32]: (1) harmonizing the exposure data and outcome data by matching the SNPs; (2) using the inverse-variance-weighted (IVW) method to test for between-SNP heterogeneity, with a p value greater than 0.05 for the Q-statistic indicating an absence of heterogeneity; (3) employing the MR-Egger regression intercept test to identify horizontal pleiotropy; (4) using the IVW method to examine the genetic association between exposure and outcome. We also conducted sensitivity analyses using MR-Egger regression, weighted-median, and weighted-mode methods. The MR-Egger regression is based on the InSIDE (INstrument Strength Independent of Direct Effect) assumption and consists of three parts: (i) a test for directional pleiotropy, (ii) a test for a causal effect, and (iii) an estimate of the causal effect [33]. The weighted-median and weighted-mode methods are more robust than IVW and MR-Egger methods when over 50% of SNPs are invalid instruments [34,35]. We also calculated the statistical power for MR analysis using the mRnd website (https://shiny.cnsgenomics.com/mRnd/, accessed on 20 March 2023) [36].
For inflammatory cytokines, we first assessed their relationship with liver cancer using the IVW method with multiple IVs or the Wald ratio test with only one IV. Cytokines that showed significant associations with liver cancer after correcting for multiple testing were further validated using MR-Egger regression, weighted-median, and weighted-mode methods. To validate the results, we performed a repeated analysis using GWAS of liver cancer from the UK Biobank, in which 539 cases and 419,992 controls were included. (https://pan.ukbb.broadinstitute.org/phenotypes/index.html, accessed on 16 May 2023).
All statistical analyses were performed using the R program (v4.1.1). MR analysis was performed using TwoSampleMR and MendelianRandomization packages. The Bonferroni method was employed to correct for multiple testing.
3. Results
3.1. Association between Immune-Mediated Diseases and Liver Cancer
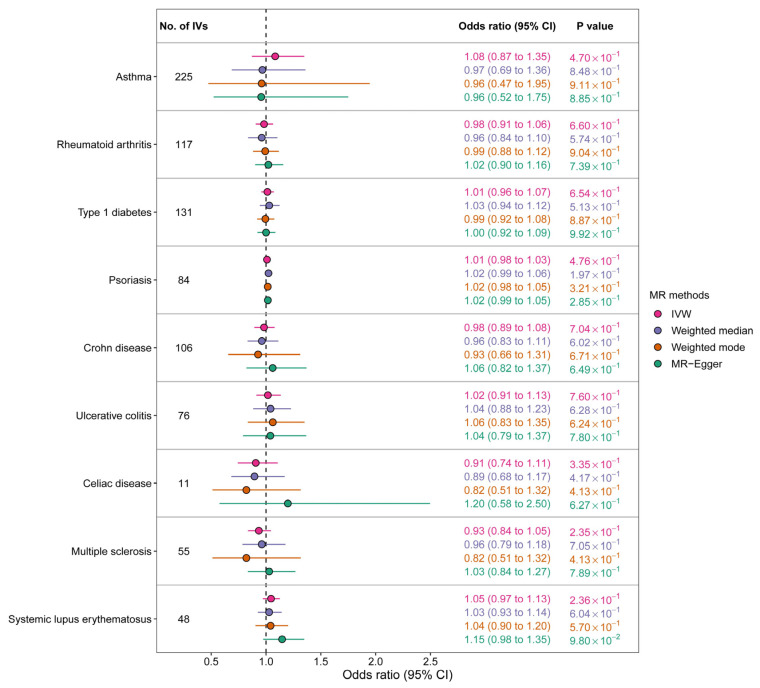
In this study, we utilized a different number of IVs for each immune-mediated disease, with 225, 117, 131, 84, 106, 76, 11, 55, and 48 IVs used for asthma, rheumatoid arthritis, type 1 diabetes, psoriasis, Crohn’s disease, ulcerative colitis, celiac disease, multiple sclerosis, and systemic lupus erythematosus, respectively (Table 1). We excluded periodontitis from the analysis due to the unavailability of valid IVs. All nine exposures had mean-F statistics greater than 10, suggesting a low probability of weak IV bias. Additionally, there was no between-SNP heterogeneity or horizontal pleiotropy detected for any of the exposures using the IVW method or the MR-Egger regression intercept test (Table 1). The statistical power was greater than 95% for detecting an odds ratio (OR) less than 0.9 or greater than 1.1, which decreased to 24–88% when identifying an OR between 0.9 and 1.1.
Table 1.
Statistics of Mendelian randomization analysis for immune-mediated diseases and liver cancer.
| Exposures | No. of IV | F-Statistics | Between-SNP Heterogeneity | Horizontal Pleiotropy |
Statistical Power to Detect OR <0.9 or >1.1 (%) |
Statistical Power to Detect OR between 0.9 and 1.1 (%) | |||
|---|---|---|---|---|---|---|---|---|---|
| Q-Value | p Value | Egger- Intercept |
p Value | ||||||
| Immune-mediated diseases | Asthma | 225 | 588.9 | 217.8 | 0.453 | 0.0043 | 0.785 | 100 | 88 |
| Rheumatoid arthritis | 117 | 98.5 | 114.4 | 0.470 | −0.0093 | 0.431 | 97 | 24 | |
| Type 1 diabetes | 131 | 674.5 | 112.0 | 0.809 | 0.0038 | 0.732 | 100 | 80 | |
| Psoriasis | 84 | 255.6 | 79.7 | 0.104 | −0.019 | 0.331 | 99 | 41 | |
| Crohn’s disease | 106 | 322.1 | 100.4 | 0.306 | −0.0189 | 0.385 | 100 | 64 | |
| Ulcerative colitis | 76 | 98.5 | 73.1 | 0.284 | 0.0019 | 0.936 | 100 | 84 | |
| Celiac disease | 11 | 21.5 | 7.7 | 0.655 | −0.0635 | 0.465 | 100 | 32 | |
| Multiple sclerosis | 55 | 266.8 | 42.3 | 0.852 | −0.0361 | 0.217 | 100 | 55 | |
| Systemic lupus erythematosus | 48 | 198.9 | 54.0 | 0.225 | −0.0352 | 0.218 | 100 | 62 | |
| Circulating inflammatory biomarkers | C-reactive protein | 291 | 458.9 | 365.2 | 0.002 | −0.0036 | 0.674 | 100 | 85 |
| Leukocyte count | 185 | 225.3 | 209.1 | 0.099 | 0.0224 | 0.095 | 100 | 87 | |
| Eosinophil count | 208 | 198.5 | 203.5 | 0.556 | −0.0028 | 0.822 | 100 | 90 | |
| Basophil count | 83 | 110.3 | 97.8 | 0.112 | −0.0027 | 0.883 | 100 | 71 | |
| Neutrophil count | 162 | 196.6 | 194.3 | 0.038 | −0.0024 | 0.871 | 100 | 80 | |
| Lymphocyte count | 193 | 288.3 | 186.8 | 0.592 | 0.0013 | 0.922 | 100 | 84 | |
| Monocyte count | 266 | 300.7 | 282.3 | 0.223 | −0.0070 | 0.447 | 100 | 92 | |
IV, instrumental variables; OR, odds ratio.
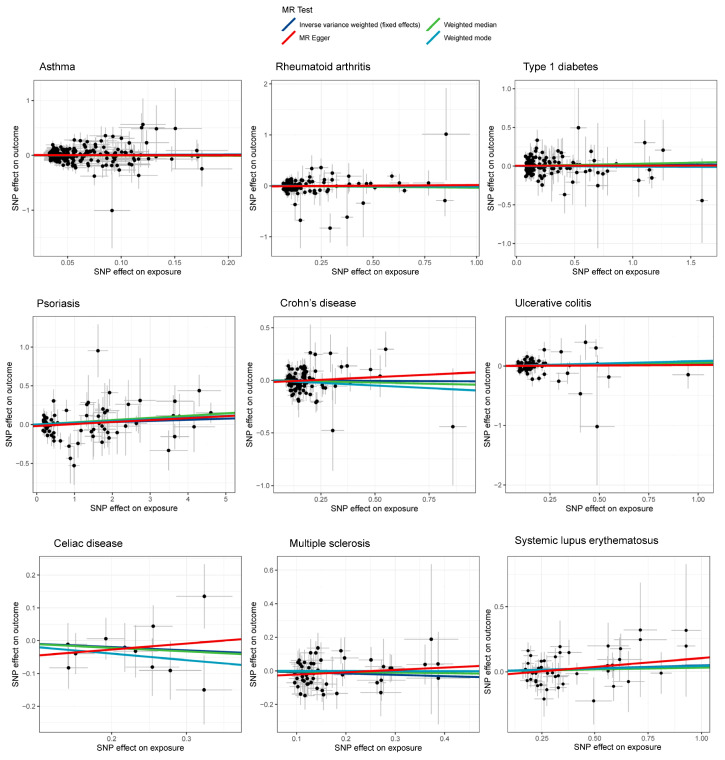
According to the IVW method, there was no significant association between any of the nine immune-mediated diseases and the risk of liver cancer. The OR estimates were as follows: 1.08 (95% CI 0.87–1.35) for asthma, 0.98 (95% CI 0.91–1.06) for rheumatoid arthritis, 1.01 (95% CI 0.96–1.07) for type 1 diabetes, 1.01 (95% CI 0.98–1.03) for psoriasis, 0.98 (95% CI 0.89–1.08) for Crohn’s disease, 1.02 (95% CI 0.91–1.13) for ulcerative colitis, 0.91 (95% CI 0.74–1.11) for celiac disease, 0.93 (95% CI 0.84–1.05) for multiple sclerosis, and 1.05 (95% CI 0.97–1.13) for systemic lupus erythematosus (Figure 1). The results obtained using the other three MR methods, namely MR-Egger regression, weighted-median, and weighted-mode, were consistent with those of the IVW method. Figure 2 displays the scatter plots depicting the SNP effects on both the exposures and the outcome.
Figure 1.
Genetic association between immune-mediated diseases and liver cancer according to Mendelian randomization analysis (IV, instrumental variable; IVW, inverse-variance-weighted method).
Figure 2.
Scatter plot showing the SNP effects on both immune-mediated diseases and liver cancer (The gray error bars denote the 95% confidence intervals of the effects).
3.2. Association between Circulating Inflammatory Biomarkers and Liver Cancer
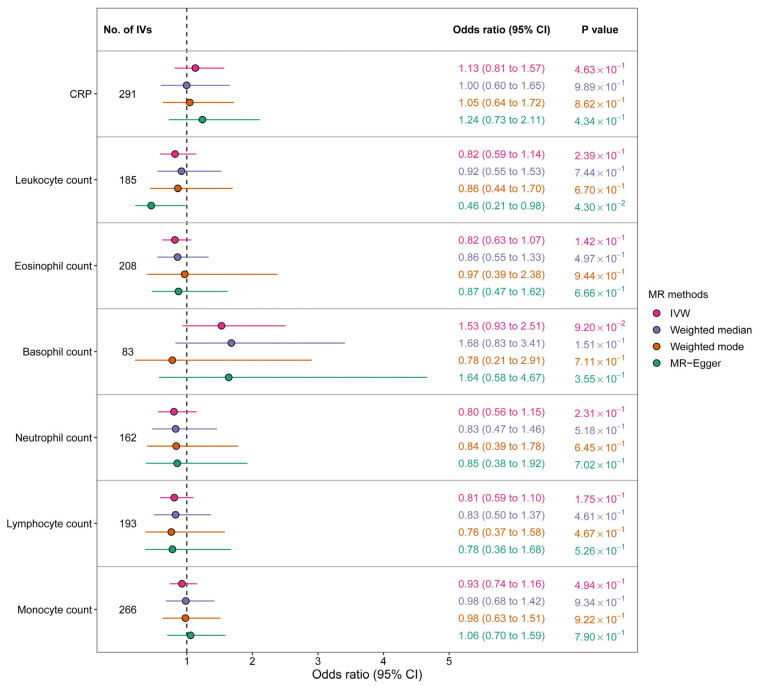
Table 1 displays the number of instrumental variables (IVs) used for each inflammatory biomarker in the MR analysis: 291 for CRP, 185 for leukocyte count, 208 for eosinophil count, 83 for basophil count, 162 for neutrophil count, 193 for lymphocyte count, and 266 for monocyte count. We observed no evidence of weak-IV bias based on the mean F-statistics, and there was no significant horizontal pleiotropy for any of the exposures. However, we detected significant between-SNP heterogeneity for CRP (p = 0.002) and neutrophil count (p = 0.038). The statistical power was high, with >70% power to detect an odds ratio (OR) between 0.9 and 1.1 and >95% power to detect an OR > 1.1 or <0.9.
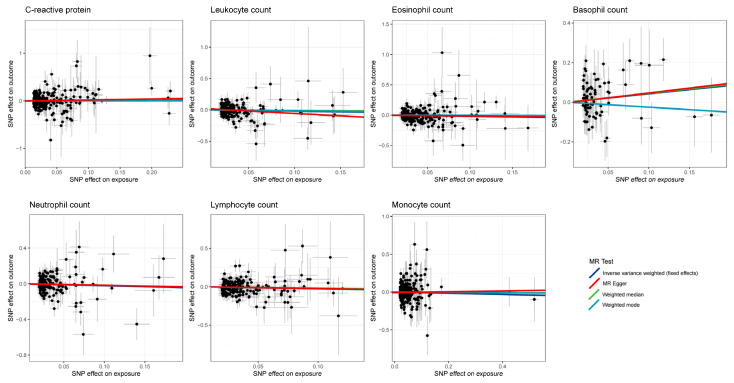
Our MR analysis did not reveal any significant associations between circulating inflammatory biomarkers and liver cancer, with an OR of 1.13 (95% CI 0.81–1.57) for CRP, 0.82 (95% CI 0.59–1.14) for leukocyte count, 0.82 (95% CI 0.63–1.07) for eosinophil count, 1.53 (95% CI 0.93–2.51) for basophil count, 0.80 (95% CI 0.56–1.15) for neutrophil count, 0.81 (95% CI 0.59–1.10) for lymphocyte count, and 0.93 (95% CI 0.74–1.16) for monocyte count (Figure 3). The results were consistent across the other three MR methods. Figure 4 displays scatter plots of SNP effects on both exposures and outcomes.
Figure 3.
Genetic association between circulating inflammatory biomarkers and liver cancer according to Mendelian randomization analysis (CRP, C-reactive protein; IV, instrumental variable; IVW, inverse-variance-weighted method).
Figure 4.
Scatter plot showing the SNP effects on both circulating inflammatory biomarkers and liver cancer (The gray error bars denote the 95% confidence intervals of the effects).
3.3. Association between Circulating Inflammatory Cytokines and Liver Cancer
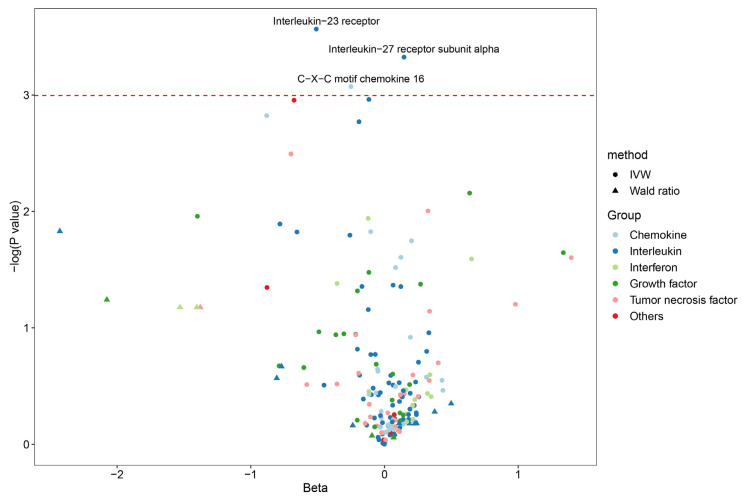
After performing quality control, we included 187 inflammatory cytokines (30 chemokines, 72 interleukins, 31 growth factors, 17 interferons, 31 TNFs, and 6 others) for MR analysis (Supplementary Table S3). Among these, 166 cytokines had two or more valid genetic variants, while the remaining 21 cytokines only had one valid IV. We observed significant associations between the risk of liver cancer and three cytokines: interleukin-23 receptor (OR = 0.60, 95% CI 0.38–0.95, p = 0.028), interleukin-27 receptor subunit alpha (OR = 1.16, 95% CI 1.01–1.13, p = 0.036), and C-X-C motif chemokine 16 (OR = 0.78, 95% CI 0.61–1.00, p = 0.046) (Figure 5; Supplementary Table S3). However, these associations were not statistically significant after Bonferroni correction for multiple testing (0.05/187). The repeated MR analysis using GWAS summary data from the UK Biobank yielded consistent results compared to our main results (Supplementary Table S4). Although a few suggestive association was detected, for example, CXCL17 (OR = 1.45, 95 CI% 1.06–1.79, p = 0.011), no significant association remained after correcting for multiple testing.
Figure 5.
Genetic association between circulating inflammatory cytokines and liver cancer according to Mendelian randomization analysis (We only show the point estimate in this plot. The red dotted line denotes the threshold of p value < 0.05).
4. Discussion
In this study, we conducted a comprehensive analysis of the genetic association between a range of chronic inflammatory diseases, biomarkers, and cytokines with the risk of liver cancer. Our study included nine extrahepatic inflammatory diseases, seven circulating inflammatory biomarkers, and 187 inflammatory cytokines, making it the most comprehensive investigation to date on the impact of systemic inflammation on liver cancer. Although we found three cytokines that showed a potential association with liver cancer at a significance threshold of p < 0.05, these associations did not remain significant after correcting for multiple testing using the Bonferroni method. Therefore, the genetic evidence did not support any causal relationship between inflammatory traits and liver cancer risk. These findings suggest that the previously reported correlations from observational studies might be confounded.
Numerous risk factors for liver cancer have been extensively investigated and well-determined [37], which has greatly contributed to the prevention of liver cancer in the general population. However, it is important to note that risk factors are not equivalent to etiologies, and only a few risk factors, such as viral hepatitis and aflatoxin, have been defined as etiologies for liver cancer. Understanding the etiologies for liver cancer is crucial to comprehending the disease pathogenesis and developing cost-effective approaches to prevent the development of this lethal disease. Nevertheless, conventional observational studies, including prospective cohort studies, may have inherent limitations in discovering etiology. Confounding, reverse causation, and various biases can affect the associations to varying degrees, and even with careful study design and statistical adjustment, incorrect causal inference is possible [38]. In comparison, MR analysis has several strengths: it is immune to confounders since genotypes are allocated during meiosis, less affected by information bias as genotype information can be accurately obtained through sequencing, and easy to perform as it only requires GWAS summary data instead of individual data [39]. Furthermore, MR interpretation of a statistically significant association as evidence that the exposure has a causal effect on the outcome is an important feature [40]. In this regard, MR analysis can serve as a valuable complement to observational studies.
Since liver cancer is closely linked to chronic inflammation [41], we applied a set of MR methods to examine the association between inflammatory traits and liver cancer and aimed to determine potential causal inflammatory factors for liver cancer. Previous population-based studies have investigated the association between certain inflammatory traits and liver cancer. For instance, a systematic review and meta-analysis of epidemiological studies reported a 90% increased risk of liver cancer in patients with psoriasis compared to healthy controls [42]. An umbrella meta-analysis reported that Crohn’s disease was associated with a 2.18-fold increased risk of liver cancer [43]. Similar positive associations were observed for type 1 diabetes and systemic lupus erythematosus [44,45]. Kim et al. reported a significantly negative association (multivariable-adjusted hazard ratio = 0.80) between asthma and liver cancer [46]. However, using MR analyses, we did not detect a genetic association between these inflammatory diseases and liver cancer. Given that complex diseases such as liver cancer and Crohn’s disease are partly determined by genetic factors, the null genetic association suggests that there may be no overlap in the genetic architecture between these two conditions.
A similar null association was observed between genetically predicted inflammatory biomarkers and cytokines and liver cancer. For example, our MR analysis did not find a genetic association between genetically predicted CRP and liver cancer, although previous epidemiological studies have identified CRP as an independent risk factor for liver cancer [7,47]. Our MR estimate for CRP was consistent with a previous MR study [7]. Similarly, we found no genetic association between vascular endothelial growth factor (VEGF) and liver cancer, consistent with the results of a study by Wu et al. [17], though this cytokine has been demonstrated to be involved in hepatic tumorigenesis [48]. The lack of association between circulating inflammatory traits and liver cancer suggests that these biomarkers were unlikely to be the cause of liver cancer, but rather acted as response markers to environmental risk factors (such as smoking, alcohol consumption, aging, and obesity), which can induce chronic low-grade inflammation. However, this hypothesis requires further validation because only a small proportion of the variance of the biomarkers can be explained by the genetic instrumental variable [7].
The main strength of our study is that it is the most comprehensive study to date using MR analysis to examine the association between over 200 inflammatory traits and liver cancer. However, we acknowledge several limitations of our study. First, we obtained GWAS summary data from the FinnGen project, which includes only 518 liver cancer cases. While the sample size was large, a small number of cases can limit the statistical power of GWAS, potentially leading to missed genetic signals [39]. We should bear in mind that the MR estimates were largely depended on robust IVs. Unfortunately, there is no large-scale GWAS with a standardized design for liver cancer to date. Second, only participants of European ancestry were included in this study, which compromise the generalization of our results to other ancestry populations. Third, the associations between inflammatory traits and liver cancer may be varied across liver cancer etiologies. Due to the lack of GWAS summary data for etiology-specific liver cancer, we did not assess the influence of liver cancer etiologies in this study. Moreover, we cannot assess the associations between exposures and liver cancer according to its histological subtypes due to the data unavailability. Finally, our estimates might also be subject to the inherent shortcomings of MR analysis such as selection bias [49]. Genetic variants which are related to specific phenotypes might also be related to participation [50]. As such, individuals at high genetic risk for inflammatory diseases may be more likely to drop out of the cohort due to a higher susceptibility to these conditions than those at low genetic risk.
5. Conclusions
To conclude, our comprehensive MR analysis did not reveal any evidence of a causal effect between genetically predicted immune-mediated diseases and circulating inflammatory biomarkers and cytokines and liver cancer. However, due to the limitations of our study, including a relatively small number of liver cancer cases and the use of only European ancestry populations, our findings should be confirmed by further studies using larger-scale GWAS summary data and more genetic instruments.
Abbreviations
MR: Mendelian Randomization; GWAS: Genome-wide association study; IVW: Inverse variance weighted.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/cancers15112930/s1, Table S1. GWAS information for eight immune-mediated diseases. Table S2. GWAS information for circulating inflammatory biomarkers. Table S3. Associations of circulating inflammatory cytokines with liver cancer according to Mendelian randomization analysis. Table S4. Associations of inflammatory traits with liver cancer (UKBB) according to Mendelian randomization analysis.
Author Contributions
U.D.K., Y.Z. and Q.Y. (Qiushi Yin) conceived the idea for the study. Q.Y. (Qiushi Yin), X.H. and Q.Y. (Qiuxi Yang) obtained the genetic data. Q.Y. (Qiushi Yin), X.H. and Q.Y. (Qiuxi Yang) performed the data analyses. Z.L., S.L. and W.S. performed the data visualization. U.D.K., Y.Z., Q.S., W.F., L.W. and X.H. interpreted the results of the data analyses. Q.Y. (Qiushi Yin), X.H. and Q.Y. (Qiuxi Yang) wrote the manuscript. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
This study was based on publicly available data and the ethics approval is waived.
Informed Consent Statement
All methods were carried out following STROBE-MR guidelines and regulations.
Data Availability Statement
The datasets analyzed during the current study are available in Supplementary Tables S1 and S2.
Conflicts of Interest
The authors declare that they have no competing interest.
Funding Statement
This research received no external funding.
Footnotes
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
References
- 1.Sung H., Ferlay J., Siegel R.L., Laversanne M., Soerjomataram I., Jemal A., Bray F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021;71:209–249. doi: 10.3322/caac.21660. [DOI] [PubMed] [Google Scholar]
- 2.Li X., Ramadori P., Pfister D., Seehawer M., Zender L., Heikenwalder M. The immunological and metabolic landscape in primary and metastatic liver cancer. Nat. Rev. Cancer. 2021;21:541–557. doi: 10.1038/s41568-021-00383-9. [DOI] [PubMed] [Google Scholar]
- 3.Chen Y., Yang Y.C., Zhu B.L., Wu C.C., Lin R.F., Zhang X. Association between periodontal disease, tooth loss and liver diseases risk. J. Clin. Periodontol. 2020;47:1053–1063. doi: 10.1111/jcpe.13341. [DOI] [PubMed] [Google Scholar]
- 4.Helenius-Hietala J., Suominen A.L., Ruokonen H., Knuuttila M., Puukka P., Jula A., Meurman J.H., Åberg F. Periodontitis is associated with incident chronic liver disease-A population-based cohort study. Liver Int. 2019;39:583–591. doi: 10.1111/liv.13985. [DOI] [PubMed] [Google Scholar]
- 5.Ogdie A., Grewal S.K., Noe M.H., Shin D.B., Takeshita J., Chiesa Fuxench Z.C., Carr R.M., Gelfand J.M. Risk of Incident Liver Disease in Patients with Psoriasis, Psoriatic Arthritis, and Rheumatoid Arthritis: A Population-Based Study. J. Investig. Dermatol. 2018;138:760–767. doi: 10.1016/j.jid.2017.10.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.He M.M., Lo C.H., Wang K., Polychronidis G., Wang L., Zhong R., Knudsen M.D., Fang Z., Song M. Immune-Mediated Diseases Associated with Cancer Risks. JAMA Oncol. 2022;8:209–219. doi: 10.1001/jamaoncol.2021.5680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zhu M., Ma Z., Zhang X., Hang D., Yin R., Feng J., Xu L., Shen H. C-reactive protein and cancer risk: A pan-cancer study of prospective cohort and Mendelian randomization analysis. BMC Med. 2022;20:301. doi: 10.1186/s12916-022-02506-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Aleksandrova K., Bamia C., Drogan D., Lagiou P., Trichopoulou A., Jenab M., Fedirko V., Romieu I., Bueno-De-Mesquita H.B., Pischon T., et al. The association of coffee intake with liver cancer risk is mediated by biomarkers of inflammation and hepatocellular injury: Data from the European Prospective Investigation into Cancer and Nutrition. Am. J. Clin. Nutr. 2015;102:1498–1508. doi: 10.3945/ajcn.115.116095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Birney E. Mendelian Randomization. Cold Spring Harb. Perspect. Med. 2022;12:a041302. doi: 10.1101/cshperspect.a041302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Liu Z., Song C., Suo C., Fan H., Zhang T., Jin L., Chen X. Alcohol consumption and hepatocellular carcinoma: Novel insights from a prospective cohort study and nonlinear Mendelian randomization analysis. BMC Med. 2022;20:413. doi: 10.1186/s12916-022-02622-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hartwig F.P., Borges M.C., Horta B.L., Bowden J., Davey Smith G. Inflammatory Biomarkers and Risk of Schizophrenia: A 2-Sample Mendelian Randomization Study. JAMA Psychiatry. 2017;74:1226–1233. doi: 10.1001/jamapsychiatry.2017.3191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Liu Z., Suo C., Fan H., Zhang T., Jin L., Chen X. Dissecting causal relationships between nonalcoholic fatty liver disease proxied by chronically elevated alanine transaminase levels and 34 extrahepatic diseases. Metabolism. 2022;135:155270. doi: 10.1016/j.metabol.2022.155270. [DOI] [PubMed] [Google Scholar]
- 13.Yuan S., Larsson S.C. An atlas on risk factors for type 2 diabetes: A wide-angled Mendelian randomisation study. Diabetologia. 2020;63:2359–2371. doi: 10.1007/s00125-020-05253-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bouras E., Karhunen V., Gill D., Huang J., Haycock P.C., Gunter M.J., Johansson M., Brennan P., Key T., Lewis S.J., et al. Circulating inflammatory cytokines and risk of five cancers: A Mendelian randomization analysis. BMC Med. 2022;20:3. doi: 10.1186/s12916-021-02193-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wang Q., Shi Q., Lu J., Wang Z., Hou J. Causal relationships between inflammatory factors and multiple myeloma: A bidirectional Mendelian randomization study. Int. J. Cancer. 2022;151:1750–1759. doi: 10.1002/ijc.34214. [DOI] [PubMed] [Google Scholar]
- 16.Xiang M., Wang Y., Gao Z., Wang J., Chen Q., Sun Z., Ling J., Xu J. Exploring causal correlations between inflammatory cytokines and systemic lupus erythematosus: A Mendelian randomization. Front. Immunol. 2022;13:985729. doi: 10.3389/fimmu.2022.985729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wu H., Ma T., Li D., He M., Wang H., Cui Y. Circulating vascular endothelial growth factor and cancer risk: A bidirectional mendelian randomization. Front. Genet. 2022;13:981032. doi: 10.3389/fgene.2022.981032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Han Y., Jia Q., Jahani P.S., Hurrell B.P., Pan C., Huang P., Gukasyan J., Woodward N.C., Eskin E., Gilliland F.D., et al. Genome-wide analysis highlights contribution of immune system pathways to the genetic architecture of asthma. Nat. Commun. 2020;11:1776. doi: 10.1038/s41467-020-15649-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ha E., Bae S.C., Kim K. Large-scale meta-analysis across East Asian and European populations updated genetic architecture and variant-driven biology of rheumatoid arthritis, identifying 11 novel susceptibility loci. Ann. Rheum. Dis. 2021;80:558–565. doi: 10.1136/annrheumdis-2020-219065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chiou J., Geusz R.J., Okino M.L., Han J.Y., Miller M., Melton R., Beebe E., Benaglio P., Huang S., Korgaonkar K., et al. Interpreting type 1 diabetes risk with genetics and single-cell epigenomics. Nature. 2021;594:398–402. doi: 10.1038/s41586-021-03552-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tsoi L.C., Spain S.L., Knight J., Ellinghaus E., Stuart P.E., Capon F., Ding J., Li Y., Tejasvi T., Gudjonsson J.E., et al. Identification of 15 new psoriasis susceptibility loci highlights the role of innate immunity. Nat. Genet. 2012;44:1341–1348. doi: 10.1038/ng.2467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.de Lange K.M., Moutsianas L., Lee J.C., Lamb C.A., Luo Y., Kennedy N.A., Jostins L., Rice D.L., Gutierrez-Achury J., Ji S.G., et al. Genome-wide association study implicates immune activation of multiple integrin genes in inflammatory bowel disease. Nat. Genet. 2017;49:256–261. doi: 10.1038/ng.3760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dubois P.C., Trynka G., Franke L., Hunt K.A., Romanos J., Curtotti A., Zhernakova A., Heap G.A., Adány R., Aromaa A., et al. Multiple common variants for celiac disease influencing immune gene expression. Nat. Genet. 2010;42:295–302. doi: 10.1038/ng.543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Beecham A.H., Patsopoulos N.A., Xifara D.K., Davis M.F., Kemppinen A., Cotsapas C., Shah T.S., Spencer C., Booth D., Goris A., et al. Analysis of immune-related loci identifies 48 new susceptibility variants for multiple sclerosis. Nat. Genet. 2013;45:1353–1360. doi: 10.1038/ng.2770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bentham J., Morris D.L., Graham D.S.C., Pinder C.L., Tombleson P., Behrens T.W., Martín J., Fairfax B.P., Knight J.C., Chen L., et al. Genetic association analyses implicate aberrant regulation of innate and adaptive immunity genes in the pathogenesis of systemic lupus erythematosus. Nat. Genet. 2015;47:1457–1464. doi: 10.1038/ng.3434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Shungin D., Haworth S., Divaris K., Agler C.S., Kamatani Y., Keun Lee M., Grinde K., Hindy G., Alaraudanjoki V., Pesonen P., et al. Genome-wide analysis of dental caries and periodontitis combining clinical and self-reported data. Nat. Commun. 2019;10:2773. doi: 10.1038/s41467-019-10630-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sinnott-Armstrong N., Tanigawa Y., Amar D., Mars N., Benner C., Aguirre M., Venkataraman G.R., Wainberg M., Ollila H.M., Kiiskinen T., et al. Genetics of 35 blood and urine biomarkers in the UK Biobank. Nat. Genet. 2021;53:185–194. doi: 10.1038/s41588-020-00757-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Astle W.J., Elding H., Jiang T., Allen D., Ruklisa D., Mann A.L., Mead D., Bouman H., Riveros-Mckay F., Kostadima M.A., et al. The Allelic Landscape of Human Blood Cell Trait Variation and Links to Common Complex Disease. Cell. 2016;167:1415–1429.e19. doi: 10.1016/j.cell.2016.10.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ferkingstad E., Sulem P., Atlason B.A., Sveinbjornsson G., Magnusson M.I., Styrmisdottir E.L., Gunnarsdottir K., Helgason A., Oddsson A., Halldorsson B.V., et al. Large-scale integration of the plasma proteome with genetics and disease. Nat. Genet. 2021;53:1712–1721. doi: 10.1038/s41588-021-00978-w. [DOI] [PubMed] [Google Scholar]
- 30.Zhu G., Zhou S., Xu Y., Gao R., Li H., Zhai B., Liu X., He Y., Wang X., Han G., et al. Mendelian randomization study on the causal effects of omega-3 fatty acids on rheumatoid arthritis. Clin. Rheumatol. 2022;41:1305–1312. doi: 10.1007/s10067-022-06052-y. [DOI] [PubMed] [Google Scholar]
- 31.Sanderson E., Spiller W., Bowden J. Testing and correcting for weak and pleiotropic instruments in two-sample multivariable Mendelian randomization. Stat. Med. 2021;40:5434–5452. doi: 10.1002/sim.9133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wang X., Wang X., Wang H., Yang M., Dong W., Shao D. Association between psoriasis and lung cancer: Two-sample Mendelian randomization analyses. BMC Pulm. Med. 2023;23:4. doi: 10.1186/s12890-022-02297-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Burgess S., Thompson S.G. Interpreting findings from Mendelian randomization using the MR-Egger method. Eur. J. Epidemiol. 2017;32:377–389. doi: 10.1007/s10654-017-0255-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bowden J., Davey Smith G., Haycock P.C., Burgess S. Consistent Estimation in Mendelian Randomization with Some Invalid Instruments Using a Weighted Median Estimator. Genet. Epidemiol. 2016;40:304–314. doi: 10.1002/gepi.21965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hartwig F.P., Davey Smith G., Bowden J. Robust inference in summary data Mendelian randomization via the zero modal pleiotropy assumption. Int. J. Epidemiol. 2017;46:1985–1998. doi: 10.1093/ije/dyx102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Brion M.J., Shakhbazov K., Visscher P.M. Calculating statistical power in Mendelian randomization studies. Int. J. Epidemiol. 2013;42:1497–1501. doi: 10.1093/ije/dyt179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Llovet J.M., Kelley R.K., Villanueva A., Singal A.G., Pikarsky E., Roayaie S., Lencioni R., Koike K., Zucman-Rossi J., Finn R.S. Hepatocellular carcinoma. Nat. Rev. Dis. Prim. 2021;7:6. doi: 10.1038/s41572-020-00240-3. [DOI] [PubMed] [Google Scholar]
- 38.Fewell Z., Davey Smith G., Sterne J.A. The impact of residual and unmeasured confounding in epidemiologic studies: A simulation study. Am. J. Epidemiol. 2007;166:646–655. doi: 10.1093/aje/kwm165. [DOI] [PubMed] [Google Scholar]
- 39.Davey Smith G., Hemani G. Mendelian randomization: Genetic anchors for causal inference in epidemiological studies. Hum. Mol. Genet. 2014;23:R89–R98. doi: 10.1093/hmg/ddu328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zuber V., Grinberg N.F., Gill D., Manipur I., Slob E.A.W., Patel A., Wallace C., Burgess S. Combining evidence from Mendelian randomization and colocalization: Review and comparison of approaches. Am. J. Hum. Genet. 2022;109:767–782. doi: 10.1016/j.ajhg.2022.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Yang Y.M., Kim S.Y., Seki E. Inflammation and Liver Cancer: Molecular Mechanisms and Therapeutic Targets. Semin. Liver Dis. 2019;39:26–42. doi: 10.1055/s-0038-1676806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Pouplard C., Brenaut E., Horreau C., Barnetche T., Misery L., Richard M.A., Aractingi S., Aubin F., Cribier B., Joly P., et al. Risk of cancer in psoriasis: A systematic review and meta-analysis of epidemiological studies. J. Eur. Acad. Dermatol. Venereol. 2013;27((Suppl. 3)):36–46. doi: 10.1111/jdv.12165. [DOI] [PubMed] [Google Scholar]
- 43.Piovani D., Hassan C., Repici A., Rimassa L., Carlo-Stella C., Nikolopoulos G.K., Riboli E., Bonovas S. Risk of Cancer in Inflammatory Bowel Diseases: Umbrella Review and Reanalysis of Meta-analyses. Gastroenterology. 2022;163:671–684. doi: 10.1053/j.gastro.2022.05.038. [DOI] [PubMed] [Google Scholar]
- 44.Sona M.F., Myung S.K., Park K., Jargalsaikhan G. Type 1 diabetes mellitus and risk of cancer: A meta-analysis of observational studies. Jpn. J. Clin. Oncol. 2018;48:426–433. doi: 10.1093/jjco/hyy047. [DOI] [PubMed] [Google Scholar]
- 45.Zhang M., Wang Y., Wang Y., Bai Y., Gu D. Association between Systemic Lupus Erythematosus and Cancer Morbidity and Mortality: Findings from Cohort Studies. Front. Oncol. 2022;12:860794. doi: 10.3389/fonc.2022.860794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kim J.A., Park S.J., Choi S., Chang J., Jeong S., Ahn J.C., Lee G., Son J.S., Park S.M. Association of the presence of allergic disease with subsequent risk of liver cancer in a nationwide retrospective cohort among Koreans. Sci. Rep. 2022;12:9856. doi: 10.1038/s41598-022-14147-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Song M., Liu T., Liu H., Zhang Q., Zhang Q., Wang Y., Ma X., Cao L., Shi H. Association between metabolic syndrome, C-reactive protein, and the risk of primary liver cancer: A large prospective study. BMC Cancer. 2022;22:853. doi: 10.1186/s12885-022-09939-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ma L., Hernandez M.O., Zhao Y., Mehta M., Tran B., Kelly M., Rae Z., Hernandez J.M., Davis J.L., Martin S.P., et al. Tumor Cell Biodiversity Drives Microenvironmental Reprogramming in Liver Cancer. Cancer Cell. 2019;36:418–430.e6. doi: 10.1016/j.ccell.2019.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Taylor A.E., Jones H.J., Sallis H., Euesden J., Stergiakouli E., Davies N.M., Zammit S., Lawlor D.A., Munafò M.R., Davey Smith G., et al. Exploring the association of genetic factors with participation in the Avon Longitudinal Study of Parents and Children. Int. J. Epidemiol. 2018;47:1207–1216. doi: 10.1093/ije/dyy060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Wang J., Zhuge J., Feng D., Zhang B., Xu J., Zhao D., Fei Z., Huang X., Shi W. Mendelian randomization study of circulating lipids and biliary tract cancer among East Asians. BMC Cancer. 2022;22:273. doi: 10.1186/s12885-022-09382-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets analyzed during the current study are available in Supplementary Tables S1 and S2.