Abstract
Simple Summary
We prospectively analyzed COVID-19 morbidity and severity in 200 consecutive patients with CLL. Increased COVID-19 morbidity was observed in vaccinated patients with CLL. With a median follow-up of 23.4 months, 41% of patients experienced COVID-19. Most patients, 36.5%, experienced COVID-19 during the Omicron pandemic, 26% of patients were hospitalized, and 4% died. Moreover, 10% had re-infections. In multivariate analysis of elderly patients, TP53 disrupted, heavily pre-treated, and those in early treatment with targeted agents showed increased vulnerability to COVID-19. Given the persistent risk of infection due to the continuous emergence of SARS-CoV-2 variants, our results support the importance of new vaccines and protective measures to prevent and mitigate COVID-19 in patients with CLL.
Abstract
High morbidity and mortality due to COVID-19 were described in the pre-vaccination era in patients with chronic lymphocytic leukemia (CLL). To evaluate COVID-19 morbidity after the SARS-CoV-2 vaccine, we carried out a prospective study in 200 CLL patients. The median age of patients was 70 years; 35% showed IgG levels ≤ 550 mg/dL, 61% unmutated IGHV, and 34% showed TP53 disruption. Most patients, 83.5%, were previously treated, including 36% with ibrutinib and 37.5% with venetoclax. The serologic response rates to the second and third dose of the vaccine were 39% and 53%, respectively. With a median follow-up of 23.4 months, 41% of patients experienced COVID-19, 36.5% during the Omicron pandemic, and 10% had subsequent COVID-19 events. Severe COVID-19 requiring hospitalization was recorded in 26% of patients, and 4% died. Significant and independent factors associated with the response to the vaccine and vulnerability to COVID-19 were age (OR: 0.93; HR: 0.97) and less than 18 months between the start of targeted agents and vaccine (OR: 0.17; HR: 0.31). TP53 mutation and ≥two prior treatments also emerged as significant and independent factors associated with an increased risk of developing COVID-19 (HR: 1.85; HR: 2.08). No statistical difference in COVID-19 morbidity was found in patients with or without antibody response to the vaccine (47.5% vs. 52.5%; p = 0.21). Given the persistent risk of infection due to the continuous emergence of SARS-CoV-2 variants, our results support the importance of new vaccines and protective measures to prevent and mitigate COVID-19 in CLL patients.
Keywords: chronic lymphocytic leukemia, COVID-19, Omicron
1. Introduction
During the early stages of the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) pandemic in 2020, before the vaccination strategy, patients with hematologic malignancies paid a high toll in terms of rates of patients with severe and fatal cases of coronavirus disease 2019 (COVID-19) [1,2,3]. Given the impaired humoral and cellular immunity due to the disease and the immunosuppressive effects of treatments, significant morbidity and mortality rates were recorded in patients with chronic lymphocytic leukemia (CLL) in 2020 in the early phases of the pandemic [4,5,6,7]. Impaired immunity was also responsible for suboptimal responses to the SARS-CoV-2 vaccine in CLL patients [8,9,10,11,12,13,14]. The global use of the SARS-CoV-2 vaccines and the emergence of mutated variants have modified the current pandemic scenario. Little is known about the long-term benefit of the vaccination strategy in patients with CLL and how the spread of new, highly transmissible variants impacted this patient population. We carried out a multicenter study in vaccinated patients with CLL to prospectively evaluate the morbidity and severity of symptomatic COVID-19 during the pandemic’s different phases, including the Omicron pandemic.
2. Patients and Methods
2.1. Patients and Methods
During the SARS-CoV-2 pandemic, the main hematology centers of our country were invited to participate in this study during an online meeting and ten agreed to participate. This study included 200 consecutive CLL patients who were vaccinated against SARS-CoV-2 between 1 February 2021, and 16 July 2021.
Eligibility criteria included CLL diagnosis [15], age ≥18 years, no clinical nor serologic signs of prior SARS-CoV-2 infection, and at least two doses of the BNT162b2 mRNA SARS-CoV-2 vaccine, the first vaccine authorized in Italy and widely available at vaccination centers.
The primary endpoint of this study was to evaluate the incidence, severity, and mortality of COVID-19 in vaccinated patients with CLL. The secondary endpoints were factors predicting the serologic response to the vaccine, the impact of the humoral response, and baseline clinical factors on the occurrence of COVID-19.
Clinical observation started from the first dose of the vaccine. The database was locked on 15 March 2023.Clinical characteristics of patients were extracted from medical records. They included demographic data, baseline characteristics, and treatment of CLL (lymphocyte count, Rai stage, immunoglobulin levels, beta-2microglobulin, disease status, prior treatment, number and type of previous treatments, IGHV and TP53 mutational status, TP53 deletion). Data about COVID-19 severity, management (hospitalization, home care), treatment, and outcome were also recorded.
Patients received at least two BNT162b2 mRNA SARS-CoV-2 vaccine doses three weeks apart. A third dose of the same vaccine was offered after at least three months from the second dose. Patients were informed about the importance of preventive measures, early symptom of COVID-19, and the need for immediate diagnostic screening. COVID-19 diagnosis was based on the positivity of respiratory specimens for SARS-CoV-2 by PCR testing in patients with symptomatic infection.
A centralized assessment of the antibody response was made at the Istituto Superiore di Sanità (I.S.S.) of Rome. Blood samples were taken before the first dose of the vaccine and three weeks after the second and third doses. Additional samples were taken six months from the second dose of the vaccine to evaluate the persistence of IgG antibodies to the SARS-CoV-2 virus. Details about methods to assess COVID-19 IgG levels are reported in the Supplementary material.
2.2. Statistical Analysis
We analyzed the impact of the following variables on the serologic response to the second dose of the SARS-CoV-22 vaccine: CLL duration, gender, age (<70 vs. ≥70 years), CIRS (<6 vs. ≥6), IgG levels (<550 vs. ≤550 mg/dL), lymphocytes count (<5 vs. ≥5 × 109/L), beta-2microglobulin (<3.5 vs. ≥3.5mg/dL), Rai stage (0–II vs. III–IV), progressive disease at the time of vaccination (present vs. absent), prior treatment (present vs. absent), number of previous treatments (0 + 1 vs. ≥2), IGHV (mutated vs. unmutated), deletion and, or mutation of TP53 (present vs. absent), the interval between last rituximab administration and the SARS-CoV-2 vaccine (≤12 months vs. >12 months or never given). In the cohort of patients who received targeted agents, in addition to these variables, we also tested the type of inhibitor administered and the interval (<18 vs. ≥18 months) between the start of treatment and the SARS-CoV-2 vaccine. The clinical and serologic characteristics of patients who developed COVID-19 were also analyzed. Moreover, we evaluated the impact of the same variables tested to identify risk factors associated with the development of COVID-19. Survival curves were calculated according to the Kaplan and Meier method. The Cox regression model was implemented to estimate the Hazard Ratio (H.R.) for each patient’s characteristics. In multivariable analysis, we tested only items with a p < 0.10 at the univariate evaluation. The same approach was used for assessing the impact of tested variables on the serological response (positive vs. negative); in this case, the logistic regression model was implemented to evaluate Odds Ratios (OR). Confidence intervals (C.I.s) were calculated at the 95% level. Unless otherwise specified, a p-value of less than 0.05 was considered significant. All analyses were performed using the IBM SPSS v.21.0 statistical software (IBM Corp., Armonk, NY, USA).
This study has been carried out according to ethical and scientific quality standards of Good clinical practice (GCP) and approved by the Ethics Committees of the participating centers. Patients were asked to sign an informed consent.
3. Results
3.1. Baseline Characteristics of Patients
Patient disposition is summarized in Supplementary Figure S1, and baseline characteristics of patients are described in Table 1.
Table 1.
Baseline characteristics of patients at the time of the first dose of the SARS-CoV-2 vaccine.
| N = 200 (%) | |
|---|---|
| Median age at the time of the anti-SARS-CoV-2 vaccine, years (range, IQR) |
70 (38–90) (61–76) |
| Sex M/F | 113/87 |
| Median time from CLL to the anti-SARS-CoV-2 vaccine, months, (range) | 92 (1–387) |
| Median follow-up from the first dose of anti-SARS-CoV-2 vaccine, months, (range) | 23.4 (4.5–25.7) |
| CIRS ≥ 6 | 69 (34.5) |
| IgG levels ≤ 550 mg/dL | 70 (35) |
| Median lymphocyte count ×109/L, (range) | 3.12 (0.4–218.0) |
| Beta2-microglobulin ≥ 3.5 mg/dL | 34/176 (19.3) |
| Rai stage III–IV | 16 (8.0) |
| Clinical signs of progressive disease | 32 (16.0) |
|
31/175 (17.7) |
|
29/175 (16.6) |
|
30/175 (17.1) |
|
34/175 (19.4) |
| TP53 mutation | 50/179 (27.9) |
| Del17p and/or TP53 mutation | 58/172 (34) |
|
111/183 (61) |
|
72/183 (39) |
Abbreviations: Ig, immunoglobulins; CIRS, Cumulative Illness Rating Scale; Del, deletion; Tris, trisomy; TP53, tumor protein p53; IGHV, immunoglobulin heavy chain variable region mutations.
The median follow-up of patients from the first dose of the SARS-CoV-2 vaccine was 23.4 months (range, 4.5–25.7). The median age of patients was 70 years (range, 38–90), 34.5% had a CIRS ≥6, and 35% had IgG levels ≤550 mg/dL. Rai stage III–IV was present in 8% of patients, and clinical signs of active disease in 16%. Unmutated IGHV was recorded in 61% of the cases, and TP53 deletion and, or mutation in 34%. Details about prior or ongoing treatment are reported in Table 2.
Table 2.
Prior and ongoing treatments at the time of the first dose of the SARS-CoV-2 vaccine.
| N = 200 (%) | |
|---|---|
| The median number of prior treatments (range) | 1 (0–8) |
| Treatment naïve patients | 33 (16.5) |
| Previously treated patients | 167 (83.5) |
| Front-line chemoimmunotherapy only | 20 (10.3) |
| Targeted agents | 147 (73.5) |
| Ibrutinib-based treatment (1) | 72 (36) |
| Front-line | 42 (21.0) |
|
28 (14) |
|
14 (7) |
| Advanced-line Ibrutinib (1) | 30 (15.0) |
| Venetoclax-based treatment | 75 (37.5) |
| Front-line venetoclax + rituximab (2) | 27 (13.5) |
| Advanced-line venetoclax ± rituximab | 48 (24.0) |
|
21 (10.5) |
|
27 (13.5) |
| Median number of months (range) between vaccine and start of: | |
|
54.5 (9–210) |
|
18 (3–90.5) |
|
36 (2–90.3) |
|
13 (0.5–44) |
| Patients previously treated with rituximab | 135 (77.5) |
|
33 (16.5) |
|
102 (51–0) |
(1) Ongoing ibrutinib-based treatment: 72/72 patients. (2) Ongoing treatment with front-line venetoclax + rituximab: 17/27 patients, median time from venetoclax + rituximab discontinuation: 12.5 months (range, 5–17 months). (3) Ongoing treatment with advanced-line venetoclax+ rituximab, 21/21 patients.
Most patients, 167 (83.5%), were previously treated. Twenty patients (10%) were in remission after front-line chemoimmunotherapy, and 147 (73.5%) were previously treated with targeted agents. Among them, 72 (36%) patients were on an ibrutinib-based treatment (front-line therapy, 21%; advanced-line, 15%), and 75 (37.5%) had received a venetoclax-based therapy (front-line, 13.5%, advanced-line, 24%) including 10 in remission and off treatment after front-line venetoclax + rituximab. The median time between vaccination and the start of chemoimmunotherapy was 54.5 months (range, 9–210 months), and 18 months for targeted therapy (ibrutinib-based treatment, 36 months; venetoclax-based therapy, 13 months). A total of 135 (77.5%) patients had been previously exposed to rituximab, including 33 (16.5%) within one year before the first dose of the vaccine.
3.2. Serological Response and Factors Predicting the Serologic Response to the Vaccine
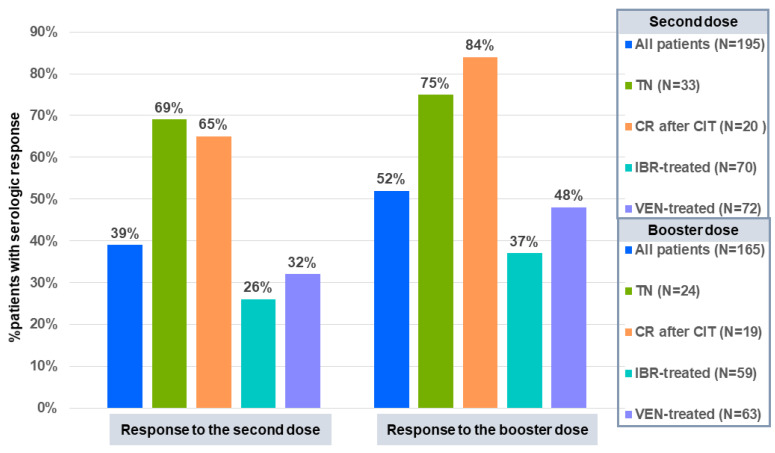
The serologic response was analyzed in 195 patients while five were excluded (high levels of anti-SARS-CoV-2 antibodies at baseline, 3; one dose only of the vaccine, 2). A total of 76 of 195 (39%) patients developed an adequate antibody response after the second dose of the vaccine with a median titer of 90 Au/mL (range, 5.87–160 Au/mL) anti-SARS-CoV-2 IgG. The rates of serologic response according to treatment were: treatment-naive patients, 69%; remission after chemoimmunotherapy, 65%; ibrutinib, 26%; venetoclax, 32% (Figure 1). A serologic response was recorded in 3/33 (9%) patients who had the last administration of anti-CD20 monoclonal antibodies within 12 months before vaccination.
Figure 1.
Serologic response of patients with CLL to the SARS-CoV-2 vaccine by treatment. Abbreviations: CLL, chronic lymphocytic leukemia, SARS-CoV-2, severe acute respiratory syndrome coronavirus 2; TN, treatment naïve; CR, complete response; CIT, chemoimmunotherapy; IBR, ibrutinib; VEN, venetoclax.
Age (OR: 0.93 [95% CI: 0.90–0.96] p < 0.0001), IgG levels (OR: 0.28 [95% CI: 0.13–0.58], p = 0.001), and the time between the previous administration of CD20 monoclonal antibodies and vaccination (OR: 0.10 [95% CI: 0.03–0.37], p = 0.001), had a significant and independent impact on the serologic response in multivariable analysis, (Supplementary Table S1). The analysis was then restricted to the 143 patients who received targeted therapy. No serologic responses were recorded in patients with progressive disease at the time of the vaccine. The type of targeted agent, ibrutinib or venetoclax, did not impact response, while the interval (<18 vs. ≥18 months) between the start of treatment and the vaccine revealed an independent impact on the serologic response (OR: 0.17 [95% CI: 0.06–0.44], p < 0.0001) together with age (OR: 0.96 [95% CI: 0.92–0.99], p = 0.038) and IgG levels (OR: 0.31 [95% CI: 0.12–0.79], p = 0.014) (Supplementary Table S1). A total of 182 patients received the third dose of the same vaccine after a median time of six months from the second dose (range, 3–9 months), and 165 were tested for the serologic response. A response was detected in 52% of patients tested after the third dose of the vaccine (Figure 1). A total of 27 of the 103 (26%) patients who had failed to respond to the second dose responded to the booster dose, while 76 (74%) maintained seronegativity. Even with the limit of patients not tested after the third dose, we could count at least 103 (76 + 27; 53%) patients who developed an antibody response to two or three doses of the vaccine, while 92 (119–27; 47%) maintained seronegativity. The response to the third dose of the vaccine according to previous treatment is described in Figure 1.
3.3. Clinical Characteristics and Outcomes of Patients with COVID-19
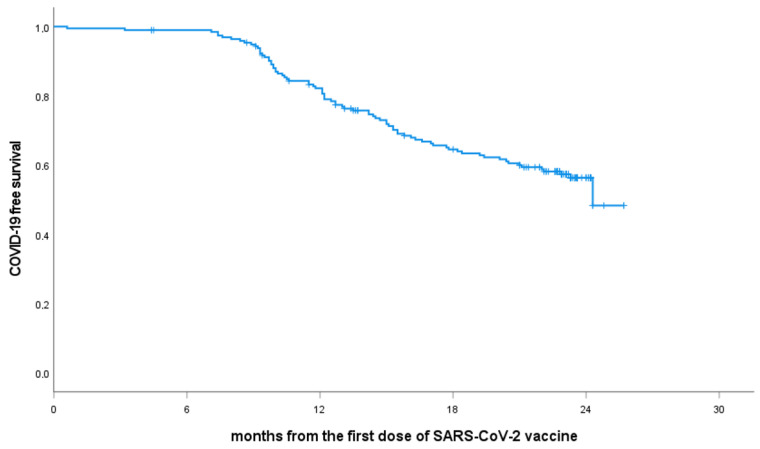
As of 1 March 2023, 80 (41%) patients included in this study have developed COVID-19. For patients who reported typical signs of COVID-19 and required home care, the diagnosis was validated by RT-PCR positivity for SARS-CoV-2 certified by a microbiology laboratory. For hospitalized patients, the diagnosis was based on discharge reports. The 24-month COVID-19-free survival rate was 56.3%, (Figure 2). The clinical characteristics of patients who experienced COVID-19 are summarized in Table 3 and Figure 3.
Figure 2.
COVID-19-free survival from the SARS-CoV-2 vaccine. Abbreviations: COVID-19, Coronavirus disease 2019; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2.
Table 3.
Clinical characteristics and outcomes of patients who developed COVID-19.
| N = 80 (%) | |
| Median age, years (range) | 69.1 (39–89) |
| CIRS ≥ 6 | 20 (25) |
| IgG levels < 550 mg/dL | 27 (34) |
| Rai stage III–IV | 5 (6) |
| Unmutated IGHV | 41 (51) |
| TP53 mutation/deletion | 15 (19) |
| More than one COVID-19 event (1) | 8 (10) |
| Treatment naive | 14 (17.5) |
| Prior treatment | 66 (82.5) |
|
6 (7.5) |
|
31 (39) |
| Venetoclax based (2) | 29 (36) |
| Last rituximab administration within six months | 3 (4) |
| Number of doses of the vaccine before COVID-19 | |
|
4 (5) |
|
61 (76) |
|
12 (15) |
|
3 (4) |
| Known serologic response to the last dose of the vaccine | |
|
42 (52.5) |
|
38 (47.5) |
| Pre-exposure prophylaxis with tixagevimab/cilgavimab | 50 (62.5) |
|
19/34 (56) |
|
0/16 (0) |
| Pandemic phase of COVID-19 diagnosis (3) | |
|
1 (1) |
|
8 (10) |
|
71 (89) |
| Discontinued ibrutinib or venetoclax-based treatment during COVID-19 | 76/80 (95) |
| Clinical management of COVID-19 | |
|
59 (74) |
|
21 (26%) [9] |
| Treatment | |
|
16 (20) |
|
35 (44) |
|
14 (17.5) |
|
15 (19) |
| COVID-19-related deaths | 3 (4) |
| Recovered from COVID-19 and resumed targeted therapy | 74/80 (92.5) |
Abbreviations: COVID-19, Coronavirus disease 2019; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2; Ig, immunoglobulins; TP53 gene, tumor protein p53 gene; Del., deletion; IGHV, immunoglobulin heavy chain variable region gene. (1) Two COVID-19 events, 6 patients; three COVID-19 events, 1. (2) Eight patients with CR and treatment-free from a median number of 8.5 months from venetoclax + rituximab discontinuation. (3) SARS-CoV-2 variants dominant in our country: alpha variant before July 2021, delta variant between July and December 2021, and omicron variants from January 2022.
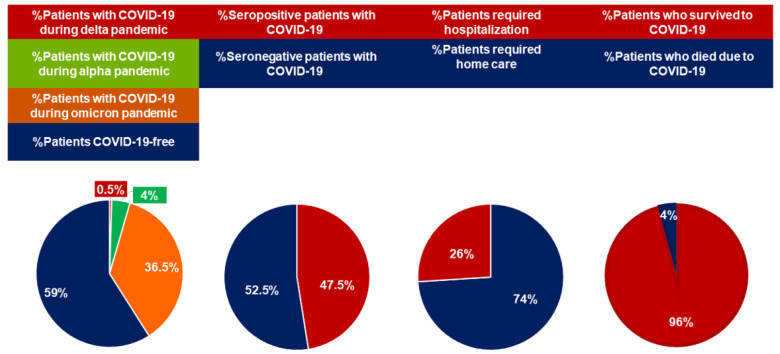
Figure 3.
Epidemiologic and clinical characteristics of COVID-19 in vaccinated patients with CLL. Abbreviations: COVID-19, Coronavirus disease 2019; CLL, chronic lymphocytic leukemia.
COVID-19 was diagnosed in one patient (1/80, 1%; 1/195, 0.5%) before July 2021, when the alpha variant was dominant in our country, in 8 (8/80, 10%; 8/195, 4%) between July and December 2021, during the delta pandemic, and in 71 (71/80, 89%; 71/195, 36.5%) and after January 2022 when Omicron variants became dominant. Eight (10%) patients experienced subsequent COVID-19 events during the Omicron pandemic (two COVID-19 events, seven patients; three COVID-19 events, 1).
The proportion of infected patients previously treated with ibrutinib or venetoclax was similar, 39% and 36%. All patients but four had received at least three doses of the vaccine (range 2–5), including 12 patients who had four and three who received five doses. Most patients (76/80; 95%) interrupted targeted agents at the time of COVID-19. Mild COVID-19-related symptoms were reported by 59 (74%) patients who received home care only. Twenty-one (26%) of patients with COVID-19 showed a severe infection requiring supplemental oxygen and hospitalization, including nine who needed care in intensive care units.
COVID-19 treatment varied widely according to the severity of the infection, local treatment protocol, and time of diagnosis. Treatment consisted of anti-inflammatory agents with or without antibiotics in 16 (20%) patients. Anti-viral drugs or anti-SARS-CoV-2 monoclonal antibodies were given to 35 (44%) and 14 (17.5%) patients. In addition, hospitalized patients received oxygen, antibiotics, dexamethasone, and nine mechanical ventilation. Home care could not be defined in 15 (19%) cases.
A total of 555 of the 60 (92%) patients on targeted therapy at the time of COVID-19 resumed targeted therapy. Three (3/200; 1.5%; 3/80, 4%) patients died due to COVID-19 during the Omicron pandemic. The three dead patients were previously treated with chemoimmunotherapy and were on active treatment with venetoclax at the time of COVID-19. Moreover, they had received three vaccine doses without developing an antibody response and showed an additional factor of increased frailty for severe COVID-19 (age > 70 years, hypogammaglobulinemia).
3.4. Risk Factors of COVID-19
The same baseline variables tested for their impact on the serologic response were also analyzed for their effect on the risk of developing COVID-19. In multivariate analysis, three baseline factors emerged as significant and independent risk factors for developing COVID-19, age (HR: 0.97 [95% CI: 0.95–0.9995], p = 0.046), the number of prior treatments, ≥2 vs. 1 (HR: 2.08 [95% CI: 1.27–3.40], p = 0.004), the presence of TP53 deletion and, or mutation (HR: 1.85 [95% CI: 1.06–3.25], p = 0.032), (Supplementary Table S2). When the analysis was restricted to patients treated with targeted agents, the same factors maintained a significant independent impact on the risk of developing COVID-19 together with the interval, ≥ 18 vs. <18 months, between vaccination and the start of prior targeted therapy (HR:0.31 [95% CI: 0.15–0.63], p = 0.001) (Supplementary Table S2).
COVID-19 was diagnosed in 42/92 (46%) patients who failed to respond to the last dose of the vaccine, and in 38/103 (37%) patients who developed an antibody response. The difference in the incidence of COVID-19 between seronegative and seropositive patients did not reach significance (p = 0.21).
In February 2022, the Italian Medicines Agency (AIFA) authorized pre-exposure prophylaxis of the SARS-CoV-2 infection with a 150/150 mg dose of two long-acting anti-Spike monoclonal antibodies, tixagevimab/cilgavimab, in immunocompromised patients regardless the response to the prior anti-SARS-CoV-2 vaccine. Fifty (62.5%) patients included in this study received pre-exposure prophylaxis with tixagevimab/cilgavimab, 34 patients without a prior COVID-19 event (COVID19-free patients) and 16 with a previous event (COVID-19 experienced patients). COVID-19 was diagnosed in 19 (56%) COVID-19-free patients (including 13 seropositive patients) after a median of 2.75 months (range, 0.25–11) from the tixagevimab/cilgavimab administration. No COVID-19-related deaths were recorded in these patients, but 8 (8/19, 42%) required hospitalization. Patients who had received tixagevimab/cilgavimab after a prior COVID-19 event did not experience subsequent COVID-19 events.
4. Discussion
This multicenter study provides a prospective long-term scenario about the morbidity, severity, and mortality of COVID-19 during the different phases of the pandemic in patients with CLL who received the SARS-CoV-2 vaccine.
Data from the first analysis of this study showed a serological response to two doses of the anti-SARS-CoV-2 vaccine in only 39% of patients. Other studies have described suboptimal responses in patients with similar characteristics [8,9,10,11,12,13,14]. Moreover, we also observed lower rates of responses in patients treated with ibrutinib or venetoclax, 26%, and 31.5%, respectively [8,9,10,11,12,13,14,16,17,18]. This finding was not surprising as low humoral responses to other vaccines were also described in ibrutinib-treated patients [19,20,21]. Interestingly, a longer time, ≥18 months, between the start of ibrutinib or venetoclax and vaccination was associated with a better chance of developing an antibody response. This finding suggests that more prolonged treatment with targeted agents could produce better clinical and humoral responses. As described by Herishanu et al. [22], the third dose of the vaccine promoted an antibody response in 26% of patients with stable disease who had failed to respond to the second dose.
We evaluate with a two-year follow-up the protective impact of the vaccine and the humoral response to the vaccine in terms of COVID-19-related morbidity and mortality.
During the first pandemic, two doses of the BNT162b2 vaccine conferred protection against COVID-19 in 94–95% of healthy subjects [23,24]. Despite the low rate of serologic responses, 99% of vaccinated patients included in this study remained COVID-19-free during the first pandemic dominated by the alpha variant. This observation further indicates that antibody titers should not be considered an absolute benchmark for the adequate immune protection of vaccines [25]. Interestingly, Shen et al. detected a T-cell response to a SARS-CoV-2 peptide pool in approximately 80% of vaccinated patients, regardless of their antibody response, suggesting a role of cellular immunity promoted by the vaccine [16].
In line with the Italian epidemiological data [26], we recorded a rapid increase in COVID-19 cases during the Omicron pandemic, with 36.5% of vaccinated patients experiencing COVID-19. This study’s prospective design led us to capture all symptomatic COVID-19 events, reducing the bias of selecting severe cases only. However, the proportion of asymptomatic infections could not be estimated as serology was not assessed in all patients beyond the second dose of the vaccine. The increased rate of COVID-19 cases during the Omicron pandemic is a well-known epidemiological data representing the result of the antibody escape by variants carrying mutated spike proteins. The immune escape developed by mutant variants raises concern about the efficacy of antibodies induced by prior vaccination or infection [27,28,29]. In this study, the proportion of patients who developed COVID-19 with or without a serologic response to the vaccine was not statistically different. About half of the patients who developed COVID-19 had an antibody response to the prior vaccine. In addition, 9% of patients with COVID-19 experienced a subsequent COVID-19 event.
A detrimental effect of mutated variants is the decreased efficacy of monoclonal antibodies designed on previous variants and developed for therapeutic or prophylactic use.
During the Omicron pandemic, 19/34 (56%) COVID-free patients who received pre-exposure prophylaxis tixagevimab/cilgavimab developed a not-fatal COVID-19 but eight required hospitalization.
The continuous spreading of new mutant variants will likely expose, despite vaccination, immunocompromised subjects to recurrent and potentially severe COVID-19.
We found that older age and two or more prior treatments emerged as significant and independent factors associated with an increased risk of developing COVID-19. In addition to being associated with reduced antibody responsiveness, less than 18 months of treatment with venetoclax or ibrutinib appeared to be associated with a higher vulnerability to COVID-19. This finding again suggests the impact on immunological reconstitution produced by more prolonged treatment with targeted agents. Surprisingly, a TP53 disruption also showed a significant and independent effect on an increased risk of COVID-19. The increased vulnerability of patients with TP53 disruption to symptomatic SARS-CoV-2 infection could be explained by recent data suggesting the role of p53 in anti-viral immunity [30]. Mice lacking the TP53 gene die due to malignancies and approximately one-quarter due to infection. It has been argued that p53 could exert anti-viral immunity in response to the viral DNA or RNA by triggering apoptosis of infected cells [30,31]. In this way, the apoptosis of the infected cells may limit the infection of neighboring cells. In addition, the increased risk of symptomatic infections could also be due to the role played by p53 in pathways, including factors involved in the anti-viral response, such as IFN-1 [32].
During the early pandemic, over 80% of non-vaccinated patients with CLL who developed COVID-19 were hospitalized, and a third died [4,5].
Despite the lack of effective antibodies against spike-mutant variants, we recorded a relatively low rate of patients who required hospitalization and fatal COVID-19, 26% and 4%, respectively. This finding is in line with the consensus that Omicron variants are highly transmissible, but COVID-19 is less severe among vaccinated people [33,34,35]. The lower intrinsic virulence of mutated variants, the availability of better therapeutic approaches, and the more limited use of steroids have played a relevant role in improving the outcome of COVID-19also in vaccinated patients with hematologic diseases, including those with CLL [36,37,38,39,40,41,42]. Interestingly, Parry et al. [17] showed that cellular immunity induced by prior vaccines produced responses against Omicron variants equivalent to those seen against the ancestral strain.
In our study, 26% of vaccinated developed severe COVID-19 requiring hospitalization. Although during the Omicron pandemic, fatal COVID-19 are rare, the potential severity of COVID-19 in immunocompromised subjects should not be underestimated, particularly in elderly and actively treated patients with hematologic disorders, including CLL [36,37,38,39]. Compared to other observational studies, including those that enrolled patients from our country, our prospective study had a long follow-up extended to the Omicron pandemic. The results of our study provided new insights into the impact of clinical, biologic and treatment characteristics on the long-term outcomes of vaccinated patients with CLL.
Our data show an increased vulnerability to COVID-19 in CLL patients during the Omicron pandemic, associated with age, TP53 disruption, prior treatment, and earlier phase of treatment with targeted agents. Moreover, COVID-19 was clinically severe in a not negligible proportion of patients. Given the persistent risk of infection due to the continuous emergence of SARS-CoV-2 variants, our results support the importance of new vaccines and protective measures to prevent and mitigate COVID-19 in patients with CLL.
Acknowledgments
The authors thank AbbVie for the unconditional grant and all patients included in this study. The authors also wish to thank Michele Porro Lurà and Daniele Focosi for the critical review of the paper, Armando Pellegrino for his assistance, and the study nurse Roberta Cecchini for their valuable contribution.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/cancers15112993/s1, Figure S1: Patient disposition; Table S1: Factors with an impact on serologic response; Table S2: Factors with an impact on the development of COVID-19.
Author Contributions
F.R.M. and D.G. designed and managed the research, collected, analyzed, interpreted data, and wrote the manuscript. C.M.G. and S.B. collected blood samples, assessed the response to the vaccine, analyzed data, and reviewed the manuscript. A.V., A.M.F., P.S., C.V., G.R., M.G., L.L., R.M., D.A., M.C.M., G.P., F.D.F., V.M., L.B., R.A., M.C., A.T., C.G., L.T., S.P. and R.M. enrolled and cared for the patients, collected data, and reviewed the manuscript. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
The present study was recorded on the Italian Register of Observational Studies (ROS) of the Italian Medicines Agency (AIFA)—8 March 2021.
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
De-identified individual data supporting the presented findings will be available upon reasonable request. Proposals for access should be sent to mauro@.bce.uniroma1.it.
Conflicts of Interest
The authors declare no competing financial interest directly related to the present paper.
Funding Statement
This project was supported by AbbVie.
Footnotes
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
References
- 1.Vijenthira A., Gong I.Y., Fox T.A., Booth S., Cook G., Fattizzo B., Martín-Moro F., Razanamahery J., Riches J.C., Zwicker J., et al. Outcomes of Patients with Hematologic Malignancies and COVID-19: A Systematic Review and Meta-Analysis of 3377 Patients. Blood. 2020;136:2881–2892. doi: 10.1182/blood.2020008824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Passamonti F., Cattaneo C., Arcaini L., Bruna R., Cavo M., Merli F., Angelucci E., Krampera M., Cairoli R., Della Porta M.G., et al. ITA-HEMA-COV Investigators. Clinical Characteristics and Risk Factors Associated with COVID-19 Severity in Patients with Haematological Malignancies in Italy: A Retrospective, Multicentre, Cohort Study. Lancet Haematol. 2020;7:e737–e745. doi: 10.1016/S2352-3026(20)30251-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pagano L., Salmanton-García J., Marchesi F., Busca A., Corradini P., Hoenigl M., Klimko N., Koehler P., Pagliuca A., Passamonti F., et al. EPICOVIDEHA working group. COVID-19 Infection in Adult Patients with Hematological Malignancies: A European Hematology Association Survey (EPICOVIDEHA) J. Hematol. Oncol. 2021;14:168. doi: 10.1186/s13045-021-01177-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Scarfò L., Chatzikonstantinou T., Rigolin G.M., Quaresmini G., Motta M., Vitale C., Garcia-Marco J.A., Hernández-Rivas J.Á., Mirás F., Baile M., et al. COVID-19 Severity and Mortality in Patients with Chronic Lymphocytic Leukemia: A Joint Study by ERIC, the European Research Initiative on CLL, and CLL Campus. Leukemia. 2020;34:2354–2363. doi: 10.1038/s41375-020-0959-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mato A.R., Roeker L.E., Lamanna N., Allan J.N., Leslie L., Pagel J.M., Patel K., Osterborg A., Wojenski D., Kamdar M., et al. Outcomes of COVID-19 in Patients with CLL: A Multicenter International Experience. Blood. 2020;136:1134–1143. doi: 10.1182/blood.2020006965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chatzikonstantinou T., Kapetanakis A., Scarfò L., Karakatsoulis G., Allsup D., Cabrero A.A., Andres M., Antic D., Baile M., Baliakas P., et al. COVID-19 Severity and Mortality in Patients with CLL: An Update of the International ERIC and Campus CLL Study. Leukemia. 2021;35:3444–3454. doi: 10.1038/s41375-021-01450-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Roeker L.E., Knorr D.A., Pessin M.S., Ramanathan L.V., Thompson M.C., Leslie L.A., Zelenetz A.D., Mato A.R. Anti-SARS-CoV-2 Antibody Response in Patients with Chronic Lymphocytic Leukemia. Leukemia. 2020;34:3047–3049. doi: 10.1038/s41375-020-01030-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Herishanu Y., Avivi I., Aharon A., Shefer G., Levi S., Bronstein Y., Morales M., Ziv T., Shorer Arbel Y., Scarfò L., et al. Efficacy of the BNT162b2 MRNA COVID-19 Vaccine in Patients with Chronic Lymphocytic Leukemia. Blood. 2021;137:3165–3173. doi: 10.1182/blood.2021011568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Roeker L.E., Knorr D.A., Thompson M.C., Nivar M., Lebowitz S., Peters N., Deonarine I., Momotaj S., Sharan S., Chanlatte V., et al. COVID-19 Vaccine Efficacy in Patients with Chronic Lymphocytic Leukemia. Leukemia. 2021;35:2703–2705. doi: 10.1038/s41375-021-01270-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Parry H., McIlroy G., Bruton R., Ali M., Stephens C., Damery S., Otter A., McSkeane T., Rolfe H., Faustini S., et al. Antibody Responses after First and Second Covid-19 Vaccination in Patients with Chronic Lymphocytic Leukaemia. Blood Cancer J. 2021;11:136. doi: 10.1038/s41408-021-00528-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bagacean C., Letestu R., Al-Nawakil C., Brichler S., Lévy V., Sritharan N., Delmer A., Dartigeas C., Leblond V., Roos-Weil D., et al. Humoral Response to MRNA Anti-COVID-19 Vaccines BNT162b2 and MRNA-1273 in Patients with Chronic Lymphocytic Leukemia. Blood Adv. 2022;6:207–211. doi: 10.1182/bloodadvances.2021006215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Benjamini O., Rokach L., Itchaki G., Braester A., Shvidel L., Goldschmidt N., Shapira S., Dally N., Avigdor A., Rahav G., et al. Safety and Efficacy of the BNT162b MRNA COVID-19 Vaccine in Patients with Chronic Lymphocytic Leukemia. Haematologica. 2022;107:625–634. doi: 10.3324/haematol.2021.279196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Haydu J.E., Maron J.S., Redd R.A., Gallagher K.M.E., Fischinger S., Barnes J.A., Hochberg E.P., Johnson P.C., Takvorian R.W., Katsis K., et al. Humoral and Cellular Immunogenicity of SARS-CoV-2 Vaccines in Chronic Lymphocytic Leukemia: A Prospective Cohort Study. Blood Adv. 2022;6:1671–1683. doi: 10.1182/bloodadvances.2021006627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chang A., Akhtar A., Linderman S.L., Lai L., Orellana-Noia V.M., Valanparambil R., Ahmed H., Zarnitsyna V.I., McCook-Veal A.A., Switchenko J.M., et al. Humoral Responses Against SARS-CoV-2 and Variants of Concern After MRNA Vaccines in Patients With Non-Hodgkin Lymphoma and Chronic Lymphocytic Leukemia. J. Clin. Oncol. 2022;40:3020–3031. doi: 10.1200/JCO.22.00088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hallek M., Cheson B.D., Catovsky D., Caligaris-Cappio F., Dighiero G., Döhner H., Hillmen P., Keating M., Montserrat E., Chiorazzi N., et al. IwCLL Guidelines for Diagnosis, Indications for Treatment, Response Assessment, and Supportive Management of CLL. Blood. 2018;131:2745–2760. doi: 10.1182/blood-2017-09-806398. [DOI] [PubMed] [Google Scholar]
- 16.Shen Y., Freeman J.A., Holland J., Solterbeck A., Naidu K., Soosapilla A., Downe P., Tang C., Kerridge I., Wallman L., et al. COVID-19 Vaccine Failure in Chronic Lymphocytic Leukaemia and Monoclonal B-Lymphocytosis; Humoural and Cellular Immunity. Br. J. Haematol. 2022;197:41–51. doi: 10.1111/bjh.18014. [DOI] [PubMed] [Google Scholar]
- 17.Parry H., Bruton R., Roberts T., McIlroy G., Damery S., Sylla P., Dowell A.C., Tut G., Lancaster T., Bone D., et al. COVID-19 Vaccines Elicit Robust Cellular Immunity and Clinical Protection in Chronic Lymphocytic Leukemia. Cancer Cell. 2022;40:584–586. doi: 10.1016/j.ccell.2022.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fürstenau M., Langerbeins P., De Silva N., Fink A.M., Robrecht S., von Tresckow J., Simon F., Hohloch K., Droogendijk J., van der Klift M., et al. COVID-19 among Fit Patients with CLL Treated with Venetoclax-Based Combinations. Leukemia. 2020;34:2225–2229. doi: 10.1038/s41375-020-0941-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sun C., Gao J., Couzens L., Tian X., Farooqui M.Z., Eichelberger M.C., Wiestner A. Seasonal Influenza Vaccination in Patients With Chronic Lymphocytic Leukemia Treated With Ibrutinib. JAMA Oncol. 2016;2:1656–1657. doi: 10.1001/jamaoncol.2016.2437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Douglas A.P., Trubiano J.A., Barr I., Leung V., Slavin M.A., Tam C.S. Ibrutinib May Impair Serological Responses to Influenza Vaccination. Haematologica. 2017;102:e397–e399. doi: 10.3324/haematol.2017.164285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mauro F.R., Giannarelli D., Galluzzo C.M., Vitale C., Visentin A., Riemma C., Rosati S., Porrazzo M., Pepe S., Coscia M., et al. Response to the Conjugate Pneumococcal Vaccine (PCV13) in Patients with Chronic Lymphocytic Leukemia (CLL) Leukemia. 2021;35:737–746. doi: 10.1038/s41375-020-0884-z. [DOI] [PubMed] [Google Scholar]
- 22.Herishanu Y., Rahav G., Levi S., Braester A., Itchaki G., Bairey O., Dally N., Shvidel L., Ziv-Baran T., Polliack A., et al. Efficacy of a Third BNT162b2 MRNA COVID-19 Vaccine Dose in Patients with CLL Who Failed Standard 2-Dose Vaccination. Blood. 2022;139:678–685. doi: 10.1182/blood.2021014085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Polack F.P., Thomas S.J., Kitchin N., Absalon J., Gurtman A., Lockhart S., Perez J.L., Pérez Marc G., Moreira E.D., Zerbini C., et al. Safety and Efficacy of the BNT162b2 MRNA Covid-19 Vaccine. N. Engl. J. Med. 2020;383:2603–2615. doi: 10.1056/NEJMoa2034577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Baden L.R., El Sahly H.M., Essink B., Kotloff K., Frey S., Novak R., Diemert D., Spector S.A., Rouphael N., Creech C.B., et al. Efficacy and Safety of the MRNA-1273 SARS-CoV-2 Vaccine. N. Engl. J. Med. 2021;384:403–416. doi: 10.1056/NEJMoa2035389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sun C. COVID-19 Vaccine Response in Chronic Lymphocytic Leukaemia Is More than Just Seroconversion. Br. J. Haematol. 2022;197:11–12. doi: 10.1111/bjh.18047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Prevalenza e Distribuzione Delle Varianti di SARS-CoV-2 di Interesse Per la Sanità Pubblica in Italia-Rapporto n. 24 del 29 Settembre. 2022. [(accessed on 17 March 2023)]. Available online: https://www.epicentro.iss.it/coronavirus/pdf/sars-cov-2-monitoraggio-varianti-rapporti-periodici-29-settembre-2022.pdf.
- 27.Hachmann N.P., Miller J., Collier A.-R.Y., Ventura J.D., Yu J., Rowe M., Bondzie E.A., Powers O., Surve N., Hall K., et al. Neutralization Escape by SARS-CoV-2 Omicron Subvariants BA.2.12.1, BA.4, and BA.5. N. Engl. J. Med. 2022;387:86–88. doi: 10.1056/NEJMc2206576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tuekprakhon A., Nutalai R., Dijokaite-Guraliuc A., Zhou D., Ginn H.M., Selvaraj M., Liu C., Mentzer A.J., Supasa P., Duyvesteyn H.M.E., et al. Antibody Escape of SARS-CoV-2 Omicron BA.4 and BA.5 from Vaccine and BA.1 Serum. Cell. 2022;185:2422–2433.e13. doi: 10.1016/j.cell.2022.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Callaway E. What Omicron’s BA.4 and BA.5 Variants Mean for the Pandemic. Nature. 2022;606:848–849. doi: 10.1038/d41586-022-01730-y. [DOI] [PubMed] [Google Scholar]
- 30.Harford J.B., Kim S.S., Pirollo K.F., Chang E.H. TP53 Gene Therapy as a Potential Treatment for Patients with COVID-19. Viruses. 2022;14:739. doi: 10.3390/v14040739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Teodoro J.G., Branton P.E. Regulation of Apoptosis by Viral Gene Products. J. Virol. 1997;71:1739–1746. doi: 10.1128/jvi.71.3.1739-1746.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Takaoka A., Hayakawa S., Yanai H., Stoiber D., Negishi H., Kikuchi H., Sasaki S., Imai K., Shibue T., Honda K., et al. Integration of Interferon-Alpha/Beta Signalling to P53 Responses in Tumour Suppression and Antiviral Defence. Nature. 2003;424:516–523. doi: 10.1038/nature01850. [DOI] [PubMed] [Google Scholar]
- 33.Ward I.L., Bermingham C., Ayoubkhani D., Gethings O.J., Pouwels K.B., Yates T., Khunti K., Hippisley-Cox J., Banerjee A., Walker A.S., et al. Risk of Covid-19 Related Deaths for SARS-CoV-2 Omicron (B.1.1.529) Compared with Delta (B.1.617.2): Retrospective Cohort Study. BMJ. 2022;378:e070695. doi: 10.1136/bmj-2022-070695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Nyberg T., Ferguson N.M., Nash S.G., Webster H.H., Flaxman S., Andrews N., Hinsley W., Bernal J.L., Kall M., Bhatt S., et al. Comparative Analysis of the Risks of Hospitalisation and Death Associated with SARS-CoV-2 Omicron (B.1.1.529) and Delta (B.1.617.2) Variants in England: A Cohort Study. Lancet. 2022;399:1303–1312. doi: 10.1016/S0140-6736(22)00462-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.World Health Organization Severity of Disease Associated with Omicron Variant as Compared with Delta Variant in Hospitalized Patients with Suspected or Confirmed SARS-CoV-2 Infection. 2022. [(accessed on 17 March 2023)]. Available online: https://apps.who.int/iris/bitstream/handle/10665/354794/9789240051829-eng.pdf.
- 36.Bronstein Y., Gat R., Levi S., Cohen Y.C., Luttwak E., Benyamini N., Shragai T., Vitkon R., Neaman M., Eilaty N., et al. COVID-19 in Patients with Lymphoproliferative Diseases during the Omicron Variant Surge. Cancer Cell. 2022;40:578–580. doi: 10.1016/j.ccell.2022.04.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Niemann C.U., da Cunha-Bang C., Helleberg M., Ostrowski S.R., Brieghel C. Patients with CLL Have a Lower Risk of Death from COVID-19 in the Omicron Era. Blood. 2022;140:445–450. doi: 10.1182/blood.2022016147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mikulska M., Testi D., Russo C., Balletto E., Sepulcri C., Bussini L., Dentone C., Magne F., Policarpo S., Campoli C., et al. Outcome of Early Treatment of SARS-CoV-2 Infection in Patients with Haematological Disorders. Br. J. Haematol. 2023;201:628–639. doi: 10.1111/bjh.18690. [DOI] [PubMed] [Google Scholar]
- 39.Infante M.S., Salmanton-García J., Fernández-Cruz A., Marchesi F., Jaksic O., Weinbergerová B., Besson C., Duarte R.F., Itri F., Valković T., et al. B-cell malignancies treated with targeted drugs and SARS-CoV-2 infection: A European Hematology Association Survey (EPICOVIDEHA) Front. Oncol. 2022;12:992137. doi: 10.3389/fonc.2022.992137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Roeker L.E., Eyre T.A., Thompson M.C., Lamanna N., Coltoff A.R., Davids M.S., Baker P.O., Leslie L., Rogers K.A., Allan J.N., et al. COVID-19 in Patients with CLL: Improved Survival Outcomes and Update on Management Strategies. Blood. 2021;138:1768–1773. doi: 10.1182/blood.2021011841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Pagano L., Salmanton-García J., Marchesi F., López-García A., Lamure S., Itri F., Gomes-Silva M., Dragonetti G., Falces-Romero I., van Doesum J., et al. COVID-19 in Vaccinated Adult Patients with Hematological Malignancies: Preliminary Results from EPICOVIDEHA. Blood. 2022;139:1588–1592. doi: 10.1182/blood.2021014124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Piñana J.L., López-Corral L., Martino R., Vazquez L., Pérez A., Martin-Martin G., Gago B., Sanz-Linares G., Sanchez-Salinas A., Villalon L., et al. Infectious Complications Subcommittee of the Spanish Hematopoietic Stem Cell Transplantation and Cell Therapy Group (GETH-TC). SARS-CoV-2 Vaccine Response and Rate of Breakthrough Infection in Patients with Hematological Disorders. J. Hematol. Oncol. 2022;15:54. doi: 10.1186/s13045-022-01275-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
De-identified individual data supporting the presented findings will be available upon reasonable request. Proposals for access should be sent to mauro@.bce.uniroma1.it.