Abstract
The reaction mechanism for the formation of 2′-deoxyoxanosine from 2′-deoxyguanosine by nitrous acid was explored using methyl derivatives of guanosine and an isolated intermediate of the reaction. When 1-methylguanosine was incubated with NaNO2 under acidic conditions, N5-methyloxanosine and 1-methylxanthosine were generated, whereas the same treatment of N2,N2-dimethylguanosine generated no product. In a similar experiment without NO2–, participation of a Dimroth rearrangement was ruled out. In the guanosine–HNO2 reaction system, an intermediate with a half-life of 5.6 min (pH 7.0, 20°C) was isolated and tentatively identified as a diazoate derivative of guanosine. The diazoate intermediate was converted into oxanosine and xanthosine at a molar ratio (oxanosine:xanthosine) of 0.26 at pH 7.0 and 20°C. The ratio was not affected by the incubation pH between 2 and 10, but increased linearly with temperature from 0.22 (0°C) to 0.32 (50°C). The addition of acetone also increased the ratio up to 0.85 (98% acetone). Based on these results, a con-ceivable pathway for the formation of 2′-deoxyoxanosine from 2′-deoxyguanosine by HNO2 is proposed.
INTRODUCTION
Oxanosine (Oxo), 5-amino-3-β-(d-ribofuranosyl)-3H-imidazo- [4,5-d][1,3]oxazin-7-one, was isolated as a novel antibiotic in 1981 from the culture broth of Streptomyces capreolus MG265-CF3 and characterized by X-ray crystallography (1,2). Oxo manifests a wide spectrum of biological effects including in vitro cytotoxicity against HeLa cells, antibacterial activity against Escherichia coli K-12 and Proteus mirabilis IFM OM-9, and induction of reversions to the normal phenotype of K-ras-transformed rat kidney cells (1,3). 2′-Deoxyoxanosine (dOxo), a 2′-deoxyribonucleoside counterpart of Oxo, has been synthesized from Oxo and found to exhibit stronger antiviral and antineoplastic activities than Oxo (4). Oxo and its analogs have been chemically synthesized by several defined routes using 4-aminoimidazole derivatives (5–8) or inosine (9) as starting materials.
We have reported that the reaction of 2′-deoxyguanosine (dGuo) with nitrous acid (HNO2) results in the formation of dOxo together with 2′-deoxyxanthosine (dXao), a deaminated product of dGuo (10). The maximum yield of dOxo was 21.5%, which corresponded to ~1/3 of the deamination products. dOxo is also formed in a single-stranded oligodeoxynucleotide and double-stranded calf thymus DNA by HNO2 treatment with a comparable yield. Furthermore, dOxo is generated from dGuo with nitric oxide (NO) at acidic and neutral pH. We have also demonstrated that the N-glycosidic bond of dOxo is as stable as that of dGuo and is hydrolyzed 44-fold more slowly than that of dXao (11). 2′-Deoxyoxanosine 5′-triphosphate (dOTP) is more efficiently incorporated opposite cytosine in a DNA template than 2′-deoxyxanthosine 5′-triphosphate (dXTP) (12). Furthermore, dOTP is incorporated opposite thymine in the template, while dXTP is not incorporated under the same conditions. These results imply that the dOxo moiety generated from a dGuo moiety in the nucleotide pool or DNA may have important and unique roles in mutagenic and lethal events in cells.
dOxo has a six-membered ring in the base moiety in which the imino group at N1 of the pyrimidine ring of dGuo is substituted by an oxygen atom. The formation of dOxo requires opening and closing of the pyrimidine ring of dGuo. Recent ab initio molecular orbital calculations predict that a ring-opened cation is formed synchronously with release of a nitrogen molecule from the diazonium ion of guanine (13–15). However, direct evidence as to the reaction mechanism for the formation of dOxo from dGuo by HNO2 has not been shown so far.
Recently, we reported the detection and isolation of an intermediate in the reaction of 2′-deoxycytidine (dCyd) with HNO2 or NO (16). The intermediate was identified from spectrometric data as a diazoate derivative of dCyd. Under physiological conditions (pH 7.4, 37°C), the diazoate intermediate was fairly stable (t = 330 h) and was exclusively converted into 2′-deoxyuridine. However, there is no information on the intermediates yielding dOxo and dXao in the reaction of dGuo with HNO2. If the intermediates could be isolated, further insights into the reaction mechanism could be obtained.
We report herein an analysis of the products formed by the reaction of methyl-substituted guanosines with HNO2. Furthermore, the diazoate intermediate of Guo was isolated and the influences of pH, temperature and solvent on the formation of Oxo and Xao from the intermediate were studied. The reaction mechanism of dOxo formation by HNO2 is discussed based on these results.
MATERIALS AND METHODS
Materials
Guanosine (Guo) was purchased from Kohjin (Tokyo, Japan). N2,N2-Dimethylguanosine (N2,N2-diMe-Guo), N2-methylguanosine (N2-Me-Guo), 1-methlyguanosine (1-Me-Guo) and xanthosine (Xao) were obtained from Sigma (St Louis, MO). All other chemicals of reagent grade were purchased from Wako Pure Chemicals (Osaka, Japan) or Nacalai Tesque (Osaka, Japan), and used without further purification. Water was purified with a Millipore Milli-QII deionizer.
HPLC conditions
The HPLC system consisted of Shimadzu LC-6A pumps and a CTO-6A system controller. On-line UV spectra were obtained with a Shimadzu SPD-M6A UV-Vis photodiode array detector. For reversed phase (RP-)HPLC, a Hypersil ODS-5 octadesylsilane column (4.6 × 150 mm, particle size 5 µm; GL Sciences) was used. The eluant was 100 mM triethylammonium acetate (TEAA) buffer (pH 7.0) containing acetonitrile (CH3CN). For measurement of the hydrolysis rate constant of the intermediate only, 50 mM sodium phosphate buffer (pH 7.0) containing CH3CN was used as the eluant. The CH3CN concentration was increased from 0 to 20% over 20 min as a linear gradient. RP-HPLC analysis was performed at a flow rate of 1.0 ml/min and ambient temperature.
Spectrometric measurements
NMR spectra were measured on a Bruker ARX-500 NMR spectrometer (500 MHz) at 30°C. The chemical shifts were referenced to sodium 3-(trimethylsilyl)[2,2,3,3-d4]propionate (TSP) in D2O or tetramethylsilane (TMS) in DMSO-d6 as an internal standard. The high resolution (HR) EI mass spectrum was recorded on a JEOL DX-300 at 30 eV. Negative ion APCI (atmospheric pressure chemical ionization)-LC mass spectra were obtained with a Hitachi M-2000 MS system. The sample was directly injected into the MS system by an HPLC pump without separation columns. The LC/MS conditions were as follows: eluant, 100% CH3CN (isocratic); flow rate, 1 ml/min; vaporization temperature, 300°C; desolvation temperature, 300–370°C; drift voltage, –195 V. UV spectra for hydrolysis of the intermediate were measured on a Shimadzu UV-260 UV-Vis spectrophotometer equipped with an SPR-5 temperature controller.
Spectrometric data
N2-Methyl-N2-nitrosoguanosine (N2-Me-N2-nitroso-Guo). 1H NMR (500 MHz, DMSO-d6 at 30°C) δ (p.p.m./TMS) 12.86 (br, 1H, NH), 8.35 (s, 1H, H-8), 5.55 (dd, 1H, H-1′), 5.48 (br, 1H, OH), 5.21 (br, 1H, OH), 4.97 (br, 1H, OH), 4.55 (m, 1H, H-2′), 4.16 (ddd, 1H, H-3′), 3.95 (ddd, 1H, H-4′), 3.61 (ABX, 2H, H-5′,5′′), 3.41 (s, 3H, CH3); UV λmax 240 nm, 293 nm (pH 7); APCI-LC/MS (negative) m/z 325 (M – H)–, 296 (M – NO)–.
1-Methylxanthosine (1-Me-Xao). 1H NMR (500 MHz, DMSO-d6 at 30°C) δ (p.p.m./TMS) 7.82 (s, 1H, H-8), 5.73 (dd, 1H, H-1′), 5.42 (br, 1H, 5′- or 2′-OH), 5.19 (br, 1H, 3′-OH), 4.30 (m, 1H, H-2′), 4.07 (ddd, 1H, H-3′), 4.00 (ddd, 1H, H-4′), 3.64 (ABX, 2H, H-5′,5′′), 3.17 (d, 3H, CH3). [We measured the 1H NMR spectrum in the range –1 to 17 p.p.m. but could not observe the signal of the NH proton. In H2O at neutral pH, 1-Me-Xao exists as the 6-keto-2-enol anion form (17). We suspect that 1-Me-Xao existed as the 6-keto-2-enol anion form in DMSO-d6 due to a trace of H2O present in DMSO or the sample, thus obscuring the NH signal. We observed only two OH proton signals derived from the ribose moiety. One (5.19 p.p.m.) was identified as the 3′-OH proton by COSY measurement. However, for another (5.42 p.p.m., a fairly broadened signal), it could not be determined whether it arose from the 2′-OH or 5′-OH since no correlation was observed for this resonance in the COSY spectrum. It is likely that the third OH signal was completely obscured by further broadening.] UV λmax 251 nm, 277 nm (pH 7); APCI-LC/MS (negative) m/z 297 (M – H)–, 165 (Mbase fragment)–.
N5-Methyloxanosine (N5-Me-Oxo). 1H NMR (500 MHz, DMSO-d6 at 30°C) δ (p.p.m./TMS) 8.28 (br, 1H, NH), 8.01 (s, 1H, H-2), 5.68 (dd, J = 6.0 Hz, 1H, H-1′), 5.41 (d, J = 6.1 Hz, 1H, 2′-OH), 5.17 (d, J = 4.9 Hz, 1H, 3′-OH), 4.93 (dd, J = 4.1 Hz, J = 4.1 Hz, 1H, 5′-OH), 4.47 (m, 1H, H-2′), 4.11 (ddd, 1H, H-3′), 3.90 (ddd, 1H, H-4′), 3.60 (ABX, 2H, H-5′,5′′), 2.81 (d, J = 3.9 Hz, 3H, CH3). [A correlation between the NH proton signal (8.28 p.p.m.) and the CH3 proton signal (2.81 p.p.m.) was observed in the COSY measurement.] UV λmax 248 nm, 292 nm (pH 7); APCI-LC/MS (negative) m/z 297 (M – H)–, 165 (Mbase fragment)–; HR-EI (positive) m/z 166.049 72 (Mbase fragment + H)+ (calculated for C6H6N4O2, 166.049 08).
Quantitative procedures
Guo or 1-Me-Guo (10 mM) was incubated with NaNO2 (100 mM) in 1 ml sodium acetate buffer (3.0 M, pH 3.7) at 37°C for 6 h. For the Guo–HNO2 system, the concentration of each reaction product was determined from the peak area of the RP-HPLC chromatogram monitored at 260 nm and the molar extinction coefficient. The molar extinction coefficients [ɛ260: Guo, 11 500; Oxo, 5100; Xao, 7800] were taken from the literature (4,18,19). The initial RP-HPLC peak area was used as a standard. Since the molar extinction coefficients of the methyl derivatives (1-Me-Guo, N5-Me-Oxo and 1-Me-Xao) were not available, the amounts of these products were determined using 1H NMR integration signals as follows. After reaction with HNO2, 1-Me-Guo solution was neutralized with concentrated NaOH. Then the sample was lyophilized and dissolved in 400 µl of D2O (98%). The 1H NMR spectrum of the sample was measured with a 45° pulse with a delay time of 10 s. The yields of products were determined by the relative intensities of the integral values for the methyl proton resonances of 1-Me-Guo (3.48 p.p.m./TSP), N5-Me-Oxo (2.96 p.p.m./TSP) and 1-Me-Xao (3.35 p.p.m./TSP).
Kinetics
pH dependence. The RP-HPLC column eluant containing the diazoate (20 µl) was directly dropped into 100 mM buffer (480 µl) and incubated at 37°C. The buffers used were sodium acetate buffer (pH 2–3.7, concentrated HCl was added for pH 2 and 3), sodium phosphate buffer (pH 5–8) and sodium carbonate buffer (pH 9 and 10). The incubation periods were 10 min for pH 2 and 3, 30 min for pH 4–6, 1 h for pH 7 and 8, 1.5 h for pH 9 and 2 h for pH 10. The samples in acidic or alkaline buffer were neutralized by addition of 1 M NaOH or HCl and analyzed by RP-HPLC.
Temperature dependence. The RP-HPLC column eluant containing the diazoate (20 µl) was directly dropped into temperature controlled 100 mM sodium phosphate buffer (480 µl) at pH 7.0. The incubation periods were 4 h for 0°C, 2 h for 10°C, 1 h for 20 and 30°C and 30 min for 37 and 50°C. The samples were analyzed by RP-HPLC.
Solvent effect. The RP-HPLC column eluant containing the diazoate (20 µl) was directly dropped into the mixture of acetone and H2O (480 or 980 µl) at 20°C. The incubation periods were 30 min for 0% (acetone % v/v), 1 h for 25%, 1.5 h for 50%, 2 h for 75% and 48 h for 96 and 98%. Acetone was removed from each sample by aspirator and the samples were analyzed by RP-HPLC.
RESULTS AND DISCUSSION
Determination of the reaction site using methyl derivatives of Guo
The reaction site leading to the conversion of dGuo into dOxo by HNO2 was explored using Guo (the ribonucleoside counterpart of dGuo) and its methyl derivatives to avoid cleavage of the labile N-glycosidic bond of deoxyribonucleosides. Guo (10 mM) was incubated with 100 mM NaNO2 in 3.0 M sodium acetate buffer (pH 3.7) at 37°C for 6 h and analyzed by RP-HPLC (data not shown). The first peak (retention time tR = 9.1 min) was identified as Xao by agreement with the retention time and the on-line UV spectrum of the authentic sample. The second peak (tR = 10.6 min) was unreacted Guo. The third peak (tR = 12.0 min) was identified as Oxo by coincidence with the UV spectrum in a previous paper (1). The yields of reaction products were 17.9% Oxo and 79.3% Xao with 4.0% unreacted Guo. Figure 1A shows the structures of these compounds with atomic numbering. It should be noted that the atomic numbering of the base moiety of Oxo and its analog is different from that of purines. Although the numbering of purines is customary, a systematic numbering following the IUPAC rules is applied to Oxa and its analog since they are not purines (2,20). For dGuo, it has been reported that the yields were 21.5% dOxo and 58.7% dXao and xanthine (a base moiety of dXao) with 7.6% unreacted dGuo under the same reaction conditions (10). Accordingly, substitution of a hydroxyl group (Guo) for a hydrogen atom (dGuo) at the 2′ sugar position had little influence on the formation of Oxo derivatives.
Figure 1.
Reaction products of HNO2-treated (A) guanosine (Guo), with the atomic numbering, (B) N2,N2-dimethylguanosine (N2,N2-diMe-Guo), (C) N2-methylguanosine (N2-Me-Guo) and (D) 1-methylguanosine (1-Me-Guo). Note that the atomic numbering of the base moieties of oxanosine (Oxo) and its analog is different from that of purines (see text). The numbers in parentheses denote percentage yields of products at a reaction time of 6 h.
Concerning the mechanism of dOxo formation, two conceivable pathways exist in the initial step of the reaction (Scheme 1). In Path I, the reaction is initiated by attack of NOx (probably N2O3) produced by protonation of nitrite on the N1-imino group of dGuo. In this case, dGuo bearing N1 substituents like nitroso, nitro or diazo groups are potential precursors en route to dOxo. dOxo is formed by subsequent attack of a water molecule on N1 of the intermediates. To obtain 15N-labeled purine nucleosides, an N1-nitropurine compound has been employed as a precursor for N1 substitution by 15N compounds (21). Path II includes attack of N2O3 on the exocyclic amino group of dGuo. In this case, a nitroso group is introduced on a N2 atom. Subsequently several intermediates are formed, such as diazohydroxide, diazoate and diazonium derivatives, based on the diazo chemistry of aromatic primary amines (Scheme 2; 22,23). To determine which reaction pathway is responsible for dOxo formation, N2,N2-diMe-Guo, N2-Me-Guo and 1-Me-Guo were treated with HNO2 and the products were analyzed. If Path I is involved, substitution of N1 by an oxygen atom will not occur for 1-Me-Guo since the methyl group protects N1 against the attack of N2O3. If Path II is involved, the reaction will not proceed for N2,N2-diMe-Guo since the methyl groups protect the exocyclic amino group on C2 against attack by N2O3. The reaction of N2-Me-Guo carrying a secondary amine will stop at the step of the nitroso derivative.
Scheme 1. Two possible reaction pathways for the formation of 2′-deoxyoxanosine (dOxo) from 2′-deoxyguanosine (dGuo) by HNO2 treatment. dR in the structures denotes a 2′-deoxyribose moiety.
Scheme 2. Proposed reaction pathways for the nitrosative deamination of aromatic amines by HNO2.
N2,N2-diMe-Guo (1.0 mM), N2-Me-Guo (10 mM) and 1-Me-Guo (10 mM) were incubated with NaNO2 (100 mM) in sodium acetate buffer (3.0 M, pH 3.7) for 6 h at 37°C, and the reactions monitored by RP-HPLC. No product was observed in the reaction of N2,N2-diMe-Guo (Fig. 1B). One product was detected in the reaction of N2-Me-Guo and isolated by RP-HPLC. From spectrometric data, the product was identified as a nitroso derivative, N2-methyl-N2-nitrosoguanosine (N2-Me-N2-nitroso-Guo) (Fig. 1C). N2-Me-Guo was completely converted to N2-Me-N2-nitroso-Guo after incubation for 6 h. When 1-Me-Guo was incubated with HNO2, two major product peaks with tR = 10.2 and 13.7 min appeared in the RP-HPLC chromatogram (data not shown). The UV spectra of the peaks were similar to those of Xao and Oxo, respectively. From the spectrometric data, these products were identified as 1-Me-Xao, a deamination product of 1-Me-Guo, and N5-methyloxanosine (N5-Me-Oxo), an Oxo analog bearing an exocyclic methyl amino group (Fig. 1D). The yields of the products (HNO2 treatment for 6 h) were 38.0% N5-Me-Oxo and 22.3% 1-Me-Xao with 39.7% unreacted 1-Me-Guo. Unlike the results for Guo and dGuo, treatment of 1-Me-Guo with HNO2 resulted in a higher yield of the Oxo derivative than the Xao derivative.
Consideration of rearrangements
In the formation of N5-Me-Oxo from 1-Me-Guo (Fig. 1D), the N1-methyl group shifted from the endocyclic to exocyclic position. This type of methyl group shift, which is known as a Dimroth rearrangement, can take place in heterocyclic compounds when an exocyclic amino or imino group has a neighboring N-alkyl group in a ring (24,25). Dimroth rearrangement proceeds under alkaline or acidic conditions for pyrimidines, purines and pteridine derivatives (26–31). If Dimroth rearrangement is involved in the present reaction system, generation of N5-Me-Oxo from 1-Me-Guo would require initial formation of N2-Me-Guo from 1-Me-Guo followed by substitution of the N1 atom by an oxygen atom. To examine this possibility, 1-Me-Guo (10 mM) was incubated in 3.0 M sodium acetate buffer (pH 3.7) in the absence of NO2– for 48 h at 37°C. No peak other than the starting 1-Me-Guo was observed in the RP-HPLC chromatogram. Consequently, it is evident that intramolecular rearrangement from 1-Me-Guo to N2-Me-Guo is not involved in the present system.
Since the base moieties of the Oxo and Xao derivatives are structural isomers of each other, we also explored the possibility of interconversion between these derivatives. An aqueous solution containing Xao or 1-Me-Xao was incubated in the presence or absence of 100 mM NaNO2 in 3.0 M sodium acetate buffer (pH 3.7) at 37°C for 48 h. However, Oxo or N5-Me-Oxo was not observed in the RP-HPLC chromatograms (data not shown). In similar experiments, no conversion from Oxo and N5-Me-Oxo to Xao and 1-Me-Xao occurred, respectively.
Intramolecular rearrangement from 1-Me-Guo to N2-Me-Guo and conversion between Oxo and Xao derivatives were not involved in the present system. These results clearly show that the formation of dOxo is initiated by the attack of N2O3 on the exocyclic amino group of dGuo. It is also evident that the amino group of dOxo was derived from the N1-imino group of dGuo. Thus, cleavage of the C6–N1 amido bond in the pyrimidine ring of dGuo occurs during the reaction.
Detection and separation of a reaction intermediate
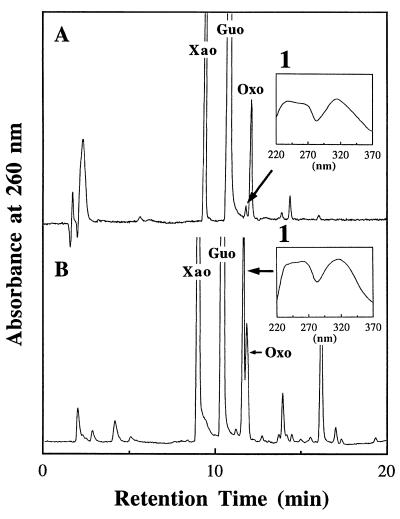
To obtain further information on the reaction mechanism of dOxo formation, we attempted to identify the reaction intermediates by RP-HPLC analysis. Guo (10 mM) and NaNO2 (100 mM) was incubated in sodium acetate buffer (3.0 M, pH 3.7) at 37°C for a short period (5 min). Immediately after neutralization, the reaction mixture was analyzed by RP-HPLC (Fig. 2A). In addition to the major products, several small peaks were detected. Among these peaks, only the peak with tR = 11.7 min, which showed a UV spectrum with λmax = 315 nm (Fig. 2A, inset), decreased at an incubation time of 30 min (data not shown). Therefore, a compound contained in this peak (referred to as 1) was assumed to be a reaction intermediate. In order to obtain 1 in large scale, another reaction system was employed. Guo (0.5 M) in 10 µl of 5 M H2SO4 was added to 1.2 µl of 4 M NaNO2 and incubated at 0°C for 5 s. The solution was immediately neutralized with 10 M NaOH (10 µl) and 100 mM phosphate buffer (pH 7.0, 100 µl) and analyzed by RP-HPLC. As shown in Figure 2B and its inset, a large peak appeared which had the same retention time and UV spectrum as 1, indicating that the peak contained 1. The RP-HPLC fraction of 1 obtained by the above method was used in the following experiments. The fraction also contained elution buffer (100 mM TEAA buffer and ~10% CH3CN).
Figure 2.
(A) RP-HPLC profile of the reaction mixture of guanosine (Guo) when 10 mM Guo and 100 mM NaNO2 were incubated in 3.0 M sodium acetate buffer (pH 3.7) for 5 min at 37°C. (B) RP-HPLC profile of the reaction mixture of Guo when 0.5 M Guo in 10 µl of 5 M H2SO4 was added to 1.2 µl of 4 M NaNO2 and incubated at 0°C for 5 s. The inset shows the UV spectrum of 1 indicated by the arrow. The CH3CN concentration was increased in the linear gradient mode (0 min; 0%, 20 min; 20%).
Identification of the intermediate
To identify the intermediate, several measurements were performed. 1 was trapped completely by an anion exchange resin (Sephadex A-25, OH– form) but passed directly through the same bed volume of a cation exchange resin (Bio-Rad AG50W-X8, H+ form). Among all of the expected intermediates in the reaction of Guo with HNO2, only the diazoate derivative is an anion (Scheme 2). Negative ion APCI-LC/MS using 100% CH3CN as eluant showed a signal with m/z = 283 for 1. However, the signal at m/z = 311 expected for the intact diazoate anion could not be detected in the LC/MS measurements, probably due to its instability (vide infra). In general, aromatic diazoate compounds (R–NNO–) are known to give rise to a strong signal of the [M– – 28] fragment which is produced by loss of a molecular nitrogen through four-center skeletal rearrangement (32,33). Thus, the signal with m/z = 283 was attributed to the –N2 product. The on-line UV spectrum of 1 (λmax = 315 nm) was similar to those of the diazoate derivatives of 2′-deoxycytidine (dCyd) (λmax = 305 nm) (16) and 9-alkyladenine (λmax = ~310 nm) (23). Combining these data, we have tentatively assigned 1 as a diazoate derivative of Guo, 9-(β-d-2′-ribofuranosyl)-6-oxopurine-2-diazoate (Fig. 3).
Figure 3.
Structure of 1, a diazoate derivative of guanosine [9-(β-d-2′-ribofuranosyl)-6-oxopurine-2-diazoate].
Hydrolysis of the diazoate intermediate
The RP-HPLC fraction of the diazoate (100 µl) was incubated in 50 mM phosphate buffer (pH 7.0, 500 µl) at 37°C for 20 min and analyzed by RP-HPLC. The peak of the diazoate disappeared and two peaks of Oxo and Xao appeared (data not shown). The ratio of the yield of Oxo to Xao was 0.29. This result clearly shows that the diazoate is the common precursor of Oxo and Xao in the Guo–HNO2 system. Incubation of the diazoate in 0.5 M H2SO4 at 37°C for 20 min resulted in Guo as the main product with small amounts of Oxo and Xao. In a previous study on the 9-alkyladenine diazoate derivative, similar conversion from the diazoate group to the starting amino group has been reported under strongly acidic conditions (23). No change in the UV spectrum was observed when the diazoate derivative of Guo was incubated in 1 M NaOH at 37°C for 2 h. Therefore, the diazoate is converted to Oxo and Xao at neutral pH, but to the starting Guo under acidic conditions. The diazoate is stable under basic conditions.
The rate constant for hydrolysis of this intermediate was measured at neutral pH. To avoid the influence of co-existing TEAA contained in the RP-HPLC eluant on the reaction rate, the diazoate was RP-HPLC purified using 50 mM phosphate buffer (pH 7.0) as eluant. The RP-HPLC fraction of the diazoate (200 µl) was diluted with 50 mM phosphate buffer (400 µl, pH 7.0) and then UV spectra were measured at intervals of 3 min at 20°C. The diazoate showed an absorption band at >310 nm, whereas Oxo and Xao showed no absorption. Thus, as shown in the inset to Figure 4, the absorption at >310 nm due to the diazoate rapidly decreased with incubation time. The plot of absorbance at 340 nm against incubation time followed first order kinetics (Fig. 4). The rate constant of the reaction was evaluated as 2.1 × 10–3 s–1 (half life t1/2 = 5.6 min) from the slope.
Figure 4.
Plot of the absorbance at 340 nm against incubation time. The diazoate was incubated at 20°C in 50 mM sodium phosphate buffer containing ~3% CH3CN. The inset is the change in the UV spectrum of the reaction mixture measured at 3 min intervals.
Ring-closed and ring-opened cations
Generally, diazonium salts generate cations by dissociative loss of a molecular nitrogen (34–36). Although aromatic diazonium compounds form ring-closed cations exclusively, some aliphatic ring compounds generate not only ring-closed but also ring-opened cations (37–39). Two types of reaction mechanism have been proposed for the ring opening process on the basis of kinetic and stereochemical criteria. One is the one-step reaction mechanism including synchronous ring opening with elimination of a nitrogen molecule. Bicycloalkanediazonium ions undergo a synchronous ring opening reaction (37). The other is the multi-step reaction mechanism, including formation of the ring-closed cation and sequential ring opening. Decomposition of the phenyl-substituted cyclopropanediazonium ion occurs via a multi-step reaction (39). In recently published papers by Glaser and colleagues, the reaction mechanism for the formation of dOxo was explored using ab initio calculation (13–15). According to their calculations, cleavage of the C6–N1 bond occurs when the length of the C2–N2+ bond of the guanine diazonium ion is >2.2 Å. All attempts to search the reaction channel connecting the diazonium ion to the ring-closed cation failed. The ring-opened cation was more stable (63.0 kcal/mol) than the ring-closed cation. These results suggest that release of a nitrogen molecule from the diazonium ion generates the ring-opened cation directly without formation of the ring-closed cation, supporting the one-step mechanism.
If the one-step reaction mechanism is involved in the present system, only the ring-opened cation would be generated (Scheme 3A). Attack by H2O on the carbonyl and carbodiimide groups of the ring-opened cation generate Oxo and Xao, respectively, after ring closure. In this case the ratio Oxo:Xao is determined only by the relative reactivity of the carbonyl and carbodiimide groups with H2O. If the multi-step mechanism is involved, the ring-closed cation exclusively generating Xao by reaction with H2O will be present in addition to the ring-opened cation (Scheme 3B). To elucidate which reaction mechanism is operating in the present system, hydrolysis of the diazoate was carried out under various conditions and the influences of pH, temperature and solvent on the product ratio (Oxo:Xao) were determined. The products were quantified by RP-HPLC analysis. No products other than Oxo and Xao were detected in all reactions. The RP-HPLC fraction containing the diazoate was incubated at 20°C in the pH range 2–10. The Oxo:Xao ratio was independent of pH within the range tested, suggesting that no acid–base equilibrium was present for the intermediate cation(s). To clarify the temperature effect, the diazoate was incubated in 100 mM sodium phosphate buffer at pH 7.0 in the temperature range 0–50°C. The Oxo:Xao ratio increased linearly with temperature from 0.22 (0°C) to 0.32 (50°C) (Fig. 5A). The previous ab initio calculations showed that the ring-opened cation was preferred over the ring-closed cation by 63.0 kcal/mol (13). If an equilibrium exists between these two cations (Scheme 3B) and the ring-opened cation is more stable than the ring-closed one, a rise in reaction temperature drives the equilibrium in the endothermic direction, hence increasing the fraction of the ring-closed cation. In this case, a rise in incubation temperature will lead to a reduction in the Oxo:Xao ratio. Thus, the present results showing an opposite tendency do not support the multi-step reaction. The observed increase in the Oxo:Xao ratio with a temperature rise might be the result of the activation energy of hydrolysis of the carbonyl group being greater than that of the carbodiimide group on the ring-opened cation. To examine the solvent effect, the Oxo:Xao ratio was measured by incubating the diazoate in solutions containing 0–98% (v/v) acetone at 20°C. The ratio increased with increasing acetone content from 0.26 (0% acetone) to 0.85 (98% acetone) (Fig. 5B). If an equilibrium exists between the two cations and the ring-closed cation is preferentially stabilized by reducing the dielectric constant of the solvent, addition of acetone should increase the fraction of the ring-closed cation. In this case, the addition of acetone would reduce the Oxo:Xao ratio. However, the observed effect of acetone was the opposite, not supporting the multi-step reaction. The increase in the Oxo:Xao ratio might result from the frequency of access of H2O to the cationic carbonyl group relative to the carbodiimide group of the ring-opened cation increasing with a rise in acetone content.
Scheme 3. Two conceivable reaction pathways for the formation of 2′-deoxyoxanosine (dOxo) and 2′-deoxyxanthosine (dXao) from the diazonium intermediate.
Figure 5.
(A) The temperature dependence of the Oxo:Xao ratio at pH 7.0. (B) The dependence of the Oxo:Xao ratio on acetone content at 20°C.
All the results concerning the effects of pH, temperature and solvent failed to support the existence of the ring-closed cation. It is very likely that the one-step mechanism dominates in the reaction of Guo with HNO2.
Overall reaction mechanism
A possible overall reaction pathway of dGuo with nitrous acid is summarized in Scheme 4 on the basis of the present results. First, a reactive species NOx (probably N2O3) generated from protonated NO2– attacks the amino group on C2 of dGuo, resulting in the N2-nitroso intermediate. The N2-nitroso intermediate is converted immediately to the diazoate intermediate, which appears to be the most stable intermediate throughout the reaction pathway. Then the diazoate intermediate is converted to the diazonium intermediate and the ring-opened cation is formed synchronously by the release of a nitrogen molecule from the diazonium. Attack by H2O on the carbodiimide of the ring-opened cation and subsequent ring closure and tautomerization generates dXao. On the other hand, attack by H2O on the carbonyl group of the ring-opened cation and subsequent ring closure generates dOxo.
Scheme 4. The proposed overall mechanism for the reaction of 2′-deoxyguanosine (dGuo) with HNO2.
Acknowledgments
ACKNOWLEDGEMENTS
We thank Dr M. Nishi (Setsunan University) for acquiring the HR-EI MS spectrum. This work was supported partly by Grants-in-Aid for Scientific Research from the Ministry of Education, Science and Culture, Japan [to K.M. (11101001, 10151219 and 10878092), K.K. (09780545) and H.I.].
REFERENCES
- 1.Shimada N., Yagisawa,N., Naganawa,H., Takita,T., Hamada,M., Takeuchi,T. and Umezawa,H. (1981) J. Antibiot. (Tokyo), 34, 1216–1218. [DOI] [PubMed] [Google Scholar]
- 2.Nakamura H., Yagisawa,N., Shimada,N., Takita,T., Umezawa,H. and Iitaka,Y. (1981) J. Antibiot. (Tokyo), 34, 1219–1221. [DOI] [PubMed] [Google Scholar]
- 3.Itoh O., Kuroiwa,K., Atsumi,S., Umezawa,K., Takeuchi,T. and Hori,M. (1989) Cancer Res., 49, 996–1000. [PubMed] [Google Scholar]
- 4.Kato K., Yagisawa,N., Shimada,N., Hamada,M., Takita,T., Maeda,K. and Umezawa,H. (1984) J. Antibiot. (Tokyo), 37, 941–942. [DOI] [PubMed] [Google Scholar]
- 5.Yagisawa N., Takita,T. and Umezawa,H. (1983) Tetrahedron Lett., 24, 931–932. [Google Scholar]
- 6.Bhattacharya B.K., Robins,R.K. and Revankar,G.R. (1990) J. Heterocyclic Chem., 27, 787–793. [Google Scholar]
- 7.Matsumoto H., Kaneko,C., Mori,T. and Mizuno,Y. (1990) J. Heterocyclic Chem., 27, 1307–1311. [Google Scholar]
- 8.Luk K.-C., Moore,D.W. and Keith,D.D. (1994) Tetrahedron Lett., 35, 1007–1010. [Google Scholar]
- 9.DeNapoli L., DiFrabio,G., Messere,A., Montesarchio,D, Piccialli,G. and Varra,M. (1998) Tetrahedron Lett., 39, 7397–7400. [Google Scholar]
- 10.Suzuki T., Yamaoka,R., Nishi,M., Ide,H. and Makino,K. (1996) J. Am. Chem. Soc., 118, 2515–2516. [Google Scholar]
- 11.Suzuki T., Matsumura,Y., Ide,H., Kanaori,K., Tajima,K. and Makino,K. (1997) Biochemistry, 36, 8013–8019. [DOI] [PubMed] [Google Scholar]
- 12.Suzuki T., Yoshida,M., Yamada,M., Ide,H., Kobayashi,M., Kanaori,K., Tajima,K. and Makino,K. (1998) Biochemistry, 37, 11592–11598. [DOI] [PubMed] [Google Scholar]
- 13.Glaser R. and Son,M.-S. (1996) J. Am. Chem. Soc., 118, 10942–10943. [Google Scholar]
- 14.Glaser R. and Lewis,M. (1999) Organic Lett., 1, 273–276. [DOI] [PubMed] [Google Scholar]
- 15.Glaser R., Rayat,S., Lewis,M., Son,M.-S. and Meyer,S. (1999) J. Am. Chem. Soc., 121, 6108–6119. [Google Scholar]
- 16.Suzuki T., Nakamura,T., Yamada,M., Ide,H., Kanaori,K., Tajima,K., Morii,T. and Makino,K. (1999) Biochemistry, 38, 7151–7158. [DOI] [PubMed] [Google Scholar]
- 17.Roy K.B. and Miles,T. (1983) Nucl. Nucl., 2, 231–242. [Google Scholar]
- 18.Sambrook J., Fritsch,E.F. and Maniatis,T. (1989) Molecular Cloning: A Laboratory Manual. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY.
- 19.McClure T.D., Scharm,K.H., Nakano,K. and Yasaka,T. (1989) Nucl. Nucl., 8, 1399–1415. [Google Scholar]
- 20.Rigaudy J. and Klesney,S.P. (1979) Nomenclature of Organic Chemistry: Sections A, B, C, D, E, F and H. Pergamon Press, Oxford, UK.
- 21.Ariza X., Bou,V. and Vilarrasa,J. (1995) J. Am. Chem. Soc., 117, 3665–3673. [Google Scholar]
- 22.Zollinger H. (1994) Diazo Chemistry I—Aromatic and Heteroaromatic Compounds. VCH, Weinheim, Germany.
- 23.Bunton C.A. and Wolfe,B.B. (1974) J. Am. Chem. Soc., 96, 7747–7752. [Google Scholar]
- 24.Dimroth O. (1909) Annalen, 364, 183–226. [Google Scholar]
- 25.Dimroth O. (1910) Annalen, 373, 336–370. [Google Scholar]
- 26.Brown D.J. (1961) Nature, 189, 828–829. [Google Scholar]
- 27.Brookes P. and Lawley,P.D. (1960) J. Chem. Soc., 539–545. [Google Scholar]
- 28.Jones J.W. and Robins,R.K. (1963) J. Am. Chem. Soc., 85, 193–201. [Google Scholar]
- 29.Brookes P., Dipple,A. and Lawley,P.D. (1968) J. Chem. Soc. (C), 2026–2028. [DOI] [PubMed] [Google Scholar]
- 30.Brown D.J. and Jacobsen,N.W. (1960) J. Chem. Soc., 1978–1985. [Google Scholar]
- 31.Brown D.M. (1974) In Ts’o,P.O.P. (ed.), Basic Principles in Nucleic Acid Chemistry. Academic Press, New York, NY, Vol. 2, pp. 1–91.
- 32.Broxton T.J., Colton,R. and Traeger,J.C. (1995) J. Mass Spectrom., 30, 319–323. [Google Scholar]
- 33.Broxton T.J. and Rowe,J.E. (1977) Org. Mass Spectrom., 12, 185–190. [Google Scholar]
- 34.Gasper S.M., Devadoss,C. and Schuster,G.B. (1995) J. Am. Chem. Soc., 117, 5206–5211. [Google Scholar]
- 35.Ambroz H.B. and Kemp,T. (1979) J. Chem. Soc. Rev., 8, 353–365. [Google Scholar]
- 36.Zollinger H. (1978) Angew. Chem. Int. Ed. Engl., 17, 141–150. [Google Scholar]
- 37.Kirmse W. and Scheidt,F. (1970) Chem. Ber., 103, 3711–3721. [Google Scholar]
- 38.Kirmse W. and Schütte,H. (1968) Chem. Ber., 101, 1674–1688. [Google Scholar]
- 39.Kirmse W. (1976) Angew. Chem. Int. Ed. Engl., 15, 251–261. [Google Scholar]