Abstract
This study aimed to compare the clinical and biochemical profiles as well as the complications in males and females with type 2 diabetes (T2DM) presenting to a private tertiary diabetes care centre in India. This is a retrospective study, conducted between 1 January 2017 and 31 December 2019, and included 72,980 individuals with T2DM, aged ≥ 18 years (age and sex-matched—males—36,490; females—36,490). Anthropometric measurements, blood pressure, fasting plasma glucose (FPG), post-prandial plasma glucose (PPPG), glycated haemoglobin (HbA1c), lipids, urea, and creatinine were measured. Retinopathy was screened using retinal photography, neuropathy using biothesiometry, nephropathy measuring urinary albumin excretion, peripheral vascular disease (PVD) using Doppler, and coronary artery disease (CAD) based on the history of myocardial infarction and/or drug treatment for CAD and/or electrocardiographic changes. Obesity (73.6% vs. 59.0%) rates were significantly higher in females compared to males. FPG, PPPG, and HbA1c were higher among younger age groups among both sexes, with males having higher values compared to females. However, after the age of 44 years, control of diabetes was worse among females. In addition, only 18.8% of the females achieved glycemic control (HbA1c < 7%) compared to 19.9% in males (p < 0.001). Males had higher prevalence of neuropathy (42.9% vs. 36.9%), retinopathy (36.0% vs. 26.3%), and nephropathy (25.0% vs. 23.3%) compared to females. Males had 1.8- and 1.6-times higher risk of developing CAD and retinopathy compared to females. Hypothyroidism (12.5% vs. 3.5%) and cancers (1.3% vs. 0.6%) were significantly higher in females compared to males. In this large sample of T2DM seen at a chain of private tertiary diabetes centres, females had higher prevalence of metabolic risk factors and poorer diabetes control compared to males, emphasizing the need for better control of diabetes in females. However, males had higher prevalence of neuropathy, retinopathy, nephropathy, and CAD compared to females.
Keywords: gender, women, type 2 diabetes, clinical profile, complications, India
1. Introduction
Diabetes is a rapidly emerging public health problem that has reached alarming proportions in low- and middle-income countries like India [1]. The global burden of diabetes currently stands at 537 million people, of which India alone accounts for 74 million [2,3].
A slightly higher prevalence of diabetes has been noted in males compared to females (10.8% vs. 10.2%) [3]. A similar trend is observed in the Indian population in the National Family Health Survey (NHFS) with a higher proportion of males than females having random blood glucose > 140 mg/dL (30.1% vs. 25.9%) [4]. The Indian Council of Medical Research—India Diabetes (ICMR-INDIAB) study also reported that the prevalence of diabetes was significantly higher in males than in females between the ages of 35 and 65 years; however, beyond this age group, the prevalence was slightly higher in females, probably reflecting survivor bias [5].
Sex differences could be due to biological differences between males and females that are produced by variations in chromosomes, autosomal gene expression, sex hormones, and their impact on various body systems [6]. Gender is a multifaceted construct, and various gender-related traits may have varying effects on health-related behaviour and other factors, such as susceptibility to stress [7]. There are differences in the behaviour of females and males, exposure to certain environmental factors, and differences in lifestyle factors like physical activity, diet, or stress that leads to gender disparities in the risk of diabetes [6]. Studies of gender differences are also essential for planning awareness programs and understanding the burden of diabetes in females [6,8].
Current evidence suggests there are clinically significant sex variations in type 2 diabetes mellitus (T2DM) rates in youth and middle age [9,10,11,12,13,14]. There are also gender disparities in the risk of cancer, dementia, and kidney diseases in those with T2DM [15,16,17]. A recent national study has revealed that compared to men, women did not achieve lipid treatment targets, suggesting gender disparities in affordability and accessibility of treatment [18]. Hence, we hypothesize that women are likely to have suboptimal diabetes management and thereby greater propensity to develop complications. Thus, the objective of this study was to analyse the sex-based differences in demography, lifestyle, glycemic and biochemical parameters, and complications profiles in Asian Indian adults with T2DM seen at a private tertiary diabetes care centre with branches across India.
2. Materials and Methods
This was a retrospective study conducted on individuals with T2DM, who were registered at a tertiary diabetes care centre in the private sector with branches across India. The centre has clinical records for over 550,000 diabetes patients. For this study, data of individuals with T2DM seen between 1 January 2017 and 31 December 2019 were analysed. The inclusion criteria for this study were individuals with T2DM belonging to both sexes, aged ≥ 18 years and in whom glycemic parameters and details of complications were available for analysis. Pregnant and lactating women and individuals who had not given prior informed consent to use their data for research purposes were excluded from this study. Thus, this study included the de-identified data of 72,980 individuals with T2DM (male: 36,490; female: 36,490) who were age and gender-matched. T2DM was diagnosed by the absence of ketosis, good b-cell reserve as shown by fasting [C-peptide assay] [>0.6 pmol/mL], and stimulated C peptide > 1.0 pmol/mL and response to oral hypoglycemic agents (OHA’s) for at least 2 years [19].
On enrolment, demographic profiles and anthropometric measurements such as height, weight, and blood pressure were measured using standard methods. Height was measured in centimetres using a stadiometer and weight in kilograms using an electronic weighing scale. Weight in kg/(height in m2) was used to determine the body mass index (BMI). Blood pressure was measured in the right arm in the sitting posture using an electronic blood pressure apparatus. A comprehensive medical history, including current medicines and family history of diabetes, was also collected. During the first visit, biochemical parameters including fasting plasma glucose (FPG) and postprandial plasma glucose (PPPG), HbA1c, and lipid profile were measured, and diabetes complications were also were assessed as described below. At each visit, all of these parameters are archived in the diabetic electronic medical record (DEMR) [20,21].
After an overnight fast of 8 to 10 h, a fasting blood sample was taken. Participants were given a meal (carbohydrates around 60 g) and a venous blood sample was obtained after 90 min for the post-prandial glucose sample. The samples were analyzed at the clinical laboratory of the centre, which is accredited by the College of American Pathologists (CAP) and the National Accreditation Board for Testing and Calibration Laboratories (NABL). The Beckman Coulter AU 2700/480 Autoanalyzer [Beckman AU(Olympus), Ireland] was used to measure plasma glucose (glucose oxidase-peroxidase method), serum cholesterol (cholesterol oxidase phenol 4-aminoantipyrine peroxidase method), serum triglycerides (glycerol phosphate oxidase-peroxidase-amidopyrine), and high-density lipoprotein [HDL] cholesterol (direct immunoinhibition method). The low-density lipoprotein (LDL) cholesterol levels were calculated using the Friedewald algorithm. The HPLC technique was used to calculate HbA1c using a Variant machine (Bio-Rad, Hercules, CA, USA).
The complications screening included retinopathy, nephropathy, neuropathy, peripheral vascular disease [PVD], and coronary artery disease [CAD]. To identify retinopathy, seven-field stereo colour retinal photography (Zeiss FF450 Plus Digital Fundus Camera, Carl Zeiss Meditec, Inc. Dublin, Ireland,) was performed and the images were graded by an ophthalmologist using the Early Treatment Diabetic Retinopathy Study criteria [22]. The presence of at least one distinct microaneurysm was the minimum requirement for diagnosing diabetic retinopathy. Nephropathy was defined as urine albumin excretion of ≥30 µg/mg creatinine [23]. Vibratory perception thresholds (VPT) of both great toes were measured using a biothesiometer, and neuropathy was diagnosed if the mean VPT was ≥20 V [24]. PVD was diagnosed if the ankle-brachial index was ≤0.9 using Doppler investigations [25]. To assess CAD, a previous history of documented myocardial infarction and/or drug treatment for CAD (aspirin or nitrates) and/or suggestive electrocardiographic changes such as ST segment depression and/or Q-wave changes and/or T-wave changes [26].
The study was approved by the institutional Ethics Committee of the Madras Diabetes Research Foundation. All patients were requested to sign a consent form at their initial visit giving permission for their anonymised data to be used for research purposes. Only data from those patients who had provided written informed consent was included in this study.
3. Statistical Analysis
The data were analysed using the SAS statistical package (version 9.0; SAS Institute, Inc., Cary, NC, USA). Estimates were expressed as mean ± standard deviation or proportions. A Z test was used to compare groups for continuous variables and a chi-square test was used to compare proportions between two groups. Logistic regression analysis was used to assess the risk for complications with respect to sex, adjusting for age, HbA1c, and duration of diabetes. p-value < 0.05 was considered statistically significant.
4. Results
From the electronic records, a total of 99,192 individuals with T2DM (male: 62,691; female: 36,501) who visited the main centre or its branches from January 2017 to December 2019 and met the inclusion criteria were identified. For the final analysis, 72,980 individuals (i.e., males: 36,490; females 36,490) who were age and gender-matched were included in the study so that the two groups were comparable.
The sex-wise demographic and clinical characteristics of the study population are depicted in Table 1. Females had a higher BMI than males. Smoking and alcohol consumption were significantly higher among males compared to females. More females than males were sedentary (58.7% vs. 48.0%, p < 0.001). Moreover, only 12.9% of females performed ≥ 150 min/week of physical activity compared to 24.9% of males (p < 0.001). Dietary intake of carbohydrates, proteins, and fat were significantly lower among females compared to males. More than 85% of the study population were on OHA’s with very little sex difference (females: 85.3%; males: 85.9%). Obesity was twice as common in females as compared to males (31.4% vs. 15.6%, p < 0.001). Hypothyroidism was noted to be significantly higher in females compared to males (12.5% vs. 3.5%). The frequency of all cancers was higher in females compared to their counterparts (females 1.3% vs. males 0.6%).
Table 1.
Sex-wise demographic, clinical, and biochemical characteristics of the study population.
| Males (n = 36,490) | Females (n = 36,490) | |
|---|---|---|
| Age (years) | 53 ± 10 | 53 ± 10 |
| Age at onset of diabetes (years) | 45 ± 9 | 46 ± 10 * |
| Height (cms) | 167.2 ± 6.5 | 153.4 ± 6 * |
| Weight (kgs) | 73.5 ± 12.9 | 66.5 ± 12.4 * |
| Body mass index (kg/m2) | 26.2 ± 4.1 | 28.2 ± 4.9 * |
| BMI Categories n (%) | ||
| <18.5 | 400 (1.1) | 264 (0.7) * |
| 18.5–24.9 | 14,534 (39.8) | 9393 (25.7) * |
| 25.0–29.9 | 15,853 (43.4) | 15,390 (42.2) * |
| ≥30 | 5703 (15.6) | 11,443 (31.4) * |
| Systolic blood pressure (mmHg) | 130 ± 16 | 130 ± 17 |
| Diastolic blood pressure (mmHg) | 79 ± 8.4 | 78.5 ± 8.1 |
| Duration of diabetes (years) | 7.7 ± 6.8 | 6.8 ± 6.3 * |
| Occupation n (%) | ||
| Home-maker | 0 | 4801 (13.2) |
| Private employee | 14,375 (39.7) | 27,601 (76.1) * |
| Government employee | 3019 (8.3) | 1654 (4.6) * |
| Self-employed/Business | 13,329 (36.8) | 484 (1.3) * |
| Retired | 5417 (14.9) | 1662 (4.6) * |
| Un-employed/Student | 105 (0.3) | 66 (0.2) ** |
| Physical activity | ||
| None | 17,518 (48.0) | 21,436 (58.7) * |
| <150 min/week | 9892 (27.1) | 10,357 (28.4) * |
| ≥150 min/week | 9080 (24.9) | 4697 (12.9) * |
| Smoking n (%) | 11,718 (32.1) | 348 (1.0) * |
| Alcohol n (%) | 14,399 (39.5) | 64 (0.2) * |
| Non-Vegetarians n (%) | 28,862 (79.7) | 26,115 (72.1) * |
| Dietary intake | ||
| Total calories (Kcals/day) | 1367 ± 425 | 1211 ± 349 * |
| Carbohydrate (gms /day) | 224 ± 72 | 200 ± 60 * |
| Protein (gms/ day) | 48 ± 14 | 43 ± 12 * |
| Fat (gms/day) | 32 ± 19 | 27 ± 16 * |
| Management n (%) | ||
| Oral hypoglycemic agents | 31,332 (85.9) | 31,114 (85.3) ** |
| Insulin | 235 (0.6) | 189 (0.5) ** |
| Oral hypoglycemic agents + insulin | 4923 (13.5) | 5187 (14.2) ** |
| Other comorbidities n (%) | ||
| Cancers | 216 (0.6) | 476 (1.3) * |
| Asthma/ chronic obstructive pulmonary disease | 180 (0.5) | 122 (0.3) ** |
| Hypothyroidism | 1292 (3.5) | 4549 (12.5) * |
* p < 0.001; ** p < 0.05.
Table 2 presents the sex-wise biochemical characteristics of the study population. There were significant differences in glycemic and lipid parameters between females and males. Females had higher FPG, PPPG, HbA1c, serum cholesterol, HDL cholesterol and LDL cholesterol than males. The sex-wise distribution of glycemic parameters stratified based on age group is presented in Figure 1. Among younger individuals, overall, glucose levels were higher than in older individuals, and younger males had higher levels of glucose compared to younger females. However, after the age of 44 years, the control of diabetes was worse among females. Overall, only 18.8% of the females achieved glycemic control (HbA1c < 7%) compared to 19.9% of males (p < 0.001).
Table 2.
Gender-wise biochemical characteristics of the study population.
| Biochemical Parameters | Males (n = 36,490) | Females (n = 36,490) |
|---|---|---|
| Fasting plasma glucose (mg/dL) | 176 ± 68 | 180 ± 73 * |
| Post prandial plasma glucose (mg/dL) | 273 ± 94 | 274 ± 97 ** |
| HbA1c (%) | 8.8 ± 2 | 8.9 ± 2 * |
| Serum cholesterol (mg/dL) | 177 ± 44 | 187 ± 45 * |
| Serum triglycerides (mg/dL) | 179 ± 133 | 167 ± 105 * |
| Serum HDL cholesterol (mg/dL) | 39 ± 9 | 44 ± 10 * |
| Serum LDL cholesterol (mg/dL) | 102 ± 38 | 109 ± 38 * |
| Total cholesterol/HDL cholesterol ratio | 4.7 ± 1.3 | 4.4 ± 1.2 * |
| Blood urea (mg/dL) | 25 ± 11 | 23 ± 10 * |
| Serum creatinine (mg/dL) | 0.9 ± 0.4 | 0.7 ± 0.6 * |
* p < 0.001; ** p < 0.05.
Figure 1.
Sex-wise distribution of glycemic parameters stratified based on age group. (a) Fasting Plasma Glucose; (b) Post Prandial Plasma Glucose; and (c) Glycated hemoglobin. *, p < 0.01.
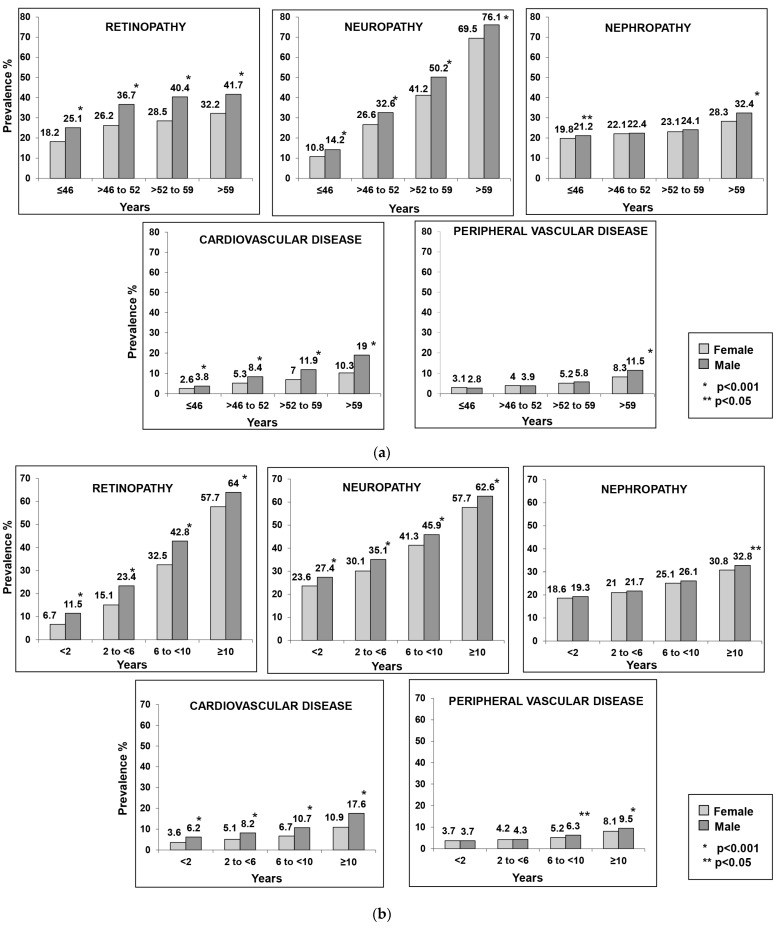
The most common complication observed in both females and males was neuropathy (females 36.9% vs. males 42.9%, p < 0.001), followed by retinopathy (females 26.3% vs. males 36.0%, p < 0.001), and nephropathy (females 23.3% vs. males 25.0%, p < 0.001). Figure 2a presents the sex-wise frequency of diabetes-related complications stratified based on quartiles of age (years). It was observed that retinopathy, neuropathy, and CVD were higher in males in all age groups. PVD prevalence was generally low in both genders and at >59 years it was higher in males compared to females. The prevalence of nephropathy was significantly higher among males in the ≤46 and >59 years age groups compared to females.
Figure 2.
(a) Sex-wise frequency of diabetes complications stratified based on quartiles of age (years); (b) Sex-wise frequency of diabetes complications stratified based on quartiles of duration of diabetes (years).
When the prevalence of complications was assessed based on quartiles of duration of diabetes, a similar trend of higher prevalence of retinopathy, neuropathy, and CVD among males was observed (Figure 2b). The prevalence of nephropathy was significantly higher in males with ≥10 years duration of diabetes compared to females, and the prevalence of PVD was higher in males compared to females when the duration was >6 years.
Multiple logistic regression analysis (Table 3) showed that compared to females, males had a higher risk of developing microvascular complications, except nephropathy and macrovascular complications, even after adjusting for confounding factors. Males had a 1.8 times higher risk of developing cardiovascular disease and a 1.6 times higher risk for retinopathy compared to females.
Table 3.
Risk for micro- and macrovascular complications in relation to sex.
| Complications | Adjusted Odds Ratio * (95% CI) |
|---|---|
| Taking females as reference = 1 | |
| Microvascular | |
| Retinopathy (yes) | 1.59 (1.52–1.66), p < 0.001 |
| Nephropathy (yes) | 1.03 (0.99–1.08), p = 0.169 |
| Neuropathy (yes) | 1.35 (1.30–1.40), p < 0.001 |
| Macrovascular | |
| Cardiovascular disease(yes) | 1.75 (1.64–1.87), p < 0.001 |
| Peripheral vascular disease (yes) | 1.16 (1.08–1.26), p < 0.001 |
* Adjusted for HbA1c, duration of diabetes, Smoking, Alcohol, Physical activity level, and energy intake.
5. Discussion
The present study reports the clinical and biochemical profiles, as well as the associated complications of diabetes in a large series of 72,980 age- and gender-matched individuals with T2DM who reported between 2017 and 2019 at a private tertiary diabetes care centre in India. The main findings from this study are as follows: (i) Overall, obesity and hypothyroidism rates were higher in females compared to males; (ii) Physical activity levels were lower among females compared to males; (iii) Control of diabetes was worse among females, especially at older age groups; (iv) Overall, complications of diabetes were lower in females; and (v) Males had a 1.8- and 1.6-times higher risk of developing CAD and retinopathy, respectively, compared to females.
Diabetes is a worldwide epidemic that is rising steadily in both advanced and emerging economies [27]. A successful management outcome for diabetes mellitus is dependent on several factors, including early diagnosis, dietary modification, exercise regimens, maintaining optimal blood glucose levels, and the quick detection and treatment of complications. We found that females had significantly higher HbA1c, FPG, and PPPG levels than males. Earlier studies also reported that females are less likely than males to meet their glycemic targets [28,29]. This can be attributed to the difference in glucose homeostasis, sex hormones, drug response, and psychosocial reasons [30,31]. Poor glycemic control observed in females could also be attributed to the need to balance their treatment with familial responsibilities, which leads to poor treatment adherence as compared to males [32,33].
Obesity and overweight play an important role in the development of T2DM and its complications, which leads to an increased risk of mortality [34,35]. Females with obesity are more likely to develop diabetes, exposing them to a higher risk for cardiovascular disease (CVD) [35]. A recent study from India predicts that the prevalence of overweight and obesity in India is expected to double and triple, respectively, among Indians aged 20–69 years between the years 2010 and 2040 and by 2040, 13.9% of females and 9.5% of males would be affected by obesity in India [36]. Our study also reports a significantly higher prevalence of obesity in females as compared to males (p < 0.001).
Efforts for obesity prevention are mostly centered on healthy eating and physical activity [37]. Physical activity aids in weight reduction and subsequently mitigates the risk of having metabolic and orthopaedic conditions [38]. It is well documented that females are less physically active than their male counterparts (31.7% vs. 23.4%) [39]. Females, right from their preschool years, devote less time to physical activity and this persists into their adult years [40]. This not only increases the risk of T2DM but also elevates the risk for cardiovascular disease and cancer, which are the first and second most common cause of mortality in females. Diabetes is currently the seventh most common cause of mortality among females, indicating a link between physical inactivity and disease development [40]. In our study, almost half of the females reported performing no physical activity at all.
Dietary modification plays an important role in the prevention and management of T2DM [41,42]. Evidence suggests that low-carbohydrate diets are effective in the management of diabetes, by significantly reducing body weight and also effectively improving HbA1c, fasting plasma glucose, blood lipid, and insulin resistance [43,44]. In addition, it has been shown that in individuals with diabetes, high-protein diets can significantly decrease HbA1c levels [45], and low-fat intake could improve total cholesterol and LDL cholesterol and lower HDL cholesterol [46]. The ICMR INDIAB national study reported that the total calorie intake was higher in males with newly diagnosed diabetes, while the carbohydrate, protein, and fat intake did not significantly differ by sex [47]. Data from the present study shows that intakes of all macronutrients were lower in females compared to males.
Micro- and macrovascular complications are the major cause of diabetes-related morbidity and mortality [48,49]. These complications pose a great disease burden. Cardiovascular disease is the major macrovascular complication observed with diabetes and is two to three times more common in people with diabetes than those without diabetes [50]. Our study reports that males are at 1.8 times higher risk of developing cardiovascular diseases as compared to females. This is in contrast with various previous studies where an increased frequency of cardiovascular events has been reported in females [51,52,53]. According to some estimates, compared to males, females have a 44% and 27% higher risk of incident coronary heart disease (CHD) and stroke, respectively [54]. Additionally, we report that males were 1.6 times and 1.4 times more prone to develop diabetic retinopathy and diabetic neuropathy, respectively, when compared to their female counterparts in spite of their diabetes control being better. Similar findings have been reported by other researchers as well; however, it is uncertain what causes sex-specific disparities in diabetic microvascular disease [55,56]. It has been hypothesized that differences in the production of inflammatory cytokines along with other factors such as biological dissimilarity in general, lifestyle choices, and treatment adherence could lead to an increased frequency of such complications in males [56].
With the increase in life expectancy in view of advances in diabetes management, there is a shift in complications from conventional ones to unconventional ones like cancer, infections, liver disease, affective disorders, and functional and cognitive disability [57]. Several studies have reported heightened cancer risk in those with diabetes [58]. When compared to the general population, people with diabetes have higher chances of developing cancers of the urinary system, liver and biliary tract, pancreas, colon, endometrium, and kidney [59]. There is also a strong link between obesity and cancer, although this risk varies for different organs [60]. In our study, there was a significantly higher frequency of cancer among females. Future studies should look at the cancer risk in females with T2DM.
We observed a higher frequency of hypothyroidism in females compared to males (12.5% vs. 3.5%). This supports previous studies where a higher prevalence of hypothyroidism was noted among females, and this mandates a more extensive screening for hypothyroidism [61,62,63].
We report that females have poor glycemic control. The period of menopausal transition known as perimenopause, which may begin anywhere from the mid-30s to the mid-50s, may contribute to the worsening of diabetes control [64]. Most females in their mid-life engage in less physical activity and have poor eating habits, which can contribute to weight gain [65]. The menopause transition is associated with a 20–25% weight gain, with the majority of the fat mass concentrated in the abdominal area [66]. During perimenopause, there is an increase in visceral fat, which increases the production of proinflammatory cytokines, circulating free fatty acids, and reactive oxygen species, all of which contribute to insulin resistance [67]. As a result, the menopausal transition is characterised by an increase in total body weight, visceral adiposity, and impairment of glucose homeostasis [67].
In this study, more females received a combination of both oral agents and insulin as compared to males. This finding is in line with an Italian multicentric study, which reported that females were more often managed with combination therapy than their male counterparts [68]. Another study done by Kramer et al. [69] reported that males had less intense pharmacological intervention and contact with their physicians. Other studies have reported contradictory findings and reported that males have more intense pharmacological management compared to females [70,71].
This study highlights female-specific considerations such as the high prevalence of obesity, less physical activity, and concerns about diabetes diagnosis. Interventions that could modify these lifestyle factors can result in better outcomes for T2DM management in women. Considering the trend of poor glucose control during the perimenopausal period, glycemic management must be intensified during this period to prevent or delay diabetes complications in females. The study also points to the need for more frequent screening for cancers in women.
Our study has some limitations. Firstly, being a retrospective study, there was limited information regarding some of the confounding factors (e.g., case-mix and missing data), which could have affected the results. Secondly, as this is a clinic-based study, the findings may not be generalizable to the whole of India, as there could be some referral bias being a private diabetes centre. Despite these limitations, the study has several strengths, including the large sample size and the use of standardized methods to assess diabetes and its complications. The study points to the need for studying gender-related variables in clinical studies. Improving the knowledge regarding diabetes in females is clearly based on the study results.
It would be helpful to examine potential sex variations in various risk factor levels related to glucose metabolism status and across levels of glycemic control in subsequent investigations. This emphasises the critical requirement for sex- and gender-specific risk assessment methodologies and therapeutic interventions that focus on diabetes management in the light of preventing diabetes-related complications. These findings may help to identify specific high-risk groups in clinical settings.
6. Conclusions
In conclusion, in this large sample of T2DM, females had higher prevalence of metabolic risk factors and poorer diabetes control compared to males, emphasising the need for better control of diabetes and other metabolic risk factors in females. With regard to complications, males had a higher prevalence of several complications, suggesting that male sex, per se, might be a risk factor for the development of diabetes-associated complications. The study also points to a need for opportunistic screening for hypothyroidism and cancer, especially in females, which can be done at the diabetes clinic to reduce the overall morbidity and mortality among females with diabetes.
Acknowledgments
We thank the study participants who took part in this study.
Author Contributions
Conceptualization, R.P., R.M.A., O.C.S. and V.M.; methodology, R.P., R.M.A., O.C.S. and V.M.; software, S.J.; validation, S.J.; formal analysis, U.V. and N.K.R.; resources, V.M.; data curation, R.P. and L.S.; writing—original draft preparation, R.P. and L.S.; writing—review and editing, R.M.A., O.C.S. and V.M.; visualization, O.C.S. and V.M.; supervision, R.M.A. and V.M.; project administration, R.P.; funding acquisition, O.C.S. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki, and approved by the Institutional Review Board (or Ethics Committee) of Madras Diabetes Research Foundation (protocol code: MDRF/NCT/09-04/2021and date of approval 30 September 2021).
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
The data presented in this study are available on request from the corresponding author. The data are not publicly available due to containing information that could compromise the privacy of research participants.
Conflicts of Interest
The authors declare no competing interest associated with this manuscript. Shreya Lal and Onkar C. Swami are full-time employees of Emcure Pharmaceuticals Limited, Pune, India, which supported this research project.
Funding Statement
The work was commissioned and supported by Emcure Pharmaceuticals Limited (066/21-22) in order to provide data on diabetes in women.
Footnotes
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
References
- 1.Mathur P., Leburu S., Kulothungan V. Prevalence, Awareness, Treatment and Control of Diabetes in India From the Countrywide National NCD Monitoring Survey. Front. Public Health. 2022;10:205. doi: 10.3389/fpubh.2022.748157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Singer M.E., Dorrance K.A., Oxenreiter M.M., Yan K.R., Close K.L. The type 2 diabetes ‘modern preventable pandemic’ and replicable lessons from the COVID-19 crisis. Prev. Med. Rep. 2022;25:101636. doi: 10.1016/j.pmedr.2021.101636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.International Diabetes Federation IDF Diabetes Atlas, 10th Edition. 2021. [(accessed on 20 May 2022)]. Available online: https://diabetesatlas.org/idfawp/resource-files/2021/07/IDF_Atlas_10th_Edition_2021.pdf.
- 4.International Institute for Population Sciences (IIPS) and ICF National Family Health Survey (NFHS-5), 2019–2021: India. Mumbai: IIPS. 2021. [(accessed on 20 May 2022)]. Available online: http://rchiips.org/nfhs/NFHS-5_FCTS/India.pdf.
- 5.Anjana R.M., Deepa M., Pradeepa R., Mahanta J., Narain K., Das H.K., Adhikari P., Rao P.V., Saboo B., Kumar A., et al. Prevalence of diabetes and prediabetes in 15 states of India: Results from the ICMR–INDIAB population-based cross-sectional study. Lancet Diabetes Endocrinol. 2017;5:585–596. doi: 10.1016/S2213-8587(17)30174-2. [DOI] [PubMed] [Google Scholar]
- 6.Kautzky-Willer A., Harreiter J., Pacini G. Sex and Gender Differences in Risk, Pathophysiology and Complications of Type 2 Diabetes Mellitus. Endocr. Rev. 2016;37:278–316. doi: 10.1210/er.2015-1137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Muilwijk M., Bolijn R., Galenkamp H., Stronks K., van Charante E.M., van Valkengoed I.G. The association between gender-related characteristics and type 2 diabetes risk in a multi-ethnic population: The HELIUS study. Nutr. Metab. Cardiovasc. Dis. 2022;32:142–150. doi: 10.1016/j.numecd.2021.09.015. [DOI] [PubMed] [Google Scholar]
- 8.Hammarström A., Annandale E. A conceptual muddle: An empirical analysis of the use of ‘sex’ and ‘gender’ in ‘gender-specific medicine’ journals. PLoS ONE. 2012;7:e34193. doi: 10.1371/journal.pone.0034193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Schober E., Holl R.W., Grabert M., Thon A., Rami B., Kapellen T., Seewi O., Reinehr T., Rami-Merhar B. Diabetes mellitus type 2 in childhood and adolescence in Germany and parts of Austria. Eur. J. Pediatr. 2005;164:705–707. doi: 10.1007/s00431-005-1709-9. [DOI] [PubMed] [Google Scholar]
- 10.Fu J., Prasad H.C. Changing epidemiology of metabolic syndrome and type 2 diabetes in Chinese youth. Curr. Diab. Rep. 2014;14:447. doi: 10.1007/s11892-013-0447-z. [DOI] [PubMed] [Google Scholar]
- 11.Mayer-Davis E.J., Dabelea D., Lawrence J.M. Incidence Trends of Type 1 and Type 2 Diabetes among Youths, 2002–2012. N. Engl. J. Med. 2017;377:301. doi: 10.1056/NEJMoa1610187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Li J., Ni J., Wu Y., Zhang H., Liu J., Tu J., Cui J., Ning X., Wang J. Sex Differences in the Prevalence, Awareness, Treatment, and Control of Diabetes Mellitus Among Adults Aged 45 Years and Older in Rural Areas of Northern China: A Cross-Sectional, Population-Based Study. Front. Endocrinol. 2019;10:147. doi: 10.3389/fendo.2019.00147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sattar N. Gender aspects in type 2 diabetes mellitus and cardiometabolic risk. Best Pract. Res. Clin. Endocrinol. Metab. 2013;27:501–507. doi: 10.1016/j.beem.2013.05.006. [DOI] [PubMed] [Google Scholar]
- 14.Choi Y.J., Kim H.C., Kim H.M., Park S.W., Kim J., Kim D.J. Prevalence and management of diabetes in Korean adults: Korea National Health and Nutrition Examination Surveys 1998–2005. Diabetes Care. 2009;32:2016–2020. doi: 10.2337/dc08-2228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Shen Y., Cai R., Sun J., Dong X., Huang R., Tian S., Wang S. Diabetes mellitus as a risk factor for incident chronic kidney disease and end-stage renal disease in women compared with men: A systematic review and meta-analysis. Endocrine. 2017;55:66–76. doi: 10.1007/s12020-016-1014-6. [DOI] [PubMed] [Google Scholar]
- 16.Ohkuma T., Peters S.A.E., Woodward M. Sex differences in the association between diabetes and cancer: A systematic review and meta-analysis of 121 cohorts including 20 million individuals and one million events. Diabetologia. 2018;61:2140–2154. doi: 10.1007/s00125-018-4664-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chatterjee S., Peters S.A.E., Woodward M., Arango S.M., Batty G.D., Beckett N., Beiser A., Borenstein A.R., Crane P.K., Haan M.N., et al. Type 2 Diabetes as a Risk Factor for Dementia in Women Compared with Men: A Pooled Analysis of 2.3 Million People Comprising More Than 100,000 Cases of Dementia. Diabetes Care. 2016;39:300–307. doi: 10.2337/dc15-1588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Anjana R.M., Unnikrishnan R., Deepa M., Venkatesan U., Pradeepa R., Joshi S., Saboo B., Das A.K., Bajaj S., Bhansali A., et al. Achievement of guideline recommended diabetes treatment targets and health habits in people with self-reported diabetes in India (ICMR-INDIAB-13): A national cross-sectional study. Lancet Diabetes Endocrinol. 2022;10:430–441. doi: 10.1016/S2213-8587(22)00072-9. [DOI] [PubMed] [Google Scholar]
- 19.Snehalatha C., Ramachandran A., Mohan V., Viswanathan M. Pancreatic beta cell response in insulin treated NIDDM patients limitations of a random C-peptide measurement. Diabete Metab. 1987;13:27–30. [PubMed] [Google Scholar]
- 20.Amutha A., Datta M., Unnikrishnan I.R., Anjana R.M., Rema M., Narayan K.M.V., Mohan V. Clinical profile of diabetes in the young seen between 1992 and 2009 at a specialist diabetes centre in south India. Prim. Care Diabetes. 2011;5:223–229. doi: 10.1016/j.pcd.2011.04.003. [DOI] [PubMed] [Google Scholar]
- 21.Pradeepa R., Prabu A.V., Jebarani S., Subhashini S., Mohan V. Use of a Large Diabetes Electronic Medical Record System in India: Clinical and Research Applications. J. Diabetes Sci. Technol. 2011;5:543–552. doi: 10.1177/193229681100500309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Early Treatment Diabetic Retinopathy Study Research Group Early Treatment Diabetic Retinopathy Study Design and Baseline Patient Characteristics: ETDRS Report Number 7. Ophthalmology. 1991;98:741–756. doi: 10.1016/S0161-6420(13)38009-9. [DOI] [PubMed] [Google Scholar]
- 23.Unnikrishnan R.I., Rema M., Pradeepa R., Deepa M., Shanthirani C.S., Deepa R., Mohan V. Prevalence and risk factors of diabetic nephropathy in an urban South Indian population: The Chennai Urban Rural Epidemiology Study (CURES 45) Diabetes Care. 2007;30:2019–2024. doi: 10.2337/dc06-2554. [DOI] [PubMed] [Google Scholar]
- 24.Pradeepa R., Rema M., Vignesh J., Deepa M., Deepa R., Mohan V. Prevalence and risk factors for diabetic neuropathy in an urban south Indian population: The Chennai Urban Rural Epidemiology Study (CURES-55) Diabet. Med. 2008;25:407–412. doi: 10.1111/j.1464-5491.2008.02397.x. [DOI] [PubMed] [Google Scholar]
- 25.Pradeepa R., Chella S., Surendar J., Indulekha K., Anjana R.M., Mohan V. Prevalence of peripheral vascular disease and its association with carotid intima-media thickness and arterial stiffness in type 2 diabetes: The Chennai Urban Rural Epidemiology Study (CURES 111) Diabetes Vasc. Dis. Res. 2014;11:190–200. doi: 10.1177/1479164114524584. [DOI] [PubMed] [Google Scholar]
- 26.Rose G.A., Blackburn H., Gillum R.F., Prineas R.J. Minnesota Code for Resting Electrocardiograms. 2nd ed. Springer; London, UK: 1982. Cardiovascular Survey Methods; pp. 124–143. [Google Scholar]
- 27.Sherwani S.I., Khan H.A., Ekhzaimy A., Masood A., Sakharkar M.K. Significance of HbA1c Test in Diagnosis and Prognosis of Diabetic Patients. Biomark. Insights. 2016;11:BMI-S38440. doi: 10.4137/BMI.S38440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Misra R., Lager J. Ethnic and gender differences in psychosocial factors, glycemic control, and quality of life among adult type 2 diabetic patients. J. Diabetes Its Complicat. 2009;23:54–64. doi: 10.1016/j.jdiacomp.2007.11.003. [DOI] [PubMed] [Google Scholar]
- 29.Arnetz L., Ekberg N.R., Alvarsson M. Sex differences in type 2 diabetes: Focus on disease course and outcomes. Diabetes Metab. Syndr. Obes. Targets Ther. 2014;7:409–420. doi: 10.2147/DMSO.S51301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Duarte F.G., da Silva Moreira S., Maria da Conceição C.A., de Souza Teles C.A., Andrade C.S., Reingold A., Moreira E.D., Jr. Sex differences and correlates of poor glycaemic control in type 2 diabetes: A cross-sectional study in Brazil and Venezuela. BMJ Open. 2019;9:e023401. doi: 10.1136/bmjopen-2018-023401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mauvais-Jarvis F. Gender differences in glucose homeostasis and diabetes. Physiol. Behav. 2018;187:20–23. doi: 10.1016/j.physbeh.2017.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Pond N., Sturock N., Jeffcoate W. Age related changes in glycosylated haemoglobin in patients with IDDM. Diabet. Med. 1996;13:510–513. doi: 10.1002/(SICI)1096-9136(199606)13:6<510::AID-DIA122>3.0.CO;2-W. [DOI] [PubMed] [Google Scholar]
- 33.Raum E., Krämer H.U., Rüter G., Rothenbacher D., Rosemann T., Szecsenyi J., Brenner H. Medication non-adherence and poor glycaemic control in patients with type 2 diabetes mellitus. Diabetes Res. Clin. Pract. 2012;97:377–384. doi: 10.1016/j.diabres.2012.05.026. [DOI] [PubMed] [Google Scholar]
- 34.Sonmez A., Yumuk V., Haymana C., Demirci I., Barcin C., Kıyıcı S., Güldiken S., Örük G., Saydam B.O., Baldane S., et al. Impact of Obesity on the Metabolic Control of Type 2 Diabetes: Results of the Turkish Nationwide Survey of Glycemic and Other Metabolic Parameters of Patients with Diabetes Mellitus (TEMD Obesity Study) Obes. Facts. 2019;12:167–178. doi: 10.1159/000496624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hu F.B. Overweight and Obesity in Women: Health Risks and Consequences. J. Women’s Health. 2003;12:163–172. doi: 10.1089/154099903321576565. [DOI] [PubMed] [Google Scholar]
- 36.Luhar S., Timæus I.M., Jones R., Cunningham S., Patel S.A., Kinra S., Clarke L., Houben R. Forecasting the prevalence of overweight and obesity in India to 2040. PLoS ONE. 2020;15:e0229438. doi: 10.1371/journal.pone.0229438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wiklund P. The role of physical activity and exercise in obesity and weight management: Time for critical appraisal. J. Sport Health Sci. 2016;5:151–154. doi: 10.1016/j.jshs.2016.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Nantel J., Mathieu M.-E., Prince F. Physical Activity and Obesity: Biomechanical and Physiological Key Concepts. J. Obes. 2010;2011:650230. doi: 10.1155/2011/650230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.The Lancet Public Health Time to tackle the physical activity gender gap. Lancet Public Health. 2019;4:e360. doi: 10.1016/S2468-2667(19)30135-5. [DOI] [PubMed] [Google Scholar]
- 40.Forouhi N.G. Nutrition and Type 2 Diabetes: Computational Optimization Modeling to Expand the Evidence Base for South Asians. Diabetes Care. 2022;45:2811–2813. doi: 10.2337/dci22-0033. [DOI] [PubMed] [Google Scholar]
- 41.Ley S.H., Hamdy O., Mohan V., Hu F.B. Prevention and management of type 2 diabetes: Dietary components and nutritional strategies. Lancet. 2014;383:1999–2007. doi: 10.1016/S0140-6736(14)60613-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wang L.-L., Wang Q., Hong Y., Ojo O., Jiang Q., Hou Y.-Y., Huang Y.-H., Wang X.-H. The Effect of Low-Carbohydrate Diet on Glycemic Control in Patients with Type 2 Diabetes Mellitus. Nutrients. 2018;10:661. doi: 10.3390/nu10060661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Scientific Advisory Commission on Nutrition Lower Carbohydrate Diets for Adults with Type 2 Diabetes. [(accessed on 12 May 2023)];2021 London, Scientific Advisory Commission on Nutrition. Available online: https://assets.publishing.service.gov.uk/government/uploads/system/uploads/attachment_data/file/1014673/SACN_report_on_lower_carbohydrate_diets_for_type_2_diabetes.pdf.
- 44.Ajala O., English P., Pinkney J. Systematic review and meta-analysis of different dietary approaches to the management of type 2 diabetes. Am. J. Clin. Nutr. 2013;97:505–516. doi: 10.3945/ajcn.112.042457. [DOI] [PubMed] [Google Scholar]
- 45.Wheeler M.L., Dunbar S.A., Jaacks L.M., Karmally W., Mayer-Davis E.J., Wylie-Rosett J., Yancy W.S. Response to Comment on: Wheeler et al. Macronutrients, Food Groups, and Eating Patterns in the Management of Diabetes: A Systematic Review of the Literature, 2010. Diabetes Care. 2012;35:434–445. doi: 10.2337/dc11-2216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Anjana R.M., Srinivasan S., Sudha V., Joshi S.R., Saboo B., Tandon N., Das A.K., Jabbar P.K., Madhu S.V., Gupta A., et al. Macronutrient Recommendations for Remission and Prevention of Diabetes in Asian Indians Based on a Data-Driven Optimization Model: The ICMR-INDIAB National Study. Diabetes Care. 2022;45:2883–2891. doi: 10.2337/dc22-0627. [DOI] [PubMed] [Google Scholar]
- 47.Edwards E., Sackett S.C. Psychosocial Variables Related to Why Women are Less Active than Men and Related Health Implications. Clin. Med. Insights Women’s Health. 2016;9:47–56. doi: 10.4137/CMWH.S34668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Afaya R.A., Bam V., Azongo T.B., Afaya A. Knowledge of chronic complications of diabetes among persons living with type 2 diabetes mellitus in northern Ghana. PLoS ONE. 2020;15:e0241424. doi: 10.1371/journal.pone.0241424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Deshpande A.D., Harris-Hayes M., Schootman M. Epidemiology of Diabetes and Diabetes-Related Complications. Phys. Ther. 2008;88:1254–1264. doi: 10.2522/ptj.20080020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Roman G., Pantea Stoian A. Type 2 Diabetes—From Pathophysiology to Cyber Systems. IntechOpen; London, UK: 2021. Cardiovascular Risk/Disease in Type 2 Diabetes Mellitus. [Google Scholar]
- 51.Madonna R., Balistreri C.R., De Rosa S., Muscoli S., Selvaggio S., Selvaggio G., Ferdinandy P., De Caterina R. Impact of Sex Differences and Diabetes on Coronary Atherosclerosis and Ischemic Heart Disease. J. Clin. Med. 2019;8:98. doi: 10.3390/jcm8010098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Malmborg M., Schmiegelow M.D.S., Nørgaard C.H., Munch A., Gerds T., Schou M., Kistorp C., Torp-Pedersen C., Hlatky M.A., Gislason G. Does type 2 diabetes confer higher relative rates of cardiovascular events in women compared with men? Eur. Heart J. 2020;41:1346–1353. doi: 10.1093/eurheartj/ehz913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ballotari P., Venturelli F., Greci M., Rossi P.G., Manicardi V. Sex Differences in the Effect of Type 2 Diabetes on Major Cardiovascular Diseases: Results from a Population-Based Study in Italy. Int. J. Endocrinol. 2017;2017:6039356. doi: 10.1155/2017/6039356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.de Ritter R., de Jong M., Vos R.C., van der Kallen C.J.H., Sep S.J.S., Woodward M., Stehouwer C.D.A., Bots M.L., Peters S.A.E. Sex differences in the risk of vascular disease associated with diabetes. Biol Sex Differ. 2020;11:1. doi: 10.1186/s13293-019-0277-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Vashist P., Senjam S., Gupta V., Manna S., Gupta N., Shamanna B., Bhardwaj A., Kumar A., Gupta P. Prevalence of diabetic retinopahty in India: Results from the National Survey 2015–2519. Indian J. Ophthalmol. 2021;69:3087. doi: 10.4103/ijo.IJO_1310_21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Qian J., Haq Z., Yang D., Stewart J.M. Male sex increases the risk of diabetic retinopathy in an urban safety-net hospital population without impacting the relationship between axial length and retinopathy. Sci. Rep. 2022;12:9780. doi: 10.1038/s41598-022-13593-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Tomic D., Shaw J.E., Magliano D.J. The burden and risks of emerging complications of diabetes mellitus. Nat. Rev. Endocrinol. 2022;18:525–539. doi: 10.1038/s41574-022-00690-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Ramteke P., Deb A., Shepal V., Bhat M.K. Hyperglycemia Associated Metabolic and Molecular Alterations in Cancer Risk, Progression, Treatment, and Mortality. Cancers. 2019;11:1402. doi: 10.3390/cancers11091402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Habib S.L., Rojna M. Diabetes and risk of cancer. ISRN Oncol. 2013;2013:583786. doi: 10.1155/2013/583786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.De Pergola G., Silvestris F. Obesity as a Major Risk Factor for Cancer. J. Obes. 2013;2013:291546. doi: 10.1155/2013/291546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Taylor P.N., Albrecht D., Scholz A., Gutierrez-Buey G., Lazarus J.H., Dayan C.M., Okosieme O.E. Global epidemiology of hyperthyroidism and hypothyroidism. Nat. Rev. Endocrinol. 2018;14:301–316. doi: 10.1038/nrendo.2018.18. [DOI] [PubMed] [Google Scholar]
- 62.Meng Z., Liu M., Zhang Q., Liu L., Song K., Tan J., Jia Q., Zhang G., Wang R., He Y., et al. Gender and Age Impacts on the Association Between Thyroid Function and Metabolic Syndrome in Chinese. Medicine. 2015;94:e2193. doi: 10.1097/MD.0000000000002193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Unnikrishnan A., Kalra S., Sahay R., Bantwal G., John M., Tewari N. Prevalence of hypothyroidism in adults: An epidemiological study in eight cities of India. Indian J. Endocrinol. Metab. 2013;17:647–652. doi: 10.4103/2230-8210.113755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Cleveland Clinic. [(accessed on 15 June 2022)]. Available online: https://my.clevelandclinic.org/health/diseases/21608-perimenopause.
- 65.Chedraui P., Pérez-López F.R. Metabolic syndrome during female midlife: What are the risks? Climacteric. 2019;22:127–132. doi: 10.1080/13697137.2018.1561666. [DOI] [PubMed] [Google Scholar]
- 66.Slopien R., Wender-Ozegowska E., Rogowicz-Frontczak A., Meczekalski B., Zozulinska-Ziolkiewicz D., Jaremek J.D., Cano A., Chedraui P., Goulis D.G., Lopes P., et al. Menopause and diabetes: EMAS clinical guide. Maturitas. 2018;117:6–10. doi: 10.1016/j.maturitas.2018.08.009. [DOI] [PubMed] [Google Scholar]
- 67.Muka T., Asllanaj E., Avazverdi N., Jaspers L., Stringa N., Milic J., Ligthart S., Ikram M.A., Laven J.S.E., Kavousi M., et al. Age at natural menopause and risk of type 2 diabetes: A prospective cohort study. Diabetologia. 2017;60:1951–1960. doi: 10.1007/s00125-017-4346-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Penno G., Solini A., Bonora E., Fondelli C., Orsi E., Zerbini G., Trevisan R., Vedovato M., Gruden G., Laviola L., et al. Gender differences in cardiovascular disease risk factors, treatments and complications in patients with type 2 diabetes: The RIACE Italian multicentre study. J. Intern. Med. 2013;274:176–191. doi: 10.1111/joim.12073. [DOI] [PubMed] [Google Scholar]
- 69.Krämer H.U., Rüter G., Schöttker B., Rothenbacher D., Rosemann T., Szecsenyi J., Brenner H., Raum E. Gender differences in healthcare utilization of patients with diabetes. Am. J. Manag. Care. 2012;18:362–369. [PubMed] [Google Scholar]
- 70.Rossi M.C., Cristofaro M.R., Gentile S., Lucisano G., Manicardi V., Mulas M.F., Napoli A., Nicolucci A., Pellegrini F., Suraci C., et al. Sex disparities in the quality of diabetes care: Biological and cultural factors may play a different role for different outcomes: A cross-sectional observational study from the AMD annals initiative. Diabetes Care. 2013;36:3162–3168. doi: 10.2337/dc13-0184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Krämer H.U., Raum E., Rüter G., Schöttker B., Rothenbacher D., Rosemann T., Szecsenyi J., Brenner H. Gender disparities in diabetes and coronary heart disease medication among patients with type 2 diabetes: Results from the DIANA study. Cardiovasc. Diabetol. 2012;11:88. doi: 10.1186/1475-2840-11-88. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data presented in this study are available on request from the corresponding author. The data are not publicly available due to containing information that could compromise the privacy of research participants.