Abstract
As the number of reports of post-acute COVID-19 musculoskeletal manifestations is rapidly rising, it is important to summarize the current available literature in order to shed light on this new and not fully understood phenomenon. Therefore, we conducted a systematic review to provide an updated picture of post-acute COVID-19 musculoskeletal manifestations of potential rheumatological interest, with a particular focus on joint pain, new onset of rheumatic musculoskeletal diseases and presence of autoantibodies related to inflammatory arthritis such as rheumatoid factor and anti-citrullinated protein antibodies. We included 54 original papers in our systematic review. The prevalence of arthralgia was found to range from 2% to 65% within a time frame varying from 4 weeks to 12 months after acute SARS-CoV-2 infection. Inflammatory arthritis was also reported with various clinical phenotypes such as symmetrical polyarthritis with RA-like pattern similar to other prototypical viral arthritis, polymyalgia-like symptoms, or acute monoarthritis and oligoarthritis of large joints resembling reactive arthritis. Moreover, high figures of post-COVID-19 patients fulfilling the classification criteria for fibromyalgia were found, ranging from 31% to 40%. Finally, the available literature about prevalence of rheumatoid factor and anti-citrullinated protein antibodies was largely inconsistent. In conclusion, manifestations of rheumatological interest such as joint pain, new-onset inflammatory arthritis and fibromyalgia are frequently reported after COVID-19, highlighting the potential role of SARS-CoV-2 as a trigger for the development of autoimmune conditions and rheumatic musculoskeletal diseases.
Keywords: COVID, post-COVID, musculoskeletal, pain, arthralgia, arthritis, autoantibodies, fibromyalgia
1. Introduction
Viral infections are a well-recognized cause of joint pain and of inflammatory arthritis [1]. Several viruses, such as parvovirus B19, hepatitis viruses, enteroviruses, rubella, flaviviruses, chikungunya virus and HIV, have been associated with different forms of arthritis, ranging from transient self-limiting episodes of joint inflammation to chronic disabling conditions [1]. Joint pain, muscle pain and fatigue are also present in a high proportion of patients with COVID-19, the disease caused by the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), both as initial presentation or during the course of the illness [2]. Although patients with COVID-19 can recover completely, a considerable number of individuals experience persistent symptoms after the acute phase of the infection and may develop a broad spectrum of sequelae [3]. Beyond pulmonary complications, frequent COVID-19 sequelae include cardiovascular, neurological or hematological manifestations and other less common long-term effects [4]. Angiotensin-converting enzyme 2, the host entry receptor of SARS-CoV-2 in human cells [5], is expressed in the musculoskeletal system, in particular in skeletal muscle and synovial tissue cells [6,7]. Not surprisingly, musculoskeletal symptoms are reported in a significant proportion of post-COVID-19 patients [8]. A growing number of articles described new-onset rheumatic musculoskeletal diseases developing in close temporal association with COVID-19, including rheumatoid arthritis (RA) [9], polymyalgia rheumatica (PMR) [10], reactive arthritis [11], axial spondyloarthritis [12], polyenthesitis [13] and connective tissue diseases [14,15,16]. Furthermore, current evidence suggests not only that various autoantibodies can be found in the sera of individuals recovered from SARS-CoV-2 infection [17,18,19], but also that the long-lasting presence of autoantibodies might be associated with persisting symptoms and residual inflammation [20]. From the rheumatologist’s perspective, the impact of musculoskeletal manifestations in post-COVID-19 patients is a rapidly evolving field. Since data about the long-term effects of SARS-CoV-2 infection are continuously rising, it is important to summarize the available literature. Therefore, the aim of the present systematic review is to provide a more granular picture of post-acute COVID-19 musculoskeletal manifestations of rheumatological interest, with particular attention to: (1) joint pain; (2) new onset of rheumatic musculoskeletal diseases including RA, spondyloarthritis, PMR, gout, myositis and fibromyalgia; (3) presence of autoantibodies related to inflammatory arthritis such as rheumatoid factor (RF) and anti-citrullinated protein antibodies (ACPA).
2. Materials and Methods
2.1. Literature Search
MedLine (through PubMed) and Web of Science (WOS) databases were searched up to October 1, 2022. The string used to perform the search in MedLine was (“COVID-19” OR “covid*” OR “2019 novel coronavirus*” OR “2019 ncov*” OR “sars cov 2*” OR “coronavirus disease*” OR “severe acute respiratory syndrome coronavirus 2” OR “wuhan coronavirus*”) AND (“Joint Diseases” [MeSH Terms] OR “arthralgia” OR “Arthralgia” [MeSH Terms] OR “musculoskeletal” OR “tenosynovitis” OR “synovitis” OR “enthesitis” OR “dactylitis” OR “autoantibodies” [MeSH Terms] OR “rheumatoid factor” OR “anticitrullinated peptide antibodies” OR “arthritis” [MeSH Terms] OR “arthri*” OR “polyarthri*” OR “spondylarthropathies” [MeSH Terms] OR “spondyloarthro*” OR “spondylarthro*” OR “myositis” [MeSH Terms] OR “myosit*” OR “polymyositis” OR “dermatomyositis” OR “inflammatory muscle*” OR “inflammatory myopath*” OR “idiopathic inflammatory myo*” OR “still s disease, adult onset” [MeSH Terms] OR “adult onset still*” OR “adult onset still*” OR “fibromy*” OR “fibrositi*” OR “secondary fibromy*” OR “gout” OR “polymyalgia rheumatica”).
The string used to perform the search in WOS was (“COVID-19" OR “covid*" OR “2019 novel coronavirus*" OR “2019 ncov*” OR “sars cov 2” OR “coronavirus disease*” OR “severe acute respiratory syndrome coronavirus 2” OR “wuhan coronavirus*”) AND (“Joint Diseases” OR “arthralgia” OR “musculoskeletal” OR “tenosynovitis” OR “synovitis” OR “enthesitis” OR “dactylitis” OR “autoantibodies” OR “rheumatoid factor” OR “anticitrullinated peptide antibodies” OR “arthri*” OR “polyarthri*” OR “Ankylosing Spondyloarthritis” OR “spondyloarthro*” OR “spondylarthro*” OR “myosit*” OR “polymyositis” OR “dermatomyositis” OR “inflammatory muscle*” OR “inflammatory myopath*” OR “idiopathic inflammatory myo*” OR “adult onset still*” OR “adult onset still*” OR “fibromy*” OR “fibrositi*” OR “secondary fibromy*” OR “gout” OR “polymyalgia rheumatica”).
The search strategy was designed by one reviewer (EV) and a supervision was granted by a senior investigator (FU). The final manuscript was prepared following the Preferred Reporting Items for Systematic Review and Meta-Analysis (PRISMA) guidelines [http://www.prisma-statement.org/ (accessed on 24 April 2023)].
2.2. Eligibility Criteria and Study Selection
Studies were selected if they: (1) were published in the English language; (2) were full-text original articles published in international, peer-reviewed journals; (3) were non-randomized studies or case series detailing 5 or more cases. For the purpose of this review, we defined post-acute COVID-19 as persistent or delayed-onset joint pain or new onset of inflammatory rheumatic musculoskeletal conditions at least 4 weeks after SARS-CoV-2 infection. Articles in which the timing of assessment from SARS-CoV-2 infection was not clearly stated or could not be derived from the available information were excluded, as well as conference proceedings and non-original publications. Moreover, for the evaluation of joint pain, only studies including more than 100 patients were considered eligible. The population, intervention, comparator, outcome (PICO) framework [21] was used to formulate the research question. Studies meeting the following criteria were included in the review:
Population: patients with a previous confirmed diagnosis of SARS-CoV-2 infection;
Intervention: assessment of presence of symptoms of rheumatological interest following COVID-19 infection;
Comparison: no comparison was considered necessary;
Outcome: prevalence of arthralgia or of autoantibody of rheumatological interest or of an established diagnosis of rheumatic musculoskeletal diseases.
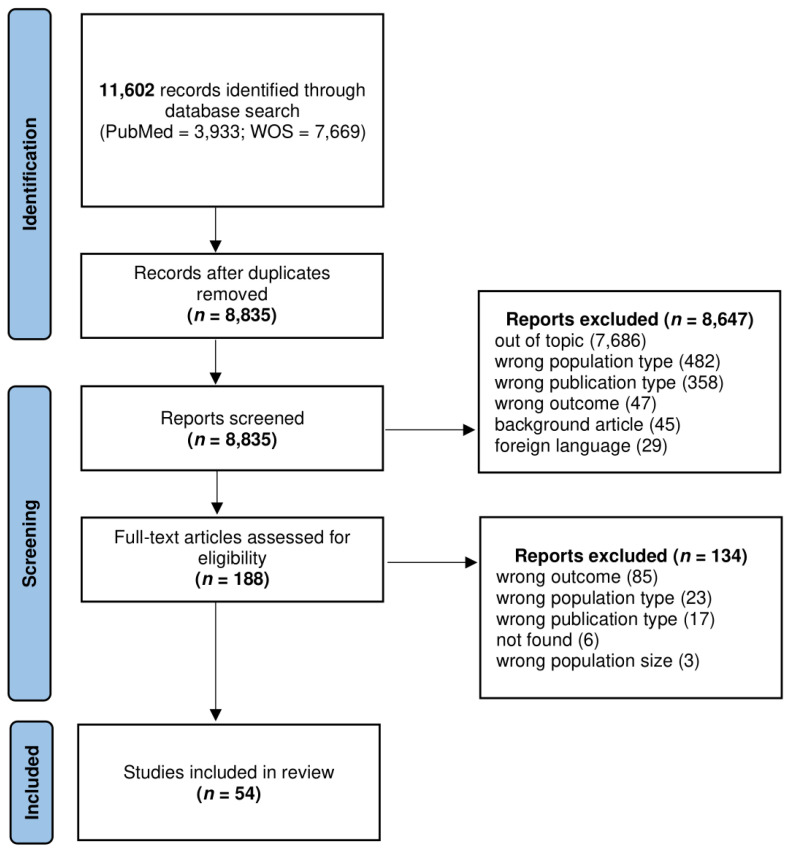
A flow diagram of the study selection process is shown in Figure 1. Study identification and data extraction.
After the removal of duplicates, title and abstract screening was performed by two authors (EV and JC) independently. Full-text reading of potentially eligible articles was then performed. References and related citations were also examined to identify additional pertinent papers. Each selected article was summarized and the following information was recorded: first author; year of publication; country; study design; sample size; prevalence of musculoskeletal manifestation of potential rheumatological interest or main findings. The Newcastle–Ottawa scale [22] and the Joanna Briggs Institute critical appraisal tools [23] were used to ensure the quality of non-randomized studies and case series, respectively.
Figure 1.
Prisma 2020 flow diagram [24].
3. Results
The search strategy identified 3933 articles from PubMed and 7669 from WOS. After removal of duplicates, 8835 unique articles were selected for title and abstract screening and 188 were considered potentially relevant for full-text evaluation. The full article review identified 54 studies that proceeded to data extraction and analysis. The geographical origin of the included studies was heterogeneous, with a contribution of 23 publications from European centers, 19 studies conducted by Asian teams, 2 undertaken in North America, 5 in South or Central America and 4 in Africa. One study included patients from the United Kingdom, United States and other countries. Characteristics of the selected studies are shown in Table 1. Of the 47 articles evaluated through the Newcastle–Ottawa scale, 18 were rated of good quality, 24 of fair quality and 5 of poor quality (Table 2). Finally, seven case series were deemed eligible after the application of Joanna Briggs critical appraisal tools.
Table 1.
Characteristics of the studies included in the review.
| First Author; Year | Study Design | Country | Rheumatic Disease | Time between COVID-19 and RMD | Number of Post-COVID-19 Patients |
Percentage of Patients with Arthralgia or RMD or Main Findings about Autoantibodies |
|---|---|---|---|---|---|---|
| Acosta-Ampudia; 2022 [25] | Prospective observational study | Colombia | Arthralgia and autontibodies (RF, ACPA) | Median of 288 days | 33 | Frequency of RF and ACPA did not differ from healthy subjects. |
| Akova; 2022 [26] | Cross-sectional observational study | Turkey | Arthralgia | 12 months | 151 | 46% |
| Al-Aly; 2021 [27] | Prospective observational study (electronic health records) | USA | Arthralgia and inflammatory arthritis | >1 month | 73,435 | N/R |
| Aly; 2021 [28] | Retrospective cross-sectional observational study | Egypt | Arthralgia | >1 month | 115 | 21% |
| Anaya; 2021 [29] | Case series | Colombia | Arthralgia | Median of 219 days | 100 | 65% |
| Ardakani; 2022 [30] | Case series | Iran | Septic arthritis | Mean of 42 days | 5 | 100% |
| Bakılan; 2021 [31] | Retrospective cross-sectional observational study | Turkey | Arthralgia | >4 weeks | 280 | 36% |
| Bileviciute-Ljungar; 2022 [32] | Cross-sectional observational study | Sweden | Fibromyalgia | >12 weeks | 100 | 40% |
| Buonsenso; 2022 [33] | Cross-sectional observational study | UK/USA/other countries | Arthralgia | >1 month | 510 children (in 41% COVID-19 was suspected, not confirmed) | 61% |
| Carfì; 2020 [34] | Cross-sectional observational study | Italy | Arthralgia | 60 days | 143 | 27% |
| Carvalho-Schneider; 2021 [35] | Prospective observational study | France | Arthralgia | 1 month and 2 months | 150 at 1 month and 130 at 2 months | 10% at 1 month and 16% at 2 months |
| Chudzik; 2022 [36] | Prospective observational study | Poland | Arthralgia | >4 weeks | 218 | 2% |
| Chudzik; 2022 [37] | Retrospective observational study | Poland | Arthralgia | 3 months | 2,218 | 4% |
| Cui; 2022 [38] | Prospective observational study | China | Arthralgia | 12 months | 1,296 | 12% |
| Derksen; 2021 [9] | Case series | Netherlands | Inflammatory arthritis and autoantibodies (ACPA) | 5 weeks for determination of ACPA, mean of 7 weeks for onset of arthritis | 61 for determination of ACPA; patients across various Dutch rheumatology clinics for RA | 0 new ACPA;5 RA |
| Galal; 2021 [39] | Cross-sectional observational study | Egypt | Arthralgia | Mean of 176 days | 430 | 57% |
| Gamal; 2022 [40] | Prospective observational study | Egypt | Arthralgia | 1.5 months | 170 | 19% |
| Ghosn; 2021 [41] | Prospective observational study | France | Arthralgia | 6 months | 1,137 | Around 16% at 3 months and 18% at 6 months |
| Gulzar; 2022 [42] | Cross-sectional observational study | Pakistan | Arthralgia and inflammatory arthritis | 6 months | 367 | 25% for arthralgia and 1% for arthritis |
| Heesakkers; 2022 [43] | Prospective observational study | Netherlands | Arthralgia | 12 months | 243 | 26% |
| Huang C.; 2021 [44] | Ambidirectional observational study | China | Arthralgia | Median of 186 days | 1,733 | 9% |
| Huang L.; 2021 [45] | Ambidirectional observational study | China | Arthralgia | >6 months | 1,225 at 6 months, 1,272 at 12 months | 11% at 6 months and 12% at 12 months |
| Irisson-Mora; 2022 [46] | Cross-sectional observational study | Mexico | Arthralgia | 6 months | 280 | 12% |
| Karaarslan; 2021 [47] | Prospective observational study | Turkey | Arthralgia | 1 month | 300 | 22% |
| Karaarslan; 2022 [48] | Prospective observational study | Turkey | Arthralgia | 3 months and 6 months | 291 at 3 months and 285 at 6 months | 39% at 3 months and 19% at 6 months |
| Kenny; 2022 [49] | Prospective observational study | Ireland | Arthralgia | >4 weeks | 233 | 23% |
| Kiatkittikul; 2022 [50] | Retrospective observational study | Thailand | Myositis | Median of 32 days | 13 | 62% |
| Kim; 2022 [51] | Prospective observational study | Korea | Arthralgia | 12 months | 170 | 12% |
| Lingel; 2021 [52] | Prospective observational study | Germany | Autoantibodies (RF, ACPA) | 8 months | 68 | ACPA were elevated in post-COVID-19 patients compared to unexposed donors and remained elevated in convalescents even after 8 months post infection. |
| Lombardo; 2021 [53] | Prospective observational study | Italy | Arthralgia | 12 months | 303 | 48% |
| Maestre-Muniz; 2021 [54] | Cross-sectional observational study | Spain | Inflammatory arthritis | ≥12 weeks | 543 | 2% |
| Martone; 2022 [55] | Cross-sectional observational study | Italy | Arthralgia | Mean of 88 days | 541 (20% sarcopenic) | 36% of non-sarcopenic patients and 24% of sarcopenic patients |
| Mukarram; 2021 [56] | Case series | Pakistan | Inflammatory arthritis | Mean of 8 weeks | 5 | 100% |
| Muñoz-Corona; 2022 [57] | Ambidirectional observational study | Mexico | Arthralgia | 3 months | 141 | 47% |
| Petersen; 2021 [58] | Prospective observational study | Denmark | Arthralgia | Mean of 93 days | 180 | Around 11% |
| Sarda; 2022 [59] | Cross-sectional observational study | India | Arthralgia | >4 weeks | 251 | 4% |
| Schultheiss; 2022 [60] | Cross-sectional observational study | Germany | Autoantibodies (RF) | >4 weeks | 201 | Around 20% |
| Taha; 2021 [61] | Cross-sectional observational study | Egypt | Inflammatory arthritis, autoantibodies (RF, ACPA) | 6 months | 100 | 37% for arthritis; 19% for RF; 39% for ACPA |
| Tiwari; 2021 [62] | Cross-sectional observational study | Nepal | Arthralgia | 2 months | 132 | 6% |
| Tleyjeh; 2022 [63] | Cross-sectional observational study | Saudi Arabia | Arthralgia | >4 weeks | 5,946 | 31% |
| Tosato; 2021 [64] | Cross-sectional observational study | Italy | Arthralgia | Mean of 77 days | 165 | 22% |
| Tuzun; 2022 [65] | Cross-sectional observational study | Turkey | Arthralgia | >4 weeks | 113 | 40% |
| Uniyal; 2022 [66] | Cross-sectional observational study | India | Arthralgia | >6 weeks | 360 | 21% at 6 weeks—3 months, 29% over 3 months |
| Ursini; 2021 [67] | Case series | Italy | Inflammatory arthritis and autoantibodies (FR, ACPA) | Mean of 33 days | 23 | 100% |
| Ursini; 2021 [68] | Cross-sectional observational study | Italy | Fibromyalgia | Mean of 6 months | 616 | 31% |
| Vaira; 2022 [69] | Cross-sectional observational study | Italy | Arthralgia | >6 Months | 431 | 18% |
| Vogler; 2022 [70] | Case series | Germany/Italy | Inflammatory arthritis | >4weeks | 10 | 10 (6 without underlying RMD, 4 with underlying RMD) |
| Wang; 2021 [71] | Cross-sectional observational study (electronic health records) | USA | Arthralgia | >50 days | 26,117 | 21% |
| Whittaker; 2021 [72] | Population based study | UK | Arthralgia | >4 weeks | 456,002 | 3% |
| Wong-Chew; 2022 [73] | Prospective observational study | Mexico | Arthralgia | >30 days | 4,670 | 11% |
| Xu; 2021 [74] | Case series | China | Autoantibodies (RF) | Several months | 129 | 20% |
| Yaksi; 2022 [75] | Retrospective observational study | Turkey | Arthralgia | 4 months | 133 | 25% |
| Zayet; 2021 [76] | Retrospective observational study | France | Arthralgia | Mean of 289 days | 127 | 24% |
| Zuschlag; 2022 [77] | Retrospective observational study | Germany | Arthralgia | 12 months | 162 | 7% |
Legend: ACPA: anti-citrullinated protein antibodies; RF: rheumatoid factor; RMD: rheumatic musculoskeletal disease.
Table 2.
Newcastle-Ottawa scale for quality appraisal of non-randomized observational studies.
| First Author; Year | Representativeness of the Exposed Cohort | Selection of the Non-Exposed Cohort | Ascertainment of Exposure | Demonstration That Outcome of Interest Was Not Present at Study Entry | Comparability of Cohorts on the Basis of the Design or Analysis | Assessment of Outcome | Was Follow-Up Long Enough for Outcomes to Occur | Adequacy of Follow-Up of Cohorts | Total Quality Score | Quality |
|---|---|---|---|---|---|---|---|---|---|---|
| Acosta-Ampudia; 2022 [25] | 1 | 0 | 1 | 0 | 1 | 1 | 1 | 1 | 6 | fair |
| Akova; 2022 [26] | 0 | 1 | 1 | 0 | 1 | 1 | 0 | 0 | 5 | fair |
| Al-Aly; 2021 [27] | 1 | 1 | 1 | 1 | 2 | 1 | 1 | 1 | 9 | good |
| Aly; 2021 [28] | 1 | 0 | 1 | 0 | 1 | 1 | 1 | 0 | 5 | fair |
| Bakılan; 2021 [31] | 1 | 0 | 1 | 0 | 0 | 1 | 0 | 0 | 3 | poor |
| Bileviciute-Ljungar; 2022 [32] | 1 | 1 | 1 | 0 | 1 | 1 | 1 | 0 | 6 | fair |
| Buonsenso; 2022 [33] | 1 | 1 | 0 | 1 | 0 | 0 | 1 | 1 | 5 | fair |
| Carfì; 2020 [34] | 1 | 1 | 1 | 1 | 2 | 1 | 1 | 1 | 9 | good |
| Carvalho-Schneider; 2021 [35] | 1 | 1 | 1 | 0 | 1 | 1 | 1 | 1 | 7 | good |
| Chudzik; 2022 [36] | 1 | 1 | 0 | 0 | 1 | 1 | 1 | 0 | 5 | fair |
| Chudzik; 2022 [37] | 1 | 0 | 0 | 1 | 1 | 1 | 1 | 0 | 5 | fair |
| Cui; 2022 [38] | 1 | 1 | 1 | 1 | 0 | 0 | 1 | 0 | 6 | fair |
| Galal; 2021 [39] | 1 | 0 | 0 | 1 | 0 | 1 | 0 | 0 | 3 | poor |
| Gamal; 2022 [40] | 1 | 0 | 1 | 1 | 0 | 1 | 1 | 0 | 5 | fair |
| Ghosn; 2021 [41] | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 8 | good |
| Gulzar; 2022 [42] | 1 | 0 | 1 | 0 | 1 | 0 | 0 | 0 | 3 | poor |
| Heesakkers; 2022 [43] | 1 | 1 | 1 | 1 | 2 | 1 | 1 | 1 | 9 | good |
| Huang C.; 2021 [44] | 1 | 1 | 1 | 1 | 2 | 1 | 1 | 1 | 9 | good |
| Huang, L.; 2021 [45] | 1 | 1 | 1 | 1 | 2 | 1 | 1 | 1 | 9 | good |
| Irisson-Mora; 2022 [46] | 1 | 0 | 1 | 0 | 0 | 1 | 0 | 1 | 4 | fair |
| Karaarslan; 2021 [47] | 1 | 0 | 1 | 0 | 0 | 1 | 0 | 1 | 4 | fair |
| Karaarslan; 2022 [48] | 1 | 1 | 1 | 0 | 1 | 1 | 0 | 0 | 5 | fair |
| Kenny; 2022 [49] | 0 | 1 | 1 | 1 | 2 | 0 | 0 | 1 | 6 | fair |
| Kiatkittikul; 2022 [50] | 1 | 1 | 1 | 0 | 0 | 0 | 0 | 1 | 4 | fair |
| Kim; 2022 [51] | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 0 | 7 | good |
| Lingel; 2021 [52] | 1 | 1 | 1 | 1 | 2 | 1 | 1 | 1 | 9 | good |
| Lombardo; 2021 [53] | 1 | 0 | 1 | 1 | 1 | 1 | 1 | 1 | 7 | good |
| Maestre-Muniz; 2021 [54] | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 8 | good |
| Martone; 2022 [55] | 1 | 0 | 1 | 1 | 2 | 1 | 1 | 0 | 7 | good |
| Muñoz-Corona; 2022 [57] | 1 | 0 | 1 | 0 | 0 | 1 | 1 | 0 | 4 | fair |
| Petersen; 2021 [58] | 1 | 1 | 0 | 1 | 1 | 0 | 1 | 1 | 6 | fair |
| Sarda; 2022 [59] | 1 | 0 | 1 | 0 | 0 | 1 | 1 | 0 | 4 | fair |
| Schultheiss; 2022 [60] | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 0 | 7 | good |
| Taha; 2021 [61] | 0 | 1 | 1 | 0 | 1 | 1 | 1 | 1 | 6 | fair |
| Tiwari; 2021 [62] | 1 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 3 | poor |
| Tleyjeh; 2022 [63] | 1 | 1 | 1 | 1 | 1 | 1 | 0 | 1 | 7 | good |
| Tosato; 2021 [64] | 1 | 1 | 1 | 0 | 1 | 1 | 1 | 1 | 7 | good |
| Tuzun; 2022 [65] | 1 | 0 | 1 | 1 | 0 | 1 | 0 | 0 | 4 | fair |
| Uniyal; 2022 [66] | 1 | 1 | 0 | 0 | 0 | 0 | 1 | 0 | 3 | poor |
| Ursini; 2021 [68] | 1 | 1 | 1 | 1 | 2 | 1 | 1 | 1 | 9 | good |
| Vaira; 2022 [69] | 0 | 1 | 1 | 1 | 1 | 1 | 1 | 0 | 6 | fair |
| Wang; 2021 [71] | 1 | 0 | 1 | 1 | 1 | 0 | 1 | 0 | 6 | fair |
| Whittaker; 2021 [72] | 1 | 1 | 1 | 1 | 2 | 1 | 1 | 1 | 9 | good |
| Wong-Chew; 2022 [73] | 1 | 0 | 1 | 1 | 0 | 1 | 0 | 0 | 4 | fair |
| Yaksi; 2022 [75] | 1 | 1 | 1 | 0 | 0 | 0 | 1 | 1 | 5 | fair |
| Zayet; 2021 [76] | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 8 | good |
| Zuschlag; 2022 [77] | 1 | 1 | 1 | 0 | 0 | 1 | 1 | 1 | 6 | fair |
3.1. Joint Pain in Post-COVID-19 Patients
We included 39 studies describing the prevalence of joint pain in post-acute COVID-19 patients. Of these, 7 studies, accounting for a total of 460,716 post-COVID cases, reported a prevalence lower than 10% [36,37,44,59,62,72,77]. The largest was a population-based study involving 456,002 individuals from the United Kingdom at least 4 weeks after COVID-19, where arthralgia was present in 2% to 3% of the patients [72]. In 10 other studies, including 9756 patients overall, a prevalence of joint pain ranging from 10% to 19% was described [35,38,40,41,45,46,51,58,69,73], while 11 studies assessing post-COVID-19 symptoms in 28,303 patients showed higher figures, between 20% and 29% [28,34,42,43,47,49,64,66,71,75,76]. Most of these patients were contributed by Wang et al. [71]. Extracting data from the electronic health records of 26,117 post-COVID-19 individuals, the authors found that arthralgia was one of the most common symptoms in the cohort, having a frequency of 21%. Additionally, 4 studies evaluating data from 7058 patients reported a prevalence between 30% and 39% [31,48,55,63]. Estimates of 40% or above were described in 7 studies on 1748 post-COVID-19 individuals [26,29,33,39,53,57,65]. Finally, data from the U.S. Department of Veterans Affairs electronic health databases showed that, in 73,435 non-hospitalized patients with COVID-19, after a median follow-up of 126 days, there was an excess burden of joint pain (hazard ratio of 5.16, 95% CI 3.18–7.01) compared to almost 5-million users of the Veterans Health Administration who did not have COVID-19 and were not hospitalized [27].
3.2. Inflammatory Arthritis in Post-COVID-19 Patients
Inflammatory arthritis in post-COVID-19 patients was reported in eight studies. In a case series of 23 patients with a median of 33 days from COVID-19 diagnosis, Ursini et al. described 15 patients with asymmetric monoarthritis or oligoarthritis, 3 with manifestations suggestive of PMR, 2 with RA-like polyarthritis, 2 with enthesitis and 1 with predominantly axial involvement [67]. Analyzing data from various Dutch rheumatology clinics, Derksen et al. identified 5 patients who developed RA-like polyarthritis after a mean of 7 weeks from infection. Three patients had a clinical presentation compatible with regular patients with new-onset RA and four fulfilled the classification criteria for RA [9]. Similarly, Mukarram et al. reported 5 cases of patients presenting on average 8 weeks after COVID-19 with polyarticular symmetrical inflammatory arthritis involving the small joints of the hands and the wrists, resembling the clinical presentation of RA [56]. Vogler et al. described 4 patients with newly diagnosed arthritis timely related to COVID-19 developing 4–16 weeks after COVID-19. An amount of 1 patient had a polyarthritis, 2 had an oligoarthritis of the upper and lower limb, and in 1 case, a late-onset RA was diagnosed [70]. Gulzar et al. described 5 patients developing arthritis in the 6 months after COVID-19 [42]. Two were patients with arthritis of the ankle joint and one of them had experienced a first gout episode identified by synovial aspirate. Two other patients had a monoarthritis, respectively, of a wrist and of a knee, while one patient with symmetrical arthritis of the small joints of both hands was eventually diagnosed with RA. After 1 year of follow-up on 543 post-COVID-19 patients, Maestre-Muniz found that 13 (2%) had developed a new inflammatory arthritis, but no additional detail was provided [54]. Finally, evaluating a cohort of 100 post-COVID-19 patients and excluding those with pre-existing autoimmune conditions or rheumatic diseases, Taha et al. found that arthritis (not better specified) was the most common post-COVID-19 manifestation of rheumatological interest, with a prevalence of 37%, even higher than arthralgia or fatigue. Knee (72%), ankle (67%) and wrist (50%) were the most commonly affected joints [61].
3.3. Fibromyalgia in Post-COVID-19 Patients
Two studies evaluated the prevalence of fibromyalgia in post-COVID-19 patients. The results of an Italian web-based cross-sectional survey including 616 patients showed that 31% fulfilled the classification criteria for fibromyalgia after an average of 6 months from the diagnosis of COVID-19, whose strongest predictors were obesity and male gender [68]. Using a similar online survey methodology in Sweden, based on self-reported data collected after a mean of 47 weeks from infection, 40% of the participants fulfilled the 2016 criteria for fibromyalgia and 22% reported to be heathy before COVID-19 [32].
3.4. Rheumatoid Factor and Anti-Citrullinated Protein Antibodies in Post-COVID-19 Patients
Data about RF and ACPA in the post-COVID-19 period were found in seven articles. To determine the seroprevalence of ACPA after COVID-19, Derksen et al. tested 61 patients visiting their post-COVID-19 outpatient clinic. With the exception of two patients previously diagnosed with ACPA-positive RA, none of the patients tested positive for ACPA [9]. Similarly, in the case series from Ursini et al., the authors concluded that autoantibodies were usually absent. None of the 23 patients with post-COVID-19 inflammatory arthritis presented RF or ACPA positivity [67]. Comparing the prevalence of RF and ACPA in a sample of 33 subjects from a Colombian post-COVID-19 clinic, Acosta-Ampudia et al. noticed that the autoantibody positivity did not change from acute disease to post-COVID-19 assessment at a median time of 266 days and that the frequency of these autoantibodies was not different compared with a control group of healthy individuals [25]. In a cohort of 201 patients with prior COVID-19, Schultheiss et al. described a prevalence of autoantibody positivity close to 20% for RF (data derived from plots – numbers not available) [60]. Comparably, Xu et al. collected sera from 129 COVID-19 patients admitted to hospital [74] and, after excluding patients with a history of RA, RF was detected in 20% of cases. Dynamic changes were further investigated in five patients. Tracking for several months the levels of RF, the authors suggested that RF antibodies peak in the later phase of the disease and last for a long time [74]. Moreover, Lingel et al. also analyzed serum levels of RF and ACPA in specimens from convalescent patients after SARS-CoV-2 infection. The authors found that there was no difference between post-COVID-19 patients and healthy donors or those with acute disease regarding levels of RF, while ACPA were elevated specifically in convalescents when compared to unexposed donors and remained elevated after 8 months [52]. Finally, assessing the rheumatologic manifestations of 100 patients 6 months after the infection and excluding patients with previous autoimmune or rheumatic diseases, Taha et al. found a prevalence of 19% for RF and of 39% for ACPA in Egypt [61].
3.5. Other Musculoskeletal Inflammatory Conditions of Rheumatological Interest
Ardakani et al. described 5 patients developing septic arthritis with concomitant avascular necrosis of the femoral head, on average 42 days after the infection [30], while performing 18F fluorodeoxyglucose (FDG) positron emission tomography (PET) on 13 post-COVID-19 patients, Kiatkittikul et al. showed radiotracer uptake in the skeletal muscle of 8 patients (62%), compatible with myositis [50].
4. Discussion
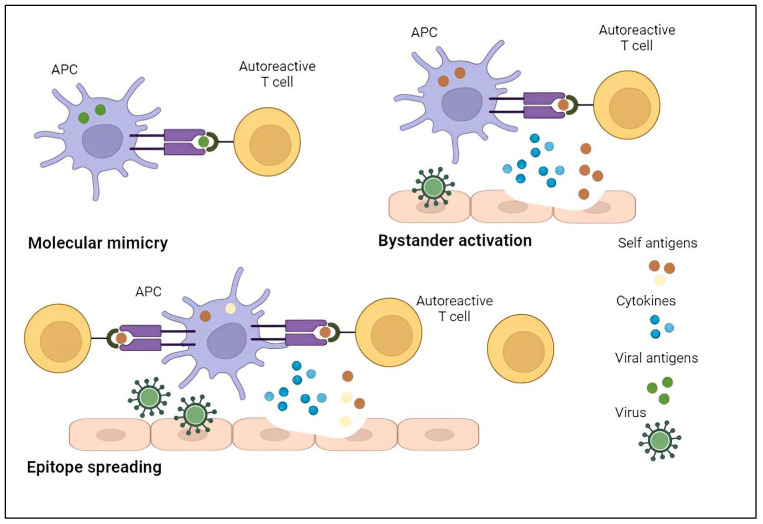
We performed a systematic review with the aim of shedding light on post-acute COVID-19 musculoskeletal manifestations of rheumatological interest. Most of the included literature reported data about prevalence of joint pain in post-COVID-19 patients. The results were widely heterogeneous, ranging from 2% to 65% with a time frame after acute infection varying from 4 weeks to 12 months, therefore precluding the possibility to perform a quantitative synthesis of the data. Regarding inflammatory arthritis, the available literature described different clinical phenotypes. A symmetrical polyarthritis involving wrists and hands has been reported, with an RA-like pattern reminiscent of other prototypical viral arthritis, while in other patients a different pattern has been observed, with monoarthritis or oligoarthritis of large joints, more similar to reactive arthritis, or PMR-like clinical presentations. Since treatment with non-steroidal anti-inflammatory drugs (NSAIDs) and local steroids has been used for post-COVID-19 arthralgia, a potential explanation of the pain origin could be the activation of peripheral nociceptors, suggesting a nociceptive phenotype [78,79]. Nevertheless, it has also been hypothesized that the damage to connective tissue caused by SARS-CoV-2 leads to widespread pain with nociplastic features, in particular in patients with joint hypermobility [78,79]. Although the precise underlying mechanisms remain to be fully elucidated, growing evidence suggests the association between COVID-19 and the development of autoimmune conditions [17,79]. The role of pathogens in the onset of autoimmune diseases has been demonstrated for different viruses, such as influenza, Ebola, Zika and Chikungunya [80]. Molecular mimicry, bystander killing, epitope spreading, clearance deficiency and viral persistence have all been proposed as factors that increase the risk of autoreactivity and reduce self-tolerance, potentially contributing to the autoimmune phenomena observed after viral infections [81,82,83]. A prototypical example of molecular mimicry underlying virus-induced autoimmunity is the association between Epstein–Barr virus and a variety of autoimmune diseases, such as RA and systemic lupus erythematosus (SLE) [80,84]. Bystander activation is believed to be the responsible mechanism for the association between Hepatitis C virus and Sjögren’s syndrome [80,85]. Additionally, the formation of neutrophilic extracellular traps (NETosis) in COVID-19 can act as a trigger for the development of humoral autoimmunity, especially against intra-nuclear antigens leading to the formation of anti-nuclear antibodies [86]. Finally, in severe COVID-19, the hyper-inflammatory state triggered by exaggerated immune response and activation of multiple inflammatory pathways may lead to the generation of autoimmunity [18]. The main mechanisms that have been postulated to be implied in the self-tolerance disruption induced by viruses are represented in Figure 2.
Figure 2.
Molecular mimicry is characterized by the presence of epitopes on SARS-CoV-2 spike glycoprotein that cross-react with host antigens leading to the activation of cellular and humoral autoreactivity, due to the similarity to self-epitopes. Bystander activation implies the activation of a non-specific and over-reactive antiviral immune response. This phenomenon leads to the liberation of self-antigens from the damaged tissue and the release of cytokines from the antigen presenting cells (APCs). Self-antigen is then taken up by APCs and presented to T cells. This way, APCs activate autoreactive T cells, exacerbating the tissue destruction. A persistent viral infection can lead to the phenomenon of epitope spreading. In fact, the perpetuation of tissue damage can promote the release of new self-antigens that may activate more autoreactive T cells when taken up by APCs [83].
Moreover, we found studies focusing on fibromyalgia and it should be noticed that, although the available literature is still limited, the proportion of post-COVID-19 patients fulfilling the classification criteria for fibromyalgia is high, ranging from 31% to 40%. Regarding the prevalence of RF and ACPA, largely inconsistent results were found, precluding the possibility to draw conclusions. Interestingly, besides arthralgia or inflammatory rheumatic diseases, a few cases of avascular necrosis of the femoral head or of transient osteoporosis of the hip have been described, suggesting potential vascular implications caused by the endothelial dysfunction induced by SARS-CoV-2 [30,87,88]. The early recognition of patients at higher risk of developing post-COVID-19 musculoskeletal diseases could lead to a better treatment of these conditions. One of the suggested responsible factors is pre-existing musculoskeletal pain. Although patients with rheumatic diseases have demonstrated a strongly resilient attitude during COVID-19 pandemic [89,90], these individuals are at higher risk of exacerbation of the pre-existing pain after COVID-19, but also of developing new onset COVID-19-related pain. Moreover, the presence of myalgia during the acute phase of SARS-CoV-2 infection has also been suggested to be related to a higher risk of developing post-COVID-19 musculoskeletal diseases [91]. The approaches that have been used to contain COVID-related rheumatic musculoskeletal diseases mostly consisted of repurposed pre-existing immunomodulatory drugs used in autoimmune diseases, including corticosteroids, intravenous immunoglobulins and biologic agents [92]. In particular, some studies reporting COVID-19-related acute arthritis proposed a variety of treatments, including corticosteroids, NSAIDs and baricitinib [18]. Other biologic therapies that have been used in critically ill COVID-19 patients include anakinra, an IL-1 receptor antagonist, and tocilizumab, a monoclonal antibody that inhibits the IL-6 receptor, which have both been proven to be beneficial in macrophage activation syndrome (MAS) [80,93]. Nevertheless, none of these treatments has reached sufficiently robust evidence to be introduced in routine practice [86]. An interesting point to consider is the possible effect of anti-SARS-CoV-2 drugs on the development of musculoskeletal and autoimmune conditions [86]. The temporary use of immunosuppressant drugs and their late withdrawal has indeed been related to the paradoxical development of autoimmunity due to the inappropriate immune reconstitution [94]. Additionally, some of the first antiviral drugs used against COVID-19, such as favipiravir and lopinavir-ritonavir combination, may have represented a trigger for gout manifestations [80]. On the other hand, studies have also hypothesized that the use of hydroxychloroquine (HCQ) in the early phase of the pandemic may have prevented or weakened inflammatory joint manifestations [95].
The main findings of our review can be summarized as follows: (1) joint pain is arguably one of the most prevalent complaints in post-COVID-19 patients, with potential long-term implications on quality of life and functional disability; (2) post-COVID-19 inflammatory arthritis and PMR might represent a new entity of musculoskeletal conditions temporally related to SARS-CoV-2 infection, whose disease course and response to treatment need to be determined in prospective studies; (3) SARS-CoV-2 infection might induce loss of tolerance and autoantibody formation triggering RF and ACPA responses, but their pathogenetic role in the risk of developing rheumatic diseases is unclear, constituting an emerging challenge for the rheumatologist.
Several limitations of our review must be considered. The follow-up after acute infection was heterogeneous, but also the definition of post-COVID varied, ranging from onset of first COVID-19 symptoms to first positive nasopharyngeal swab, end of quarantine, negative nasopharyngeal swab, hospital admission or hospital discharge. Furthermore, the vaccination status of these patients was not always reported. Information about COVID-19 vaccination might be relevant when assessing the development of rheumatic musculoskeletal conditions because, for instance, cases of new-onset inflammatory arthritis and PMR after COVID-19 vaccine administration have been described [96,97,98,99]. Even though no relevant changes in the prevalence of long-COVID symptoms between vaccinated and non-vaccinated hospitalized COVID-19 survivors has been observed [100], the topic is still controversial and other studies suggest that a reduction of risk of long-COVID manifestations could be attributed to vaccines [101]. Finally, the heterogeneity of the different populations considered in the studies represented a major limitation to the evaluation of comorbidities and medication use, which are strongly associated with the severity of the disease but could also be able to mitigate the symptoms of covid-related manifestations, thus acting as confounding factors [86,102].
5. Conclusions
In conclusion, notwithstanding the abovementioned limitations, our systematic review suggests that manifestations of potential rheumatological interest such as arthralgia, but also established musculoskeletal rheumatic diseases, are frequently reported in post-COVID-19 patients, both early during the convalescent period or later in the recovery course. However, large epidemiological studies are needed to elucidate the association between SARS-CoV-2 exposure and the development of rheumatic musculoskeletal diseases, quantify the true incidence, and explore the pathophysiological mechanism linking SARS-CoV-2 infection to the development of rheumatic diseases.
Author Contributions
Conceptualization, J.C., E.V., L.M., V.B., L.L., F.P., S.N. (Susanna Naldi), E.A., S.N. (Simona Neri), M.R., C.F. and F.U.; methodology, J.C., E.V., V.B. and F.U.; validation, J.C., M.R., C.F. and F.U.; formal analysis, J.C., L.M., L.L. and F.U.; resources, F.P., S.N. (Susanna Naldi), E.A., S.N. (Simona Neri) and M.R.; data curation, J.C., E.V., L.L., F.P. and C.F.; writing—original draft preparation, J.C., E.V. and L.M.; writing—review and editing, J.C., E.V., L.M., V.B., L.L., F.P., S.N. (Susanna Naldi), E.A., S.N. (Simona Neri), M.R., C.F. and F.U.; visualization, V.B. and L.L.; supervision, M.R., C.F. and F.U.; project administration, J.C. and F.U. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
No new data were created or analyzed in this study. Data sharing is not applicable to this article.
Conflicts of Interest
The authors declare no conflict of interest.
Funding Statement
This research was supported by project 5x1000 year 2020—income 2019 (progetto 5x1000 anno 2020—redditi 2019).
Footnotes
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
References
- 1.Marks M., Marks J.L. Viral arthritis. Clin. Med. 2016;16:129–134. doi: 10.7861/clinmedicine.16-2-129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ciaffi J., Meliconi R., Ruscitti P., Berardicurti O., Giacomelli R., Ursini F. Rheumatic manifestations of COVID-19: A systematic review and meta-analysis. BMC Rheumatol. 2020;4:65. doi: 10.1186/s41927-020-00165-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gavriilaki E., Kokoris S. COVID-19 sequelae: Can long-term effects be predicted? Lancet Infect. Dis. 2022;22:1651–1652. doi: 10.1016/S1473-3099(22)00529-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Joshee S., Vatti N., Chang C. Long-Term Effects of COVID-19. Mayo Clin. Proc. 2022;97:579–599. doi: 10.1016/j.mayocp.2021.12.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hoffmann M., Kleine-Weber H., Schroeder S., Krüger N., Herrler T., Erichsen S., Schiergens T.S., Herrler G., Wu N.-H., Nitsche A., et al. SARS-CoV-2 Cell Entry Depends on ACE2 and TMPRSS2 and Is Blocked by a Clinically Proven Protease Inhibitor. Cell. 2020;181:271–280.e8. doi: 10.1016/j.cell.2020.02.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Li M.Y., Li L., Zhang Y., Wang X.S. Expression of the SARS-CoV-2 cell receptor gene ACE2 in a wide variety of human tissues. Infect. Dis. Poverty. 2020;9:45. doi: 10.1186/s40249-020-00662-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.dos Santos A.A.C., Rodrigues L.E., Alecrim-Zeza A.L., Ferreira L.D.A., Trettel C.D.S., Gimenes G.M., da Silva A.F., Sousa-Filho C.P.B., Serdan T.D.A., Levada-Pires A.C., et al. Molecular and cellular mechanisms involved in tissue-specific metabolic modulation by SARS-CoV-2. Front. Microbiol. 2022;13:1037467. doi: 10.3389/fmicb.2022.1037467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fernández-de-las-Peñas C., Navarro-Santana M., Plaza-Manzano G., Palacios-Ceña D., Arendt-Nielsen L. Time course prevalence of post-COVID pain symptoms of musculoskeletal origin in patients who had survived severe acute respiratory syndrome coronavirus 2 infection: A systematic review and meta-analysis. Pain. 2022;163:1220–1231. doi: 10.1097/j.pain.0000000000002496. [DOI] [PubMed] [Google Scholar]
- 9.Derksen V.F.A.M., Kissel T., Lamers-Karnebeek F.B.G., van der Bijl A.E., Venhuizen A.C., Huizinga T.W.J., Toes R.E.M., Roukens A.H.E., van der Woude D. Onset of rheumatoid arthritis after COVID-19: Coincidence or connected? Ann. Rheum. Dis. 2021;80:1096–1098. doi: 10.1136/annrheumdis-2021-219859. [DOI] [PubMed] [Google Scholar]
- 10.Metyas S., Chen C., Aung T., Ballester A., Cheav S. Rheumatologic Manifestations of Post SARS-CoV-2 Infection: A Case Series. Curr. Rheumatol. Rev. 2022;18:346–351. doi: 10.2174/1573397118666220211155716. [DOI] [PubMed] [Google Scholar]
- 11.Slouma M., Abbes M., Mehmli T., Dhahri R., Metoui L., Gharsallah I., Louzir B. Reactive arthritis occurring after COVID-19 infection: A narrative review. Infection. 2023;51:37–45. doi: 10.1007/s15010-022-01858-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wong J.R.Y., Zhu L., Shah S., Gadikoppula S. A Case of Axial Spondyloarthritis Triggered by SARS-CoV-2 Infection. Cureus. 2022;14:e22860. doi: 10.7759/cureus.22860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ciaffi J., Mancarella L., Borlandelli E., Facchini G., Meliconi R., Ursini F. May polyenthesitis follow COVID-19? Jt. Bone Spine. 2021;88:105158. doi: 10.1016/j.jbspin.2021.105158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fineschi S. Case Report: Systemic Sclerosis After Covid-19 Infection. Front. Immunol. 2021;12:686699. doi: 10.3389/fimmu.2021.686699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ramachandran L., Dontaraju V.S., Troyer J., Sahota J. New onset systemic lupus erythematosus after COVID-19 infection: A case report. AME Case Rep. 2022;6:14. doi: 10.21037/acr-21-55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ciaffi J., Meliconi R., Ruscitti P., Iagnocco A., Ferri C., Giacomelli R., Ursini F. Isolated ‘puffy hands’ following COVID-19: Clue to a long-term capillary leakage syndrome? Clin. Rheumatol. 2021;40:3863–3864. doi: 10.1007/s10067-021-05835-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gracia-Ramos A.E., Martin-Nares E., Hernández-Molina G. New Onset of Autoimmune Diseases Following COVID-19 Diagnosis. Cells. 2021;10:3592. doi: 10.3390/cells10123592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tang K.T., Hsu B.C., Chen D.Y. Autoimmune and Rheumatic Manifestations Associated With COVID-19 in Adults: An Updated Systematic Review. Front. Immunol. 2021;12:645013. doi: 10.3389/fimmu.2021.645013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rojas M., Rodríguez Y., Acosta-Ampudia Y., Monsalve D.M., Zhu C., Li Q.-Z., Ramírez-Santana C., Anaya J.-M. Autoimmunity is a hallmark of post-COVID syndrome. J. Transl. Med. 2022;20:129. doi: 10.1186/s12967-022-03328-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Son K., Jamil R., Chowdhury A., Mukherjee M., Venegas C., Miyasaki K., Zhang K., Patel Z., Salter B., Yuen A.C.Y., et al. Circulating anti-nuclear autoantibodies in COVID-19 survivors predict long-COVID symptoms. Eur. Respir. J. 2022;61:2200970. doi: 10.1183/13993003.00970-2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Schardt C., Adams M.B., Owens T., Keitz S., Fontelo P. Utilization of the PICO framework to improve searching PubMed for clinical questions. BMC Med. Inform. Decis. Mak. 2007;7:16. doi: 10.1186/1472-6947-7-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wells G.A., Shea B., O’Connell D., Pereson J., Welch V., Losos M., Tugwell P. The Newcastle-Ottawa Scale (NOS) for Assessing the Quality of Nonrandomised Studies in Meta-Analyses. [(accessed on 17 November 2022)]. Available online: http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp.
- 23.Munn Z., Barker T.H., Moola S., Tufanaru C., Stern C., McArthur A., Stephenson M., Aromataris E. Methodological quality of case series studies: An introduction to the JBI critical appraisal tool. JBI Evid. Synth. 2020;18:2127–2133. doi: 10.11124/JBISRIR-D-19-00099. [DOI] [PubMed] [Google Scholar]
- 24.Page M.J., McKenzie J.E., Bossuyt P.M., Boutron I., Hoffmann T.C., Mulrow C.D., Shamseer L., Tetzlaff J.M., Akl E.A., Brennan S.E., et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ. 2021;372:n71. doi: 10.1136/bmj.n71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Acosta-Ampudia Y., Monsalve D.M., Rojas M., Rodríguez Y., Zapata E., Ramírez-Santana C., Anaya J.-M. Persistent Autoimmune Activation and Proinflammatory State in Post-Coronavirus Disease 2019 Syndrome. J. Infect. Dis. 2022;225:2155–2162. doi: 10.1093/infdis/jiac017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Akova I. Determination of Ongoing Symptoms, Quality of Life Levels, and Risk Factors in Post-COVID-19 Patients. J. Clin. Pract. Res. 2022;44:208–215. doi: 10.14744/etd.2021.99825. [DOI] [Google Scholar]
- 27.Al-Aly Z., Xie Y., Bowe B. High-dimensional characterization of post-acute sequelae of COVID-19. Nature. 2021;594:259–264. doi: 10.1038/s41586-021-03553-9. [DOI] [PubMed] [Google Scholar]
- 28.Aly M.A.E.G., Saber H.G. Long COVID and chronic fatigue syndrome: A survey of elderly female survivors in Egypt. Int. J. Clin. Pract. 2021;75:e14886. doi: 10.1111/ijcp.14886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Anaya J.-M., Rojas M., Salinas M.L., Rodríguez Y., Roa G., Lozano M., Rodríguez-Jiménez M., Montoya N., Zapata E., Monsalve D.M., et al. Post-COVID syndrome. A case series and comprehensive review. Autoimmun. Rev. 2021;20:102947. doi: 10.1016/j.autrev.2021.102947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ardakani M.V., Parviz S., Ghadimi E., Zamani Z., Salehi M., Firoozabadi M.A., Mortazavi S.M.J. Concomitant septic arthritis of the hip joint and femoral head avascular necrosis in patients with recent COVID-19 infection: A cautionary report. J. Orthop. Surg. 2022;17:302. doi: 10.1186/s13018-022-03192-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bakılan F., Gökmen I.G., Ortanca B., Uçan A., Güvenç E., Mutlu F., Gökmen H.M., Ekim A. Musculoskeletal symptoms and related factors in postacute COVID-19 patients. Int. J. Clin. Pract. 2021;75:e14734. doi: 10.1111/ijcp.14734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bileviciute-Ljungar I., Norrefalk J.R., Borg K. Pain Burden in Post-COVID-19 Syndrome following Mild COVID-19 Infection. J. Clin. Med. 2022;11:771. doi: 10.3390/jcm11030771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Buonsenso D., Pujol F.E., Munblit D., Pata D., McFarland S., Simpson F.K. Clinical characteristics, activity levels and mental health problems in children with long coronavirus disease: A survey of 510 children. Future Microbiol. 2022;17:577–588. doi: 10.2217/fmb-2021-0285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Carfì A., Bernabei R., Landi F., for the Gemelli Against COVID-19 Post-Acute Care Study Group Persistent Symptoms in Patients after Acute COVID-19. JAMA. 2020;324:603. doi: 10.1001/jama.2020.12603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Carvalho-Schneider C., Laurent E., Lemaignen A., Beaufils E., Bourbao-Tournois C., Laribi S., Flament T., Ferreira-Maldent N., Bruyère F., Stefic K., et al. Follow-up of adults with noncritical COVID-19 two months after symptom onset. Clin. Microbiol. Infect. 2021;27:258–263. doi: 10.1016/j.cmi.2020.09.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Chudzik M., Lewek J., Kapusta J., Banach M., Jankowski P., Bielecka-Dabrowa A. Predictors of Long COVID in Patients without Comorbidities: Data from the Polish Long-COVID Cardiovascular (PoLoCOV-CVD) Study. J. Clin. Med. 2022;11:4980. doi: 10.3390/jcm11174980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Chudzik M., Babicki M., Kapusta J., Kałuzińska-Kołat Ż., Kołat D., Jankowski P., Mastalerz-Migas A. Long-COVID Clinical Features and Risk Factors: A Retrospective Analysis of Patients from the STOP-COVID Registry of the PoLoCOV Study. Viruses. 2022;14:1755. doi: 10.3390/v14081755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Cui D., Wang Y., Huang L., Gu X., Huang Z., Mu S., Wang C., Cao B. Rheumatic Symptoms Following Coronavirus Disease 2019 (COVID-19): A Chronic Post–COVID-19 Condition. Open Forum Infect. Dis. 2022;9:ofac170. doi: 10.1093/ofid/ofac170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Galal I., Hussein A.A.R.M., Amin M.T., Saad M.M., Zayan H.E.E., Abdelsayed M.Z., Moustafa M.M., Ezzat A.R., Helmy R.E.D., Abd_Elaal H.K., et al. Determinants of persistent post-COVID-19 symptoms: Value of a novel COVID-19 symptom score. Egypt J. Bronchol. 2021;15:10. doi: 10.1186/s43168-020-00049-4. [DOI] [Google Scholar]
- 40.Gamal D.M., Ibrahim R.A., Samaan S.F. Post COVID-19 syndrome in a prospective cohort study of Egyptian patients. Egypt Rheumatol. Rehabil. 2022;49:12. doi: 10.1186/s43166-021-00104-y. [DOI] [Google Scholar]
- 41.Ghosn J., Piroth L., Epaulard O., Le Turnier P., Mentré F., Bachelet D., Laouénan C., French COVID Cohort Study and Investigators Groups Persistent COVID-19 symptoms are highly prevalent 6 months after hospitalization: Results from a large prospective cohort. Clin. Microbiol. Infect. 2021;27:1041.e1–1041.e4. doi: 10.1016/j.cmi.2021.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Gulzar R., Mahmud T.e.H., Rasheed A., Riaz S., Adnan W.A., Hafeez U., Malik A.M. Musculoskeletal Symptoms in Patients Recovering from COVID-19. Muscle Ligaments Tendons J. 2022;12:09. doi: 10.32098/mltj.01.2022.02. [DOI] [Google Scholar]
- 43.Heesakkers H., van der Hoeven J.G., Corsten S., Janssen I., Ewalds E., Simons K.S., Westerhof B., Rettig T.C.D., Jacobs C., van Santen S., et al. Clinical Outcomes Among Patients with 1-Year Survival Following Intensive Care Unit Treatment for COVID-19. JAMA. 2022;327:559. doi: 10.1001/jama.2022.0040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Huang C., Huang L., Wang Y., Li X., Ren L., Gu X., Kang L., Guo L., Liu M., Zhou X., et al. 6-month consequences of COVID-19 in patients discharged from hospital: A cohort study. Lancet. 2021;397:220–232. doi: 10.1016/S0140-6736(20)32656-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Huang L., Yao Q., Gu X., Wang Q., Ren L., Wang Y., Hu P., Guo L., Liu M., Xu J., et al. 1-year outcomes in hospital survivors with COVID-19: A longitudinal cohort study. Lancet. 2021;398:747–758. doi: 10.1016/S0140-6736(21)01755-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Irisson-Mora I., Salgado-Cordero A.M., Reyes-Varón E., Cataneo-Piña D.J., Fernández-Sánchez M., Buendía-Roldán I., Salazar-Lezama M.A., on behalf of the Occupational Health and Preventive Medicine Consortium Comparison between the persistence of post COVID-19 symptoms on critical patients requiring invasive mechanical ventilation and non-critical patients. Yemul Golhar SR, editor. PLoS ONE. 2022;17:e0273041. doi: 10.1371/journal.pone.0273041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Karaarslan F., Demircioğlu Güneri F., Kardeş S. Postdischarge rheumatic and musculoskeletal symptoms following hospitalization for COVID-19: Prospective follow-up by phone interviews. Rheumatol. Int. 2021;41:1263–1271. doi: 10.1007/s00296-021-04882-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Karaarslan F., Güneri F.D., Kardeş S. Long COVID: Rheumatologic/musculoskeletal symptoms in hospitalized COVID-19 survivors at 3 and 6 months. Clin. Rheumatol. 2022;41:289–296. doi: 10.1007/s10067-021-05942-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kenny G., McCann K., O’brien C., Savinelli S., Tinago W., Yousif O., Lambert J.S., O’broin C., Feeney E.R., De Barra E., et al. Identification of Distinct Long COVID Clinical Phenotypes Through Cluster Analysis of Self-Reported Symptoms. Open Forum Infect. Dis. 2022;9:ofac060. doi: 10.1093/ofid/ofac060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kiatkittikul P., Promteangtrong C., Kunawudhi A., Siripongsatian D., Siripongboonsitti T., Ruckpanich P., Thongdonpua S., Jantarato A., Piboonvorawong C., Fonghoi N., et al. Abnormality Pattern of F-18 FDG PET Whole Body with Functional MRI Brain in Post-Acute COVID-19. Nucl. Med. Mol. Imaging. 2022;56:29–41. doi: 10.1007/s13139-021-00730-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kim Y., Kim S.W., Chang H.H., Kwon K.T., Hwang S., Bae S. One Year Follow-Up of COVID-19 Related Symptoms and Patient Quality of Life: A Prospective Cohort Study. Yonsei Med. J. 2022;63:499. doi: 10.3349/ymj.2022.63.6.499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Lingel H., Meltendorf S., Billing U., Thurm C., Vogel K., Majer C., Prätsch F., Roggenbuck D., Heuft H.-G., Hachenberg T., et al. Unique autoantibody prevalence in long-term recovered SARS-CoV-2-infected individuals. J. Autoimmun. 2021;122:102682. doi: 10.1016/j.jaut.2021.102682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Lombardo M.D.M., Foppiani A., Peretti G.M., Mangiavini L., Battezzati A., Bertoli S., Boneschi F.M., Zuccotti G.V. Long-Term Coronavirus Disease 2019 Complications in Inpatients and Outpatients: A One-Year Follow-up Cohort Study. Open Forum Infect. Dis. 2021;8:ofab384. doi: 10.1093/ofid/ofab384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Maestre-Muñiz M.M., Arias Á., Mata-Vázquez E., Martín-Toledano M., López-Larramona G., Ruiz-Chicote A.M., Nieto-Sandoval B., Lucendo A.J. Long-Term Outcomes of Patients with Coronavirus Disease 2019 at One Year after Hospital Discharge. J. Clin. Med. 2021;10:2945. doi: 10.3390/jcm10132945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Martone A.M., Tosato M., Ciciarello F., Galluzzo V., Zazzara M.B., Pais C., Savera G., Calvani R., Marzetti E., Robles M.C., et al. Sarcopenia as potential biological substrate of long COVID-19 syndrome: Prevalence, clinical features, and risk factors. J. Cachexia Sarcopenia Muscle. 2022;13:1974–1982. doi: 10.1002/jcsm.12931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Mukarram M.S., Ishaq Ghauri M., Sethar S., Afsar N., Riaz A., Ishaq K. COVID-19: An Emerging Culprit of Inflammatory Arthritis. Case Rep. Rheumatol. 2021;2021:6610340. doi: 10.1155/2021/6610340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Muñoz-Corona C., Gutiérrez-Canales L.G., Ortiz-Ledesma C., Martínez-Navarro L.J., Macías A.E., Scavo-Montes D.A., Guaní-Guerra E. Quality of life and persistence of COVID-19 symptoms 90 days after hospital discharge. J. Int. Med. Res. 2022;50:030006052211104. doi: 10.1177/03000605221110492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Petersen M.S., Kristiansen M.F., Hanusson K.D., Danielsen M.E., Steig B., Gaini S., Strøm M., Weihe P. Long COVID in the Faroe Islands: A Longitudinal Study Among Nonhospitalized Patients. Clin. Infect. Dis. 2021;73:e4058-63. doi: 10.1093/cid/ciaa1792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Sarda R., Kumar A., Chandra A., Bir M., Kumar S., Soneja M., Sinha S., Wig N. Prevalence of Long COVID-19 and its Impact on Quality of Life Among Outpatients with Mild COVID-19 Disease at Tertiary Care Center in North India. J. Patient Exp. 2022;9:237437352211173. doi: 10.1177/23743735221117358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Schultheiß C., Willscher E., Paschold L., Gottschick C., Klee B., Henkes S.-S., Bosurgi L., Dutzmann J., Sedding D., Frese T., et al. The IL-1β, IL-6, and TNF cytokine triad is associated with post-acute sequelae of COVID-19. Cell. Rep. Med. 2022;3:100663. doi: 10.1016/j.xcrm.2022.100663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Taha S.I., Samaan S.F., Ibrahim R.A., El-Sehsah E.M., Youssef M.K. Post-COVID-19 arthritis: Is it hyperinflammation or autoimmunity? Eur. Cytokine Netw. 2021;32:83–88. doi: 10.1684/ecn.2021.0471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Tiwari B., Ghimire M., Bhatta G., Banstola H., Tiwari B., Twayana A., Shrestha K. Persistent Symptoms in Non-critical COVID-19 Patients at Two Months Follow-Up in a District Hospital: A Descriptive Cross-sectional Study. J. Nepal Med. Assoc. 2021;59 doi: 10.31729/jnma.6440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Tleyjeh I.M., Kashour T., Riaz M., Amer S.A., AlSwaidan N., Almutairi L., Halwani R., Assiri A. Persistent COVID-19 symptoms at least one month after diagnosis: A national survey. J. Infect. Public Health. 2022;15:578–585. doi: 10.1016/j.jiph.2022.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Tosato M., Carfì A., Martis I., Pais C., Ciciarello F., Rota E., Tritto M., Salerno A., Zazzara M.B., Martone A.M., et al. Prevalence and Predictors of Persistence of COVID-19 Symptoms in Older Adults: A Single-Center Study. J. Am. Med. Dir. Assoc. 2021;22:1840–1844. doi: 10.1016/j.jamda.2021.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Tuzun H., Özbaş C., Budak B., Altunay G., Baran Aksakal F. Patterns in the relationship between acute COVID-19/long COVID-19 and quality of life: A cross-sectional study of patients attending a tertiary care hospital in Turkey. Asian Pac. J. Trop. Med. 2022;15:274. doi: 10.4103/1995-7645.345943. [DOI] [Google Scholar]
- 66.Uniyal N., Sethi Y., Sharma P.C., Sayana A., Jeet N., Agarwal A., Rawat V. Post-COVID Syndrome and Severity of COVID-19: A Cross-Sectional Epidemiological Evaluation from North India. Cureus. 2022;14:e27345. doi: 10.7759/cureus.27345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Ursini F., Ruscitti P., D’Angelo S., Cacciapaglia F., De Angelis R., Campochiaro C., Caso F., De Santis M., Di Cola I., Parisi S., et al. Broad clinical spectrum of SARS-CoV-2-associated inflammatory joint disease in adults: A report of 35 cases from the COVID-19 & Autoimmune Systemic Disease Italian study group. Ann. Rheum. Dis. 2021;80:1498–1501. doi: 10.1136/annrheumdis-2021-220606. [DOI] [PubMed] [Google Scholar]
- 68.Ursini F., Ciaffi J., Mancarella L., Lisi L., Brusi V., Cavallari C., D’onghia M., Mari A., Borlandelli E., Cordella J.F., et al. Fibromyalgia: A new facet of the post-COVID-19 syndrome spectrum? Results from a web-based survey. RMD Open. 2021;7:e001735. doi: 10.1136/rmdopen-2021-001735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Vaira L.A., Gessa C., Deiana G., Salzano G., Maglitto F., Lechien J.R., Saussez S., Piombino P., Biglio A., Biglioli F., et al. The Effects of Persistent Olfactory and Gustatory Dysfunctions on Quality of Life in Long-COVID-19 Patients. Life. 2022;12:141. doi: 10.3390/life12020141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Vogler D., Ruscitti P., Navarini L., Giacomelli R., Iagnocco A., Hoff P., Burmester G.-R., Ohrndorf S. Diagnosis of COVID-19 associated arthritis in patients with or without underlying rheumatic and musculoskeletal disease supported by musculoskeletal ultrasound: A case series from three European centres. Clin. Exp. Rheumatol. 2022;41:656–666. doi: 10.55563/clinexprheumatol/an1yrh. [DOI] [PubMed] [Google Scholar]
- 71.Wang L., Foer D., MacPhaul E., Lo Y.C., Bates D.W., Zhou L. PASCLex: A comprehensive post-acute sequelae of COVID-19 (PASC) symptom lexicon derived from electronic health record clinical notes. J. Biomed. Inform. 2022;125:103951. doi: 10.1016/j.jbi.2021.103951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Whittaker H.R., Gulea C., Koteci A., Kallis C., Morgan A.D., Iwundu C., Weeks M., Gupta R., Quint J.K. GP consultation rates for sequelae after acute covid-19 in patients managed in the community or hospital in the UK: Population based study. BMJ. 2021;375:e065834. doi: 10.1136/bmj-2021-065834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Wong-Chew R.M., Cabrera E.X.R., Valdez C.A.R., Lomelin-Gascon J., Morales-Juárez L., de la Cerda M.L.R., Villa-Romero A.R., Fernández S.A., Fernandez M.S., Bello H.H., et al. Symptom cluster analysis of long COVID-19 in patients discharged from the Temporary COVID-19 Hospital in Mexico City. Ther. Adv. Infect. Dis. 2022;9:204993612110692. doi: 10.1177/20499361211069264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Xu C., Fan J., Luo Y., Zhao Z., Tang P., Yang G., Pan Y., Guo S., Liu Y., Xiong Y., et al. Prevalence and Characteristics of Rheumatoid-Associated Autoantibodies in Patients with COVID-19. J. Inflamm. Res. 2021;14:3123–3128. doi: 10.2147/JIR.S312090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Yaksi N., Teker A.G., Imre A. Long COVID in Hospitalized COVID-19 Patients: A Retrospective Cohort Study. Iran. J. Public Health. 2022;51 doi: 10.18502/ijph.v51i1.8297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Zayet S., Zahra H., Royer P.Y., Tipirdamaz C., Mercier J., Gendrin V., Lepiller Q., Marty-Quinternet S., Osman M., Belfeki N., et al. Post-COVID-19 Syndrome: Nine Months after SARS-CoV-2 Infection in a Cohort of 354 Patients: Data from the First Wave of COVID-19 in Nord Franche-Comté Hospital, France. Microorganisms. 2021;9:1719. doi: 10.3390/microorganisms9081719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Zuschlag D., Grandt D., Custodis F., Braun C., Häuser W. Spontaneously reported persistent symptoms related to coronavirus disease 2019 one year after hospital discharge: A retrospective cohort single-center study. Schmerz. 2022;36:315–325. doi: 10.1007/s00482-022-00626-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Fernández-De-Las-Peñas C., Nijs J., Neblett R., Polli A., Moens M., Goudman L., Patil M.S., Knaggs R.D., Pickering G., Arendt-Nielsen L. Phenotyping Post-COVID Pain as a Nociceptive, Neuropathic, or Nociplastic Pain Condition. Biomedicines. 2022;10:2562. doi: 10.3390/biomedicines10102562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Fernández-de-las-Peñas C., Nijs J., Giordano R., Arendt-Nielsen L. Precision management of post-COVID pain: An evidence and clinical-based approach. Eur. J. Pain. 2023. early view . [DOI] [PubMed]
- 80.Shah S., Danda D., Kavadichanda C., Das S., Adarsh M.B., Negi V.S. Autoimmune and rheumatic musculoskeletal diseases as a consequence of SARS-CoV-2 infection and its treatment. Rheumatol. Int. 2020;40:1539–1554. doi: 10.1007/s00296-020-04639-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Kanduc D., Shoenfeld Y. Molecular mimicry between SARS-CoV-2 spike glycoprotein and mammalian proteomes: Implications for the vaccine. Immunol. Res. 2020;68:310–313. doi: 10.1007/s12026-020-09152-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Muñoz L.E., Lauber K., Schiller M., Manfredi A.A., Herrmann M. The role of defective clearance of apoptotic cells in systemic autoimmunity. Nat. Rev. Rheumatol. 2010;6:280–289. doi: 10.1038/nrrheum.2010.46. [DOI] [PubMed] [Google Scholar]
- 83.Smatti M.K., Cyprian F.S., Nasrallah G.K., Al Thani A.A., Almishal R.O., Yassine H.M. Viruses and Autoimmunity: A Review on the Potential Interaction and Molecular Mechanisms. Viruses. 2019;11:762. doi: 10.3390/v11080762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Toussirot É., Roudier J. Epstein–Barr virus in autoimmune diseases. Best Pract. Res. Clin. Rheumatol. 2008;22:883–896. doi: 10.1016/j.berh.2008.09.007. [DOI] [PubMed] [Google Scholar]
- 85.Ramos-Casals M., Loustaud-Ratti V., De Vita S., Zeher M., Bosch J.A., Toussirot E., Medina F., Rosas J., Anaya J.-M., Font J., et al. Sjögren Syndrome Associated with Hepatitis C Virus: A Multicenter Analysis of 137 Cases. Medicine. 2005;84:81–89. doi: 10.1097/01.md.0000157397.30055.c9. [DOI] [PubMed] [Google Scholar]
- 86.Ahmed S., Zimba O., Gasparyan A.Y. COVID-19 and the clinical course of rheumatic manifestations. Clin. Rheumatol. 2021;40:2611–2619. doi: 10.1007/s10067-021-05691-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Agarwala S.R., Vijayvargiya M., Pandey P. Avascular necrosis as a part of ‘long COVID-19’. BMJ Case Rep. 2021;14:e242101. doi: 10.1136/bcr-2021-242101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Ciaffi J., Vanni E., Facchini G., Miceli M., Ursini F. Transient osteoporosis of the hip: A novel vascular manifestation of COVID-19? Rheumatology. 2022;62:e127–e128. doi: 10.1093/rheumatology/keac537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Ciaffi J., Giuggioli D., Spinella A., Meliconi R., Ursini F., Ferri C. Resilience of systemic sclerosis patients following the first COVID-19 wave in Italy. Scand. J. Rheumatol. 2021;50:411–412. doi: 10.1080/03009742.2020.1856407. [DOI] [PubMed] [Google Scholar]
- 90.Ciaffi J., Brusi V., Lisi L., Mancarella L., D’onghia M., Quaranta E., Bruni A., Spinella A., Giuggioli D., Landini M.P., et al. Living with arthritis: A “training camp” for coping with stressful events? A survey on resilience of arthritis patients following the COVID-19 pandemic. Clin. Rheumatol. 2020;39:3163–3170. doi: 10.1007/s10067-020-05411-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Fernández-De-Las-Peñas C., Rodríguez-Jiménez J., Fuensalida-Novo S., Palacios-Ceña M., Gómez-Mayordomo V., Florencio L.L., Hernández-Barrera V., Arendt-Nielsen L. Myalgia as a symptom at hospital admission by severe acute respiratory syndrome coronavirus 2 infection is associated with persistent musculoskeletal pain as long-term post-COVID sequelae: A case-control study. Pain. 2021;162:2832–2840. doi: 10.1097/j.pain.0000000000002306. [DOI] [PubMed] [Google Scholar]
- 92.Siddiqi H.K., Mehra M.R. COVID-19 illness in native and immunosuppressed states: A clinical–therapeutic staging proposal. J. Heart Lung Transplant. 2020;39:405–407. doi: 10.1016/j.healun.2020.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Berardicurti O., Ruscitti P., Ursini F., D’Andrea S., Ciaffi J., Meliconi R., Iagnocco A., Cipriani P., Giacomelli R. Mortality in tocilizumab-treated patients with COVID-19: A systematic review and meta-analysis. Clin. Exp. Rheumatol. 2020;38:1247–1254. [PubMed] [Google Scholar]
- 94.Cañas C.A. The triggering of post-COVID-19 autoimmunity phenomena could be associated with both transient immunosuppression and an inappropriate form of immune reconstitution in susceptible individuals. Med. Hypotheses. 2020;145:110345. doi: 10.1016/j.mehy.2020.110345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Gasparotto M., Framba V., Piovella C., Doria A., Iaccarino L. Post-COVID-19 arthritis: A case report and literature review. Clin. Rheumatol. 2021;40:3357–3362. doi: 10.1007/s10067-020-05550-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Vanni E., Ciaffi J., Mancarella L., Ursini F. An Unusual Case of “Conjugal” Polymyalgia Rheumatica after SARS-CoV-2 Vaccination. Rheumato. 2021;1:17–21. doi: 10.3390/rheumato1010004. [DOI] [Google Scholar]
- 97.Ursini F., Ruscitti P., Raimondo V., De Angelis R., Cacciapaglia F., Pigatto E., Olivo D., Di Cola I., Galluccio F., Francioso F., et al. Spectrum of short-term inflammatory musculoskeletal manifestations after COVID-19 vaccine administration: A report of 66 cases. Ann. Rheum. Dis. 2022;81:440–441. doi: 10.1136/annrheumdis-2021-221587. [DOI] [PubMed] [Google Scholar]
- 98.Ottaviani S., Juge P.A., Forien M., Ebstein E., Palazzo E., Dieudé P. Polymyalgia rheumatica following COVID-19 vaccination: A case-series of ten patients. Jt. Bone Spine. 2022;89:105334. doi: 10.1016/j.jbspin.2021.105334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Ursini F., Ruscitti P., Raimondo V., De Angelis R., Cacciapaglia F., Pigatto E., Olivo D., Di Cola I., Galluccio F., Francioso F., et al. Systemic syndromes of rheumatological interest with onset after COVID-19 vaccine administration: A report of 30 cases. Clin. Rheumatol. 2022;41:2261–2267. doi: 10.1007/s10067-022-06078-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Fernández-de-las-Peñas C., Ortega-Santiago R., Fuensalida-Novo S., Martín-Guerrero J.D., Pellicer-Valero O.J., Torres-Macho J. Differences in Long-COVID Symptoms between Vaccinated and Non-Vaccinated (BNT162b2 Vaccine) Hospitalized COVID-19 Survivors Infected with the Delta Variant. Vaccines. 2022;10:1481. doi: 10.3390/vaccines10091481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Notarte K.I., Catahay J.A., Velasco J.V., Pastrana A., Ver A.T., Pangilinan F.C., Peligro P.J., Casimiro M., Guerrero J.J., Gellaco M.M.L., et al. Impact of COVID-19 vaccination on the risk of developing long-COVID and on existing long-COVID symptoms: A systematic review. eClinicalMedicine. 2022;53:101624. doi: 10.1016/j.eclinm.2022.101624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Fernández-de-las-Peñas C., Pellicer-Valero O.J., Navarro-Pardo E., Palacios-Ceña D., Florencio L.L., Guijarro C., Martín-Guerrero J.D. Symptoms Experienced at the Acute Phase of SARS-CoV-2 Infection as Risk Factor of Long-term Post-COVID Symptoms: The LONG-COVID-EXP-CM Multicenter Study. Int. J. Infect. Dis. 2022;116:241–244. doi: 10.1016/j.ijid.2022.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
No new data were created or analyzed in this study. Data sharing is not applicable to this article.