Abstract
BACKGROUND:
Obesity is a complex health problem characterized by abnormal and excessive body weight. Globally, the epidemic of obesity is escalating, and today, around one-third of the world’s adult population is overweight or obese. Obesity is a risk factor and a predictor of poor outcomes of diabetes. This study aimed to determine the prevalence and characteristics of obesity in adults with type-2 diabetes mellitus.
MATERIALS AND METHODS:
This study was conducted at five primary care centers in Bahrain. Obesity was assessed using body mass index, while glycemic control status was assessed using glycated hemoglobin (HbA1c). Informed consent was obtained from all participants. Means and standard deviation were computed for continuous variables, while categorical variables were presented as frequencies and percentages. Student’s t-test and Mann-WhitneyU test, as appropriate, were performed to determine statistical significance between two continuous variables. Chi-square or Fisher’s Exact test were used to test for statistical significance for categorical variables.
RESULTS:
A total of 732 participants were included; the mean age was 58.4 ± 11.3 years. Hypertension was the most prevalent comorbidity (63.5%), followed by hyperlipidemia (51.9%). Most participants (59.8%) had HbA1c levels of more than 7%, 20.9% had HbA1c levels between 7% and 8%, and 38.9% had HbA1c levels of more than 8%. Of the cohort, 47.5% were obese and 35.0% were overweight. Obesity was significantly higher in Bahraini patients and females (P < 0.001). Lower obesity rates were observed among patients who exercised regularly (P < 0.001) and patients who followed diet control measures (P = 0.039). In addition, we found higher obesity rates were found in patients with uncontrolled diabetes (P = 0.004), hypertension (P = 0.032), and hyperlipidemia (P = 0.048).
CONCLUSION:
Obesity is prevalent among type-2 diabetic patients and is associated with poor glycemic outcomes. Thus, more efforts should be taken by physicians to address obesity in diabetic patients as it negatively impacts their glycemic control.
Keywords: Diabetes mellitus, glycemic control, obesity
Introduction
Obesity is a complex health problem characterized by abnormal and excessive body weight.[1] Globally, the epidemic of obesity has been rising rapidly, and today, up to one-third of the world’s population is overweight or obese.[2] Compared to the 1980s, the current prevalence of obesity is triple.[3] The prevalence of obesity in the Gulf Cooperation Council (GCC) countries is even higher than in the rest of the world. The statistics of the world atlas show that the prevalence of obesity in the GCC countries ranges between 27% and 37.9% in Oman and Kuwait, respectively. However, several national surveys have shown higher prevalence rates. For instance, the national survey in Bahrain found that as much as one-third of the Bahraini population was overweight (33.2%) and around 42.8% were obese, while around 40% and 20% of Saudi citizens were obese and overweight, respectively.[4-7]
The association between obesity and other comorbidities such as diabetes mellitus, cardiovascular diseases, essential hypertension, and dyslipidemia has consistently been proven in the literature.[8] Compared to adults with normal weight, obese patients have a higher risk of diabetes (Odds ratio [OR] = 7.37), essential hypertension (OR = 6.38), arthritis (OR = 4.41), hyperlipidemia (OR = 1.88), and poor health (OR = 4.19). In addition, obesity has a negative impact on health expenditure and outcomes.[9]
In recent literature, the term “diabesity” has been used to stress the connection between diabetes mellitus and obesity. Obesity results in insulin resistance mainly as a result of the release of nonesterified fatty acids from adipose tissues, leptins, and inflammatory cytokines. In addition, it also causes pancreatic B-cells dysfunction. Both, insulin resistance and dysfunction of pancreatic B-cells are the main characteristics of type-2 diabetes mellitus. Thus, the obesity pandemic has generated a parallel diabetes mellitus pandemic. Projection models estimate that by 2030, 9.5% of the British population and 25% of the GCC population will have diabetes.[10,11]
Although different tools are used to diagnose obesity, body mass index (BMI) is the most common tool to diagnose and classify obesity in clinical settings. According to the World Health Organization, a BMI of 30 and above defines the status of obesity.[12]
It has been noted in multiple studies that the prevalence of obesity and overweight has been increasing among patients with diabetes.[13] For instance, a large study conducted in the United Kingdom concluded that 86% of patients with type-2 diabetes mellitus were either overweight or obese.[14] Comparable rates were reported in several countries including the United Arab Emirates and Saudi Arabia. A significant parallel association has been shown between BMI and diabetes.[15]
The interrelationship between obesity and glycemic control has been assessed thoroughly in the literature. Glycemic control is assessed by measuring glycated hemoglobin level (HbA1c).[16] Several studies have reported a significant inverse relationship between obesity and glycemic control; the percentage of patients with poor glycemic control increased as the BMI increased. In addition, a relationship between HbA1c and weight changes was found in studies, in which the management of obesity in diabetic patients was linked to better diabetic outcomes and glycemic control. For instance, a systematic review of 11 long-term studies found that intentional weight reduction positively affected diabetic patients and lowered mortality by 25%.[17] The latter linear relationship is more evident in weight reduction strategies such as restrictive bariatric surgeries, in which remission of type-2 diabetes mellitus occurs in approximately 75% of the cases.[18] Our study’s aim was to determine the prevalence and characteristics of obesity in adults with type-2 diabetes mellitus in Bahrain. To the best of our knowledge, our study is the first to assess the prevalence and characteristics of obesity in adults with type-2 diabetes mellitus in Bahrain, despite the high prevalence among Bahraini adults (42.8%).[5]
Materials and Methods
We conducted a cross-sectional study at five different primary care centers in Bahrain. Bahrain’s primary healthcare system comprises five health regions and 28 primary care centers. One health center was selected randomly from each region by a lottery method. Patients attending the diabetic clinics at the selected centers were invited. In Bahrain, all governmental primary healthcare centers have diabetic clinics run by professional teams consisting of family physicians and diabetes specialist nurses. Ethical approval was obtained from the Ethics Committee of Primary Healthcare in Bahrain vide Letter No. 7 dated 28/03/2022, and informed written consent was taken from all participants in the study.
Subjects who were 18 years or older, diagnosed with type-2 diabetes mellitus, and attended the diabetic clinics were eligible for enrollment. Pregnant patients, patients with intellectual disabilities, and those with language barriers were excluded from the study. Based on an initial estimate of a 50% prevalence of obesity in type-2 diabetic patients, a 5% precision, and a 95% confidence level, a sample size of 378 was calculated.
Data was collected using a structured questionnaire comprising of four sections. Section A assessed the characteristics of the participants such as age, sex, nationality, education, marital status, comorbidities, medications, and duration of diabetes. Section B assessed the physical measurements of the patients such as weight in kilograms (Kg), height in centimeters (cm), and blood pressure; Section C dealt with the most recent laboratory results including HbA1c, fasting plasma glucose, and lipid profile, while Section D evaluated lifestyle measures such as smoking, alcohol consumption, exercises, and diet patterns.
BMI formula, weight divided by height squared, was used to calculate and categorize obesity. Specifically, BMI of 18.5–24.9 kg/m2 indicated a normal reference, a BMI of 25–29.9 kg/m2 indicated an overweight status, and a BMI of ≥30 kg/m2 indicated obesity, which was subdivided into three levels according to the BMI level. BMI of 30–34.9, BMI 35–39.9, and BMI ≥40.0 kg/m2 indicated class 1, class 2, and class 3 obesity levels, respectively. Patients’ weights and heights were measured using similar standardized and validated machines by diabetes specialist nurses.
Neurological complications were assessed by the presence of neuropathic symptoms and monofilament test, retinopathy was determined by retinal screening findings, and renal complications were determined by laboratory tests. Regular exercises were defined as the performance of weekly moderate-intensity exercises for 150 min or high-intensity exercises for 75 min.[19] The patients were asked four questions to assess their exercises as follows; do you exercise regularly? If yes, how often do you do exercises per week (daily to once weekly)? for how long do you exercise (in minutes) per week? and what type of exercises do you do, e.g., walking, running, swimming, football, bicycling, and or others? For dietary control, we asked the patients two questions; do you follow a healthy diet regimen? If yes, do you follow your healthy diet plan well? Diet control was defined as the adoption of a healthy diet regimen according to the patient. These two questions are used to assess general dietary practices by patients in the Summary of Diabetes Self-Care Activity Questionnaire.
Diabetes was diagnosed according to the American Diabetes Association diagnostic criteria. Duration of diabetes was calculated as the period, in years, between the diagnosis of diabetes and the data collection period. We assessed glycemic control according to HbA1c results. HbA1c <7 mmol/L was indicative of good control of diabetes, HbA1c >8 mmol/L indicated poor glycemic results, and HbA1c readings between 7 and 8 mmol/L were indicative of partially controlled diabetes.[16]
We obtained the medical treatments of diabetes for all patients. These treatments included oral agents such as metformin, sulfonylurea, and dipeptidyl peptidase-4 inhibitors and injectable medications such as insulin. We also included other medications such as statins, beta-blockers, and psychiatric medications.
We carried out a pilot study on 10 patients to identify possible challenges in the data collection process. The sequence of the data collection tool was modified according to the responses received.
Means with standard deviation were calculated for continuous data, while frequencies with percentages were computed for categorical data. Tests of normality were done. t-test and Mann–Whitney were performed to determine statistical significance between two continuous variables while exact Fisher and Chi-square tests were used for categorical variables. P < 0.05 was considered statistically significant. All data were analyzed by Statistical Package for the Social Sciences program for Windows (V.25.0; IBM Corp, Armonk, New York, USA).
Results
This study included 732 participants with a mean age of 58.4 ± 11.3 years. Approximately 75% of the participants (n = 545) were Bahraini and 50.7% (n = 371) were males. Essential hypertension was the most prevalent comorbidity (n = 465, 63.5%), followed by hyperlipemia and heart disease (51.9% and 13.3%, respectively). Of the cohort, 61 (8.3%) patients were smokers and 26 (3.6%) drank alcohol. The sociodemographic and clinical characteristics of the participants are summarized in Table 1.
Table 1.
Sociodemographic and clinical characteristics of patients with type-2 diabetes attending PHCCs in Bahrian, 2022
| Characteristics | N (%) |
|---|---|
| Nationality | |
| Bahraini | 545 (74.5) |
| Non-Bahraini | 187 (25.5) |
| Sex | |
| Male | 371 (50.7) |
| Female | 361 (49.3) |
| Age (years), mean±SD | 58.4±11.3 |
| Duration of diabetes mellitus (years), mean±SD | 12.7±7.8 |
| Education | |
| No primary education/others | 74 (10.1) |
| Primary | 80 (10.9) |
| Intermediate | 94 (12.8) |
| Secondary | 286 (39.1) |
| University/college | 198 (27.0) |
| Marital status | |
| Single | 48 (6.6) |
| Married | 603 (82.4) |
| Widowed | 60 (8.2) |
| Divorced | 21 (2.9) |
| Comorbidities | |
| Hypertension | 465 (63.5) |
| Hyperlipidemia | 380 (51.9) |
| Heart diseases | 97 (13.3) |
| Cerebrovascular accidents | 19 (2.6) |
| Chronic kidney disease | 12 (1.6) |
| Smoking | 61 (8.3) |
| Alcohol drinking | 26 (3.6) |
SD=Standard deviation
Around half of the participants exercised regularly (n = 356, 48.8%), but only 15% took diet control measures (n = 107, 14.6%). Regarding diabetes treatments, 91.8% of our patients were on metformin and 51.8% were on sulfonylurea. Insulin formulations were used by 41% of the cohort (n = 302). While 40% of the participants had HbA1c results of ≤7% (n = 294), most of our patients had HbA1c levels >7%, 20.9% had A1C levels between 7% and 8%, and 38.9% had HbA1c levels of at least 8%. The average systolic and diastolic blood pressure readings were 129.9 ± 17.6 mmHg and 76.0 ± 11.1 mmHg, respectively [Table 2].
Table 2.
Management measures and outcomes for patients with type-2 diabetes attending PHCCs in Bahrain, 2022
| Management measures and outcomes | N (%) |
|---|---|
| Lifestyle modifications | |
| Diet control | 107 (14.6) |
| Regular exercise | 356 (48.8) |
| Medications | |
| Oral agents | 700 (95.6) |
| Metformin | 672 (91.8) |
| Sulfonylurea | 379 (51.8) |
| Insulin | 302 (41.3) |
| Statins | 623 (85.1) |
| Beta-blockers | 145 (19.8) |
| Diabetes mellitus-related outcomes | |
| Systolic blood pressure (mmHg), mean±SD | 129.9±17.6 |
| Diastolic blood pressure (mmHg), mean±SD | 76.0±11.1 |
| HbA1c, mean±SD | 7.9±4.2 |
| HbA1c | |
| ≤7% | 294 (40.2) |
| 7.1%–7.9% | 153 (20.9) |
| ≥8% | 285 (38.9) |
| Fasting plasma glucose (mmoL/L), mean±SD | 8.0±3.3 |
| Total cholesterol (mmoL/L), mean±SD | 4.2±4.4 |
| Low-density lipoprotein (mmoL/L), mean±SD | 2.3±3.1 |
SD=Standard deviation, HbA1c=Glycated hemoglobin
Almost 50% of the cohort were obese (47.5%, n = 348), while <20% had normal and low BMI (129, 17.6%). Overweight patients constituted one-third of the sample (n = 256, 35.0%). Further classification of obesity showed that 8% of the patients (n = 58) had class 3 obesity, 12% (n = 91) had class 2 obesity, and 27% (n = 198) had class 1 obesity [Table 3 and Figure 1].
Table 3.
Obesity status and classification for patients with type-2 diabetes attending PHCCs in Bahrain, 2022
| Obesity status | N (%) |
|---|---|
| Obesity status | |
| No obesity (BMI <30 kg/m2) | 384 (52.5) |
| Obesity (BMI ≥30 kg/m2) | 348 (47.5) |
| Obesity classification | |
| Underweight (<18.5 kg/m2) | 4 (0.5) |
| Normal (18.5–24.9 kg/m2) | 125 (17.1) |
| Overweight (25–29.9 kg/m2) | 256 (35.0) |
| Class 1 obesity (30–34.9 kg/m2) | 198 (27.0) |
| Class 2 obesity (35–39.9 kg/m2) | 91 (12.4) |
| Class 3 obesity (≥40 kg/m2) | 58 (7.9) |
BMI=Body mass index
Figure 1.
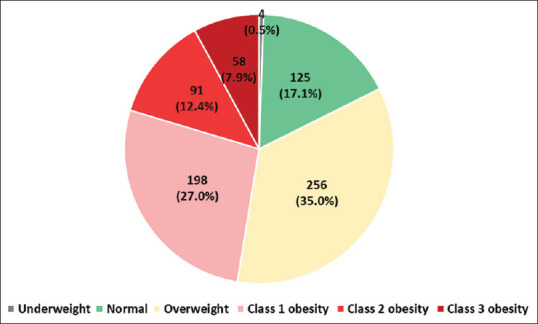
Body weight distribution for patients with type-2 diabetes
As shown in Table 4, Bahraini patients and females had higher obesity rates (P < 0.001), but the prevalence of obesity was lower in patients who exercised regularly (P < 0.001) and patients who followed diet control measures (P = 0.039). Furthermore, the rates of obesity were higher in patients with uncontrolled diabetes (P = 0.004), hypertension (P = 0.032), and hyperlipidemia (P = 0.048).
Table 4.
Association between obesity and demographic characteristics, comorbidities, and treatment outcomes among patients with type-2 diabetes attending PHCCs in Bahrain, 2022
| Characteristics | Obese (n=348) N (%) | Nonobese (n=384) N (%) | P-value |
|---|---|---|---|
| Baseline characteristics of participants | |||
| Age (years), median, mean±SD | 59.0, 58.0±11.8 | 60.0, 58.8±10.8 | 0.342 |
| Duration of diabetes (years), median, mean±SD | 10.0, 12.4±8.2 | 11.0, 13.0±7.5 | 0.315* |
| Nationality | |||
| Bahraini | 299 (54.9) | 246 (45.1) | <0.001 |
| Non-Bahraini | 49 (26.2) | 138 (73.8) | |
| Sex | |||
| Male | 119 (32.1) | 252 (67.9) | <0.001 |
| Female | 229 (63.4) | 132 (36.6) | |
| Marital status | |||
| Married | 276 (45.8) | 327 (54.2) | 0.223 |
| Unmarried | 72 (55.8) | 57 (44.2) | |
| Regular exercise | |||
| Yes | 136 (38.2) | 220 (61.8) | <0.001 |
| No | 211 (56.4) | 163 (43.6) | |
| Diet control | |||
| Yes | 41 (38.3) | 66 (61.7) | 0.039 |
| No | 307 (49.1) | 318 (50.9) | |
| Insulin use | |||
| Yes | 155 (51.3) | 147 (48.7) | 0.086 |
| No | 193 (44.9) | 237 (55.1) | |
| Sulfonylurea use | |||
| Yes | 183 (48.3) | 196 (51.7) | 0.676 |
| No | 165 (46.7) | 188 (53.3) | |
| Comorbidities and diabetes mellitus complications | |||
| Hypertension | 235 (50.5) | 230 (49.5) | 0.032 |
| Hyperlipidemia | 194 (51.1) | 186 (48.9) | 0.048 |
| Ischemic heart disease | 54 (55.7) | 43 (44.3) | 0.085 |
| Cerebrovascular accidents | 9 (47.4) | 10 (52.6) | 0.988 |
| Diabetic retinopathy | 43 (46.2) | 50 (53.8) | 0.787 |
| Diabetic nephropathy | 38 (45.2) | 46 (54.8) | 0.653 |
| Treatment outcomes | |||
| Systolic blood pressure in mmHg, (median) mean±SD | 129.0, 130.6±17.9 | 128, 129.4±17.4 | 0.368 |
| Diastolic blood pressure in mmHg, (median) mean±SD | 76, 75.4±10.4 | 77, 76.7±11.6 | 0.110 |
| HbA1c | |||
| ≤7% | 118 (40.1) | 176 (59.9) | 0.004 |
| 7.1%–7.9% | 78 (51.0) | 75 (49.0) | |
| ≥8% | 152 (53.3) | 133 (46.7) | |
| Total cholesterol (mmol/L), median, mean±SD | 4.0, 4.4±5.6 | 3.8, 4.0±2.8 | 0.202 |
| Low-density lipoprotein (mmol/L), median, mean±SD | 2.0, 2.3±2.4 | 2.4, 2.4±3.6 | 0.618 |
*Mann–Whitney test. HbA1c=Glycated hemoglobulin, SD=Standard deviation
Analysis of glycemic control of the different BMI classes was conducted and is presented in Table 5. In general, patients in the higher obesity classes had lower glycemic control (P = 0.040).
Table 5.
Glycemic control by body weight for patients with type-2 diabetes attending PHCCs in Bahrain, 2022
| Glycemic control | Underweight N (%) | Normal N (%) | Overweight N (%) | Class 1 obesity N (%) | Class 2 obesity N (%) | Class 3 obesity N (%) | P-value |
|---|---|---|---|---|---|---|---|
| Controlled | 2 (50.0) | 54 (43.2) | 121 (47.3) | 74 (37.4) | 22 (24.2) | 21 (36.2) | 0.040 |
| Partially controlled | 1 (25.0) | 22 (17.6) | 52 (20.3) | 45 (22.7) | 21 (23.1) | 12 (20.7) | |
| Uncontrolled | 1 (25.0) | 49 (39.2) | 83 (32.4) | 79 (39.9) | 48 (52.7) | 25 (43.1) |
Discussion
Our results showed that the prevalence rates of obesity and overweight (82.4%) of type-2 diabetic patients were significantly higher in females, Bahraini, and patients who did not follow specific diet and exercise programs. In addition, obesity was associated with a significantly higher prevalence of hypertension, dyslipidemia, and poor glycemic control in participants.
The high prevalence of obesity (47.5%) and overweight (35.0%) of type-2 diabetic patients in this present study is comparable to what has been reported in the literature. Three studies conducted in Saudia Arabia showed that the frequency rates of obesity and overweight in the cohorts of diabetic patients ranged between 77% and 86%.[20-22] Furthermore, a study of 1275 type-2 diabetic patients in Uganda revealed the prevalence of overweight and obesity as 36% and 27%, respectively.[13] A Tanzanian study which aimed to determine the prevalence of overweight and obesity in patients with type 2 diabetes revealed that the majority of patients (85.0%) were overweight (44.9%) or obese (40.1%).[23]
The significantly higher prevalence of obesity in females with diabetes is consistent with the findings of several surveys. A survey in Bahrain, for instance, revealed that females had a higher prevalence of obesity than males (47.2% vs. 39.2%).[5] Similarly, a national survey in the Emirates found that females were more likely to be obese than males (30.6% and 25.1%, respectively).[24] Several studies have also found higher rates of obesity in females. In Kuwait, a study showed that obesity in females was higher than in males (44.0% compared to 36.5%).[25] The higher rates of obesity in females with diabetes and Bahraini citizens can also be attributed to lifestyle, food options, physical inactivity, cultural beliefs, and attitudes toward obesity and overweight.
A significant association between poor glycemic control and obesity has also been reported in the literature. Compared to normal weight, higher obesity classes were associated with poor glycemic control. This relationship is supported by several international data.[26] For example, Boye et al., revealed in a large study that higher BMI classes were significantly associated with higher levels of HbA1c.[27] Our research results were similar and support this hypothesis and revealed poorer glycemic control in obese patients. However, these findings were inconsistent in the literature.
In parallel to increasing the risk of diabetes, the risk of developing hypertension and cardiovascular diseases is higher in obese patients. Here, we found higher obesity rates in diabetic patients who were hypertensive and hyperlipidemic (P = 0.032 and P = 0.048, respectively). Linked to this association, guidelines for hypertension and hyperlipidemia recommend weight loss for all overweight and obese patients as a part of disease management.[28-30] According to American Heart Association guidelines, weight reduction is recommended to reduce blood pressure, control hyperlipidemia, and decrease the risk of atherosclerotic cardiovascular diseases in overweight and obese individuals.[28-30]
Our findings showed that rates of obesity were lower in patients who exercised regularly (P < 0.001) and those who followed diet control measures (P < 0.010). The importance of weight control, regular exercise, and diet control should be emphasized at every clinical encounter with diabetic patients. Diabetes care providers should advise their patients that higher BMI levels are linked to a higher risk of cardiovascular diseases and mortality. In addition, the link between weight control and improvements in glycemic control should be utilized to facilitate behavior change. In general, weight reduction is a key therapeutic goal in the management of type-2 diabetes mellitus.[31]
It should be noted that the approach to treating diabetes in the past decade has changed dramatically. Weight status has been addressed and weight-lowering medications have been incorporated in the first-line management of cases. Thus, there is a careful selection of patients before the selection of certain drug classes.[32] In addition, obesity is a complex problem that occurs as a result of many obesogenic factors including but not limited to medications. This could partially explain the lack of association between the use of sulfonylurea and obesity in our study.
This study has several strengths. It is the first nationally representative study of the Bahraini population to assess the prevalence rates of obesity in patients diagnosed with type-2 diabetes. The prevalence of obesity in patients with different comorbidities and outcomes of diabetes was assessed. Several determinants including lifestyle and diet were examined. However, the study has some limitations as well. An overview of different drug types and their observed trends in weight were not evaluated in our study. In addition, only patients visiting primary care were included. Although we believe that all patients with diabetes have regular follow-ups with their primary care doctors, some patients do not. Further studies are needed to determine the impact of weight loss on glycemic control, blood pressure, and hyperlipidemia. Studies to evaluate the prevalence and impact of obesity on glycemic control in patients with type-1 diabetes are also needed.
Conclusion
The prevalence rates of obesity and overweight are high in diabetic patients, particularly in patients who do not follow a dietary regimen, Bahraini patients, and inactive participants. More efforts should be taken by physicians to address obesity in diabetic patients as it negatively impacts their glycemic control; patients with obesity had higher glycemic measures. Physicians should encourage all patients with type-2 diabetes to control their weight and monitor it regularly. Weight reduction interventions are also recommended for diabetic patients.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- 1.Müller MJ, Geisler C. Defining obesity as a disease. Eur J Clin Nutr. 2017;71:1256–8. doi: 10.1038/ejcn.2017.155. [DOI] [PubMed] [Google Scholar]
- 2.Chooi YC, Ding C, Magkos F. The epidemiology of obesity. Metabolism. 2019;92:6–10. doi: 10.1016/j.metabol.2018.09.005. [DOI] [PubMed] [Google Scholar]
- 3.Boutari C, Mantzoros CS. A 2022 update on the epidemiology of obesity and a call to action: As its twin COVID-19 pandemic appears to be receding, the obesity and dysmetabolism pandemic continues to rage on. Metabolism. 2022;133:155217. doi: 10.1016/j.metabol.2022.155217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ministry of Health. World Health Survey – Saudi Arabia; 2019. [[Last accessed on 2022 Nov 16]]. Available from: https://www.moh.gov.sa/en/Ministry/Statistics/Population-Health-Indicators/Documents/World-Health-Survey-Saudi-Arabia.pdf .
- 5.Information and Egovernment Authority. Bahrain National Health Survey; 2018. [[Last accessed on 2022 Nov 20]]. Available from: https://www.iga.gov.bh/Media/Agencies/Bahrain%20National%20Health%20Survey%202018%20English.pdf .
- 6.Dillinger J. The Most Obese Countries in the World. [[Last accessed on 2022 Feb 16]]. Available from: https://www.worldatlas.com/articles/29-most-obesecountries-in-the-world.html .
- 7.Khalil AB, Beshyah SA, Abdella N, Afandi B, Al-Arouj MM, Al-Awadi F, et al. Diabesity in the Arabian Gulf: Challenges and opportunities. Oman Med J. 2018;33:273–82. doi: 10.5001/omj.2018.53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Babu GR, Murthy GV, Ana Y, Patel P, Deepa R, Neelon SE, et al. Association of obesity with hypertension and type 2 diabetes mellitus in India: A meta-analysis of observational studies. World J Diabetes. 2018;9:40–52. doi: 10.4239/wjd.v9.i1.40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Apovian CM. Obesity: Definition, comorbidities, causes, and burden. Am J Manag Care. 2016;22:S176–85. [PubMed] [Google Scholar]
- 10.Wondmkun YT. Obesity, insulin resistance, and type 2 diabetes: Associations and therapeutic implications. Diabetes Metab Syndr Obes. 2020;13:3611–6. doi: 10.2147/DMSO.S275898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Al-Goblan AS, Al-Alfi MA, Khan MZ. Mechanism linking diabetes mellitus and obesity. Diabetes Metab Syndr Obes. 2014;7:587–91. doi: 10.2147/DMSO.S67400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nimptsch K, Konigorski S, Pischon T. Diagnosis of obesity and use of obesity biomarkers in science and clinical medicine. Metabolism. 2019;92:61–70. doi: 10.1016/j.metabol.2018.12.006. [DOI] [PubMed] [Google Scholar]
- 13.Tino S, Mayanja BN, Mubiru MC, Eling E, Ddumba E, Kaleebu P, et al. Prevalence and factors associated with overweight and obesity among patients with type 2 diabetes mellitus in Uganda-a descriptive retrospective study. BMJ Open. 2020;10:e039258. doi: 10.1136/bmjopen-2020-039258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Daousi C, Casson IF, Gill GV, MacFarlane IA, Wilding JP, Pinkney JH. Prevalence of obesity in type 2 diabetes in secondary care: Association with cardiovascular risk factors. Postgrad Med J. 2006;82:280–4. doi: 10.1136/pmj.2005.039032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ganz ML, Wintfeld N, Li Q, Alas V, Langer J, Hammer M. The association of body mass index with the risk of type 2 diabetes: A case-control study nested in an electronic health records system in the United States. Diabetol Metab Syndr. 2014;6:50. doi: 10.1186/1758-5996-6-50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.ElSayed NA, Aleppo G, Aroda VR, Bannuru RR, Brown FM, Bruemmer D, et al. 6. Glycemic targets: Standards of care in diabetes-2023. Diabetes Care. 2023;46:S97–110. doi: 10.2337/dc23-S006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Aucott L, Poobalan A, Smith WC, Avenell A, Jung R, Broom J, et al. Weight loss in obese diabetic and non-diabetic individuals and long-term diabetes outcomes –A systematic review. Diabetes Obes Metab. 2004;6:85–94. doi: 10.1111/j.1462-8902.2004.00315.x. [DOI] [PubMed] [Google Scholar]
- 18.Vetter ML, Ritter S, Wadden TA, Sarwer DB. Comparison of bariatric surgical procedures for diabetes remission: Efficacy and mechanisms. Diabetes Spectr. 2012;25:200–10. doi: 10.2337/diaspect.25.4.200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.ElSayed NA, Aleppo G, Aroda VR, Bannuru RR, Brown FM, Bruemmer D, et al. 5. Facilitating positive health behaviors and well-being to improve health outcomes: Standards of care in diabetes-2023. Diabetes Care. 2023;46:S68–96. doi: 10.2337/dc23-S005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mugharbel KM, Al-Mansouri MA. Prevalence of obesity among type 2 diabetic patients in Al-Khobar primary health care centers. J Family Community Med. 2003;10:49–53. [PMC free article] [PubMed] [Google Scholar]
- 21.Bakhotmah AB. Prevalence of obesity among type 2 diabetic patients: Non-smokers housewives are the most affected in Jeddah, Saudi Arabia. Open J Endocr Metab Dis. 2013;3:2530. [Google Scholar]
- 22.AlShahrani MS. Prevalence of obesity and overweight among type 2 diabetic patients in Bisha, Saudi Arabia. J Family Med Prim Care. 2021;10:143–8. doi: 10.4103/jfmpc.jfmpc_1349_20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Damian DJ, Kimaro K, Mselle G, Kaaya R, Lyaruu I. Prevalence of overweight and obesity among type 2 diabetic patients attending diabetes clinics in Northern Tanzania. BMC Res Notes. 2017;10:515. doi: 10.1186/s13104-017-2861-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.United Arab Emirates Ministry of Health and Prevention. UAE National Health Survey Report; 2017-2018. [[Last accessed on 2022 Dec 20]]. Availablefrom: https://cdn.who.int/media/docs/default-source/ncds/ncd-surveillance/data-reporting/united-arab-emirates/uae-national-health-survey-report-2017-2018.pdf .
- 25.Weiderpass E, Botteri E, Longenecker JC, Alkandari A, Al-Wotayan R, Al Duwairi Q, et al. The prevalence of overweight and obesity in an adult Kuwaiti population in 2014. Front Endocrinol (Lausanne) 2019;10:449. doi: 10.3389/fendo.2019.00449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bae JP, Lage MJ, Mo D, Nelson DR, Hoogwerf BJ. Obesity and glycemic control in patients with diabetes mellitus: Analysis of physician electronic health records in the US from 2009-2011. J Diabetes Complications. 2016;30:212–20. doi: 10.1016/j.jdiacomp.2015.11.016. [DOI] [PubMed] [Google Scholar]
- 27.Boye KS, Lage MJ, Shinde S, Thieu V, Bae JP. Trends in HbA1c and body mass index among individuals with type 2 diabetes: Evidence from a US database 2012-2019. Diabetes Ther. 2021;12:2077–87. doi: 10.1007/s13300-021-01084-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Flack JM, Adekola B. Blood pressure and the new ACC/AHA hypertension guidelines. Trends Cardiovasc Med. 2020;30:160–4. doi: 10.1016/j.tcm.2019.05.003. [DOI] [PubMed] [Google Scholar]
- 29.Arnett DK, Blumenthal RS, Albert MA, Buroker AB, Goldberger ZD, Hahn EJ, et al. 2019 ACC/AHA guideline on the primary prevention of cardiovascular disease: Executive summary: A report of the American college of cardiology/American heart association task force on clinical practice guidelines. Circulation. 2019;140:e563–95. doi: 10.1161/CIR.0000000000000677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Grundy SM, Stone NJ, Bailey AL, Beam C, Birtcher KK, Blumenthal RS, et al. 2018 AHA/ACC/AACVPR/AAPA/ABC/ACPM /ADA/AGS/APhA/ASPC/NLA/PCNA guideline on the management of blood cholesterol: Executive summary: A report of the American college of cardiology/American heart association task force on clinical practice guidelines. J Am Coll Cardiol. 2019;73:3168–209. doi: 10.1016/j.jacc.2018.11.002. [DOI] [PubMed] [Google Scholar]
- 31.ElSayed NA, Aleppo G, Aroda VR, Bannuru RR, Brown FM, Bruemmer D, et al. 8. Obesity and weight management for the prevention and treatment of type 2 diabetes: Standards of care in diabetes-2023. Diabetes Care. 2023;46:S128–39. doi: 10.2337/dc23-S008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.American Diabetes Association Professional Practice Committee. 9. Pharmacologic approaches to glycemic treatment: Standards of medical care in diabetes-2022. Diabetes Care. 2022;45:S125–43. doi: 10.2337/dc22-S009. [DOI] [PubMed] [Google Scholar]


