Abstract
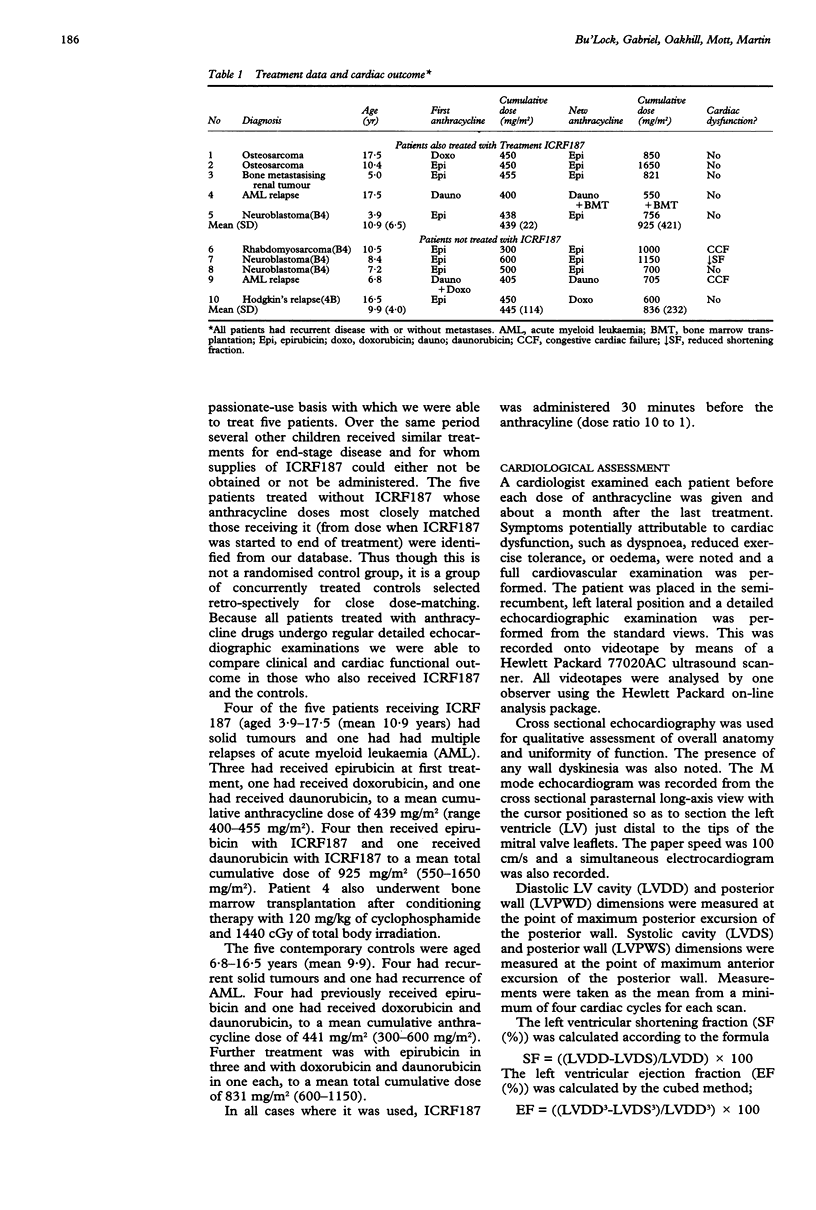
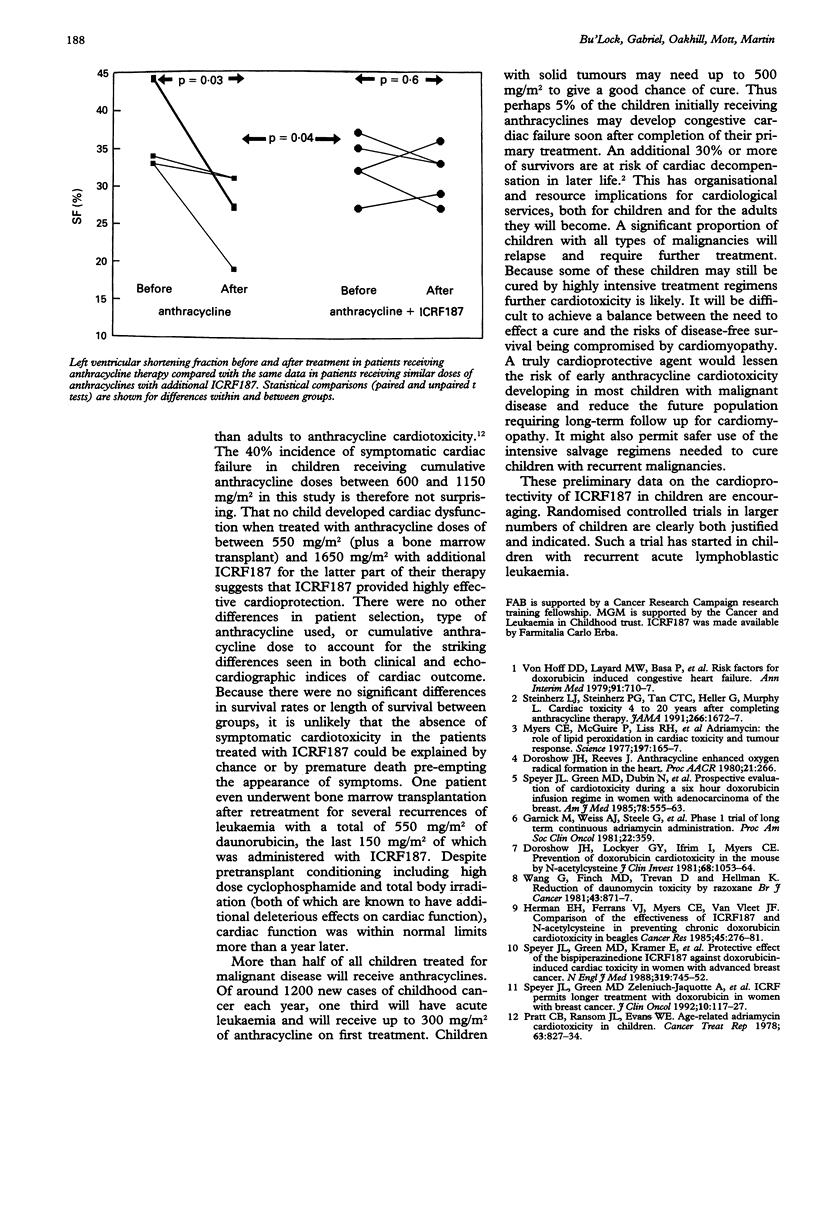
OBJECTIVE--A pilot study to assess the efficacy of ICRF187 as a protective agent against the cardiotoxic effects of anthracycline drugs used to treat childhood malignancies. DESIGN--A study of cardiac function in children treated receiving ICRF187 ((s)-(+)-1,2 bis (3,5-dioxopiperazenyl) propane) in addition to anthracycline therapy compared with contemporary controls selected retrospectively on the basis of anthracycline dose matching. PATIENTS--Five children in whom recurrence of malignant disease was re-treated with chemotherapy containing anthracycline drugs and additional ICRF187 (supplied on a compassionate-use basis) (cumulative anthracycline doses 550-1650 mg/m2). Five more children with recurrence of malignant disease were re-treated to similar cumulative anthracycline doses (600-1150 mg/m2) without ICRF187. METHODS--Cardiac function was assessed clinically and echocardiographically throughout treatment. Clinical and echocardiographic state were compared before treatment and after completion of therapy within and between groups treated with and without ICRF187. RESULTS--Two patients treated without ICRF187 developed symptomatic congestive cardiac failure from which one died. Another developed considerable but as yet asymptomatic left ventricular dysfunction. No patient receiving additional ICRF187 developed cardiac failure or left ventricular dysfunction. There were no significant differences in cumulative anthracycline dose, dose increase, type of anthracycline used, survival rate, or length of survival between groups. Left ventricular shortening fraction fell by a mean of 1.0% in patients receiving ICRF187 and by a mean of 11% in the patients treated without it (p = 0.04). CONCLUSIONS--ICRF187 seems to have provided highly effective cardioprotection to this small group of children with end-stage malignancy. Severe cardiotoxicity was seen in a similar group treated with comparable anthracycline doses but without ICRF187.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Doroshow J. H., Locker G. Y., Ifrim I., Myers C. E. Prevention of doxorubicin cardiac toxicity in the mouse by N-acetylcysteine. J Clin Invest. 1981 Oct;68(4):1053–1064. doi: 10.1172/JCI110328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herman E. H., Ferrans V. J., Myers C. E., Van Vleet J. F. Comparison of the effectiveness of (+/-)-1,2-bis(3,5-dioxopiperazinyl-1-yl)propane (ICRF-187) and N-acetylcysteine in preventing chronic doxorubicin cardiotoxicity in beagles. Cancer Res. 1985 Jan;45(1):276–281. [PubMed] [Google Scholar]
- Myers C. E., McGuire W. P., Liss R. H., Ifrim I., Grotzinger K., Young R. C. Adriamycin: the role of lipid peroxidation in cardiac toxicity and tumor response. Science. 1977 Jul 8;197(4299):165–167. doi: 10.1126/science.877547. [DOI] [PubMed] [Google Scholar]
- Speyer J. L., Green M. D., Dubin N., Blum R. H., Wernz J. C., Roses D., Sanger J., Muggia F. M. Prospective evaluation of cardiotoxicity during a six-hour doxorubicin infusion regimen in women with adenocarcinoma of the breast. Am J Med. 1985 Apr;78(4):555–563. doi: 10.1016/0002-9343(85)90395-x. [DOI] [PubMed] [Google Scholar]
- Steinherz L. J., Steinherz P. G., Tan C. T., Heller G., Murphy M. L. Cardiac toxicity 4 to 20 years after completing anthracycline therapy. JAMA. 1991 Sep 25;266(12):1672–1677. [PubMed] [Google Scholar]
- Von Hoff D. D., Layard M. W., Basa P., Davis H. L., Jr, Von Hoff A. L., Rozencweig M., Muggia F. M. Risk factors for doxorubicin-induced congestive heart failure. Ann Intern Med. 1979 Nov;91(5):710–717. doi: 10.7326/0003-4819-91-5-710. [DOI] [PubMed] [Google Scholar]
- Wang G., Finch M. D., Trevan D., Hellmann K. Reduction of daunomycin toxicity by razoxane. Br J Cancer. 1981 Jun;43(6):871–877. doi: 10.1038/bjc.1981.127. [DOI] [PMC free article] [PubMed] [Google Scholar]


