Abstract
Transcriptional determinants in the skeletal muscle that govern exercise capacity, while poorly defined, could provide molecular insights into how exercise improves fitness. Here, we have elucidated the role of nuclear receptors, estrogen-related receptor alpha and gamma (ERRα/γ) in regulating myofibrillar composition, contractility, and exercise capacity in skeletal muscle. We used muscle-specific single or double (DKO) ERRα/γ knockout mice to investigate the effect of ERRα/γ deletion on muscle and exercise parameters. Individual knockout of ERRα/γ did not have a significant impact on the skeletal muscle. On the other hand, DKO mice exhibit pale muscles compared to wild-type (WT) littermates. RNA-seq analysis revealed a predominant decrease in expression of genes linked to mitochondrial and oxidative metabolism in DKO versus WT muscles. DKO muscles exhibit marked repression of oxidative enzymatic capacity, as well as mitochondrial number and size compared to WT muscles. Mitochondrial function is also impaired in single myofibers isolated from DKO versus WT muscles. In addition, mutant muscles exhibit reduced angiogenic gene expression and decreased capillarity. Consequently, DKO mice have a significantly reduced exercise capacity, further reflected in poor fatigue resistance of DKO mice in in vivo contraction assays. These results show that ERRα and ERRγ together are a critical link between muscle aerobic capacity and exercise tolerance. The ERRα/γ mutant mice could be valuable for understanding the long-term impact of impaired mitochondria and vascular supply on the pathogenesis of muscle-linked disorders.
Keywords: angiogenesis, estrogen-related receptors, exercise, mitochondria, skeletal muscle
1 |. INTRODUCTION
Exercise positively impacts metabolic and cardiovascular health, general wellness, and resistance to obesity and diabetes.1,2 Exercise capacity is partly governed by cardiovascular and pulmonary fitness, but more critically by skeletal muscle aerobic capacity constituting mitochondrial content and function, fiber type composition, and vascular supply.1 Inactivity, aging, and obesity impair aerobic ability in the skeletal muscle, causing mitochondrial dysfunction and vascular regression, ultimately leading to exercise intolerance and cardiometabolic complications.3 These complications can be delayed, or generally fitness can be improved by regular exercise, highlighting the plasticity of the skeletal muscle and its potential amenability to improving function through molecular regulation. In this regard, there has been a long-standing interest in delineating the molecular determinants that link muscle aerobic metabolism and contractile function that directly impact exercise capacity.
Estrogen-related receptors (ERRs) belonging to the orphan nuclear receptor superfamily have emerged as master-metabolic regulators in multiple organs including liver and adipose tissue contributing to energy homeostasis.4–6 These receptors generally control glucose and fatty acid metabolism, and are also involved in adipose tissue browning and thermogenesis. Among the three isoforms, ERRα is highly and ubiquitously expressed in the skeletal muscle.7 ERRγ is also highly expressed but has preferential expression in oxidative muscles.8,9 ERRβ is undetectable in the skeletal muscle.8 ERRs are regulated by exercise, hypoxia/ischemia, and diet-induced obesity in skeletal muscle,8,10,11 conditions that are known to affect mitochondrial function and tissue vascularity, suggesting a potential link between ERR expression and aerobic capacity. In support of this notion, several studies with ectopically overexpressed ERRα or ERRγ indicate that these receptors can drive oxidative myofiber type regulation, mitochondrial metabolism, and even paracrine vascularization in the skeletal muscle. Muscle-specific overexpression of ERRγ or VP16-ERRγ promotes mitochondrial biogenesis, fatty acid oxidation, and myofiber type switch to type IIA and IIX myofibers, known to have high oxidative metabolic capacity.8,9 Similarly, ERRα overexpression also promotes an oxidative myofiber type switch to type IIA and IIX in the skeletal muscle.10 Both receptors drive basal skeletal muscle vascularization and neo-vascularization in ischemia, underscoring the overlapping functions of ERRs in aerobic adaptation.8,10 These observations are further corroborated by other studies in cultured muscle cells demonstrating that both ERRα and ERRγ regulate metabolic pathways.12–16 Despite these reports on the potential critical role of ERRs in muscle aerobic regulation, to date there has been no study that has analyzed in depth the contribution of endogenous ERRα and ERRγ expressed in the muscle to aerobic capacity, contractile function, and whole-body endurance.
Here we have generated muscle-specific ERRα and ERRγ double knockout mice and investigated the impact of simultaneous loss of the two ERRs on muscle architecture and function, as well as whole-body exercise tolerance. Our rationale for using the double knockout strategy and the focus on ERRα and ERRγ is based on the high expression of these two isoforms in the skeletal muscle, undetectable ERRβ (third isoform) levels,8 the potentially overlapping gene regulatory effects of ERRα and ERRγ,5,17 as well as initial observation in this study that conditional knockout of individual receptors had a mild impact on the skeletal muscle. We report that ERRα/γ together are indispensable for muscle aerobic capacity in terms of mitochondrial function, capillary supply, contractile function, and exercise tolerance predominantly through mitochondrial metabolic gene programming and angiogenesis. Our findings suggest that muscle ERRs are an important transcriptional node of aerobic regulation in exercise adaptation and likely in pathogenesis of metabolic disease and myopathy.
2 |. MATERIALS AND METHODS
2.1 |. Animal husbandry
ERRα floxed mice targeting exons 3 and 4 and ERRγ floxed mice targeting exon 3 have been described.16,18 The ERRα and ERRγ floxed mice were originally generated at Institut Clinique de la Souris (ICS, Strasbourg, France). Muscle-specific human alpha skeletal actin-driven Cre recombinase transgenic mice (HSA-Cre, B6.Cg-Tg (ACTA1-cre) 79Jme/J) were obtained from The Jackson Laboratory. All the mice were housed in a temperature-controlled room (20–22°C) with ad libitum access to water and food (Pico Lab rodent diet 20; 13.2% fat) under a 12:12 h light–dark cycle. Mice were individually housed in cages for the voluntary wheel running studies, and up to 5 mice were otherwise housed in a single cage. Age-matched 4- to 5-month-old mice were used for the experiments. Animals were maintained and treated in accordance with the U.S. National Institute of Health Guide for Care and Use of Laboratory Animals. The procedures were approved by the Animal Welfare Committee at The University of Texas Health Science Center in Houston (UTHealth).
2.2 |. Generation of muscle-specific ERRα/γ single or double knockout mice
Muscle-specific ERRα (MEAKO) or ERRγ (MEGKO) knockout mice were generated by crossing the respective floxed mice with HSA-Cre mice for initial analysis. ERRα floxed (f/f) mice were cross-bred with ERRγ floxed (f/f) mice to generate ERRα/γ f/f mice. Initially, muscle-specific ERRα or ERRγ knockout mice were crossed with ERRα/γ f/f mice to obtain muscle-specific ERRα/γ double knockout (DKO) mice. Routine breeding for experimental cohorts was maintained by crossing HSA-Cre::ERRα/γ f/f mice and the ERRα/γ f/f mice to generate the ERRα/γ f/f mice (WT controls) and the HSA-Cre::ERRα/γ f/f mice (DKO). Age-matched male and female knockout and littermate WT mice were used for the experiments.
2.3 |. Tissue collection and preparation
Mice were euthanized by isoflurane inhalation followed by cervical dislocation, and muscle tissues were rapidly dissected. For RNA and protein analyses, muscles were freeze-clamped in liquid nitrogen. For immunofluorescence, tibialis anterior (TA) muscle was mounted in OCT and frozen on melting isopentane in liquid nitrogen.
2.4 |. Western immunoblotting and ELISA
Muscle as well as other tissues were homogenized in RIPA buffer (Thermo Scientific, Waltham, MA) containing protease and phosphatase inhibitors, using Tissue Lyser (Qiagen, Hilden, Germany) at setting 50, for 3 cycles of 1 min each followed by centrifugation at 10 000 × g for 15 min at 4°C. The Pierce BCA protein assay kit (Thermo Scientific) was used to quantify protein in the super-natant, which was then stored at −80°C. Samples were separated by SDS-PAGE, transferred onto nitrocellulose membrane, stained using Ponceau S, blocked with 5% BSA in phosphate-buffered saline with 0.1% Tween20 (PBST), and incubated overnight at 4°C with primary antibodies for ERRα (# 13826, Cell signaling, MA) at 1:1000 dilution, ERRγ (kindly provided by Ron Evans), CD31 (# 77699, Cell Signaling, MA) at 1:1000 dilution, Cytochrome C (# sc13561, Santa Cruz Biotechnology, TX) at 1:500 dilution or total OXPHOS rodent antibody cocktail (# ab110413, Abcam, MA) at 1:1000 dilution. Membranes were then washed with PBST and incubated with the appropriate secondary antibodies (Cell Signaling: Anti-rabbit IgG # 7074 at 1:5000 dilution for ERRα, ERRγ, and CD31 primary antibodies; anti-mouse IgG # 7076 at 1:4000 dilution for Cytochrome C and OXPHOS cocktail primary antibodies) for 1 h at room temperature, washed with PBST. Bands were visualized using chemiluminescence western blotting detection reagents.
VEGFA protein levels were measured using mouse VEGF DuoSet ELISA (# DY493 R&D Systems, Minneapolis, MN) in gastrocnemius, quadriceps, and TA protein lysates (100 μg) as per the manufacturer’s instructions.
2.5 |. Quantitative real-time PCR
Quantitative PCR (QPCR) analysis was performed using SYBR Green PCR Master Mix (Applied Biosystems, Waltham, MA) with a BioRad CFX96 cycler (BioRad, Hercules, CA) using cDNA reverse transcribed from muscle mRNA. Primer sequences are given in Table S1. All data were normalized to Tbp and Hsp90.
2.6 |. Immunohistology
TA muscle was cryosectioned (10 μm) in the mid-belly region. The sections were blocked in 10% goat serum in PBS. Myosin heavy chains (MHC) type 2A and 2B were stained using the mouse monoclonal antibodies SC71 (5 μg/ml) and BF.F3 (4 μg/ml), respectively (Developmental Studies Hybridoma Bank, Iowa City, IA), as we previously described.19 Laminin was stained using antibody L9393 from Sigma, St. Louis, MO at 3.5 μg/mL dilution to visualize sarcolemma. Capillaries were stained using CD31 (# MCA2388, Bio-Rad, Hercules, CA) at 10 μg/ml. All primary antibodies were visualized using suitable Alexa Fluor® secondary antibodies (Molecular Probes, Eugene, OR). Immunostained cryosections were examined using confocal microscopy. Myofiber number and area was quantified using Amira software version 6.3 from FEI Hillsboro, OR. CD31 quantification was performed using ImageJ.
2.7 |. Nicotinamide adenine dinucleotide-tetrazolium reductase and Succinate dehydrogenase activity staining
Nicotinamide adenine dinucleotide-tetrazolium reductase (NADH-TR) and succinate dehydrogenase activity (SDH) staining was performed on 10 μm TA cryosections. Briefly, the cryosections were incubated at 37°C for 30 min in substrate buffer (0.05 M Tris– HCl buffer pH 7.4 containing nitro blue tetrazolium—[NBT, Sigma N6876 (10 mg/10 ml) and NADH (8 mg/10 ml) for NADH-TR assay, or NBT (10 mg/10 ml) and sodium succinate (27 mg/mL) for SDH assay]). Sections were then washed three times with water followed by 2-min incubation with increasing and decreasing concentrations of acetone (30%, 60%, and 90%). Finally, the sections were washed three times with water, mounted in an aqueous mounting medium, and visualized under a light microscope. NADH and SDH densitometry was performed using ImageJ.
2.8 |. RNA sequencing and data analysis
Total RNA from WT and DKO gastrocnemius was extracted with Purelink Kit (Ambion, Life technologies, Carlsbad, CA) and was further reverse-transcribed to cDNA with SuperScript III Reverse Transcriptase (Invitrogen, Waltham, MA) and used for performing RNA-seq analysis. The RNA-seq library was prepared using enriched poly (A)-tailed mRNA in the UT Cancer Genomics Center by following the instructions in KAPA mRNA HyperPrep Kit (KK8581, Roche, Holding AG, Switzerland) and KAPA Unique Dual-indexed Adapter kit (KK8727, Roche). Illumina Nextseq550 was used for performing RNA-seq with the 75 bp pair-ended running mode. Cutadapt (v1.15) was used to process raw mRNA sequence reads and to remove bases with quality scores <20 and adapter sequences,20 followed by the alignment of clean RNA-seq reads to GRCm38 with STAR (v2.5.3a).21 Uniquely mapped reads overlapping genes were counted by HTseq-count with default parameter using annotation from GencodeM15. Only genes with >5 reads in at least one sample were retained. The raw read counts of retained genes were submitted for differential expression analysis of cases compared with controls with DESeq2 software,22 which uses a model based on the negative binomial distribution. The resulting P-values were adjusted using the Benjamini and Hochberg’s approach23 to control for the false discovery rate (FDR). Genes with fold change (FC) >1.5 (or FC <0.667) and FDR <0.05 were assigned as differentially expressed genes (DEGs). Standard gene set enrichment analysis was performed with a hypergeometric test using WebGestalt (v 0.4.3).23 The resulting p-values were also adjusted using the Benjamini and Hochberg’s approach.23
2.9 |. Seahorse mitochondrial stress test
The seahorse mitochondrial stress test was performed on single myofibers isolated from flexor digitorum brevis (FDB) muscles of the WT and DKO mice, following the manufacturer’s (Agilent, Santa Cruz, CA) instructions. The FDB muscles were isolated from the mice, immediately given three washes in sterile PBS and transferred to 0.22-micron filter sterilized digestion medium (DMEM containing 1% Pen/Strep and 400 U/ml Collagenase II). The FDB muscles were incubated overnight at 37°C in 5% CO2 incubator. FDB muscles were transferred to F10 media with 5% FBS and gently triturated in 2% Horse serum-coated plates. Approximately, equal number of FDB myofibers were plated in 10% Matrigel-coated XFe96 seahorse cell culture plate and returned to the incubator. Seahorse XF cell Mito stress test kit (# 103010–100 Agilent Technologies, Santa Clara, CA) was used following the manufacturer’s instructions. The following reagents were loaded into injection ports of calibrant plate to achieve a final concentration of 2 μM Oligomycin, 2.5 μM FCCP, and 1.5 μM mix of Antimycin A and Rotenone. One hour before the assay, the myofiber media was replaced with XFe assay medium containing 10 mM glucose, 1 mM pyruvate, and 2 mM glutamine. The number of myofibers were counted in each well and mitochondrial stress test assay was then performed using Seahorse XFe96 analyzer, where basal oxygen consumption rate (OCR), oligomycin-treated OCR, FCCP-treated OCR, and Antimycin A/Rotenone-treated OCR was recorded. The OCR data are presented after normalization to per 100 FDB myofibers.
2.10 |. Transmission electron microscopy
WT and DKO (n = 3) TA muscle longitudinal sections were used for transmission electron microscopy (TEM). The TA were collected and cut longitudinally into 1 mm strip and stored in 2% glutaraldehyde solution overnight at 4°C. The following day, samples for electron microscopy were fixed in Trump’s 2% paraformaldehyde 3% phosphate-buffered glutaraldehyde. Samples were post fixed in 1% buffered osmium tetroxide, dehydrated in a graded series of ethanol, and embedded in Polybed resin. Thin sections were generated using a Leica UC7 ultramicrotome, stained with Uranyless and lead citrate. Samples were viewed with a FEI Tecnai Spirit TEM equipped with an Eagle digital camera. Data were analyzed from 4 × 9000 magnification micrographs, each for subsarcolemmal and intermyofibrillar regions for each mouse, and a total of ~500 mitochondria were analyzed. The mitochondria were manually outlined using ImageJ software and the following parameters were analyzed: mitochondrial size (mean area, μm2), distribution (number per μm2), density (total mitochondrial area/area of field), perimeter (μm), circularity, and cristae number per mitochondrion (total number of cristae/total number of mitochondria per image).24
2.11 |. Treadmill running test
The maximal running speed of the mice was determined by treadmill sprint running test following previously published protocol.25 WT and DKO mice were acclimatized to the treadmill running over 3 days. Mice ran at a speed of 10 m/min for 10 min with 0° incline on day 1, 5° incline on day 2, and 10° incline on day 3. On day 5, the mice were placed on the treadmill and the speed, duration, and incline were changed as follows: 0 m/min, 5 min, 0° incline; 6 m/min, 5 min, 0° incline; 7, 8, 9, and 10 m/min, 30 sec each, 20° incline; and +1 m/min increment every min, 20° incline after that until exhaustion (5 s off the treadmill).
One week after the sprint test, the endurance test was performed following a previously published protocol.25 The speed, incline, and time were changed as follows: 6 m/min, 0° incline for 5 min; 6–14 m/min, 0° incline, +1 m/min increment every min; 14 m/min, 5° incline for 30 min; 16 m/min, 10° incline for 30 min; and 18 m/min, 15° incline until exhaustion. Exhaustion was defined as 10 s continuously off the treadmill on the platform, at which point the run was terminated.
2.12 |. Voluntary wheel running test
Mice were housed individually in cages with an upright (9.5-inch diameter) Tecniplast 1284 L mouse-size running wheel (Starr Life Sciences). The wheels had a magnetic sensor attached that was connected to a data acquisition board (VitalView® Activity Software), which was connected to a computer. The mice had ad libitum access to food and water and 12 h:12 h light:dark cycle. The mice were acclimatized for 1 week and readings were recorded for subsequent 2 weeks. The following data were recorded: (i) the number of times the mice got on to the running wheel (bouts), (ii) the duration of time they spent on the running wheel during each bout, and (iii) the revolutions of the running wheel during each bout (counts per bout). The data were analyzed using ClockLab (Actimetrics) software.
2.13 |. In vivo muscle contraction
As previously described, the plantar flexion force measurements of the posterior right leg muscles were performed using 1300A 3-in-1 Whole Animal System (Aurora Scientific).26 The mean specific twitch force was generated from five stimulations at 150 Hz. The muscle was stimulated from 25 to 300 Hz in 25 Hz intervals resulting in 12 specific tetanic forces, which were normalized to body weight and plotted as the force-frequency graph. Following a 5-min rest period, a fatigue protocol was performed where 180 Hz stimulation was given every second for 1 min for a total of 60 stimuli. The integral force generated during each stimulus was normalized to bodyweight. Contractile events were recorded with the ASI611A Dynamic Muscle Control software (Aurora Scientific) and were calculated with the accompanying ASI611A Dynamic Muscle Analysis software (Aurora Scientific).
2.14 |. Statistics
Unpaired Student’s t-test were used as indicated in the figure legends to compare the different groups. Data in figures are presented as mean ± standard error of mean, such that N used in each experiment was sufficient to achieve a power of 0.8 and Type 1 error rate of 5%. Data were analyzed and plotted using GraphPad Prism 5. The analysis of RNA-seq data is described above.
3 |. RESULTS
3.1 |. Muscle-specific ERRα/γ double knockout in mice results in pale musculature compared to WT littermates
First, we generated muscle-specific ERRα and ERRγ single receptor knockout mice by crossing the ERRα or ERRγ floxed mice with HSA-Cre mice, respectively. Cre-mediated recombination results in muscle-specific deletion of exons 3 and 4 in ERRα (MEAKO), and exon 3 in ERRγ (MEGKO),16,18,27 leading to decreased receptor expression (Figure S1A,F). QPCR analysis of WT and MEAKO gastrocnemius showed that loss of ERRα in the skeletal muscle reduced the expression of Tfam and Cycs (mitochondria related genes) but not the expression of genes related to fatty acid oxidation and angiogenesis (Figure S1A, Right Panel). Furthermore, myofiber type distribution was similar in WT and MEAKO TA (Figure S1B). Other parameters such as capillary staining (Figure S1C) and NADH-TR activity (Figure S1D) were also not majorly affected by ERRα deletion in the skeletal muscle, with the exception that capillary-to-fiber ratio showed a slight but statistically significant decrease. Finally, exercise capacity on running wheel was comparable between WT and MEAKO mice (Figure S1E). Therefore, selective knockout of ERRα in the skeletal muscle does not have a major impact on muscle architecture or exercise capacity. Likewise, muscle-specific knockout of ERRγ (Figure S1F) did not affect the expression of known ERRγ target genes (Cycs, Pdk4, Vegfa) in soleus muscle, where it is predominantly expressed, as seen in QPCR analysis of WT and MEGKO mice (Figure S1G). Paradoxically, PDK4 expression (a known ERR target) was induced in MEGKO muscle, pointing to compensation via an alternative pathway, potentially ERRα. Therefore, we did not further analyze the muscle-specific ERRγ knockout mice, as single knockout of either ERRα or ERRγ were likely to have a mild impact on muscle function.
Consequently, to study the compound role of ERRα and ERRγ innately expressed in the skeletal muscle, we generated muscle-specific ERRα/γ double knockout (DKO) mice, as described in Section 2 and Figure 1A. Deletion of ERRα and ERRγ protein expression is shown in gastrocnemius of DKO compared to WT mice (Figure 1B). Decrease in ERRα and ERRγ transcripts were also measured and confirmed by QPCR in gastrocnemius (Figure 1C). Deletion of ERRs at the protein level was additionally confirmed in other skeletal muscles like FDB, extensor digitorum longus (EDL), soleus, TA, and quadriceps (Figure S2A). Selectivity of ERRα/γ deletion in the skeletal muscle was confirmed by measuring ERRα and ERRγ protein expression in non-muscle tissues including heart, liver, kidney, and adipose tissue (Figure S2B), which was found to be comparable between WT and DKO mice. Initial gross characterization shows that muscle-specific knockout of ERRα and ERRγ neither affects body weight nor percent lean mass in DKO mice versus WT mice (Figure 1D). Lack of difference in lean mass was reflected in the individual muscle weights, where there was no difference between WT and DKO mice across different muscles including pre-dominantly fast-twitch (e.g., EDL, TA, plantaris, and quadriceps), pre-dominantly slow-twitch (e.g., soleus), and mixed fiber type (e.g., gastrocnemius) muscles (Figure 1E, upper panel). Notably, DKO muscles were pale in color compared to the corresponding WT muscles (Figure 1E, lower panel), potentially indicating an aerobic or a metabolic shift resulting from the loss of ERR expression.
FIGURE 1.
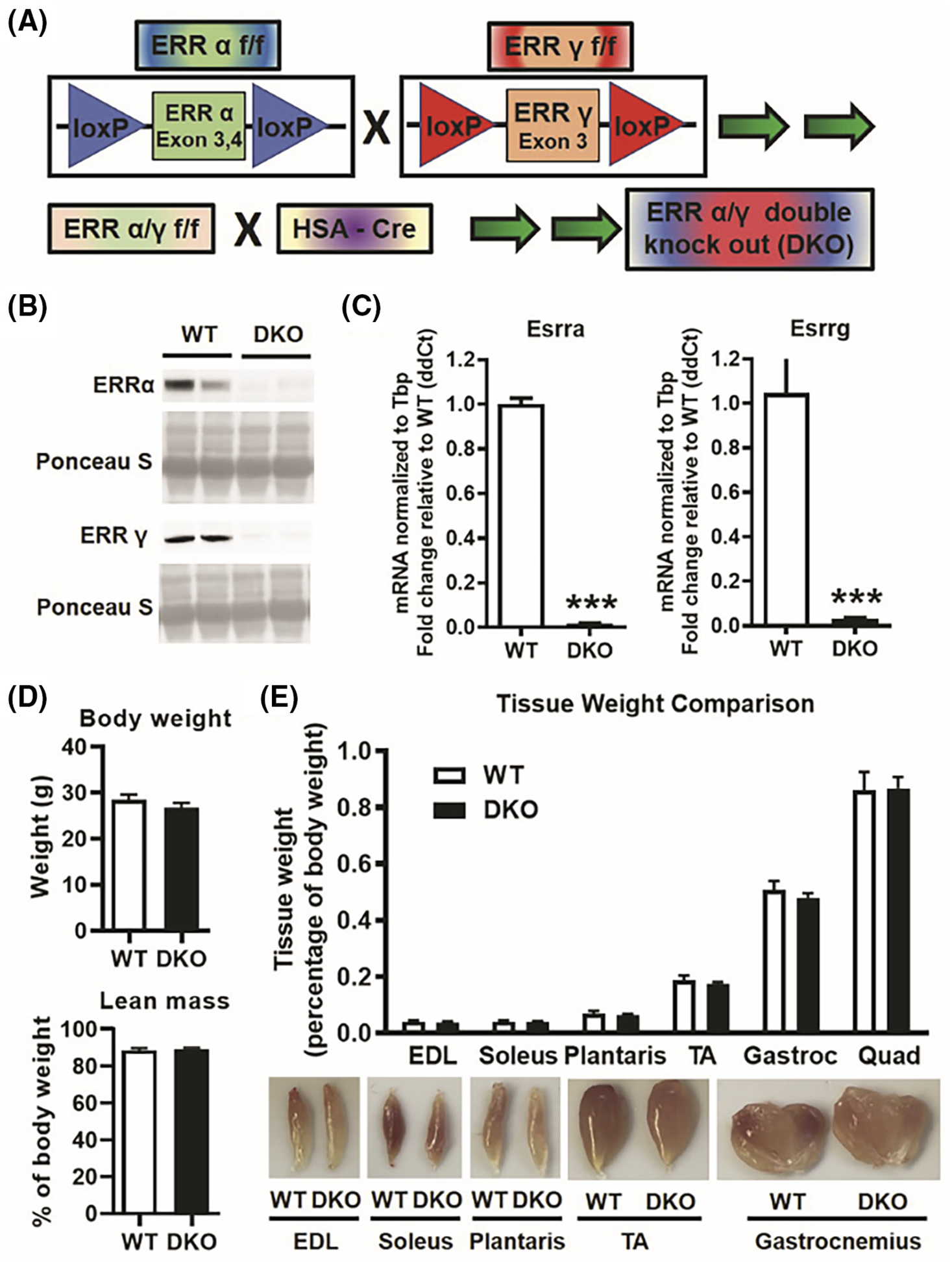
Skeletal muscle-specific ERRα/γ double knockout (DKO) mice. (A) Scheme showing generation of muscle-specific ERRα/γ DKO mice. HSA, human skeletal alpha actin. (B) Representative western blots of ERRα and ERRγ protein expression in gastrocnemius muscle of DKO versus wild type (WT) mice (N = 5). (C) QPCR showing Esrra and Esrrg transcript expression in DKO versus WT gastrocnemius muscle (N = 6). (D) Body weight and lean mass (measured by Echo MRI) in DKO versus WT mice (N = 5). (E) Upper panel: individual muscle weight comparison between DKO and WT mice (N = 6). Lower panel: representative images of different muscles. EDL, extensor digitorum longus; Gastroc, gastrocnemius; Quad, quadriceps; TA, Tibialis anterior. ***p < .001. Unpaired Student’s t-test.
We also quantitatively examined the myofiber size distribution of TA in WT and DKO mice. We found that WT TA has significantly more myofibers in the 250–500 μm2 size range compared to DKO TA (Figure S3A,B). On the other hand, DKO TA has significantly more myofibers in the 750–1000 μm2 size range compared to WT TA (Figure S3B). Therefore, there is a rightward shift in myofiber size in DKO TA in the size range of 250–1000 μm2, suggestive of a switch to larger myofiber size in DKO mice. However, the overall average myofiber area was comparable between WT and DKO TA (Figure S3C).
3.2 |. Muscle-specific loss of ERRα/γ predominantly downregulates oxidative metabolism and mitochondrial gene program
We performed RNA-seq analysis on gastrocnemius to measure the impact of ERRα/γ loss in the skeletal muscle on global gene expression changes in the skeletal muscle. Gastrocnemius was selected because of its mixed myofiber type composition. Loss of ERRα/γ results in differential expression of 422 genes by over twofold (computed using a FDR of p < .05) in DKO versus WT gastrocnemius (Figure 2A). Of the 422 genes, 232 genes were downregulated in DKO and 189 genes were upregulated compared to WT (Figure 2B).
FIGURE 2.
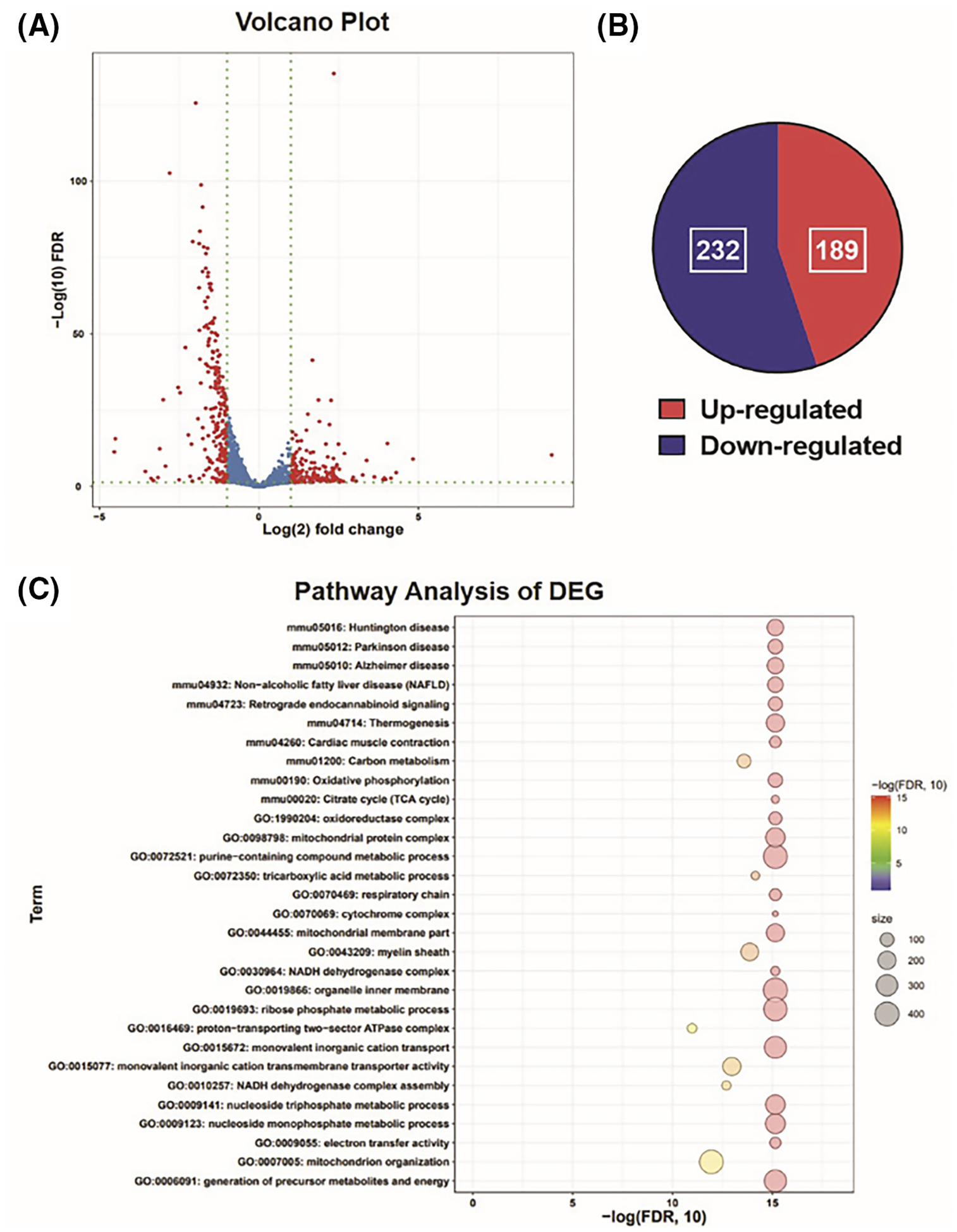
Global muscle gene expression. (A) Volcano plot from RNA-seq analysis of DKO versus WT gastrocnemius muscle showing the differentially expressed genes (Red dots) with twofold change and a false discovery rate (FDR) <0.05 (N = 3). (B) Pie chart representing up-regulated and downregulated genes in DKO gastrocnemius muscle compared to WT. (C) Functional enrichment analysis of the differentially expressed gene sets (DEG) in DKO gastrocnemius muscle using gene ontology (GO) and Kyoto Encyclopedia of Genes and Genomes (KEGG) annotations showing top 30 gene sets.
Functional enrichment analysis of the DEGs using gene ontology (GO) and Kyoto Encyclopedia of Genes and Genomes (KEGG) annotations showed that several gene sets related to mitochondrial homeostasis and cellular energy dynamics were downregulated in DKO compared to WT muscle (Figure 2C). We found a significant decline in the expression of genes related to oxidative phosphorylation (Figure 3A), respiratory chain, cytochrome complex, and tricarboxylic acid cycle (Figure S4A–C). QPCR analysis of biomarker genes for mitochondrial biogenesis (Tfam), mitochondrial respiration/OxPhos (Cycs, Cox4a, Cox6a, Ndufa5), mitochondrial reactive oxygen species sequestration (Sod2), and muscle oxygen delivery (Mb) confirmed that genes related to aerobic mitochondrial function were significantly downregulated in the DKO gastrocnemius compared to WT (Figure 3B). In addition, metabolic genes related to fatty acid oxidation (Cpt1a, Fabp3, Pfkfb2, and Pdk4) were also downregulated in the DKO muscle (Figure 3C and Figure S4D). These data suggest a downregulation in mitochondrial function and oxidative metabolism in the skeletal muscles of DKO mice lacking both ERRα/γ expression.
FIGURE 3.
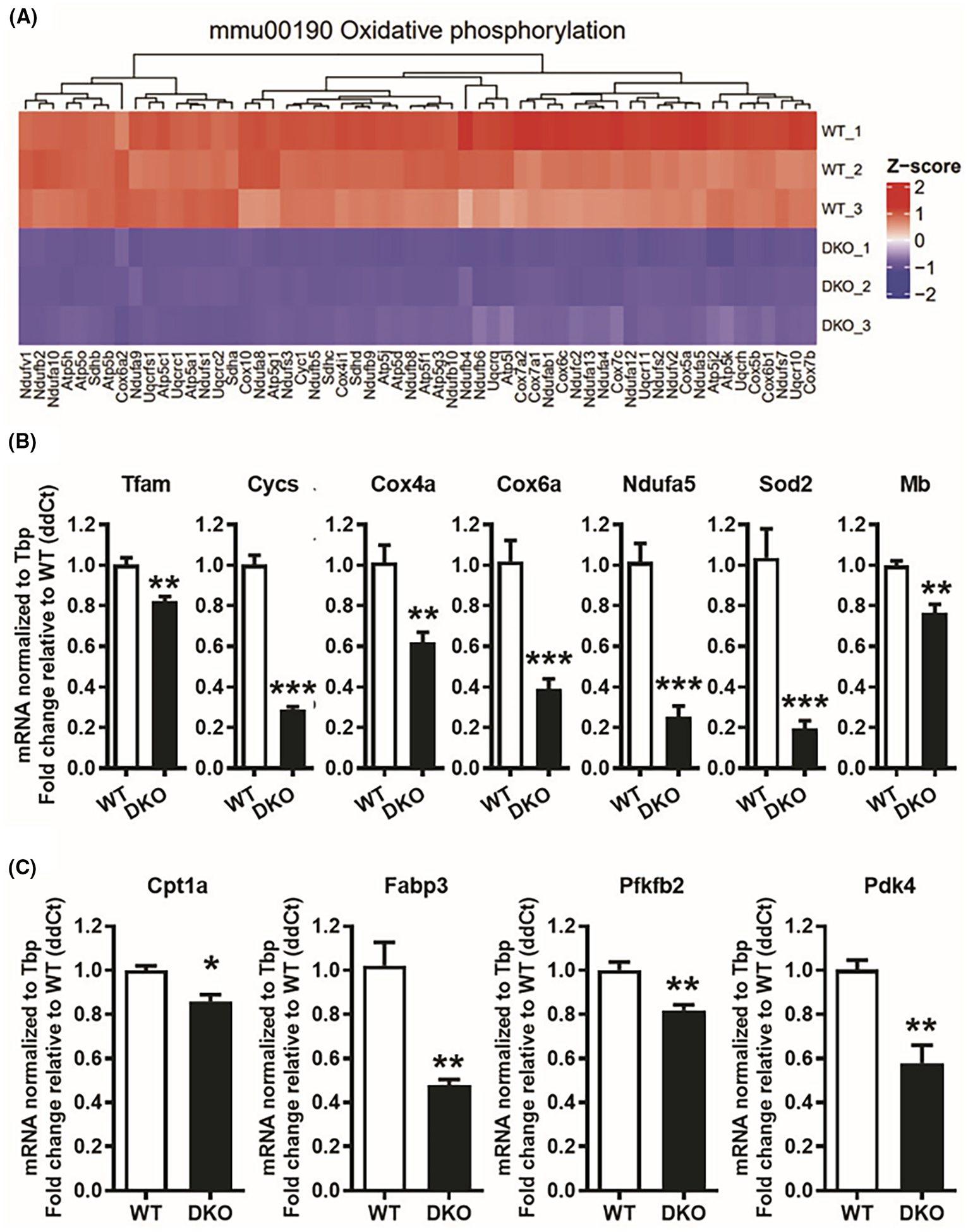
Decreased expression of oxidative phosphorylation genes in ERRα/γ deficient muscles. (A) Heat map showing the downregulation (Z-score scaled) of oxidative phosphorylation-linked genes in DKO versus WT gastrocnemius muscles (N = 3). (B) QPCR analysis of mitochondrial gene expression in DKO compared to WT gastrocnemius muscle (N = 5). (C) QPCR analysis of fatty acid oxidation genes in DKO versus WT muscle (N = 5). *p < .05, **p < .01, and ***p < .001. Unpaired Student’s t-test.
3.3 |. Loss of muscle ERRα/γ causes mitochondrial dysfunction in the skeletal muscle
To further investigate the effect of ERRα/γ double knockout in muscle on mitochondrial function, we performed several measurements starting with protein expression of mitochondrial electron transport chain (ETC) subunits. We found that protein levels of various ETC subunits and cytochrome c were significantly downregulated in the DKO versus WT gastrocnemius (Figure 4A, upper panel), with statistically significant differences shown (Figure 4A, lower panel). Furthermore, functional analysis of complex I and II using NADH-TR (Figure 4B, left panel) and SDH (Figure 4C, Left Panel) enzymatic activity immunostaining, respectively, of DKO and WT TA muscle revealed a significantly lower activity of both complexes in muscles of DKO versus WT mice (Figure 4B,C, right panel).
FIGURE 4.
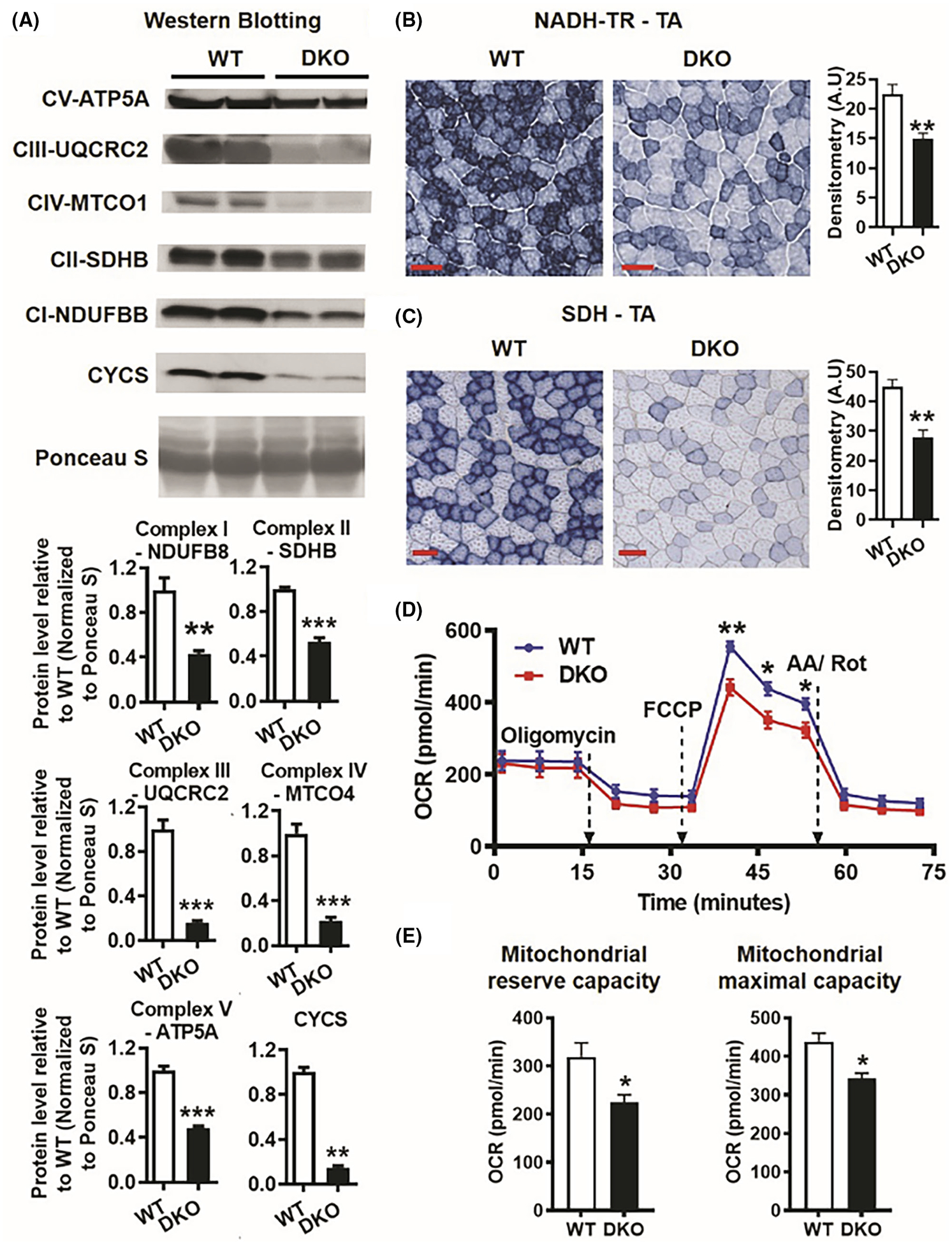
Mitochondrial dysfunction in ERRα/γ deficient muscles. (A) Western blotting and quantification of subunits of mitochondrial electron transport chain complexes I-V and CYCS in DKO versus WT gastrocnemius (N = 5). (B) NADH-TR activity staining in tibialis anterior (TA) cross-sections in DKO compared to WT mice (N = 5). Scale bar (red) = 100 μm. (C) SDH activity staining in TA cross-sections in DKO compared to WT mice (N = 5). Scale bar (red) = 100 μm. Left panel—representative images. Right panel—quantification. (D) Seahorse respirometry assay using mitochondrial stress test in FDB singe fibers in DKO versus WT mice (N = 6 mice). (E) Quantification of mitochondrial reserve capacity and maximal capacity in WT and DKO FDB myofibers by Seahorse respirometry. (N = 6). **p < .01 and ***p < .001. Unpaired Student’s t-test.
Next, we performed Seahorse analysis of mitochondrial respiration in enzymatically dissociated DKO and WT FDB myofibers. The baseline OCR was not different between DKO and WT myofibers (Figure 4D). In response to oligomycin (inhibitor of mitochondrial complex V), the OCR reduced similarly in both WT and DKO myofibers. After the addition of FCCP (mitochondrial uncoupler) the OCR increased in both WT and DKO myofibers, but the increase in WT was significantly more than DKO (Figure 4D), suggesting that the mitochondrial reserve capacity (difference between baseline and maximal OCR) is considerably higher in WT compared to DKO myofibers (Figure 4E). Subsequent addition of Antimycin A and Rotenone (inhibitors of mitochondrial complexes I and II) decreased the OCR in both WT and DKO myofibers indicating no difference in non-mitochondrial OCR between the two groups. This shows that maximal mitochondrial capacity (difference between maximal OCR and non-mitochondrial OCR) is significantly reduced in DKO versus WT myofibers (Figure 4E). Collectively, these data reveal that the DKO muscles have reduced mitochondrial ETC proteins and peak respiratory function, confirming that ERRs are critical for maintaining normal mitochondrial function in the skeletal muscle.
3.4 |. Loss of muscle ERRα/γ results in fewer and smaller mitochondria
Transmission electron micrographs of WT and DKO TA showed altered mitochondrial properties in the subsarcolemmal region (Figure 5A). In Figure 5A, the white boxed region in the top panel is magnified in the bottom panel. Indeed, quantification of the TEM images showed significantly smaller size and perimeter of individual mitochondria in the DKO versus WT TA (Figure 5B). Although the number of mitochondria per unit area was similar in DKO and WT TA, the mitochondrial density (total mitochondrial area per image) was significantly lower in DKO versus WT TA (Figure 5B). A closer look at the cristae network showed DKO mitochondria seemed to have less organized and regular cristae (Figure 5A, lower panel—solid black arrows). This was confirmed by a significantly lower number of cristae per mitochondrion in DKO versus WT TA (Figure 5B). The circularity of mitochondria was not different between WT and DKO TA (Figure 5B). Analysis of intermyofibrillar images revealed that compared to WT, DKO TA has fewer longitudinal mitochondria (Figure 5C, open white arrows) as well as triadic mitochondria (Figure 5C, solid white arrows), and more triadic mitochondrial-deficient spots (Figure 5C, open black arrows). Note that in Figure 5C, the white boxed region in the top panel is magnified in the bottom panel. Similar quantification of intermyofibrillar images showed the DKO mitochondria parameters are significantly lower than WT across all measurements, including number of mitochondria per unit area (Figure 5D). Similar to the subsarcolemmal region the circularity of intermyofibrillar mitochondria is similar for DKO and WT TA (Figure 5D).
FIGURE 5.
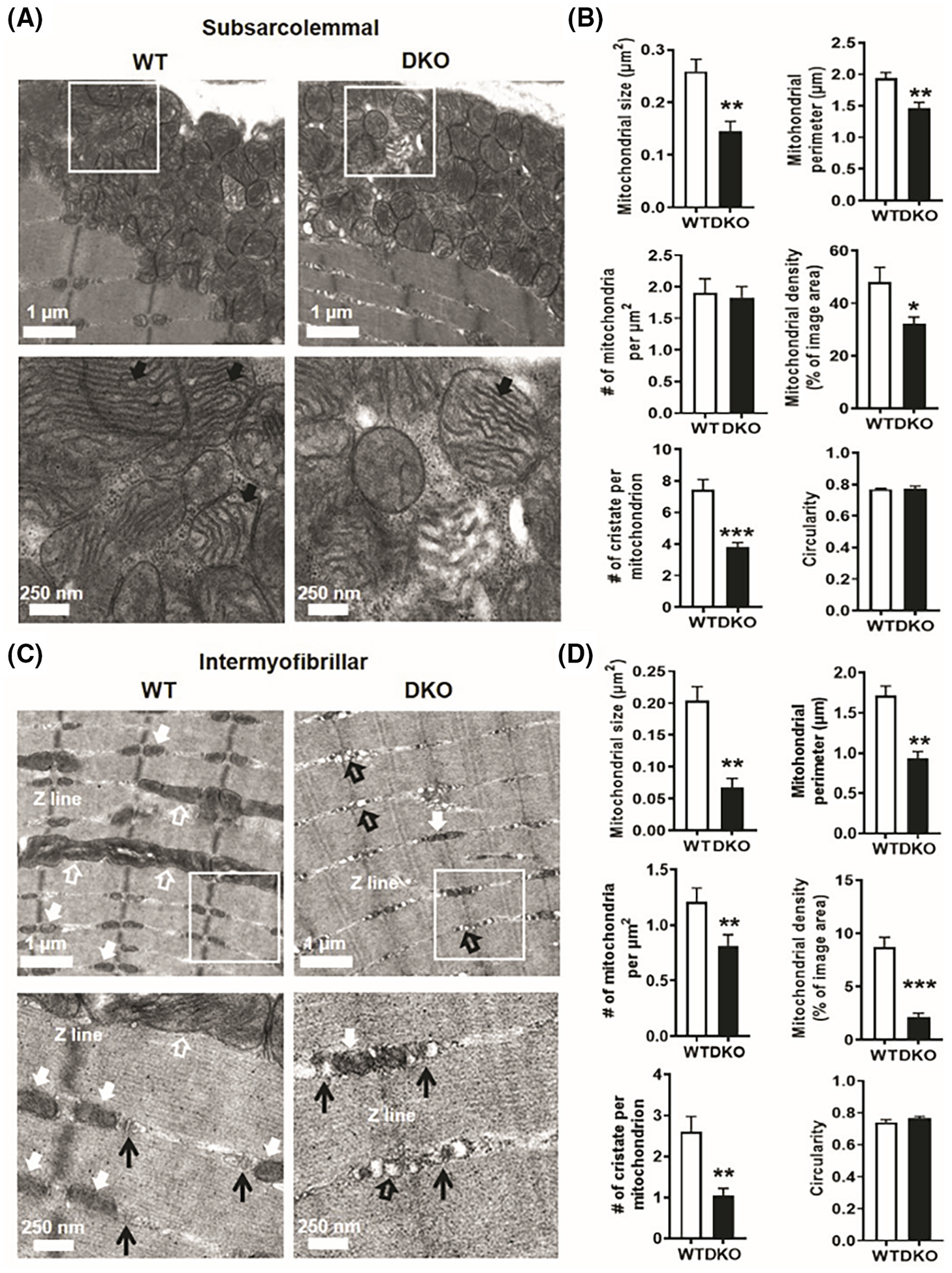
ERRα/γ deficient muscle has smaller and fewer mitochondria. (A) Representative transmission electron micrographs of TA from WT and DKO mice showing subsarcolemmal mitochondria. White box—specified area in the top panel magnified in the bottom panel. Solid black arrows in the bottom panel showing cristae within the mitochondria (N = 3). (B) Quantification of subsarcolemmal images from WT and DKO TA for mitochondrial size, perimeter, distribution, density, cristae number, and circularity (N = 3). (C) Representative transmission electron micrographs of TA from WT and DKO mice showing intermyofibrillar mitochondrial images. White box—specified area in the top panel magnified in the bottom panel. Solid white arrows—triadic mitochondria, open white arrows—longitudinal mitochondria, Open black arrow—triadic mitochondria-deficient spots, black arrow heads—T tubule and triad. (N = 3). (D) Quantification of intermyofibrillar images from WT and DKO TA for mitochondrial size, perimeter, distribution, density, cristae number, and circularity (N = 3). *p < .05, **p < .01, and ***p < .001. Unpaired Student’s t-test.
3.5 |. Myofiber type regulation
Skeletal muscle fiber type is thought to be intricately linked to oxidative capacity in the skeletal muscle. Type 1, 2A, and 2X myofibers are oxidative in nature and rely predominantly on mitochondrial metabolism. On the other hand, type 2B myofibers are glycolytic in nature and primarily depend on anaerobic glucose metabolism. MHC immunostaining revealed that DKO TA muscles have higher type 2A, lower type 2X, and comparable proportion of type 2B myofibers compared to WT TA (Figure S5A,B).
3.6 |. Loss of muscle ERRα/γ results in lower capillary supply
A complementary component of oxidative mitochondrial respiration and aerobic adaptation is tissue vascularization for adequate oxygen delivery. For example, endurance exercise increases both muscle mitochondrial biogenesis as well as vascularization, thus optimizing adaptation in both respiratory capacity and oxygen delivery. Moreover, our previous studies with ERRα and ERRγ muscle-specific ectopic overexpression mouse models showed that these receptors co-regulate muscle oxidative capacity and angiogenesis.8,10 Therefore, we analyzed the mRNA expression of known markers of angiogenesis and capillarity in WT and DKO gastrocnemius and found that pro-angiogenic genes (Vegfa, Vegfb), and Dll4 (endothelial cell marker) were significantly lower in DKO compared to WT gastrocnemius (Figure 6A). Reciprocally, the level of anti-angiogenic gene Mmp14 was markedly higher in DKO versus WT muscle (Figure 6A). We also measured VEGFA protein expression in gastrocnemius, quadriceps, and TA muscles using ELISA, and found that DKO muscle lysates had significantly lower VEGFA protein levels compared to WT (Figure 6B). In agreement with gene and protein expression of angiogenic factors, CD31 (endothelial cell marker) immunostaining of DKO and WT TA cryosections revealed a considerably lower capillary-to-myofiber ratio in DKO muscles (Figure 6C), indicative of lower vascular supply in the skeletal muscle. Because capillary density was slightly lower in MEAKO muscles, we performed a direct statistical comparison of capillary-to-myofiber ratio in DKO and MEAKO muscles, which showed that suppression of capillary density was statistically greater in DKO versus MEAKO muscles (data not shown), likely the compounding effect of the double receptor knockout.
FIGURE 6.
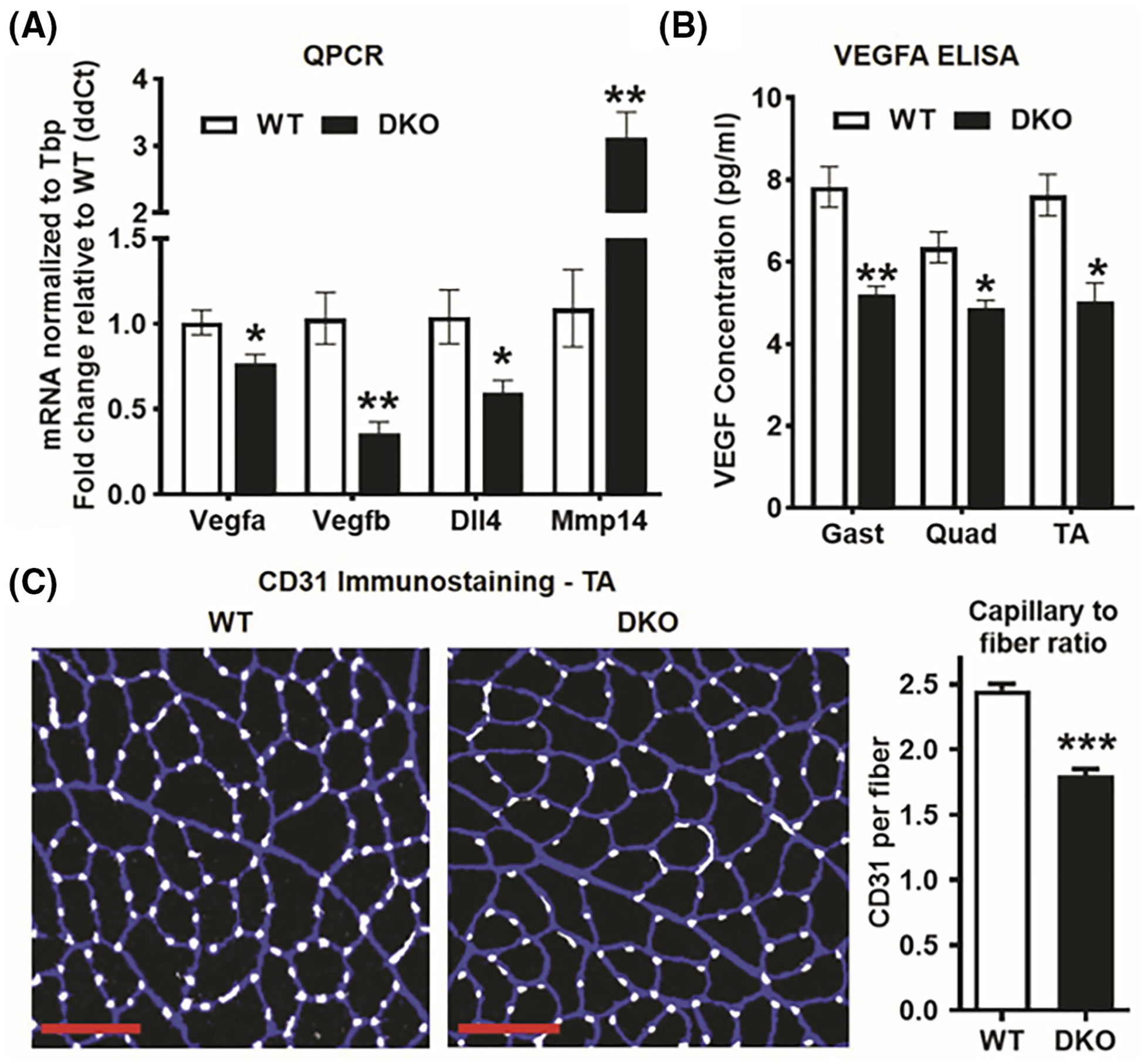
Decreased capillarity in ERRα/γ deficient muscle. (A) QPCR analysis of pro-angiogenic (Vegfa, Vegfb, DLL4) and anti-angiogenic gene (Mmp14) expression in DKO versus WT gastrocnemius (N = 5). (B) VEGFA protein levels in DKO versus WT Gastrocnemius (Gast), Quadriceps (Quad), and TA muscles measured by ELISA (N = 3–4). (C) CD31 immunostaining of TA cross-sections (Left panel) and capillary-to-myofiber ratio quantification (Right panel) in DKO compared to WT mice (N = 5). Scale bar (red) = 100 μm. *p < .05, **p < .01, and ***p < .001. Unpaired Student’s t-test.
3.7 |. Loss of muscle ERRα/γ impairs running capacity and contractile function
We next investigated whether the reduced mitochondrial function and vascular supply in muscles lacking ERRα and ERRγ results in muscle functional deficiency. First, we performed an incremental speed sprint test, where we found that the DKO mice could not keep pace beyond 10 m/min on average, thus resulting in a significantly lower running time and distance, as well as work performed in these mice (Figure 7A). Similarly, in the endurance running test at constant sub-maximal speed, DKO mice had dramatically reduced running time and distance and performed less work (Figure 7B). In addition, we used cage running wheels to measure voluntary exercise in DKO and WT mice. In Figure 7C, the representative running actograms for 2 weeks of voluntary wheel running show that the DKO mice run less than WT mice, as represented by the higher density of black bars (indicating wheel usage). The quantification of the wheel running data shows that although the WT and DKO mice have similar numbers and lengths of bouts (running wheel events), the DKO mice have significantly lower counts per bout (Figure 7D). In other words, the revolutions of running wheel during each usage (distance run) is substantially lower in DKO versus WT mice.
FIGURE 7.
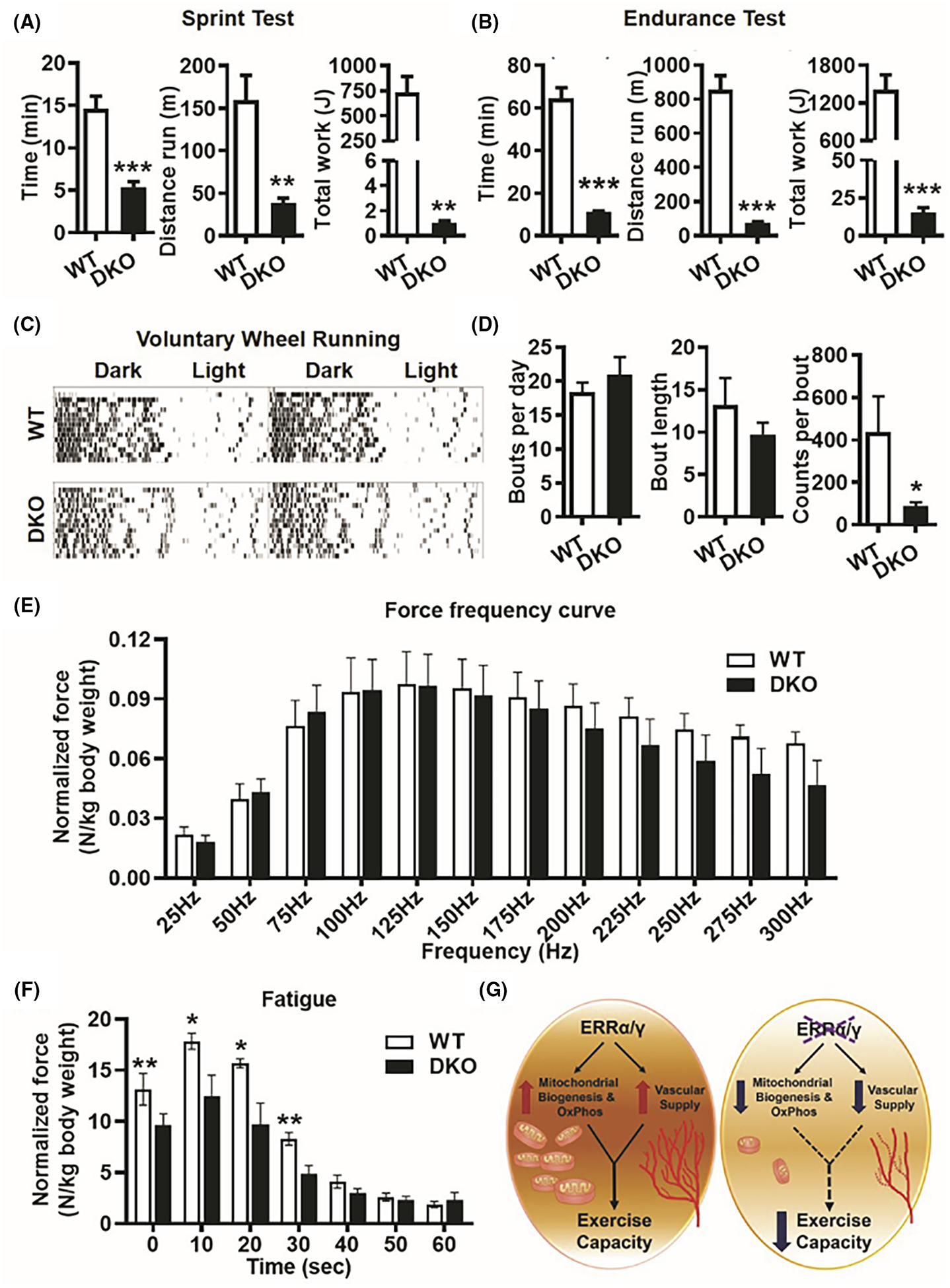
Decreased exercise tolerance and muscle fatigue resistance in ERRα/γ deficient mice. (A) Treadmill running sprint test showing running time and distance, and total work performed in DKO versus WT mice (N = 5). (B) Treadmill running endurance test showing running time and distance, and total work performed in DKO versus WT mice (N = 5). (C) Representative actograms of voluntary wheel running in WT and DKO mice over 2 weeks. (D) Quantification of voluntary wheel running data shown in (C) measuring parameters such as bouts per day, bout length and counts per minute in DKO versus WT mice (N = 5). (E) In vivo plantar flexion test showing force-frequency curve is similar between WT and DKO muscle (N = 5). (F) Plantar flexion fatigue analysis at 180 Hz frequency stimulation over 60 sec showing DKO muscle produces significantly lesser integral force per stimulus than WT (N = 5). *p < .05, **p < .01, and ***p < .001. Unpaired Student’s t-test. (G) Scheme depicting that intact endogenous expression of ERRα/γ in skeletal muscle is required for mitochondrial biogenesis, oxidative phosphorylation, and vascular supply, which determines contractile fatigue resistance and exercise capacity.
Lastly, we performed in vivo plantar flexion contraction studies in WT and DKO mice. While the force-frequency curve was similar between DKO and WT mice, the DKO mice trended toward lower peak force generation at higher frequencies (Figure 7E). Thus, we performed a fatigue test at 180 Hz of stimulation/second for 1 min and found that the DKO muscle generates significantly less integral force per stimulus than WT muscle over the course of the protocol (Figure 7F).
3.8 |. Comparison of DKO versus WT female mice show similar phenotype as male mice
To determine whether there are gender-based differences in DKO phenotype, we performed key experiments in female mice. Body weight, lean and fat mass, as well as individual muscle weights were comparable between female DKO versus WT mice (Figure S6A,B); however, the muscles were pale in DKO compared to the WT mice (Figure S6C). Expression of ERRα and ERRγ protein/gene expression in muscle was significantly impaired in DKO versus WT females (Figure S6D,E). Likewise, expression of mitochondrial (Figure S6D), metabolic (Figure S6E), and angiogenic (Figure S6E) gene and/or protein expression was also suppressed in DKO versus WT female mice. Furthermore, exercise tolerance measured using sprint and endurance testing was also compromised in DKO versus WT females (Figure S6F,G), suggesting no gender-based differences in the double receptor knockout phenotype.
Collectively, these data indicate that loss of muscle ERRα/γ results in impaired exercise capacity associated with a decrease in mitochondrial oxidative phosphorylation and vascular supply (Figure 7G) in gender independent fashion.
4 |. DISCUSSION
Decoding the transcriptional regulators linking muscle aerobic capacity to muscle contractile function and exercise fitness is critical for understanding the adaptability of skeletal muscle to training, inactivity and muscle wasting diseases. Here we show that compound deletion of skeletal muscle ERRα and ERRγ results in perturbed oxidative metabolic gene program leading to loss of mitochondria, cell autonomous decrease in myofibrillar respiration, and decreased capillary supply to the muscle. The loss of aerobic capacity results in remarkable exercise intolerance and muscle fatigue at the level of myofibrillar contraction. Therefore, ERRα and ERRγ together are indispensable for muscle aerobic capacity and exercise performance, and loss of ERR signaling node in the skeletal muscle could mediate inactivity and muscle-debilitating disorders.
Early indication of the role of ERRs in skeletal muscle metabolism emerged through in vitro studies, where ERRs (particularly ERRα) were identified as downstream mediators of metabolic gene regulatory effects of transcriptional co-activators PGC1α and β in muscle cells.13,28 ERRα is also responsible for PGC1α-mediated regulation of Vegfa angiogenic growth factor expression.29,30 Studies with muscle-specific transgenic mouse lines ectopically overexpressing ERRs8–10 further linked these orphan receptors to skeletal muscle mitochondrial metabolic and myofiber type remodeling. Despite being informative, the drawback of these models was supra-physiological overexpression of ERRs, thus limiting interpretation regarding the role of innately expressed ERRs in the skeletal muscle. Interestingly, global knockout of ERRα impaired exercise tolerance and caused metabolic gene deregulation in mice.7 Yet, the specific contribution of muscle ERRα signaling to exercise and metabolic phenotype cannot be estimated from a global knockout strategy considering the critical role of ERRα in other tissues such as adipose, liver, and heart.5,17,31–34 Germline ERRγ knockout mice die at birth due to cardiac defects,35 negating the possibility of measuring skeletal muscle performance in these mice. Muscle-specific ERRα knockout mice have been generated and examined in the context of muscle regeneration; however, the effect on muscle contractile function and exercise tolerance was not reported in this study.18 Muscle-specific ERRβ/γ knockout mice were also previously generated to investigate the regulation of microRNA27; however, the effect on exercise capacity was mild in this knockout. As such, ERRβ expression is undetectable in the skeletal muscle. Therefore, despite the generation of several mouse models, the contribution of ERRs endogenously expressed in the skeletal muscle to contractility and exercise fitness has remained unmeasured. Our study describes the role of native ERRα and ERRγ in the skeletal muscle. While the individual tissue-specific knockout of ERRα or ERRγ seems to have minimal effect on the skeletal muscle, compound deletion of these two receptors has a profound impact on muscle function and exercise capacity, indicating a mutually compensatory but highly critical role of these two ERRs in the skeletal muscle.
Global gene expression analysis by RNA-seq of muscle-specific ERRα/γ DKO mice shows that oxidative metabolic pathways are the predominant top gene-sets under the inherent control of ERRs in the skeletal muscle. GO classification identified pathways including mitochondrial organization (GO:0007005), electron transfer activity (GO: 0009055), NADH dehydrogenase complex (GO: 0010257, 0030964), cytochrome complex (GO: 0070069), tricarboxylic acid cycle (GO: 0072350), and respiratory chain (GO: 0070469) among the top 30 downregulated pathways in the ERRα/γ DKO muscles. Interestingly, several downregulated genes classified under GO terms for neurological diseases (Huntington (mmu05016), Parkinson (mmu05012), Alzheimer (mmu05010)) are linked to mitochondrial quality control, indicating that in addition to the direct regulation of oxidative metabolism and mitochondrial biogenesis, ERRs may also be involved in mitochondrial quality control. This hypothesis is to an extent supported by a previous study in hepatocytes that showed that ERRα is involved in the regulation of both mitochondrial biogenesis and fission/mitophagy.36 Genes involved in fatty acid metabolism (GO: 0006631) and amino acid biosynthesis (mmu01230) were also downregulated by ERR loss, and these pathways are likely to contribute to muscle homeostasis and fitness. Surprisingly, despite previous reports that paracrine angiogenic genes are one of the significant gene sets activated by ERRα/γ overexpression in the skeletal muscle,10,19 only selective angiogenic genes were downregulated in DKO muscle. This suggests that ERRs may not be required for the expression of most but a few critical pro-angiogenic genes (e.g., Vegfa and Vegfb) under basal conditions. Interestingly, expression of anti-angiogenic genes (e.g., Mmp14) was induced in DKO muscles, suggesting repression of negative angiogenic factors as an alternative potential mechanism through which ERRs may maintain vascular supply in the muscle tissue.
Skeletal muscle phenotype closely mimicked changes in gene expression. Protein expression of key components in complex I-V was downregulated in muscle lacking ERRα/γ. Similarly, complex I and II enzyme activities were also decreased in ERRα/γ null muscles. Electron microscopy revealed that loss of ERRs leads to a decrease in the number and size of mitochondria, further supporting the protein and biochemical data. ERRα/γ DKO affects intermyofibrillar mitochondria more than the subsarcolemmal mitochondria. The intermyofibrillar mitochondria express higher levels of oxidative phosphorylation enzymes. They have higher respiratory chain complex activity than subsarcolemmal mitochondria, indicating that the mitochondria in this location are conducive to energy production under increased energy demand during exercise.37,38 Furthermore, while both triadic and longitudinal mitochondria within the intermyofibrillar region are lower in DKO, there seems to be an absence of stretches of longitudinal mitochondria similar to those seen in the WT muscle. The interconnected networks of the longitudinal mitochondria are more efficient and have higher oxidative capacity.39 This spatial disparity in mitochondrial arrangement between subsarcolemmal and intermyofibrillar regions, and between triadic and longitudinal mitochondria within the intermyofibrillar region observed in the DKO muscle could contribute to metabolic impairment in addition to a general decrease in mitochondrial number/size in DKO muscle.
Impaired respiration in isolated myofibers from the DKO muscle underscores the cell autonomous critical role of ERRα/γ in regulating myocellular respiration. The loss of mitochondria and respiratory capacity in the skeletal muscle did not lead to myopathy or decreased muscle mass. Peculiarly, there was a shift in myofiber cross-section area-to-number proportion, with the ERRα/γ null muscle having significantly larger myofibers in the range of 750–1000 μm2. However, the average cross-sectional area remained the same between the WT and the mutant mice. Nevertheless, the long-term impact of chronic loss in mitochondrial capacity on muscle mass in DKO versus WT mice remains to be measured, as young mice in the age range of 4–5 months were used in our studies. This will be interesting to study in the future, as mitochondrial health has been increasingly linked to maintaining muscle mass. For example, alterations in mitochondrial DNA are associated with myofiber atrophy and decreased muscle regenerative capacity in sarcopenia.40,41 Furthermore, muscle wasting in several types of muscular dystrophies is related to loss of mitochondrial and oxidative gene expression and mitochondrial dysfunction.42–44
Previous transgenic overexpression studies showed that both ERRs positively regulated the formation of type IIA and IIX (oxidative) myofibers in the skeletal muscle, and suppress the formation of type IIB (glycolytic) myofibers.10,19 In contrast, deletion of ERRs in the DKO muscles results in an increased type IIA and decreased type IIX myofibers without affecting type IIB myofibers. The discrepancy in the regulation of fiber type under gain-of-function versus loss-of-function scenarios by ERRs is interesting. It seems unlikely that type IIB myofibers are under the predominant control of endogenous ERRs. An increase in type IIA myofibers in response to the loss of ERRs may be a compensatory effect through a different transcriptional pathway. For example, we found aerobic master-regulator PGC1α is induced in the DKO muscles (in RNA-seq data), which could be responsible for type IIA myofiber upregulation. PGC1α is considered as a key co-activator of ERRα,28 and it is likely induced as a compensatory response to loss of ERRs. PGC1α could also regulate fiber type through its interaction with PPARδ,45,46 and it can be speculated that increased type IIA myofibers in DKO muscles may involve the PGC1α-PPARδ axis. On the other hand, type IIX myofibers seem to be under a more direct control of ERRs in the skeletal muscle, as they were the only myofiber type downregulated in the DKO muscles. Indeed, type IIX myofibers, although fast-twitch, are known to be highly oxidative in nature,47 loss of which is likely to contribute to the observed phenotype. Similar uncoupling between mitochondrial/oxidative metabolic and myofiber type regulation in the DKO mice was observed in another study involving PGC1α/β double knockout mice,48 where the knockout effect was more prominent on mitochondrial function, as opposed to myofiber type remodeling. Therefore, it is possible that the mitochondrial and metabolic function may be more amenable to molecular regulation than the myofiber type.
Vasculature is in part responsible for the effects of ERR loss on muscle function. In variation from the overexpression studies, loss of ERRs results in decreased expression of far fewer angiogenic genes. Nevertheless, DKO muscles have decreased capillarity, associated with suppressed expression of key angiogenic factors such as Vegfa and Vegfb. Notably, regulation of skeletal muscle vascularization is much poorly elucidated compared to metabolism and myofiber type.49 Despite a much-pronounced effect on mitochondria compared to vasculature, ERRs are highlighted here as key paracrine regulators of muscle angiogenesis. Interestingly, ERRs have been shown to interact with the hypoxia-inducible factor 1 (HIF1) pathway.10,50 Moreover, previous studies have shown that both ERRα and ERRγ overexpression promotes muscle revascularization in models of peripheral arterial (ischemic) disease.10,19 It will be interesting to see in subsequent studies whether loss of ERRs in DKO mice impairs HIF signaling and ischemic revascularization in the skeletal muscle, and therefore whether ERRs are critical for post-hypoxic tissue revascularization.
Exclusive loss of mitochondrial oxidative capacity and vasculature was sufficient to cause muscle contractile fatigue and exercise intolerance. Notably, exercise intolerance was evident in both voluntary and forced exercise, highlighting the critical role of aerobic capacity in determining exercise tolerance, possibly over myofiber type composition. It remains to be seen whether the loss of aerobic capacity in the DKO muscles lacking ERRs can be surmounted by long-term exercise training, or whether the adaptation of the skeletal muscles to exercise training is lost in mice lacking muscle ERRs. Interestingly, both ERRα and ERRγ protein expression were induced by 4 weeks of exercise training in the muscle (Figure S7), raising the possibility that these orphan receptors are involved in skeletal muscle adaptation to exercise.
Our reporting of the significant impact of double ERRα/γ loss on muscle mitochondrial capacity, vasculature, and exercise capacity raises several interesting questions. What is the relative contribution of ERRα and ERRγ transcriptional programs to the muscle phenotype in DKO mice? Previous studies have shown that both ERRα and ERRγ have overlapping transcriptomes.5 Furthermore, both ERRs seem to compensate for each other in context of loss-of-function studies for each receptor (Figure S1). Along these lines, ERRs bind to the same hormone response elements,17 and are known to function as monomers as well as homodimers or heterodimers.5,51–53 The nature of ERR complexes (monomer vs. homodimer vs. heterodimer) expressed in the skeletal muscle remains to be deciphered and could be critical for understanding the relative contribution of each ERR to muscle function. Comparative ChIP sequencing analysis for ERRα and ERRγ will also reveal both overlapping and exclusive muscle genes directly targeted by these two receptors. As much as ERRs are known to be regulated under various conditions in the skeletal muscle, transcriptional as well as post-translational modulation of ERRs remains poorly defined in the muscle. Our findings highlighting the impact of ERRs on muscle function should propel new studies on how ERRs are regulated. In this context, studies in other cellular systems already suggest regulation of ERRs through phosphorylation, acetylation, o-glycosylation, and ubiquitination.34,54–57 In turn, ERRs are likely downstream transcriptional mediators of AMPK and PGC1α,11,14,28,58,59 two master regulators of muscle homeostasis. The ERRα and ERRγ muscle-specific DKO mice reported here would be a valuable tool to examine the role of ERRs downstream of the two master-regulators in a physiological setting. Similarly, the role of double ERRα and ERRγ knockout and mitochondrial loss on skeletal muscle regeneration would be another potential area to explore. This is particularly important as several recent studies have reported downregulation of ERRs, mitochondrial markers, and oxidative metabolic genes in the skeletal muscles of dystrophic and aging mice.41,60,61 The impact of muscle ERRα/γ and mitochondrial loss on the development of diet-induced obesity and insulin resistance also remains to be determined.
Overall, our study underscores the critical role of ERRα/γ in maintaining muscle mitochondrial metabolic capacity, capillary supply, muscle contractility, and exercise tolerance, and simultaneously reports on a transgenic mouse model that will be critical for studying the long-term impact of metabolic and ERR deregulation on muscle health.
Supplementary Material
ACKNOWLEDGMENTS
The ERRα floxed mice were kindly provided by Janice Huss (City of Hope). ERRγ floxed mice were obtained from ICS (Strasburg, France). Both ERRα and ERRγ floxed mice were originally generated at ICS (Strasburg, France), and were used in this study under the auspices of material transfer agreement between ICS and UTHealth. We would like to thank Dr. Zhengmei Mao in the UTHealth Institute of Molecular Medicine microscopy core facility for assistance with microscopy, imaging, and analysis. We would like to thank Mr. James P. Barrish at Department of Pathology, Texas Children’s Hospital, Houston, TX for performing transmission electron microscopy of skeletal muscle samples.
FUNDING INFORMATION
This research was supported in parts by NIH/NHLBI grants (R01HL152108, R01HL129191), American Heart Association transformational research award (20TPA35410038), and Hamman Foundation Endowment in Cardiovascular Research to V.A.N, as well as NIH/NIAMS grant (RO1AR059810) to Ashok Kumar. D.H.S. was supported by a post-doctoral fellowship from American Heart Association (19POST34380771). Z.Z. was partially supported by the Cancer Prevention and Research Institute of Texas grants (CPRIT RP180734 and RP210045). The funders had no role in the study design, data collection, and analysis, decision to publish or preparation of the manuscript.
Abbreviations:
- ERRs
estrogen-related receptors
- MEAKO
muscle-specific ERRα knockout mice
- MEGKO
muscle-specific ERRγ knockout mice
- DKO
muscle-specific ERRα/γ knockout mice
- WT
wild type mice
- QPCR
quantitative polymerase chain reaction
- MHC
myosin heavy chain
- NADH-TR
nicotinamide adenine dinucleotide-tetrazolium reductase
- SDH
succinate dehydrogenase
- FDB
flexor digitorum brevis
- OCR
oxygen consumption rate
- TEM
transmission electron microscopy
Footnotes
DISCLOSURES
The authors declare that they have no conflict of interest.
SUPPORTING INFORMATION
Additional supporting information can be found online in the Supporting Information section at the end of this article.
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available on request from the corresponding author.
REFERENCES
- 1.Maessen MF, Verbeek AL, Bakker EA, Thompson PD, Hopman MT, Eijsvogels TM. Lifelong exercise patterns and cardiovascular health. Mayo Clin Proc. 2016;91:745–754. [DOI] [PubMed] [Google Scholar]
- 2.Pinckard K, Baskin KK, Stanford KI. Effects of exercise to improve cardiovascular health. Front Cardiovasc Med. 2019;6:69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hyatt HW, Powers SK. Mitochondrial dysfunction is a common denominator linking skeletal muscle wasting due to disease, aging, and prolonged inactivity. Antioxidants (Basel). 2021;10:588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Audet-Walsh E, Giguere V. The multiple universes of estrogen-related receptor alpha and gamma in metabolic control and related diseases. Acta Pharmacol Sin. 2015;36:51–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dufour CR, Wilson BJ, Huss JM, et al. Genome-wide orchestration of cardiac functions by the orphan nuclear receptors ERRalpha and gamma. Cell Metab. 2007;5:345–356. [DOI] [PubMed] [Google Scholar]
- 6.Giguere V Transcriptional control of energy homeostasis by the estrogen-related receptors. Endocr Rev. 2008;29:677–696. [DOI] [PubMed] [Google Scholar]
- 7.Perry MC, Dufour CR, Tam IS, B’Chir W, Giguere V. Estrogen-related receptor-alpha coordinates transcriptional programs essential for exercise tolerance and muscle fitness. Mol Endocrinol. 2014;28:2060–2071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Narkar VA, Fan W, Downes M, et al. Exercise and PGC-1alpha-independent synchronization of type I muscle metabolism and vasculature by ERRgamma. Cell Metab. 2011;13:283–293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rangwala SM, Wang X, Calvo JA, et al. Estrogen-related receptor gamma is a key regulator of muscle mitochondrial activity and oxidative capacity. J Biol Chem. 2010;285:22619–22629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sopariwala DH, Likhite N, Pei G, et al. Estrogen-related receptor alpha is involved in angiogenesis and skeletal muscle revascularization in hindlimb ischemia. FASEB J. 2021;35:e21480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sopariwala DH, Rios AS, Park MK, Song MS, Kumar A, Narkar VA. Estrogen-related receptor alpha is an AMPK-regulated factor that promotes ischemic muscle revascularization and recovery in diet-induced obese mice. FASEB BioAdv. 2022;4:602–618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cho Y, Hazen BC, Russell AP, Kralli A. Peroxisome proliferator-activated receptor gamma coactivator 1 (PGC-1)- and estrogen-related receptor (ERR)-induced regulator in muscle 1 (Perm1) is a tissue-specific regulator of oxidative capacity in skeletal muscle cells. J Biol Chem. 2013;288:25207–25218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Huss JM, Torra IP, Staels B, Giguere V, Kelly DP. Estrogen-related receptor alpha directs peroxisome proliferator-activated receptor alpha signaling in the transcriptional control of energy metabolism in cardiac and skeletal muscle. Mol Cell Biol. 2004;24:9079–9091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wende AR, Huss JM, Schaeffer PJ, Giguere V, Kelly DP. PGC-1alpha coactivates PDK4 gene expression via the orphan nuclear receptor ERRalpha: a mechanism for transcriptional control of muscle glucose metabolism. Mol Cell Biol. 2005;25:10684–10694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sladek R, Bader JA, Giguere V. The orphan nuclear receptor estrogen-related receptor alpha is a transcriptional regulator of the human medium-chain acyl coenzyme A dehydrogenase gene. Mol Cell Biol. 1997;17:5400–5409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Murray J, Auwerx J, Huss JM. Impaired myogenesis in estrogen-related receptor gamma (ERRgamma)-deficient skeletal myocytes due to oxidative stress. FASEB J. 2013;27:135–150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wang T, McDonald C, Petrenko NB, et al. Estrogen-related receptor alpha (ERRalpha) and ERRgamma are essential co-ordinators of cardiac metabolism and function. Mol Cell Biol. 2015;35:1281–1298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.LaBarge S, McDonald M, Smith-Powell L, Auwerx J, Huss JM. Estrogen-related receptor-alpha (ERRalpha) deficiency in skeletal muscle impairs regeneration in response to injury. FASEB J. 2014;28:1082–1097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Matsakas A, Yadav V, Lorca S, Evans RM, Narkar VA. Revascularization of ischemic skeletal muscle by estrogen-related receptor-gamma. Circ Res. 2012;110:1087–1096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Martin M Cutadapt removes adapter sequences from high-throughput sequencing reads. EMBnet Journal. 2011;2011(17): 10–12. [Google Scholar]
- 21.Dobin A, Davis CA, Schlesinger F, et al. STAR: ultrafast universal RNA-seq aligner. Bioinformatics. 2013;29:15–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Anders S, Huber W. Differential expression analysis for sequence count data. Genome Biol. 2010;11:R106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Benjamini Y, Hochberg Y. Controlling the false discovery rate: a practical and powerful approach to multiple testing. J R Stat Soc B Methodol. 1995;57:289–300. [Google Scholar]
- 24.Lam J, Katti P, Biete M, et al. A universal approach to analyzing transmission electron microscopy with ImageJ. Cell. 2021;10:2177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sopariwala DH, Pant M, Shaikh SA, et al. Sarcolipin overexpression improves muscle energetics and reduces fatigue. J Appl Physiol. 2015;118:1050–1058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gallot YS, Bohnert KR, Straughn AR, Xiong G, Hindi SM, Kumar A. PERK regulates skeletal muscle mass and contractile function in adult mice. FASEB J. 2019;33:1946–1962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gan Z, Rumsey J, Hazen BC, et al. Nuclear receptor/microRNA circuitry links muscle fiber type to energy metabolism. J Clin Invest. 2013;123:2564–2575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mootha VK, Handschin C, Arlow D, et al. Erralpha and Gabpa/b specify PGC-1alpha-dependent oxidative phosphorylation gene expression that is altered in diabetic muscle. Proc Natl Acad Sci U S A. 2004;101:6570–6575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Arany Z, Foo SY, Ma Y, et al. HIF-independent regulation of VEGF and angiogenesis by the transcriptional coactivator PGC-1alpha. Nature. 2008;451:1008–1012. [DOI] [PubMed] [Google Scholar]
- 30.Chinsomboon J, Ruas J, Gupta RK, et al. The transcriptional co-activator PGC-1alpha mediates exercise-induced angiogenesis in skeletal muscle. Proc Natl Acad Sci U S A. 2009;106:21401–21406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.B’Chir W, Dufour CR, Ouellet C, et al. Divergent role of estrogen-related receptor alpha in lipid-and fasting-induced hepatic steatosis in mice. Endocrinology. 2018;159:2153–2164. [DOI] [PubMed] [Google Scholar]
- 32.Brown EL, Hazen BC, Eury E, et al. Estrogen-related receptors mediate the adaptive response of Brown adipose tissue to adrenergic stimulation. iScience. 2018;2:221–237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sakamoto T, Matsuura TR, Wan S, et al. A critical role for estrogen-related receptor signaling in cardiac maturation. Circ Res. 2020;126:1685–1702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Xia H, Scholtes C, Dufour CR, Ouellet C, Ghahremani M, Giguere V. Insulin action and resistance are dependent on a GSK3beta-FBXW7-ERRalpha transcriptional axis. Nat Commun. 2022;13:2105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Alaynick WA, Kondo RP, Xie W, et al. ERRgamma directs and maintains the transition to oxidative metabolism in the postnatal heart. Cell Metab. 2007;6:13–24. [DOI] [PubMed] [Google Scholar]
- 36.Singh BK, Sinha RA, Tripathi M, et al. Thyroid hormone receptor and ERRalpha coordinately regulate mitochondrial fission, mitophagy, biogenesis, and function. Sci Signal. 2018;11:eaam5855. [DOI] [PubMed] [Google Scholar]
- 37.Ferreira R, Vitorino R, Alves RM, et al. Subsarcolemmal and intermyofibrillar mitochondria proteome differences disclose functional specializations in skeletal muscle. Proteomics. 2010;10:3142–3154. [DOI] [PubMed] [Google Scholar]
- 38.Kras KA, Langlais PR, Hoffman N, et al. Obesity modifies the stoichiometry of mitochondrial proteins in a way that is distinct to the subcellular localization of the mitochondria in skeletal muscle. Metabolism. 2018;89:18–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Westermann B Bioenergetic role of mitochondrial fusion and fission. Biochim Biophys Acta. 2012;1817:1833–1838. [DOI] [PubMed] [Google Scholar]
- 40.Kimoloi S, Sen A, Guenther S, et al. Combined fibre atrophy and decreased muscle regeneration capacity driven by mitochondrial DNA alterations underlie the development of sarcopenia. J Cachexia Sarcopenia Muscle. 2022;13:2132–2145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kan J, Hu Y, Ge Y, et al. Declined expressions of vast mitochondria-related genes represented by CYCS and transcription factor ESRRA in skeletal muscle aging. Bioengineered. 2021;12:3485–3502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Aksu-Menges E, Eylem CC, Nemutlu E, et al. Reduced mitochondrial fission and impaired energy metabolism in human primary skeletal muscle cells of megaconial congenital muscular dystrophy. Sci Rep. 2021;11:18161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Liu F, Lou J, Zhao D, et al. Dysferlinopathy: mitochondrial abnormalities in human skeletal muscle. Int J Neurosci. 2016;126:499–509. [DOI] [PubMed] [Google Scholar]
- 44.Moore TM, Lin AJ, Strumwasser AR, et al. Mitochondrial dysfunction is an early consequence of partial or complete dystrophin loss in mdx mice. Front Physiol. 2020;11:690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Koh JH, Hancock CR, Terada S, Higashida K, Holloszy JO, Han DH. PPARbeta is essential for maintaining Normal levels of PGC-1alpha and mitochondria and for the increase in muscle mitochondria induced by exercise. Cell Metab. 2017;25:1176–1185.e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Schuler M, Ali F, Chambon C, et al. PGC1alpha expression is controlled in skeletal muscles by PPARbeta, whose ablation results in fiber-type switching, obesity, and type 2 diabetes. Cell Metab. 2006;4:407–414. [DOI] [PubMed] [Google Scholar]
- 47.Arany Z, Lebrasseur N, Morris C, et al. The transcriptional co-activator PGC-1beta drives the formation of oxidative type IIX fibers in skeletal muscle. Cell Metab. 2007;5:35–46. [DOI] [PubMed] [Google Scholar]
- 48.Zechner C, Lai L, Zechner JF, et al. Total skeletal muscle PGC-1 deficiency uncouples mitochondrial derangements from fiber type determination and insulin sensitivity. Cell Metab. 2010;12:633–642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Gorski T, De Bock K. Metabolic regulation of exercise-induced angiogenesis. Vasc Biol. 2019;1:H1–H8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Li Y, Padmanabha D, Gentile LB, Dumur CI, Beckstead RB, Baker KD. HIF-and non-HIF-regulated hypoxic responses require the estrogen-related receptor in Drosophila melanogaster. PLoS Genet. 2013;9:e1003230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Barry JB, Laganiere J, Giguere V. A single nucleotide in an estrogen-related receptor alpha site can dictate mode of binding and peroxisome proliferator-activated receptor gamma co-activator 1alpha activation of target promoters. Mol Endocrinol. 2006;20:302–310. [DOI] [PubMed] [Google Scholar]
- 52.Gearhart MD, Holmbeck SM, Evans RM, Dyson HJ, Wright PE. Monomeric complex of human orphan estrogen related receptor-2 with DNA: a pseudo-dimer interface mediates extended half-site recognition. J Mol Biol. 2003;327:819–832. [DOI] [PubMed] [Google Scholar]
- 53.Huppunen J, Aarnisalo P. Dimerization modulates the activity of the orphan nuclear receptor ERRgamma. Biochem Biophys Res Commun. 2004;314:964–970. [DOI] [PubMed] [Google Scholar]
- 54.Misra J, Kim DK, Jung YS, et al. O-GlcNAcylation of orphan nuclear receptor estrogen-related receptor gamma promotes hepatic gluconeogenesis. Diabetes. 2016;65:2835–2848. [DOI] [PubMed] [Google Scholar]
- 55.Tremblay AM, Wilson BJ, Yang XJ, Giguere V. Phosphorylation-dependent sumoylation regulates estrogen-related receptor-alpha and -gamma transcriptional activity through a synergy control motif. Mol Endocrinol. 2008;22:570–584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Wilson BJ, Tremblay AM, Deblois G, Sylvain-Drolet G, Giguere V. An acetylation switch modulates the transcriptional activity of estrogen-related receptor alpha. Mol Endocrinol. 2010;24:1349–1358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Yang Y, Li S, Li B, et al. FBXL10 promotes ERRalpha protein stability and proliferation of breast cancer cells by enhancing the mono-ubiquitylation of ERRalpha. Cancer Lett. 2021;502:108–119. [DOI] [PubMed] [Google Scholar]
- 58.Araki M, Motojima K. Identification of ERRalpha as a specific partner of PGC-1alpha for the activation of PDK4 gene expression in muscle. FEBS J. 2006;273:1669–1680. [DOI] [PubMed] [Google Scholar]
- 59.Salatino S, Kupr B, Baresic M, Omidi S, van Nimwegen E, Handschin C. The genomic context and corecruitment of SP1 affect ERRalpha coactivation by PGC-1alpha in muscle cells. Mol Endocrinol. 2016;30:809–825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Banerji CRS, Panamarova M, Pruller J, et al. Dynamic transcriptomic analysis reveals suppression of PGC1alpha/ERRalpha drives perturbed myogenesis in facioscapulohumeral muscular dystrophy. Hum Mol Genet. 2019;28:1244–1259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Hardee JP, Martins KJB, Miotto PM, et al. Metabolic remodeling of dystrophic skeletal muscle reveals biological roles for dystrophin and utrophin in adaptation and plasticity. Mol Metab. 2021;45:101157. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data that support the findings of this study are available on request from the corresponding author.


