Abstract
Powdery mildew is an apple disease caused by the obligate trophic fungus Podosphaera leucotricha. Basic helix–loop–helix (bHLH) transcription factors play important roles in plant development and stress responses, and they have been widely studied in model plants such as Arabidopsis thaliana. However, their role in the stress response of perennial fruit trees remains unclear. Here, we investigated the role of MdbHLH093 in the powdery mildew of apples. The expression of MdbHLH093 was significantly induced during the infection of apples with powdery mildew, and the allogenic overexpression of MdbHLH093 in A. thaliana enhanced the resistance to powdery mildew by increasing the accumulation of hydrogen peroxide (H2O2) and activating the salicylic acid (SA) signaling pathway. The transient overexpression of MdbHLH093 in apple leaves increased the resistance to powdery mildew. Conversely, when MdbHLH093 expression was silenced, the sensitivity of apple leaves to powdery mildew was increased. The physical interaction between MdbHLH093 and MdMYB116 was demonstrated by yeast two-hybrid, bi-molecular fluorescence complementation, and split luciferase experiments. Collectively, these results indicate that MdbHLH093 interacts with MdMYB116 to improve apple resistance to powdery mildew by increasing the accumulation of H2O2 and activating the SA signaling pathway, as well as by providing a new candidate gene for resistance molecular breeding.
Keywords: apple, MdbHLH093, powdery mildew, disease resistance, salicylic acid signal, hydrogen peroxide
1. Introduction
During growth, development, and reproduction, plants inevitably suffer from various biotic and abiotic stresses [1]. Powdery mildew is an apple disease caused by the obligate trophic fungus Podosphaera leucotricha [2], which is widespread in all apple-producing areas of the world and causes immeasurable losses to farmers [3]. The fungus damages the different tissues and organs of the apple tree, including new shoots, leaves, flowers, and young fruits. The symptoms of powdery mildew first appear on leaves, and the disease is characterized by leaf curling, white powder production and deposition, dry leaf tips, and leaf shedding, which greatly affect the tree’s potential and fruit quality [4]. In current agricultural practices, powdery mildew in apples is mainly controlled through the use of bacteriostatic agents. Although the loss of apple yield can be controlled to a certain extent, long-term use is cost-prohibitive, adversely affects the environment [5], and fails to fundamentally address the cause of the disease. Therefore, it is important to identify and characterize the disease-resistance genes and to reveal their molecular mechanisms of action, which will help to improve the disease resistance of apples.
Plant life activities depend on the regulation of various transcription factors, among which the bHLH family of transcription factors is the second largest in plants [6]. These transcription factors are named for their 50 to 60-amino-acid bHLH domains, which consist of two highly conserved but distinct regions: namely, the N-terminal basic amino acid region and the C-terminal helix–loop–helix (HLH) [7]. Previous studies have reported that bHLH-family transcription factors play important roles in plant growth and development, as well as in resisting biotic and abiotic stresses. Numerous studies have demonstrated that the members of this transcription factor family are involved in the development of tissues and organs such as the carpel, anther, epidermal cells [8], stomata [9], and root [10]. Additionally, the bHLH-family transcription factors enhance plant resistance to abiotic stresses, and they can also promote tolerance to drought, high salt stress, low temperature, iron deficiency, heavy metal stress, and osmotic conditions [11,12]. Members of this transcription factor family are also important in the plant responses to various biotic stresses. For example, in Arabidopsis thaliana, the bHLH-family transcription factor HBI1 is a negative regulator of the defense response, and the loss of HBI1 increases resistance to bacterial infection, whereas overexpression of HBI1 reduces the pathogen-induced immune response [13]. The transient overexpression of StCHL1 significantly increases the ability of Phytophthora to infect tobacco leaves, which is consistent with the finding that its congeners HBI1 and CIB1 are negative regulators of the immune response [14]. The overexpression of TabHLH060 in A. thaliana significantly increases sensitivity to Pseudomonas clove DC3000 and decreases the transcriptional levels of the resistant genes PR1, PR2, and PR5 that are involved in the salicylate (SA) signaling pathway; whereas, the transcriptional levels of the PDF1.2 and ORA59 involved in jasmonic acid and ethylene signaling pathways are upregulated, indicating TabHLH060 negatively regulates plant disease resistance through jasmonic acid and ethylene signaling pathways [15]. In soybean, GmPIB1 inhibits the expression of SPOD1, a key enzyme in the production of reactive oxygen species—which can reduce their production and improve soybean resistance to phytophthora disease [16].
However, the regulatory mechanisms of the bHLH family of transcription factors that are involved in powdery mildew are poorly understood. A study performed in wheat showed that the expression levels of 28 TabHLH genes and 6 TabHLH genes were significantly downregulated and upregulated in powdery mildew, respectively [17]. The heterologous expression of the pumpkin bHLH-family transcription factor CmbHLH87 in tobacco plants alleviates powdery mildew symptoms in leaves, accelerates cell necrosis, and enhances H2O2 accumulation. The expression levels of PR1a, PR5, and NPR1 in transgenic plants were also significantly higher than those in control plants [18]. A differentially expressed gene was screened from the transcriptomic data of apple inoculated with P. leucotricha, and according to the study of Yang et al., its homologous gene in A. thaliana was AtbHLH093 [19], so the gene was named MdbHLH093. When apple leaves were infected with P. leucotricha, the expression level of MdbHLH093 was significantly increased [20]. We hypothesized that MdbHLH093 may have a certain regulatory effect on powdery mildew infection in apples. The Arabidopsis bHLH-family transcription factor AtbHLH93 was observed to promote flowering in plants under short-day conditions by regulating the expression of genes associated with erythrominin biosynthesis and metabolism [21]. The knockdown of bHLH93 in tobacco significantly weakens the allergic response induced by the bacterial wilt effector protein RipI and reduces the expression of the defense gene PDF1.2 [22]. For the transcription factor MdbHLH093, a previous study demonstrated that it directly activates the transcription of MdSAG18, promotes the expression of age-related genes, and regulates the plant-aging process [23]. To clarify the relationship between MdbHLH093 expression and powdery mildew in apples, we performed functional analyses through the allogenic transformation of A. thaliana and the transient transformation of apple leaves. In subsequent experiments, we observed that MdbHLH093 physically interacts with MdMYB116 and plays an active role in the SA-mediated powdery mildew defense. The aim of this study is to identify the candidate genes for breeding apple varieties resistant to powdery mildew and to provide a foundation for studying the molecular mechanism of apple resistance to powdery mildew.
2. Results
2.1. Sequence Analysis and Evolutionary Tree Construction of MdbHLH093
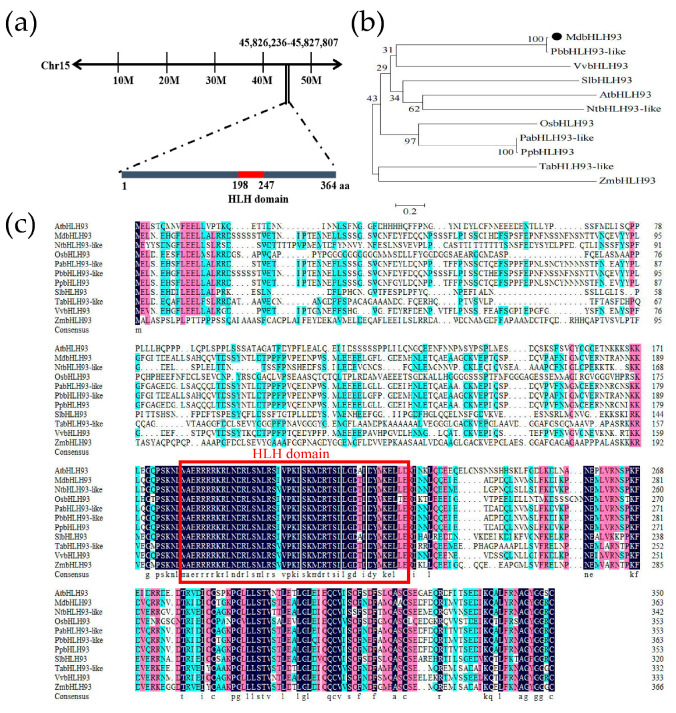
In the apple genome, MdbHLH093 is located on chromosome 15 (https://www.rosaceae.org, accessed on 5 March 2022). The coding sequence (CDS) of MdbHLH093 is 1095 bp in length, which encodes a 364-amino acid (aa) protein. The MdbHLH093 protein contains a highly conserved HLH domain (198–247 aa) (Figure 1a). The phylogenetic tree of MdbHLH093 and its homologous proteins in 10 species—namely, A. thaliana, rice, wheat, corn, tobacco, tomato, grape, pear, cherry, and peach—was constructed. The MdbHLH093 in apple was closely related to the homologous genes in pear (Figure 1b). The amino acid sequences of these genes were compared using DNAMAN 6.0 software, all genes were contained in an HLH domain and were highly conserved (Figure 1c).
Figure 1.
Sequence analysis of the MdbHLH093 isolated from the apple cultivar Gala. (a) Chromosomal location of MdbHLH093. (b) Phylogenetic analysis of MdbHLH093 and its homologs in 10 different species. The conserved HLH domain is outlined in red. The accession numbers of the bHLHs are as follows: Arabidopsis thaliana, AtbHLH93 (NP_569014.1); Nicotiana tabacum, NtbHLH93-like (XP_016490472.1); Oryza sativa, OsbHLH93 (XP_015629094.1); Prunus avium, PabHLH93-like (XP_021832105.1); Pyrus bretschneideri, PbbHLH93-like (XP_009349682.1); Prunus persica, PpbHLH93 (XP_007226892.2); Solanum lycopersicum, SlbHLH93 (XP_004233470.1); Triticum aestivum, TabHLH93-like (XP_044425383.1); Vitis vinifera, VvbHLH93 (XP_002285595.1); and Zea mays, ZmbHLH93 (AQK67253.1). (c) Multiple sequence alignment of MdbHLH093 and its homologs. Black means 100 percent homology, red means more than 75 percent homology, and blue means more than 50 percent homology.
2.2. Expression Pattern Analysis of MdbHLH093
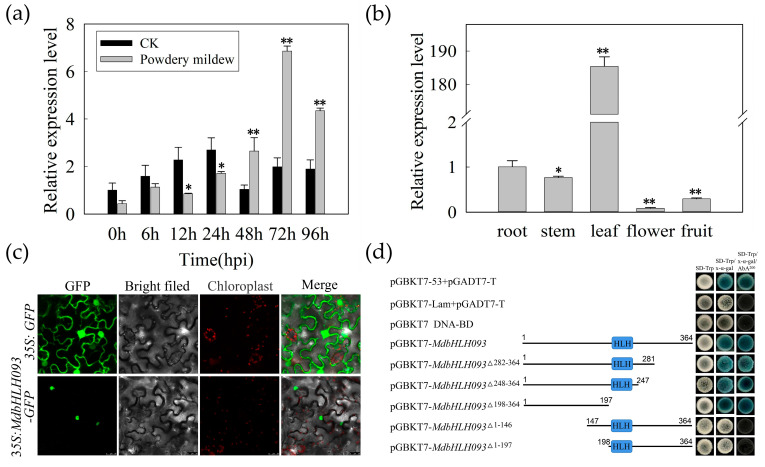
To evaluate the potential regulatory effect of the MdbHLH093 transcription factor on apple powdery mildew, we analyzed the expression pattern of MdbHLH093 in apple leaves inoculated with powdery mildew. The MdbHLH093 level was upregulated after the inoculation of apples with powdery mildew. However, it was significantly lower than that of the control group at 12 h post-inoculation (hpi) and 24 hpi, whereas after 48 hpi, the expression level was significantly upregulated, peaking at 72 hpi, and it was significantly increased at 48 hpi, 72 hpi, and 96 hpi when compared to the controls (Figure 2a). To analyze the spatial regulation where MdbHLH093 may play a major role, we analyzed the expression of MdbHLH093 in roots, stems, leaves, flowers, and fruits. MdbHLH093 was expressed in all tissues, but mainly in leaves, and its level in leaves was more than 100-fold higher that of the other tissues (Figure 2b), indicating MdbHLH093 mainly functions in leaves.
Figure 2.
Expression pattern analysis of MdbHLH093. (a) Quantitative real-time PCR (qRT–PCR) analysis of MdbHLH093 in plants infected with powdery mildew. (b) Relative expression of MdbHLH093 in apple root, stem, leaf, flower and fruit. MdTubulin was used as an internal reference gene. Error bars are calculated from three biological experiments and show the standard deviation of the mean. Asterisks indicate statistical significance (*, p < 0.05; **, p < 0.01; Student’s t-test). (c) Subcellular localization of MdbHLH093 in tobacco leaves. Green fluorescent protein (GFP) signals were detected with a laser confocal microscope. Scale bar = 25 μm. (d) The MdbHLH093 Yeast autoactivation transformation experiment, co-transformation of AD/T with BD/P53 or BD/Lam into yeast cells was used as positive and negative controls, respectively.
To determine the specific location of the MdbHLH093 protein in cells, the open reading frame (ORF) of MdbHLH093 was cloned downstream of the cauliflower mosaic virus (CaMV) 35S promoter with green fluorescent protein (GFP), and the plasmid was heterogeneously expressed in tobacco. Confocal microscopy was used to detect the GFP in the nucleus, indicating MdbHLH093 was localized in this ultrastructure (Figure 2c). To determine whether the transcription factor had transcriptional activation activity, we introduced the pGBKT7 vector containing the MdbHLH093 gene into yeast receptor cells by the yeast two-hybrid method and observed that it could reduce tryptophan deficiency. The cells inoculated in the medium containing X-α-Gal and aureobasidin A (SD/-Trp/X-α-Gal/AbA) grew normally and the medium turned blue, indicating MdbHLH093 had transcriptional activation activity. To confirm the presence of the transcriptional activation region and to avoid self-activation in subsequent experiments, we truncated MdbHLH093 by intercepting 83, 117, and 167 aa at the C-terminus, and 143 and 197 aa at the N-terminus, respectively. The truncated fragments were fused into the pGBKT7 vector and transformed into yeast receptor cells. The truncated fragments at the C-terminus showed transcriptional activation activity, whereas the truncated fragments at the N-terminus eliminated self-activation (Figure 2d), indicating the transcriptional activation region may be located within the N-terminus (1–146 aa). Thus, MdbHLH093△1−146 was selected for follow-up studies.
2.3. Heterologous Expression of MdbHLH093 in A. thaliana Enhances Resistance to Powdery Mildew
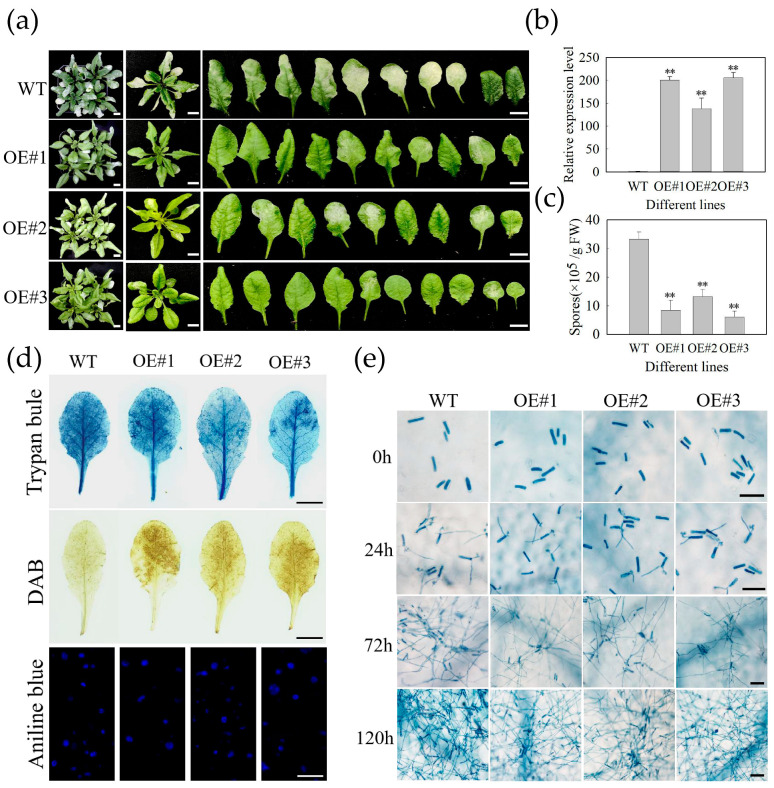
To determine whether MdbHLH093 has a regulatory effect on apple resistance to powdery mildew, we generated transgenic A. thaliana plants expressing MdbHLH093 under the control of a strong CaMV 35S promoter (35S: MdbHLH093) and selected three transgenic lines with significantly upregulated expression levels to evaluate the apple resistance to powdery mildew (Figure 3b). After 7 days of infection, compared to wild type (WT) plants, the three transgenic lines had a significantly lower incidence of infection, fewer infected leaves, and no leaf chlorosis (Figure 3a). The number of spores on the leaves of WT and transgenic A. thaliana plants was counted, and the number of spores on the leaves of transgenic lines was significantly lower than those on the wild type leaves (Figure 3c). Additionally, staining with trypan blue, diaminoaniline (DAB), and aniline blue was performed on WT and transgenic Arabidopsis leaves to observe the lesion area, as well as H2O2 and callose accumulation. From trypan blue staining, the WT plants had larger lesions and a more severe disease. From DAB staining, the H2O2 accumulation in transgenic A. thaliana plants was higher than those in WT plants, which may be one of the mechanisms of enhanced disease resistance. Aniline blue staining showed no significant difference between the WT and transgenic lines, indicating MdbHLH093 overexpression does not affect the accumulation of callose to regulate disease resistance (Figure 3d). In addition, A. thaliana leaves infected with powdery mildew were stained with trypan blue at 0 hpi, 24 hpi, 72 hpi, and 120 hpi to observe pathogenic growth and disease development. Compared to WT plants, the germination of spores was inhibited in transgenic A. thaliana plants, as well as the growth and elongation of mycelia and the generation of secondary spores to a certain extent (Figure 3e); this indicates that MdbHLH093 overexpression enhances the resistance of plants to powdery mildew.
Figure 3.
Overexpression of MdbHLH093 in A. thaliana enhances resistance to powdery mildew. (a) Phenotypes of wild type and transgenic lines (OE # 1,2,3) after 7 days of infection with powdery mildew, Scale bar = 1 cm. (b) Relative gene expression of MdbHLH093 in A. thaliana transgenic lines. (c) Number of spores per gram of fresh leaves at 7 dpi (days post-inoculation). Error bars are calculated from three biological experiments and show the standard deviation of the mean. Asterisks indicate statistical significance (**, p < 0.01; Student’s t-test). (d) Lesion area, H2O2, and callose accumulation after inoculation with pathogenic bacteria. Scale bar = 1 cm. (e) Comparison of the growth and development of powdery mildew on leaves of WT and transgenic A. thaliana. Scale bar = 50 μm.
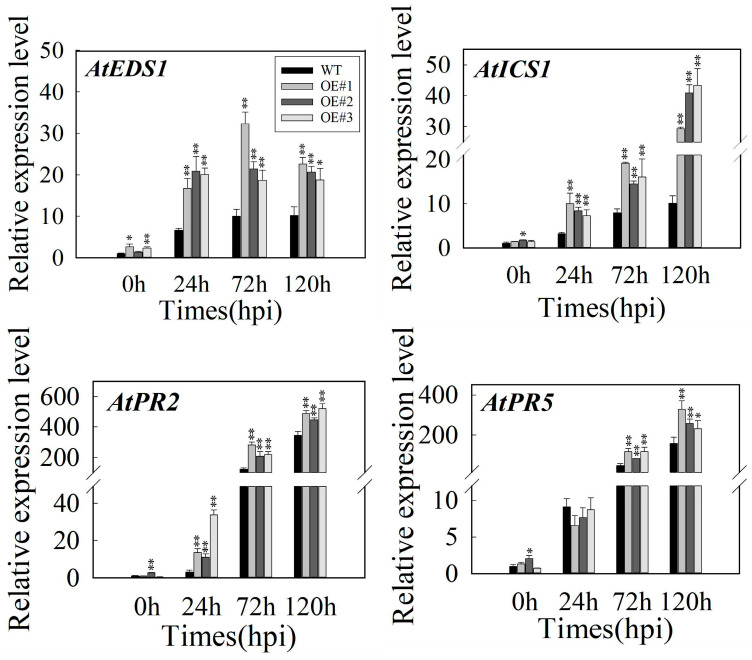
2.4. MdbHLH093-Overexpressing Plants Increase the Expression of Genes Related to the SA Signaling Pathway
The SA-regulated defense response plays an important role in defending plants against eutrophic pathogens. Therefore, we analyzed the transcriptional levels of SA synthesis-related genes (AtEDS1, AtICS1) and SA signaling pathway-related genes (AtPR2, AtPR5). In transgenic plants, AtEDS1 was significantly increased at 24 hpi, and its expression level remained elevated, whereas AtICS1 expression increased gradually in both WT and transgenic plants, and the expression levels of AtEDS1 and AtICS1 were significantly higher than those in WT plants at 24 hpi, 72 hpi, and 120 hpi. Furthermore, AtPR2, a downstream gene of the SA signaling pathway, was significantly upregulated in powdery mildew. Specifically, the response was more intense after 72 hpi, the transcriptional level was significantly increased, and the extent of the increase was higher in transgenic lines than that in WT plants. AtPR5 was also upregulated in powdery mildew, but the extent of the increase was lower than that of AtPR2. There was no significant difference compared to inoculated control plants at 24 hpi, and the expression level in transgenic lines at 72 hpi and 120 hpi was significantly higher than that in WT plants. Collectively, these results indicate MdbHLH093 positively regulates apple resistance to powdery mildew by promoting SA biosynthesis and signaling pathways (Figure 4).
Figure 4.
Expression analysis of disease resistance genes in transgenic Arabidopsis. Transcription levels of SA-pathway-related genes (AtEDS1, AtICS1, AtPR2, AtPR5) were analyzed after the 0, 24, 72, and 120 hpi infection of the MdbHLH093 overexpression’s strain and wild type. AtActin2 was used as an internal reference gene. Error bars are calculated from three biological experiments, and show the standard deviation of the mean. Asterisks indicate statistical significance (*, p < 0.05; **, p < 0.01; Student’s t-test).
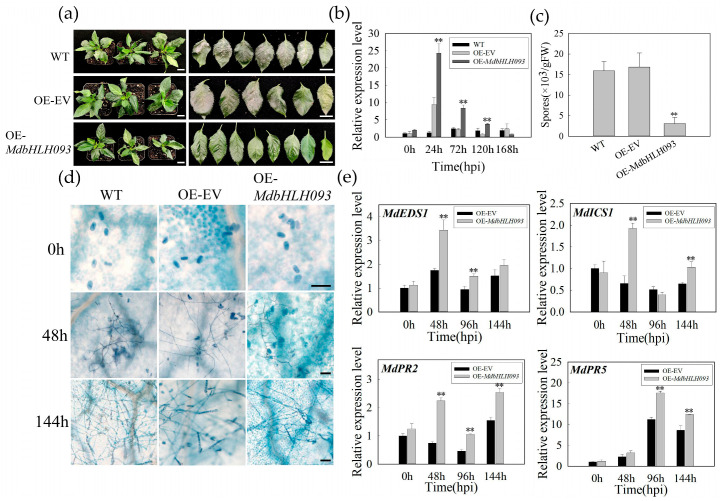
2.5. Transient MdbHLH093 Overexpression Enhances Apple Leaf Resistance to Powdery Mildew
To confirm the regulatory effect of MdbHLH093 in powdery mildew in apples, we transiently transformed the overexpression vector containing MdbHLH093 into Gala-3 apple tissue culture seedlings and inoculated P. leucotricha 24 hpi after penetration. We analyzed the MdbHLH093 level at 0 hpi, 24 hpi, 72 hpi, 120 hpi, and 168 hpi after osmosis. The expression level increased and then decreased, peaking at 24 hpi, and the expression level was more than 2-fold higher than that in seedlings transformed with the empty vector (OE-EV). Furthermore, the transcription level at 24 hpi, 72 hpi, and 120 hpi was significantly increased compared to the controls, indicating MdbHLH093 was successfully overexpressed (Figure 5b). The disease degree of the transient overexpression of MdbHLH093 in inoculated plants was significantly less severe than that in WT plants and osmotic no-load controls (Figure 5a). Quantitative analysis of the number of spores revealed that the overexpressed strains had fewer spores (Figure 5c). Trypan blue staining was performed on apple leaves at 0 hpi, 48 hpi, and 144 hpi after inoculation, and the growth of P. leucotricha was inhibited, especially in terms of the production of secondary spores (Figure 5d). Additionally, we quantitatively analyzed the transcriptional levels of the key SA pathway genes MdEDS1, MdICS1, MdPR2, and MdPR5 by real-time fluorescence. Upon MdbHLH093 overexpression, the levels of both MdEDS1 and MdICS1 peaked at 48 hpi and increased gradually after 96 hpi, in which the MdEDS1 level at 48 hpi and 96 hpi was significantly higher than that in the controls, and the MdICS1 level at 48 hpi and 144 hpi was significantly higher than that in the controls. PR2, a protein related to the course of powdery mildew, was significantly higher in overexpressing plants than that in the controls at 48 hpi, 96 hpi, and 144 hpi. MdPR5 showed an increasing and then a decreasing trend during infection, and its expression level was higher after 96 hpi, with significant differences at 96 hpi and 144 hpi (Figure 5e), indicating that MdbHLH093 overexpression enhances apple resistance to powdery mildew through the SA signaling pathway.
Figure 5.
The transient overexpression of MdbHLH093 in tissue culture plantlet Gala-3 could enhance resistance to powdery mildew. (a) Disease symptoms on infiltrated leaves (wild-type [WT] empty overexpression vector [OE-EV], OE-MdbHLH093) after P. leucotricha inoculation. Scale bar = 1 cm. (b) Analysis of MdbHLH093 expression after transient transformation. The asterisk indicates a significant difference between OE-MdbHLH093 and OE-EV or WT. (c) Number of spores per gram of fresh leaves at 7 dpi. (d) Comparison of growth and development of powdery mildew on leaves of WT, OE-EV, and OE-MdbHLH093. Scale bar = 50 μm. (e) Analysis of transcription levels of SA-pathway-related genes (MdEDS1, MdICS1, MdPR2, and MdPR5). Error bars are from three biological experiments, and show the standard deviation of the mean. Asterisks indicate statistical significance (**, p < 0.01; Student’s t-test).
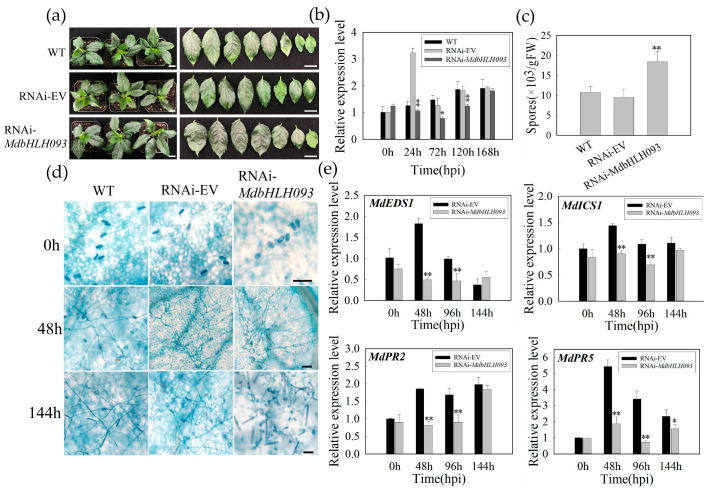
2.6. Transient Silencing of MdbHLH093 Reduces Apple Resistance to Powdery Mildew
We constructed the MdbHLH093 RNAi vector, transiently transformed it into apple plants, and examined the MdbHLH093 expression level. The results showed that, compared with WT, the expression level of MdbHLH093 was obviously increased in plants that were transformed into empty vectors (RNAi-EV); we speculated that this might be caused by vacuum stress. Compared with RNAi-EV, the expression of MdbHLH093 in the experimental group (RNAi-MdbHLH093) was reduced by approximately two-thirds at 24 hpi, and by approximately one-third at 72 hpi and 120 hpi, indicating successful MdbHLH093 knockdown (Figure 6b). Furthermore, the knockdown of MdbHLH093 increased the incidence of P. leucotricha infection at 168 hpi in apple leaves (Figure 6a). Quantitative analysis showed that MdbHLH093 knockdown significantly increased the number of spores in leaves (Figure 6c) and the sensitivity of plants to powdery mildew. Trypan blue staining also indicated that P. leucotricha growth on the leaves of MdbHLH093-silenced plants was enhanced, and the production of secondary spores was higher than that in the controls (Figure 6d). Additionally, we examined the transcriptional levels of key genes of the SA pathway; the results showed that after MdbHLH093 knockdown, MdEDS1 expression was significantly inhibited, its expression at 48 hpi was one-third that of the controls, and its expression at 96 hpi was reduced by approximately 50%. The expression levels of MdICS1 and MdPR2 at 48 hpi and 96 hpi were significantly decreased compared to the controls, whereas MdPR5 expression was significantly decreased at 48 hpi, 96 hpi, and 144 hpi compared to the controls (Figure 6e). This indicates that the inhibition of the SA signaling pathway after MdbHLH093 knockdown reduces disease resistance.
Figure 6.
Transient silencing of MdbHLH093 reduces resistance to apple powdery mildew. (a) Disease symptoms in infiltrated leaves (wild-type [WT], empty silencing vector [RNAi-EV], RNAi-MdbHLH093) after P. leucotricha inoculation. Scale bar = 1 cm. (b) Analysis of MdbHLH093 expression after transient transformation. Asterisks indicate a significant difference between RNAi-MdbHLH093 and RNAi-EV or WT. (c) Number of spores per gram of fresh leaves at 7 dpi. (d) Comparison of growth and development of powdery mildew on leaves of WT, RNAi-EV, and RNAi-MdbHLH093. Scale bar = 50 μm. (e) Analysis of transcriptional levels of SA pathway-related genes (MdEDS1, MdICS1, MdPR2, and MdPR5). Error bars are from three biological experiments and show the standard deviation of the mean. Asterisks indicate statistical significance (*, p < 0.05; **, p < 0.01; Student’s t-test).
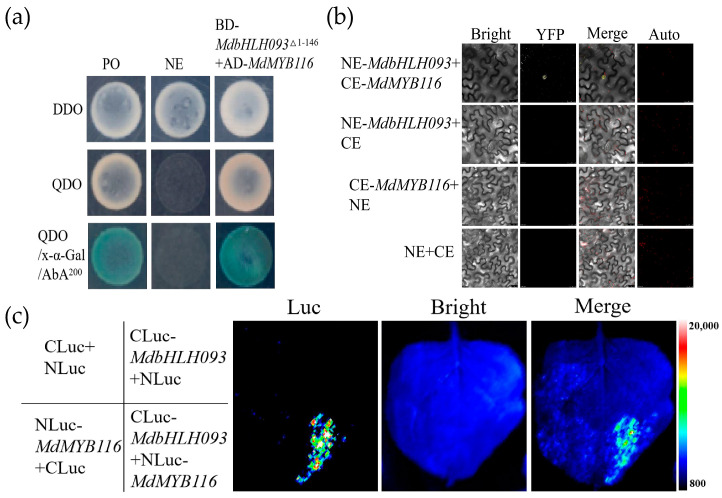
2.7. MdbHLH093 Interacts with MdMYB116
In our previous studies, we identified the MdMYB116 transcription factor as a positive regulator of powdery mildew infection in apples and observed that it could enhance the resistance of apple to powdery mildew by promoting reactive oxygen species accumulation and SA signaling [24]. Other studies have reported that bHLH and MYB families can form dimers, thereby functioning in a synergistic manner [25]; thus, we hypothesized that MdbHLH093 and MdMYB116 may also be involved in a similar interaction. We used the yeast two-hybrid method to verify the presence of protein-protein interactions. Yeast colonies containing pGBKT7-MdbHLH093△1−146 and pGADT7-MdMYB116 grew in a SD/−Ade−His−Leu−Trp medium. In the presence of X-α-Gal and AbA, the colonies grew normally and appeared blue; this was similar to the positive control (Figure 7a), indicating MdbHLH093 specifically interacts with the MdMYB116 in yeast cells. To verify this interaction in plants, we conducted bimolecular fluorescence complementation experiments (Figure 7b). Compared to the control, only NE-MdbHLH093+CE-MdMYB116 produced a fluorescent signal in the nucleus. Split-luciferase assay was performed on tobacco leaves, and luciferase activity was detected at the site of co-injection of Nluc-MdMYB116 and Cluc-MdbHLH093, whereas no luciferase activity was detected in the control leaves (Figure 7c), indicating MdbHLH093 interacts and co-localizes with MdMYB116 in the nucleus.
Figure 7.
Verification of the interaction between MdbHLH093 and MdMYB116. (a) Yeast two-hybrid assay. pGBKT7-MdbHLH093Δ1−146 and pGADT7-MdMYB116 plasmids were co-transformed into yeast two-hybrid gold cells. pGBKT7-53 + pGADT7-T and pGBKT7-Lam + pGADT7-T served as positive (PO) and negative controls (NE), respectively. (b) Bimolecular fluorescence complementation assay. Merged fluorescent and visible light images. Scale bar = 25 μm. (c) Interaction between MdbHLH093 and MdMYB116 was verified by split-luciferase assay. CLuc/NLuc, CLuc-MdbHLH093/NLuc and CLuc/NLuc-MdMYB116 served as controls.
3. Discussion
Apple, one of the most popular edible fruits worldwide, has important economic value, but its production often suffers from various biotic stresses, such as powdery mildew. At present, powdery mildew in apples is mainly prevented through pesticide use, which not only increases costs, but also creates food safety and environmental pollution issues [26]. A better sustainable solution is to enhance the resistance of apples to powdery mildew in order to control the disease and to breed resistant varieties by exploring genes with specific resistance to powdery mildew or broad-spectrum resistance to fungal diseases. It was observed that NPR1, a major regulatory factor of the SA-mediated disease resistance pathway, enhances broad-spectrum resistance to diseases by altering the expression levels of disease-course-related proteins in plants [27,28]. Additionally, MhNPR1 is overexpressed in the Fuji apple, where it regulates the expression of MdPR and MdMLO and improves the resistance to powdery mildew [29]. Thus, the loss of MLO can improve plant resistance to powdery mildew, which has been demonstrated in barley, Arabidopsis, tomato, pea, wheat, and cucumber [30], as well as confirmed in apple. It was observed that the knockout of MdMLO19 significantly improves the resistance of apples to powdery mildew [5]. Previous studies have reported that some bHLH-family transcription factors control disease resistance through various pleiotropic mechanisms. For example, in tomato, the overexpression of bHLH132, a transcription factor, greatly improves resistance to Xanthomonas euvesicatoria [31]. In rice, the bHLH-family transcription factor RERJ1 is involved in the jasmonate-mediated expression of stress response genes through a mechanism that involves its interaction with OsMYC2, which improves plant defense against herbivores and bacterial infection [32]. At present, most studies of biotic stress induced by bHLH-family transcription factors focus on model plants and bacterial diseases; as such, there are only a few studies on powdery mildew in apples.
In this study, the qPCR analysis showed that the MdbHLH093 level was significantly decreased at 12 hpi and 24 hpi compared to the controls, and significantly increased at 48 hpi, 72 hpi, and 96 hpi, indicating MdbHLH093 can be induced by P. leucotricha (Figure 2a). In addition, MdbHLH093 overexpression in A. thaliana and apple leaves enhanced resistance to powdery mildew (Figure 3, Figure 4 and Figure 5), whereas MdbHLH093 knockdown in apple leaves increased sensitivity to powdery mildew (Figure 6), indicating MdbHLH093 has a positive regulatory effect on the resistance of apple to powdery mildew. Hydrogen peroxide plays an important role in plant resistance to stress. It kills pathogenic microorganisms, inhibits the germination of pathogenic spores and the production of plant protectants and antibacterial proteins, as well as induces the hypersensitivity of cell death system-acquired resistance [33]. Additionally, it regulates the secondary growth of cell wall structures, facilitates the lignification of cell walls after cell injury, and upregulates the expression of disease-resistance-related genes [34,35]. The overexpression of MdbHLH093 in A. thaliana visibly increased the H2O2 content compared to the controls (Figure 3d), which may be one of the mechanisms involved in disease resistance. Callose is rapidly deposited between plasma membranes and cell walls after plants are attacked by disease agents, where it induces a specific defense mechanism. Thus, callose is an important index for evaluating the resistance of plants to disease [36]. Cui et al. demonstrated that the transient silencing of VvCSN5 in grape leaves increases callose deposition, and thus induces resistance to powdery mildew [37]. However, in our study, no obvious change in the callose content was observed after MdbHLH093 overexpression, indicating MdbHLH093 does not increase the callose content in disease resistance (Figure 3d).
Plant hormones are important regulatory factors required for plant defense, and the complex signaling network composed of hormones enables plants to activate appropriate and effective defense responses against pathogens [38]. Salicylate plays an important role in the resistance of plants to eutrophic and semi-eutrophic pathogens [39], which allows plants to resist powdery mildew. A previous study has shown that VqWRKY31 improves grape resistance to powdery mildew by activating SA biosynthesis and SA-related immune signaling pathways [40]. The wheat bHLH-family transcription factor TabHLH60 negatively regulates the resistance of plants to disease by inhibiting the transcriptional levels of resistant genes PR1, PR2, and PR5 in the SA signaling pathway [15]. Furthermore, the CmbHLH87 overexpression in pumpkins increases the accumulation of H2O2, upregulates the transcriptional levels of PR1a, PR5, and NPR1, as well as enhances resistance to powdery mildew [18], indicating bHLH-family transcription factors regulate disease resistance by regulating hormonal pathways. Upon MdbHLH093 overexpression in A. thaliana, the transcriptional levels of four SA signaling-related defense genes were upregulated compared to the controls (Figure 4). AtEDS1 and AtICS1 are the key genes of the SA biosynthesis pathway [41], whereas AtPR2 and AtPR5 are the key defense genes induced by SA [42]. In our study, the transient overexpression of MdbHLH093 in apple leaves upregulated the transcriptional levels of four SA signaling-related defense genes, which is consistent with the study of A. thaliana. Whereas the knockdown of MdbHLH093 downregulated the transcriptional levels of these genes, indicating MdbHLH093 overexpression enhances resistance to powdery mildew through the SA signaling pathway.
The bHLH proteins co-regulate plant disease resistance by interacting with other proteins. For example, the rice bHLH-family transcription factor RERJ1 interacts with members of the OsMYC2 and JAZ families to enhance the resistance of plants to insects and pathogenic bacteria through the regulation of the jasmonate pathway [32]. Apple MdbHLH92 interacts with MdERF100 to regulate the resistance of apple to powdery mildew [4]. In our previous study, MdMYB116 increased H2O2 accumulation and SA signaling-related gene expressions to regulate the resistance to powdery mildew [24]. Furthermore, members of the bHLH family can interact and co-function with those of the MYB family. For example, the bHLH transcription factor MYC5 interacts with MYB transcription factors MYB21 and MYB24 to form the bHLH–MYB transcription complex, which regulates stamen development [43]. In our study, the interaction between MdbHLH093 and MdMYB116 was verified by yeast two-hybrid, bi-molecular fluorescence complementation, and split luciferase experiments (Figure 7), and it was observed that this interaction had a synergistic effect on the positive regulation of powdery mildew resistance.
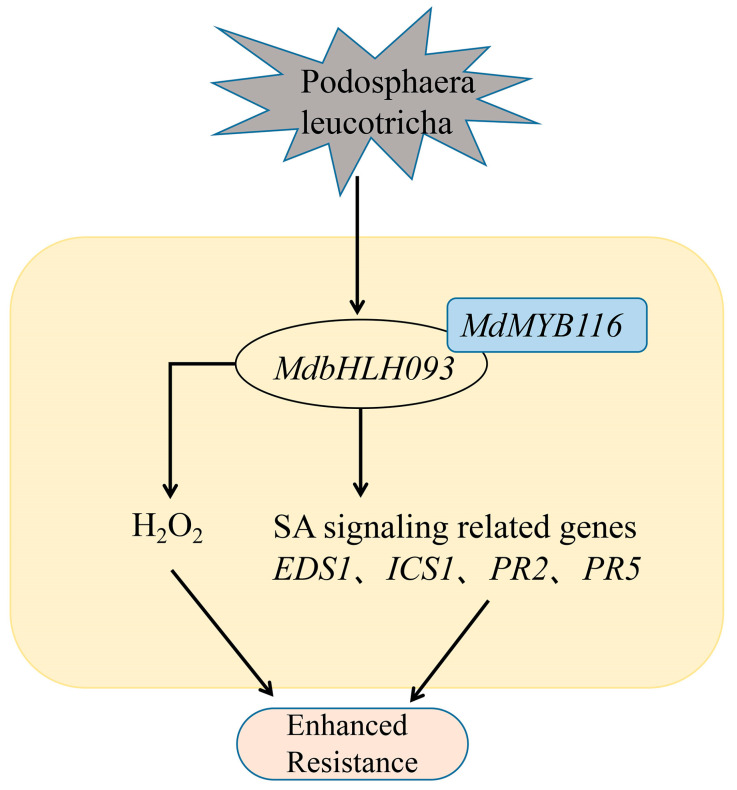
In conclusion, we found that MdbHLH093 enhances resistance to powdery mildew by regulating H2O2 accumulation and SA signaling, and it interacts with the resistance gene MdMYB116 to elicit synergistic effects (Figure 8). Further studies are needed to explore whether there is an association or a regulatory mechanism between H2O2 production and SA signaling. Our results provide new options for breeding disease-resistant plants and offer new insights into the functions and mechanisms of bHLH in response to pathogen inoculation.
Figure 8.
Hypothetical model depicting how MdbHLH093 functions as a positive regulator of responses to powdery mildew infection. The transcription factor MdbHLH093 can interact with MdMYB116 to enhance resistance to P. leucotricha through hydrogen peroxide (H2O2) and salicylic acid (SA) signaling pathways, as well as improves the transcription levels of defense-related genes. Arrows represent positive effects.
4. Materials and Methods
4.1. Plant Materials and Growth Conditions
Apple (Gala-3) tissue culture seedlings rooted for 45 days were used, and the rooting medium was composed of 2.22 g/L Murashige and Skoog (MS), 20 g/L of sucrose, 500 μL of Indole-3-acetic Acid (IAA, 1 mg/mL), 500 μL of Indole-3-butyric Acid (IBA, 1 mg/mL), and 7.5 g/L of agar, pH 6.1. Seedlings were cultivated at a temperature of 25 °C and a photoperiod of 16 h light/8 h dark. Arabidopsis Columbia ecotype (Col-0) plants were grown in an incubator with a 16 h/8 h photoperiod and 70% relative humidity. Nicotiana benthamiana (line NC89) was grown in a chamber at 25 °C. Apple root, stem, leaf, flower, and fruit tissues were acquired from the White Water Apple Test Station of Northwest A&F University, Shaanxi, China.
4.2. Pathogen Inoculation
A 100 mL spore suspension (1 × 106 spores/mL) was prepared using material collected from the farm of Northwest A&F University and was evenly sprayed on the leaves of rooting seedlings that were previously transplanted into the substrate for one month. Water served as the control. Temperature and humidity were maintained at 25 °C and 70%, respectively, and the seedlings were exposed to 16 h of light and 8 h of dark. Leaves were sampled at 0 hpi, 6 hpi, 12 hpi, 24 hpi, 48 hpi, 72 hpi, and 96 hpi for expression analysis.
4.3. Bioinformatics Analysis
Chromosome positions of MdbHLH093 were predicted using Blast-Search in the Apple Genome Browser, and the conserved protein domains were predicted using the simple modular architecture research tool (SMART; http://smart.embl-heidelberg.de/, accessed on 5 March 2022). Ten homologous genes of MdbHLH093 from NCBI (https://www.ncbi.nlm.nih.gov/, accessed on 5 March 2022) were downloaded, and the multi-sequence alignment of MdbHLH093 was performed using DNAMAN 6.0 software. A phylogenetic tree of the two genes and their homologous genes was generated using the neighbor joining method, and 1000 bootstrap replicates were performed by MEGA 5.0 software.
4.4. Subcellular Localization Analysis
The full-length CDS of MdbHLH093 (without stop codons) was cloned from Gala-3 apple cDNA by using primers designed by Primer Premier 5.0 software (Table S1). In addition, the cloned fragment was inserted into the pCAMBIA2300-GFP recombinant vector. The MdbHLH093-GFP plasmid was transformed into Agrobacterium tumefaciens Gv3101 and penetrated into tobacco leaves [44]. Tobacco plants were grown for 2 days at a temperature of 25 °C, a relative humidity of 60%, and a photoperiod of 16 h light/8 h dark. Green fluorescent protein was observed with a laser scanning confocal microscope (model FV1000MPE; Olympus, Tokyo, Japan).
4.5. Arabidopsis Transformation and Disease Analysis
The pCAMBIA2300-MdbHLH093-GFP vector was heterogenetically transformed into A. thaliana by the inflorescence infection method [45]. T0 plant seeds were selected on an MS medium supplemented with 75 mg/L of kanamycin. Homozygous T3 strains were selected from 3 independent T1 strains for subsequent experiments. After the inoculation of wild type and transgenic strains with Golovinomyces cichoracearum UCSC1, the leaves were collected at 0 hpi, 24 hpi, 72 hpi, and 120 hpi for qPCR, and the infected leaves were stained with trypan blue to observe the growth of pathogenic bacteria. In brief, A. thaliana leaves were immersed in boiling trypan blue solution for 4 min and rinsed with water 2–3 times. Leaves were decolorized with 2.5 g/mL of chloral hydrate for 4–6 h and stored in 50% glycerol. At 168 hpi, the number of spores on leaves was counted by the blood cell counting plate method; the infected A. thaliana leaves were collected, added to 5 mL of sterile water, and mixed for 1 min until the spores were completely released. The number of spores was counted using a hematocytometer, and the leaves were stained with trypan blue, DAB, and aniline blue. For the DAB staining, the leaves were immersed in 1 mg/mL of DAB (pH 3.8) for 8 h, and then soaked in 95% ethanol to observe the accumulation of H2O2. Aniline blue staining was used to assess the deposition of the callose. In brief, leaves were decolorized with 95% ethanol, stained with aniline blue for 24 h, and observed with a fluorescence microscope (model BX63; Olympus).
4.6. Instantaneous Transformation of Apple Leaves Mediated by Agrobacterium tumefaciens and Inoculation with Powdery Mildew
Overexpression and RNA interference (RNAi) vectors (pK7GWIWG2D-MdbHLH093) were transformed into Agrobacterium Gv3101, cultured in liquid Luria-Bertani medium at 28 °C for 16 h in a 180 rpm shaking incubator, and centrifuged at 5000 rpm for 10 min. Bacteria were suspended in an infiltrating buffer (10 mM MES, 10 mM MgCl2, 200 μM acetyl syringone, pH 5.6) to OD600 = 0.8. Apple (Gala-3) tissue culture seedlings, which had been rooted for 45 days, were washed free of the root medium, placed in the resuspension solution upside down, soaked under vacuum conditions of 0.08 MPa for 15–20 min until the backs of leaves were completely water soaked, and then transplanted into substrate for growth at a temperature of 25 °C and a relative humidity of 90%. Each treatment consisted of at least 10 seedlings.
After vacuum osmosis for 24 h, the P. leucotricha were inoculated and maintained at a temperature of 25 °C, a relative humidity of 90%, and a photoperiod of 16 h light/8 h dark. Leaves were collected at 0 hpi, 48 hpi, 96 hpi, and 144 hpi for qPCR. Leaves inoculated at 0 hpi, 48 hpi, and 144 hpi were stained with trypan blue to observe the growth of the pathogenic bacteria. In brief, leaves were immersed in a boiling trypan blue solution for 10 min, soaked in trypan blue solution at room temperature for 15–20 h, rinsed with water 2–3 times, decolorized with 2.5 g/mL of chloral hydrate for 24 h, and then observed with a fluorescence microscope. Three biological replicates were performed to determine the number of spores in the infected leaves.
4.7. Yeast Two-Hybrid Assay
For the yeast transcriptional activation test, the full-length CDS of MdbHLH093 and its truncated fragments were inserted into the pGBKT7 vector, and the plasmids were transformed into Y2H receptor cells, which were cultured in media (SD/Trp, SD/Trp + 200 ng/mL AbA, SD/Trp + 200 ng/mL AbA, and 50 μg/mL X-α-Gal) at 30 °C for 2–3 days. Colony growth and color changes were observed. The pGBKT7-53 combined with pGADT7 served as a positive control, whereas pGBKT7-Lam combined with pGADT7 or empty pGBKT7 served as a negative control.
To explore the interaction in yeast, the full-length CDS of MdMYB116 was inserted into the pGADT7 vector and transformed into Y2H receptor cells together with the pGBKT7-MdbHLH093△1−146 vector. Cells were cultured in media (SD/−Leu−Trp [DDO], SD/−Ade−His−Leu−Trp [QDO], and SD/−Ade−His−Leu−Trp [QDO] + 200 ng/mL AbA + 50 μg/mL X-α-Gal) at 30 °C for 2–3 days. Colony growth and color changes were observed. Blue colonies indicated an interaction between the two proteins.
4.8. Bimolecular Fluorescence Complementary Analysis
The full-length CDS of MdbHLH093 and MdMYB116 (without their respective stop codons) were cloned into pSPYNE and pSPYCE vectors, respectively, and the plasmids were transformed into Agrobacterium Gv3101. pSPYNE-MdbHLH093/pSPYCE-MdMYB116 was expressed instantaneously in the tobacco leaves by Agrobacterium-mediated transformation. pSPYNE+pSPYCE, pSPYNE-MdbHLH093+pSPYCE and pSPYNE+pSPYCE-MdMYB116 served as the controls. After 48 h of conversion, the fluorescence signals were observed with a laser scanning confocal microscope (model TCS SP8; Leica, Wetzlar, Germany). The primers used to prepare the constructs are listed in Table S1.
4.9. Split Luciferase Assay
The full-length CDS of MdbHLH093 and MdMYB116 (without their respective stop codons) were inserted into pCB1300-Cluc and pCB1300-Nluc vectors, respectively. The plasmids were transformed into Agrobacterium Gv3101 and then penetrated into 4-week-old tobacco leaves. After 2 days of infiltration, the firefly luciferase substrate (0.3 mg/mL) was evenly applied to the backs of leaves, which were then placed in the dark for 10 min. A charge-coupled device camera (iKonM 934, Andor Technology, Belfast, Northern Ireland) and Winview 32 software were used for luciferase imaging. pCB1300-MdbHLH093-Cluc+pCB1300-Nluc, pCB1300-MdMYB116-Nluc+pCB1300-Cluc, and pCB1300-Cluc+pCB1300-Nluc served as the controls.
4.10. Gene Expression Analysis by RT-qPCR
Total RNA was extracted from A. thaliana and apples with the E.Z.N.A. plant RNA kit (Omega Bio-tek, Norcross, GA, USA). The Hifair III 1st Strand cDNA Synthesis SuperMix kit for qPCR (gDNA digester plus) (Yeasen, Shanghai, China) was used for reverse transcription, and operated according to the manufacturer’s instructions. Thereafter, the synthesized cDNA was diluted six-fold to serve as the qPCR template, and fluorescence quantification was performed using the Taq SYBR Green qPCR Premix kit (universal) (Yugong Biotech, Nanjing, China) in a 20 µL reaction system. The cycling conditions were as follows: 95 °C for 30 s, followed by 40 cycles of 95 °C for 5 s and 60 °C for 30 s. Relative expression was analyzed by the 2−∆∆Ct method [46]. SigmaPlot 12.0 (Systat Software, San Jose, CA, USA) was used to generate the graphs.
4.11. Statistical Analysis
Statistical analysis was conducted using Student’s two-tailed t test (* p < 0.05, ** p < 0.01). Data were generated from three biological repeats. Error bars indicate standard error of the mean.
5. Conclusions
The transcription factor MdbHLH093, which is localized in the nucleus, has transcriptional activation activity. MdbHLH093 can positively regulate the resistance of apples to powdery mildew by regulating the accumulation of H2O2 and the expression of genes related to the SA signaling pathway, and it can interact with the transcription factor of the resistance gene MdMYB116 to control plant disease resistance. Therefore, this study provides important information on the role of MdbHLH093 in powdery mildew resistance.
Acknowledgments
The authors thank all editors and reviewers for their comments on this manuscript.
Supplementary Materials
The supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/ijms24119390/s1.
Author Contributions
Conceptualization, H.M., X.W. and H.G.; methodology, Z.Z. and H.G.; formal analysis, H.M. and F.Z.; investigation, H.M., F.Z., D.L., Y.W. and Y.Z.; resources, X.W. and H.G.; writing—original draft preparation, H.M.; writing—review and editing, H.M., F.Z., D.L. and Y.Z.; funding acquisition, H.G. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data supporting the findings of this study are available within the article and its Supplementary Materials.
Conflicts of Interest
The authors declare no conflict of interest.
Funding Statement
The work was supported by the Shaanxi Provincial Key Research and Development (R&D) Program (2020zdzx03-06-02-02) and the China Agriculture Research System (CARS-27).
Footnotes
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
References
- 1.Guo T., Liu C., Meng F., Hu L., Fu X., Yang Z., Wang N., Jiang Q., Zhang X., Ma F. The m6 A reader MhYTP2 regulates MdMLO19 mRNA stability and antioxidant genes translation efficiency conferring powdery mildew resistance in apple. Plant Biotechnol. J. 2022;20:511–525. doi: 10.1111/pbi.13733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gañán L., White R.A., Friesen M.L., Peever T.L., Amiri A. A genome resource for the apple powdery mildew pathogen Podosphaera leucotricha. Phytopathology. 2020;110:1756–1758. doi: 10.1094/PHYTO-05-20-0158-A. [DOI] [PubMed] [Google Scholar]
- 3.Papp D., Király I., Tóth M. Suitability of old apple varieties in organic farming, based on their resistance against apple scab and powdery mildew. Org. Agric. 2016;6:183–189. doi: 10.1007/s13165-015-0126-2. [DOI] [Google Scholar]
- 4.Zhang Y., Zhang L., Ma H., Zhang Y., Zhang X., Ji M., Van Nocker S., Ahmad B., Zhao Z., Wang X., et al. Overexpression of the apple (Malus × domestica) MdERF100 in Arabidopsis increases resistance to powdery mildew. Int. J. Mol. Sci. 2021;22:5713. doi: 10.3390/ijms22115713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pessina S., Angeli D., Martens S., Visser R.G., Bai Y.L., Salamini F., Velasco R., Schouten H.J., Malnoy M. The knock-down of the expression of MdMLO19 reduces susceptibility to powdery mildew (Podosphaera leucotricha) in apple (Malus domestica) Plant Biotechnol. J. 2016;14:2033–2044. doi: 10.1111/pbi.12562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zhou Z., Wang J., Zhang S., Yu Q., Lan H. Investigation of the nature of CgCDPK and CgbHLH001 interaction and the function of bHLH transcription factor in stress tolerance in Chenopodium glaucum. Front. Plant Sci. 2020;11:603298. doi: 10.3389/fpls.2020.603298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Feller A., Machemer K., Braun E.L., Grotewold E. Evolutionary and comparative analysis of MYB and bHLH plant transcription factors. Plant J. 2011;66:94–116. doi: 10.1111/j.1365-313X.2010.04459.x. [DOI] [PubMed] [Google Scholar]
- 8.Groszmann M., Bylstra Y., Lampugnani E.R., Smyth D.R. Regulation of tissue-specific expression of SPATULA, a bHLH gene involved in carpel development, seedling germination, and lateral organ growth in Arabidopsis. J. Exp. Bot. 2010;61:1495–1508. doi: 10.1093/jxb/erq015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pillitteri L.J., Sloan D.B., Bogenschutz N.L., Torii K.U. Termination of asymmetric cell division and differentiation of stomata. Nature. 2007;445:501–505. doi: 10.1038/nature05467. [DOI] [PubMed] [Google Scholar]
- 10.Zhang Y., Mitsuda N., Yoshizumi T., Horii Y., Oshima Y., Ohme-Takagi M., Matsui M., Kakimoto T. Two types of bHLH transcription factor determine the competence of the pericycle for lateral root initiation. Nat. Plants. 2021;7:633–643. doi: 10.1038/s41477-021-00919-9. [DOI] [PubMed] [Google Scholar]
- 11.Guo J., Sun B., He H., Zhang Y., Tian H., Wang B. Current understanding of bHLH transcription factors in plant abiotic stress tolerance. Int. J. Mol. Sci. 2021;22:4921. doi: 10.3390/ijms22094921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Qian Y., Zhang T., Yu Y., Gou L., Yang J., Xu J., Pi E. Regulatory mechanisms of bHLH transcription factors in plant adaptive responses to various abiotic stresses. Front. Plant Sci. 2021;12:677611. doi: 10.3389/fpls.2021.677611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fan M., Bai M.Y., Kim J.G., Wang T., Oh E., Chen L., Park C.H., Son S.H., Kim S.K., Mudgett M.B. The bHLH transcription factor HBI1 mediates the trade-off between growth and pathogen-associated molecular pattern-triggered immunity in Arabidopsis. Plant Cell. 2014;26:828–841. doi: 10.1105/tpc.113.121111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Turnbull D., Yang L., Naqvi S., Breen S., Welsh L., Stephens J., Morris J., Boevink P.C., Hedley P.E., Zhan J., et al. RXLR effector AVR2 up-regulates a brassinosteroid responsive bHLH transcription factor to suppress immunity. Plant Physiol. 2017;174:356–369. doi: 10.1104/pp.16.01804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wang F., Lin R., Feng J., Qiu D., Chen W., Xu S. Wheat bHLH transcription factor gene, TabHLH060, enhances susceptibility of transgenic Arabidopsis thaliana to Pseudomonas syringae. Physiol. Mol. Plant Pathol. 2015;90:123–130. doi: 10.1016/j.pmpp.2015.04.007. [DOI] [Google Scholar]
- 16.Cheng Q., Dong L., Gao T., Liu T., Li N., Wang L., Chang X., Wu J., Xu P., Zhang S. The bHLH transcription factor GmPIB1 facilitates resistance to Phytophthora sojae in Glycine max. J. Exp. Bot. 2018;69:2527–2541. doi: 10.1093/jxb/ery103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wei K.F., Chen H.Q. Comparative functional genomics analysis of bHLH gene family in rice, maize and wheat. BMC Plant Biol. 2018;18:309. doi: 10.1186/s12870-018-1529-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Guo W.L., Chen B.H., Guo Y.Y., Chen X.J., Li Q.F., Yang H.L., Li X.Z., Zhou J.G., Wang G.Y. Expression of pumpkin CmbHLH87 gene improves powdery mildew resistance in tobacco. Front. Plant Sci. 2020;11:163. doi: 10.3389/fpls.2020.00163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yang J., Gao M., Huang L., Wang Y., Van Nocker S., Wan R., Guo C., Wang X., Gao H. Identification and expression analysis of the apple (Malus × domestica) basic helix-loop-helix transcription factor family. Sci. Rep. 2017;7:28. doi: 10.1038/s41598-017-00040-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tian X., Zhang L., Feng S., Zhao Z., Wang X., Gao H. Transcriptome analysis of apple leaves in response to powdery mildew (Podosphaera leucotricha) infection. Int. J. Mol. Sci. 2019;20:2326. doi: 10.3390/ijms20092326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sharma N., Xin R., Kim D.H., Sung S., Lange T., Huq E. NO FLOWERING IN SHORT DAY (NFL) is a bHLH transcription factor that promotes flowering specifically under short-day conditions in Arabidopsis. Development. 2016;143:682–690. doi: 10.1242/dev.128595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhuo T., Wang X., Chen Z., Cui H., Zeng Y., Chen Y., Fan X., Hu X., Zou H. The Ralstonia solanacearum effector RipI induces a defence reaction by interacting with the bHLH93 transcription factor in Nicotiana benthamiana. Mol. Plant Pathol. 2020;21:999–1004. doi: 10.1111/mpp.12937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.An J.P., Zhang X.W., Bi S.Q., You C.X., Wang X.F., Hao Y.J. MdbHLH93, an apple activator regulating leaf senescence, is regulated by ABA and MdBT2 in antagonistic ways. New Phytol. 2019;222:735–751. doi: 10.1111/nph.15628. [DOI] [PubMed] [Google Scholar]
- 24.Ji M., Wan Y., Zhang Y., Ma H., Wang X., Gao H. Studies on the resistance of apple MdMYB116 to powdery mildew. Acta Hortic. Sin. 2021;48:2133–2145. doi: 10.16420/j.issn.0513-353x.2021-0060. (In Chinese) [DOI] [Google Scholar]
- 25.Pesch M., Schultheiß I., Klopffleisch K., Uhrig J.F., Koegl M., Clemen C.S., Simon R., Weidtkamp-Peters S., Hülskamp M. Transparent testa GLABRA1 and GLABRA1 compete for binding to GLABRA3 in Arabidopsis. Plant Physiol. 2015;168:584–597. doi: 10.1104/pp.15.00328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Höfer M., Flachowsky H., Schröpfer S., Peil A. Evaluation of scab and mildew resistance in the gene bank collection of apples in dresden-pillnitz. Plants. 2021;10:1227. doi: 10.3390/plants10061227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cao H., Li X., Dong X. Generation of broad-spectrum disease resistance by overexpression of an essential regulatory gene in systemic acquired resistance. Proc. Natl. Acad. Sci. USA. 1998;95:6531–6536. doi: 10.1073/pnas.95.11.6531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Thomma B.P., Eggermont K., Penninckx I.A., Mauch-Mani B., Vogelsang R., Cammue B.P., Broekaert W.F. Separate jasmonate-dependent and salicylate-dependent defense-response pathways in Arabidopsis are essential for resistance to distinct microbial pathogens. Proc. Natl. Acad. Sci. USA. 1998;95:15107–15111. doi: 10.1073/pnas.95.25.15107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chen X.K., Zhang J.Y., Zhang Z., Du X.L., Du B.B., Qu S.C. Overexpressing MhNPR1 in transgenic Fuji apples enhances resistance to apple powdery mildew. Mol. Biol. Rep. 2012;39:8083–8089. doi: 10.1007/s11033-012-1655-3. [DOI] [PubMed] [Google Scholar]
- 30.Pessina S., Palmieri L., Bianco L., Gassmann J., van de Weg E., Visser R.G.F., Magnago P., Schouten H.J., Bai Y.L., Velasco R.R., et al. Frequency of a natural truncated allele of MdMLO19 in the germplasm of Malus domestica. Mol. Breed. 2017;37:7. doi: 10.1007/s11032-016-0610-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kim J.G., Mudgett M.B. Tomato bHLH132 transcription factor controls growth and defense and is activated by xanthomonas euvesicatoria effector XopD during pathogenesis. Mol. Plant Microbe Interact. 2019;32:1614–1622. doi: 10.1094/MPMI-05-19-0122-R. [DOI] [PubMed] [Google Scholar]
- 32.Valea I., Motegi A., Kawamura N., Kawamoto K., Miyao A., Ozawa R. The rice wound-inducible transcription factor RERJ1 sharing same signal transduction pathway with OsMYC2 is necessary for defense response to herbivory and bacterial blight. Plant Mol. Biol. 2021;107:1402–1409. doi: 10.1007/s11103-021-01186-0. [DOI] [PubMed] [Google Scholar]
- 33.Yuan H.M., Liu W.C., Lu Y.T. CATALASE2 coordinates SA-mediated repression of both auxin accumulation and JA biosynthesis in plant defenses. Cell Host Microbe. 2017;21:143–155. doi: 10.1016/j.chom.2017.01.007. [DOI] [PubMed] [Google Scholar]
- 34.Djébali N., Jauneau A., Ameline-Torregrosa C., Chardon F., Jaulneau V., Mathé C., Bottin A., Cazaux M., Pilet-Nayel M.L., Baranger A., et al. Partial resistance of Medicago truncatula to Aphanomyces euteiches is associated with protection of the root stele and is controlled by a major QTL rich in proteasome-related genes. Mol. Plant Microbe Interact. 2009;22:1043–1055. doi: 10.1094/MPMI-22-9-1043. [DOI] [PubMed] [Google Scholar]
- 35.Bi Y.M., Kenton P., Mur L., Darby R., Draper J. Hydrogen peroxide does not function downstream of salicylic acid in the induction of PR protein expression. Plant J. 1995;8:235–245. doi: 10.1046/j.1365-313X.1995.08020235.x. [DOI] [PubMed] [Google Scholar]
- 36.Ton J., Jakab G., Toquin V., Flors V., Iavicoli A., Maeder M.N., Métraux J.P., Mauch-Mani B. Dissecting the beta-aminobutyric acid-induced priming phenomenon in Arabidopsis. Plant Cell. 2005;17:987–999. doi: 10.1105/tpc.104.029728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Cui K.C., Liu M., Ke G.H., Zhang X.Y., Mu B., Zhou M., Hu Y., Wen Y.Q. Transient silencing of VvCSN5 enhances powdery mildew resistance in grapevine (Vitis vinifera) Plant Cell Tissue Organ Cult. 2021;3:146. doi: 10.1007/s11240-021-02098-z. [DOI] [Google Scholar]
- 38.Derksen H., Rampitsch C., Daayf F. Signaling cross-talk in plant disease resistance. Plant Sci. 2013;207:79–87. doi: 10.1016/j.plantsci.2013.03.004. [DOI] [PubMed] [Google Scholar]
- 39.Burra D.D., Mühlenbock P., Andreasson E. Salicylic and jasmonic acid pathways are necessary for defence against Dickeya solani as revealed by a novel method for Blackleg disease screening of in vitro grown potato. Plant Biol. 2015;17:1030–1038. doi: 10.1111/plb.12339. [DOI] [PubMed] [Google Scholar]
- 40.Yin W., Wang X., Liu H., Wang Y., Nocker S., Tu M., Fang J., Guo J., Li Z., Wang X. Overexpression of VqWRKY31 enhances powdery mildew resistance in grapevine by promoting salicylic acid signaling and specific metabolite synthesis. Hortic. Res. 2022;9:uhab064. doi: 10.1093/hr/uhab064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Venugopal S.C., Jeong R.D., Mandal M.K., Zhu S., Chandra-Shekara A., Xia Y., Hersh M., Stromberg A.J., Navarre D., Kachroo A. Enhanced disease susceptibility 1 and salicylic acid act redundantly to regulate resistance gene-mediated signaling. PLoS Genet. 2009;5:e1000545. doi: 10.1371/journal.pgen.1000545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Dai L., Wang D., Xie X., Zhang C., Wang X., Xu Y., Wang Y., Zhang J. The Novel Gene VpPR4-1 from Vitis pseudoreticulata increases powdery mildew resistance in transgenic Vitis vinifera L. Front. Plant Sci. 2016;7:695. doi: 10.3389/fpls.2016.00695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Qi T., Huang H., Song S., Xie D. Regulation of jasmonate-mediated stamen development and seed production by a bHLH-MYB complex in Arabidopsis. Plant Cell. 2015;27:1620–1633. doi: 10.1105/tpc.15.00116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zhang X., Zhang L., Ji M., Wu Y., Zhang S., Zhu Y., Yao J., Li Z., Gao H., Wang X. Genome-wide identification and expression analysis of the B-box transcription factor gene family in grapevine (Vitis vinifera L.) BMC Genomics. 2021;22:221. doi: 10.1186/s12864-021-07479-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Clough S.J., Bent A.F. Floral dip: A simplified method for Agrobacterium-mediated transformation of Arabidopsis thaliana. Plant J. 1998;16:735–743. doi: 10.1046/j.1365-313x.1998.00343.x. [DOI] [PubMed] [Google Scholar]
- 46.Livak K.J., Schmittgen T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCt Method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data supporting the findings of this study are available within the article and its Supplementary Materials.