Abstract
Introduction: There are several pathologic mechanisms involved in diabetic nephropathy, but the role of oxidative stress seems to be one of the most important. Sodium-glucose cotransporter 2 (SGLT2) inhibitors are a relatively new class of antidiabetic drugs that might also have some other effects in addition to lowering glucose. The aim of this study was to evaluate the possible effects of the SGLT2 inhibitor empagliflozin on oxidative stress and renal function in diabetes. Methods: Male Wistar rats were randomly divided into four groups: control, control-treated, diabetic, and diabetic-treated (n = 8 per group). Diabetes was induced by a single intraperitoneal dose of streptozotocin (50 mg/kg). The treated animals received empagliflozin for 5 weeks (20 mg/kg/day/po). All groups were sacrificed on the 36th day, and blood and tissue samples were collected. Serum levels of urea, uric acid, creatinine, and glucose levels were determined. The level of malondialdehyde (MDA) and glutathione (GLT), as well as the activity of catalase (CAT) and superoxide dismutase (SOD), was measured in all groups. Data were analyzed using one-way Anova and paired T-tests, and p ≤ 0.05 was considered significant. Results: Diabetes significantly increased urea (p < 0.001), uric acid (p < 0.001), and creatinine (p < 0.001) in the serum, while the activities of CAT (p < 0.001) and SOD (p < 0.001) were reduced. GLT was also reduced (p < 0.001), and MDA was increased (p < 0.001) in non-treated animals. Treatment with empagliflozin improved renal function, as shown by a reduction in the serum levels of urea (p = 0.03), uric acid (p = 0.03), and creatinine (p < 0.001). Empagliflozin also increased the antioxidant capacity by increasing CAT (p = 0.035) and SOD (p = 0.02) activities and GLT content (p = 0.01) and reduced oxidative damage by lowering MDA (p < 0.001). Conclusions: It seems that uncontrolled diabetes induces renal insufficiency by decreasing antioxidant defense mechanisms and inducing oxidative stress. Empagliflozin might have additional benefits in addition to lowering glucose—-reversing these processes, improving antioxidative capacity, and improving renal function.
Keywords: diabetes mellitus, diabetic nephropathy, oxidative stress, SGLT2 inhibitor, empagliflozin, malondialdehyde
1. Introduction
The prevalence of diabetes mellitus (DM) is growing worldwide [1]. This chronic disease has negative effects on different metabolic pathways and induces pathophysiologic processes involved in diabetic complications [2]. Diabetic nephropathy, that develops in many patients with diabetes, is a frequent complication of this disease [3]. It is considered the leading cause of end-stage renal disease (ESRD) and is the second cause of mortality in patients with diabetes [4]. Therefore, preventing or reducing its progression and improving renal function is one of the major goals in the treatment of patients with diabetes [4]. Although its exact pathophysiology is so far unclear, the role of oxidative damage seems to be the most important [2,5]. Oxidative damage can be associated with endothelial dysfunction in patients with diabetes, and endothelial dysfunction could be measured through the flow-mediated dilation method [6,7]. Oxidative stress is a pathologic process which develops as a result of an imbalance between reactive oxygen species (ROS) and intrinsic antioxidant defense (ADS) and can be directed toward ROS [8]. ROS are short-living but highly active biomolecules with important physiologic roles in the control of gene expression, synaptic plasticity, cell growth, memory formation, transcription factor activation, etc. [9]. They are produced as normal by-products of metabolic pathways, and because they have unpaired electron(s), they are able to bind to biological targets such as DNA, proteins, and lipids [9]. In a normal milieu, antioxidative defense systems neutralize an excess of ROS and prevent subsequent oxidative injuries [9]. However, in the diabetic milieu, they are hyper-produced and often overwhelm ADS, and induce extensive oxidative damage in biomolecules [9]. Glucose metabolism disorders such as pre-diabetes and DM are important elements in the pathogenesis of insulin resistance which is itself a potent inducer of oxidative stress [10]. It has been shown that diabetes-induced oxidative stress can be closely associated with diabetic nephropathy and that induces it through several pathophysiologic pathways [5]. Therefore, the antioxidative agents may reverse these processes and could prevent or delay renal failure development in the diabetic milieu [11].
Sodium-glucose cotransporter 2 (SGLT2) inhibitors are a relatively new class of antidiabetic drugs that effectively reduce blood glucose levels [8]. They have inhibitory effects on urinary glucose reabsorption by blocking SGLT2s activity in renal proximal tubules and, therefore, induce massive urinary glucose excretion [8]. In vitro experiments (which are not influenced by changes in the systemic milieu) with one of these drugs—empagliflozin—also suggest that SGLT2 inhibitors may directly attenuate inflammation and oxidative stress, determining a reduction in the superoxide production and enhanced expression of glutathione s-reductase and catalase in the leukocytes of diabetic patients and downregulating IKK/NFκB, MKK7/JNK and JAK2/STST1 signaling pathways in LPS-stimulated macrophages, which could represent another pathway involved in this process [12]. Recent evidence has suggested that SGLT2 inhibitors might have additional benefits due to their antioxidative effects and that they could reduce oxidative damage in the kidneys [13,14,15,16,17,18,19]. Some studies have suggested that treatment with SGLT2 inhibitors could provide dual benefits: glucose lowering and nephroprotective effects [13]. However, there is not enough evidence to confirm their benefits from the kidneys. Therefore, the aim of this study was to evaluate the possible antioxidative effects of SGLT2 inhibitors on the kidneys and to assess their antioxidant effects on the markers of renal function in the diabetic milieu, thus providing more evidence to support the renal benefits of SGLT2 inhibitors.
2. Methods
2.1. Animals
Male healthy Wistar rats (200–220 g) were purchased from the animal house of the physiology research center in Semnan, Iran. They were kept in standard polyester cages (three rats per cage) in a laboratory room under a standard temperature (22 ± 2 °C) and humidity (55 ± 5%) with 12 h of light and dark cycles. They had free access to water and standard rodents’ chow throughout the study except before measuring the glucose levels. They were divided randomly into four groups: control (C), control + empagliflozin (CE), diabetes (D), and diabetes + empagliflozin (DE) (n = 8 per each group).
2.2. Diabetes Induction and Treatment
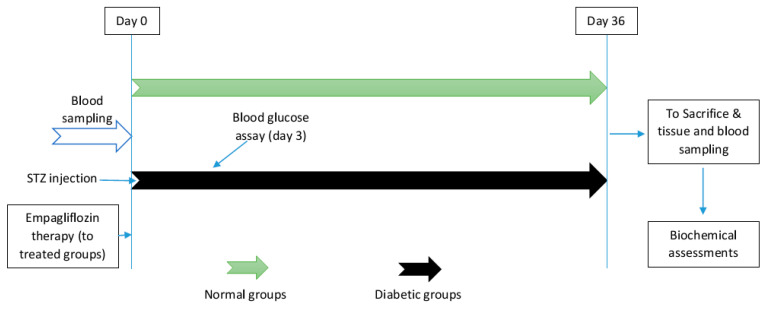
Diabetes was induced by an intraperitoneal injection of streptozotocin (STZ) (Sigma Aldrich, St. Louis, MO, USA) (50 mg/kg). After 72 h, blood samples were collected from the rat’s tail vein for a blood glucose assessment using a standard glucometer (Easy Gluco, Anyang-si, Republic of Korea), and rats with blood glucose above 220 mg/dL were considered diabetic before being randomly divided into two diabetic groups. Empagliflozin was dissolved daily in a suspension (5%) of CMC (carboxy methyl cellulose) and then was prescribed daily (20 mg/kg) by intragastric gavage with the two treated groups (CE and DE) for 5 weeks (Figure 1). The dose and duration of empagliflozin therapy were determined based on previous studies [20,21].
Figure 1.
Timeline of the current study. Final biochemical assays were performed on the collected blood samples and kidney tissues. STZ: streptozotocin.
2.3. Blood and Tissue Sampling
Blood samples were collected from the animals twice. At day zero, it was collected from the rats’ tail veins after 10 h of overnight fasting by inducing deep anesthesia through an intraperitoneal injection of ketamine (80 mg/kg) and xylazine (10 mg/kg). At the end of the 5th week, and after 10 h of overnight fasting, all rats were anesthetized, again by drugs, and were sacrificed by carbon dioxide. Then, blood (directly from the heart) and kidney tissue samples were collected immediately. Blood serum was separated immediately by centrifugation (3000 rpm for 10 min), and the samples were kept at −20 °C for biochemical tests, including glucose, creatinine, urea, and uric acid concentration measurements, which were all performed using standard commercial kits. The removed kidneys were kept at −20 °C until oxidative stress indicators such as the malondialdehyde (MDA) and glutathione (GLT) contents could be assessed, as well as the activities of the enzyme catalase (CAT) and superoxide dismutase (SOD).
2.4. Tissue Preparation
The collected renal tissues were weighed and homogenized on ice by a specialized electric tool after adding a homogenization medium (phosphate buffer (0.1 mol, pH = 7.4)). Homogenized mixtures were then centrifuged (at 4 °C and 4000 rpm for 20 min), and the supernatants (as the cytosolic extract of the renal tissues) were removed and stored at −80 °C for oxidative stress indicators. The following indicators were measured.
2.4.1. Superoxide Dismutase (SOD) Activity
SOD is the main element of the enzymatic antioxidant defense system. The activity of this enzyme can be assessed through the method of Winterbourn et al., which is based on SOD’s ability to inhibit the reduction in nitro-blue tetrazolium by superoxide anion [22]. This method was performed as follows: a 0.067 mole of potassium phosphate buffer (pH = 7.8) was added to 0.1 moles of EDTA (ethylenediaminetetraacetic acid) containing 0.3 mM sodium cyanide, 1.5 mM nitro-blue tetrazolium and 0.1 mL of the sample. Then, 0.12 mM riboflavin was added to each sample to activate the reaction and was incubated for 10 min. The sample optical absorbance was recorded at 560 nm for 5 min on a spectrophotometer. The amount of enzyme required to produce a 50% inhibition was taken as 1 Unit (U), and the results were expressed as U/mL.
2.4.2. Catalase (CAT) Activity
CAT is another enzyme that is involved in cellular antioxidant defense. The activity of this enzyme was calculated using the method of Abebi [23]. A mixture containing 0.85 mL of the potassium phosphate buffer 50 mM, pH 7.0, and 0.1 mL of the homogenate was incubated for 10 min at room temperature. The reaction was triggered by the addition of 0.05 mL H2O2 (30 mM prepared in potassium phosphate buffer 50 mM, pH = 7.0). Then, the optic absorbance reduction was recorded by a spectrophotometer for 3 min at 240 nm spectrum. Enzyme activity was expressed as U/mL (1 µmole H2O2).
2.4.3. Glutathione (GLT) Content
The GLT content was measured through the method of Tietze [24]. The cellular protein content of the collected supernatant was precipitated by the addition of sulfosalicylic acid (5%), which was added and removed by centrifugation (4000× g for 15 min). Then, the GLT content was assessed as follows: 100 µL of the protein-free supernatant was added to 810 µL of 0.3 mM Na2HPO4 and 90 µL of DTNB (5,5′-dithiobis(2-nitrobenzoic acid) in 0.1% sodium citrate. The DTNB absorbance was recorded at 412 nm for 5 min. A standard curve for GLT was recorded, and the sensitivity of the measurement was determined between 1 and 100 µM. The level of the GLT was estimated in µMol/mL.
2.4.4. Malondialdehyde (MDA) Content
MDA is a toxic byproduct and marker of lipid peroxidation and oxidative damage. Its concentration was assessed through the method of Satoh et al. [25]. In total, 0.5 mL of the supernatant and 1.5 mL of 10% trichloroacetic acid were mixed by centrifugation (4000× g for 10 min). Then, 1.5 mL of the supernatant and 2 mL of thiobarbituric acid (0.67%) were added and placed in a hot water bath in sealed tubes for 30 min and afterward allowed to chill at room temperature. Then, 2 mL of N-butanol was added, and the mixture was centrifuged (2000× g for 5 min). The resulting supernatant was removed, and its optic absorbance was measured at 532 nm on a spectrophotometer. The MDA content was determined using 1,1,3,3-tetraethoxypropane as a standard. MDA’s concentration was expressed in nMol/mL.
2.5. Data Analyses
The Kolmogorov–Smirnov test was used to examine the normal distribution of collected data. Then, one-way ANOVA was used to assess the possible differences among the analyzed factors between groups. A paired sample T-test was also used to assess the possible differences in the groups before and after the interventions. Tukey’s test was applied post hoc. Data were expressed as the mean ± SD (standard deviation), and p < 0.05 was considered to be a significant difference.
2.6. Ethical Considerations
All ethical protocols regarding animal rights and complying with the demands of local and global ethics committees as well as NIH Guidelines for the care and use of experimental animals were followed throughout the study. This study was approved by the research ethics committee of Semnan University of medical sciences (IR.SEMUMS.RC.1400.263).
3. Results
Table 1 shows the values of serum glucose (mg/dL) in all experimental groups at days 0 and 36 of the study. The STZ injection significantly increased it to 268 ± 22 (p = 0.002), causing diabetes, but empagliflozin significantly decreased it to 131 ± 11 mg/dl (Table 1).
Table 1.
Mean values of blood glucose in mg/dL (±SD) for all experimental groups at days 0 and 36 of the study.
| Groups | Serum Glucose | |
|---|---|---|
| Day 0 | Day 36 | |
| Control | 97 ± 11 | 103 ± 8 |
| Control + Empagliflozin | 96 ± 12 | 100 ± 9 |
| Diabetes | 268 ± 22 | 160 ± 13 |
| Diabetes + Empagliflozin | 268 ± 22 | 131 ± 11 |
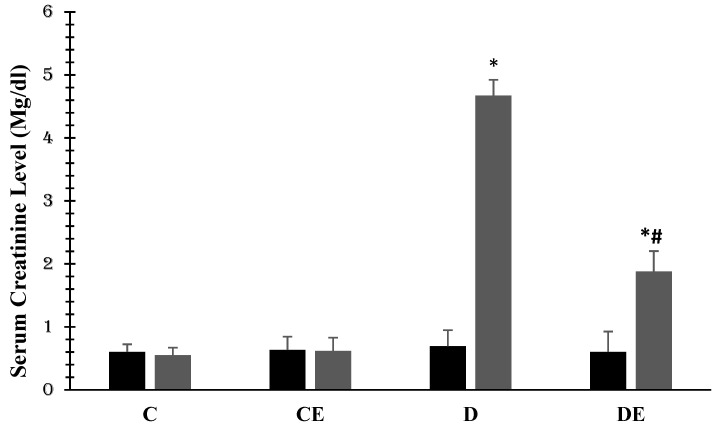
Figure 1 shows the changes in the mean value of serum creatinine (mg/dL) in all the groups. In the control group, this value was at the beginning and at the end 0.605 ± 0.12 and 0.55 ± 0.15, respectively. In diabetes, the value was significantly increased to 4.67 ± 0.254 (p < 0.001, compared with the control group). Empagliflozin had no significant effect on the blood creatinine in normal animals, but it reduced serum creatinine in diabetic animals to 1.88 ± 0.213 (p < 0.001, compared with the diabetic group) (Figure 2).
Figure 2.
Mean values of serum creatinine (mg/dL) in all experimental groups. Diabetes induction increased this value (p < 0.001), but empagliflozin therapy reduced it (p < 0.001) at days 36th (* (p < 0.001)) showing a significant difference when compared with the control (C) group; # (p < 0.001) and a significant difference when compared with the diabetic (D) group (1 = day 0 or first examination, 2 = day 36). (C = control, CE = control plus empagliflozin, D = diabetes, DE = diabetes plus empagliflozin).
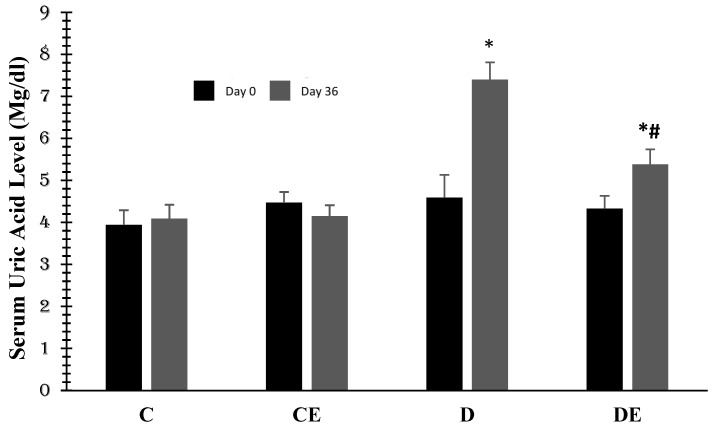
Figure 3 demonstrates the changes in the mean values of serum uric acid (mg/dL) in all the groups. There were no significant differences in the control and control-treated groups. The induction of diabetes significantly increased serum uric acid to 7.4 ± 0.41 (p < 0.001 compared to the control group). However, empagliflozin reduced it to 5.38 ± 0.36 (p = 0.03) when compared with the D group of diabetic rats.
Figure 3.
Mean values of serum uric acid (mg/dL) in all experimental groups; diabetes induction increased it (p < 0.001) in the D group, but empagliflozin therapy reduced it (p < 0.03) at days 36th. (* (p < 0.001)) with a significant difference when compared to the control (C); # (p = 0.03) and a significant difference when compared to the diabetic (D) group (1 = day 0 or first examination, 2 = day 36). (C = control, CE = control plus empagliflozin, D = diabetes, DE = diabetes plus empagliflozin).
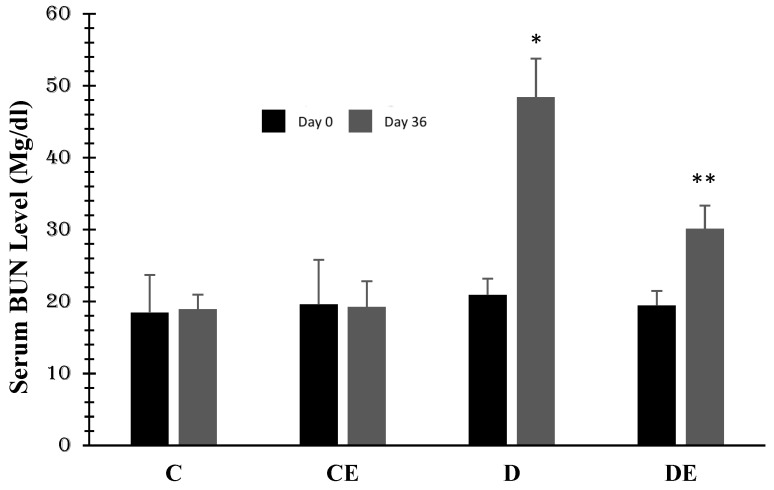
Changes in serum BUN (mg/dL) in all experimental groups are presented in Figure 3. There were no significant differences in BUN levels in the control and control-treated groups. Diabetes induction significantly increased BUN to 48.41 ± 8.21 (p < 0.001, compared to the control group) on the 36th day. However, empagliflozin therapy decreased it to 30.1 ± 6.59 (p = 0.03) when compared with the D group of diabetic rats (Figure 4).
Figure 4.
Mean values of serum BUN (mg/dL) in all groups. Diabetes induction increased it in the D group when compared with the control group (*, p < 0.001), but empagliflozin reduced it when compared with the D group (**, p = 0.03) (1 = day 0 or first examination, 2 = day 36). (C = control, CE = control plus empagliflozin, D = diabetes, DE = diabetes plus empagliflozin).
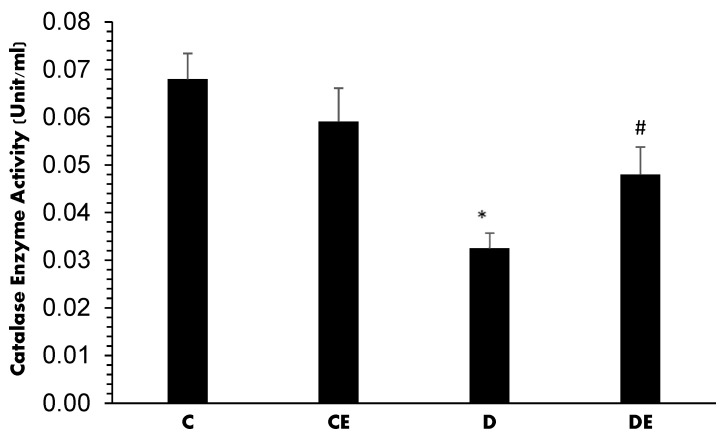
Figure 5 shows the CAT activity expressed as unit/mL in the kidney from all experimental groups. In normal and normal-treated animals, it was 0.068 ± 0.005 and 0.059 ± 0.007, respectively, with no significant difference. Diabetes induction significantly decreased to 0.0325 ± 0.01 (p = 0.01) when compared with the control (C) group. However, empagliflozin therapy increased it to 0.048 ± 0.005 (p = 0.01) when compared with the diabetic (D) group (Figure 5).
Figure 5.
Mean CAT activity (Unit/mL) in all experimental groups. Diabetes induction decreased this parameter significantly when compared with the C group (*, p < 0.001)), but empagliflozin improved it when compared with the D group (#, p = 0.035). (C = control, CE = control plus empagliflozin, D = diabetes, DE = diabetes plus empagliflozin).
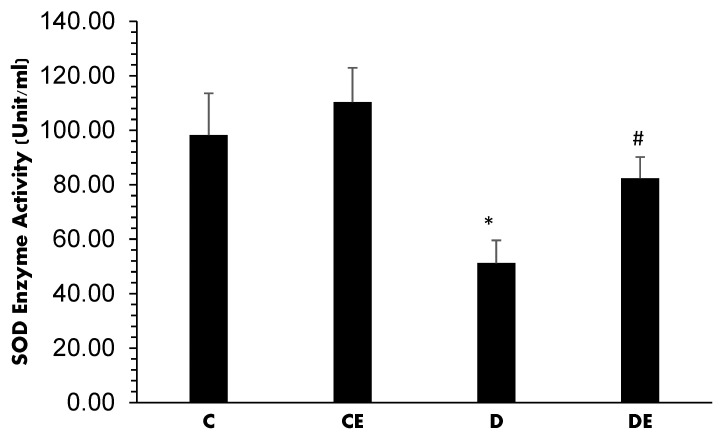
The changes in SOD activity (expressed as Units/mL) are presented in Figure 5. SOD activity was 98.23 ± 15.3 and 108.25 ± 12.6 in the control and control-treated groups, respectively, with no significant difference. Diabetes decreased significantly to 51.24 ± 8.32 (p < 0.001). However, empagliflozin significantly increased it to 82.36 ± 7.84 (p = 0.02) when compared with the diabetic (D) group (Figure 6).
Figure 6.
Mean values of SOD activity (Unit/mL) levels in all experimental groups. Diabetes induction decreased it when compared with the control group (*, p < 0.001), but empagliflozin increased it when compared with the D group (#, p = 0.02). (C = control, CE = control plus empagliflozin, D = diabetes, DE = diabetes plus empagliflozin).
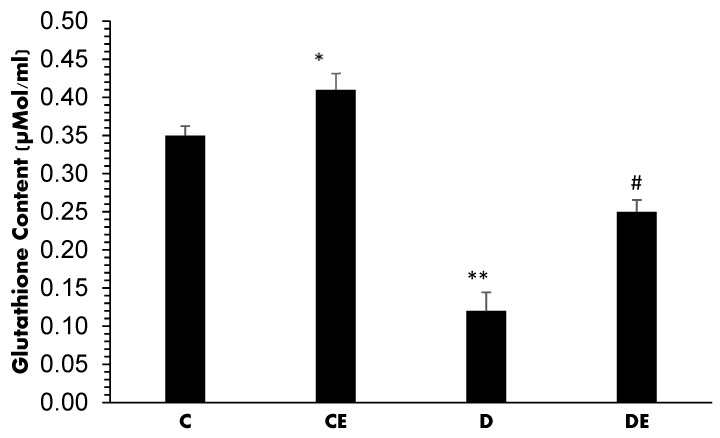
The changes in GLT concentrations (expressed as µMol/mL) are presented in Figure 7. In normal (C) and normally treated (CE) rats, the GLT content was 0.35 ± 0.012 and 0.41 ± 0.0215, respectively, with a significant difference (p = 0.045). Diabetes induction significantly decreased this value to 0.12 ± 0.024 (p < 0.001). However, treatment with empagliflozin increased it to 0.25 ± 0.014 (p = 0.01); however, this was still lower compared to the control group (p < 0.001).
Figure 7.
Mean values of GLT contents (µMol/mL) in all experimental groups. Diabetes induction decreased the GLT content when compared with the C group (**, p < 0.001). Empagliflozin improved it in non-diabetic (CE) (*, p = 0.045) and diabetic (DE) (#, p = 0.01) animals. (C = control, CE = control plus empagliflozin, D = diabetes, DE = diabetes plus empagliflozin).
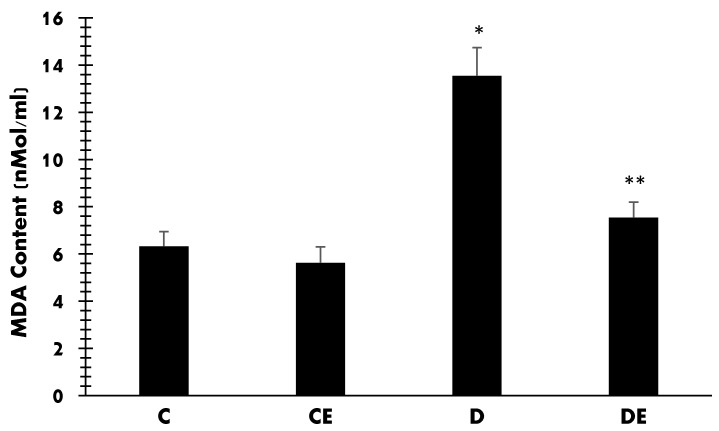
The MDA contents (nMol/mL) in all groups are shown in Figure 8. In control (C) and control-treated (CE) animals, the MDA contents were 6.32 ± 0.62 and 5.62 ± 0.68, respectively, with no significant difference. Diabetes increased MDA significantly to 13.54 ± 1.25 (p < 0.001). However, empagliflozin reduced it to 7.54 ± 0.65 (p < 0.001).
Figure 8.
Mean values of MDA content (nMol/mL) in all groups. Diabetes induction increased it when compared with the control group (*, p < 0.001), but empagliflozin therapy reduced it when compared with the D group (**, p = 0.04). (C = control, CE = control plus empagliflozin, D = diabetes, DE = diabetes plus empagliflozin).
4. Discussion
This study demonstrated that the antidiabetic drug empagliflozin could have dual benefits—not only lowering glucose but also providing benefits for the kidneys in diabetes. It also showed that chronic uncontrolled hyperglycemia suppresses ADS capacity and induces oxidative stress and toxic byproduct generation leading to renal insufficiency and diabetic nephropathy. However, the SGLT2 inhibitor empagliflozin reversed these processes and improve the renal function by increasing ADS and protecting against oxidative injury in renal tissues.
Nephropathy induced by diabetes is the leading cause of ESRD and kidney failure in patients of hemodialysis [26,27] with diabetes after a median follow-up of 15 years [28,29]. This chronic complication of diabetes was primarily characterized by an increased urinary albumin excretion or decreased estimated glomerular filtration rate (eGFR), or both [29]. It is often accompanied by biochemical changes in the serum, such as increased creatinine, BUN, and uric acid levels, as well as a reduced albumin concentration [30,31]. The exact pathophysiology of this entity is so far not clear; however, the role of oxidative stress seems to be the most important [5]. As was stated before, the diabetic milieu is usually accompanied by different levels of oxidative stress due to the activation of different pathophysiologic pathways and pro-oxidant enzymes, e.g., NOX (nicotinamide adenine dinucleotide phosphate oxidase) [9]. Oxidative stress has a negative impact on podocytes, and it destroys the negative charges of the filtration barrier, allowing proteins (mostly albumin) to cross this barrier [5]. Oxidative stress also has a significant effect on the extensive vascular network of the kidneys, inducing vascular injury by activating the renin-angiotensin system and causing hemodynamic changes alongside the formation of atheromatous plaques in big vessels [5,32]. Therefore, suppressing it and reducing free radical levels by using antioxidants could be an effective adjuvant approach for the treatment of diabetic nephropathy [11].
In this study, the induction of diabetes was accompanied by suppressing ADS in the renal tissue. It reduced the activity of SOD, CAT, and GLT concentrations which are the major elements of ADS in the kidneys. The level of MDA as a toxic byproduct of oxidative damage was also higher in diabetic kidneys compared with non-diabetic kidneys. This finding was in accordance with the results of our previous studies [33,34,35] as well as some other reports [36,37]. Diabetes has negative impacts on ADS at several steps: at the transcriptional, post-transcriptional, and functional levels [9]. It can change the regulation and expression of enzymes such as SOD and CAT [38,39]; it can also reduce GLT synthesis [40] and increase its utilization [41], which all leads to reduced levels of antioxidants and decreased ADS which, in turn, increases the risk of oxidative stress [9]. The findings of this study showed that the diabetes-induced decrease in ADS caused oxidative stress in the kidneys [5]. These changes were accompanied by renal insufficiency, which was marked by an increase in BUN, uric acid, and creatinine levels in the serum. Treatment with the SGLT2 inhibitor empagliflozin improved diabetes-induced diabetic nephropathy in these animals to a certain extent. In addition to improving the serum glucose level, empagliflozin improved the ADS capacity and decreased oxidative injury (as shown by MDA levels) in diabetic kidneys. This was in accordance with the findings of some previous studies, which suggested that empagliflozin might increase ADS capacity [42,43]. However, since there was little evidence concerning its effects on diabetic kidneys, this study provided such evidence. Based on the current evidence, SGLT2 inhibition by empagliflozin increased ADS capacity and provided antioxidative beneficial effects in diabetic kidneys. These effects were accompanied by an improvement in renal function, as shown by the improved serum levels of BUN, uric acid, and creatinine. However, the net effect of empagliflozin in non-diabetic animals was not significant. Diabetes induction increased uric acid, and empagliflozin reduced it. This is important to mention since in the plasma, uric acid has antioxidant properties while, on the contrary, in the cytoplasm or in the atherogenic plaque, it has a pro-oxidative role promoting oxidative stress and thus contributing to the development of cardiovascular disease. [44]. The limitations of this study include a lack of assessment of the evaluated factors using PCR, Western blotting, or ELISA. Nevertheless, previous studies [45,46] have indicated these effects at molecular levels. There is also a need for additional evaluations using genetic techniques, which should be used in further studies which are already planned. However, the findings of this study have demonstrated the effects of diabetes induction and empagliflozin therapy on the examined parameters. Another limitation is that the streptozotocin model is not the best one to evaluate the development of diabetic nephropathy in type 2 diabetes. The duration of the study was short, and the changes observed in such a limited time interval might not reflect the long-term changes in type 2 diabetes. The strength of this study is that its results suggest the anti-oxidative properties of SGLT2 inhibitor empagliflozin and its benefits on the kidneys in the diabetic milieu.
5. Conclusions
Uncontrolled diabetes significantly decreased ADS and induced renal insufficiency. However, treatment with empagliflozin could provide dual benefits, not only in serum glucose reduction but also with beneficial effects on the kidneys. Empagliflozin improved ADS in the kidney tissue and protected it against oxidative damage. It could improve the parameters of renal function by providing antioxidative benefits in the diabetic milieu.
Author Contributions
Conceptualization, H.Y., Ž.R. and A.S.; Methodology, H.Y., M.A.H., A.S. and Ž.R.; Formal analysis, M.A.H., M.M. and I.R.; Investigation, H.Y., M.A.H., F.N., M.M., T.J. and I.R.; Data curation, H.Y., F.N., T.J. and I.R.; Writing—original draft, H.Y., Ž.R. and A.S.; Writing—review & editing, M.A.H., F.N., M.M., T.J., I.R., Ž.R. and A.S.; Supervision, H.Y., Ž.R. and A.S. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
This study was approved by the research ethics committee of Semnan University of medical sciences (IR.SEMUMS.RC.1400.263).
Informed Consent Statement
Not applicable.
Data Availability Statement
Data associated with this study could be accessed from the authors on a reasonable request.
Conflicts of Interest
The authors declare no conflict of interest.
Funding Statement
This study was conducted using a grant provided by the Deputy of Research and Technology of Semnan University of Medical Sciences (No: 1401/16/1200).
Footnotes
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
References
- 1.Mobasseri M., Shirmohammadi M., Amiri T., Vahed N., Fard H.H., Ghojazadeh M. Prevalence and incidence of type 1 diabetes in the world: A systematic review and meta-analysis. Health Promot. Perspect. 2020;10:98–115. doi: 10.34172/hpp.2020.18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Forbes J.M., Cooper M.E. Mechanisms of Diabetic Complications. Physiol. Rev. 2013;93:137–188. doi: 10.1152/physrev.00045.2011. [DOI] [PubMed] [Google Scholar]
- 3.Donate-Correa J., Luis-Rodríguez D., Martín-Núñez E., Tagua V.G., Hernández-Carballo C., Ferri C., Rodríguez-Rodríguez A.E., Mora-Fernández C., Navarro-González J.F. Inflammatory Targets in Diabetic Nephropathy. J. Clin. Med. 2020;9:458. doi: 10.3390/jcm9020458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jorsal A., Tarnow L., Frystyk J., Lajer M., Flyvbjerg A., Parving H.-H., Vionnet N., Rossing P. Serum adiponectin predicts all-cause mortality and end stage renal disease in patients with type I diabetes and diabetic nephropathy. Kidney Int. 2008;74:649–654. doi: 10.1038/ki.2008.201. [DOI] [PubMed] [Google Scholar]
- 5.Yaribeygi H., Farrokhi F.R., Rezaee R., Sahebkar A. Oxidative stress induces renal failure: A review of possible molecular pathways. J. Cell. Biochem. 2018;119:2990–2998. doi: 10.1002/jcb.26450. [DOI] [PubMed] [Google Scholar]
- 6.Capellini V.K., Celotto A.C., Baldo C.F., Olivon V.C., Viaro F., Rodrigues A.J., Evora P.R.B. Diabetes and vascular disease: Basic concepts of nitric oxide physiology, endothelial dysfunction, oxidative stress and therapeutic possibilities. Curr. Vasc. Pharmacol. 2010;8:526–544. doi: 10.2174/157016110791330834. [DOI] [PubMed] [Google Scholar]
- 7.Mućka S., Miodońska M., Jakubiak G.K., Starzak M., Cieślar G., Stanek A. Endothelial Function Assessment by Flow-Mediated Dilation Method: A Valuable Tool in the Evaluation of the Cardiovascular System. Int. J. Environ. Res. Public Health. 2022;19:11242. doi: 10.3390/ijerph191811242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yaribeygi H., Sathyapalan T., Atkin S.L., Sahebkar A. Molecular Mechanisms Linking Oxidative Stress and Diabetes Mellitus. Oxidative Med. Cell. Longev. 2020;2020:8609213. doi: 10.1155/2020/8609213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yaribeygi H., Atkin S.L., Sahebkar A. A review of the molecular mechanisms of hyperglycemia-induced free radical generation leading to oxidative stress. J. Cell. Physiol. 2019;234:1300–1312. doi: 10.1002/jcp.27164. [DOI] [PubMed] [Google Scholar]
- 10.Jakubiak G.K., Osadnik K., Lejawa M., Osadnik T., Goławski M., Lewandowski P., Pawlas N. “Obesity and Insulin Resistance” Is the Component of the Metabolic Syndrome Most Strongly Associated with Oxidative Stress. Antioxidants. 2021;11:79. doi: 10.3390/antiox11010079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tavafi M. Diabetic nephropathy and antioxidants. J. Nephropathol. 2013;2:20–27. doi: 10.5812/nephropathol.9093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Salvatore T., Galiero R., Caturano A., Rinaldi L., Di Martino A., Albanese G., Di Salvo J., Epifani R., Marfella R., Docimo G., et al. An Overview of the Cardiorenal Protective Mechanisms of SGLT2 Inhibitors. Int. J. Mol. Sci. 2022;23:3651. doi: 10.3390/ijms23073651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Winiarska A., Knysak M., Nabrdalik K., Gumprecht J., Stompór T. Inflammation and Oxidative Stress in Diabetic Kidney Disease: The Targets for SGLT2 Inhibitors and GLP-1 Receptor Agonists. Int. J. Mol. Sci. 2021;22:10822. doi: 10.3390/ijms221910822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yaribeygi H., Ashrafizadeh M., Henney N.C., Sathyapalan T., Jamialahmadi T., Sahebkar A. Neuromodulatory effects of an-ti-diabetes medications: A mechanistic review. Pharmacol. Res. 2020;152:104611. doi: 10.1016/j.phrs.2019.104611. [DOI] [PubMed] [Google Scholar]
- 15.Yaribeygi H., Atkin S.L., Butler A.E., Sahebkar A. Sodium–glucose cotransporter inhibitors and oxidative stress: An update. J. Cell. Physiol. 2019;234:3231–3237. doi: 10.1002/jcp.26760. [DOI] [PubMed] [Google Scholar]
- 16.Yaribeygi H., Atkin S.L., Jamialahmadi T., Sahebkar A. A review on the effects of new anti-diabetic drugs on platelet function. Endocr. Metab. Immune Disord.-Drug Targets (Former. Curr. Drug Targets-Immune Endocr. Metab. Disord.) 2020;20:328–334. doi: 10.2174/1871530319666191014110414. [DOI] [PubMed] [Google Scholar]
- 17.Yaribeygi H., Maleki M., Butler A.E., Jamialahmadi T., Sahebkar A. Sodium-glucose cotransporter 2 inhibitors and mitochondrial functions: State of the art. EXCLI J. 2023;22:53–66. doi: 10.17179/excli2022-5482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yaribeygi H., Maleki M., Butler A.E., Jamialahmadi T., Sahebkar A. Sodium-glucose co-transporter-2 inhibitors and epicardial adiposity. Eur. J. Pharm. Sci. 2022;180:106322. doi: 10.1016/j.ejps.2022.106322. [DOI] [PubMed] [Google Scholar]
- 19.Yaribeygi H., Maleki M., Nasimi F., Butler A.E., Jamialahmadi T., Sahebkar A. Sodium-glucose co-transporter 2 inhibitors and hematopoiesis. J. Cell. Physiol. 2022;237:3778–3787. doi: 10.1002/jcp.30851. [DOI] [PubMed] [Google Scholar]
- 20.Botros S.R., Matouk A.I., Anter A., Khalifa M.M., Heeba G.H. Protective effect of empagliflozin on gentamicin-induced acute renal injury via regulation of SIRT1/NF-κB signaling pathway. Environ. Toxicol. Pharmacol. 2022;94:103907. doi: 10.1016/j.etap.2022.103907. [DOI] [PubMed] [Google Scholar]
- 21.Kolkhof P., Hartmann E., Freyberger A., Pavkovic M., Mathar I., Sandner P., Droebner K., Joseph A., Hüser J., Eitner F. Effects of finerenone combined with empagliflozin in a model of hypertension-induced end-organ damage. Am. J. Nephrol. 2021;52:1–11. doi: 10.1159/000516213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Winterbourn C.C., E Hawkins R., Brian M., Carrell R.W. The estimation of red cell superoxide dismutase activity. Transl. Res. 1975;85:337–341. [PubMed] [Google Scholar]
- 23.Aebi H. Methods in Enzymology. Volume 105. Academic Press; Cambridge, MA, USA: 1984. Catalase in vitro; pp. 121–126. [DOI] [PubMed] [Google Scholar]
- 24.Tietze F. Enzymic method for quantitative determination of nanogram amounts of total and oxidized glutathione: Applications to mammalian blood and other tissues. Anal. Biochem. 1969;27:502–522. doi: 10.1016/0003-2697(69)90064-5. [DOI] [PubMed] [Google Scholar]
- 25.Satoh M., Fujimoto S., Haruna Y., Arakawa S., Horike H., Komai N., Sasaki T., Tsujioka K., Makino H., Kashihara N. NAD(P)H oxidase and uncoupled nitric oxide synthase are major sources of glomerular superoxide in rats with experimental diabetic nephropathy. Am. J. Physiol. Physiol. 2005;288:F1144–F1152. doi: 10.1152/ajprenal.00221.2004. [DOI] [PubMed] [Google Scholar]
- 26.Kakio Y., A Uchida H., Takeuchi H., Okuyama Y., Okuyama M., Umebayashi R., Wada K., Sugiyama H., Sugimoto K., Rakugi H., et al. Diabetic nephropathy is associated with frailty in patients with chronic hemodialysis. Geriatr. Gerontol. Int. 2018;18:1597–1602. doi: 10.1111/ggi.13534. [DOI] [PubMed] [Google Scholar]
- 27.Sagoo M.K., Gnudi L. Diabetic Nephropathy: An Overview. Diabet. Nephrop. 2019;2067:3–7. doi: 10.1007/978-1-4939-9841-8_1. [DOI] [PubMed] [Google Scholar]
- 28.Retnakaran R., Cull C.A., Thorne K.I., Adler A.I., Holman R.R., UKPDS Study Group Risk factors for renal dysfunction in type 2 diabetes: UK Prospective Diabetes Study 74. Diabetes. 2006;55:1832–1839. doi: 10.2337/db05-1620. [DOI] [PubMed] [Google Scholar]
- 29.Hussain S., Jamali M.C., Habib A., Hussain S., Akhtar M., Najmi A.K. Diabetic kidney disease: An overview of prevalence, risk factors, and biomarkers. Clin. Epidemiology Glob. Health. 2020;9:2–6. doi: 10.1016/j.cegh.2020.05.016. [DOI] [Google Scholar]
- 30.Idowu A.A., Ajose A.O., Adedeji A.T., Adegoke A.O., Jimoh K.A. Microalbuminuria, other markers of nephropathy and biochemical derangements in type 2 diabetes mellitus: Relationships and determinants. Ghana Med. J. 2017;51:56–63. [PMC free article] [PubMed] [Google Scholar]
- 31.Schleicher E.D. The Kidney and Hypertension in Diabetes Mellitus. Springer; Berlin/Heidelberg, Germany: 1998. Biochemical aspects of diabetic nephropathy; pp. 269–279. [Google Scholar]
- 32.Kamiyama M., Urushihara M., Morikawa T., Konishi Y., Imanishi M., Nishiyama A., Kobori H. Oxidative Stress/Angiotensinogen/Renin-Angiotensin System Axis in Patients with Diabetic Nephropathy. Int. J. Mol. Sci. 2013;14:23045–23062. doi: 10.3390/ijms141123045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yaribeygi H., Mohammadi M.T., Sahebkar A. Crocin potentiates antioxidant defense system and improves oxidative damage in liver tissue in diabetic rats. Biomed. Pharmacother. 2018;98:333–337. doi: 10.1016/j.biopha.2017.12.077. [DOI] [PubMed] [Google Scholar]
- 34.Yaribeygi H., Mohammadi M.T., Sahebkar A. PPAR-α Agonist Improves Hyperglycemia-Induced Oxidative Stress in Pancreatic Cells by Potentiating Antioxidant Defense System. Drug Res. 2018;68:355–360. doi: 10.1055/s-0043-121143. [DOI] [PubMed] [Google Scholar]
- 35.Yaribeygi H., Mohammadi M.T., Rezaee R., Sahebkar A. Crocin improves renal function by declining Nox-4, IL-18, and p53 expression levels in an experimental model of diabetic nephropathy. J. Cell. Biochem. 2018;119:6080–6093. doi: 10.1002/jcb.26806. [DOI] [PubMed] [Google Scholar]
- 36.Gilani S.J., Bin-Jumah M.N., Al-Abbasi F.A., Nadeem M.S., Afzal M., Sayyed N., Kazmi I. Fustin ameliorates hyperglycemia in streptozotocin induced type-2 diabetes via modulating glutathione/Superoxide dismutase/Catalase expressions, suppress lipid peroxidation and regulates histopathological changes. Saudi J. Biol. Sci. 2021;28:6963–6971. doi: 10.1016/j.sjbs.2021.07.070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sanad F.A.-A., Ahmed S.F., El-Tantawy W.H. Antidiabetic and hypolipidemic potentials of Solidago virgaurea extract in alloxan-induced diabetes type 1. Arch. Physiol. Biochem. 2022;128:716–723. doi: 10.1080/13813455.2020.1722705. [DOI] [PubMed] [Google Scholar]
- 38.Sadi G., Yılmaz, Güray T., Yilmaz O. Effect of vitamin C and lipoic acid on streptozotocin-induced diabetes gene expression: mRNA and protein expressions of Cu–Zn SOD and catalase. Mol. Cell. Biochem. 2008;309:109–116. doi: 10.1007/s11010-007-9648-6. [DOI] [PubMed] [Google Scholar]
- 39.Sindhu R.K., Koo J.R., Roberts C.K., Vaziri N.D. Dysregulation of hepatic superoxide dismutase, catalase and glutathione peroxidase in diabetes: Response to insulin and antioxidant therapies. Clin. Exp. Hypertens. 2004;26:43–53. doi: 10.1081/CEH-120027330. [DOI] [PubMed] [Google Scholar]
- 40.Lutchmansingh F.K., Hsu J.W., Bennett F.I., Badaloo A., McFarlane-Anderson N., Gordon-Strachan G.M., Wright-Pascoe R.A., Jahoor F., Boyne M.S. Glutathione metabolism in type 2 diabetes and its relationship with microvascular complications and glycemia. PLoS ONE. 2018;13:e0198626. doi: 10.1371/journal.pone.0198626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Darmaun D., Smith S.D., Sweeten S., Sager B.K., Welch S., Mauras N. Evidence for Accelerated Rates of Glutathione Utilization and Glutathione Depletion in Adolescents With Poorly Controlled Type 1 Diabetes. Diabetes. 2005;54:190–196. doi: 10.2337/diabetes.54.1.190. [DOI] [PubMed] [Google Scholar]
- 42.Jigheh Z.A., Haghjo A.G., Argani H., Roshangar L., Rashtchizadeh N., Sanajou D., Ahmad S.N.S., Rashedi J., Dastmalchi S., Abbasi M.M. Empagliflozin alleviates renal inflammation and oxidative stress in streptozotocin-induced diabetic rats partly by repressing HMGB1-TLR4 receptor axis. Iran J. Basic Med. Sci. 2019;22:384–390. doi: 10.22038/IJBMS.2019.31788.7651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Iannantuoni F.M., De Marañon A., Diaz-Morales N., Falcon R., Bañuls C., Abad-Jimenez Z., Victor V.M., Hernandez-Mijares A., Rovira-Llopis S. The SGLT2 inhibitor empagliflozin ameliorates the inflammatory profile in type 2 diabetic patients and promotes an antioxidant response in leukocytes. J. Clin. Med. 2019;8:1814. doi: 10.3390/jcm8111814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Nevola R., Alfano M., Pafundi P.C., Brin C., Gragnano F., Calabrò P., Adinolfi L.E., Rinaldi L., Sasso F.C., Caturano A. Cardiorenal Impact of SGLT-2 Inhibitors: A Conceptual Revolution in The Management of Type 2 Diabetes, Heart Failure and Chronic Kidney Disease. Rev. Cardiovasc. Med. 2022;23:0106. doi: 10.31083/j.rcm2303106. [DOI] [PubMed] [Google Scholar]
- 45.Oelze M., Kröller-Schön S., Welschof P., Jansen T., Hausding M., Mikhed Y., Stamm P., Mader M., Zinßius E., Agdauletova S., et al. The sodium-glucose co-transporter 2 inhibitor empagliflozin improves diabetes-induced vascular dysfunction in the strepto-zotocin diabetes rat model by interfering with oxidative stress and glucotoxicity. PLoS ONE. 2014;9:e112394. doi: 10.1371/journal.pone.0112394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Steven S., Oelze M., Hanf A., Kröller-Schön S., Kashani F., Roohani S., Welschof P., Kopp M., Gödtel-Armbrust U., Xia N., et al. The SGLT2 inhibitor empagliflozin improves the primary diabetic complications in ZDF rats. Redox Biol. 2017;13:370–385. doi: 10.1016/j.redox.2017.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data associated with this study could be accessed from the authors on a reasonable request.