Abstract
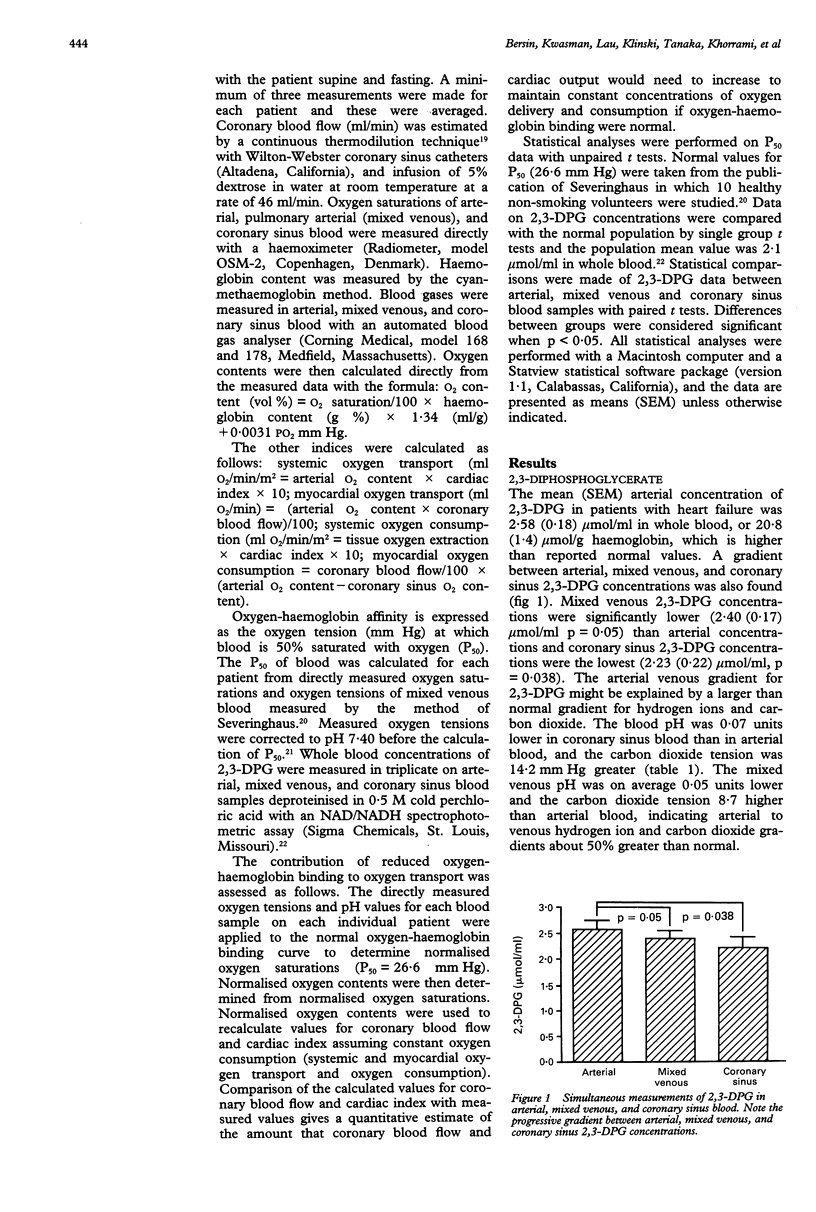
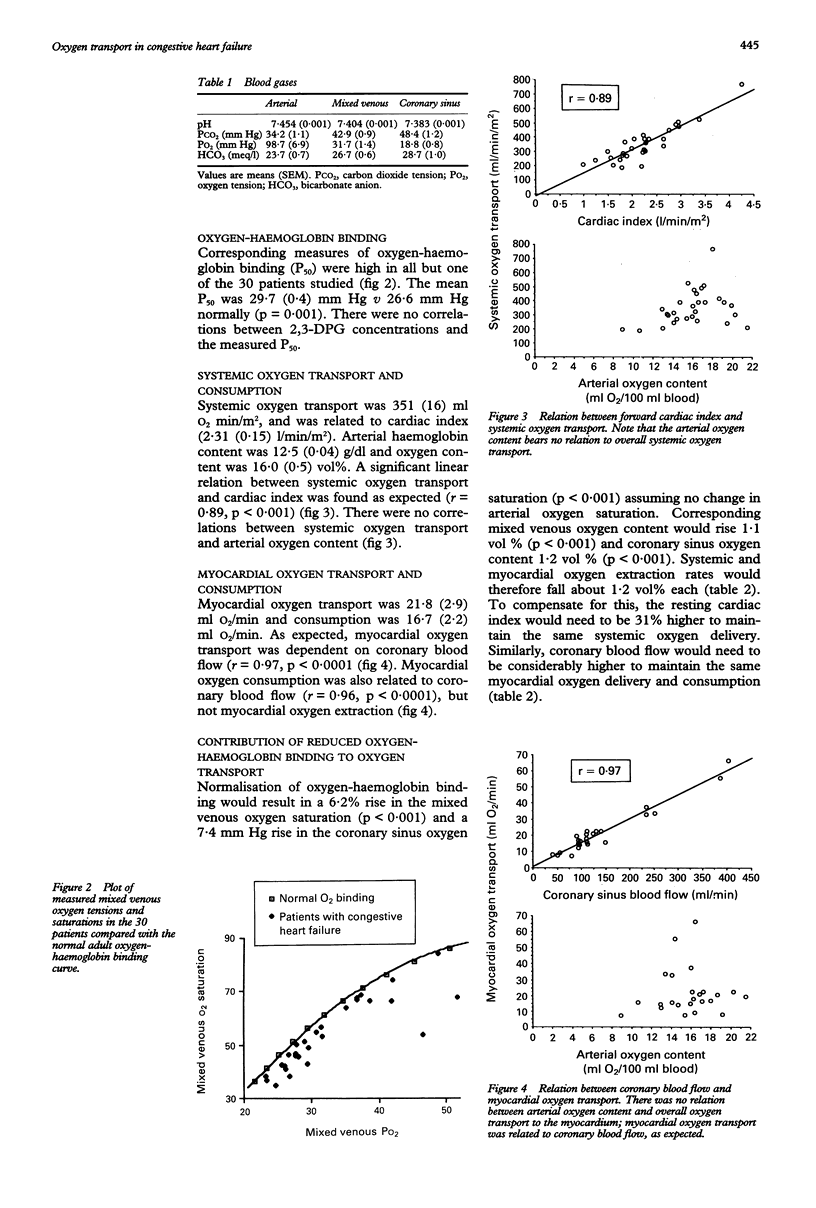
OBJECTIVE--To assess the importance of 2,3-diphosphoglycerate (2,3-DPG) and oxygen-haemoglobin binding to oxygen transport in patients with congestive heart failure. METHODS--In 30 patients with severe congestive heart failure, arterial, mixed venous, and coronary sinus venous blood concentrations of 2,3-DPG were measured and systemic output and coronary sinus blood flow were measured by a thermodilution technique. Oxygen-haemoglobin affinity was expressed as the oxygen tension in mm Hg at which blood is 50% saturated with oxygen (P50). RESULTS--Compared with normal values, 2,3-DPG was high in arterial blood (2.58 mumol/ml, p = 0.01; 20.8 mumol/g haemoglobin, p < 0.0001). Significant gradients between arterial, mixed venous, and coronary sinus blood 2,3-DPG concentrations were also found (mixed venous = 2.40 mumol/ml, p = 0.05 v arterial blood; coronary sinus venous blood = 2.23 mumol/ml, p < 0.04 v arterial blood). P50 was correspondingly high compared with the accepted normal value (mean 29.7 mm Hg, normal 26.6 mm Hg, p < 0.001). Systemic oxygen transport (351 ml O2/min/m2) varied directly with the forward cardiac index (r = 0.89, p < 0.0001). There was no relation between systemic oxygen transport and arterial oxygen content. Similarly, myocardial oxygen transport was found to vary directly with coronary sinus blood flow. Calculations of changes in cardiac index and coronary sinus blood flow at normal oxygen-haemoglobin binding indicate that a considerable increase in cardiac index and coronary blood flow would be required to maintain similar systemic and myocardial oxygen transport. CONCLUSIONS--In patients with severe heart failure increased 2,3-DPG and reduced oxygen-haemoglobin binding may be compensatory mechanisms that maintain adequate systemic and delivery of oxygen to myocardial tissue.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ALELLA A., WILLIAMS F. L., BOLENE-WILLIAMS C., KATZ L. N. Interrelation between cardiac oxygen consumption and coronary blood flow. Am J Physiol. 1955 Dec;183(3):570–582. doi: 10.1152/ajplegacy.1955.183.3.570. [DOI] [PubMed] [Google Scholar]
- Baim D. S., Rothman M. T., Harrison D. C. Simultaneous measurement of coronary venous blood flow and oxygen saturation during transient alterations in myocardial oxygen supply and demand. Am J Cardiol. 1982 Mar;49(4):743–752. doi: 10.1016/0002-9149(82)91954-3. [DOI] [PubMed] [Google Scholar]
- Benesch R., Benesch R. E., Yu C. I. Reciprocal binding of oxygen and diphosphoglycerate by human hemoglobin. Proc Natl Acad Sci U S A. 1968 Feb;59(2):526–532. doi: 10.1073/pnas.59.2.526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berman W., Jr, Wood S. C., Yabek S. M., Dillon T., Fripp R. R., Burstein R. Systemic oxygen transport in patients with congenital heart disease. Circulation. 1987 Feb;75(2):360–368. doi: 10.1161/01.cir.75.2.360. [DOI] [PubMed] [Google Scholar]
- Bristow J. D., Metcalfe J., Krall M. A., Welch J. E., Black J. A., Dhindsa D. S. Reduction of blood oxygen affinity in dogs by infusion of glycolytic intermediates. J Appl Physiol Respir Environ Exerc Physiol. 1977 Jul;43(1):102–106. doi: 10.1152/jappl.1977.43.1.102. [DOI] [PubMed] [Google Scholar]
- Cain S. M. Oxygen delivery and uptake in dogs during anemic and hypoxic hypoxia. J Appl Physiol Respir Environ Exerc Physiol. 1977 Feb;42(2):228–234. doi: 10.1152/jappl.1977.42.2.228. [DOI] [PubMed] [Google Scholar]
- De Marco T., Chatterjee K., Rouleau J. L., Parmley W. W. Abnormal coronary hemodynamics and myocardial energetics in patients with chronic heart failure caused by ischemic heart disease and dilated cardiomyopathy. Am Heart J. 1988 Apr;115(4):809–815. doi: 10.1016/0002-8703(88)90883-6. [DOI] [PubMed] [Google Scholar]
- Eaton J. W., Brewer G. J. The relationship between red cell 2,3-diphosphoglycerate and levels of hemoglobin in the human. Proc Natl Acad Sci U S A. 1968 Oct;61(2):756–760. doi: 10.1073/pnas.61.2.756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FINCH S., HASKINS D., FINCH C. A. Iron metabolism; hematopoiesis following phlebotomy; iron as a limiting factor. J Clin Invest. 1950 Aug;29(8):1078–1086. doi: 10.1172/JCI102339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flenley D. C., Fairweather L. J., Cooke N. J., Kirby B. J. Changes in haemoglobin binding curve and oxygen transport in chronic hypoxic lung disease. Br Med J. 1975 Mar 15;1(5958):602–604. doi: 10.1136/bmj.1.5958.602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ganz W., Donoso R., Marcus H. S., Forrester J. S., Swan H. J. A new technique for measurement of cardiac output by thermodilution in man. Am J Cardiol. 1971 Apr;27(4):392–396. doi: 10.1016/0002-9149(71)90436-x. [DOI] [PubMed] [Google Scholar]
- Ganz W., Tamura K., Marcus H. S., Donoso R., Yoshida S., Swan H. J. Measurement of coronary sinus blood flow by continuous thermodilution in man. Circulation. 1971 Aug;44(2):181–195. doi: 10.1161/01.cir.44.2.181. [DOI] [PubMed] [Google Scholar]
- KENNEDY A. C., VALTIS D. J. The oxygen dissociation curve in anemia of various types. J Clin Invest. 1954 Oct;33(10):1372–1381. doi: 10.1172/JCI103014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kilmartin J. V. Interaction of haemoglobin with protons, CO2 and 2,3-diphosphoglycerate. Br Med Bull. 1976 Sep;32(3):209–212. doi: 10.1093/oxfordjournals.bmb.a071364. [DOI] [PubMed] [Google Scholar]
- Lenfant C., Torrance J., English E., Finch C. A., Reynafarje C., Ramos J., Faura J. Effect of altitude on oxygen binding by hemoglobin and on organic phosphate levels. J Clin Invest. 1968 Dec;47(12):2652–2656. doi: 10.1172/JCI105948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lenfant C., Ways P., Aucutt C., Cruz J. Effect of chronic hypoxic hypoxia on the O2-Hb dissociation curve and respiratory gas transport in man. Respir Physiol. 1969 Jun;7(1):7–29. doi: 10.1016/0034-5687(69)90066-8. [DOI] [PubMed] [Google Scholar]
- Mehmel H. C., Duvelleroy M. A., Laver M. B. Response of coronary blood flow to pH-induced changes in hemoglobin-O2 affinity. J Appl Physiol. 1973 Oct;35(4):485–489. doi: 10.1152/jappl.1973.35.4.485. [DOI] [PubMed] [Google Scholar]
- Metcalfe J., Dhindsa D. S., Edwards M. J., Mourdjinis A. Decreased affinity of blood for oxygen in patients with low-output heart failure. Circ Res. 1969 Jul;25(1):47–51. doi: 10.1161/01.res.25.1.47. [DOI] [PubMed] [Google Scholar]
- Mulhausen R., Astrup P., Kjeldsen K. Oxygen affinity of hemoglobin in patients with cardiovascular diseases, anemia, and cirrhosis of the liver. Scand J Clin Lab Invest. 1967;19(3):291–297. doi: 10.3109/00365516709090641. [DOI] [PubMed] [Google Scholar]
- Oski F. A., Gottlieb A. J., Delivoria-Papadopoulos M., Miller W. W. Red-cell 2,3-diphosphoglycerate levels in subjects with chronic hypoxemia. N Engl J Med. 1969 May 22;280(21):1165–1166. doi: 10.1056/NEJM196905222802108. [DOI] [PubMed] [Google Scholar]
- Pantely G. A., Oyama A. A., Metcalfe J., Lawson M. S., Welch J. E. Improvement in the relationship between flow to ischemic myocardium and the extent of necrosis with glycolytic intermediates that decrease blood oxygen affinity in dogs. Circ Res. 1981 Aug;49(2):395–404. doi: 10.1161/01.res.49.2.395. [DOI] [PubMed] [Google Scholar]
- Rose Z. B. Enzymes controlling 2,3-diphosphoglycerate in human erythrocytes. Fed Proc. 1970 May-Jun;29(3):1105–1111. [PubMed] [Google Scholar]
- Rose Z. B., Liebowitz J. Direct determination of 2,3-diphosphoglycerate. Anal Biochem. 1970 May;35(1):177–180. doi: 10.1016/0003-2697(70)90023-0. [DOI] [PubMed] [Google Scholar]
- Rude R. E., Tumas J., Gunst M., Kloner R. A., DeBoer L. W., Maroko P. R. Effects of ortho-iodo sodium benzoate on acute myocardial ischemia, hemodynamic function, and infarct size after coronary artery occlusion in dogs. Am J Cardiol. 1983 May 1;51(8):1422–1427. doi: 10.1016/0002-9149(83)90323-5. [DOI] [PubMed] [Google Scholar]
- Severinghaus J. W. Blood gas calculator. J Appl Physiol. 1966 May;21(3):1108–1116. doi: 10.1152/jappl.1966.21.3.1108. [DOI] [PubMed] [Google Scholar]
- Severinghaus J. W. Simple, accurate equations for human blood O2 dissociation computations. J Appl Physiol Respir Environ Exerc Physiol. 1979 Mar;46(3):599–602. doi: 10.1152/jappl.1979.46.3.599. [DOI] [PubMed] [Google Scholar]
- Torrance J., Jacobs P., Restrepo A., Eschbach J., Lenfant C., Finch C. A. Intraerythrocytic adaptation to anemia. N Engl J Med. 1970 Jul 23;283(4):165–169. doi: 10.1056/NEJM197007232830402. [DOI] [PubMed] [Google Scholar]
- Weil M. H., Rackow E. C., Trevino R., Grundler W., Falk J. L., Griffel M. I. Difference in acid-base state between venous and arterial blood during cardiopulmonary resuscitation. N Engl J Med. 1986 Jul 17;315(3):153–156. doi: 10.1056/NEJM198607173150303. [DOI] [PubMed] [Google Scholar]
- Wilkinson P. L., Graham B. H., Moyers J. R., Hamilton W. K., Ports T. A., Ullyot D. J., Chatterjee K. Changes in mixed venous and coronary sinus P50 secondary to anesthesia and cardiac disease. Anesth Analg. 1980 Oct;59(10):751–758. [PubMed] [Google Scholar]
- Woodson R. D., Torrance J. D., Shappell S. D., Lenfant C. The effect of cardiac disease on hemoglobin-oxygen binding. J Clin Invest. 1970 Jul;49(7):1349–1356. doi: 10.1172/JCI106351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Planta M., Weil M. H., Gazmuri R. J., Bisera J., Rackow E. C. Myocardial acidosis associated with CO2 production during cardiac arrest and resuscitation. Circulation. 1989 Sep;80(3):684–692. doi: 10.1161/01.cir.80.3.684. [DOI] [PubMed] [Google Scholar]


