Abstract
Background:
Due to the involvement of opioids and benzodiazepines in rising pharmaceutical overdoses, a reduction in co-prescribing of these medications is a national priority, particularly among patients with substance use disorders and other high-risk conditions. However, little is known about primary care (PC) and mental health (MH) prescribers’ perspectives on these medications and efforts being implemented to reduce co-prescribing.
Design:
An anonymous survey.
Setting:
One multisite VA health care system.
Subjects:
Participants were 55 PC and 31 MH prescribers.
Methods:
Survey development was guided by the Promoting Action on Research Implementation in Health Services (PARIHS) conceptual framework. PC and MH prescribers of opioids or benzodiazepines were invited to complete an anonymous electronic survey. Responses were collapsed to highlight agreement, disagreement and neutrality and summarized with means and percentages.
Results:
Over 80% of both prescriber groups reported concern about concurrent use and >75% strongly agreed with clinical practice guidelines (CPG) that recommend caution in co-prescribing among patients with high-risk conditions. Greater than 40% of both prescriber groups indicated that co-prescribing continues because of beliefs that patients appear stable without adverse events and tapering/discontinuation is too difficult. Over 70% of prescribers rated strategies for addressing patients who refuse to discontinue, more time with patients, and identification of high-risk patients as helpful in reducing co-prescribing.
Conclusion:
Despite strong agreement with CPGs, prescribers reported several barriers that contribute to co-prescribing of opioids and benzodiazepines and challenge their ability to taper these medications. Multiple interventions are likely needed to reduce opioid and benzodiazepine co-prescribing.
Keywords: Chronic Pain, Concurrent Opioid and Benzodiazepine Use, Primary Care, Mental Health, Beliefs, Attitudes, High-risk conditions
Introduction
Despite clinical practice guidelines (CPG) that discourage opioid analgesic and benzodiazepine co-prescribing, 27% of Veterans enrolled in VA care and who are prescribed opioids are also prescribed benzodiazepines.1 Opioids and benzodiazepines are the most common prescription classes involved in pharmaceutical overdoses, with opioids implicated in 75.2% and benzodiazepines in 29.4% of all pharmaceutical overdoses in 2010. Further, benzodiazepines were involved in 30% of opioid-related overdoses in 2011.2 For those with problematic alcohol or drug use, suicide risk or impaired breathing-related conditions such as sleep apnea,3, 4 as well as the elderly (age ≥65), the potentially lethal risks associated with concurrent use of these medications may be even greater.5–8
As a result of the substantial increase in unintentional drug overdose fatalities involving opioid analgesics and benzodiazepines over the last decade,9 the VA has prioritized an overall reduction in non-recommended opioid prescribing practices, including concurrent use of opioids and benzodiazepines.10 Conceptual frameworks of implementation strategies in health services such as the Promoting Action on Research Implementation in Health Services (PARIHS) model highlight the importance of assessing prescribers’ knowledge and experience of evidence in support of the recommended practices and the receptivity of the setting to adopting such practices.11 Given that in VA most opioids are prescribed in primary care while the majority of benzodiazepines are prescribed in mental health clinics,12 obtaining the perspectives of prescribers from both settings is critical. Although prior studies have identified primary care prescribers’ attitudes and practices regarding the use of opioids or benzodiazepines separately, little is known about primary care and mental health prescribers’ perspectives on concurrent use of these medications and, in particular, on efforts to reduce co-prescribing of opioids and benzodiazepines.
Very little information is available on factors that may facilitate reductions in opioid prescribing. However, provider identified concerns regarding opioid use for the management of chronic pain may prove helpful in facilitating adherence to guideline recommendations. With regards to opioids, prescribers have expressed several concerns, including potential for addiction,13–15 potential for abuse or misuse,13, 15, 16 diversion,15, 16 and patient harm from adverse effects.17 Barriers to tapering or discontinuing opioids include lack of prescriber awareness regarding prescribing guidelines,17 threats to provider/patient relationship,16 and additional workload or insufficient time.17 With regards to the use of benzodiazepines, prior research has identified factors that facilitate adherence to guideline recommendations, including prescribers’ concerns about the potential for abuse or dependence, concerns about negative consequences, and beliefs that cessation will bring benefits.18, 19 Barriers to recommended benzodiazepine prescribing practices identified in prior studies are numerous and have included limited availability of effective alternatives,19–22 few side effects observed by providers,23–25 negative impact on provider/patient relationship,21 limited time,19, 20 perception that discontinuation is harsh,19, 20 futility of discontinuation/tapering,20 and expected resistance from patients.19
This project consists of an anonymous survey of primary care (PC) and mental health (MH) prescribers at one large VA health care system and is the first phase of a larger quality improvement project to evaluate the implementation of an advanced medication alert designed to identify patients who are co-prescribed opioids and benzodiazepines and who have comorbid substance use or other high risk conditions. Primary goals of this survey include identification of factors affecting implementation of the medication alert and reductions in opioid and benzodiazepine co-prescribing. To address these goals, we assessed PC and MH prescribers’ beliefs regarding 1) CPG recommendations discouraging opioid and benzodiazepine co-prescribing among patients with substance use and other high-risk conditions; 2) the effect of these medications on patient functioning and quality of life; 3) barriers to tapering or discontinuing concurrent use of these medications; and 4) prescribers perspectives on medication alerts.
Methods:
Conceptual framework for evaluating implementation
The PARIHS model was selected as the theoretical framework to guide survey development, as well as the overall evaluation of the implementation of the medication alert.26 The PARIHS framework was selected for its multi-dimensional focus on the complexities of implementing evidence into practice26 and because it has been adapted for evidence-based practice implementation efforts in the VA.27, 28 The PARIHS framework proposes that successful implementation is a function of three interacting elements – the strength of the evidence supporting the intervention, the quality of the implementation context, and the facilitation of the change process.29 Survey items focused on the evidence and context domains of the PARIHS model and assessed prescribers’ perceptions of the evidence associated with the risks of co-prescribing opioids and benzodiazepines, their overall receptivity to the medication alert and the goal of reducing concurrent use of opioid and benzodiazepines, and contextual factors that may potentially affect the adoption of the medication alert and modification of prescribers’ prescribing practices.
Setting:
The VA Puget Sound Health Care System (VAPSHCS) is a multisite health care system that includes two medical centers, one in Seattle and one in American Lake, and seven community-based outpatient clinics (including clinics staffed by contracting services rather than VA employees) providing care to over 80,000 Veterans living in the Pacific Northwest. This project, including development and approval of the medication alert, was a partnership between several VAPSHCS services, including mental health, primary care, pharmacy, and specialty-pain, and VA central office, with a primary goal of improving the quality and safety of clinical care; therefore the project was considered a quality improvement evaluation and did not require human subjects’ approval from the VAPSHCS Institutional Review Board.
Sample:
All prescribers working in PC and MH settings (including specialty mental health and substance use disorder services) were eligible to participate in this project. Utilizing pharmacy data extracted from the VA Northwest Veterans Integrated Service Network (VISN 20) Data Warehouse, we identified providers who ordered at least one opioid and/or benzodiazepine prescription between January 1, 2014 and March 31, 2014. This list was confirmed with leaders from eligible clinics. The final sample of eligible prescribers included 114 PC and 67 MH prescribers, all of whom were invited to complete the electronic survey anonymously.
Survey Development:
The PARIHS model was used to develop a 30-item survey (Appendix A) organized into 10 content domains. Table 1 provides an overview of the content domains and the associated questions on the survey. A 5-point Likert scale was used for most questions and included response options 1) not at all/never/extremely negative, 2) slightly/rarely/negative, 3) moderately/sometimes/neither disagree or agree, 4) quite a bit/often/positive or 5) extremely/always/extremely positive. Several questions included an ‘If other, please specify option’ for respondents to provide narrative responses. Results of these responses will be analyzed and presented in a subsequent manuscript. The survey was pilot tested by two PC and two MH prescribers for clarity, wording and content. Prior to piloting the survey, testers were instructed to measure the time required to complete the survey and to identify questions that were poorly worded, unclear or redundant. After piloting the survey, the lead author briefly interviewed each tester to identify questions that could be deleted, revised to improve clarity or added to address content, with responses used to iteratively revise the survey.
Table 1.
30-Item Survey Organized into 10 Content Domains
| PARIHS Domains | Survey Content Domains | Associated Questions |
|---|---|---|
| Evidence | 1. Background information | 1-8 |
| 2. Opioid and benzodiazepine prescribing practices | 9-13 | |
| 3. Knowledge of pain management and insomnia clinical practice guidelines | 14-15 | |
| 4. Level of agreement with clinical practice guidelines that caution against concurrent prescribing of opioids and benzodiazepines among high risk groups | 16 | |
| 5. Beliefs that contribute to opioid and benzodiazepine co-prescribing | 17-18 | |
| 6. Beliefs about discontinuing co-prescribed opioids and benzodiazepines | 19-20 | |
| 7. Typical outcomes for patients co-prescribed opioids and benzodiazepines | 21-25 | |
| Context | 8. Team support/encouragement around reducing opioid and benzodiazepine co-prescribing | 26 |
| 9. Resources that would support efforts to discontinue or reduce co-prescribing of opioids and benzodiazepines | 27-29 | |
| 10. Potential benefits of and barriers to using medication alerts | 30 |
Survey Distribution:
The anonymous electronic survey was distributed to PC and MH prescribers across VAPSHCS using an individualized email with an embedded survey link from April through June 2014. Following the initial email, three subsequent emails were sent, at 1-week intervals, to all prescribers as reminders to complete the survey.
Data analysis:
We first examined the data to determine whether the age and gender of survey respondents were representative of the age and gender of all of the prescribers who were invited to participate in the survey. Questions regarding age and gender were included on the survey and for the full sample of invited participants age and gender were obtained from the VISN 20 Data Warehouse. To identify perspectives of respondents that reflected agreement, disagreement or neutrality, we collapsed the ends of the response sets. For example, for items with the response set “not at all,” “slightly,” “moderately,” “quite a bit” and “extremely,” responses were collapsed to “not at all or slightly,” “moderately” and “quite a bit or extremely.” Descriptive statistics were used to summarize responses, with means calculated for continuous variables and percentages for categorical variables. Chi-square statistics were used to compare responses of PC and MH prescribers. Responses to item 23 were not analyzed because of an error in the response set for this item. All analyses were conducted using Stata MP, version 14.30
Results:
Participant characteristics
Table 2 shows the demographic characteristics of the PC and MH participants who responded to the survey. Overall, 55 of 115 (47.8%) PC and 31 of 67 (46.3%) MH prescribers responded to the survey. The prescriber groups were similar with respect to age and gender, with women representing over half of both PC and MH prescribers. A large majority of both the PC and MH prescribers were white. MH prescribers had more years in practice in the VA compared to PC prescribers. Physicians represented the most common discipline in both prescriber groups, followed by nurse practitioners.
Table 2.
Demographic Characteristics of Survey Respondents
| Primary Care | Mental Health | |
|---|---|---|
| (n=55) | (n=31) | |
| Characteristic | n (%) | n (%) |
| Gender | ||
| Women | 30 (54.6) | 16 (51.6) |
| Men | 25 (45.5) | 15 (48.4) |
| Age, M (SD) | 51.6 (9.0) | 51.2 (9.7) |
| Race/Ethnicity | ||
| White/Caucasian | 43 (78.2) | 24 (77.4) |
| Non White | 12 (21.8) | 6 (19.4) |
| Missing | 1 (3.2) | |
| Years of VA Practice M (SD) | 10.1 (9.2) | 14.5 (9.4)* |
| Discipline | ||
| Physician | 32 (59.3) | 22 (75.9) |
| Nurse Practitioner | 20 (37.0) | 7 (24.1) |
| Physician’s Assistant | 2 (3.7) | 0 (0.0) |
| Missing | 1 (1.8) | 2 (6.7) |
| Primary Facility Role | ||
| Clinician – Outpatient | 44 (80.0) | 24 (77.4) |
| Clinician – Inpatient | 5 (9.1) | 4 (12.9) |
| Leadership (Defined as Clinic Director) | 6 (10.9) | 3 (9.7) |
P = <0.05
There were no meaningful differences by gender (PC, women: 54.6% vs. 58.6%; MH, women: 51.6% vs. 47.7%) or age (PC: 51.6 vs. 51.8 years; MH: 51.2 vs. 52.8 years) among those who responded to the survey and the full sample of those who were invited to complete the survey.
In response to the question, “For what percent of your panel do you prescribe opioids?”, 64.8% of PC and 12.9% of MH prescribers reported ≥10%. For the question, “For what percent of your panel do you prescribe benzodiazepines?”, 5.6% of PC and 45.9% of MH prescribers reported ≥10%. Twenty-six percent of PC prescribers and 32.3% of MH prescribers indicated that ≥10% of their panel was co-prescribed opioids and benzodiazepines.
Knowledge of VA/Department of Defense 31 31 clinical practice guidelines
A larger percentage of PC than MH prescribers rated their knowledge of the 2010 VA/DoD clinical practice guidelines for the Management of Opioid Therapy for Chronic Pain32 as very good to excellent (31.5% vs. 19.4%, p<0.05), with only 11.1% of PC and 16.1% of MH prescribers reporting that they had not seen the clinical practice guidelines, and 3.7% of PC and 25.8% of MH prescribers reporting very poor to poor ratings of their knowledge. Nineteen percent of PC and 12.9% of MH prescribers reported very good to excellent knowledge of the VA Pharmacy Benefit Management Services clinical guidance for insomnia treatments.33 In contrast, 31.5% of PC and 22.6% of MH prescribers rated their knowledge of this guidance as very poor to poor and 20.4% of PC and 38.7% of MH prescribers reported that they had never seen the clinical guidance on insomnia. Table 3 shows that most PC and MH respondents reported strong concerns about prescribing opioids for patients who are also prescribed benzodiazepines and about prescribing benzodiazepines for patients who are also prescribed opioids. Most PC and MH prescribers also agreed with clinical practice guidelines that recommend caution in the co-prescribing of opioids and benzodiazepines among patients with substance use disorders, high suicide risk, sleep apnea and ages ≥ 65.
Table 3.
Primary Care and Mental Health Prescribers’ Concerns about Concurrent Use of Opioids and Benzodiazepines and Agreement with Clinical Practice Guidelines (CPG) Recommending Against Concurrent Use of Opioids and Benzodiazepines Among Patients with High Risk Conditions
| Primary Care, % | Mental Health, % | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| n | Strongly Disagree/Disagree | Neither Agree or Disagree | Strongly Agree/Agree | N/A | n | Strongly Disagree/Disagree | Neither Agree or Disagree | Strongly Agree/Agree | N/A | |
| I have concerns about prescribing | ||||||||||
| Opioids if benzodiazepines also prescribed | 54 | 0.0 | 5.6 | 94.4 | 0.0 | 31 | 3.2 | 6.5 | 80.7 | 9.7 |
| Benzodiazepines if opioids also prescribed | 54 | 0.0 | 5.6 | 92.6 | 1.9 | 31 | 3.2 | 6.5 | 90.3 | 0.0 |
| Extent of agreement with CPG that recommend caution in concurrent use amonga | ||||||||||
| Substance Use Disorders | 54 | 1.9 | 11.1 | 83.3 | 3.7 | 31 | 0.0 | 0.0 | 96.8 | 3.2 |
| High Suicide Risk | 53 | 1.9 | 3.8 | 88.7 | 5.7 | 31 | 3.2 | 3.2 | 90.3 | 3.2 |
| Sleep Apnea | 54 | 3.7 | 14.8 | 75.9 | 5.6 | 31 | 3.2 | 0.0 | 93.6 | 3.2 |
| Age ≥ 65 | 54 | 7.4 | 11.1 | 75.9 | 5.6 | 31 | 3.2 | 3.2 | 90.3 | 3.2 |
Response scale for extent of agreement with CPG: Strongly Disagree/Disagree is Not at all/Slightly, Moderately is Neither Agree or Disagree, Quite a bit/Extremely is Strongly Agree/Agree, Not applicable is Not sure
Beliefs that contribute to continuation of opioid and benzodiazepine co-prescribing
PC and MH prescribers endorsed many reasons for opioid and benzodiazepine co-prescribing (Table 4). Beliefs endorsed most often among PC and MH prescribers as often/always contributing to co-prescribing included tapering/discontinuing would be too difficult (47.2% and 61.3%, respectively), not enough time to negotiate discontinuing or tapering with patients (50.0% and 48.4%, respectively), and patients are stable on these medications with no adverse effects (43.3% and 50.0%, respectively). Although endorsed less often, 34.0% of PC and 45.2% of MH prescribers also indicated that when the medications were prescribed by different prescribers, it was often/always too difficult to coordinate with the other prescriber to taper/discontinue one or both of the medications. MH prescribers were nearly two times more likely than PC prescribers to endorse the belief that the benefits of opioids and benzodiazepines often/always exceed the risks (41.9% vs. 21.6%, respectively, ns) and the belief that discontinuation of these medications often/always causes suffering (54.8% vs. 26.9%, respectively, p< 0.05).
Table 4.
Beliefs that contribute to continued co-prescribing of opioids and benzodiazepines
| Primary Care, % | Mental Health, % | |||||||
|---|---|---|---|---|---|---|---|---|
| n | Never/Rarely | Sometimes | Always/Often | n | Never/Rarely | Sometimes | Always/Often | |
| Opioids and benzodiazepines are co-prescribed because: | ||||||||
| Benefits of opioids and benzodiazepines exceed risks | 51 | 29.4 | 49.0 | 21.6 | 31 | 16.1 | 41.9 | 41.9 |
| Difficult to say no to patients who expect them | 51 | 45.1 | 23.5 | 31.4 | 31 | 25.8 | 29.0 | 45.2 |
| Not enough time to negotiate discontinuation or tapering with patients who expect them | 52 | 26.9 | 23.1 | 50.0 | 31 | 22.6 | 29.0 | 48.4 |
| No treatment alternatives exist | 52 | 40.8 | 25.0 | 26.9 | 31 | 38.7 | 45.2 | 16.1 |
| Risk of particular patient abusing these medications is low | 52 | 32.7 | 51.9 | 15.4 | 30 | 30.0 | 46.7 | 23.3 |
| Patient is stable on medication with no adverse effects | 51 | 9.8 | 47.1 | 43.1 | 28 | 3.6 | 46.4 | 50.0 |
| Lack information on how to taper these medications | 52 | 26.0 | 44.0 | 30.0 | 30 | 34.5 | 31.0 | 34.5 |
| Lack information on medication alternatives to these medications | 52 | 32.7 | 38.5 | 28.9 | 30 | 30.0 | 46.7 | 23.3 |
| Lack information on behavioral alternatives to these medications | 50 | 26.9 | 30.8 | 42.3 | 29 | 23.3 | 40.0 | 36.7 |
| Discontinuing or tapering these medications will: | ||||||||
| Cause patients to suffer* | 52 | 23.1 | 50.0 | 26.9 | 31 | 9.7 | 34.5 | 54.8 |
| Be too difficult | 53 | 20.8 | 32.1 | 47.2 | 31 | 12.9 | 25.8 | 61.3 |
| Cause patients to obtain illicit drugs. | 52 | 51.9 | 38.5 | 9.6 | 31 | 51.6 | 38.7 | 9.7 |
| When these medications are prescribed by different prescribers: | ||||||||
| It is too difficult to coordinate a plan to taper/discontinue one or both of these medications with the other prescriber | 53 | 20.8 | 45.3 | 34.0 | 31 | 9.7 | 45.2 | 45.2 |
| The prescribers disagree about which medication should be tapered/discontinued. | 51 | 27.5 | 56.9 | 15.7 | 31 | 32.3 | 51.6 | 16.1 |
P<0.05
Beliefs regarding patient functioning and quality of life
Figure 1a presents prescribers’ responses to questions regarding a patient’s functioning (e.g., physical and psychosocial functioning) and quality of life (e.g. health and happiness) while co-prescribed opioids and benzodiazepines. Responses to the question about typical outcome for patient functioning were spread somewhat evenly across negative/extremely negative, positive/extremely positive and no change response options. A substantial minority, 15.4% of PC and 22.6% of MH prescribers, were not sure about the functioning of these patients. Prescribers’ responses regarding the typical outcome for patient quality of life were more varied, with no change (34.6%) the most common rating among PC prescribers and equal endorsement of negative/extremely negative and positive/extremely positive (32.3% and 32.3%, respectively) among MH prescribers. The response not sure was indicated by 11.5% of PC and 22.6% of MH prescribers.
Figure 1a.
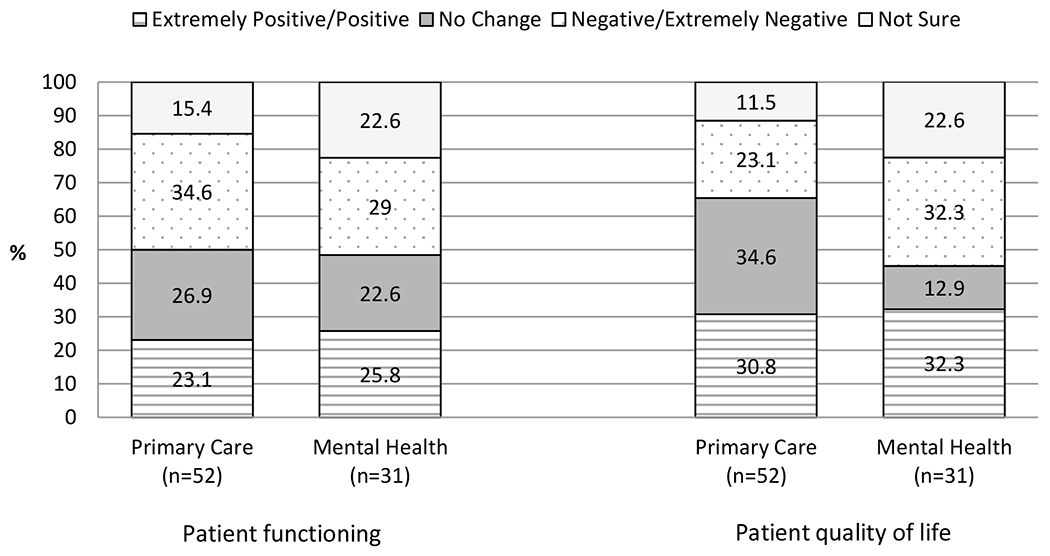
Typical functioning and quality of life outcome for patients prescribed opioids and benzodiazepines concurrently
Figure 1b shows prescribers’ responses to the questions about changes in patient functioning when long-term opioid or benzodiazepine therapy is discontinued. Approximately one-third of respondents from both groups rated the typical change in patient functioning after discontinuing long-term opioid therapy as positive to extremely positive, with another one-third of both groups rating the change as negative to extremely negative. Of note, 2 in 10 MH prescribers were not sure about the typical patient’s functioning after discontinuation of long-term opioid therapy. Responses were similar to the question about changes in patient functioning after discontinuation of long-term benzodiazepine therapy, with an equal percentage (30.8%) of PC prescribers rating the typical change in functioning as negative/extremely negative and positive/extremely positive. For MH prescribers, the most frequent response was negative/extremely negative (41.9%), followed by positive/extremely positive (29.0%). Further, 17.3% of PC and 19.4% of MH prescribers were not sure about the typical change in functioning after discontinuation of long-term benzodiazepine therapy.
Figure 1b.
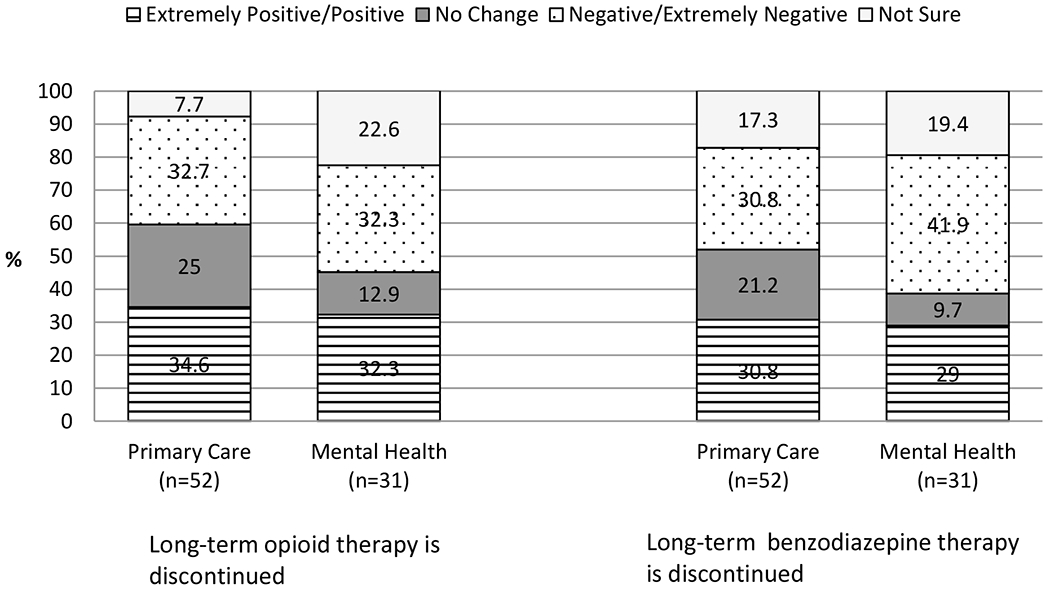
Typical change in patient functioning when long-term opioid or benzodiazepine therapy is discontinued
Resources to reduce co-prescribing of opioids and benzodiazepines among patients with high risk conditions
Table 5 shows resources that prescribers indicated would be helpful in efforts to reduce concurrent use of opioids and benzodiazepines. Of all of the resources, a need for approved strategies to guide interactions with patients who refuse to consider discontinuation of opioid and/or benzodiazepine medications was rated quite to extremely helpful by more than 80% of both prescriber groups. Access to alternative behavioral interventions, more time with patients and identification of patients who are co-prescribed these medications were rated as quite to extremely helpful by more than 70% of both prescriber groups. Firm policies that support decisions to discontinue opioid and/or benzodiazepine medications and improved communication between prescribers in cases where medications are prescribed by multiple prescribers were rated quite to extremely helpful by more than 60% of prescribers. PC prescribers were more likely to rate access to alternative medication interventions (80.4% vs.61.3%, p< 0.05) and consultation with experts (76.5% vs. 48.4%, p<0.05) as quite to extremely helpful compared to MH prescribers.
Table 5.
Ratings of resources considered helpful efforts to reduce concurrent use of opioids and benzodiazepines among patients with high risk conditions
| Primary Care, % | Mental Health, % | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| n | Not at all/Slightly | Moderately | Extremely/Quite a bit | N/A | n | Not at all/Slightly | Moderately | Extremely/Quite a bit | N/A | |
| Tapering guidelines | 51 | 11.8 | 19.6 | 66.7 | 2.0 | 31 | 25.8 | 9.7 | 58.1 | 6.5 |
| Consultation with experts* | 51 | 9.8 | 13.7 | 76.5 | 0.0 | 31 | 22.6 | 22.6 | 48.4 | 6.5 |
| Continuing education/skills training | 51 | 13.7 | 29.4 | 56.9 | 0.0 | 30 | 26.7 | 20.0 | 50.0 | 3.3 |
| More time with patients | 51 | 9.8 | 17.7 | 72.6 | 0.0 | 31 | 6.5 | 9.7 | 80.7 | 3.2 |
| Identification of patients who are prescribed these medications | 51 | 10.0 | 18.0 | 72.0 | 0.0 | 30 | 10.0 | 13.3 | 73.3 | 3.3 |
| Firm policies that support decisions to discontinue these medications | 51 | 9.8 | 9.8 | 80.4 | 0.0 | 31 | 29.0 | 9.7 | 61.3 | 0.0 |
| Improved communication with prescribers | 50 | 12.0 | 20.0 | 68.0 | 0.0 | 31 | 3.2 | 25.8 | 71.0 | 0.0 |
| Accessible clinical practice guidelines | 51 | 17.7 | 23.5 | 56.9 | 2.0 | 31 | 25.8 | 9.7 | 61.3 | 3.2 |
| Approved strategies for patients who refuse to consider discontinuation of opioid/bzd medications | 50 | 4.0 | 10.0 | 86.0 | 0.0 | 31 | 3.2 | 9.7 | 87.1 | 0.0 |
| Access to alternative medication interventions* | 51 | 11.8 | 7.8 | 80.4 | 0.0 | 31 | 9.7 | 19.4 | 61.3 | 9.7 |
| Access to alternative behavioral interventions | 49 | 4.0 | 10.0 | 86.0 | 0.0 | 31 | 3.2 | 16.1 | 74.2 | 6.5 |
N/A – (resource is available)
p< 0.05
Team support around reducing opioid and benzodiazepine co-prescribing
The majority of PC and MH prescribers agreed or strongly agreed with the statement that their team (e.g., clinic or patient aligned care team) frequently discussed risks associated with concurrent opioid and benzodiazepine use (53.9% and 61.3%, respectively), while 23.1% of PC and 16.1% of MH prescribers strongly disagreed or disagreed. Most PC (73.1%) and MH (77.4%) prescribers also agreed or strongly agreed that their team encouraged the use of alternatives, with only 3.8% of PC and 0.0% of MH prescribers reporting strong disagreement or disagreement. Approximately one-quarter of both prescriber groups strongly disagreed or disagreed that their team provided information to taper patients off of opioids and/or benzodiazepines (PC, 25.5% and MH, 22.6%).
Beliefs about medication alerts
As shown in Table 6, both PC and MH prescribers held positive views of medication alerts, with >70% agreeing/strongly agreeing that medication alerts can identify patient risks that might otherwise go unnoticed, prevent serious adverse outcomes, and help providers prescribe more safely. Less than one-quarter of both prescriber groups agreed or strongly agreed that medication alerts waste more time than they save, while even fewer (<20%) agreed or strongly agreed with a statement indicating they ignore medication alerts.
Table 6.
Primary care and mental health providers’ beliefs about medication alerts
| Primary Care, % | Mental Health,% | |||||||
|---|---|---|---|---|---|---|---|---|
| n | Disagree/Strongly Agree | Neither Agree or Disagree | Strongly Agree/Agree | n | Disagree/Strongly Agree | Neither Agree or Disagree | Strongly Agree/Agree | |
| Medication alerts identifying high risk conditions may prevent serious adverse events | 51 | 2.0 | 17.7 | 80.4 | 31 | 6.5 | 16.1 | 77.4 |
| Medication alerts waste more time than they save | 51 | 43.1 | 37.3 | 19.6 | 31 | 32.3 | 41.9 | 25.8 |
| Medication alerts may identify patient risks that I might otherwise not be aware of | 51 | 3.9 | 11.8 | 84.3 | 31 | 6.5 | 6.5 | 87.1 |
| I typically ignore medication alerts | 51 | 66.7 | 19.6 | 13.7 | 31 | 58.1 | 25.8 | 16.1 |
| Medication alerts can help me prescribe more safely | 51 | 6.5 | 5.9 | 72.6 | 31 | 6.5 | 19.4 | 74.2 |
Discussion
Despite the rising rates of opioid overdose in the United States over the last decade and the contribution of benzodiazepines to this trend, there is surprisingly little information about the perspectives of PC and MH providers, who often prescribe these medications and are increasingly being expected to change such prescribing practices. This survey, designed to assess the knowledge, beliefs and experiences of PC and MH providers prior to the implementation of an advanced medication alert, found that both prescriber groups were concerned about the concurrent use of opioids and benzodiazepines among their patients and agreed with clinical practice guidelines that recommend caution in prescribing these medications among those diagnosed with substance use disorders, sleep apnea, suicide risk and those aged 65 and older. Furthermore, survey results suggest there is a culture in prescribers’ clinics or patient aligned care teams to discuss the risks of these medications and encourage alternative treatments. Several beliefs identified in the survey suggest that co-prescription persists because of logistical or resource constraints, such as the difficulty of tapering/discontinuing and lack of time to discuss a taper. However substantial minorities of prescribers also note their clinical experience as contributing to co-prescribing of these medications, including that the benefits of opioids and benzodiazepines outweigh the risks, that patients have tolerated these medications without prior adverse events, and that discontinuation would cause patients to suffer. Providers opinions regarding the functioning and quality of life of patients co-prescribed these medications, as well as those discontinued from the medications, are clearly mixed, with similar percentages of prescribers reporting improved, worsened and no change in or uncertainty about patients’ status. Over 70% of prescribers identified a need for approved strategies for treating patients who refuse to consider discontinuation of opioids and/or benzodiazepines, medication and behavioral alternatives, more time with patients, and identification of patients who are prescribed these medications in combination to assist in their efforts to reduce co-prescribing of opioids and benzodiazepines. Overall, beliefs about medication alerts were largely favorable, with most prescribers agreeing or strongly agreeing with the ability of medication alerts to identify patients at risk of adverse events and promote safe prescribing.
Many of the most common beliefs identified in this project as contributing to the continued co-prescribing of opioids and benzodiazepines have been reported in prior studies assessing prescribers’ perspectives of opioid or benzodiazepine medications. For instance, limited prescriber time18, 20, 34, 35 and beliefs that tapering/discontinuation is too difficult18, 20, 35, 36 have been reported as factors contributing to providers continuing medications in the opioid, benzodiazepine and polypharmacy literatures, as has the belief that co-prescribing is safe and effective for patients receiving stable doses over extended periods.20, 35 In our sample, a significant minority of prescribers also identified a lack of information on medication and behavioral alternatives to opioids and benzodiazepines. This belief not often reported in the literature and might be relatively easy to address with additional education focused on the clinical efficacy and local availability of behavioral treatments for chronic pain,37 anxiety38 and sleep disorders.39 Another less commonly reported finding in the polypharmacy literature identified by this project is prescribers’ belief that it is too difficult to coordinate a plan to discontinue or taper when the medications are prescribed by different prescribers. As opioids and benzodiazepines are often prescribed by different prescribers in the VA, this poses a considerable challenge to reducing this medication combination without strategies that facilitate prescriber communication.
Our finding that one-third of prescribers in both settings rated the typical outcomes for patient functioning and quality of life as positive to extremely positive for patients who are co-prescribed opioids and benzodiazepines suggests that a substantial minority of prescribers may hold complex views about the therapeutic benefits and potential harms associated with these medications. For instance, prescribers may agree in principle with the clinical practice guidelines and research findings recommending caution, but in their clinical experience have observed the benefits of these medications to exceed the potential risks among patients they treat with the medication combination. Because changing the prescribing practices of this subset of prescribers is likely to be challenging without identifying the reasons such a view is held, further research is needed to identify the specific beliefs and clinical experiences that contribute to the understanding that this medication combination improves functioning and quality of life. It is also possible that co-prescribing is considered clinically justifiable under certain conditions, such as when treatment is of limited duration, palliative or the prescriber intends to closely monitor the patient for adverse events.40 Changing non-recommended opioid and benzodiazepine prescribing practices will be further complicated by the nearly one-third of PC and MH prescribers who rated the change in functioning of a typical patient to be negative or extremely negative, after discontinuing long-term opioid or benzodiazepine therapy. Doubt about the benefits of discontinuing long-term opioid or benzodiazepine treatment among these PC and MH prescribers represents a significant barrier to reducing co-prescribing. Somewhat surprisingly, more than 1 in 10 PC and approximately 1 in 5 MH prescribers were not sure about the typical patient functioning and quality of life outcomes of patients who are co-prescribed these medications. Our survey was not able to identify the source of these prescribers’ uncertainty, but it may be due to a lack of time or experience to measure such outcomes, difficulty in recalling the typical outcomes of patients, witnessed variation in outcomes or concerns about the validity of patient-reported functioning or quality of life. As traditional education approaches such as passive dissemination of materials and educational meetings alone are minimally effective,41 multifaceted interventions such as medication alerts or reminders,42 audit and feedback 43, 44 also may be necessary to effect change in this subset of prescribers. Our findings also suggest that health care systems will likely need to increase access to medication and behavioral alternatives to opioids and benzodiazepines and present additional information about their effectiveness to facilitate clinical decision making.
Consistent with studies on determinants of prescribing, prescribers’ responses to our survey suggest patients’ expectations and demands influence their prescribing practices as well.34, 45, 46 Nearly half of prescribers reported there was not enough time to negotiate discontinuing or tapering of opioid and benzodiazepine medications with patients who expect to continue receiving them. Furthermore, nearly 90% of all prescribers rated a need for approved strategies to address the challenge that occurs when patients refuse to consider discontinuation of opioids and/or benzodiazepines, a barrier not explicitly identified in the opioid, benzodiazepine or polypharmacy literatures. However, a concern that is commonly reported in the opioid and benzodiazepine literatures is the effect that prescribing decisions can have on the provider-patient relationship.16, 21, 35 Denying patients the medications they expect or have been prescribed long-term, particularly if the decision appears prescriber driven, may have a detrimental effect on the prescriber-patient relationship and represent a source of prescribers’ frustration and burn-out.16 Such strong endorsement for the need for approved strategies may reflect prescribers’ belief that patients’ frustration or hostility would be less impactful to the relationship if decisions to deny or discontinue their medications were supported by policies of the larger health care system. A healthy provider-patient relationship is the cornerstone of healthcare, the means by which information is gathered, diagnoses and treatment plans are developed and treatment progress or outcomes are assessed.47 Thus, identification of clinical strategies to repair or prevent ruptures in the provider-patient relationship is an important area for research. Qualitative data collection approaches provide opportunities to obtain in depth information that are likely needed to advance our understanding of patients and providers’ concerns and reactions to these challenging health encounters. A subsequent stage of this larger quality improvement project included semi-structured interviews with prescribers and analyses of these data are currently underway.
With few exceptions, most of the commonly reported beliefs and helpful resources were reported consistently across both prescriber groups. However, MH prescribers were two times more likely than PC prescribers to identify the belief that the benefits of opioids and benzodiazepines exceed their risks and the belief that discontinuing or tapering these medications will cause patients to suffer. In contrast, PC prescribers were more likely to identify access to alternative medication interventions and consultation with experts as quite/extremely helpful. Such differences speak to the need to be mindful of differences in clinical experiences across prescriber groups and to tailor interventions appropriately.
Several of the study’s findings suggest changes in policy may be needed at the organization level to assist providers’ efforts to reduce non-recommended co-prescribing practices. Patient expectations can shape provider-patient interactions and lead to dissatisfaction and distrust among patients if they are not met, especially if providers are perceived as responsible.48, 49 According to attribution theory, patients attribute changes in prescribing behaviors to either the prescriber (internal) or to the situation (external)50 . For instance, prior research has found that patients who are dissatisfied with restrictions on their opioid medications tended to attribute the cause to their prescribers, whereas those who are not dissatisfied by such restrictions tended to attribute the changes to factors external to the prescriber (e.g., potential harm caused by medications).49 Although prescribers may benefit from additional training to negotiate these difficult interactions, another approach might be to shift the reason for tapering these medication classes from the provider to the health care organization through formal clinical policy. In this context, prescribers are less likely to become the target of patients’ frustration and dissatisfaction. Furthermore, for the more complex and challenging interactions with patients, the time restrictions created by brief visits typical of most healthcare systems can lead to diminished satisfaction among patients and burn-out among providers.16 Traditional time for health encounters may have to be extended to allow for a full discussion of patients concerns, risks of these medication classes and pharmacological and behavioral alternatives. Changes to these policies may be particularly helpful for providers who are tasked with tapering opioids and/or benzodiazepines and who have limited history with patients who have been co-prescribed these medications long-term by another provider.
Our finding that communication is difficult between prescribers of these medications is part of larger communication problem observed in healthcare. Communication failures have been identified as the leading cause of harm to patients by the Joint Commission.51 Several barriers potentially contribute to breakdowns in healthcare communication, including differences in communication styles,52 uncertainty about which provider is ultimately responsible for a patient’s care53 and lack of standardized communication practices..54 While strategies to improve efficient communication and reduce adverse events have been found to be effective in various healthcare settings,55–58 additional work is needed by researchers and/or quality improvement experts to assess whether such strategies lead to meaningful improvements in communication among outpatient PC and MH prescribers.
Our quality improvement project has several limitations. Data were obtained from prescribers at one VA health care system limiting the generalizability of survey results to other VA or non-VA facilities. Overall, the survey response rate of 47% is low, resulting in potential for response bias. Though no meaningful gender or age differences were noted between those who were invited to participate and those who completed a survey, it is possible that other important differences were not detected. The small sample size may have limited our ability to detect differences between PC and MH prescribers. Furthermore, the survey was developed specifically for the project and has not been validated. Information collected from prescribers was limited by the survey’s response options, and thus it may lack the detail and depth of information collected by alternative approaches.
Conclusion.
Although most prescribers had concerns about concurrent opioid and benzodiazepine use and agreed with clinical guidelines recommending caution in co-prescribing medications in patients with comorbid risk conditions, they endorsed several factors that contribute to co-prescribing of opioids and benzodiazepines, as well as barriers to discontinuing/tapering these medications. Perspectives on medication alerts, which have the potential to efficiently identify patients who are co-prescribed these medications, were mostly favorable. However, nearly one-third of both prescriber groups view more harm than benefit from discontinuing long-term opioid and benzodiazepine therapies, suggesting that for some patients and prescribers, changing practice will likely require multifaceted interventions. While plans to taper any medication must take the circumstances and health of the individual patient into account in both the timing and rate of the taper and should not be applied simply as an automatic response to all patients because of organizational policy, healthcare systems’ policies that place formal restrictions on co-prescription of opioids or benzodiazepines may aid prescribers’ efforts to reduce co-prescribing, while simultaneously maintaining the trust of their patients.
Funding Source
This material is based upon work supported by the U.S. Department of Veterans Affairs, Veterans Health Administration, the VA Center of Excellence in Substance Abuse Treatment & Education, the VA Health Services Research and Development (HSR&D) Quality Enhancement Research Initiative Rapid Response Project (RRP) # 12-527 and the Quality Enhancement Research Initiative for Substance Use Disorders (SUD QUERI). Supporting organizations had no further role in the study design; in the collection, analysis and interpretation of data; in the writing of the report; or in the decision to submit the paper for publication.
Appendix A. Survey
Note: Due to an error in the Likert scale, question #23 was not analyzed.
Footnotes
Disclosures/Conflicts of Interest
The views expressed in this article are those of the authors and do not necessarily reflect the position or policy of the Department of Veterans Affairs or University of Washington.
References
- 1.Park TW, et al. , Benzodiazepine prescribing patterns and deaths from drug overdose among US veterans receiving opioid analgesics: case-cohort study. BMJ, 2015. 350: p. h2698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Jones CM, Mack KA, and Paulozzi LJ, Pharmaceutical overdose deaths, United States, 2010. JAMA, 2013. 309(7): p. 657–9. [DOI] [PubMed] [Google Scholar]
- 3.Spitzer C, et al. , Association of airflow limitation with trauma exposure and post-traumatic stress disorder. Eur Respir J, 2011. 37(5): p. 1068–75. [DOI] [PubMed] [Google Scholar]
- 4.Williams SG, et al. , Sleep disorders in combat-related PTSD. Sleep Breath, 2014. [DOI] [PubMed] [Google Scholar]
- 5.Sehgal N, Colson J, and Smith HS, Chronic pain treatment with opioid analgesics: benefits versus harms of long-term therapy. Expert Rev Neurother, 2013. 13(11): p. 1201–20. [DOI] [PubMed] [Google Scholar]
- 6.Jacobson IG, et al. , Alcohol use and alcohol-related problems before and after military combat deployment. JAMA, 2008. 300(6): p. 663–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bullman TA and Kang HK, The risk of suicide among wounded Vietnam veterans. Am J Public Health, 1996. 86(5): p. 662–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jakupcak M, et al. , Posttraumatic stress disorder as a risk factor for suicidal ideation in Iraq and Afghanistan War veterans. J Trauma Stress, 2009. 22(4): p. 303–6. [DOI] [PubMed] [Google Scholar]
- 9.Centers for Disease Control and Prevention Vital Signs: Overdoses of Prescription Opioid Pain Relievers—United States, 1999-2008. Morbidity and Mortality Weekly Report 2011. 60(43): p. 1487–1492. [PubMed] [Google Scholar]
- 10.Veteran Affairs, Office of Public and Intergovernmental Affairs, VA Initiative Shows Early Promise in Reducing Use of Opioids for Chronic Pain, 2014.
- 11.Kitson A, Harvey G, and McCormack B, Enabling the implementation of evidence based practice: a conceptual framework. Qual Health Care, 1998. 7(3): p. 149–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Seal KH, et al. , Association of mental health disorders with prescription opioids and high-risk opioid use in US veterans of Iraq and Afghanistan. JAMA, 2012. 307(9): p. 940–7. [DOI] [PubMed] [Google Scholar]
- 13.Hooten WM and Bruce BK, Beliefs and attitudes about prescribing opioids among healthcare providers seeking continuing medical education. J Opioid Manag, 2011. 7(6): p. 417–24. [DOI] [PubMed] [Google Scholar]
- 14.Morley-Forster PK, et al. , Attitudes toward opioid use for chronic pain: a Canadian physician survey. Pain Res Manag, 2003. 8(4): p. 189–94. [DOI] [PubMed] [Google Scholar]
- 15.Lin JJ, Alfandre D, and Moore C, Physician attitudes toward opioid prescribing for patients with persistent noncancer pain. Clin J Pain, 2007. 23(9): p. 799–803. [DOI] [PubMed] [Google Scholar]
- 16.Matthias MS, et al. , The patient-provider relationship in chronic pain care: providers’ perspectives. Pain Med, 2010. 11(11): p. 1688–97. [DOI] [PubMed] [Google Scholar]
- 17.Alford DP, Weighing in on opioids for chronic pain: the barriers to change. JAMA, 2013. 310(13): p. 1351–2. [DOI] [PubMed] [Google Scholar]
- 18.Iliffe S, et al. , Attitudes to long-term use of benzodiazepine hypnotics by older people in general practice: findings from interviews with service users and providers. Aging Ment Health, 2004. 8(3): p. 242–8. [DOI] [PubMed] [Google Scholar]
- 19.Parr JM, et al. , Views of general practitioners and benzodiazepine users on benzodiazepines: a qualitative analysis. Soc Sci Med, 2006. 62(5): p. 1237–49. [DOI] [PubMed] [Google Scholar]
- 20.Cook JM, et al. , Physicians’ perspectives on prescribing benzodiazepines for older adults: a qualitative study. J Gen Intern Med, 2007. 22(3): p. 303–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sirdifield C, et al. , General practitioners’ experiences and perceptions of benzodiazepine prescribing: systematic review and meta-synthesis. BMC Fam Pract, 2013. 14: p. 191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Anthierens S, et al. , Barriers to nonpharmacologic treatments for stress, anxiety, and insomnia: family physicians’ attitudes toward benzodiazepine prescribing. Can Fam Physician, 2010. 56(11): p. e398–406. [PMC free article] [PubMed] [Google Scholar]
- 23.Dybwad TB, et al. , Why are some doctors high-prescribers of benzodiazepines and minor opiates? A qualitative study of GPs in Norway. Fam Pract, 1997. 14(5): p. 361–8. [DOI] [PubMed] [Google Scholar]
- 24.Damestoy N, Collin J, and Lalande R, Prescribing psychotropic medication for elderly patients: some physicians’ perspectives. CMAJ, 1999. 161(2): p. 143–5. [PMC free article] [PubMed] [Google Scholar]
- 25.Rogers A, et al. , Prescribing benzodiazepines in general practice: a new view of an old problem. Health (London), 2007. 11(2): p. 181–98. [DOI] [PubMed] [Google Scholar]
- 26.Kitson AL, et al. , Evaluating the successful implementation of evidence into practice using the PARiHS framework: theoretical and practical challenges. Implement Sci, 2008. 3: p. 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Stetler CB, et al. , A Guide for applying a revised version of the PARIHS framework for implementation. Implement Sci, 2011. 6: p. 99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Helfrich CD, et al. , A critical synthesis of literature on the promoting action on research implementation in health services (PARIHS) framework. Implement Sci, 2010. 5: p. 82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Harvey G and Kitson A, PARIHS Re-visited: Introducing the i-PARHIS Framework, in Implementing evidence-based practice in healthcare: A facilitation guide., Harvey G and Kitsonv A, Editors. 2015, Routledge: London. [Google Scholar]
- 30.StataCorp., Stata Statistical Software: Release 14.0, 2015, Stata Corporation: College Station TX. [Google Scholar]
- 31.Gallo JJ, et al. , Primary care clinicians evaluate integrated and referral models of behavioral health care for older adults: results from a multisite effectiveness trial (PRISM-e). Ann Fam Med, 2004. 2(4): p. 305–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Department of Veteran Affairs and Department of Defense, VA/DoD Clinical Practice Guideline for Management of Opioid Therapy for Chronic Pain, 2010.
- 33.Department of Veterans Affairs Pharmacy Benefits Management Service and Medical Advisory Panel, Guidance for Treatment of Insomnia in Veterans in the Primary Care Setting, March, 2013, Archived.
- 34.Moen J, et al. , GPs’ perceptions of multiple-medicine use in older patients. J Eval Clin Pract, 2010. 16(1): p. 69–75. [DOI] [PubMed] [Google Scholar]
- 35.Anderson K, et al. , Prescriber barriers and enablers to minimising potentially inappropriate medications in adults: a systematic review and thematic synthesis. BMJ Open, 2014. 4(12): p. e006544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Anthierens S, et al. , Qualitative insights into general practitioners views on polypharmacy. BMC Fam Pract, 2010. 11: p. 65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Williams AC, Eccleston C, and Morley S, Psychological therapies for the management of chronic pain (excluding headache) in adults. Cochrane Database Syst Rev, 2012. 11: p. CD007407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bandelow B, et al. , Efficacy of treatments for anxiety disorders: a meta-analysis. Int Clin Psychopharmacol, 2015. 30(4): p. 183–92. [DOI] [PubMed] [Google Scholar]
- 39.Trauer JM, et al. , Cognitive Behavioral Therapy for Chronic Insomnia: A Systematic Review and Meta-analysis. Ann Intern Med, 2015. 163(3): p. 191–204. [DOI] [PubMed] [Google Scholar]
- 40.Weingart SN, et al. , Physicians’ decisions to override computerized drug alerts in primary care. Arch Intern Med, 2003. 163(21): p. 2625–31. [DOI] [PubMed] [Google Scholar]
- 41.Grimshaw J, et al. , Effectiveness and efficiency of guideline dissemination and implementation strategies. Health Technology Assessment NHS, 2004. 8(6): p. 4. [DOI] [PubMed] [Google Scholar]
- 42.Kawamoto K, et al. , Improving clinical practice using clinical decision support systems: a systematic review of trials to identify features critical to success. BMJ, 2005. 330(7494): p. 765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Jamtvedt G, et al. , Does telling people what they have been doing change what they do? A systematic review of the effects of audit and feedback. Qual Saf Health Care, 2006. 15(6): p. 433–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Jamtvedt G, et al. , Audit and feedback: effects on professional practice and health care outcomes. Cochrane Database Syst Rev, 2006(2): p. CD000259. [DOI] [PubMed] [Google Scholar]
- 45.Lewis PJ and Tully MP, The discomfort caused by patient pressure on the prescribing decisions of hospital prescribers. Res Social Adm Pharm, 2011. 7(1): p. 4–15. [DOI] [PubMed] [Google Scholar]
- 46.Little P, et al. , Importance of patient pressure and perceived pressure and perceived medical need for investigations, referral, and prescribing in primary care: nested observational study. BMJ, 2004. 328(7437): p. 444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Dorr Goold S and Lipkin M Jr., The doctor-patient relationship: challenges, opportunities, and strategies. J Gen Intern Med, 1999. 14 Suppl 1: p. S26–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Esquibel AY and Borkan J, Doctors and patients in pain: Conflict and collaboration in opioid prescription in primary care. Pain, 2014. 155(12): p. 2575–82. [DOI] [PubMed] [Google Scholar]
- 49.Matthias MS, et al. , Communicating about opioids for chronic pain: a qualitative study of patient attributions and the influence of the patient-physician relationship. Eur J Pain, 2014. 18(6): p. 835–43. [DOI] [PubMed] [Google Scholar]
- 50.Fiske ST and Taylor SE, Social Cognition 1991: McGraw-Hill. [Google Scholar]
- 51.Leonard M, Graham S, and Bonacum D, The human factor: the critical importance of effective teamwork and communication in providing safe care. Qual Saf Health Care, 2004. 13 Suppl 1: p. i85–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Greenfield LJ, Doctors and nurses: a troubled partnership. Ann Surg, 1999. 230(3): p. 279–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Thomas EJ, Sexton JB, and Helmreich RL, Discrepant attitudes about teamwork among critical care nurses and physicians. Crit Care Med, 2003. 31(3): p. 956–9. [DOI] [PubMed] [Google Scholar]
- 54.Dayton E and Henriksen K, Communication failure: basic components, contributing factors, and the call for structure. Jt Comm J Qual Patient Saf, 2007. 33(1): p. 34–47. [DOI] [PubMed] [Google Scholar]
- 55.Compton J, et al. , Implementing SBAR across a large multihospital health system. Jt Comm J Qual Patient Saf, 2012. 38(6): p. 261–8. [DOI] [PubMed] [Google Scholar]
- 56.Haig KM, Sutton S, and Whittington J, SBAR: a shared mental model for improving communication between clinicians. Jt Comm J Qual Patient Saf, 2006. 32(3): p. 167–75. [DOI] [PubMed] [Google Scholar]
- 57.Randmaa M, et al. , SBAR improves communication and safety climate and decreases incident reports due to communication errors in an anaesthetic clinic: a prospective intervention study. BMJ Open, 2014. 4(1): p. e004268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.De Meester K, et al. , SBAR improves nurse-physician communication and reduces unexpected death: a pre and post intervention study. Resuscitation, 2013. 84(9): p. 1192–6. [DOI] [PubMed] [Google Scholar]


