Abstract
Background
Immunologically cold tumors with an ‘immune desert’ phenotype lack tumor-infiltrating lymphocytes (TILs) and are typically impervious to systemic immune checkpoint blockade (ICB). Intratumoral treatment of tumors with immunomodulatory agents can promote local tumor inflammation leading to improved T cell responses in injected tumors. Addition of systemic ICB increases response frequency and immune-mediated clearance of injected and distal non-injected lesions, and this promising approach is being widely investigated clinically. In this work, we evaluate and characterize the local and systemic antitumor immunotherapeutic activity of VAX014, a novel non-viral targeted oncolytic agent based on recombinant bacterial minicells, following intratumoral administration and in combination with systemic ICB.
Methods
The immunotherapeutic activity of VAX014 following weekly intratumoral administration was investigated in multiple preclinical tumor models with B16F10 murine melanoma serving as the primary model for evaluation of immune desert tumors. Mice bearing a single intradermal tumor were used to evaluate tumor response and overall survival (OS), assess changes in immune cell populations, and explore global changes to immunotranscriptomes of injected tumors. Mice bearing bilateral intradermal tumors were then used to evaluate non-injected tumors for changes in TIL populations and phenotypes, compare immunotranscriptomes across treatment groups, and assess distal non-injected tumor response in the context of monotherapy or in combination with ICB.
Results
VAX014 demonstrated strong immune-mediated tumor clearance of injected tumors coinciding with significantly elevated CD8+ TILs and upregulation of multiple immune pathways essential for antitumor immune responses. Modest activity against distal non-injected immune desert tumors was observed despite elevated levels of systemic antitumor lymphocytes. Combination with systemic CTLA-4 blockade improved survival and elevated TILs but did not improve clearance rates of non-injected tumors. Immunotranscriptomes of non-injected tumors from this treatment combination group exhibited upregulation of multiple immune pathways but also identified upregulation of PD-1. Further addition of systemic PD-1 blockade led to rapid clearance of non-injected tumors, enhanced OS, and provided durable protective immunological memory.
Conclusions
Intratumoral administration of VAX014 stimulates local immune activation and robust systemic antitumor lymphocytic responses. Combination with systemic ICB deepens systemic antitumor responses to mediate clearance of injected and distal non-injected tumors.
Keywords: immunotherapy; therapies, investigational; tumor microenvironment; lymphocytes, tumor-infiltrating; translational medical research
WHAT IS ALREADY KNOWN ON THIS TOPIC
Local administration of VAX014 elicits systemic immune-mediated clearance in orthotopic bladder tumor models. This study was conducted to investigate the broader utility of VAX014 as an in situ immunization agent after direct injection into solid tumors.
WHAT THIS STUDY ADDS
This study demonstrates for the first time that local intratumoral administration of VAX014 generates systemic immune-mediated clearance of tumors and is particularly effective in treating local and disseminated tumors with poor immune infiltration when combined with immune checkpoint blockade.
HOW THIS STUDY MIGHT AFFECT RESEARCH, PRACTICE OR POLICY
This study lays the groundwork for clinical investigation of VAX014 and may change practice for intralesional administered oncolytic therapies because VAX014 facilitates oncolysis within hours of administration and can be administered weekly as opposed to every 3 weeks, as is the case for oncolytic virotherapy.
Background
New discoveries elucidating the molecular mechanisms underlying the subverted role of the immune system in tumor pathobiology and immune evasion have led to the rapid development of new immunotherapeutic agents for the treatment of solid tumors.1 Systemically administered immune checkpoint blockade (ICB) directed against PD-1/PD-L1 and CTLA-4 lymphocyte immune checkpoint pathways have demonstrated remarkable durable complete responses (CRs) but, unfortunately, only in a minority of patients.2 A common immunopathological feature associated with a lack of response to ICB is a paucity of tumor-infiltrating lymphocytes (TILs) in the tumor microenvironment (TME).3 4 These immunologically ‘cold’ tumors employ various immunosuppressive mechanisms to thwart T cell priming, activation, expansion, and/or trafficking to tumor sites, leading to a low number of TILs in tumor and stroma (immune desert histology) or lack of penetration of TILs into tumors from tumor stroma (immune excluded histology).5
New locally delivered intratumoral immunotherapies designed to stimulate tumor inflammatory signals that support T cell priming, maturation, and recruitment to the tumor sites have provided encouraging results and important insights. The therapeutic premise of this emerging treatment paradigm is to facilitate in situ immunization against the injected tumor following intratumoral administration, the goal of which is to stimulate systemic antitumor immunity.6 Oncolytic viruses (OVs) have shown particular promise in this area, and while only a limited number of OV therapies have been approved to date, several others are in various stages of development.7–9 Despite adequate preclinical evidence that OVs facilitate in situ immunization, the clinical activity of OVs as monotherapy for solid tumors has been relatively limited.10 More recent experience has led to an appreciation for the potential therapeutic cooperation between intratumoral administered OV therapy with systemic ICB. For example, increased overall response rates in injected and non-injected tumors have been observed in exploratory studies combining talimogene laherparepvec (T-VEC) with ipilimumab (CTLA-4 blockade) or pembrolizumab (PD-1 blockade) in patients with treatment refractory melanoma.11–13 While improved, reported durable response rates and overall survival (OS) rates still remain relatively low, making the development of new, more effective oncolytic immunotherapies of critical importance.
In this work, we investigate and characterize the immunotherapeutic activity of VAX014, a novel clinical stage non-viral tumor targeted oncolytic agent based on recombinant bacterial minicells (rBMCs), following intratumoral treatment of established solid tumors. VAX014 has been described extensively elsewhere but can be summarized as an rBMC designed to target tumor-associated integrins using a protein called invasin (Inv) to deliver an oncolytic protein toxin payload, perfringolysin O (PFO).14–16 Here, we primarily used both single and bilateral tumor variations of the immune desert B16F10 murine melanoma model to explore the effectiveness of VAX014 as an oncolytic immunotherapy following intratumoral administration as monotherapy and in combination with systemically administered ICB. We demonstrate that shortly following injection into tumors, VAX014 facilitates rapid oncolysis while stimulating multiple immune pathways supporting adaptive antitumor immunity in the TME. Antitumor activity was entirely dependent on CD8+ T cells (and to a lesser extent on Natural Killer (NK) cells), and tumors actively responding to therapy had significantly elevated CD8+ TILs. Using a bilateral intradermal tumor variation of the B16F10 model, we demonstrated VAX014 promotes systemic immune-mediated reduction in distal non-injected tumors (abscopal effect) that did not lead to tumor clearance. Addition of systemic CTLA-4 blockade (αCTLA-4) improved abscopal responses, markedly increased CD8+ TILs in non-injected tumors, and extended survival, but rarely led to complete tumor clearance. Further addition of systemic PD-1 blockade (αPD-1) to the combination treatment regimen led to durable tumor clearance of both injected and non-injected tumors with an increase in CD8+ effector T cells, cytotoxic cell gene signature, presence of effector memory cells in distal non-injected tumors, and durable protective immunological memory.
The results presented here serve as a prelude for clinical investigation of VAX014 as an intratumoral delivered oncolytic immunotherapy, particularly in combination with ICBs, to enhance responses in patients with injectable ICB refractory tumors.
Methods
Mice
C57BL/6 female mice aged 6–8 weeks were purchased from Jackson Laboratory. Mice were housed in microisolators and in accordance with National Institute of Health and American Association of Laboratory Animal Care regulations. All mouse experiments and procedures were approved by the San Diego State University Institutional Animal Care and Use Committee.
Cell lines and reagents
The B16F10, CT26, and EL4 murine cell lines were purchased from American Type Culture Collection. The MB49 murine urothelial carcinoma cell line was obtained from Dr. W T Godbey at Tulane University. VAX014 rBMCs and vector control VAX-I rBMCs (PFO− and Inv+) were produced by Vaxiion Therapeutics (San Diego, California, USA), and the former was confirmed to meet the product specification for clinical use.
Statistics
All data were analyzed using GraphPad Prism V.9.0. Two-tailed Student’s t-test or Mann-Whitney U test was used to determine statistical significance between treatment groups, cytotoxic cell gene signatures, and TIL immunophenotyping analysis as indicated. Log-rank test was used to compare Kaplan-Meier survival curves. Error bars represent means ±SEM unless indicated otherwise. Any p value ≤0.05 was considered statistically significant. For all figures, *P≤0.05, **P≤0.01, ***P≤0.001, ****P≤0.0001.
Supplementary materials
Detailed method descriptions for in vitro studies, in vivo tumor models, flow cytometry, cytotoxic T lymphocyte (CTL) activity, TIL immunophenotyping analysis, and immunotranscriptome analysis are included in the online supplemental materials.
jitc-2023-006749supp001.pdf (109.8KB, pdf)
Results
Intratumoral treatment of B16F10 tumors with VAX014 mediates immune-dependent tumor clearance
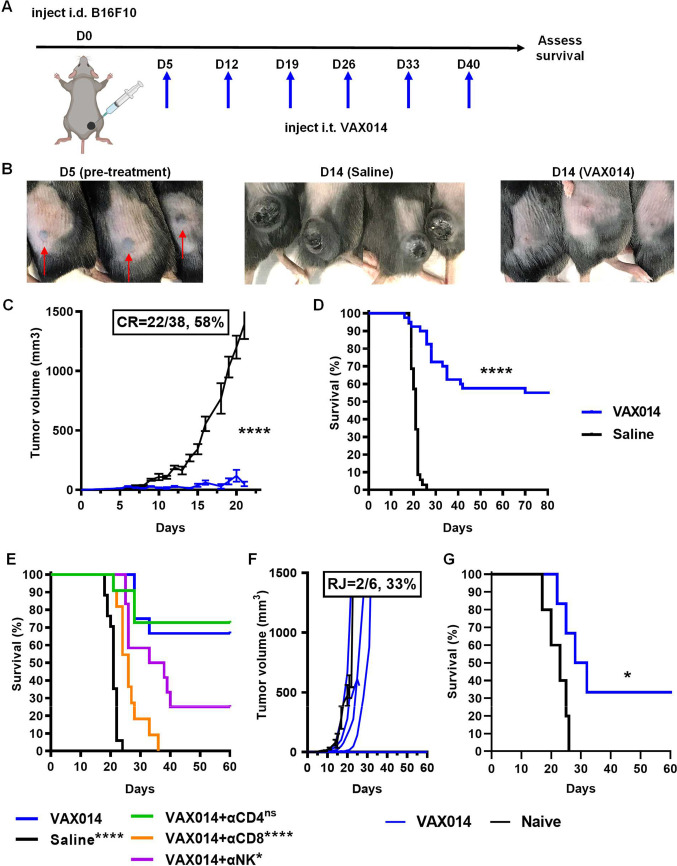
To evaluate the antitumor effects of VAX014 on tumors with an immune desert phenotype, we used the intradermal variation of the B16F10 model. The advantage of the intradermal model over the subcutaneous model is that intradermal implantation results in more accessible tumors for injection while yielding both cutaneous and subcutaneous tumor histology, which may be considered more clinically relevant. Prior to conducting in vivo experiments, the B16F10 cell line was characterized for integrin expression, rBMC uptake, VAX014 potency and oncolysis, and VAX014-induced apoptosis (online supplemental figure S1). Using a standardized experimental set-up (illustrated in figure 1A), we initiated intratumoral treatment of B16F10 tumors with VAX014 on day 5 as tumors approached 4 mm in length. Starting intratumoral treatment at this tumor size in the intradermal B16F10 model was essential for proper evaluation of pharmacodynamic activity of VAX014 after intratumoral administration because it insured that the entire injected dose volume (30 µL) was retained in injected tumors, which had a propensity to spontaneously cavitate and leak shortly after cresting 4 mm in length (online supplemental figure S2). We observed a rapid response to intratumoral administration of B16F10 tumors, which typically led to marked flattening of treated tumors within 24–72 hours of first treatment, with many tumors exhibiting near-complete regression after a second intratumoral dose of VAX014 on day 14 (figure 1B). Weekly intratumoral treatment of B16F10 tumors with VAX014 led to a significant reduction in tumor growth with frequent durable CRs (figure 1C), resulting in a significant OS advantage (figure 1D). Mice that achieved CR were tumor-free for as long as 364 days (longest time point observed), and individual tumor growth rates are provided in online supplemental figure S3. Similar antitumor responses from weekly intratumoral treatment with VAX014 were also observed in other syngeneic intradermal tumor models where treatments could be initiated on larger starting tumor sizes (less spontaneous ulceration) (online supplemental figures S4 and S5). Importantly, these additional models have differing immunophenotypes from each other and B16F10. The CT26 model is widely considered an immune exhausted model whereas the MB49 model is considered immune excluded.
Figure 1.
Weekly intratumoral administration of VAX014 leads to immune-dependent clearance of injected tumors. (A) Experimental design of single intradermal tumor model and dosing schedule. Treatments (30 µL) were initiated when tumors approached 4 mm in length. (B) Intradermal B16F10 tumors prior to treatment initiation (D5) and 2 days following second VAX014 intratumoral treatment (D14) in comparison with saline-treated controls. Red arrows indicate the location of intradermal tumors. (C) Mean B16F10 tumor growth rate and CR frequency from VAX014 (n=38) or saline-treated tumors (n=35). (D) Comparative survival curves for VAX014 versus saline-treated controls. (E) Survival curves of B16F10 tumor-bearing mice treated weekly via the intratumoral route with VAX014 after depletion of CD8+ T cells, CD4+ T cells, or NK cells (n=11–17/group). (F) Individual tumor growth rates following RC of long-term VAX014 treated survivors with a single intradermal B16F10 tumor (n=6) performed a minimum of 35–45 days post CR. Mean tumor growth rate of B16F10 tumor-naïve control mice is shown for comparison (n=5, black line). Individual tumor growth curves for naïve control are provided in online supplemental figure S3. (G) Survival curves of VAX014-treated rechallenged survivors and naïve control. Significance was determined using Student’s t-test for mean tumor growth rate and log-rank test for survival curves. Where present, error bars represent ±SEM. CR, complete response; i.d., intradermal; i.t., intratumoral; NK, Natural Killer cells; ns, not significant; RC, rechallenge; RJ, rejection of rechallenged tumor.
jitc-2023-006749supp002.pdf (5MB, pdf)
Previous studies demonstrate that the antitumor activity of VAX014 following topical intravesical treatment of orthotopic MB49 bladder tumors in mice is dependent on T lymphocytes.16 Therefore, we investigated the role of CD8+ T cells, CD4+ T cells, or NK cells in the antitumor activity of VAX014 following intratumoral administration by depleting these immune cell subsets throughout treatment (figure 1E). The antitumor activity of VAX014 following intratumoral administration was entirely dependent on CD8+ T cells, dependent to a lesser degree on NK cells, and independent of CD4+ T cells. Next, to evaluate if the antitumor lymphocytic response following intratumoral administration of VAX014 afforded immunological protection, we rechallenged mice that had achieved a CR with a second round of B16F10 tumors (figure 1F, G). Complete tumor rejection was seen in a number of mice, and most exhibited a significant reduction in tumor growth rate and improved survival compared with naïve controls. Despite the complete dependence on CD8+ T cells for VAX014 antitumor activity following intratumoral treatment, not all mice achieving CR were capable of complete tumor control following rechallenge. In contrast, the activity of VAX014 in the intradermal CT26 and MB49 models was also completely dependent on CD8+ T cells (online supplemental figures S4 and S5), and in both those models, strong protective antitumor memory was observed. Coincidentally, the MB49 cell line, which is syngeneic to B16F10, was found to express detectable levels of major histocompatibility complex-I (MHC-I) in vitro, whereas B16F10 did not (online supplemental figure S6). Comparatively higher expression of MHC-I in the MB49 model versus the B16F10 model was preserved in vivo (online supplemental figure S7), which may explain why T cell mediated memory responses were not as robust in the B16F10 model.
Intratumoral treatment with VAX014 increases TILs, results in tumor-specific CTL activity, and upregulates multiple immune networks
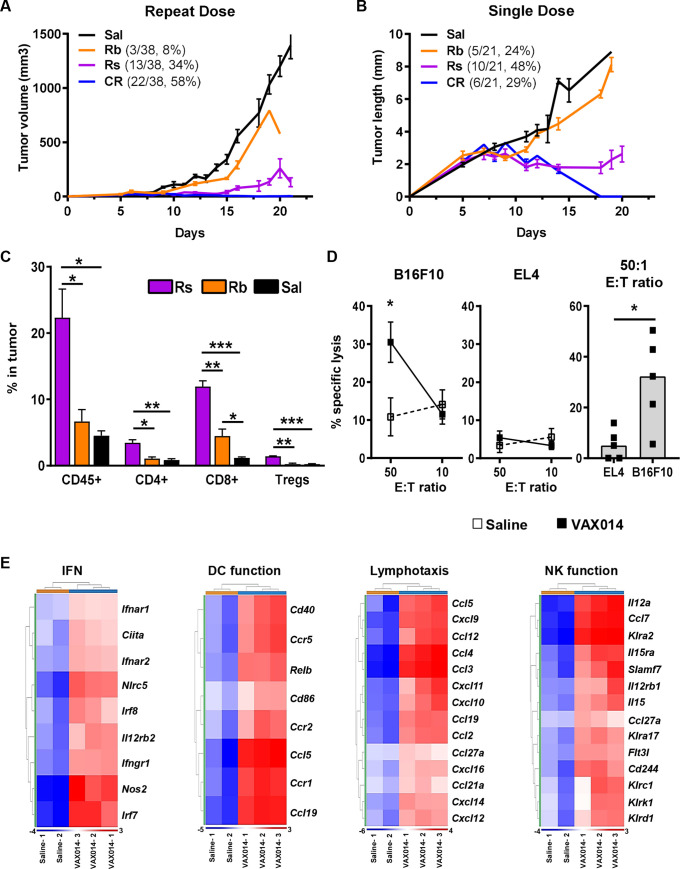
The B16F10 model has been generally accepted as an immunologically cold model with an immune desert phenotype.17 18 This concept was supported by a comparison of immunotranscriptomes from saline-treated B16F10 tumors to the immune excluded MB49 model (online supplemental figure S8). The B16F10 model has also been shown to have increasing levels of myeloid-derived suppressor cells (MDSCs) in the periphery as tumors increase in size, which is known to contribute to immunosuppression both systemically and within tumors.19 20 Increasing levels of MDSCs as a function of tumor growth were confirmed before using the model for these studies (online supplemental figures S9 and S10). To evaluate whether intratumoral administration of VAX014 could turn cold tumors ‘hot’, we evaluated changes in TIL levels, splenic CTL activity, and immunotranscriptomes from B16F10 tumors actively responding to VAX014 (figure 2). First, a deeper analysis of individual tumor growth curves shown in figure 1C revealed 100% of treated tumors had an initial response to weekly intratumoral treatment with VAX014 but would eventually diverge into response subgroup categories of “actively responding” or “rebounding” tumors (figure 2A). We took advantage of this response divergence to study differences in TIL levels in actively responding tumors in comparison to rebounding and saline-treated control tumors. To allow for sufficient response divergence to occur and to obtain larger tumors for TIL analysis, we manipulated the model to provide a single, lower dose of VAX014 via the intratumoral route (figure 2B). Remarkably, several mice achieved CR after a single intratumoral dose of VAX014 (individual growth curves provided in online supplemental figure S11). Using this experimental set-up, we then used flow cytometry to analyze the immune compartment of actively responding tumors to those that were rebounding or treated with saline (figure 2C). Actively responding tumors had a significant increase in the total CD45+ leukocyte population, most of which were CD8+ T cells. CD4+ T cells and Tregs were also significantly increased in responding tumors in comparison to rebounding tumors or saline-treated controls. Reduced tumor size did not contribute to the observed increase in proportion of immune cells within tumors actively responding to VAX014 as immune cell proportions of size-matched saline-treated tumors were similar to larger saline-treated tumors (online supplemental figure S12).
Figure 2.
Intratumoral administration of VAX014 increases TILs, upregulates multiple immune gene networks, and results in systemic tumor-specific cellular immunity. (A) Mean tumor growth rates of injected B16F10 tumors following weekly intratumoral treatment with saline (n=35) or VAX014 divided into disparate response groups defined as Rs, Rb, or CR. (B) Mean tumor growth rates of injected B16F10 tumors after a single lower intratumoral dose with saline (n=10) or VAX014 and divided into disparate response groups (described in A). (C) Percentage of total leukocytes (CD45+), CD8+ T cells, CD4+ T cells, and Tregs (CD4+Foxp3+) in actively responding B16F10 tumors versus rebounding and saline-treated tumors following a single intratumoral treatment with VAX014 (tumors analyzed by flow cytometry on days 20–28, n=3/group). (D) Splenic CTL activity against B16F10 target cells or EL4 non-specific haplotype matched control target cells following a single intratumoral treatment with VAX014 versus saline-treated control (splenocyte isolation/expansion performed on day 14 post tumor implantation, n=4–5/group). (E) Heatmaps of immune gene networks for IFN response, DC function, lymphotaxis, and NK function from immunotranscriptomes of B16F10 tumors 24 hours following intratumoral injection with VAX014 (n=3) versus saline-treated tumors (n=2). Statistical significance for mean CTL activity was analyzed using Student’s t-test, and median VAX014 CTL activity at 50:1 E:T ratio was analyzed using Mann-Whitney U test. Where present, error bars represent ±SEM. CR, complete response; CTL, cytotoxic T lymphocyte; DC, dendritic cell; E:T, effector-to-target; IFN, interferon; NK, Natural Killer cells; Rb, rebounding tumor; Rs, actively responding tumor; Sal, saline.
Building on these observations, we explored the potential development of systemic tumor-specific lymphocytic responses by evaluating CTL activity of splenocytes isolated from mice treated with a single dose of VAX014 using B16F10 as target cells (figure 2D). Splenocytes from saline-treated control mice exhibited limited CTL activity, whereas those isolated from mice treated with VAX014 demonstrated a CTL response specific for B16F10 target cells, but not syngeneic haplotype matched murine EL4 thymoma cells (non-specific target cell control). In comparison to the robust CTL activity against B16F10 target cells (which contains all tumor specific epitopes), only marginal systemic CTL responses were mounted against two well-characterized B16F10-specific MHC-I restricted peptide epitopes (gp100 and TRP-2), and this coincided with very few TRP-2 specific CD8+ TILs in injected tumors (online supplemental figure S12).
Finally, comparative immunotranscriptome analysis of B16F10 tumors conducted 24 hours following intratumoral administration of VAX014 revealed upregulation of multiple immune gene networks. Among others, these included gene networks supporting type I and type II interferon (IFN) response, dendritic cell (DC) activation/function, T cell recruitment, and NK cell function (figure 2E). Each of these immune pathways/functions are known requirements for initiation, priming, and maturation of an adaptive cellular antitumor immune response and provide molecular evidence supporting the lymphocyte and NK cell-dependent activity of VAX014 following intratumoral administration.21 22
Combination of VAX014 with systemic ICB against CTLA-4 or PD-1 increases injected tumor clearance and improves non-injected tumor responses
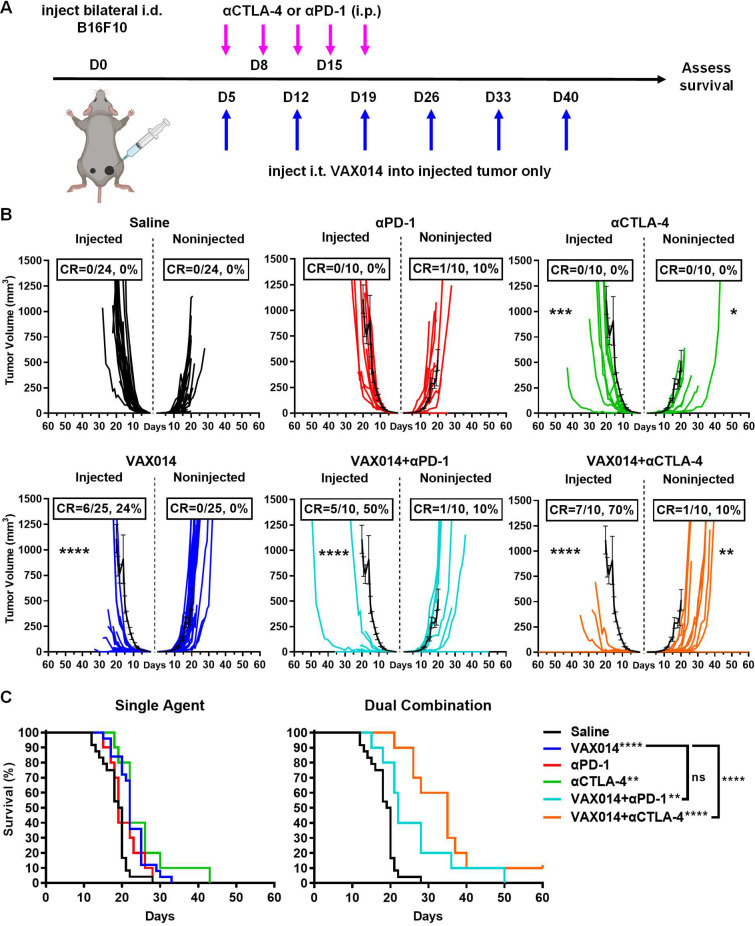
Recent clinical data provide evidence that combining intratumoral administered oncolytic virotherapy with systemic ICB against either PD-1 or CTLA-4 enhances abscopal effects in distal non-injected lesions.11 12 Therefore, we used a bilateral intradermal variation of the B16F10 model to assess abscopal effects engendered by intratumoral administration of VAX014 as monotherapy or in combination with either PD-1 or CTLA-4 blockade (figure 3). In this experimental model, one tumor is designated for weekly intratumoral treatment with VAX014 (injected tumor), and the other was designated as the distal non-injected tumor (figure 3A). Results from these studies demonstrated that intratumoral administration of VAX014 resulted in antitumor activity against injected B16F10 tumors but had limited effect on non-injected tumors (figure 3B, C). However, this was only observed in the immune desert bilateral intradermal B16F10 model. In the immune excluded bilateral intradermal MB49 model, appreciable abscopal effects were observed, and activity was both tumor-specific and CD8+ T cell-dependent (online supplemental figures S13 and S14). In addition, the antitumor immune response to B16F10 was specific for the treated tumor type in vivo as evidenced by lack of abscopal effect when a different syngeneic tumor cell line was used as the non-injected tumor in the bilateral tumor model (online supplemental figure S15). Consistent with previous reports, single-agent ICB controls demonstrated limited treatment effect in this model (figure 3B, C).23 24
Figure 3.
Weekly intratumoral administration of VAX014 in combination with systemic αPD-1 or αCTLA-4 improves injected tumor responses with variable impact on distal non-injected tumors in the bilateral intradermal B16F10 tumor model. (A) Experimental design of the bilateral intradermal B16F10 tumor model and dosing schedule. (B) Individual tumor growth rates and CR frequencies (n=10–25/treatment group) for injected and distal non-injected tumors compared with the mean tumor growth rate of saline-treated tumors (black line). (C) Comparative survival curves of VAX014/αCTLA-4 and VAX014/αPD-1 combination treatment groups and single-agent controls. Statistical significance was determined by Student’s t-test for mean tumor growth rate and log-rank test for survival curves. Where present, error bars represent ±SEM. CR, complete response; i.d., intradermal; i.p., intraperitoneal; i.t., intratumoral; ns, not significant.
The combination of VAX014 with systemic αPD-1 had a discernible additive treatment effect in injected tumors but only a modest treatment effect in non-injected tumors, suggestive of an established immunosuppressive environment in distal non-injected tumors (figure 3B). A slight increase in survival over VAX014 monotherapy was observed but was not significant, and there were no long-term survivors using this combination (figure 3C). The combination of VAX014 with systemic αCTLA-4 represented a significant improvement in response of both injected and non-injected tumors (figure 3B). Most importantly, survival was significantly extended with this combination, and the first instance of a durable CR of both tumors affording long-term survival was observed (figure 3C).
Activation of multiple inflammatory immune pathways in injected and non-injected tumors
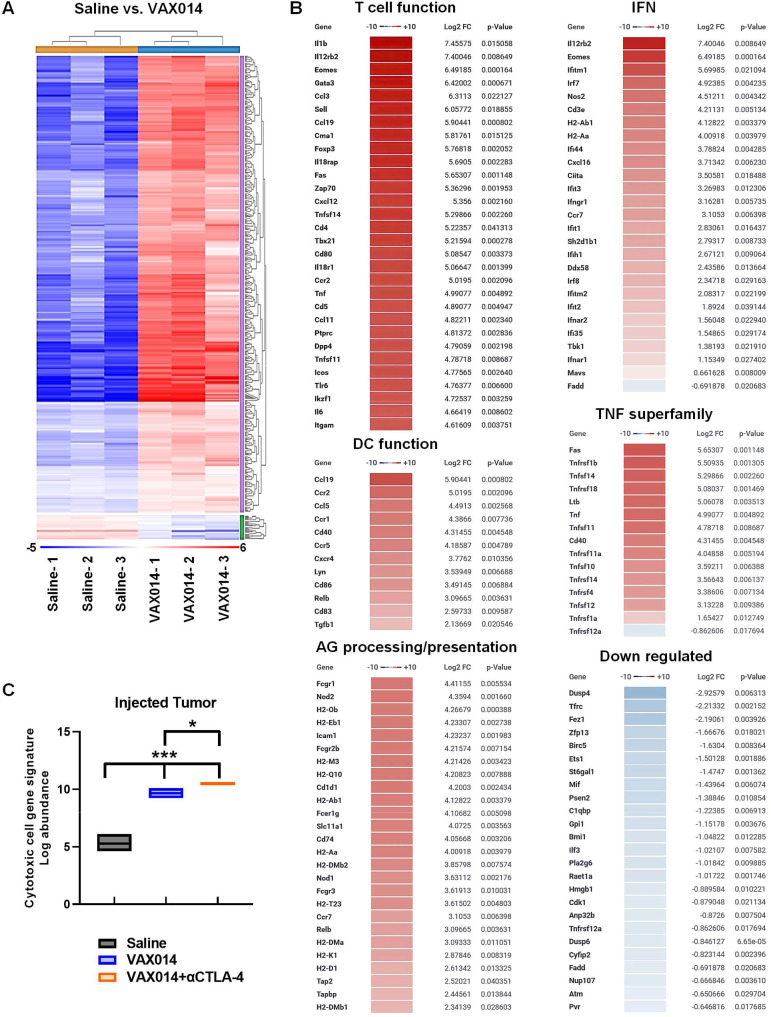
To gain a fundamental understanding of why the VAX014/αCTLA-4 combination improved responses in both tumors, we next performed comparative immunotranscriptome analysis of injected tumors (figure 4) and non-injected tumors (figure 5) using the bilateral intradermal B16F10 model (individual tumor growth rates used for immunotranscriptome analysis shown in online supplemental figure S16). Immunotranscriptomes of injected tumors from the VAX014 monotherapy group exhibited upregulation of 462 genes spanning multiple immune networks in comparison to saline-treated control tumors (figure 4A, B). Upregulated immune activation pathways included those involved in T cell lymphotaxis and effector function (non-exhaustive list of T cell genes is shown; for the full list, see online supplemental figure S17), IFN production/signaling, DC activation/function, tumor necrosis factor superfamily members, and antigen (AG) processing/presentation (figure 4B). Downregulation occurred in a limited number of genes primarily categorized as tumor-promoting or melanoma-specific. These included antiapoptotic, cell cycle regulation, epithelial mesenchymal transition, and melanin synthesis pathway genes. Only four additional genes were found to be upregulated/downregulated in injected tumors from the VAX014/αCTLA-4 combination treatment group compared with VAX014 as monotherapy (online supplemental figure S17). Comprehensive cytotoxic cell gene network signatures increased in VAX014 injected tumors, and this gene signature was further intensified in injected tumors from the VAX014/αCTLA-4 combination treatment group (figure 4C).
Figure 4.
Immunologically cold B16F10 tumors injected with VAX014 upregulate multiple inflammatory immune activation pathways in the bilateral intradermal B16F10 tumor model. (A) Complete comparative heat maps of immunotranscriptomes from total RNA extracted from injected B16F10 tumors after weekly intratumoral treatment with VAX014 as monotherapy versus saline (n=3/group). Immunotranscriptome analysis was performed on total RNA isolated from tumors 2 days following the second weekly intratumoral treatment. (B) Hierarchical list of fold change in individual gene transcripts among select innate and adaptive immune pathway gene networks in injected B16F10 tumors following treatment with VAX014 versus saline. (C) Comprehensive cytotoxic cell gene network signatures of injected B16F10 tumors following intratumoral treatment with saline, VAX014 monotherapy, or VAX014/αCTLA-4 combination (n=3/group). Bar graph represents median values, and statistical significance of cytotoxic cell log abundance was analyzed using Student’s t-test. AG, antigen; DC, dendritic cell; IFN, interferon; log2 FC, log2 fold change; TNF, tumor necrosis factor.
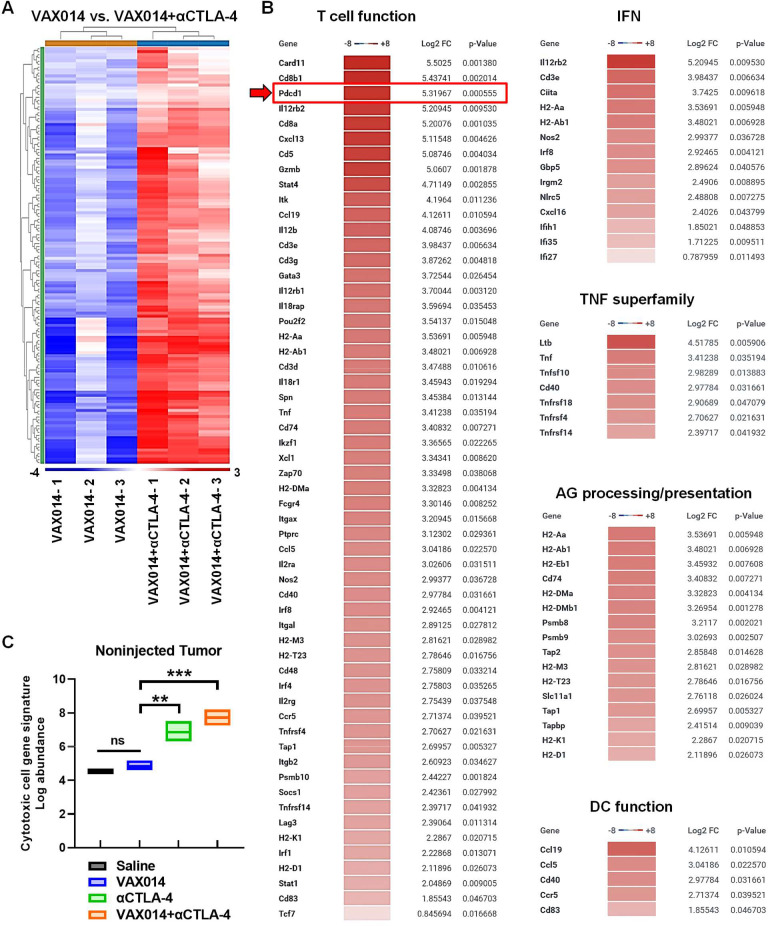
Figure 5.
Combination of VAX014 with systemic αCTLA-4 in the bilateral intradermal B16F10 model leads to upregulation of multiple immune pathways in distal non-injected tumors. (A) Complete comparative heat maps of immunotranscriptomes from total RNA extracted from distal non-injected B16F10 tumors after weekly intratumoral treatment of the contralateral B16F10 tumor designated for injection with VAX014 as monotherapy versus VAX014/αCTLA combination (n=3/group). Immunotranscriptome analysis was performed on total RNA isolated from non-injected tumors 2 days following the second weekly intratumoral treatment of the contralateral injected tumor with VAX014. (B) Hierarchical list of fold change in individual gene transcripts among select innate and adaptive immune pathway gene networks in non-injected B16F10 tumors from the VAX014/αCTLA-4 combination treatment group versus VAX014 as monotherapy. Red arrow highlights PD-1 gene (Pdcd1) in the T cell function gene network. (C) Comprehensive cytotoxic cell gene network signatures of non-injected B16F10 tumors following intratumoral treatment of the contralateral injected B16F10 tumor with saline, VAX014 monotherapy, systemic αCTLA-4 monotherapy, or VAX014/αCTLA-4 combination (n=3/group). Bar graph represents median values, and statistical significance of cytotoxic cell log abundance was analyzed using Student’s t-test. AG, antigen; DC, dendritic cell; IFN, interferon; log2 FC, log2 fold change; ns, not significant; TNF, tumor necrosis factor.
Immunotranscriptomes of distal non-injected tumors revealed VAX014 treatment did not impact immune gene expression in comparison to saline-treated tumors (ie, no heat map could be generated), whereas the VAX014/αCTLA-4 combination led to the upregulation of 137 genes in non-injected tumors in comparison to VAX014 monotherapy spanning multiple antitumor immune response networks (figure 5A, B). Interestingly, Pdcd1 (PD-1) was found to be upregulated, ranking third highest among upregulated transcripts within the T cell function network (figure 5B). The comprehensive cytotoxic cell gene network signatures of non-injected tumors were similar between VAX014 monotherapy and saline-treated tumors, increased in the systemic αCTLA-4 monotherapy group, and further intensified in the VAX014/αCTLA-4 combination treatment group (figure 5C).
Tripartite combination of VAX014 with both CTLA-4 and PD-1 blockade improves systemic tumor clearance
Upregulation of PD-1 in the distal non-injected tumors of VAX014/αCTLA-4 combination-treated mice provided rationale for evaluating the treatment effect of tripartite combination by further addition of PD-1 blockade (figure 6). Following the same treatment schedule as shown in figure 3, the tripartite combination resulted in a significant decrease in injected and non-injected tumor growth rates with the majority of mice achieving CR in both tumors (figure 6A). Furthermore, the tripartite VAX014/αCTLA-4/αPD-1 combination resulted in a significant increase in median survival and improved the OS rate (figure 6B) in comparison to the systemic αCTLA-4/αPD-1 treatment control group. No difference in the intensity of comprehensive cytotoxic cell gene network signatures of injected tumors was observed between the VAX014/αCTLA-4 combination and the tripartite VAX014/αCTLA-4/αPD-1 combination groups (figure 6C). In non-injected tumors, the change in cytotoxic cell gene networks followed an increase in intensity progressing stepwise from systemic αCTLA-4 monotherapy to VAX014/αCTLA-4 combination to tripartite VAX014/αCTLA-4/αPD-1 combination group.
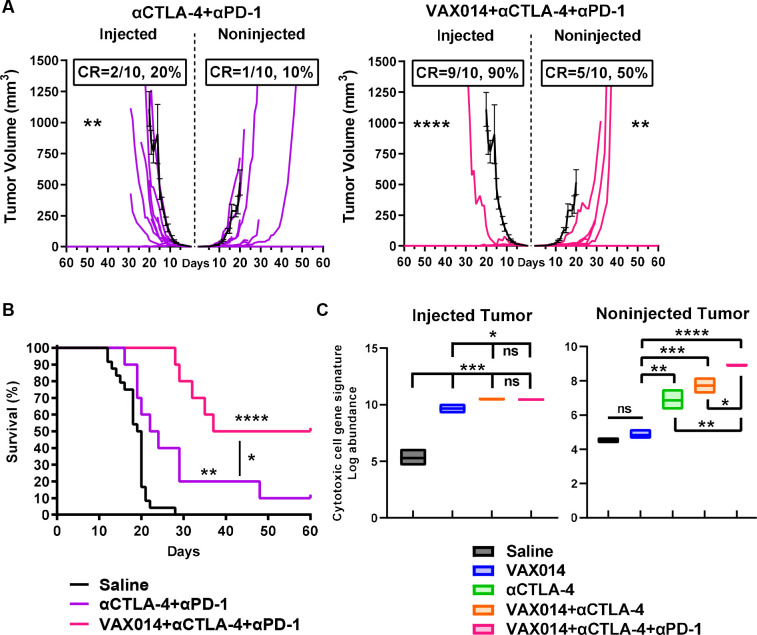
Figure 6.
Tripartite combination of VAX014 with systemic CTLA-4 and PD-1 blockade effectively controls injected and non-injected immunologically cold tumors. (A) Individual tumor growth rates and CR frequencies (n=10/treatment group) for injected and distal non-injected B16F10 tumors from the systemic αCTLA-4/αPD-1 treatment control group (left panel) versus the tripartite VAX014/αCTLA-4/αPD-1 combination group (right panel). Mean tumor growth rate of saline-treated tumors is shown for comparison (black line). (B) Survival curves from the tripartite VAX014/αCTLA-4/αPD-1 combination group in comparison to the systemic αCTLA-4/αPD-1 treatment control group. (C) Comprehensive cytotoxic cell gene network signatures of distal non-injected B16F10 tumors following intratumoral treatment of the contralateral injected B16F10 tumor with saline, VAX014 monotherapy, systemic αCTLA-4 monotherapy, VAX014/αCTLA-4 combination, or tripartite VAX014/αCTLA-4/αPD-1 combination (n=3/treatment group). Bar graph represents median values. Statistical significance for mean tumor growth rates and cytotoxic cell gene signature log abundance was determined by Student’s t-test. Log-rank test was used for survival curves. Where present, error bars represent ±SEM. CR, complete response; ns, not significant.
Effector TILs increase in distal non-injected tumors after treatment with tripartite combination
We next performed a comparative evaluation of the lymphocyte compartment of distal non-injected tumors among different treatment groups on day 14 post tumor implantation (figure 7, individual tumor growth rates used for TIL analysis shown in online supplemental figure S18). The percentage of CD45+ leukocytes within the total live cell population of non-injected tumors increased slightly in response to different treatment combinations in comparison to saline-treated control tumors (figure 7A). Notably, the percentage of CD3+ TILs among the total leukocyte population significantly increased in stepwise fashion in non-injected tumors following intratumoral treatment of the opposing tumor with VAX014 monotherapy, VAX014/αCTLA-4 combination, or tripartite VAX014/αCTLA-4/αPD-1 combination (figure 7B), and CD8+ TILs were the predominant infiltrating lymphocyte subset (figure 7C–E). In any treatment regimen containing VAX014, nearly all CD8+ TILs in the non-injected tumor had a cytotoxic phenotype (figure 7F). As a result of the large increase in CD8+ TILs, the percentage of CD4+ T cells decreased among the lymphocyte population, leading to a positive change in the overall CD8+/CD4+ TIL ratio (figure 7G–H). Additional analyses of tumor lymphocytes are provided in online supplemental figure S19. Finally, a significant increase in CD8+ T effector memory (TEM) cells was observed in non-injected tumors from the tripartite combination (figure 7I), which is consistent with the ability of surviving mice that achieved CR of both tumors to either completely reject B16F10 tumor rechallenge or significantly delay tumor growth (figure 7J).
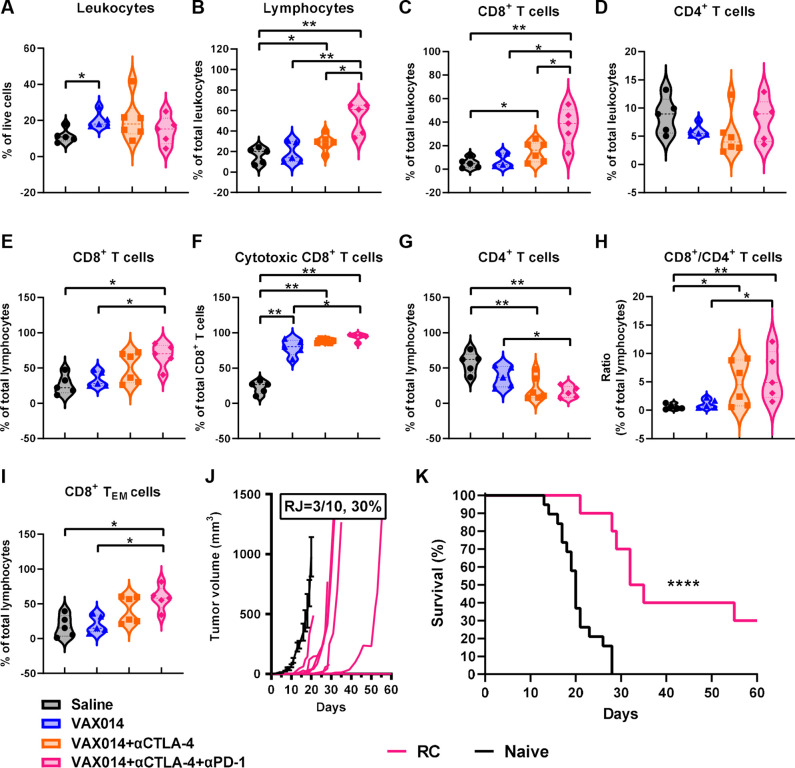
Figure 7.
Tripartite combination of VAX014 with systemic CTLA-4 and PD-1 blockade increases cytotoxic CD8+ and effector memory TILs in distal non-injected tumors and protects from tumor rechallenge. On day 14 post tumor implantation, non-injected tumors were extracted and evaluated for (A) percentage of total leukocytes (CD45+) among live tumor cells. (B) Percentage of total lymphocytes (CD45+CD3+) among total leukocytes. (C) Percentage of CD8+ T cells (CD45+CD3+CD8+) among total leukocytes. (D) Percentage of CD4+ T cells (CD45+CD3+CD4+) among total leukocytes. (E) Percentage of CD8+ T cells among total lymphocytes. (F) Percentage of cytotoxic effector CD8+ T cells (CD45+CD3+CD8+GzmB+) among total CD8+ lymphocytes. (G) Percentage of CD4+ T cells among total lymphocytes. (H) Ratio of CD8+ T cells to CD4+ T cells. (I) Percentage of CD8+ TEM cells (CD45+CD3+CD8+CD44+CD62L−) among total lymphocytes. Median values from each treatment group are plotted (n=5–6/treatment group). (J) Individual tumor growth rates following RC of long-term survivors from the tripartite VAX014/αCTLA-4/αPD-1 combination with a single intradermal B16F10 tumor (n=10) performed 35–45 days post CR. Mean tumor growth rate of naïve control tumors (n=19) is shown for comparison (black line). Error bars represent±SEM. (K) Survival curves of tripartite VAX014/αCTLA-4/αPD-1 combination rechallenged survivors and naïve controls. Statistical significance of immune cell data was analyzed using Mann-Whitney U-test, and log-rank test was used to determine significance in survival. RC, rechallenge; RJ, rejection of rechallenged tumor; TEM, T effector memory; TIL, tumor-infiltrating lymphocyte.
Discussion
In this study, we characterize the immunotherapeutic antitumor activity of VAX014, a novel non-viral oncolytic agent based on bacterial minicells, following direct injection into poorly immune infiltrated tumors. The B16F10 murine melanoma model was selected as the central tumor model for these studies as it is well characterized, largely non-immunogenic, exhibits ‘immune desert’ histology with minimal presence of TILs, is resistant to systemic ICB, and can be manipulated to evaluate abscopal effects in distal non-injected tumors.17 18 25–28 In mice bearing a single intradermal B16F10 tumor, local intratumoral administration of VAX014 led to rapid tumor inflammation, upregulation of multiple immune activation gene networks associated with antitumor responses, and observable antitumor effects (eg, flattening of palpable tumors) shortly following the first administered dose. Rapid flattening of tumors after the initial intratumoral administration of VAX014 occurred in every solid tumor model tested. This observation could simply be a function of the established rapid oncolytic mechanism of VAX014 yet also suggests VAX014 may quickly promote local innate antitumor effector functions after intratumoral administration.14 The diminished antitumor activity of VAX014 injected B16F10 tumors in mice depleted of NK cells provides some evidence to support this, although ultimately, complete tumor clearance was dependent on CD8+ T cells. Together, these two observations may support a model where innate antitumor effector functions provide a first wave of immune-mediated antitumor activity after intratumoral administration of VAX014, which keeps tumors at bay until a secondary lymphocytic response sufficient to completely clear tumors can be mounted. While more work remains to elucidate this potential mechanism, this biphasic model of immune events aligns with known mechanisms of immune-mediated tumor clearance.22 Injected tumors actively responding to VAX014 consistently exhibited elevated leukocytes, the majority of which were CD8+ TILs, whereas rebounding and saline-treated control tumors did not, a finding consistent with what one would expect, given the CD8+ T cell-dependent activity of VAX014. The adaptive response was systemic and specific for the injected tumor type as determined in vitro by CTL activity and in vivo by a lack of abscopal response when a different syngeneic tumor was used as the non-injected tumor in the bilateral tumor model. Notably, in comparison to the strong CTL activity against B16F10 target cells, only marginal CTL activity was observed against either gp100 or TRP-2 B16F10 tumor-selective MHC-I restricted peptides.29 30 This may provide evidence that intratumoral administration of VAX014 confers a T cell response against a broad repertoire of tumor epitopes (ie, epitope spread), which is considered a positive attribute with respect to curtailing tumor immune escape.31 32 While outside the scope of this report, future work incorporating a longitudinal evaluation of T cell evolution over time using deep T cell receptor sequencing following treatment with VAX014 is warranted.
We also demonstrated intratumoral administration with VAX014 led to appreciable abscopal effects including complete tumor clearance of distal non-injected tumors in the immune excluded MB49 model. However, and not unexpectedly, abscopal effects were more subdued in the immune desert B16F10 model, where TMEs are bereft of TILs. Interestingly, in both bilateral intradermal tumor models, we observed reduced CR rates in response to VAX014 in injected tumors. This phenomenon only occurred when using the same tumor in each flank. In contrast, when two different tumors were implanted in opposite flanks, there was no change in CR rate in response to VAX014 in injected tumors (online supplemental figures S14 and S15) in comparison to when a single intradermal tumor was used. This observation suggests some form of tumor-promoting crosstalk occurs, which may be related to increased tumor burden when identical tumors are used in bilateral tumor models. One intriguing possibility is that these tumors produce factors that promote aberrant myelopoiesis of MDSCs from the bone marrow of immune competent mice. Doubling the tumor burden in the bilateral models would presumably enhance the global production of these factors, accelerate myelopoiesis of MDSCs, and may lead to additive systemic and/or peripheral immune suppression. Based on the known immunosuppressive role of MDSCs in preclinical tumor models, enhanced myelopoiesis owing to increased systemic tumor burden may represent a possible explanation for the reduced CR rates seen in tumors injected with VAX014 in both bilateral tumor models. While further investigation is required to evaluate the role of MDSCs in the bilateral tumor models, we did observe a marked increase of MDSCs in the periphery in relation to tumor size in mice bearing a single B16F10 tumor when characterizing the model for use in these studies (online supplemental figures S9 and S10).19 20 Clinically, elevated MDSCs in peripheral blood are associated with more aggressive disease coinciding with worse prognosis.33
Based on limited abscopal effects following intratumoral administration of VAX014 alone in the immune desert B16F10 model, we hypothesized any established immune suppressive mechanisms in distal non-injected B16F10 tumors may have prevented TIL infiltration and/or effector function at the non-injected tumor site and that combination with ICB against either PD-1 or CTLA-4 would help to overcome immunosuppression and improve immune-mediated clearance of non-injected tumors. To explore this, we again used the bilateral intradermal B16F10 model to evaluate treatment regimens combining intratumoral VAX014 with systemic ICB against either PD-1 or CTLA-4 and found combination with either αPD-1 or αCTLA-4 led to improved responses in injected tumors, but only the VAX014/αCTLA-4 combination led to improved control of distal non-injected tumors. Improved tumor control following addition of αCTLA-4 was associated with higher levels of cytotoxic CD8+ TILs in non-injected tumors as well as a decrease in the number of CD4+ Tregs (online supplemental figure S19). Indeed, immunotranscriptome analysis of non-injected tumors from the VAX014/αCTLA-4 combination treatment group confirmed upregulation of multiple immune pathways associated with development of an antitumor T cell response but also identified marked upregulation of PD-1. Addition of PD-1 blockade to the VAX014/αCTLA-4 combination treatment regimen further improved abscopal effects, led to frequent durable CRs of both injected and non-injected tumors, enhanced overall long-term survival, and led to antitumor immunological memory. Interestingly, immunotranscriptome analysis showed no difference in the number of individual genes upregulated in tumors from the tripartite VAX014/αCTLA-4/αPD-1 combination treatment group in comparison to the VAX014/αCTLA-4 combination. However, the comprehensive cytotoxic cell gene network intensified significantly in non-injected tumors from the tripartite combination treatment group, indicating more robust immune activation relative to the VAX014/αCTLA-4 combination. Immunophenotyping of TILs in non-injected tumors after tripartite treatment combination indicated higher levels of effector CD8+ TILs as well as a significant increase in CD8+ TEM cells, which was consistent with the finding that mice exhibiting CR of both tumors in this treatment group developed immunological memory against tumor rechallenge. It is interesting to note that complete tumor control following rechallenge was observed in only 35% of mice in the B16F10 model after clearance of primary tumors in the bilateral model (and the single-tumor model), despite a robust tumor-specific CTL response, complete dependency on CD8+ T cells for tumor clearance, elevated levels of CD8+ T cells in actively responding tumors, and elevated CD8+ TEM cells in non-injected tumors of the bilateral model after tripartite treatment. This calls into question whether protective antitumor memory is established in the CD8+ T cell compartment in response to VAX014 in the B16F10 model. There is evidence to suggest that the lower tumor rejection rate in the B16F10 model may be related to the lack of detectable MHC-I expression in this tumor cell line in comparison to the other models used in this study. For example, MHC-I expression was detectable in the MB49 model both in vitro and in vivo, and in that model, which is syngeneic to B16F10, 100% of mice demonstrated complete tumor control on rechallenge. Similarly high rechallenge rejection rates were also observed in the CT26/Balb/c model. A more detailed comparative analysis looking specifically at potentially disparate memory responses between models will require further investigation.
This study serves as a prelude for the clinical investigation of VAX014 and provides compelling evidence this novel non-viral oncolytic agent facilitates in situ immunization following intratumoral administration while bolstering the activity of ICB to enhance clearance of distal non-injected tumors, even those with an immune desert phenotype. Clinical data from first generation OV therapies administered by direct tumor injection in combination with systemic ICB have provided encouraging results and have paved the way for this emerging treatment paradigm, but it is clear the activity of OVs remains marginal, and a straightforward path to improvement of OVs is not presently clear.7 34 35 VAX014 differentiates from OV-based therapies in several ways. First, VAX014 induces rapid oncolysis by delivering a potent pore-forming protein toxin directly to tumor cells.15 16 At the same time, VAX014 potently stimulates multiple immune activation networks, including an IFN response. The close juxtaposition of these two critical events is unique to VAX014 and may better ensure the availability of tumor antigens at the peak of immune activation, which may lead to more robust cross-presentation and T cell priming. This is different from OVs, which activate the immune response on infection but do not generate an oncolytic event until viral burst, which occurs days later, following completion of the viral life cycle. Second, unlike OVs, VAX014 does not require replication for oncolysis and therefore may not be negatively affected by a type I IFN response, a known barrier to viral replication and, therefore, OV-mediated oncolysis.36 In addition, VAX014 is derived from bacterial minicells with immune-attenuated lipopolysaccharide and contains multiple pathogen-associated molecular pattern molecules that can engage and activate different supportive innate pathways.15 16 37–39 Finally, the weekly dosing regimen reported in these studies is the same that will be used clinically. This is in stark contrast to OVs, where preclinical reports typically use a dosing frequency of three to four intratumoral administrations a week, yet clinically are given once every 3 weeks.10 40–44 Taken together, VAX014 represents a new oncolytic treatment modality primed for clinical investigation alone and in combination with ICB in advanced solid tumor indications.
Conclusions
VAX014 facilitates in situ immunization following local intratumoral administration. The combination of VAX014 with ICB in preclinical immunologically cold ICB-resistant solid tumor models led to enhanced immune-mediated clearance of injected and distal non-injected tumors, coinciding with durable long-term survival and protective immunological memory. Results of this study provide rationale for the clinical investigation of VAX014 for the intralesional treatment of advanced solid tumors.
jitc-2023-006749supp003.pdf (76.8KB, pdf)
jitc-2023-006749supp004.pdf (100.3KB, pdf)
Acknowledgments
Thank you to Steven Fiering at Dartmouth Geisel School of Medicine, Trevor Hallam at Sutro Biopharma, and Stanley Maloy at San Diego State University for their thoughtful review of the manuscript. We thank the UC San Diego Stem Cell Genomics Core, the UC San Diego Stem Cell Program, La Jolla, California, and Cristian Quintero for his assistance with the NanoString assay. We also thank Amanda Parikh for her technical assistance.
Footnotes
KLM and MJG contributed equally.
Contributors: Conceptualization: MJG, KLM, KAR, and ST. Methodology: KAR, MJG, KLM, ST, KLN, and EM. Investigation: KAR, ST, KLN, and EM. Visualization: KAR. Funding acquisition, project administration, and supervision: KLM and MJG. Writing (original draft): MJG and KAR. Writing (review and editing): MJG, KLM, KAR, ST, KLN and EM. Guarantor: MG accepts full responsibility for the work.
Funding: This work was funded in part by National Cancer Institute Phase I SBIR (grant number 1R43CA22450-01A1).
Competing interests: MJG, KAR, ST, and KLN are employees of Vaxiion Therapeutics. MJG, KAR, ST, and KLM hold an equity interest in Vaxiion Therapeutics. MJG is a listed inventor on patents covering VAX014 and assigned to Vaxiion Therapeutics.
Provenance and peer review: Not commissioned; externally peer reviewed.
Supplemental material: This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.
Data availability statement
All data relevant to the study are included in the article or uploaded as supplementary information.
Ethics statements
Patient consent for publication
Not applicable.
Ethics approval
All animal use protocols were approved by the Institutional Animal Care and Use Committee at San Diego State University under protocol 22-05-004Mc prior to conducting animal studies.
References
- 1. Waldman AD, Fritz JM, Lenardo MJ. A guide to cancer Immunotherapy: from T cell basic science to clinical practice. Nat Rev Immunol 2020;20:651–68. 10.1038/s41577-020-0306-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Morad G, Helmink BA, Sharma P, et al. Hallmarks of response, resistance, and toxicity to immune checkpoint blockade. Cell 2021;184:5309–37. 10.1016/j.cell.2021.09.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Barnes TA, Amir E. HYPE or HOPE: the prognostic value of infiltrating immune cells in cancer. Br J Cancer 2017;117:451–60. 10.1038/bjc.2017.220 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Bonaventura P, Shekarian T, Alcazer V, et al. Cold tumors: a therapeutic challenge for Immunotherapy. Front Immunol 2019;10:168. 10.3389/fimmu.2019.00168 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Hegde PS, Chen DS. Top 10 challenges in cancer immunotherapy. Immunity 2020;52:17–35. 10.1016/j.immuni.2019.12.011 [DOI] [PubMed] [Google Scholar]
- 6. Hammerich L, Bhardwaj N, Kohrt HE, et al. In situ vaccination for the treatment of cancer. Immunotherapy 2016;8:315–30. 10.2217/imt.15.120 [DOI] [PubMed] [Google Scholar]
- 7. Twumasi-Boateng K, Pettigrew JL, Kwok YYE, et al. Oncolytic viruses as engineering platforms for combination immunotherapy. Nat Rev Cancer 2018;18:419–32. 10.1038/s41568-018-0009-4 [DOI] [PubMed] [Google Scholar]
- 8. Russell SJ, Barber GN. Oncolytic viruses as antigen-agnostic cancer vaccines. Cancer Cell 2018;33:599–605. 10.1016/j.ccell.2018.03.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Hamid O, Hoffner B, Gasal E, et al. Oncolytic immunotherapy: unlocking the potential of viruses to help target cancer. Cancer Immunol Immunother 2017;66:1249–64. 10.1007/s00262-017-2025-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Andtbacka RHI, Kaufman HL, Collichio F, et al. Talimogene laherparepvec improves durable response rate in patients with advanced melanoma. J Clin Oncol 2015;33:2780–8. 10.1200/JCO.2014.58.3377 [DOI] [PubMed] [Google Scholar]
- 11. Puzanov I, Milhem MM, Minor D, et al. Talimogene laherparepvec in combination with Ipilimumab in previously untreated, unresectable stage IIIB-IV melanoma. J Clin Oncol 2016;34:2619–26. 10.1200/JCO.2016.67.1529 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Ribas A, Dummer R, Puzanov I, et al. Oncolytic virotherapy promotes intratumoral T cell infiltration and improves anti-PD-1 immunotherapy. Cell 2017;170:1109–1119. 10.1016/j.cell.2017.08.027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Sivanandam V, LaRocca CJ, Chen NG, et al. Oncolytic viruses and immune checkpoint inhibition: the best of both worlds. Molecular Therapy - Oncolytics 2019;13:93–106. 10.1016/j.omto.2019.04.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Hancock BM, McGuire KL, Tsuji S, et al. A single intravesical instillation of Vax014 inhibits orthotopic superficial bladder tumor implantation to increase survival. Anticancer Res 2016;36:6243–8. 10.21873/anticanres.11218 [DOI] [PubMed] [Google Scholar]
- 15. Tsuji S, Chen X, Hancock B, et al. Preclinical evaluation of VAX-IP, a novel bacterial Minicell-based biopharmaceutical for nonmuscle invasive bladder cancer. Mol Ther Oncolytics 2016;3:16004. 10.1038/mto.2016.4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Tsuji S, Reil K, Nelson K, et al. Intravesical VAX014 synergizes with PD-L1 blockade to enhance local and systemic control of bladder cancer. Cancer Immunol Res 2022;10:978–95. 10.1158/2326-6066.CIR-21-0879 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Lechner MG, Karimi SS, Barry-Holson K, et al. Immunogenicity of murine solid tumor models as a defining feature of in vivo behavior and response to immunotherapy. J Immunother 2013;36:477–89. 10.1097/01.cji.0000436722.46675.4a [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Yu JW, Bhattacharya S, Yanamandra N, et al. Tumor-immune profiling of murine syngeneic tumor models as a framework to guide mechanistic studies and predict therapy response in distinct tumor Microenvironments. PLoS One 2018;13:e0206223. 10.1371/journal.pone.0206223 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Li Y, Qiao K, Zhang X, et al. Targeting myeloid-derived suppressor cells to attenuate vasculogenic mimicry and synergistically enhance the anti-tumor effect of PD-1 inhibitor. IScience 2021;24:103392. 10.1016/j.isci.2021.103392 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Youn J-I, Nagaraj S, Collazo M, et al. Subsets of myeloid-derived suppressor cells in tumor-bearing mice. J Immunol 2008;181:5791–802. 10.4049/jimmunol.181.8.5791 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Fuertes MB, Kacha AK, Kline J, et al. Host type I IFN signals are required for antitumor CD8+ T cell responses through Cd8{Alpha}+ dendritic cells. J Exp Med 2011;208:2005–16. 10.1084/jem.20101159 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Liu C, Lou Y, Lizée G, et al. Plasmacytoid dendritic cells induce NK cell-dependent, tumor antigen-specific T cell cross-priming and tumor regression in mice. J Clin Invest 2008;118:1165–75. 10.1172/JCI33583 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Lin H, Wei S, Hurt EM, et al. Host expression of PD-L1 determines efficacy of PD-L1 pathway blockade-mediated tumor regression. J Clin Invest 2018;128:805–15. 10.1172/JCI96113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Simpson TR, Li F, Montalvo-Ortiz W, et al. Fc-dependent depletion of tumor-infiltrating regulatory T cells co-defines the efficacy of anti-CTLA-4 therapy against melanoma. J Exp Med 2013;210:1695–710. 10.1084/jem.20130579 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Baird JR, Byrne KT, Lizotte PH, et al. Immune-mediated regression of established B16F10 melanoma by Intratumoral injection of attenuated toxoplasma gondii protects against rechallenge. J Immunol 2013;190:469–78. 10.4049/jimmunol.1201209 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Khalil DN, Suek N, Campesato LF, et al. In situ vaccination with defined factors overcomes T cell exhaustion in distant tumors. J Clin Invest 2019;129:3435–47. 10.1172/JCI128562 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Vijayakumar G, Palese P, Goff PH. Oncolytic Newcastle disease virus expressing a checkpoint inhibitor as a radioenhancing agent for murine melanoma. EBioMedicine 2019;49:96–105. 10.1016/j.ebiom.2019.10.032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Zamarin D, Ricca JM, Sadekova S, et al. PD-L1 in tumor microenvironment mediates resistance to oncolytic immunotherapy. J Clin Invest 2018;128:5184. 10.1172/JCI125039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Bloom MB, Perry-Lalley D, Robbins PF, et al. Identification of tyrosinase-related protein 2 as a tumor rejection antigen for the B16 melanoma. J Exp Med 1997;185:453–9. 10.1084/jem.185.3.453 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Overwijk WW, Tsung A, Irvine KR, et al. Gp100/Pmel 17 is a murine tumor rejection antigen: induction of "self"-reactive, tumoricidal T cells using high-affinity, altered peptide ligand. J Exp Med 1998;188:277–86. 10.1084/jem.188.2.277 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Brossart P. The role of antigen spreading in the efficacy of Immunotherapies. Clin Cancer Res 2020;26:4442–7. 10.1158/1078-0432.CCR-20-0305 [DOI] [PubMed] [Google Scholar]
- 32. Gulley JL, Madan RA, Pachynski R, et al. Role of antigen spread and distinctive characteristics of immunotherapy in cancer treatment. J Natl Cancer Inst 2017;109. 10.1093/jnci/djw261 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Jordan KR, Amaria RN, Ramirez O, et al. Myeloid-derived Suppressor cells are associated with disease progression and decreased overall survival in advanced-stage melanoma patients. Cancer Immunol Immunother 2013;62:1711–22. 10.1007/s00262-013-1475-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Breitbach CJ, Lichty BD, Bell JC. Oncolytic viruses: therapeutics with an identity crisis. EBioMedicine 2016;9:31–6. 10.1016/j.ebiom.2016.06.046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Raja J, Ludwig JM, Gettinger SN, et al. Oncolytic virus immunotherapy: future prospects for oncology. J Immunother Cancer 2018;6:140. 10.1186/s40425-018-0458-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Rasmussen SB, Sørensen LN, Malmgaard L, et al. Type I interferon production during herpes simplex virus infection is controlled by cell-type-specific viral recognition through toll-like receptor 9, the mitochondrial antiviral signaling protein pathway, and novel recognition systems. J Virol 2007;81:13315–24. 10.1128/JVI.01167-07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Coats SR, Pham T-TT, Bainbridge BW, et al. MD-2 mediates the ability of tetra-acylated and penta-acylated lipopolysaccharides to antagonize escherichia coli lipopolysaccharide at the TLR4 signaling complex. J Immunol 2005;175:4490–8. 10.4049/jimmunol.175.7.4490 [DOI] [PubMed] [Google Scholar]
- 38. Low KB, Ittensohn M, Le T, et al. Lipid A mutant salmonella with suppressed virulence and TNFalpha induction retain tumor-targeting in vivo. Nat Biotechnol 1999;17:37–41. 10.1038/5205 [DOI] [PubMed] [Google Scholar]
- 39. Somerville JE, Cassiano L, Bainbridge B, et al. A novel Escherichia coli lipid A mutant that produces an antiinflammatory lipopolysaccharide. J Clin Invest 1996;97:359–65. 10.1172/JCI118423 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Beasley GM, Nair SK, Farrow NE, et al. Phase I trial of Intratumoral PVSRIPO in patients with unresectable, treatment-refractory melanoma. J Immunother Cancer 2021;9:e002203. 10.1136/jitc-2020-002203 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Kaufman HL, Kim DW, DeRaffele G, et al. Local and distant immunity induced by Intralesional vaccination with an oncolytic herpes virus encoding GM-CSF in patients with stage Iiic and IV melanoma. Ann Surg Oncol 2010;17:718–30. 10.1245/s10434-009-0809-6 [DOI] [PubMed] [Google Scholar]
- 42. Liu BL, Robinson M, Han Z-Q, et al. ICP34.5 deleted herpes simplex virus with enhanced oncolytic, immune stimulating, and anti-tumour properties. Gene Ther 2003;10:292–303. 10.1038/sj.gt.3301885 [DOI] [PubMed] [Google Scholar]
- 43. Mosaheb MM, Dobrikova EY, Brown MC, et al. Genetically stable poliovirus vectors activate dendritic cells and prime antitumor CD8 T cell immunity. Nat Commun 2020;11:524. 10.1038/s41467-019-13939-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Thomas S, Kuncheria L, Roulstone V, et al. Development of a new fusion-enhanced oncolytic immunotherapy platform based on herpes simplex virus type 1. J Immunother Cancer 2019;7:214. 10.1186/s40425-019-0682-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
jitc-2023-006749supp001.pdf (109.8KB, pdf)
jitc-2023-006749supp002.pdf (5MB, pdf)
jitc-2023-006749supp003.pdf (76.8KB, pdf)
jitc-2023-006749supp004.pdf (100.3KB, pdf)
Data Availability Statement
All data relevant to the study are included in the article or uploaded as supplementary information.