Abstract
Background
KN046 is a novel bispecific antibody targeting programmed death ligand 1 (PD-L1) and cytotoxic T lymphocyte-associated protein 4 (CTLA-4). This multicenter phase I trial investigated the safety, tolerability, pharmacokinetics (PK), and efficacy of KN046 in patients with advanced solid tumors.
Methods
Patients who failed standard treatment were included. KN046 was administered at doses of 1, 3, and 5 mg/kg every 2 weeks (Q2W), 5 mg/kg every 3 weeks (Q3W), and 300 mg Q3W based on the modified toxicity probability interval method in the dose-escalation phase; the recommended dose was used in the expansion phase. Primary objectives were maximum tolerated dose (MTD) and recommended phase II dose (RP2D) in escalation and preliminary efficacy in expansion. Secondary objectives included PK, pharmacodynamics, safety, and tolerability of KN046. We also explored biomarkers based on PD-L1 expression, multiplex immunofluorescence (mIF) staining, and RNAseq-derived nCounter platform.
Results
Totally, 100 eligible patients were enrolled, including 59 with nasopharyngeal carcinoma (NPC), 36 with epidermal growth factor receptor (EGFR)-mutated non-small-cell lung cancer (NSCLC), and those with other advanced solid tumors. The most common treatment-related adverse events (TRAEs) were rash (33.0%), pruritus (31.0%), and fatigue (20.0%). Grade ≥3 TRAEs were observed in 14.0% of participants. No dose-limiting toxicity occurred in the dose-escalation phase, and the MTD was not reached. The RP2D was determined as 5 mg/kg Q2W according to the pharmacokinetic–pharmacodynamic model, the preliminary exposure–response analysis, and the overall safety profile. Among 88 efficacy-evaluable participants, the objective response rate (ORR) was 12.5%, and the median duration of response was 16.6 months. In the NPC subgroup, the ORR was 15.4%, and the median overall survival (OS) was 24.7 (95% CI 16.3 to not estimable) months. In the EGFR-mutant NSCLC subgroup, the ORR was 6.3%. mIF analysis results showed patients with high CD8 expression showed longer median OS (27.1 vs 9.2 months, p=0.02); better prognosis was observed in patients with high CD8 and PD-L1 expression.
Conclusions
KN046 was well tolerated and showed promising antitumor efficacy in advanced solid tumors, especially in patients with NPC. The combination of both CD8 and PD-L1 expression improved the prediction of KN046 response.
Trial registration numbers
Keywords: Clinical Trials as Topic; Antibodies, Neoplasm; Immunohistochemistry; Tumor Biomarkers
WHAT IS ALREADY KNOWN ON THIS TOPIC
A combination of programmed death 1/programmed death ligand 1(PD-L1) and cytotoxic T lymphocyte-associated protein 4 (CTLA-4) blockade showed favorable outcomes in advanced solid tumors, while such combinations are associated with more severe adverse events and a lack of putative biomarkers.
WHAT THIS STUDY ADDS
KN046 is a novel bispecific antibody targeting PD-L1 and CTLA-4. This is the first reported KN046 study that showed a relatively lower grade of ≥3 treatment-related adverse events rate and promising efficacy in advanced solid tumors, especially for patients with nasopharyngeal carcinoma (NPC). Biomarker analysis demonstrated that patients with high CD8 expression and high PD-L1 expression had longer median overall survival.
HOW THIS STUDY MIGHT AFFECT RESEARCH, PRACTICE OR POLICY
This study provided evidence for a further clinical trial of KN046 in patients with NPC and may lay the foundation of biomarker discovery related to advantage population from bispecific antibody.
Background
In recent years, the advent of immunotherapy has changed the treatment strategy for solid tumors.1 Several anti-programmed death 1 (PD-1)/programmed death ligand 1 (PD-L1) and anti-cytotoxic T lymphocyte-associated protein 4 (CTLA-4) immune checkpoint inhibitors (ICIs) have been developed, with proven efficacy and safety in many tumor types.2 3 One of the most promising strategies for improving the outcome of immunotherapy is the combination of two ICIs or the use of bispecific antibodies with dual checkpoint blockade.4 5 Recent studies support that inhibiting PD-1/PD-L1 and CTLA-4 simultaneously achieves favorable outcomes in urothelial carcinoma, non-small-cell lung cancer (NSCLC), and melanoma.6–11 However, such combinations are expensive and associated with a higher rate of adverse events compared with single-agent immunotherapy. The development of bispecific antibodies by binding two distinct epitopes on the same or different antigens provides an option for enhancing the immune response and potentially minimizing toxicity.5 12 13 Several bispecific antibodies have been approved for global marketing, including blinatumomab (CD3×CD19), tebentafusp (gp100×CD3), cadonilimab (PD-1×CTLA-4), and amivantamab (estimated glomerular filtration rate (EGFR)×cellular-mesenchymal epithelial transition factor (c-MET)), and numerous advances have been observed for bispecific antibody drugs.12–14 However, bispecific antibodies targeting PD-L1 and CTLA-4 in solid tumors have not been applied clinically.
KN046, produced from Chinese hamster ovary cells, is a novel bispecific domain antibody fused with human IgG1 Fc, which blocks PD-L1 interaction with PD-1 and CTLA-4 interaction with CD80/CD86. PD-1-mediated immune suppression occurs in the antigen-elimination phase in the tumor, while CTLA-4-mediated suppression of T-cell activation occurred in the antigen-presentation phase; therefore, blockade of both PD-L1 and CTLA-4 might result in synergistic antitumor effects.15 The wild-type IgG1 Fc portion of KN046 preserves the intact effector functions, including antibody-dependent cell-mediated cytotoxicity and complement-dependent cytotoxicity. A preclinical study indicated KN046 targets PD-L1 and CTLA-4, showed higher affinity for PD-L1, and mediated the depletion of regulatory T cells in the tumor microenvironment,16 which could enhance the antitumor immune response and reduce immunosuppressive effects. Compared with dual ICI therapy, KN046 might also reduce side effects and treatment costs.
Here, we conducted this multicenter phase I trial to assess the safety, maximum tolerated dose (MTD), recommended phase II dose (RP2D), and preliminary efficacy of KN046 in patients with advanced solid tumors who failed standard treatment. We also explored potential biomarkers for efficacy including PD-L1 expression, multiplex immunofluorescence (mIF) staining, and the nCounter PanCancer IO 360 panel.
Patients and methods
Study design
This study included a dose-escalation phase and a schedule-expansion phase. The dose-escalation phase followed the modified toxicity probability interval (mTPI) method.17 A scientific monitoring committee discussed and adjusted the schedule-expansion phase. KN046 was administered at doses of 1, 3, and 5 mg/kg every 2 weeks (Q2W), 5 mg/kg every 3 weeks (Q3W) and 300 mg Q3W based on the mTPI method in the dose-escalation phase and the recommended dose was used in the expansion phase.
KN046 was administered intravenously until image-proven disease progression, significant clinical deterioration, unacceptable toxicity, and loss to follow-up, or administered for 2 years. In case of dose-limiting toxicity (DLT) or grade of ≥3 toxicity related to KN046, KN046 administration was discontinued until the treatment-related toxicity returned to a grade of ≤1. The specific adjustment scheme is presented in the online supplemental methods.
jitc-2022-006654supp001.pdf (1.8MB, pdf)
For the dose-escalation phase, the primary objectives were safety, MTD, and/or the RP2D. For the dose-expansion phase, the primary objective was preliminary efficacy. MTD is defined as the dose at which the DLT occurred in no more than 33% of the patients during 28 days in the Q2W dosing group or 21 days in the Q3W dosing group. DLT was defined as a severe adverse event related to KN046 treatment, including severe hematological or non-hematological toxicities, detailed in the online supplemental methods. The RP2D was determined as the MTD if the criteria was met. Otherwise, the R2PD was determined according to the pharmacokinetic–pharmacodynamic model.
Secondary objectives included pharmacokinetics (PK), pharmacodynamics (PD), safety, and tolerability of KN046. The exploratory endpoint was the associations of biomarkers (including PD-L1 expression, mIF, and RNAseq-derived nCounter platform) with efficacy.
Patients
This multicenter phase I trial included patients with advanced solid tumors who failed standard treatment. The inclusion and exclusion criteria are detailed in the online supplemental methods. The key inclusion criteria were (1) ≥18 years of age; (2) histologically or cytologically confirmed advanced unresectable or metastatic solid tumors, progression after standard treatment, no standard treatment option available, or could not tolerate the standard treatment; (3) measurable lesions at baseline; (4) an Eastern Cooperative Oncology Group performance status score of 0–1. The key exclusion criteria were (1) untreated active brain metastasis or (2) received radical radiotherapy within 3 months before enrollment.
Assessments
Treatment-emergent adverse events (TEAEs) graded according to National Cancer Institute Common Terminology Criteria for Adverse Events V.5.0, treatment-related adverse events (TRAEs) were judged by investigators. Objective response rate (ORR), duration of response (DOR), disease control rate (DCR, complete response (CR), partial response (PR) or stable disease (SD) for ≥6 weeks), clinical benefit rate (CBR, CR, PR, or SD for ≥12 weeks), 12-month progression-free survival (PFS), 12-month overall survival (OS), and pharmacokinetic parameters. Disease progression was evaluated according to Response Evaluation Criteria in Solid Tumors V.1.1.
CT or MRI examination was performed every 6 weeks in the first year and every 12 weeks thereafter. The safety was followed up to 90 days after the end of the treatment or the start of new antitumor treatment, whichever occurred first.
Pharmacokinetic–pharmacodynamic model development
Blood samples for pharmacokinetic analysis were collected predose, post dose (15 min after infusion), and 0.5, 1.0, 4.0, 24.0, 48.0, 72.0, 168.0, 336.0, and 504.0 hours after the administration of the first dose. For robustness, the pharmacokinetic data of KN046 in another phase I trial in Australia (NCT03529526) were included.18 The pharmadynamic model was developed based on ex vivo interleukin-2 (IL-2) data. As an indirect measure of target KN046 occupancy on T cells, the ex vivo IL-2 stimulation ratio was measured predose and post dose.19 Blood samples were drawn before dosing, and 1, 7, 14, and 21 days after the administration of the first dose. Different concentrations of KN046 and 800 ng/mL of staphylococcal enterotoxin-B were added into the peripheral blood mononuclear cell solutions to evaluate endogenous IL-2 levels by the ELISA method.
A two-compartmental first-order elimination model was fitted to multiple dose pharmacokinetic data in humans to build a population pharmacokinetic model (online supplemental methods). The relationship between KN046 concentration and the reduction of IL-2 stimulation ratio was described using a Sigmoid Imax model (online supplemental methods). Target trough concentration (Ctrough_target) was defined as the KN046 concentration reaching 95% inhibition of ex vivo IL-2 release (IC95).19 Simulations were performed to predict KN046 concentrations over time under different dose levels based on the population pharmacokinetic model. Patients in different dose groups predicted to attain the Ctrough_target were analyzed, and the percentage (preset ≥80% for RP2D) of individuals whose trough concentration exceeded the target concentration was calculated based on the simulation results.
Biomarker analyses
Archived or fresh tumor samples were retrieved for immunohistochemical staining of PD-L1 expression in tumor cells with SP263 antibody (Abcam) in a central laboratory.20 mIF staining of immune-related biomarkers including CD86, CD206, CD11B, CD4, CD8, FOXP3, GZMB, and PANCK was also conducted in a central laboratory. The expression of immune-related biomarkers was digitally measured and quantified as percentages of positive expression. We used the median percentage as a cut-off.
Gene expression analysis was performed on all available baseline formalin-fixed paraffin-embedded samples by the nCounter PanCancer IO 360 panel.21 Immune cell abundance was evaluated according to the algorithm of the average log-transformed expression values of marker genes. The details of mIF and the nCounter PanCancer IO 360 panel are shown in the online supplemental methods.
Statistical analysis
The safety analysis set included all participants who received KN046 at least once. The efficacy analysis set included all participants who had at least one efficacy evaluation. The DLT analysis set included participants who received ≥80% of the planned KN046 dosing in the dose-escalation phase. The pharmacokinetic analysis set included participants who had at least one pharmacokinetic sample available. Pharmacokinetic parameters were calculated using Phoenix WinNonlin V.8.3 (Certara, Princeton, New Jersey, USA).
For ORR, DCR, and CBR, the 95% CI calculated by the Clopper-Pearson method was reported in the total population and different doses and/or tumor species. For DOR, PFS, and OS, the median and 95% CI were calculated using the Kaplan-Meier method. Univariable Cox regression analysis was applied to explore the associations of immune-related biomarkers with OS. The correlation was examined with the Spearman rank correlation coefficients. All statistical analyses were performed using SAS V.9.4 or R V.4.0.3.
Results
Patients
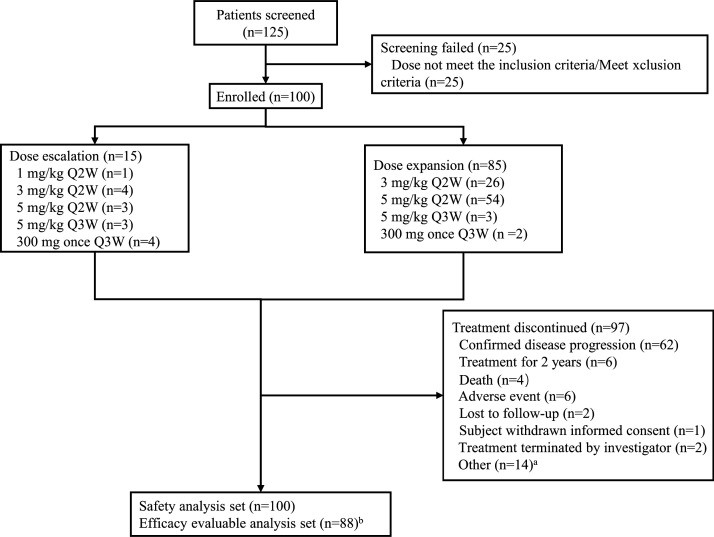
From November 2018 to April 2020, a total of 100 patients were enrolled (figure 1), including 59 with nasopharyngeal carcinoma (NPC), 36 with EGFR-mutated NSCLC, 2 with gastric cancer, 1 with small-cell lung cancer (SCLC), 1 with melanoma, and 1 with external auditory canal carcinoma. There were 24 (40.7%) participants with NPC and 9 (25%) participants with NSCLC who failed prior immunotherapy. The data cut-off date was August 31, 2021. The median follow-up was 22.6 (range 19.9–25.3) months. Patient characteristics are listed in table 1.
Figure 1.
Flowchart. a. 14 patients discontinued treatment (seven clinical disease progression, seven withdrawn from participants). b. twelve excluded from efficacy analysis set (two lost to follow-up, one AE, three death, one withdrawal informed consent, five clinical disease progression). AE, adverse event; Q2W, every 2 weeks; Q3W, every 3 weeks.
Table 1.
Baseline characteristics of the participants
| All (N=100) | 1 mg/kg Q2W (n=1) |
3 mg/kg Q2W (n=30) |
5 mg/kg Q2W (n=57) |
5 mg/kg Q3W (n=6) |
300 mg Q3W (n=6) |
|
| Age (years), median (range) | 51.5 (25–73) | 63.0 (63–63) | 54.0 (25–73) | 48.0 (31–70) | 53.0 (38–73) | 60.0 (45–71) |
| Age ≥60 years, n (%) | 30 (30.0) | 1 (100.0) | 12 (40.0) | 12 (21.1) | 2 (33.3) | 3 (50.0) |
| Male, n (%) | 82 (82.0) | 1 (100.0) | 24 (80.0) | 47 (82.5) | 4 (66.7) | 6 (100.0) |
| BMI, mean±SD | 21.8±3.2 | 21.1 | 22.5±3.6 | 21.8±3.0 | 21.6±2.5 | 19.9±2.9 |
| ECOG score of 1, n (%) | 47 (47.0) | 0 | 17 (56.7) | 26 (45.6) | 2 (33.3) | 2 (33.3) |
| Smoking, n (%) | ||||||
| Former smoker | 32 (32.0) | 1 (100.0) | 13 (43.3) | 13 (22.8) | 1 (16.7) | 4 (66.7) |
| Current smoker | 10 (10.0) | 0 | 4 (13.3) | 5 (8.8) | 1 (16.7) | 0 |
| Diagnosis, n (%) | ||||||
| Nasopharyngeal carcinoma | 59 (59.0) | 0 | 12 (40.0) | 44 (77.2) | 3 (50.0) | 0 |
| NSCLC (all EGFR-mutated) | 36 (36.0) | 0 | 16 (53.3) | 11 (19.3) | 3 (50.0) | 6 (100.0) |
| Gastric cancer | 2 (2.0) | 0 | 0 | 2 (3.5) | 0 | 0 |
| Right external auditory canal carcinoma | 1 (1.0) | 0 | 1 (3.3) | 0 | 0 | 0 |
| Small cell lung cancer | 1 (1.0) | 1 (100.0) | 0 | 0 | 0 | 0 |
| Melanoma | 1 (1.0) | 0 | 1 (3.3) | 0 | 0 | 0 |
| Pathological classification, n (%) | ||||||
| Non-keratinizing carcinoma | 47 (79.7) | 0 | 9 (75.0) | 35 (79.5) | 3 (100.0) | 0 |
| Squamous cell carcinoma | 5 (8.5) | 0 | 1 (8.3) | 4 (9.1) | 0 | 0 |
| Unknown | 3 (5.1) | 0 | 1 (8.3) | 2 (4.5) | 0 | 0 |
| Other | 4 (6.8) | 0 | 1 (8.3) | 3 (6.8) | 0 | 0 |
| Brain metastasis | 14 (14.0) | 1 (100.0) | 4 (13.3) | 6 (10.5) | 1 (16.7) | 2 (33.3) |
| Baseline PD-L1 expression, n (%) | ||||||
| <1% | 19 (19.0) | 1 (100.0) | 6 (20.0) | 9 (15.8) | 1 (16.7) | 2 (33.3) |
| 1%–49% | 42 (42.0) | 0 | 11 (36.7) | 25 (43.9) | 3 (50.0) | 3 (50.0) |
| ≥50% | 32 (32.0) | 0 | 9 (30.0) | 21 (36.8) | 1 (16.7) | 1 (16.7) |
| Lost | 7 (7.0) | 0 | 4 (13.3) | 2 (3.5) | 1 (16.6) | 0 |
| Time from diagnosis to enrollment (months), median (range) | 28.6 (3.7 –137.7) | 10.1 | 24.1 (6.6 –137.7) | 34.3(6.1 –128.5) | 34.4 (15.3–49.5) | 17.1 (3.7–31.90) |
| Prior surgery, n (%) | 19 (19.0) | 0 | 6 (20.0) | 12 (21.1) | 1 (16.7) | 0 |
| Prior radiotherapy, n (%) | 64 (64.0) | 1 (100.0) | 13 (43.3) | 44 (77.2) | 4 (66.7) | 2 (33.3) |
| Prior immunotherapy, n (%) | ||||||
| Anti-CTLA-4 | 1 (1.0) | 0 | 0 | 1 (1.8) | 0 | 0 |
| Anti-PD-1 | 31 (31.0) | 0 | 3 (10.0) | 24 (42.1) | 3 (50.0) | 1 (16.7) |
| Anti-OX40 | 3 (3.0) | 0 | 0 | 1 (1.8) | 1 (16.7) | 1 (16.7) |
Data with n (%) are displayed.
BMI, body mass index; CTLA-4, cytotoxic T lymphocyte-associated protein 4; ECOG, Eastern Cooperative Oncology Group; EGFR, estimated glomerular filtration rate; NSCLC, non-small-cell lung cancer; OX40, Tumor necrosis factor receptor superfamily member 4; PD-1, programmed death 1; PD-L1, programmed death ligand 1; Q2W, every 2 weeks; Q3W, every 3 weeks.
Safety
A total of 100 patients were included in the safety analysis. Of these, 12 cases were included in the DLT analysis set. No DLT occurred in the dose-escalation phase. TRAEs were observed in 83.0% (83/100) of all participants, including 14 (14.0%) participants with grade ≥3 TRAEs, and 9 (9.0%) with serious TRAEs. The most common TRAEs were rash (33.0%), pruritus (31.0%), and fatigue (20.0%) (table 2). The most common TEAEs are listed in online supplemental table S1. Immune-related adverse events (irAEs) and grade≥3 irAEs were observed in 47.0% and 4.0% of participants, respectively (table 2). The median onset time of irAEs was 2 weeks (range 0.1–74.3). The incidence rate of irAEs in 0–6 weeks was 35.0%. Among all participants, 14 (14.0%) had TRAEs leading to treatment suspension; 7 (7.0%) had TRAEs leading to treatment termination; and 1 (1.0%) had TRAE leading to death (table 2). One participant in the 300 mg Q3W dose group died of unknown reasons related to KN046.
Table 2.
TRAE grouped by dose level
| Total (N=100) |
1 mg/kg Q2W (n=1) |
3 mg/kg Q2W (n=30) |
5 mg/kg Q2W (n=57) |
5 mg/kg Q3W (n=6) |
300 mg Q3W (n=6) |
|
| Any TRAE | 83 (83.0) | 0 | 27 (90.0) | 45 (78.9) | 6 (100) | 4 (66.7) |
| Grade ≥3 TRAE | 14 (14.0) | 0 | 6 (20.0) | 5 (8.8) | 2 (33.3) | 1 (16.7) |
| Serious | 9 (9.0) | 0 | 5 (16.7) | 2 (3.5) | 1 (16.7) | 1 (16.7) |
| Lead to suspension | 14 (14.0) | 0 | 8 (26.7) | 5 (8.8) | 1 (16.7) | 0 |
| Lead to termination | 7 (7.0) | 0 | 3 (10.0) | 4 (7.0) | 0 | 0 |
| Lead to death | 1 (1.0) | 0 | 0 | 0 | 0 | 1 (16.7) |
| irAE | 47 (47.0) | 0 | 21 (70.0) | 20 (35.1) | 5 (83.3) | 1 (16.7) |
| Grade ≥3 irAE | 4 (4.0) | 0 | 3 (10.0) | 1 (1.8) | 0 | 0 |
| Infusion-related reaction | 20 (20.0) | 0 | 9 (30.0) | 9 (15.8) | 2 (33.3) | 0 |
| Grade ≥3 infusion-related reaction | 5 (5.0) | 0 | 3 (10.0) | 2 (3.5) | 0 | 0 |
| Most common TRAE (≥5% of the total population) | ||||||
| Rash | 33 (33.0) | 0 | 14 (46.7) | 15 (26.3) | 3 (50.0) | 1 (16.7) |
| Pruritus | 31 (31.0) | 0 | 11 (36.7) | 15 (26.3) | 4 (66.7) | 1 (16.7) |
| Fatigue | 20 (20.0) | 0 | 8 (26.7) | 8 (14.0) | 3 (50.0) | 1 (16.7) |
| Increased AST | 19 (19.0) | 0 | 9 (30.0) | 8 (14.0) | 1 (16.7) | 1 (16.7) |
| Increased ALT | 15 (15.0) | 0 | 6 (20.0) | 6 (10.5) | 2 (33.3) | 1 (16.7) |
| Hypothyroidism | 15 (15.0) | 0 | 4 (13.3) | 9 (15.8) | 2 (33.3) | 0 |
| Fever | 11 (11.0) | 0 | 4 (13.3) | 7 (12.3) | 0 | 0 |
| Joint pain | 9 (9.0) | 0 | 5 (16.7) | 4 (7.0) | 0 | 0 |
| Increased TSH | 5 (5.0) | 0 | 1 (3.3) | 2 (3.5) | 1 (16.7) | 1 (16.7) |
ALT, alanine aminotransferase; AST, aspartate aminotransferase; irAE, immune-related adverse event; Q2W, every 2 weeks; Q3W, every 3 weeks; TRAE, treatment-related adverse event; TSH, thyroid stimulating hormone.
Pharmacokinetics
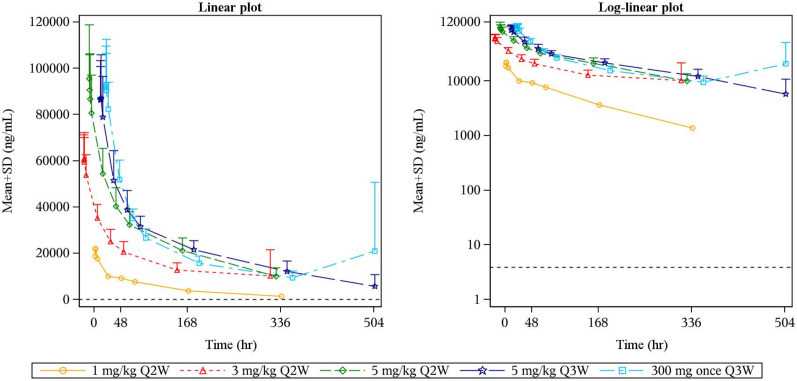
A total of 62 subjects were included in the systemic pharmacokinetic analysis. Following KN046 administration at 1 mg/kg Q2W, 3 mg/kg Q2W, 5 mg/kg Q2W, 5 mg/kg Q3W, and 300 mg Q3W, no significant changes were observed in half-life (t1/2, 111.0–137.4 hours), the volume of distribution (4400.0–6138.8 mL), and clearance (27.4–34.65 mL/hour) in the different dose cohorts (n=62). Maximum concentration and area under the time–concentration curve0–t increased in an approximately dose-proportional manner (online supplemental table S2 and figure 2). In popPK analysis, two-compartmental first-order elimination combined with a proportional and additive residual error model well described the observed human pharmacokinetic data (online supplemental figure S1 and table S3).
Figure 2.
KN046 plasma time–concentration curve. Mean KN046 plasma time–concentration curve (ng/mL) by dose level after administration of a single dose. The ordinate of the left graph is a linear scale, and the ordinate of the right graph is a log-linear scale. Q2W, every 2 weeks; Q3W, every 3 weeks.
RP2D decision
As no DLT was observed, the MTD was not determined. RP2D determination was based on the pharmacokinetic–pharmacodynamic model and the preliminary exposure–response analysis from the dose-escalation phase and the Australian cohort.
Data obtained from blood samples in 16 patients were included in the pharmacokinetic–pharmacodynamic model. The KN046 concentration required to cause IC95 was estimated to be 2629 ng/mL, which was set as a Ctrough_target to reach the maximum target engagement. Due to the relatively small sample size, to provide uncertainty in estimates of the target engagement, the upper bound of 95% CI estimation of the corresponding calculated IC95 (8683 ng/mL) was considered as a worst-case scenario (online supplemental table S4). To improve the robustness of the estimation for the target concentration, the association of plasma concentration with objective response was analyzed. Preliminary exposure–response for efficacy showed a median Ctrough of 8703 ng/mL (lower quartile, 7150 ng/mL) in patients who achieved disease control (CR, PR, or SD), which was higher than the median Ctrough (4048 ng/mL) in patients with progressive disease. The results indicated that efficacy might be correlated with Ctrough.
To increase the DCR, the selected RP2D should have a Ctrough over the preset Ctrough-target. Using the popPK simulation model, we applied the estimated Ctrough_target in the simulated concentration–time curves of 3 mg/kg Q2W, 5 mg/kg Q2W, and 5 mg/kg Q3W (online supplemental figure S2). The percentage of patients predicted to reach or exceed the set Ctrough-target was simulated. There were 73.5%, 85.1%, and 62.3% probability of Ctrough over Ctrough_target (2629 ng/mL) in doses of 3 mg/kg Q2W, 5 mg/kg Q2W, and 5 mg/kg Q3W, respectively (online supplemental table S5). When applying the upper limit of the 95% CI of the Ctrough_target (8683 ng/mL), the highest probability (51.4%) of Ctrough over Ctrough_target was observed at 5 mg/kg Q2W (online supplemental table S5). Considering the highest probability of Ctrough over Ctrough_target, along with the overall safety and tolerability of KN046, we determined R2PD to be 5 mg/kg Q2W.
Efficacy
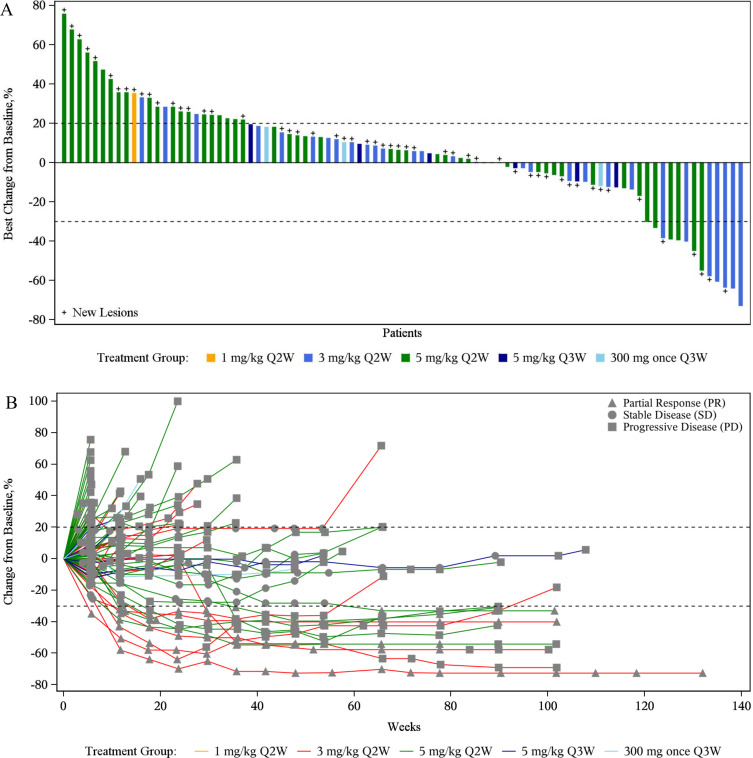
Eighty-eight participants were evaluable for treatment response, including 11 who achieved a PR with an ORR of 12.5% (95% CI 6.4% to 21.3%) (online supplemental table S6 and figure 3). With a median follow-up of 23.2 (range 20.5–25.9) months, the median DOR was 16.6 (95% CI 4.2 to not estimable (NE)) months, the median PFS was 1.7 (95% CI 1.3 to 2.7) months, and the median OS was 16.6 (95% CI 10.6 to 20.2) months. The efficacy for immunotherapy-naïve and treated patients with NPC and NSCLC is described in online supplemental table S7. In the NPC subgroup, the ORR was 15.4% (95% CI 6.9% to 28.1%); the median DOR was 8.31 (95% CI 2.83 to NE) months with a 1-year DOR rate of 46.9% (95% CI 12.0% to 76.3%). The median PFS was 1.5 (95% CI 1.3 to 2.7) months, and the median OS was 24.7 (95% CI 16.3 to NE) months with a 1 year OS rate of 66.1% (95% CI 52.1% to 76.9%). In the NSCLC subgroup, all patients were with EGFR mutation. the ORR was 6.3% (95% CI 0.8% to 20.8%), and the median DOR was 17.3 (95% CI 16.6 to NE) months with a 1-year DOR rate of 100.0%. The median PFS was 2.5 (95% CI 1.3 to 2.8) months; the median OS was 7.8 (95% CI 5.3 to 14.8) months with a 1-year OS rate of 41.7% (95% CI 25.6% to 57.0%) (online supplemental figure S3).
Figure 3.
Treatment response to KN046. (A) Optimum change of target lesions from baseline in target lesion size; (B) longitudinal change of target lesions from baseline. The dashed lines at 20% and −30% represent the boundary for the determination of PD and PR. PD, progressive disease; PR, partial response. Q2W, every 2 weeks; Q3W, every 3 weeks.
Biomarkers
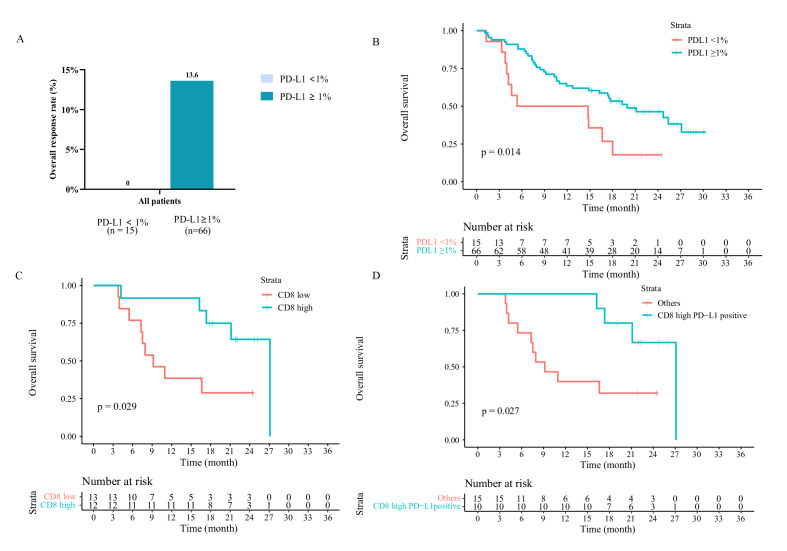
Tumor PD-L1 expression was assessed in 93 patients (NPC, n=57; NSCLC, n=32; SCLC, n=1; gastric cancer, n=2; and melanoma, n=1) (online supplemental table S8). Among them, 81 participants were available for efficacy evaluation; 15 were PD-L1− (PD-L1+ staining on <1% of tumor cells), and 66 were PD-L1+ (online supplemental figure S4). The ORRs were 0% and 13.6% (95% CI 6.4 to 24.3) in patients with PD-L1 expressions of <1% and ≥1%, respectively (figure 4A). Prolonged median PFS was observed in patients with PD-L1+ (mPFS 2.5 months, 95% CI 2.0 to 3.1) than those with PD-L1− (mPFS 1.3 months, 95% CI 1.3 to 1.4, p=0.0098; online supplemental table S9). In addition, longer median OS was observed in patients with PD-L1+ (19.9 months, 95% CI 12.6 to 27.2) than those with PD-L1− (5.4 months, 95% CI 0.0 to 23.9, p=0.014; figure 4B).
Figure 4.
Associations of PD-L1 and CD8 expression with response to KN046 therapy. (A) treatment response across different PD-L1 expression; (B) OS of patients with different PD-L1 expression; (C) OS of patients with different CD8 expression (median positive expression percentage as a cut-off); (D) OS among different patient groups, which was defined by a composite variable including PD-L1 expression (≥1%) and CD8 expression (above median). PD-L1, programmed death ligand 1; ORR, objective response rate; OS, overall survival.
mIF staining was carried out in 25 patients with PD-L1 tested (NPC, n=13; NSCLC, n=12) (online supplemental figure S4), and the relative expression of immune-related biomarkers was analyzed (online supplemental figure S5). Univariate Cox regression analysis identified CD8 expression was associated with better OS (online supplemental figure S6; HR=0.22, 95% CI 0.051 to 0.94, p=0.04). No significant associations were observed between OS and other immune-related biomarkers (online supplemental figure S6). Patients with high CD8 expression (>median, n=12) showed longer OS, with a median OS of 27.1 vs 9.2 months in the low-CD8 expression group (n=13, p=0.029; figure 4C). The ORRs were 8.3% and 7.7% in patients with high and low CD8 expressions, respectively; also, no significant difference in median PFS (p=0.33) was observed (online supplemental table S9). We then investigated the potential predictive value of combined biomarkers. Patients with both PD-L1+ and high CD8 expression (n=10) had better OS compared with the remaining patients (27.1 vs 9.2 months, p=0.027; figure 4D). Prior applicability test indicated no correlation between PD-L1 and CD8 expression (Spearman’s p=0.166). We also tested samples from 4 patients (among 25 with mIF) using the nCounter PanCancer IO360 panel for RNAseq-derived analysis (2 NPC and 2 NSCLC, all immunotherapy-treated). Consistent with the mIF results, higher CD8 T-cell infiltration was observed in patients with OS exceeding 24 months (online supplemental table S10 and figure S7). One patient with high PD-L1 expression but low CD8 expression had a relatively poor OS (patient number 1101062, OS=7.52 months; online supplemental table S10).
Discussion
To our knowledge, this is the first large phase I trial of a bispecific antibody with dual blockade of PD-L1 and CTLA-4 in advanced solid tumors. Our results indicate that KN046 monotherapy was well tolerated and had a satisfactory safety profile with a low rate of grade 3 TRAEs. Also, KN046 showed potential survival benefits in different tumor types (median DOR of 16.6 months and prolonged OS). The RP2D of KN046 monotherapy was determined as 5 mg/kg Q2W using a comprehensive strategy based on pharmacokinetic–pharmacodynamic model, preliminary exposure–response analysis, and overall safety profile. Biomarker analysis demonstrated that high PD-L1 expression combined with high CD8 expression was associated with a good prognosis after KN046 treatment.
Previous studies have reported promising clinical effects for the combination of two ICIs, while high toxicity and treatment interruption were also observed.7 9 15 22 23 The incidence rate of grade ≥3 TRAEs was numerically low for the KN046 treatment (14.0%), with 27%–53% for two ICI combination treatments in advanced solid tumors.7 9 15 23 Furthermore, we also found that the incidence of TRAEs leading to treatment termination was relatively low (7.0%) after KN046 treatment, with 7.1%–22.5% reported in anti-PD-1/PD-L1 and anti-CTLA-4 antibody combinations.7 22 The t1/2 of KN046 is about 5 days, much shorter compared with 11–15 days reported for ipilimumab,24 and intermittent continuous exposure to anti-CTLA-4 may be attributed to relatively lower toxicity. Based on the aforementioned data, the bispecific antibody KN046 showed the advantage of good safety compared with the combination of the two ICIs. Considering the lower toxicity and improved tolerability, long period treatment of KN046 may translate into long-term disease control and sustained survival benefits.
Traditional RP2D determination based on DLT observation is commonly not optimal for PD-1/PD-L1 antibodies or bispecific antibodies due to DLT seldom occurred during the first treatment cycle.25 26 A previous study integrated clinical PK and IL-2 stimulation data from the KEYNOTE-001 trial to establish a model to reveal the relationship between PK and PD of monoclonal antibody therapy.19 In this study, as an indirect measure of target KN046 occupancy on T cells, which are the major participant of immune reactions in the tumor microenvironment, the ex vivo IL-2 stimulation ratio was measured, and the simulated pharmacokinetic–pharmacodynamic model was used. This is also the first time an IL2-based pharmacokinetic–pharmacodynamic model was used in the analysis of a bispecific antibody. As no DLT was observed in this study, the R2PD of KN046 was recommended as 5 mg/kg Q2W based on the consideration of overall safety, tolerability, and preliminary efficacy, alongside the results of the pharmacokinetic–pharmacodynamic model. Similarly, the RP2D of bintrafusp alfa, a bifunctional fusion protein targeting transforming growth factor beta and PD-L1 was determined based on PK–PD, the population pharmacokinetic model, and exposure–response for efficacy and safety.27 This novel and constructive pharmacokinetic–pharmacodynamic model and RP2D determination method may provide a reference for the application of a model-based design in the dose selection of bispecific antibody in the future.
The efficacy of KN046 was promising, especially for patients with NPC. Although the ORR and PFS were relatively low in patients with NPC, the median DOR (8.3 months) and the 1-year DOR rate (46.9%) were prolonged, suggesting that antitumor effects in responders might be sustained over time. Besides, the median OS of pretreated patients with NPC was 21.1 months (online supplemental table S7), suggesting potential survival benefit from KN046 treatment. As a reference, the median OS reported were 17.2 and 17.4 months for toripalimab28 and camrelizumab,29 respectively. To our knowledge, this is the first report on the efficacy of bispecific antibodies in patients with NPC. Sustained efficacy observed in responsive patients suggested KN046 may provide a new treatment option for patients with metastatic or recurrent NPC. It also needs to be noted that the median PFS was only 1.5 months; further biomarker studies are wanted to find responsive patients. Objective response was observed in patients with EGFR-mutated NSCLC who failed standard therapies. For patients with EGFR-mutated NSCLC who progressed after treatment with tyrosine kinase inhibitors, further clinical trials of KN046 combined with chemotherapy or angiogenesis inhibitor should be considered.30
Currently, studies identifying populations with potential benefits from bispecific antibodies targeting PD-L1 and CTLA-4 are limited. Recently, Song et al provided a signature consisting of 18 protein candidates associated with treatment response based on spatial multiomics analysis in patients treated with KN046 (n=18).31 In our study, we found that PD-L1 positivity was associated with better ORR, PFS, and OS after KN046 treatment, suggesting that PD-L1 expression is a predictor of the bispecific antibody. This part of our results was consistent with the research of Song et al, who also found that the high expression of PD-L1 is related to the treatment efficacy.31 In our mIF analysis, we selected immune-related biomarkers, including CD86, CD206, CD11B, CD4, CD8, FOXP3, and GZMB.32 Regardless of PD-L1 expression, CD8 was independently associated with prognosis. Studies have found that the combination of mIF-based markers and PD-L1 could help more accurately identify people who may benefit from immunotherapy.29 Similarly, we also found that using CD8 expression jointly with PD-L1 expression may help precisely identify patients who could potentially respond to KN046 treatment. This provides insights for the identification of patients with optimal eligibility for KN046 treatment. It should be noted that the biomarker analysis was a preliminary exploration which needs to be validated in further clinical trials.
This study had some limitations. Major limitations included this study being a preliminary safety and efficacy evaluation of a phase I study, with a lack of randomization; the trial included patients with multiple tumor types; and the generalizability of efficacy data is limited. The post hoc biomarker analysis was conducted in a quite small cohort, which may confer bias.
In conclusion, in this phase I trial, KN046, a novel bispecific antibody targeting PD-L1 and CTLA-4, was well tolerated and showed promising antitumor efficacy in advanced solid tumors. Potential OS benefit may be favored through KN046 treatment, especially in patients with pretreated advanced NPC. Besides, patients with high PD-L1 and CD8 expression might benefit from KN046 treatment. Investigations of KN046-based regimens with larger sample sizes in various solid tumors are ongoing.
Acknowledgments
We thank all investigators for their efforts in this trial, including Dr Qin Lin from The First Affiliated Hospital of Xiamen University and Dr Jianhua Shi from Linyi Cancer Hospital. We also thank all patients and their caregivers who participated in this trial.
Footnotes
YXM, JHX and YYZ contributed equally.
Contributors: Conception and design: YXM, LZ, and HYZ; development of methodology: YXM, YYZ, YZ, YH, YPY, WFF, LZ, and HYZ; data curation: YXM, JHX, YYZ, YZ, YH, YPY, WFF, YG, QL, XXG, LZ, and HYZ; analysis and interpretation of data: YXM, JHX, JS, BYZ, YHZ, and JYX; writing (review and editing): YXM, JHX, YYZ, YZ, YH, YPY, WFF, YG, QL, XXG, JS, BYZ, YHZ, JYX, LZ, and HYZ; study supervision: YXM, LZ, and HYZ. Guarantor: LZ and HYZ
Funding: This study was supported by the National Nature Science Foundation of China (grant number 82002409 for YXM, grant number 82073396 for HYZ, grant number 82272789 for LZ, and grant number 82072558 for YPY), the Guangdong Basic and Applied Basic Research Foundation (grant number 2020A1515010020 for YXM and grant number 2018A0303130243 for HYZ), and Jiangsu Alphamab Biopharmaceuticals Co., Ltd.
Competing interests: LZ reports receiving research support from Jiangsu Hengrui Pharmaceuticals, Eli Lilly and Company, Novartis, Roche, and Bristol-Myers Squibb. JS and BZ are employees of Jiangsu Alphamab Biopharmaceuticals Co., Ltd. The other authors have no conflicts of interest to declare.
Provenance and peer review: Not commissioned; externally peer reviewed.
Supplemental material: This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.
Data availability statement
Data are available upon reasonable request. The data of this study have been deposited into the research data deposit (https://www.researchdata.org.cn, ID: RDDA2022604579). All requests should be submitted to the corresponding authors and are available on reasonable request.
Ethics statements
Patient consent for publication
Not applicable.
Ethics approval
This study involves human participants and was approved by the ethics committee of Sun Yat-sen University Cancer Center and Shanghai East Hospital (ethics approval number A2018-055-01). This trial was registered at ClinicalTrials.gov. Written informed consent was obtained from all patients before any study procedure. This trial was conducted according to the tenets of the Declaration of Helsinki and Good Clinical Practices.
References
- 1. Bagchi S, Yuan R, Engleman EG. Immune checkpoint inhibitors for the treatment of cancer: clinical impact and mechanisms of response and resistance. Annu Rev Pathol 2021;16:223–49. 10.1146/annurev-pathol-042020-042741 [DOI] [PubMed] [Google Scholar]
- 2. Jacob JB, Jacob MK, Parajuli P. Review of immune checkpoint inhibitors in immuno-oncology. Adv Pharmacol 2021;91:111–39. 10.1016/bs.apha.2021.01.002 [DOI] [PubMed] [Google Scholar]
- 3. Robert C. A decade of immune-checkpoint inhibitors in cancer therapy. Nat Commun 2020;11:3801.:3801. 10.1038/s41467-020-17670-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Carloni R, Rizzo A, Ricci AD, et al. Dual immune checkpoint blockade in hepatocellular carcinoma: where do we stand? Future Oncology 2022;18:1023–34. 10.2217/fon-2021-0905 [DOI] [PubMed] [Google Scholar]
- 5. Burton EM, Tawbi HA. Bispecific antibodies to PD-1 and CTLA4: doubling down on T cells to decouple efficacy from toxicity. Cancer Discov 2021;11:1008–10. 10.1158/2159-8290.CD-21-0257 [DOI] [PubMed] [Google Scholar]
- 6. Gao J, Navai N, Alhalabi O, et al. Neoadjuvant PD-L1 plus CTLA-4 blockade in patients with cisplatin-ineligible operable high-risk urothelial carcinoma. Nat Med 2020;26:1845–51. 10.1038/s41591-020-1086-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Perets R, Bar J, Rasco DW, et al. Safety and efficacy of quavonlimab, a novel anti-CTLA-4 antibody (mk-1308), in combination with pembrolizumab in first-line advanced non-small-cell lung cancer. Ann Oncol 2021;32:395–403. 10.1016/j.annonc.2020.11.020 [DOI] [PubMed] [Google Scholar]
- 8. Larkin J, Chiarion-Sileni V, Gonzalez R, et al. Combined nivolumab and ipilimumab or monotherapy in untreated melanoma. N Engl J Med Overseas Ed 2015;373:23–34. 10.1056/NEJMoa1504030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Olson DJ, Eroglu Z, Brockstein B, et al. Pembrolizumab plus ipilimumab following anti-PD-1/L1 failure in melanoma. J Clin Oncol 2021;39:2647–55. 10.1200/JCO.21.00079 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Paz-Ares LG, Ramalingam SS, Ciuleanu T-E, et al. First-Line nivolumab plus ipilimumab in advanced NSCLC: 4-year outcomes from the randomized, open-label, phase 3 checkmate 227 Part 1 trial. J Thorac Oncol 2022;17:289–308. 10.1016/j.jtho.2021.09.010 [DOI] [PubMed] [Google Scholar]
- 11. Yi M, Zheng X, Niu M, et al. Combination strategies with PD-1/PD-L1 blockade: current advances and future directions. Mol Cancer 2022;21:28. 10.1186/s12943-021-01489-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Rader C. Bispecific antibodies in cancer immunotherapy. Curr Opin Biotechnol 2020;65:9–16. 10.1016/j.copbio.2019.11.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Esfandiari A, Cassidy S, Webster RM. Bispecific antibodies in oncology. Nat Rev Drug Discov 2022;21:411–2. 10.1038/d41573-022-00040-2 [DOI] [PubMed] [Google Scholar]
- 14. Keam SJ. Cadonilimab: first approval. Drugs 2022;82:1333–9. 10.1007/s40265-022-01761-9 [DOI] [PubMed] [Google Scholar]
- 15. Wolchok JD, Kluger H, Callahan MK, et al. Nivolumab plus ipilimumab in advanced melanoma. N Engl J Med Overseas Ed 2013;369:122–33. 10.1056/NEJMoa1302369 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Jiang C, Zhang L, Xu X, et al. Engineering a smart agent for enhanced immunotherapy effect by simultaneously blocking PD-L1 and CTLA-4. Adv Sci (Weinh) 2021;8:2102500. 10.1002/advs.202102500 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Ji Y, Wang SJ. Modified toxicity probability interval design: a safer and more reliable method than the 3 + 3 design for practical phase I trials. J Clin Oncol 2013;31:1785–91. 10.1200/JCO.2012.45.7903 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Coward J, Ganju V, Behzadigohar R, et al. Preliminary safety, efficacy, and pharmacokinetics (pK) results of KN046 (bispecific anti-PD-L1/CTLA4) from a first-in-human study in subjects with advanced solid tumors. JCO 2019;37(15_suppl):2554. 10.1200/JCO.2019.37.15_suppl.2554 [DOI] [Google Scholar]
- 19. Elassaiss-Schaap J, Rossenu S, Lindauer A, et al. Using model-based "learn and confirm'' to reveal the pharmacokinetics-pharmacodynamics relationship of pembrolizumab in the keynote-001 trial. CPT Pharmacometrics Syst Pharmacol 2017;6:21–8. 10.1002/psp4.12132 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Gaule P, Smithy JW, Toki M, et al. A quantitative comparison of antibodies to programmed cell death 1 ligand 1. JAMA Oncol 2017;3:256–9. 10.1001/jamaoncol.2016.3015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Cristescu R, Mogg R, Ayers M, et al. Pan-tumor genomic biomarkers for PD-1 checkpoint blockade-based immunotherapy. Science 2018;362:eaar3593. 10.1126/science.aar3593 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. O'Malley DM, Neffa M, Monk BJ, et al. Dual PD-1 and CTLA-4 checkpoint blockade using balstilimab and zalifrelimab combination as second-line treatment for advanced cervical cancer: an open-label phase II study. JCO 2022;40:762–71. 10.1200/JCO.21.02067 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Hellmann MD, Paz-Ares L, Bernabe Caro R, et al. Nivolumab plus ipilimumab in advanced non-small-cell lung cancer. N Engl J Med 2019;381:2020–31. 10.1056/NEJMoa1910231 [DOI] [PubMed] [Google Scholar]
- 24. Horinouchi H, Yamamoto N, Fujiwara Y, et al. Phase I study of ipilimumab in phased combination with paclitaxel and carboplatin in Japanese patients with non-small-cell lung cancer. Invest New Drugs 2015;33:881–9. 10.1007/s10637-015-0243-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Finn RS, Ikeda M, Zhu AX, et al. Phase Ib study of lenvatinib plus pembrolizumab in patients with unresectable hepatocellular carcinoma. JCO 2020;38:2960–70. 10.1200/JCO.20.00808 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Clertant M. Early-Phase oncology trials: why so many designs? J Clin Oncol 2022;40:3529–36. 10.1200/JCO.21.02493 [DOI] [PubMed] [Google Scholar]
- 27. Vugmeyster Y, Wilkins J, Koenig A, et al. Selection of the recommended phase 2 dose for bintrafusp alfa, a bifunctional fusion protein targeting TGF‐β and PD‐L1. Clin. Pharmacol. Ther. 2020;108:566–74. 10.1002/cpt.1776 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Wang F-H, Wei X-L, Feng J, et al. Efficacy, safety, and correlative biomarkers of Toripalimab in previously treated recurrent or metastatic nasopharyngeal carcinoma: a phase II clinical trial (POLARIS-02). J Clin Oncol 2021;39:704–12. 10.1200/JCO.20.02712 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Yang Y, Zhou T, Chen X, et al. Efficacy, safety, and biomarker analysis of Camrelizumab in previously treated recurrent or metastatic nasopharyngeal carcinoma (CAPTAIN study). J Immunother Cancer 2021;9:e003790. 10.1136/jitc-2021-003790 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Lu S, Wu L, Jian H, et al. Sintilimab plus bevacizumab biosimilar IBI305 and chemotherapy for patients with EGFR-mutated non-squamous non-small-cell lung cancer who progressed on EGFR tyrosine-kinase inhibitor therapy (ORIENT-31): first interim results from a randomised, double-blind, multicentre, phase 3 trial. Lancet Oncol 2022;23:1167–79. 10.1016/S1470-2045(22)00382-5 [DOI] [PubMed] [Google Scholar]
- 31. Song X, Xiong A, Wu F, et al. Spatial multi-omics revealed the impact of tumor ecosystem heterogeneity on immunotherapy efficacy in patients with advanced non-small cell lung cancer treated with bispecific antibody. J Immunother Cancer 2023;11:11.:e006234. 10.1136/jitc-2022-006234 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Parra ER, Ferrufino-Schmidt MC, Tamegnon A, et al. Immuno-profiling and cellular spatial analysis using five immune oncology multiplex immunofluorescence panels for paraffin tumor tissue. Sci Rep 2021;11:8511. 10.1038/s41598-021-88156-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
jitc-2022-006654supp001.pdf (1.8MB, pdf)
Data Availability Statement
Data are available upon reasonable request. The data of this study have been deposited into the research data deposit (https://www.researchdata.org.cn, ID: RDDA2022604579). All requests should be submitted to the corresponding authors and are available on reasonable request.