Abstract
We received a call from a transplant coordinator about the availability of a consented deceased donor. En-bloc kidneys with the aorta and IVC (inferior vena cava) were harvested from a toddler weighing 8 kg. The recipient was of early childhood weighing 14 kg who had been on haemodialysis for the last 3 years for end-stage kidney disease. He received anti-thymocyte globulin as an induction immunosuppressant. The kidneys were transplanted en bloc in the right lower quadrant retroperitoneal region; an anastomosis was performed to the recipient’s aorta and IVC, and two separate neocystoureterostomies were created. His serum creatinine reached 0.5 mg/dL on the seventh postoperative day, following a few days of delayed graft function. In this study, we describe the surgical and non-surgical challenges that we faced while performing en-bloc kidney transplant to the youngest recipient and how a multidisciplinary team approach helped us overcome them.
Keywords: General surgery, Transplantation, Renal transplantation, Chronic renal failure, Dialysis
Background
Global scenario
Worldwide, the prevalence of paediatric (<14 years) chronic kidney disease (CKD) is 15–74/million cases. Most of these patients progress rapidly to end-stage renal disease (ESRD). The median time period from CKD to ESRD is 4.5 years.1 Kidney transplantation is the treatment of choice for these patients with ESRD. The kidney transplant waiting list increases by 10% each year. Five per cent to 7% of those on the waiting list die each year. In 2020, 1083 paediatric patients were added to the kidney transplant waiting list, of which 21% were below 6 years old. However, the mortality rate among paediatric patients on the transplant waiting list is low (1.4%).2
India scenario
ESRD is an increasing concern in developing countries like India. There is a paucity of literature regarding the data on paediatric CKD. A retrospective study done at a tertiary care centre revealed that the annual paediatric renal transplant rate was 8.3.3 Congenital abnormalities of the kidney and urinary tract and glomerular diseases were the primary causes of ESRD in children.3 Organ donation rate in India is only 0.26%, and paediatric deceased organ donation can aid in increasing the donor pool.4
There is a stark contrast in donor pools between India and worldwide. Around 55% of kidney transplants performed worldwide are from deceased donors, whereas only 9% of kidney transplants performed in our country are cadaveric.5 There is an acute shortage of organs, mainly in small children. The main reasons are the scarcity of deceased paediatric donors and the absence of paediatric living donors. Furthermore, due to the risk of vascular and urological complications, kidneys from very small paediatric deceased donors are being transplanted en bloc, mainly into adults.6 7 Prolonged paediatric dialysis is fraught with issues related to dialysis access, nutrition, growth, developmental and neurocognitive delays, and a paediatric patient on dialysis does not survive for long.8 Here, we report the challenges faced in transplanting en-bloc kidneys harvested from a very small toddler donor weighing 8 kg into a very small child weighing 13 kg. There are very few case reports on paediatric en-bloc kidney transplants in India. To our knowledge, this is the youngest en-bloc kidney transplant recipient.
Case presentation
Donor’s details
The donor was an 8 kg toddler who had suffered injuries after falling from a great height and had been declared brain dead by the certifying authorities. Multiple organs were retrieved after taking consent from the child’s parents. At the time of donation, the patient was on inotropic support with 10 mL/hour urine output. His urine culture showed growth of Candida sp, and serum procalcitonin was elevated. The donor had no comorbidities or known diseases.
Recipient’s details
The recipient was of early childhood weighing 14 kg with a history of decreased urine output and generalised body swelling for 4 years. He was diagnosed with steroid-resistant nephrotic syndrome, and the genetic evaluation revealed a TRPC6 (homozygous) mutation. He progressed to CKD within 6 months. He had been on four times a week maintenance haemodialysis for the previous 3 years. He was admitted under paediatric nephrology, and all routine blood workups and imaging were done. Preoperatively, the patient underwent haemodialysis, was started on broad-spectrum antibiotics, and anti-thymocyte globulin (ATG) was given for induction therapy.
Organ retrieval
Under general anaesthesia (GA), a midline laparotomy was performed, and the liver was harvested by the surgical gastroenterology team. Subsequently, bilateral ureters were identified and cut. The aorta and the inferior vena cava (IVC) were cut after ensuring adequate length. The bilateral kidneys were mobilised en bloc and extracted with the aorta and IVC in toto (figure 1).
Figure 1.

En-bloc kidneys with inferior vena cava (IVC) and aorta and two separate ureters (red arrow—aorta, blue arrow—IVC, black arrows—ureters, K1 and K2—kidneys).
Bench surgery
Bench dissection was done, and perinephric fat was removed from both kidneys. The aorta and IVC were dissected, and all the small branches around them were identified and ligated. The aorta and IVC were closed at their proximal ends with 6-0 prolene.
The kidneys were reperfused with custodial fluid, and a leak test was done twice. No leak was found. En-bloc kidneys were stored in organ bags with the custodial solution, which were then placed in an icebox and transferred to the operating theatre (OT) where the recipient was.
Investigations
The recipient’s haemograms, liver function tests and kidney function tests were done. Preoperative urea and creatinine were 88 mg/dL and 3.2 mg/dL, respectively. Preoperative haemoglobin was 90 g/L. The rest of the blood parameters tested were normal. The coagulation profile was also normal. A bilateral iliofemoral Doppler showed patent iliac vessels with normal flow. The echocardiogram was normal, with an ejection fraction of 60%. The micturating cystourethrogram was normal. Complement dependent cytotoxicity and flow cytometry test reports were negative. The blood group of both the donor and the recipient was B positive.
Treatment
Recipient’s surgery
The patient was intubated in the supine position under GA. The patient was catheterised using an 8 Fr two-way Foley catheter. Right-sided modified Gibson’s incision was made and deepened. The peritoneum was reflected medially, and a space in the retroperitoneum was created. A self-retaining Omni-Tract retractor was applied. Because the iliac vessels were found to be of poor quality, the IVC and aorta were skeletonised until the origin of the inferior mesenteric artery. The graft aorta was anastomosed to the recipient’s aorta, and the graft IVC was anastomosed to the recipient’s IVC in an end-to-side fashion using 6-0 prolene. Additionally, the donor’s ureters were anastomosed to the recipient’s urinary bladder over double J stents (figures 2 and 3). The abdomen was closed in layers. The total operative time was 140 min.
Figure 2.

Graphic representation of graft anastomosis: graft aorta to recipient aorta in end-to-side fashion, graft inferior vena cava (IVC) to the recipient’s IVC in end-to-side fashion and two separate neoureterostomies. (Drawn by Dr Jagadeep Ajmera and Dr Mallika Yerukala)
Figure 3.

Pink turgid kidneys post-anastomosis (green arrow—aorta, blue arrow—inferior vena cava, light blue arrow—common iliac artery, black arrows—ureters, K1 and K2—kidneys).
Postoperative management
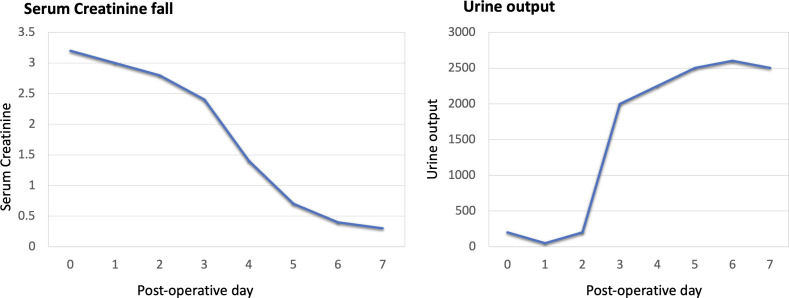
Post-procedure, the patient was transferred to the transplant intensive care unit and was resuscitated accordingly. He received ATG for 2 days and weight-based fixed immunosuppressive therapy (prednisolone, mycophenolate mofetil and tacrolimus) for maintenance. He had delayed graft function with decreased urine output on postoperative days (PODs) 0, 1 and 2, during which he required haemodialysis. On PODs 0 and 3, renal Doppler was done, which was normal, with a peak systolic velocity of 120 cm/s at the anastomotic site and a resistive index of 0.5. He was started on intravenous fluconazole as the donor had Candida sp on the urine culture. On POD 2, he had one episode of fever. His serum procalcitonin was elevated. Hence, antibiotics were upgraded to meropenem and teicoplanin. Prophylactic antiviral drug valganciclovir was given because the patient received ATG as the induction drug. Titration of doses of immunosuppressants and haemodialysis for delayed graft function were provided by the paediatric nephrologists. His urine output improved from POD 3, and a gradual fall in creatinine was seen, reaching 0.7 by POD 5 (figure 4). His diethylenetriaminepentaacetic acid done on POD 4 showed normal-functioning kidneys with non-obstructive clearance.
Figure 4.
Graphs showing trends of decreasing creatinine and increasing urine output in the postoperative period.
His abdominal drain and Foley catheter were removed on POD 5. The internal jugular vein permacath was removed on POD 11 in the OT under GA and sent for culture.
Outcome and follow-up
The patient was checked on a regular basis. His creatinine level was 0.3 at the end of a week, and his urine output was 2.5 L per day. On POD 21, a cystoscopy-assisted double J stent removal was performed by paediatric surgery. The patient was discharged the next day and instructed to follow up regularly at 1, 3 and 6 months.
Six-month follow-up
We just completed a 6-month follow-up, and the outcome is encouraging. At 6 months, the patient had no clinical symptoms, his urine output had been adequate and his serum creatinine level was 0.4 mg/dL. His quality of life has improved significantly, and he is now leading a normal life (free from dialysis). He is playing actively and attending his school regularly.
The patient is being followed by paediatric nephrology and surgery departments at regular intervals.
Discussion
Transplant recipients demonstrate better survival and quality of life when compared with those on dialysis.4 In many developing nations, children with kidney failure have limited access to a dedicated transplant team. They do not have the option of a kidney transplant, reflecting the dearth of financial and social support for most families in low/middle-income countries. Small paediatric kidneys had previously been underused due to the risk of vascular thrombosis and urological complications.7 Therefore, paediatric deceased donor kidneys are being transplanted into adult recipients. All this has led to widespread organ shortages in very small children.
The organ shortage is a grave threat to children’s growth potential, quality of life, prolonged life and neurocognitive development.2 Successful en-bloc transplantation in very small children helps in decreasing these problems.
Recipient selection criteria
The recipient is selected as per the kidney transplant waiting list. All the patients with CKD who want to undergo kidney transplantation surgery are put on the deceased donor waiting list by the paediatric nephrologists. The Organ Retrieval Banking Organization team coordinator informs a paediatric nephrologist consultant about the availability of a consented deceased donor, who checks the waitlist and calls the top three to four patients with CKD (also known as potential recipients) waiting for a kidney transplant. The nephrologists then send all three patients’ blood for a cross-matching test and initiate haemodialysis. Meanwhile, information about the three possible recipients is shared with the operating surgeons, who go through their clinical details. Following that, the nephrologists send the patients’ information about the patients having negative crossmatch tests to the NOTTO (National Organ & Tissue Transplant Organization) team. Patients who have a positive crossmatch will be counselled about the risks and benefits and will most likely choose to remain on the waiting list and try again the next time. Afterward, the NOTTO, through its computerised randomisation, allocates one potential recipient and sends their details to the surgeon for kidney transplant surgery.
When to transplant kidneys en bloc?
There are controversies regarding whether to allocate paediatric donor kidneys to children of the same age and weight or to adults. Few studies have shown that minimising donor–recipient size differences improves long-term graft survival.9 Paediatric kidneys are transplanted either as a single kidney or en bloc. The term ‘en bloc’ refers to the transplant of both kidneys along with the donor’s aorta and an IVC anastomosis to the recipient’s vessels. Graft vessels during en-bloc kidney transplantation in children are usually anastomosed to the recipient’s iliac vessels. However, due to the poor calibre of the recipient’s iliac vessels (that is, the size of the recipient’s iliac vessels was significantly smaller when compared with the graft aorta), an anastomosis was made to the recipient’s aorta and IVC in this case.
There are several criteria considered for en-bloc kidney transplantation. Few have used donor kidney size (6 cm) and donor age (5 years) as parameters.10 A few surgeons have considered donor weight as an important criterion; en-bloc kidney transplantation involving kidneys from deceased donors weighing less than 10 kg was associated with graft failure when they were transplanted separately into two different recipients. Furthermore, paediatric kidneys from donors weighing more than 20 kg have been safely transplanted as a single kidney. Kidneys harvested from donors weighing between 10 and 20 kg constitute a grey area.10 Therefore, it is a combined decision taken by the transplant team and the paediatric nephrologists.
Vascular complications and controversies
Meakins et al reported the first successful en-bloc kidney transplant in adults in 1972.11 Following this, en-bloc kidney transplantation in adults became a well-established technique. However, paediatric en-bloc kidney transplantation remains controversial due to technical difficulties and vascular complications. The results of paediatric kidney transplants in adult recipients were suboptimal in the early years. En-bloc kidney transplantation is done to increase the nephron mass and avoid hyperfiltration injury. It also helps to ease surgical anastomosis as the calibre of the aorta and IVC is larger. However, the risk of vascular thrombosis remains high.4
Bhayana et al reported that the risk of graft thrombosis is 10% among adult recipients who received en-bloc kidneys from children aged 1–5 years.12 Hobart et al reported an 18% (6 of 33) risk of vascular thrombosis in the group with en-bloc kidney transplant.13
Our case was one of the few where the graft aorta was anastomosed to the recipient’s aorta. This was done to avoid the risk of steal phenomenon due to discrepancy in lumen diameters (graft aorta was 6 mm, and the recipient’s common iliac artery was 5 mm). The clamping time of the aorta was kept limited due to the risk of lower limb ischaemia, which was one of the major challenges we faced.
Role of anticoagulation
Literature shows that kidney transplant outcomes have improved in recent years, most likely due to improved surgical techniques and anticoagulation.14
A few studies have shown that anticoagulation with low-dose heparin and aspirin may decrease the risk of post-transplant vascular thrombosis without increasing bleeding risk.15 However, in the absence of concrete evidence, the use of anticoagulation mostly depends on the surgeon’s preference. A European survey combining the responses of transplant professionals from 21 countries revealed that thromboprophylaxis was preferred by 78% of the respondents.16 Anticipated bleeding was the main reason for stopping anticoagulation, as given by 92% of the respondents.16 In our case, during the anastomosis, both the artery and vein were flushed with heparinised saline. Postoperatively, anticoagulants were avoided due to the bleeding risk.
Positive results in the recent past
Kizilbash et al investigated paediatric en-bloc kidney transplants performed between 1987 and 2017. He compared the outcomes of paediatric en-bloc kidney transplant (n=149) and paediatric non-en bloc kidney transplant (n=581) recipients and found that en-bloc kidney recipients had a better 10-year graft (51.6% vs 39.9%; p=0.04) and patient survival (89.0% vs 80.4%; p=0.04). The high risk of graft loss at 1 year was seen in the old era (1987–1997). Superior results of paediatric en-bloc kidney transplants, when compared with a single-kidney transplant, are due to improved kidney function; additionally, the same is reflected in the improved growth of the recipient.14
Pape et al conducted a retrospective study and noticed that the grafts from paediatric donors doubled in size at 1 year post-transplant and that their glomerular filtration rate at 5 years was significantly higher than those of adult donors.17
Kim et al, in 2019, published a case report of two paediatric en-bloc kidney transplants to adult recipients with two different ureterovesical anastomotic techniques: ureteroneocystostomy in one and bladder patch transplantation in another.7 In our case, two separate neoureterostomies were done to the bladder over two separate double J stents. The short length, small calibre and high placement of kidneys made ureter anastomosis difficult.
Warm and cold ischaemia time in en-bloc kidney transplant
Jain et al published a similar case report in describing the surgical steps of en-bloc kidney transplantation from a male toddler to a man in his 40s. Total ischaemia time was 267 min, and total operative time was 170 min. Intraoperative and postoperative courses were uneventful, with creatinine reaching 1.7 mg/dL by POD 12.4
The cold ischaemia time in our case was 4 hours and 50 min, and the warm ischaemia time was 48 min.
Approach: intra-abdominal versus extraperitoneal
En-bloc kidney transplantation is technically challenging, particularly in very small children. In such cases, an intra-abdominal approach is generally preferred (especially in children under 20 kg), as it provides adequate space for the en-bloc kidneys. A transverse incision is preferred over a midline incision in infants and young children, as they have a circular abdomen compared with an oval one in adults. The intra-abdominal approach gives the advantage of native kidney nephrectomy, which is required in many cases. Still, it has its limitations, including the formation of intra-abdominal adhesions, muscle injury, bleeding and delayed detection of urine leakage. However, the retroperitoneal approach is a conventional technique with easy accessibility to the bladder and iliac vessels. It also gives the advantage of a better view for a renal biopsy.18
Yang et al published a case report in 2020 describing the retroperitoneal en-bloc kidney transplant of a female donor in early childhood (13.3 kg) into a female recipient in early childhood (13.1 kg).18 We also used the extraperitoneal approach owing to its advantage of a better view for the subsequent renal biopsy. This approach was associated with less space for both kidneys. Moreover, the recipient’s growth restriction made it even worse. We simply reflected the peritoneum until the subhepatic region to visualise the IVC, which is slightly more than we do in a typical kidney transplant surgery case. Aside from that, nothing was done to make more room.
The challenges faced by both the surgical team (surgical) and paediatric nephrology (medical) were summarised in table 1.
Table 1.
Surgical and medical challenges
| Surgical challenges | Medical challenges |
| Less space for implanting kidneys en bloc | Patient in early childhood with 3 years of maintenance hemodialysis |
| Rare case of graft vessels anastomosis to aorta (due to small calibre of iliac vessels) | Catheter patency issues in view of hypoalbuminaemia |
| Narrow window for blood loss | Catheter-related blood stream infections |
| Limited time period for clamping aorta | History of parvovirus infection |
| Difficult neoureterostomies due to small calibre, short length of ureters and high placement of kidneys | History of SARS-COVID-19 infection twice |
| Non-availability of 3-way Foley catheters in very small sizes | |
| Small-sized double J stents not readily available |
Patient's perspective.
Our son had been suffering from chronic kidney disease for a long time and had been on maintenance hemodialysis for the previous 3 years. We had difficulty getting him dialysis because of our low socioeconomic status. He required many admissions due to fluid overload in his lungs. When compared to his peers, his development was very slow. His social life was very affected due to repeated infections and dialysis. He wasn’t even going to school. But after this renal transplant, our son is going to school and playing well. Most of the above-stated difficulties were resolved post-kidney transplant. We thank all the doctors who were involved in treating our son. We are also grateful that our son is the youngest en bloc kidney transplant recipient in India. We hope that he becomes an example for many more to come.
Learning points.
End-stage kidney failure in very small children seriously threatens their growth potential, quality of life and neurocognitive development.
Although en-bloc kidney transplantation in very small children is technically challenging and fraught with complications, it remains a viable option for reducing organ shortages.
En-bloc kidneys with anastomoses to the aorta and inferior vena cava could be required if the calibres of the iliac vessels are small.
With meticulous surgical techniques, long-term immunosuppressive therapies and a multidisciplinary team approach, the outcomes of an en-bloc kidney transplant in small children are good.
Acknowledgments
Dr Deepgupta and Mr Balram played a major role in counselling the donor's parents for organ donation.
Footnotes
Twitter: @Aditisinha04
Contributors: Dr Sai Kaustubh and Md Fawaz were involved in donor surgery along with JA and MMP. Dr Srinivas, AB and AS admitted the recipient and prepared him for the surgery. JA, Md Fawaz and Dr Srinivas did preoperative workup. Dr Sai Kaustubh, Md Fawaz, Dr Arun, Dr Ratnakar, Dr Ravi Ranjan, MMP and JA were scrubbed in the recipient’s surgery. JA, AB, AS and MMP were involved in the postoperative care of the recipient. JA and Dr Sai Kaustubh compiled the case report. JA, Dr Ratnakar and MMP did the literature review. JA compiled the final case report, which was edited by MMP. AB, AS and MMP were involved in further follow-up of the patient. The following authors were responsible for drafting of the text, sourcing and editing of clinical images, investigation results, drawing original diagrams and algorithms, and critical revision for important intellectual content—JA, MMP, AB and AS. The following author gave final approval of the manuscript—MMP.
Funding: The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Case reports provide a valuable learning resource for the scientific community and can indicate areas of interest for future research. They should not be used in isolation to guide treatment choices or public health policy.
Competing interests: None declared.
Provenance and peer review: Not commissioned; externally peer reviewed.
Ethics statements
Patient consent for publication
Parental/guardian consent obtained.
References
- 1.Kamath N, Iyengar A, George N, et al. Risk factors and rate of progression of CKD in children. Kidney Int Rep 2019;4:1472–7. 10.1016/j.ekir.2019.06.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.US department of health and human services . The scientific registry of transplant recipients. 2021. Available: https://srtr.transplant.hrsa.gov/annual_reports/2020/Kidney.aspx#KI_adult_waiting_new_kili_b64
- 3.Sharma A, Khandelwal P, Sinha A, et al. Hypertension with metabolic alkalosis. Asian J Pediatr Nephrol 2018;1:90. 10.4103/AJPN.AJPN_20_18 [DOI] [Google Scholar]
- 4.Jain V, Jain S, Singhal P, et al. Surgical illustration of en-bloc (dual) kidney transplant from a 16-month old brain-dead donor to an adult recipient. Indian J Urol 2017;33:85–9. 10.4103/0970-1591.194788 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Transplant observatory. 2021. Available: http://www.transplant-observatory.org/summary/
- 6.Nghiem DD, Schlosser JD, Hsia S, et al. En bloc transplantation of infant kidneys: ten-year experience. J Am Coll Surg 1998;186:402–7. 10.1016/s1072-7515(98)00046-5 [DOI] [PubMed] [Google Scholar]
- 7.Kim SH, Yu HC, Hwang HP, et al. En-bloc pediatric kidney transplant to adult recipient with two different ureterovesical anastomosis techniques. Am J Case Rep 2019;20:517–21. 10.12659/AJCR.914290 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Crittenden MR, Holliday MA, Piel CF, et al. Intellectual development of children with renal insufficiency and end stage disease. Int J Pediatr Nephrol 1985;6:275–80. [PubMed] [Google Scholar]
- 9.Bretan PN, Banafsche R, Vapnek E, et al. Minimizing recipient-donor size differences improves long-term graft survival using single pediatric cadaveric kidneys. Transplant Proc 1994;26:28–9. [PubMed] [Google Scholar]
- 10.Al-Shraideh Y, Farooq U, El-Hennawy H, et al. Single vs dual (en bloc) kidney transplants from donors ≤ 5 years of age: a single center experience. World J Transplant 2016;6:239–48. 10.5500/wjt.v6.i1.239 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Meakins JL, Smith EJ, Alexander JW. En bloc transplantation of both kidneys from pediatric donors into adult patients. Surgery 1972;71:72–5. [PubMed] [Google Scholar]
- 12.Bhayana S, Kuo Y-F, Madan P, et al. Pediatric en bloc kidney transplantation to adult recipients: more than suboptimal? Transplantation 2010;90:248–54. 10.1097/TP.0b013e3181e641f8 [DOI] [PubMed] [Google Scholar]
- 13.Hobart MG, Modlin CS, Kapoor A, et al. Transplantation of pediatric en bloc cadaver kidneys into adult recipients. Transplantation 1998;66:1689–94. 10.1097/00007890-199812270-00020 [DOI] [PubMed] [Google Scholar]
- 14.Kizilbash SJ, Evans MD, Chinnakotla S, et al. Survival benefit of en bloc transplantation of small pediatric kidneys in children. Transplantation 2020;104:2435–43. 10.1097/TP.0000000000003158 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Salerno DM, Tsapepas D, Papachristos A, et al. Direct oral anticoagulant considerations in solid organ transplantation: a review. Clin Transplant 2017;31. 10.1111/ctr.12873 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.van den Berg TAJ, Lisman T, Dor FJMF, et al. Antithrombotic management in adult kidney transplantation-a European survey study. Eur Surg Res 2021;63:176–83. 10.1159/000521327 Available: https://www.karger.com/Article/FullText/521327 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pape L, Hoppe J, Becker T, et al. Superior long-term graft function and better growth of grafts in children receiving kidneys from paediatric compared with adult donors. Nephrol Dial Transplant 2006;21:2596–600. 10.1093/ndt/gfl119 [DOI] [PubMed] [Google Scholar]
- 18.Yang K, Kim D, Kim S, et al. En bloc kidney transplant from a pediatric donor to a pediatric recipient through a total extraperitoneal approach: a case report. Exp Clin Transplant 2020;18:834–7. 10.6002/ect.2020.0295 [DOI] [PubMed] [Google Scholar]