Abstract
Postural orthostatic tachycardia syndrome (POTS) has been previously described after SARS-CoV-2 infection; however, limited data is available on the relation of POTS with COVID-19 vaccination. Here we show in a cohort of 284,592 COVID-19 vaccinated individuals using a sequence-symmetry analysis, that the odds of POTS are higher 90 days after vaccine exposure than 90 days prior to exposure, and that the odds for POTS are higher than referent conventional primary care diagnoses, but lower than the odds of new POTS diagnosis after SARS-CoV-2 infection. Our results identify a possible association between COVID-19 vaccination and incidence of POTS. Notwithstanding the probable low incidence of POTS after COVID-19 vaccination, particularly when compared to SARS-Cov-2 post-infection odds which were five times higher, our results suggest that further studies, are needed to investigate the incidence and etiology of POTS occurring after COVID-19 vaccination.
Editor summary:
Through the analysis of electronic medical records of 284,592 vaccinated patients, using a sequence-symmetry analysis, Kwan et al. show that the risk of postural orthostatic tachycardia syndrome (POTS) is increased following COVID-19 vaccination compared to a 90-day control period prior to exposure, although 5.35 times lower than the risk of POTS occurrence post- SARS-Cov-2 infection.
COVID-19 vaccination has been shown to be safe and effective in multiple trials.1–4 Vaccine pharmacovigilance has revealed diverse rare side-effects in the setting of population-wide administration,5,6 including off-target cardiovascular effects with the most well-characterized being myocarditis.7,8 Reports have emerged regarding cases of postural orthostatic tachycardic syndrome (POTS) following vaccination.9 Recognized as a clinical syndrome that manifests with orthostatic intolerance and postural tachycardia, POTS is diagnosed based on clinical features, such as orthostatic dizziness, palpitations and pre-syncope, and a 10-minute stand test or a tilt table test that demonstrate a heart rate elevation of at least 30 beats per minute from supine to standing position.10–12 Given that POTS may be associated with small fiber or autonomic neuropathy, further diagnostic evaluation with autonomic function tests and/or a skin biopsy for the assessment of small fiber neuropathy may be performed. POTS is now known as one of many possible features of post-acute COVID-19 syndromes that can develop after SARS-CoV-2 infection.13–16 Given that COVID-19 vaccination elicits an immunological response to SARS-CoV-2 spike protein, there is biological plausibility for a similar even if attenuated systemic response to vaccine when compared to that seen from viral exposure. Therefore, in this study we evaluated the relation between COVID-19 vaccination and new POTS-related diagnoses by assessing the odds of diagnosis in the baseline 90 days before first vaccine exposure versus the subsequent 90 days after vaccine exposure in a sequence-symmetry analysis.17 We first compared new POTS-related diagnosis odds to those for myocarditis and for common primary care (CPC) diagnoses to provide benchmarks accounting for potential confounding from changes in patient engagement with the healthcare system during the pandemic as well as detection bias from the provider standpoint. We then compared risks of new POTS diagnoses arising after vaccination compared to new POTS diagnoses arising after natural infection, to provide a broader context for interpreting results.
RESULTS
For the post-vaccination analysis, we studied 284,592 patients (age 52±20 years; 57% female; 63% white, 10% Asian, and 8.9% African American; 12% Hispanic ethnicity). The types of vaccinations received included: 62% Pfizer-BioNTech (BNT162b2); 31% Moderna (mRNA-1273); 6.9% Johnson & Johnson/Janssen (Ad26.COV2.S); and <0.1% other vaccines including Astrazeneca (ChAdOx1-S), Novavax (NVX-CoV2373), and Sinovac (CoronaVac).
For new diagnoses made following vaccination, we found that the five conditions with the highest post-vaccination odds of new diagnoses were myocarditis, dysautonomia, POTS, mast cell activation syndrome, and urinary tract infection. Two POTS-associated conditions had lower odds, with fatigue demonstrating a moderate ratio and EDS having second from the lowest ratio (Figure 1a, Table 1). Overall, the post-vaccination odds of new POTS-associated diagnoses (n=4,526, odds: 1.33 [1.25–1.41], p<0.001) was higher than for CPC diagnoses (n=33,590, odds: 1.21 [1.18–1.23], p<0.001), but lower than for myocarditis (n=25, odds: 2.57 [1.02–6.77], p=0.046). When we repeated analyses around receipt of second (rather than the first) vaccination dose, we observed overall similar findings (Supplemental Table 1). The OR of post-vaccine diagnoses of POTS-associated versus CPC conditions was 1.10 (1.03–1.17), p=0.003, with similar results observed from analyses conducted using clustered bootstrapping (OR 1.10 [1.02–1.17]). Patients with POTS-associated diagnoses (n=1,924) after vaccination had similar demographics and vaccine types compared to the overall population (age 56±20 years; 59% female; 67% white, 9% Asian, and 11% African American; 12% Hispanic ethnicity; 59% Pfizer-BioNTech, 35% Moderna, 6.0% Johnson & Johnson/Janssen). We conducted sex-stratified analyses and found similar between-sex results for POTS-associated diagnoses, although EDS was rarely diagnosed in males (n=5) compared to females (n=35) (Figure 1b and 1c).
Figure 1. Post-vaccination odds by diagnosis.
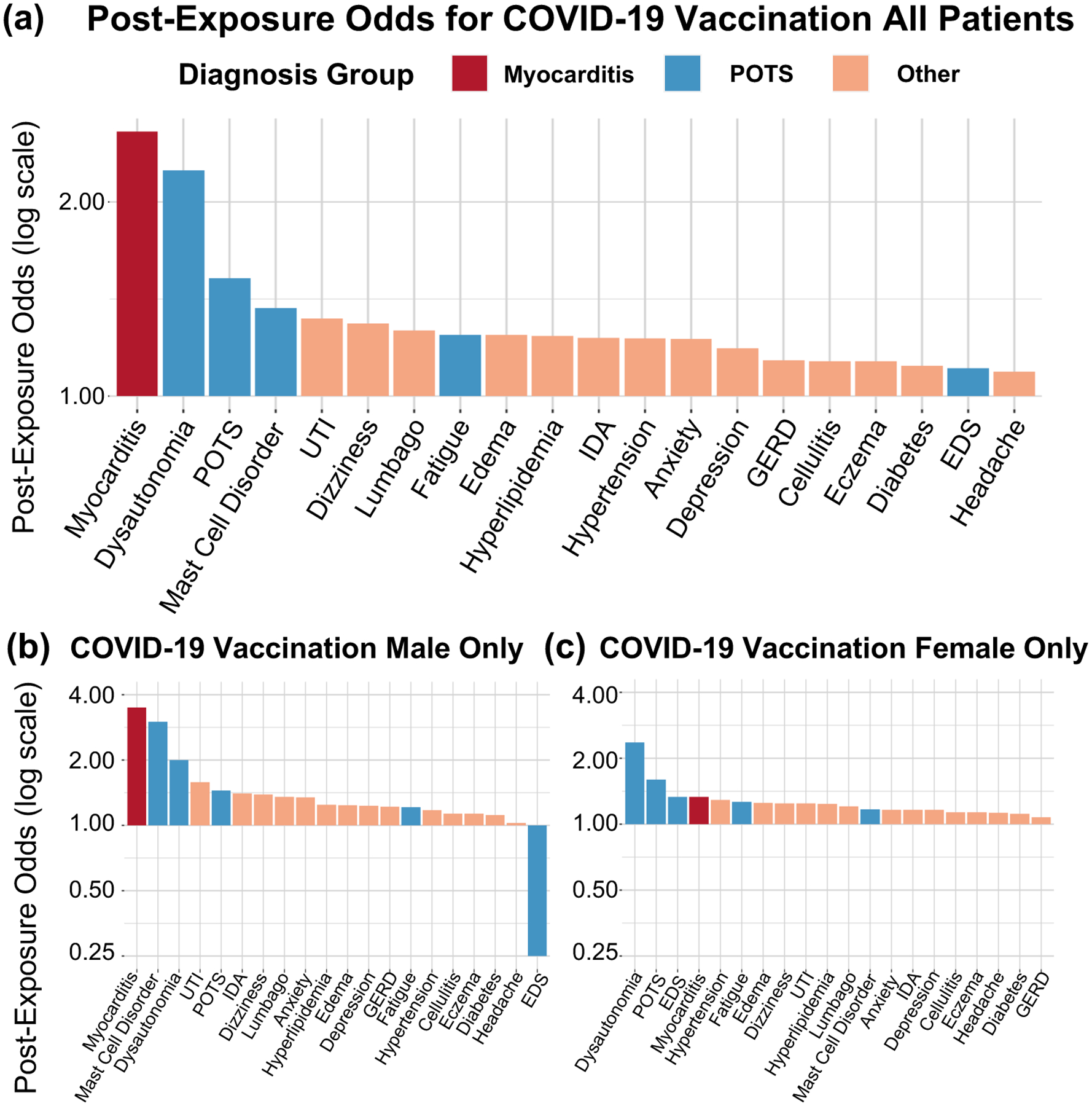
A) All patients, post-vaccination, B) Male patients only, post-vaccination, C) Female patients only, post-vaccination.
POTS: Postural Orthostatic Tachycardia Syndrome; GERD: Gastroesophageal Reflux Disease; EDS: Ehlers-Danlos Syndrome; UTI: Urinary tract infection; IDA: Iron deficiency anemia
Table 1.
Diagnoses within 90 days of exposure for study sample with documented COVID-19 vaccination (N=284,592).
| Diagnosis | No. New Diagnoses N (No. per 100,000) |
New Diagnosis Before Exposure N (No. per 100,000) |
New Diagnosis After Exposure N (No. per 100,000) |
Post-Exposure Risk | Diagnostic Group | |
|---|---|---|---|---|---|---|
| Odds (95% CI) | P value | |||||
| Myocarditis | 25 (8.78) | 7 (2.46) | 18 (6.32) | 2.57 (1.02–6.77)* | 0.046 | Myocarditis |
| Dysautonomia | 68 (23.89) | 21 (7.38) | 47 (16.51) | 2.24 (1.30–3.87)† | 0.002 | POTS |
| POTS | 1264 (444.14) | 501 (176.04) | 763 (268.10) | 1.52 (1.36–1.71)‡ | <0.001 | POTS |
| Mast Cell Disorders | 64 (22.49) | 27 (9.49) | 37 (13.00) | 1.37 (0.81–2.32) | 0.26 | POTS |
| Urinary Tract Infection | 2038 (716.11) | 879 (308.86) | 1159 (407.25) | 1.32 (1.21–1.44)‡ | <0.001 | CPC |
| Dizziness | 2191 (769.87) | 954 (335.22) | 1237 (434.66) | 1.30 (1.19–1.41)‡ | <0.001 | CPC |
| Lumbago | 2845 (999.68) | 1256 (441.33) | 1589 (558.34) | 1.27 (1.17–1.36)‡ | <0.001 | CPC |
| Fatigue | 3090 (1085.76) | 1377 (483.85) | 1713 (601.91) | 1.24 (1.16–1.34)‡ | <0.001 | POTS |
| Edema | 1196 (420.25) | 533 (187.29) | 663 (232.97) | 1.24 (1.11–1.40)‡ | <0.001 | CPC |
| Hyperlipidemia | 4373 (1536.59) | 1952 (685.89) | 2421 (850.69) | 1.24 (1.17–1.32)‡ | <0.001 | CPC |
| Hypertension | 4639 (1630.05) | 2080 (730.87) | 2559 (899.18) | 1.23 (1.16–1.30)‡ | <0.001 | CPC |
| Iron Deficiency Anemia | 1688 (593.13) | 757 (265.99) | 931 (327.13) | 1.23 (1.12–1.36)‡ | <0.001 | CPC |
| Anxiety | 2929 (1029.19) | 1316 (462.42) | 1613 (566.78) | 1.23 (1.14–1.32)‡ | <0.001 | CPC |
| Depression | 1737 (610.35) | 795 (279.35) | 942 (331.00) | 1.18 (1.08–1.30)‡ | <0.001 | CPC |
| GERD | 2795 (982.11) | 1308 (459.61) | 1487 (522.50) | 1.14 (1.05–1.23)‡ | <0.001 | CPC |
| Cellulitis | 1799 (632.13) | 844 (296.56) | 955 (335.57) | 1.13 (1.03–1.24)* | 0.01 | CPC |
| Eczema | 1799 (632.13) | 844 (296.56) | 955 (335.57) | 1.13 (1.03–1.24)* | 0.01 | CPC |
| Diabetes Mellitus | 1269 (445.90) | 600 (210.83) | 669 (235.07) | 1.12 (1.00–1.25) | 0.06 | CPC |
| Ehlers Danlos Syndrome | 40 (14.06) | 19 (6.68) | 21 (7.38) | 1.11 (0.57–2.14) | 0.87 | POTS |
| Headache | 2292 (805.36) | 1096 (385.11) | 1196 (420.25) | 1.09 (1.00–1.19)* | 0.039 | CPC |
POTS: Postural Orthostatic Tachycardia Syndrome; GERD: Gastroesophageal Reflux Disease; CPC: Common Primary Care.
Odds of post-exposure diagnosis were estimated using 1-sample proportions testing with continuity correction, and two-sided P values are shown without correction for multiple testing while noting that a conservative Bonferroni threshold of 0.05/20 = 0.0025 may be considered for aiding interpretation of results.
P<0.05,
P<0.01,
P<0.001
For new diagnoses made following SARS-Cov-2 infection, we conducted separate analyses in 12,460 patients with documented SARS-Cov-2 infection (age 47±23 years; 50% female; 54% white, 6% Asian, and 20% African American; 29% Hispanic ethnicity). Overall, the post-infection odds of new POTS-associated diagnoses (n=1,004, odds: 1.52 [1.33–1.72], p<0.001) was numerically higher than that for CPC diagnoses (n=3,325, odds: 1.4 [1.31–1.50], p<0.001) (Figure 2, Table 2); however, the OR was not significantly higher (1.08 [0.93–1.25], p=0.29), potentially related to limited sample size. Similar results were observed when analyses were conducted using clustered bootstrapping (OR 1.08 [0.94–1.26]). Patients who received POTS-associated diagnoses (n=686) after infection had similar demographics to the overall COVID-19 population but were slightly older (47% female; 59% white, 6.1% Asian, and 22% African American; 26% Hispanic ethnicity; mean age 60±20 years). Similar sex-stratified analyses showed similar results, with the slightly higher rate of myocarditis in men being non-significant likely due to the low rate of new outpatient new diagnoses (three in men and two in women) (Figure 2b and 2c).
Figure 2. Post-infection odds by diagnosis.
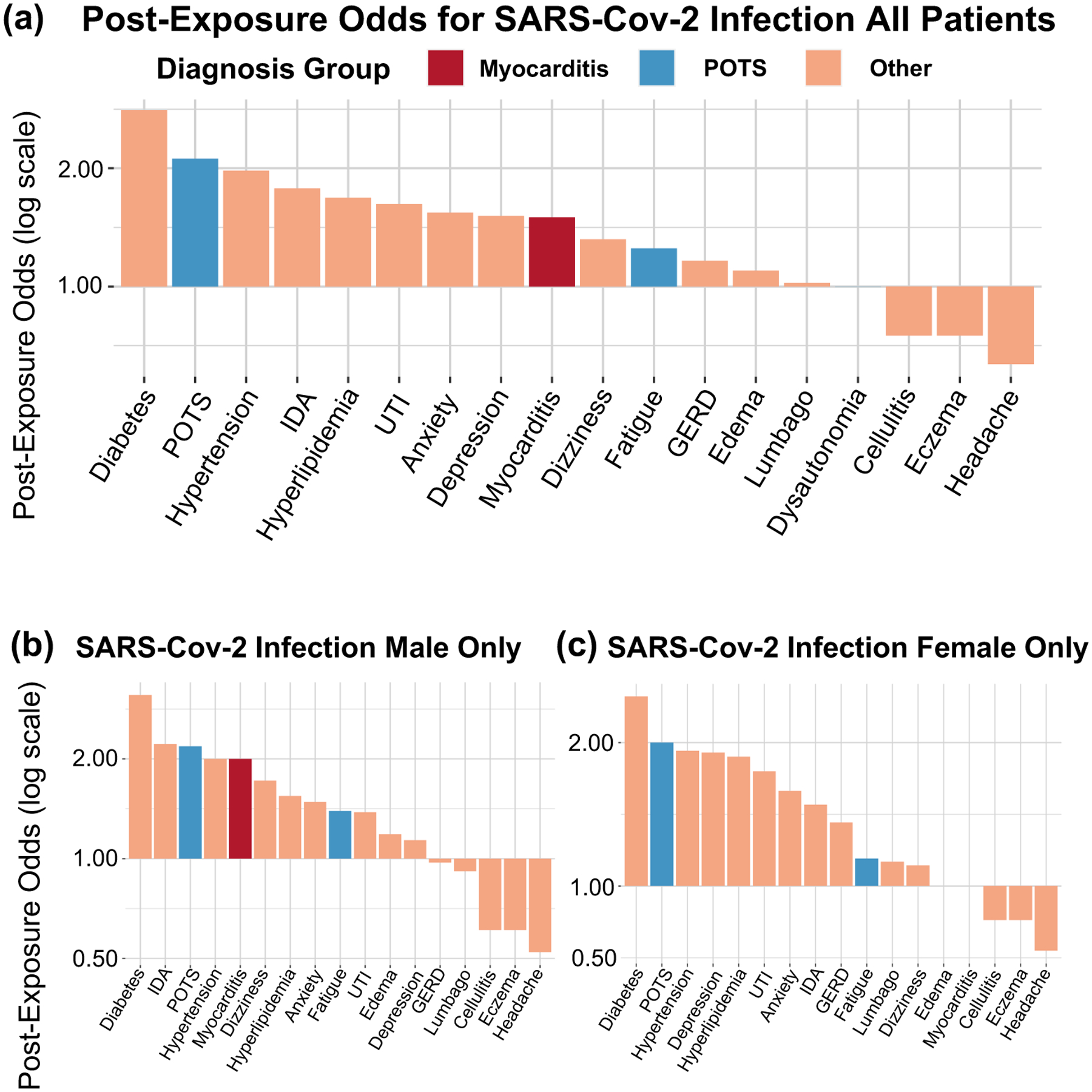
A) All patients, post-infection, B) Male patients only, post-infection, C) Female patients only, post-infection.
POTS: Postural Orthostatic Tachycardia Syndrome; GERD: Gastroesophageal Reflux Disease; EDS: Ehlers-Danlos Syndrome; UTI: Urinary tract infection; IDA: Iron deficiency anemia.
Table 2.
Diagnoses within 90 days of exposure for study sample with documented SARS-Cov-2 infection (N=12,460).
| Diagnosis | No. New Diagnoses N (No. per 100,000) |
New Diagnosis Before Exposure N (No. per 100,000) |
New Diagnosis After Exposure N (No. per 100,000) |
Post-Exposure Risk | Diagnostic Group | |
|---|---|---|---|---|---|---|
| Odds (95% CI) | P value | |||||
| Diabetes Mellitus | 328 (2632.42) | 86 (690.21) | 242 (1942.22) | 2.81 (2.19–3.63)‡ | <0.001 | CPC |
| POTS | 383 (3073.84) | 123 (987.16) | 260 (2086.68) | 2.11 (1.70–2.63)‡ | <0.001 | POTS |
| Hypertension | 642 (5152.49) | 216 (1733.55) | 426 (3418.94) | 1.97 (1.67–2.33)‡ | <0.001 | CPC |
| Iron Deficiency Anemia | 125 (1003.21) | 45 (361.16) | 80 (642.05) | 1.78 (1.22–2.60)† | 0.002 | CPC |
| Hyperlipidemia | 244 (1958.27) | 91 (730.34) | 153 (1227.93) | 1.68 (1.29–2.20)‡ | <0.001 | CPC |
| Urinary Tract Infection | 438 (3515.25) | 167 (1340.29) | 271 (2174.96) | 1.62 (1.33–1.98)‡ | <0.001 | CPC |
| Anxiety | 211 (1693.42) | 83 (666.13) | 128 (1027.29) | 1.54 (1.16–2.05)† | 0.002 | CPC |
| Depression | 108 (866.77) | 43 (345.10) | 65 (521.67) | 1.51 (1.01–2.26)* | 0.043 | CPC |
| Myocarditis | 5 (40.13) | 2 (16.05) | 3 (24.08) | 1.50 (0.21–12.78) | 1.00 | Myocarditis |
| Dizziness | 167 (1340.29) | 72 (577.85) | 95 (762.44) | 1.32 (0.96–1.81) | 0.09 | CPC |
| Fatigue | 619 (4967.90) | 275 (2207.06) | 344 (2760.83) | 1.25 (1.06–1.47)† | 0.006 | POTS |
| GERD | 160 (1284.11) | 74 (593.90) | 86 (690.21) | 1.16 (0.84–1.60) | 0.39 | CPC |
| Edema | 107 (858.75) | 51 (409.31) | 56 (449.44) | 1.10 (0.74–1.63) | 0.70 | CPC |
| Lumbago | 192 (1540.93) | 95 (762.44) | 97 (778.49) | 1.02 (0.76–1.37) | 0.94 | CPC |
| Dysautonomia | 2 (16.05) | 1 (8.03) | 1 (8.03) | 1.00 (0.10–9.58) | 1.00 | POTS |
| Cellulitis | 98 (786.52) | 56 (449.44) | 42 (337.08) | 0.75 (0.49–1.14) | 0.19 | CPC |
| Eczema | 98 (786.52) | 56 (449.44) | 42 (337.08) | 0.75 (0.49–1.14) | 0.19 | CPC |
| Headache | 407 (3266.45) | 249 (1998.39) | 158 (1268.06) | 0.63 (0.52–0.78)‡ | <0.001 | CPC |
| Ehlers Danlos Syndrome | 0 (0) | 0 (0) | 0 (0) | - | - | POTS |
| Mast Cell Disorders | 0 (0) | 0 (0) | 0 (0) | - | - | POTS |
POTS: Postural Orthostatic Tachycardia Syndrome; GERD: Gastroesophageal Reflux Disease; CPC: Common Primary Care.
Odds of post-exposure diagnosis were estimated using 1-sample proportions testing with continuity correction, and two-sided P values are shown without correction for multiple testing while noting that a conservative Bonferroni threshold of 0.05/20 = 0.0025 may be considered for aiding interpretation of results.
P<0.05,
P<0.01,
P<0.001
To interpret post-exposure odds of new diagnoses in the context of their overall frequency, we plotted both post-exposure odds and absolute rates of new diagnosis occurrence for all studied conditions (Figure 3). For the post-vaccination cohort, the odds of new POTS, dysautonomia, and myocarditis diagnoses were elevated but with variably low rates of occurrence. For the post-infection cohort, both the odds of new diagnoses and their rate of occurrence tended to be elevated particularly for conditions such as diabetes, POTS, and hypertension. For most conditions studied, post-infection rates were higher than post-vaccination rates. For POTS-associated diagnoses, in particular, the post-infection risk was 5.35 (5.05–5.68 p<0.001) times higher after exposure to SARS-Cov-2 infection than after exposure to vaccination.
Figure 3. Central Illustration.
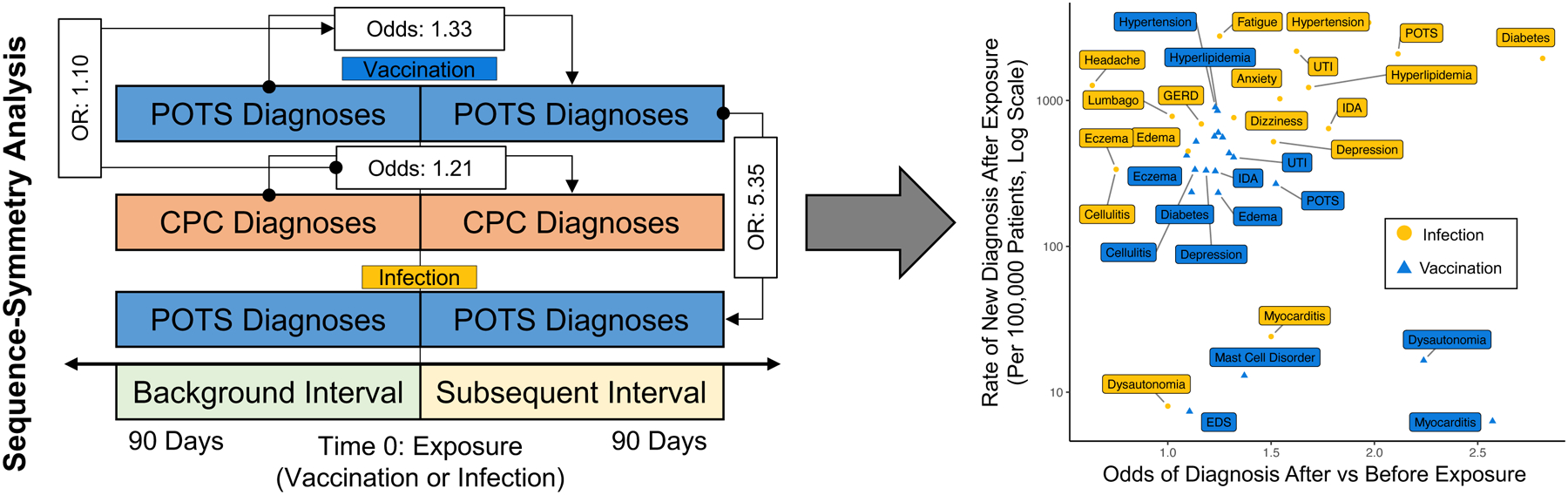
Study design (left) and odds of post-exposure diagnosis versus rate per 100,000 for SARS-Cov-2 infection and COVID-19 vaccination. (right). For odds and odds ratios in left panel, the numerator is designated by the arrow, denominator by the circle.
POTS: Postural Orthostatic Tachycardia Syndrome; GERD: Gastroesophageal Reflux Disease; EDS: Ehlers-Danlos Syndrome; UTI: Urinary tract infection; IDA: Iron deficiency anemia; OR: Odds Ratio
DISCUSSION
In our large and diverse population, using a sequence-symmetry analysis, we found apparent evidence of POTS-associated diagnoses occurring more frequently after COVID-19 vaccination than before vaccination. These new POTS diagnoses occurred at a more frequent rate than did new CPC diagnoses after vaccination. However, the rate of new POTS diagnoses made after vaccination was much less frequent the rate of new POTS diagnoses made after SARS-Cov-2 infection – indicating that excess risks remain higher after infection than after vaccination. This same general trend of proportionately higher rates of new diagnosis after infection compared to after vaccination was consistently seen for myocarditis, which we considered the benchmark condition, as well as for other more common diagnoses, which we considered the referent conditions.
POTS occurring after SARS-Cov-2 infection has been described, but reports of POTS or other neuropathies after COVID-19 vaccination have only started to emerge in case reports.9,18 Historically similar reports of post-vaccination POTS have appeared in the context of human papillomavirus vaccination,19,20 although without sufficient follow-up or validating data to establish causality.21,22 Similarly, our results should not be interpreted as definitive for any causal links between COVID-19 vaccination and POTS due to the observational design of the study. However, the concordant observations of elevated albeit less frequent risks for the same types of diagnoses made after vaccination when compared to those made after infection are suggestive, with the prototypical example represented by myocarditis that presented in our cohorts at frequencies matching those reported by other studies.7,8,23 In addition, we observed similar effects in patients receiving primarily but not exclusively mRNA vaccines. Because heterogeneity is seen in the beneficial responses to COVID-19 vaccination, as well as in clinical responses to natural viral exposure, it is not surprising that heterogeneity would be seen for off-target effects of vaccination.24
There is biological plausibility for the association between POTS and COVID-19 vaccination, in particular. Prior to the pandemic, mRNA vaccination had been administered in small trials predominantly involving cancer therapy, demonstrating rare off-target neurological effects such as Bell’s Palsy, which has also been seen with COVID-19 vaccination.25,26 In SARS-Cov-2 infection, multiple reports of post-infection POTS invoke the possibility of an immune-mediated mechanism triggered by an antigenic component of the spike protein shared with vaccination.13,24,27 Given the broad expression of ACE2 preceptors, inflammasome activation by synthetic spike protein could result in multisystemic effects, including neurocardiogenic targets and potential induction of variable types of autoimmunity.28–30 Additionally, the lipid nanoparticle coating in mRNA vaccine formulations is known to be highly inflammatory, though effects related to the lipid coating appear less likely contributors than spike-protein mediated effects.31 Further research is needed to clarify potential mechanisms related to either vaccine formulation or vaccine target. Fortunately, in our study, POTS-related diagnoses were seen at a substantially lower rate in post-vaccination than in post-infection scenarios. We have observed that POTS in either scenario may respond to conventional therapies. In our experience, patients are managed according to standard-of-care guidelines11,12 for treatment of POTS which involves initially conservative therapies such as salt tablets and hydration, structured exercise programs, and compressive stockings. When clinically indicated, usually for substantial or persistent symptoms, medication therapy such as beta blockers or ivabradine were prescribed as tolerated for tachycardic response and midodrine for orthostatic intolerance. In patients with hyperadrenergic variants, clonidine was given or considered. Accordingly, patients studied received clinical care that was reviewed to be consistent with guidelines recommendations and referral to local experts in managing POTS was often pursued in cases that warranted consideration for more specialized evaluation and therapies.11,12
In summary, POTS-related diagnoses appear to be acquired with increased frequency after, compared to before COVID-19 vaccination, particularly when compared to more commonly diagnosed conditions, but at a rate that is approximately five times lower than after SARS-Cov-2 infection. Additional research regarding the relation between COVID-19 vaccination and POTS is needed. By further developing the evidence base and augmenting our understanding around emerging vaccine side effects, clinical researchers may work to enhance medical trust and improve quality of care as well as communications around vaccines – with the ultimate goal of optimizing vaccine uptake.
Study Limitations
Our study has several limitations. We focused on data collection from outpatient encounters and excluded data from inpatient encounters in a single medical center, which minimizes confounding but limits external validity. Because patients may also receive care outside of our health system, there is a possibility that some unrecorded exposures could have led to misclassification. However, given the time period of the study, during which vaccinations tended to be delayed by 90 days after infection and during which any vaccine history tended to be diligently documented, the effects of any unrecorded exposures are expected to be minimal. Additionally, our separate populations of vaccinated and infected patients were mutually exclusive; recognizing that these populations may have inherent differences, the comparisons between the populations should be interpreted more cautiously than the comparisons within the populations. We did not formally adjudicate all diagnoses due to the large number of events, and an adjudicated sub-sample did show that a significant degree of non-POTS diagnoses were captured within our ICD codes; however, given that this would likely result in non-differential misclassification biasing towards the null, we believe that our relative comparisons remain valid. Our analyses, based on medical records data, may have captured vaccinations more effectively than SARS-Cov-2 infections, thus limiting the sample size for the infection-related analyses. Our exclusion criteria limit the generalizability of our results in patients who have had both vaccination and infection, in either order. We did not specifically assess for interactions between infection and vaccination, or temporal effects potentially arising from seasonal variation or dynamic factors that evolved over the course of the pandemic (e.g. infections caused by Delta versus Omicron variants). Given that POTS is recognized as a condition that is commonly underdiagnosed as well as misdiagnosed,32,33 our records-based search may have underestimated true prevalence. Conversely, the lack of a standard single ICD code for capturing a formal diagnosis of POTS can lead to overlap with other medical conditions and variation in the application of available ICD codes including in the choice of which POTS-associated codes are used. Thus, prospective studies using more specific methods for identifying POTS and associated conditions are needed to clarify absolute post-exposure diagnosis rates, as opposed to the relative comparisons primarily featured in the current study. Finally, because we focused on data derived from outpatient encounters occurring at a single medical center, additional studies in ideally larger and more diverse external cohorts are needed to assess the generalizability of our findings.
METHODS
This study complies with all relevant ethical regulations. The Cedars Sinai IRB approved the study and waived informed consent for this retrospective study. No compensation was given to participants.
Study Cohorts
Our study cohorts were derived from the diverse patient population of Cedars-Sinai Health System in Los Angeles County, California between the dates of 2020–2022. Our study design includes two sequence-symmetry analyses17 within separate retrospective cohorts of patients with COVID-19 vaccination and patients with SARS-Cov-2 infection.
Post-Vaccine Cohort.
In our primary cohort investigating the relation of COVID-19 vaccination with POTS diagnoses, the primary exposure was first COVID-19 vaccination, as documented in the electronic health record. Of all patients who had at least one COVID-19 vaccination dose documented (n=289,662), we excluded those with SARS-Cov-2 infection prior to and within 90 days after the first vaccination dose (n=5,070). We identified new diagnoses occurring within 90 days of exposure, associated with an outpatient encounter and defined by ICD-9 and ICD-10 code or grouping by phecode (Supplemental Table 2).34 We considered three groups of diagnoses: POTS-associated diagnoses, myocarditis, and CPC diagnoses. Given the lack of single ICD code for POTS, we garnered expert opinion from clinical specialists to define a POTS-associated group of diagnoses that includes dysautonomia, other specified cardiac dysrhythmias (the primary ICD code, herein referred to as POTS), mast cell activation syndrome and related disorders, Ehlers-Danlos syndrome (EDS), and fatigue. The CPC diagnoses were prospectively selected from ICD codes frequently documented in primary care,35 excluding diagnoses with strong biological plausibility for being directly related to COVID-19 (e.g. upper respiratory infection, cough, fever).
To assess the validity of our approach to identifying possible POTS diagnoses, we conducted clinical adjudication of 50 sequentially encountered patients identified has having both the I49.8 and G90.9 codes. From this adjudication process, we observed that 40 (80%) were either formally confirmed POTS through comprehensive diagnostic testing or with signs and symptoms consistent with guidelines definitions of POTS but still awaiting full diagnostic testing for confirmation. We used limited but available ICD codes in attempts to identify POTS diagnoses with optimal sensitivity and specificity while recognizing that misclassification can result from both variable ICD coding patterns and the prior absence of unique ICD code for POTS. Notwithstanding the acceptable results of having clinically adjudicated a subset of our identified cases, we recognize that our analyses of EHR data are intrinsically subject to non-differential misclassification that generally tends to bias results towards the null.
Post-Infection Cohort.
The secondary cohort investigated the relation of SARS-Cov-2 infection with POTS diagnoses for contextual comparison. We included all patients with documented SARS-Cov-2 infection (n=20,390) and excluded those with vaccination prior to or within 90 days after infection (n=7,930). The primary exposure for the secondary cohort was first SARS-Cov-2 infection. We analyzed the same diagnoses and diagnosis groups occurring within 90 days of first SARS-Cov-2 infection. In designing our study, we observed increases in multiple post-COVID-19 CPC diagnosis odds, particularly for diabetes and hypertension (unadjusted for other CPC diagnoses). Increase in diabetes and cardiometabolic risk has been previously reported from separate cohorts36–40. Thus, we recognized the importance of including these diagnoses within the CPC group, given they represent conditions that are commonly diagnosed in primary care settings even if elevated in the post-exposure setting for reasons that are not yet entirely clear. We also recognized that the increased risk ratio for these diagnoses would conservatively bias our primary comparative results toward the null.
Statistical Analyses
The study was designed to address multiple potential confounding factors at the outset. Given the medical-records based data source with certain intrinsic limits to query-able patient-level data, we recognized that a self-controlled design would allow at least some ability to control for time-invariant confounders such as age and sex, or latent but time-invariant confounders that could reflect differences in healthcare interaction between vaccinated patients and those unvaccinated at time of infection. We also recognized that the exposure itself could influence healthcare behavior, e.g. patients may feel more comfortable visiting physicians after vaccination. To this end, we compared the events of new diagnoses of POTS with new diagnoses of myocarditis (the benchmark event) and with new diagnoses of other conditions commonly made during primary care visits (referent events). The comparisons between populations with two distinct but discernible exposures (vaccination and SARS-Cov-2 infection) could permit controlling for detection bias after exposure. Because our source dataset includes patients who may have had SARS-Cov-2 infection or vaccination events occurring outside of our health system, potentially influencing the outcomes of interest, we were careful to restrict our analyses to data collected within a specific and limited timeframe before and after the exposure ‘event’ (i.e. infection or vaccination) given that unrecorded (i.e. unmeasured) exposures could otherwise have more opportunity to exert confounding effects. For this reason, we employed a sequence-symmetry analysis along with pre-specified narrow timeframes around documented exposures to help minimize the possibility that unrecorded and potentially confounding or interacting additional exposures could have occurred during the same narrow time period.17 We note that because our pre-specified separate populations of vaccinated and infected patients were mutually exclusive, the results of comparison analyses conducted between the populations should be interpreted more cautiously than the results of comparison analyses conducted within the populations.
We expressed new diagnosis events as a rate per 100,000 exposures, rather than rate per number of sequence-symmetry exposure periods (e.g. two per exposure), given that the rate per exposure is more readily clinically interpretable. We used these rates to calculate two sets of primary outcomes. The first was the diagnosis-specific odds that the new diagnosis occurred after exposure versus before exposure. The second was the odds ratio (OR) of acquiring a post-exposure new POTS-group diagnosis versus a new CPC diagnosis. Odds of post-exposure diagnosis were estimated using 1-sample proportions testing with continuity correction; OR’s were estimated with logistic regression with cluster-robust standard errors to account for possible repeated measures (e.g. multiple diagnoses) between patients. With these comparisons, we sought to assess not only the relative odds of developing a new diagnosis after versus before a given exposure, but also assess whether any new POTS-related post-exposure may be disproportionately more common when compared to other newly occurring diagnoses, given potential for the frequency of new diagnoses to temporally vary during the pandemic (Figure 4). In secondary analyses, we repeated the main analyses after exchanging the first dose of vaccine with the second dose of vaccine as the index exposure. We also repeated primary OR analyses using clustered bootstrapping (2000 replications with ordinary non-parametric bootstrapping). Additionally, we performed manual adjudication of a subset of 50 events. Data query was performed using DBeaver Enterprise Database Manager v22.0.0.202203131528 with data formatting by python Python 3.9.0 in Jupyter-notebook 6.0.3. Analyses were performed using R/R Studio 4.1.1/2022.02.041 with open source packages tidyverse v1.3.1, janitor v2.1.0, lubridate v1.8.0, gtsummary v1.6.1, knitr 1.39, and ggrepel 0.9.1.
Figure 4. Study Design.
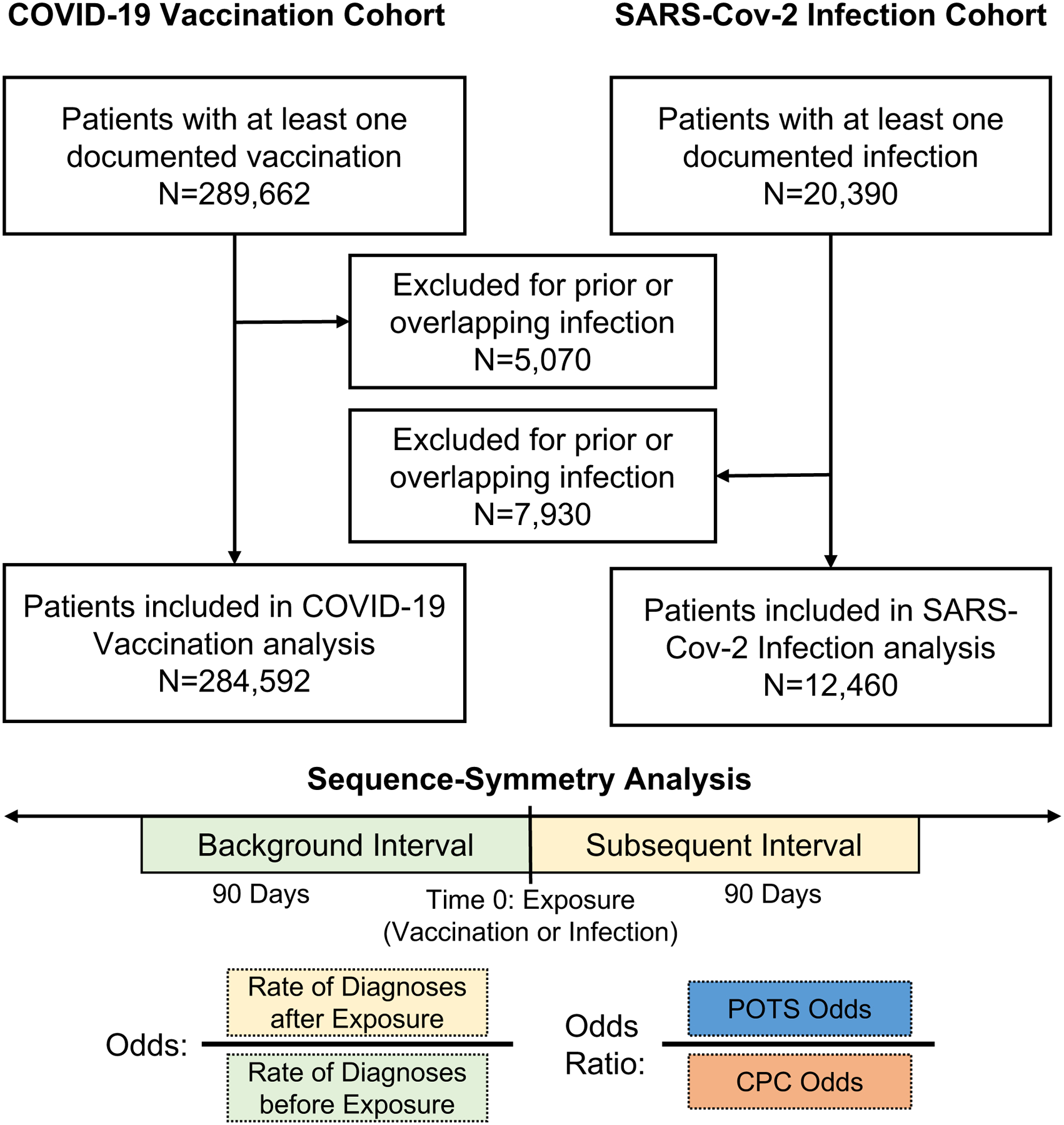
Participant flow and study design.
POTS: Postural Orthostatic Tachycardia Syndrome; CPC: Common Primary Care
Supplementary Material
ACKNOWLEDGEMENTS
This study was supported in part by NIH Grants R01HL139829 (PSC), 1OT2OD028190 (PSC), K23HL153888 (JEE), K23HL159276 (BNW), R01HL153500 (JW), R01HL151828 (SC), R01HL131532 (SC); The Doris Duke Charitable Foundation Grant 2020059 (ACK), AHA 21CDA851511 (BNW), ASNC 2021 IANC Research Award (BNW), The Smidt Heart Institute (ACK) and the Burns & Allen Chair in Cardiology Research (PSC), Cedars-Sinai Medical Center, Los Angeles, California. The authors thank the members of the BioDataCore Lab, CORALE and EMBARC research groups, and COVID-19 Recovery Program at Cedars-Sinai for their support and thoughtful ideas around COVID-19 research.
ABBREVIATIONS:
- CPC
Common primary care
- EDS
Ehlers-Danlos syndrome
- OR
Odds Ratio
- POTS
postural orthostatic tachycardia syndrome
Footnotes
CODE AVAILABILITY
Code for the analysis conducted for the manuscript is available at https://github.com/biodatacore/pots_vax_covid
COMPETING INTERESTS
The authors declare no competing interests.
DATA AVAILABILITY
The clinical data that support the findings of this study are available from Cedars-Sinai Medical Center, upon reasonable request. The data are not publicly available due to the contents including information that could compromise research participant privacy/consent. Information regarding data access requests can be found at https://github.com/biodatacore/pots_vax_covid and all inquiries also be directed to biodatacore@cshs.org. The timeframe for response to requests from the authors is 4 weeks. Source data for figures and ICD codes are included in supplementary materials.
References
- 1.Kim JH, Marks F & Clemens JD Looking beyond COVID-19 vaccine phase 3 trials. Nature medicine 27, 205–211 (2021). [DOI] [PubMed] [Google Scholar]
- 2.Polack FP, et al. Safety and efficacy of the BNT162b2 mRNA Covid-19 vaccine. New England Journal of Medicine (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Baden LR, et al. Efficacy and safety of the mRNA-1273 SARS-CoV-2 vaccine. New England journal of medicine (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sadoff J, et al. Safety and efficacy of single-dose Ad26. COV2. S vaccine against Covid-19. New England Journal of Medicine 384, 2187–2201 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Goss AL, Samudralwar RD, Das RR & Nath A ANA investigates: neurological complications of COVID‐19 vaccines. Annals of neurology 89, 856 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kadali RA, Janagama R, Peruru S & Malayala SV Side effects of BNT162b2 mRNA COVID-19 vaccine: A randomized, cross-sectional study with detailed self-reported symptoms from healthcare workers. International Journal of Infectious Diseases 106, 376–381 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mevorach D, et al. Myocarditis after BNT162b2 mRNA vaccine against Covid-19 in Israel. New England Journal of Medicine 385, 2140–2149 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bozkurt B, Kamat I & Hotez PJ Myocarditis With COVID-19 mRNA Vaccines. Circulation 144, 471–484 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Reddy S, Reddy S & Arora M A case of postural orthostatic tachycardia syndrome secondary to the messenger RNA COVID-19 vaccine. Cureus 13(2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Vernino S, et al. Postural orthostatic tachycardia syndrome (POTS): State of the science and clinical care from a 2019 National Institutes of Health Expert Consensus Meeting-Part 1. Autonomic Neuroscience 235, 102828 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Raj SR, et al. Canadian Cardiovascular Society Position Statement on Postural Orthostatic Tachycardia Syndrome (POTS) and Related Disorders of Chronic Orthostatic Intolerance. Canadian Journal of Cardiology 36, 357–372 (2020). [DOI] [PubMed] [Google Scholar]
- 12.Sheldon RS, et al. 2015 heart rhythm society expert consensus statement on the diagnosis and treatment of postural tachycardia syndrome, inappropriate sinus tachycardia, and vasovagal syncope. Heart rhythm 12, e41–e63 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Raj SR, et al. Long-COVID postural tachycardia syndrome: an American Autonomic Society statement. Clinical Autonomic Research 31, 365–368 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jamal SM, et al. Prospective Evaluation of Autonomic Dysfunction in Post-Acute Sequela of COVID-19. Journal of the American College of Cardiology 0(2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rai NS, Everin O & Manohar VA POSTURAL ORTHOSTATIC TACHYCARDIA SYNDROME DUE TO COVID-19. Journal of the American College of Cardiology 79, 2197–2197 (2022). [Google Scholar]
- 16.Johansson M, et al. Long-haul post–COVID-19 symptoms presenting as a variant of postural orthostatic tachycardia syndrome: the Swedish experience. Case Reports 3, 573–580 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Takeuchi Y, Shinozaki T & Matsuyama Y A comparison of estimators from self-controlled case series, case-crossover design, and sequence symmetry analysis for pharmacoepidemiological studies. BMC medical research methodology 18, 1–15 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Safavi F, et al. Neuropathic symptoms with SARS-CoV-2 vaccination. medRxiv, 2022.2005.2016.22274439 (2022). [Google Scholar]
- 19.Blitshteyn S P ostural tachycardia syndrome following human papillomavirus vaccination. European Journal of Neurology 21, 135–139 (2014). [DOI] [PubMed] [Google Scholar]
- 20.Blitshteyn S & Brook J Postural tachycardia syndrome (POTS) with anti-NMDA receptor antibodies after human papillomavirus vaccination. Immunologic research 65, 282–284 (2017). [DOI] [PubMed] [Google Scholar]
- 21.Agency EM HPV vaccines: EMA confirms evidence does not support that they cause CRPS or POTS. (2016).
- 22.Barboi A, et al. Human papillomavirus (HPV) vaccine and autonomic disorders: a position statement from the American Autonomic Society. Clinical Autonomic Research 30, 13–18 (2020). [DOI] [PubMed] [Google Scholar]
- 23.Witberg G, et al. Myocarditis after Covid-19 vaccination in a large health care organization. New England Journal of Medicine (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Teijaro JR & Farber DL COVID-19 vaccines: modes of immune activation and future challenges. Nature Reviews Immunology 21, 195–197 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Verbeke R, Lentacker I, De Smedt SC & Dewitte H Three decades of messenger RNA vaccine development. Nano Today 28, 100766 (2019). [Google Scholar]
- 26.Cirillo N Reported orofacial adverse effects of COVID‐19 vaccines: The knowns and the unknowns. Journal of Oral Pathology & Medicine 50, 424–427 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Goldstein DS The possible association between COVID-19 and postural tachycardia syndrome. Heart rhythm 18, 508 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Conceicao C, et al. The SARS-CoV-2 Spike protein has a broad tropism for mammalian ACE2 proteins. PLoS biology 18, e3001016 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Angeli F, Spanevello A, Reboldi G, Visca D & Verdecchia P SARS-CoV-2 vaccines: Lights and shadows. Eur J Intern Med 88, 1–8 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chen Y, et al. New‐onset autoimmune phenomena post‐COVID‐19 vaccination. Immunology 165, 386–401 (2022). [DOI] [PubMed] [Google Scholar]
- 31.Ndeupen S, et al. The mRNA-LNP platform’s lipid nanoparticle component used in preclinical vaccine studies is highly inflammatory. iScience 24, 103479–103479 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Schmidt LL, Karabin BL & Malone AC Postural Orthostatic Tachycardia Syndrome (POTS): Assess, Diagnose, and Evaluate for POTS Treatment (ADEPT). Integrative Medicine International 4, 142–153 (2017). [Google Scholar]
- 33.Kavi L, Gammage MD, Grubb BP & Karabin BL Postural tachycardia syndrome: multiple symptoms, but easily missed. Br J Gen Pract 62, 286–287 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wei W-Q, et al. Evaluating phecodes, clinical classification software, and ICD-9-CM codes for phenome-wide association studies in the electronic health record. PloS one 12, e0175508 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Coders A.A.o.P. Fast Forward Top 50 Codes. Vol. 2022 (2015). [Google Scholar]
- 36.Ayoubkhani D, et al. Post-covid syndrome in individuals admitted to hospital with covid-19: retrospective cohort study. bmj 372(2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Barrett CE, et al. Risk for newly diagnosed diabetes> 30 days after SARS-CoV-2 infection among persons aged< 18 years—United States, March 1, 2020–June 28, 2021. Morbidity and Mortality Weekly Report 71, 59 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Daugherty SE, et al. Risk of clinical sequelae after the acute phase of SARS-CoV-2 infection: retrospective cohort study. Bmj 373(2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Xie Y & Al-Aly Z Risks and burdens of incident diabetes in long COVID: a cohort study. The Lancet Diabetes & Endocrinology 10, 311–321 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Xie Y, Xu E, Bowe B & Al-Aly Z Long-term cardiovascular outcomes of COVID-19. Nature medicine 28, 583–590 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Team RCR: A Language and Environment for Statistical Computing. R Foundation for Statistcial Computing. 2021. (2021). [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The clinical data that support the findings of this study are available from Cedars-Sinai Medical Center, upon reasonable request. The data are not publicly available due to the contents including information that could compromise research participant privacy/consent. Information regarding data access requests can be found at https://github.com/biodatacore/pots_vax_covid and all inquiries also be directed to biodatacore@cshs.org. The timeframe for response to requests from the authors is 4 weeks. Source data for figures and ICD codes are included in supplementary materials.


