Abstract
Objective
Sedentary behaviour is associated with increased cancer risk. We aim to assess the associations of domain-specific and total sedentary behaviour with risk of endometrial cancer, with additional attention paid to potential differences in adjustment strategy for obesity and physical activity.
Design
A systematic review and meta-analysis was conducted in accordance with the guidelines of Preferred Reporting Items for Systematic Reviews (PRISMA) and Meta-Analyses and the Meta-analysis of Observational Studies in Epidemiology (MOOSE).
Data sources
PubMed, Embase and MEDLINE databases were searched up to 28 February 2023, supplemented by grey literature searches.
Eligibility criteria for selecting studies
Observational human studies evaluating the association between sedentary behaviour and endometrial cancer.
Data extraction and synthesis
Two reviewers extracted data and conducted the quality assessment based on Newcastle-Ottawa Scale (NOS) independently. We used a random-effects model with inverse variance approach to pool the estimates. The extent of heterogeneity was quantified with the I2 statistics.
Results
Sixteen studies were included in the systematic review. Fourteen studies involving 882 686 participants were included in the meta-analysis. The pooled relative risks (RRs) for high versus low level of overall sedentary behaviour was 1.28 (95% CI: 1.14 to 1.43; I2=34.8%). The increased risk regarding specific domains was 1.22 (95% CI: 1.09 to 1.37; I2=13.4%, n=10) for occupational domain, 1.34 (95% CI: 0.98 to 1.83; I2=53.7%, n=6) for leisure-time domain and 1.55 (95% CI: 1.27 to 1.89; I2=0.0%, n=2) for total sedentary behaviour. Larger pooled RRs were observed among studies with adjustment for physical activity and studies without adjustment for body mass index.
Conclusions
Higher levels of sedentary behaviour, total and occupational sedentary behaviour in particular, increase the risk of endometrial cancer. Future studies are needed to verify domain-specific associations based on objective quantification of sedentary behaviour, as well as the interaction of physical activity, adiposity and sedentary time on endometrial cancer.
Keywords: epidemiology, gynaecological oncology, public health
STRENGTHS AND LIMITATIONS OF THIS STUDY.
The present systematic review and meta-analysis was conducted following the registered proposal, Preferred Reporting Items for Systematic Reviews and Meta-Analyses and Meta-analysis of Observational Studies in Epidemiology guidelines, and Newcastle-Ottawa Scale to report results and evaluate study quality, respectively.
Previous studies reported inconsistent associations between sedentary behaviour and endometrial cancer.
Little is known regarding the association between specific domains of sedentary behaviour and endometrial cancer, as well as the potential role of obesity and physical activity in the association.
The results would add to the existing evidence by showing a possible domain-specific effect of sedentary behaviour on endometrial cancer.
The review highlighted the importance of evaluating the interaction of sedentary behaviour with other lifestyle factors when analysing the association between sedentary behaviour and endometrial cancer.
Introduction
According to the updated global cancer burden estimates from Global Cancer Statistics 2020,1 endometrial cancer ranks the sixth most common cancer in women worldwide, and the most common gynaecologic cancer in several developed regions, including North America and Eastern and Northern Europe. A worrying trend is that, since the late 1990s, the incidence of endometrial cancer has rapidly increased in several developing countries during urbanisation, including some Asian countries (Japan, Singapore, China and the Philippines) and South Africa.2 It is suggested that this phenomenon may be explained, at least partly, by changing environmental and lifestyle risk factors in these regions, such as the epidemic of obesity, lack of physical activity and long-time sitting.3 4 Although obesity is a known risk factor for endometrial cancer, the association between sedentary behaviour and endometrial cancer remains largely unclear. Sedentary behaviour includes sitting, reclining or lying behaviour characterised by low energy expenditure.5 During the past decades, technological innovation has influenced how people work and spend leisure-time, and has led to inevitably prolonged sitting time, particularly for desk-based office work and screen-based recreation. According to the WHO Guidelines on Physical Activity and Sedentary Behaviour (2020), long sedentary time is associated with various deleterious health outcomes, including all-cause mortality, cardiovascular diseases, obesity and more recently total cancer morbidity.5
Three previous meta-analyses investigating the association between sedentary behaviour and several types of cancers,6–8 reported a 28–36% increased risk of endometrial cancer among individuals with higher levels of sedentary behaviour by summarising 3–11 individual studies. However, some evidence on sedentary behaviour and endometrial cancer has not yet been included in existing review and meta-analyses, the level of evidence for cancer-specific incidence remains unclear.9 10 Given inconsistent results reported, an up-to-date review of current evidence is in urgent need to clarify the association between sedentary behaviour and endometrial cancer risk.
No distinction in domains of sedentary behaviour is a likely source of the discrepancy in previous findings. The WHO Guidelines 2020 has operationalised the definition of sedentary behaviour to further include self-reported sitting that can be assessed in various domains (including leisure-time and occupational domain) and total sedentary behaviour.5 Meanwhile, the association with adverse health outcomes may differ in certain domains of sedentary behaviour.11 It is increasingly recognised that confounding factors may vary greatly across domains of sedentary behaviour, and contribute to varied associations with health-related outcomes.12 For example, while occupational sedentary behaviour is related to education and socioeconomic variables, leisure-time sedentary behaviour is likely linked to lifestyle factors such as diet and obesity.13 Moreover, these two domains are often inversely correlated to physical activity. However, current evidence has been derived mostly from studies that have broadly categorised sedentary behaviour according to the level of sitting time involved.7 14 Domain-specific analyses, taking account of variability in study characteristics, may help to further clarify the investigated association and to refine the prevention strategy of endometrial cancer.
Besides, the complex interplay within lifestyle factors, including obesity, physical activity and sedentary behaviour, needs to be taken into consideration within the context. Obesity is a known risk factor for endometrial cancer, with a clear dose-response relationship (the higher the body mass index (BMI), the greater the risk), detailed documented by the International Agency for Research on Cancer (IARC) working group.15 Given that prolonged sitting is likely to be related with high BMI, obesity thus may be a potential mediator linking sedentary behaviour to cancer incidence. Under this circumstance, studies adjusting for BMI as a confounding factor may attenuate the true effects of sedentary behaviour when evaluating its impacts on endometrial cancer. A few studies have probably recognised this issue and provided results without and with additional adjustment for BMI.16–19 In addition, although less evidence presented, similar concerns have been raised with regard to physical activity, which has a potential protective effect on cancer risk.4 20 21
In this systematic review and meta-analysis, the primary aim was to analyse comprehensively the existing studies of the associations between domain-specific (occupational and leisure-time) and total sedentary behaviour and endometrial cancer risk, with additional attention paid to potential difference of the findings related to different adjustment strategies for BMI and physical activity.
Methods
We performed this systematic review and meta-analysis in accordance with the 2020 guidance of the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA)22 and guidelines of the Meta-analysis of Observational Studies in Epidemiology (MOOSE) as well.23 Reported items in this systematic review and meta-analysis strictly followed the checklist of PRISMA 2020 and MOOSE (online supplemental tables 1 and 2). The full review protocol was registered with the international prospective register of systematic reviews (PROSPERO) under the registration number CRD42021246283.
bmjopen-2022-069042supp001.pdf (152.9KB, pdf)
bmjopen-2022-069042supp002.pdf (48.5KB, pdf)
bmjopen-2022-069042supp003.pdf (65.5KB, pdf)
Search strategy and selection criteria
We conducted a comprehensive literature search of the electronic databases, including Embase, MEDLINE and PubMed. The search was updated on 28 February 2023, and publication language was limited to English. The search combined Medical Subject Headings with text search using varied terms related to ‘sedentary behavior’ and ‘endometrial cancer’. Detailed search terms and strategy used are listed in the online supplemental file 1. Terms associated with physical activity and physical inactivity (insufficient or low levels of physical activity) were also searched since some sedentary behaviour studies were conducted in the name of physical activity. In addition, we screened and manually checked reference lists from selected articles and relevant reviews to identify other potentially eligible studies.
The inclusion criteria for the studies included in the systematic review are listed as follows: (1) observational human study that published in English; (2) evaluated the association between sedentary behaviour (total sitting time, leisure-time sedentariness including sitting, television or screen viewing and occupational sedentary behaviour) and incidence of endometrial cancer. Apart from all criteria for systematic review, the studies further included in the meta-analysis should also meet the following criteria: report a relative risk (RR), odds ratio (OR), hazard ratio (HR) or standardised incidence ratio (SIR) with 95% CI for highest versus lowest level of sedentary behaviour or provide sufficient data to calculate them.
Studies were excluded if they were published as conference abstracts or papers, letters and short surveys. We also excluded studies for physical activity that used terms ‘sedentary’ or ‘sitting’ to represent the lowest or reference level of physical activity categories.
Data extraction and quality assessment
Two authors (LY and JN) independently performed the literature search and reviewed potential studies in compliance with the selection criteria. The disagreements were resolved through discussion. The authors were contacted by email for full text or additional information when needed. Extracted information from each study included: (a) name of the first author and publication year; (b) study design; (c) study area; (d) enrolment period for cohort study, or study period for case–control study; (e) age at baseline; (f) follow-up length for cohort study; (g) study population; (h) sample size; (i) case number; (j) sedentary behaviour type and its assessment; (k) diagnostic criteria of endometrial cancer, and if available, its specific cancer classification; (l) results and if possible, reported risk estimates and their 95% CI; (m) adjusted covariates, if possible, particular attention to adjustment for BMI and physical activity.
In the main analysis, we prioritised risk estimates that were adjusted for physical activity, and unadjusted for BMI in studies with a separate step of BMI adjustment, or other adiposity-related factors when available, due to the potential intermediate role of obesity. If study populations overlapped between included studies, we selected the article that contained the most comprehensive data.24 25
Quality assessment of the studies included in the meta-analysis was assessed based on the validated Newcastle-Ottawa Scale (NOS) for observational studies,26 27 where each study was evaluated based on three categories: participant selection (four items, one star for each item); comparability of study groups (one item, up to two stars); exposure or outcome assessment (three items, one star for each item). Thus, a study can be awarded up to a maximum of nine stars.27 We used the comparability category of the NOS to determine whether the crucial confounders had been adjusted, that is, the study can be awarded one star for adjusting for age, two stars for also controlling for physical activity. The quality of the study was classified as poor (≤4 stars), fair (4–6 stars) and good (≥7 stars). We also extracted confounders adjusted by each study, and evaluated whether the study had adequate adjustment for potential confounders, that is, adjustment for at least five of seven confounders: age; diabetes, blood glucose; hypertension, blood pressure; age at menarche, menopausal status and age, parity; smoking; oral use of contraceptives, use of hormone replacement therapy; and physical activity.28
Statistical analysis
Given underlying methodological heterogeneity across studies including study design, participants’ characteristics and adjusted confounders, random effects models were applied to summarise domain-specific (occupational and leisure-time), and total RRs and their 95% CIs for the highest level versus the lowest level of sedentary behaviour, regardless of whether statistically significant heterogeneity was found. The highest and lowest values were defined by individual studies with different underlying definitions and different measurements of sedentary behaviour. Detailed definition and assessment of sedentary behaviour in individual study were summarised in online supplemental table S3. The natural logarithms of the study-specific RR and corresponding SEs were calculated using the inverse variance approach.29 Employing random effects models, the RR of each study was weighted using random effects weights and was further combined to obtain an overall estimate. When studies reported subgroup-specific results such as estimates of different calendar periods, we fitted a fixed effects model to combine the separate results to obtain the overall estimates for the main analysis.25 For studies not using the lowest category as the reference category of sedentary behaviour,9 10 17 30 31 we used the method by Hamling to recalculate the estimates through changing the lowest category as the reference category.32 We used the I2 statistics to test for heterogeneity between included studies. I2 values of more than 25%, 50% and 75% were deemed to indicate low, moderate and high level of heterogeneity, separately. Potential publication bias was assessed by inspection of funnel plots, and further evaluated using Egger’s regression test as well as Begg’s correlation test. Asymmetry in the funnel plots or p value<0.1 indicated publication bias.
Subgroup analyses were performed according to study design (cohort study, case–control study), study area (Asia, Europe and North America), sample size (≥ 5000 and <5000), number of cases (≥ 500 and<500), study quality (good, fair, poor) and adjustment for potential confounding factors (adequate, not adequate). In addition, sedentary behaviour, obesity and physical activity are lifestyle factors that are complexly associated and interacted. As obesity potentially mediates the association between sedentary behaviour and endometrial cancer risk, in which case the adjustment for BMI would over adjust the association, we conducted subgroup analyses stratified by whether BMI was adjusted.28 Similarly, we also conducted subgroup analyses by adjusting the physical activity.
Associations with total sedentary behaviour were reported in only two studies. Therefore, we also included all studies in the analysis to assess the effects of overall sedentary behaviour. If a study reported results at a specific domain, we extracted the results as the nearest estimate for overall sedentariness. If a study reported results at multiple domains, we used fixed effects models to combine the separate results to obtain the overall estimates as the total level. Random-effects meta-regression analyses were then conducted to explore whether the estimates differed by main characteristics of the included study. The analyses were unavailable for domain-specific sedentary behaviour analysis due to a limited number of studies (n≤10). The Tau2 was used to evaluate between-study variance of each covariate.
We also performed sensitivity analyses to test the robustness of the results in the main analysis. We first conducted analyses by omitting one study at each time to recalculate the pooled results to ensure the stability of the results. Second, we fitted the trim-and-fill analysis to inspect the impact of publication bias correction on the pooled outcomes. The statistical analyses were performed using Stata V.12.0 software (Stata Corp, College Station, Texas, USA). A two-tailed p value<0.05 was deemed statistically significant.
Patient and public involvement
This issue is not applicable to our research since the data collected in this study is secondary data without any personal information and not transferable.
Results
Studies retrieved and characteristics
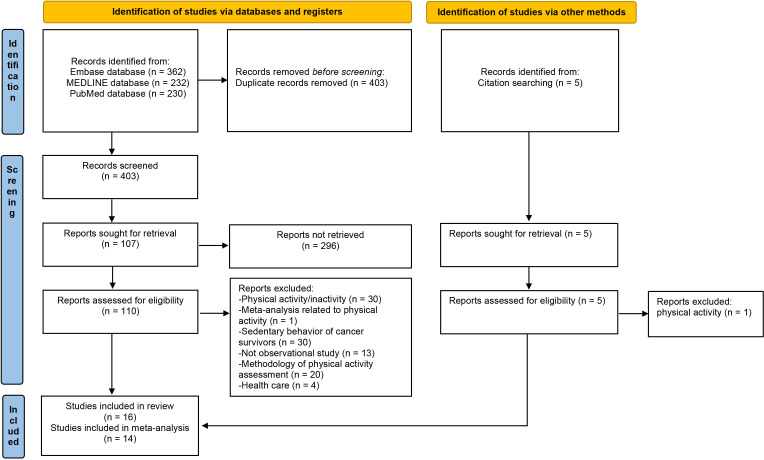
Our initial search identified 749 records. After screening and selection (figure 1), 16 studies were included in the systematic review of sedentary behaviour and risk of endometrial cancer. The main characteristics of the included studies are shown in table 1. Of these 16 studies, 6 were from Europe,9 17 24 25 31 33 5 from Asia10 16 34–36 and 5 from North America.18 19 30 37 38 All included studies assessed self-reported sedentary levels based on questionnaires, interviews or occupations (online supplemental table S4). Detailed data and characteristics of study participants, diagnostic criteria of the outcome and the assessment of sedentary behaviour are provided in online supplemental tables S3 and S4.
Figure 1.
Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) 2020 flow diagram of literature search and selection. From: Page et al.22 For more information, visit: http://www.prisma-statement.org/.
Table 1.
Study characteristics of the included studies in systematic review
| Publication | Study design | Study area | Enrolment/ study period | Age at baseline (years) | Subject (controls/ cases) | Sedentary behaviour | Results | NOS study quality |
| Dosemeci et al34 | Case–control study | Turkey | 1979–1984 | — | 275/31 | Occupational sedentary | OR (sedentary >6 hours/day) = 0.50 (0.10–4.40) | Fair (6) |
| Shu et al16 | Case–control study | China | 1988–1990 | 18–74 | 536/268 | Occupational sedentary | OR=1.20 (0.70–2.00) | Fair (5) |
| Zheng et al35* | Cross-sectional study | China | 1980–1984 | ≥30 | 452/452 | Occupational sedentary | SIR (long sitting time) = 110 | — |
| Olson et al30 | Case–control study | USA | 1986–1991 | 40–85 | 631/232 | Occupational sedentary | OR=0.93 (0.55–1.56) | Good (7) |
| Moradi et al24* | Cohort study | Sweden | 1960 | 16–95 | Sub-cohort A (1960): 704 904/4462 | Occupational sedentary | RR (1960) = 1.13 (0.99–1.29) | — |
| 1970 | Sub-cohort B (1970): 982 270/5287 | RR (1970) = 1.32 (1.17–1.50) | ||||||
| 1960 and 1970 | Sub-cohort C (1960 and 1970): 253 336/1949 | RR (1960 and 1970) = 1.30 (1.03–1.65) | ||||||
| Moradi et al25 | Case–control study | Sweden | 1994–1995 | 50–74 | 3368/709 | Occupational sedentary | OR (1960) = 1.30 (0.80–2.20) | Good (7) |
| OR (1970) = 1.20 (0.80–1.90) | ||||||||
| OR (1980) = 1.40 (1.00–1.90) | ||||||||
| OR (1990) = 1.30 (0.90–1.90) | ||||||||
| Weiderpass et al33 | Cohort study | Finland | 1970 | 25–64 | 413 877/2833 | Occupational sedentary | RR (high level of sedentary work) = 1.30 (1.10–1.50) | Fair (5) |
| Furberg and Thune31 | Cohort study | Norway | 1974–1981 | 20–49 | 24 460/130 | Leisure-time sedentary | RR (grade1-sedentary activity) = 1.27 (0.69–2.32) | Good (9) |
| Occupational sedentary | RR (grade1-sedentary work) = 1.64 (0.95–2.84) | |||||||
| Matthews et al36 | Case–control study | China | 1997–2001 | 30–69 | 846/832 | Occupational sedentary | OR (sitting Q4) = 0.93 (0.67–1.30) | Fair (5) |
| Friberg et al17 | Cohort study | Sweden | 1997 | 50–83 | 33 723/199 | Occupational sedentary | RR (work/occupation activity, low, mostly sitting down and sitting down more than half of the time) = 1.03 (0.76–1.39); additional adjustment for BMI: RR=0.99 (0.73–1.34) | Good (8) |
| Leisure-time sedentary | RR (watching TV/sitting, high, ≥5 hours/day) = 1.80 (1.14–2.83); additional adjustment for BMI: RR=1.66 (1.05–2.61) | |||||||
| Patel et al18 | Cohort study | USA | 1992 | 50–74 | 42 672/466 | Leisure-time sedentary | RR (sitting ≥6 hours/day) = 1.40 (1.03–1.89); additional adjustment for BMI: RR=1.18 (0.87–1.59) | Good (7) |
| Gierach et al19 | Cohort study | USA | 1995–1996 | 50–71 | 70 351/650 | Leisure-time sedentary | RR (≥7 hours) = 1.66 (1.20–2.88); additional adjustment for BMI: RR=1.21 (0.87–1.67) | Good (7) |
| Total sedentary | RR (≥7 hours) = 1.56 (1.22–1.99); additional adjustment for BMI: RR=1.26 (0.99–1.62) | |||||||
| Friedenreich et al37 | Case–control study | Canada | 2002–2006 | 30–79 | 1032/542 | Occupational sedentary | OR (lifetime occupational sedentary activity, >16.94 hours/week/years) = 1.28 (0.89–1.83) | Fair (6) |
| Arem et al38 | Case–control study | USA | 2004–2008 | Cases: 61.1; controls: 62.1 | 662/667 | Total sedentary | OR (≥8 hours/day) =1.52 (1.07–2.16) | Fair (5) |
| Hunter et al9 | Cohort study | UK | 2006–2010 | 40–69 | 253 171/872 | Leisure-time sedentary | HR (daily TV viewing time, >5 hours) = 0.59 (0.40–0.88) | Fair (6) |
| HR (daily computer use time, >3 hours) = 0.82 (0.55–1.22) | ||||||||
| HR (daily total screen time, >8 hours) = 0.57 (0.31–1.03) | ||||||||
| Miyata et al10 | Cohort study | Japan | 1988–1990 | 40–79 | 33 801/79 | Leisure-time sedentary | HR (TV viewing, ≥4 hours/day) = 2.10 (0.57–7.71) | Good (8) |
| Occupational sedentary | HR (occupational activity, mainly sitting) = 2.17 (1.04–4.56) |
*Not included in the meta-analysis and NOS study quality assessment; table values are mean (SD) for continuous variables.
BMI, body mass index; NOS, Newcastle-Ottawa Scale; RR, relative risk; SIR, standardised incidence ratio; TV, television.
The meta-analysis included 14 studies after excluding 2 studies, in which one failed to provide 95% CI for risk estimates35 and the other one was based on less comprehensive data among overlapped study participants.24 In total, 882 686 participants from seven cohort studies and seven case–control studies were involved. In the meta-analysis, 2 studies (71 680 participants, table 1) investigated the association between total sedentary behaviour and risk of endometrial cancer, 10 studies (515 163 participants) investigated the association with the assessment of occupational sedentary behaviour and 6 studies (458 178 participants) with the assessment of leisure-time sedentariness. Three studies (91 984 participants) have adjusted for physical activity. Nine studies (321 757 participants) have adjusted for BMI in the multivariate model, and three studies (146 746 participants) took a separate step for additional BMI adjustment. Based on the Newcastle-Ottawa quality assessment scale,27 seven studies were evaluated as having fair quality, and seven as having good quality. Detailed information on the NOS quality assessment of meta-analysed studies is provided in online supplemental tables S5 and S6. Details of confounders adjusted by each study are presented in online supplemental table S7.
Occupational sedentary behaviour
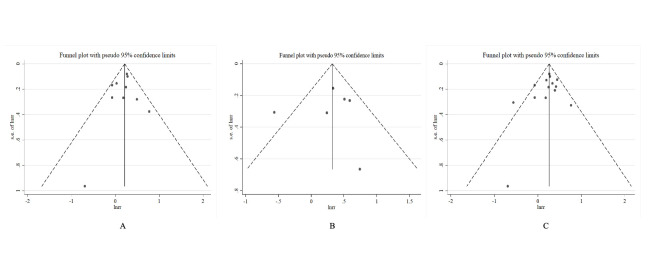
Twelve studies have investigated impacts of sedentary behaviour during work on endometrial cancer, and five of them reported statistically significant association between occupational sedentary behaviour and increased risk of endometrial cancer.10 24 25 33 35 However, seven studies did not observe a similar significant effect.16 17 30 31 34 36 37 Among these studies, the meta-analysis for occupational domain included 10 eligible studies, involving 515 163 participants and 5855 cases. The summary RR for high versus low occupational sedentary level was 1.22 (95% CI: 1.09 to 1.37, p<0.01; I2=13.4%, pHeterogeneity=0.30) (figure 2). Consistent with the inspection of the funnel plot, the results of Begg’s test (p=0.72) and Egger’s test (p=0.59) suggested no publication bias (figure 3A).
Figure 2.
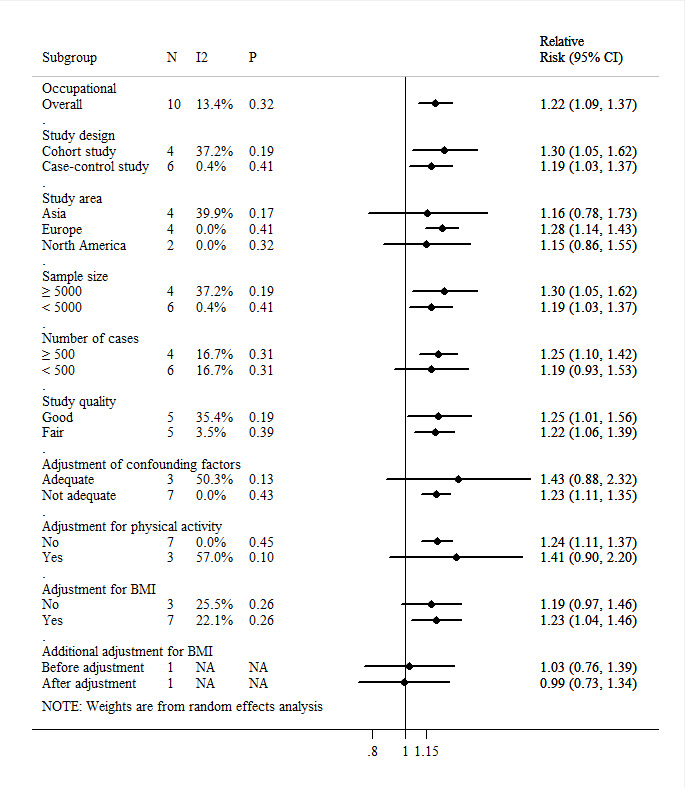
Pooled association between occupational sedentary behaviour and endometrial cancer. I2 for heterogeneity between studies; p value for heterogeneity in subgroups; NA: not applicable. Adequate adjustment denotes adjustment for at least five of seven confounders: age; diabetes, blood glucose; hypertension, blood pressure; age at menarche, menopausal status and age, parity; smoking; oral use of contraceptives, use of hormone replacement therapy; and physical activity. Adjustment for BMI denotes adjustment for BMI in the multivariate model. BMI, body mass index.
Figure 3.
Pooled association between leisure-time sedentary behaviour and endometrial cancer. I2 for heterogeneity between studies; p value for heterogeneity in subgroups; NA: not applicable. Adequate adjustment denotes adjustment for at least five of seven confounders: age; diabetes, blood glucose; hypertension, blood pressure; age at menarche, menopausal status and age, parity; smoking; oral use of contraceptives, use of hormone replacement therapy; and physical activity. Adjustment for BMI denotes adjustment for BMI in the multivariate model. BMI, body mass index.
The adverse effects of occupational sedentary behaviour on endometrial cancer incidence persisted in nearly all subgroup analyses stratified by study design, study area, number of participants and cases, study quality, adjustment for confounders including BMI and physical activity (figure 2). The association between occupational sedentary behaviour and endometrial cancer was stronger among studies that were cohort study (RRSummary=1.30, 95% CI: 1.05 to 1.62, p=0.02; I2=37.2%, pHeterogeneity=0.19), studies conducted in European areas (RRSummary=1.28, 95% CI: 1.14 to 1.43, p<0.01; I2=0.0%, pHeterogeneity=0.41), studies with large number of participants (≥5000; RRSummary=1.30, 95% CI: 1.05 to 1.62, p=0.02; I2=37.2%, pHeterogeneity=0.19) or cases (≥500; RRSummary=1.25, 95% CI: 1.10 to 1.42, p<0.01; I2=16.7%, pHeterogeneity=0.31), and studies with good quality (RRSummary=1.25, 95% CI: 1.01 to 1.56, p=0.04; I2=35.4%, pHeterogeneity=0.19). There was moderate heterogeneity in the studies with adequate adjustment and with physical activity adjustment (adequate adjustment for confounders: I2=50.3%, pHeterogeneity=0.13; adjustment for physical activity: I2=57.0%, pHeterogeneity=0.10). Compared with studies without adequate adjustment or physical activity adjustment, the associations observed in these two groups were slightly attenuated, showing greater estimates and wider CIs. There was only one study adjusting for BMI separately,17 and no statistically significant risk estimates were exhibited before and after adjustment (before adjustment: RR=1.03, 95% CI: 0.76 to 1.39; after adjustment: RR=0.99, 95% CI: 0.73 to 1.34).
The sensitivity analyses suggested that the association between occupational sedentary behaviour and endometrial cancer risk did not change when recalculating the pooled estimates by omitting one study at a time (online supplemental table S8). After excluding the most influential research, the summarised RR ranged from 1.19 (95% CI: 1.04 to 1.37) when excluding the study conducted by Moradi et al to 1.27 (95% CI: 1.15 to 1.40) when excluding the study by Matthews et al.25 36
Leisure-time sedentary behaviour
Six prospective cohort studies (458 178 participants, 2396 cases) have assessed the relationship between endometrial cancer and time spent sitting outside of work, including watching television (TV), videos or computer, reading and other sedentary activities. Three of these studies found statistically significant associations between leisure-time sedentary behaviour and risk of endometrial cancer,17–19 and the rest indicated non-statistically significant associations.9 10 31 The pooled RR for high versus low level of leisure-time sedentary behaviour was 1.34 (95% CI: 0.98 to 1.83, p=0.07; I2=53.7%, pHeterogeneity=0.06), with moderate and non-statistically significant heterogeneity (figure 4). However, these results seemed to be driven by a large study (253 171 participants, 872 cases) that reported inconsistent results with other studies (RR=0.57, 95% CI: 0.31 to 1.03).9 After excluding this study, no potential heterogeneity remained in the analysis, and the summarised association between leisure-time sedentary behaviour and endometrial cancer turned out to be statistically significant (RRSummary=1.53, 95% CI: 1.24 to 1.87, p<0.01; I2=0.0%, pHeterogeneity=0.82). No evidence of publication bias was revealed according to visual inspection of the funnel plot, Begg’s test (p=0.85) or Egger’s test (p=0.78) (figure 3B).
Figure 4.
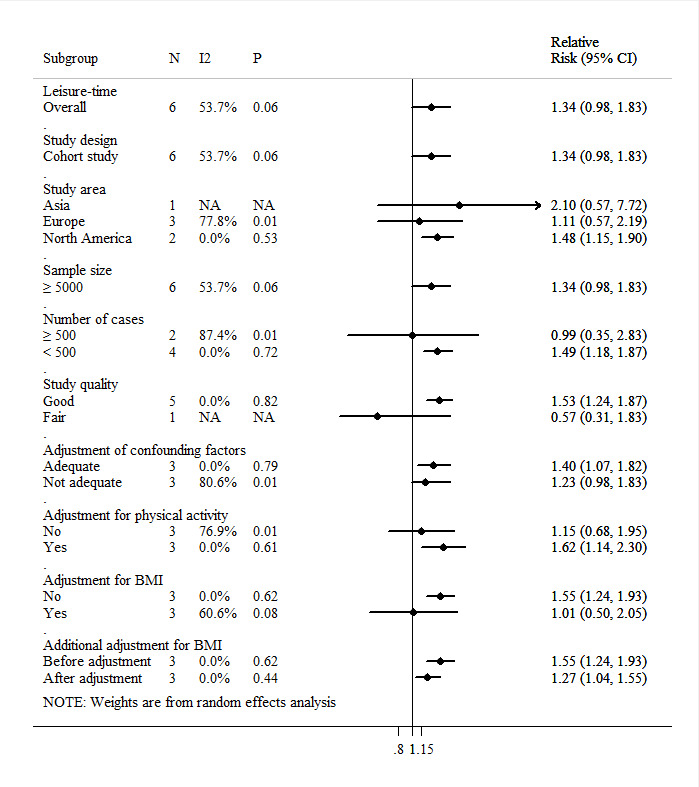
Pooled association between total sedentary behaviour and endometrial cancer. I2 for heterogeneity between studies; p value for heterogeneity in subgroups; NA: not applicable. Adequate adjustment denotes adjustment for at least five of seven confounders: age; diabetes, blood glucose; hypertension, blood pressure; age at menarche, menopausal status and age, parity; smoking; oral use of contraceptives, use of hormone replacement therapy; and physical activity. Adjustment for BMI denotes adjustment for BMI in the multivariate model. BMI, body mass index.
In subgroup analyses, the significance of the associations across the stratified groups also appeared to be driven by the study reported by Hunter et al. Statistically significant positive associations were observed among studies in North America (RRSummary=1.48, 95% CI: 1.15 to 1.90, p<0.01; I2=0.0%, pHeterogeneity=0.53), studies with good quality (RRSummary=1.53, 95% CI: 1.24 to 1.87, p<0.01; I2=0.0%, pHeterogeneity=0.82), studies with small number of cases (RRSummary=1.49, 95% CI: 1.18 to 1.87, p<0.01; I2=0.0%, pHeterogeneity=0.72), studies without adjustment for BMI (RRSummary=1.55, 95% CI: 1.24 to 1.93, p<0.01; I2=0.0%, pHeterogeneity=0.62) and studies adjusted for physical activity (RRSummary=1.62, 95% CI: 1.14 to 2.30, p=0.01; I2=0.0%, pHeterogeneity=0.61). In three studies with additional adjustment for BMI, despite a decreased effect size, the association remained significant after adjusting for BMI (before adjustment: RRSummary=1.55, 95% CI: 1.24 to 1.93, p<0.01; I2=0.0%, pHeterogeneity=0.62, vs, after adjustment: RRSummary=1.27, 95% CI: 1.04 to 1.55, p=0.02; I2=0.0%, pHeterogeneity=0.44).
In sensitivity analyses, after excluding the most influential research, the summary RRs ranged from 1.24 (95% CI: 0.86 to 1.79) when excluding the study conducted by Friberg et al to 1.53 (95% CI: 1.24 to 1.87) after excluding the study by Hunter et al9 17 (online supplemental table S8).
Total sedentary behaviour
Two studies from the USA, one large cohort study,19 and one case–control study,38 including 71 680 participants and 1317 cases in total, have investigated the effect of total sedentary behaviour (evaluated as total time spent sitting during a 24-hour day) on endometrial cancer risk, and both proved significantly adverse effect. The pooled RR for high versus low analysis of total sedentary behaviour and endometrial cancer risk was 1.55 (95% CI: 1.27 to 1.89, p<0.01; I2=0.0%, pHeterogeneity=0.91) (figure 5). After combining all included studies as evaluating overall sedentary behaviour, the pooled RR for high versus low analysis was 1.28 (95% CI: 1.14 to 1.43, p<0.01; I2=34.8%, pHeterogeneity=0.10) (figure 5). No evidence of publication bias was indicated through visual inspection of the funnel plot (figure 3C), which was supported by Begg’s test (p=0.38), and Egger’s test (p=0.29).
Figure 5.
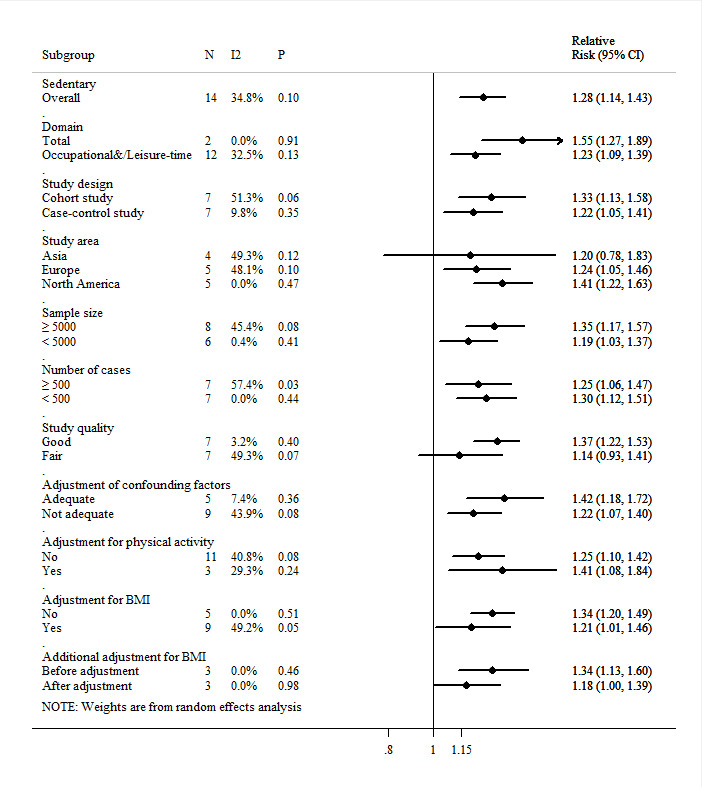
Funnel plot of overall sedentary behaviour and endometrial cancer. BMI, body mass index.
The meta-regression analyses showed that all prespecified study characteristics explained little of the heterogeneity for overall sedentary behaviour (online supplemental table S9). There was weak evidence that associations were stronger for cohort studies, studies conducted in North America, studies with large sample size (n≥5000), good quality and adequate adjustment of confounding factors as well as adjustment for physical activity (figure 5).
Discussion
In this systematic review and comprehensive meta-analysis, 55% increased risk of endometrial cancer was observed among individuals with higher levels of total sedentary behaviour (RR=1.55, 95% CI: 1.27 to 1.89), 22% among those with higher levels of occupational sedentary behaviour (RR=1.22, 95% CI: 1.09 to 1.37) and 34% with borderline significancy among those with higher levels of leisure-time sedentary behaviour (RR=1.34, 95% CI: 0.98 to 1.83). The overall increased risk disregarding specific domains was 27% (RR=1.28, 95% CI: 1.14 to 1.43). The pooled associations were consistent within subgroups stratified according to study design, sample size and adjustment strategy for physical activity and BMI.
The present results added to the existing evidence by showing a possible domain-specific association between sedentary behaviour and endometrial cancer, particularly for total and occupational domain. Subgroup analyses were generally supportive of the overall estimates. Our results are partially in line with three previous meta-analyses that focused on the effect of sedentary behaviour on all-site cancers.6–8 Including eight studies, Schmid et al6 reported a 36% increased risk of endometrial cancer among participants with higher levels of overall sedentary behaviour. However, this research did not find a statistically significant association for occupational domain, which could be attributed to the limited number of studies included (n=4) and their heterogeneous quality. Including three prospective studies, Shen et al7 reported a 66% increased risk of endometrial cancer for the defined sedentary behaviour that was assessed by total sitting and TV viewing time. With limited number of studies included, this research did not discuss potential heterogeneity of the studies. The most recent meta-analysis was an umbrella review based on 11 case–control and cohort studies, their results showed that higher overall sedentary behaviour was associated with a 29% higher risk of endometrial cancer.8 Moreover, differences in geographical region and study design were found to have larger impacts on the results. However, caution is needed when interpreting their findings, as the investigated outcomes were more than one specific site, and the authors called into attention the importance of adjusting for obesity in the context, which could be misleading given its mediating role. Our research found that the positive associations between sedentariness and endometrial cancer were more pronounced in studies with high quality, prospective design and large sample size. These studies were more prone to reveal the true association between sedentary behaviour and endometrial cancer by reducing the possibility of misclassification and selection, recall and confounding bias.
While we found a statistically significant increased risk of endometrial cancer related to higher levels of occupational sedentary behaviour, the results related to leisure-time sedentary behaviour was borderline significant. Possible explanations for domain-specific differences may be attributed to changes of sedentary behaviour over time, susceptible population and exposure window across the life span.12 Compared with leisure-time sedentary behaviour, occupational sedentary behaviour is more frequently and closely associated with stable biological accumulation of early-onset and long-term exposure of prolonged, uninterrupted sitting.39 Moreover, leisure-time sedentary behaviour interacts in a complex way with other lifestyle factors, such as diet, physical activity and obesity in association with health outcomes.13 Failure to account for these factors in research is likely to yield biased results. Besides, the domain-specific differences may be explained, at least partly, by the small number as well as heterogeneity of studies within leisure-time domain, in which the pooled estimates were dominated by a study with a large sample size showing contrasting findings.9 Further longitudinal studies incorporating the measures of different domains are needed to better clarify the domain-specific association and the difference across domains.
Subgroup-analyses suggested greater effect size in studies with adjustment for physical activity. Emerging evidence has shown that the sedentary behaviour is distinct from lack of physical activity because of its unique postural and intervenable health hazards effects that cannot be offset by physical activity.40 Without proper adjustment for physical activity, the real correlation between sedentary behaviour and endometrial cancer could be attenuated due to the role of physical activity in reducing cancer risk by healthy body weight maintenance and obesity prevention.12 41 However, most included studies in the analysis did not adjust for physical activity. Our findings highlight the importance of considering the interactive effects of sedentary behaviour and other lifestyle factors may have on endometrial cancer in future studies. Novel analytical methods, such as marginal structural models with time-varying exposure assessment, may be particularly important in evaluating the interactive effects of sedentary behaviour, physical activity and obesity in association with endometrial cancer, as well as identifying critical exposure windows.42–44
It is widely hypothesised that sedentary behaviour may increase the risk of cancers due to low energy expenditure and by inducing obesity, a well-understood risk factor for endometrial cancer.45 Under this circumstance, adjusting for obesity indices (mostly BMI) may lead to overadjustment of the association and produce a less pronounced risk estimate. Realising this issue, three studies included in the meta-analysis have reported respective results with and without adjustment for BMI.17–19 The pooled estimates of these studies showed that the association between sedentary behaviour and endometrial cancer attenuated but remained statistically significant after adjusting for BMI, suggesting that other mechanisms distinct from obesity-related pathways likely exist.
The biological mechanisms by which sedentary behaviour increases endometrial cancer risk remains unclear. Several pathways related to metabolic abnormalities and insulin sensitivity, chronic systemic inflammation and endogenous sex hormones are suggested as the main hypothesis linking physical activity, sedentary behaviour and obesity to cancer incidence.41 45 46 Besides, long-time sitting posture might also contribute through its adverse effect on mitochondrial and endothelial function.41 Given the complex mechanisms, further analysis may help better understand the potential mechanisms through rating evidence separately among different study populations, particularly in non-obese and obese, pre-menopausal and post-menopausal women, populations with different intensity of physical activity and for different histological subtypes.42
Strengths of this systematic review and meta-analysis include strictly following the uniform criteria for study selection, quality evaluation and reporting. Also, our meta-analysis included substantial numbers of participants and cancer cases, ensuring sufficient statistical power to yield precise associations. Furthermore, our meta-analysis revealed some novel insights not previously investigated, such as varied effects of sedentary behaviour on endometrial cancer across different domains. This is also the first study taking the complex interaction between obesity, physical activity and sedentary behaviour into account in the association. Additional merits include the robustness of the pooled associations in multiple subgroups and sensitivity analyses within different sedentary behaviour domains.
There are some limitations in our review at the level of the meta-analysis and at the level of included studies that need to be noticed. At the review level, we observed evidence of heterogeneity between subgroups especially within the leisure-time domain. However, this seems to be mainly driven by one large-sampled study with contradicting conclusion. After excluding the study, no more indication of heterogeneity was shown. Also, the pooled associations showed little evidence of heterogeneity across different domains of sedentary behaviour and endometrial cancer. Second, the small number of studies included in our meta-analysis could lower the statistical power and limit the ability to examine the existence of small study effects and excess significance bias. For total domain of sedentary behaviour, only two studies estimated the association with endometrial cancer. In such case, the reliability of the pooling may be influenced, and the results should be interpreted with caution.47 Third, it should be emphasised that there could be wide interindividual variation in level of sedentary behaviour, with all studies assessing self-reported levels of sedentariness based on questionnaires, interviews or job titles, and neither of these studies applied repeated measures or corrected for measurement errors. Most included studies compared high versus low level of sedentary behaviour and thus, the effect estimate may be inflated compared with a linear analysis. Moreover, definitions of high versus low levels of sedentary behaviour varied greatly in the included studies. For example, the highest level of sedentary behaviour in some studies may vary from more than 3–8 hours/day,9 38 which may decrease the comparability among studies. There is therefore an urgent need for the combination of self-report assessment, objective quantitative monitors in further prospective cohort studies, to study these associations and improve understanding of benefits brought by reductions in sedentary time. Caution is warranted in interpreting our findings, as despite the association between sedentary behaviour and increased endometrial cancer risk, the relatively low cancer incidence means that higher relative risks observed may only lead to slight increases in absolute risk.
Conclusion
Despite the little evidence on domain-specific effect of sedentary behaviour on endometrial cancer, we found, in general, higher levels of total and occupational sedentary behaviour increase the risk of endometrial cancer. The association between leisure-time sedentary behaviour and endometrial cancer is borderline significant. The pooling may be influenced by limited studies and variations in assessment of sedentary behaviour and should be interpreted with caution. Future longitudinal studies employing objective physical activity monitors may help to clarify the quantitative association between total and domain-specific sedentary behaviour and endometrial cancer. The interactive effects of physical activity, obesity and sedentary behaviour on endometrial cancer warrant further investigation as well.
Supplementary Material
Footnotes
Contributors: The literature reviews were conducted by LY and JN. LY drafted the manuscript based on discussion involving all the authors, and contributed to the integrity of the data and statistical analysis. ZL and XW contributed to study concept and design, and critical revision of the manuscript and administrative support. WL and QY supervised the study, critically reviewed the draft and approved the final version before submission. ZL is responsible for the overall content as the guarantor.
Funding: This study is funded by the National Natural Science Foundation of China (grant number 81972438 to XW), two grants from the Shanghai Hospital Development Center (grant number SHDC12020107 to XW and SHDC2020CR5003 to XW). The funding bodies have not participated in the design of the study and collection, analysis, interpretation of data or in writing the manuscript.
Competing interests: None declared.
Patient and public involvement: Patients and/or the public were not involved in the design, or conduct, or reporting, or dissemination plans of this research.
Provenance and peer review: Not commissioned; externally peer reviewed.
Supplemental material: This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.
Data availability statement
All data relevant to the study are included in the article or uploaded as supplementary information.
Ethics statements
Patient consent for publication
Not applicable.
Ethics approval
Not applicable.
References
- 1.Sung H, Ferlay J, Siegel RL, et al. Global cancer Statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 2021;71:209–49. 10.3322/caac.21660 [DOI] [PubMed] [Google Scholar]
- 2.Lortet-Tieulent J, Ferlay J, Bray F, et al. International patterns and trends in endometrial cancer incidence, 1978–2013. JNCI J Natl Cancer Inst 2018;110:354–61. 10.1093/jnci/djx214 [DOI] [PubMed] [Google Scholar]
- 3.Katzmarzyk PT, Friedenreich C, Shiroma EJ, et al. Physical inactivity and non-Communicable disease burden in low-income, middle-income and high-income countries. Br J Sports Med 2022;56:101–6. 10.1136/bjsports-2020-103640 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yasin HK, Taylor AH, Ayakannu T. A narrative review of the role of diet and lifestyle factors in the development and prevention of endometrial cancer. Cancers (Basel) 2021;13:2149. 10.3390/cancers13092149 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.World Health Organization . WHO guidelines on physical activity and sedentary behaviour, 2020. [PubMed] [Google Scholar]
- 6.Schmid D, Leitzmann MF. Television viewing and time spent sedentary in relation to cancer risk: a meta-analysis. JNCI 2014;106. 10.1093/jnci/dju098 [DOI] [PubMed] [Google Scholar]
- 7.Shen D, Mao W, Liu T, et al. Sedentary behavior and incident cancer: A meta-analysis of prospective studies. PLoS ONE 2014;9:e105709. 10.1371/journal.pone.0105709 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hermelink R, Leitzmann MF, Markozannes G, et al. Sedentary behavior and cancer–an umbrella review and meta-analysis. Eur J Epidemiol 2022;37:447–60. 10.1007/s10654-022-00873-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hunter RF, Murray JM, Coleman HG. The association between recreational screen time and cancer risk: Findings from the UK Biobank, a large prospective cohort study. Int J Behav Nutr Phys Act 2020;17:97. 10.1186/s12966-020-00997-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Miyata H, Shirai K, Muraki I, et al. Associations of body mass index, weight change, physical activity, and sedentary behavior with endometrial cancer risk among Japanese women: The Japan collaborative cohort study. J Epidemiol 2021;31:621–7. 10.2188/jea.JE20200145 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Moore SC, Gierach GL, Schatzkin A, et al. Physical activity, sedentary Behaviours, and the prevention of endometrial cancer. Br J Cancer 2010;103:933–8. 10.1038/sj.bjc.6605902 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mahmood S, MacInnis RJ, English DR, et al. Domain-specific physical activity and sedentary behaviour in relation to colon and Rectal cancer risk: A systematic review and meta-analysis. Int J Epidemiol 2017;46:1797–813. 10.1093/ije/dyx137 [DOI] [PubMed] [Google Scholar]
- 13.Hobbs M, Pearson N, Foster PJ, et al. Sedentary behaviour and diet across the LifeSpan: An updated systematic review. Br J Sports Med 2015;49:1179–88. 10.1136/bjsports-2014-093754 [DOI] [PubMed] [Google Scholar]
- 14.Jochem C, Wallmann-Sperlich B, Leitzmann MF. The influence of sedentary behavior on cancer risk: Epidemiologic evidence and potential molecular mechanisms. Curr Nutr Rep 2019;8:167–74. 10.1007/s13668-019-0263-4 [DOI] [PubMed] [Google Scholar]
- 15.Lauby-Secretan B, Scoccianti C, Loomis D, et al. Body Fatness and cancer — viewpoint of the IARC working group. N Engl J Med 2016;375:794–8. 10.1056/NEJMsr1606602 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Shu XO, Hatch MC, Zheng W, et al. Physical activity and risk of endometrial cancer. Epidemiology 1993;4:342–9. 10.1097/00001648-199307000-00010 [DOI] [PubMed] [Google Scholar]
- 17.Friberg E, Mantzoros CS, Wolk A. Physical activity and risk of endometrial cancer: A population-based prospective cohort study. Cancer Epidemiol Biomarkers Prev 2006;15:2136–40. 10.1158/1055-9965.EPI-06-0465 [DOI] [PubMed] [Google Scholar]
- 18.Patel AV, Feigelson HS, Talbot JT, et al. The role of body weight in the relationship between physical activity and endometrial cancer: Results from a large cohort of US women. Int J Cancer 2008;123:1877–82. 10.1002/ijc.23716 [DOI] [PubMed] [Google Scholar]
- 19.Gierach GL, Chang S-C, Brinton LA, et al. Physical activity, sedentary behavior, and endometrial cancer risk in the NIH-AARP diet and health study. Int J Cancer 2009;124:2139–47. 10.1002/ijc.24059 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Saint-Maurice PF, Sampson JN, Michels KA, et al. Physical activity from adolescence through Midlife and associations with body mass index and endometrial cancer risk. JNCI Cancer Spectr 2021;5:pkab065. 10.1093/jncics/pkab065 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kitson SJ, Aurangzeb O, Parvaiz J, et al. Quantifying the effect of physical activity on endometrial cancer risk. Cancer Prevention Research 2022;15:605–21. 10.1158/1940-6207.CAPR-22-0129 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Page MJ, McKenzie JE, Bossuyt PM, et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021;372:n71. 10.1136/bmj.n71 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Stroup DF. Meta-analysis of observational studies in Epidemiologya proposal for reporting. JAMA 2000;283:2008. 10.1001/jama.283.15.2008 [DOI] [PubMed] [Google Scholar]
- 24.Moradi T, Nyrén O, Bergström R, et al. Risk for endometrial cancer in relation to occupational physical activity: A nationwide cohort study in Sweden. Int J Cancer 1998;76:665–70. [DOI] [PubMed] [Google Scholar]
- 25.Moradi T, Weiderpass E, Signorello LB, et al. Physical activity and postmenopausal endometrial cancer risk (Sweden). Cancer Causes Control 2000;11:829–37. 10.1023/A:1008919717930 [DOI] [PubMed] [Google Scholar]
- 26.Stang A. Critical evaluation of the Newcastle-Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur J Epidemiol 2010;25:603–5. 10.1007/s10654-010-9491-z [DOI] [PubMed] [Google Scholar]
- 27.Wells GA, Shea B, O’Connell D, et al. The Newcastle-Ottawa scale (NOS) for assessing the quality of Nonrandomised studies in meta-analyses. n.d. Available: https://www.ohri.ca/programs/clinical_epidemiology/oxford.asp
- 28.Cai X, Zhang Y, Li M, et al. Association between prediabetes and risk of all cause mortality and cardiovascular disease: updated meta-analysis. BMJ 2020;370:m2297. 10.1136/bmj.m2297 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Higgins JPT, Green S, eds. Cochrane handbook for systematic reviews of interventions version 5.1.0. The Cochrane Collaboration, 2011. Available: https://training.cochrane.org/handbook [Google Scholar]
- 30.Olson SH, Vena JE, Dorn JP, et al. Exercise, occupational activity, and risk of endometrial cancer. Ann Epidemiol 1997;7:46–53. 10.1016/s1047-2797(96)00071-3 [DOI] [PubMed] [Google Scholar]
- 31.Furberg AS, Thune I. Metabolic abnormalities (hypertension, hyperglycemia and overweight), Lifestyle (high energy intake and physical inactivity) and endometrial cancer risk in a Norwegian cohort. Int J Cancer 2003;104:669–76. 10.1002/ijc.10974 [DOI] [PubMed] [Google Scholar]
- 32.Hamling J, Lee P, Weitkunat R, et al. Facilitating meta-analyses by deriving relative effect and precision estimates for alternative comparisons from a set of estimates presented by exposure level or disease category. Stat Med 2008;27:954–70. 10.1002/sim.3013 [DOI] [PubMed] [Google Scholar]
- 33.Weiderpass E, Pukkala E, Vasama-Neuvonen K, et al. Occupational exposures and cancers of the Endometrium and Cervix uteri in Finland. Am J Ind Med 2001;39:572–80. 10.1002/ajim.1056 [DOI] [PubMed] [Google Scholar]
- 34.Dosemeci M, Hayes RB, Vetter R, et al. Occupational physical activity, socioeconomic status, and risks of 15 cancer sites in Turkey. Cancer Causes Control 1993;4:313–21. 10.1007/BF00051333 [DOI] [PubMed] [Google Scholar]
- 35.Zheng W, Shu XO, McLaughlin JK, et al. Occupational physical activity and the incidence of cancer of the breast, corpus uteri, and ovary in Shanghai. Cancer 1993;71:3620–4. [DOI] [PubMed] [Google Scholar]
- 36.Matthews CE, Xu WH, Zheng W, et al. Physical activity and risk of endometrial cancer: A report from the Shanghai endometrial cancer study. Cancer Epidemiol Biomarkers Prev 2005;14:779–85. 10.1158/1055-9965.EPI-04-0665 [DOI] [PubMed] [Google Scholar]
- 37.Friedenreich CM, Cook LS, Magliocco AM, et al. Case–control study of lifetime total physical activity and endometrial cancer risk. Cancer Causes Control 2010;21:1105–16. 10.1007/s10552-010-9538-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Arem H, Irwin ML, Zhou Y, et al. Physical activity and endometrial cancer in a population-based case–control study. Cancer Causes Control 2011;22:219–26. 10.1007/s10552-010-9689-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Gilchrist SC, Howard VJ, Akinyemiju T, et al. Association of sedentary behavior with cancer mortality in middle-aged and older US adults. JAMA Oncol 2020;6:1210–7. 10.1001/jamaoncol.2020.2045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lavie CJ, Ozemek C, Carbone S, et al. Sedentary behavior, exercise, and cardiovascular health. Circ Res 2019;124:799–815. 10.1161/CIRCRESAHA.118.312669 [DOI] [PubMed] [Google Scholar]
- 41.Kerr J, Anderson C, Lippman SM. Physical activity, sedentary behaviour, diet, and cancer: An update and emerging new evidence. Lancet Oncol 2017;18:e457–71. 10.1016/S1470-2045(17)30411-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kalliala I, Markozannes G, Gunter MJ, et al. Obesity and gynaecological and obstetric conditions: Umbrella review of the literature. BMJ 2017;359:j4511. 10.1136/bmj.j4511 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Bodnar LM, Davidian M, Siega-Riz AM, et al. Marginal structural models for analyzing causal effects of time-dependent treatments: An application in perinatal epidemiology. Am J Epidemiol 2004;159:926–34. 10.1093/aje/kwh131 [DOI] [PubMed] [Google Scholar]
- 44.Mansournia MA, Etminan M, Danaei G, et al. Handling time varying confounding in observational research. BMJ 2017;359:j4587. 10.1136/bmj.j4587 [DOI] [PubMed] [Google Scholar]
- 45.Wiseman AJ, Lynch BM, Cameron AJ, et al. Associations of change in television viewing time with biomarkers of postmenopausal breast cancer risk: The Australian diabetes, obesity and lifestyle study. Cancer Causes Control 2014;25:1309–19. 10.1007/s10552-014-0433-z [DOI] [PubMed] [Google Scholar]
- 46.Friedenreich CM, Ryder-Burbidge C, McNeil J. Physical activity, obesity and sedentary behavior in cancer etiology: epidemiologic evidence and biologic mechanisms. Mol Oncol 2021;15:790–800. 10.1002/1878-0261.12772 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Borenstein M, Hedges LV, Higgins JPT, et al. Introduction to meta‐analysis, 1st edn. Wiley, 2009. 10.1002/9780470743386 [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
bmjopen-2022-069042supp001.pdf (152.9KB, pdf)
bmjopen-2022-069042supp002.pdf (48.5KB, pdf)
bmjopen-2022-069042supp003.pdf (65.5KB, pdf)
Data Availability Statement
All data relevant to the study are included in the article or uploaded as supplementary information.