Abstract
Background
The COVID-19 pandemic required science to provide answers rapidly to combat the outbreak. Hence, the reproducibility and quality of conducting research may have been threatened, particularly regarding privacy and data protection, in varying ways around the globe. The objective was to investigate aspects of reporting informed consent and data handling as proxies for study quality conduct.
Methods
A systematic scoping review was performed by searching PubMed and Embase. The search was performed on November 8th, 2020. Studies with hospitalised patients diagnosed with COVID-19 over 18 years old were eligible for inclusion. With a focus on informed consent, data were extracted on the study design, prestudy protocol registration, ethical approval, data anonymisation, data sharing and data transfer as proxies for study quality. For reasons of comparison, data regarding country income level, study location and journal impact factor were also collected.
Results
972 studies were included. 21.3% of studies reported informed consent, 42.6% reported waivers of consent, 31.4% did not report consent information and 4.7% mentioned other types of consent. Informed consent reporting was highest in clinical trials (94.6%) and lowest in retrospective cohort studies (15.0%). The reporting of consent versus no consent did not differ significantly by journal impact factor (p=0.159). 16.8% of studies reported a prestudy protocol registration or design. Ethical approval was described in 90.9% of studies. Information on anonymisation was provided in 17.0% of studies. In 257 multicentre studies, 1.2% reported on data sharing agreements, and none reported on Findable, Accessible, Interoperable and Reusable data principles. 1.2% reported on open data. Consent was most often reported in the Middle East (42.4%) and least often in North America (4.7%). Only one report originated from a low-income country.
Discussion
Informed consent and aspects of data handling and sharing were under-reported in publications concerning COVID-19 and differed between countries, which strains study quality conduct when in dire need of answers.
Keywords: COVID-19, Review, Study design
WHAT IS ALREADY KNOWN ON THIS TOPIC
The quality of COVID-19 studies has been negatively influenced by fast tracking publications and the use of non-peer-review platforms.
WHAT THIS STUDY ADDS
Informed consent and aspects of data handling for privacy, as proxies for study quality conduct, were structurally under-reported in publications concerning COVID-19.
Publications from lower-income countries were sparse, showing research equity issues between high-income and low-income countries, which could potentially create blind spots in evidence.
HOW THIS STUDY MIGHT AFFECT RESEARCH, PRACTICE OR POLICY
Transparency in reporting on informed consent and other aspects of data handling should markedly improve.
We recommend the development of a framework to advise concerns of informed consent and other ethics regulations during times of crisis and in situations with limited resources.
International and intercontinental inequalities in resources should be considered, with academic journals setting the standard to improve the reporting of study quality conduct, while taking inequalities regarding resources into account to avoid selective publication of data from high-income countries.
Introduction
The unknown nature of COVID-19 unleashed an enormous drive for research. Based on the search term ‘COVID-19’ alone, the number of 283 PubMed citations in 2019 increased to 91 634 in 2020 and 208 994 in 2021. In these publications, patient data were investigated and shared to increase the understanding of the disease to support physicians globally.1 2 However, fast-track publications and publications by non-peer-review platforms of patient data were often used and have negatively influenced study quality for COVID-19.2 3 As a result, a high risk of bias was, for example, reported in diagnostic and prognostic prediction models and other observational studies.4–6 Furthermore, inferior intervention study designs were used, and retraction of randomised controlled trial study results for COVID-19 occurred.5 7
Current reporting and research quality guidelines nevertheless aim for study quality conduct improvement, reproducibility transparency with a focus on informed consent, and guaranteeing appropriate and effective data sharing.8–12 However, it is unclear how informed consent, and aspects of data handling for privacy, as proxies for study quality conduct, were reported in publications concerning COVID-19.13
For research in general, legal rules and regulations surrounding ethics apply to guide responsible conduct in a way that contributes to research quality.14 Although legal rules might differ between regions of the world, the scientific community embraces general ethical regulations globally.13 15–17 One main facet of responsible and ethical research conduct is asking for consent. According to General Data Protection Regulation (GDPR) and Health Insurance Portability and Accountability Act (HIPAA), consent must be specific, unambiguous, freely given, and most importantly, informed, that is, the patients know what data are being processed, and the purpose of the data processing, and each patient may withdraw their consent at any time.16 However, several aspects, such as the level of comprehension or the person’s capacity to consent, varying study design requirements (ie, more obligatory reporting in intervention than observational studies), as well as health inequalities between low-income and high-income countries, for example, could influence the process of obtaining consent.18 19 Another facet of good research conduct is the application of Open Science, which aims to increase responsible (re)use of data for research. The key to achieving this is through the application of Findable, Accessible, Interoperable and Reusable (FAIR) data and applying Open Science to share open data.20 21 However, legal and ethical challenges relating to these principles can likewise be identified.22–25 Moreover, facets of informed consent and responsible data use are potentially compromised during a more urgent need for clinical answers, as was the case during the COVID-19 pandemic.18 24 26 Informed consent procedures and adherence to data-sharing principles are nevertheless a prerequisite to assure high-quality and responsible conduct of clinical studies, especially in challenging eras such as the COVID-19 pandemic.27 Although there have been publications in the past that evaluated aspects such as informed consent reporting and reporting of review board approval,27–30 it is currently unknown how these aspects were handled during the pandemic. Particularly the urging need for new information during a pandemic might have affected informed consent reporting. This evidence gap is the focus of this study.
We hypothesise that reporting on prestudy protocol registration, informed consent, data handling and sharing aspects during the COVID-19 pandemic were compromised due to the need for fast information and the shortened review procedures. Therefore, we conducted a systematic scoping review of observational studies and clinical trials during the COVID-19 pandemic to investigate the above-mentioned aspects of reporting informed consent and data handling as proxies for study quality conduct.31–38 Additionally, we compared those aspects between predefined regions of the world.
Methods
We designed a study protocol to systematically identify studies meeting the inclusion criteria. Consequently, wherever possible, this study adheres to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines for reporting systematic reviews (online supplemental table S1).39 We could not register our review due to its design and therefore our review was not-preregistered.
bmjgh-2023-012007supp001.pdf (196KB, pdf)
PubMed (NCBI) and Embase (Ovid) were systematically searched, and studies published in English that reported on SARS-CoV-2 infection and COVID-19 disease published before 8 November 2020 were identified.40 41 Search terms included the MeSH (Medical Subject Headings) terms: “Coronavirus”. The second component of the search included study designs, that is, observational studies, randomized controlled trials (RCTs), cohort studies and cross-sectional studies. These were all entered as ‘publication type’ or as a MeSH term. Furthermore, the MeSH term ‘humans’ was included. Finally, the search was designed to exclude case reports, reviews, meta-analyses and animal studies as publication types. The search in Embase included all components mentioned above but was refined with an expert search offered by the Ovid team at the time. The expert search was designed to remove publications on previous Sars-CoV-1 and Middle East respiratory syndrome-virus outbreaks and animal-related studies. The expert search also included non-indexed publications. More detailed information on the Embase and PubMed search can be found in online supplemental table S2.
After identifying and excluding duplicates, studies were screened based on title and abstract to include studies with hospitalised patients 18 years and older, and a PCR confirmed SARS-CoV-2 infection. We excluded publications in languages other than English and studies with healthcare professionals as the study subjects.
Title and abstract screening were performed in duplicate by two investigators (CWEH and NW). Reasons for study exclusion were registered, and any discrepancies between the two investigators on reasons for exclusion were resolved by discussion and mutual agreement.
After screening, detailed data on the following reported variables were extracted from full papers: prestudy protocol registration, ethical approval, informed consent type and legal data handling for privacy. For multicentre studies, we extracted reporting of data transfer and sharing agreements, whether data were FAIR and whether data were classified as open data.20
Furthermore, data on study characteristics, such as publication date and journal, sample size, study design, the country where the study was conducted, and its income level organised according to the World Banks classification of country income, were collected.42 43 More information on what data were extracted is available in table 1. These variables, while not exhaustive, were predefined and chosen as multidimensional proxies for study quality conduct. We discuss each of these aspects in table 1. Data extraction was performed independently by four investigators (CWEH, CTAV, NW and SJWMC) and was not performed in duplicate.
Table 1.
Study variables, including a non-exhaustive list of proxy variables for study conduct quality
| Variable | Relevance | Data were collected |
| Study designs | To be able to establish a link between the other variables and study designs. | The following designs were classified and, as such, collected:
|
| Study protocol | In contrast to RCTs reporting intervention effects, many observational studies report associations investigated within a single study. Therefore, the publication of hypotheses in a study protocol, or a registration in a trial registry, enhances the validity of intervention effects respectively observational results by reducing publication bias. Protocol registration for RCTs is a requirement in Europe, and the USA66 67 and bias reduction in reported observational study results also benefits from prestudy protocol registration.31 | Data were collected on whether prestudy protocol registration was mentioned anywhere in the manuscript. Scored as either ‘yes’ or ‘no’. |
| Ethical approval | According to the Declaration of Helsinki, studies on humans should be approved by scientific, ethical institutions and this approval should be reported.68 The declaration states that studies not executed in accordance with the principles in the declaration should not be published. Evidence suggests that studies describing ethical aspects have a higher methodological quality than those that do not.69 | Whether the manuscript provided any information on the ethical approval of their respective review boards. Scored as either ‘yes’ or ‘no’. |
| Informed consent | When patients participate in research, they must provide informed consent. According to the GDPR, informed consent has to be specific, unambiguous, freely given and informed. Informed means that patients know what data are being processed and in what manner, the purpose of the data processing, and that they can withdraw their consent at any time.16 | The following types of consent were classified, defined and collected:
|
| Legal data handling for privacy | Anonymisation and pseudoanonymisation, also called de-identification, protect the patients’ privacy by uncoupling healthcare data from data that traces back to individuals. Pseudoanonymised data hold a key identifier, so data can be enriched by adding variables by coupling using the key. Therefore, pseudoanonymisation is somewhat more vulnerable to privacy breach than anonymisation. In contrast, anonymisation aims to fully de-identify data, making these data independent from legal rules and regulations, such as GDPR in Europe and HIPAA in the USA.16 17 | The following types of anonymisation were classified and collected:
|
| Data transfer and sharing | A data transfer is defined as the transfer of pseudoanonymised patient data between at least two different centres. It should be done with a data transfer (DTA, unidirectional) or data sharing (DSA, bidirectional) agreement to protect the rights and obligations of both the sending and receiving parties. Generally, a DTA/DSA is accompanied by a study protocol explaining the data-sharing goals, hence our investigation of prestudy protocol registration. | Data were collected on whether the manuscript provided any information on a DTA or DSA. Scored ‘yes’ when the manuscript mentioned DTA/DSA data and ‘no’ when it was not mentioned. |
| The FAIR principles | Improving the Findability, Accessibility, Interoperability and Reuse (FAIR) of digital health data will improve data quality and collaboration between parties. Therefore, we collected data on this subject. | Data were collected on whether the manuscript made any mention of FAIR principles. Scored ‘yes’ when the manuscript mentioned FAIR data and ‘no’ when it was not mentioned. Extraction was limited as we did not score whether any of the individual traits ‘Findability’, ‘Accessibility’, ‘Interoperability’ or ‘Reuse’ were reported. |
| Open data | Open data can contribute to future improvements in research for healthcare. Therefore, these items were additionally investigated for multicentre studies in particular. Open data are anonymised, fully de-identified data that can be used free of rules and regulations, such as GDPR and HIPAA. In fact, these data are not sensitive data anymore. Open data are increasingly recognised to create equity between investigators, particularly if data curation is outsourced to users of the open data. Hence, our collection of data on this subject. | Data were collected on whether data were open. Scored ‘yes’ when the manuscript mentioned open data and ‘no’ when it was not mentioned. |
| Regions and countries | To be able to establish a link between the other variables and the geographical location of the studies. | We defined the following regions in the world to compare: Southern Europe, Northern Europe, the Middle East, Asia, North America, South America, Africa, Australia and Oceania (online supplemental table S4). |
| Journal impact factor | To establish a link between the other variables and the impact factors of the journals, the included studies were published. | Data were collected on the 5-year impact factors of included studies. Those data were obtained from either the journal website or, if unavailable at the journal website, from Academic Accelerator.70 No impact factor was entered for new journals (established less than 5 years ago). |
GDPR, General Data Protection Regulation; HIPAA, Health Insurance Portability and Accountability Act.
Patient and public involvement
The Intensive Care Unit’s patient panel of Maastricht University Medical Centre+ supports transparent reporting of health data.
Statistical analysis
Data are presented as numbers and percentages according to categories of variables under investigation, using SPSS Statistics V.28.0 (International Business Machines). Furthermore, we used R (R Foundation for Statistical Computing, V.4.1.3) to construct a world map to illustrate the sample size of studies included per country. Results for informed consent are stratified according to study design, journal impact factor and country income categories. Each country’s income was classified as high income, upper middle income, lower middle income or low income, as defined by The World Bank.44 The statistical tests were carried out for the binary variable ‘any kind of consent reported’ versus ‘no consent reported’, for reasons of power and instead of testing each separate consent kind (ie, informed consent, deferred consent, verbal consent, waiver of consent, opt-out and other type of consent vs no consent reported). The journal impact factor was categorised into three classes: 0–5, 5–10 and >10 for the purpose of illustration. The Mann-Whitney U test tested whether the continuous journal impact factor differed significantly between ‘any kind of consent reported’ versus ‘no consent reported’. χ2 test tested whether ‘any kind of consent reported’ versus ‘no consent reported’ differed significantly across study design categories. A p<0.05 was considered statistically significant.
Results
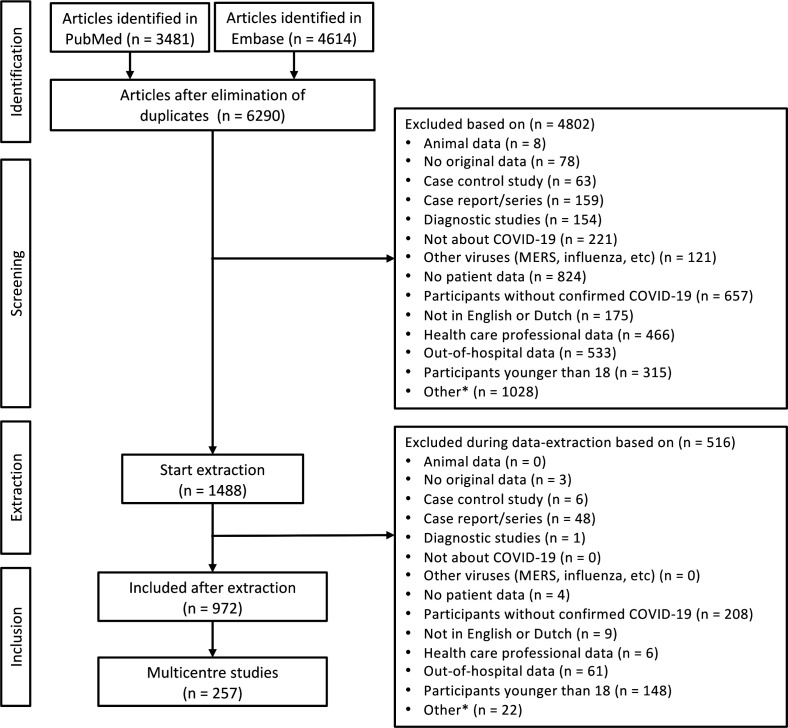
The initial search on 8 November 2020, identified 3481 publications in PubMed and 4614 publications in Embase, resulting in 6290 publications after removing duplicates. Of these, 1488 were examined in detail, and 972 were included for data extraction (figure 1). In total, these publications included international data from 618 598 individual patients (figure 2).
Figure 1.
Flow diagram of study selection. *pilot studies, letters, abstracts, protocols, non-peer-reviewed and/or retracted studies.
Figure 2.
World map with numbers of included patients at country level. The world map shows the amount of included hospitalized COVID-19 studies across our included 972 studies per country, with greater circle magnitudes indicating more patients.
Only 3.8% of 972 studies were clinical trials (randomised and non-randomised clinical trials), of which 94.6% reported informed consent. Sixteen per cent were prospective cohort studies, of which 27.6% reported informed consent, 34.6% reported waiver of consent, whereas 29.5% did not report information on informed consent. 21.3% of all included studies reported informed consent, 42.6% reported waiver of consent, whereas 31.4% did not report information on informed consent (online supplemental table S3). Other forms of consent, such as deferred consent, opt-out or verbal consent, were reported in <5% of studies. Ethical approval was reported in 90.9% of studies. Eighty-three per cent of studies did not report any information on pseudoanonymisation or anonymisation of the data. Of 972 studies, 16.8% reported a prestudy protocol registration or design of the study (table 2).
Table 2.
Reported informed consent type, legal data handling for privacy, prestudy protocol registration and ethical approval in 972 COVID-19 studies
| Type of consent | n | % |
| Informed consent | 207 | 21.3 |
| Deferred consent | 2 | 0.2 |
| Verbal consent | 35 | 3.6 |
| Waiver of consent | 414 | 42.6 |
| Opt-out | 8 | 0.8 |
| Other types of consent | 1 | 0.1 |
| No consent reported | 305 | 31.4 |
| Legal data handling for privacy | ||
| Full anonymisation | 30 | 3.1 |
| Pseudoanonymisation/de-identification | 48 | 4.9 |
| Unknown | 87 | 9.0 |
| Not mentioned | 807 | 83.0 |
| Study protocol | ||
| Prestudy registration | 163 | 16.8 |
| No registration | 809 | 83.2 |
| Ethical approval | ||
| Reported | 884 | 90.9 |
| Not reported | 88 | 9.1 |
Data are numbers (n) and percentages (%).
The majority of studies (74.0%) described retrospective data, of which 15.0% reported informed consent, 48.0% reported waiver of consent, whereas 33.5% did not report informed consent (figure 3, online supplemental table S3). The remaining 6.2% were cross-sectional studies, of which 35.0% reported informed consent. ‘Any kind of consent reported’ versus ‘no consent reported’ was significantly different between study design categories (p=0.003). Impact factor as a continuous variable was not significantly different for ‘any kind of consent reported’ versus ‘no consent reported’ (p=0.159) (table 3).
Figure 3.
Study designs and reported consent (n=972).
Table 3.
Journal impact factor and reported informed consent type in COVID-19 studies
| Impact factor | 0–5 | 5–10 | >10 | Data not available | ||||
| Total, n | 669 | 220 | 54 | 29 | ||||
| n | % | n | % | n | % | n | % | |
| Informed consent | 148 | 22.1 | 43 | 19.5 | 11 | 20.4 | 5 | 17.2 |
| Deferred consent | 2 | 0.3 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 |
| Verbal consent | 21 | 3.1 | 11 | 5.0 | 3 | 5.6 | 0 | 0.0 |
| Waiver of consent | 273 | 40.8 | 103 | 46.8 | 24 | 44.4 | 14 | 48.3 |
| Opt-out | 6 | 0.9 | 1 | 0.5 | 0 | 0.0 | 1 | 3.4 |
| Other consent types | 0 | 0.0 | 1 | 0.5 | 0 | 0.0 | 0 | 0.0 |
| No consent reported | 219 | 32.7 | 61 | 27.7 | 16 | 29.6 | 9 | 31.0 |
Data are numbers (n) and percentages (%).
We observed only one ‘other consent type’ that was not predefined, which was family consent.
Of the included 972 publications, 257 publications reported on multicentre data. Only 1.2% reported on data sharing or data transfer agreements, none reported on FAIR principles, and only 1.2% reported on open data (table 4). Taken together, none of the multicentre studies reported on all three.
Table 4.
Multicentre data sharing reporting in 257 COVID-19 studies: data transfer/sharing, FAIR data and open data
| n | % | |
| Data transfer/sharing agreement | ||
| Reported | 3 | 1.2 |
| Not reported | 254 | 98.8 |
| FAIR data | ||
| Reported | 0 | 0 |
| Not Reported | 257 | 100 |
| Open data | ||
| Reported | 3 | 1.2 |
| Not Reported | 254 | 98.8 |
Data are numbers (n) and percentages (%).
FAIR, Findable, Accessible, Interoperable and Reusable.
When we organised reporting on informed consent according to predefined regions in the world (excluding regions with a minimum of 10 publications and publications with an unknown location of the study population), informed consent reporting ranged from 4.7% in North America to 42.4% in the Middle East (p<0.001) (online supplemental table S4, S5).
51.2% of studies were conducted in countries classified as high-income, 44.7% of studies were conducted in countries classified as upper-middle-income and 3.9% of studies were conducted in lower-middle-income countries (table 5). Only one of the included studies originated from a low-income country (the Democratic Republic of the Congo). Informed consent was most often mentioned in studies conducted in lower-middle-income countries. A waiver of consent was most often reported in studies from upper-middle-income countries. Verbal consent and not reporting on informed consent happened most often in studies conducted in high-income countries.
Table 5.
Consent types stratified by studies from high, upper-middle, lower-middle and low-income countries (n=968)*.
| Types of consent | High-income countries | Upper-middle-income countries | Lower-middle-income countries | Low-income countries | Total | |||||
| n | % | n | % | n | % | n | % | n | % | |
| Informed consent | 76 | 15.3 | 109 | 25.2 | 20 | 52.6 | 0 | 0.0 | 205 | 21.2 |
| Deferred consent | 1 | 0.2 | 1 | 0.2 | 0 | 0.0 | 0 | 0.0 | 2 | 0.2 |
| Verbal consent | 20 | 4.0 | 14 | 3.2 | 1 | 2.6 | 0 | 0.0 | 35 | 3.6 |
| Waiver of consent | 199 | 40.1 | 207 | 47.8 | 7 | 18.4 | 0 | 0.0 | 413 | 42.7 |
| Opt-out | 5 | 1.0 | 2 | 0.5 | 0 | 0.0 | 1 | 100.0 | 8 | 0.8 |
| Other types of consent | 1 | 0.2 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 1 | 0.1 |
| No consent reported | 194 | 39.1 | 100 | 23.1 | 10 | 26.3 | 0 | 0.0 | 304 | 31.4 |
| Total | 496 | 100.0 | 433 | 100.0 | 38 | 100.0 | 1 | 100.0 | 968 | 100.0 |
Data are numbers (n) and percentages (%).
*In four studies, it was unclear in which countries they were conducted; therefore, these four studies were not considered for this analysis.
Discussion
This systematic scoping review has three main findings. First, of the 972 studies included, prestudy protocol registration was reported in only 16.8%, ethical approval in 90.1% and consent in 68.6%, with a waiver of consent being the most common. Informed consent (also when assessing all kinds of consent combined) was most often reported in clinical trials. Overall, 31.4% of studies did not report consent. Second, regarding aspects of data handling for privacy, data anonymisation was mentioned in 17.0% of publications. Other aspects, such as reporting on data sharing or transfer, or FAIR and open data, were mentioned in 1.2%, 0%, and 1.2% of multicentre publications, respectively. Taken together, none of the studies reported on all aspects, namely, prestudy protocol registration, ethical approval, informed consent, data handling for privacy, data anonymisation, data sharing and transfer, irrespective of FAIR and open data. Third, differences exist globally, suggesting that inequalities and legislation play a role in study conduct and reporting. Consent was most often reported in the Middle East (42.4%) and least often in North America (4.7%). Furthermore, only one report originated from a low-income country, suggesting that data from individuals living in these countries are not published with the same frequency as data collected from those residing in higher-income countries. This latest is consistent with current literature, which states the need to include low-income countries in research and the global pandemic response regarding preventing potential blind spots and new hotspots of a global pandemic.45 Our findings suggest that overall research conduct could be substantially improved. This is in line with previous evidence showing that study quality in COVID-19 research was compromised.46 47 Although our results can be explained by the societal need for rapid answers early in the COVID-19 pandemic, we cannot exclude that the pandemic has exposed and magnified pre-existing trends focused on the quantity of publications instead of high-quality research. Supportive of the latter hypothesis is that almost 20 years ago, it was recognised that we perhaps need fewer publications but more of superior quality.48 49
Informed consent is a legal and ethical construct used in a state-of-the-art investigation of patient data, where legal and ethical rules and regulations apply. Although legal rules vary between regions and countries, which has likely driven the differences in reporting across regions of the world, the scientific community embraces general ethical regulations globally.15–17 50 Obtaining informed consent during the COVID-19 pandemic, however, came with significant challenges. For example, study personnel was at risk of infection when contacting patients to obtain a signature for written informed consent.3 51 Also, scarce personnel resources were likely to be employed clinically instead of in research activities. Importantly, informed consent protects the patients’ autonomy, particularly regarding study risks, such as interventions under study and sharing of sensitive personal health data.18 52 Therefore, the Council for International Organizations of Medical Sciences states that obtaining informed consent should continue, even in situations of duress and other methods than written informed consent are possible.15 53 For example, verbally asking for consent with a witness present is an accepted alternative, as well as asking a legally authorised representative of the patient.51 When a representative is unavailable, asking for consent at a later point (deferred consent) could also be considered.25 This may explain why many studies did not explicitly report written informed consent during the COVID-19 pandemic. We found that a waiver of consent was most often applied for observational studies, apparently balancing individual risks versus the overall general gain of data investigation. Taken together, we feel that reporting informed consent is at least a proxy for study quality conduct, although informed consent is neither an obstacle nor a guarantee for good-quality data.54 To our knowledge, no quantitative data are available about the effect of acquiring informed consent on the quality of study results. Although the rapid growth of COVID-19 publications appeared to affect journal impact factors, we found that consent reporting did not differ according to the journal impact factor.55
Rapid data sharing during the COVID-19 pandemic has changed science. The vast need for sharing was acknowledged and can be integrated into study protocols.13 Legal rules to protect individuals’ privacy were often experienced as boundaries restraining the benefits of data sharing.13 This urges the transition to more FAIR and open data to tackle future pandemics.2 20 26 56–59 However, sharing data too rapidly, for example, relying on data analysed and reported without peer review on preprint servers, might spread misinformation.24 Nevertheless, data sharing needs to happen according to specific rules and regulations, such as GDPR in Europe and HIPAA in the USA.16 22 23 27 Data transfer or sharing agreements form a legal basis for sharing data for a predefined purpose.60 The scarce reporting on data sharing and transfer agreements hampers transparency of whether legal rules are met to protect data privacy for individuals. The aim of Open Science is transparent and accessible knowledge sharing in collaborative networks and acts of FAIR principles and open data.20 57 61 Open Science increases the reproducibility, transparency and quality of scientific results.57 62 63 On the one hand, FAIR data improve the findability and reuse of data and enhances federated data analyses, whereas storing data in public archives enables public investigation of health data.64 Indeed, it has been reported that 16.0% of clinical trials in COVID-19 indicated wanting to share their raw data and many scientific journals and national research grant providers have adopted policies requesting scientists to share their raw data by default.65 66 We, however, observe that opening data for sharing has not been widely implemented in COVID-19 research. Most likely, the complexities of ethical and legal aspects obstruct the implementation of opening data for wide sharing beyond the parties involved in data transfer and sharing agreements. This must be overcome to implement suggestions like sharing raw data by default or using trusted third parties to accommodate privacy concerns.63
The results only include data from one low-income country. In fact, this shows that the current research world makes it difficult for low-income countries to publish, and we cannot exclude that strict criteria, such as informed consent or data handling for privacy, play a role. Inadequate resources, especially high rates of COVID-19 related hospitalisations and fatalities, are conditions that may have prevented researchers in low-income countries from collecting informed consent.67 Thus, the data may not adequately reflect the systematic challenges that COVID-19 researchers in low-income countries have faced due to higher rates of severe COVID-19 cases, limiting their ability to publish research in high-impact journals. These factors contribute to ongoing equity challenges in the kind of research conducted, knowledge generated and interventions developed to address health outcomes. Future research should identify more factors limiting researchers from obtaining consent and provide a comprehensive framework for approaching crises and limited resources. Journals play a crucial role in promoting complete reporting. This study, for example, identified whether consent was simply not obtained or obtained but not reported as a general unclarity. We recommend that structural reporting on ethical aspects, such as consent, anonymisation, ethical approval and legal data handling for privacy in studies involving humans, should be done, acknowledging high-quality research. This study shows researchers, who play a crucial role, that ethics regulations are more than just rules to comply with as both relevant public questions require investigation, while individuals should be respected. Clear reporting has been shown to improve the quality of research markedly.8 11 39
This study has several strengths and limitations. First, the search was comprehensive, minimising missed publications on COVID-19 during the period of investigation. Although screening was done in duplicate, which is a strength, data extraction was limited to one person and used a standardised data extraction form. This approach was chosen to minimise the risk of missing relevant studies, while optimising accurate data extraction efficiency. Another strength was using the pandemic as a case study. However, this was also a weakness as we have no information on informed consent reporting and aspects of data handling for privacy beyond COVID-19, thus limiting the generalisability of the results. Although our review was extensive, we did not include genomic surveillance studies based on viral samples and focused on patients and their consent.45 Hence, no conclusions can be drawn from surveillance data. When looking at reporting on informed consent, it is possible that mainly resource-rich countries were selected, with informed consent serving as an additional barrier in a pandemic setting. This has led to under-reporting data from low-income countries, further increasing health inequalities. Indeed, bias due to consent has been reported.31 To tackle this issue in the future, data sharing governance initiatives in low-income parts of the world that aim for high-quality data collaboration, including cross-border consent, should be supported.50 In addition, we found that some publications did not provide sufficient information on the type of study being conducted; thus, misclassification of study types may have occurred. Another limitation is that only articles in English were included. In addition, we could not differentiate between instances where consent was not obtained at all or situations where consent was obtained but not reported, potentially leading to an underestimation of reported consent. A final limitation was that during the extraction regarding FAIR data, we searched whether the term ‘FAIR’ was mentioned, not if its separate components were presented. Hence, we could have missed studies that possibly partly complied with the FAIR principles. This might have resulted in an underestimation of our results regarding the compliance of included studies with reference to FAIR data principles.
Conclusion
Informed consent, and aspects of data handling for privacy, as proxies for study quality conduct, were structurally under-reported in publications concerning COVID-19. Furthermore, publications from lower-income countries were sparse. To move Open Science ahead to support physicians’ needs based on clinical investigation and data in general and in future pandemics specifically, transparency in reporting on informed consent and aspects of data handling should markedly improve. We recommend the development of a comprehensive framework to advise concerns of informed consent and other ethics regulations during times of crisis, such as a pandemic, and in situations with limited resources. Finally, international and intercontinental inequalities in resources likely affect the possibilities of complying with consent and data sharing. Here, academic journals should set the standards in a way to incorporate data inclusively to avoid selective reporting throughout the world, while improving the reporting of study quality conduct.
Footnotes
Handling editor: Seye Abimbola
NW and CWEH contributed equally.
Contributors: NW: performed the search. NW, CWEH, SJWMC, CTAV: study selection and data extraction. NW, CWEH: analysed the data. NW, CWEH: wrote the initial draft of the paper, revised the paper and finalised the manuscript. NW, CWEH: wrote the paper. BvB, IvdH: initiated the project, developed the idea and coordinated the writing process. NW, WECH, CTAV, SJWMC, WKAvM, JCB, LAC, NM-M, JG, CW, FB-R, LW, ICCvdH, BCTvB: wrote the paper and critically reviewed the content. The corresponding author attests that all listed authors meet authorship criteria and that no others meeting the criteria have been omitted. BCTvB is responsible for the overall content as the guarantor.
Funding: LAC is funded by the National Institute of Health through the NIBIB R01 grant EB01720.
Map disclaimer: The inclusion of any map (including the depiction of any boundaries therein), or of any geographic or locational reference, does not imply the expression of any opinion whatsoever on the part of BMJ concerning the legal status of any country, territory, jurisdiction or area or of its authorities. Any such expression remains solely that of the relevant source and is not endorsed by BMJ. Maps are provided without any warranty of any kind, either express or implied.
Competing interests: LAC is funded by the National Institute of Health through the NIBIB R01 grant EB01720.
Patient and public involvement: Patients and/or the public were involved in the design, or conduct, or reporting, or dissemination plans of this research. Refer to the Methods section for further details.
Provenance and peer review: Not commissioned; externally peer reviewed.
Supplemental material: This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.
Data availability statement
Data used can be made available on request to the corresponding author.
Ethics statements
Patient consent for publication
Not applicable.
References
- 1.Topol E. It’s not too late. Science 2022;375:245. 10.1126/science.abo1074 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Watson C. Rise of the preprint: how rapid data sharing during COVID-19 has changed science forever. Nat Med 2022;28:2–5. 10.1038/s41591-021-01654-6 [DOI] [PubMed] [Google Scholar]
- 3.Hashem H, Abufaraj M, Tbakhi A, et al. Obstacles and considerations related to clinical trial research during the covid-19 pandemic. Front Med (Lausanne) 2020;7:598038. 10.3389/fmed.2020.598038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Elgendy IY, Nimri N, Barakat AF, et al. A systematic bias assessment of top-cited full-length original clinical investigations related to COVID-19. Eur J Intern Med 2021;86:104–6. 10.1016/j.ejim.2021.01.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ramiro S, Mostard RLM, Magro-Checa C, et al. Historically controlled comparison of glucocorticoids with or without tocilizumab versus supportive care only in patients with COVID-19-associated cytokine storm syndrome: results of the chiC study. Ann Rheum Dis 2020;79:1143–51. 10.1136/annrheumdis-2020-218479 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wynants L, Van Calster B, Collins GS, et al. Prediction models for diagnosis and prognosis of covid-19: systematic review and critical appraisal. BMJ 2020;369:m1328. 10.1136/bmj.m1328 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mehra MR, Desai SS, Ruschitzka F, et al. Retracted: hydroxychloroquine or chloroquine with or without a macrolide for treatment of COVID-19: a multinational registry analysis. Lancet 2020. 10.1016/S0140-6736(20)31180-6 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 8.Collins GS, Reitsma JB, Altman DG, et al. Transparent reporting of a multivariable prediction model for individual prognosis or diagnosis (TRIPOD). Circulation 2015;131:211–9. 10.1161/CIRCULATIONAHA.114.014508 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Equator-Network . Enhancing the quality and transparency of health research (EQUATOR). 2022. Available: https://www.equator-network.org/ [Accessed 13 Sep 2022].
- 10.Pollock D, Peters MDJ, Khalil H, et al. Recommendations for the extraction, analysis, and presentation of results in scoping reviews. JBI Evidence Synthesis 2023;21:520–32. 10.11124/JBIES-22-00123 [DOI] [PubMed] [Google Scholar]
- 11.Elm E von, Altman DG, Egger M, et al. Strengthening the reporting of observational studies in epidemiology (STROBE) statement: guidelines for reporting observational studies. BMJ 2007;335:806–8. 10.1136/bmj.39335.541782.AD [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wolff RF, Moons KGM, Riley RD, et al. PROBAST: a tool to assess the risk of bias and applicability of prediction model studies. Ann Intern Med 2019;170:51. 10.7326/M18-1376 [DOI] [PubMed] [Google Scholar]
- 13.RDA COVID-19 Working Group . Recommendations and Guidelines on data sharing. Research Data Alliance, [Google Scholar]
- 14.Khalil H, Peters MDJ, McInerney PA, et al. The role of scoping reviews in reducing research waste. J Clin Epidemiol 2022;152:30–5. 10.1016/j.jclinepi.2022.09.012 [DOI] [PubMed] [Google Scholar]
- 15.Council for International Organizations of Medical Sciences (CIOMS) . International Ethical Guidelines for Health-related Research Involving Humans. Geneva, 2016. [Google Scholar]
- 16.European Commission . General Data Protection Regulation (GDPR). 2018. [Google Scholar]
- 17.United States . The Health Insurance Portability and Accountability Act (HIPAA). Washington, D.C: U.S. Dept. of Labor EBSA, 2004. [Google Scholar]
- 18.Longo DL, Grady C. Enduring and emerging challenges of informed consent. N Engl J Med 2015;372:855–62. 10.1056/NEJMra1411250 [DOI] [PubMed] [Google Scholar]
- 19.Cassell J, Young A. Why we should not seek individual informed consent for participation in health services research. J Med Ethics 2002;28:313–7. 10.1136/jme.28.5.313 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wilkinson MD, Dumontier M, Aalbersberg IJJ, et al. The fair guiding principles for scientific data management and stewardship. Sci Data 2016;3:160018. 10.1038/sdata.2016.18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.European Commission Directorate-General for Research Innovation . Open innovation, open science, open to the world: a vision for Europe. Publications Office, 2016. [Google Scholar]
- 22.Becker R, Thorogood A, Ordish J, et al. COVID-19 research: Navigating the European General data protection regulation. J Med Internet Res 2020;22:e19799. 10.2196/19799 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fegan G, Cheah PY, Data Sharing Working Group. Electronic address: WorkingGroups@covid19crc.org . Solutions to covid-19 data sharing. Lancet Digit Health 2021;3. 10.1016/S2589-7500(20)30273-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Khalil H, Tamara L, Rada G, et al. Challenges of evidence synthesis during the 2020 COVID pandemic: a scoping review. J Clin Epidemiol 2022;142:10–8. 10.1016/j.jclinepi.2021.10.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bhatt A. Clinical trials during the COVID-19 pandemic: challenges of putting scientific and ethical principles into practice. Perspect Clin Res 2020;11:59. 10.4103/picr.PICR_77_20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cosgriff CV, Ebner DK, Celi LA. Data sharing in the era of COVID-19. Lancet Digit Health 2020;2:e224. 10.1016/S2589-7500(20)30082-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gudi N, Kamath P, Chakraborty T, et al. Regulatory frameworks for clinical trial data sharing: Scoping review. J Med Internet Res 2022;24:e33591. 10.2196/33591 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Finlay KA, Fernandez CV. Failure to report and provide commentary on research ethics board approval and informed consent in medical journals. J Med Ethics 2008;34:761–4. 10.1136/jme.2007.023325 [DOI] [PubMed] [Google Scholar]
- 29.Jacobsen KH. Reporting of ethics-related methods in epidemiological research. J Med Ethics 2009;35:262–7. 10.1136/jme.2008.026815 [DOI] [PubMed] [Google Scholar]
- 30.Sibai AM, Arawi T, Al Faisal W, et al. Ethics reporting practices in aging research from the Arab region. J Appl Gerontol 2021;40:105–9. 10.1177/0733464819886453 [DOI] [PubMed] [Google Scholar]
- 31.Flaatten H, Guidet B, Jung C, et al. Consent is a confounding factor in a prospective observational study of critically ill elderly patients. PLoS One 2022;17:e0276386. 10.1371/journal.pone.0276386 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gergely M, Dankar FK, Alrabaee S. Misconduct and consent: the importance of informed consent in medical research. In: Faintuch J, Faintuch S, eds. Integrity of Scientific Research: Fraud, Misconduct and Fake News in the Academic, Medical and Social Environment. Cham: Springer International Publishing, 2022: 81–91. [Google Scholar]
- 33.Takai Y, Matsui K. Pseudoscience during the COVID-19 pandemic. In: Faintuch J, Faintuch S, eds. Integrity of Scientific Research: Fraud, Misconduct and Fake News in the Academic, Medical and Social Environment. Cham: Springer International Publishing, 2022: 61–8. 10.1007/978-3-030-99680-2 [DOI] [Google Scholar]
- 34.Siwek J, Gourlay ML, Slawson DC, et al. How to write an evidence-based clinical review article. Am Fam Physician 2002;65:251–8. [PubMed] [Google Scholar]
- 35.Pautasso M, Bourne PE. Ten simple rules for writing a literature review. PLOS Comput Biol 2013;9:e1003149. 10.1371/journal.pcbi.1003149 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Munn Z, Pollock D, Barker TH, et al. n.d. The Pandora’s box of evidence synthesis and the case for a living evidence synthesis taxonomy. BMJ EBM:bmjebm–2022 10.1136/bmjebm-2022-112065 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Héroux ME, Butler AA, Cashin AG, et al. Quality output checklist and content assessment (quocca): a new tool for assessing research quality and reproducibility. BMJ Open 2022;12:e060976. 10.1136/bmjopen-2022-060976 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mahtani KR. All health researchers should begin their training by preparing at least one systematic review. J R Soc Med 2016;109:264–8. 10.1177/0141076816643954 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Page MJ, McKenzie JE, Bossuyt PM, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ 2021;372:n71. 10.1136/bmj.n71 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Carvalho T, Krammer F, Iwasaki A. The first 12 months of COVID-19: a timeline of immunological insights. Nat Rev Immunol 2021;21:245–56. 10.1038/s41577-021-00522-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wuhan City Health Committee (WCHC) . Wuhan municipal health and health Commission’s briefing on the current pneumonia epidemic situation in our City. 2019. Available: http://wjw.wuhan.gov.cn/front/web/showDetail/2019123108989 [Accessed 13 Sep 2022].
- 42.Hamadeh NvR C, Metreau E, Eapen SG. New World Bank country classifications by income level: 2022-2023. World bank, 2022. [Google Scholar]
- 43.World Bank . Country and Lending Groups. 2021. [Google Scholar]
- 44.World bank . The world by income. 2021. Available: https://datatopics.worldbank.org/world-development-indicators/the-world-by-income-and-region.html [Accessed 4 Nov 2022].
- 45.Wilkinson E, Giovanetti M, Tegally H, et al. A year of genomic surveillance reveals how the SARS-cov-2 pandemic unfolded in Africa. Science 2021;374:423–31. 10.1126/science.abj4336 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Dobler CC. Poor quality research and clinical practice during COVID-19. Breathe 2020;16:200112. 10.1183/20734735.0112-2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Salluh JIF, Arabi YM, Binnie A. COVID-19 research in critical care: the good, the bad, and the ugly. Intensive Care Med 2021;47:470–2. 10.1007/s00134-021-06367-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Altman DG. The scandal of poor medical research. BMJ 1994;308:283–4. 10.1136/bmj.308.6924.283 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Alayche M, Cobey KD, Ng JY, et al. Evaluating prospective study registration and result reporting of trials conducted in Canada from 2009-2019. Health Policy [Preprint] 2022. 10.1101/2022.09.01.22279512 [DOI] [Google Scholar]
- 50.Brand D, Singh JA, McKay AGN, et al. Data sharing governance in sub-Saharan Africa during public health emergencies: gaps and guidance. S Afr J Sci 2022;118. 10.17159/sajs.2022/13892 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ma X, Wang Y, Gao T, et al. Challenges and strategies to research ethics in conducting COVID-19 research. J Evid Based Med 2020;13:173–7. 10.1111/jebm.12388 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Beesley SJ, Powell A, Groat D, et al. Evaluating the balance between privacy and access in digital information sharing. Crit Care Med 2022;50:e109–16. 10.1097/CCM.0000000000005234 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Tuttle KR. Impact of the COVID-19 pandemic on clinical research. Nat Rev Nephrol 2020;16:562–4. 10.1038/s41581-020-00336-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Crow G, Wiles R, Heath S, et al. Research ethics and data quality: the implications of informed consent. International Journal of Social Research Methodology 2006;9:83–95. 10.1080/13645570600595231 [DOI] [Google Scholar]
- 55.Delardas O, Giannos P. How COVID-19 affected the Journal impact factor of high impact medical journals: bibliometric analysis. J Med Internet Res 2022;24:e43089. 10.2196/43089 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Ercole A, Brinck V, George P, et al. Guidelines for data acquisition, quality and curation for observational research designs (DAQCORD). J Clin Transl Sci 2020;4:354–9. 10.1017/cts.2020.24 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Vicente-Saez R, Martinez-Fuentes C. Open science now: a systematic literature review for an integrated definition. Journal of Business Research 2018;88:428–36. 10.1016/j.jbusres.2017.12.043 [DOI] [Google Scholar]
- 58.Calling all coronavirus researchers: keep sharing, stay open. Nature 2020;578:7. 10.1038/d41586-020-00307-x [DOI] [PubMed] [Google Scholar]
- 59.Moradian N, Ochs HD, Sedikies C, et al. The urgent need for integrated science to fight covid-19 pandemic and beyond. J Transl Med 2020;18:205. 10.1186/s12967-020-02364-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Gao F, Tao L, Huang Y, et al. Management and data sharing of COVID-19 pandemic information. Biopreservation and Biobanking 2020;18:570–80. 10.1089/bio.2020.0134 [DOI] [PubMed] [Google Scholar]
- 61.European Commission . Open research Europe, how it works. Available: https://open-research-europe.ec.europa.eu/about [Accessed 9 Jul 2022].
- 62.Munafò MR, Nosek BA, Bishop DVM, et al. A manifesto for reproducible science. Nat Hum Behav 2017;1:0021. 10.1038/s41562-016-0021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Besançon L, Peiffer-Smadja N, Segalas C, et al. Open science saves lives: lessons from the COVID-19 pandemic. BMC Med Res Methodol 2021;21:117. 10.1186/s12874-021-01304-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.de Carvalho Junior MA, Bandiera-Paiva P. Strengthen electronic health records system (EHR-S) access-control to cope with GDPR explicit consent. J Med Syst 2020;44:172. 10.1007/s10916-020-01631-5 [DOI] [PubMed] [Google Scholar]
- 65.Miyakawa T. No RAW data, no science: another possible source of the reproducibility crisis. Mol Brain 2020;13:24. 10.1186/s13041-020-0552-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Li R, von Isenburg M, Levenstein M, et al. COVID-19 trials: declarations of data sharing intentions at trial registration and at publication. Trials 2021;22:153. 10.1186/s13063-021-05104-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.McGowan VJ, Bambra C. COVID-19 mortality and deprivation: pandemic, syndemic, and endemic health inequalities. Lancet Public Health 2022;7:e966–75. 10.1016/S2468-2667(22)00223-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.World Medical Association, World Medical Association . World Medical Association Declaration of Helsinki: Ethical principles for medical research involving human subjects. JAMA 2013;310:2191–4. 10.1001/jama.2013.281053 [DOI] [PubMed] [Google Scholar]
- 69.Ruiz-Canela M, de Irala-Estevez J, Martínez-González MA, et al. Methodological quality and reporting of ethical requirements in clinical trials. J Med Ethics 2001;27:172–6. 10.1136/jme.27.3.172 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Academic Accelerator. 2022. Available: https://academic-accelerator.com
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
bmjgh-2023-012007supp001.pdf (196KB, pdf)
Data Availability Statement
Data used can be made available on request to the corresponding author.