Abstract
Immunotherapies, such as immune checkpoint inhibitors, cellular therapies, and T-cell engagers, have fundamentally changed our approach to treating cancer. However, successes with cancer vaccines have been more difficult to realize. While vaccines against specific viruses have been widely adopted to prevent the development of cancer, only two vaccines can improve survival in advanced disease: sipuleucel-T and talimogene laherparepvec. These represent the two approaches that have the most traction: vaccinating against cognate antigen and priming responses using tumors in situ. Here, we review the challenges and opportunities researchers face in developing therapeutic vaccines for cancer.
Keywords: Vaccination; Immunity; Antigens, Neoplasm; Adjuvants, Immunologic
Introduction
Cancer immunotherapy has significantly improved outcomes for a wide range of cancers. It is an important new development in our therapeutic approach to treating both hematological and solid malignancies. One of the most noteworthy milestones that prompted a decade of progress in cancer immunotherapy was the US Food and Drug Administration’s (FDA) approval in 2011 of ipilimumab, an anti-CTLA-4 monoclonal antibody, for the treatment of unresectable or metastatic melanoma. Subsequently, anti-PD-1/L1 antibodies emerged and transformed the oncological landscape, leading to FDA approvals in over 20 unique tissue-specific cancer indications between 2014 and 2022.1 Pembrolizumab was also granted tissue-agnostic approval in 2020 for any tumors with high mutational burden and for any mismatch repair-deficient solid cancers in 2021. Currently, multiple anti-PD-1/L1 antibodies, each developed by a different manufacturer, have been approved by the FDA for the treatment of various cancers. In addition to checkpoint inhibitors, many kinds of T cell-based immunotherapy have demonstrated clinically significant benefit. Cellular therapy, most notably adoptively transferred CD19-targeting CAR-T cells, was approved in 2017 for the treatment of B-cell lymphoma and acute lymphoblastic leukemia, while in 2021 anti-BMCA-targeting CAR-T cells were approved for relapsed/refractory multiple myeloma. CD3-targeted bispecific antibodies were approved in 2017 for the treatment of pediatric B-cell acute lymphoblastic leukemia. Besides T cell-directed therapies that target the adaptive immune system, innovations in immunomodulatory agents, oncolytic viruses, and cancer vaccines that activate immunity have also received FDA approval. These critical therapeutic modalities have high potential for combination with chemotherapy, radiotherapy, and other immunotherapies.
Therapeutic cancer vaccines have a long history. The FDA approved the first immunotherapy for cancer more than 20 years before the first checkpoint inhibitor was approved. In 1990, intravesical BCG was approved for the treatment and prophylaxis of urothelial carcinoma in situ of the urinary bladder and for prophylaxis of primary or recurrent stage Ta and/or T1 urothelial carcinoma following transurethral resection.2 The approval was based on various open-label studies that demonstrated 50% complete histological response in patients with bladder carcinoma in situ treated with intravesical BCG. The use of prophylactic intravesical BCG for stage Ta/T1 urothelial carcinoma was supported by two open-label, randomized, phase 3 studies that demonstrated favorable 2-year event-free survival.2 3 Although the precise antitumor mechanism of BCG is unclear, some have proposed that BCG is internalized by bladder cancer cells, which then activates tumor-cell antigen presentation and cytokine release. This leads to recruitment of immune cells, including T lymphocytes, natural killer (NK) cells, and macrophages to the tumor bed and, together with cytokine production, elicits immune cell-mediated tumor cytotoxicity.4
Vaccine approaches can correct or salvage critical dysfunctions in T-cell antitumor immunity such as defective antigen presentation, inadequate priming, biased immunity toward non-relevant truncal mutations, immunosuppression in the tumor microenvironment, or permanent exhaustion of antigen-primed T cells. These defects can lead to tumor equilibrium, partial tumor killing, and eventual tumor growth escape (figure 1A). Therapeutic cancer vaccines deserve particular attention. These treatments elicit antitumor immune responses by delivering immune adjuvants and frequently, but not necessarily, codelivering tumor antigens. Proper vaccination can lead to improved antitumor immunity through better antigen presentation, robust priming, forced presentation of tumor-relevant antigens, and generation of non-exhausted cytotoxic T cells (figure 1B).
Figure 1.
Therapeutic cancer vaccines correct defective antitumor immunity. (A) Defective antigen presentation or biased endogenous immunity toward non-relevant truncal mutations compromises the activation of naïve T cells by dendritic cells (DC). Inadequate priming results in suppressed T-cell activation. Activated T cells differentiate into memory T cells and eventually effector T cells which encounter an immunosuppressive tumor microenvironment that leads only to partial tumor killing or equilibrium of tumor mass. Activated T cells also lead to the differentiation of terminally exhausted T cells which express high exhaustion markers, low proliferative potential, and low cytotoxicity. Permanent exhaustion of antigen-primed T cells leads to tumor escape. (B) Cancer vaccines can elicit more effective antigen presentation and force presentation or more relevant truncal mutations or differentiation antigens by DCs. Immune agonistic properties of vaccines lead to more robust T-cell priming and activation. The regeneration of non-exhausted cytotoxic effector T cells leads to more effective tumor killing.
Tumor-associated antigen vaccines
Tumor-associated antigens (TAAs) can be self-antigens that are preferentially overexpressed on tumor cells but can also be displayed by normal healthy cells or cancer testes antigens that are only expressed by tumor cells and adult reproductive tissues. Examples of TAAs include CEA, CA-125, MUC-1, PSA, PAP, PSMA, TERT, WT1, NY-ESO1, Her-2/neu, mesothelin, survivin, MAGE-A1, MAGE-A3, and gp100. T and B cells with high affinity toward these self-antigens are often removed from the immune repertoire by central and peripheral tolerance. Thus, a potent vaccine must break tolerance by stimulating lower affinity and rare TAA-reactive T cells.5–7 These types of antigens may be specifically incorporated into a vaccine to elicit a TAA-specific antitumor immune response in treated subjects.
In 2010, the FDA approved sipuleucel-T, an autologous cellular immunotherapy, for the treatment of asymptomatic or minimally symptomatic metastatic castration-resistant prostate cancer (mCRPC). Sipuleucel-T is a dendritic cell (DC) vaccine that elicits an immune response against prostatic acid phosphatase (PAP), which is expressed on most prostate cancers.8 Sipuleucel-T was the first autologous cell therapy for cancer approved by the FDA based on results from three phase 3 trials. In the pivotal randomized phase 3 IMPACT study, sipuleucel-T reduced the risk of death by 22.5% compared with control and improved survival by 4.1 months, demonstrating a median overall survival (OS) of 25.8 months compared with 21.7 months in the control arm. Similar results were also reported from 2 randomized phase 3 D9901 and D9902A trials which showed that patients treated with sipuleucel-T had a 33% reduction in risk of death compared with patients in the control group. In an integrated analysis, patients treated with sipuleucel-T achieved a 23.2-month OS compared with 18.9 months in the control group.8–10 As would be expected with a vaccine, this treatment has been shown to not only induce T-cell and B-cell responses to PAP, but to other antigens as well in a phenomenon known as antigen spreading.11 This treatment also elicits significant changes in the T-cell repertoire, with treatment-induced clonotypes migrating to the tumor microenvironment.12 13 Sipuleucel-T also alters the B-cell repertoire, with treatment-induced clones persisting for years.14 Multiple combination trials of sipuleucel-T with IL-7, anti-CTLA-4, and anti-PD-1 have been performed.15 16 While these treatments can induce changes in T-cell responses, these combinations have not resulted in significant objective response rates. Sipuleucel-T has also been combined with radium-223, another FDA-approved treatment for prostate cancer that targets bone metastases.17 This trial demonstrated improved efficacy with the combination, suggesting that altering the microenvironment in bone metastases could help sensitize prostate cancer to an immunotherapy.
Several other studies of TAA vaccines have demonstrated immunological and clinical activity. A folate receptor-alpha (FRα) peptide vaccine with Granulocyte-macrophage colony-stimulating factor (GM-CSF) adjuvant generated durable T-cell immunity against FRα antigen. In one study, all 22 treated patients (8 with breast cancer; 14 with ovarian cancer) were alive 2 years postimmunization.18 GP2 is a peptide derived from the transmembrane domain of HER2/neu. When coadministered with GM-CSF to disease-free patients with breast cancer, GP2 peptide vaccines induced GP2-specific CD8+T cell responses.19 Galinpepimut-S, a multivalent WT1 peptide vaccine, was studied in 22 patients with acute myeloid leukemia. Most patients (68%) relapsed; however, patients who achieved an immune response experienced improved disease-free survival from time of complete response (CR) and OS from time of diagnosis compared with those who did not achieve an immune response.20 In a randomized phase 2 study (n=190) of VX-001, a cancer vaccine targeting telomerase reverse transcriptase, demonstrated no improvement in OS in stage IV non-small cell lung cancer (NSCLC). Similarly, post hoc analysis showed that patients who experienced an immunological response had longer OS than those who did not.21 Endogenous viral elements have been shown to be a source of targetable immunogenic tumor antigens. In a case of renal cell carcinoma (RCC) regression following allogeneic stem cell transplantation, researchers detected RCC-reactive donor-derived CD8+ T cells that target a 10-mer peptide called CR-RCC-1. This antigen was found to be derived from human endogenous retrovirus group E. It selectively overexpresses unique transcripts in clear cell RCC that elicit T cell-mediated antitumor immunity.22 These early-stage studies highlight a correlation between antigen-specific T-cell immunological response and clinical efficacy. The heterogeneous and unpredictable immune activation are practical challenges that need to be overcome.
PROSTVAC-VF is a prostate cancer vaccine regimen consisting of a recombinant vaccinia vector as a prime, followed by multiple boosts with a recombinant fowlpox vector. Each vector contains the transgenes for prostate-specific antigen (PSA) and multiple T-cell costimulatory molecules. PROSTVAC promotes the expression of PSA on antigen-presenting cells and subsequently elicits a T cell-mediated antitumor response.23 24 Studies of PROSTVAC-VF have demonstrated that humoral response to the viral glycan Forssman disaccharide (GalNAcα1-3GalNAcβ) correlates with improved survival.25 Although early-stage clinical studies showed the vaccine was safe and effective in generating an immune response, a phase 3 study evaluating PROSTVAC in mCRPC was terminated early due to futility and concern for treatment-related cardiac arrhythmias.26–29 Trials of neoadjuvant PROSTVAC and PROSTVAC in combination with other immunotherapies are currently underway.30 31 The minimal clinical benefit of TAA vaccines demonstrated to date may be explained by the challenge of achieving a potent threshold of high-affinity antigen-specific T-cell activation and expansion while avoiding collateral toxicities stemming from TAAs expressed on normal cells.
Tumor-specific antigen vaccines
Tumor-specific antigens (TSAs) are de novo epitopes expressed by oncoviruses and shared, or private neoantigens encoded by somatic mutations. TSAs are truly tumor-specific with no central tolerance. Therefore, high-affinity TSA-specific T cells may be more prevalent in patients with cancer. Discovering effective neoantigens is highly complex and typically involves sophisticated genetic sequencing and bioinformatics technologies that add to the cost and time required to manufacture these individualized vaccines.32 Here, we discuss both cognate TSA vaccines and non-cognate tumor-neoantigen vaccines.
A prime/boost vaccine containing a heterologous chimpanzee adenovirus and self-amplifying RNA vector that encodes shared KRAS neoantigens has also been studied in combination with checkpoint blockade in patients with tumors harboring KRAS mutations. Although several of the 18 evaluable patients achieved a molecular response as measured by reduction in KRAS ctDNA variant allele frequency, and some patients had reductions in serum tumor markers, no confirmed radiographic responses were observed. A trend toward improved survival in patients with NSCLC treated with the KRAS vaccines was observed in those patients who had achieved a molecular response compared with those who did not.33
VGX-3100 is a DNA plasmid vaccine encoding the E6 and E7 genes of human papillomavirus (HPV)-16 and HPV-18. It is delivered by intramuscular injection followed by electroporation for the treatment of cervical intraepithelial neoplasia (CIN) 2/3. The vaccine induced robust HPV-16 and HPV-18 E6, E7 antigen-specific adaptive T-cell and humoral responses. Furthermore, promising data from a mid-stage trial showed that patients treated with VGX-3100 experienced higher rates of histopathological regression and clearance of CIN 2/3.34 Vaccination with an HPV DNA vaccine also resulted in enhanced specific immunity to virus-derived TSAs in patients previously treated for HPV-associated head and neck cancer.35
NOUS-209 is based on a heterologous prime/boost regimen composed of the great ape adenovirus GAd20-209-FSP used for priming and modified vaccinia virus Ankara MVA-209-FSP used for boosting. It encodes 209 shared tumor-specific frameshift peptides, which are tumor-specific neoantigens shared across patients with mismatch repair (MMR)-deficient cancer.36 NOUS-209 was studied in combination with pembrolizumab as first-line or second-line treatment in patients with tumors with deficiency in MMR or microsatellite instability (dMMR/MSI) in a phase 1 trial. Of 12 evaluable patients with dMMR/MSI, seven partial responses (PRs) were achieved, and there was dose responsiveness in vaccine immunogenicity as measured by ex vivo interferon-gamma ELISpot assay across the two dose cohorts.37
Neon Therapeutics (Cambridge, Massachusetts, USA) has reported results of a trial of NeoVax, which contains up to 20 neoantigen peptides personalized to patients based on target selection by whole exome sequencing and RNA-seq prediction of HLA binders, TLR3, and poly-ICLC in treatment-naïve patients with stage IIIB/C and IVM1a/b melanoma after surgical resection with curative intent. Of 10 enrolled patients, 6 were vaccinated. After a median follow-up of 25 months postvaccination, four of six vaccinated patients had no disease recurrence; the other two patients received pembrolizumab after disease recurrence and both subsequently experienced CRs. Vaccination with the personalized peptide vaccine induced strong multifunctional CD4 and CD8 T-cell responses in which T cells were shown to be tumor neoantigen-reactive.38 Based on a similar technology, phase I/Ib studies personalized neoantigen vaccines for patients with newly diagnosed methylguanine methyltransferase (MGMT)-unmethylated glioblastoma, from whom surgically resected tumors and matched normal cells were analyzed to identify neoantigens. Patients in each study received vaccines that contained up to 20 peptides split into 4 pools of 3–5 distinct peptides admixed with poly-ICLC. Vaccination induced circulating neoantigen-specific memory T-cell responses as well as increased T-cell infiltration. However, there were no tumor responders. All eight study participants experienced disease progression and subsequently died.39
Rosenberg et al reported a novel mRNA vaccine encoding up to 20 neoantigens selected based on expression on autologous cancer cells and validated as recognized by patients’ own tumor-infiltrating lymphocytes. The vaccine backbone contains any mutation in TP53, KRAS, or PIK3CA identified by exome sequencing of the autologous tumor and up to 15 HLA class I candidate neoantigens that were predicted to bind to a patient’s MHC alleles. Only 15.7% of potential neoantigens were immunogenic, and vaccinated patients (n=4) exhibited inconsistent neoantigen-specific CD4 and CD8 T-cell responses. Interestingly, KRASG12D mutation-specific T-cell receptors were isolated in circulation after vaccination, but no objective responses were observed in the four vaccinated patients with metastatic gastrointestinal tumors.40
In a collaboration between BioNTech (Germany) and Immatics (Germany), TSA vaccines were personalized based on mutations and analyses of the transcriptomes and immunopeptidomes of individual tumors. In a phase 1 study, 15 HLA-A*02:01– or HLA-A*24:02-restricted patients with glioblastoma multiforme (GBM) were treated with a vaccine (APVAC1) derived from a premanufactured library of non-mutated GBM-associated antigens followed by treatment with APVAC2, which contains preferentially targeted personalized neoepitopes. Each personalized vaccine contained up to 84 non-synonymous mutations along with poly-ICLC and GM-CSF as adjuvants. Vaccine safety was favorable, and vaccination induced sustained responses of central memory CD8 T cells and type 1 T helper CD4 T-cell responses in 80% of treated patients. The median OS was 29 months with a progression-free survival (PFS) of 14.2 months, including one patient who had an OS>38.9 months.41
Patients with cancer treated with both cognate and non-cognate TSA vaccines exhibited minimal tumor response. This may be explained by the inherent challenges of identifying tumor-relevant antigens and inducing a robust tumor neoantigen T-cell response in patients who have compromised endogenous immunity from being heavily pretreated with cytotoxic systemic therapy or due to advanced-stage disease. Administering cancer vaccines to patients with earlier-stage disease who have relatively more intact immune systems may yield better clinical outcomes.
Moderna and Merck announced promising results of a personalized mRNA cancer vaccine in combination with a checkpoint inhibitor in KEYNOTE-942, a randomized, prospective, open-label phase 2b study. mRNA-4157/V970 is a novel mRNA-based personalized cancer vaccine consisting of a synthetic mRNA encoding up to 34 neoantigens that is designed and produced based on the unique mutational signature of the DNA sequence of the patient’s tumor. Following complete surgical resection of high-risk stage III/IV melanoma, patients received adjuvant mRNA-4157/V940 combined with pembrolizumab versus adjuvant pembrolizumab alone for 1 year until disease recurrence or unacceptable toxicity. The primary endpoint of recurrence-free survival was statistically significant with an HR of 0.56 (p=0.0266) favoring mRNA-4157/V940 combined with pembrolizumab.42 This is the first prospective randomized study of a cancer neoantigen vaccine that has demonstrated statistically significant clinical efficacy.
Oncolytic virus vaccines
Talimogene laherparepvec (T-VEC), a first-in-class oncolytic virus therapy, was FDA approved in 2015 for the local treatment of unresectable cutaneous, subcutaneous, and nodal lesions in patients with melanoma recurrent after initial surgery. T-VEC is a herpes simplex virus genetically engineered to incorporate GM-CSF and delete ICP34.5 and ICP47. It is designed to preferentially replicate in tumors, produce GM-CSF, and stimulate antitumor immune responses.43 Intratumoral injection of T-VEC is thought to trigger both local and systemic immunological responses leading to cell lysis, the release of TAAs, and subsequent activation of innate and adaptive immune systems to induce tumor antigen-specific effector T-cell antitumor immunity. T-VEC’s approval was based on the pivotal phase 3 OPTiM study, which showed significant improvements in durable response rate (16.3% vs 2.1%), overall response rate (ORR) (26.4% vs 5.7%), and CR rate (11% vs 1%) compared with GM-CSF-treated patients. Patients treated with T-VEC experienced an OS of 23.3 months compared with 18.9 months in GM-CSF-treated patients. Clinical benefit in both responder rate and OS was observed in treatment-naïve, advanced-stage melanoma.44–46
In December 2022, the FDA approved nadofaragene firadenovec, the first oncolytic virus therapy, for treatment of high-risk, BCG-refractory non-muscle-invasive bladder cancer (NMIBC) carcinoma in situ with or without papillary tumors. Nadofaragene firadenovec, a non-replicating adenovirus delivered intravesically, was evaluated in a multicenter clinical study where it achieved a CR rate of 51% with a 9.7-month median duration of response in patients with high-risk BCG-refractory NMIBC.47 48
Other viruses have been studied as systemically or intratumorally administered in situ vaccines. Coxsackievirus A21 (CAVATAK) oncolytic virus administered intratumorally into melanoma lesions elicited abscopal responses in non-injected metastatic lesions, suggesting induction of systemic antitumor immunity. However, melanoma patients treated with CAVATAK as monotherapy had a confirmed ORR of only 28.1% and a 75.4% 12-month OS, which appear to be lower than rates in historical T-VEC studies.49 50
Reolysin is an intravenously administered reovirus serotype 3-Dearing strain, a double-stranded, replication-competent RNA non-enveloped icosahedral virus that induces antitumor activity by activating Ras through inhibition of dsRNA-activated protein kinase. Clinical benefit has been limited to date. Overall, 1 of 8 patients had a PR in a single-center, monotherapy, dose-escalation trial, while in another trial 3/19 patients had an objective response in intralesionally treated tumors.51 52 No responses were seen in a metastatic melanoma trial (n=21) and a pediatric solid tumor study (n=29), which may be explained by the fact that many patients had pre-existing neutralizing antireovirus antibodies.53 54 Reolysin in combination with checkpoint inhibition or chemotherapy also failed to demonstrate meaningful improvement in clinical outcomes in settings of melanoma, lung, pancreatic, breast, and ovarian cancers.55–61
JX-594 is a re-engineered vaccinia virus that disrupts thymidine kinase genes and inserts GM-CSF and beta-galactosidase transgenes. It is designed to induce viral replication-dependent oncolysis and antitumor immunity in hepatocellular carcinoma and melanoma.62–65 In a phase 2 study, 30 patients had either high-dose or low-dose JX-594 infused into liver tumors. A statistically significant improvement in median survival of 14.1 months compared with 6.7 months was seen with the high and low dose, respectively. Evidence of induction of humoral and cellular antitumoral immunity was seen in ex vivo assays.66
PV701, a replication-competent strain of Newcastle disease virus, has been studied across multiple tumor types. In a phase 1 study, intravenous administration of PV701 achieved one CR and one PR out of 62 evaluable patients across multiple tumor types. Post-treatment tumor biopsies showed histological evidence of increased inflammation within the tumor microenvironment.67 In another phase 1/2 study, an oncolytic HUJ strain of Newcastle disease virus was studied as an intravenous monotherapy in patients with recurrent glioblastoma. Of 11 treated patients, only 1 had a CR.68
Overall, oncolytic virus monotherapy has yielded very limited clinical benefit. Combinations of oncolytic viruses with checkpoint inhibitors are proving to be more promising. Multiple studies combining T-VEC with checkpoint inhibitors have been performed to try to improve antitumor efficacy. In an open-label phase 1b study of T-VEC combined with ipilimumab in the front-line treatment of unresectable stage IIIB–IV melanoma, 50% of treated patients had an objective response, with most patients experiencing a durable 18-month PFS and OS of 50% and 67%, respectively.69 Similarly, in an open-label phase 2 study, T-VEC combined with ipilimumab in early treatment of unresectable melanoma demonstrated a 39% objective response compared with 18% in the ipilimumab-only arm. Notably, an abscopal effect was observed in non-injected visceral lesions in 52% of patients receiving T-VEC combined with ipilimumab compared with 23% in the ipilimumab-only arm.70
T-VEC has also been combined with pembrolizumab. In a phase 1b study, recurrent or metastatic squamous cell carcinoma of the head and neck treated with T-VEC and pembrolizumab had a 13.9% ORR. Yet there were significant adverse events, including fatal arterial hemorrhage related to T-VEC, and the ORR was not better than pembrolizumab monotherapy in historical studies.71 In another study involving 20 patients with locally advanced or metastatic sarcoma treated with T-VEC and pembrolizumab, the ORR was 35% with a tolerable safety profile.72 Pembrolizumab combined with T-VEC in 21 melanoma patients resulted in an ORR of 62% and a CR rate of 33%.73 However, in a phase 3 study (n=692) in patients with stage IIIB-IVM1c unresectable melanoma naïve to PD-1, T-VEC combined with pembrolizumab failed to significantly improve PFS or OS compared with placebo combined with pembrolizumab.74 T-VEC appears to have activity in the neoadjuvant setting for surgically resectable melanoma. Neoadjuvant T-VEC showed a 2-year recurrence-free survival rate of 29.5% vs 16.5% and a 2-year OS rate of 88.9% vs 77.4% compared with surgery only. Interestingly, increased tumor infiltration by CD8 T cells was associated with improved clinical outcomes.73 75
CG0070 is a replication-competent oncolytic adenovirus genetically modified to express GM-CSF under control of the human E2F-1 promoter. The virus is being developed for bladder cancer due to loss of retinoblastoma tumor suppressor activity commonly seen in that disease, which leads to upregulation of the E2F-1 transcription factor. Promising results were reported in an early phase 1/2 trial of intravesical CG0070 in patients with recurrent T1, Ta, and Tcis bladder cancer after BCG treatment. The CR rate was 23% and 64% in the single-dose and multidose cohorts, respectively. Durable responses of up to 38.2 months were observed in some patients in the multidose cohort. In subsequent studies, CG0070 monotherapy in 45 patients with BCG-refractory high-grade NMIBC led to a 58% 6-month CR rate in pure CIS patients and an overall 6-month CR rate of 47% with good tolerability. More recently, CG0070 in combination with pembrolizumab was studied in BCG-refractory NMIBC. Of 24 treated patients, 22 achieved a 3-month CR that persisted up to 12 months; 6/8 evaluable patients remain in CR.76
Vusolimogene oderparepvec (RP1) is a novel engineered HSV-1 oncolytic virus that expresses GM-CSF and GALV-GP R–. Intratumoral RP1 has been studied in combination with systemic nivolumab in patients with melanoma. ORR was 36.1% (13/36) in melanoma patients and, notably, 37.5% (6/16) in patients who had failed treatment with anti-PD1/anti-PDL-1+anti-CTLA-4.77 Intratumoral RP1 plus nivolumab was studied in a larger phase 2 clinical trial (IGNYTE; NCT03767348) in patients with cutaneous melanoma who failed previous anti-PD-1 therapy. At a median follow-up of 9.96 months, the first 75 patients enrolled on the trial achieved an ORR of 36%, including a CR rate of 20%.78
To date, clinical studies have shown that oncolytic viruses are most effective when delivered in combination with a checkpoint inhibitor. However, intralesional or local administration is necessary due to the prevalence of circulating neutralizing antibodies.79 This drastically limits the types of tumors that may be treated by oncolytic viruses because most cancers are not easily accessible cutaneously or by minimally invasive procedures. Innovations in viral capsid engineering and viral drug delivery technologies may enable systemic delivery to a wider range of tumor types. While oncolytic viruses elicit immunological responses against a heterogeneous set of de novo tumor and non-tumor antigens released by replication-induced tumor-cell lysis, autologous-cell vaccines have the benefit of inducing a more focused immune response against defined antigens.
Autologous-cell vaccines
Autologous-cell vaccines that use either killed cancer cells or cancer antigen-primed antigen-presenting cells have been studied clinically. While sipuleucel-T is engineered to react to one specific antigen, DCs can be primed with different or multiple antigens to treat additional types of cancers. GVAX vaccines are GM-CSF-secreting cell vaccines prepared with different vectors and vector targets, including autologous tumor cells, allogeneic tumor-cell lines, and bystander third-party tumor-cell lines. They promote DC antigen presentation, activation, and survival. Studies testing GVAX in melanoma, glioma, prostate, and lung cancer have demonstrated limited efficacy despite being able to stimulate an immune response; a phase 3 study of GVAX for prostate cancer failed to show benefit.80–85 Below we discuss autologous-cell vaccine results from early phase 1 studies.
Hirschowitz et al reported that a minority of patients with lung cancer receiving an autologous DC vaccine pulsed with Her2, CEA, WT1, MAGE2, and survivin-expressing apoptotic bodies of an allogeneic lung cancer cell line induced antigen-specific immune responses.86 Indeed, the heterogeneity of patient baseline immune profiles makes it challenging to control the quality of autologous DC vaccines and even harder to optimize their immunological impact in response to the infusion of such a bespoke vaccine product.
Autologous DCs may also be pulsed with tumor lysates to prime them for a broader array of TSAs and neoantigens. This type of DC vaccine was studied in combination with IL-2 and IL-alpha2a for the treatment of metastatic RCC. Of 18 treated patients, 50% achieved a confirmed response, including 3 who experienced a CR, while median survival was not reached after a follow-up of more than 37 months. When investigators analyzed responding patients’ immunological profiles, NK cells and Th2 T cells were significantly increased, and T-regulatory cells were markedly reduced compared with non-responders.87 In a further effort to induce activation of innate immune cells, eight patients with high-risk surgically resected stages II–IV melanoma were treated with autologous DCs loaded with the NKT-cell agonist α-GalCer and peptides derived from NY-ESO-1. Vaccination induced NKT-cell activation and peptide-specific T-cell response. However, the study did not report on any clinical outcomes.88 In a similar approach, autologous tumor lysate-pulsed DC vaccines in combination with cytokine-induced NK cells administered after surgery with or without chemoradiotherapy in gastric and colorectal cancer reduced the risk of postoperative disease progression and improved OS. Vaccinated patients also had measurably higher levels of IFN-γ and IL-12 proinflammatory cytokines.89 Another group administered Wilms’ tumor antigen 1 (WT1)-expressing artificial adjuvant vector cells into nine patients with relapsed/refractory acute myelogenous leukemia. Immunological activation of iNKT and/or NK cells was observed in all treated patients. Five of the patients who generated WT1-specific T-cell responses also experienced leukemic regression.90
A DC vaccine targeting cancer stem cells (CSCs) for the treatment of glioblastoma was studied in seven patients in combination with postoperative chemoradiotherapy. This individualized vaccine was produced by dissociating brain tumor biopsies into single-cell suspensions, followed by in vitro expansion of autologous CSCs into tumor spheres and, finally, amplification and transfection of CSC-mRNA into monocyte-derived autologous DCs. A vaccine-induced immune response was identified in all seven treated patients. Compared with matched historical controls, PFS was statistically longer in vaccinated patients (median 694 vs 236 days; p=0.0018).91
Other autologous-cell vaccines have been studied in larger randomized phase 2 trials with some promising results in multiple tumor types. DC vaccines loaded with tumor lysates were studied for their ability to delay disease relapse in patients with colon cancer liver metastasis. All 19 randomized patients were treated surgically with neoadjuvant and/or adjuvant chemotherapy. Patients treated with DC vaccines had longer disease-free survival compared with patients in the observation-only arm. Like other reported studies of tumor lysate-pulsed DC vaccines, serum IL-12 levels in patients were higher after vaccination.92 A phase 2 study compared autologous DC vaccines to autologous tumor-cell vaccines in metastatic melanoma. Patients treated with a DC vaccine (n=42) demonstrated a longer median OS than patients receiving a tumor-cell vaccine (43.4 vs 20.5 months, respectively), with a statistically significant HR of 0.304.93
In a study by Levy et al, patients with mantle cell lymphoma in remission postimmunochemotherapy were vaccinated with irradiated CpG-activated tumor cells. Vaccine-primed lymphocytes were collected and reinfused after standard autologous stem cell transplantation. Vaccinated patients who generated a memory CD8 T-cell response experienced a significantly longer PFS after autologous stem cell transplantation. Higher PD-L1 expression in tumor cells following CpG induction was associated with poor outcomes but not with failure to elicit vaccine-induced memory CD8 T-cell response.94
Another report studied gemogenovatucel-T (Vigil), a vaccine manufactured from harvested tumor tissue and transfected with hGM-CSF and a bifunctional short-hairpin RNA construct targeting furin and TGF-β1 and TGF-β2. The vaccine was administered as maintenance therapy in patients with stage III/IV ovarian cancer who achieved a clinical CR after surgery and chemotherapy. Patients vaccinated with Vigil had a recurrence-free survival of 11.5 months compared with 8.4 months for patients treated with placebo. Patients with BRCA wild-type tumors had better outcomes, which may be explained by a more concentrated clonal neoantigen exposure compared with BRCA-mutated tumors. Vaccine-induced GM-CSF increases and TGF-β1 knockdown levels did not correlate with improved outcomes as expected and may require further investigation.95 Follow-on studies are now investigating Vigil in combination with checkpoint inhibitors.96
In a larger double-blind, placebo-controlled phase 2 trial, patients newly diagnosed with glioblastoma were randomized 2:1 to receive adjuvant ICT-107, a DC vaccine pulsed with six synthetic peptide epitopes targeting the GBM tumor/stem cell-associated antigens MAGE-1, HER-2, AIM-2, TRP-2, gp100, and IL13Rα2, or a matching unpulsed DC control after radiotherapy with concurrent temozolomide. Patients receiving the adjuvant DC vaccine demonstrated a trend toward improved median OS in the intent-to-treat population while posting a 2.2-month statistically significant improvement in PFS. In particular, PFS for HLA-A2+ patients with MGMT promoter methylation was significantly increased in the ICT-107 group (24.1 months) compared with the control group (8.5 months). IFN-γ ELISpot was used to detect immune responders. HLA-A2+ patients vaccinated with ICT-107 had a much higher rate of immune response compared with control (86% vs 33%, respectively). Importantly, immune responders experienced improved OS compared with non-responders. Investigators suggested that unpulsed DCs may not have been an appropriate negative control since they may have processed free tumor antigen in tumor-draining lymph nodes to prime T cells.97 Overall, autologous-cell vaccines have demonstrated promising clinical outcomes and predictive biomarkers in phase 2 studies and warrant further investigation.
Innate immune agonists
Immunophenotyping assays from therapeutic cancer vaccines have shed light on the importance of the innate immune system in orchestrating potent adaptive immunity. Innate immune cells such as DCs are involved in the presentation of TAAs or TSAs and may be further activated through sensing of pathogen-associated or damage-associated molecular patterns followed by the release of proinflammatory cytokines, while engaging with adaptive immunity by priming and activating antigen-specific T cells within the tumor microenvironment. Furthermore, innate immune cells such as NK cells and macrophages also play a pivotal role in antigen-independent phagocytic tumor lysis and processing of antigens.98 Thus, therapeutic strategies aimed at invigorating innate immunity using STimulator of INterferon Genes (STING), toll-like-receptors (TLRs), and retinoic acid-inducible gene-I (RIG-I)-like receptors are being investigated for their potential to augment cancer vaccines and other immunotherapeutic modalities.
MK-1454 and ADU-S100 are intratumorally delivered STING agonists that have been studied as monotherapy or in combination with checkpoint inhibitors. In a phase 1 study, MK-1454 combined with pembrolizumab induced PRs in multiple tumor types, with observed elevations in serum cytokines IL-6 and IP-10 and STING-induced gene expressions.99 ADU-S100 also induced confirmed tumor responses when combined with checkpoint inhibitors but not as monotherapy in early-stage trials.100–102 Numerous other STING-agonistic agents are currently under clinical investigation.103
PF-3512676, a synthetic cytosine-phosphate-guanine oligodeoxynucleotide TLR9 agonist, has been well studied. In two open-label phase 1 studies, objective responses including CR were observed in basal-cell carcinoma and melanoma with PF-3512676 as monotherapy and in lung cancer when PF-3512676 was combined with carboplatin and paclitaxel.104 105 A subsequent phase 2 study of PF-3512676 in combination with first-line chemotherapy for advanced NSCLC showed significantly improved objective responses and a trend toward improved OS compared with chemotherapy only.106 Despite the promising results, a confirmatory phase 3 study was terminated prematurely due to futility as the combination therapy failed to show improvement in median PFS or OS.107 SD-101, another TLR9 agonist, showed promising antitumor efficacy when combined with pembrolizumab. Patients with advanced melanoma and head and neck cancer had confirmed response rates of 78% and 30.4%, respectively. As expected, lower activity was observed in patients who had received prior anti-PD-1 therapy. RNA profiling of tumor biopsies demonstrated increased immune activation within the tumor microenvironment.108–110 One patient with gastric cancer treated with the RIG-I agonist MK-4621 plus bevacizumab had a durable CR of >560 days.111
The promising activity of innate immune agonists in early-stage phase 1 studies has generally failed to translate into later-stage trials. Many of these agents are restricted to intratumoral injections, which may make it more difficult to achieve consistent activity in later-stage trials involving multiple tumor sites. Optimal therapeutic sequencing, combination, formulation, and tumor indication all warrant further investigation.
Cytotoxic therapy as priming therapy
Conventional cytotoxic therapy, including chemotherapy and radiotherapy, has an important role in cytoreduction and release of tumor antigens, which may be an effective priming therapy for cancer vaccines. Patients who were pretreated with a PSA-expressing recombinant vaccinia virus vaccine had higher PSA-specific T-cell responses and longer PFS when subsequently treated with docetaxel than patients in a historical control who received docetaxel alone.112 In another study, a vaccine composed of a plasmid DNA of CYP1B1 encapsulated in biodegradable poly-DL-lactide-coglycolide microparticles was administered to patients with advanced cancer, which led to meaningful durable clinical responses to subsequent salvage chemotherapy.113 Radiation therapy has been shown to induce type 1 interferon in the treated tumor and promote activation of antitumor T-cell immunity and abscopal tumor responses by augmenting exposure to immunogenic mutations.114–116 In chemorefractory metastatic NSCLC, radiation therapy and CTLA-4 blockade induced systemic antitumor T cells and led to an 18% ORR and a 31% disease control rate.117 Intriguingly, functional analysis in one of the responders demonstrated in vivo expansion of KPNA2-reactive CD8 T cells that recognize a neoantigen derived from a gene that is upregulated by radiation therapy.118
Discussion
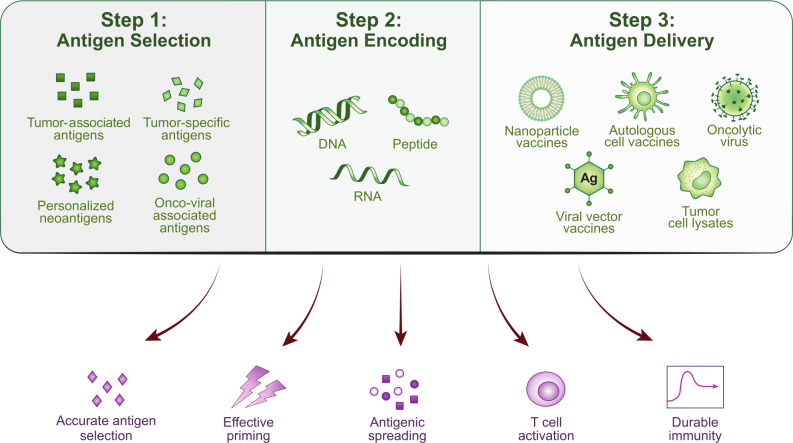
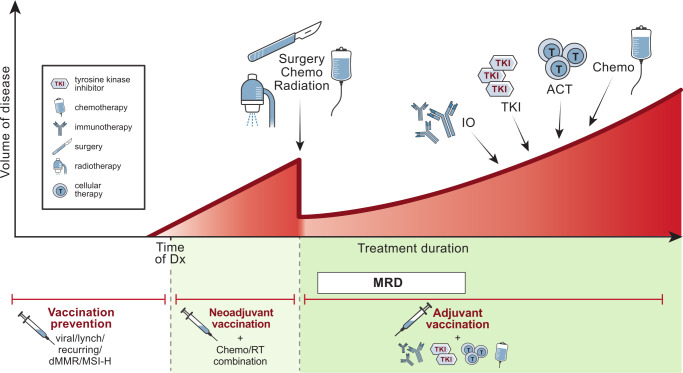
The field of therapeutic cancer vaccines has seen vibrant innovation in the last decade, as evidenced by the diverse therapeutic vaccines undergoing clinical studies (table 1). However, after earlier FDA approvals in autologous-cell vaccines and oncolytic viruses, we have yet to see any follow-on agents in this class demonstrate compelling clinical benefit in late-stage trials. That said, the wealth of clinical lessons derived from these studies, along with new insights into immunophenotyping, have laid fertile groundwork for investigators to produce the next breakthrough. Designing a cancer vaccine involves careful planning and starts with selection of an antigen, followed by choosing a method of antigen encoding, and finally deciding on how the antigen can best be delivered. mRNA encoding of antigen would necessitate use of specific delivery modalities, such as nanoparticles, for example. An effective vaccine should promote immunological properties which include relevant antigen selection, effective priming, antigenic spreading, T-cell activation, and durable immunity (figure 2). We have seen that checkpoint blockade combinations with personalized cancer vaccines have not yielded compelling clinical responses to date, which may support the hypothesis of inadequate or defective antigenic priming as one reason for failure.119–121 A diverse T-cell repertoire appears to be an important common factor among various cancer vaccines. Immunophenotyping data have revealed clonotypic diversification of intraprostatic T cells following treatment with sipuleucel-T, suggesting that T cells are being recruited into the tumor microenvironment.13 Similarly, in melanoma patients treated postresection with an autologous IL-12p70-producing DC vaccine, vaccination promoted a diverse neoantigen-specific T-cell receptor repertoire in terms of both T-cell receptor-β usage and clonal composition.122 Novel combination approaches such as a CLND6-encoding mRNA vaccine combined with CLD6 CAR-T cells has shown clinical responses in 45% of 11 treated patients with treatment-refractory ovarian and testicular cancers.123 These findings, along with the excellent safety profile of cancer vaccines, pave the way for studies of more complex combinations of chemotherapy, radiotherapy, immunotherapy, cell therapy, or surgery to augment antitumor immunity. Finally, we need to consider whether certain cancer vaccines could demonstrate greater clinical benefit if used as upfront interventions in the neoadjuvant/adjuvant setting (figure 3).
Table 1.
Summary of cancer vaccines
| Vaccine name | Delivery | Antigen | Antigen type | Antigen encoding | Clinical setting | Tumor type | Phase | Clinical outcome |
| Sipuleucel-T9 10 | Autologous cell | PAP | TAA | Protein | Advanced/metastatic | Prostate | 3 | OS benefit |
| PROSTVAC-VF27 | Poxvirus | PSA | TAA | DNA | Advanced/metastatic | Prostate | 3 | Terminated due to futility and treatment-related cardiac arrythmias |
| FRα peptide vaccine18 | Peptide | FRα | TAA | Peptide | Advanced/metastatic | Breast, ovarian | 1 | Well tolerated; efficacy non evaluable |
| GP2 peptide vaccine19 | peptide | GP2 (Her2/neu) | TAA | Peptide | Advanced/metastatic | Breast | 1 | Well tolerated; efficacy non evaluable |
| Galinpepimut-S20 | Peptide | WT1 | TAA | Peptide | Advanced/metastatic | AML | 2 | Majority relapsed, improved DFS in those who had immune response |
| VX-00121 | Peptide | TERT | TAA | peptide | Advanced/metastatic | NSCLC | 2 | No OS benefit |
| Allo-SCT22 | – | CT-RCC-1 HERV-E | TAA | Peptide | Adjuvant | RCC | 1 | Tumor regression |
| NeoVax38 39 | Peptide | Personalized Neoantigen | TSA | Peptide | Adjuvant | Melanoma, Glioma | 1 | No tumor responders |
| mRNA-465040 | mRNA | Personalized Neoantigen | TSA | RNA | Metastatic | GI | 1 | No tumor responders |
| GAPVAC-10141 | Peptide | Personalized Neoantigen | TSA | Peptide | Adjuvant | Glioma | 1 | Well tolerated; efficacy non evaluable |
| mRNA-4157/V97042 | mRNA | Personalized Neoantigen | TSA | RNA | Adjuvant | Melanoma | 2 | RFS benefit in anti- PD-1 combo vs anti-PD-1 monotherapy arm |
| NOUS-20937 | Adenovirus | Personalized Neoantigen | TSA | Peptide | Advanced/metastatic | dMMR/MSI tumors | 1 | Partial responses seen in combo with anti-PD1 |
| GRT-C903/GRT-R90433 | Adenovirus | KRAS neoantigens | TSA | RNA | Advanced/metastatic | KRAS tumors - NSCLC, CRC | 1 | ctDNA molecular response; no radiographic tumor responders |
| VGX-310034 | DNA plasmid | E6, E7 HPV | TSA | DNA | Neoadjuvant | HPV cancers - Cervical, Head and neck | 2 | Higher rates of histopathological regression and clearance |
| Talimogene laherparepvec44 46 | Oncolytic virus | None | n/a | n/a | Advanced/metastatic | Melanoma | 3 | OS benefit |
| CAVATAK49 | Oncolytic virus | None | n/a | n/a | Advanced/metastatic | Melanoma | 2 | 28.1% ORR |
| Reolysin50 53 55 57 | Oncolytic virus | None | n/a | n/a | Advanced/metastatic | Melanoma, lung, pancreatic, breast, and ovarian cancers | 2 | Limited responders and clinical benefit |
| JX-59462 63 | Oncolytic virus | None | n/a | n/a | Advanced/metastatic | Hepatocellular carcinoma, melanoma | 2 | OS benefit in high dose vs low dose |
| CG007076 | Oncolytic virus | None | n/a | n/a | BCG refractory | NMIBC | 2 | Combo with anti-PD1: 92% CR |
| PV70167 | Oncolytic virus | None | n/a | n/a | Advanced/metastatic | multiple | 1 | Limited ORR% |
| Vusolimogene oderparepvec (RP1)77 78 | Oncolytic virus | None | n/a | n/a | Advanced/metastatic | Melanoma | 2 | 36% ORR, 20% CR |
| Nadofaragene firadenovec47 48 | Oncolytic virus | None | n/a | n/a | BCG refractory | NMIBC | 3 | 51% CR, 9.7 mo DoR |
| GVAX80–85 | Autologous cell | None | n/a | n/a | Advanced/metastatic | Melanoma, glioma, prostate and lung | 3 | No OS benefit in prostate study |
| Autologous DC vaccine97 | Autologous cell | Her2/neu, CEA, WT1, Mage2, survivin | TAA | Protein | Adjuvant | NSCLC | 1 | Efficacy non evaluable |
| Tumor lysate DC vaccine87 | Autologous cell | None | n/a | n/a | Advanced/metastatic | RCC, gastric, CRC | 2 | 50% ORR in RCC in combo with IL2 and IFN-α2a |
| Cancer stem cell DC vaccine91 | Autologous cell | None | n/a | n/a | Adjuvant | Glioma | 1 | Improved PFS compared with matched historical control |
| Tumor lysate DC vaccine89 | Autologous cell | None | n/a | n/a | Advanced/metastatic | CRC, melanoma | 2 | Longer OS in DC vaccine compared with tumor cell vaccine in melanoma |
| Irradiated CpG-activated tumor cells94 | Autologous cell | None | n/a | n/a | Advanced/metastatic | Mantle cell lymphoma | 2 | Efficacy non evaluable |
| Gemogenovatucel-T95 | Autologous cell | None | n/a | n/a | Maintenance | Ovarian | 2 | Improved RFS compared with placebo |
| aAVC-WT190 | Autologous cell | WT1 | TAA | Protein | Advanced/metastatic | AML | 1 | Best response CRi |
| DC (α-GalCer, NY-ESO-1)88 | Autologous cell | NY-ESO-1 | TAA | Protein | Adjuvant | Melanoma | 1 | Efficacy non evaluable |
| ICT-10797 | Autologous cell | MAGE-1, HER-2, AIM-2, TRP-2, gp100, and IL13Rα2 | TAA | Protein | Adjuvant | Glioma | 2 | DFS benefit |
| MK-145499 | Innate Immune Agonists | None | n/a | n/a | Advanced/metastatic | Multiple tumor types | 1 | Partial responses seen in anti-PD1 combination |
| ADU-S100101 102 | Innate Immune Agonists | None | n/a | n/a | Advanced/metastatic | Multiple tumor types | 2 | Partial responses seen in anti-PD1 combination |
| PF-3512676104–107 | Innate Immune Agonists | None | n/a | n/a | Advanced/metastatic | Basal cell carcinoma, melanoma, lung, | 3 | Terminated due to futility: failed to improve PFS, OS in NSCLC compared with chemo |
| SD-101108–110 | Innate Immune Agonists | None | n/a | n/a | Advanced/metastatic | Melanoma, head and neck | 2 | Tumor responses seen with anti-PD1 combination |
| MK-4621111 | Innate Immune Agonists | None | n/a | n/a | Advanced/metastatic | Multiple tumor types | 2 | Limited tumor responses |
CR, complete response; CRC, colorectal cancer; DC, dendritic cell; DFS, disease-free survival; dMMR/MSI, deficiency in mismatch repair or microsatellite instability; DoR, Duration of response; HPV, Human papilloma virus; n/a, not available; NMIBC, non-muscle-invasive bladder cancer; NSCLC, non-small cell lung cancer; ORR, overall response rate; OS, overall survival; PFS, progression-free survival; PSA, prostate-specific antigen; RCC, Renal cell carcinoma; RFS, recurrence-free survival; TAA, tumor-associated antigen; TSA, tumor-specific antigen.
Figure 2.
Stepwise design of cancer vaccines. Step 1: Selection of antigens which may be cognate tumor-associated antigens or tumor-specific antigens such as oncoviral-associated antigens, non-cognate tumor-specific antigens, or personalized neoantigens. Step 2: Encoding tumor antigens using either DNA, RNA, or peptides. Step 3: Packaging tumor antigens into delivery systems such as nanoparticles, autologous immune cells, oncolytic viruses, viral vectors, or tumor-cell lysates. Downstream immunological efficacy is measured by accurate antigen selection, effective immune priming, antigenic spreading, antigen-specific T-cell activation, and durable immunity.
Figure 3.
Cancer vaccine combination strategies for early-stage cancer. Conventional modalities (chemotherapy, surgery, radiotherapy (RT), tyrosine kinase inhibitors (TKI), cell therapy) of treatment according to tumor volume are illustrated by the red graph (top half). Introducing tumor vaccines combined with other modalities in the adjuvant, neoadjuvant, and prevention stages of cancer is illustrated in the green graph (bottom half). IO, immune modulators; MRD, minimal residual disease; ACT, adoptive cell therapy; dMMR, deficiency in mismatch repair; MSI, microsatellite instability.
Footnotes
Twitter: @gulleyj1
Contributors: All authors wrote, revised the manuscript and figures. All authors have read and approved the final manuscript.
Funding: LF was supported by the Parker Institute of Cancer Immunotherapy, the Prostate Cancer Foundation, and NIH U01CA233100, U01CA244452, and R35CA253175.
Competing interests: LF has received research support from Roche/Genentech, Abbvie, Bavarian Nordic, Bristol Myers Squibb, Dendreon, Janssen, Merck, and Partner Therapeutics. LF has served on the scientific advisory boards of Actym, Allector, Astra Zeneca, Atreca, Bioalta, Bolt, Bristol Myer Squibb, Daiichi Sankyo, Immunogenesis, Innovent, Merck, Merck KGA, Nutcracker, RAPT, Scribe, Senti, Soteria, Sutro, and Roche/Genentech.
Provenance and peer review: Not commissioned; externally peer reviewed.
Ethics statements
Patient consent for publication
Not applicable.
References
- 1. Cancer Research Institute . PD-1/PD-L1 landscape. Available: https://www.cancerresearch.org/pd-1-pd-l1-landscape [Accessed 21 Mar 2023].
- 2. US Food and Drug Administration . BCG LIVE (for intravesical use). Organon package insert. Available: https://www.fda.gov/media/76396/download [Accessed 21 Mar 2023].
- 3. Lamm DL, Blumenstein BA, David Crawford E, et al. Randomized Intergroup comparison of Bacillus Calmette-Guerin Immunotherapy and mitomycin C chemotherapy prophylaxis in superficial transitional cell carcinoma of the bladder a Southwest oncology group study. Urol Oncol 1995;1:119–26. 10.1016/1078-1439(95)00041-f [DOI] [PubMed] [Google Scholar]
- 4. Redelman-Sidi G, Glickman MS, Bochner BH. The mechanism of action of BCG therapy for bladder cancer--a current perspective. Nat Rev Urol 2014;11:153–62. 10.1038/nrurol.2014.15 [DOI] [PubMed] [Google Scholar]
- 5. Simpson AJG, Caballero OL, Jungbluth A, et al. Cancer/Testis antigens, Gametogenesis and cancer. Nat Rev Cancer 2005;5:615–25. 10.1038/nrc1669 [DOI] [PubMed] [Google Scholar]
- 6. Hollingsworth RE, Jansen K. Turning the corner on therapeutic cancer vaccines. NPJ Vaccines 2019;4:7. 10.1038/s41541-019-0103-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Pedersen SR, Sørensen MR, Buus S, et al. Comparison of vaccine-induced Effector Cd8 T cell responses directed against Self- and non-self-tumor antigens: implications for cancer Immunotherapy. J Immunol 2013;191:3955–67. 10.4049/jimmunol.1300555 [DOI] [PubMed] [Google Scholar]
- 8. Kantoff PW, Higano CS, Shore ND, et al. Sipuleucel-T Immunotherapy for Castration-resistant prostate cancer. N Engl J Med 2010;363:411–22. 10.1056/NEJMoa1001294 [DOI] [PubMed] [Google Scholar]
- 9. Small EJ, Schellhammer PF, Higano CS, et al. Placebo-controlled phase III trial of immunologic therapy with Sipuleucel-T (Apc8015) in patients with metastatic, asymptomatic hormone refractory prostate cancer. J Clin Oncol 2006;24:3089–94. 10.1200/JCO.2005.04.5252 [DOI] [PubMed] [Google Scholar]
- 10. Higano CS, Schellhammer PF, Small EJ, et al. Integrated data from 2 randomized, double-blind, placebo-controlled, phase 3 trials of active cellular Immunotherapy with Sipuleucel-T in advanced prostate cancer. Cancer 2009;115:3670–9. 10.1002/cncr.24429 [DOI] [PubMed] [Google Scholar]
- 11. GuhaThakurta D, Sheikh NA, Fan L-Q, et al. Humoral immune response against Nontargeted tumor antigens after treatment with Sipuleucel-T and its association with improved clinical outcome. Clin Cancer Res 2015;21:3619–30. 10.1158/1078-0432.CCR-14-2334 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Fong L, Carroll P, Weinberg V, et al. Activated lymphocyte recruitment into the tumor Microenvironment following preoperative Sipuleucel-T for localized prostate cancer. J Natl Cancer Inst 2014;106:dju268. 10.1093/jnci/dju268 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Sheikh N, Cham J, Zhang L, et al. Clonotypic diversification of Intratumoral T cells following Sipuleucel-T treatment in prostate cancer subjects. Cancer Res 2016;76:3711–8. 10.1158/0008-5472.CAN-15-3173 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Zhang L, Kandadi H, Yang H, et al. Long-term Sculpting of the B-cell repertoire following cancer Immunotherapy in patients treated with Sipuleucel-T. Cancer Immunol Res 2020;8:1496–507. 10.1158/2326-6066.CIR-20-0252 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Pachynski RK, Morishima C, Szmulewitz R, et al. IL-7 expands lymphocyte populations and enhances immune responses to Sipuleucel-T in patients with metastatic Castration-resistant prostate cancer (mCRPC). J Immunother Cancer 2021;9:e002903. 10.1136/jitc-2021-002903 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Sinha M, Zhang L, Subudhi S, et al. Pre-existing immune status associated with response to combination of Sipuleucel-T and Ipilimumab in patients with metastatic Castration-resistant prostate cancer. J Immunother Cancer 2021;9:e002254. 10.1136/jitc-2020-002254 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Marshall CH, Fu W, Wang H, et al. Randomized phase II trial of Sipuleucel-T with or without Radium-223 in men with bone-metastatic Castration-resistant prostate cancer. Clin Cancer Res 2021;27:1623–30. 10.1158/1078-0432.CCR-20-4476 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Kasi PM, Kalli K, Block MS, et al. A phase I trial of the safety and Immunogenicity of a multi-EPITOPE folate receptor alpha peptide vaccine used in combination with cyclophosphamide in subjects previously treated for breast or ovarian cancer. JCO 2015;33:e14028. 10.1200/jco.2015.33.15_suppl.e14028 [DOI] [Google Scholar]
- 19. Carmichael MG, Benavides LC, Holmes JP, et al. Results of the first phase 1 clinical trial of the HER-2/Neu peptide (Gp2) vaccine in disease-free breast cancer patients: United States military cancer Institute clinical trials group study I-04. Cancer 2010;116:292–301. 10.1002/cncr.24756 [DOI] [PubMed] [Google Scholar]
- 20. Maslak PG, Dao T, Bernal Y, et al. Phase 2 trial of a multivalent Wt1 peptide vaccine (Galinpepimut-S) in acute myeloid leukemia. Blood Adv 2018;2:224–34. 10.1182/bloodadvances.2017014175 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Gridelli C, Ciuleanu T, Domine M, et al. Clinical activity of a Htert (Vx-001) cancer vaccine as post-chemotherapy maintenance Immunotherapy in patients with stage IV non-small cell lung cancer: final results of a randomised phase 2 clinical trial. Br J Cancer 2020;122:1461–6. 10.1038/s41416-020-0785-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Takahashi Y, Harashima N, Kajigaya S, et al. Regression of human kidney cancer following allogeneic stem cell transplantation is associated with recognition of an HERV-E antigen by T cells. J Clin Invest 2008;118:1099–109. 10.1172/JCI34409 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Madan RA, Arlen PM, Mohebtash M, et al. Prostvac-VF: a vector-based vaccine targeting PSA in prostate cancer. Expert Opinion on Investigational Drugs 2009;18:1001–11. 10.1517/13543780902997928 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Mandl SJ, Rountree RB, Dela Cruz TB, et al. Elucidating immunologic mechanisms of PROSTVAC cancer Immunotherapy. J Immunother Cancer 2014;2:34. 10.1186/s40425-014-0034-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Campbell CT, Gulley JL, Oyelaran O, et al. Humoral response to a viral Glycan correlates with survival on PROSTVAC-VF. Proc Natl Acad Sci USA 2014;111:E1749–58. 10.1073/pnas.1314722111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. DiPaola RS, Plante M, Kaufman H, et al. A phase I trial of Pox PSA vaccines (PROSTVAC-VF) with B7-1, ICAM-1, and LFA-3 Co-stimulatory molecules (TRICOM) in patients with prostate cancer. J Transl Med 2006;4:1. 10.1186/1479-5876-4-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Gulley JL, Borre M, Vogelzang NJ, et al. Phase III trial of PROSTVAC in asymptomatic or minimally symptomatic metastatic Castration-resistant prostate cancer. J Clin Oncol 2019;37:1051–61. 10.1200/JCO.18.02031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Gulley JL, Heery CR, Madan RA, et al. Phase I study of Intraprostatic vaccine administration in men with locally recurrent or progressive prostate cancer. Cancer Immunol Immunother 2013;62:1521–31. 10.1007/s00262-013-1448-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Madan RA, Mohebtash M, Arlen PM, et al. Ipilimumab and a Poxviral vaccine targeting prostate-specific antigen in metastatic Castration-resistant prostate cancer: a phase 1 dose-escalation trial. Lancet Oncol 2012;13:501–8. 10.1016/S1470-2045(12)70006-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Abdul Sater H, Marté JL, Donahue RN, et al. Neoadjuvant PROSTVAC prior to radical Prostatectomy enhances T-cell infiltration into the tumor immune Microenvironment in men with prostate cancer. J Immunother Cancer 2020;8:e000655. 10.1136/jitc-2020-000655 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Maughan BL, Sanchez A, O’Neil BB, et al. A phase IB/II trial of perioperative Intratumoral MVA-BN-Brachyury (MVA) plus systemic PROSTVAC and Atezolizumab (Atezo) for intermediate-risk and high-risk localized prostate cancer (Atezovax). JCO 2020;38:TPS382. 10.1200/JCO.2020.38.6_suppl.TPS382 [DOI] [Google Scholar]
- 32. Blass E, Ott PA. Advances in the development of personalized Neoantigen-based therapeutic cancer vaccines. Nat Rev Clin Oncol 2021;18:215–29. 10.1038/s41571-020-00460-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Kyi CK, Spira A, Carbone DP, et al. 736Mo personalized, off-the-shelf KRAS Neoantigen-specific Immunotherapy for the treatment of advanced solid tumors: clinical benefit associated with decreases in ctDNA (SLATE-KRAS). Annals of Oncology 2022;33:S880. 10.1016/j.annonc.2022.07.862 [DOI] [Google Scholar]
- 34. Trimble CL, Morrow MP, Kraynyak KA, et al. Safety, efficacy, and Immunogenicity of VGX-3100, a therapeutic synthetic DNA vaccine targeting human Papillomavirus 16 and 18 E6 and E7 proteins for Cervical intraepithelial Neoplasia 2/3: a randomised, double-blind, placebo-controlled phase 2B trial. Lancet 2015;386:2078–88. 10.1016/S0140-6736(15)00239-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Chandra J, Woo WP, Finlayson N, et al. A phase 1, single centre, open label, escalating dose study to assess the safety, tolerability and Immunogenicity of a therapeutic human Papillomavirus (HPV) DNA vaccine (Amv002) for HPV-associated head and neck cancer (HNC). Cancer Immunol Immunother 2021;70:743–53. 10.1007/s00262-020-02720-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. D’Alise AM, Brasu N, De Intinis C, et al. Adenoviral-based vaccine promotes Neoantigen-specific Cd8(+) T cell Stemness and tumor rejection. Sci Transl Med 2022;14:eabo7604. 10.1126/scitranslmed.abo7604 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Overman M, Fakih M, Le D, et al. 410 phase I interim study results of Nous-209, an off-the-shelf Immunotherapy, with Pembrolizumab, for the treatment of tumors with a deficiency in mismatch repair/Microsatellite instability (dMMR/MSI). J Immunother Cancer 2021;9:A441. 10.1136/jitc-2021-SITC2021.410 [DOI] [Google Scholar]
- 38. Hu Z, Leet DE, Allesøe RL, et al. Personal Neoantigen vaccines induce persistent memory T cell responses and EPITOPE spreading in patients with Melanoma. Nat Med 2021;27:515–25. 10.1038/s41591-020-01206-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Keskin DB, Anandappa AJ, Sun J, et al. Neoantigen vaccine generates Intratumoral T cell responses in phase IB glioblastoma trial. Nature 2019;565:234–9. 10.1038/s41586-018-0792-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Cafri G, Gartner JJ, Zaks T, et al. mRNA vaccine-induced Neoantigen-specific T cell immunity in patients with gastrointestinal cancer. J Clin Invest 2020;130:5976–88. 10.1172/JCI134915 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Hilf N, Kuttruff-Coqui S, Frenzel K, et al. Actively personalized vaccination trial for newly diagnosed glioblastoma. Nature 2019;565:240–5. 10.1038/s41586-018-0810-y [DOI] [PubMed] [Google Scholar]
- 42. MODERNA AND MERCK ANNOUNCE MRNA-4157/V940 . An investigationalpersonalized mrna cancer vaccine, in combination with keytruda(r)(pembrolizumab), met primary efficacy endpoint in phase 2b keynote-942trial [press release]. 2022. [Google Scholar]
- 43. Liu BL, Robinson M, Han Z-Q, et al. Icp34.5 deleted herpes Simplex virus with enhanced Oncolytic, immune stimulating, and anti-tumour properties. Gene Ther 2003;10:292–303. 10.1038/sj.gt.3301885 [DOI] [PubMed] [Google Scholar]
- 44. Andtbacka RHI, Kaufman HL, Collichio F, et al. Talimogene Laherparepvec improves durable response rate in patients with advanced Melanoma. J Clin Oncol 2015;33:2780–8. 10.1200/JCO.2014.58.3377 [DOI] [PubMed] [Google Scholar]
- 45. Chesney J, Awasthi S, Curti B, et al. Phase IIIB safety results from an expanded-access protocol of Talimogene Laherparepvec for patients with Unresected, stage IIIB-Ivm1C Melanoma. Melanoma Res 2018;28:44–51. 10.1097/CMR.0000000000000399 [DOI] [PubMed] [Google Scholar]
- 46. Andtbacka RHI, Collichio F, Harrington KJ, et al. Final analyses of Optim: a randomized phase III trial of Talimogene Laherparepvec versus granulocyte-macrophage colony-stimulating factor in Unresectable stage III-IV Melanoma. J Immunother Cancer 2019;7:145. 10.1186/s40425-019-0623-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. US Food and Drug Administration . FDA Approves First Gene Therapy for the Treatment of High-Risk, Non-Muscle-Invasive Bladder Cancer [press release]. 2022. [Google Scholar]
- 48. Boorjian SA, Alemozaffar M, Konety BR, et al. Intravesical Nadofaragene Firadenovec Gene therapy for BCG-unresponsive non-muscle-invasive bladder cancer: a single-arm, open-label, repeat-dose clinical trial. Lancet Oncol 2021;22:107–17. 10.1016/S1470-2045(20)30540-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Andtbacka RH, Shafren DR, Grose M, et al. CAVATAK-mediated Oncolytic Immunotherapy in advanced Melanoma patients. Cancer Res 2014;74:2939. 10.1158/1538-7445.AM2014-2939 [DOI] [Google Scholar]
- 50. Andtbacka RHI, Curti B, Daniels GA, et al. Clinical responses of Oncolytic Coxsackievirus A21 (V937) in patients with Unresectable Melanoma. J Clin Oncol 2021;39:3829–38. 10.1200/JCO.20.03246 [DOI] [PubMed] [Google Scholar]
- 51. Gollamudi R, Ghalib MH, Desai KK, et al. Intravenous administration of Reolysin, a live replication competent RNA virus is safe in patients with advanced solid tumors. Invest New Drugs 2010;28:641–9. 10.1007/s10637-009-9279-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Morris DG, Feng X, DiFrancesco LM, et al. REO-001: A phase I trial of percutaneous Intralesional administration of Reovirus type 3 Dearing (Reolysin®) in patients with advanced solid tumors. Invest New Drugs 2013;31:696–706. 10.1007/s10637-012-9865-z [DOI] [PubMed] [Google Scholar]
- 53. Galanis E, Markovic SN, Suman VJ, et al. Phase II trial of intravenous administration of Reolysin(®) (Reovirus Serotype-3-Dearing strain) in patients with metastatic Melanoma. Molecular Therapy 2012;20:1998–2003. 10.1038/mt.2012.146 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Kolb E. A, Sampson V, Stabley D, et al. A phase I trial and viral clearance study of Reovirus (Reolysin) in children with Relapsed or refractory extra-cranial solid tumors: a children’s oncology group phase I consortium report. Pediatr Blood Cancer 2015;62:751–8. 10.1002/pbc.25464 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Noonan AM, Farren MR, Geyer SM, et al. Randomized phase 2 trial of the Oncolytic virus Pelareorep (Reolysin) in Upfront treatment of metastatic Pancreatic adenocarcinoma. Mol Ther 2016;24:1150–8. 10.1038/mt.2016.66 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Mahalingam D, Fountzilas C, Moseley J, et al. A phase II study of REOLYSIN(®) (Pelareorep) in combination with carboplatin and paclitaxel for patients with advanced malignant Melanoma. Cancer Chemother Pharmacol 2017;79:697–703. 10.1007/s00280-017-3260-6 [DOI] [PubMed] [Google Scholar]
- 57. Cohn DE, Sill MW, Walker JL, et al. Randomized phase IIB evaluation of weekly paclitaxel versus weekly paclitaxel with Oncolytic Reovirus (Reolysin®) in recurrent ovarian, Tubal, or peritoneal cancer: an NRG oncology/gynecologic oncology group study. Gynecologic Oncology 2017;146:477–83. 10.1016/j.ygyno.2017.07.135 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Mahalingam D, Goel S, Aparo S, et al. n.d. A phase II study of Pelareorep (REOLYSIN(®)) in combination with Gemcitabine for patients with advanced Pancreatic adenocarcinoma. Cancers;10:160. 10.3390/cancers10060160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Bradbury PA, Morris DG, Nicholas G, et al. Canadian cancer trials group (CCTG) Ind211: A randomized trial of Pelareorep (Reolysin) in patients with previously treated advanced or metastatic non-small cell lung cancer receiving standard salvage therapy. Lung Cancer 2018;120:142–8. 10.1016/j.lungcan.2018.03.005 [DOI] [PubMed] [Google Scholar]
- 60. Bernstein V, Ellard SL, Dent SF, et al. A randomized phase II study of weekly paclitaxel with or without Pelareorep in patients with metastatic breast cancer: final analysis of Canadian cancer trials group IND.213. Breast Cancer Res Treat 2018;167:485–93. 10.1007/s10549-017-4538-4 [DOI] [PubMed] [Google Scholar]
- 61. Mahalingam D, Chen S, Xie P, et al. Treatment with Pembrolizumab in combination with the Oncolytic virus Pelareorep promotes anti-tumor immunity in patients with advanced Pancreatic adenocarcinoma. JCO 2021;39:4144. 10.1200/JCO.2021.39.15_suppl.4144 [DOI] [Google Scholar]
- 62. Heo J, Breitbach CJ, Moon A, et al. Sequential therapy with JX-594, a targeted Oncolytic Poxvirus, followed by sorafenib in hepatocellular carcinoma: Preclinical and clinical demonstration of combination efficacy. Mol Ther 2011;19:1170–9. 10.1038/mt.2011.39 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Hwang T-H, Moon A, Burke J, et al. A mechanistic proof-of-concept clinical trial with JX-594, a targeted multi-mechanistic Oncolytic Poxvirus, in patients with metastatic Melanoma. Molecular Therapy 2011;19:1913–22. 10.1038/mt.2011.132 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Parato KA, Breitbach CJ, Le Boeuf F, et al. The Oncolytic Poxvirus JX-594 selectively Replicates in and destroys cancer cells driven by genetic pathways commonly activated in cancers. Molecular Therapy 2012;20:749–58. 10.1038/mt.2011.276 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Kim JH, Oh JY, Park BH, et al. Systemic armed Oncolytic and immunologic therapy for cancer with JX-594, a targeted Poxvirus expressing GM-CSF. Molecular Therapy 2006;14:361–70. 10.1016/j.ymthe.2006.05.008 [DOI] [PubMed] [Google Scholar]
- 66. Heo J, Reid T, Ruo L, et al. Randomized dose-finding clinical trial of Oncolytic Immunotherapeutic Vaccinia JX-594 in liver cancer. Nat Med 2013;19:329–36. 10.1038/nm.3089 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Pecora AL, Rizvi N, Cohen GI, et al. Phase I trial of intravenous administration of Pv701, an Oncolytic virus, in patients with advanced solid cancers. JCO 2002;20:2251–66. 10.1200/JCO.2002.08.042 [DOI] [PubMed] [Google Scholar]
- 68. Freeman AI, Zakay-Rones Z, Gomori JM, et al. Phase I/II trial of intravenous NDV-HUJ Oncolytic virus in recurrent glioblastoma multiforme. Molecular Therapy 2006;13:221–8. 10.1016/j.ymthe.2005.08.016 [DOI] [PubMed] [Google Scholar]
- 69. Puzanov I, Milhem MM, Minor D, et al. Talimogene Laherparepvec in combination with Ipilimumab in previously untreated, Unresectable stage IIIB-IV Melanoma. JCO 2016;34:2619–26. 10.1200/JCO.2016.67.1529 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Chesney J, Puzanov I, Collichio F, et al. Randomized, open-label phase II study evaluating the efficacy and safety of Talimogene Laherparepvec in combination with Ipilimumab versus Ipilimumab alone in patients with advanced, Unresectable Melanoma. J Clin Oncol 2018;36:1658–67. 10.1200/JCO.2017.73.7379 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Harrington KJ, Kong A, Mach N, et al. Talimogene Laherparepvec and Pembrolizumab in recurrent or metastatic squamous cell carcinoma of the head and neck (MASTERKEY-232): A multicenter, phase 1b study. Clin Cancer Res 2020;26:5153–61. 10.1158/1078-0432.CCR-20-1170 [DOI] [PubMed] [Google Scholar]
- 72. Kelly CM, Antonescu CR, Bowler T, et al. Objective response rate among patients with locally advanced or metastatic sarcoma treated with Talimogene Laherparepvec in combination with Pembrolizumab: A phase 2 clinical trial. JAMA Oncol 2020;6:402–8. 10.1001/jamaoncol.2019.6152 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Ribas A, Dummer R, Puzanov I, et al. Oncolytic Virotherapy promotes Intratumoral T cell infiltration and improves anti-PD-1 Immunotherapy. Cell 2017;170:1109–1119. 10.1016/j.cell.2017.08.027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Chesney JA, Ribas A, Long GV, et al. Randomized, double-blind, placebo-controlled, global phase III trial of Talimogene Laherparepvec combined with Pembrolizumab for advanced Melanoma. J Clin Oncol 2023;41:528–40. 10.1200/JCO.22.00343 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Dummer R, Gyorki DE, Hyngstrom J, et al. Neoadjuvant Talimogene Laherparepvec plus surgery versus surgery alone for Resectable stage IIIB-Ivm1A Melanoma: a randomized, open-label, phase 2 trial. Nat Med 2021;27:1789–96. 10.1038/s41591-021-01510-7 [DOI] [PubMed] [Google Scholar]
- 76. Li R, Steinberg GD, Uchio EM, et al. Core1: phase 2, single-arm study of Cg0070 combined with Pembrolizumab in patients with Nonmuscle-invasive bladder cancer (NMIBC) unresponsive to Bacillus Calmette-Guerin (BCG). JCO 2022;40:4597. 10.1200/JCO.2022.40.16_suppl.4597 [DOI] [Google Scholar]
- 77. Milhem MM, Vanderwalde AM, Bowles TL, et al. Updated results from the skin cancer cohorts from an ongoing phase 1/2 Multicohort study of Rp1, an enhanced potency Oncolytic HSV, combined with Nivolumab (IGNYTE). JCO 2022;40:9553. 10.1200/JCO.2022.40.16_suppl.9553 [DOI] [Google Scholar]
- 78. Replimune . Replimune ANNOUNCES positive initial data from the anti-Pd1 failed Melanoma cohort of the IGNYTE clinical trial & an Rp2/3 program update. Available: https://ir.replimune.com/news-releases/news-release-details/replimune-announces-positive-initial-data-anti-pd1-failed/ [Accessed 21 Mar 2023].
- 79. Filley AC, Dey M. Immune system, friend or foe of Oncolytic Virotherapy? Front Oncol 2017;7:106. 10.3389/fonc.2017.00106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Small EJ, Sacks N, Nemunaitis J, et al. Granulocyte macrophage colony-stimulating factor--Secreting allogeneic cellular Immunotherapy for hormone-refractory prostate cancer. Clin Cancer Res 2007;13:3883–91. 10.1158/1078-0432.CCR-06-2937 [DOI] [PubMed] [Google Scholar]
- 81. Nemunaitis J, Jahan T, Ross H, et al. Phase 1/2 trial of Autologous tumor mixed with an allogeneic GVAX vaccine in advanced-stage non-small-cell lung cancer. Cancer Gene Ther 2006;13:555–62. 10.1038/sj.cgt.7700922 [DOI] [PubMed] [Google Scholar]
- 82. Dranoff G, Jaffee E, Lazenby A, et al. Vaccination with irradiated tumor cells engineered to Secrete murine granulocyte-macrophage colony-stimulating factor stimulates potent, specific, and long-lasting anti-tumor immunity. Proc Natl Acad Sci U S A 1993;90:3539–43. 10.1073/pnas.90.8.3539 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Curry WT, Gorrepati R, Piesche M, et al. Vaccination with irradiated Autologous tumor cells mixed with irradiated GM-K562 cells stimulates antitumor immunity and T lymphocyte activation in patients with recurrent malignant glioma. Clin Cancer Res 2016;22:2885–96. 10.1158/1078-0432.CCR-15-2163 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Soiffer R, Lynch T, Mihm M, et al. Vaccination with irradiated Autologous Melanoma cells engineered to Secrete human granulocyte-macrophage colony-stimulating factor generates potent antitumor immunity in patients with metastatic Melanoma. Proc Natl Acad Sci U S A 1998;95:13141–6. 10.1073/pnas.95.22.13141 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Higano C, Saad F, Somer B, et al. A phase III trial of GVAX Immunotherapy for prostate cancer vs Docetaxel plus prednisone in asymptomatic, Castration-resistant prostate cancer (CRPC). Genitourinary Cancer Symposium Proc Am Soc Clin Oncol 2009. [Google Scholar]
- 86. Hirschowitz EA, Foody T, Kryscio R, et al. Autologous Dendritic cell vaccines for non-small-cell lung cancer. J Clin Oncol 2004;22:2808–15. 10.1200/JCO.2004.01.074 [DOI] [PubMed] [Google Scholar]
- 87. Schwaab T, Schwarzer A, Wolf B, et al. Clinical and immunologic effects of Intranodal Autologous tumor Lysate-Dendritic cell vaccine with Aldesleukin (interleukin 2) and IFN-{Alpha}2A therapy in metastatic renal cell carcinoma patients. Clin Cancer Res 2009;15:4986–92. 10.1158/1078-0432.CCR-08-3240 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Gasser O, Sharples KJ, Barrow C, et al. A phase I vaccination study with Dendritic cells loaded with NY-ESO-1 and Α-Galactosylceramide: induction of Polyfunctional T cells in high-risk Melanoma patients. Cancer Immunol Immunother 2018;67:285–98. 10.1007/s00262-017-2085-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Gao D, Li C, Xie X, et al. Autologous tumor Lysate-pulsed Dendritic cell Immunotherapy with cytokine-induced killer cells improves survival in gastric and colorectal cancer patients. PLoS ONE 2014;9:e93886. 10.1371/journal.pone.0093886 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Fujii S, Kawamata T, Shimizu K, et al. Reinvigoration of innate and adaptive immunity via therapeutic cellular vaccine for patients with AML. Molecular Therapy - Oncolytics 2022;27:315–32. 10.1016/j.omto.2022.09.001 [DOI] [Google Scholar]
- 91. Vik-Mo EO, Nyakas M, Mikkelsen BV, et al. Therapeutic vaccination against Autologous cancer stem cells with mRNA-Transfected Dendritic cells in patients with glioblastoma. Cancer Immunol Immunother 2013;62:1499–509. 10.1007/s00262-013-1453-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Rodriguez J, Castañón E, Perez-Gracia JL, et al. A randomized phase II clinical trial of Dendritic cell vaccination following complete resection of colon cancer liver metastasis. J Immunotherapy Cancer 2018;6. 10.1186/s40425-018-0405-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Dillman RO, Cornforth AN, Nistor GI, et al. Randomized phase II trial of Autologous Dendritic cell vaccines versus Autologous tumor cell vaccines in metastatic Melanoma: 5-year follow up and additional analyses. J Immunother Cancer 2018;6:19. 10.1186/s40425-018-0330-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Frank MJ, Khodadoust MS, Czerwinski DK, et al. Autologous tumor cell vaccine induces antitumor T cell immune responses in patients with Mantle cell lymphoma: A phase I/II trial. J Exp Med 2020;217:e20191712. 10.1084/jem.20191712 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Rocconi RP, Grosen EA, Ghamande SA, et al. Gemogenovatucel-T (vigil) Immunotherapy as maintenance in frontline stage III/IV ovarian cancer (VITAL): a randomised, double-blind, placebo-controlled, phase 2B trial. Lancet Oncol 2020;21:1661–72. 10.1016/S1470-2045(20)30533-7 [DOI] [PubMed] [Google Scholar]
- 96. Rocconi RP, Stevens EE, Bottsford-Miller JN, et al. A phase I combination study of vigil and Atezolizumab in recurrent/refractory advanced-stage ovarian cancer: efficacy assessment in Brca1/2-WT patients. JCO 2020;38:3002. 10.1200/JCO.2020.38.15_suppl.3002 [DOI] [Google Scholar]
- 97. Wen PY, Reardon DA, Armstrong TS, et al. A randomized double-blind placebo-controlled phase II trial of Dendritic cell vaccine ICT-107 in newly diagnosed patients with glioblastoma. Clin Cancer Res 2019;25:5799–807. 10.1158/1078-0432.CCR-19-0261 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Chen DS, Mellman I. Oncology meets Immunology: the cancer-immunity cycle. Immunity 2013;39:1–10. 10.1016/j.immuni.2013.07.012 [DOI] [PubMed] [Google Scholar]
- 99. Harrington KJ, Brody J, Ingham M, et al. Preliminary results of the first-in-human (FIH) study of MK-1454, an agonist of Stimulator of interferon genes (STING), as monotherapy or in combination with Pembrolizumab (Pembro) in patients with advanced solid tumors or Lymphomas. Annals of Oncology 2018;29:viii712. 10.1093/annonc/mdy424.015 [DOI] [Google Scholar]
- 100. Meric-Bernstam F, Sandhu SK, Hamid O, et al. Phase IB study of Miw815 (ADU-S100) in combination with Spartalizumab (Pdr001) in patients (Pts) with advanced/metastatic solid tumors or Lymphomas. JCO 2019;37:2507. 10.1200/JCO.2019.37.15_suppl.2507 [DOI] [Google Scholar]
- 101. Meric-Bernstam F, Sweis RF, Hodi FS, et al. Phase I dose-escalation trial of Miw815 (ADU-S100), an Intratumoral STING agonist, in patients with advanced/metastatic solid tumors or Lymphomas. Clin Cancer Res 2022;28:677–88. 10.1158/1078-0432.CCR-21-1963 [DOI] [PubMed] [Google Scholar]
- 102. Zandberg DP, Ferris R, Laux D, et al. 71P A phase II study of ADU-S100 in combination with Pembrolizumab in adult patients with PD-L1+ recurrent or metastatic HNSCC: preliminary safety, efficacy and PK/PD results. Annals of Oncology 2020;31(suppl 7):S1446–7. 10.1016/j.annonc.2020.10.559 [DOI] [Google Scholar]
- 103. Amouzegar A, Chelvanambi M, Filderman JN, et al. STING agonists as cancer Therapeutics. Cancers (Basel) 2021;13:2695. 10.3390/cancers13112695 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Hofmann MA, Kors C, Audring H, et al. Phase 1 evaluation of Intralesionally injected Tlr9-agonist PF-3512676 in patients with basal cell carcinoma or metastatic Melanoma. J Immunother 2008;31:520–7. 10.1097/CJI.0b013e318174a4df [DOI] [PubMed] [Google Scholar]
- 105. Yamada K, Nakao M, Fukuyama C, et al. Phase I study of Tlr9 agonist PF-3512676 in combination with carboplatin and paclitaxel in patients with advanced non-small-cell lung cancer. Cancer Sci 2010;101:188–95. 10.1111/j.1349-7006.2009.01361.x Available: http://blackwell-synergy.com/doi/abs/10.1111/cas.2009.101.issue-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Manegold C, Gravenor D, Woytowitz D, et al. Randomized phase II trial of a toll-like receptor 9 agonist Oligodeoxynucleotide, PF-3512676, in combination with first-line Taxane plus platinum chemotherapy for advanced-stage non-small-cell lung cancer. J Clin Oncol 2008;26:3979–86. 10.1200/JCO.2007.12.5807 [DOI] [PubMed] [Google Scholar]
- 107. Manegold C, van Zandwijk N, Szczesna A, et al. A phase III randomized study of Gemcitabine and cisplatin with or without PF-3512676 (Tlr9 agonist) as first-line treatment of advanced non-small-cell lung cancer. Ann Oncol 2012;23:72–7. 10.1093/annonc/mdr030 [DOI] [PubMed] [Google Scholar]
- 108. Ribas A, Medina T, Kummar S, et al. SD-101 in combination with Pembrolizumab in advanced Melanoma: results of a phase IB, multicenter study. Cancer Discov 2018;8:1250–7. 10.1158/2159-8290.CD-18-0280 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Cohen EEW, Algazi A, Laux D, et al. Phase IB/II, open label, multicenter study of Intratumoral SD-101 in combination with Pembrolizumab in anti-PD-1 treatment Naïve patients with recurrent or metastatic head and neck squamous cell carcinoma (HNSCC). Annals of Oncology 2018;29:viii375. 10.1093/annonc/mdy287.006 [DOI] [Google Scholar]
- 110. Cohen EEW, Algazi A, Laux D, et al. Phase 1B/2, open label, multicenter study of Intratumoral SD-101 in combination with Pembrolizumab in anti-PD-1 treatment Naïve patients with recurrent or metastatic head and neck squamous cell carcinoma (HNSCC). Annals of Oncology 2018;29:viii375. 10.1093/annonc/mdy287.006 [DOI] [Google Scholar]
- 111. Middleton MR, Wermke M, Calvo E, et al. Phase I/II, multicenter, open-label study of Intratumoral/Intralesional administration of the retinoic acid–inducible Gene I (RIG-I) activator MK-4621 in patients with advanced or recurrent tumors. Annals of Oncology 2018;29:viii712. 10.1093/annonc/mdy424.016 [DOI] [Google Scholar]
- 112. Arlen PM, Gulley JL, Parker C, et al. A randomized phase II study of concurrent Docetaxel plus vaccine versus vaccine alone in metastatic androgen-independent prostate cancer. Clin Cancer Res 2006;12:1260–9. 10.1158/1078-0432.CCR-05-2059 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113. Gribben JG, Ryan DP, Boyajian R, et al. Unexpected association between induction of immunity to the universal tumor antigen Cyp1B1 and response to next therapy. Clin Cancer Res 2005;11:4430–6. 10.1158/1078-0432.CCR-04-2111 [DOI] [PubMed] [Google Scholar]
- 114. Vanpouille-Box C, Alard A, Aryankalayil MJ, et al. DNA Exonuclease Trex1 regulates radiotherapy-induced tumour Immunogenicity. Nat Commun 2017;8:15618. 10.1038/ncomms15618 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115. Deng L, Liang H, Xu M, et al. STING-dependent cytosolic DNA sensing promotes radiation-induced type I interferon-dependent antitumor immunity in Immunogenic tumors. Immunity 2014;41:843–52. 10.1016/j.immuni.2014.10.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116. Burnette BC, Liang H, Lee Y, et al. The efficacy of radiotherapy relies upon induction of type I interferon-dependent innate and adaptive immunity. Cancer Res 2011;71:2488–96. 10.1158/0008-5472.CAN-10-2820 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117. Formenti SC, Rudqvist N-P, Golden E, et al. Radiotherapy induces responses of lung cancer to CTLA-4 blockade. Nat Med 2018;24:1845–51. 10.1038/s41591-018-0232-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118. Song K-H, Jung S-Y, Kang S-M, et al. Induction of Immunogenic cell death by radiation-upregulated Karyopherin alpha 2 in vitro. Eur J Cell Biol 2016;95:219–27. 10.1016/j.ejcb.2016.04.002 [DOI] [PubMed] [Google Scholar]
- 119. Palmer CD, Rappaport AR, Davis MJ, et al. Individualized, heterologous Chimpanzee adenovirus and self-amplifying mRNA Neoantigen vaccine for advanced metastatic solid tumors: phase 1 trial interim results. Nat Med 2022;28:1619–29. 10.1038/s41591-022-01937-6 [DOI] [PubMed] [Google Scholar]
- 120. Sahin U, Oehm P, Derhovanessian E, et al. An RNA vaccine drives immunity in Checkpoint-inhibitor-treated Melanoma. Nature 2020;585:107–12. 10.1038/s41586-020-2537-9 [DOI] [PubMed] [Google Scholar]
- 121. Awad MM, Govindan R, Balogh KN, et al. Personalized Neoantigen vaccine NEO-PV-01 with chemotherapy and anti-PD-1 as first-line treatment for non-squamous non-small cell lung cancer. Cancer Cell 2022;40:1010–26. 10.1016/j.ccell.2022.08.003 [DOI] [PubMed] [Google Scholar]
- 122. Carreno BM, Magrini V, Becker-Hapak M, et al. Cancer Immunotherapy. A Dendritic cell vaccine increases the breadth and diversity of Melanoma Neoantigen-specific T cells. Science 2015;348:803–8. 10.1126/science.aaa3828 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123. Mackensen A, Haanen J, Koenecke C, et al. Bnt211-01: A phase I trial to evaluate safety and efficacy of Cldn6 CAR T cells and Cldn6-Encoding mRNA vaccine-mediated in vivo expansion in patients with Cldn6-positive advanced solid tumours. Annals of Oncology 2022;33:S1404–5. 10.1016/j.annonc.2022.08.035 [DOI] [Google Scholar]