Abstract
Significance:
Given their capacity for self-renewal, multilineage differentiation, and immunomodulatory potential, mesenchymal stem cells (MSCs) represent a promising modality of clinical therapy for both regenerative medicine and immune diseases. In this study, we review the key approaches and popular methods utilized to boost potency and modify functions of MSCs for clinical purposes as well as their associated limitations.
Recent Advances:
Several major domains of cell modification strategies are currently employed by investigators to overcome these deficits and augment the therapeutic potential of MSCs. Priming MSCs with soluble factors or pharmacologic agents as well as manipulating oxygen availability in culture have been demonstrated to be effective biochemical methods to augment MSC potential. Distinct genetic and epigenetic methods have emerged in recent years to modify the genetic expression of target proteins and factors thereby modulating MSCs capacity for differentiation, migration, and proliferation. Physical methods utilizing three-dimensional culture methods and alternative cell delivery systems and scaffolds can be used to recapitulate the native MSC niche and augment their engraftment and viability for in vivo models.
Critical Issues:
Unmodified MSCs have demonstrated only modest benefits in many preclinical and clinical studies due to issues with cell engraftment, viability, heterogeneity, and immunocompatibility between donor and recipient. Furthermore, unmodified MSCs can have low inherent therapeutic potential for which intensive research over the past few decades has been dedicated to improving cell functionality and potency.
Keywords: mesenchymal stem cell, stem cell therapy, cell-based therapy

Omaida C. Velazquez, MD

Zhao Jun-Liu, MD, PhD
SCOPE AND SIGNIFICANCE
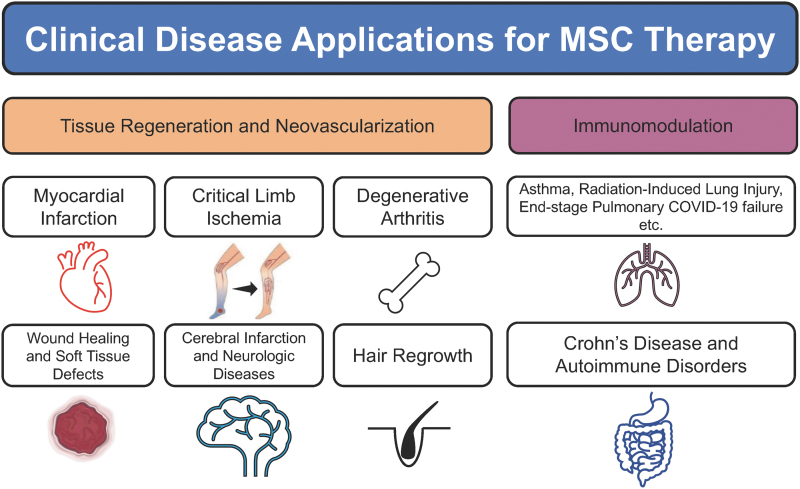
Mesenchymal stem/stromal cells (MSCs) are pluripotent cells with a wide variety of independent functions and modulatory effects on other cells in their tissue microenvironment.1 Thus, MSCs are a major source of preclinical and clinical study to improve treatment options for diseases ranging from ischemic pathologies such as myocardial infarction and critical limb ischemia, respiratory diseases such as pulmonary failure from COVID-19, and autoimmune diseases, including Crohn's disease and multiple sclerosis (Fig. 1).1–7
Figure 1.
Examples of clinical disease applications for MSC therapy. MSC, mesenchymal stem cell.
The aim of this review is to discuss popular strategies employed to augment MSC therapeutic actions for preclinical and clinical applications and their associated limitations.
TRANSLATIONAL RELEVANCE
MSC tissue regenerative functions, encompassing their ability for self-renewal and differentiation, have profound implications for future clinical applications. MSCs further enact a variety of paracrine effects in their local environment through the secretion of soluble factors and extracellular vesicles.1 Moreover, their immunosuppressive properties and low immunogenicity contribute to a reduced or weakened immune response elicited by the implantation of allogeneic MSCs compared with other cell types.8,9 Any method to improve stem cell potency and therapeutic efficacy as well as their survival and viability can have profound implications for improving stem cell-based therapy for a myriad of disease applications.
CLINICAL RELEVANCE
Despite their numerous benefits and acceptable safety profiles, unmodified MSCs have demonstrated only modest benefit in clinical trials compared to placebo treatments.10–12 For example, MSC therapy for patients with critical limb ischemia has demonstrated little to no improvement in outcomes such as amputation-free survival rates, which many theorize may be due to low potency in the MSCs given.10–12 Therefore, optimizing modifications of MSCs to overcome these challenges has become an intensified area of medical research in recent years.
BACKGROUND
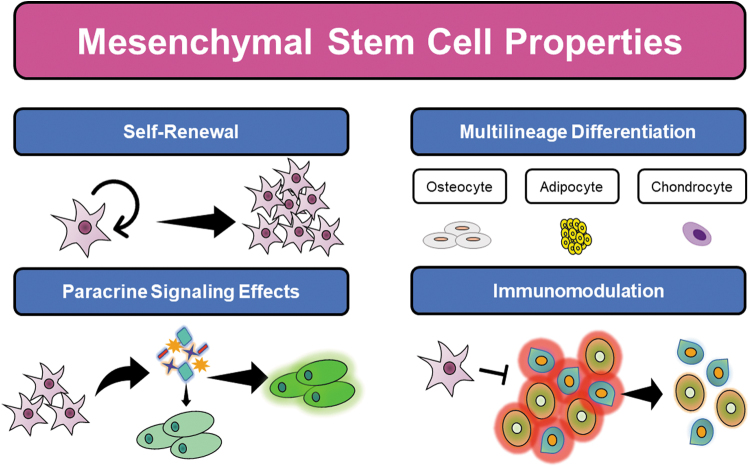
Given their capacity for self-renewal, multilineage differentiation, and immunosuppressive properties, MSCs represent a promising modality of clinical therapy for both regenerative medicine and immune disease treatments (Fig. 2). These features of MSCs aid in the endogenous tissue repair machinery by replenishing resident cells in the wound bed, secreting a variety of soluble factors, and recruiting other cells involved in immunomodulation and tissue repair.13 However, the effectiveness of stem cell therapy depends on efficient engraftment of cells to sites of disease to restore homeostasis and function, and thereby accomplish the desired therapeutic effect. Even when administered locally to diseased tissue sites, MSC therapy is significantly limited by the retention of administered cells, poor survival and viability, and indiscriminate or decreased migratory capacity.13 Furthermore, significant heterogeneity in MSC donor cell populations can exist, which can result in issues with immunocompatibility between the donor and recipient.1
Figure 2.
Overview of mesenchymal stem cell properties.
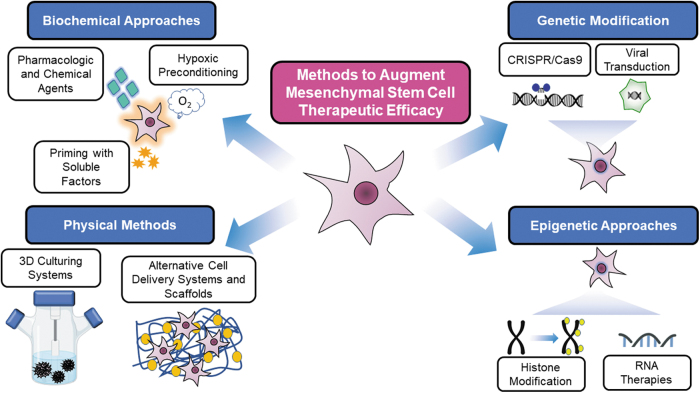
These challenges are further compounded by the local tissue microenvironment, which in diseased states can be inhospitable to MSCs due to reduced blood flow, tissue hypoxia, and widespread local inflammation in many disease states.13,14 As a result, current methods have attempted to optimize both MSCs' nascent properties and their delivery conditions with consideration of the host environment to which they are transplanted to maximize their efficacy (Fig. 3).
Figure 3.
Schematic of common methods to augment mesenchymal stem cells for therapeutic applications.
BIOCHEMICAL APPROACHES
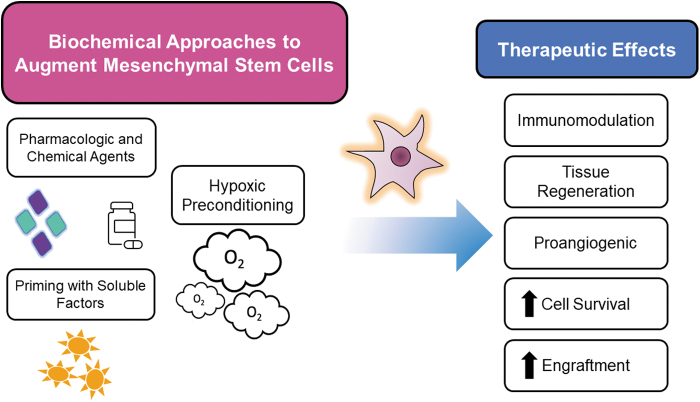
Priming with soluble factors
A well-studied strategy for improving MSCs potential is cell priming through exposure to bioactive molecules before use (Fig. 4). For example, one limitation of adult stem cell treatment in the realm of cardiac disease has been due to these cells' limited plasticity and difficulty in isolating cardiac stem cells.15 One study demonstrated that priming MSCs through culture in cardiogenic media enhanced MSC differentiation toward cardiomyogenic lineages thought to be related to the upregulation of molecular targets, including proteins Cx-43 and sarcomeric alpha-actinin.2 Infusion of MSCs primed toward a cardiomyogenic program promoted myocardial protection compared to unprimed cells in a rodent model of acute myocardial infarction.2 Priming MSCs can also be an effective method to improve MSC therapeutic potential in inhospitable or diseased tissue microenvironments.
Figure 4.
Effects of MSCs modified by differential priming methods.
Castilla et al16 demonstrated that culturing bone marrow-derived MSCs (BM-MSCs) from diabetic mice in the presence of stromal cell-derived factor 1 alpha (SDF1-α) improved neovascularization, endothelial progenitor cell recruitment, and the rate of wound healing compared to unprimed BM-MSCs in a murine model of diabetic wound healing. Similarly, others have demonstrated that adipose-derived MSCs (AD-MSCs) from diabetic mice exposed to platelet-derived growth factor (PDGF) displayed improvement in their trilineage differentiation potential and migratory capacity through PDGF-associated signaling receptor pathways.17
Recruitment of bone marrow-derived circulating stem/progenitor cells, including MSCs, to diseased tissue relies on critical interactions between circulating stem/progenitor cells and hyaluronan (HA) present in the extracellular matrix of wound tissues, which can also be improved through priming of molecular targets by exposure to soluble factors.18 One previous preclinical study demonstrated that inducing overexpression of CD44 in rat-derived MSCs during exposure to PDGF enhanced in vitro migration through CD44-HA binding interactions and could be inhibited by anti-CD44 neutralizing antibodies.19
Modulation of the inflammatory milieu can be another method to prime MSCs toward their anti-inflammatory and immunomodulatory paracrine actions.9 Previous studies have demonstrated that in vitro exposure of MSCs with interferon gamma can result in protrophic and anti-inflammatory changes to the MSC secretome.9,20 Stimulation with similar cytokines can also improve MSC ability to empower resident cells through their paracrine effects.21
One study utilizing BM-MSCs demonstrated that preconditioning cells with interleukin 1 (IL-1) resulted in an upregulation of granulocyte colony-stimulating factor.21 Exposure of microglial cells to the culture media of IL-1-preconditioned human MSCs resulted in an upregulation of the anti-inflammatory factor IL-10 compared to exposure with media from unprimed MSCs.21 Thus, manipulating MSC exposure to differential inflammatory conditions and soluble factors can prove an effective strategy to augment their therapeutic potential.
Hypoxic preconditioning
An alternative method of MSC priming is through stimulation in hypoxic conditions. Unlike the normoxic conditions that MSCs are typically cultured in, the native MSC tissue microenvironment in adipose and bone marrow has an oxygen tension as low as 1–7%.22 Furthermore, MSCs in clinical therapeutics are often conveyed to ischemic and hypoxic tissue sites that can be inhospitable to unmodified MSCs.23 As a result, it is reasonable to expect that recapitulating the hypoxic MSC niche can optimize MSCs before cell-based therapy applications. One study found that incubation of MSCs under low oxygen tension stimulated the expression of glucose-regulated protein 78 through hypoxia-inducible factor-1 alpha (HIF-1α) molecular signaling, which resulted in improved proliferation and migratory potential.24 Hypoxic preconditioning of MSCs further promoted their survival and angiogenic cytokine secretion when transplanted into ischemic tissues in a murine model of hindlimb ischemia.24
HIF-1α can also enhance MSCs' therapeutic effect by augmenting their resistance to hypoxic stress and decrease the generation of reactive oxygen species in hypoxic-ischemic tissue such as in applications for radiation-induced lung injury.25 Others have demonstrated that hypoxic stimulation of MSCs improve cell retention and viability by augmenting their metabolic potential, which can improve their functional capacity in tissue environments with limited nutrition and oxygen availability.26,27 As a result, hypoxia serves as a simple method to increase the survival and retention of transplanted MSCs.
Chemical and pharmacological treatment
Another promising strategy of altering MSC function to improve their therapeutic actions and engraftment is through pretreatment of cells with pharmacologic and chemical agents before transplantation. An additional advantage of using such agents in vitro is achieving a modification of MSCs at doses that would be toxic or produce off-target side effects if administered systemically. For example, one significant obstacle to utilizing MSCs is the culture and expansion of sufficient quantities of cells to produce clinically relevant effects.
All-trans retinoic acid (ATRA) is a vitamin A metabolite and retinoic acid isomer and acts as a significant transcriptional regulator that has been used for a variety of diseases.28 Pourjafar et al28 demonstrated that pharmacologic treatment of MSCs with ATRA at varying concentrations improved MSC survival and proliferation in vitro through the upregulation of genes and trophic factors, including HIF-1α, C-X-C chemokine receptor type 4 (CXCR4), vascular endothelial growth factor (VEGF), and angiopoietin-2 and angiopoietin-4. Subsequent exposure of MSCs to ATRA stimulated their ability to induce angiogenesis and tube formation of endothelial cells derived from the modulation of these genes, as well as upregulation of prostaglandin E2 production and signaling pathways.28 These proangiogenic and proliferative effects translated to increased collagenization and epithelialization, as well as increased vascular density of wounds treated with MSCs preconditioned with ATRA in a rat model of excisional wound healing.28
Other studies have demonstrated the benefit of pharmacologic agents to modulate the immunomodulatory potential of MSCs. Using a mouse model of asthma through constitutive overexpression of IL-13, one study demonstrated that human umbilical cord-derived MSCs pretreated with a ferroptosis inhibitor, Liproxstatin-1, were able to downregulate T helper Type 2 cells to reduce macrophage infiltration and chronic airway inflammation.29 Although the exact mechanism was not described, Liproxstatin-1-primed MSCs were demonstrated to have multiple direct interactions with the inflammatory milieu, including suppression of M2 macrophage activation and reduced eosinophil infiltration.29 As more pharmacologic and chemical agents continue to be discovered, future studies should characterize their effects on MSCs as potential targets for cell-based therapy applications.
Limitations
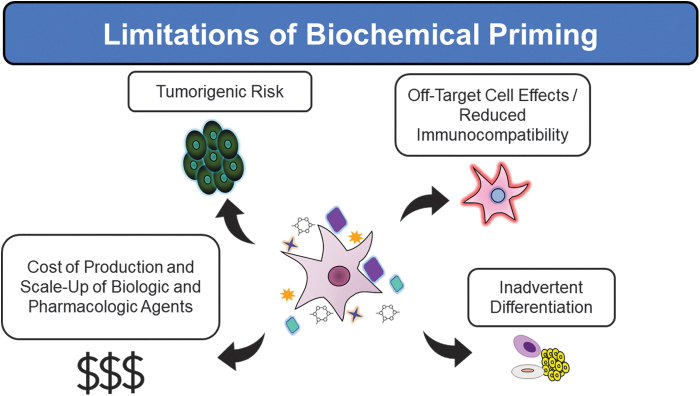
Unfortunately, priming with bioactive soluble factors, hypoxic culture conditions, and chemical agents can produce unintended, off-target downstream effects in cell populations and can be associated with only transient expression of the therapeutic targets (Fig. 5).30 One such off-target effect can be the induction of differential major histocompatibility complex II expression, which can result in reduced immunocompatibility and rejection of transplanted MSCs.8,31 Furthermore, priming strategies can often be costly due to the price of producing soluble factors and pharmacologic agents and can be difficult to scale-up to manufacture sufficient quantities of cells for clinical applications.32
Figure 5.
Drawbacks of utilizing biochemical methods to prime MSCs.
GENETIC MODIFICATION
Supercharging MSCs with therapeutic molecules by viral transduction
One of the most common methods of genetic engineering of MSCs is through the use of viral transduction. Adeno-associated viruses (AAV) have a favorable therapeutic profile and are one such safe FDA-approved vector currently utilized for gene therapy in humans, which have been successfully applied for MSCs.33 Quiroz et al14 demonstrated that modifying MSCs to overexpress an adhesion molecule, E-selectin, through an AAV vector improved their ability to induce neovascularization and functional recovery, and reduce muscle atrophy in a murine model of hindlimb ischemia. E-selectin overexpression as a target not only improved MSC engraftment but may also play roles in augmenting MSC ability to induce angiogenesis and beneficial immunomodulation in ischemic tissue.14
Other targets to augment MSC proliferative capacity have included viral transduction to overexpress growth regulators such as thioredoxin-1.34 Suresh et al34 demonstrated that MSCs transduced with AAV containing thioredoxin-1 displayed augmented cardiac function and angiogenesis for the treatment of myocardial infarction. Numerous studies have demonstrated promise of AAV for modification of MSCs to develop therapeutic properties for clinical applications.
Boosting MSCs with therapeutic factors by CRISPR/Cas9 genome editing
Another strategy becoming increasingly popular for genetic modification is the use of CRISPR/Cas9 technology. For stem cell therapy applications, CRISPR/Cas9 allows for highly specific and efficient genetic manipulation of MSCs for both gene silencing and activation.35 Previous investigators have performed CRISPR/Cas9 gene editing to induce upregulation of HIF-1α, to improve the viability and potency of MSCs before transplantation into recipient sites with variable or low oxygen tension.36 This improved the therapeutic efficacy of HIF-1α-modified MSCs and decreased disease burden in a murine model of Alzheimer's disease without affecting these cells' trilineage differentiation potential or viability thought to be derived from the improved retention and survival of these MSCs.36 Others have demonstrated that targeted gene knock-in of factors such as PDGF of MSCs can improve their differentiation and regenerative capacity.37
Limitations
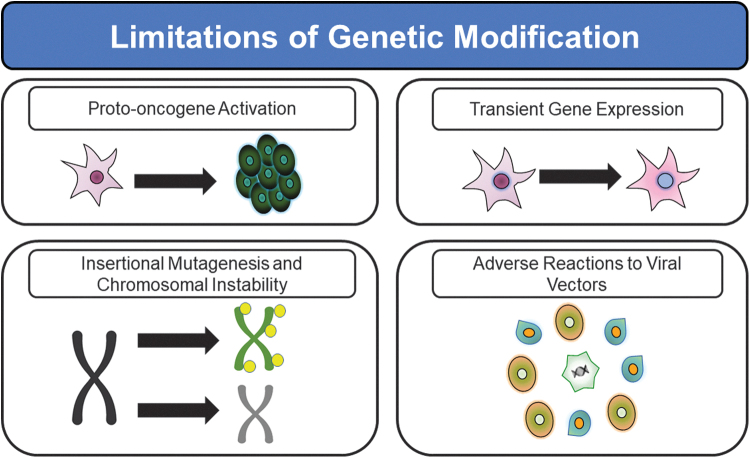
Several significant drawbacks exist with the use of genetic modification (Fig. 6). Both viral vectors and transfection/transduction methods to deliver CRISPR/Cas9 constructs can result in low or transient gene expression, as well as risks associated with introducing genetic packages, including insertional mutagenesis and chromosomal instability.38 However, for some clinical applications such as the induction of wound healing and augmentation of tissue vascularity, a transient genetic effect such as AAV-induced overexpression of MSC surface membrane-bound E-selectin can be a clinically desirable therapeutic design.14,39 This is due to the fact that transient E-selectin overexpression avoids long-term chronic activation of healing and angiogenesis pathways.14,39
Figure 6.
Common limitations of genetically modifying MSCs for clinical therapy using viral vectors and genome editing.
Such persistent activation beyond the point of complete healing and/or normal tissue perfusion would be counterproductive to the desired regenerative medicine benefit in MSC therapies and could lead to therapy with unwanted side effects. Genetic insertion can further result in epigenetic changes and inadvertent proto-oncogene activation.38 Viral vectors can also propagate adverse immune reactions, which can limit the stability of transgene expression.40,41
EPIGENETIC APPROACHES
Histone modification
As more advanced technologies and understanding of genomic alterations emerge, epigenetic targets are becoming another area that holds major potential to augment MSCs (Fig. 7). For example, the role of Wnt signaling in regulating MSC differentiation in osteoblastic cell lineages has been previously characterized and implicated as a possible therapeutic target for orthopedic diseases such as osteoporosis where Wnt signaling in MSCs is suppressed.42,43 To exploit this signaling pathway, Jing et al42 demonstrated that augmenting GCN5 expression, a histone acetyltransferase, improved Wnt gene expression. In turn, this resulted in restored BM-MSC osteogenic differentiation and attenuated bone loss in mice with osteoporosis.42
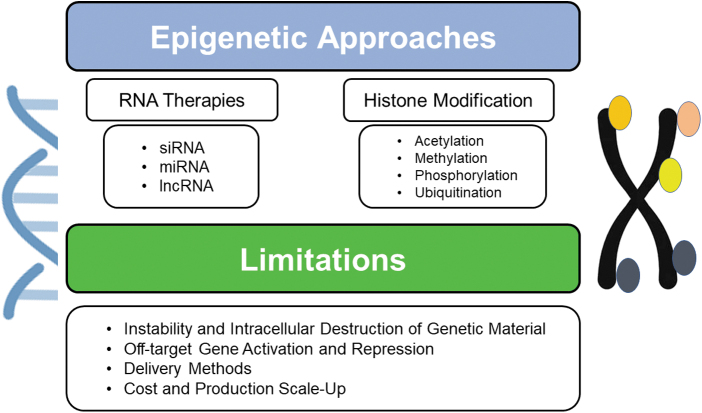
Figure 7.
Epigenetic approaches to modify MSCs with their associated limitations.
Other strategies such as chemical inhibitors of histone demethylases have been utilized to rescue the osteogenic differentiation ability of MSCs and selectively induce their differentiation toward osteoblast instead of osteoclast populations in murine models of osteoporosis to prevent disease progression.44 Influencing epigenetic regulation has the additional advantages of being reversible and stably passed down to subsequent cell lineages.43,45
RNA therapies
Another mechanism to potentiate MSCs' actions and therapeutic efficacy is through modulation of inhibitory RNAs such as small interfering, long noncoding, and microRNAs (miRNAs). This has been investigated in the arena of cardiac disease to examine miRNA targets related to VEGF signaling, to promote postmyocardial infarction angiogenesis.46 Wen et al46 demonstrated knockdown of miRNA-377 in MSCs can promote VEGF upregulation and postnatal vascularization in diseased myocardial infarction tissue in rats. Others have applied this methodology in experimental models of vascular insult, including intracerebral hemorrhage. Engineering MSCs to overexpress miRNA-21 resulted in augmented MSC survival and recovery of neurological function in rats after intracerebral hemorrhage by activation of the nuclear factor-kappa B signaling pathway.47
Limitations
Shortcomings associated with epigenetic methods to modify MSCs can include their instability and destruction by in vivo nucleases (Fig. 7).43 This can yield reduced treatment efficiency of carrier-free gene therapies due to difficulty with intracellular trafficking and degradation within lysosomal compartments.43 As a result, modulation of epigenetic targets can often still require delivery by viral vectors or exosomes to achieve their therapeutic effects, and significant scale-up and manufacturing costs can still be incurred with these approaches.48 Furthermore, epigenetic strategies must be carefully selected and constructed as they can bind multiple effector domains and promoter regions, which can result in off-target genetic activation and repression.43
PHYSICAL METHODS
Three-dimensional culturing systems
One emerging alternative to improve expansion and functionality of MSCs is 3D in vitro cell culture methods.49 One drawback of traditional MSC-based therapy is the extensive 2D in vitro culture methods and resources needed to cultivate a sufficient quantity of cells required to induce a therapeutic effect. Furthermore, the time and typical protocols utilized to expand MSCs can result in cell populations with decreased stem cell potency, which may have reduced clinical efficacy. To more closely mimic the native MSC tissue microenvironment, newer 3D culturing methods have been introduced in the past decade. MSCs possess the ability to organize into 3D spheroid aggregates in vivo, which may recapitulate their function as limb precursors during mesenchymal condensation for early skeletal development.49
Cell aggregation in this manner enhances cell-cell interactions, which can trigger differential adhesion molecule expression, altered cell morphology and polarization among cells.50 For example, Lee et al51 demonstrated E-cadherin as a major calcium-dependent adhesion molecule playing a pivotal function in the formation of MSC spheroids. Subsequent E-cadherin activation was demonstrated to regulate both paracrine functions and self-renewal of MSCs in spheroids through ERK/AKT signaling.51 Cadherin expression has been shown to mediate the immunomodulatory effects of MSCs upon fibroblasts during inflammatory reactions.52,53 Furthermore, modified cadherin surfaces in 2D culture have been demonstrated to regulate MSC differentiation and cell-cell adhesion in vitro.54
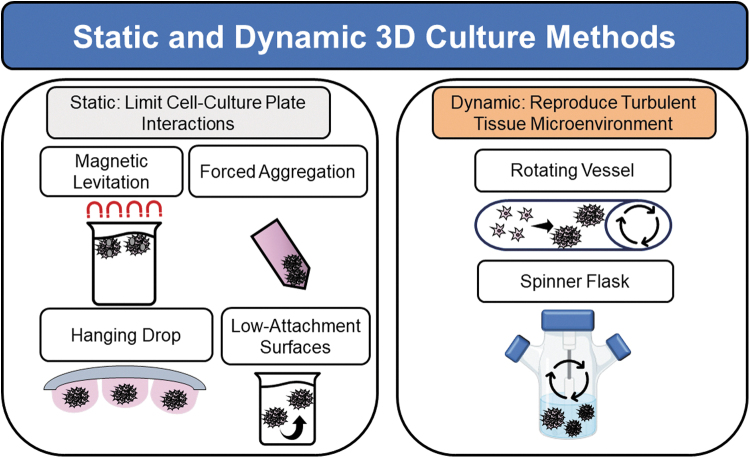
Three-dimensional culture methods include both static and dynamic culture, and typically rely more closely on manipulation of external gravitational forces than manipulation of cell contact with specific substrates (Fig. 8). Static culture environments utilize low-attachment surfaces, hanging-drop, forced aggregation, or magnetic levitation methods to promote MSCs to coalesce into spheroids by decreasing their interaction with tissue culture plate surfaces.21,55,56 Dynamic culture methods attempt to recapitulate the natural turbulent tissue microenvironment through continuous mixing using spinner flasks or rotating wall vessels.
Figure 8.
Three-dimensional culture methods to produce MSC spheroids in vitro.
One study comparing 2D culture versus dynamic culture systems demonstrated improved adipogenic and osteogenic differentiation capacity of MSC spheroids cultured in dynamic 3D environments.57 However, osteogenic differentiation potential was reduced in these cells due to decreased type I collagen expression and reduced integrin/type I collagen signaling interactions between cell and the extracellular matrix.57,58 Future advances and therapies utilizing MSCs should carefully consider the advantages associated with 3D culture systems to augment their intended clinical effects.
Alternative cell delivery systems and scaffolds
Another method of augmenting MSC potential by physical means is through cell delivery systems to improve their engraftment and viability. Scaffold-based platforms utilizing both natural and synthetic biomaterials can be seeded with MSCs to improve their integration in tissue sites where they are applied, particularly if the recipient tissue microenvironment is diseased or inhospitable to donor MSCs. MSCs cultured on chitosan-based scaffolds have been demonstrated to have increased chondrogenic differentiation potential, while still preserving markers of MSC stemness, including Oct4, Sox2, and Nanog.59 However, these substrates should be carefully selected as they can also decrease the stemness of MSCs.
Wang et al60 demonstrated that MSCs exposed to micropatterned polyethylene glycol demonstrated upregulated genetic expression of markers related to osteogenesis and adipogenesis, but downregulated expression of genes related to stemness such as THY1 and MEST. Chitosan and other molecular substrates can also be utilized to create hydrogels to enhance MSC delivery and survival. A protein-based hydrogel (SHIELD-2.5) was created by one group with a heightened degree of mechanical stiffness to improve protection of induced pluripotent stem cells from shear forces experienced during injection.61 Utilizing this hydrogel delivery system increased stem cell engraftment and arteriogenesis.61
More recently, small-molecule hydrogels have begun to show early promise with the advantages of being more readily biodegradable and compatible compared to standard hydrogels.62,63 In a murine model of hindlimb ischemia, Huang et al demonstrated that the use of a small-molecule hydrogel using disulfide bond links improved engraftment of human placenta-derived MSCs as well as their paracrine and proangiogenic functions, which resulted in the regeneration of muscle cells and restored perfusion.59
Utilizing other autologous substrates such as platelet-rich plasma (PRP) and fat grafting has shown significant promise to improve the tissue regenerative functions of MSCs. PRP holds potential as a substrate, given the regenerative properties of hundreds of signaling factors and bioactive proteins contained in platelet alpha granules, including PDGF and VEGF, which can augment the environment and tissue repair function of MSCs.64–67
Moreover, tissue scaffolds containing HA and/or collagen to approximate the cutaneous dermal bed have long been utilized for surgical reconstruction of soft tissue defects and wound repair and represent another strategy to augment substrates such as PRP.68–71 For example, PRP has been combined with both HA and fat grafting for clinical applications ranging from wound repair to inflammatory diseases such as Hidradenitis suppurativa.72–77 PRP can be further prepared as leukocyte poor and with low or high levels of fibrin, which can be optimized for the survival of hair follicle stem cells to treat conditions such as hair loss and androgenetic alopecia.78–85
To explore potential solutions for the treatment of osteochondral lesions related to trauma and joint degeneration, Scioli et al found that administration of PRP and recombinant insulin could enhance the osteogenic and chondrogenic differentiation capacity of AD-MSCs independent of insulin-like growth factor-1 receptor and mammalian target of rapamycin signaling.86 Fat grafting has long been recognized for its clinical applications in tissue regeneration and reconstruction, in part, due to its significance as a source of and delivery method for AD-MSCs.87–95 AD-MSC phenotype and viability can be influenced by the method of fat graft preparation (enzymatic versus mechanical), as well as delivery with the stromal-vascular fraction (SVF) from fat containing a heterogeneous cell population of pericytes, mast cells, endothelial cells, smooth muscle cells, preadipocytes, and AD-MSCs.95–98
In particular, isolation and delivery of the SVF offer the concerted benefits of these heterogeneous cell types in maintaining an environment capable of sustaining AD-MSCs, which may contribute to their clinical uses for tissue reconstruction and rejuvenation.99–103 Utilizing a similar concept to deliver MSCs with their native tissue environment, others have demonstrated the potential of skin micrografts containing HF-MSCs as a targeted delivery method to regenerate hair and follicle growth in patients with hair loss and androgenetic alopecia.104–106 Overall, research dedicated to optimizing scaffolds and delivery methods for cell-based therapy hold significant potential as another promising strategy to utilize MSCs for a wide variety of clinical applications.
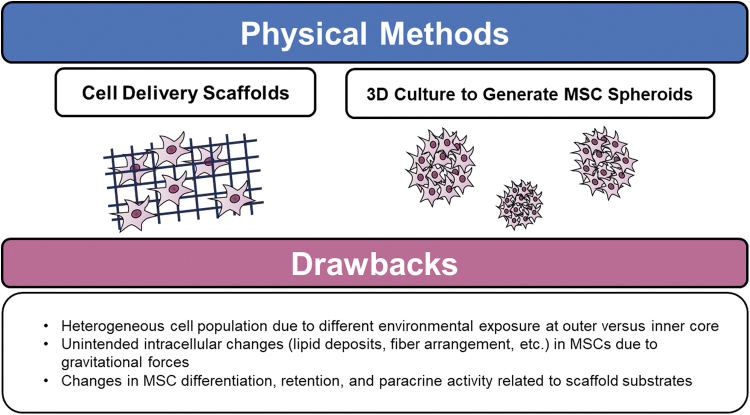
Limitations
Despite their novelty and functional resemblance to native MSCs, MSC spheroids generated by 3D culture methods have several drawbacks (Fig. 9). Given their spherical arrangement, the environmental exposure to both oxygen and nutrient supply is unevenly distributed between cells at the outer versus inner layers, which can result in necrosis of spheroid cores.107–109 This can result in heterogenous morphology, cellular adhesion molecule expression, paracrine activity, and decreased cellular metabolism among coalesced cells.107,108,110,111
Figure 9.
Recognized limitations of MSCs exposed to physical methods of modification.
Culturing with the effect of microgravitational forces as in these static and dynamic culture systems can also cause unintended changes in MSCs such as increased intracellular lipid deposition, decrease in RhoA activity, and interrupted F-actin fibers.112 Similar challenges are faced by cell delivery scaffolds. The substrates utilized for cell delivery systems must be carefully selected with regard to their ability to stimulate cell differentiation, proliferation, retention, polarization, and cell-cell communication.
CONCLUSIONS & PERSPECTIVES
Although many MSC therapies have not yielded definitive, pronounced benefits in many clinical trials, intensive clinical and preclinical investigations are underway to optimize aspects of stem cell regenerative and immunomodulatory potential. As more functional targets and pathways continue to be discovered and refined to augment cell therapy potency, strategies to modify MSCs through biochemical, physical, genetic, and epigenetic methods will need to be continually optimized to achieve these intended clinical applications.
Modification of molecular targets to optimize the functional tissue repair and immunomodulatory machinery of MSCs may uncover the optimal method of utilizing MSCs to be a combination of these aforementioned strategies. One study demonstrated that the combination of a viral vector to induce overexpression of CXCR4 on umbilical cord-derived MSCs improved these cells' ability to bind a chitosan scaffold crosslinked to a bioactive factor (brain-derived neurotrophic factor) (114).
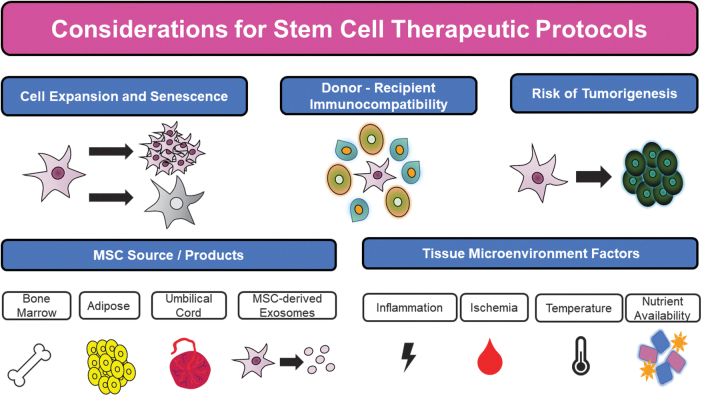
The overexpression of CXCR4 substantially augmented the ability of these MSCs to be seeded onto this scaffold, and the transplantation of this cell-scaffold implant into the brain cavity of rats with traumatic brain injury resulted in improved migratory and neuronal differentiation capacity of MSCs at the site of injury.113 Similar strategies as this one combining multiple approaches may serve as an elegant method to harness the maximum therapeutic potential of MSCs and provide future hope for innumerable clinical therapeutics. However, future studies are still needed to address limitations associated with cell-based therapy, despite these modifications (Fig. 10). For all in vitro manipulation and attempts at expansion of stem cells, an important concern is the lifespan and viability of MSCs before senescence.
Figure 10.
Factors for consideration in designing future MSC modification protocols for clinical applications.
The accumulation of age-related genomic changes can result in incremental loss of cellular functions and homeostasis with increasing passage numbers that future approaches could attempt to limit through specific genetic targets.43 An equally important concern of cell-based therapy is the associated tumorigenic risk associated with implantation of modified, ectopic cells. Previous work has acknowledged the ability of MSCs to form teratomas at injection sites, and future work should consider the risks of insertional mutagenesis and alteration associated with priming of native cells to limit the generation of tumorigenesis.114
The tissue source from which MSCs are derived for therapy is another important technical consideration for any future experimental investigations into MSC modification. Stem cells derived from common sources such as umbilical, adipose, and bone marrow tissue contain heterogeneous genetic landscapes and functional characteristics that may not respond equivalently to the same therapeutic alterations.49 As a result, the optimal conditions and stimuli must be tailored to each distinct origin of stem cells to establish cell-specific protocols and ensure the consistency of delivered stem cells.
As important as the origin of the stem cells utilized, clinical applications must also consider the tissue microenvironment to which cells are delivered. For example, application to ischemic tissue sites in extremities with critical limb ischemia should consider the relative hypothermia and lack of nutrient and blood supply in these areas.60 Cell-specific manipulations to improve MSC proangiogenic effects and cell engraftment as well as the scaffolds with which they are delivered in can be optimized to overcome these factors in diseased host tissue.14,60,115
These alterations should also take into account the resultant immunogenic profile of MSCs to produce the most immunocompatible profile of donor cells to limit a robust recipient immune response.31 One such emerging arena of medical research, given their favorable immunologic phenotype and lack of genomic materials, is the use of MSC-derived exosomes from modified MSCs.47 As future investigations continue to delineate the advantages and disadvantages of both MSC and MSC-derived exosome therapy for both regenerative medicine and immunomodulatory applications, standard application-based assays will need to be developed to measure their desired clinical responses. Despite the significant limitations that still need to be overcome in scaling up and applying these methods to augment MSCs, MSC-based therapy holds tremendous promise in improving patient outcomes for a wide variety of regenerative purposes and disease treatments.
TAKE-HOME MESSAGES
MSCs represent a promising modality of clinical therapy for both regenerative medicine and immune diseases.
Unmodified MSCs have demonstrated only modest benefits in many preclinical and clinical studies due to issues with cell engraftment, viability, heterogeneity, and immunocompatibility between donor and recipient.
Priming cells with soluble factors or pharmacologic agents as well as changing oxygen availability are effective biochemical methods to augment MSC potential.
Both genetic and epigenetic methods have emerged in recent years to modify the genetic expression of target proteins and factors to modify or “supercharge” MSC capacity for differentiation, migration, proliferation, angiogenesis, and tissue repair.
3D culture methods and alternative cell delivery scaffolds can be utilized to recapitulate the native MSC environment and augment their retention and viability for clinical therapy.
Future studies are still needed to address limitations associated with cell-based therapy, including cell expansion, immunocompatibility, tumorigenic risks, tissue sources of MSCs, and the host microenvironment to which MSCs are delivered.
Abbreviations and Acronyms
- 3D
three dimensional
- AAV
adeno-associated virus
- AD-MSC
adipose-derived mesenchymal stem cell
- ATRA
all-trans retinoic acid
- BM-MSC
bone marrow-derived MSC
- CXCR4
C-X-C chemokine receptor type 4
- HA
hyaluronan
- HIF-1α
hypoxia-inducible factor-1 alpha
- IL
interleukin
- miRNA
microRNAs
- MSC
mesenchymal stem cell
- PDGF
platelet-derived growth factor
- PRP
platelet-rich plasma
- SDF1-α
stromal cell-derived factor 1 alpha
- VEGF
vascular endothelial growth factor
ACKNOWLEDGMENTS AND FUNDING SOURCES
This article is supported by grants from the National Institutes of Health R01 HL149452 and HL156152-01A1 Catalyze.
AUTHOR DISCLOSURE AND GHOSTWRITING
The authors declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: The E-selectin gene modification technologies described were developed in our research laboratory and patented/licensed by the University of Miami. This technology is currently under preclinical development by Ambulero, Inc., a new start-up company out of the University of Miami that focuses on developing new vascular treatments for ischemic conditions. Co-authors, Z.J.L. and O.C.V., serve as consultants and scientific and medical advisory officers.
ABOUT THE AUTHORS
Carlos Theodore Huerta, MD, is a general surgery resident currently completing a postdoctoral research fellowship in the Velazquez/Liu laboratory at the University of Miami. Yulexi Y. Ortiz, BS, is a research associate in the Velazquez/Liu laboratory at the University of Miami. Zhao-Jun Liu, MD, PhD, is Professor and Director of the Vascular Research Laboratory within the University of Miami Department of Surgery. Omaida C. Velazquez, MD, is Professor and Chair of the DeWitt Daughtry Family Department of Surgery within the University of Miami as well as the David Kimmelman Endowed Chair in Vascular Surgery and Surgeon-in-Chief for the UHealth/Jackson Health System.
REFERENCES
- 1. Zhou T, Yuan Z, Weng J, et al. Challenges and advances in clinical applications of mesenchymal stromal cells. J Hematol Oncol 2021;14(1):24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Carvalho JL, Braga VBA, Melo MB, et al. Priming mesenchymal stem cells boosts stem cell therapy to treat myocardial infarction. J Cell Mol Med 2013;17(5):617–625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Lanzoni G, Linetsky E, Correa D, et al. Umbilical cord mesenchymal stem cells for COVID-19 acute respiratory distress syndrome: A double-blind, phase 1/2a, randomized controlled trial. Stem Cells Transl Med 2021;10(5):660–673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Gentile P, Sterodimas A. Adipose-derived stromal stem cells (ASCs) as a new regenerative immediate therapy combating coronavirus (COVID-19)-induced pneumonia. Expert Opin Biol Ther 2020;20(7):711–716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Gentile P, Sterodimas A, Pizzicannella J, et al. Research progress on mesenchymal stem cells (MSCs), adipose-derived mesenchymal stem cells (AD-MSCs), drugs, and vaccines in inhibiting COVID-19 disease. Aging Dis 2020;11(5):1191–1201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Gentile P, Sterodimas A. Adipose stem cells (ASCs) and stromal vascular fraction (SVF) as a potential therapy in combating (COVID-19)-disease. Aging Dis 2020;11(3):465–469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Gentile P. SARS-CoV-2: The “Uncensored” truth about its origin and adipose-derived mesenchymal stem cells as new potential immune-modulatory weapon. Aging Dis 2021;12(2):330–344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Barrachina L, Remacha AR, Romero A, et al. Priming equine bone marrow-derived mesenchymal stem cells with proinflammatory cytokines: Implications in immunomodulation-immunogenicity balance, cell viability, and differentiation potential. Stem Cells Dev 2017;26(1):15–24. [DOI] [PubMed] [Google Scholar]
- 9. Najar M, Krayem M, Merimi M, et al. Insights into inflammatory priming of mesenchymal stromal cells: Functional biological impacts. Inflamm Res 2018;67(6):467–477. [DOI] [PubMed] [Google Scholar]
- 10. Abdul Wahid SF, Ismail NA, Wan Jamaludin WF, et al. Autologous cells derived from different sources and administered using different regimens for “no-option” critical lower limb ischaemia patients. Cochrane Database Syst Rev 2018;2018(8):CD010747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Qadura M, Terenzi DC, Verma S, et al. Concise review: Cell therapy for critical limb ischemia: An integrated review of preclinical and clinical studies. Stem Cells 2018;36(2):161–171. [DOI] [PubMed] [Google Scholar]
- 12. Duscher D, Rennert RC, Januszyk M, et al. Aging disrupts cell subpopulation dynamics and diminishes the function of mesenchymal stem cells. Sci Rep 2014;4:7144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Liu ZJ, Daftarian P, Kovalski L, et al. Directing and potentiating stem cell-mediated angiogenesis and tissue repair by cell surface E-selectin coating. PLoS One 2016;11(4):e0154053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Quiroz HJ, Valencia SF, Shao H, et al. E-Selectin-overexpressing mesenchymal stem cell therapy confers improved reperfusion, repair, and regeneration in a murine critical limb ischemia model. Front Cardiovasc Med 2022;8:826687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Steinhauser ML, Lee RT. Regeneration of the heart. EMBO Mol Med 2011;3(12):701–712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Castilla DM, Liu ZJ, Tian R, et al. A novel autologous cell-based therapy to promote diabetic wound healing. Ann Surg 2012;256(4):560–572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Capilla-González V, López-Beas J, Escacena N, et al. PDGF restores the defective phenotype of adipose-derived mesenchymal stromal cells from diabetic patients. Mol Ther 2018;26(11):2696–2709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Misra S, Hascall VC, Markwald RR, et al. Interactions between hyaluronan and its receptors (CD44, RHAMM) regulate the activities of inflammation and cancer. Front Immunol 2015;6:201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Zhu H, Mitsuhashi N, Klein A, et al. The role of the hyaluronan receptor CD44 in mesenchymal stem cell migration in the extracellular matrix. Stem Cells 2006;24(4):928–935. [DOI] [PubMed] [Google Scholar]
- 20. Wang Y, Chen X, Cao W, et al. Plasticity of mesenchymal stem cells in immunomodulation: Pathological and therapeutic implications. Nat Immunol 2014;15(11):1009–1016. [DOI] [PubMed] [Google Scholar]
- 21. Redondo-Castro E, Cunningham C, Miller J, et al. Interleukin-1 primes human mesenchymal stem cells towards an anti-inflammatory and pro-trophic phenotype in vitro. Stem Cell Res Ther 2017;8(1):79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Spencer JA, Ferraro F, Roussakis E, et al. Direct measurement of local oxygen concentration in the bone marrow of live animals. Nature 2014;508(7495):269–273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Huang YC, Parolini O, Deng L, et al. Should hypoxia preconditioning become the standardized procedure for bone marrow MSCs preparation for clinical use? Stem Cells 2016;34(7):1992–1993. [DOI] [PubMed] [Google Scholar]
- 24. Lee JH, Yoon YM, Lee SH. Hypoxic preconditioning promotes the bioactivities of mesenchymal stem cells via the HIF-1α-GRP78-Akt Axis. Int J Mol Sci 2017;18(6):E1320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Li B, Li C, Zhu M, et al. Hypoxia-induced mesenchymal stromal cells exhibit an enhanced therapeutic effect on radiation-induced lung injury in mice due to an increased proliferation potential and enhanced antioxidant ability. Cell Physiol Biochem 2017;44(4):1295–1310. [DOI] [PubMed] [Google Scholar]
- 26. Beegle J, Lakatos K, Kalomoiris S, et al. Hypoxic preconditioning of mesenchymal stromal cells induces metabolic changes, enhances survival, and promotes cell retention in vivo. Stem Cells 2015;33(6):1818–1828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Zhu H, Sun A, Zou Y, et al. Inducible metabolic adaptation promotes mesenchymal stem cell therapy for ischemia: A hypoxia-induced and glycogen-based energy prestorage strategy. Arterioscler Thromb Vasc Biol 2014;34(4):870–876. [DOI] [PubMed] [Google Scholar]
- 28. Pourjafar M, Saidijam M, Mansouri K, et al. All-trans retinoic acid preconditioning enhances proliferation, angiogenesis and migration of mesenchymal stem cell in vitro and enhances wound repair in vivo. Cell Prolif 2016;50(1):e12315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Kim RL, Bang JY, Kim J, et al. Mesenchymal stem cells exert their anti-asthmatic effects through macrophage modulation in a murine chronic asthma model. Sci Rep 2022;12:9811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Sobacchi C, Palagano E, Villa A, et al. Soluble factors on stage to direct mesenchymal stem cells fate. Front Bioeng Biotechnol 2017;5:32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Chan JL, Tang KC, Patel AP, et al. Antigen-presenting property of mesenchymal stem cells occurs during a narrow window at low levels of interferon-gamma. Blood 2006;107(12):4817–4824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Guess AJ, Daneault B, Wang R, et al. Safety profile of good manufacturing practice manufactured interferon γ-primed mesenchymal stem/stromal cells for clinical trials. Stem Cells Transl Med 2017;6(10):1868–1879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Research C for BE and. LUXTURNA. FDA [Internet]. 2019. Apr 5 [cited 2022 Apr 19]; Available from: https://www.fda.gov/vaccines-blood-biologics/cellular-gene-therapy-products/luxturna [Last accessed: April 19, 2022].
- 34. Suresh SC, Selvaraju V, Thirunavukkarasu M, et al. Thioredoxin-1 (Trx1) engineered mesenchymal stem cell therapy increased pro-angiogenic factors, reduced fibrosis and improved heart function in the infarcted rat myocardium. Int J Cardiol 2015;201:517–528. [DOI] [PubMed] [Google Scholar]
- 35. Golchin A, Shams F, Karami F. Advancing mesenchymal stem cell therapy with CRISPR/Cas9 for clinical trial studies. Adv Exp Med Biol 2020;1247:89–100. [DOI] [PubMed] [Google Scholar]
- 36. Enhancing the therapeutic potential of mesenchymal stem cell-based therapy via CRISPR/Cas9-based genome editing - ClinicalKey [Internet]. [cited 2022 Jun 1]. Available from: https://www-clinicalkey-com.access.library.miami.edu/#!/content/playContent/1-s2.0-S1465324920307258?returnurl=null&referrer=null [Last accessed: April 19, 2022].
- 37. Miwa H, Era T. Tracing the destiny of mesenchymal stem cells from embryo to adult bone marrow and white adipose tissue via Pdgfrα expression. Development 2018;145(2):dev155879. [DOI] [PubMed] [Google Scholar]
- 38. Cheng S, Nethi SK, Rathi S, et al. Engineered mesenchymal stem cells for targeting solid tumors: Therapeutic potential beyond regenerative therapy. J Pharmacol Exp Ther 2019;370(2):231–241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Parikh PP, Lassance-Soares RM, Shao H, et al. Intramuscular E-selectin/adeno-associated virus gene therapy promotes wound healing in an ischemic mouse model. J Surg Res 2018;228:68–76. [DOI] [PubMed] [Google Scholar]
- 40. Muhuri M, Maeda Y, Ma H, et al. Overcoming innate immune barriers that impede AAV gene therapy vectors. J Clin Invest 2021;131(1):143780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Mingozzi F, High KA. Immune responses to AAV vectors: Overcoming barriers to successful gene therapy. Blood 2013;122(1):23–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Jing H, Su X, Gao B, et al. Epigenetic inhibition of Wnt pathway suppresses osteogenic differentiation of BMSCs during osteoporosis. Cell Death Dis 2018;9(2):176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Zhao Y, He J, Qiu T, et al. Epigenetic therapy targeting bone marrow mesenchymal stem cells for age-related bone diseases. Stem Cell Res Ther 2022;13:201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Lv L, Ge W, Liu Y, et al. Lysine-specific demethylase 1 inhibitor rescues the osteogenic ability of mesenchymal stem cells under osteoporotic conditions by modulating H3K4 methylation. Bone Res 2016;4:16037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Sui BD, Zheng CX, Li M, et al. Epigenetic Regulation of Mesenchymal Stem Cell Homeostasis. Trends Cell Biol 2020;30(2):97–116. [DOI] [PubMed] [Google Scholar]
- 46. Wen Z, Huang W, Feng Y, et al. MicroRNA-377 regulates mesenchymal stem cell-induced angiogenesis in ischemic hearts by targeting VEGF. PLoS One 2014;9(9):e104666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Zhang H, Wang Y, Lv Q, et al. MicroRNA-21 Overexpression Promotes the Neuroprotective Efficacy of Mesenchymal Stem Cells for Treatment of Intracerebral Hemorrhage. Front Neurol 2018;9:931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Dobrovolskaia MA, Bathe M. Opportunities and challenges for the clinical translation of structured DNA assemblies as gene therapeutic delivery and vaccine vectors. Wiley Interdiscip Rev Nanomed Nanobiotechnol 2021;13(1):e1657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Kouroupis D, Correa D. Increased mesenchymal stem cell functionalization in three-dimensional manufacturing settings for enhanced therapeutic applications. Front Bioeng Biotechnol 2021;9:621748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Foty RA, Steinberg MS. The differential adhesion hypothesis: A direct evaluation. Dev Biol 2005;278(1):255–263. [DOI] [PubMed] [Google Scholar]
- 51. Lee EJ, Park SJ, Kang SK, et al. Spherical bullet formation via E-cadherin promotes therapeutic potency of mesenchymal stem cells derived from human umbilical cord blood for myocardial infarction. Mol Ther 2012;20(7):1424–1433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Chang SK, Noss EH, Chen M, et al. Cadherin-11 regulates fibroblast inflammation. Proc Natl Acad Sci U S A 2011;108(20):8402–8407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Agarwal SK, Brenner MB. Role of adhesion molecules in synovial inflammation. Curr Opin Rheumatol 2006;18(3):268–276. [DOI] [PubMed] [Google Scholar]
- 54. Alimperti S, Andreadis ST. CDH2 and CDH11 act as regulators of stem cell fate decisions. Stem Cell Res 2015;14(3):270–282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Foty R. A simple hanging drop cell culture protocol for generation of 3D spheroids. J Vis Exp 2011;(51):2720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Mackay AM, Beck SC, Murphy JM, et al. Chondrogenic differentiation of cultured human mesenchymal stem cells from marrow. Tissue Eng 1998;4(4):415–428. [DOI] [PubMed] [Google Scholar]
- 57. Meyers VE, Zayzafoon M, Gonda SR, et al. Modeled microgravity disrupts collagen I/integrin signaling during osteoblastic differentiation of human mesenchymal stem cells. J Cell Biochem 2004;93(4):697–707. [DOI] [PubMed] [Google Scholar]
- 58. Frith JE, Thomson B, Genever PG. Dynamic three-dimensional culture methods enhance mesenchymal stem cell properties and increase therapeutic potential. Tissue Eng Part C Methods 2010;16(4):735–749. [DOI] [PubMed] [Google Scholar]
- 59. Huang A, Liu D, Qi X, et al. Self-assembled GFFYK peptide hydrogel enhances the therapeutic efficacy of mesenchymal stem cells in a mouse hindlimb ischemia model. Acta Biomater 2019;85:94–105. [DOI] [PubMed] [Google Scholar]
- 60. Wang R, Yao X, Li T, et al. Reversible thermoresponsive hydrogel fabricated from natural biopolymer for the improvement of critical limb ischemia by controlling release of stem cells. Adv Healthcare Mater 2019;8(20):e1900967. [DOI] [PubMed] [Google Scholar]
- 61. Foster AA, Dewi RE, Cai L, et al. Protein-engineered hydrogels enhance the survival of induced pluripotent stem cell-derived endothelial cells for treatment of peripheral arterial disease. Biomater Sci 2018;6(3):614–622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Wu Z, Chen G, Zhang J, et al. Treatment of myocardial infarction with gene-modified mesenchymal stem cells in a small molecular hydrogel. Sci Rep 2017;7(1):15826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Miao X, Cao W, Zheng W, et al. Switchable catalytic activity: Selenium-containing peptides with redox-controllable self-assembly properties. Angew Chem Int Ed Engl 2013;52(30):7781–7785. [DOI] [PubMed] [Google Scholar]
- 64. Gentile P, Garcovich S. Systematic review-the potential implications of different platelet-rich plasma (PRP) concentrations in regenerative medicine for tissue repair. Int J Mol Sci 2020;21(16):E5702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Gentile P, Garcovich S. Systematic review: Adipose-derived mesenchymal stem cells, platelet-rich plasma and biomaterials as new regenerative strategies in chronic skin wounds and soft tissue defects. Int J Mol Sci 2021;22(4):1538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Gentile P, Bottini DJ, Spallone D, et al. Application of platelet-rich plasma in maxillofacial surgery: Clinical evaluation. J Craniofac Surg 2010;21(3):900–904. [DOI] [PubMed] [Google Scholar]
- 67. Cervelli V, Gentile P. Use of cell fat mixed with platelet gel in progressive hemifacial atrophy. Aesthetic Plast Surg 2009;33(1):22–27. [DOI] [PubMed] [Google Scholar]
- 68. De Angelis B, Gentile P, Tati E, et al. One-stage reconstruction of scalp after full-thickness oncologic defects using a dermal regeneration template (Integra). Biomed Res Int 2015;2015:698385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Gentile P, Colicchia GM, Nicoli F, et al. Complex abdominal wall repair using a porcine dermal matrix. Surg Innov 2013;20(6):NP12–NP15. [DOI] [PubMed] [Google Scholar]
- 70. De Angelis B, Orlandi F, Morais D'Autilio MFL, et al. Vasculogenic chronic ulcer: Tissue regeneration with an innovative dermal substitute. J Clin Med 2019;8(4):E525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. De Angelis B, Orlandi F, Fernandes Lopes Morais D'Autilio M, et al. Long-term follow-up comparison of two different bi-layer dermal substitutes in tissue regeneration: Clinical outcomes and histological findings. Int Wound J 2018;15(5):695–706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. De Angelis B, D'Autilio MFLM, Orlandi F, et al. Wound healing: In vitro and in vivo evaluation of a bio-functionalized scaffold based on hyaluronic acid and platelet-rich plasma in chronic ulcers. J Clin Med 2019;8(9):E1486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Cervelli V, Lucarini L, Spallone D, et al. Use of platelet-rich plasma and hyaluronic acid in the loss of substance with bone exposure. Adv Skin Wound Care 2011;24(4):176–181. [DOI] [PubMed] [Google Scholar]
- 74. Gentile P, Calabrese C, De Angelis B, et al. Impact of the different preparation methods to obtain autologous non-activated platelet-rich plasma (A-PRP) and activated platelet-rich plasma (AA-PRP) in plastic surgery: Wound healing and hair regrowth evaluation. Int J Mol Sci 2020;21(2):E431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Cervelli V, Bocchini I, Di Pasquali C, et al. P.R.L. platelet rich lipotransfert: Our experience and current state of art in the combined use of fat and PRP. Biomed Res Int 2013;2013:434191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Nicoli F, Balzani A, Lazzeri D, et al. Severe hidradenitis suppurativa treatment using platelet-rich plasma gel and Hyalomatrix. Int Wound J 2015;12(3):338–343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Gentile P, Di Pasquali C, Bocchini I, et al. Breast reconstruction with autologous fat graft mixed with platelet-rich plasma. Surg Innov 2013;20(4):370–376. [DOI] [PubMed] [Google Scholar]
- 78. Gentile P, Alves R, Cole JP, et al. AIRMESS - Academy of International Regenerative Medicine & Surgery Societies: Recommendations in the use of platelet-rich plasma (PRP), autologous stem cell-based therapy (ASC-BT) in androgenetic alopecia and wound healing. Expert Opin Biol Ther 2021;21(11):1443–1449. [DOI] [PubMed] [Google Scholar]
- 79. Gentile P, Garcovich S. Advances in regenerative stem cell therapy in androgenic alopecia and hair loss: Wnt pathway, growth-factor, and mesenchymal stem cell signaling impact analysis on cell growth and hair follicle development. Cells 2019;8(5):E466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Gentile P, Garcovich S. Systematic review: The platelet-rich plasma use in female androgenetic alopecia as effective autologous treatment of regenerative plastic surgery. J Plast Reconstr Aesthet Surg 2022;75(2):850–859. [DOI] [PubMed] [Google Scholar]
- 81. Gentile P, Garcovich S. Systematic review of platelet-rich plasma use in androgenetic alopecia compared with Minoxidil®, Finasteride®, and adult stem cell-based therapy. Int J Mol Sci 2020;21(8):E2702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Gentile P, Garcovich S, Bielli A, et al. The effect of platelet-rich plasma in hair regrowth: A randomized placebo-controlled trial. Stem Cells Transl Med 2015;4(11):1317–1323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Cervelli V, Garcovich S, Bielli A, et al. The effect of autologous activated platelet rich plasma (AA-PRP) injection on pattern hair loss: Clinical and histomorphometric evaluation. Biomed Res Int 2014;2014:760709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Gentile P, Cole JP, Cole MA, et al. Evaluation of not-activated and activated PRP in hair loss treatment: Role of growth factor and cytokine concentrations obtained by different collection systems. Int J Mol Sci 2017;18(2):E408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Gentile P, Garcovich S. Autologous activated platelet-rich plasma (AA-PRP) and non-activated (A-PRP) in hair growth: A retrospective, blinded, randomized evaluation in androgenetic alopecia. Expert Opin Biol Ther 2020;20(3):327–337. [DOI] [PubMed] [Google Scholar]
- 86. Scioli MG, Bielli A, Gentile P, et al. Combined treatment with platelet-rich plasma and insulin favours chondrogenic and osteogenic differentiation of human adipose-derived stem cells in three-dimensional collagen scaffolds. J Tissue Eng Regen Med 2017;11(8):2398–2410. [DOI] [PubMed] [Google Scholar]
- 87. Scioli MG, Bielli A, Gentile P, et al. The biomolecular basis of adipogenic differentiation of adipose-derived stem cells. Int J Mol Sci 2014;15(4):6517–6526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Gentile P, Sterodimas A, Calabrese C, et al. Systematic review: Advances of fat tissue engineering as bioactive scaffold, bioactive material, and source for adipose-derived mesenchymal stem cells in wound and scar treatment. Stem Cell Res Ther 2021;12(1):318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Gentile P, Garcovich S. Concise review: Adipose-derived stem cells (ASCs) and adipocyte-secreted exosomal microRNA (A-SE-miR) modulate cancer growth and proMote wound repair. J Clin Med 2019;8(6):E855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Gentile P, Piccinno MS, Calabrese C. Characteristics and potentiality of human adipose-derived stem cells (hASCs) obtained from enzymatic digestion of Fat Graft. Cells 2019;8(3):E282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Gentile P. New strategies in plastic surgery: Autologous adipose-derived mesenchymal stem cells contained in fat grafting improves symptomatic scars. Front Biosci (Landmark Ed) 2021;26(8):255–257. [DOI] [PubMed] [Google Scholar]
- 92. Grimaldi M, Gentile P, Labardi L, et al. Lipostructure technique in Romberg syndrome. J Craniofac Surg 2008;19(4):1089–1091. [DOI] [PubMed] [Google Scholar]
- 93. Araco A, Gravante G, Araco F, et al. Breast asymmetries: A brief review and our experience. Aesthetic Plast Surg 2006;30(3):309–319. [DOI] [PubMed] [Google Scholar]
- 94. Gentile P. Breast silicone gel implants versus autologous fat grafting: Biomaterials and bioactive materials in comparison. J Clin Med 2021;10(15):3310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Gentile P, Kothari A, Casella D, et al. Fat graft enhanced with adipose-derived stem cells in aesthetic breast augmentation: Clinical, histological, and instrumental evaluation. Aesthet Surg J 2020;40(9):962–977. [DOI] [PubMed] [Google Scholar]
- 96. Gentile P, Calabrese C, De Angelis B, et al. Impact of the different preparation methods to obtain human adipose-derived stromal vascular fraction cells (AD-SVFs) and human adipose-derived mesenchymal stem cells (AD-MSCs): Enzymatic digestion versus mechanical centrifugation. Int J Mol Sci 2019;20(21):E5471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Gentile P, Scioli MG, Bielli A, et al. Concise review: The use of adipose-derived stromal vascular fraction cells and platelet rich plasma in regenerative plastic surgery. Stem Cells 2017;35(1):117–134. [DOI] [PubMed] [Google Scholar]
- 98. Gentile P, Sterodimas A, Pizzicannella J, et al. Systematic review: Allogenic use of stromal vascular fraction (SVF) and decellularized extracellular matrices (ECM) as advanced therapy medicinal products (ATMP) in tissue regeneration. Int J Mol Sci 2020;21(14):E4982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Gentile P, De Angelis B, Pasin M, et al. Adipose-derived stromal vascular fraction cells and platelet-rich plasma: Basic and clinical evaluation for cell-based therapies in patients with scars on the face. J Craniofac Surg 2014;25(1):267–272. [DOI] [PubMed] [Google Scholar]
- 100. Gentile P, Sterodimas A, Calabrese C, et al. Regenerative application of stromal vascular fraction cells enhanced fat graft maintenance: Clinical assessment in face rejuvenation. Expert Opin Biol Ther 2020;20(12):1503–1513. [DOI] [PubMed] [Google Scholar]
- 101. Gentile P, Casella D, Palma E, et al. Engineered fat graft enhanced with adipose-derived stromal vascular fraction cells for regenerative medicine: Clinical, histological and instrumental evaluation in breast reconstruction. J Clin Med 2019;8(4):E504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Gentile P, Scioli MG, Orlandi A, et al. Breast reconstruction with enhanced stromal vascular fraction fat grafting: What is the best method? Plast Reconstr Surg Glob Open 2015;3(6):e406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Gentile P, Scioli MG, Bielli A, et al. Comparing different nanofat procedures on scars: Role of the stromal vascular fraction and its clinical implications. Regen Med 2017;12(8):939–952. [DOI] [PubMed] [Google Scholar]
- 104. Gentile P, Scioli MG, Cervelli V, et al. Autologous micrografts from scalp tissue: Trichoscopic and long-term clinical evaluation in male and female androgenetic alopecia. Biomed Res Int 2020;2020:7397162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Gentile P. Autologous cellular method using micrografts of human adipose tissue derived follicle stem cells in androgenic alopecia. Int J Mol Sci 2019;20(14):E3446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Gentile P, Scioli MG, Bielli A, et al. Stem cells from human hair follicles: First mechanical isolation for immediate autologous clinical use in androgenetic alopecia and hair loss. Stem Cell Invest 2017;4:58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. Baraniak PR, Cooke MT, Saeed R, et al. Stiffening of human mesenchymal stem cell spheroid microenvironments induced by incorporation of gelatin microparticles. J Mech Behav Biomed Mater 2012;11:63–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Sart S, Tsai AC, Li Y, et al. Three-dimensional aggregates of mesenchymal stem cells: Cellular mechanisms, biological properties, and applications. Tissue Eng Part B Rev 2014;20(5):365–380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Alvarez-Pérez J, Ballesteros P, Cerdán S. Microscopic images of intraspheroidal pH by 1H magnetic resonance chemical shift imaging of pH sensitive indicators. MAGMA 2005;18(6):293–301. [DOI] [PubMed] [Google Scholar]
- 110. Bhang SH, Cho SW, La WG, et al. Angiogenesis in ischemic tissue produced by spheroid grafting of human adipose-derived stromal cells. Biomaterials 2011;32(11):2734–2747. [DOI] [PubMed] [Google Scholar]
- 111. Murphy KC, Hung BP, Browne-Bourne S, et al. Measurement of oxygen tension within mesenchymal stem cell spheroids. J R Soc Interface 2017;14(127):20160851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112. Meyers VE, Zayzafoon M, Douglas JT, et al. RhoA and cytoskeletal disruption mediate reduced osteoblastogenesis and enhanced adipogenesis of human mesenchymal stem cells in modeled microgravity. J Bone Miner Res 2005;20(10):1858–1866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113. Huang C, Zhao L, Gu J, et al. The migration and differentiation of hUC-MSCs(CXCR4/GFP) encapsulated in BDNF/chitosan scaffolds for brain tissue engineering. Biomed Mater 2016;11(3):035004. [DOI] [PubMed] [Google Scholar]
- 114. Lin YT, Wang CK, Yang SC, et al. Elimination of undifferentiated human embryonic stem cells by cardiac glycosides. Sci Rep 2017;7(1):5289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115. Quiroz HJ, Valencia SF, Liu ZJ, et al. Increasing the therapeutic potential of stem cell therapies for critical limb ischemia. HSOA J Stem Cells Res Dev Ther 2020;6(1):024. [DOI] [PMC free article] [PubMed] [Google Scholar]