Abstract
Background
Immune checkpoint inhibitors have significantly improved outcomes in first line cutaneous melanoma. However, there is a high unmet need for patients who progress on these therapies and combination therapies are being explored to improve outcomes. Tebentafusp is a first-in-class gp100×CD3 ImmTAC bispecific that demonstrated overall survival (OS) benefit (HR 0.51) in metastatic uveal melanoma despite a modest overall response rate of 9%. This phase 1b trial evaluated the safety and initial efficacy of tebentafusp in combination with durvalumab (anti-programmed death ligand 1 (PDL1)) and/or tremelimumab (anti-cytotoxic T lymphocyte-associated antigen 4) in patients with metastatic cutaneous melanoma (mCM), the majority of whom progressed on prior checkpoint inhibitors.
Methods
In this open-label, multicenter, phase 1b, dose-escalation trial, HLA-A*02:01-positive patients with mCM received weekly intravenous tebentafusp with increasing monthly doses of durvalumab and/or tremelimumab starting day 15 of each cycle. The primary objective was to identify the maximum tolerated dose (MTD) or recommended phase 2 dose for each combination. Efficacy analyses were performed in all tebentafusp with durvalumab±tremelimumab treated patients with a sensitivity analysis in those who progressed on prior anti-PD(L)1 therapy.
Results
85 patients were assigned to receive tebentafusp in combination with durvalumab (n=43), tremelimumab (n=13), or durvalumab and tremelimumab (n=29). Patients were heavily pretreated with a median of 3 prior lines of therapy, including 76 (89%) who received prior anti-PD(L)1. Maximum target doses of tebentafusp (68 mcg) alone or in combination with durvalumab (20 mg/kg) and tremelimumab (1 mg/kg) were tolerated; MTD was not formally identified for any arm. Adverse event profile was consistent with each individual therapy and there were no new safety signals nor treatment-related deaths. In the efficacy subset (n=72), the response rate was 14%, tumor shrinkage rate was 41% and 1-year OS rate was 76% (95% CI: 70% to 81%). The 1-year OS for triplet combination (79%; 95% CI: 71% to 86%) was similar to tebentafusp plus durvalumab (74%; 95% CI: 67% to 80%).
Conclusion
At maximum target doses, the safety of tebentafusp with checkpoint inhibitors was consistent with safety of each individual therapy. Tebentafusp with durvalumab demonstrated promising efficacy in heavily pretreated patients with mCM, including those who progressed on prior anti-PD(L)1.
Trial registration number
Keywords: Melanoma, Immune Checkpoint Inhibitors, T-Lymphocytes, Immunotherapy
WHAT IS ALREADY KNOWN ON THIS TOPIC
Tebentafusp monotherapy showed promising activity in adult HLA-A2+ patients with metastatic cutaneous melanoma (mCM) in a phase 1 study and overall survival benefit in a randomized phase 3 study in metastatic uveal melanoma, with tumor shrinkage in 39% of patients despite a modest overall response rate of 9%. Due to the complementary mechanisms of action, we conducted a dose escalation trial to determine the safety and efficacy of tebentafusp in combination with immune checkpoint inhibitors durvalumab and/or tremelimumab in heavily pretreated patients with mCM.
WHAT THIS STUDY ADDS
Tebentafusp in combination with anti-programmed death ligand 1 (PDL1) and/or anti-cytotoxic T lymphocyte-associated antigen 4 was well tolerated. The signals of efficacy were similar to those observed in patients with metastatic uveal melanoma, including response rate of 14%, tumor shrinkage in 41% of patients, 1-year survival of 76% and median overall survival of 18.7 months. These data suggest clinically meaningful antitumor activity with tebentafusp+durvalumab in patients with mCM, including those who have previously progressed on prior anti-PD(L)1 therapy.
HOW THIS STUDY MIGHT AFFECT RESEARCH, PRACTICE OR POLICY
The prognosis for patients with mCM with progressive disease on anti-programmed death 1 therapy is poor. Tebentafusp in combination with immune checkpoint inhibitors may be a novel treatment option for these patients.
Introduction
Cutaneous melanoma (CM) is the most aggressive skin cancer and the cause of the vast majority of skin cancer-related mortality.1 With an estimated 106,110 new cases in 2021,2 the incidence of CM is increasing dramatically, especially in men.2–6 Following primary tumor excision, up to one-third of patients go on to develop metastatic disease.7
Currently available therapies for the first-line treatment of metastatic cutaneous melanoma (mCM) include anti-programmed death 1 (PD1) monotherapies (pembrolizumab, nivolumab) or anti-PD1 in combination with a cytotoxic T lymphocyte-associated antigen 4 (CTLA4) or lymphocyte-activation gene 3 (LAG3) inhibitor.8 Combined BRAF and MEK inhibition is used for tumors harboring a BRAF V600 mutation.9 In pivotal studies, 5-year overall survival (OS) rates were ~43% for anti-PD1 monotherapy, 52% for combination anti-PD1 and anti-CTLA4, and ~33% for combined BRAF/MEK inhibitors.10–14 For patients who progress on these therapies, there are few options beyond the (re)use of anti-PD1 or, in the event of specific gene aberrations (BRAF and KIT mutations), targeted therapies.9 In these patients, prognosis is poor, with recent studies in patients with anti-PD(L)1-resistant/refractory mCM reporting 1-year OS rates of ~55% and median OS of 14 months.15–18
The success of immune checkpoint inhibitors in CM relies on an active immune inhibitory pathway including the PD1/PDL1 axis (ie, expression of PD1 by tumor infiltrating T cells together with high PDL1 expression by tumor cells), and thus requires the presence of an intratumoral T cell infiltrate which has been associated with the high mutational burden and immunogenicity of this particular tumor type.19–22 However, two-thirds of patients reveal either a primary or secondary resistance to anti-PD(L)1 therapy with many failing to recruit tumor-reactive T cells that mediate tumor cell killing. Consequently, new therapeutic approaches and combinations that recruit effector T cells into the tumor may overcome resistance to checkpoint blockade.19 23
Glycoprotein of 100 kDa (gp100), a melanocyte-associated antigen, is an intracellular protein overexpressed in melanoma24 25 but with limited expression in normal cells.26 ImmTAC (Immune mobilizing monoclonal T cell receptor (TCR) Against Cancer) molecules are a unique class of bispecific proteins that use a TCR for the effective targeting of any protein, including cancer specific intracellular targets, that is, processed and presented as peptide on the cell surface in the context of HLA. Tebentafusp, a gp100×CD3 bispecific ImmTAC, is a fusion protein comprizing a soluble affinity-enhanced HLA-A*02:01-restricted TCR specific for the gp100 peptide, YLEPGPVTA, fused to an anti-CD3 single-chain variable fragment. Once bound to its target peptide-HLA, these ImmTAC bispecifics can redirect and activate polyclonal CD3+T cells to mediate target cell lysis.24 27
In a randomized, phase 3 trial in adult HLA-A*02:01 patients with previously untreated metastatic uveal melanoma (mUM; NCT03070392), tebentafusp significantly prolonged OS in comparison to investigator’s choice of therapy (HR 0.51, 95% CI: 0.37 to 0.71; p<0.0001).27 One remarkable observation was the disconnect between low Response Evaluation Criteria In Solid Tumors (RECIST) response, 9%, and the 49% improvement in OS. The OS benefit appears to be driven by prolonged stable disease including among the 39% of patients with any tumor shrinkage, and even from patients with apparent progressive disease (PD) by radiographic assessment.28
Tebentafusp was well tolerated with most patients experiencing manageable cytokine-mediated (eg, cytokine release syndrome) or skin-related adverse events (AEs) (eg, rash, pruritus) consistent with the mechanism of action (gp100 is expressed in skin melanocytes). The first-in-human trial (NCT01211262) of tebentafusp monotherapy in advanced metastatic melanoma (mostly cutaneous),25 showed a 1-year OS of ~74% in anti-PD(L)1 therapy-naïve patients.29 In that study, serial tumor biopsies showed an increase in CD3+T cells in both the tumor and the skin, which correlated with increased expression of PDL1 and LAG3 in the tumor. Tebentafusp treatment was also associated with an increase in serum cytokines and chemokines and T-cell trafficking from the blood.25 30
Based on their distinct and complementary mechanisms of action and toxicity profile, a combination of tebentafusp with checkpoint inhibitors could overcome resistance and loss of response to PD(L)1 therapy. Here we present proof of concept for the combination of tebentafusp with immune checkpoint inhibitor and complete safety and efficacy results of the phase 1b portion of study IMCgp100-201 in patients who progressed on prior anti-PD(L)1.
Methods
Study design
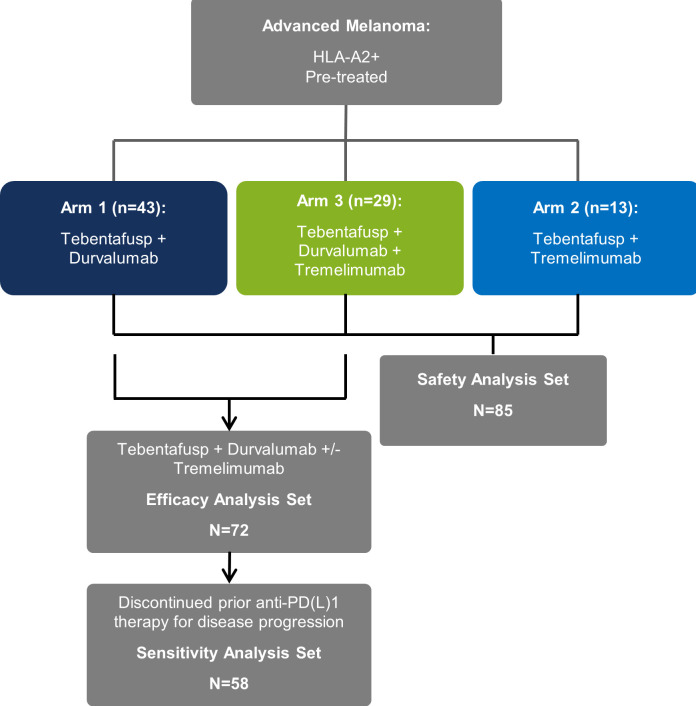
The phase 1b/2 dose-escalation trial was designed to evaluate the safety, tolerability, preliminary efficacy, pharmacokinetics, and pharmacodynamics of tebentafusp in combination with durvalumab (anti-PDL1; Arm 1), tremelimumab (anti-CTLA4; Arm 2) or with durvalumab and tremelimumab (Arm 3) in patients with advanced melanoma (figure 1). The phase 1b portion of the study was an open-label, uncontrolled, multicenter trial that used a standard 3+3 design. Doses of tebentafusp, durvalumab, and/or tremelimumab were escalated in cohorts of 3–6 patients. Dose escalation continued until the maximum tolerated dose (MTD) or recommended phase 2 dose (RP2D) was reached for each arm.
Figure 1.
Flowchart for a phase 1b study of tebentafusp in combination with durvalumab and/or tremelimumab in patients with metastatic cutaneous melanoma. All patients who received at least one full or partial dose of study drug are in the safety analysis set. All patients assigned to Arm 1 (tebentafusp+durvalumab) and Arm 3 (tebentafusp+durvalumab+tremelimumab) who received at least one full or partial dose of study drug are in the efficacy analysis set (EAS). Patients in the EAS who discontinued prior anti-PD(L)1 therapy due to disease progression are in sensitivity analysis set.
A trial safety committee that comprised study investigators and representatives from the sponsor convened at regular intervals to review the safety of trial participants and dose-limiting toxicities (DLTs), and to determine the MTD and the RP2D.
Patients
Eligible patients for the phase 1b study were age ≥18 years, HLA-A*02:01-positive with unresectable stage III or metastatic stage IV non-uveal melanoma, and had an Eastern Cooperative Oncology Group (ECOG) performance status ≤1. There were no restrictions on prior therapy. Patients with uveal melanoma or untreated/symptomatic central nervous system metastases were excluded from the study. Patients who had previously experienced an immune-related adverse event (irAE) leading to permanent discontinuation of their most recent treatment, Grade ≥3 irAE within the past 16 weeks, any Grade 4 irAE (regardless of duration), neurologic or ocular irAE of any grade, or unresolved irAEs (ie, Grade >1; except endocrinopathy) at screening were also excluded.
Treatment
Patients received once weekly intravenous tebentafusp in combination with increasing doses of durvalumab and/or tremelimumab administered intravenously monthly (Q4W) starting on day 15 of each cycle with each cycle consisting of 4 weeks. In the first cycle, patients first received two step-up doses of single-agent tebentafusp on Cycle 1 Day 1 (C1D1) and C1D8, with doses capped at 20 µg and 30 µg, respectively (online supplemental figure S1). Combination dosing started at C1D15 with doses of tebentafusp (up to a maximum of 68 µg in Arm 1 and 50 µg in Arms 2 and 3), durvalumab (up to a maximum of 20 mg/kg) and/or tremelimumab (up to a maximum of 10 mg/kg in Arm 2 and 1 mg/kg in Arm 3) determined by the cohort level within each arm. Full details of the treatment cohorts and dosing levels can be found in online supplemental table S1.
jitc-2023-006747supp001.pdf (2.4MB, pdf)
Continuation of treatment beyond PD according to RECIST V.1.1 was permitted in the absence of clinical symptomatic progression. Treatment was discontinued in the event of confirmed disease progression, initiation of alternative anticancer therapy, unacceptable toxicity, withdrawal of consent by the patient or investigator, completion of 25 cycles of treatment, or if the patient was lost to follow-up.
Objectives and assessments
The primary objectives of the phase 1b study were to identify the MTD and RP2D of durvalumab and/or tremelimumab when administered with tebentafusp. Secondary objectives of the study were characterization of the safety and tolerability of tebentafusp when administered in combination with durvalumab and/or tremelimumab, and to assess the preliminary antitumor efficacy of tebentafusp in combination with durvalumab and/or tremelimumab.
Investigator-reported AEs, collected from C1D1 up to the safety follow-up visit (ie, 30 days after the last dose of study drug), were coded using the Medical Dictionary for Regulatory Activities, V.23.1 and graded according to Common Terminology Criteria for Adverse Events (CTCAE) V.4.03. DLTs were defined as any AE of CTCAE Grade ≥3 occurring during the DLT observation period with selected exceptions (see online supplemental table S2), for which relationship to study treatment cannot be ruled out. The MTD was defined as the highest dose level with an observed incidence of DLTs in <33% of patients in the cohort, and the RP2D was defined as the dose level selected for testing in phase 2 based on all available safety, tolerability, pharmacokinetic, and pharmacodynamic data.
Efficacy was evaluated using RECIST V.1.1 for clinical response and time-to-event estimates of OS. CT/MRI scans were performed at Weeks 8, 16, 24, 32, 40 (±1 week), then every 12 weeks (±1 week) until PD or withdrawal. Tumor shrinkage was based on observing a decrease in the sum of the longest diameters for all target lesions while on treatment relative to baseline measurements regardless of the appearance of new lesions. Patients were followed for survival every 3 months until death or the end of the study.
Statistical analyses
The safety analysis set comprised all patients who received ≥1 full or partial dose of tebentafusp, durvalumab, or tremelimumab, with patients classified according to their assigned arm. The efficacy analysis set comprised patients assigned to treatment in Arms 1 (tebentafusp+durvalumab) and 3 (triplet therapy) and who received ≥1 full or partial dose of tebentafusp, durvalumab, or tremelimumab. Arm 2 (tebentafusp+tremelimumab) was excluded from efficacy analyses due to too few patients for meaningful analyses (n=13). Sensitivity analyses of patients in the efficacy analysis set who progressed on prior anti-PD(L)1 therapy were also conducted (sensitivity analysis set).
Time-to-event estimates of OS, duration of response, and duration of tumor shrinkage were calculated using Kaplan-Meier methods.
Results
Patients
All patients enrolled, N=85, received at least one dose of study treatment and contributed to the safety analysis. This included 43 patients who were assigned to receive tebentafusp+durvalumab (Arm 1), 13 assigned to receive tebentafusp+tremelimumab (Arm 2), and 29 assigned to receive triplet therapy (Arm 3). Among patients enrolled, 81 (95%) had CM and 4 (5%) had mucosal melanoma. The median age was 58 (range 28–79), 76% were ECOG 0, and 38% had baseline lactate dehydrogenase (LDH) above the upper limit of normal (ULN) (table 1). Thirty-three (39%) patients had mutated BRAF, of whom 30 had BRAFV600 mutations and 25 had received prior BRAF/MEK inhibitors (7 single agents and 18 combinations). The median number of prior metastatic lines of therapy was 3 (range 1–4) with nearly all patients (76/85 (89%)) receiving prior anti-PD(L)1 therapy; of whom 80% also received prior ipilimumab. Of the 72 patients who were assigned to receive tebentafusp and durvalumab±tremelimumab (Arms 1 and 3), 63 received prior anti-PD(L)1 therapy, of which 58 were reported as having discontinued prior anti-PD(L)1 therapy due to disease progression. Thirty-four of these 58 patients (59%) were documented as relapsed (best overall response (BOR)=stable disease (SD)/partial response (PR)/complete response (CR)) and 19/58 (33%) as primary refractory (BOR=PD) to prior anti-PD(L)1 therapy.
Table 1.
Baseline patient demographics and clinical characteristics
| Arm 1: tebentafusp +durvalumab (N=43) | Arm 2: tebentafusp +tremelimumab (N=13) | Arm 3: tebentafusp +durvalumab +tremelimumab (N=29) | All patients (N=85) | Efficacy population*(N=72) | Sensitivity population†(N=58) | |
| Median age (range), years | 59 (28–79) | 52 (30–74) | 58 (30–79) | 58 (28–79) | 59 (28–79) | 58 (29–79) |
| ECOG performance status, n (%) | ||||||
| 0 | 34 (79) | 5 (38) | 26 (90) | 65 (76) | 60 (83) | 47 (81) |
| 1 | 9 (21) | 8 (62) | 3 (10) | 20 (24) | 12 (17) | 11 (19) |
| Lactate dehydrogenase, n (%) | ||||||
| ≤ULN | 16 (37) | 5 (38) | 13 (45) | 34 (40) | 29 (40) | 24 (41) |
| >ULN | 18 (42) | 3 (23) | 11 (38) | 32 (38) | 29 (40) | 23 (40) |
| Missing | 9 (21) | 5 (38) | 5 (17) | 19 (22) | 14 (19) | 11 (19) |
| BRAFm, n (%) | 19 (44) | 6 (46) | 8 (28) | 33 (39) | 27 (38) | 22 (38) |
| BRAFm pts who received inhibitors | 13 (68) | 6 (100) | 6 (75) | 25 (76) | 19 (70) | 16 (73) |
| Prior lines of metastatic therapy, n (%) | ||||||
| 1L | 11 (26) | 1 (8) | 5 (17) | 17 (20) | 16 (22) | 10 (17) |
| 2L | 9 (21) | 1 (8) | 7 (24) | 17 (20) | 16 (22) | 16 (28) |
| 3L | 5 (12) | 5 (38) | 8 (28) | 18 (21) | 13 (18) | 12 (21) |
| 4L+ | 13 (30) | 6 (46) | 6 (21) | 25 (30) | 19 (26) | 18 (31) |
| Prior immunotherapy | ||||||
| Checkpoint inhibitors | ||||||
| Anti-PD(L)1 | 36 (84) | 13 (100) | 27 (93) | 76 (89) | 63 (88) | 58 (100) |
| Anti-CTLA4 | 32 (74) | 13 (100) | 19 (66) | 64 (75) | 51 (71) | 44 (76) |
| TIL therapy | 1 (2) | 2 (15) | 3 (10) | 6 (7) | 4 (6) | 4 (7) |
| Tebentafusp | 0 | 0 | 1 (3) | 1 (1) | 1 (1) | 1 (2) |
| Other | 18 (42) | 3 (23) | 8 (28) | 29 (34) | 26 (36) | 22 (38) |
| BOR to prior anti-PD(L)1 therapy, n (%) | ||||||
| CR/PR/SD (ie, relapsed) | 21 (46) | 10 (77) | 16 (55) | 47 (55) | 37 (51) | 34 (59) |
| PD (ie, refractory) | 11 (26) | 3 (23) | 10 (34) | 24 (28) | 21 (29) | 19 (33) |
| Missing | 4 (9) | 0 | 1 (3) | 5 (6) | 5 (7) | 5 (9) |
*The efficacy analysis population consists of patients treated with tebentafusp and durvalumab±tremelimumab (Arms 1 and 3).
†The sensitivity analysis population consists of patients treated with tebentafusp and durvalumab±tremelimumab who discontinued prior anti-PD(L)1 due to disease progression.
BOR, best overall response; BRAFm, BRAF mutation; CR, complete response; CTLA4, cytotoxic T-lymphocyte-associated antigen 4; ECOG, Eastern Cooperative Oncology Group; 1L, first line; 2L, second line; 3L, third line; 4L+, fourth line and beyond; PD, progressive disease; PD(L)1, programmed death (ligand) 1; PD(L)1, programmed death (ligand) 1; PR, partial response; SD, stable disease; TIL, tumor infiltrating lymphocyte; ULN, upper limit of normal.
Safety
MTDs were not formally identified in any of the treatment arms. The maximum administered doses were as follows: Arm 1 (tebentafusp 68 mcg+durvalumab 20 mg/kg); Arm 2 (tebentafusp 30 mcg+tremelimumab 1 mg/kg); Arm 3 (tebentafusp 50 mcg+durvalumab 10 mg/kg+tremelimumab 1 mg/kg). There were two DLTs reported including prolonged Grade 3 rash (Arm 1; tebentafusp 68 mcg and durvalumab 20 mg/kg) and Grade 2 diarrhea leading to treatment delay (Arm 2; tebentafusp 30 mcg and tremelimumab 1 mg/kg).
All patients experienced a tebentafusp-related adverse event (TRAE); however, few TRAEs led to treatment discontinuation (table 2). Two TRAEs led to discontinuation from Arm 3 and included Grade 2 pancreatitis and Grade 3 rash with Grade 2 generalized edema. There were no treatment-related deaths (table 2).
Table 2.
Summary of treatment-related adverse events
| n (%) | Arm 1: tebentafusp + durvalumab (N=43) |
Arm 2: tebentafusp + tremelimumab (N=13) |
Arm 3: tebentafusp + durvalumab+ tremelimumab(N=29) | All patients (N=85) |
| Any related | 43 (100) | 13 (100) | 29 (100) | 85 (100) |
| Grade ≥3 | 19 (44) | 3 (23) | 12 (41) | 34 (40) |
| Leading to discontinuation | 0 | 0 | 2* (7) | 2* (2) |
| Resulting in death | 0 | 0 | 0 | 0 |
| Any, tebentafusp-related | 43 (100) | 13 (100) | 29 (100) | 85 (100) |
| Grade ≥3, tebentafusp-related | 19 (44) | 3 (23) | 11 (38) | 33 (39) |
| Leading to discontinuation | 0 | 0 | 2* (7) | 2* (2) |
| Resulting in death | 0 | 0 | 0 | 0 |
*Related adverse events that led to discontinuation: Grade 2 pancreatitis (Arm 3); Grade 3 rash with Grade 2 generalized edema (Arm 3).
AEs were generally consistent with those previously reported for the individual components of each treatment arm; no AEs were synergistic or exacerbated (online supplemental tables S3 and S4). The most common AEs related to tebentafusp in patients who received tebentafusp plus durvalumab (Arm 1) or triplet therapy (Arm 3) included rash (67% and 79%, respectively), pruritus (65% and 59%, respectively), fever (51% and 38%, respectively), and fatigue (47% and 45%, respectively). No patient experienced Grade 3–4 cytokine release syndrome or immune effector cell-associated neurotoxicity syndrome.
Efficacy
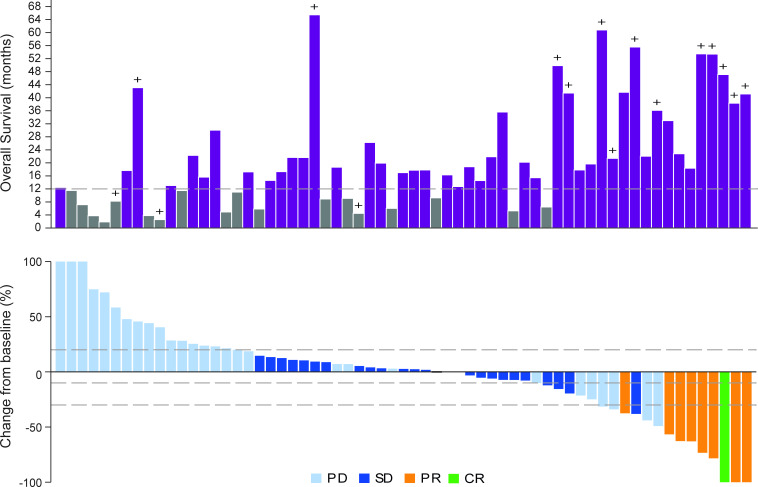
Among evaluable patients receiving tebentafusp in combination with durvalumab±tremelimumab (Arms 1 and 3), the overall response rate was 14%. One patient had a confirmed CR (Arm 1) and eight patients had a confirmed PR (four in Arm 1 and four in Arm 3), with a median duration of response of 19.6 months (range 5.6–not reached months). One patient has an ongoing response at 38.7 months. Forty-one per cent of evaluable patients (26/63) had tumor shrinkage as their best overall change in tumor size (figure 2); of whom 92% were alive at 1 year.
Figure 2.
Overall survival and best change in tumor size from baseline in patients receiving tebentafusp in combination with durvalumab±tremelimumab (A) Overall survival in months is plotted for each evaluable patient (n=63). + denotes censored. (A–B) Data are presented only for those patients for whom best overall response to previous anti- anti-PD(L)1 therapy was known and only patients with at least one evaluable post baseline target lesion scan were included. Nine patients overall were not included due to non-measurable disease at baseline or no evaluable post-baseline target lesion scans. Evaluable post-baseline scans must be on or prior to disease progression or starting subsequent alternative cancer therapy to be considered. (B) Waterfall plot showing the best change in tumor size (n=63). Forty-one per cent of patients had tumor reduction at any time. Tumor size was measured as the sum of longest diameters or short axis of the target lesions according to Response Evaluation Criteria In Solid Tumors V.1.1 by investigator assessment. Best percent change in target lesion size was the maximum percent reduction from baseline or the minimum percent increase from baseline (in the absence of a reduction), up until disease progression or starting subsequent alternative cancer therapy. Tumor shrinkage is shown regardless of whether new lesions identified. Reference lines at 20% and −30% mark target lesion response criteria for disease progression (PD), partial response (PR), respectively. CR, complete response; SD, stable disease.
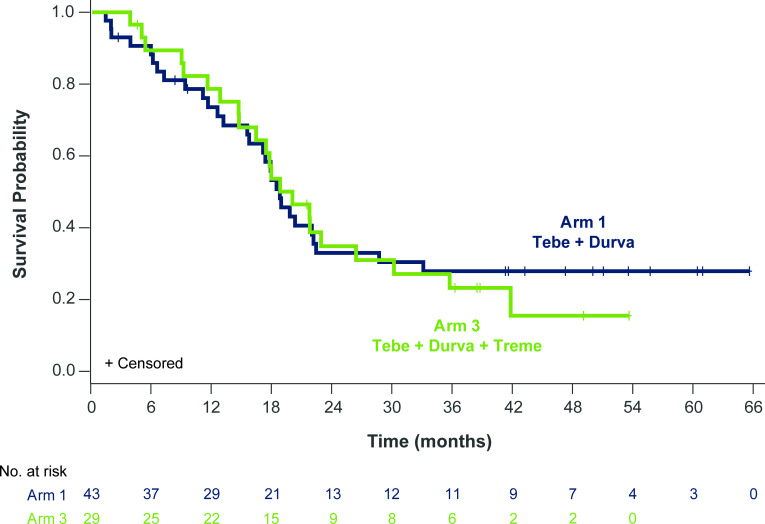
The median OS was 18.7 (95% CI: 17 to 21.9) months and overall 1-year and 2-year OS rates were 76% (95% CI: 70% to 81%) and 34% (95% CI: 28% to 40%), respectively, and were similar for patients receiving tebentafusp+durvalumab (Arm 1) or triplet therapy (Arm 3) (figure 3), with 1-year OS rates of 74% (95% CI: 67% to 80%) and 79% (95% CI: 71% to 86%), respectively, and 2-year rates of 33% (95% CI: 26% to 40%) and 35% (95% CI: 26% to 44%), respectively.
Figure 3.
Overall survival in patients who received tebentafusp and durvalumab±tremelimumab. (A) Kaplan-Meier estimates of overall survival (OS) for patients treated with tebentafusp in combination with durvalumab (Arm 1; n=43; blue) or durvalumab and tremelimumab (Arm 3; n=29; green). Events are deaths due to any cause. Patients not known to have died at the time of analysis are censored. For patients receiving combination tebentafusp and durvalumab (Arm 1), the median OS was 18.7 (95% CI: 15.4 to 22.3) months with a 1-year OS rate of 74% (95% CI: 67% to 80%). For patients receiving triplet therapy (Arm 3), the median OS was 19.9 (95% CI: 14.6 to 26.3) months with a 1-year OS rate of 79% (95% CI: 71% to 86%).
In order to compare these results to recent clinical trials, a sensitivity analysis was limited to patients with documented progression on prior anti-PD(L)1 therapy (n=58). In this subset of patients, the overall response rate was 10%. Five patients had a confirmed PR (three in Arm 1 and two in Arm 3), with a median duration of response of 11.8 months (range 5.6–34.5 months with the response of one patient ongoing at 34.5 months at time of data cut-off). Thirty-seven per cent of those evaluable (19/52) had tumor shrinkage as their best overall change in tumor size (online supplemental figure S2). Some patients had tumor shrinkage that was durable despite the appearance of new lesions. Sustained durable tumor shrinkage was seen both in patients who were primary refractory, defined as best response of PD to prior anti-PD(L)1 therapy, or relapsed, defined as best response of SD, PR, or CR followed by disease progression while on prior anti-PD(L)1 (online supplemental figure S3).
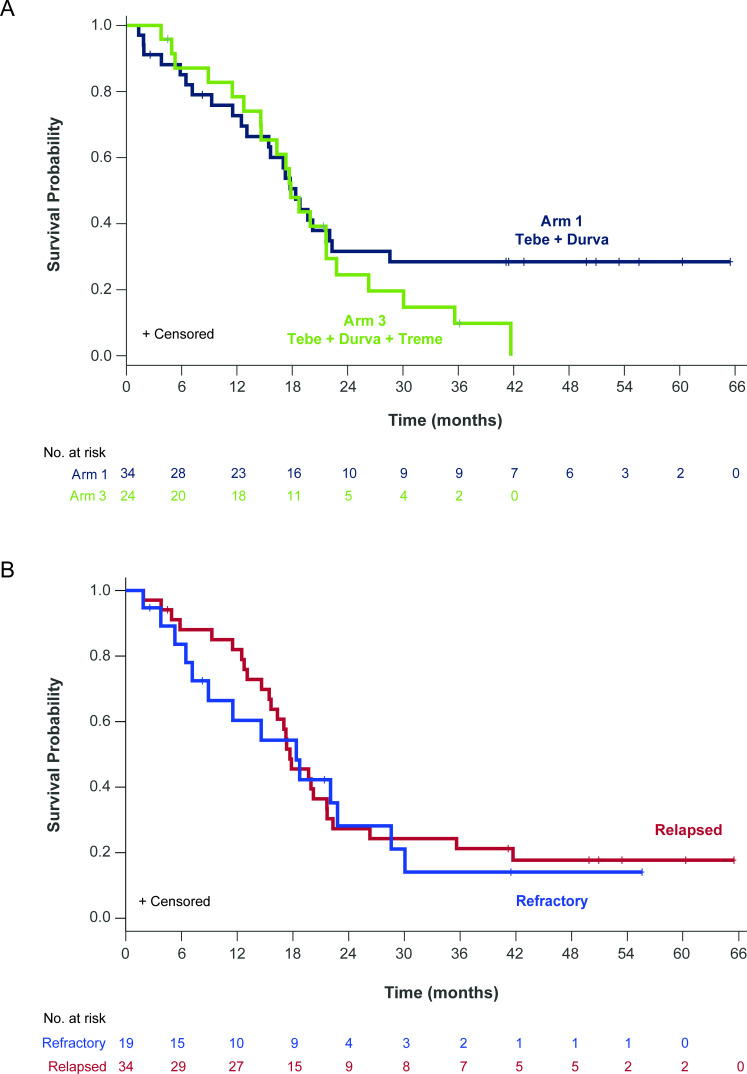
The OS in the sensitivity analysis, restricted to patients who progressed on prior anti-PD(L)1, was similar to that of the efficacy evaluable patients. The overall 1-year and 2-year OS rates were 75% (95% CI: 70% to 87%) and 32% (95% CI: 23% to 40%), respectively, and were similar between treatment arms (figure 4A). OS with tebentafusp and durvalumab±tremelimumab was not influenced by the timing of prior anti-PD(L)1 relative to the start of tebentafusp as patients receiving anti-PD(L)1 therapy immediately prior to enrollment (median 2 months earlier) had the same 1-year OS rate of 75% as those patients who had their most recent anti-PD(L)1 more remotely (median 9 months earlier) (online supplemental figure S4). Intriguingly, even patients with tumor increase as best change in tumor size from baseline had promising 1-year OS of 58% (31/52).
Figure 4.
Overall survival in patients who progressed on prior anti-PD(L)1. (A) Kaplan-Meier estimates of overall survival (OS) for patients who progressed on prior anti-PD(L)1 therapy treated with tebentafusp in combination with durvalumab (Arm 1; n=34; blue) or durvalumab and tremelimumab (Arm 3; n=24; green). Events are deaths due to any cause. Patients not known to have died at the time of analysis are censored. For patients receiving combination tebentafusp and durvalumab (Arm 1), the median OS was 18.4 (95% CI: 13.1 to 22.3) months with a 1-year OS rate of 73% (95% CI: 65% to 80%). For patients receiving triplet therapy (Arm 3), the median OS was 17.8 (95% CI: 14.6 to 21.7) months with a 1-year OS rate of 78% (95% CI: 70% to 87%). (B) Kaplan-Meier estimates of OS for patients who had a best response of progressive disease while on prior anti-PD(L)1 (refractory; n=19; blue) or had a best response of either stable disease (n=17) or partial response/complete response (n=17) and then had disease progression on prior anti-PD(L)1 (relapsed n=34; red). For patients refractory to prior anti-PD(L)1, the median OS was 18.4 (95% CI: 7.2 to 28.6) months with a 1-year OS rate of 60% (95% CI: 49% to 72%). For patients relapsed on anti-PD(L)1, the median OS was 17.7 (95% CI: 15.4 to 21.7) months with a 1-year OS rate of 82% (95% CI: 75% to 89%).
Promising OS was observed in patients who relapsed or were refractory to prior anti-PD(L)1 (figure 4B). Among 34 evaluable patients who relapsed on prior anti-PD(L)1, the median OS was 17.7 (95% CI: 15.4 to 21.7) months with 1-year and 2-year OS rates of 82% (95% CI: 75% to 89%) and 27% (95% CI: 20% to 35%), respectively. Among 19 evaluable patients with disease refractory to prior anti-PD(L)1, the 1-year and 2-year OS rates were 60% (95% CI: 49% to 72%) and 28% (95% CI: 17% to 40%), respectively, with median OS of 18.4 (95% CI: 7.2 to 28.6) months.
Discussion
This phase 1 study indicates that tebentafusp can be safely combined with anti-PD(L)1 and/or anti-CTLA4 therapy and provides the first evidence of efficacy for this combination therapy. AEs were generally mild-to-moderate and consistent with the individual components of each arm. No new safety concerns were identified with either combination regimen.
OS has been demonstrated as the best endpoint to capture benefit from tebentafusp in prior uveal melanoma studies.27 31 The combination of tebentafusp and durvalumab (± tremelimumab) led to promising overall 1-year and 2-year OS rates of 76% and 34% in this heavily pretreated cohort of patients. Clinical benefit was seen in patients with melanoma that was primary refractory to prior anti-PD(L)1 as well as those who relapsed on prior PD(L)1 therapy.
Intriguingly, the efficacy endpoints observed in this tebentafusp mCM study matched those observed in the phase 3 study of tebentafusp in mUM.27 The 1-year OS rates were both approximately 75%. The RECIST response rates were roughly the same, both with promising durability, and approximately 40% of patients in both studies had any tumor shrinkage. In the mUM study, benefit was observed even in patients with an increase in the size of the index lesions.28 In this study, a similar percentage of patients had an increase in their tumor burden and the 1-year OS in these patients was similar to that from the phase 3 mUM study, approximately 60%.
While anti-PD1 with or without anti-CTLA4 has shown clear survival benefit in first line, there is a high unmet need for patients who progress on these therapies. Retrospective studies of ICI reuse following progression have reported 1 years OS rates of 38–58%, with differences in baseline characteristics across these studies (eg, baseline LDH>ULN range 26–66%) not correlated with OS benefit.16 32 33 A few other treatments have been tested in phase 1/2 trials in patients who progressed on prior anti-PD1 regimens with varying response rates although the 1-year OS rates are around 55% which is lower than the 76% reported in this study. For example, the combination of the tyrosine kinase inhibitor lenvatinib plus pembrolizumab led to a response rate of 21% with a response duration of 8 months,34 but the 1-year OS was 55%. Adoptive T cell transfer where tumor infiltrating lymphocytes (TILs) are extracted from the tumor and expanded ex vivo has shown response rates of up to 36% and durable remission in some patients with advanced melanoma.35 However, the 1-year OS rate is also approximately 55%.36 Furthermore, patients must undergo non-myeloablative lymphodepleting chemotherapy prior to infusion and receive high dose interleukin-2, which together has significant associated toxicity,37 thus limiting patient eligibility. Consequently, TIL therapy will only be possible to use in a small subset of fit patients and is presently only available in a limited number of centers worldwide.
T cell engaging bispecifics have a number of advantages over cell therapies as they are ‘off-the-shelf’ molecules with potential for repeat dosing and tunable serum kinetics, they have the ability to activate a polyclonal T cell response with potential for triggering natural secondary responses38 and, as shown in this study, they can be combined with other therapies. Antibody-based bispecifics like blinatumomab (CD19×CD3) that use antibody fragments as both targeting and effector domains are limited to targeting cell surface antigens which are also highly expressed on normal cells and, to date, have only shown benefit in liquid tumors.39 By contrast, ImmTAC bispecifics like tebentafusp use a TCR as targeting domain providing access to intracellular cancer specific/enriched proteins (eg, gp100, cancer testis antigens) that are processed and presented as peptides on the cell surface by HLA. Since most cancer specific antigens are intracellular and thus not accessible by antibodies, four new TCR bispecifics are currently in clinical development for treatment of various solid tumors.40
Conclusions
Tebentafusp can be safely combined with anti-PDL1 and anti-CTLA4 in heavily pretreated patients with mCM. Tebentafusp with anti-PDL1 demonstrated promising OS compared with other investigational therapies in a similar mCM population. These data provide rationale for a randomized study of tebentafusp with or without anti-PD1 versus standard of care in mCM.
Acknowledgments
We thank the patients and their families and caregivers for participating in the study, as well as the study teams at participating sites for their support of this trial and the following employees of Immunocore: Hannah Ryan and Michelle L McCully for assistance with preparation of the manuscript; David Berman and Mohammed Dar for critical review of the manuscript. Medical writing assistance with the writing of the first draft of this manuscript was provided by Andrea Bothwell on behalf of Ashfield MedComms, an Ashfield Health company, with funding provided by Immunocore. MRM is supported by the Oxford NIHR Biomedical Research Centre. Durvalumab and tremelimumab were provided by AstraZeneca.
Footnotes
Twitter: @PAscierto
Presented at: OH., et al. ‘546 Results from Phase Ib study of tebentafusp (tebe) in combination with durvalumab (durva) and/or tremelimumab (treme) in metastatic cutaneous melanoma (mCM).’ In Proceedings of the Society for Immunotherapy of Cancer Annual Meeting, Washington, DC, US, 10–14th November 2021. 5 MRM., et al. ‘104 Updated overall survival (OS) data from Phase 1b study of tebentafusp (tebe) as monotherapy or combination therapy with durvalumab (durva) and/or tremelimumab (treme) in metastatic cutaneous melanoma (mCM).’ In Proceedings of the American Society of Clinical Oncology Annual Meeting, Chicago, IL, US, 3–7th June 2022.
Contributors: Conception and design: OH, CH, SEA and MRM. Provision of study material or patients: OH, JCH, AS, FM, TMB, AS, JMK, PAA, PCL, CM, MO, TRJE, MRM. Collection and assembly of data: All authors. Data analysis and interpretation: All authors. Manuscript writing/review: All authors. Final approval of manuscript: All authors. Accountable for all aspects of the work: All authors. Responsible for the overall content as guarantor: OH
Funding: This study was funded by Immunocore Ltd.
Disclaimer: OH discloses Advisory/Consulting: Aduro Biotech, Akeso Biopharma, Alkermes, Amgen, BeiGene, BioAtla, BMS, Genentech, GlaxoSmithKline, Idera, Immunocore, Incyte, Iovance Biotherapeutics, Janssen, Merck, NextCure, Novartis, Pfizer, Regeneron, Roche, Sanofi, Seattle Genetics, Tempus, Zelluna; Speaker’s Bureau: BMS, Novartis, Pfizer, Sanofi/Regeneron; Honoraria: BMS, Novartis, Pfizer, Sanofi/Regeneron; Research Funding (Institute): Aduro Biotech, Akeso Biopharma, Amgen, Arcus Biosciences, Bioatla, BMS, CytomX Therapeutics, Exelixis, Genentech, GlaxoSmithKline, Idera, Immunocore, Incyte, Iovance Biotherapeutics, Merck, Merck Serono, Moderna Therapeutics, NextCure, Novartis, Pfizer, Regeneron, Roche, Rubius Therapeutics, Sanofi, Seattle Genetics, Torque, Zelluna. JCH discloses Honoraria: Amgen, Bristol Myers Squibb Foundation, GlaxoSmithKline, Immunocore, MSD, Novartis, Pierre Fabre, Sanofi, Sun Pharma; Advisory/Consulting: MSD, GSK, Pierre Fabre, Sun Pharma; Research Funding (Institute): 4SC, BioNTech, BMS, Genentech/Roche, Genmab, Huya, Idera, Immunocore, IO-Biotech, Iovance Biotherapeutics, Kartos, Nektar, Novartis, Philogen, Pierre Fabre, Regeneron, Sanofi, Seagen, Sun Pharma; Expenses: Sunpharma, Pierre Fabre. AS discloses Advisory/Consulting: BMS, Immunocore, Novartis; Research Funding (Institute): BMS, Immunocore, Novartis, Targovax, Polaris, Pfizer, Alkermes, Checkmate Pharmaceuticals, Foghorn Therapeutics, Linnaeus Therapeutics, Prelude Therapeutics. FM discloses Honoraria: Amgen, BMS, Merck, MSD, Novartis, Roche, Sanofi, Immunocore; Advisory/Consulting: Amgen, BMS, Merck, MSD, Novartis, Pierre Fabre, Roche, Sanofi, Immunocore; Research Funding: Novartis, Roche; Expenses: Amgen, BMS, Merck, MSD, Novartis, Pierre Fabre, Roche, Sanofi. TMB discloses Employment: Tennessee Oncology; Advisory/Consulting: AstraZeneca, Bayer, Loxo, Pfizer; Speakers’ Bureau: Bayer, BMS, Lilly; Research Funding (Institution): AbbVie, Amgen, Astellas Pharma, AstraZeneca, Boehringer Ingelheim, BMS, Daiichi Sankyo, Foundation Medicine, Genentech/Roche, GlaxoSmithKline, Immunocore, Immunogen, Incyte, Janssen, Lilly, Loxo, MabVax, MedImmune, Merck, Mirati Therapeutics, Moderna Therapeutics, Novartis, Onyx, Pfizer, Roche, Sanofi, Takeda, Top Alliance BioScience; Expenses: Astellas Pharma, AstraZeneca, Celgene, Clovis Oncology, EMD Serono, Genentech, Lilly, Merck, Novartis, Pfizer. AS discloses Research Funding (Institute): Ascentage, BMS, Ideaya, Immunocore, Merck, Pfizer, Regeneron Pharmaceuticals, Replimune, Seattle Genetics; Advisory Boards: Iovance Biotherapeutics, Novartis, Pfizer, Regeneron Pharmaceuticals. JMK discloses Advisory/Consulting: Amgen, Ankyra Therapeutics, AXIO Research, Becker Pharmaceutical Consulting, Checkmate Pharmaceuticals, DermTech, Fenix Group International, Harbour BioMed, Immunocore, Intellisphere LLC, Iovance Biotherapeutics, IQVIA, Istari Oncology, Merck, Natera, Novartis, Oncocyte, OncoSec, Pfizer, Replimune, Scopus BioPharma, SR One Capital Management, Takeda; Research Funding (Institute): Amgen, BMS, Castle Biosciences, Checkmate Pharmaceuticals, Harbour BioMed, Immunocore, Immvira, Iovance Biotherapeutics, Iovance Biotherapeutics, Merck, Novartis, Schering-Plough, Takeda, Verastem; Honoraria: BMS. PAA discloses Advisory/Consulting: Bristol Myers Squibb, Roche-Genentech, Merck Sharp & Dohme, Novartis, Merck Serono, Pierre-Fabre, AstraZeneca, Sun Pharma, Sanofi, Idera, Sandoz, Immunocore, 4SC, Italfarmaco, Nektar, Boehringer-Ingelheim, Eisai, Regeneron, Daiichi Sankyo, Pfizer, Oncosec, Nouscom, Lunaphore, Seagen, iTeos, Medicenna, Bio-Al Health, ValoTX, Replimmune, Bayer; Research Funding (Institute): Pfizer, BMS, Roche/Genentech, Sanofi; Expenses: Pfizer, Bio-Al Health, Replimmune. PCL discloses Honoraria: Amgen, BMS, Merck, MSD, Nektar, NeraCare GmbH, Novartis, Oncology Education, Pierre Fabre, Roche; Advisory/Consulting: Amgen, BMS, MSD, Nektar, Novartis, Pierre Fabre; Speakers’ Bureau: BMS, MSD, Novartis, Pierre Fabre; Research Funding: BMS; Expenses: BMS, MSD. CM discloses Honoraria: BMS, Roche, Sanofi; Expenses: BMS, Sanofi. MO discloses Speaker’s Bureau: Immunocore; Advisory/Consulting: IDEAYA Biosciences, Immunocore, Trisalus Life Sciences, Delcath; Research Funding (Institute): BMS, Delcath Systems, IDEAYA Biosciences, Immunocore, Linnaeus Therapeutics, Ascentage, Trisalus, Foghorn. TRJE discloses Honoraria (Institute): Ascelia, AstraZeneca, Bicycle Therapeutics, BMS, Eisai, Medivir, MSD, Nucana, Roche/Genentech; Advisory/Consulting (Institute): Karus Therapeutics; Speakers’s Bureau (Institute): AstraZeneca, BMS, Eisai, Medivir, MSD, Nucana, Roche/Genentech, United Medical; Research Funding (Institute): Adaptimmune, Astellas Pharma, AstraZeneca, Athenex, Basilea, Beigene, Berg Pharma, Bicycle Therapeutics, BiolineRx, Boehringer Ingelheim, BMS, Celgene, Clovis Oncology, CytomX Therapeutics, Eisai, GlaxoSmithKline, Halozyme, Immunocore, iOnctura, Iovance Biotherapeutics, Janssen, Johnson & Johnson, Lilly, Medivir, Merck Serono, MSD, MiNA Therapeutics, Modulate Pharma, Novartis, Nucana, Plexxikon, Roche/Genentech, Sanofi/Aventis, Sapience Therapeutics, Seattle Genetics, Sierra Pharma, Starpharma, UCB, Verastem, Vertex; Expert Testimony (Institute): Medivir; Expenses: BMS, Eisai, MSD, Nucana, Pierre Fabre; Other Relationship (Institute): Genmab. CH, RE and SEA are employees of Immunocore, which could benefit from commercialization of these results. MRM discloses Advisory/Consulting: Alkermes, Bayer, BiolineRx, Boehringer Ingelheim, BMS, Immunocore, Kineta, Merck, Silicon Therapeutics, Vaccitech; Research Funding to Institution: Amgen, AstraZeneca, BiolineRx, BMS, GRAIL, Immunocore, Medivir, Merck, Novartis, Pfizer, Regeneron, Replimune, Roche, Salvarx, Vaccitech; Uncompensated relationship: GenesisCare.
Provenance and peer review: Not commissioned; externally peer reviewed.
Supplemental material: This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.
Data availability statement
Data are available upon reasonable request. Access to pre-existing summary outputs (tables or figures) of trial level data may be granted to qualified academic researchers in the field upon request and approval by the study management committee and subject to appropriate data sharing and transfer agreements. Requesters should submit a proposal including purpose, data format (e.g. sas files), hypothesis and specific rationale to info@immunocore.com.
Ethics statements
Patient consent for publication
Not applicable.
Ethics approval
The protocol and proposed informed consent form were reviewed and approved by all relevant Institutional Review Boards, Independent Ethics Committees and/or Research Ethics Boards prior to study commencement. There is no number provided as we did not receive one. Participants gave informed consent to participate in the study before taking part.
References
- 1. Davey MG, Miller N, McInerney NM. A review of epidemiology and cancer biology of malignant Melanoma. Cureus 2021;13. 10.7759/cureus.15087 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. SEER cancer STAT facts: Melanoma of the skin [Internet]. 2022. Available: https://seer.cancer.gov/statfacts/html/melan.html#ref11
- 3. Feigelson HS, Powers JD, Kumar M, et al. Melanoma incidence, recurrence, and mortality in an integrated Healthcare system: A retrospective cohort study. Cancer Med 2019;8:4508–16. 10.1002/cam4.2252 Available: https://onlinelibrary.wiley.com/toc/20457634/8/9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Ghazawi FM, Le M, Lagacé F, et al. Incidence, mortality, and Spatiotemporal distribution of cutaneous malignant Melanoma cases across Canada. J Cutan Med Surg 2019;23:394–412. 10.1177/1203475419852048 [DOI] [PubMed] [Google Scholar]
- 5. Jemal A, Saraiya M, Patel P, et al. Recent trends in cutaneous Melanoma incidence and death rates in the United States, 1992-2006. J Am Acad Dermatol 2011;65(5 Suppl 1):S17–25. 10.1016/j.jaad.2011.04.032 [DOI] [PubMed] [Google Scholar]
- 6. Rigel DS. Epidemiology of Melanoma. Semin Cutan Med Surg 2010;29:204–9. 10.1016/j.sder.2010.10.005 [DOI] [PubMed] [Google Scholar]
- 7. Essner R, Lee JH, Wanek LA, et al. Contemporary surgical treatment of advanced-stage Melanoma. Arch Surg 2004;139:961–6. 10.1001/archsurg.139.9.961 [DOI] [PubMed] [Google Scholar]
- 8. Tawbi HA, Schadendorf D, Lipson EJ, et al. Relatlimab and Nivolumab versus Nivolumab in untreated advanced Melanoma. N Engl J Med 2022;386:24–34. 10.1056/NEJMoa2109970 Available: 10.1056/NEJMoa2109970 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Michielin O, van Akkooi ACJ, Ascierto PA, et al. Cutaneous Melanoma: ESMO clinical practice guidelines for diagnosis, treatment and follow-up†. Ann Oncol 2019;30:1884–901. 10.1093/annonc/mdz411 [DOI] [PubMed] [Google Scholar]
- 10. Hamid O, Robert C, Daud A, et al. Five-year survival outcomes for patients with advanced Melanoma treated with Pembrolizumab in KEYNOTE-001. Ann Oncol 2019;30:582–8. 10.1093/annonc/mdz011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Larkin J, Chiarion-Sileni V, Gonzalez R, et al. Five-year survival with combined Nivolumab and Ipilimumab in advanced Melanoma. N Engl J Med 2019;381:1535–46. 10.1056/NEJMoa1910836 [DOI] [PubMed] [Google Scholar]
- 12. Dummer R, Flaherty KT, Robert C, et al. COLUMBUS 5-year update: a randomized, open-label, phase III trial of Encorafenib plus Binimetinib versus Vemurafenib or Encorafenib in patients with BRAF V600-mutant Melanoma. J Clin Oncol 2022;40:4178–88. 10.1200/JCO.21.02659 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Robert C, Grob JJ, Stroyakovskiy D, et al. Five-year outcomes with Dabrafenib plus Trametinib in metastatic Melanoma. N Engl J Med 2019;381:626–36. 10.1056/NEJMoa1904059 [DOI] [PubMed] [Google Scholar]
- 14. Ascierto PA, Dréno B, Larkin J, et al. 5-year outcomes with Cobimetinib plus Vemurafenib in Brafv600 Mutation–positive advanced Melanoma: extended follow-up of the coBRIM study. Clin Cancer Res 2021;27:5225–35. 10.1158/1078-0432.CCR-21-0809 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Arance AM, de la Cruz-Merino L, Petrella TM, et al. Lenvatinib (Len) plus Pembrolizumab (Pembro) for patients (Pts) with advanced Melanoma and confirmed progression on a PD-1 or PD-L1 inhibitor: updated findings of LEAP-004. JCO 2021;39.(15_suppl) 10.1200/JCO.2021.39.15_suppl.9504 [DOI] [Google Scholar]
- 16. Zimmer L, Apuri S, Eroglu Z, et al. Ipilimumab alone or in combination with Nivolumab after progression on anti-PD-1 therapy in advanced Melanoma. Eur J Cancer 2017;75:47–55. 10.1016/j.ejca.2017.01.009 [DOI] [PubMed] [Google Scholar]
- 17. Pires Da Silva I, Ahmed T, Lo S, et al. Ipilimumab (IPI) alone or in combination with anti-PD-1 (IPI+Pd1) in patients (Pts) with metastatic Melanoma (MM) resistant to Pd1 monotherapy. JCO 2020;38.(15_suppl) 10.1200/JCO.2020.38.15_suppl.10005 [DOI] [Google Scholar]
- 18. Weichenthal M, Ugurel S, Leiter UM, et al. Salvage therapy after failure from anti-PD-1 single agent treatment: a study by the German Adoreg Melanoma Registry. JCO 2019;37.(15_suppl) 10.1200/JCO.2019.37.15_suppl.9505 [DOI] [Google Scholar]
- 19. Tumeh PC, Harview CL, Yearley JH, et al. PD-1 blockade induces responses by inhibiting adaptive immune resistance. Nature 2014;515:568–71. 10.1038/nature13954 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Snyder A, Makarov V, Merghoub T, et al. Genetic basis for clinical response to CTLA-4 blockade in Melanoma. N Engl J Med 2014;371:2189–99. 10.1056/NEJMoa1406498 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Hugo W, Zaretsky JM, Sun L, et al. Genomic and Transcriptomic features of response to anti-PD-1 therapy in metastatic Melanoma. Cell 2017;168:542. 10.1016/j.cell.2017.01.010 [DOI] [PubMed] [Google Scholar]
- 22. Samstein RM, Lee C-H, Shoushtari AN, et al. Tumor mutational load predicts survival after Immunotherapy across multiple cancer types. Nat Genet 2019;51:202–6. 10.1038/s41588-018-0312-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Chen DS, Mellman I. Oncology meets Immunology: the cancer-immunity cycle. Immunity 2013;39:1–10. 10.1016/j.immuni.2013.07.012 [DOI] [PubMed] [Google Scholar]
- 24. Damato BE, Dukes J, Goodall H, et al. Tebentafusp: T cell redirection for the treatment of metastatic Uveal Melanoma. Cancers (Basel) 2019;11:971. 10.3390/cancers11070971 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Middleton MR, McAlpine C, Woodcock VK, et al. A TCR/anti-Cd3 Bispecific fusion protein targeting Gp100, Potently activated antitumor immune responses in patients with metastatic Melanoma. Clin Cancer Res 2020;26:5869–78. 10.1158/1078-0432.CCR-20-1247 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. He Q, Jiang X, Zhou X, et al. Targeting cancers through TCR-peptide/MHC interactions. J Hematol Oncol 2019;12:139. 10.1186/s13045-019-0812-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Nathan P, Hassel JC, Rutkowski P, et al. Overall survival benefit with Tebentafusp in metastatic Uveal Melanoma. N Engl J Med 2021;385:1196–206. 10.1056/NEJMoa2103485 [DOI] [PubMed] [Google Scholar]
- 28. Joshua AM, Baurain J-F, Piperno-Neumann S, et al. Overall survival benefit from Tebentafusp in patients with best response of progressive disease. JCO 2021;39.(15_suppl) 10.1200/JCO.2021.39.15_suppl.9509 [DOI] [Google Scholar]
- 29. Hamid O, Hassel J, Shoushtari A, et al. 546 results from phase IB study of Tebentafusp (Tebe) in combination with Durvalumab (Durva) and/or Tremelimumab (Treme) in metastatic cutaneous Melanoma (mCM). J Immunother Cancer 2021;9(Suppl 2):A576. 10.1136/jitc-2021-SITC2021.546 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Carvajal RD, Nathan P, Sacco JJ, et al. Phase I study of safety, tolerability, and efficacy of Tebentafusp using a step-up dosing regimen and expansion in patients with metastatic Uveal Melanoma. J Clin Oncol 2022;40:1939–48. 10.1200/JCO.21.01805 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Carvajal RD, Butler MO, Shoushtari AN, et al. Clinical and molecular response to Tebentafusp in previously treated patients with metastatic Uveal Melanoma: a phase 2 trial. Nat Med 2022;28:2364–73. 10.1038/s41591-022-02015-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Pires Da Silva I, Ahmed T, Lo S, et al. Ipilimumab (IPI) alone or in combination with anti-PD-1 (IPI+Pd1) in patients (Pts) with metastatic Melanoma (MM) resistant to Pd1 monotherapy. JCO 2020;38(15_suppl):10005. 10.1200/JCO.2020.38.15_suppl.10005 [DOI] [Google Scholar]
- 33. Weichenthal M, Ugurel S, Leiter UM, et al. Salvage therapy after failure from anti-PD-1 single agent treatment: A study by the German Adoreg Melanoma Registry. JCO 2019;37(15_suppl):9505. 10.1200/JCO.2019.37.15_suppl.9505 [DOI] [Google Scholar]
- 34. Arance AM, de la Cruz-Merino L, Petrella TM, et al. Lenvatinib (Len) plus Pembrolizumab (Pembro) for patients (Pts) with advanced Melanoma and confirmed progression on a PD-1 or PD-L1 inhibitor: updated findings of LEAP-004. JCO 2021;39(15_suppl):9504. 10.1200/JCO.2021.39.15_suppl.9504 [DOI] [Google Scholar]
- 35. Dafni U, Michielin O, Lluesma SM, et al. Efficacy of adoptive therapy with tumor-infiltrating lymphocytes and Recombinant Interleukin-2 in advanced cutaneous Melanoma: a systematic review and meta-analysis. Ann Oncol 2019;30:1902–13. 10.1093/annonc/mdz398 [DOI] [PubMed] [Google Scholar]
- 36. Sarnaik AA, Hamid O, Khushalani NI, et al. Lifileucel, a tumor-infiltrating lymphocyte therapy, in metastatic Melanoma. J Clin Oncol 2021;39:2656–66. 10.1200/JCO.21.00612 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Rohaan MW, van den Berg JH, Kvistborg P, et al. Adoptive transfer of tumor-infiltrating lymphocytes in Melanoma: a viable treatment option. J Immunother Cancer 2018;6:102. 10.1186/s40425-018-0391-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Lowe KL, Cole D, Kenefeck R, et al. Novel TCR-based Biologics: Mobilising T cells to warm ‘cold’ tumours. Cancer Treat Rev 2019;77:35–43. 10.1016/j.ctrv.2019.06.001 [DOI] [PubMed] [Google Scholar]
- 39. Middelburg J, Kemper K, Engelberts P, et al. Overcoming challenges for Cd3-Bispecific antibody therapy in solid tumors. Cancers (Basel) 2021;13:287. 10.3390/cancers13020287 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Killock D. Tebentafusp for Uveal Melanoma. Nat Rev Clin Oncol 2021;18:747. 10.1038/s41571-021-00572-3 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
jitc-2023-006747supp001.pdf (2.4MB, pdf)
Data Availability Statement
Data are available upon reasonable request. Access to pre-existing summary outputs (tables or figures) of trial level data may be granted to qualified academic researchers in the field upon request and approval by the study management committee and subject to appropriate data sharing and transfer agreements. Requesters should submit a proposal including purpose, data format (e.g. sas files), hypothesis and specific rationale to info@immunocore.com.