Abstract
Introduction
The combination of checkpoint inhibition and cisplatin-based chemotherapy is investigated in muscle invasive bladder cancer (MIBC) and results from phase 2 trials have been presented. Intravesical BCG has been used for non-MIBC (NMIBC) in patients with carcinoma in situ and high-grade Ta/T1 tumours. BCG induces innate and adapted immune response and upregulation of PD-L1 in preclinical models. The proposed trial is intended to implement a new immuno-immuno-chemotherapy induction therapy for MIBC. The combination of BCG and checkpoint inhibition with chemotherapy aims at higher intravesical responses and better local and systemic control of disease.
Methods and analysis
SAKK 06/19 is an open-label single-arm phase II trial for patients with resectable MIBC T2-T4a cN0-1. Intravesical recombinant BCG (rBCG: VPM1002BC) is applied weekly for three instillations followed by four cycles of neoadjuvant cisplatin/gemcitabine every 3 weeks. Atezolizumab 1200 mg every 3 weeks is started together with rBCG and given for four cycles. All patients then undergo restaging and radical cystectomy and pelvic lymphadenectomy. Atezolizumab is continued as maintenance therapy after surgery every 3 weeks for 13 cycles. Pathological complete remission is the primary endpoint. Secondary endpoints include pathological response rate (<ypT2 N0), event-free survival, recurrence-free survival, overall survival, feasibility and toxicity. An interim safety analysis will be performed after the first 12 patients have completed neoadjuvant treatment specifically assessing toxicity possibly associated with intravesical rBCG application.
The study has received approval by ethical committee Zurich, Switzerland, BASEC-No. 2021–01872. Results will be made available by publication.
Trial registration number
Keywords: IMMUNOLOGY, Urological tumours, CHEMOTHERAPY, Urological tumours, ONCOLOGY
STRENGTHS AND LIMITATIONS OF THIS STUDY.
The completed predecessor study used the same therapeutic multimodality backbone.
The study combines local immunotherapy with chemotherapy, immune checkpoint blockade and radical cystectomy.
This is an open-label, single-arm phase II study.
The primary endpoint is pathological complete remission (pCR).
The population included consists of patients with muscle invasive bladder cancer cT2-T4a cN0-1 cM0.
Introduction
Beside bladder sparing chemoradiation therapy, radical cystectomy is the accepted standard curative treatment modality for patients with muscle invasive bladder cancer (MIBC) without evidence of metastatic disease (cM0).1 Despite the radical surgical approach, stage independent cure rates are, however, only around 50% at 5 years. Two phase III trials using cisplatin-based neoadjuvant chemotherapy demonstrated a significant improvement of overall survival (OS) of MIBC of approximately 5% compared with radical cystectomy alone.2 3 These results were confirmed in a meta-analysis demonstrating that the addition of neoadjuvant cisplatin-based chemotherapy can improve OS by around 5%.4 Therefore, according to international guidelines, the use of cisplatin-based neoadjuvant chemotherapy is considered standard of care in all patients with localised MIBC with planned curative local treatment.1
For a long time, there was no consensus which cisplatin-combination regimen (cisplatin/gemcitabine vs dose dense methotrexate, vinblastine, adriamycin and cisplatin (ddMVAC)) should be administered in the neoadjuvant setting. Recently, a phase III clinical trial (VESPER) suggested improved OS for the ddMVAC regimen compared with cisplatin/gemcitabine.5
There remains a high unmet need to improve the cure rate for patients with localised MIBC. Moreover, establishment of a treatment with high local control omitting the need for either complete resection or irradiation of the bladder would substantially improve quality of life for those patients. Early results from clinical trials support the feasibility of bladder preserving approaches after immune-chemo-therapy (HCRN GU16-257)6
In recent years, immunotherapy using PD-1 or PD-L1 targeting immune checkpoint inhibitors (ICIs) proved to be beneficial for patients with metastatic bladder cancer and a significant improvement in OS was shown for pembrolizumab in the second-line setting.7 The first results have been presented and published using ICIs as neoadjuvant treatment for localised MIBC. Two monotherapy studies using either pembrolizumab (PURE-01) or atezolizumab (ABACUS) demonstrated pathological complete remission (pCR) rates of 30%–40%.8 9
Atezolizumab is a human monoclonal antibody (mAb) of the IgG 1 kappa subclass that inhibits binding of PD-L1. Atezolizumab was the first ICI to be tested in patients with urothelial carcinoma (UC). The published study programme of atezolizumab in UC is broad, comprising phases I–IV trials in metastatic pretreated patients10–13 and a phase II trial in metastatic treatment naïve cisplatin-ineligible patients.14 In the phase I trial, 95 pretreated metastatic UC patients received atezolizumab achieving a 40% response rate.10 The phase II trial included 310 platinum-pretreated patients and achieved a response rate of 15% including 5% complete remissions (CR).11 A total of 931 patients were randomised in the phase III trial comparing atezolizumab against chemotherapy of physician’s choice (either docetaxel, paclitaxel or vinflunine). While the primary endpoint of improved OS for patients with high PD-L1 expression was not reached, the OS was numerically higher in the intention to treat population.12 Atezolizumab had a better safety profile than chemotherapy with 20% grade 3/4 toxicity as compared with 43% on chemotherapy. The efficacy and safety were confirmed in a large real-world population (N=1004) safety trial also including patients usually not eligible for immunotherapy trials such as patients with brain metastasis, autoimmune disease, renal insufficiency, HIV positivity as well as frail patients.13 Moreover, atezolizumab monotherapy demonstrated interesting efficacy in the first-line treatment of cisplatin-ineligible patients with a response rate of 23% (9% CR) and an OS of 15.9 months.14
The combination of cisplatin/gemcitabine chemotherapy with atezolizumab has been demonstrated to be effective and safe in a large phase III trial.15 The trial was positive for the primary endpoint of progression-free survival without unexpected toxicity from the chemoimmunotherapy combination.
Intravesical instillation of BCG is the recommended standard of care treatment for patients with intermediate/high risk for progression non-MIBC (NMIBC) after complete transurethral resection of the bladder tumour (TURB).16 BCG was shown to cure carcinoma in situ (CIS) and prevent recurrence of high grade NMIBC and to prolong survival compared with TURB alone.16 17 While the exact mechanism of BCG effect is not entirely understood, it is clear that intravesical BCG induces a local inflammation leading to induction of the innate immune system allowing for a tumour-specific immunity (adaptive immune response.18 19 Several different BCG strains have been developed and used for intravesical therapy. It has been recognised that there might be differences in terms of immunogenicity and efficacy between strains.20 This has increased interest in developping novel BCG formulations.
A far developed and promising new BCG-derived vaccine is the recombinant Mycobacterium bovis (M. bovis) BCGΔureC::hly. rBCGΔureC::hly which was formulated as VPM1002BC for intravesical immunotherapy against NMIBC. This recombinant BCG (rBCG) VPM1002BC leads to translocation of proteins to the cytosol of infected host or cancer cells by perforation of the phagosome.21 22 In preclinical models, these changes induce macrophage apoptosis, T cell priming and proinflammatory cytokine expression, leading to CD4+ and CD8+ T cell responses that are superior compared with the parental BCG subtype Prague. These observations are potentially leading to an improved immune response. VPM1002BC has been used for intravesical therapy in patients with BCG refractory NMIBC in a clinical phase I/II trial (SAKK06/14). The phase I part demonstrated very good tolerance of the compound without need for dose modifications or grade 3 or 4 adverse events (AEs).23 The phase II part including 42 patients clearly met the primary endpoint resulting in a recurrence-free survival (RFS) rate in the bladder at 60 weeks in 49.3% of patients,24 while historical data from second-line treatment with conventional BCG results in an RFS rate of 12.5%.25 Only two patients (5%) did tolerate less than five instillations and this was not directly related to VPM1002BC. Over the whole course of therapy, treatment-related grades 1, 2 and 3 AEs were observed in 14.3%, 54.8% and 4.8% of the patients, respectively.
Methods/design
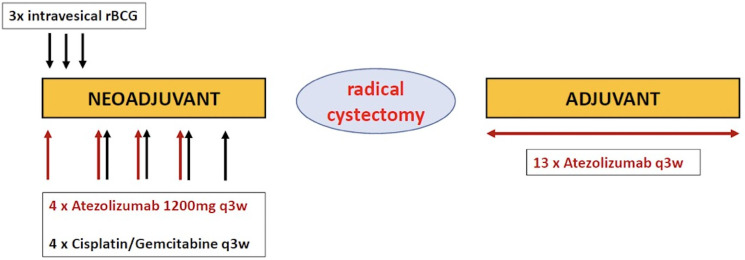
The trial aims to implement a new immuno-immuno-chemotherapy induction therapy for MIBC combining rBCG intravesical installations and ICI followed by neoadjuvant ICI in combination with chemotherapy, followed by radical cystectomy and adjuvant ICI (figure 1).
Figure 1.
Study schedule SAKK 06/19: intravesical rBCG followed by perioperative chemoimmunotherapy for patients with muscle-invasive bladder cancer. A multicentre, single-arm phase 2 trial. rBCG, recombinant BCG.
The trial is a single arm phase 2 trial including patients with histologically proven urothelial cell carcinoma of the bladder (pT2 or cT2, cT3 or cT4a and ≤cN1 (defined as a solitary lymph node ≤2 cm in the greatest dimension) and cM0 and be considered suitable for curative multimodality treatment including radical cystectomy by a multidisciplinary tumour board. Furthermore, location of tumour must allow placement of catheter without risk of bleeding. All histological subtypes are eligible with the exception of small cell neuroendocrine carcinoma. The renal function must be estimated to reach a glomerular filtration rate of >50 mL/min/1.73 m² to allow the use of cisplatin. Patients with prior intravesical BCG, with macrohaematuria and those unable to retain BCG instillation for less than 1 hour are excluded.
The protocol includes additional research questions such as preoperative assessment of treatment response using MRI and circulating cell-free tumour DNA (ctDNA) and correlation with the pathological outcome, the tumour immunome before and after neoadjuvant chemotherapy and immunotherapy, tissue expression of PD-L1 and its relation to efficacy endpoints, biomarkers for anti-PD-L1 treatment and their relation to efficacy endpoints, the effect of the gut microbiota on the response to immunotherapy, immune parameters in urine samples and their relation to efficacy endpoints.
Patients receive intravesical rBCG (VPM1002BC) by three weekly instillations of rBCG with single dose of VPM1002BC, live, 1–19.2×108 colony-forming units (CFU) on days 1, 8 and 15 of the protocol. Atezolizumab 1200 mg fixed dose is started with the first instillation of rBCG (+/- 1 day) and continued in combination with the chemotherapy every 3 week for four cycles. Chemotherapy consists of cisplatin and gemcitabine for four cycles and is started on day 22 after the first rBCG instillation. Cisplatin is used at a dose of 70 mg/m2 intravenously on day 1 every 3 weeks and gemcitabine is used at a dose of 1000 mg/m2 intravenously on day 1 and day 8 every 3 weeks. Radical cystectomy with extensive lymph node dissection according to actual EAU guidelines is performed 4–8 weeks after completion of the last chemoimmunotherapy cycle. Adjuvant atezolizumab is given 1200 mg fixed dose every 3 weeks for 13 cycles starting 4–16 weeks after date of surgery.
Endpoints
The primary endpoint of the trial is pathological complete remission (pCR) after neoadjuvant treatment defined as ypT0 ypN0 and no evidence of NMIBC (low grade, high grade or CIS). The primary analysis will be based on the results from central pathology review. This endpoint will only be calculated for patients in the resected patients set.
The secondary endpoints are the following:
Event-free survival (EFS)
EFS is defined as the time from treatment start until one of the following events, whichever comes first:
Progression during neoadjuvant treatment leading to inoperability.
Recurrence or progression (in case of disease persistence) of locoregional disease after surgery.
Appearance of metastases at any localisation.
Death.
Patients without event at the time of analysis and patients starting a subsequent treatment in the absence of an event will be censored at the date of the last available assessment showing no event before the start of the subsequent treatment, if any. This endpoint will be calculated for patients in the full analysis set (FAS).
Recurrence-free survival
RFS after R0 resection is defined as the time from surgery until one of the following events, whichever comes first:
Recurrence of locoregional disease.
Appearance of metastases at any localisation.
Death.
Patients without event at the time of analysis and patients starting a subsequent treatment in the absence of an event will be censored at the date of the last available assessment showing no event before the start of the subsequent treatment, if any.
This endpoint will only be calculated for patients in the R0 resection set.
Overall survival
OS is defined as the time from treatment start until death from any cause. Patients not experiencing an event will be censored at the last date they were known to be alive. This endpoint will be calculated for patients in the full analysis set (FAS).
Quality of resection
The quality of resection will be assessed in the following way:
Complete resection (R0) defined as free resection margins proved microscopically.
Completeness of the lymphadenectomy and surgery using the photo documentation and histopathology.
Postoperative complications will be assessed using the Clavien-Dindo classification.
This endpoint will only be calculated for patients in the resected patients set.
Pathological response rate
Pathological response rate (PaR) is defined as pathological downstaging to<ypT2 N0M0. The proportion of patients with PaR will be calculated for patients in the resected patients set. This endpoint will only be calculated for patients in the resected patients set.
Pattern of recurrence
Pattern of recurrence is defined as location of first tumour recurrence. Patterns can be locoregional or distant or any combination of these patterns.
Patients with secondary malignancies or patients with no recurrence will not be taken into consideration for this endpoint.
Feasibility
The following treatment feasibility criteria will be assessed:
Completion of three instillations of intravesical VPM1002BC.
Completion of four cycles of neoadjuvant chemotherapy.
Completion of four cycles of neoadjuvant atezolizumab treatment.
Timely admission to and completion of planned surgery.
Timely initiation and completion of 13 cycles of adjuvant atezolizumab treatment.
Adverse events
AEs will be assessed according to NCI CTCAE V.5.0.
This endpoint will be calculated for patients in the safety set.
The protocol includes additional research questions such as preoperative assessment of treatment response using MRI and circulating ctDNA and correlation with the pathological outcome, the tumour immunome before and after neoadjuvant chemotherapy and immunotherapy, tissue expression of PD-L1 and its relation to efficacy endpoints, biomarkers for anti-PD-L1 treatment and their relation to efficacy endpoints, the effect of the gut microbiota on the response to immunotherapy, immune parameters in urine samples and their relation to efficacy endpoints.
Statistics
The sample size is based on the primary endpoint pCR. The null hypothesis is a pCR rate ≤35% (based on reference 26) and the alternative hypothesis a pCR rate ≥55%. Using Simon’s minimax two-stage design with a type I error of 5% and a power of 80%, 39 resected patients are needed. With an estimated drop-out rate of 15% (7 patients), we plan to recruit a total of 46 patients.
After the first 12 patients have completed neoadjuvant treatment, an interim safety analysis will be performed. AEs and SAEs will be analysed descriptively. Special focus will be given to CTCAE grade ≥3 directly related to intravesical rBCG.
After neoadjuvant therapy and resection of the first 21 patients an interim efficacy analysis will be performed. If the number of patients with pCR is 8 or less, the trial will be stopped for futility. If, however, the number of patients with pCR is 9 or more, the trial will continue to stage 2.
The primary analysis will take place after all patients have completed neoadjuvant therapy and had surgery, if applicable. The secondary analysis will be performed when all patients have reached a follow-up of at least 2 years.
For the primary endpoint, the point estimate of the pCR rate will be calculated using the uniformly minimum variance unbiased estimator and the corresponding two-sided 90% CI will be calculated using the ‘stage-wise ordering’ based method. If the lower bound of the CI is above 35%, the null hypothesis can be rejected.
For all other binary endpoints the point estimate and exact 95% Clopper-Pearson CI of the proportion will be calculated.
For the primary analysis of the primary endpoint, the results from the central pathology review will be used. Supportive analyses are planned based on the following results:
Local pathology.
MRI (local and central assessment) before surgery.
Cystoscopy and biopsy before surgery.
ctDNA.
The following subgroup analyses are planned for the primary endpoint:
High PD-L1 expression (assessed by standardised immunohistochemistry on tumour cells and tumour-associated immune cells (IC) using a≥5% positivity on IC (ie, IC2) as cut-off) versus no or low expression.
ypT0 versus rest.
ypN0 versus rest.
Resection status of TUR-B (complete vs incomplete).
All time-to-event endpoints will have the median value estimated using the Kaplan-Meier method. The number and type of events of each endpoint will be presented descriptively by frequency and percentage.
Categorical variables will be summarised with frequency and percentage. The denominator for percentages will be the number of patients within the set of interest, unless otherwise specified. Continuous variables will be summarised using median and range.
Laboratory values will be expressed as the absolute values and as grading according to NCI CTCAE V.5.0. AE grading will be presented by type, grade and relation showing frequency and percentage of the within-patient worst grade. In addition, grade ≥3 AEs and AEs with relation to treatment ≥3 will be summarised separately.
Patient and public involvement
The protocol was developed within the SAKK network involving multiple stakeholders including physicians specialised in uro-onocology, nurses and the patient advisory board. The design of the trial is aimed to improve cure rates and to pave a scientific way to avoid radical cystectomy in the future, both clear aims to improve quality of live. Patients will be recruited within the SAKK network and the trial is accessible to the public via the SAKK webpage (https://www.sakk.ch/en/news/new-trial-patients-bladder-cancer-sakk-0619). After closing and analysis of the trial results will be published in scientific journals. A lay abstract will be uploaded on the SAKK webpage.
Discussion
The presented clinical trial SAKK 06/19 is the further development of immunochemotherapies for MIBC within the SAKK network. SAKK has performed a predecessor single arm phase II trial using neoadjuvant chemoimmunotherapy with cisplatin/gemcitabine in combination with the PD-L1 inhibitor durvalumab (SAKK 06/17). In this trial a total of 61 patients were included in Switzerland and in one German centre between May 2018 and September 2019. We presented the primary analysis at ASCO 202226 as first trial in MIBC to report a primary endpoint of EFS (manuscript in preparation).
The rationale of the SAKK 06/17 trial was the addition of neo-adjuvant chemotherapy with cisplatin and gemcitabine to checkpoint inhibition to support the development of a therapeutic immune response by reducing the influence of the chronic inflammation caused by the immune suppressive innate cell network. Predominantly myeloid derived suppressor cells (MDSCs, including macrophages and neutrophils) are responsible for chronic inflammation hampering the immune response. Gemcitabine is known to reduce MDSCs and is therefore the ideal partner for an immunochemotherapy.27 As a consequence of immune activation, IFN-gamma is released resulting in TH1 T cell response. However, IFN-gamma also induces PD-1 expression on TH1 T cells leading to adaptive immune suppression aiming to stop the T-cell response.28 The use of ICIs is intended to block this negative feedback loop to allow a prolonged T-cell response. Furthermore, the ddMVAC protocol was avoided to not allow methotrexate to built up its known T cell suppressive capacity counteracting the immune activating intention of this protocol.
Several similar neoadjuvant studies in MBIC using immunotherapy or the combination of immunochemotherapy have reported pCR rates in the same range of 30%–40% and in addition, residual NMIBC can be found in approximately 15%–20%.8 9 29 30 Therefore, there is hardly any improvement in the pCR rate compared with cisplatin-based chemotherapy, especially when compared with the more active regimen of ddMVAC.5
In view of these rather modest results so far, strategies to further augment the immune response need to be evaluated. Beside concomitant application of radiotherapy and immune checkpoint blockade, BCG appears to be a promising combination partner. BCG has been used for treatment of NMIBC for decades with very good success. It induces initial CR in 70%–75% of patients with CIS and prevents recurrence in 55%–65% of patients with high-risk papillary tumours.16 17 However, 25%–45% of patients do not respond initially and up to 40% experience relapse after initial response. BCG induces an intense local inflammatory response that mediates tumour immunity. Several steps are involved in mounting the inflammatory response including attachment to the urothelium with uptake by antigen presenting cells and putative internalisation into urothelial cells followed by a boost of the innate immune response and induction of adaptive responses.18 Preclinical experiments demonstrated that intravesical BCG instillations induce a robust infiltration of T cells (CD4+ and CD8+) in the bladder wall.31 Moreover, a systemic immune response arises following intravesical BCG demonstrated by increased levels of different cytokines and chemokines including IFNγ, IL-1, IL-2, Il-8, TNF, CCL2, CCL5.32
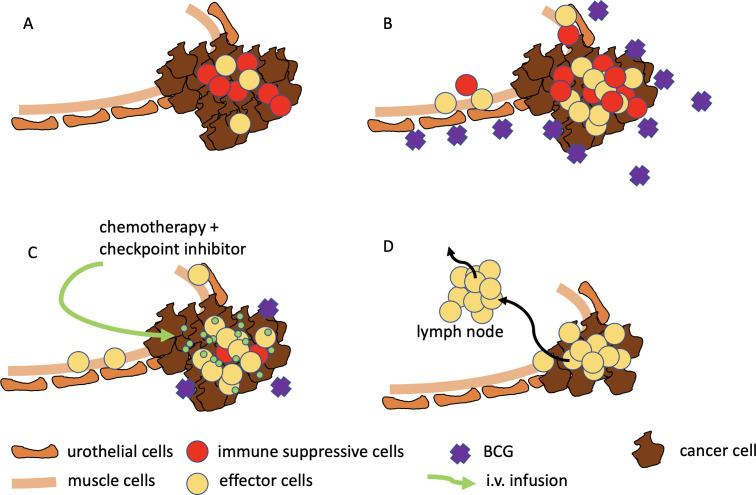
Resistance mechanisms to BCG are not entirely understood but interestingly, granulomata found in patients not responding to BCG were found to be highly expressing PD-L128 suggesting a T-cell exhaustion resulting from checkpoint activation. Patients with ARIDA1A mutation and CCNE1 amplification also appear to be at higher risk of relapse after BCG treatment.33 The immune response induced by intravesical BCG is, however, not solely restricted to the superficial urothelial layer but affects the whole bladder wall and also induces a systemic immune response.20 Therefore, the next logical step appears to use intravesical BCG also in patients with MIBC as an adjuvant to prime and boost the immune response (both innate and adaptive) when using systemic immunotherapy with checkpoint inhibitors (figure 2). To avoid clinically relevant delay three installations of BCG were considered to be enough to prime and boost. This intended priming of the immune system might be better achieved by using the novel rBCG strain VPM1002BC which appears to have improved safety21 immunogenicity.22 This is mediated by the exchange of the urease C gene with the lsteriolysin gene in rBCG VPM1002BC leading to a stronger adoptive and innate immune response. Furthermore, increased autophagy likely contributes to more rapid elimination of rBCG in the host and because listeriolysin is only active at acidic pH it is rapidly degraded in the cytosol of the host cell and its effects are short-lived.
Figure 2.
(A) The urothelial cancer (brown) is infiltrated by immune cells (yellow and red), (B) BCG enhances the local inflammation by IFNγ release resulting in increased number of immune suppressive immune cells (MDSC) and upregulation of PD-L1, (C) chemotherapy diminishes MDSC, checkpoint inhibition blocks PD1-PD-L1 axis, (D) due to blocked immune suppressive network immune effector cells (T cells) expand and kill tumour cells, additional cytotoxic effect of chemotherapy kills tumour cells, activated T cells can cause systemic antitumour immune response. MDSC, myeloid-derived suppressor cell.
Our trial includes a broad translational research programme evaluating different possible markers of treatment efficacy. We hope to help identify molecular predicitive biomarkers to tailor treatment more efficiently towards patients who are more likely to benefit and to spare the others unnecessary systemic treatment and proceed directly to radical local therapy.
In conclusion, this trial tests the hypothesis if a addition of a novel intravesical rBCG can enhance the local and systemic immune response in the context of ICI and chemotherapy and thereby increase pCR rate and consequently also EFS. Improving pCR rate would be a next step to the ultimate goal of omitting radical surgery or extensive local radiotherapy to the bladder for these patients.
Ethics and dissemination
The study has received approval by ethical committee Zurich, Switzerland, BASEC-No. 2021–01872. Results will be made available by publication.
Trial status
Recruitment started on May 2022, estimated closure of accrual April 2025.
Supplementary Material
Acknowledgments
We are thankful to the work of all members of the Competence Center of SAKK.
Footnotes
UP and MS contributed equally.
Contributors: UP performed the study design and wrote the manuscript, MSpahn performed the study design, MSchneider submitted the protocol to authorities and ethical committee, SH performed the study design and did all statistical planning, CAR performed the study design, SR planned all translational research and will perform the analysis, AO performed the study design and coordinated all centres for patient accrural and RC performed the study design and wrote the manuscript.
Funding: The trial is supported by F. Hoffmann-La Roche.
Competing interests: UP: advisory board (compensated, institutional) for Astellas, Astra Zeneca, BMS, Merck, Pfizer, Roche, MSD, Janssen, Novartis; RC: advisory board (compensated, institutional) for Astellas, Astra Zeneca, BMS, Merck, Pfizer, Roche, MSD, Ipsen, Janssen, Novartis; Honoraria (compensated, institutional) for Janssen, Astellas; MSpahn: None; SH: None; CAR: None; SR: Honoraria (compensated, institutional) from Roche, Astra Zeneca, BMS, Boehringer Ingelheim, MSD, Novartis, Amgen, Eli Lilly, Eisai, Merck Serono, Pfizer, Takeda, Bayer, Janssen, Otsuka, PharmaMar, Sanofi; Advisory role (institutional, compensated): Astea Zeneca, Boehringer Ingelheim, BMS, Pfizer, Eisai, Eli Lilly, Merck Serono, MSD, Roche, Novartis, Takeda, Amgen, Otsuka; Research Funding (institutional): Abbvie, BMS, Astra Zeneca, Boehringer Ingelheim, Merck Serono, Roche; MSchneider: None; AO: advisory role (compensated, institutional): Astra Zeneca, Astellas, Bayer, Janssen, Molecular Partners, MSD, Pfizer, Roche, Sanofi Aventis (compensated, institutional). Novartis, Janssen, Bayer, MSD, AstraZeneca, Merck, Astellas (compensated). Research support (institutional): TEVA, Janssen. Travel support Astellas, Bayer, Janssen, Sanofi Aventis. Speakers Bureau (compensated, institutional): Astellas, Bayer, Janssen.
Patient and public involvement: Patients and/or the public were not involved in the design, or conduct, or reporting, or dissemination plans of this research.
Provenance and peer review: Not commissioned; externally peer reviewed.
Ethics statements
Patient consent for publication
Not applicable.
References
- 1.Witjes JA, Bruins HM, Cathomas R, et al. European Association of Urology guidelines on muscle-invasive and metastatic bladder cancer: summary of the 2020 guidelines. Eur Urol 2021;79:82–104. 10.1016/j.eururo.2020.03.055 [DOI] [PubMed] [Google Scholar]
- 2.Grossman HB, Natale RB, Tangen CM, et al. Neoadjuvant chemotherapy plus cystectomy compared with cystectomy alone for locally advanced bladder cancer. N Engl J Med 2003;349:859–66. 10.1056/NEJMoa022148 [DOI] [PubMed] [Google Scholar]
- 3.Griffiths G, Hall R, Sylvester R, et al. International phase III trial assessing neoadjuvant cisplatin, methotrexate, and vinblastine chemotherapy for muscle-invasive bladder cancer: long-term results of the BA06 30894 trial. J Clin Oncol 2011;29:2171–7. 10.1200/JCO.2010.32.3139 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Advanced Bladder Cancer (ABC) Meta-analysis Collaboration . Neoadjuvant chemotherapy in invasive bladder cancer: update of a systematic review and meta-analysis of individual patient data advanced bladder cancer (ABC) meta-analysis collaboration. Eur Urol 2005;48:202–5. 10.1016/j.eururo.2005.04.006 [DOI] [PubMed] [Google Scholar]
- 5.Pfister C, Gravis G, Fléchon A, et al. Dose-dense methotrexate, vinblastine, doxorubicin, and cisplatin or Gemcitabine and cisplatin as perioperative chemotherapy for patients with Nonmetastatic muscle-invasive bladder cancer: results of the GETUG-AFU V05 VESPER trial. J Clin Oncol 2022;40:2013–22. 10.1200/JCO.21.02051 [DOI] [PubMed] [Google Scholar]
- 6.Galsky MD, Daneshmand S, Chan KG, et al. Phase 2 trial of gemcitabine, cisplatin, plus nivolumab with selective bladder sparing in patients with muscle- invasive bladder cancer (MIBC): HCRN GU 16-257. JCO 2021;39:4503. 10.1200/JCO.2021.39.15_suppl.4503 [DOI] [Google Scholar]
- 7.Bellmunt J, de Wit R, Vaughn DJ, et al. Pembrolizumab as second-line therapy for advanced urothelial carcinoma. N Engl J Med 2017;376:1015–26. 10.1056/NEJMoa1613683 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Necchi A, Anichini A, Raggi D, et al. Pembrolizumab as neoadjuvant therapy before radical cystectomy in patients with muscle-invasive urothelial bladder carcinoma (PURE-01): an open-label, single-arm, phase II study. J Clin Oncol 2018;36:3353–60. 10.1200/JCO.18.01148 [DOI] [PubMed] [Google Scholar]
- 9.Powles T, Kockx M, Rodriguez-Vida A, et al. Clinical efficacy and biomarker analysis of neoadjuvant atezolizumab in operable urothelial carcinoma in the ABACUS trial. Nat Med 2019;25:1706–14. 10.1038/s41591-019-0628-7 [DOI] [PubMed] [Google Scholar]
- 10.Powles T, Eder JP, Fine GD, et al. MPDL3280A (anti-PD-L1) treatment leads to clinical activity in metastatic bladder cancer. Nature 2014;515:558–62. 10.1038/nature13904 [DOI] [PubMed] [Google Scholar]
- 11.Rosenberg JE, Hoffman-Censits J, Powles T, et al. Atezolizumab in patients with locally advanced and metastatic urothelial carcinoma who have progressed following treatment with platinum-based chemotherapy: a single-arm, multicentre, phase 2 trial. Lancet 2016;387:1909–20. 10.1016/S0140-6736(16)00561-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Powles T, Durán I, van der Heijden MS, et al. Atezolizumab versus chemotherapy in patients with platinum-treated locally advanced or metastatic urothelial carcinoma (Imvigor211): a multicentre, open-label, phase 3 randomised controlled trial. Lancet 2018;391:748–57. 10.1016/S0140-6736(17)33297-X [DOI] [PubMed] [Google Scholar]
- 13.Sternberg CN, Loriot Y, James N, et al. Primary results from SAUL, a multinational single-arm safety study of atezolizumab therapy for locally advanced or metastatic urothelial or nonurothelial carcinoma of the urinary tract. European Urology 2019;76:73–81. 10.1016/j.eururo.2019.03.015 [DOI] [PubMed] [Google Scholar]
- 14.Balar AV, Galsky MD, Rosenberg JE, et al. Atezolizumab as first-line treatment in cisplatin-ineligible patients with locally advanced and metastatic urothelial carcinoma: a single-arm, multicentre, phase 2 trial. Lancet 2017;389:67–76. 10.1016/S0140-6736(16)32455-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Galsky MD, Arija JÁA, Bamias A, et al. Atezolizumab with or without chemotherapy in metastatic urothelial cancer (Imvigor130): a multicentre, randomised, placebo-controlled phase 3 trial. Lancet 2020;395:1547–57. 10.1016/S0140-6736(20)30230-0 [DOI] [PubMed] [Google Scholar]
- 16.Babjuk M, Burger M, Compérat EM, et al. European Association of Urology guidelines on non-muscle-invasive bladder cancer (Tat1 and carcinoma in situ) - 2019 update. European Urology 2019;76:639–57. 10.1016/j.eururo.2019.08.016 [DOI] [PubMed] [Google Scholar]
- 17.Herr HW, Schwalb DM, Zhang ZF, et al. Oettgen HF: intravesical Bacillus Calmette-Guerin therapy prevents tumor progression and death from superficial bladder cancer: ten-year follow-up of a prospective randomized trial. J Clin Oncol 1995;13:1404–8. 10.1200/JCO.1995.13.6.1404 [DOI] [PubMed] [Google Scholar]
- 18.Pettenati C, Ingersoll MA. Mechanisms of BCG Immunotherapy and its outlook for bladder cancer. Nat Rev Urol 2018;15:615–25. 10.1038/s41585-018-0055-4 [DOI] [PubMed] [Google Scholar]
- 19.Biot C, Rentsch CA, Gsponer JR, et al. Preexisting BCG-specific T cells improve intravesical immunotherapy for bladder cancer. Sci Transl Med 2012;4:137. 10.1126/scitranslmed.3003586 [DOI] [PubMed] [Google Scholar]
- 20.Rentsch CA, Birkhäuser FD, Biot C, et al. Bacillus Calmette-Guerin strain differences have an impact on clinical outcome in bladder cancer immunotherapy. Eur Urol 2014;66:677–88. 10.1016/j.eururo.2014.02.061 [DOI] [PubMed] [Google Scholar]
- 21.Grode L, Seiler P, Baumann S, et al. Increased vaccine efficacy against tuberculosis of recombinant Mycobacterium bovis bacille Calmette-Guerin Mutants that Secrete Listeriolysin. J Clin Invest 2005;115:2472–9. 10.1172/JCI24617 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nieuwenhuizen NE, Kulkarni PS, Shaligram U, et al. The Recombinant bacille calmette-guerin vaccine Vpm1002: ready for clinical efficacy testing. Front Immunol 2017;8:1147. 10.3389/fimmu.2017.01147 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rentsch CA, Bosshard P, Mayor G, et al. Results of the phase I open label clinical trial SAKK 06/14 assessing safety of intravesical instillation of Vpm1002Bc, a recombinant mycobacterium Bacillus Calmette Guerin (BCG), in patients with non-muscle invasive bladder cancer and previous failure of conventional BCG therapy. Oncoimmunology 2020;9:1748981. 10.1080/2162402X.2020.1748981 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rentsch CA, Thalmann GN, Lucca I, et al. A phase 1/2 single-arm clinical trial of recombinant Bacillus Calmette-Guérin (BCG) Vpm1002Bc immunotherapy in non-muscle-invasive bladder cancer recurrence after conventional BCG therapy: SAKK 06/14. Eur Urol Oncol 2022;5:195–202. 10.1016/j.euo.2021.12.006 [DOI] [PubMed] [Google Scholar]
- 25.Di Lorenzo G, Perdonà S, Damiano R, et al. Gemcitabine versus Bacille Calmette-Guerin after initial Bacille Calmette-Guerin failure in non-muscle-invasive bladder cancer: a multicenter prospective Randomizedtrial. Cancer 2010;116:1893–900. 10.1002/cncr.24914 [DOI] [PubMed] [Google Scholar]
- 26.Cathomas R, Rothschild S, Hayoz S, et al. Perioperative chemoimmunotherapy with durvalumab for operable muscle-invasive urothelial carcinoma (MIUC): primary analysis of the single arm phase II trial SAKK 06/17. JCO 2022;40:4515. 10.1200/JCO.2022.40.16_suppl.4515 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Eriksson E, Wenthe J, Irenaeus S, et al. Gemcitabine reduces MDSCs, Tregs and TGFbeta-1 while restoring the Teff/Treg ratio in patients with pancreatic cancer. J Transl Med 2016;14:282. 10.1186/s12967-016-1037-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Inman BA, Sebo TJ, Frigola X, et al. PD-L1 (B7-H1) expression by urothelial carcinoma of the bladder and BCG induced granulomata: associations with localized stage progression. Cancer 2007;109:1499–505. 10.1002/cncr.22588 [DOI] [PubMed] [Google Scholar]
- 29.Funt SA, Lattanzi M, Whiting K, et al. Neoadjuvant Atezolizumab with Gemcitabine and cisplatin in patients with muscle-invasive bladder cancer: a multicenter, single-arm. J Clin Oncol 2022;40:1312–22. 10.1200/JCO.21.01485 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rose TL, Harrison MR, Deal AM, et al. Phase II study of Gemcitabine and split-dose cisplatin plus Pembrolizumab as Neoadjuvant therapy before radical cystectomy in patients with muscle-invasive bladder cancer. J Clin Oncol 2021;39:3140–8. 10.1200/JCO.21.01003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Biot C, Rentsch CA, Gsponer JR, et al. Preexisting BCG-specific T cells improve intravesical Immunotherapy for bladder cancer. Sci Transl Med 2012;4:137ra72. 10.1126/scitranslmed.3003586 [DOI] [PubMed] [Google Scholar]
- 32.Taniguchi K, Koga S, Nishikido M, et al. Systemic immune response after intravesical instillation of Bacilli Calmette-Guerin (BCG) for superficial bladder cancer. Clin Exp Immunol 1999;115:131–5. 10.1046/j.1365-2249.1999.00756.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bacon JVW, Müller DC, Ritch E, et al. Somatic features of response and relapse in non-muscle-invasive bladder cancer treated with Bacillus Calmette-Guérin Immunotherapy. Eur Urol Oncol 2022;5:677–86. 10.1016/j.euo.2021.11.002 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.