Abstract
Background
Treatment of some blood cancers with T cells that express a chimeric antigen receptor (CAR) against CD19 have shown remarkable results. In contrast, CAR-T cell efficacy against solid tumors has been difficult to achieve.
Methods
To examine the potential of CAR-T cell treatments against ovarian cancers, we used the mouse ovarian cancer cell line ID8 in an intraperitoneal model that exhibits disseminated solid tumors in female C57BL/6J mice. The CAR contained a single-chain Fv from antibody 237 which recognizes a Tn-glycopeptide-antigen expressed by ID8 due to aberrant O-linked glycosylation in the absence of the transferase-dependent chaperone Cosmc. The efficacy of four Tn-dependent CARs with varying affinity to Tn antigen, and each containing CD28/CD3ζ cytoplasmic domains, were compared in vitro and in vivo in this study.
Results
In line with many observations about the impact of aberrant O-linked glycosylation, the ID8Cosmc knock-out (ID8Cosmc-KO) exhibited more rapid tumor progression compared with wild-type ID8. Despite the enhanced tumor growth in vivo, 237 CAR and a mutant with 30-fold higher affinity, but not CARs with lower affinity, controlled advanced ID8Cosmc-KO tumors. Tumor regression could be achieved with a single intravenous dose of the CARs, but intraperitoneal administration was even more effective. The CAR-T cells persisted over a period of months, allowing CAR-treated mice to delay tumor growth in a re-challenge setting. The most effective CARs exhibited the highest affinity for antigen. Antitumor effects observed in vivo were associated with increased numbers of T cells and macrophages, and higher levels of cleaved caspase-3, in the tumor microenvironment. Notably, the least therapeutically effective CAR mediated tonic signaling leading to antigen-independent cytokine expression and it had higher levels of the immunosuppressive cytokine interleukin10.
Conclusion
The findings support the development of affinity-optimized CAR-T cells as a potential treatment for established ovarian cancer, with the most effective CARs mediating a distinct pattern of inflammatory cytokine release in vitro. Importantly, the most potent Tn-dependent CAR-T cells showed no evidence of toxicity in tumor-bearing mice in a syngeneic, immunocompetent system.
Keywords: Receptors, Chimeric Antigen; T-Lymphocytes; Immunotherapy; Antigens, Tumor-Associated, Carbohydrate; Cell Engineering
WHAT IS ALREADY KNOWN ON THIS TOPIC.
Solid tumors have been difficult to treat with chimeric antigen receptor (CAR)-T cells because of a lack of suitable targets. Despite the tremendous promise of CAR-T cell therapy against solid tumors, a majority of preclinical studies with CARs have used human tumors in immune-deficient mouse models.
WHAT THIS STUDY ADDS
We established an immunocompetent, syngeneic murine model to characterize Tn-antigen specific CAR-T cells targeting advanced stages of ovarian solid tumors. We find evidence of direct CAR T-cell mediated cytotoxicity against tumor cells and exhibited long-term delay of tumor progression when administered to mice with disseminated tumors.
HOW THIS STUDY MIGHT AFFECT RESEARCH, PRACTICE OR POLICY
In this study, we developed guidelines for successful CAR-T cell treatment of advanced ovarian cancer. We demonstrate that a single dose of Tn-dependent CAR with sufficient affinity for a Tn-glycoprotein is able to control well-established tumors in this model, and was most effective with intraperitoneal administration. The study also provides additional evidence of the role of CAR affinity in the optimization of solid tumor targeting.
Background
Adoptive therapies with T cells that express chimeric antigen receptors (CARs) against hematopoietic cancers have shown tremendous promise.1 2 The success of these CAR-T cell therapies has stemmed in part from the targeting of the normal B cell-specific differentiation antigen, CD19, which allows elimination of malignant B cells. In contrast, solid tumors have been difficult to treat with CAR-T cells because of a lack of suitable targets and unique immunosuppressive environments.3 4 Hematopoietic cancers offer other B cell-specific differentiation antigens, but targeting self-antigens (eg, HER2, folate receptor-α, mesothelin, MUC16) (reviewed in study by Rodriguez-Garcia et al 5) expressed on non-hematopoietic cancers has been limited by toxicity to normal tissues that express the same targets.
Ovarian cancer is a particularly devastating disease, with a poor prognosis due to the lack of early diagnostics.6 Treatment involves surgery and chemotherapy (typically platinum-based drugs) and, more recently, inhibitors of angiogenesis and poly(ADP-ribose) polymerase-1 (PARP-1). Despite these approaches, 5-year survival statistics have seen minimal improvement. While there are CARs in development against various ovarian cancer antigens (reviewed in study by Rodriguez-Garcia et al 5), to date there have not been clinically effective results. Although preclinical effective CARs have been identified, these typically involved immunodeficient mouse models that did not express the human target.7 8
Recently, we focused on CARs 237 and 5E5 that recognize specific Tn-glycopeptide-antigens (GalNAcα1-O-Ser/Thr-protein).9–13 The most studied Tn-antigens are the heavily O-glycosylated, repeat proteins of the Mucin family.14–17 MUC1 has recently been shown to induce affinity-matured antibodies,18 but these and other antibodies19 are bound only to non-glycosylated MUC1. In contrast, antibodies 237 and 5E5 are cancer-specific and require GalNAc in combination with peptide epitopes. Targeting Tn-peptide antigens has the potential to avoid antigen-loss variants as observed when targeting protein epitopes such as CD19,20 as the glycosylation defect is often critical for tumor cell function and Tn-dependent CARs that recognize multiple different protein backbones can be generated.12 13
The 237 and 5E5 antibodies were generated against the Tn-antigen linked to their cognate proteins, mouse podoplanin (also known as OTS8)9 and human MUC1,21 respectively. The cancer-specific expression of Tn-antigens can result from various dysregulated steps of the pathway, including the glycosyltransferase enzymes or the chaperone Cosmc.22 23 While COSMC (also known as C1GALT1C1) mutations are found in approximately 1–5% of cancers,12 13 other mechanisms of O-linked glycosylation dysregulation appear to contribute to estimates that 85% of ovarian cancers express the epitope recognized by the Tn-MUC1 antibody 5E5.17
Many previous studies have used the mouse tumor line ID824 as a model for human ovarian cancer.6 25 Various treatment approaches, including surgery, cisplatin, checkpoint inhibitors, oncolytic viruses, and PARP-1 inhibitors have been tested in the ID8 model (reviewed in study by McMullen et al 26), but tumor control in these studies has required that treatments be given early, before the presence of established, disseminated tumors. Our approaches here, with mouse CAR-T cells against ID8, focus on treatment when there are well-established solid tumors27 in a syngeneic system that is intended to resemble the scenario encountered in human disease.
We show that a single 237 CAR-T cell treatment of ID8Cosmc knock-out (ID8Cosmc-KO)-bearing mice, intravenously on day 40 after tumor transplantation, resulted in long-term delay of tumor growth. Interestingly, intraperitoneal administration of the CAR-T cells resulted in improved efficacy, with delayed progression when given as late as 60 days after tumor inoculation. At this point, mice exhibited extensive dissemination of solid tumors in the peritoneal cavity. A comparison of four Tn-dependent CARs showed that maximal efficacy required a minimal affinity for the Tn-OTS8 antigen. Among the four CARs, there was a distinct profile of inflammatory cytokine release, highlighted by the observation that the least effective CAR (5E5) exhibited tonic signaling leading to a higher basal level of cytokine expression and antigen-induced secretion of interleukin (IL)-10. We also describe long-term persistence of the CAR-T cells, and their ability to delay growth of an ID8Cosmc-KO tumor re-challenge. These findings support the potential of treating ovarian cancer with affinity-optimized CAR-T cells, using intraperitoneal routes of administration for optimal effectiveness.
Methods
Cell lines
Murine ovarian cancer cell line, ID8, and its mutant line with Cosmc gene deletion (ID8Cosmc-KO) were maintained in complete Dulbecco’s Modified Eagle Medium supplemented with insulin-transferrin-sodium-selenite supplement at 1:5000 dilution. The generation of ID8Cosmc-KO using clustered regularly interspaced short palindromic repeats and CRISPR-associated protein 9 (CRISPR/Cas9) gene editing technique has been described.12 13 Cell lines were submitted to IDEXX BioAnalytics for authentication, and were tested free of pathogens. All cell lines and primary cells used in this study were cultured at 37°C with 5% CO2.
Chimeric antigen receptors
The 237 single-chain Fv (scFv) was fused to CD8α hinge-CD28-CD3ζ and cloned into the pMP71 retroviral vector to generate 237 CAR plasmid.10 Structure-guided engineering of the 237 scFv fragment13 yielded CAR variants that bound with higher affinity to the cognate murine Tn-OTS8 peptide (237 scFv mutant WE, designated WE CAR here) or exhibited binding to both murine Tn-OTS8 and human Tn-MUC1 (237 scFv mutant TNGK, designated TNGK CAR here). The 5E5 scFv CAR that binds to human Tn-MUC1 was characterized in human T cells previously11 and was subcloned into the pMP71 for mouse T-cell studies.13
T cell transduction and activation
The protocol for retroviral transduction of primary T cells isolated from splenocytes of donor mice has been described.10 13 A detailed protocol is outlined (DOI: dx.doi.org/10.17504/protocols.io.6qpvr4jwpgmk/v1). Mock-transduced T cells went through the retroviral transduction process but without recombinant virus. Transduction efficiency was measured using Tn-OTS8 tetramer for 237, WE and TNGK CARs or Tn-MUC-1 tetramer for 5E5 CARs.
Animal studies
Animal studies were approved by the UIUC Institutional Animal Care and Use Committee (IACUC) under protocol numbers 21041 and 20176. Female C57BL/6J mice (8–10 weeks old) were purchased from Jackson Laboratories, maintained under pathogen-free conditions in a barrier facility, ear-tagged, and assigned to treatment groups. ID8 and ID8Cosmc-KO tumor cells (107) were inoculated intraperitoneally. After various days, 5×106 CAR-T cells (or mock-transduced T cells) were administered intravenously or intraperitoneally. At 24 hours prior to T-cell transfer, cyclophosphamide (100 mg/kg) was administered intraperitoneally. Tumor growth and progression of disease were monitored by measuring body weight on a regular basis. Mice were euthanized when their body weights reached the endpoint criterion of 36 g or a gain of 50% of their weight from the start of treatment. For some experiments, blood samples (~70 µL) were collected by submandibular vein puncture or retro-orbital bleeding. Tissue samples were collected postmortem, fixed in 10% buffered formalin for 24 hours, and subsequently transferred to 70% ethanol prior to routine tissue sectioning at 5 micron thickness and staining with H&E.
Antibody detection agents
Antibodies used for flow cytometry were purchased from BioLegend or BD Bioscience: CD3 (clone 17A2), CD8α (53–6.7), CD4 (RM4–5), CD45 (30-F11), and TCR Cβ (H57-597). Fixable Viability Dye eFluor 780 was purchased from eBioscience (Invitrogen 50-169-66). Data were acquired on a BD LSRII and analyzed with FlowJo software. The Tn-specific monoclonal antibody 5F4 (IgM), a kind gift from Dr Henrik Clausen (University of Copenhagen, Denmark),28 was used as culture supernatant (for flow cytometry) or purified using LigaTrap Microspin Kit (LT-145KIT) and biotinylated (Thermo Fisher/Pierce EZ-Link NHS-PEG4 biotinylation kit 21455) (for immunohistology). The 237 scFv mutant WE, cloned into pET28a with a 6X histidine and AviTag, was purified from inclusion bodies and biotinylated using BirA ligase from Biotin-Protein Ligase Reaction Kits (Avidity BIRA-500). Fluorescently-labeled WE scFv tetramers were prepared by combining biotinylated WE scFv with streptavidin-PE (BD Bioscience CN554061) or streptavidin-Alexa Fluor 647 (Invitrogen CNS32357) at a 4:1 molar ratio. Biotinylated Tn-OTS8 and biotinylated Tn-MUC1 peptides were prepared as described in study by Sharma et al.13
Processing and analysis of tissues and tumors
Protocols for processing mouse tissues and staining for flow cytometry are described in DOI: dx.doi.org/10.17504/protocols.io.6qpvr4jwpgmk/v1. H&E staining and immunohistochemistry of tissue sections from formalin-fixed paraffin embedded samples were performed by the University of Chicago Human Tissue Resource Center core facility. Stained slides were scanned using a Hamamatsu NanoZoomer slide scanner. Protocols for staining with biotinylated agents (anti-Tn antibody 5F4 or WE scFv), anti-CD3 (Abcam ab135372, clone SP162), anti-F4/80 (BioRad MCA497GA, clone A3-1), anti-Ly6G (BioLegend 127602, clone 1A8), and anti-cleaved caspase 3 (Cell Signaling Technology 9661) are described in DOI: dx.doi.org/10.17504/protocols.io.6qpvr4jwpgmk/v1.
In vitro co-culture assays
ID8 wild-type (WT) or ID8Cosmc-KO cells were co-cultured with CAR-T cells in 96-well plates at different seeding density. In one approach, the number of effector T cells was kept constant (12,000 cells/well) while the number of tumor cells was varied (tumor:effector ratios). In another, tumor cell number was constant while effector T-cell numbers were varied. After co-culturing for 24 hours at 37°C, supernatants were transferred to V-bottom 96-well plates and supernatants were stored at −20°C.
For cytokine analysis, 25 µL supernatants were used in a customized mouse cytokine array (Milliplex MCYTOMAG-70K) following the manufacturer’s protocol. The panels included interferon (IFN)-γ, IL-2, IL-4, IL-6, IL-10, and tumor necrosis factor (TNF)α (Milliplex MCYTOMAG-70K-6plex). Plates were read on Luminex 200, with quality controls and standards included.
T-cell cytotoxicity of tumor cells was measured using the sulforhodamine B colorimetric assay29 following a protocol described in DOI: DOI: dx.doi.org/10.17504/protocols.io.6qpvr4jwpgmk/v1. IFN-γ ELISpot assays were performed using splenic T cells and a mouse IFN-γ ELISpot kit (CTL ImmunoSpot), following 24-hour co-culture.
T-cell proliferation assays were performed by labeling activated mock or CAR-T cells with CFSE (Invitrogen C34554) following the manufacturer’s protocol. Post-transduction (72 hours), T cells were washed, resuspended in phosphate-buffered saline, and incubated with 10 µM CFSE for 20 min at 37°C in the dark. Cells were washed with culture media, and resuspended to a final cell density of 1×106 cells/mL. 100 µL cell suspension was aliquoted in a 96-well plate precoated with streptavidin followed by biotinylated peptides (described in studies by He et al and Sharma et al 12 13). Following 72-hour incubation, T cells were harvested, washed, and analyzed in a BD Accuri flow cytometer.
Results
ID8Cosmc-KO as a model for CAR treatment of ovarian cancer
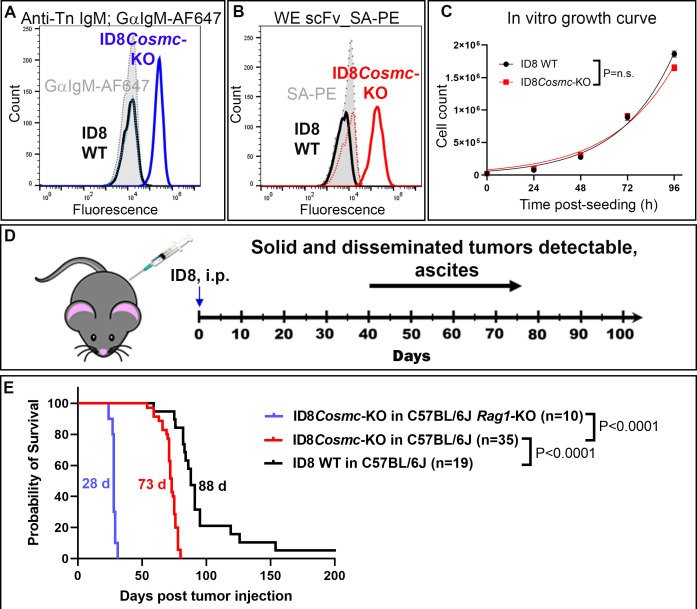
The ovarian cancer cell line ID8, with a deletion of the gene for the chaperone Cosmc, expresses high levels of Tn-antigen as detected with anti-Tn antibody 5F4 (figure 1A). ID8Cosmc-KO is also recognized by Tn-dependent antibody 237 and its CAR derivatives.13 For detection, the high-affinity variant called 237-WE (WE from here-on) was expressed as a soluble biotinylated scFv. Tetramers of the WE scFv were used to validate the expression of the Tn-OTS8-antigen in the ID8Cosmc-KO line (figure 1B). Notably, the parental ID8 (ID8 WT) and ID8Cosmc-KO lines exhibited the same rate of growth in vitro, with doubling times of 19.2 hours and 21.8 hours, respectively (figure 1C).
Figure 1.
ID8Cosmc-KO as a model for ovarian cancer in C57BL/6J mice. Tn antigen expression on ID8Cosmc-KO was measured by flow cytometry using either (A) staining with monoclonal mouse IgM anti-Tn antibody 5F4 followed by goat anti-mouse IgM conjugated to Alexa Fluor 647 or (B) biotinylated anti-Tn-OTS8 WE scFv tetramer containing streptavidin-PE. (C) ID8 WT and ID8Cosmc-KO cell doubling times (19.2 hours and 21.8 hours, respectively) in vitro were measured over time by manual cell counting. Growth curves from two different experiments (total n=6) were combined and plotted using GraphPad Prism V.9.4.1 and cell doubling times were calculated using the exponential growth equation. (D) Timeline for ID8Cosmc-KO tumor growth in syngeneic C57BL/6J mice after intraperitoneal injection of 107 tumor cells. (E) Survival curves of C57BL/6J mice inoculated intraperitoneally with 107 ID8 WT or ID8Cosmc-KO. Median survival was 88 days and 73 days for ID8 WT (n=19) and ID8Cosmc-KO (n=35), respectively. For C57BL/6 Rag1-KO mice inoculated with 107 ID8Cosmc-KO (n=10), the median survival was 28 days. Survival curves were plotted using GraphPad Prism V.9.4.1 and effects were calculated using the log-rank (Mantel-Cox) test. i.p., intraperitoneal; KO, knock-out; scFv, single-chain Fv; WT, wild-type.
To evaluate the ID8Cosmc-KO as a model for 237 CAR treatment, we inoculated 107 tumor cells intraperitoneally into syngeneic C57BL/6 mice (figure 1D). We used ID8 lines without luciferase for imaging to minimize the potential for immunogenicity of foreign proteins in immunocompetent mice.30 31 Consistent with previous ID8 studies, mice with ID8 WT began to develop ascites and gained weight to the point of euthanasia criterion (36 g or >50% weight gain) within 10–12 weeks (online supplemental figure S1A). Mice with ID8Cosmc-KO tumors developed slightly earlier signs of ascites (8 weeks; Online supplemental figure S1B), and the median survival was 73 days, with 100% tumor take (figure 1E). By comparison, ID8 WT had a median survival of 88 days (figure 1E). To assess the possible role of B or T cells in ID8Cosmc-KO growth, immunodeficient Rag1-KO mice were inoculated with 107 ID8Cosmc-KO cells (figure 1E). Growth of the tumor was rapid, with a median survival of 28 days, indicating a significant contribution of acquired immune responses in immunocompetent mice. Mice showed signs of lethargy but no ascites-related weight gain (online supplemental figure S1C).
jitc-2022-006509supp001.pdf (16.1MB, pdf)
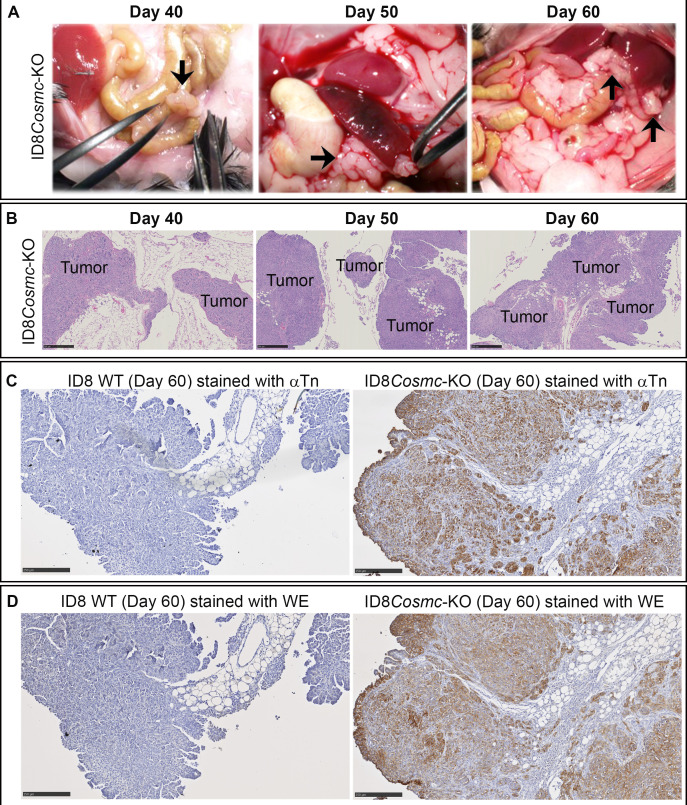
At the endpoint criterion, immunocompetent C57BL/6 mice exhibited extensive tumor growth distributed throughout the peritoneal cavity, typically with the presence of ascites fluid (median, 17 mL) (online supplemental figure S1D–F). Solid tumors were most often located in association with adipose tissues that surround organs (figure 2A). Pathological and histochemical analyses of tissue sections from the peritoneal cavity revealed the presence of tumor cell nodules and masses in adipose tissues by day 40 with progression to more extensive intraperitoneal spread (figure 2B). Tumor masses were identified along capsular and serosal surfaces of most major abdominal organs, with no parenchymal invasion. Tumor cells formed solid nests supported by a fine collagenous matrix as well as occasional small, irregular glandular structures. The omentum and mesentery were extensively colonized by tumor cells. The largest masses were present in the cranial abdomen (bordering the stomach, duodenum and pancreas). Dissemination of the peritoneal masses was attributed to transcoelomic spread given the general absence of organ involvement. There was no histologic evidence of tumor cells in distant organs such as heart or brain tissues.
Figure 2.
Pathological and histochemical analyses of tissue sections from ID8Cosmc-KO and ID8 WT mice at various times after tumor challenge. (A) At indicated days post-inoculation with 107 tumor cells, mice were euthanized, and organs in the peritoneal cavity were examined visually for tumor nodules (black arrows). (B) Formalin-fixed paraffin embedded tissue sections from mice inoculated with ID8Cosmc-KO cells at the indicated time points were stained with H&E. Tumor foci are labeled. Scale bar=500 µm. (C, D) ID8 WT or ID8Cosmc-KO tumors at day 60 post-tumor challenge were examined by immunohistochemistry staining using biotinylated anti-Tn IgM antibody 5F4 (C) or biotinylated WE scFv (D). Scale bar=250 µm. KO, knock-out; scFv, single-chain Fv; WT, wild-type.
Tissue sections were stained with biotinylated antibody 5F4 (figure 2C)28 and WE scFv (figure 2D) to verify Tn-antigen expression of ID8Cosmc-KO tumors. Consistent with the survival times of mice containing ID8 WT and ID8Cosmc-KO tumors, the extent of tumor spread and size observed grossly and histologically was 10–20 days sooner for ID8Cosmc-KO (online supplemental figure S1G, H).
Efficacy of CAR-T cells in mice with established ID8 tumors: intravenous versus intraperitoneal treatments
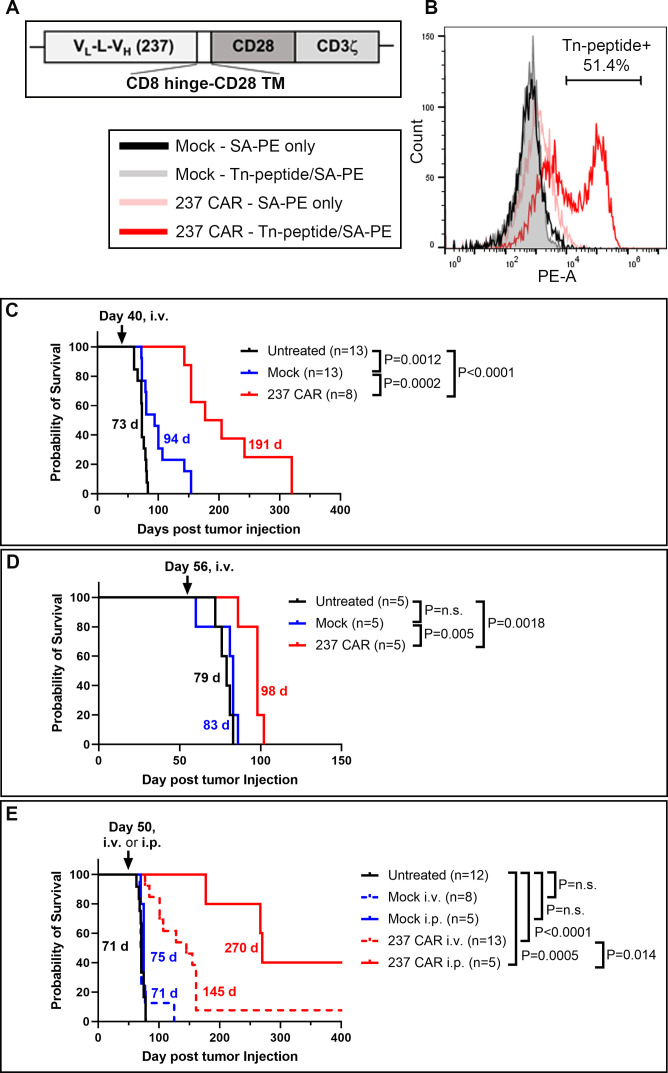
We examined if 237 CAR-T cell treatment could delay the growth of ID8Cosmc-KO tumors, at times when solid tumors were well established. Activated splenic T cells (total CD4 and CD8) from C57BL/6 mice were transduced with the pMP71 vector containing 237 CAR/CD28/CD3ζ (figure 3A). Transduced T cells were analyzed by flow cytometry for CAR expression with biotinylated Tn-glycopeptide (figure 3B). Among multiple experiments, this analysis showed that 30–50% of transduced activated T cells were positive.
Figure 3.
CAR-T cell treatment of mice with established ID8Cosmc-KO tumors. (A) Schematic of 237 single-chain Fv linked by a CD8 hinge-CD28 transmembrane domain to the CD28 and CD3ζ cytoplasmic domains, cloned into the pMP71 retroviral vector. (B) Primary T cells isolated from splenocytes of naïve donor C57BL/6J mice were transduced with 237 CAR for 72 hours. Transduction efficiency was measured by flow cytometry using tetramers of biotinylated/Tn-glycosylated OTS8 peptide made with streptavidin-PE. Mock-transduced T cells were included as negative control. (C, D) Survival curves of ID8Cosmc-KO-bearing C57BL/6J mice either untreated (black), treated with mock-transduced T cells (blue) or 237 CAR-T cells (red) administered by retro-orbital intravenous injection of 5×106 T cells at day 40 (C) or day 56 (D) post-tumor inoculation. Note that five of the mice in the 237 CAR group (C) were used at day 160 for a re-challenge experiment (figure 7B), such that their survival times may have been even longer without this re-challenge. (E) Comparison of intravenous (dashed red line) and intraperitoneal (solid red line) delivery of 237 CAR-T cells at day 50 post-tumor inoculation. All survival curves were plotted with GraphPad Prism V.9.4.1 using cumulative data across multiple experiments (two combined experiments for C and D, four combined experiments for E), with the indicated total n for each treatment arm. Median survival times are indicated; p values were calculated using the log-rank (Mantel-Cox) test. CAR, chimeric antigen receptor; i.p., intraperitoneal; i.v., intravenous; KO, knock-out.
Initial experiments involved intravenous administration of CAR-T cells or mock-transduced T cells around day 40 after ID8Cosmc-KO transfer, 1 day after treatment with cyclophosphamide (figure 3C). Treatment with 237 CAR resulted in long-term control of tumors, with a median survival of 191 days (figure 3C). Mice treated with mock-transduced T cells exhibited a delay in tumor growth to a median survival of 94 days, presumably due to the presence of activated polyclonal T cells. Based on the exceptional result with CAR treatment on day 40, mice were treated on day 56 after tumor transfer (figure 3D). The 237 CAR delayed growth compared with the untreated and mock controls (p=0.0018 and p=0.0050, respectively).
Ovarian cancer therapy can benefit from treatments that can be delivered directly to the peritoneal cavity, especially since tumors often develop on the surface of abdominal organs. To examine if intraperitoneal administration of CAR-T cells could control tumor growth better than intravenous delivery, we treated mice on day 50 after ID8Cosmc-KO transfer. Intravenous treatment with 237 CAR on day 50 slowed the growth of the tumor to a median survival of 145 days (figure 3E). Intraperitoneal delivery of the same CAR-T cells prolonged survival to 270 days (figure 3E). Importantly, no treatment-related adverse events were observed following intraperitoneal injection of mock-T or 237 CAR-T cells in either tumor bearing or non-tumor bearing mice, based on gross pathological and histological examination of major organs (including peritoneal tissues) from CAR-treated mice (online supplemental figure S2 and online supplemental tables S1 and S2).
Efficacy of different Tn-dependent CARs in treatment of ID8Cosmc-KO tumors
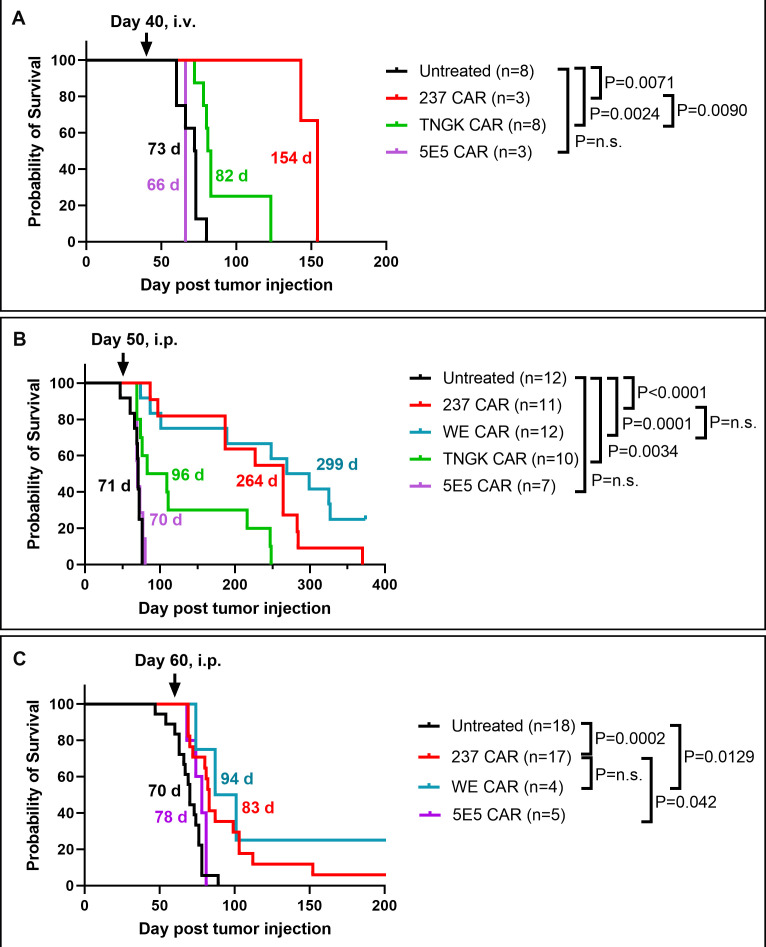
We recently engineered a Tn-dependent CAR called TNGK, using the 237 scFv antibody backbone, that was selected with the human antigen Tn-MUC1.13 The TNGK scFv bound with higher affinity to Tn-MUC1 than 237 scFv, but it exhibited lower affinity binding to Tn-OTS8, the cognate mouse antigen recognized by 2379 (online supplemental figure S3A). Another Tn-dependent CAR called 5E5 was originally developed against Tn-MUC111 21 and has high affinity for Tn-MUC1 but low affinity for the mouse antigen Tn-OTS813 (online supplemental figure S3A, B). TNGK and 5E5 CARs have about 10-fold less sensitivity than 237 CAR using immobilized Tn-OTS8 antigen to stimulate IFN-γ.13 Both of these CARs were tested in an experiment where mice with ID8Cosmc-KO were treated intravenously on day 40 (figure 4A). Although TNGK CAR delayed growth of the ID8 tumor, 5E5 showed no effect.
Figure 4.
Efficacy of engineered CAR T constructs with different affinities for the mouse Tn-OTS8 antigen in the ID8Cosmc-KO ovarian tumor model. (A) Survival curves of ID8Cosmc-KO-bearing C57BL/6J mice treated with 237 CAR T (red), TNGK CAR T (green), or 5E5 CAR T (purple) by intravenous injection of 5×106 transduced T cells at day 40 post-tumor inoculation. (B, C) Survival curves of ID8Cosmc-KO-bearing C57BL/6J mice treated with different CAR-T cells by intraperitoneal injection of 5×106 transduced T cells at day 50 (B) or day 60 (C) post tumor-inoculation. All survival curves were plotted with GraphPad Prism V.9.4.1 using cumulative data across multiple experiments (two combined experiments for A and C, four for B) with the indicated total n for each treatment arm. Median survival times are indicated; p values were calculated using the log-rank (Mantel-Cox) test. CAR, chimeric antigen receptor; i.p., intraperitoneal; i.v., intravenous; KO, knock-out.
To extend these studies with additional Tn-dependent CARs, we also examined WE CAR-T cells that contained the scFv variant with 30-fold higher affinity than 237 (KD values of 120 nM and 4 nM for 237 and WE scFv, respectively). WE, 237, TNGK, and 5E5 CARs were administered intraperitoneally at day 50 after ID8Cosmc-KO inoculation (figure 4B). The 5E5 CAR showed no effect, TNGK CAR significantly extended median survival by 17 days (p=0.0034), and both 237 and WE CARs mediated long-term control of tumors, to median survivals of 264 and 299 days, respectively. There was no significant difference in tumor control between 237 and WE CARs, despite the 30-fold higher affinity of the latter.
To understand the limits of the CAR-T cell treatments, we performed an experiment in which mice were treated with 237 and WE CAR-T cells administered intraperitoneally at day 60, the phase at which some mice reach the weight criterion for euthanasia. Not surprisingly, there was reduced efficacy of the CAR-T cell treatment at this advanced disease stage (figure 4C), but there was a significant delay in disease progression mediated by 237 and WE CARs (p=0.0009 and p=0.0095, respectively).
In vitro analyses comparing 237, WE, TNGK and 5E5 CARs
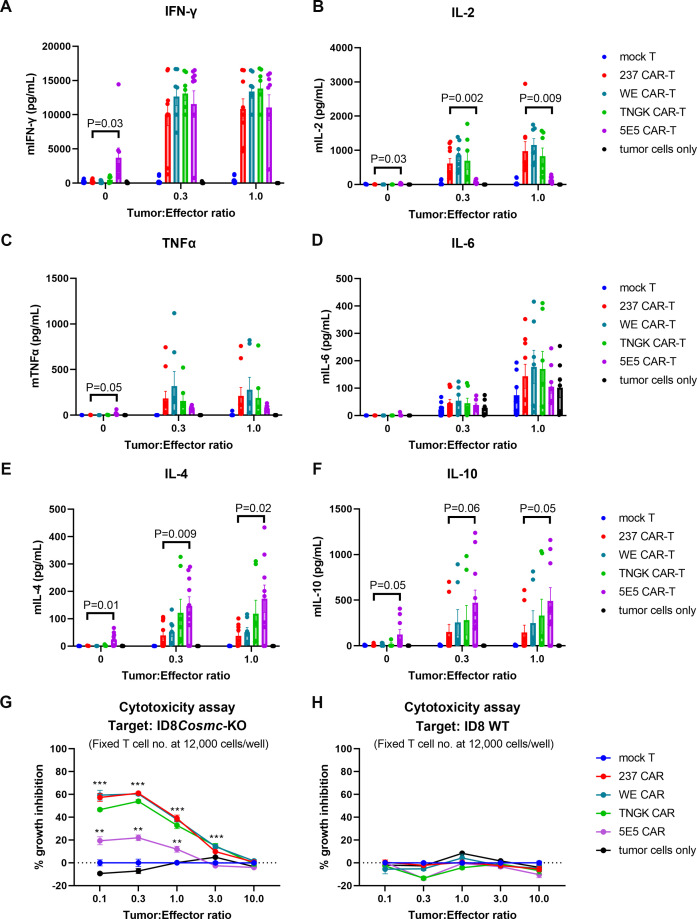
Given that all four CARs mediated Tn-dependent IFN-γ release in vitro when stimulated by ID8Cosmc-KO cells,13 the question remained what might explain their distinct differences in efficacy. To examine if there was a correlation between in vivo effects and in vitro cytokine release, we assayed in vitro-stimulated CAR-T cells for secretion of various cytokines, including IFN-γ, IL-2, TNFα, IL-6, IL-4 and IL-10 (figure 5A–F, online supplemental figure S4A–F). To present antigen, we used various ratios of ID8Cosmc-KO tumor cells. Consistent with our previous findings, all four CARs were effective in mediating release of IFN-γ, even at the lowest tumor:CAR-T cell ratio of 0.1:1 (online supplemental figure S4A). Of particular note, among multiple experiments, the 5E5 CAR showed a level of tonic cytokine release, especially with IFN-γ, in the absence of antigen. While all four CARs mediated release of the other cytokines, there was a distinct quantitative difference among them. The two most effective CARs in vivo, 237 and WE, exhibited greater stimulation of IL-2 compared with 5E5. Interestingly, the reciprocal was observed for the release of IL-4 and IL-10, where 5E5 CAR exhibited higher levels than 237 and WE. However, 5E5 CAR-T cells also exhibited higher tonic levels of IL-4 and IL-10 than the other CARs, and significant levels of antigen-independent TNFα release. The release of IL-10 mediated by 5E5 CAR was also observed when it was co-cultured with Jurkat, a human tumor line with a known Cosmc mutation (online supplemental figure S4F).
Figure 5.
In vitro cytokine and killing analyses of different CAR T cells. Mock, 237-CAR, WE-CAR, TNGK-CAR, or 5E5-CAR transduced primary T cells from C57BL/6J donor mice were co-cultured with ID8Cosmc-KO cells in a 96-well plate for 24 hours at various tumor:effector ratios. The number of effector T cells were kept constant at 12,000 cells/well across all conditions tested. After 24 hours, 25 uL culture supernatants were analyzed for multiple cytokines using a Luminex plate reader to measure IFN-γ (A), IL-2 (B), TNFα (C), IL-6 (D), IL-4 (E), and IL-10 (F). Plots shown are combined cytokine array data points from five experiments. Bar graphs were plotted using GraphPad Prism V.9.4.1. Error bars are SEM. To simplify the presentation, p values are shown only for 237 versus 5E5 CAR. A complete set of tumor:effector ratio titration plots for each of the five cytokine array experiments are shown in online supplemental figure S5. (G, H) The remaining ID8Cosmc-KO (G) or ID8 WT (H) adherent tumor cells on plates were fixed in ice-cold 10% trichloroacetic acid for the SRB viability assay. The percent growth inhibition was calculated using the formula outlined in the Methods section. SRB experiments were performed twice, each with a technical replicate of n=4. Bar graphs were plotted using GraphPad Prism V.9.4.1. Error bars are SEM. P values for 237, WE, TNGK were calculated against either mock or 5E5 at <0.0001 (or denoted by ***); p values for 5E5 against either mock T or TNGK are at <0.001 (**). CAR, chimeric antigen receptor; IFN, interferon; IL, interleukin; KO, knock-out; TNF, tumor necrosis factor; WT, wild-type.
We also assessed in vitro tumor killing mediated by CAR-T cells. All four CARs mediated killing of ID8Cosmc-KO cells (figure 5G and online supplemental figure S5A) but not ID8 WT cells (figure 5H and online supplemental figure S5B). However, 5E5 CAR-T cells exhibited lower cytotoxic activity among the four CARs tested.
Finally, we examined antigen-dependent proliferation using CFSE-labeled T cells stimulated with various concentrations of OTS8, Tn-OTS8, MUC-1, and Tn-MUC1 peptides for 72 hours by flow cytometry (online supplemental figure S6). Gating on viable cells using forward/side scatter, we observed that 237, WE, and TNGK CAR exhibited background levels (mock T cells) of proliferation in the absence of the cognate antigen, with about 0.5% in the lymphocyte gate (online supplemental figure S6A, C). In contrast, 5E5 CAR exhibited almost 5% proliferating cells with the control peptides OTS8 and MUC1 (online supplemental figure S6B, C). All of the CARs, including 5E5, exhibited antigen-dependent proliferation, and for 237 and WE this level was highest with the mouse antigen Tn-OTS8. Similar results were observed when monitoring proliferation by flow cytometry for CFSE, where reduced mean fluorescent intensity correlates with greater proliferation (online supplemental figure S6D, E). The extent of proliferation was greatest for 237 and WE CARs with Tn-OTS8, whereas TNGK showed extensive proliferation with both Tn-OTS8 and Tn-MUC1. Again, 5E5 showed a high level of Tn-antigen-independent proliferation.
Immunophenotyping ascites and TILs from ID8Cosmc-KO mice
To assess CAR T cell distribution immediately after CAR treatment, we performed immunophenotyping by flow cytometry on samples from blood, spleen, pancreas, and ascites fluid recovered from ID8Cosmc-KO-bearing mice (online supplemental figure S7). The 237 or 5E5 CAR-T cells were administered intraperitoneally on day 54 post-tumor challenge, and mice were euthanized 3 days post-T cell transfer. Although ascites at this time point is typically not large, the volume collected from 237 CAR-treated mice was higher relative to that collected from untreated mice (online supplemental table S3). Ascites from 237 CAR-treated mice was ‘milky off-white’, compared with ‘red’ typical in untreated and 5E5 CAR-treated mice and the surface of pancreases from CAR-treated mice appeared more viscous compared with untreated controls.
We examined the distribution of CAR-T cells in circulation and within the pancreatic tissue using Tn-OTS8 tetramers (gating strategy, online supplemental figure S7A). Both 237 and 5E5 CARs (CD3+, CD8+, and CD4+ double-positive cell populations) were detectable and significantly elevated in tumor tissues and ascites samples from CAR-treated tumor-bearing groups (online supplemental figure S7B), indicating that CAR-T cells, administered to mice 3 days prior, have migrated to target sites.
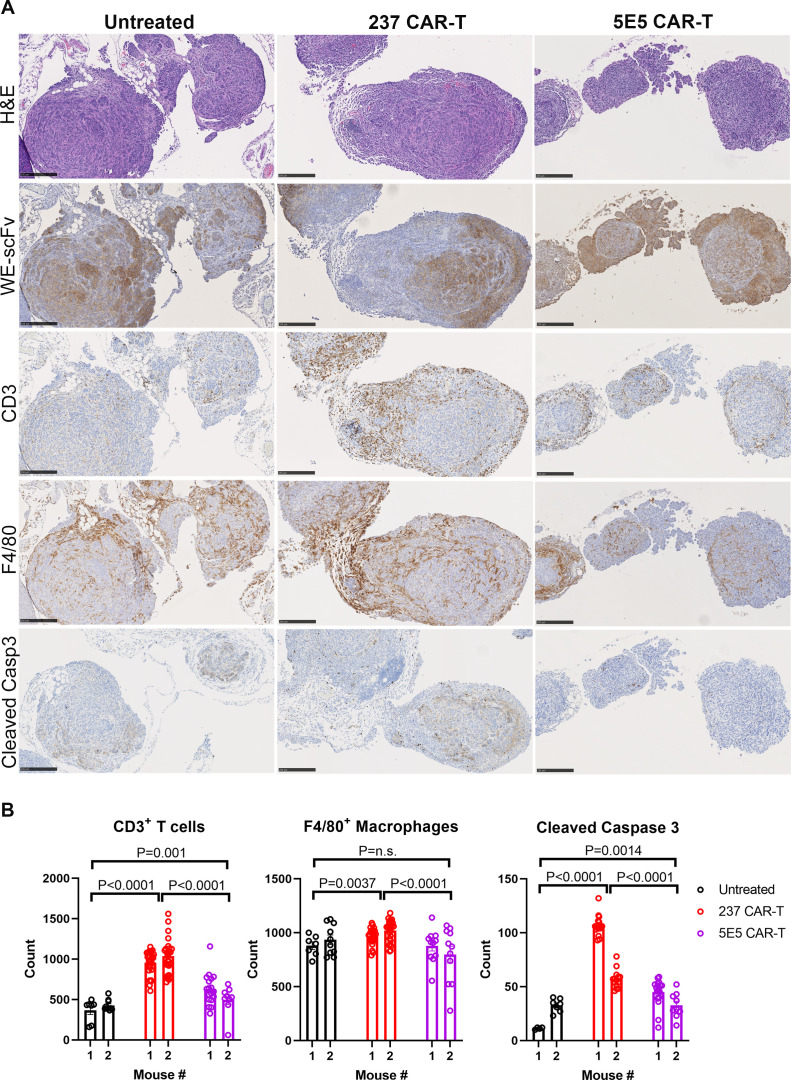
To further assess immune cell infiltration in tumors 3 days after CAR treatment, immunohistochemistry was performed with biotinylated WE-scFv, anti-CD3, anti-F4/80, anti-Ly6G, and anti-cleaved caspase 3 antibodies (figure 6A and online supplemental figure S8). CAR-treated tumor sections contained prominent cellular infiltrates that outnumbered tumor cells. In the absence of CAR-treatment, few CD3+ T cells were detected in tumors. Following either 237 or 5E5 CAR administration, tumor nodules and masses were extensively infiltrated by CD3+ T cells, with 237 CAR-treated tissues showing significantly higher CD3+ T cell infiltration compared with 5E5 CAR-treated tissues (figure 6B, left panel). Based on flow analysis of TILs, it appears that only 5–10% of these CD3+ T cells represent CAR-T cells (online supplemental figure S7B). The high frequency of infiltrating immune cells could partly explain the presence of sections that appeared negative for WE immunostaining, compared with tumors from untreated mice. While most of the small infiltrating cells had lymphocyte morphology and reacted with anti-CD3, there was also an increase in F4/80+ macrophage infiltration in all tumor sections examined, with 237 CAR-treated tissues exhibiting highest levels compared with either untreated or 5E5 CAR-treated tissues (figure 6B, middle panel). Rare to small numbers of Ly6G+ cells (neutrophils) were observed in the tumor sections (online supplemental figure S8). Consistent with high levels of T cell and macrophage infiltration, 237 CAR-treated tissues also had significantly higher numbers of apoptotic cells as shown in cleaved-caspase 3 immunoreactive tissue sections (figure 6B, right panel). We also examined the tumor stroma using trichrome staining and found scant amounts of intratumoral fibrous tissue/collagen, arranged in thin, delicate strands (positive blue stain in bottom row of online supplemental figure S8), but not to an extent that we believe would interfere with CAR migration. Taken together, our results show that 237 CAR treatment recruited more CD3+ and F4/80+ macrophages to the tumor microenvironment, resulting in higher cleaved caspase 3-positive apoptotic cells compared with both 5E5 CAR treated and untreated tissues.
Figure 6.
Peritoneal organs were harvested from ID8Cosmc-KO inoculated mice, 3 days after intraperitoneal treatment with either 237 CAR-T or 5E5 CAR-T at day 54 post-tumor inoculation. Untreated mice were used as control. (A) Immunohistochemistry was performed to visualize Tn-OTS8-glycopeptide+ tumors, CD3+ T cells, F4/80+ macrophages, and apoptotic cells from adjacent FFPE tissue sections stained with biotinylated WE-scFv, anti-CD3, anti-F4/80, and anti-cleaved caspase 3 antibodies, respectively. H&E stained tissue sections were included as additional reference. Scale bar=250 µm. (B) ImageJ-quantified CD3+ T cells, F4/80+ macrophages, and cleaved caspase-3+ apoptotic cells from tissue sections of untreated, 237 CAR-T, 5E5 CAR-T treated mice. A total of 5–20 images at 20× magnification corresponding to tumors spotted lining the fatty tissues near stomach, small and large intestines of two mice per treatment group were analyzed and quantified using ImageJ. Dot plots and p values were generated using GraphPad Prism V.9.4.1. Error bars are SEM. Each dot represent one field of view at 20× magnification. CAR, chimeric antigen receptor; FFPE, formalin-fixed paraffin embedded; KO, knock-out; scFv, single-chain Fv.
Persistence of CAR-T cells in treated mice
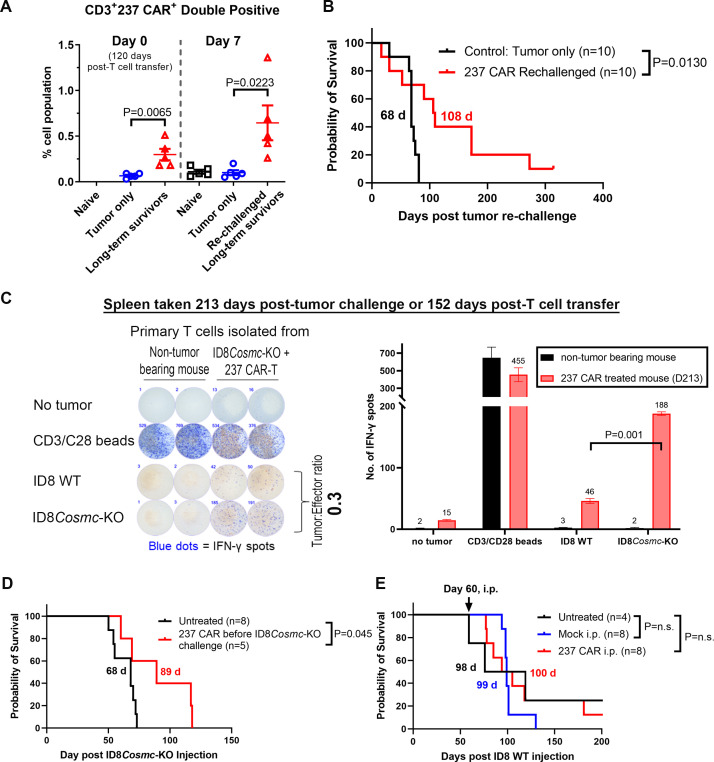
One of the metrics associated with efficacy of CAR-T cell treatments is persistence of CAR-T cells.32 To assess persistence, we tested the ability of CAR-T cell-treated mice to mount a response to re-challenge with ID8Cosmc-KO tumor, using mice that had controlled progression of tumor for a period of 120 days post T-cell administration. Prior to re-challenge, blood cells were co-stained with anti-CD3 and Tn-OTS8 tetramer, showing that CAR-T cells persisted, compared with age-matched non-tumor bearing mice (figure 7A, left panel). Seven days after re-challenge with ID8Cosmc-KO, the population of CD3+ 237 CAR T increased further (figure 7A, right panel). Survival data of re-challenged, CAR-treated mice (figure 7B) indicated that some mice were able to delay tumor progression, with a median survival of 108 days after re-challenge (compared with 68 days for naïve mice) (p=0.0130). In addition, analysis of blood 55 days post-re-challenge suggested that levels of CD4+CAR-T remained elevated in treated mice compared with control (online supplemental figure S9A).
Figure 7.
Evidence for in vivo persistence of 237 CAR T cells. (A) 237 CAR T-treated ID8Cosmc-KO-bearing mice were re-challenged with 107 ID8Cosmc-KO cells at 120 days post-T cell transfer. Age-matched naïve mice were inoculated with 107 ID8Cosmc-KO cells and used as control. Blood samples were collected from some mice immediately before intraperitoneal injection of re-challenged tumors and 7 days after re-challenge; processed and stained with anti-CD3 antibody and Tn-glycosylated OTS8 peptide tetramer, and analyzed by flow cytometry. The percentage of CD3+ 237 CAR T cells were plotted using GraphPad Prism V.9.4.1. Each dot represents one mouse. Error bars are SEM. (B) Survival curve of 237 CAR T treated mice re-challenged with ID8Cosmc-KO at either 120 (n=5) or 160 days (n=5) after initial tumor injection, compared with age-matched C57BL/6 naïve mice that were inoculated with the same number of tumor cells. (C) Spleens were removed from 237 CAR T-treated ID8Cosmc-KO bearing mice up to 5 months after CAR treatment (152 days for the data shown). Spleen cells were co-cultured with either ID8 WT or ID8Cosmc-KO tumor cells in a 96-well plate at a tumor:effector ratio of 0.3. ELISpot assays were performed to measure IFN-gamma spots 24 hours after co-culture experiment. Splenocytes from age-matched mice were used as a control, while CD3/CD28 Dynabeads were added to T cells as a positive control for T cell activation. Plates were scanned with automated ImmunoSpot analyzer. Bar graph of quantified IFN-γ spots are plotted on the right panel. Results representative of five independent experiments. (D) Non-tumor bearing mice were injected intraperitoneally with 237 CAR T (see online supplemental figure S2B) or untreated. At day 60, mice were challenged with 107 ID8Cosmc-KO cells and the survival curve was compared with that of mice inoculated with tumor that did not receive prior 237 CAR T injections (untreated). (E) Survival curve of ID8 WT-bearing C57BL/6J mice either untreated (black), treated with mock-transduced T cells (blue), or treated with 237 CAR T (red), administered by intraperitoneal injection of 5×106 T cells at day 60. Data for D and E are from two combined experiments. All survival curves were plotted using GraphPad Prism V.9.4.1 with the indicated total n for each treatment arm. Median survival times are indicated; p values were calculated using the log-rank (Mantel-Cox) test. CAR, chimeric antigen receptor; IFN, interferon; i.p., intraperitoneal; KO, knock-out; WT, wild-type.
To further assess T-cell persistence, we performed IFN-γ ELISpot of spleen cells from 237 CAR-treated mice. Splenic T cells were co-cultured with ID8 WT or ID8Cosmc-KO tumor cells at various tumor-to-effector ratios. More cells from 237 CAR-treated mice were stimulated to secrete IFN-γ by ID8Cosmc-KO compared with ID8 WT cells (figure 7C), indicating that Tn-specific T cells persisted in treated mice. Presence of Tn-dependent CAR T cells was also shown by the stimulation of IFN-γ secreting T cells by the Cosmc-deletion line AG104A, but not other mouse tumor lines (online supplemental figure S9B).
To explore whether antigen was necessary for CAR-T cell persistence, we examined whether CAR-T cells administered 60 days prior to tumor inoculation could delay tumor progression (figure 7D). CAR-T pretreated mice survived to a median of 89 days compared with 68 days for untreated naïve mice, indicating that sufficient CAR-T cells were present, even in the absence of antigen, to delay growth (p=0.045).
Tn-antigen requirement in CAR-T cell treated mice
To verify that Tn-antigen was required for 237 CAR efficacy, we treated ID8 WT-bearing mice with 237 CAR (figure 7E). The 237 CAR-treated mice did not show a delay in survival compared with untreated or mock-treated controls, indicating that the in vivo potency of the 237 CAR requires Tn-antigens such as Tn-OTS8 generated by the ID8Cosmc-KO line.
Finally, to determine if long-term, relapsed mice treated with the 237 or WE CARs showed evidence of loss of the Tn-antigen, we analyzed the Tn-expression status of tumor cells collected from ascites of relapsed mice. The frequency of Tn-expressing tumor cells in ascites collected from 237-treated and WE-CAR-treated mice that reached endpoint was reduced compared with untreated and mock T treated groups (online supplemental figure S10A). To directly address if the tumor cells in ascites from 237 CAR-treated mice contained lower level of Tn-antigen, we cultured cells from untreated mice (n=4), short-term treated mice (n=2), and long-term treated, relapsed mice (n=6). Tumor cell lines from untreated and short-term treated mice all expressed the same surface level of Tn-antigen (WE+) as the parental ID8Cosmc-KO line (online supplemental figure S10B). In contrast, all six of the tumor lines derived from relapsed long-term survivors showed either lower levels of the antigen, or complete absence of detectable Tn-antigen. Immunohistochemistry of tissue sections from two long-term relapsed 237 CAR treated mice also demonstrated heterogeneity in Tn-expression in tumors (online supplemental figure S10C).
Discussion
The mouse ovarian cancer line ID8 has been used in many previous studies to examine the efficacy of various therapies. However, successful treatment of this tumor has been difficult, requiring early treatments (ie, often less than 2 weeks after tumor inoculation) to observe delayed tumor growth. In contrast, human ovarian cancer is rarely diagnosed at such early stages of disease development. Our most important finding is that late stage treatments with CAR-T cells, when solid tumors are detectable by gross and histologic analysis, yielded long-term control of the tumor. Our studies also contrast with studies using human tumors transplanted into immunodeficient mice, which often do not express the human target antigen on normal mouse tissues which would be needed to assess toxicity.
Successful treatment was accomplished with just a single dose of 237 or WE CAR-T cells, even when ID8Cosmc-KO tumors were disseminated throughout the peritoneal cavity. Gross visual and histochemical analyses revealed tumor nodules and masses in adipose tissues by day 40 with progression to more extensive tumor spread by the time of CAR-T cell treatments on day 50 or 60. Several days after treatment, extensive T-cell infiltration into tumors was identified, yet there was no sign of CAR-T cell-related injury to neighboring organ systems. Although edema was observed in pancreas sections of CAR-treated mice (online supplemental table S2), there was no evidence of toxicity in either tumor-bearing or non-tumor bearing mice in this syngeneic, immunocompetent system. The potency of the 237 CAR was associated with 237 CAR-driven immune cell infiltration which conferred an antitumor phenotype. This is consistent with a previous study showing that adoptively transferred T cells are capable of overcoming immune suppressive cells.33 It was also interesting that the ID8Cosmc-KO tumor grew rapidly in Rag1-KO mice, indicating that endogenous B and/or T cells are capable of controlling the tumor. Whether these cells are, or could be, recruited through the activity of 237 CAR-T cells remains to be examined.
Our results have relevance to the human disease, as CARs are in clinical development against several human ovarian cancer antigens, including folate receptor alpha (NCT03585764), mesothelin (NCT03916679, NCT03799913, NCT03814447, NCT05372692, NCT03054298, NCT05057715), B7-H3 (NCT05211557, NCT04670068), TAG72 (NCT05225363), MUC16 (NCT03907527), placental alkaline phosphatase, PLAP (NCT04627740), and P-MUC1 (NCT05239143).5 Preclinical studies against other targets, such as the Mullerian inhibiting substance type II receptor, have also shown promise.34 The optimal antigen target has yet to be determined, but development of multiple CARs against different targets provides opportunities to mitigate immune-evasive strategies employed by cancer cells such as antigen loss. In the present study, the potency of the Tn-dependent CARs ultimately resulted in reduced antigen expression in long-term survivors that eventually relapsed, but we have not yet elucidated the mechanism associated with the loss or reduction of Tn-antigen.
A trial using CAR T-cells expressing the humanized 5E5 scFv against Tn-MUC1 has been initiated (NCT04025216). In this regard, it is worth comparing the binding features of the 5E5 CAR and the 237, WE, and TNGK CARs. While all of these CARs are Tn-dependent, each has a different affinity and/or peptide fine-specificity. Surface plasmon resonance (SPR) has been used to determine the affinity of the parental antibodies and their antigens: 5E5 has an affinity (KD) of 2 nM for human Tn-MUC135 and 237 has an affinity (KD) of 140 nM for mouse Tn-OTS8.36 The WE scFv was derived from 237 using in vitro affinity maturation to yield an affinity increase of 30-fold, to an affinity of 4 nM for Tn-OTS8 (ie, similar to 5E5 for Tn-MUC1). The TNGK scFv was engineered to bind to Tn-MUC1.13 WE and TNGK CAR differ in their CDRs from 237 CAR by only two and four amino acids, respectively.
While affinities using SPR have not been determined for these scFv:Tn-glycopeptide pairs, based on antigen titrations the order of binding to the predominant mouse antigen in ID8Cosmc-KO, Tn-OTS8, is WE>237>TNGK>5E5 (online supplemental figure S3A). The order of binding to human Tn-MUC1 is 5E5>TNGK>237>WE. Although all these CARs exhibit a degree of cross-reactivity with different Tn-peptide backbones, we show in this report that affinities of 140 nM (237 for Tn-OTS8) or higher may be equally optimal for in vivo efficacy, at least in the context of antigen densities represented with ID8Cosmc-KO. The reduced in vivo efficacy of the TNGK CAR may be accounted for by its lower affinity than 237 and WE for the Tn-OTS8 antigen on ID8Cosmc-KO. An affinity threshold for the scFv fragments used in CARs has been previously described.37
Another observation of interest is that despite the ability of all four CARs to mediate in vitro release of IFN-γ, stimulation of other cytokines differed among the CARs. It was particularly notable that the most efficacious CARs in vivo showed higher IL-2 but lower IL-10 and IL-4. In contrast, 5E5 CAR-T cells secreted higher relative levels of IL-10 and IL4. This observation is consistent with the well-known immunosuppressive abilities of IL-10.38
Perhaps most decisive with regard to the mechanism underlying the lack of efficacy of the 5E5 CAR was our observation that it yielded a low level of tonic activity, unlike the other three CARs. A head-to-head comparison of 237-CAR and 5E5-CAR (both with CD28 signaling domain) showed that the 5E5 CAR used in this study exhibited basal tonic activity and proliferation, and about a twofold lower level of surface expression compared with other CARs (online supplemental figure S3B). Tonic, antigen-independent activity of CARs has been reported to be due to aggregation of scFv. The VH and VL domains of 5E5 are quite different from the VH and VL domains of the 237, WE, and TNGK CARs, which have identical framework regions. Importantly, tonic activity has been correlated with ineffective CARs, and one way to mitigate this tonic stimulation is through CAR engineering.39–41 It is worth noting that two previous studies by Posey et al and He et al 11 12 used 4-1BB instead of CD28 in their 5E5 CAR constructs, which could explain why these 5E5 CAR constructs did not yield tonic activity.42 The use of the TNGK-CD28 CAR for human translational studies, on the other hand, may be advantageous given its favorable properties of low tonic release and its recognition of human Tn-MUC1.
The question remains what surface level of Tn-protein antigen in human cancers is minimally sufficient to elicit CAR T-cell efficacy, as described here. The 5E5 CAR has been shown to mediate killing of, and IFN-γ stimulation by, a panel of diverse human cell lines (6 out of 10 cell lines).11 This activity was correlated with upregulation of the ST6GalNAc1 transferase, or in one case (Jurkat) the mutation of COSMC. Among the limited tumor cell lines that we have examined with 237 or its derivatives (including WE and TNGK) using transduced mouse T cells, a Cosmc deletion was necessary for Tn-dependent activity.10 12 13 The high density of Tn-targets found in Cosmc deletion mutants may correlate with the optimal in vitro cytokine stimulation and proliferative activity, and in vivo tumor infiltration of the 237 and WE CARs, as described here. Nevertheless, the frequency of human tumors that exhibit COSMC mutations may make treatment with Tn-dependent CARs a valuable option for some patients. Endometrial cancers are reported to have a 5% prevalence of COSMC-mutations, and other cancer types including ovarian cancer are in the range of 0.5–1%.12 13 While this may seem low, current efforts to target a specific driver mutation (eg, in p53 or KRAS), combined with the requirement for HLA-restriction brings these frequencies to the same range.
Our findings that intraperitoneal administration of the CAR-T cells was more effective than intravenous administration supports such an approach in clinical trials of CAR-T cells against ovarian cancer. Furthermore, the potency of a single CAR T-cell treatment at days 40 to 50 after tumor transplant indicates that post-surgical treatment, when there might be minimal but significant disease, could be a preferred time for optimal therapeutic benefit. This is also particularly relevant to a recent study in which ID8 was implanted into the ovaries43; despite surgical debulking at different times, all mice exhibited ID8 tumor development with pathology and dissemination much like the ID8Cosmc-KO model described here.
A notable exception for later treatment of the ID8 model involved the use of an ‘armored’ CAR that expressed a synthetic IL-12 gene.44 It remains to be seen if CAR-T cells engineered to secrete IL-12 or other cytokines have appropriate safety profiles, as IL-12 has shown serious toxicities clinically.45 Inducible systems have been designed to mitigate potential cytokine toxicity associated with these types of CARs.46 While the induction of some cytokines in the latter system46 delayed tumor growth, the exact cytokine balance that will drive optimal CAR-T cell efficacy in different solid tumors is unknown.
As with CAR or TCR-based cellular approaches and other immunotherapies, combination therapies that target other cancer antigens or pathways would be expected to reduce the chances of loss of any single tumor antigen. Combination with oncolytic viruses for induction of innate and natural killer cell responses47 48 and vaccines that elicit endogenous T-cell activity could be key combination therapies to sustain tumor control, without the emergence of CAR-antigen loss variants.49–52 There is some evidence that endogenous T cells can be recruited in the ID8 tumor model,50 53 but their existence in the ID8 CAR system described here remains to be seen. Nevertheless, our findings using a single dose of CAR T-cells are even more notable given that other strategies would likely provide greater benefit through synthetic engineering, recipient T cell selections, or combination treatments.1 2 54
Footnotes
Contributors: Conceptualization—DRER, DMK. Funding—HS, TMF, PJH, DMK. Data curation and formal analysis—DRER, DMK. Supervision—DRER, PJH, DMK. Validation—DRER, PS, CPS, ANL, EV, HS, KB, EJR, DMK. Investigation—DRER, PS, CPS, ANL, EV, VVVRM, MVH, WM, SPW, KS, HS, KB, EJR, DMK. Methodology—DRER, PS, EJR, DMK. Writing-original draft—DRER, DMK. Writing-review and editing—DRER, PS, CPS, ANL, EV, SPW, KS, HS, KB, TMF, PJH, DMK. Guarantor—DMK.
Funding: This work was supported by NIH grants CA238628 (to DMK), CA120439 (to PJH, TMF, and DMK), CA256746 (to PJH and TMF), and CA022677 (to HS), and the Harriet and Allan Wulfstat, and the Gerald O. Mann Foundation (to HS). We thank the Roy J. Carver Biotechnology Center Flow Cytometry Facility at the University of Illinois Urbana-Champaign (UIUC), Terri Li and Can Gong at the University of Chicago Human Tissue Resource Center for their assistance with immunohistochemistry of tissue sections, Division of Animal Resources at the UIUC (especially Jamie Reed and Raegan Carter), Dewi Nurmalasari and Kingsley Boateng at the Carl Woese Institute for Genomic Biology Core Facilities, Dylan Blaha, Veronika Wallace, and Ainhoa Arina for technical assistance, Henrik Clausen and Ulla Mandel for discussions and the anti-Tn monoclonal antibody 5F4.
Competing interests: DMK is a member of the scientific advisory board of Affini-T Therapeutics and a consultant for Tempus. PS, HS, KS, and DMK are co-inventors on a patent application related to these chimeric antigen receptors. No potential conflicts of interest were disclosed by the other authors.
Provenance and peer review: Not commissioned; externally peer reviewed.
Supplemental material: This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.
Data availability statement
All data relevant to the study are included in the article or uploaded as supplementary information.
Ethics statements
Patient consent for publication
Not applicable.
Ethics approval
Not applicable.
References
- 1. June CH, Riddell SR, Schumacher TN. Adoptive cellular therapy: a race to the finish line. Sci Transl Med 2015;7:280. 10.1126/scitranslmed.aaa3643 [DOI] [PubMed] [Google Scholar]
- 2. Finck AV, Blanchard T, Roselle CP, et al. Engineered cellular Immunotherapies in cancer and beyond. Nat Med 2022;28:678–89. 10.1038/s41591-022-01765-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Anderson KG, Stromnes IM, Greenberg PD. Obstacles posed by the tumor Microenvironment to T cell activity: A case for synergistic therapies. Cancer Cell 2017;31:311–25. 10.1016/j.ccell.2017.02.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Philip M, Schietinger A. Cd8+ T cell differentiation and dysfunction in cancer. Nat Rev Immunol 2022;22:209–23. 10.1038/s41577-021-00574-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Rodriguez-Garcia A, Minutolo NG, Robinson JM, et al. T-cell target antigens across major gynecologic cancers. Gynecol Oncol 2017;145:426–35. 10.1016/j.ygyno.2017.03.510 [DOI] [PubMed] [Google Scholar]
- 6. Bast RC, Matulonis UA, Sood AK, et al. Critical questions in ovarian cancer research and treatment: Report of an American Association for cancer research special conference. Cancer 2019;125:1963–72. 10.1002/cncr.32004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Textor A, Listopad JJ, Wührmann LL, et al. Efficacy of CAR T-cell therapy in large tumors relies upon Stromal targeting by Ifngamma. Cancer Res 2014;74:6796–805. 10.1158/0008-5472.CAN-14-0079 [DOI] [PubMed] [Google Scholar]
- 8. Textor A, Grunewald L, Anders K, et al. Cd28 Co-stimulus achieves superior CAR T cell effector function against solid tumors than 4-1BB Co-stimulus. Cancers n.d.;13:1050. 10.3390/cancers13051050 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Schietinger A, Philip M, Yoshida BA, et al. A mutant chaperone converts a wild-type protein into a tumor-specific antigen. Science 2006;314:304–8. 10.1126/science.1129200 [DOI] [PubMed] [Google Scholar]
- 10. Stone JD, Aggen DH, Schietinger A, et al. A sensitivity scale for targeting T cells with Chimeric antigen receptors (CAR) and Bispecific T cell Engagers (bite). Oncoimmunology 2012;1:863–73. 10.4161/onci.20592 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Posey AD, Schwab RD, Boesteanu AC, et al. Engineered CAR T cells targeting the cancer-associated Tn-Glycoform of the membrane Mucin Muc1 control adenocarcinoma. Immunity 2016;44:1444–54. 10.1016/j.immuni.2016.05.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. He Y, Schreiber K, Wolf SP, et al. Multiple cancer-specific antigens are targeted by a Chimeric antibody receptor on a single cancer cell. JCI Insight 2019;4:21.:e135306. 10.1172/jci.insight.135306 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Sharma P, Marada VVVR, Cai Q, et al. Structure-Guided engineering of the affinity and specificity of cars against Tn-glycopeptides. Proc Natl Acad Sci U S A 2020;117:15148–59. 10.1073/pnas.1920662117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Khodarev NN, Pitroda SP, Beckett MA, et al. Muc1-induced transcriptional programs associated with tumorigenesis predict outcome in breast and lung cancer. Cancer Res 2009;69:2833–7. 10.1158/0008-5472.CAN-08-4513 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Kufe DW. Mucins in cancer: Function, prognosis and therapy. Nat Rev Cancer 2009;9:874–85. 10.1038/nrc2761 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Kufe DW. Muc1-C Oncoprotein as a target in breast cancer: Activation of signaling pathways and therapeutic approaches. Oncogene 2013;32:1073–81. 10.1038/onc.2012.158 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Van Elssen CHMJ, Frings PWH, Bot FJ, et al. Expression of Aberrantly Glycosylated Mucin-1 in ovarian cancer. Histopathology 2010;57:597–606. 10.1111/j.1365-2559.2010.03667.x [DOI] [PubMed] [Google Scholar]
- 18. Lohmueller JJ, Sato S, Popova L, et al. Antibodies elicited by the first non-viral prophylactic cancer vaccine show tumor-specificity and Immunotherapeutic potential. Sci Rep 2016;6:31740. 10.1038/srep31740 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Thie H, Toleikis L, Li J, et al. Rise and fall of an anti-Muc1 specific antibody. PLoS One 2011;6:e15921. 10.1371/journal.pone.0015921 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Sadelain M. Tales of antigen evasion from CAR therapy. Cancer Immunol Res 2016;4:473. 10.1158/2326-6066.CIR-16-0089 [DOI] [PubMed] [Google Scholar]
- 21. Sørensen AL, Reis CA, Tarp MA, et al. Chemoenzymatically synthesized Multimeric Tn/Stn Muc1 Glycopeptides elicit cancer-specific anti-Muc1 antibody responses and override tolerance. Glycobiology 2006;16:96–107. 10.1093/glycob/cwj044 [DOI] [PubMed] [Google Scholar]
- 22. Sun X, Ju T, Cummings RD. Differential expression of Cosmc, T-synthase and mucins in Tn-positive colorectal cancers. BMC Cancer 2018;18. 10.1186/s12885-018-4708-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Coelho R, Marcos-Silva L, Mendes N, et al. Mucins and TRUNCATED O-Glycans unveil Phenotypic discrepancies between Serous ovarian cancer cell lines and primary tumours. Int J Mol Sci 2018;19:2045. 10.3390/ijms19072045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Roby KF, Taylor CC, Sweetwood JP, et al. Development of a Syngeneic mouse model for events related to ovarian cancer. Carcinogenesis 2000;21:585–91. 10.1093/carcin/21.4.585 [DOI] [PubMed] [Google Scholar]
- 25. Magnotti E, Marasco WA. The latest animal models of ovarian cancer for novel drug discovery. Expert Opin Drug Discov 2018;13:249–57. 10.1080/17460441.2018.1426567 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. McMullen M, Karakasis K, Rottapel R, et al. Advances in ovarian cancer, from biology to treatment. Nat Cancer 2021;2:6–8. 10.1038/s43018-020-00166-5 [DOI] [PubMed] [Google Scholar]
- 27. Wolf SP, Wen FT, Schreiber H. Criteria to make animal studies more relevant to treating human cancer. Curr Opin Immunol 2022;74:25–31. 10.1016/j.coi.2021.09.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Rømer TB, Aasted MKM, Dabelsteen S, et al. Mapping of TRUNCATED O-Glycans in cancers of epithelial and non-epithelial origin. Br J Cancer 2021;125:1239–50. 10.1038/s41416-021-01530-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Vichai V, Kirtikara K. Sulforhodamine B Colorimetric assay for cytotoxicity screening. Nat Protoc 2006;1:1112–6. 10.1038/nprot.2006.179 [DOI] [PubMed] [Google Scholar]
- 30. Grzelak CA, Goddard ET, Lederer EE, et al. Elimination of fluorescent protein Immunogenicity permits modeling of metastasis in immune-competent settings. Cancer Cell 2022;40:1–2. 10.1016/j.ccell.2021.11.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Kaiser J. Glowing tumor marker hampers mouse cancer studies. Science 2022;375:6583. 10.1126/science.ada1554 [DOI] [PubMed] [Google Scholar]
- 32. Melenhorst JJ, Chen GM, Wang M, et al. Decade-Long leukaemia remissions with persistence of CD4+ CAR T cells. Nature 2022;602:503–9. 10.1038/s41586-021-04390-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Arina A, Schreiber K, Binder DC, et al. Adoptively transferred immune T cells eradicate established tumors despite cancer-induced immune suppression. J Immunol 2014;192:1286–93. 10.4049/jimmunol.1202498 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Rodriguez-Garcia A, Sharma P, Poussin M, et al. CAR T cells targeting MISIIR for the treatment of ovarian cancer and other gynecologic malignancies. Mol Ther 2020;28:548–60. 10.1016/j.ymthe.2019.11.028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Lavrsen K, Madsen CB, Rasch MG, et al. Aberrantly Glycosylated Muc1 is expressed on the surface of breast cancer cells and a target for antibody-dependent cell-mediated cytotoxicity. Glycoconj J 2013;30:227–36. 10.1007/s10719-012-9437-7 [DOI] [PubMed] [Google Scholar]
- 36. Brooks CL, Schietinger A, Borisova SN, et al. Antibody recognition of a unique tumor-specific Glycopeptide antigen. Proc Natl Acad Sci U S A 2010;107:10056–61. 10.1073/pnas.0915176107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Chmielewski M, Hombach A, Heuser C, et al. T cell activation by antibody-like Immunoreceptors: Increase in affinity of the single-chain fragment domain above threshold does not increase T cell activation against antigen-positive target cells but decreases selectivity. J Immunol 2004;173:7647–53. 10.4049/jimmunol.173.12.7647 [DOI] [PubMed] [Google Scholar]
- 38. Neumann C, Scheffold A, Rutz S. Functions and regulation of T cell-derived Interleukin-10. Semin Immunol 2019;44:101344. 10.1016/j.smim.2019.101344 [DOI] [PubMed] [Google Scholar]
- 39. Labanieh L, Mackall CL. CAR immune cells: Design principles, resistance and the next generation. Nature 2023;614:635–48. 10.1038/s41586-023-05707-3 [DOI] [PubMed] [Google Scholar]
- 40. Salter AI, Rajan A, Kennedy JJ, et al. Comparative analysis of TCR and CAR signaling informs CAR designs with superior antigen sensitivity and in vivo function. Sci Signal 2021;14:eabe2606. 10.1126/scisignal.abe2606 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Kouro T, Himuro H, Sasada T. Exhaustion of CAR T cells: Potential causes and solutions. J Transl Med 2022;20:239. 10.1186/s12967-022-03442-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Long AH, Haso WM, Shern JF, et al. 4-1Bb Costimulation ameliorates T cell exhaustion induced by tonic signaling of Chimeric antigen receptors. Nat Med 2015;21:581–90. 10.1038/nm.3838 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Morse CB, Voillet V, Bates BM, et al. Development of a clinically relevant ovarian cancer model incorporating surgical Cytoreduction to evaluate treatment of micro-metastatic disease. Gynecol Oncol 2021;160:427–37. 10.1016/j.ygyno.2020.11.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Yeku OO, Purdon TJ, Koneru M, et al. Armored CAR T cells enhance antitumor efficacy and overcome the tumor Microenvironment. Sci Rep 2017;7:10541. 10.1038/s41598-017-10940-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Zhang L, Morgan RA, Beane JD, et al. Tumor-Infiltrating lymphocytes genetically engineered with an inducible gene encoding interleukin-12 for the immunotherapy of metastatic melanoma. Clin Cancer Res 2015;21:2278–88. 10.1158/1078-0432.CCR-14-2085 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Lin RJ, Nager AR, Park S, et al. Design and validation of inducible Turbocarstm with Tunable induction and Combinatorial cytokine signaling. Cancer Immunol Res 2022;10:1069–83. 10.1158/2326-6066.CIR-21-0253 [DOI] [PubMed] [Google Scholar]
- 47. van Vloten JP, Matuszewska K, Minow MAA, et al. Oncolytic Orf virus licenses NK cells via Cdc1 to activate innate and adaptive antitumor mechanisms and extends survival in a murine model of late-stage ovarian cancer. J Immunother Cancer 2022;10:e004335. 10.1136/jitc-2021-004335 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Nounamo B, Liem J, Cannon M, et al. Myxoma virus Optimizes cisplatin for the treatment of ovarian cancer in vitro and in a Syngeneic murine dissemination model. Mol Ther Oncolytics 2017;6:90–9. 10.1016/j.omto.2017.08.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Odunsi K, Qian F, Matsuzaki J, et al. Vaccination with an NY-ESO-1 peptide of HLA class I/II Specificities induces integrated humoral and T cell responses in ovarian cancer. Proc Natl Acad Sci U S A 2007;104:12837–42. 10.1073/pnas.0703342104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Tanyi JL, Chiang CL-L, Chiffelle J. Personalized cancer vaccine strategy elicits polyfunctional T cells and demonstrates clinical benefits in ovarian cancer. NPJ Vaccines 2021;6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Chiang CL-L, Rovelli R, Sarivalasis A, et al. Integrating cancer vaccines in the standard-of-care of ovarian cancer: Translating Preclinical models to human. Cancers (Basel) 2021;13:4553. 10.3390/cancers13184553 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Block MS, Dietz AB, Gustafson MP, et al. Th17-inducing Autologous Dendritic cell vaccination promotes antigen-specific cellular and humoral immunity in ovarian cancer patients. Nat Commun 2020;11:5173. 10.1038/s41467-020-18962-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Spear P, Barber A, Sentman CL. Collaboration of Chimeric antigen receptor (CAR)-Expressing T cells and host T cells for optimal elimination of established ovarian tumors. Oncoimmunology 2013;2:e23564. 10.4161/onci.23564 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Anderson KG, Oda SK, Bates BM, et al. Engineering adoptive T cell therapy to Co-opt Fas ligand-mediated death signaling in ovarian cancer enhances therapeutic efficacy. J Immunother Cancer 2022;10:e003959. 10.1136/jitc-2021-003959 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
jitc-2022-006509supp001.pdf (16.1MB, pdf)
Data Availability Statement
All data relevant to the study are included in the article or uploaded as supplementary information.