Abstract
Objectives
We sought to validate, or refute, the common belief that bedtime diuretics are poorly tolerated due to nocturia.
Design
Prespecified prospective cohort analysis embedded within the randomised BedMed trial, in which hypertensive participants are randomised to morning versus bedtime antihypertensive administration.
Setting
352 community family practices across 4 Canadian provinces between March 2017 and September 2020.
Participants
552 hypertensive patients (65.6 years old, 57.4% female) already established on a single once-daily morning antihypertensive and randomised to switch that antihypertensive to bedtime. Of these, 203 used diuretics (27.1% thiazide alone, 70.0% thiazide/non-diuretic combinations) and 349 used non-diuretics.
Intervention
Switching the established antihypertensive from morning to bedtime, and comparing the experience of diuretic and non-diuretic users.
Primary and secondary outcome measures
Primary outcome: Adherence to bedtime allocation time at 6 months (defined as the willingness to continue with bedtime use, not an assessment of missed doses). Secondary 6-month outcomes: (1) nocturia considered to be a major burden and (2) increase in overnight urinations/week. All outcomes were self-reported and additionally collected at 6 weeks.
Results
At 6 months: Adherence to bedtime allocation time was lower in diuretic users than non-diuretic users (77.3% vs 89.8%; difference 12.6%; 95% CI 5.8% to 19.8%; p<0.0001; NNH 8.0), and more diuretic users considered nocturia a major burden (15.6% vs 1.3%; difference 14.2%; 95% CI 8.9% to 20.6%; p<0.0001; NNH 7.0). Compared with baseline, diuretic users experienced 1.0 more overnight urinations/week (95% CI 0.0 to 1.75; p=0.01). Results did not differ between sexes.
Conclusions
Switching diuretics to bedtime did promote nocturia, but only 15.6% found nocturia a major burden. At 6 months, 77.3% of diuretic users were adherent to bedtime dosing. Bedtime diuretic use is viable for many hypertensive patients, should it ever become clinically indicated.
Trial registration number
Keywords: Hypertension, CLINICAL PHARMACOLOGY, PRIMARY CARE, Cardiology
STRENGTHS AND LIMITATIONS OF THIS STUDY.
Our study question arises directly from members of the public who participated in the design of the BedMed trial.
Intervention and comparison groups were randomly selected from the same clinical trial population.
Our data represent the first prospective evaluation of the tolerability of bedtime diuretics.
Those who previously tried and failed morning diuretics due to nocturia would be absent from the diuretic cohort, which could bias towards better bedtime diuretic tolerance.
Introduction
Although consensus is lacking,1–3 two randomised trials by the same principal investigator suggest large reductions in major adverse cardiovascular events occur if blood pressure (BP) medications are taken at bedtime, as compared with conventional morning use.4 5 This finding, however, may be difficult to implement for those using diuretics—common first-line therapeutics, with a unique and important role in volume control and natriuresis.6 7 This is because diuretics are widely believed to promote nocturia, and typically recommended for morning use only as a result.8 9
Nocturia occurs in roughly two-thirds of men and women over the age of 70 years10 and is believed to disrupt sleep, impair quality of life and increase the risk of night-time falls and fractures.11 12 However, there are no randomised trials examining diuretic timing and adverse effects. The concern that bedtime diuretics could produce troublesome nocturia, being based on opinion and observational data, could be incorrect. Morning diuretics (typically thiazides) are generally well tolerated, and cross-sectional analysis of diuretic-using populations, without accounting for administration time, does not support a strong association between diuretic use and nocturia.13 14 Whether clinicians can recommend diuretics for bedtime use is, therefore, unclear.
To determine how well diuretics are tolerated at bedtime we conducted a prespecified prospective cohort study embedded within the ongoing BedMed trial. BedMed randomises Canadian primary care patients with hypertension to take their existing antihypertensive medications either in the morning, or at bedtime, and examines mortality and morbidity outcomes.15 Recruitment started in March 2017 and the trial is ongoing, with follow-up continuing until late 2023. This paper examines those participants with a single morning antihypertensive at baseline who were randomised to switch that antihypertensive to bedtime. Our goal was to compare adherence with bedtime allocation, and self-reported nocturia burden, between those switching a diuretic to bedtime and those switching other types of BP lowering medication to bedtime. Note, our definition of adherence to allocation time differs from the conventional notion. When we refer to adherence to bedtime allocation, we are talking about the participant’s intention to use their antihypertensive at bedtime. This study is not evaluating the extent to which individual doses are missed. As such, we did not compare bedtime diuretic use to morning diuretic use because morning medication use was already well established for all participants. As we have defined it, we would expect virtually everyone allocated to morning antihypertensives to be adherent to their administration time, as a morning allocation meant no change of any kind was needed.
Methods
Study design and sample size
BedMed is an ongoing prospective, randomised, open, blinded-endpoint16 trial. Recruitment is registry-like, with participating family physicians using their usual-care electronic medical records to identify their eligible patients, and then mailing those patients information about the study. Interested patients call the study team and, if eligible and consenting, are randomised to take all their regular BP medication (as tolerated) either in the morning, or at bedtime. Participants received their allocation, using the REDCap17 server’s central randomisation module, directly from a research assistant with no prior clinical interactions, achieving irreversible, independent and concealed allocation.
The prospective cohort study reported in this manuscript is a prespecified interim analysis of BedMed data, carried out as part of an adaptive trial design. The analysis was triggered on the allocation to bedtime dosing of 203 participants whose only baseline antihypertensive included a morning diuretic (whether a diuretic only, or a diuretic/non-diuretic combination pill). If adherence with bedtime diuretic use had been poor, the BedMed trial’s inclusion criteria would have been altered to exclude future such individuals from enrolling. This sample size gave a 90% chance of detecting a 20% relative reduction in adherence to bedtime allocation if (1) morning adherence was 75% and (2) there were an equal number of participants switching a non-diuretic antihypertensive to bedtime with whom to compare.
Setting and participants
In Canada’s publicly funded healthcare system, residents are not billed directly for physician services, but medication costs are either paid for privately, or partially or completely covered by either employer-sponsored health insurance, or government subsidised programmes (including coverage for seniors). The vast majority of Canadians have family physicians, who are normally the sole prescriber of their patient’s hypertension medications.
BedMed recruitment began in March 2017, with the final participant included in this analysis enrolling in September 2020. Over this period, participants were being recruited by 352 family physicians (typical practice panel ~1500 patients, with 20% hypertension prevalence among adults) in the Canadian provinces of Alberta, Manitoba, British Columbia and Saskatchewan. Some BedMed participants (22% of those randomised) also learnt about the study through social media, or other sources, and were enrolled with their family physician’s consent, but without their family physician actively recruiting them. To be eligible for BedMed, participants needed to be community dwelling (including assisted living) and to have a physician diagnosis of hypertension for which they used one or more BP-lowering medications. BedMed excluded anyone with a personal history of glaucoma because of an association between nocturnal hypotension and ischaemic optic neuropathy in such individuals.18–21 For this substudy, we intentionally kept our eligibility criteria as broad as possible (including participants with potentially nocturia-modifying conditions such as diabetes, sleep apnoea and congestive heart failure (CHF)) so as to most closely resemble, and be generalisable to, a hypertensive primary care population.
For this substudy, the following inclusion and exclusion criteria defined the study cohort.
Inclusion criteria
Physician diagnosis of hypertension.
Only one antihypertensive pill in use at baseline (combination antihypertensive pills permitted).
That single baseline antihypertensive pill was used in the morning at baseline, and only once a day.
The participant was randomised to switch that morning antihypertensive pill to bedtime.
Exclusion criteria
Participant did not attempt a medication timing change*.
-
Physician changed the type of antihypertensive prior to the timing change*.
*We made both these exclusions since, for the diuretic group, including patients who were not actually attempting to switch a diuretic to bedtime would have lessened any potential nocturia, and biased the groups towards looking more similar. When looking at adverse effects of an intervention, such a ‘modified intention-to-treat’ analysis is the more conservative analytic option, given a full intention-to-treat analysis, for the reason described above, is more likely to underestimate nocturia-related problems.
Procedures
Assistance changing antihypertensive medication to bedtime
Participants had their choice of being assisted in making their timing change by their family physician, who applied their own judgement as to how to make the change or, if they described no heart disease, by the research assistant with whom they were dialoging. Exceptions included those whose BP-lowering medication was Tiazac XC or Diltiazem XC (which have delayed-release kinetics), and furosemide, isosorbide mononitrate/dinitrate, or alpha blockers (medications whose timing decision may be more complicated). Such participants had their family physician guide their timing change. Advice from research assistants was to delay the next morning dose until bedtime, and to continue all future doses at bedtime. If bedtime use proved problematic, and there were concern participants would switch back to morning, switching to dinnertime was suggested. As a memory aid, participants were advised to place pill bottles near objects they use when getting ready for bed (eg, toothbrush, denture case, alarm clock), or to use an AM/PM dosette. If medication type, dosage or timing needed to be changed, for any reason, those decisions were at the sole discretion of the prescribing physician.
Follow-up interviews
Baseline characteristics were collected directly from participants through telephone interview prior to randomisation. The first follow-up with a research assistant occurred by telephone 7 days post-timing change to encourage adhering to the timing change, and to troubleshoot participant concerns. Another telephone follow-up took place at 6 weeks to obtain self-reported adherence to bedtime antihypertensive use (‘Are you taking your blood pressure medication at bedtime?’; and if ‘no’—the reason for not doing so), and to assess nocturia. Participants could report nocturia as ‘no’, ‘minor’ or ‘major’ burden (subjective overall assessment, no itemised criteria), and they were asked to quantify the number of overnight urinations per week by estimating the number of nights they rose to urinate, and the number of times per night they urinated on those evenings. The same follow-up questions were asked again at 6 months, either by telephone or by email questionnaire (participant’s choice), and again every 6 months thereafter.
Note: this study was not designed to explore whether or not individual medication doses were missed, something which could be better assessed with electronic devices, or pill counting. We were instead assessing each participant’s willingness to persist with bedtime antihypertensive use. As such, self-report more accurately reflects the patient feedback prescribers could expect, were they to recommend diuretics be administered at bedtime.
Administrative health claims data
Comorbidities were also collected as baseline characteristics (coronary artery disease, diabetes, sleep apnoea, chronic kidney disease, chronic obstructive pulmonary disease, CHF and stroke). For non-Alberta residents, these comorbidities were self-reported. For Alberta residents, the vast majority of participants (83%), these comorbidities were derived from physician diagnoses submitted to Alberta Health in the normal course of care, specifically, by extracting comorbidities from linked governmental databases recording community physician billings and hospital separations. Access and analysis of this administrative data was performed by Alberta Health Services, the governmental data steward. These data, and this linking process, have been widely used in other studies and have been identified as valid.22–25 Most comorbidities were considered present if there were two community visits with that diagnosis, or one hospital diagnosis, from any physician. However, only one such diagnosis was required for stroke (since the diagnosis might not repeat outside of the acute event), and for chronic kidney disease (which, in our experience, is infrequently recorded by primary care providers).
Outcomes (as self-reported by participants)
Primary
Adherence to bedtime allocation at 6 months (non-adherence=changing back to morning, stopping altogether or switching antihypertensives).
Secondary
Adherence to bedtime allocation at 6 weeks.
Nocturia considered to be a ‘major burden’ at 6 weeks, and at 6 months (includes those who report a major burden, and those who failed the timing change because of nocturia).
Number of overnight urinations per week at 6 weeks and at 6 months.
Statistical analysis
Our inclusion and exclusion criteria created one prospective cohort with two exposures: (1) an established morning diuretic medication being switched to bedtime and (2) an established morning non-diuretic medication being switched to bedtime. Participants using a combination pill with two or more antihypertensive components were considered diuretic users if at least one of those components was a diuretic. The analysis was by modified intention to treat and consisted of descriptive statistics, comparing proportions using Fisher’s exact test (primary outcome analysis), comparing the number of overnight urinations/week using Mann-Whitney, and using Hodges-Lehmann estimation for difference in medians. All analyses used GraphPad Prism V.9.1.2.
Modified intention-to-treat assumptions
Missing data: Missing variables were imputed using the value from the subsequent follow-up interview. For example, if 6-week data were missing, adherence and nocturia burden were assigned the 6-month value. If no subsequent data were available for imputation (ie, lost to follow-up or study drop-out) participants were excluded from analysis. We did not impute missing values for lost or dropped out participants because we were looking to demonstrate potential harm (harm constituting a difference in non-adherence or major nocturia burden) and imputing missing values, being reasonably balanced between groups, would have biased the groups towards looking more similar. Baseline characteristics of those excluded from the primary analysis were compared to assess whether analysis exclusion appeared random.
Medication changes: If nocturia resulted in participants switching medications, or medication timing, we considered them non-adherent and to have a ‘major nocturia burden’, even if a lesser degree of nocturia burden was reported at their follow-up interview. Data from these non-adherent individuals were not used for assessment of nocturia frequency. If medication or timing changes were made for reasons other than nocturia, participants were excluded from analysis. We made this exclusion because including such individuals would have biased the groups towards appearing more similar, and could have led us to underestimate the nocturia burden in diuretic users. If physicians changed the participant’s medication to twice daily (with the second dose at bedtime or dinnertime) we considered them to still experience the effects of a bedtime dose, and included them in the analysis.
Patient and public involvement
Patient working group
BedMed has a 10-member patient working group which began meeting in 2016 prior to the recruitment of any participants. Working group members have participated in (1) the construction of all participant facing materials, (2) the wording of research assistant follow-up scripts, (3) decisions as to what data to collect and (4) the hiring of research assistants.
Patient-driven question
The draft BedMed protocol was presented to a group of ~25 seniors in 2015, prior to grant application and study registration. The question pursued in this manuscript derived directly from this group’s feedback, where concern was expressed that bedtime diuretics would be poorly tolerated due to nocturia.
Results
Deidentified patient-level outcome data are available for download on the Pragmatic Trials Collaborative’s website (www.PragmaticTrials.ca).26 Of 579 eligible participants, 552 (95.3%) had analysable data at 6 weeks, and 533 (92.1%) had analysable data at 6 months. This included, for our 6-month adherence primary outcome, 198/210 (94.3%) of the eligible diuretic users and 335/369 (90.8%) of the eligible non-diuretic users. Individual reasons for exclusion are shown in figure 1. A comparison of baseline characteristics (online supplemental table 1) shows no notable differences between those excluded from the primary outcome analysis, and those analysed. At 6 months, of those considered compliant with allocation in the diuretic group, 147/153 (96.1%) took their medication at bedtime, 5/153 (3.3%) took it at dinner and 1/153 (0.7%) had their diuretic split into twice daily dosing. This compares to the non-diuretic group, of whom 282/301 (93.7%) took their medication at bedtime, 12/301 (4.0%) took it at dinner and 7/301 (2.3%) had been split into twice daily dosing.
Figure 1.
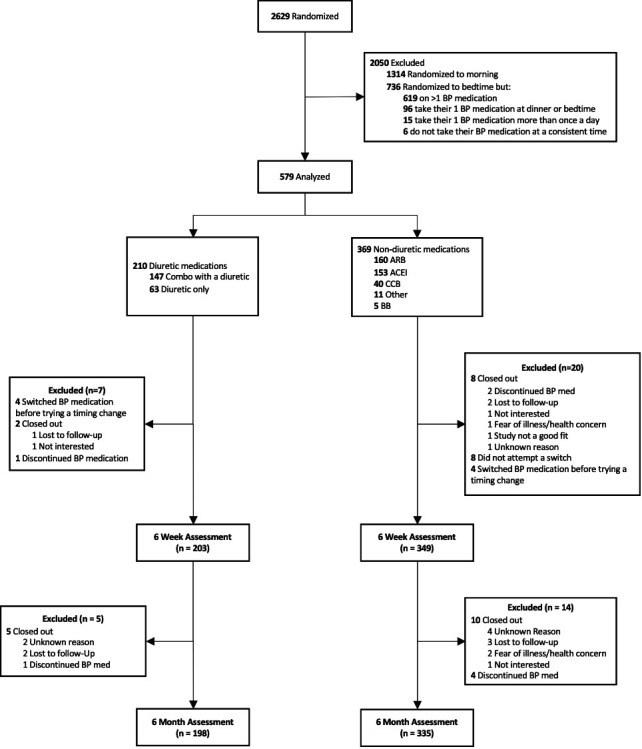
Study flow diagram for analysis of adherence. ACEI, ACE inhibitor; BB, beta-blockers; BP, blood pressure; CCB, calcium channel blocker.
bmjopen-2022-068188supp001.pdf (1.4MB, pdf)
Baseline
Overall, most participants were from Alberta (83.0%), 94.7% identified as white and they were a mean 65.6 (SD 10.0) years of age. Baseline characteristics were comparable between groups (table 1) although slightly more diuretic users were female (65.5% vs 52.7%). They were largely non-smokers (92.4%), exercised a median 3 (IQR 0–5) days/week and had a median body mass index of 28.3 (IQR 25.5–32.3). The most common comorbidities were coronary artery disease (19.2%), sleep apnoea (18.3%) and diabetes (17.2%). The cohort-defining medications used are broken down in figure 2. Of the diuretic users, 142 (70.0%) used a thiazide containing combination pill, and 42 (20.7%) used hydrochlorothiazide alone. Although BedMed does not collect information on drug dosage, it would be unusual for Canadians to be prescribed hydrochlorothiazide outside a range of 12.5–25 mg/day. Of the non-diuretic cohort, 150 (43.0%) used angiotensin receptor blockers, 148 (42.4%) used ACE inhibitors, 38 (10.9%) used calcium channel blockers, 4 (1.1%) used beta-blockers and 9 (2.6%) used combination pills that did not include diuretics. Baseline nocturia was similar between diuretic versus non-diuretic users, in terms of the number of overnight urinations per week (median 5.5 vs 6.0), and the percentage of participants perceiving nocturia to be a major burden (1.5% vs 2.3%). However, slightly more diuretic users felt nocturia was a minor burden (30.5% vs 23.5%). Overall, three-fourths of participants did experience nocturia at least once per week. Of these, 62.8% considered it ‘not a problem’, 34.5% considered it ‘a minor burden’ and 2.6% considered it ‘a major burden’.
Table 1.
Baseline characteristics
| Characteristics | Diuretic (n=203) N (%) | Non-diuretic (n=349) N (%) | P value |
| Sex, female | 133 (65.5) | 184 (52.7) | 0.004 |
| Age, mean (SD), years | 65.4 (8.9) | 65.6 (10.6) | 0.81 |
| Province | |||
| Alberta | 169 (83.3) | 289 (82.8) | 0.99 |
| British Columbia | 14 (6.9) | 37 (10.6) | 0.17 |
| Manitoba | 16 (7.9) | 19 (5.4) | 0.23 |
| Saskatchewan | 4 (2.0) | 4 (1.1) | 0.47 |
| Ethnicity | |||
| White | 195 (96.1) | 328 (94.0) | 0.33 |
| South east asian | 2 (1.0) | 10 (2.9) | 0.23 |
| Asian | 0 | 3 (0.8) | 0.30 |
| First nation | 0 | 6 (1.7) | 0.09 |
| Black | 0 | 0 | 0.99 |
| Other | 5 (2.5) | 2 (0.6) | 0.11 |
| Decline to answer | 1 (0.5) | 0 | 0.37 |
| Comorbidities* | |||
| Coronary artery disease | 39 (19.2) | 67 (19.2) | 0.99 |
| Diabetes | 31 (15.3) | 64 (18.3) | 0.41 |
| Sleep apnoea | 38 (18.7) | 63 (18.1) | 0.91 |
| Chronic kidney disease | 14 (6.9) | 39 (11.2) | 0.13 |
| COPD | 16 (7.9) | 35 (10.0) | 0.45 |
| Stroke | 11 (5.4) | 18 (5.2) | 0.99 |
| Heart failure | 4 (2.0) | 6 (1.7) | 0.99 |
| Cigarette smoker (current) | 14 (6.9) | 28 (8.0) | 0.74 |
| Physical exercise, median (IQR), days per week† | 3 (1–5) | 3 (0–5) | 0.14 |
| BMI, median (IQR), kg/m2 | 28.9 (26–33) | 28.0 (25–32) | 0.15 |
| Underweight (<18.5) | 1 (0.5) | 2 (0.6) | 0.99 |
| Normal weight (18.5–24.9) | 35 (17.2) | 74 (21.2) | 0.27 |
| Overweight (25–29.9) | 84 (41.4) | 143 (41.0) | 0.93 |
| Obese (≥30) | 83 (40.9) | 130 (37.2) | 0.42 |
| Nocturia, median (IQR), nocturnal urinations/wk | 5.5 (1–10.5) | 6.0 (1–10.5) | 0.91 |
| Does not experience nocturia | 49 (24.1) | 86 (24.6) | 0.92 |
| Nocturia occurs but ‘not a problem’ | 89 (43.8) | 173 (49.6) | 0.22 |
| Nocturia ‘a minor burden’ | 62 (30.5) | 82 (23.5) | 0.07 |
| Nocturia ‘a major burden’ | 3 (1.5) | 8 (2.3) | 0.75 |
*Derived from Alberta provincial health claims data for 454 participants, and self-reported for 98.
†'How many days in the past week have you exercised for 30 min or more, vigorously enough to raise your breathing rate?’.
BMI, body mass index; COPD, chronic obstructive pulmonary disease.
Figure 2.
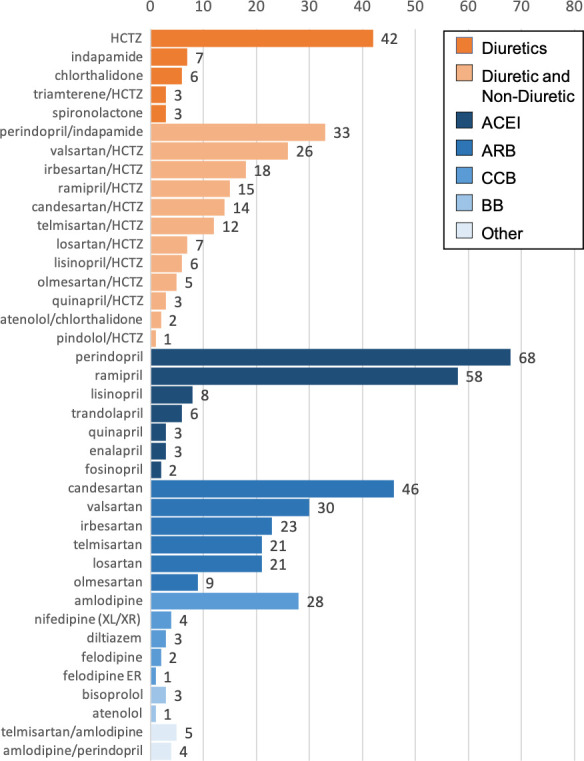
Medication frequency at baseline. ACEI, angiotensin converting enzyme inhibitor; ARB, angiotensin receptor blocker; CCB, calcium channel blocker; BB, beta blocker; HCTZ, hydrochlorothiazide; ER/XL/XR, extended release.
Six weeks
Adherence with bedtime medication use was lower in diuretic users (88.7% vs 94.6%), but still high in both cohorts (difference 5.8%; 95% CI 1.0% to 11.6%; p=0.02; NNH 17.0) (table 2). Change in the number of overnight urinations per week could be calculated for 180 diuretic users, and 330 non-diuretic users (online supplemental figure 1). Compared with baseline, there was a median 1.0 more overnight urinations per week in diuretic users (95% CI 0.0 to 1.5; p<0.0001), and 9.9% more diuretic users perceived nocturia to be a major burden as compared with those who did not use diuretics (11.3% vs 1.4%; 95% CI Diff, 5.5% to 15.4%; p<0.0001; NNH 10.1) (table 3).
Table 2.
Non-adherence to bedtime allocation and major nocturia burden, no (%)
| Diuretic | Non-diuretic | Attributable risk* (%) (95% CI) | P value | |
| 6 weeks | ||||
| Major burden† | 23/198 (11.6) | 5/330 (1.5) | 10.1 (5.6 to 15.7) | <0.0001 |
| Non-adherence‡ | 23/203 (11.3) | 19/349 (5.4) | 5.9 (1.0 to 11.6) | 0.02 |
| 6 months | ||||
| Major burden† | 28/180 (15.6) | 4/301 (1.3) | 14.2 (8.9 to 20.6) | <0.0001 |
| Non-adherence‡ | 45/198 (22.7) | 34/335 (10.2) | 12.6 (5.8 to 19.8) | <0.0001 |
*Excess risk of the outcome (noncompliance or major burden) for those in the diuretic group, compared with the non-diuretic group.
†Includes those reporting major burden while using a bedtime diuretic, and those non-adherent due to nocturia. Data are number (%).
‡Non-adherent for any reason. Data are number (%).
Table 3.
Change in number of overnight urinations per week, median (IQR)
| No of overnight urinations per Week | Median change from baseline | Between group difference median (95% CI) |
P value | |||
| Diuretic | Non-diuretic | Diuretic | Non-diuretic | |||
| Baseline | 5.5 (1.0 to 10.5) | 6.0 (1.0 to 10.5) | – | – | 0.0*† (0.0 to 0.0) | 0.92 |
| 6 weeks | 7.0 (2.6 to 11.6) | 7.0 (1.5 to 10.5) | 0.0 (0.0 to 3.5) | 0.0 (−1.0 to 1.0) | 1.0*‡ (0.0 to 1.5) | <0.0001 |
| 6 months | 6.2 (0.0 to 10.5) | 5.0 (0.0 to 10.5) | 0.5 (−1.5 to 4.0) | 0.0 (−2.0 to 2.0) | 1.0*§ (0.0 to 1.8) | 0.01 |
*The between-group difference in medians is by Hodges-Lehmann estimation, hence this value differs from a simple subtraction of the diuretic and non-diuretic group medians provided.
†Between-group difference for the median number of urinations per week at baseline.
‡Between-group difference for the median change from baseline at 6 weeks.
§Between-group difference for the median change from baseline at 6 months.
Six months
At 6 months, adherence to bedtime medication use (our primary outcome) had fallen somewhat in both groups (77.3% vs 89.8%), and the difference in adherence had widened (difference 12.6%; 95% CI Diff, 5.8% to 19.8%; p<0.0001; NNH 8.0). However, most diuretic users were still adherent to bedtime medication use. Nocturia was given as the reason for non-adherence by 25/45 (55.6%) diuretic users, compared with 0/34 (0%) non-diuretic users, whose main reasons for non-adherence were forgetting to take their pill (10/34, 29.4%), worsening of BP control (8/34, 23.5%), and non-symptom driven medication changes (6/34, 17.6%). Of those still adherent to allocation (153 diuretic users and 301 non-diuretic users), the median difference in overnight urinations compared with baseline remained 1.0 urinations per week higher in diuretic users, compared with non-diuretic users (95% CI 0.0 to 1.75; p=0.012). Including those who had stopped adhering because of nocturia, 14.2% more diuretic users had perceived nocturia to be a major burden (15.6% vs 1.3%; 95% CI Diff, 8.9% to 20.6%; p<0.0001; NNH 7.0).
Sex differences
Given slightly more diuretic users were female, we conducted a post hoc analysis to determine whether nocturia burden or adherence differed between sexes. Using the Mann-Whitney test, there was no difference, male versus female, in the number of overnight urinations at 6 weeks for the diuretic group (median difference 0.0; 95% CI Diff −1.0 to 1.0; p=0.96), nor for the non-diuretic group (median difference 0.0; 95% CI Diff, 0.0 to 0.0; p=0.98). Adherence with bedtime antihypertensive use, diuretic versus non-diuretic users, was also similar between males and females at 6 months (male: 76.5% vs 90.5%; female: 77.7% vs 89.3%; p=0.86 for the difference in adherence between male and female diuretic users). The same was true for major nocturia burden at 6 months, which was no different between sexes (male: 14.8% vs 0%; female: 16.0% vs 2.5%; p>0.99 for the difference in major nocturia burden between male and female diuretic users).
Discussion
In this prospective cohort of hypertensive primary care patients, 1.5% of morning diuretic users experienced nocturia as a major burden at baseline. When these morning diuretic users switched their diuretic to bedtime, nocturia was more frequent, becoming a major burden for 15.6% of participants over a period of 6 months. Similar primary care patients simultaneously switching other types of antihypertensives from morning to bedtime experienced no increase in nocturia, but they still failed to adhere to bedtime use 10.2% of the time. Due to the extra burden of nocturia, non-adherence in diuretic users was higher, at 22.7%. Hence, one in eight diuretic users, compared with non-diuretic users, will fail the switch to bedtime, with one in four diuretic users failing the switch overall.
Our findings are limited by the potential for selection bias, given some BedMed participants may have previously tried diuretics (morning or evening) and, if they experienced troublesome nocturia, may have stopped using diuretics altogether prior to enrolling. Morning diuretic users may also have avoided enrolling in BedMed if they were concerned about the possibility of needing to switch their diuretics to bedtime. The subjective nature of our outcomes might also be considered a limitation, in that nocturia burden could be interpreted differently by different people. However, self-reporting of nocturia burden integrates the patient’s perceptions and values, and our use of it prioritises the individual’s own assessment of their experience. Similarly, while our allowing patients struggling with bedtime use to switch their diuretic to dinnertime might lessen nocturia, this would likely reflect real-world practice. Our findings are simultaneously strengthened by the prospective nature of the design, and by the cohort selection process, which ensured diuretic and non-diuretic users were all recruited from the same practices, using the same approach, and meeting the same inclusion and exclusion criteria.
To our knowledge, our study is the first to prospectively evaluate the link between bedtime diuretic use and nocturia. Our finding the majority of diuretic users able to adhere to bedtime use is consistent with the generally weak and variable association of diuretics and nocturia in cross-sectional studies,11 13 the inability of baseline diuretics to predict future nocturia (2-year incidence) in 1289 community dwelling respondents 60 years and older in the Medical, Epidemiologic, and Social Aspects of Aging (MESA) study,27 and the number of participants changing the timing of a diuretic in the Treatment In Morning versus Evening (TIME) antihypertensive timing trial.28 While 22.5% of TIME subjects used a diuretic at baseline, postrandomisation diuretic timing changes were made by 5.2% of the evening group versus 0.7% of the morning group (p<0.0001), suggesting 5.2%/22.5%=¼ of diuretic users chose not to continue bedtime use. Although bedtime antihypertensive use might offer an advantage so far as cardiovascular risk reduction,4 5 our clinical experience is that most BP-lowering medication is still administered in the morning. In a 2017 survey of hypertensive primary care patients (single centre in Ohio, 139 respondents), 75.5% used all of their antihypertensive medication in the morning.29 Of the same population, 21 of 22 thiazide-diuretic users (95.5%) took that thiazide in the morning.
Although roughly 14% of hypertensive primary care patients will newly experience nocturia as a major burden after switching a thiazide diuretic from morning to bedtime, the vast majority of morning diuretic users can successfully make the switch to bedtime should it become clinically indicated to do so. The key remaining question is whether or not an attempt to switch diuretics to bedtime is clinically indicated for cardiovascular risk reduction, as the MAPEC (Monitorizacion Ambulatoria para Prediccion de Eventos Cardiovasculares, i.e. Ambulatory Blood Pressure Monitoring for Prediction of Cardiovascular Events) and Hygia trials suggest.4 5 Three confirmatory trials, of which BedMed is one, are looking to evaluate this,15 28 30 with the first of these, the TIME trial,28 recently reporting neither benefit, nor harm, to bedtime prescribing. Final results of the remaining two trials, BedMed15 and BedMed-Frail,30 are expected in mid 2024.
Supplementary Material
Footnotes
Twitter: @WhatScottSays, @Bakal_Jeff, ABSPORU, @DrAlexSinger, @PadwalRaj, @MiHill68, @kimchspr, KimCHSPR, @DPManca, @DeeMangin, DeeMangin, @KirkyKirks, @MedMyths
Contributors: SRG conceptualised the study. SRG, TK, MRK, GMA, JB, AS, AK, FM, RSP, RL, MDH, KM, DPM, STW, JPM and LG designed the study and obtained grant funding. SRG, TK, MRK, GMA, AS, BO'N, MG, DPM, DM, JEMK and JPM recruited physicians for the study. SRG supervised the conduct of the trial. MK and JMSY prepared the study data. SRG and MK analysed the data. SRG and MK wrote the draft manuscript. All authors contributed to critical revision of the manuscript, and all authors approved the final manuscript. SRG is the guarantor. The corresponding author attests that all listed authors meet authorship criteria and that no others meeting the criteria have been omitted.
Funding: BedMed is funded by a Support for Patient Oriented Research (SPOR) Innovative Clinical Trial Multi-Year Grant (REF#: 151212) from the Canadian Institutes of Health Research (CIHR), and from a Partnership for Research and Innovation in the Health System (PRIHS) Grant from Alberta Innovates (REF#: 201 500 912 G2019000450). It also received in-kind research assistance from Enhancing Alberta Primary Care Research Networks (EnACt), which itself is funded entirely by Alberta Innovates, and received pilot funding from the Northern Alberta Family Medicine Fund (University of Alberta Dept of Family Medicine funding).
Competing interests: All authors have completed the ICMJE uniform disclosure form at https://www.icmje.org/disclosure-of-interest/ and declare: SRG is the nominated principal applicant of two grants from governmental sources that are funding the BedMed trial (from Alberta Innovates, and the Canadian Institutes of Health Research); RSP is CEO of 'mmHg', a digital health company and maker of software solutions for BP monitoring; MDH is the recipient of several grants from pharmaceutical and device companies related to interventions geared at stroke treatment. He holds two device patents related to stroke imaging, has chaired or sat on the data safety monitoring boards of five other cardiovascular trials, is the President of the Canadian Neurological Sciences Federation, a member of the board of the Canadian Stroke Consortium, and has private stock ownership in two companies targeting imaging interventions (Circle and PureWeb).
Patient and public involvement: Patients and/or the public were involved in the design, or conduct, or reporting, or dissemination plans of this research. Refer to the Methods section for further details.
Provenance and peer review: Not commissioned; externally peer reviewed.
Supplemental material: This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.
Data availability statement
Data are available in a public, open access repository. Coincident with publication, deidentified patient-level data on which this manuscript’s analyses are based will be freely available for download on the Pragmatic Trials Collaborative’s website (www.PragmaticTrials.ca). Downloadable data will include age at study entry, sex, specific BP medication used, corresponding cohort assignment (ie, diuretic/non-diuretic), baseline nocturia measures, and all primary and secondary outcomes.
Ethics statements
Patient consent for publication
Not applicable.
Ethics approval
This study involves human participants and all procedures and methods were approved by the clinical research ethics boards of the Universities of Alberta (Pro00045958), Calgary (REB17-1887), British Columbia (H21-00523), Saskatchewan (1421) and Manitoba (HS20852:B2017:08). Participants gave informed consent to participate in the study before taking part.
References
- 1.Turgeon RD, Althouse AD, Cohen JB, et al. Lowering nighttime blood pressure with bedtime dosing of antihypertensive medications: controversies in hypertension - con side of the argument. Hypertension 2021;78:871–8. 10.1161/HYPERTENSIONAHA.121.16501 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kreutz R, Kjeldsen SE, Burnier M, et al. Disregard the reported data from the Hygia project: blood pressure medication not to be routinely dosed at bedtime. J Hypertens 2020;38:2144–5. 10.1097/HJH.0000000000002671 [DOI] [PubMed] [Google Scholar]
- 3.Carlsberg B, Brunstrom B. Is bedtime the best time of day? International society of hypertension March 2020 newsletter. Available: https://ish-world.com/data/uploads/2003-1.pdf#page=19 [Accessed 17 Dec 2021].
- 4.Hermida RC, Ayala DE, Mojón A, et al. Influence of circadian time of hypertension treatment on cardiovascular risk: results of the Mapec study. Chronobiology International 2010;27:1629–51. 10.3109/07420528.2010.510230 [DOI] [PubMed] [Google Scholar]
- 5.Hermida RC, Crespo JJ, Domínguez-Sardiña M, et al. Bedtime hypertension treatment improves cardiovascular risk reduction: the Hygia Chronotherapy trial. Eur Heart J 2020;41:4565–76. 10.1093/eurheartj/ehz754 [DOI] [PubMed] [Google Scholar]
- 6.Rabi DM, McBrien KA, Sapir-Pichhadze R, et al. Hypertension Canada's 2020 comprehensive guidelines for the prevention, diagnosis, risk assessment, and treatment of hypertension in adults and children. Can J Cardiol 2020;36:596–624. 10.1016/j.cjca.2020.02.086 [DOI] [PubMed] [Google Scholar]
- 7.Wright JM, Musini VM, Gill R, et al. First-line drugs for hypertension. Cochrane Database Syst Rev 2018;2018. 10.1002/14651858.CD001841.pub3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.WebMD . Diuretics (water pills) for high blood pressure. Available: https://www.webmd.com/hypertension-high-blood-pressure/guide/diuretic-treatment-high-blood-pressure [Accessed 1 Jul 2021].
- 9.Heart and stroke foundation of Canada. Available: https://www.heartandstroke.ca/heart-disease/treatments/medications/diuretics [Accessed 1 Jul 2021].
- 10.Bosch JLHR, Weiss JP. The prevalence and causes of nocturia. Journal of Urology 2010;184:440–6. 10.1016/j.juro.2010.04.011 [DOI] [PubMed] [Google Scholar]
- 11.Asplund R. Nocturia in relation to sleep, health, and medical treatment in the elderly. BJU Int 2005;96 Suppl 1:15–21. 10.1111/j.1464-410X.2005.05653.x [DOI] [PubMed] [Google Scholar]
- 12.Ali A, Snape J. Nocturia in older people: A review of causes, consequences, assessment and management. Int J Clin Pract 2004;58:366–73. 10.1111/j.1368-5031.2004.00086.x [DOI] [PubMed] [Google Scholar]
- 13.Rembratt A, Norgaard JP, Andersson KE. Nocturia and associated morbidity in a community-dwelling elderly population. BJU Int 2003;92:726–30. 10.1046/j.1464-410X.2003.04467.x [DOI] [PubMed] [Google Scholar]
- 14.Asplund R, Aberg H. Health of the elderly with regard to sleep and nocturnal micturition. Scand J Prim Health Care 1992;10:98–104. 10.3109/02813439209014044 [DOI] [PubMed] [Google Scholar]
- 15.Garrison SR, Kolber MR, Allan GM, et al. Bedtime versus morning use of Antihypertensives for cardiovascular risk reduction (Bedmed): protocol for a prospective, randomised, open-label, blinded end-point pragmatic trial. BMJ Open 2022;12:e059711. 10.1136/bmjopen-2021-059711 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hansson L, Hedner T, Dahlöf B. Prospective randomized open blinded end-point (probe) study. A novel design for intervention trials. Blood Pressure 1992;1:113–9. 10.3109/08037059209077502 [DOI] [PubMed] [Google Scholar]
- 17.Harris PA, Taylor R, Thielke R, et al. Research electronic data capture (Redcap)--A Metadata-driven methodology and Workflow process for providing Translational research Informatics support. J Biomed Inform 2009;42:377–81. 10.1016/j.jbi.2008.08.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Grieshaber MC, Flammer J. Blood flow in glaucoma. Curr Opin Ophthalmol 2005;16:79–83. 10.1097/01.icu.0000156134.38495.0b [DOI] [PubMed] [Google Scholar]
- 19.Hayreh SS. Role of nocturnal arterial hypotension in the development of ocular manifestations of systemic arterial hypertension. Curr Opin Ophthalmol 1999;10:474–82. 10.1097/00055735-199912000-00017 [DOI] [PubMed] [Google Scholar]
- 20.Hayreh SS, Zimmerman MB, Podhajsky P, et al. Nocturnal arterial hypotension and its role in optic nerve head and ocular ischemic disorders. Am J Ophthalmol 1994;117:603–24. 10.1016/s0002-9394(14)70067-4 [DOI] [PubMed] [Google Scholar]
- 21.Krasińska B, Karolczak-Kulesza M, Krasiński Z, et al. Effects of the time of antihypertensive drugs administration on the stage of primary open-angle glaucoma in patients with arterial hypertension. Blood Press 2012;21:240–8. 10.3109/08037051.2012.666423 [DOI] [PubMed] [Google Scholar]
- 22.Tonelli M, Wiebe N, Fortin M, et al. Methods for identifying 30 chronic conditions: application to administrative data. BMC Med Inform Decis Mak 2015;15:31. 10.1186/s12911-015-0155-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Roos LL, Gupta S, Soodeen RA, et al. Data quality in an information-rich environment: Canada as an example. Can J Aging 2005;24 Suppl 1:153–70. 10.1353/cja.2005.0055 [DOI] [PubMed] [Google Scholar]
- 24.Kokotailo RA, Hill MD. Coding of stroke and stroke risk factors using International classification of diseases, revisions 9 and 10. Stroke 2005;36:1776–81. 10.1161/01.STR.0000174293.17959.a1 [DOI] [PubMed] [Google Scholar]
- 25.Quan H, Sundararajan V, Halfon P, et al. Coding Algorithms for defining Comorbidities in Icd-9-cm and Icd-10 administrative data. Med Care 2005;43:1130–9. 10.1097/01.mlr.0000182534.19832.83 [DOI] [PubMed] [Google Scholar]
- 26.Garrison SR, Kelmer MD, Korownyk T, et al. Tolerability of bedtime diuretics: A prospective cohort analysis pragmatic trials collaborative Website. 2023. Available: https://www.PragmaticTrials.ca/ [DOI] [PMC free article] [PubMed]
- 27.Johnson TM, Sattin RW, Parmelee P, et al. Evaluating potentially Modifiable risk factors for prevalent and incident nocturia in older adults. J Am Geriatr Soc 2005;53:1011–6. 10.1111/j.1532-5415.2005.53321.x [DOI] [PubMed] [Google Scholar]
- 28.Mackenzie IS, Rogers A, Poulter NR, et al. Cardiovascular outcomes in adults with hypertension with evening versus morning dosing of usual Antihypertensives in the UK (TIME study): a prospective, randomised, open-label, blinded-Endpoint clinical trial. Lancet 2022;400:1417–25. 10.1016/S0140-6736(22)01786-X [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Perry L, Surowiec S, Danso D, et al. Evaluation of administration time and adherence rates of morning vs. bedtime dosing of antihypertensive medications. J Contemp Pharm Pract 2019;66:11–6. 10.37901/jcphp18-00010 [DOI] [Google Scholar]
- 30.Garrison SR. Bedmed-frail: does the potential benefit of bedtime antihypertensive Prescribing extend to frail populations? National Institutes of health clinical trials Registry. 2023. Available: https://www.clinicaltrials.gov/ct2/show/NCT04054648/
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
bmjopen-2022-068188supp001.pdf (1.4MB, pdf)
Data Availability Statement
Data are available in a public, open access repository. Coincident with publication, deidentified patient-level data on which this manuscript’s analyses are based will be freely available for download on the Pragmatic Trials Collaborative’s website (www.PragmaticTrials.ca). Downloadable data will include age at study entry, sex, specific BP medication used, corresponding cohort assignment (ie, diuretic/non-diuretic), baseline nocturia measures, and all primary and secondary outcomes.


