Abstract
Breast cancer has become the most common malignancy among women, posing a severe health risk to women worldwide and creating a heavy social burden. Based on current observational studies, the dietary factor may have a causal relationship with breast cancer. Therefore, exploring how dietary composition affects breast cancer incidence will provide nutrition strategies for clinicians and women. We performed a two-sample Mendelian randomization (MR) analysis to find the causal effect of four kinds of relative macronutrient intake (protein, carbohydrate, sugar, and fat) on the risk of breast cancer and its subtypes [Luminal A, Luminal B, Luminal B HER2-negative, HER2-positive, Triple-negative, Estrogen receptor (ER) positive, and ER-negative breast cancer]. The Mendelian randomization pleiotropy residual sum and outlier (MR-PRESSO) test, MR-Egger intercept test, Cochran’s Q statistic, funnel plot, and leave-one-out (Loo) analysis were all used in a sensitivity analysis to test the robustness of MR. Genetically, a higher relative protein intake was found as a protective factor for Luminal A and overall breast cancer, which was inconsistent with recent findings. A higher relative sugar intake could genetically promote the risk of Luminal B and HER2-positive breast cancer. Conclusions: A higher protein proportion in diet genetically reduces the risk of breast cancer, while higher relative sugar intake does the opposite.
Keywords: Mendelian randomization, breast cancer, protein, carbohydrate, fat, sugar
1. Introduction
Breast cancer in women has overtaken lung cancer as the most commonly diagnosed cancer worldwide. Approximately 2.3 million new cases of female breast cancer were diagnosed, which represents almost 12% of all cancer cases globally [1]. Furthermore, according to the GLOBOCAN Tomorrow Cancer prediction tool, the incidence of breast cancer is expected to increase by more than 46% by 2040, meaning breast cancer will bring a huge burden to society [2]. Meanwhile, compared to other malignancies, breast cancer leads to more disability-adjusted life years lost by women [3].
Dietary pattern has been testified as being associated with different diseases. Previous studies have also suggested that dietary factors could influence the risk of breast cancer in different ways [4,5,6]. For example, a higher dairy and total sugar intake could promote the risk of female breast cancer and other malignancies [7,8,9]. Furthermore, healthy dietary patterns, such as a higher vegetable, fruit, and soy product intake, can help reduce breast cancer risk [8,10]. Long-term observational studies have found an inverse association between breast cancer and the Mediterranean diet, characterized by a dietary pattern with abundant vegetables, fruits, fish, and olive oil [11,12]. As for the causal effect of dietary factors on breast cancer, the Global Cancer Update Programme has claimed that no causality can be inferred from current statistical correlations [6]. Therefore, figuring out the causal effect is a helpful measurement in conducting dietary intervention studies for women.
Randomized controlled trials (RCTs) are still the gold standard for identifying causal relationships [13]. Randomization enables studies to eliminate differences between subgroups to reduce bias. However, RCTs can neither eliminate all the confounders nor avoid the “reverse causation” that may influence the outcomes [14,15]. Mendelian randomization uses genetic variation as an instrumental variable (IV) to testify to the potential causal effects between exposures and outcomes [16]. It is important to note that the causality of genetic variation and traits is the foundation of MR. Because genetic variants are randomly assigned at conception, MR is not influenced by confounders that observational studies find difficult to avoid, making them a good proxy for cause-and-effect relationships [17].
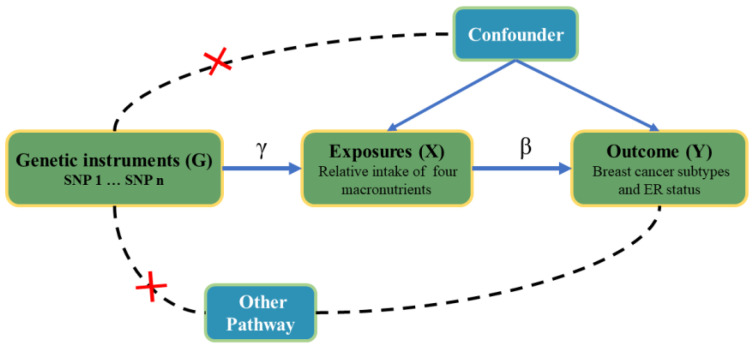
Environmental and genetic factors can influence dietary habits [18,19]. In addition, the surrounding environment, including social and cultural factors, home and work environments, economic factors, and social support, can affect an individual’s sensitivity and preference for particular tastes, thereby influencing their dietary choices. In genetics, polymorphisms of some specific genes, such as fat mass and obesity-associated (FTO), melanocortin 4 receptor (MC4R), leptin receptor (LEPR), peroxisome proliferator-activated receptor-gamma (PPARG), and Adiponectin, have shown effects on weight gain, suppression of appetite, and oncogenesis [20,21,22,23,24,25]. The FTO gene displayed the most robust genetic correlation with polygenic obesity. FTO is commonly dysregulated and exerts significant effects on different categories of cancer. Meanwhile, FTO has the ability to stimulate cancer cell proliferation, enhance the self-renewal of cancer stem cells, and alter the immune and metabolic characteristics of cancer cells by eliminating the m6A modification from its target mRNAs and regulating their stability [24,26]. Based on the above facts, MR analysis can effectively analyze the causal relationship between dietary patterns and breast cancer risk from a genetic perspective. Several studies have already assessed the causal relationship between micronutrients (vitamin D, vitamin C, and vitamin E) and cancers [27,28,29], showing that dietary factors may contribute to the development of breast cancer. In comparison, there have been recent developments in the field of nutrition science indicating that the impact of diet on non-communicable diseases can be better explained by considering overall food consumption and dietary patterns rather than focusing solely on individual nutrients [30]. In this study, we have used the MR to investigate the potential causal relationship between the risk of breast cancer and four macronutrients in order to find robust genetic and phenotypic associations (Figure 1), giving some valuable suggestions for nutritional policies in the clinic.
Figure 1.
Study design overview and the hypothetical relationship between genetic variant, exposure, and outcome of the Mendelian randomization design. Allowed relationships between the variables are indicated by solid arrows, while dashed lines and red cross indicate relationships that are not permitted for G to qualify as a valid instrumental variable. The G–X and X–Y arrows are parameterized by γ and β, with the latter denoting the causal effect of X on Y.
2. Methods and Materials
2.1. Breast Cancer Data
We obtained the breast cancer risk genome-wide association studies (GWASs) summary data from the Breast Cancer Association Consortium (BCAC), which recruited over 100 groups with data on more than 200,000 individuals. Summary data on breast cancer risk came from these GWASs, including 133,384 breast cancer cases and 113,789 controls of European ancestry. In one GWAS, Zhang et al. used a novel two-stage polytomous regression method to characterize tumor heterogeneity by ER, progesterone receptor (PR), and human epidermal growth factor receptor 2 (HER2) status and tumor grade [31]. Moreover, the summary data for overall breast cancer and subtype-specific breast cancer (including Luminal A, Luminal B, Luminal B HER2-negative, HER2-positive, and Triple-negative breast cancer) risk were downloaded for free from the BCAC data resource [https://bcac.ccge.medschl.cam.ac.uk/bcacdata/oncoarray/oncoarray-and-combined-summary-result/gwas-summary-associations-breast-cancer-risk-2020/ (accessed on 13 February 2023)]. As for ER status, we used the summary data obtained from the GWAS conducted by Michailidou et al., including 21,468 ER-negative cases, 69,501 ER-positive cases, and 105,974 controls [32]. In addition, summary data with different ER statuses were also obtained from BCAC [https://bcac.ccge.medschl.cam.ac.uk/bcacdata/oncoarray/oncoarray-and-combined-summary-result/gwas-summary-results-breast-cancer-risk-2017/ (accessed on 13 February 2023)].
2.2. Relative Intake of Macronutrients Data
The IVs for the relative intake of the macronutrient data were obtained from the lead single-nucleotide polymorphisms (SNPs) of the GWAS conducted by Meddens et al. [33], which was performed on more than 235,000 individuals of European ancestry. Researchers got all participants’ dietary habits according to self-reports from the 24 h dietary recall (24HDR) questionnaire and food-frequency questionnaire (FFQ) [34,35]. In the discovery analyses, all the dietary information of the United Kingdom biobank (UKB) cohort was from the 24HDR. All participants with a verified email address were sent the questionnaire via email. They were requested to complete the questionnaire four times over a period of approximately one year (February 2011–April 2012) (https://biobank.ctsu.ox.ac.uk/crystal/refer.cgi?id=118240, accessed on 19 February 2023). In contrast, FFQ was used by all replication cohorts. The “macronutrient densities” are acquired by dividing the macronutrient intake by total energy intake. However, suppose the relative intake of macronutrients does not increase linearly with the total energy intake. In that case, the simple construction of macronutrient proportions may not be the optimal correction for total energy intake. As a result, the macronutrient intakes may need to be properly corrected for total energy intake, leading to residual correlations between macronutrient and total energy intake, which may vary by macronutrient. Meddens et al. [33] adopted corrected “macronutrient densities” () with the correction factor to measure the relative intake of macronutrient fat, protein, carbohydrates, and sugar.
2.3. Selection of Instrumental Variables
We used R (version 4.2.2) with the “TwoSampleMR” (version 0.5.6) package to perform the two-sample MR analysis. In this study, the summary data of exposure (relative intake of macronutrients) and outcome (breast cancer risk) came from different GWASs, which helped to reduce bias and improve precision. IVs are the only bridge to communicate exposure and outcome. Those SNPs regarded as IVs must satisfy the three following conditions: (i) they must exhibit strong associations with exposure (p < 5 × 10−8); (ii) they must only affect the outcome by exposure; (iii) they must have no relationship with the confounders [36]. According to the criteria mentioned above, IVs were clumped (p < 5 × 10−8, linkage disequilibrium (LD) r2 < 0.001, window size = 10,000 kb) from the lead SNP (Table S1), summarized by Meddens et al. [33,37]. Then, we harmonized the clumped data with the assistance of effect allele frequencies (EAF > 0.42), and palindromic variants would be deleted. Moreover, the outcome-related SNPs (P value of breast cancer risk < 5 × 10−8) were also removed from MR analysis. The variance of exposure [R2 = ] was explained by the IVs of each macronutrient and calculated with (genetic effect of each IV in exposure) and effect allele frequency (EAF). We calculated F statistics [()] with R2, sample size (N), and the number of instruments (K) (Table S1), considered as the index used to measure IV strength for MR analysis [38]. We used the online tool to calculate MR power (https://shiny.cnsgenomics.com/mRnd/, accessed on 13 February 2023). The power of MR analysis ranged from 5% to 94%, as shown in Table S2. The MR’s power values for relative protein intake between overall, Luminal A, and ER-positive breast cancer were 0.82, 0.20, and 0.94, respectively. As for sugar, the power of HER2-positive breast cancer was 0.83, while for the Luminal B breast cancer, no specific value was conducted using the online tool (for possible reasons, see the Discussion).
2.4. Mendelian Randomization Analysis
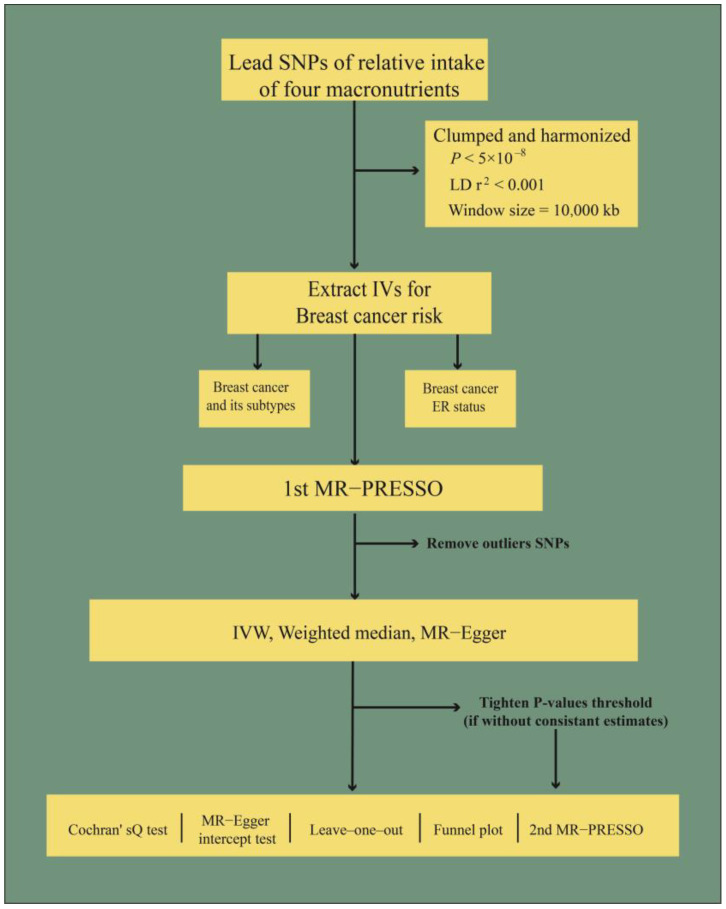
To avoid the potential pleiotropic effect of the IVs, we performed different MR analysis methods to investigate the causal effect between the relative intake of four macronutrients and breast cancer risk in this study. Inverse variance weighted (IVW) estimates were considered as the primary methodology. IVW uses the Wald ratio from each variant to obtain the pooled causal effect. At the same time, there is the worry that it will underestimate the actual variation in the estimate, especially when the IV is weak [39]. Egger regression is used to detect bias from pleiotropy, and its slope coefficient estimates the causal effect. Egger regression can provide a consistent causal effect estimate even when IVs are invalid [40]. A weighted median estimator can combine data on multiple genetic variants into a single causal estimate and provide robust results, even with the number of invalid IVs being as high as 50% [41]. Moreover, the MR analysis flow chart is shown in Figure 2.
Figure 2.
The flow chart of the MR analysis used in this study. Note: LD: linkage disequilibrium; ER: estrogen receptor; IV: instrumental variable; MR-PRESSO: Mendelian randomization pleiotropy residual sum and outlier test; IVW: inverse variance weighted.
2.5. Sensitivity Analysis
Sensitivity analysis was performed to ensure the MR results’ robustness and reduce bias due to IV pleiotropy. Mendelian randomization pleiotropy residual sum and outlier (MR-PRESSO) test was used to test the horizontal pleiotropy (number of bootstrap replications = 10,000) and deleted the horizontal pleiotropic outliers to retest differences in the causal estimates of MR [42]. The intercept of Egger regression indicated the average pleiotropic effect across the IVs, manifesting overall directional pleiotropy (if p value < 0.05) [40]. Furthermore, Cochran’s Q statistic, funnel plot, and leave-one-out (LOO) analyses were all conducted to detect the presence of pleiotropy and assess the robustness of the results. Heterogeneity was considered when the p value of Cochran’s Q statistic was smaller than 0.05.
For the MR of the relative intake of macronutrients and risk of breast cancer, the putative causal effect would be considered if the P of MR met the Bonferroni correction (p < 0.0125, set as 0.05/4), and p < 0.05 was considered as nominally significant.
2.6. Sample Overlap
There were two partially overlapped studies [European Prospective Investigation into Cancer and Nutrition (EPIC) and Women’s Health Initiative (WHI)] included in the macronutrient composition and breast cancer risk GWAS. We considered the smallest sample size as an overlap in the same study. The EPIC and WHI sample overlap was 3.03% (7491/247,173) and 3.47% (8566/247,173) in the GWAS conducted by Zhang et al., respectively. The EPIC and WHI sample overlap was 3.08% (7057/228,951) and 3.74% (8566/228,951) in the GWAS conducted by Michailidou et al., respectively. Moreover, the proportion of sample overlap for the breast cancer risk GWAS conducted by Zhang et al. and Michailidou et al. was 6.50% (16,057/247,173) and 6.82% (15,623/228,951), respectively. The cohort information of the three studies and the overlapped samples are all shown in Table S3.
3. Results
3.1. Causal Effects
As for the risk of overall breast cancer, only relative intake of protein showed a strong genetically protective effect [Odds ratio (OR) = 0.64; 95% confidence interval (CI) = 0.45–0.89; p = 8.46 × 10−3, Table 1 and Figure S1a]. In the subtype analysis, the relative intake of protein also showed a genetically pronounced causal effect on lower incidences of Luminal A (OR = 0.50, 95% CI = 0.32–0.78, p = 2.21 × 10−3, Table 1 and Figure S1b) and ER-positive breast cancer (OR = 0.49, 95% CI = 0.32–0.74, p = 7.91 × 10−4, Table 1 and Figure S1c). On the contrary, relative intake of sugar would genetically increase the incidences of Luminal B (OR = 8.72, 95% CI = 2.31–32.88, p = 1.4 × 10−3, Table 1 and Figure S2a) and HER2-positive breast cancer (OR = 4.40, 95% CI = 1.44–13.43, p = 9.2 × 10−3, Table 1 and Figure S2b). Furthermore, the IVs for MR analysis were all shown in Figures S3d–f and S4c,d. Additionally, we observed that relative intake of carbohydrates can genetically promote the risk of breast cancer (OR = 1.61, 95% CI = 1.09–2.40, p = 1.79 × 10−2). However, the causal relationship cannot be testified via MR analysis because the P value failed to pass the Bonferroni correction (p = 1.25 × 10−2). Further sensitivity analyses have found no pleiotropy and heterogeneity for the above estimates. In the subgroup MR analysis of the relative intake of protein and HER2-enriched breast cancer, the IVW showed a protective trend in breast cancer incidence (OR = 0.31, 95% CI = 0.11–0.90, p = 3.13 × 10−2), while MR-Egger manifested an inconsistent nonsignificant estimate. Therefore, we tightened the p value of IVs to 5 × 10−9, and SNP rs445551 was removed. Further analyses showed inconsistent but nonsignificant estimates using three methods (Table S4). Finally, the MR results from MR-Egger and the weight median are shown in Table S5.
Table 1.
Mendelian randomization results derived from IVW for macronutrient composition and breast cancer.
| Exposure | Outcome (Breast Cancer) |
Number of IVs | p | OR (95%CI) | Cochran’s Q Test | MR-Egger Intercept Test |
|---|---|---|---|---|---|---|
| Relative intake of carbohydrate | Overall | 6 | 0.19 | 1.26 (0.89–1.80) | 0.16 | 0.41 |
| Luminal A | 5 | 1.79 × 10−2 | 1.61 (1.09–2.40) | 0.44 | 0.76 | |
| Luminal B | 7 | 0.14 | 2.07 (0.78–5.50) | 0.14 | 0.04 | |
| Luminal B HER2-negative | 8 | 0.87 | 0.95 (0.49–1.83) | 0.34 | 0.64 | |
| HER2-positive | 8 | 0.89 | 1.10 (0.27–4.55) | 0.07 | 0.91 | |
| Triple-negative | 8 | 0.61 | 0.83 (0.41–1.69) | 0.25 | 0.51 | |
| ER-negative | 6 | 0.38 | 0.79 (0.46–1.34) | 0.78 | 0.45 | |
| ER-positive | 7 | 0.85 | 1.04 (0.72–1.51) | 0.26 | 0.33 | |
| Relative intake of fat | Overall | 4 | 0.68 | 0.91 (0.57–1.44) | 0.13 | 0.13 |
| Luminal A | 4 | 0.54 | 0.86 (0.52–1.40) | 0.16 | 0.17 | |
| Luminal B | 4 | 0.83 | 1.17 (0.29–4.80) | 0.47 | 0.15 | |
| Luminal B HER2-negative | 4 | 0.52 | 0.74 (0.29–1.87) | 0.13 | 0.15 | |
| HER2-positive | 4 | 0.16 | 0.51 (0.20–1.30) | 0.76 | 0.94 | |
| Triple-negative | 4 | 0.15 | 1.50 (0.87–2.58) | 0.98 | 0.90 | |
| ER-negative | 4 | 0.12 | 1.40 (0.92–2.12) | 0.95 | 0.80 | |
| ER-positive | 4 | 0.67 | 0.89 (0.50–1.57) | 0.26 | 0.11 | |
| Relative intake of protein | Overall | 4 | 8.46 × 10−3 | 0.64 (0.45–0.89) | 0.43 | 0.42 |
| Luminal A | 4 | 2.21 × 10−3 | 0.50 (0.32–0.78) | 1.00 | 0.96 | |
| Luminal B | 5 | 0.09 | 0.48 (0.21–1.13) | 0.26 | 0.19 | |
| Luminal B HER2-negative | 6 | 0.99 | 1.00 (0.56–1.79) | 0.56 | 0.32 | |
| HER2-positive | 6 | 0.06 | 0.31 (0.09–1.07) | 0.16 | 0.42 | |
| Triple-negative | 5 | 0.87 | 0.94 (0.45–1.95) | 0.79 | 0.95 | |
| ER-negative | 5 | 0.07 | 0.60 (0.35–1.04) | 0.70 | 0.44 | |
| ER-positive | 4 | 7.91 × 10−4 | 0.49 (0.32–0.74) | 0.54 | 0.34 | |
| Relative intake of sugar | Overall | 3 | 0.27 | 1.36 (0.79–2.35) | 0.17 | 0.31 |
| Luminal A | 3 | 0.38 | 1.28 (0.74–2.18) | 0.52 | 0.55 | |
| Luminal B | 4 | 1.39 × 10−3 | 8.72 (2.31–32.88) | 0.06 | 1.00 | |
| Luminal B HER2-negative | 5 | 0.15 | 2.41 (0.72–8.03) | 0.01 | 0.24 | |
| HER2-positive | 5 | 9.19 × 10−3 | 4.40 (1.44–13.43) | 0.60 | 0.56 | |
| Triple-negative | 3 | 0.94 | 1.04 (0.37–2.89) | 0.95 | 0.84 | |
| ER-negative | 4 | 0.90 | 0.96 (0.55–1.68) | 0.73 | 0.62 | |
| ER-positive | 4 | 0.21 | 1.26 (0.88–1.82) | 0.70 | 0.59 |
Note: IVW: inverse variance weighted; 95%CI: 95% confidence interval; IVs: instrumental variables; p = p value; outcome = risk of breast cancer.
3.2. Sensitivity Analysis
To guarantee the robustness of causal estimates, we performed sensitivity analyses after each MR analysis (Table S5). Horizontal pleiotropy and outliers were found in the first MR-PRESSO test between the relative intake of protein and Luminal A as well as overall and ER-positive breast cancer (p < 1.00 × 10−4). When outliers (rs13146907, rs55872725, rs838133, as shown in Table S5) were removed, the pleiotropy was corrected. The same situation was also observed in the MR-PRESSO test between relative intake of sugar and Luminal B breast cancer. After outlier rs7012814 was deleted, no pleiotropy was detected. As for the HER2-positive breast cancer and relative intake of sugar, the first MR-PRESSO did not find any outliers. All the p values of Cochran’s Q and MR-Egger intercept tests were greater than 0.05 in the five significant estimates mentioned above. Egger intercepts did not detect any pleiotropy (Figures S1 and S2). Furthermore, the LOO analysis demonstrated that no SNP drove the findings (Figures S3d–f and S4c,d), and the funnel plots (Figures S3a–c and S4a,b) displayed a symmetrical distribution.
4. Discussion
In our study, we used MR analysis to explore the genetic causal relationship between the relative intake of four macronutrients and the risk of breast cancer. A higher relative intake of protein was found to be a protective factor against breast cancer. At the same time, a higher relative intake of sugar had shown a significant causal effect on breast cancer. As for the sensitivity analysis, MR-PRESSO was performed secondly, unless IVs were less than four or no outliers were detected (Table S5). Moreover, no IV pleiotropy and heterogeneity were found in the significant estimates after the correction of MR-PRESSO (Figures S3 and S4). Therefore, almost all the power values of the significant estimates were robust. As for the power calculation of sugar and HER2-positive breast cancer, there might be a limiting value instead of no value. Based on the original parameters, we have artificially set the ORs as 2.5, 3.5, 4.5, and 5.5, and the calculating power values as 0.37, 0.82, 1.00, and 1.00, respectively. There was a trend that when the OR value was increasing, the power was infinitely close to 1.00. Therefore, when the OR was equal to 8.72, the power value was robust enough to support the MR analysis.
Because the macronutrients are the primary energy source of the human body, there may be a possibility that the four macronutrients can influence the incidence of breast cancer through the pathway of obesity, which has been considered a risk factor for breast cancer [43]. Dennis et al. found that a low relative carbohydrate proportion and a high intake of fat genetically contribute to higher body mass index (BMI) and a higher waist circumference (WC); they found that there was no causal relationship between relative intake of protein and sugar and BMI and WC [44].
In this study, dietary structure has been considered a form of oncogenesis [45]. Macronutrients, such as fat, protein, and carbohydrate, provide almost all the energy and essential components required to satisfy human physiological activities. However, outcomes derived from observational studies of meat consumption and the incidence of breast cancer were still controversial [46,47,48]. Our results are inconsistent with most observational studies and meta-analyses that the red or processed meat was believed to induce breast cancer for the following reasons: (1) carcinogenic compounds, such as N-nitroso compounds, heterocyclic amines, and polycyclic aromatic hydrocarbons that can dose-dependently generate DNA adducts [49,50]; (2) inflammation and oxidative stress may arise from animal fat and iron-enriched red meat [51]. On the contrary, a recent meta-analysis suggested that a higher soy food intake would help decrease the risk of breast cancer [52]. Furthermore, a long-time large-scale observational study has also found that a higher soy intake could reduce the risk of breast cancer in postmenopausal women [53]. Soy is an essential source of vegetable protein. Its positive effect on breast cancer risk and breast cancer survival was attributed to isoflavone, a phytoestrogen, and selective estrogen receptor modulator [54]. Given the relationship between soy consumption and the risk of breast cancer mentioned above, it somewhat validated our MR finding that women would benefit from a higher dietary protein proportion.
A higher relative sugar intake is strongly associated with a higher risk of breast cancer in our MR analysis. Recently, a fasting-mimicking diet (FMD) has been testified to enhance the efficacy of standard cancer therapy. FMD, a dietary structure low in calories, sugars, and protein but relatively high in fat content, could strengthen endocrine therapeutics tamoxifen and fulvestrant by lowering circulating IGF1, insulin, and leptin by upregulating EGR1 and PTEN to inhibit the AKT–mTOR signal pathway [55]. Claudio et al. revealed that an FMD could lead to a consistent decrease in blood glucose and growth factor concentration, remodeling anticancer immunity via changes to peripheral and intratumor cellular components [56]. Notably, a higher dietary proportion of sugar could result in high glucose levels and promote high insulin levels in parallel, leading to cancer growth [57]. As a subtype of carbohydrates, sugar can be more rapidly absorbed and affect plasma glucose levels than other sources of carbohydrates, such as starch and dietary fiber. Potentially, it can lead to breast cancer via higher plasma glucose levels, which can, in turn, promote potential pathways. At the same time, the association between the risk of breast cancer and relative intake of carbohydrates was unclear in our study, which was consistent with current findings. In a prospectively observational study, the vegetable-based, low-carbohydrate diet habit was inversely associated with a reduced risk of ER-negative breast cancer [58]. Bahareh et al. have found that adherence to a low-carbohydrate diet may increase the risk of breast cancer in postmenopausal women. Long-term, large-cohort studies with a precise definition of a low-carbohydrate diet and scientific study design should be further performed.
We have failed to find the causal relationship between the risk of breast cancer and the relative intake of fat. At the same time, a large-scale, case–control study has revealed that replacing fat intake with carbohydrates of equal calories could lower the risk of breast cancer. Moreover, the same association was also shown for fat and protein [59]. Sabina et al. found that high total and saturated fat intakes were associated with a greater risk of hormone-receptor-positive breast cancer. Meanwhile, a high saturated fat intake was significantly associated with a greater risk of HER2-negative breast cancer [60]. Compared to protein and carbohydrates, fat can provide more energy per gram. Therefore, it means that a higher relative intake of fat is more likely to lead to obesity, which has already been considered a risk factor for breast cancer.
The strength of our study is that we, for the first time, investigated with the MR analysis the causal relationship between the relative intake of four macronutrients and the risk of breast cancer. In addition, the proportion of sample overlap in our study was small, guaranteeing the independence of exposure and outcome and making the estimates of MR more robust. Moreover, we used different methods to perform sensitivity analysis to detect outliers and correct potential pleiotropy and heterogeneity. On the other hand, most current diet-related research has concentrated on specific foods or ingredients, which may not offer comprehensive insights into the optimal diet for overall health. Instead, dietary patterns, which encompass a range of foods, nutrients, and beverages, can be valuable tools for assessing the overall impact of diet on health outcomes. The exposure information in our study is derived from large cohorts via dietary questionnaires, a process that reflects, to the greatest extent, the dietary structure of the participants in the cohorts. In addition, it indirectly describes the proportion of the four macronutrients in the dietary pattern using energy density.
Our study also had some limitations. Firstly, the original dietary information obtained from UK Biobank was collected via a 24HDR questionnaire. Moreover, participants were asked to recall as accurately as possible how many portions of each food item they had consumed the previous day. Compared with the FFQ used by other cohorts, feedback from the 24HDR may have more random variation since the FFQ can obtain dietary intakes for a standard day in the previous week or month. Secondly, the IV numbers for relative fat, protein, and sugar intake were too small, potentially weakening the strength of the MR analysis due to the convergence bias. Thirdly, only European ancestry was tested in our study, so it is unreasonable to interpret our findings in other populations. Finally, the findings in our study are based on the current background of GWASs, which have identified the causal relationship between macronutrient intake and the risk of breast cancer in genetics. This can provide some reference for the formulation of clinical nutrition strategies but still requires large-scale clinical trials for validation.
5. Conclusions
This study utilized the genetic method to investigate the causal relationship between the relative intake of macronutrients and the risk of breast cancer. We found that a higher relative intake of protein was inversely associated with the risk of Luminal A and overall breast cancer. On the contrary, a higher relative intake of sugar would promote incidences of Lumina B and HER2-positive breast cancer. However, what we have found was partially inconsistent with the current finding, so further validation needs to be performed via clinical trials.
Acknowledgments
We sincerely thank the Breast Cancer Association Consortium and Meddens et al. Additionally, we want to acknowledge the participants and investigators of the Breast Cancer Association Consortium.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/nu15112586/s1. Supplementary tables; Table S1: lead SNPs identified across diet composition GWAS; Table S2: the power of Mendelian randomization analysis in the study; Table S3: sample overlap of exposure and outcome genome-wide association studies; Table S4: details for Mendelian randomization studies with tightened instrument variables selection; Table S5: results of MR analysis and sensitivity analysis. Supplementary figures; Figure S1. scatter plots from genetically predicted protective effects assessed the relative intake of protein’s effect on breast cancer risk; Figure S2. scatter plots from genetically predicted protective effects assessed the relative intake of sugar on breast cancer risk; Figure S3. funnel plot and leave-one-out sensitivity analysis of MR estimate assessed the relative intake of protein and its effect on overall breast cancer risk; Figure S4. funnel plot and leave-one-out sensitivity analysis of MR estimate assessed the relative intake of sugar and its effect on overall breast cancer risk.
Author Contributions
Conceptualization, H.D. and J.W.; Formal analysis, H.D. and X.K.; Funding acquisition, Y.F. and J.W.; Methodology, Q.L.; Resources, X.W. and Q.L.; Software, X.K.; Supervision, Y.F. and J.W.; Writing—original draft, H.D. and Y.F.; Writing—review and editing, H.D., X.K., X.W., Q.L., Y.F. and J.W. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
Not applicable. This study used publicly available de-identified data from previous studies approved by an ethical standards committee. Therefore, no further ethical approval was required in this study.
Informed Consent Statement
Not applicable. This study used publicly available de-identified data from previous studies approved by an ethical standards committee. Therefore, no further informed consent was required in this study.
Data Availability Statement
Publicly available GWAS data on breast cancer survival/susceptibility were obtained from https://bcac.ccge.medschl.cam.ac.uk/bcacdata (accessed on 14 February 2023). Genetic data on the relative intake of macronutrients were obtained from GWAS reported by Meddens et al. [33].
Conflicts of Interest
The authors declare no conflict of interest.
Funding Statement
This study was supported by the China National Key R&D (or Research and Development) Program (No. 2020AAA0105000, 2020AAA0105004 to YF), the Natural Science Foundation of China (No. 82173328 to JW), the Natural Science Foundation of China (No. 81872160 to JW), the Beijing Municipal Natural Science Foundation (Key Project) (No. 7191009 to JW), the CAMS Innovation Fund for Medical Sciences (CIFMS) (No. 2021-I2M-C&T-B-044 to YF), the CAMS Innovation Fund for Medical Sciences (CIFMS) (No. 2022-I2M-C&T-B-087 to XK), and the Non-profit Central Research Institute Fund of Chinese Academy of Medical Sciences (No. 2022-JKCS-04 to XK).
Footnotes
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
References
- 1.Sung H., Ferlay J., Siegel R.L., Laversanne M., Soerjomataram I., Jemal A., Bray F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021;71:209–249. doi: 10.3322/caac.21660. [DOI] [PubMed] [Google Scholar]
- 2.Heer E., Harper A., Escandor N., Sung H., McCormack V., Fidler-Benaoudia M.M. Global burden and trends in premenopausal and postmenopausal breast cancer: A population-based study. Lancet Glob. Health. 2020;8:e1027–e1037. doi: 10.1016/S2214-109X(20)30215-1. [DOI] [PubMed] [Google Scholar]
- 3.Fitzmaurice C., Abate D., Abbasi N., Abbastabar H., Abd-Allah F., Abdel-Rahman O., Abdelalim A., Abdoli A., Abdollahpour I., Abdulle A.S.M., et al. Global, Regional, and National Cancer Incidence, Mortality, Years of Life Lost, Years Lived with Disability, and Disability-Adjusted Life-Years for 29 Cancer Groups, 1990 to 2017: A Systematic Analysis for the Global Burden of Disease Study. JAMA Oncol. 2019;5:1749–1768. doi: 10.1001/jamaoncol.2019.2996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chlebowski R.T., Aragaki A.K., Anderson G.L., Pan K., Neuhouser M.L., Manson J.E., Thomson C.A., Mossavar-Rahmani Y., Lane D.S., Johnson K.C., et al. Dietary Modification and Breast Cancer Mortality: Long-Term Follow-Up of the Women’s Health Initiative Randomized Trial. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2020;38:1419–1428. doi: 10.1200/JCO.19.00435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Prentice R.L., Aragaki A.K., Howard B.V., Chlebowski R.T., Thomson C.A., Van Horn L., Tinker L.F., Manson J.E., Anderson G.L., Kuller L.E., et al. Low-Fat Dietary Pattern among Postmenopausal Women Influences Long-Term Cancer, Cardiovascular Disease, and Diabetes Outcomes. J. Nutr. 2019;149:1565–1574. doi: 10.1093/jn/nxz107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tsilidis K.K., Cariolou M., Becerra-Tomás N., Balducci K., Vieira R., Abar L., Aune D., Markozannes G., Nanu N., Greenwood D.C., et al. Postdiagnosis body fatness, recreational physical activity, dietary factors and breast cancer prognosis: Global Cancer Update Programme (CUP Global) summary of evidence grading. Int. J. Cancer. 2023;152:635–644. doi: 10.1002/ijc.34320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chen M., Li S., Arora I., Yi N., Sharma M., Li Z., Tollefsbol T.O., Li Y. Maternal soybean diet on prevention of obesity-related breast cancer through early-life gut microbiome and epigenetic regulation. J. Nutr. Biochem. 2022;110:109119. doi: 10.1016/j.jnutbio.2022.109119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kazemi A., Barati-Boldaji R., Soltani S., Mohammadipoor N., Esmaeilinezhad Z., Clark C.C.T., Babajafari S., Akbarzadeh M. Intake of Various Food Groups and Risk of Breast Cancer: A Systematic Review and Dose-Response Meta-Analysis of Prospective Studies. Adv. Nutr. 2021;12:809–849. doi: 10.1093/advances/nmaa147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Debras C., Chazelas E., Srour B., Kesse-Guyot E., Julia C., Zelek L., Agaësse C., Druesne-Pecollo N., Galan P., Hercberg S., et al. Total and added sugar intakes, sugar types, and cancer risk: Results from the prospective NutriNet-Santé cohort. Am. J. Clin. Nutr. 2020;112:1267–1279. doi: 10.1093/ajcn/nqaa246. [DOI] [PubMed] [Google Scholar]
- 10.Wei Y., Lv J., Guo Y., Bian Z., Gao M., Du H., Yang L., Chen Y., Zhang X., Wang T., et al. Soy intake and breast cancer risk: A prospective study of 300,000 Chinese women and a dose-response meta-analysis. Eur. J. Epidemiol. 2020;35:567–578. doi: 10.1007/s10654-019-00585-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Toledo E., Salas-Salvadó J., Donat-Vargas C., Buil-Cosiales P., Estruch R., Ros E., Corella D., Fitó M., Hu F.B., Arós F., et al. Mediterranean Diet and Invasive Breast Cancer Risk among Women at High Cardiovascular Risk in the PREDIMED Trial: A Randomized Clinical Trial. JAMA Intern. Med. 2015;175:1752–1760. doi: 10.1001/jamainternmed.2015.4838. [DOI] [PubMed] [Google Scholar]
- 12.Castelló A., Pollán M., Buijsse B., Ruiz A., Casas A.M., Baena-Cañada J.M., Lope V., Antolín S., Ramos M., Muñoz M., et al. Spanish Mediterranean diet and other dietary patterns and breast cancer risk: Case-control EpiGEICAM study. Br. J. Cancer. 2014;111:1454–1462. doi: 10.1038/bjc.2014.434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Klungel O.H., Martens E.P., Psaty B.M., Grobbee D.E., Sullivan S.D., Stricker B.H., Leufkens H.G., de Boer A. Methods to assess intended effects of drug treatment in observational studies are reviewed. J. Clin. Epidemiol. 2004;57:1223–1231. doi: 10.1016/j.jclinepi.2004.03.011. [DOI] [PubMed] [Google Scholar]
- 14.Sekula P., Del Greco M.F., Pattaro C., Köttgen A. Mendelian Randomization as an Approach to Assess Causality Using Observational Data. J. Am. Soc. Nephrol. JASN. 2016;27:3253–3265. doi: 10.1681/ASN.2016010098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lawlor D.A., Harbord R.M., Sterne J.A., Timpson N., Davey Smith G. Mendelian randomization: Using genes as instruments for making causal inferences in epidemiology. Stat. Med. 2008;27:1133–1163. doi: 10.1002/sim.3034. [DOI] [PubMed] [Google Scholar]
- 16.Davey Smith G. Capitalizing on Mendelian randomization to assess the effects of treatments. J. R. Soc. Med. 2007;100:432–435. doi: 10.1177/014107680710000923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ba D.M., Ssentongo P., Beelman R.B., Muscat J., Gao X., Richie J.P. Higher Mushroom Consumption Is Associated with Lower Risk of Cancer: A Systematic Review and Meta-Analysis of Observational Studies. Adv. Nutr. 2021;12:1691–1704. doi: 10.1093/advances/nmab015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wansink B. Environmental factors that increase the food intake and consumption volume of unknowing consumers. Annu. Rev. Nutr. 2004;24:455–479. doi: 10.1146/annurev.nutr.24.012003.132140. [DOI] [PubMed] [Google Scholar]
- 19.Frayling T.M., Timpson N.J., Weedon M.N., Zeggini E., Freathy R.M., Lindgren C.M., Perry J.R., Elliott K.S., Lango H., Rayner N.W., et al. A common variant in the FTO gene is associated with body mass index and predisposes to childhood and adult obesity. Science. 2007;316:889–894. doi: 10.1126/science.1141634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chung S.J., Nagaraju G.P., Nagalingam A., Muniraj N., Kuppusamy P., Walker A., Woo J., Győrffy B., Gabrielson E., Saxena N.K., et al. ADIPOQ/adiponectin induces cytotoxic autophagy in breast cancer cells through STK11/LKB1-mediated activation of the AMPK-ULK1 axis. Autophagy. 2017;13:1386–1403. doi: 10.1080/15548627.2017.1332565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Muntean C., Sasaran M.O., Crisan A., Banescu C. Effects of PPARG and PPARGC1A gene polymorphisms on obesity markers. Front. Public Health. 2022;10:962852. doi: 10.3389/fpubh.2022.962852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chen L., Zhang J., Zou Y., Wang F., Li J., Sun F., Luo X., Zhang M., Guo Y., Yu Q., et al. Kdm2a deficiency in macrophages enhances thermogenesis to protect mice against HFD-induced obesity by enhancing H3K36me2 at the Pparg locus. Cell Death Differ. 2021;28:1880–1899. doi: 10.1038/s41418-020-00714-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Clément K., van den Akker E., Argente J., Bahm A., Chung W.K., Connors H., De Waele K., Farooqi I.S., Gonneau-Lejeune J., Gordon G., et al. Efficacy and safety of setmelanotide, an MC4R agonist, in individuals with severe obesity due to LEPR or POMC deficiency: Single-arm, open-label, multicentre, phase 3 trials. Lancet Diabetes Endocrinol. 2020;8:960–970. doi: 10.1016/S2213-8587(20)30364-8. [DOI] [PubMed] [Google Scholar]
- 24.Azzam S.K., Alsafar H., Sajini A.A. FTO m6A Demethylase in Obesity and Cancer: Implications and Underlying Molecular Mechanisms. Int. J. Mol. Sci. 2022;23:3800. doi: 10.3390/ijms23073800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mosialou I., Shikhel S., Liu J.M., Maurizi A., Luo N., He Z., Huang Y., Zong H., Friedman R.A., Barasch J., et al. MC4R-dependent suppression of appetite by bone-derived lipocalin 2. Nature. 2017;543:385–390. doi: 10.1038/nature21697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Li Y., Su R., Deng X., Chen Y., Chen J. FTO in cancer: Functions, molecular mechanisms, and therapeutic implications. Trends Cancer. 2022;8:598–614. doi: 10.1016/j.trecan.2022.02.010. [DOI] [PubMed] [Google Scholar]
- 27.Xin J., Jiang X., Ben S., Yuan Q., Su L., Zhang Z., Christiani D.C., Du M., Wang M. Association between circulating vitamin E and ten common cancers: Evidence from large-scale Mendelian randomization analysis and a longitudinal cohort study. BMC Med. 2022;20:168. doi: 10.1186/s12916-022-02366-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Fu Y., Xu F., Jiang L., Miao Z., Liang X., Yang J., Larsson S.C., Zheng J.S. Circulating vitamin C concentration and risk of cancers: A Mendelian randomization study. BMC Med. 2021;19:171. doi: 10.1186/s12916-021-02041-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Dimitrakopoulou V.I., Tsilidis K.K., Haycock P.C., Dimou N.L., Al-Dabhani K., Martin R.M., Lewis S.J., Gunter M.J., Mondul A., Shui I.M., et al. Circulating vitamin D concentration and risk of seven cancers: Mendelian randomisation study. BMJ (Clin. Res. Ed.) 2017;359:j4761. doi: 10.1136/bmj.j4761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mozaffarian D., Rosenberg I., Uauy R. History of modern nutrition science-implications for current research, dietary guidelines, and food policy. BMJ (Clin. Res. Ed.) 2018;361:k2392. doi: 10.1136/bmj.k2392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zhang H., Ahearn T.U., Lecarpentier J., Barnes D., Beesley J., Qi G., Jiang X., O’Mara T.A., Zhao N., Bolla M.K., et al. Genome-wide association study identifies 32 novel breast cancer susceptibility loci from overall and subtype-specific analyses. Nat. Genet. 2020;52:572–581. doi: 10.1038/s41588-020-0609-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Michailidou K., Lindström S., Dennis J., Beesley J., Hui S., Kar S., Lemaçon A., Soucy P., Glubb D., Rostamianfar A., et al. Association analysis identifies 65 new breast cancer risk loci. Nature. 2017;551:92–94. doi: 10.1038/nature24284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Meddens S.F.W., de Vlaming R., Bowers P., Burik C.A.P., Linnér R.K., Lee C., Okbay A., Turley P., Rietveld C.A., Fontana M.A., et al. Genomic analysis of diet composition finds novel loci and associations with health and lifestyle. Mol. Psychiatry. 2021;26:2056–2069. doi: 10.1038/s41380-020-0697-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Liu B., Young H., Crowe F.L., Benson V.S., Spencer E.A., Key T.J., Appleby P.N., Beral V. Development and evaluation of the Oxford WebQ, a low-cost, web-based method for assessment of previous 24 h dietary intakes in large-scale prospective studies. Public Health Nutr. 2011;14:1998–2005. doi: 10.1017/S1368980011000942. [DOI] [PubMed] [Google Scholar]
- 35.Cade J.E., Burley V.J., Warm D.L., Thompson R.L., Margetts B.M. Food-frequency questionnaires: A review of their design, validation and utilisation. Nutr. Res. Rev. 2004;17:5–22. doi: 10.1079/NRR200370. [DOI] [PubMed] [Google Scholar]
- 36.Boef A.G., Dekkers O.M., le Cessie S. Mendelian randomization studies: A review of the approaches used and the quality of reporting. Int. J. Epidemiol. 2015;44:496–511. doi: 10.1093/ije/dyv071. [DOI] [PubMed] [Google Scholar]
- 37.Yavorska O.O., Burgess S. MendelianRandomization: An R package for performing Mendelian randomization analyses using summarized data. Int. J. Epidemiol. 2017;46:1734–1739. doi: 10.1093/ije/dyx034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hao Y., Xiao J., Liang Y., Wu X., Zhang H., Xiao C., Zhang L., Burgess S., Wang N., Zhao X., et al. Reassessing the causal role of obesity in breast cancer susceptibility: A comprehensive multivariable Mendelian randomization investigating the distribution and timing of exposure. Int. J. Epidemiol. 2023;52:58–70. doi: 10.1093/ije/dyac143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Burgess S., Thompson S.G. Improving bias and coverage in instrumental variable analysis with weak instruments for continuous and binary outcomes. Stat. Med. 2012;31:1582–1600. doi: 10.1002/sim.4498. [DOI] [PubMed] [Google Scholar]
- 40.Bowden J., Davey Smith G., Burgess S. Mendelian randomization with invalid instruments: Effect estimation and bias detection through Egger regression. Int. J. Epidemiol. 2015;44:512–525. doi: 10.1093/ije/dyv080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bowden J., Davey Smith G., Haycock P.C., Burgess S. Consistent Estimation in Mendelian Randomization with Some Invalid Instruments Using a Weighted Median Estimator. Genet. Epidemiol. 2016;40:304–314. doi: 10.1002/gepi.21965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Verbanck M., Chen C.Y., Neale B., Do R. Detection of widespread horizontal pleiotropy in causal relationships inferred from Mendelian randomization between complex traits and diseases. Nat. Genet. 2018;50:693–698. doi: 10.1038/s41588-018-0099-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Protani M., Coory M., Martin J.H. Effect of obesity on survival of women with breast cancer: Systematic review and meta-analysis. Breast Cancer Res. Treat. 2010;123:627–635. doi: 10.1007/s10549-010-0990-0. [DOI] [PubMed] [Google Scholar]
- 44.Freuer D., Meisinger C., Linseisen J. Causal relationship between dietary macronutrient composition and anthropometric measures: A bidirectional two-sample Mendelian randomization analysis. Clin. Nutr. 2021;40:4120–4131. doi: 10.1016/j.clnu.2021.01.047. [DOI] [PubMed] [Google Scholar]
- 45.Kerr J., Anderson C., Lippman S.M. Physical activity, sedentary behaviour, diet, and cancer: An update and emerging new evidence. Lancet Oncol. 2017;18:e457–e471. doi: 10.1016/s1470-2045(17)30411-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Farvid M.S., Cho E., Chen W.Y., Eliassen A.H., Willett W.C. Adolescent meat intake and breast cancer risk. Int. J. Cancer. 2015;136:1909–1920. doi: 10.1002/ijc.29218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Farvid M.S., Cho E., Chen W.Y., Eliassen A.H., Willett W.C. Dietary protein sources in early adulthood and breast cancer incidence: Prospective cohort study. BMJ (Clin. Res. Ed.) 2014;348:g3437. doi: 10.1136/bmj.g3437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Pouchieu C., Deschasaux M., Hercberg S., Druesne-Pecollo N., Latino-Martel P., Touvier M. Prospective association between red and processed meat intakes and breast cancer risk: Modulation by an antioxidant supplementation in the SU.VI.MAX randomized controlled trial. Int. J. Epidemiol. 2014;43:1583–1592. doi: 10.1093/ije/dyu134. [DOI] [PubMed] [Google Scholar]
- 49.Turteltaub K.W., Dingley K.H., Curtis K.D., Malfatti M.A., Turesky R.J., Garner R.C., Felton J.S., Lang N.P. Macromolecular adduct formation and metabolism of heterocyclic amines in humans and rodents at low doses. Cancer Lett. 1999;143:149–155. doi: 10.1016/S0304-3835(99)00116-0. [DOI] [PubMed] [Google Scholar]
- 50.Sinha R., Rothman N., Salmon C.P., Knize M.G., Brown E.D., Swanson C.A., Rhodes D., Rossi S., Felton J.S., Levander O.A. Heterocyclic amine content in beef cooked by different methods to varying degrees of doneness and gravy made from meat drippings. Food Chem. Toxicol. Int. J. Publ. Br. Ind. Biol. Res. Assoc. 1998;36:279–287. doi: 10.1016/s0278-6915(97)00162-2. [DOI] [PubMed] [Google Scholar]
- 51.Hodgson J.M., Ward N.C., Burke V., Beilin L.J., Puddey I.B. Increased lean red meat intake does not elevate markers of oxidative stress and inflammation in humans. J. Nutr. 2007;137:363–367. doi: 10.1093/jn/137.2.363. [DOI] [PubMed] [Google Scholar]
- 52.Shin S., Fu J., Shin W.K., Huang D., Min S., Kang D. Association of food groups and dietary pattern with breast cancer risk: A systematic review and meta-analysis. Clin. Nutr. 2023;42:282–297. doi: 10.1016/j.clnu.2023.01.003. [DOI] [PubMed] [Google Scholar]
- 53.Wada K., Nakamura K., Tamai Y., Tsuji M., Kawachi T., Hori A., Takeyama N., Tanabashi S., Matsushita S., Tokimitsu N., et al. Soy isoflavone intake and breast cancer risk in Japan: From the Takayama study. Int. J. Cancer. 2013;133:952–960. doi: 10.1002/ijc.28088. [DOI] [PubMed] [Google Scholar]
- 54.Messina M. Soy foods, isoflavones, and the health of postmenopausal women. Am. J. Clin. Nutr. 2014;100((Suppl. S1)):423s–430s. doi: 10.3945/ajcn.113.071464. [DOI] [PubMed] [Google Scholar]
- 55.Caffa I., Spagnolo V., Vernieri C., Valdemarin F., Becherini P., Wei M., Brandhorst S., Zucal C., Driehuis E., Ferrando L., et al. Fasting-mimicking diet and hormone therapy induce breast cancer regression. Nature. 2020;583:620–624. doi: 10.1038/s41586-020-2502-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Vernieri C., Fucà G., Ligorio F., Huber V., Vingiani A., Iannelli F., Raimondi A., Rinchai D., Frigè G., Belfiore A., et al. Fasting-Mimicking Diet Is Safe and Reshapes Metabolism and Antitumor Immunity in Patients with Cancer. Cancer Discov. 2022;12:90–107. doi: 10.1158/2159-8290.CD-21-0030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Hopkins B.D., Pauli C., Du X., Wang D.G., Li X., Wu D., Amadiume S.C., Goncalves M.D., Hodakoski C., Lundquist M.R., et al. Suppression of insulin feedback enhances the efficacy of PI3K inhibitors. Nature. 2018;560:499–503. doi: 10.1038/s41586-018-0343-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Fung T.T., Hu F.B., Hankinson S.E., Willett W.C., Holmes M.D. Low-carbohydrate diets, dietary approaches to stop hypertension-style diets, and the risk of postmenopausal breast cancer. Am. J. Epidemiol. 2011;174:652–660. doi: 10.1093/aje/kwr148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Sasanfar B., Toorang F., Zendehdel K., Salehi-Abargouei A. Substitution of dietary macronutrients and their sources in association with breast cancer: Results from a large-scale case-control study. Eur. J. Nutr. 2022;61:2687–2695. doi: 10.1007/s00394-022-02811-4. [DOI] [PubMed] [Google Scholar]
- 60.Sieri S., Chiodini P., Agnoli C., Pala V., Berrino F., Trichopoulou A., Benetou V., Vasilopoulou E., Sánchez M.J., Chirlaque M.D., et al. Dietary fat intake and development of specific breast cancer subtypes. J. Natl. Cancer Inst. 2014;106:dju068. doi: 10.1093/jnci/dju068. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Publicly available GWAS data on breast cancer survival/susceptibility were obtained from https://bcac.ccge.medschl.cam.ac.uk/bcacdata (accessed on 14 February 2023). Genetic data on the relative intake of macronutrients were obtained from GWAS reported by Meddens et al. [33].