Abstract
Objective
This study investigated changes in kidney histology over time in patients with lupus nephritis (LN) undergoing immunosuppressive treatment.
Methods
Patients with proliferative±membranous LN were studied. After a diagnostic kidney biopsy (Bx1), patients had protocol biopsy 2 (Bx2) at 9 (6–15) months and protocol biopsy 3 (Bx3) at 42 (28–67) months. Kidney histological activity and chronicity indices (AI, CI) were measured.
Results
AI declined in a biphasic fashion, falling rapidly between Bx1 and Bx2 and then more slowly between Bx2 and Bx3. Patients were divided into those who achieved histological remission, defined as an AI=0 at Bx3 (group 1), and those with persistent histological activity (AI >0) at Bx3 (group 2). The early decline in AI was 1.6 times greater (95% CI 1.30, 1.91) in group 1 than group 2 (p=0.01). Between Bx2 and Bx3, the AI decline was 2.19-fold greater (95% CI 2.09, 2.29) in group 1 versus group 2 (p=7.34×10−5). Individual histological components of the AI resolved at different rates. Inflammatory lesions like glomerular crescents, karyorrhexis and necrosis mostly resolved by Bx2, whereas endocapillary hypercellularity, subendothelial hyaline deposits and interstitial inflammation resolved slowly, accounting for residual histological activity at biopsy 3 in group 2. In contrast, CI increased rapidly, by 0.15 units/month between Bx1 and Bx2, then plateaued. There were no differences in the rate of accumulation of chronic damage between group 1 and group 2. The increase in CI was significantly related to the severity of glomerular crescents (p=0.044), subendothelial hyaline deposits (p=0.002) and interstitial inflammation (p=0.015) at Bx1.
Conclusions
LN histological activity takes months to years to resolve, providing a rationale for the need of long-term, well-tolerated maintenance immunosuppression. Despite responding, LN kidneys accrue chronic damage early during treatment. This finding provides an explanation for the association of chronic progressive kidney disease with recurrent episodes of LN.
Keywords: lupus nephritis, inflammation, autoimmune diseases
WHAT IS ALREADY KNOWN ON THIS TOPIC
The time course of kidney repair during active immunosuppressive treatment of lupus nephritis has not previously been characterised.
WHAT THIS STUDY ADDS
This study describes, for the first time, how kidneys with lupus nephritis heal at the tissue level.
HOW THIS STUDY MIGHT AFFECT RESEARCH, PRACTICE OR POLICY
This study demonstrates that complete resolution of inflammatory kidney injury in lupus nephritis often takes years of treatment, emphasising the need for well-tolerated maintenance immunosuppression. Because kidney repair results in the rapid accumulation of chronic kidney damage, lupus nephritis may be best managed through a combined strategy of immunosuppression and mitigation of tissue fibrosis.
Introduction
The present management of proliferative lupus nephritis (LN) starts with a period of intense immunosuppression to rapidly attenuate kidney inflammation and begin to control autoimmunity, followed by long-term, less intense immunosuppression to consolidate and maintain a complete kidney response and provide ongoing control of autoimmunity. The overall duration of immunosuppression is not clearly defined. The European Alliance of Associations for Rheumatology and the European Renal Association (EULAR/ERA) LN guidelines recommend slowly withdrawing immunosuppression after patients have achieved and maintained a complete clinical response for 3–5 years.1 The Kidney Disease Improving Global Outcomes LN guidelines recommend discontinuation of immunosuppression after patients have been in complete clinical response for at least 12 months.2 Like the EULAR/ERA guidelines, this generally translates to several years of immunosuppression.3 This management paradigm seems straightforward, but the details are more complex. Many patients with LN, especially those who have had moderate-severe kidney damage during an episode of active disease, or who have had multiple LN flares, may never be able to meet current clinical criteria for a complete kidney response because they have fixed, persistent proteinuria due to glomerulosclerosis and interstitial fibrosis.4 5 Nonetheless, such patients may have no ongoing immunological or inflammatory activity in the kidney.6 Using clinical criteria alone, immunosuppression will be continued longer than needed. Alternatively, up to one-third of patients who achieve a complete clinical kidney response have ongoing intrarenal inflammation.7–9 Such patients are at high risk of developing an LN flare if medication is tapered off too soon,10 and avoiding flares is important for avoiding chronic kidney injury and preserving nephron mass.11
These observations raise the question of how rapidly one may expect the immunological injury of LN to resolve in patients who are treated with standard-of-care (SOC) immunosuppression and respond well. We postulated that histological response occurs slowly and in general takes months to years for complete resolution of immunological activity. To address this question, we examined a cohort of patients with proliferative LN who underwent serial biopsies during treatment to characterise the trajectory of resolution of active injury and the development of chronic kidney damage.
Methods
Patients
The 110 patients with proliferative or proliferative plus membranous LN reported here are followed by the Nephrology Division at Hospital Fernandez in Buenos Aires, Argentina. Clinical and histological data were systematically collected on sequential patients since 2005, and this analysis used data captured through the end of 2021. These patients were included because they achieved clinical kidney remission with treatment. These patients were all diagnosed with SLE having at least four American College of Rheumatology criteria, and were found to have biopsy-proven immune-complex glomerulonephritis consistent with proliferative or proliferative plus membranous LN. LN was de novo in all but 15 patients who had been biopsied before 2005 and were entered into this cohort at the time of an LN flare. One patient died in 2020 and one patient was lost to follow-up after 2015.
Patients were not involved in the design or conduct of this study.
Management of patients
All of the patients were treated initially with glucocorticoids and mycophenolate mofetil (MMF) or intravenous high-dose cyclophosphamide and transitioned to MMF or azathioprine for long-term immunosuppression. The management of maintenance immunosuppression was guided by a kidney biopsy-based algorithm developed by the Hospital Fernandez Nephrology Division and previously described.7 Briefly, after a diagnostic kidney biopsy (biopsy 1) and treatment for LN, a second kidney biopsy (biopsy 2) was done a median of 9 months (range 6–15 months) after diagnosis and shortly after completion of intense immunosuppression. This biopsy was done to verify that patients were improving histologically and guided subsequent treatment. To decide if maintenance immunosuppression (cytotoxic agents and glucocorticoids) could be withdrawn, a third kidney biopsy (biopsy 3) was done. Biopsy 3 occurred after at least 36 months of treatment and 12 months of having achieved a complete clinical kidney response (n=99, proteinuria ≤500 mg/day, normal or stable serum creatinine concentration, no extrarenal SLE activity), or if patients had responded well but did not quite achieve the proteinuria target and wanted to come off treatment (n=11). Biopsy 3 was done a median of 42 (28–67) months after biopsy 1. Immunosuppression was withdrawn if the National Institutes of Health (NIH) LN activity index (AI) was 0 on biopsy 3, but was continued for 2 more years if the AI was ≥1. A fourth kidney biopsy (biopsy 4) was done and if AI=0, immunosuppression was stopped. If AI was ≥1, immunosuppression was continued for 2 more years and the kidney biopsy (biopsy 5) was repeated. In summary, immunosuppression was maintained until there was no evidence of histological activity.
Acquisition of clinical and histological data
Patients were seen as clinically indicated in the outpatient setting, and at each visit, an interval history was taken and a physical examination performed. Urinalysis, serum chemistry panel, complete blood count, complement levels and 24-hour proteinuria were measured monthly during the initial 6 months of treatment and every 2 months during maintenance immunosuppression. Kidney biopsies performed with a spring-loaded biopsy gun were assessed by light, immunofluorescence microscopy, and in selected cases, electron microscopy. The International Society of Nephrology/Renal Pathology Society (ISN/RPS) LN classification system was used to describe the biopsies.12
In addition to classifying LN by ISN/RPS criteria, the revised NIH AI and chronicity indices (CI) were used to grade each biopsy.13 The AI is comprised of six histological features that reflect intrarenal inflammation and immune injury. The glomerular components of the AI are endocapillary hypercellularity (an increased number of cells, usually leucocytes that occlude the capillary lumens), cellular and fibrocellular crescents, fibrinoid necrosis (fibrin in areas of glomerular basement membrane disruption and/or mesangial matrix lysis), neutrophils/karyorrhexis (infiltration of glomerular by polymorphonuclear leucocytes or the presence of fragmented nuclei) and hyaline deposits (subendothelial immune complex deposits) in the glomerular capillaries. The tubulointerstitial component of the AI is interstitial inflammation defined as infiltration of the tubulointerstitial space by leucocytes, away from areas of sclerosis and atrophy. Using light microscopy, individual components are semiquantitatively graded on a scale of 0–3 based on presence and severity. The AI is the sum of the scores of these individual components. The AI has a maximum value of 24 because scores for crescents and fibrinoid necrosis are doubled to capture the severity of inflammation they represent. The CI has four components. The glomerular components of the CI are global and/or segmental glomerulosclerosis and fibrous crescents (crescents having more than 75% fibrous matrix), and the tubulointerstitial components are tubular atrophy and interstitial fibrosis. Grading is similar to the AI, with each component receiving a score of 0–3 leading to a maximum chronicity score of 12. A second blinded nephropathologist independently read 25% of the biopsies (chosen randomly) and calculated AI and CI. Concordance for AI was 71% and for CI 93%. The AI readings that were discordant differed by 1 AI unit.
Immunofluorescence for C3 and C1q, and the immunoglobulins IgG, IgA and IgM was routinely performed and graded for intensity on a semiquantitative scale of 0–3. Biopsies were evaluated by an experienced nephropathologist (VA). All histopathology readings were done for clinical purposes and the data later used for this observational study.
Statistical analysis
Data are presented as mean±SD or as median (range). This cohort of patients was divided into four groups. Group 1 was defined as patients who had achieved histological remission (AI=0) on their biopsy to decide if immunosuppression could be withdrawn (biopsy 3). Group 2 was comprised of patients who had not achieved histological remission (AI >0) on biopsy 3. Group 3 consisted of patients who had achieved histological remission on biopsy 3 but during follow-up experienced an LN flare. Group 4 patients were still being treated and had only undergone diagnostic and 1-year kidney biopsies. Demographics and continuous clinical variables were compared between groups using the Mann-Whitney test (two groups) or the Kruskal-Wallis test (multiple groups) followed by Dunn’s post-test for multiple comparisons. Categorical variables were compared using the Fisher’s exact test.
To evaluate the trajectory of resolution of the AI and each of its component lesions, a series of linear mixed-effects models with linear splines (LME-LS) were constructed. Trajectory analyses were restricted to patients who had all three biopsies (biopsies 1, 2 and 3) and who never had an LN flare. An interaction term between time and group was included in all LME-LS models to evaluate if patients who achieved AI=0 at biopsy 3 (group 1) resolved immunological activity differently than those who did not achieve AI=0 at biopsy 3 (group 2). Associations between changes in (1) histological damage, (2) kidney function (serum creatinine and log-transformed proteinuria), and (3) complement levels and change in AI were examined using a series of LME models that included AI as the predictor. The trajectory of increase of the CI and each component lesion of the CI was also evaluated using a series of LME-LS models. The association between AI and CI was evaluated using an LME model that included AI as the predictor. All LME and LME-LS models accounted for correlation between observations from the same subject.
Results
The 110 patients (table 1) in this cohort were managed with repeat kidney biopsies as shown in figure 1. Of note, 31 patients did not have a second, post-induction biopsy; their second biopsy was included under the heading of biopsy 3 because it was done to determine if maintenance therapy could be stopped. The cohort was followed for a median of 9 years. During follow-up, 74 patients (67%) achieved an AI=0 at biopsy 3 and immunosuppression was withdrawn. Subsequently, eight of these patients (designated patient group 3) had an LN flare (7.3%) and were retreated. Group 3 patients had a median (range) proteinuria of 4.7 g/day (0.9–9) at biopsy 1 compared with 2 g/day (0.4–7) for those (patient group 1) who remained in remission (p=0.006). Group 3 also had a higher titre of anti-double-stranded DNA (dsDNA) antibodies at biopsy 1 than group 1 (1:960 (1:160–1:1280) vs 1:320 (0–1:1280), p=0.047); however, the per cent of patients who were positive for anti-dsDNA did not differ between groups. Numerically, the flare patients were more often class III and more often had low levels of complement components at biopsy 1, but neither of these differences was statistically significant.
Table 1.
Demographic, clinical and histological characteristics of the patient cohort
| Characteristics | Entire cohort (n=110) | Patient group 1* (n=67) | Patient group 2† (n=30) | Patient group 3‡ (n=8) | Patient group 4§ (n=5) |
| Age at Bx1 (years) | 31 (17–58)¶ | 32.5 (17–56) | 31 (17–49) | 29 (22–47) | 32 (20–58) |
| Female, n (%) | 89 (81)** | 55 (84) | 23 (77) | 7 (87.5) | 3 (60) |
| Duration of follow-up (months) | 109 (34–202) | 107 (35–202) | 135 (40–192) | 111 (101–152) | 48 (34–76) |
| Initial treatment MMF, n (%) | 61 (55) | 37 (55) | 17 (57) | 4 (50) | 4 (80) |
| Initial treatment cyclophosphamide, n (%) | 49 (45) | 30 (45) | 13 (42) | 4 (50) | 1 (20) |
| Serum creatinine at Bx1 (mg/dL) | 0.8 (0.4–4) | 0.9 (0.4–4) | 0.8 (0.5–0.4) | 0.85 (0.6–1.3) | 0.7 (0.5–1.3) |
| Serum creatinine at last follow-up (mg/dL) | 0.7 (0.5–3.4) | 0.7 (0.5–3.0) | 0.7 (0.5–1.3) | 0.7 (0.6–2) | 0.8 (0.5–0.9) |
| Patients progressing to ESKD or death, n (%) | 2 (1.8) ESKD | 2 (3) ESKD | 0 | 1 (12.5) death | 0 |
| Patients with CKD†† at last follow-up, n (%) | 9 (8) | 6 (9) | 0 | 3 (37.5) | 0 |
| Proteinuria at Bx1 (g/day) | 3 (0.4–12) | 3 (0.4–12) | 3.1 (0.6–8) | 4.7 (0.9–9) | 1.2 (0.5–5) |
| Proteinuria at last follow-up (g/day) | 0.2 (0–3) | 0.2 (0–0.8) | 0.3 (0–1.9) | 0.25 (1–3) | 0.2 (0.2–0.5) |
| Anti-dsDNA positive at Bx1, n (%) | 92 (84) | 54 (81) | 26 (87) | 8 (100) | 4 (80) |
| Anti-dsDNA titre at Bx1‡‡ | 500 (80–1280) | 640 (80–1280) | 320 (80–1280) | 960 (160–1280) | 480 (160–640) |
| C3 at Bx1 (mg/dL) | 56 (20–124) | 53 (23–101) | 60 (23–124) | 53 (20–84) | 52 (44–80) |
| C3 low at Bx1, n (%) | 77 (70) | 46 (69) | 20 (67) | 7 (87.5) | 4 (80) |
| C4 at Bx1 (mg/dL) | 8 (1.6–50) | 8 (2–50) | 9 (2–29) | 5.4 (3–27) | 12 (6–15) |
| C4 low at Bx1, n (%) | 68 (62) | 42 (63) | 16 (53) | 7 (87.5) | 2 (40) |
| Bx1 ISN/RPS class III | 29 (26%) | 17 (25.4%) | 5 (16.7%) | 4 (50%) | 3 (60%) |
| Bx1 ISN/RPS class III+V | 10 (9%) | 7 (10.4%) | 3 (10%) | 0 | 0 |
| Bx1 ISN/RPS class IV | 60 (55%) | 34 (50.7%) | 21 (70%) | 3 (37.5%) | 2 (40%) |
| Bx1 ISN/RPS class IV+V | 11 (10%) | 9 (13.4%) | 1 (3.9%) | 1 (12.5%) | 0 |
| Activity index (AI) of Bx1 | 7.5 (1–16) | 8 (2–17) | 7.5 (3–16) | 8 (4–15) | 3 (1–8) |
| Chronicity index of Bx1 | 3 (0–6) | 3 (0–6) | 3 (0–6) | 3 (0–5) | 4 (1–5) |
*Group 1 patients had an AI of 0 on their biopsy done to decide whether immunosuppression could be withdrawn.
†Group 2 patients had an AI >0 on their biopsy done to decide whether immunosuppression could be withdrawn.
‡Group 3 patients had an LN flare after achieving clinical and histological remission.
§Group 4 patients are still being treated and have had only initial and 1-year biopsy.
¶Data presented as median and range.
**Data presented as number of patient and per cent of cohort of subgroup.
††This does not include those patients who have gone on to ESKD.
‡‡Titre only in those patients positive for anti-dsDNA antibodies.
Bx1, biopsy 1; CKD, chronic kidney disease; dsDNA, double-stranded DNA; ESKD, end-stage kidney disease; ISN/RPS, International Society of Nephrology/Renal Pathology Society; LN, lupus nephritis; MMF, mycophenolate mofetil.
Figure 1.
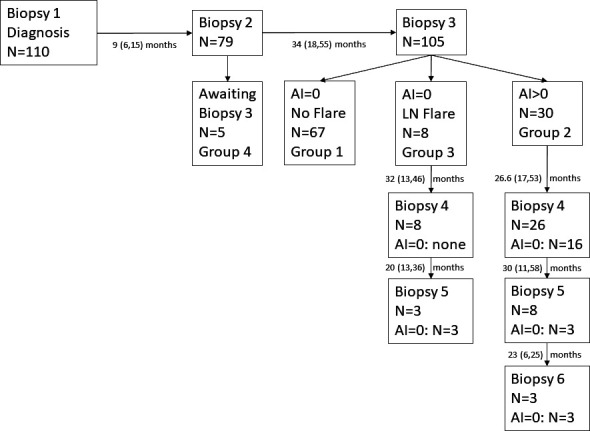
Patient kidney biopsy flow diagram. All patients had a diagnostic biopsy (biopsy 1). Patients are divided into groups corresponding with the groups listed in table 1. The number of kidney biopsies each patient group had is indicated as is the median (range) time between biopsies. AI, activity index; LN, lupus nephritis.
Of the 79 patients who had a biopsy after initial therapy (biopsy 2), 16 patients (20%) achieved an AI=0. At biopsy 3, 3 of these 16 patients (18.8%) had an AI ≥1 and continued therapy. The rest maintained no histological activity at biopsy 3.
Thirty-one patients (28%) did not reach an AI=0 by biopsy 3 and remained on immunosuppression longer. These were designated patient group 2. A fourth biopsy was done in 27 of these 31 patients and 16 were found to have no further histological activity so immunosuppression was stopped a median of 26 (17–53) months after biopsy 3. None of these patients have had an LN flare to date. A fifth biopsy was done 30 (11–58) months after biopsy 4 in eight of the nine remaining patients, and three patients had an AI=0, leading to withdrawal of immunosuppression. There were no significant clinical or serological features at diagnosis that distinguished group 1 from group 2 (table 1). There were fewer pure class IV patients in group 1 and more patients with class IV+V, but this was not significantly different than group 2. Finally, we included a few more recent patients who have only had biopsies 1 and 2 (group 4).
Overall, during long-term follow-up, this LN cohort responded very well to treatment. One patient died of a non-COVID-19-related pneumonia, two (1.8%) patients developed kidney failure requiring dialysis, and at last follow-up, nine (8%) patients had chronic kidney disease (CKD) with an estimated glomerular filtration rate (eGFR) <60 mL/min/1.73 m2. Of the patients with CKD, four had pre-existing CKD and five developed de novo CKD during treatment. Viewed another way, 11 (10%) patients had stage 2 CKD (eGFR 60–89 mL/min/1.73 m2), 6 (5.5%) patients had stage 3a CKD (eGFR 45–59 mL/min/1.73 m2) and 2 (1.8%) patients had stage 3b CKD (eGFR 30–44 mL/min/1.73 m2).
Resolution of AI and individual AI legion components
As anticipated for a group of patients responding well to treatment, the AI declined over the 42 (28–67) months between biopsy 1 and biopsy 3 from 7.5 (1–16) to 0 (0–6), p<0.0001. At biopsy 3, the only patients contributing to a non-0 AI were those of group 2 who had not achieved histological remission. The dependence of the rate of decline in AI on baseline AI was tested by calculating the linear regression line between AI slope for each patient and AI at biopsy 1. Considering only those patients who had biopsies 1, 2 and 3 and never flared (n=69), those with a higher baseline AI had a steeper rate of AI decline (r2=0.61, p<0.0001).
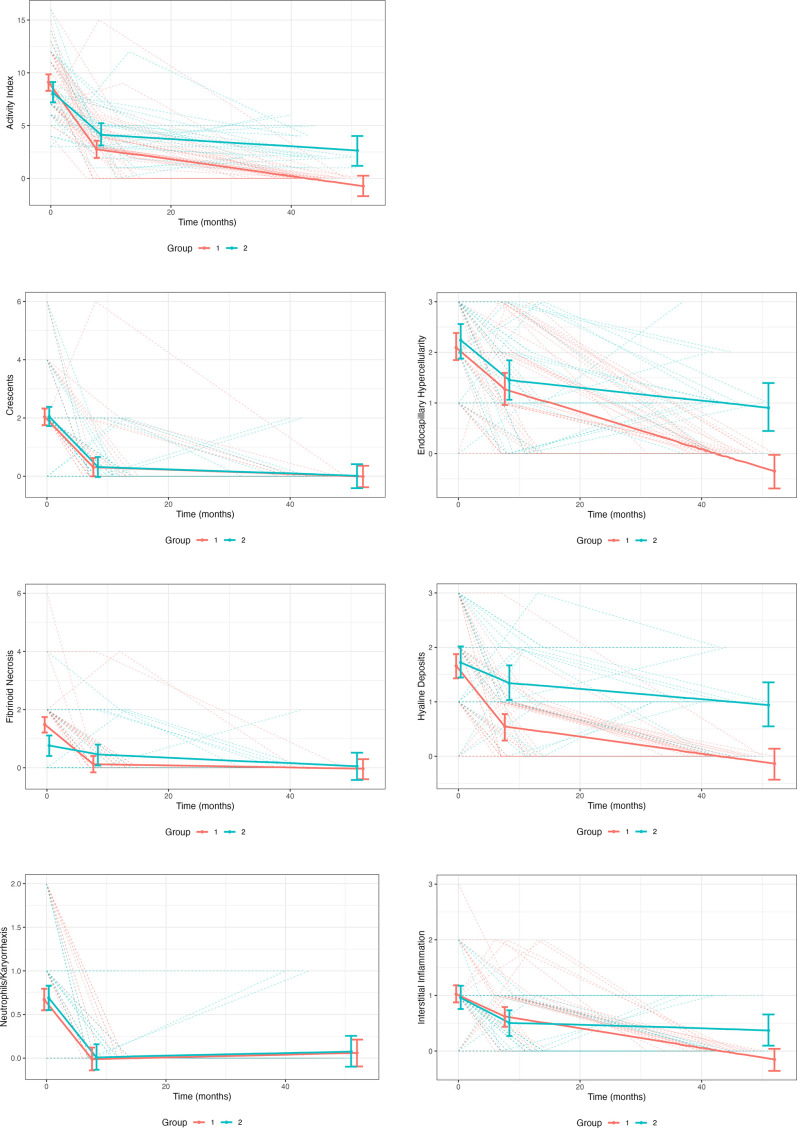
For patients who had biopsies 1, 2 and 3, the trajectory of resolution of the AI and each component lesion of the AI was visualised using predicted trend lines from LME-LS models (figure 2). These models included 69 patients from groups 1 and 2, and excluded patients who flared (n=5, group 3). The decline in AI during treatment was biphasic for both groups 1 and 2. Activity fell quickly between 0 and 8 months and then the rate of decline decreased in both groups. Overall, the rate of AI decline was significantly greater in group 1 than group 2 (p=0.0001). The initial rate of decline in group 1 was 1.60-fold (95% CI: 1.30, 1.91) greater than group 2 (0.79 units/month in group 1 vs 0.49 units/month in group 2, p=0.01). The subsequent rate of decline in group 1 was 2.19-fold (95% CI 2.09, 2.29) times greater than group 2 (0.08 units/month in group 1 vs 0.04 units/month in group 2, p=7.34×10−5). The AI components that resolved most rapidly and completely, generally within 8 months of initiating treatment, were glomerular crescents, neutrophils/karyorrhexis and fibrinoid necrosis. The rate of resolution of crescents and neutrophils/karyorrhexis was similar between group 1 and group 2 patients (p=0.94 and p=0.77, respectively); however, fibrinoid necrosis took significantly longer to resolve in group 2 (p=0.001). In contrast, glomerular endocapillary hypercellularity, subendothelial hyaline deposits and interstitial inflammation took approximately 40 months to resolve in group 1, and endocapillary hypercellularity and subendothelial hyaline deposits were the dominant ongoing lesions beyond 40 months contributing to the residual histological activity of group 2 at biopsy 3, with smaller contributions from interstitial inflammation and fibrinoid necrosis. The rate of decline in capillary hypercellularity, hyaline deposits and interstitial inflammation was significantly less in group 2 (p=0.006, p=0.0003 and p=0.019, respectively) than group 1.
Figure 2.
The decrease of active kidney injury during treatment. All patients analysed had three kidney biopsies. The patients were divided in those who achieved a complete histological remission by biopsy 3 (orange) and those who still had histological activity on biopsy 3 (green). The trajectory of the overall activity index is shown in the first graph, followed by the trajectories of the individual components of the activity index. Time is given in months after the first (diagnostic) biopsy at time 0. Linear splines that account for each patient at each time point are shown.
Increase of CI and individual CI legion components
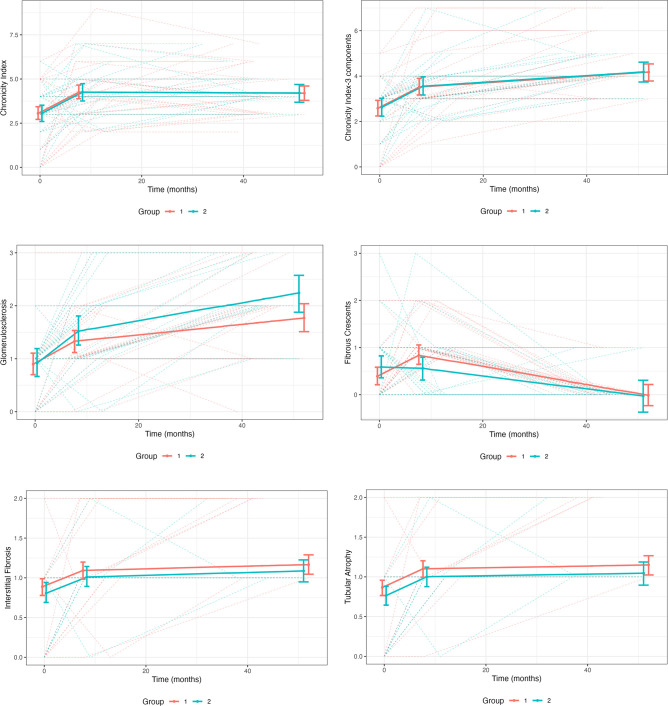
The CI of the cohort increased over the 42 (28–67) months between biopsy 1 and biopsy 3. The median CI for all patients at biopsy 1 was 3 (0–6) and at biopsy 3 was 4 (1–7), p<0.001. The increase in CI could not be attributed only to the group 2 patients who did not reach histological remission (AI=0) at biopsy 3. The median CI at biopsy 3 for the patients who achieved and did not achieve histological remission at biopsy 3 was 4 (1–7), p=0.14. The trajectory of the CI between biopsy 1 and biopsy 3 was biphasic, with an initial rapid increase followed by a plateau (figure 3). The increase in CI over time was similar in groups 1 and 2 (p=0.99). The trajectories of glomerulosclerosis, interstitial fibrosis and tubular atrophy were also biphasic, with a rapid increase during the first 8 months of treatment, followed by a slower increase up through month 52 (figure 3). The fibrous crescent score did not follow this pattern and seemed to improve overtime in both groups. This, however, is likely an artefact of the difficulty in distinguishing fibrous crescents from segmental glomerulosclerosis as these lesions age. If the fibrous crescent component is pulled from the total CI calculation, the CI curve over time shows that after the initial steep accumulation of damage, damage continues to accumulate but at a slower pace (figure 3).
Figure 3.
The increase in chronic kidney damage during treatment. All patients analysed had three kidney biopsies. The patients were divided in those who achieved a complete histological remission by biopsy 3 (orange) and those who still had histological activity on biopsy 3 (green). The trajectory of the overall chronicity index is shown in the first graph, followed by the overall chronicity index in which the component score for fibrous crescents had been removed (Chronicity Index-3 Components). This was done because fibrous crescents are difficult to distinguish from glomerulosclerosis and appear to resolve over time, flattening the trajectory of the overall chronicity index. Time is given in months after the first (diagnostic) biopsy at time 0. The trajectories of the individual components of the chronicity index are also given. Linear splines that account for each patient at each time point are shown.
Association between AI and CI
Inflammation of the kidney parenchyma is likely the driving force behind chronic kidney damage in LN. Despite resolving activity, kidney damage increased. We found a significant association between AI and CI such that for every unit decrease in AI, CI increased by 0.16 units on average (p<0.0001). Considering each component of the AI using a Pearson Χ2 test, the increase in the CI between biopsy 1 and biopsy 3 was significantly associated with baseline levels of hyaline deposits (p=0.0019), cellular crescents (p=0.044) and interstitial inflammation (p=0.015). None of the other AI components was associated with change in CI over time. None of the AI components was significantly associated with CI on biopsy 1.
Association between clinical findings and AI and CI
Serum creatinine, log (proteinuria), C3 and C4 were associated with AI. Specifically, for every unit decline in AI, proteinuria declined by 21% (p<0.0001), serum creatinine declined by 0.02 mg/dL (p<0.0001), C3 increased by 3.92 mg/dL (p<0.0001) and C4 increased by 0.81 mg/dL (p<0.0001). In contrast, there was no consistent association between increases in CI and changes in proteinuria, serum creatinine or plasma complement levels in this cohort.
Evaluation of immunoglobulins and complement components
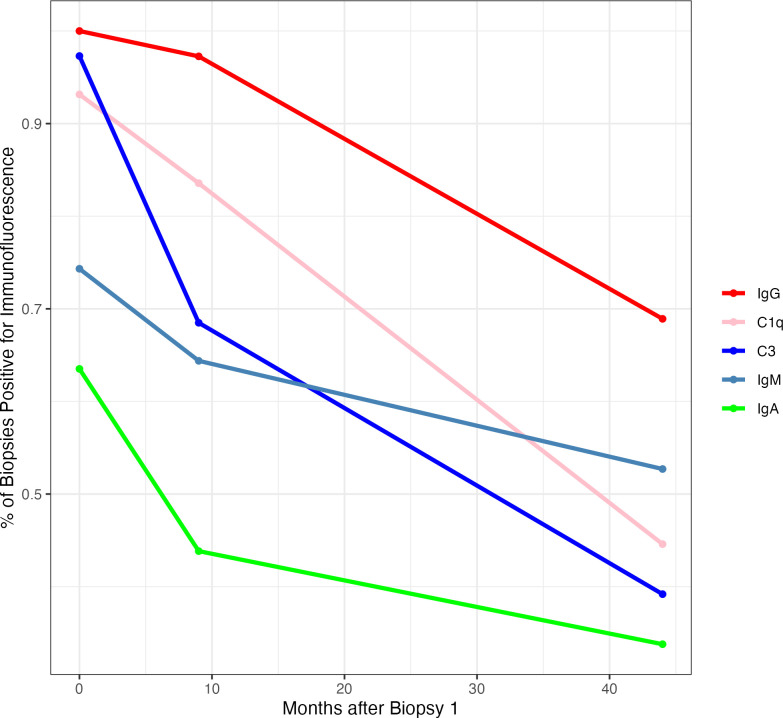
As LN improves, it is expected that C3 and C1q, and IgG, IgA, IgM in glomeruli will decline and/or disappear. To visualise this trajectory, the average proportion of biopsies that were positive for IgG, IgA, IgM, C3 and C1q immunofluorescence was determined for the cohort, and plotted against average time from biopsy 1 (figure 4). Complement components seemed to clear from glomeruli more rapidly than immunoglobulins, but even after a minimum of 3 years of therapy, complement was still present in the glomeruli of over 30% of patients with LN. Glomerular IgG, present in nearly every biopsy at the time of diagnosis, resolved more slowly than complement and other immunoglobulins. After more than 3 years of treatment, close to 70% of biopsies were still positive for glomerular IgG, and immunofluorescence remained positive more often in patients who did not achieve an AI of 0 at biopsy 3 (table 2). Of note, 20% of patients cleared all deposits and none of these patients flared. The semiquantitative intensity of IgG, C3 and C1q immunofluorescence did decline significantly between biopsies 1 and 3. At biopsy 1, the median (range) intensities of IgG, C3 and C1q were 3 (0–3), 2 (0–3) and 3 (0–3), respectively, and at biopsy 3, the median intensities were 1 (0–3), 0 (0–3) and 0 (0–3), respectively (all p<0.0001). Importantly, the persistence of glomerular complement and IgG after several years of treatment was not due entirely to patients with persistent histological activity at biopsy 3 (group 2). Rather, the patients from groups 1 and 3, all of whom had an AI=0 at biopsy 3, contributed to the ongoing presence of immune reactants in the kidney (table 2).
Figure 4.
The decrease in glomerular immunofluorescence during treatment. The presence or absence of immunofluorescence of IgG, IgA and IgM, and C1q and C3 for each patient was determined at each biopsy. The per cent of patients positive for each immune reactant at each biopsy is shown. The time between biopsies is the average time based on all of the patients.
Table 2.
Glomerular immunofluorescence at biopsy 3
| Antigen | Immunofluorescence positive | ||
| AI=0* (n=76) | AI ≥1† (n=32) | P value | |
| IgG | 55% | 94% | <0.0001 |
| IgA | 26% | 50% | 0.025 |
| IgM | 37% | 66% | 0.01 |
| C3 | 21% | 66% | <0.0001 |
| C1q | 22% | 78% | <0.0001 |
*Patients who achieved an AI of 0 at biopsy 3.
†Patients who did not achieve an AI of 0 at biopsy 3.
AI, activity index.
Discussion
During the treatment of LN, clinical response, defined by improvement in proteinuria, kidney function and autoimmune serology, assumes concomitant improvement in kidney histology. However, kidney biopsies during treatment are usually done for lack of response, so very little is known about kidney histology of patients who respond well to treatment, achieve clinical remission and maintain good long-term kidney health. To address this knowledge gap, the time course of improvement in kidney histology and inflammation in patients with SLE who were treated successfully for active LN was characterised by evaluating serial kidney biopsies. Our data show that active lesions, defined as the components of the NIH AI, decline rapidly during initial immunosuppression but do not often resolve by the end of induction therapy. Complete resolution occurs over the ensuing months to years, during long-term therapy with less intense immunosuppression. In some cases, more than 10 years may be required to achieve complete histological remission. As expected, improvement in the AI predicts improvements in proteinuria, kidney function and complement consumption. However, despite achieving good clinical response, chronic lesions, defined as the components of the NIH CI, accumulate during initial immunosuppression, and then stabilise during long-term immunosuppression. Furthermore, glomeruli remain positive for immunoglobulins, especially IgG, and complement components in a large proportion of patients even after over 3 years of continuous immunosuppressive therapy.
The decline in the AI appears to be biphasic, rapid initially and then more slowly. It is possible that this biphasic response is exaggerated because only three biopsy time points were available, and the time between biopsies 2 and 3 was almost 3 years, a limitation of this study. To verify the shape of the AI trajectory more accurately would require biopsies at intermediate time points after biopsy 2. Nonetheless, this biphasic pattern raises the question of whether a longer course of intense immunosuppression after LN is diagnosed, as opposed to the usual 3–6 months before switching to a less intense regimen, may result in more rapid resolution of active lesions. The tradeoff, of course, may be increased treatment toxicity. Ideally, an individual’s histological trajectory assessed during the first year of treatment with repeat histology, or ideally with a non-invasive biomarker of pathology, would be used to precisely tailor the approach to maintenance treatment.
Resolution of active injury also appears to be specific for each histological component. The highly inflammatory lesions (neutrophils/karyorrhexis, fibrinoid necrosis, cellular/fibrocellular crescents) mostly tend to resolve completely during initial immunosuppression. This observation, coupled with the finding that AI declines more rapidly in patients with higher baseline AI, suggests that current SOC treatment works well for highly inflammatory lesions. It is interesting to speculate that this may be due to the high-dose glucocorticoids used for most patients at the start of LN treatment. Given the side-effect profile of glucocorticoids, it would be ideal to develop a non-glucocorticoid anti-inflammatory drug for LN. In contrast to the highly inflammatory histological lesions, endocapillary hypercellularity, subendothelial hyaline deposits and interstitial inflammation seem more resistant to SOC therapy, and are still present in over 50%–60% of patients after 8–9 months of treatment, although at lower intensity. These lesions eventually resolve in many patients during the long term, less intense immunosuppression that follows initial therapy, supporting the usual practice of treating patients with LN for many months/years. Long-term immunosuppression appears to be required even for those patients who achieve complete histological remission (AI=0) after initial treatment. In our cohort, 20% of patients were in histological remission at the time of biopsy 2, but only 81% maintained complete remission in the years between biopsies 2 and 3 despite receiving ongoing immunosuppression.
Glomerular endocapillary hypercellularity and subendothelial hyaline deposits seem to be the main components of the AI that drive persistent histological activity in the individuals who do not reach an AI=0 by biopsy 3. In the context of this seemingly variable responsiveness of active lesions to SOC LN treatment, it will be interesting to determine whether newly approved LN drugs14 15 can change the trajectory of the slower-to-resolve lesions.
Accumulation of chronic damage within the kidney during LN also appears to follow a biphasic time course, with most of the increase in CI and the components of CI occurring during initial immunosuppression. Interestingly, accumulation of chronic kidney damage did not differ between patients who did or did not reach an AI of 0 by biopsy 3. This is somewhat surprising given the intensity of initial immunosuppression for LN, and suggests that fibrotic damage occurs rapidly in the presence of active inflammation despite SOC therapy. Chronic damage seems to be driven mainly by glomerular subendothelial hyaline deposits, glomerular crescents and tubulointerstitial inflammation. It remains to be seen whether the new therapies for LN14 15 mitigate this increase in CI, or whether an alternative management strategy, such as adding an anti-fibrotic agent, when available, may attenuate accumulation of chronic damage. Nonetheless, if the kidney accumulates additional chronic damage with every episode of active LN, it is easy to understand why LN flares predispose to the development of progressive CKD.16–18
The other surprising finding was the persistence of immunoglobulin and complement proteins in glomeruli. Over 30% of the patients had complement and immunoglobulin positivity despite 3 or more years of therapy, and IgG remained in the glomeruli of about 70% of patients. The implications of ongoing immunoreactants in the kidney are not known. None of the patients who cleared all their deposits developed an LN flare while under observation, but flares also did not occur in most patients with persistent immunoglobulins and complement in their kidneys.
This study has several limitations. The number of patients is relatively small, and the cohort is of homogeneous ethnicity (Hispanic), so there are concerns with generalisability. Not everyone had a repeat kidney biopsy within the first year after treatment, but our analyses focused on the (smaller) subset of patients who had biopsies 1, 2 and 3 to develop more accurate trajectories of histological change. Histological and immunological variables are only semiquantitative. Nonetheless, this type of granular examination of change in kidney histology as LN improves has never been done before and has yielded some fundamental observations concerning the nature of the kidneys’ response to treatment.
From a clinical perspective, these data raise several issues that have implications for long-term kidney health. The rapidity of resolution of the most inflammatory histological lesions begs the question of whether glucocorticoid-free regimens will be possible for LN. The rapid appearance of histological damage with current SOC drugs highlights the importance of LN flare prevention and the relevance of clinical trials designed to look at a flare endpoint, even if more time-consuming than current renal response trials. The accumulation of chronicity also suggests that anti-fibrotic agents, when available, may find a role in the initial treatment of LN to control damage along with immunomodulation to control inflammation and autoimmunity. Finally, investigation of why certain histological lesions are persistent may uncover novel pathways and targets for more rapid and complete resolution of kidney inflammation in LN.
In conclusion, successful treatment of LN does result in resolution of the active histological lesions of LN, but this takes a considerable amount of time, longer than usually appreciated. The aftermath of an episode of active LN seldom results in the return of the kidneys to a pristine state. Even in the best responders, kidneys are often left with some amount of chronic injury, and components of the immune system remain within the kidney parenchyma.
Footnotes
Twitter: @bradrovin
AM and VA contributed equally.
Contributors: This study was conceived and conducted by AM and VA. AM, VA, BL, ML, JM and LB managed the patients in this cohort and were responsible for collection of clinical data. VA, CB and JN analysed kidney histology. HNN, AK and BW performed the statistical analyses for this work. BR had access to all the data, and with AM and VA, was responsible for data analysis, data interpretation and data presentation. BR wrote the first draft, and all authors were subsequently involved in critical review and revision of the draft and approved the submitted version. AM and BR are specifically responsible for the overall content of this work and accept full responsibility for the work as guarantors, had access to the data, and controlled the decision to publish.
Funding: BR, AK and BW were supported in part by NIH (R01 AR071947).
Competing interests: None declared.
Patient and public involvement: Patients and/or the public were not involved in the design, or conduct, or reporting, or dissemination plans of this research.
Provenance and peer review: Not commissioned; externally peer reviewed.
Data availability statement
Data are available upon reasonable request. Please provide a written request and contact information to the first author or the corresponding author.
Ethics statements
Patient consent for publication
Not required.
Ethics approval
This study involves human participants. This protocol was approved by the ethics committee of Hospital Fernandez when it was initiated, but is now standard of care in the practice. All patients signed informed consent for the anonymised use of their data.
References
- 1.Fanouriakis A, Kostopoulou M, Cheema K, et al. Update of the Joint European League Against Rheumatism and European Renal Association-European Dialysis and Transplant Association (EULAR/ERA-EDTA) recommendations for the management of lupus nephritis. Ann Rheum Dis 2020;79:713–23. 10.1136/annrheumdis-2020-216924 [DOI] [PubMed] [Google Scholar]
- 2.Kidney Disease: Improving Global Outcomes (KDIGO) Glomerular Diseases Work Group . KDIGO 2021 clinical practice guideline for the management of glomerular diseases. Kidney Int 2021;100:S1–276. 10.1016/j.kint.2021.05.021 [DOI] [PubMed] [Google Scholar]
- 3.McKinley A, Park E, Spetie D, et al. Oral cyclophosphamide for lupus glomerulonephritis: an under-utilized therapeutic option. Clin J Am Soc Nephrol 2009;4:1754–60. 10.2215/CJN.02670409 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kopp JB, Factor VM, Mozes M, et al. Transgenic mice with increased plasma levels of TGF-beta 1 develop progressive renal disease. Lab Invest 1996;74:991–1003. [PubMed] [Google Scholar]
- 5.Diamond JR, Anderson S. Irreversible tubulointerstitial damage associated with chronic aminonucleoside nephrosis. Amelioration by angiotensin I converting enzyme inhibition. Am J Pathol 1990;137:1323–32. [PMC free article] [PubMed] [Google Scholar]
- 6.Malvar A, Pirruccio P, Alberton V, et al. Histologic versus clinical remission in proliferative lupus nephritis. Nephrol Dial Transplant 2017;32:1338–44. 10.1093/ndt/gfv296 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Malvar A, Alberton V, Lococo B, et al. Kidney biopsy-based management of maintenance immunosuppression is safe and may ameliorate flare rate in lupus nephritis. Kidney Int 2020;97:156–62. 10.1016/j.kint.2019.07.018 [DOI] [PubMed] [Google Scholar]
- 8.Parodis I, Adamichou C, Aydin S, et al. Per-protocol repeat kidney biopsy portends relapse and long-term outcome in incident cases of proliferative lupus nephritis. Rheumatology (Oxford) 2020;59:3424–34. 10.1093/rheumatology/keaa129 [DOI] [PubMed] [Google Scholar]
- 9.Zickert A, Sundelin B, Svenungsson E, et al. Role of early repeated renal biopsies in lupus nephritis. Lupus Sci Med 2014;1:e000018. 10.1136/lupus-2014-000018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.De Rosa M, Azzato F, Toblli JE, et al. A prospective observational cohort study highlights kidney biopsy findings of lupus nephritis patients in remission who flare following withdrawal of maintenance therapy. Kidney Int 2018;94:788–94. 10.1016/j.kint.2018.05.021 [DOI] [PubMed] [Google Scholar]
- 11.Anders H-J, Saxena R, Zhao M-H, et al. Lupus nephritis. Nat Rev Dis Primers 2020;6:7. 10.1038/s41572-019-0141-9 [DOI] [PubMed] [Google Scholar]
- 12.Weening JJ, D’agati VD, Schwartz MM, et al. The classification of glomerulonephritis in systemic lupus erythematosus revisited. Kidney International 2004;65:521–30. 10.1111/j.1523-1755.2004.00443.x [DOI] [PubMed] [Google Scholar]
- 13.Bajema IM, Wilhelmus S, Alpers CE, et al. Revision of the International society of Nephrology/renal pathology society classification of lupus nephritis: Clarification of definitions, and modified National Institutes of Health activity and Chronicity indices. Kidney International 2018;93:789–96. 10.1016/j.kint.2017.11.023 [DOI] [PubMed] [Google Scholar]
- 14.Furie R, Rovin BH, Houssiau F, et al. Two-year, randomized, controlled trial of belimumab in lupus nephritis. N Engl J Med 2020;383:1117–28. 10.1056/NEJMoa2001180 [DOI] [PubMed] [Google Scholar]
- 15.Rovin BH, Teng YKO, Ginzler EM, et al. Efficacy and safety of voclosporin versus placebo for lupus nephritis (AURORA 1): a double-blind, randomised, multicentre, placebo-controlled, phase 3 trial. Lancet 2021;397:2070–80. 10.1016/S0140-6736(21)00578-X [DOI] [PubMed] [Google Scholar]
- 16.Parikh SV, Nagaraja HN, Hebert L, et al. Renal flare as a predictor of incident and progressive chronic kidney disease in patients with lupus nephritis. Clin J Am Soc Nephrol 2014;9:279–84. 10.2215/CJN.05040513 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Enfrein A, Pirson V, Le Guern V, et al. Worse long-term renal outcome of lupus nephritis patients of African descent living in Europe. RMD Open 2022;8:e002386. 10.1136/rmdopen-2022-002386 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Perez-Arias AA, Márquez-Macedo SE, Pena-Vizcarra OR, et al. The influence of repeated flares in response to therapy and prognosis in lupus nephritis. Nephrol Dial Transplant 2023;38:884–93. 10.1093/ndt/gfac304 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data are available upon reasonable request. Please provide a written request and contact information to the first author or the corresponding author.