Abstract
Introduction
From 2018 single inhaler triple therapy (SITT) became available in France to treat moderate-to-severe chronic obstructive pulmonary disease (COPD). Given its simplified inhaler use compared with multiple inhaler triple therapy (MITT), this therapeutic option has the potential to offer benefit in terms of improved persistence and adherence. Given the lack of real-world evidence of the effectiveness of triple therapy, this study was designed to evaluate the use of MITT and SITT in France and compare persistence.
Methods
A retrospective cohort study was performed. Patients with COPD who initiated triple therapy between 1 July 2017 and 31 December 2019 were included from The Health Improvement Network, a large electronic medical database in France, which includes pharmacy data. A 60-day treatment gap defined discontinuation and thereby persistence.
Results
A total of 3134 patients initiated triple therapy for COPD in the study period, among them 485 with SITT. In 2019, the rate of use of SITT was 28.2%. The mean age (67.3 years) and sex (44.2% female) of patients initiating triple therapy was similar between MITT and SITT, and most patients had escalated from dual therapy (84.1%). However, SITT was more frequently initiated by a pulmonologist (59.8%) and a higher prevalence of comorbid asthma was observed for SITT (47.0% vs 37.9%). Persistence was assessed among patients who did not discontinue after a single dispensation of triple therapy (n=1674). Median persistence was 181 days for SITT and 135 days for MITT, and the covariate-adjusted HR for persistence was 1.47 (p<0.001) and the estimated persistence at 1 year was 33% for SITT compared with 18% for MITT.
Discussion
This study suggests that persistence was higher for the patients treated with SITT compared with MITT in France. Moreover, most patients initiated with triple therapy were previously treated with dual therapy and had exacerbations in the previous year.
Keywords: COPD Pathology, COPD Pharmacology, COPD Exacerbations, COPD epidemiology
WHAT IS ALREADY KNOWN ON THIS TOPIC
Triple therapy, comprising long-acting muscarinic antagonist, long-acting β2-agonist and inhaled corticosteroid, is a commonly used maintenance medication in chronic obstructive pulmonary disease (COPD). Triple therapies combinations are available as multiple inhaler triple therapy (‘MITT’) and, more recently, as a single inhaler triple therapy (‘SITT’). As treatment complexity increases with the number of devices used, SITT could potentially impact adherence and persistence through greater treatment simplification. An assessment of this is, therefore, warranted.
WHAT THIS STUDY ADDS
Triple therapy, whether prescribed in primary care or specialty care, tends to be initiated for patients with COPD previously on dual therapy, with a history of moderate or severe exacerbation in the 12 previous months. Clinical characteristics of new triple treatment users, whether SITT or MITT, were mainly similar. However, MITT users demonstrated lower treatment persistence rates than SITT users.
HOW THIS STUDY MIGHT AFFECT RESEARCH, PRACTICE OR POLICY
In France, the initiation of triple therapy in the clinical management of patients with COPD was similar in both the primary care and specialist settings. Simplification of the COPD therapeutic regimen for patients may have a beneficial effect on persistence and could potentially improve treatment effectiveness through single inhaler delivery.
Introduction
Chronic obstructive pulmonary disease (COPD) has a high disease burden and is the fifth-leading cause of death in high-income countries.1 2 In France, over 7% of the population lives with COPD and it currently accounts for 2.9% of all deaths and nearly 2% of all years of life lost due to ill health3 (disability-adjusted life-years (‘DALY’). Pharmacological management of COPD is aimed at improving symptoms and reducing the frequency of exacerbations.4 In France, current COPD guidelines recommend an incremental approach towards triple therapy, which is recommended for patients who remain symptomatic or suffer acute exacerbation while being treated with dual therapy5 6 and an initiation by a pulmonologist.5 Composed of long-acting muscarinic antagonist (LAMA), long-acting β2-agonist (LABA) and inhaled corticosteroid (ICS), triple therapy was only available as a multiple inhaler triple therapy (MITT) prior to 2018, that is, via two or more inhalers. In 2018, two single inhaler triple therapies (SITT) became available for COPD management in France.7 8 A third SITT was approved by the European Medicines Agency in late 2020 for maintenance treatment of COPD.9 In France, health authorities recommend that SITT should be initiated by a pulmonologist in the treatment of COPD.5
It has been previously reported that SITT treated patients could derive greater benefit than MITT through improved adherence.10 11 Poor adherence is commonplace in the clinical management of COPD and may increase the risk of exacerbation and poor outcomes.12 13 Treatment complexity, including dosing frequency, number of medications and ease of use, contributes to poor adherence.14 Also, polypharmacy, which is frequent in older patient groups, is another factor that can lead to poor adherence of COPD treatment.15 16
Treatment effectiveness is largely dependent on inhaler technique.17 Several studies have suggested that potentially as little as 40% of patients treated for COPD demonstrate a ‘correct’ inhaler technique.18 Indeed, the use of multiple inhalers may also require the patient to learn different techniques if both dry powder and pressurised metered dose inhalers are used.
A recent systematic literature review comparing single and multiple inhalers in both COPD and asthma, concluded that evidence from non-randomised studies was largely consistent in showing benefits for single inhalers including decreased health resource utilisation.19 However, they highlighted that the lack of long-term data and design differences hampered their ability to provide robust conclusions. However, additional trials comparing SITT to MITT19 20 failed to provide data on adherence or persistence differences between SITT and MITT as they were not designed for these outcomes.
Regimen simplicity, therefore, appears to be key to improving both adherence and persistence and, consequentially, clinical outcomes, and is the rationale behind the development of SITT.21 22 Treatment adherence (or compliance) and persistence are interrelated yet differing concepts. Adherence is defined as ‘the extent to which a patient acts in accordance with the prescribed interval and dose of a dosing regimen’.23 Deviations from the recommended dose and schedule can impact the benefit–risk balance. Persistence is the ‘time of continuous therapy, demarcated by the time from initiation to discontinuation of therapy’.23 Unless step down of triple therapy is a clinical objective, then persistence of chronic prevention therapy should be considered a positive outcome.18 24
Studies have shown that poor adherence and non-persistence of chronic prevention therapies are linked with increased morbidity and mortality and underpins the need to understand their roles.23 Again, this highlights the assertion that greater persistence may be associated with improved outcomes. Thus, the purpose of the current study was to provide real-world evidence of the use of triple therapy to treat COPD in France and specifically to examine the use of SITT and MITT, comparing both persistence and adherence.
Objectives
The objective of the study was to describe the characteristics of patients who initiated triple therapy, whether SITT or MITT for COPD, in France. Furthermore, this study compared persistence and adherence of patients who initiated SITT or MITT regimens.
Material and methods
Patients were included in this retrospective cohort study from The Health Improvement Network (THIN) in France, a Cegedim database. The Cegedim databases are anonymised in compliance with currently applicable data protection laws, regulations and policies.
THIN is an unobtrusive medical data collection scheme that collects anonymised patient data from its members, that is, electronic health records of GPs and specialists. This includes information about people’s health such as their diseases, test results and medication, but not their name, address or other information that could directly identify them—the study was a retrospective analysis of secondary anonymised patient data only. Data cannot be shared publicly because of legal restrictions. For the THIN database in France, several audits were conducted by Commission Nationale de l’Informatique et des Libertés—the French authority responsible for the protection of personal data and is approved for research purposes and does not require patient consent for specific studies. Data are available from the GERS SAS for researchers who meet the criteria for access to confidential data.
THIN France is collected from a pool of 2000 general practitioners and 1000 specialists including 65 pulmonologists (private practice exclusively) and is representative of the French population in terms of age, sex and geography.
The study period was from 1 July 2017 to the end of data collection for this study (20 September 2021). Patients were eligible for this study if they initiated a new triple therapy regimen (LABA, LAMA and ICS) in the study inclusion period comprising 1 July 2017 until 31 December 2019 and had a diagnosis of COPD or chronic bronchitis at or prior to the initiation of their triple therapy. Inclusion of patients coded with chronic bronchitis increases the sensitivity of the case definition and is consistent with other European database studies.25–27 Other respiratory comorbidities such as asthma or bronchiectasis when associated with COPD or chronic bronchitis were not excluded as they are common COPD comorbidities.28 Treatments were recorded from pharmacy dispensation data. A treatment dispensation corresponded to 30 days of treatment. Patients were excluded if they had used triple therapy previously and thus patients who switched triple therapy, for instance, from MITT to SITT, were therefore not included. All subjects were aged 40 years or over at date of onset of their initial triple therapy regimen and they had to have at least 12 months of medical records to facilitate the identification of existing comorbidities, history of exacerbation and to ensure that no previous triple therapy had been used for at least a year. The date of the triple therapy initiation was the patient’s index date and patients were followed up until the earliest of 20 September 2021 or lost to follow-up. Lost to follow-up occurred when there was no contact with the patient for 180 days.
Patient characteristics, treatment initiated and any previous treatment were described at the index date. Patients were classified as having initiated either on MITT or SITT. For this report, a fixed combination of therapies was denoted with “/” (eg, fixed combination of ICS with LABA is written as “ICS/LABA”) while therapies administered via separate inhalers are denoted with “+” (eg, “ICS+LABA” to reflect separate ICS and LABA inhalers). Thus, SITT was denoted as “LABA/LAMA/ICS”. MITT was defined as concomitant use of at least two different inhalers (ie, ICS/LABA+LAMA, LABA/LAMA+ICS or LABA+LAMA+ICS).
Recent exacerbation was examined in the 12 months prior to the triple therapy initiation. In accordance with the French society of pneumology (Société de Pneumologie de Langue Française), exacerbations were classified as moderate if short-term systemic corticosteroids or COPD exacerbation specific antibiotics were prescribed, and as severe if a related hospitalisation occurred.29
Patients were described, at initiation of SITT or MITT in terms of their age, sex and body mass index (BMI), their comorbidities, their COPD medication immediately prior to initiation of triple therapy and the prescribing physician: primary care physician (PCP), pulmonologist or another specialist. The Charlson Comorbidity Index (CCI) was calculated using the algorithm developed by Gasparini.30
A treatment gap greater than 60 days determined discontinuation of therapy and thereby non-persistence. Persistence was examined among those patients who received at least two dispensations of their initial triple therapy regimen and thus did not discontinue at 30 days from their index triple therapy. This allowed for the analysis of patients who initiated triple therapy with the aim of maintaining it as background treatment. Furthermore, persistence was examined only among those with at least 60 days follow-up to account for the treatment gap which defines discontinuation. Median persistence in days since initiation of triple therapy was calculated for SITT and MITT and by other factors and the log-rank test was applied to test for differences in persistence.31 A Kaplan-Meier curve of persistence was plotted for SITT and MITT whereby persistence terminated when treatment was discontinued otherwise the patient’s follow-up was censored at end of their follow-up. It should be noted that no discontinuation occurred in the first 30 days by design. The Kaplan-Meier curve was plotted with 95% CIs and thereby displayed whether a statistically significant difference in persistence occurred over the follow-up.31 Cox proportional hazards model was applied to evaluate whether any observed, univariate, differences in persistence between SITT and MITT, may have been confounded by other related factors.31 Specifically, as the event was discontinuation, the HRs, therefore, represented the relative risk of discontinuation. Thus, for this report, the HRs were inverted to provide the related chance of persisting. For these models a broad set of covariates were included and subsequently removed if they did not have a significant impact on persistence. The most parsimonious model was reported. All analyses were performed using Stata V.17.0/MP.32
Lastly, adherence was measured using the proportion of days covered (PDC).33 The PDC describes the proportion of days a person is in possession of the required medication over a defined period. Thus, the timing of dates of pharmacy dispensation should reflect this pattern and deviation from this may indicate suboptimal adherence. For this study, the PDC measured this pattern, within periods of persistent use, using the dates of pharmacy dispensation. A cut-off of 0.8 was applied to define ‘good adherence’ which is in line with previous published use of the PDC statistic in COPD and elsewhere.11 33 PDC was calculated solely among patients with greater than 90 days of persistence as such calculations can be unstable when the time denominator is short. The PDC was further stratified by duration of persistence as the persistence can impact PDC and the χ2 test was applied to evaluate differences in PDC between SITT and MITT.31
Sensitivity analyses were carried out to evaluate the impact of potential misdiagnosis. These sensitivity analyses assessed the impact on the effect of SITT, compared with MITT, on treatment persistence for patients with COPD with or without comorbid asthma and, furthermore, for patients who only had a record of only chronic bronchitis compared with those with a record of COPD. The impact was evaluated by adding, to the final model, an interaction term between triple therapy type and these strata (ie, COPD+ asthma vs COPD without asthma; only chronic bronchitis vs COPD). The magnitude of the interaction term, its statistical significance and the overall effect of triple therapy type were then assessed to conclude whether these strata may impact the findings of this study.
Results
Baseline characteristics of patients with COPD at initiation of triple therapy
From the THIN France database, a total of 3134 patients initiated a triple therapy regimen for COPD and met the inclusion and exclusion criteria in the study period. Of these, 485 patients (15.5%) initiated SITT (table 1).
Table 1.
Characteristics and comorbidities of patients initiating triple therapy for COPD by type of regimen: 1 July 2017–31 December 2019
| MITT | SITT | Total | P value | |
| All patients, n (%) | 2649 (84.5) | 485 (15.5) | 3134 | -- |
| COPD diagnosis at inclusion, n (%) | 1893 (71.5) | 375 (77.3) | 2268 (72.4) | -- |
| Chronic bronchitis diagnosis at inclusion, n (%) | 756 (28.5) | 110 (22.7) | 866 (27.6) | -- |
| All patients: 2019 only, n (%) | 891 (71.9) | 349 (28.2) | 1240 | -- |
| Age, mean (SD) (years) | 67.1 (11.7) | 68.2 (11.1) | 67.3 (11.7) | 0.059 |
| Female, n (%) | 1171 (44.2) | 214 (44.1) | 1385 (44.2) | 0.973 |
| Existing comorbidities, n (%) | ||||
| Asthma (ever) | 1245 (47.0) | 184 (37.9) | 1429 (45.6) | <0.001 |
| Asthma (previous 12 months) | 661 (25.0) | 92 (19.0) | 753 (24.0) | 0.005 |
| Hypertension | 1170 (44.2) | 220 (45.4) | 1390 (44.4) | 0.627 |
| Sleep disorder | 988 (37.3) | 165 (34.0) | 1153 (36.8) | 0.169 |
| Mood disorder | 729 (27.5) | 124 (25.6) | 853 (27.2) | 0.374 |
| Anxiety disorder | 722 (27.3) | 119 (24.5) | 841 (26.8) | 0.214 |
| Depressive disorder | 655 (24.7) | 112 (23.1) | 767 (24.5) | 0.442 |
| Diabetes | 507 (19.1) | 99 (20.4) | 606 (19.3) | 0.514 |
| Osteoporosis | 288 (10.9) | 45 (9.3) | 333 (10.6) | 0.295 |
| Oral candidiasis | 224 (8.5) | 35 (7.2) | 259 (8.3) | 0.362 |
| Heart failure | 184 (6.9) | 26 (5.4) | 210 (6.7) | 0.199 |
| Dysphonia | 136 (5.1) | 25 (5.2) | 161 (5.1) | 0.985 |
| Glaucoma | 108 (4.1) | 11 (2.3) | 119 (3.8) | 0.055 |
| Atopic dermatitis | 101 (3.8) | 17 (3.5) | 118 (3.8) | 0.744 |
| Tuberculosis (ever) | 94 (3.5) | 14 (2.9) | 108 (3.4) | 0.463 |
| Allergic conjunctivitis | 88 (3.3) | 7 (1.4) | 95 (3.0) | 0.027 |
| Cataracts (ever) | 65 (2.5) | 15 (3.1) | 80 (2.6) | 0.412 |
| Malnutrition | 34 (1.3) | 4 (0.8) | 38 (1.2) | 0.396 |
| ENT cancer | 20 (1.4) | 3 (0.6) | 0.014 (0.118) | 0.11 |
| BMI ≥30 kg/m², n (%) | 355 (30.2) | 79 (34.8) | 434 (31.0) | 0.173 |
| Charlson Comorbidity Index: ≥5, n (%) | 897 (38.0) | 172 (40.2) | 1069 (38.3) | 0.394 |
Asthma (ever) corresponds to a record of asthma on full history of patient record. Asthma (previous 12 months) corresponds to a record of asthma on the last 12 months of patient record.
BMI, body mass index; COPD, chronic obstructive pulmonary disease; ENT, ears, nose and throat; MITT, multiple inhaler triple therapy; SITT, single inhaler triple therapies.
In 2019, the first full calendar year in the study period after the introduction of SITT in France, the proportion of patients initiating SITT was 28.2% (table 1). Overall, the mean age at initiation of triple therapy was 67.3 years and 44.2% of the patients were female (table 1). These demographics were similar between MITT and SITT. The most common comorbidity was history of asthma (prevalence of 45.6% of all patients) and approximately a quarter of patients had a record of asthma in the preceding 12 months. There was no significant difference in the rate of asthma patients between pulmonologist (47.2%) and PCPs (47.5%) (result not shown). The proportion of patients with asthma was higher among those who initiated with MITT compared with SITT, 47.0% vs 37.9%, respectively (p<0.001). Other frequent comorbidities were similar between patients initiating MITT and SITT, including hypertension (44.4% overall), sleep disorder (36.8%), mood disorder (27.2%), anxiety (26.8%) and depression (24.5%). Diabetes (19.3%) and osteoporosis (10.6%) were also commonly observed (table 1). Overall, 38.3% of the study population had a CCI greater than or equal to 5% and 31% were classified as obese (BMI≥30 kg/m2). Both measures were similar between the MITT and SITT groups.
A majority of patients (74.9%) had suffered at least one moderate or severe exacerbation in the past year (table 2). This was similar between patients initiating MITT or SITT. The percentage suffering at least two moderate exacerbations or one severe exacerbation was 55.2%, again this was similar between MITT and SITT.
Table 2.
COPD exacerbations and treatment at initiation of triple therapy for COPD: 1 July 2017–31 December 2019
| MITT | SITT | Total | P value | |
| All patients: 2017–2019, n (%) | 2649 (84.5) | 485 (15.5) | 3134 | -- |
| Exacerbations in previous 12 months, n (%) | ||||
| ≥1 moderate or severe exacerbation | 1988 (75.0) | 359 (74.0) | 2347 (74.9) | 0.901 |
| ≥2 moderate or ≥1 severe exacerbation | 1457 (55.0) | 274 (56.5) | 1731 (55.2) | 0.901 |
| Initiating physician, n (%) | ||||
| Primary care physician | 1460 (55.1) | 143 (29.5) | 1603 (51.1) | <0.001 |
| Pulmonologist | 851 (32.1) | 290 (59.8) | 1141 (36.4) | |
| Other (including hospital) | 338 (12.8) | 52 (10.7) | 390 (12.4) | |
| Seen by pulmonologist in previous 90 days, n (%) | 1224 (46.2) | 328 (67.6) | 1552 (49.5) | <0.001 |
| Previous treatment, n (%) | ||||
| Naïve | 224 (8.5) | 30 (6.2) | 254 (8.1) | 0.072 |
| Monotherapy | 213 (8.0) | 30 (6.2) | 243 (7.8) | |
| Dual therapy | 2212 (83.5) | 425 (87.6) | 2637 (84.1) | |
| Type of dual therapy | ||||
| LABA/ICS | 1271 (57.5) | 196 (46.1) | 1467 (55.6) | <0.001 |
| LABA/LAMA | 536 (24.2) | 135 (31.8) | 671 (25.4) | |
| Other dual therapy | 405 (18.3) | 94 (22.1) | 499 (18.9) | |
| Initial triple therapy combination, n (%) | ||||
| MITT: LABA/ICS+LAMA | 1585 (59.8) | -- | -- | |
| MITT: LABA/ICS+LABA/LAMA | 456 (17.2) | -- | -- | |
| MITT: LABA/LAMA+ICS | 432 (16.3) | -- | -- | |
| MITT: other combination | 176 (6.6) | -- | -- | |
| SITT: without additional inhalers | -- | 433 (89.3) | -- | |
COPD, chronic obstructive pulmonary disease; ICS, inhaled corticosteroid; LABA, long-acting β2-agonist; LAMA, long-acting muscarinic antagonist; MITT, multiple inhaler triple therapy; SITT, single inhaler triple therapy.
There was a clear difference between SITT and MITT in terms of the initiating physician (p<0.001) (table 2). Specifically, a total of 59.8% of patients initiating SITT were managed by a pulmonologist, compared with 32.1% for those initiating MITT.
Treatment pathway
Most patients who initiated triple therapy had stepped up from a dual therapy regimen (84.1%), which was similar between the MITT (83.5%) and SITT (87.6%) groups. Among these, the most common regimens were LABA/ICS (55.6%) and LABA/LAMA (25.4%). There was a clear difference in the type of initial dual therapy such that LABA/ICS was more frequent in patients who proceeded to MITT (57.5%) compared with SITT (46.1%) and LABA/LAMA was more common in the SITT versus MITT group (31.8% vs 24.2%). Among those initiating MITT, the most common regimens were LABA/ICS+LAMA (59.8%), LABA/ICS+LABA/LAMA (17.2%) and LABA/LAMA+ICS (16.3%) (table 2). SITT was prescribed without any additional COPD inhalers for 89.3% of the patients.
Persistence and adherence
Persistence was examined in 1674 patients who had at least 60 days of follow-up and who did not discontinue after a single dispensation (table 3).
Table 3.
Persistence and adherence of initial TT regimen for COPD from 1 July 2017 to 31 December 2019 in patients followed for >60 days and did not discontinue in the first 30 days—median days of persistence depending on multiple factors
| Persistence* | MITT | SITT | Overall | P value* |
| Median days (N patients) | Median days (N patients) | Median days (N patients) | ||
| All† | 135 (1371) | 181 (303) | 142 (1674) | <0.001 |
| Sex | ||||
| Male | 141 (799) | 187 (176) | 147 (975) | <0.001 |
| Female | 120 (572) | 177 (127) | 135 (699) | <0.001 |
| Age | ||||
| <70 | 135 (758) | 177 (172) | 140 (930) | <0.001 |
| 70+ | 135 (613) | 204 (131) | 145 (744) | <0.001 |
| Year initiated TT | ||||
| 2017 | 123 (294) | -- | 123 (294) | -- |
| 2018 | 140 (622) | 258 (76) | 142 (698) | <0.001 |
| 2019 | 129 (455) | 171 (227) | 145 (682) | <0.001 |
| Initiating physician | ||||
| Primary care physician | 112 (649) | 173 (80) | 115 (729) | <0.001 |
| Pulmonologist | 149 (518) | 187 (183) | 155 (701) | 0.001 |
| Other/unknown | 170 (204) | 286 (40) | 170 (244) | 0.042 |
| BMI | ||||
| <30 | 120 (468) | 204 (107) | 129 (575) | <0.001 |
| 30+ | 138 (202) | 177 (53) | 141 (255) | 0.040 |
| Exacerbations | ||||
| Definition 1: | ||||
| 0 moderate or severe | 156 (409) | 209 (90) | 162 (499) | 0.019 |
| ≥1 moderate or severe | 121 (962) | 181 (213) | 136 (1175) | <0.001 |
| Definition 2: | ||||
| <2 moderate or 0 severe | 149 (671) | 194 (143) | 154 (814) | <0.001 |
| ≥2 moderate or ≥1 severe | 117 (700) | 178 (160) | 126 (860) | <0.001 |
| Previous medication | ||||
| Naïve | 134 (138) | 218 (19) | 140 (157) | 0.073 |
| Monotherapy | 128 (114) | 282 (18) | 148 (132) | <0.001 |
| Double therapy | 136 (1119) | 177 (266) | 142 (1385) | <0.001 |
| Adherence: PDC‡ | N (%) | N (%) | N (%) | |
| All: PDC≥0.8 | 736 (86.1) | 191 (85.7) | 927 (86.0) | 0.869 |
| Patients with more than 60 days follow-up | 2526 (95.4) | 453 (93.4) | 2979 (95.1) | 0.067 |
| Discontinuation after one dispensation* | 1155 (45.7) | 150 (33.1) | 1305 (43.8) | <0.001 |
| Persistence 91–270 days: PDC≥0.8 | 93 (80.2) | 468 (84.3) | 561 (83.6) | 0.272 |
| Persistence >270 days: PDC≥0.8 | 98 (91.6) | 268 (89.3) | 407 (89.9) | 0.506 |
Non-persistence is defined by a gap of more than 60 days between two dispensations of TT.
*Log-rank test for difference in persistence; χ2 test for the difference in proportion of PDC≥0.8.
†All patients had more than one dispensation of the initial therapy regimen and had more than 60 days of follow-up (n=1674).
‡PDC (adherence), n=1078 with at least 90 days persistence.
BMI, body mass index; COPD, chronic obstructive pulmonary disease; MITT, multiple inhaler triple therapy; PDC, proportion of days covered; SITT, single inhaler triple therapy; TT, triple therapy.
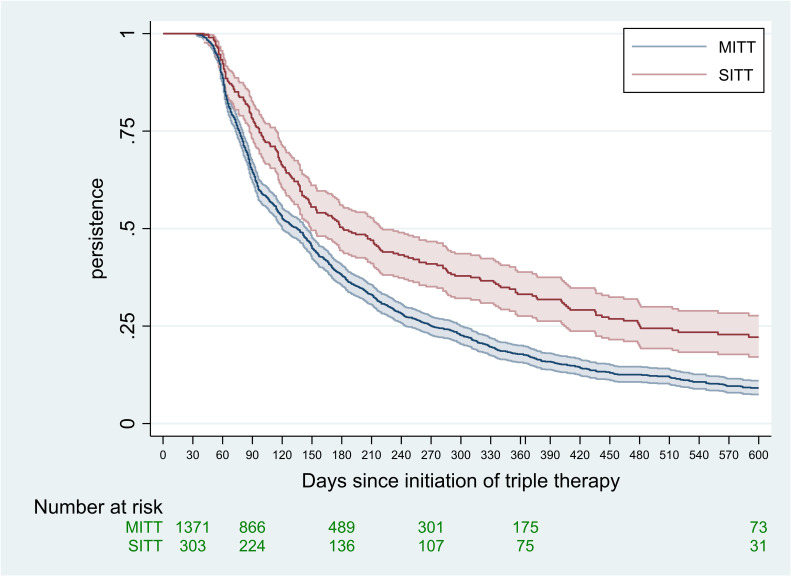
Overall, the median duration of persistence was 142 days (table 3). This includes the initial 30 days in which, by design, none of the patients could discontinue. Greater persistence for the SITT group is shown in figure 1 and this pattern continues throughout the follow-up with a median persistence of the SITT group of 181 days compared with a median of 135 days for those who initiated MITT (p<0.001). Further to this, median persistence (days) for MITT and SITT was stratified by several factors (table 3). Persistence was greater for those patients who had not suffered a moderate or severe exacerbation in the past year (162 days compared with 136 days). Persistence was also greater among those whose triple therapy was prescribed by a pulmonologist compared with a PCP (155 days vs 115 days) whether SITT or MITT. A Cox proportional hazards model was applied to examine the role of these potential confounders on the observation that SITT was associated with greater treatment persistence (table 4).
Figure 1.
Persistence of triple therapy for the treatment of COPD, 1 July 2017 to 31 December 2019: SITT versus MITT. COPD, chronic obstructive pulmonary disease; MITT, multiple inhaler triple therapy; SITT, single inhaler triple therapy.
Table 4.
Predictors of persistence beyond 30 days of initial TT for COPD in France: 1 July 2017–31 December 2019 (n=1674)
| Variables* | HR† | 95% CI | P value | |
| Lower | Upper | |||
| SITT versus MITT | 1.47 | 1.27 | 1.69 | <0.001 |
| Initiating clinician | ||||
| Pulmonologist versus primary care physician | 1.22 | 1.09 | 1.37 | 0.001 |
| Other/unknown versus primary care physician | 1.33 | 1.14 | 1.59 | 0.001 |
| Exacerbation (previous 12 months)‡ | ||||
| ≥2 moderate or ≥1 severe | 0.86 | 0.77 | 0.95 | 0.005 |
| Depressive disorder (ever) | 0.76 | 0.67 | 0.85 | <0.001 |
*Other variables considered and excluded from final model (not significant) were age, hypertension, diabetes, year-initiated TT, prior treatment, asthma (previous 12 months) and ENT cancer.
†HR is the HR of the chance that a patient persists with treatment; HR presented in this table are 1/HR calculated by the models with the discontinuation outcome.
‡≥2 moderate or ≥1 severe exacerbation vs <2 moderate and no severe exacerbations in the previous 12 months.
COPD, chronic obstructive pulmonary disease; ENT, ears, nose and throat; MITT, multiple inhaler triple therapy; SITT, single inhaler triple therapy; TT, triple therapy.
The inference from the model was that, after adjusting for potential confounders, there was an approximate 47% increase in the chance of persisting with triple therapy in the SITT group (p<0.001). The other factors independently associated with increased persistence on triple therapy were being prescribed triple therapy by a specialist (pulmonologist or another specialist), not having had any moderate or severe exacerbations and not suffering from depressive disorder.
Sensitivity analyses were first carried out according to whether a patient with COPD had a recent record of comorbid asthma. Among those patients who persisted beyond 30 days, the prevalence of asthma was 24.1%. The interaction term between type of triple therapy (SITT vs MITT) and asthma was not significant (HR=1.10; p=0.610) and the estimator for SITT versus MITT remained similar (HR=1.49). Thus, comorbid asthma did not affect the inference or magnitude of the effect of type of triple therapy on persistence. Further sensitivity analyses were conducted to assess whether the inclusion of patients with chronic bronchitis (without any record of COPD) affected persistence associated with type of triple therapy. Among those with more than 30 days persistence, the proportion of patients with only chronic bronchitis diagnostic codes was 28.5% (n=477) and again there was no evidence of a significant interaction with the type of triple therapy on treatment persistence (HR=1.13; p=0.425) and the estimator for SITT versus MITT remained similar (HR=1.35). Thus, the case definition did not impact the estimation of treatment persistence associated with type of triple therapy.
Good adherence was defined as a PDC≥0.8 and was calculated among those with a persistence of at least 90 days of persistent use of their initial triple therapy (n=1078). Overall, 86% were categorised to have good adherence to their triple therapy and it was similar between SITT and MITT (p=0.869) (table 3).
Discussion
This is the first large real-world study on patients with COPD in France who initiated triple therapy since the availability of SITT in 2018. The patients included in this study were similar to other studies conducted in France prior to 2018, in terms of age, sex and certain comorbidities which supports the robustness of this study’s findings.34 35 The patient characteristics were also in line with a study carried out using the UK primary care database, the Clinical Practice Research Datalink.36 The codes used to identifyD patients with COPD are consistent with other European database studies.25–27 Moreover, the sensitivity analyses provided no evidence that chronic bronchitis or asthma affected the finding that SITT was observed to have a greater persistence than MITT. The results of this study highlight the high disease burden of patients with COPD who require triple therapy, with frequent comorbidities comprising hypertension, depression, diabetes and asthma. The high prevalence of asthma, among this population, is in line with other studies.28 37 Misdiagnosis is a possibility given disease similarities in terms of symptoms. Physicians may also be recording history of childhood asthma. However, when restricting to recent asthma, the prevalence was still high with approximately a quarter of patients having a recent diagnostic code for asthma.
Over half of the patients who initiated triple therapy had at least one severe or two moderate exacerbations (55%) and most were stepped up from dual therapy for COPD (84%) thus indicating a good level of compliance with the French and international guidelines.4–6
The study period encompassed time before and after the availability of SITT. However, in 2019, the first full calendar year after the availability of SITT, around 28% of patients were prescribed triple therapy via a single inhaler highlighting the rapid acceptance of these new therapeutic options. This swift uptake reinforces the need, as posited by other authors, for observational studies of the effectiveness, safety and acceptability of SITT as a therapeutic option among patients requiring triple therapy.19 38
Overall, patients initiating MITT or SITT were similar in terms of age, sex, BMI, exacerbations and most comorbidities. One notable exception was that asthma was more prevalent among those initiating with MITT. This is unlikely to be due to misdiagnosis of asthma given that the prevalence of asthma was nearly identical between patients who were initially prescribed triple therapy by a pulmonologist or a PCP. Moreover, the comorbidity of asthma had no impact on the inference that persistence is greater in SITT. The proportion of patients who stepped up from dual therapy was similar between the SITT and MITT groups. Thus, the decision to prescribe either SITT or MITT does not appear to be determined by the patients’ characteristics or disease severity. However, there was a clear difference in the specific type of prior dual therapy, with a greater frequency of prior LABA/ICS for the MITT group while more LABA/LAMA was observed in the SITT group. Lastly, we observed that a majority of SITT was initiated by pulmonologists. It is aligned with French health authority guidance to limit the initiation of SITT only by pulmonologist (Haute Autorité de Santé).5 According to the health authorities, SITT should be initiated by pulmonologist in the treatment of COPD.5 We observed a rate of immediate discontinuation of triple therapy of 43.8% and this was higher in the MITT group. As long-term triple therapy may not be the intention among those who are prescribed LABA, LAMA and ICS simultaneously for a single month, the inferences on persistence precluded those that were immediately discontinued. Persistence was clearly superior in the SITT group. After 1 year since initiation of triple therapy, among those who did not discontinue in the first month, 33% of the SITT group persisted with the initial regimen compared with 18% of the MITT group. This finding was not due to chance and was independent of other factors considered, as demonstrated in the multivariable model with an HR for persisting of 1.47 in favour of the SITT group.
Persistence in observational studies of chronic prevention therapies is often considered a proxy for effectiveness, tolerability and also the ease of use of the therapeutic options.39 If any of these outcomes are worse for a treatment then it should correspond to a shorter persistence compared with other therapeutic options and regimen changes, including augmentation, discontinuation or switching, would be expected. Thus, an observed greater persistence for SITT may imply that it is more effective, safe and easy to use as the more bespoke therapeutic options offered by MITT. However, we cannot rule out whether discontinuation may reflect a desire to step down treatment. Stepping down of triple therapy, usually via the removal of ICS, may be beneficial for some patients given adverse reactions.4 6 40 However, this requires careful clinical consideration especially if patients are currently stable after previously suffering exacerbations.4 41–43 This underlines the personalised nature of the clinical decision to discontinue triple therapy and revert to dual therapy probably without ICS. However, greater persistence is likely to indicate a positive benefit–risk balance of continued triple therapy as clinicians and patients prefer to continue treatment rather than switch or step down.
Adherence was measured using the PDC and applying a cut-off at 0.8 to define ‘good adherence’. At 86%, the rate of ‘good adherence’ is higher than described elsewhere.11 Bogart et al reported a rate of ‘good adherence’ of just 19% by 6 months for patients with COPD using two inhalers11 while Zucchelli et al reported rates of 15% (in men) to 31% (in women).44 This difference was due to the definition of the time denominator in the PDC estimate. Indeed, in Zucchelli study, the time denominator includes the time after the treatment discontinuation, therefore, COPD medication prescribed by a specialist was not captured by the HSD primary care database giving rise to apparent treatment gaps. Another study from Germany (2010–2012), however, provided the estimate of patients with a medical possession ratio “MPR” >80% among persistent users.45 The observed rate of MPR>80% was 70% and thus more in line with the current study, however, only monotherapy or dual therapy were assessed.
The use of either time denominator to define PDC is appropriate although care must be taken when interpreting ‘good adherence’ and comparing studies.33 Specifically, our study demonstrated that persistent users of triple therapy tend to obtain dispensation refills at regular intervals and gaps, and that it is relatively rare for patients who initiate triple therapy to spend over 20% of days without treatment. However, while the PDC has been advocated by the Pharmacy Quality Alliance to quantify adherence in chronic diseases, the PDC remains a relatively crude statistic based on the pattern of dates of refill only and thus cannot account for whether patients use the appropriate inhaler technique to deliver the correct dose.46
The strength of this study is the use of real-world data from THIN database that is reflective of French clinical practice.47 Furthermore, prescriptions from differing specialists are obtained via the linkage with pharmacy data which is important given that triple therapy may be expected to be initiated by a specialist. However, certain limitations should be considered when interpreting the results. First, the study relies on the accuracy of recording the diagnosis of COPD with case identification based solely on the use of diagnostic codes as recorded by healthcare professionals. To better analyse real-world prescription of triple therapy in COPD, we also considered patients recorded with chronic bronchitis, and did not exclude patients with comorbidities including asthma and bronchiectasis. Our sensitivity analyses demonstrated that comorbid asthma and inclusion of chronic bronchitis in the case definition did not impact the observed difference in persistence between SITT and MITT. Another important limitation is that we do not have access to spirometry data such as forced expiratory volume in 1 s (FEV1), FEV1/forced vital capacity and postbronchodilator reversibility, a fundamental diagnostic feature for COPD.6 Thus, there is potential for misclassification of diagnosis. Furthermore, due to low collection in the database, it was not feasible to include eosinophil test results and smoking history which again are important in the history of patients with COPD. However, any misclassification would affect equally both the SITT and MITT groups and thus would not impact the conclusions of this study regarding persistence of triple therapy. Furthermore, the demographic similarity with other epidemiological studies, such as the age of the patients, is suggestive of COPD as opposed to asthma.28 Second, COPD burden is probably underestimated with the use of punctual treatments to identify moderate exacerbations and the lack of comprehensive identification of severe exacerbations. Our main outcomes of persistence and adherence require further qualification. However, even if a greater persistence can be associated with good effectiveness, safety and acceptability, we cannot know the combination of these treatment outcomes that results in greater persistence. Also, it is possible that there could be a positive outcome associated with shorter persistence such as discontinuation due to stepping down of triple therapy. Thus, a degree of caution is required when concluding better adherence associated with greater treatment persistence. For adherence, we used the PDC which as previously highlighted cannot provide information on correct inhaler technique. Lastly, this study has not considered to what extent patients on MITT may switch to SITT. However, this would represent a preference to replace the MITT regimen with a single inhaler.
Conclusion
This real-world study confirms that patients initiating SITT experience longer treatment persistence than those initiating MITT, even after adjusting for confounding factors. Moreover, the majority of patients initiated with triple therapy were previously treated with dual therapy and had exacerbations. This is likely a reflection of similar or improved ease of use of SITT compared with MITT, which could result in increased triple therapy effectiveness through single inhaler delivery, though further research is needed to confirm this. Such studies are timely given the rapid uptake of the SITT therapeutic options.
Acknowledgments
Additional clinical review was provided by Dr Henri Leleu (Public Health Expertise, France) and review and editing assistance was provided by Dr Lucy Tran, Dr Victoria Parsons and Salman Ramjaun (EpiFocus, UK).
Footnotes
Contributors: AC and AM performed data management and statistical analyses and provided the initial draft version under the guidance of NP, CVF, GD and TP. All authors were involved in the interpretation of data. All authors reviewed the manuscript, provided input and have approved the final version. AC is responsible for the overall content as the guarantor.
Funding: This study was supported by AstraZeneca, France.
Competing interests: AstraZeneca is a funder and an affiliation for some of the authors. GD reports personal fees from Nuvaira, BTG/PneumRx, Chiesi, Boehringer Ingelheim and AstraZeneca. CVF, NP and GT are employees of AstraZeneca. AC, CEP and CR are employees of Cegedim. AM is an independent consultant for Cegedim.
Patient and public involvement: Patients and/or the public were not involved in the design, or conduct, or reporting, or dissemination plans of this research.
Provenance and peer review: Not commissioned; externally peer reviewed.
Data availability statement
Data may be obtained from a third party and are not publicly available.
Ethics statements
Patient consent for publication
Not applicable.
Ethics approval
THIN is an unobtrusive medical data collection scheme that collects anonymised patient data. THIN in France is approved for research by CNIL (Commission Nationale de l’Informatique et des Libertés).
References
- 1.The top 10 causes of death. n.d. Available: https://www.who.int/news-room/fact-sheets/detail/the-top-10-causes-of-death
- 2.Chronic obstructive pulmonary disease (COPD). n.d. Available: https://www.who.int/news-room/fact-sheets/detail/chronic-obstructive-pulmonary-disease-(copd)
- 3.Fuhrman C, Delmas M-C. Épidémiologie descriptive de la Bronchopneumopathie Chronique obstructive (BPCO) en France. Revue Des Maladies Respiratoires 2010;27:160–8. 10.1016/j.rmr.2009.08.003 [DOI] [PubMed] [Google Scholar]
- 4.Global Initiative for Chronic Obstructive Lung Disease (GOLD . Global strategy for the diagnosis, management and prevention of COPD; 2022.
- 5.Haute Autorité de Santé . Guide dupParcours de soins: Bronchopneumopathie chronique obstructive; 2019.
- 6.Zysman M, Ribeiro Baptista B, Soumagne T, et al. Pharmacological treatment optimisation in patients with stale COPD. Position of the French-language respiratory society. 2021 update. Rev Mal Respir 2021;38:539–61. 10.1016/j.rmr.2021.02.070 [DOI] [PubMed] [Google Scholar]
- 7.Assessment of TRELEGY from French transparency committee. Available: https://www.has-sante.fr/upload/docs/evamed/CT-17439_TRELEGY_ELLIPTA_PIC_EI_Avis3_CT17439.pdf) [Accessed 10 Apr 2019].
- 8.Assessment of TRIMBOW from French transparency committee. Available: https://www.has-sante.fr/upload/docs/evamed/CT-17701_TRIMBOW_PIC_EI_Avis2_CT17701.pdf) [Accessed 10 Apr 2019].
- 9.European Medicines Agency (EMA) . Trixeo Aerosphere. 2020. Available: https://www.ema.europa.eu/en/medicines/human/EPAR/trixeo-aerosphere
- 10.Mannino D, Bogart M, Wu B, et al. Adherence and persistence to once-daily single-Inhaler versus multiple-Inhaler triple therapy among patients with chronic obstructive pulmonary disease in the USA: a real-world study. Respir Med 2022;197:106807. 10.1016/j.rmed.2022.106807 [DOI] [PubMed] [Google Scholar]
- 11.Bogart M, Stanford RH, Laliberté F, et al. Medication adherence and persistence in chronic obstructive pulmonary disease patients receiving triple therapy in a USA commercially insured population. Int J Chron Obstruct Pulmon Dis 2019;14:343–52. 10.2147/COPD.S184653 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Covvey JR, Mullen AB, Ryan M, et al. A comparison of medication adherence/persistence for asthma and chronic obstructive pulmonary disease in the United Kingdom. Int J Clin Pract 2014;68:1200–8. 10.1111/ijcp.12451 [DOI] [PubMed] [Google Scholar]
- 13.van Boven JFM, Chavannes NH, van der Molen T, et al. Clinical and economic impact of non-adherence in COPD: a systematic review. Respir Med 2014;108:103–13. 10.1016/j.rmed.2013.08.044 [DOI] [PubMed] [Google Scholar]
- 14.Pantuzza LL, Ceccato M das GB, Silveira MR, et al. Association between medication regimen complexity and Pharmacotherapy adherence: a systematic review. Eur J Clin Pharmacol 2017;73:1475–89. 10.1007/s00228-017-2315-2 [DOI] [PubMed] [Google Scholar]
- 15.Bourbeau J, Bartlett SJ. Patient adherence in COPD. Thorax 2008;63:831–8. 10.1136/thx.2007.086041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Vetrano DL, Bianchini E, Onder G, et al. Poor adherence to chronic obstructive pulmonary disease medications in primary care: role of age, disease burden and Polypharmacy. Geriatr Gerontol Int 2017;17:2500–6. 10.1111/ggi.13115 [DOI] [PubMed] [Google Scholar]
- 17.Melani AS, Bonavia M, Cilenti V, et al. Inhaler mishandling remains common in real life and is associated with reduced disease control. Respir Med 2011;105:930–8. 10.1016/j.rmed.2011.01.005 [DOI] [PubMed] [Google Scholar]
- 18.Restrepo RD, Alvarez MT, Wittnebel LD, et al. Medication adherence issues in patients treated for COPD. Int J Chron Obstruct Pulmon Dis 2008;3:371–84. 10.2147/copd.s3036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhang S, King D, Rosen VM, et al. Impact of single combination Inhaler versus multiple Inhalers to deliver the same medications for patients with asthma or COPD: a systematic literature review. Int J Chron Obstruct Pulmon Dis 2020;15:417–38. 10.2147/COPD.S234823 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bremner PR, Birk R, Brealey N, et al. Single-Inhaler Fluticasone Furoate/Umeclidinium/Vilanterol versus Fluticasone Furoate/Vilanterol plus Umeclidinium using two Inhalers for chronic obstructive pulmonary disease: a randomized non-inferiority study. Respir Res 2018;19:19. 10.1186/s12931-018-0724-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Marceau C, Lemière C, Berbiche D, et al. Persistence, adherence, and effectiveness of combination therapy among adult patients with asthma. J Allergy Clin Immunol 2006;118:574–81. 10.1016/j.jaci.2006.06.034 [DOI] [PubMed] [Google Scholar]
- 22.Pokorski M. Medical research and development. In: Adherence to Therapy in Chronic Obstructive Pulmonary Disease: A Systematic Review. Cham: Springer International Publishing, 2020: 37–47. [Google Scholar]
- 23.Cramer JA, Roy A, Burrell A, et al. Medication compliance and persistence: terminology and definitions. Value Health 2008;11:44–7. 10.1111/j.1524-4733.2007.00213.x [DOI] [PubMed] [Google Scholar]
- 24.López-Pintor E, Grau J, Lumbreras B. Patient’s awareness on COPD is the strongest Predictor of persistence and adherence in treatment-Naïve patients in real life: a prospective cohort study. BMC Pulm Med 2021;21:388. 10.1186/s12890-021-01754-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sivakumaran S, Alsallakh MA, Lyons RA, et al. Identifying COPD in routinely collected electronic health records: a systematic Scoping review. ERJ Open Res 2021;7. 10.1183/23120541.00167-2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Maitre T, Cottenet J, Beltramo G, et al. Increasing burden of Noninfectious lung disease in persons living with HIV: a 7-year study using the French nationwide hospital administrative database. Eur Respir J 2018;52:1800359. 10.1183/13993003.00359-2018 [DOI] [PubMed] [Google Scholar]
- 27.Chalmers JD, Poole C, Webster S, et al. Assessing the Healthcare resource use associated with inappropriate Prescribing of Inhaled corticosteroids for people with chronic obstructive pulmonary disease (COPD) in GOLD groups A or B: an observational study using the clinical practice research Datalink (CPRD). Respir Res 2018;19:63. 10.1186/s12931-018-0767-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.de Marco R, Pesce G, Marcon A, et al. The coexistence of asthma and chronic obstructive pulmonary disease (COPD): prevalence and risk factors in young, middle-aged and elderly people from the general population. PLoS One 2013;8:e62985. 10.1371/journal.pone.0062985 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jouneau S, Dres M, Guerder A, et al. Management of acute exacerbations of chronic obstructive pulmonary disease (COPD). Guidelines from the Société de Pneumologie de Langue Française (summary). Rev Mal Respir 2017;34:282–322. 10.1016/j.rmr.2017.03.034 [DOI] [PubMed] [Google Scholar]
- 30.Gasparini A. comorbidity: An R package for computing Comorbidity scores. JOSS 2018;3:648. 10.21105/joss.00648 [DOI] [Google Scholar]
- 31.Altman DG. Practical statistics for medical research, 1st ed. London: Chapman & Hall, 1991. [Google Scholar]
- 32.StataCorp . Stata Statistical Software: Release 17. College Station, TX: StataCorp LLC, 2021. [Google Scholar]
- 33.Prieto-Merino D, Mulick A, Armstrong C, et al. Estimating proportion of days covered (PDC) using real-world online medicine suppliers’ datasets. J Pharm Policy Pract 2021;14:113. 10.1186/s40545-021-00385-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Dalon F, Roche N, Belhassen M, et al. Dual versus triple therapy in patients hospitalized for COPD in France: a claims data study. Int J Chron Obstruct Pulmon Dis 2019;14:1839–54. 10.2147/COPD.S214061 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Meeraus W, Wood R, Jakubanis R, et al. COPD treatment pathways in France: a retrospective analysis of electronic medical record data from general practitioners. Int J Chron Obstruct Pulmon Dis 2019;14:51–63. 10.2147/COPD.S181224 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sansbury LB, Bains C, Lipson DA, et al. Real-world treatment patterns of multiple-Inhaler triple therapy among patients with chronic obstructive pulmonary disease in UK general practice. Int J Chron Obstruct Pulmon Dis 2021;16:1255–64. 10.2147/COPD.S290773 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hersh CP, Jacobson FL, Gill R, et al. Computed tomography phenotypes in severe, early-onset chronic obstructive pulmonary disease. COPD 2007;4:331–7. 10.1080/15412550701601274 [DOI] [PubMed] [Google Scholar]
- 38.Gaduzo S, McGovern V, Roberts J, et al. When to use single-Inhaler triple therapy in COPD: a practical approach for primary care health care professionals. Int J Chron Obstruct Pulmon Dis 2019;14:391–401. 10.2147/COPD.S173901 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Romagnoli A, Santoleri F, Costantini A. Adherence and persistence analysis after three years in real-life of inhalation therapies used in the treatment of COPD. Curr Med Res Opin 2020;36:2055–61. 10.1080/03007995.2020.1841617 [DOI] [PubMed] [Google Scholar]
- 40.Yang IA, Clarke MS, Sim EHA, et al. Inhaled corticosteroids for stable chronic obstructive pulmonary disease. Cochrane Database Syst Rev 2012;2012:CD002991. 10.1002/14651858.CD002991.pub3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Magnussen H, Lucas S, Lapperre T, et al. Withdrawal of Inhaled corticosteroids versus continuation of triple therapy in patients with COPD in real life: observational comparative effectiveness study. Respir Res 2021;22:25. 10.1186/s12931-021-01615-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Nadeem NJ, Taylor SJC, Eldridge SM. Withdrawal of Inhaled corticosteroids in individuals with COPD--a systematic review and comment on trial methodology. Respir Res 2011;12:107. 10.1186/1465-9921-12-107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Chalmers JD, Laska IF, Franssen FME, et al. Withdrawal of Inhaled corticosteroids in COPD: a European respiratory society guideline. Eur Respir J 2020;55:2000351. 10.1183/13993003.00351-2020 [DOI] [PubMed] [Google Scholar]
- 44.Zucchelli A, Vetrano DL, Bianchini E, et al. Adherence to COPD free triple Inhaled therapy in the real world: a primary care based study. Clin Respir J 2020. 10.1111/crj.13190 [DOI] [PubMed] [Google Scholar]
- 45.Mueller S, Wilke T, Bechtel B, et al. Non-persistence and non-adherence to long-acting COPD medication therapy: a retrospective cohort study based on a large German claims Dataset. Respir Med 2017;122:1–11. 10.1016/j.rmed.2016.11.008 [DOI] [PubMed] [Google Scholar]
- 46.Pharmacy Quality Alliance (PQA) . PQA measure overview. 2019. Available: https://www.pqaalliance.org/adherence-measures
- 47.Nedelec T, Couvy-Duchesne B, Monnet F, et al. Identifying health conditions associated with Alzheimer’s disease up to 15 years before diagnosis: an Agnostic study of French and British health records. Lancet Digit Health 2022;4:e169–78. 10.1016/S2589-7500(21)00275-2 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data may be obtained from a third party and are not publicly available.