Abstract
Introduction
African American women (AA), particularly those living in the Southeastern USA, experience disproportionately high rates of HIV infection. Pre-exposure prophylaxis (PrEP) is a highly effective HIV prevention tool that may circumvent barriers to traditional HIV prevention tools, such as condom use; however, very little is known about how to improve PrEP access and uptake among AA women who may benefit from PrEP use. This project aims to understand how to increase PrEP access among AA women in the rural Southern USA, which may ultimately affect HIV incidence in this population.
Methods and analysis
The goal of the current study is to systematically adapt a patient–provider communication tool to increase PrEP uptake among AA women receiving care at a federally qualified health centre in Alabama. We will use an iterative implementation process, by assessing the feasibility, acceptability and preliminary impact of the tool on PrEP uptake, using a pilot preintervention/postintervention design (N=125). We will evaluate women’s reasons for declining a referral to a PrEP provider, reasons for incomplete referrals, reasons for not initiating PrEP after a successful referral and ongoing PrEP use at 3 and 12 months after PrEP initiation among our sample. The proposed work will significantly contribute to our understanding of factors impacting PrEP uptake and use among AA women, particularly in underserved areas in the Deep South that are heavily impacted by the HIV epidemic and experience worse HIV-related health outcomes relative to other areas in the USA.
Ethics and dissemination
This protocol has been approved by the Institutional Review Board (IRB) at University of Alabama at Birmingham (Birmingham, AL; protocol 300004276). All participants will review a detailed informed consent form approved by the IRB and will provide written or verbal informed consent prior to enrolment. Results will be disseminated through peer-reviewed manuscripts, reports, and local, national and international presentations.
Trial registration number
Keywords: HIV & AIDS, Feasibility Studies, Health Equity, Health Education, SEXUAL MEDICINE, Primary Prevention
Strengths and limitations of this study.
Application of an implementation science framework facilitates rapid implementation, evaluation and modification of a novel patient–provider communication intervention to increase pre-exposure prophylaxis (PrEP) awareness and potential uptake for women in a US region where HIV transmission remains comparatively high.
Provides data on PrEP uptake and key social, behavioural, and cultural factors associated with PrEP uptake among African American women in the Southern USA, who remain under-represented in PrEP research despite being disproportionately affected by HIV.
Expands knowledge base on PrEP attitudes and experiences among healthcare providers within a federally qualified healthcare centre in the Southern USA, where many women who may benefit from PrEP enter the healthcare system.
Challenges and barriers identified by participants and providers throughout the adaptation process may include contextual, structural and system-level factors that are beyond the scope of this communication intervention.
Introduction
The Deep South region of the USA bears the greatest burden of the HIV epidemic in the USA, with rural counties disproportionately affected and underserved by both HIV care and prevention services.1 2 Of the more than 1.1 million people living with HIV in the USA in 2018, women accounted for nearly a quarter (23%) of all cases and a significant portion (19%) of new cases, with most new cases attributed to heterosexual contact (85%).3 Despite representing just 13% of the US female population, African American (AA) women accounted for 55% of new HIV diagnoses among all women in the USA in 2019.2 In 2016, the rate of new HIV diagnoses among AA women was 15 times higher than that of white women,3 and in 2018, HIV infection ranked in the top eight leading causes of death among AA women aged 20–44 in the USA.4 The elevated HIV risk profile among AA women in the south may reflect factors unique to rural areas, such as the prevalence of small sexual networks resulting in cyclical HIV transmission patterns5 and barriers to HIV prevention rooted in structural racism such as high incarceration rates among male AA populations, limited awareness of and access to effective contraception and sexual health interventions, higher rates of concurrent partnerships among AA men in AA women’s sexual networks, and financial and transportation-related barriers to accessing HIV/sexually transmitted infection (STI) screening, prevention and treatment.6–11
Traditional HIV prevention efforts, like abstinence-only education approaches that are prevalent in the rural south, have been insufficient to control high rates of STIs and HIV infections. Abstinence-only education does not provide women with comprehensive information critical for maintaining sexual health.12 13 Moreover, interventions promoting condom use, partner-based testing and/or monogamous relationships are dependent on behaviours of women’s sexual partners and, therefore, may be outside each woman’s direct control. AA women experiencing financial challenges may be financially dependent on male partners; they may also be experiencing intimate partner violence, further complicating their ability to make independent sexual health decisions.14 15 Given disproportionate diagnoses and mortality among AA women, along with limitations to non-biomedical HIV prevention methods, novel and more effective approaches to HIV prevention are necessary.
Biomedical HIV prevention tools, including pre-exposure prophylaxis (PrEP) are promising as they can potentially minimise barriers to traditional means of HIV prevention among groups who are persistently vulnerable to HIV infection. Oral PrEP has over 90% efficacy demonstrated across numerous trials, including cisgender women, but efficacy depends on consistent daily adherence as prescribed.16 Long-acting injectable PrEP is also highly efficacious for cisgender women and is now available for use in the USA.17 18 Although overall oral PrEP prescriptions in the USA increased substantially between 2012 and 2017, PrEP uptake among AA women has remained low. While biomedical prevention efforts have historically focused on men who have sex with men, women have not received equitable attention reflective of the epidemiology.19 Indeed, 94% of PrEP users in 2017 were male, indicating a substantial unmet need among PrEP-eligible women.18 In 2016, only 11% of all PrEP users with available race/ethnicity data were from AA communities.20
Regardless of geographic location, most women in the USA remain unaware of PrEP, and PrEP uptake among AA women remains low, particularly in rural areas of the USA where women may have less access to healthcare and more limited knowledge of PrEP than women in urban areas.21 In several studies, the majority of women participating in PrEP focus groups and staff at health services organisations were unfamiliar with PrEP as an HIV prevention tool and expressed concern about a broad lack of awareness within their communities; of the 10% of women who had previously heard of PrEP, none were aware of its availability and efficacy for women.22 23 However, AA women expressed being generally interested in using PrEP if available,24 especially if recommended by a trusted healthcare provider.25
Suboptimal patient–provider communication has been identified as a barrier to PrEP uptake among AA women,26 as has limited provider knowledge of PrEP. Historically, the most common reported barriers to prescribing PrEP include a perceived lack of clinical training and experience in PrEP delivery, greater time investment to monitor patients on PrEP and insufficient structural support from clinic sites.27 In a 2022 survey of 359 healthcare providers across the USA, 100% of respondents were aware of PrEP, about 97% reported willingness to prescribe PrEP, and around 80% had prescribed PrEP28; however, these statistics varied by region of practice and by race, with a higher number of providers prescribing PrEP in the west and a disproportionate number of PrEP prescriptions provided to white individuals.28 A recent qualitative study among providers in Alabama indicated uncertainty about offering PrEP to AA heterosexual, cisgender adolescent or young adult females in the absence of transactional sex or a known HIV positive partner.29
Existing literature highlights numerous barriers to effective patient–provider communication about sexual health, including time constraints, embarrassment or shame surrounding these topics, patient confidentiality concerns, and both language and cultural barriers.30–33 Moreover, PrEP services are less routinely implemented in settings where many patients who may benefit from PrEP use may receive care, such as federally qualified health centres (FQHCs).34 In rural areas and small metropolitan areas in particular, FQHCs offering a variety of health services, ranging from primary care to family planning, have been central to the provision of primary and preventive care to underserved populations.35 Almost 6 million women of reproductive age received care from FQHCs in 2012.36 Of the 30 million patients served by FQHCs in 2021, 65% were racial and/or ethnic minorities and 42% lived in rural areas.37 FQHCs are thus an important treatment setting for research geared toward increasing PrEP access among AA women in the rural south.
Study aims
This paper describes the second phase of two-phased prospective, mixed-methods pilot demonstration study. The aim of the first phase was to conduct qualitative interviews exploring preferences around patient–provider communication about HIV and PrEP services to address the needs of AA women. Participants (N=41) included FQHC patients—AA women who reported current and/or recent PrEP use (N=6) or had clinical indications for PrEP use (N=15)—as well as providers (N=20).38
The primary aims of this second phase are: (1) to systematically adapt a patient–provider PrEP communication tool developed by the Centers for Disease Control and Prevention39 to increase PrEP uptake at an FQHC serving a small metropolitan area as well as rural Alabama, using an iterative implementation process and (2) to assess feasibility, acceptability and preliminary impact of the patient–provider communication tool on PrEP uptake among AA women (up to N=125) and their providers (up to N=20) using a pilot preintervention/postintervention design.
Methods and analysis
Study design
During this second phase of the study, the qualitative data collected in the first phase has been used to adapt a patient–provider communication tool focusing on the first steps of the PrEP care cascade, notably identifying as a person who may benefit from PrEP use and being interested in using PrEP, among AA women receiving care at an FQHC in Alabama. For interested women, referrals to PrEP services within the health centre will be facilitated. The protocol will be evaluated in real-time for acceptability and feasibility using both quantitative and qualitative data. The protocol will be iteratively updated until satisfactory procedures have been designed and simultaneously tested for preliminary impact on PrEP uptake. We anticipate conducting three waves of assessments (approximately every 3–5 months). Each wave will consist of 25–40 participants at one enrolment (clinic) site with a target total of N=125. This protocol will be tested for effectiveness, including cost-effectiveness, in a larger R01 cluster randomised implementation trial at primary care and reproductive health centres serving AA women vulnerable to HIV infection in the Deep South.
Population and setting
Participants will include both patients (up to N=125) and healthcare providers (up to N=20) recruited from one FQHC in Alabama. The participating FQHC offers PrEP services, so all PrEP referrals are handled internally at the clinic. Participants will attend a total of two assessment visits (baseline and 3-month assessment) and an intervention visit following the baseline assessment.
Eligibility criteria
Inclusion criteria for patients include: (1) self-identified cisgender women; (2) AA race; (3) age 18 or older; (4) not living with HIV according to self-report; (5) any sex with male partners in past 6 months or anticipated sex in the next 6 months; (6) primary language English; and (7) willing and able to give informed consent. Inclusion criteria for healthcare providers includes: (1) fluency in English; (2) identifies as a physician, nurse practitioner, physician assistant, nurse, medical assistant, social worker/counsellor or other potential PrEP service provider; and (3) willing and able to give informed consent. Potential participants may be excluded if the principal investigators determine, on a case-by-case basis, that their participation would be medically unsafe, complicate interpretation of study findings or otherwise interfere with achieving study objectives.
Theoretical framework
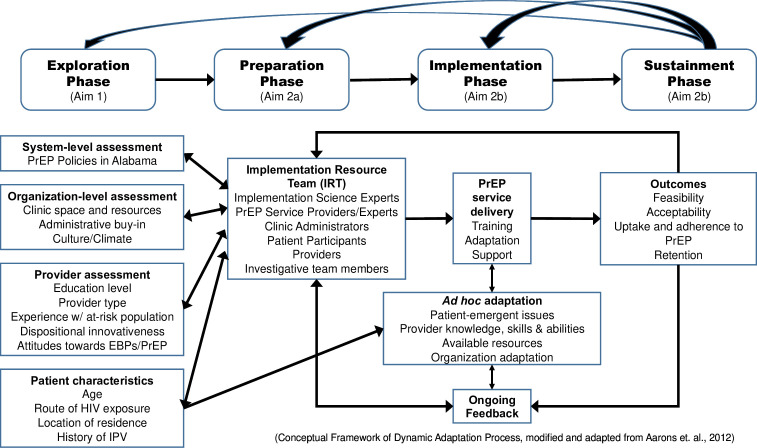
The Exploration, Preparation, Implementation and Sustainment (EPIS) Implementation Framework40 is a meta-theoretical framework that incorporates components from multiple evidence-based implementation process theories and provides a platform to guide intervention planning, adaptation and implementation (figure 1). The EPIS guided the development and evaluation of multiple implementation trials41 42 and will be used as the overarching methodological framework to guide intervention adaptation in this study. EPIS is segmented into four stages: Exploration (ie, organisation, provider and client-level factors that identify potential barriers/facilitators for PrEP uptake); Preparation (ie, adapting intervention to enhance PrEP uptake); Implementation (ie, training, coaching and active facilitation of patient–provider communication intervention); and Sustainment (ie, PrEP uptake and adherence).
Figure 1.
Overview of the Exploration, Preparation, Implementation and Sustainment (EPIS) Implementation Framework for the study. The EPIS Implementation Framework a meta-theoretical framework incorporating components from multiple implementation process theories to guide intervention planning, adaptation and implementation. EPIS provides the overarching methodological framework to guide intervention adaptation in this study. EBPs, evidence-based practices; PrEP, pre-exposure prophylaxis; IPV, intimate partner violence.
Furthermore, the dynamic adaptation process (DAP) framework, which is part of the Preparation and Implementation phases of EPIS, will be used in the adaptation of the patient–provider communication tool (figure 1). The DAP provides direction for activities during each EPIS phase and a continuously iterative, data-informed approach to support intervention implementation.43 Developed for the adaptation of evidence-based interventions (EBIs), it provides a model framework that includes adaptations tailored to specific subgroups. The DAP provides a process for pre-assessment, convening an ‘implementation resource team (IRT)’ to guide the implementation process, and use of audit and feedback data to help guide appropriate EBI adaptation.
Outcome variables
Primary outcomes will include intervention feasibility, acceptability and PrEP uptake. Secondary outcomes will include PrEP adherence and clinic visit adherence. Psychosocial factors will also be measured to characterise the sample and assess potential mediating and moderating factors associated with the outcome measures. All measures and timing of assessments are provided in table 1.
Table 1.
Measures to be administered at each assessment visit
| Patients | BL | PT | 3M | 12M |
| Recruitment: number screened; number of eligible individuals enrolled; reasons for declining enrolment or leaving study; participant contact throughout study; recruitment/scheduling strategies; feasibility of administering instruments/questions | X | X | ||
| PrEP referral: number of patients referred; number of patients accepting referral; reasons for declining referral; reasons for unsuccessful or incomplete referral | X | X | X | |
| PrEP uptake: ratio of patients initiating PrEP to the number eligible patients screened and referred (measured throughout) | X | X | ||
| PrEP adherence: self-report via Visual Analogue Scale52; reasons for discontinuing PrEP | X | X | ||
| Clinic visit adherence: calculated as PrEP visits adhered to divided by PrEP visits scheduled | X | X | ||
| Satisfaction with intervention: Client Satisfaction Questionnaire (CSQ-8)44 | X | |||
| Perceptions of study and evaluation of intervention: qualitative interview | X | |||
| Intimate partner violence: Abuse Assessment Screen (AAS)53 | X | X | ||
| Depression: Center for Epidemiologic Studies Depression (CES-D)54 | X | X | ||
| Spiritual support: Ironson-Woods Spirituality/Religiousness (SR)55 | X | |||
| Social support: Medical Outcomes Study (MOS) social support survey56 | X | |||
| Substance use: Addiction Severity Index-Lite (ASI-Lite)57 | X | X | ||
| Trauma experience: adapted from items within Project BRIgHT58 | X | X | ||
| Anxiety: State-Trait Anxiety Inventory State Form (STAI-S)59 | X | X | ||
| Identification with organisation: items drafted by study team | X | |||
| Stage of change: adapted from Stage of Change measures60 61 | X | X | X | |
| PrEP knowledge and experience: adapted from PrEP Awareness and Willingness62 | X | |||
| HIV transmission knowledge: adapted from the HIV Risk Knowledge Test63 | X | X | ||
| Sexual behaviour: number of sexual partners; alcohol/drug use before sex; vaginal/anal sex; knowledge of partners’ HIV status; condom use | X | X | ||
| Reflecting and evaluating: quantitative and qualitative feedback about progress and quality of implementation, accompanied with regular personal and team debriefing about progress and experience | Continuous | |||
| Proposed intervention modifications: both structural and didactic | Continuous | |||
| Providers | BL | PT | 3M | 12M |
| Sociodemographics: age, race, ethnicity, education, clinic position | X | |||
| Implementation readiness: adapted from the Implementation Climate Scale (ICS)64 | X | |||
| Dispositional innovativeness: Physician-Motivation-Adoption (PMA)65 | X | |||
| Culture: Organisational Culture Assessment Instrument (OCAI)66 | X | |||
| Patient needs and clinic resources: adapted subscales from Texas Christian University Organisational Readiness for Change Scale (TCU-ORC-D4)67 | X | |||
| Stigma: adapted from the attitude toward people living with HIV scale68 | X | |||
| Satisfaction with intervention: Behavioral Interventionist Satisfaction Survey (BISS)45 (12M only) and Short Survey (PT only) | X | X | ||
| Identification with organisation: qualitative interview | X | |||
| Stage of change: qualitative interview | X | |||
| Perceptions of study and evaluation of intervention: qualitative interview | X | |||
| Reflecting and evaluating: quantitative and qualitative feedback about progress and quality of implementation, accompanied with regular personal and team debriefing about progress and experience | X | |||
| Proposed intervention modifications: both structural and didactic | Continuous | |||
BL, baseline; 3M, 3-month assessment visit; 12M, 12-month data abstraction from electronic medical record (patients) or assessment visit (provider); PrEP, pre-exposure prophylaxis; PT, post-treatment.
Feasibility
We will measure the number of individuals screened, number of eligible individuals enrolled and number of enrolled participants who initiate PrEP and adhere to their prescribed regimen. We will also track reasons for declining enrolment, prematurely leaving the study, declining a referral, not attending a PrEP clinic visit and/or discontinuing PrEP. Recruitment and scheduling strategies, participant contact and feasibility of administering instruments (eg, assessment duration), will be documented.
Acceptability
Acceptability will be assessed through individual in-depth qualitative interviews at the end of the study. Interviews will explore participants’ experiences with and perceptions of the study, and their evaluations of the patient–provider communication tool to facilitate PrEP uptake. Patient and provider satisfaction with the intervention will be assessed via the Client Satisfaction Questionnaire (CSQ-8)44 and the Behavioral Interventionist Satisfaction Survey.45
PrEP uptake
PrEP uptake will be measured by calculating the ratio of patients initiating PrEP to the number of patients eligible for the study who enrolled and were referred to PrEP services.
PrEP adherence
Self-report (ie, Visual Analogue Scale) will be used to assess patients’ adherence to taking PrEP as prescribed, as is currently standard practice in the participating clinics.46 Reasons for discontinuing PrEP use, as applicable, will also be tracked. Participants will also be asked to rate, on a 6-point Likert scale, their ability to take all medications as prescribed.
Clinic visit adherence
Attendance at clinic visits will be defined as the number of PrEP visits attended divided by the number of visits scheduled. Adherence will be assessed at 3-month follow-up and 12 months via electronic medical record (EMR) abstraction.
Psychosocial factors
Psychosocial factors will include assessments of intimate partner violence, depression, anxiety, post-traumatic stress disorder (PTSD), sexual behaviours, HIV transmission knowledge, substance use, social support and spirituality/religiousness.
Intervention adaptation
Adaptation activities
Based on the formative evaluation in the first phase of this study, involving qualitative interviews with patients and providers (ie, Exploration phase), a first draft of the patient–provider communication tool was produced. An adaptation plan as described by Aarons and colleagues40 was used to document changes (ie, new activities and materials to be included) to the protocol and reasons for such changes or additions. Given the minority status of our target population, it was anticipated that cultural adaptations would include process and content changes relevant to AA women vulnerable to HIV infection.47 48 Adaptation was considered on the patient, provider and organisational levels as per the EPIS.
During the Preparation phase, an IRT was convened to review the first draft of the adapted patient–provider communication tool. The IRT was comprised experts in implementation science and PrEP delivery, representatives of the clinic administration/staff (at both the FQHCs and the PrEP clinics), potential PrEP candidates and PrEP users, providers and research team members. A second draft of the adapted communication tool integrated recommendations made and measures added by the IRT, maintaining the core elements of the patient–provider communication tool and considering the limitations and needs of the study sites.
Intervention implementation
Provider recruitment and training
Provider participants will be identified by the partnering clinic’s study research assistant (RA). The study RA will contact study research staff on participating providers’ behalf. Recruited providers will complete an informed consent form and baseline assessment. Links to the assessment battery will be sent to providers via email by research team members. Signed consent forms and baseline assessment responses will be directly entered and stored in a secure Research Electronic Data Capture (REDCap) database.
Enrolled providers will receive training in use of the patient–provider communication tool and best practices for prescribing PrEP. Training will be directed by healthcare practitioners with extensive experience in PrEP prescribing, training providers in PrEP prescribing and/or managing PrEP-related logistics. Provider training will consist of two parts: first, providers will watch two training videos, which will include an overview of PrEP basics and the study’s patient–provider communication tool; second, providers will participate in a live virtual training session with trainers, including an interactive roleplay using parts of the patient–provider communication tool, all of which has been piloted during phase I. Additional trainings and preparation sessions may be held in-person at the clinic site or by phone as needed.
Patient recruitment
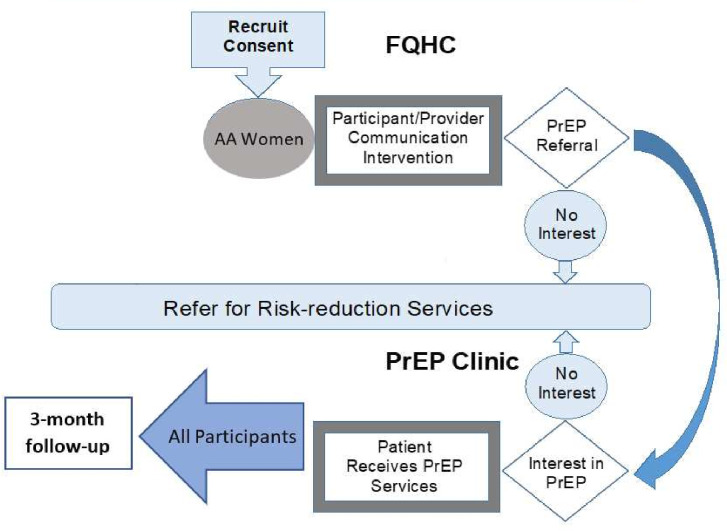
Patient participants will be recruited in three ways: (1) study RA will prescreen potential participants through their EMRs and flag any patients with upcoming clinic visits who meet study eligibility criteria; (2) flyers will be posted in clinic waiting areas and (3) healthcare providers at the participating clinic will directly refer interested patients who may be eligible to participate in the study. If a patient is recruited through EMR prescreen, they will be formally screened by the study RA during their routine clinic visit. If a patient is recruited via flyer or provider referral, they will either contact the study RA by phone (using the phone number listed on the flyer) or may provide permission to be contacted by the study RA directly, who will then screen the prospective participant by phone. All individuals who meet inclusion criteria will be invited to complete the informed consent process at the baseline visit (figure 2).
Figure 2.
Flowchart of the informed consent, assessment and intervention process for the study. AA, African American; FQHC, federally qualified health centre; PrEP, pre-exposure prophylaxis.
Informed consent
All participants will complete a baseline assessment visit either in-person or by phone, in which they will review an informed consent form, including a detailed explanation of all study procedures, information about potential risks and benefits of participation, and contact information for the study team in the event of further questions. The consent form will also state that participation is voluntary, that they can withdraw from the study at any time, and that study participation is in no way related to their healthcare, including receipt of PrEP services. Written or verbal consent will be obtained, and a copy of the signed consent form will be provided to participants.
Study assessments and intervention
Enrolled patients will complete a quantitative assessment during the baseline visit, administered via REDCap,49 which will include sociodemographics and measures of anxiety, depression, interpersonal violence, substance use, PrEP awareness, sexual behaviours, PTSD as well as spiritual/emotional support. Patients will then be scheduled to meet with a provider, who will use the newly adapted PrEP patient–provider communication tool. Patients who are interested in receiving a referral for PrEP after completing the intervention will be referred to the in-house PrEP clinic; this referral will include a ‘warm hand-off’ by a provider or PrEP navigator. Providers will document the visit in patients’ electronic health records per standard clinic practice. Referrals will be made if domestic or intimate partner violence, and/or suicidal ideation are indicated in the baseline assessment.
Patients and providers at the local PrEP clinic will then jointly decide whether to initiate PrEP after referral. Providers at the PrEP clinics will be responsible for all aspects of PrEP care, including reviewing lab results, reinforcing educational messages around adherence and additional HIV/STI prevention options, and conducting patient examinations as needed, consistent with their current PrEP delivery practices. Regardless of PrEP initiation, patients will be scheduled for a 3-month follow-up study visit conducted via phone. During implementation, quarterly feedback will be provided by the IRT (or more frequently if needed), who will evaluate whether further adaptations are needed to the patient-provider communication intervention. IRT members will be comprised of experts in implementation science and PrEP delivery, representatives of the clinic administration and staff at local FQHCs, potential PrEP candidates and users, providers and members of the investigative team. Best practices for intervention delivery process, type and frequency of communication with AA women vulnerable to HIV infection will also be assessed.
Remuneration
Patients will be remunerated a total of $100 for their participation and transportation costs, independent of PrEP uptake. Providers will receive $50 for their time and 1.5 credit hours of continuing education credit for their participation in the study training.
Patient and public involvement
Patient and PrEP healthcare provider involvement is incorporated into the design and conduct of this implementation science study. The IRT described above is comprised of FQHC patients and PrEP navigators/providers as well as research team members. The IRT guides and informs the intervention design and implementation of the study throughout, including the interpretation and dissemination of results.
Data analyses
The feasibility, acceptability and preliminary impact of this intervention will be assessed among patients and providers using this preintervention/postintervention design. Quantitative data will be analysed using SPSS or R. Feasibility will be determined by evaluation of recruitment and retention, number of PrEP referrals, PrEP initiation, PrEP adherence and clinic visit adherence. Acceptability will be measured using in-depth, individual, qualitative exit interviews and satisfaction surveys. Continuous feedback from participants and experts relevant to the population and outcomes studied will assure feasibility, acceptability and appropriateness of the adapted intervention. Structural and content related changes of the intervention will be based on the feedback provided by patients and providers.
Primary and secondary outcomes will be assessed by characterising the sample using descriptive statistics, computing CIs on these measures and exploring patient and implementation characteristics as possible moderators of these outcomes in order to inform the design of our future trial. Measures of effect size (eg, Cohen’s d, Cohen’s r, Cramer’s V, R2, etc) will be used to determine the characteristics that individually appeared to be relevantly associated with PrEP uptake and secondary outcomes in the sample.
To examine impact of the protocol adaptation, we will use calendar quarter of entry in the study as a possible moderator. Multivariable exploratory analyses will be conducted using non-parametric methods50 including penalised regression (LASSO) and random forest, to determine if the sample data suggest a smaller set of relevant characteristics based on their cross-validated predictive ability of each outcome. These data will be useful in tailoring our implementation approach based on empirical observation. On a priori conceptual considerations, we expect age, intimate partner violence and sexual risk behaviour to be supported by the data as moderators of PrEP uptake. In addition, exploratory mediation analyses will be conducted based on conceptual considerations using the procedures outlined by Hayes.51
Clinics’ EMR data be used to estimate PrEP uptake, based on the number of PrEP-eligible AA women who have been referred to PrEP in the 3 years pre-implementation and post implementation of the protocol (ie, pretest/post-test design). Binomial logistic models (events/trials syntax) will be fitted with time-period and clinic as main effects to estimate the differences in uptake proportions between preprotocol and postprotocol time periods. To determine the specific impact of the iterative process of protocol adaptation during the year of implementation, we will fit a logistic model for uptake with bimonthly assessment wave number (three waves) as a categorical predictor. A priori, we expect a positive relationship between the assessment wave number and the odds of uptake, indicating that as the protocol became more refined during the year, PrEP uptake increased.
Sample size
To examine differences in PrEP uptake rates in the participating clinic comparing 3 years before and the year after the implementation of the protocol, we estimate that there are at least 3500 AA PrEP-eligible women serviced annually. Assuming an uptake proportion of 0.0125 (44/3500=1.25%) estimated retrospectively in the 3 years prior to study implementation and a within-subject correlation of 0.65, at a significance level of 0.01, a sample size of 3500 provides 90% power to detect an increase in proportion of uptake of 0.007 (0.7%=25/3500); however, though power is high, interpretation of this inference would be restricted to the particular clinic in the study or clinics with similar characteristics.
The target sample size of N=125 is informative to provide reasonable range estimates (in the form of 95% CIs) for the measures of interest in this study. For instance, assuming that 25% (n=31/125) of the in-study clients referred to PrEP in fact initiate PrEP, the width of the CI for this percentage is 15.8%, and assuming that age has a SD of 5 years and age moderates PrEP uptake, the CI width for the mean difference in age between those who initiate PrEP (n=31) and those who do not (n=94) is 4.1 years (computations conducted using PASS V.23 software). Given that this is an exploratory study with several primary and secondary outcomes, we do not have an expected effect size, but our study is nonetheless powered to detect a moderate effect.
Ethics and dissemination
The study will be conducted under a single IRB (sIRB) at the University of Alabama at Birmingham (UAB), with reliant sites at Massachusetts General Hospital and Beth Israel Deaconess Medical Center. Ethics approval was obtained for all aspects of this study by the IRB at UAB (UAB; protocol 300004276), where the work is being conducted. All participants will receive detailed written information on the purpose and procedures of the study and will provide written or verbal informed consent prior to enrolment. Study updates, preliminary findings and final results will be disseminated through publication of manuscripts in peer-reviewed journals, as well as through reports to the National Institutes of Health, and through local, national and international presentations at HIV-focused conferences and meetings (table 2).
Table 2.
WeExPAnd trial registration data
| Data category | Information |
| Primary registry and trial identifying number | ClinicalTrials.gov NCT04373551 |
| Date of registry in primary registry | 4 May 2020 |
| Secondary identifying numbers | 300003885, 1R34MH118044-01A1 |
| Source(s) of monetary or material support | National Institute of Mental Health |
| Primary sponsor | National Institute of Mental Health |
| Secondary sponsor | N/a |
| Contact for public queries | Mirjam-Colette Kempf, PhD (mkempf@uab.edu) |
| Contact for scientific queries | Mirjam-Colette Kempf, PhD University of Alabama at Birmingham |
| Public title | PrEP Demonstration Project Among Women at Risk for HIV Infection |
| Scientific title | N/a |
| Countries of recruitment | USA |
| Health condition(s) or problem(s) studied | HIV-infection/AIDS |
| Intervention(s) | Behavioural: cultural adaptation of a patient–provider communication tool |
| Key inclusion and exclusion criteria | Ages eligible for study: ≥18 years Sexes eligible for study: cisgender female Gender-based eligibility: yes Accepts health volunteers: no |
| Patients Inclusion criteria: African American cisgender women aged 18 years or older without HIV; report HIV risk and/or recent PrEP use; English-speaking | |
| Providers Inclusion criteria: physicians, nurse practitioners, physician assistants, nurses, medical assistants, social workers/counsellors or other potential/actual PrEP service providers; English-speaking | |
| Study type | Interventional (clinical trial) |
| Allocation: N/a | |
| Primary purpose: health services research | |
| Phase: N/a | |
| Study start date | 14 April 2020 |
| Target sample size | 125 participants |
| Recruitment status | Enrolling by invitation |
| Primary outcome(s) | PrEP uptake changes (time frame: baseline, 3 months and 12 months) Intervention feasibility changes (time frame: baseline, 3 months and 12 months) Intervention acceptability (time frame: through study completion, an average of 12 months) |
| Secondary outcome(s) | PrEP adherence (time frame: 3 months and 12 months) Clinic visit adherence changes (time frame: 3 months and 12 months) Biological measures of HIV, STIs and pregnancy (time frame: baseline, 3 months and 12 months) |
PrEP, pre-exposure prophylaxis; STI, sexually transmitted infection.
Study status
At the time of writing, a total of 49 patients and nine providers have been enrolled in the study; screening and enrolment is ongoing. The PIs determined that a single FQHC site, rather than the two FQHCs initially identified, would both provide sufficient data to pilot test the patient–provider communication tool and allow for better concentration of implementation resources; as such, only one site has been activated with a target enrolment of N=125. The study will be completed by June 2024.
Supplementary Material
Footnotes
Contributors: CP and M-CK conceived and designed the pilot demonstration study and its protocol and directed protocol activities. GRG drafted the protocol manuscript and designed several manuscript tables. VWM, CO, AB and AR contributed to drafting, editing and finalising the protocol manuscript and the design of the overall project protocol. LS planned and edited the statistical methods section of the manuscript. MC and EU advised on the protocol methods and provided comments on manuscript. DK, LE, KK and KHS advised on the structure and implementation of the protocol and provided comments on the protocol manuscript.
Funding: This work is supported by the National Institute of Mental Health (NIMH; grant number 5R34MH118044-03).
Competing interests: None declared.
Patient and public involvement: Patients and/or the public were involved in the design, or conduct, or reporting, or dissemination plans of this research. Refer to the Methods section for further details.
Provenance and peer review: Not commissioned; peer reviewed for ethical and funding approval prior to submission.
Ethics statements
Patient consent for publication
Not applicable.
References
- 1.Centers for disease control and prevention . HIV in the Southern United States issue brief. 2019. Available: https://www.cdc.gov/hiv/pdf/policies/cdc-hiv-in-the-south-issue-brief.pdf
- 2.Centers for Disease Control and Prevention . Diagnoses of HIV infection in the United States and dependent areas 2019: special focus profiles. 2021. Available: https://www.cdc.gov/hiv/library/reports/hiv-surveillance/vol-32/content/special-focus-profiles.html#Women
- 3.Kaiser Family Foundation . Women and HIV in the United States. 2020. Available: https://www.kff.org/hivaids/fact-sheet/women-and-hivaids-in-the-united-states/
- 4.Centers for Disease Control and Prevention . Leading causes of death – females – non-Hispanic black – United States 2018. 2022. Available: https://www.cdc.gov/women/lcod/2018/nonhispanic-black/index.htm
- 5.Adimora AA, Schoenbach VJ, Doherty IA. HIV and African Americans in the Southern United States: sexual networks and social context. Sex Transm Dis 2006;33(7 Suppl):S39–45. 10.1097/01.olq.0000228298.07826.68 [DOI] [PubMed] [Google Scholar]
- 6.Bowleg L, Malekzadeh AN, Mbaba M, et al. Ending the HIV epidemic for all, not just some: structural racism as a fundamental but overlooked social-structural determinant of the U.S HIV epidemic. Curr Opin HIV AIDS 2022;17:40–5. 10.1097/COH.0000000000000724 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Adimora AA, Schoenbach VJ, Martinson FEA, et al. Heterosexually transmitted HIV infection among African Americans in North Carolina. J Acquir Immune Defic Syndr 2006;41:616–23. 10.1097/01.qai.0000191382.62070.a5 [DOI] [PubMed] [Google Scholar]
- 8.Adimora AA, Schoenbach VJ, Doherty IA. Concurrent sexual partnerships among men in the United States. Am J Public Health 2007;97:2230–7. 10.2105/AJPH.2006.099069 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Doherty IA, Schoenbach VJ, Adimora AA. Sexual mixing patterns and Heterosexual HIV transmission among African Americans in the southeastern United States. J Acquir Immune Defic Syndr 2009;52:114–20. 10.1097/QAI.0b013e3181ab5e10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Harawa N, Adimora A. Incarceration, African Americans, and HIV: advancing a research agenda. J Natl Med Assoc 2008;100:57–62. 10.1016/s0027-9684(15)31175-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Aaron E, Blum C, Seidman D, et al. Optimizing delivery of HIV Preexposure prophylaxis for women in the United States. AIDS Patient Care STDS 2018;32:16–23. 10.1089/apc.2017.0201 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Stanger-Hall KF, Hall DW. Abstinence-only education and teen pregnancy rates: why we need comprehensive sex education in the U.S. PLoS One 2011;6:e24658. 10.1371/journal.pone.0024658 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jozkowski KN, Crawford BL. The status of reproductive and sexual health in Southern USA: policy recommendations for improving health outcomes. Sex Res Soc Policy 2016;13:252–62. 10.1007/s13178-015-0208-7 [DOI] [Google Scholar]
- 14.Cavanaugh CE, Hansen NB, Sullivan TP. HIV sexual risk behavior among low-income women experiencing intimate partner violence: The role of Posttraumatic Stress Disorder. AIDS Behav 2010;14:318–27. 10.1007/s10461-009-9623-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Frew PM, Parker K, Vo L, et al. Socioecological factors influencing women’s HIV risk in the United States: qualitative findings from the women’s HIV Seroincidence study (HPTN 064). BMC Public Health 2016;16:803. 10.1186/s12889-016-3364-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Baeten JM, Donnell D, Ndase P, et al. Antiretroviral prophylaxis for HIV prevention in Heterosexual men and women. N Engl J Med 2012;367:399–410. 10.1056/NEJMoa1108524 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.World Health Organization . Trial results reveal that long-acting Injectable Cabotegravir as prep is highly effective in preventing HIV acquisition in women. 2020. Available: https://www.who.int/news/item/09-11-2020-trial-results-reveal-that-long-acting-injectable-cabotegravir-as-prep-is-highly-effective-in-preventing-hiv-acquisition-in-women
- 18.Delany-Moretlwe S, Hughes JP, Bock P, et al. Cabotegravir for the prevention of HIV-1 in women: results from HPTN 084, a phase 3, randomised clinical trial. Lancet 2022;399:1779–89. 10.1016/S0140-6736(22)00538-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.HIV.gov . Impact on racial and ethnic minorities. 2023. Available: https://www.hiv.gov/hiv-basics/overview/data-and-trends/impact-on-racial-and-ethnic-minorities/
- 20.Huang Y L, Zhu W, Smith DK, et al. HIV Preexposure prophylaxis, by race and Ethnicity - United States, 2014-2016. MMWR Morb Mortal Wkly Rep 2018;67:1147–50. 10.15585/mmwr.mm6741a3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sullivan PS, Mena L, Elopre L, et al. Implementation strategies to increase prep uptake in the South. Curr HIV/AIDS Rep 2019;16:259–69. 10.1007/s11904-019-00447-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Auerbach JD, Kinsky S, Brown G, et al. Knowledge, attitudes, and likelihood of pre-exposure prophylaxis (prep) use among US women at risk of acquiring HIV. AIDS Patient Care STDS 2015;29:102–10. 10.1089/apc.2014.0142 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Collier KL, Colarossi LG, Sanders K. Raising awareness of pre-exposure prophylaxis (prep) among women in New York City: community and provider perspectives. J Health Commun 2017;22:183–9. 10.1080/10810730.2016.1261969 [DOI] [PubMed] [Google Scholar]
- 24.Sales JM, Steiner RJ, Brown JL, et al. Prep eligibility and interest among Clinic- and community-recruited young black women in Atlanta. Curr HIV Res 2018;16:250–5. 10.2174/1570162X16666180731143756 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wingood GM, Dunkle K, Camp C, et al. Racial differences and correlates of potential adoption of pre-exposure prophylaxis (prep): results of a national survey. J Acquir Immune Defic Syndr 2013;63 Suppl 1:S95–101. 10.1097/QAI.0b013e3182920126 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Goparaju L, Praschan NC, Warren-Jeanpiere L, et al. Providers and costs: potential barriers to prep uptake among US women. J AIDS Clin Res 2017;8:730. 10.4172/2155-6113.1000730 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Castel AD, Feaster DJ, Tang W, et al. Understanding HIV care provider attitudes regarding intentions to prescribe prep. J Acquir Immune Defic Syndr 2015;70:520–8. 10.1097/QAI.0000000000000780 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rao S, Reed AE, Parchem B, et al. Optimizing provider Preexposure prophylaxis (prep) training: A cross-sectional analysis of recommendations from providers across the prep implementation Cascade. AIDS Behav 2022;26:218–31. 10.1007/s10461-021-03375-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pratt MC, Hill SV, Elopre L, et al. Prep prescription for black adolescent girls and young women in Alabama: findings from a survey of Healthcare providers. J Int Assoc Provid AIDS Care 2022;21:23259582221127936. 10.1177/23259582221127936 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hoopes AJ, Benson SK, Howard HB, et al. Adolescent perspectives on patient-provider sexual health communication: A qualitative study. J Prim Care Community Health 2017;8:332–7. 10.1177/2150131917730210 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Malta M, Todd CS, Stibich MA, et al. Patient-provider communication and reproductive health among HIV-positive women in Rio de Janeiro. Patient Educ Couns 2010;81:476–82. 10.1016/j.pec.2010.09.013 [DOI] [PubMed] [Google Scholar]
- 32.Calabrese SK, Mayer KH. Stigma Impedes HIV prevention by stifling patient-provider communication about U = U. J Int AIDS Soc 2020;23:e25559. 10.1002/jia2.25559 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Flynn KE, Whicker D, Lin L, et al. Sexual orientation and patient-provider communication about sexual problems or concerns among US adults. J Gen Intern Med 2019;34:2505–11. 10.1007/s11606-019-05300-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tookes H, Yao K, Chueng T, et al. Pre-exposure prophylaxis access in federally qualified health centers across 11 United States metropolitan statistical areas. Int J STD AIDS 2019;30:978–84. 10.1177/0956462419855178 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.National Association of Community Health Centers . Health centers' workforce: the backbone of our country’s Healthcare. 2022. Available: https://www.nachc.org/wp-content/uploads/2022/02/P-I-Brief-2022.pdf
- 36.Wood S, Beeson T, Bruen B, et al. Scope of family planning services available in federally qualified health centers. Contraception 2014;89:85–90. 10.1016/j.contraception.2013.09.015 [DOI] [PubMed] [Google Scholar]
- 37.National Association of Community Health Centers . America’s health centers. 2022. Available: https://www.nachc.org/wp-content/uploads/2022/08/Americas-Health-Centers-2022_final.pdf
- 38.Psaros C, Goodman G, Blyler A, et al. Barriers to expanding prep uptake among Cisgender African American women in the South. 11th International AIDS Society (IAS) Conference on HIV Science; Berlin, Germany, 2021. Available: https://www.natap.org/2021/IAS/IAS_59.htm [Google Scholar]
- 39.Centers for Disease Control and Prevention . Centers for disease control and prevention. A guide for the Healthcare professional: discussing sexual health with your patients. 2022. Available: https://www.cdc.gov/hiv/pdf/clinicians/screening/cdc-hiv-php-discussing-sexual-health.pdf
- 40.Aarons GA, Hurlburt M, Horwitz SM. Advancing a conceptual model of evidence-based practice implementation in public service sectors. Adm Policy Ment Health 2011;38:4–23. 10.1007/s10488-010-0327-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Willging CE, Green AE, Ramos MM. Implementing school nursing strategies to reduce LGBTQ adolescent suicide: a randomized cluster trial study protocol. Implement Sci 2016;11. 10.1186/s13012-016-0507-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Becan JE, Bartkowski JP, Knight DK, et al. A model for rigorously applying the exploration, preparation, implementation, Sustainment (EPIS) framework in the design and measurement of a large scale collaborative multi-site study. Health Justice 2018;6:9. 10.1186/s40352-018-0068-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Aarons GA, Green AE, Palinkas LA, et al. Dynamic adaptation process to implement an evidence-based child Maltreatment intervention. Implement Sci 2012;7:32. 10.1186/1748-5908-7-32 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Larsen DL, Attkisson CC, Hargreaves WA, et al. Assessment of client/patient satisfaction: development of a general scale. Eval Program Plann 1979;2:197–207. 10.1016/0149-7189(79)90094-6 [DOI] [PubMed] [Google Scholar]
- 45.McLean KA. Healthcare provider acceptability of a behavioral intervention to promote adherence [Master’s thesis]. University of Miami, 2013. Available: https://scholarship.miami.edu/esploro/outputs/graduate/Healthcare-Provider-Acceptability-of-a-Behavioral/991031447949202976 [Google Scholar]
- 46.Montgomery MC, Oldenburg CE, Nunn AS, et al. Adherence to pre-exposure prophylaxis for HIV prevention in a clinical setting. PLoS One 2016;11:e0157742. 10.1371/journal.pone.0157742 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Marsiglia FF, Booth JM. Cultural adaptation of interventions in real practice settings. Res Soc Work Pract 2015;25:423–32. 10.1177/1049731514535989 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Norton WE, Amico KR, Cornman DH, et al. An agenda for advancing the science of implementation of evidence-based HIV prevention interventions. AIDS Behav 2009;13:424–9. 10.1007/s10461-009-9556-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Harris PA, Taylor R, Thielke R, et al. Research electronic data capture (Redcap)--A Metadata-driven methodology and Workflow process for providing Translational research Informatics support. J Biomed Inform 2009;42:377–81. 10.1016/j.jbi.2008.08.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Efron B, Hastie T. Computer age statistical inference. In: Computer Age Statistical Inference: Algorithms, Evidence, and Data Science. Cambridge University Press, 2016. 10.1017/CBO9781316576533 [DOI] [Google Scholar]
- 51.Hayes A. Introduction to Mediation, Moderation, and Conditional Process Analysis. The Guilford Press, 2013. [Google Scholar]
- 52.Lu M, Safren SA, Skolnik PR, et al. Optimal recall period and response task for self-reported HIV medication adherence. AIDS Behav 2008;12:86–94. 10.1007/s10461-007-9261-4 [DOI] [PubMed] [Google Scholar]
- 53.Laughon K, Renker P, Glass N, et al. Revision of the abuse assessment screen to address nonlethal strangulation. JOGNN 2008;37:502–7. 10.1111/j.1552-6909.2008.00268.x [DOI] [PubMed] [Google Scholar]
- 54.Radloff LS. The CES-D scale: A self-report depression scale for research in the general population. Appl Psychol Meas 1977;1:385–401. 10.1177/014662167700100306 [DOI] [Google Scholar]
- 55.Ironson G, Solomon GF, Balbin EG, et al. The Ironson-woods spirituality/religiousness index is associated with long survival, health behaviors, less distress, and low Cortisol in people with HIV/AIDS. Ann Behav Med 2002;24:34–48. 10.1207/S15324796ABM2401_05 [DOI] [PubMed] [Google Scholar]
- 56.Sherbourne CD, Stewart AL. The MOS social support survey. Soc Sci Med 1991;32:705–14. 10.1016/0277-9536(91)90150-b [DOI] [PubMed] [Google Scholar]
- 57.McLellan AT, Luborsky L, Woody GE, et al. An improved diagnostic evaluation instrument for substance abuse patients. J Nerv Ment Dis 1980;168:26–33. 10.1097/00005053-198001000-00006 [DOI] [PubMed] [Google Scholar]
- 58.Psaros C. The bright program: building resilience in HIV together. Clinicaltrials.Gov. 2018. Available: https://clinicaltrials.gov/ct2/show/NCT03673098
- 59.Skapinakis P. Spielberger state-trait anxiety inventory. In: Michalos AC, ed. Encyclopedia of Quality of Life and Well-Being Research. Springer, 2014. 10.1007/978-94-007-0753-5_2825 [DOI] [Google Scholar]
- 60.Napper LE, Branson CM, Fisher DG, et al. Assessing the validity of a single-item HIV risk stage-of-change measure. J Drug Educ 2008;38:27–37. 10.2190/DE.38.1.c [DOI] [PubMed] [Google Scholar]
- 61.Basta TB, Reece M, Wilson MG. Predictors of exercise stage of change among individuals living with HIV/AIDS. Med Sci Sports Exerc 2008;40:1700–6. 10.1249/MSS.0b013e318173f09e [DOI] [PubMed] [Google Scholar]
- 62.Taggart T, Liang Y, Pina P, et al. Awareness of and willingness to use prep among black and Latinx adolescents residing in higher prevalence areas in the United States. PLoS One 2020;15:e0234821. 10.1371/journal.pone.0234821 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Sikkema KJ, Heckman TG, Kelly JA, et al. HIV risk behaviors among women living in low-income, inner-city housing developments. Am J Public Health 1996;86:1123–8. 10.2105/ajph.86.8_pt_1.1123 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Ehrhart MG, Aarons GA, Farahnak LR. Assessing the organizational context for EBP implementation: the development and validity testing of the implementation climate scale (ICS). Implement Sci 2014;9. 10.1186/s13012-014-0157-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Hatz MHM, Sonnenschein T, Blankart CR. The PMA scale: A measure of physicians' motivation to adopt medical devices. Value Health 2017;20:533–41. 10.1016/j.jval.2016.12.002 [DOI] [PubMed] [Google Scholar]
- 66.Heritage B, Pollock C, Roberts L. Validation of the organizational culture assessment instrument. PLoS One 2014;9:e92879. 10.1371/journal.pone.0092879 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Institute of Behavioral Research . TCU organizational readiness for change (ORC-D4).Texas Christian University. 2009. Available: https://ibr.tcu.edu/forms/organizational-staff-assessments/#:~:text=TCU%20Organizational%20Readiness%20for%20Change,individually%2C%20depending%20on%20assessment%20strategy
- 68.Stringer KL, Turan B, McCormick L, et al. HIV-related stigma among Healthcare providers in the deep South. AIDS Behav 2016;20:115–25. 10.1007/s10461-015-1256-y [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.