Abstract
We present the case of an HIV-negative man in his 50s who developed a generalised nodular rash while having first-line bortezomib–cyclophosphamide–dexamethasone chemotherapy for multiple myeloma. The rash was biopsied and proven to be Kaposi’s sarcoma. The patient’s treatment was interrupted at the sixth cycle of chemotherapy, by which time the rash had also spread to the oral mucosa and eyelid. The rash regressed spontaneously on stopping treatment. We were reluctant to restart myeloma treatment, but on the other hand, we wished to consolidate the very good partial response achieved. An autologous marrow transplant was done months later without any recurrence of his Kaposi’s with the initiation of bortezomib maintenance. Bortezomib has putative activity against Kaposi’s. The patient could benefit from imid-based (thalidomide, lenalidomide, pomalidomide) combination chemotherapy once his myeloma progresses or if there is a recurrence of Kaposi’s sarcoma.
Keywords: haematology (incl blood transfusion), oncology, malignant disease and immunosuppression
Background
It has been over a 150 years since the Hungarian dermatologist Kaposi published the article: ‘Idiopathic multiple Pigmented Sarcoma of the Skin.’1 Our case highlights the treatment decisions for the uncommon scenario of iatrogenic Kaposi’s sarcoma as a result of chemotherapy for multiple myeloma. Kaposi’s sarcoma is an angioproliferative tumour that can occur in the iatrogenic form from chronic immunosuppression. Human herpesvirus 8 (HHV8) is invariably associated with the neoplasm and can be detected by staining for the surrogate marker: latent nuclear antigen 1 in a suspected tumour. Interruption of immunosuppression and the resultant immune reconstitution caused regression of the condition, which left us with the challenge of choosing the best regimen that would effectively control the myeloma clone without causing the recurrence of the sarcoma. Our dilemma was whether to proceed with autologous transplantation with maintenance, given the theoretical risk of recurrence. This case highlights the fact that autologous stem cell transplantation (ASCT) can be successfully carried out for myeloma after immune reconstitution without necessarily provoking the recurrence of Kaposi’s sarcoma.
Case presentation
A heterosexual man in his 50s with a history of hypertension was originally referred to a rheumatologist from the community for evaluation of gradually progressive back pain causing sleep disturbance. The patient meanwhile presented with anuria, lethargy and nausea to the emergency department and was found to be in acute renal failure and metabolic acidosis. The patient underwent three sessions of emergent haemodialysis.
Serum protein electrophoresis revealed the presence of a paraprotein measuring 28.5 g/L, which was identified as an IgG kappa monoclonal band on immunofixation. There were several lytic lesions on MRI of the whole spine. The summary of investigations is listed in table 1. The patient was diagnosed with multiple myeloma with stage III disease as per the international staging system score, and he was then started on induction therapy with bortezomib, cyclophosphamide and dexamethasone. Rapid renal recovery was observed by the end of his first cycle of chemotherapy. The patient had a very good partial response with near disappearance of IgG paraprotein as per International Myeloma Working Group criteria at the end of cycle 6; however, the appearance of an eruptive, nodular rash was observed and was biopsied with findings on histology confirming the diagnosis of Kaposi’s sarcoma as shown in figure 1. The nodular rash involved also the oral mucosa as shown in figure 2.
Table 1.
Relevant blood and bone marrow investigations done at initial presentation
| Blood investigations at multiple myeloma diagnosis | Results (reference range) | |
| Blood tests | ||
| Serum protein electrophoresis | ||
| Monoclonal band (g/L) | 28.5 | |
| Immunoglobulin levels | ||
| IgG (g/L) | 36.1 (6–16) | |
| IgM (g/L) | 0.1 (0.4–2.5) | |
| IgA (g/L) | 0.37 (0.8–3.0) | |
| Immunofixation | IgG kappa monoclonal band isolated | |
| Lactate dehydrogenase (U/L) | 304 (140–280) | |
| B2-microglublin (mg/L) | 5.5 (1.1–2.4) | |
| Serum free light chains | ||
| Kappa light chain (mg/L) | 642 (3.3–19.4) | |
| Lambda light chain (mg/L) | 63 (5.71–26.3) | |
| Kappa/lambda ratio | 10.19 (0.26–1.65) | |
| Albumin (g/L) | 27 (34–54) | |
| Creatinine (µmol/L) | 504 (65.4–119) | |
| Urea (mmol/L) | 34.5 (2.1–7.8) | |
| Estimated glomerular filtration rate (mL/min) | 10 (>70) | |
| Corrected calcium (mmol/L) | 2.24 (2.2–2.6) | |
| HHV8 PCR | Not assessed | |
| HIV p24Ag/ T.Abs | Negative | |
| Haemoglobin (g/L) | 93 (13.2–16.6) | |
| White cell count (×109/L) | 14 (4.5–11) | |
| Lymphocyte count (×109/L) | 0.6 (1–4.8) | |
| Platelet count (×109/L) | 230 (165–415) | |
| Bone marrow trephine at diagnosis | Hypercellular core with a heavy infiltrate by a CD138+ve plasma cells making up 80%. Consistent with a diagnosis of multiple myeloma. | |
| Bone marrow aspirate at diagnosis | Hypercellular particles with hypercellular trails. Increased plasma cells making up 60% of nucleated cell count. | |
| Bone marrow cytogenetics | Failed karyotypic analysis and Flourescence In-Situ Hybridisation (FISH) studies. | |
| TP53 mutational status: | No detectable mutation by next generation sequencing | |
| T cell subset analysis by flow cytometry of peripheral blood | ||
| CD4 count/µL: at diagnosis of iatrogenic Kaposi’s (Reference range) | CD4 count/µL: at clinical resolution of lesions | CD4 count/µL: 3 months postautologous stem cell transplant |
| 143 (500–1400) | 662 | 128 |
T cell flow cytometry was done at Kaposi’s sarcoma diagnosis and subsequent time points.
HHV8, human herpesvirus 8.
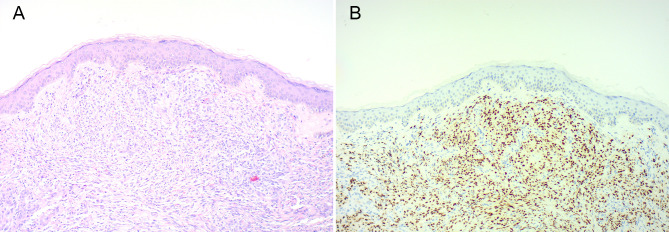
Figure 1.
Composite picture showing skin nodule with Kaposi’s sarcoma H&E (A) and human herpesvirus-8 staining (B). Note the typical nodular dermal spindle shaped cells proliferation and vascular spaces on H&E staining and positive nuclear staining for human herpesvirus-8 antibody (×100 magnification).
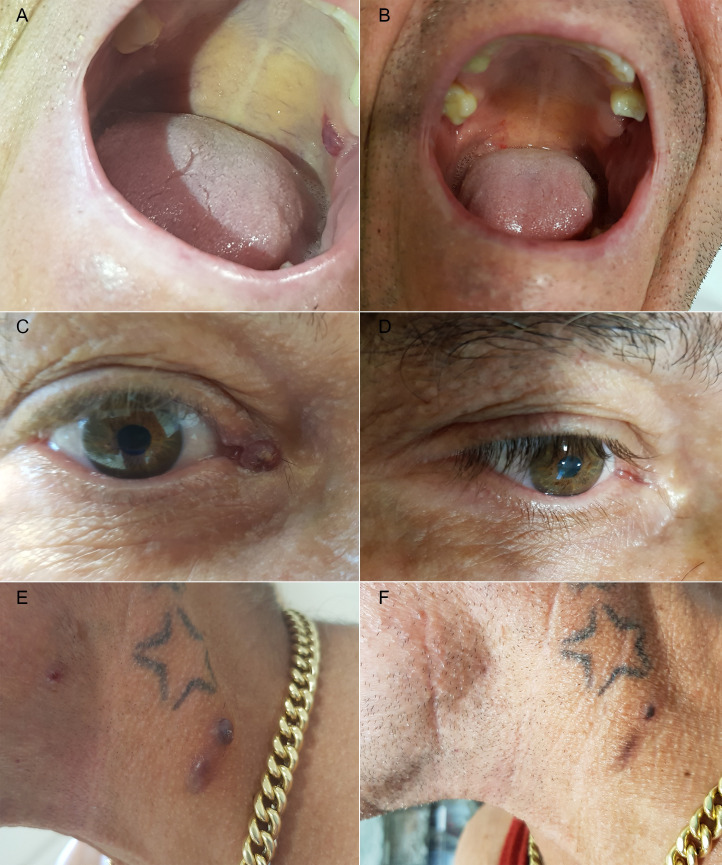
Figure 2.
Composite picture showing Kaposi’s lesions before interruption of treatment: panels on the left showing: (A) lesion in right upper gum mucosa, (C) lesion at right ocular caruncle, (E) lesions on left side of neck. Resolution of skin tumours after treatment interruption (B, D, F).
The sarcoma was deemed to be iatrogenic, and on cessation of treatment, the rash improved over 6 weeks as shown in figure 2. An urgent positron emission tomography (PET) scan was performed to exclude the presence of concomitant visceral Kaposi’s and to assess response to myeloma therapy. The lesions did not show fluorodeoxyglucose uptake on PET likely due to their small size, and the disease seemed to be limited to the skin and was cosmetically acceptable. An otolaryngology assessment and gastrointestinal endoscopy did not reveal other deposits. HHV8 testing was not performed.
A T cell subset analysis showed a depressed CD4 count during the emergence of this rash, the counts improved subsequently over several weeks with the resolution of the cutaneous lesions. The diagnosis of iatrogenic Kaposi’s during antimyeloma treatment, left us with a dilemma of whether to proceed with ASCT. After careful deliberation and discussion with the patient, it was decided to proceed with ASCT. There was an unintentional 7-month long gap from interruption of treatment to transplantation due to the COVID-19 pandemic, which presented logistical barriers to the patient being referred for the ASCT to a tertiary care centre. Stem cell mobilisation was achieved using growth factors only, given the history of iatrogenic Kaposi’s during therapy with cyclophosphamide, and the patient underwent a myeloablative conditioning with full dose melphalan at 200 mg/m2. Despite severe lymphopenia, his cutaneous lesions remained resolved. From a myeloma point of view, at 3 months postautograft, the patient had a partial response with persistence of the paraprotein (6 g/L) on serum protein electrophoresis and 5%–10% plasma cells on bone marrow biopsy. Maintenance of bortezomib was then initiated. He is currently being followed in myeloma clinic.
Treatment
For his myeloma, the patient was treated with standard of care triplet combination of cyclophosphamide, bortezomib and dexamethasone (CyBorD), keeping in mind his renal failure at presentation. He had received a total of six cycles before the emergence of Kaposi’s sarcoma. At this point, the caring oncologists were considering treatment with liposomal doxorubicin and lenalidomide. However, these were not administered as the sarcoma regressed after stopping antimyeloma treatment.
Outcome and follow-up
Our patient is currently on day 500+ postautologous transplant. There is no evidence of cutaneous or visceral recurrence of Kaposi’s and he is in partial response from his myeloma, with persistence of paraprotein and 5%–10% plasma cells on bone marrow biopsy. The myeloma flourescence in situ hybridisation panel was resent at 3 months postautograft and this failed analysis again, possibly due to low disease burden. Neither next generation sequencing nor next generation flow cytometry for detection of minimal residual disease in myeloma were available in our centre at the time. He has completed 12 months of bortezomib maintenance without re-emergence of the rash. Data from the European Group for Blood and Bone Marrow Transplantation case series shows that the median time to development of Kaposi’s post-transplant was around 7 months.2 We do not expect our patient to develop Kaposi’s this far from the transplant. Nevertheless, we remain vigilant.
Discussion
HHV8 is a lymphotropic virus linked with the aetiology of at least three haematological malignancies.3 Rettig et al described the presence of Kaposi’s sarcoma associated herpesvirus infection in bone marrow dendritic stromal cells in 1992 with the postulation that the viral homologue of interleukin 6 coded for by HHV8 might drive the progression of monoclonal gammopathy of uncertain significance to overt myeloma through paracrine stimulation.4 This hypothesis has been challenged and there have been several conflicting bodies of evidence from studies conducted in different geographical areas.4 5 This might stem from different methodologies used and also the diverse epidemiology and seroprevalence rates of HHV8 across different ethnicities and geographical locations. The link between HHV8 and myeloma remains at best, a controversial one. A recent comprehensive systematic review recommends further research on the issue.6
There is scanty literature about Kaposi’s sarcoma arising during treatment for multiple myeloma. The first case series published by Chavarot et al describe two cases of Kaposi’s both of which on cyclophosphamide and dexamethasone combination together with bortezomib, coming up with the bold suggestion that the latter drug might do little to prevent HHV-8 reactivation in combination with cyclophosphamide.7 This contrasts with the in vitro activity that bortezomib has shown in activating lytic induction in HHV8 and the drug was trialled in a phase I study for HIV associated Kaposi sarcoma.8 9 This case series does not mention whether patients were transplanted.
The occurrence of Kaposi’s sarcoma post bone marrow transplantation is a rarity with a recent important publication with data retrieved from the European Bone Marrow Transplant database including previously reported cases, reporting an incidence of 0.05% of Kaposi’s sarcoma after autologous transplantation. However, this case series about Kaposi’s in the context of bone marrow transplantation, the first of its kind, failed to identify risk factors for the development of Kaposi’s sarcoma and excluded cases of Kaposi’s happening pretransplant from their analysis.2 We do not know the outcomes of Kaposi’s in patients with pretransplant disease, but no patient with post-transplant Kaposi’s died directly from the condition in this series.
The scanty literature on the subject left us unsure about the benefits of performing the transplant. The rapid clinical response to the withdrawal of therapies, together with the absence of visceral involvement and limited disease burden in the form of nodular skin involvement, together with an excellent patient performance status, was encouraging. After liaison with our transplant specialists together with the oncology team, it was decided to proceed with the ASCT after careful patient counselling regarding the uncertainty of recurrence. The transplant was done after immune recovery and complete clinical resolution of the tumour.
Another concern is about when to consider post-transplant maintenance. Maintenance improves progression-free and overall survival post-transplant and this can be in several forms such as bortezomib or lenalidomide. The latter agent is interesting as, apart from having established activity against Kaposi’s sarcoma, it does not cause depression in post-transplant CD4 counts.10 Despite its appeal, lenalidomide could not be used for maintenance in this patient’s case as it was not available at our centre at the time of treatment due to administrative issues surrounding its approval.
For iatrogenic cases of Kaposi’s sarcoma, there is no established CD4 cut-off above which recurrence of tumour is unlikely and neither is there guidance as to whether serial HHV-8 serology or CD4 measurement should be carried out. Our patient was thus not tested for HHV-8, as the result would not have altered the management. The AIDS Clinical Trials Group staging system takes into consideration the immune status with a CD4 cut-off of less than 200/µL as poor risk for HIV associated Kaposi’s.11 Several cases of iatrogenic Kaposi’s with CD4 counts beyond 350/µL are reported.12 Bortezomib is known to reduce the CD4+T cell count and lessens CD8 T-cell toxicity.13 After recovery of platelet and neutrophil counts, vigilance is still required in similar cases as immune reconstitution and recovery of CD4+ve T-cells may persist for a year or more.14 The only reported case of fatality postautograft that was directly related to Kaposi’s sarcoma happened at 4.5 months post-transplantation.15
There are various case reports in the literature that implicate corticosteroids in the development of Kaposi’s sarcoma. These reports describe cases of Kaposi’s sarcoma in the setting of steroid use for a vast array of diseases ranging from idiopathic thrombocytopenic purpura to rheumatoid arthritis and pemphigus vulgaris.16 Corticosteroids inhibit activation of transforming growth factor beta, which is responsible for inhibition of epithelial and lymphocyte proliferation, thus allowing Kaposi’s Sarcoma cell proliferation. Cases of iatrogenic Kaposi’s sarcoma secondary to steroid use have been reported in patients with negative HIV serology and also in relation to topical corticosteroids.17
Iatrogenic Kaposi’s sarcoma occurring in a pretransplant setting presents a dilemma regarding the risk of recurrence with further treatment and from transplant immunosuppression. We demonstrate through our case that ASCT can be safely done with close monitoring in a multidisciplinary setting.
Learning points.
Although the link of human herpesvirus 8 with multiple myeloma is at best, a tenuous one, Iatrogenic Kaposi’s is an important differential for an eruptive nodular rash in a patient on chemotherapy for myeloma.
Antiangiogenic properties of certain antimyeloma drugs are an important therapeutic option in treating concurrent Kaposi’s sarcoma.
The use of a positron emission tomography scan is a useful imaging modality in assessing patients having concurrent myeloma and Kaposi’s.
The risks and benefits of antimyeloma treatment, including autologous stem cell transplantation, need to be carefully weighed against the likely possibility of a flare-up of Kaposi’s sarcoma—treatment decisions need to happen in a multidisciplinary setting with a transplant specialist, haematologist and oncologist on board.
Our case shows that the occurrence of iatrogenic Kaposi’s with limited disease burden is not an absolute contraindication to transplantation and that the procedure has been carried out once immune reconstitution and resolution of tumour has occurred.
Footnotes
Contributors: All were directly involved in patient care and have coauthored the write-up. DF has written and reviewed the manuscript. ES has reviewed and proof-read the write-up. DB has reviewed the histology and reviewed the writeup. MG has reviewed and amended the manuscript.
Funding: The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Case reports provide a valuable learning resource for the scientific community and can indicate areas of interest for future research. They should not be used in isolation to guide treatment choices or public health policy.
Competing interests: None declared.
Provenance and peer review: Not commissioned; externally peer reviewed.
Ethics statements
Patient consent for publication
Consent obtained directly from patient(s).
References
- 1.Kaposi M. Idiopathisches multiples Pigmentsarkomen der Haut. Arch f Dermat 1872;4:265–73. 10.1007/BF01830024 [DOI] [Google Scholar]
- 2.Cesaro S, Tridello G, van der Werf S, et al. Incidence and outcome of Kaposi sarcoma after hematopoietic stem cell transplantation: a retrospective analysis and a review of the literature, on behalf of infectious diseases working party of EBMT. Bone Marrow Transplant 2020;55:110–6. 10.1038/s41409-019-0644-8 [DOI] [PubMed] [Google Scholar]
- 3.Malnati MS, Dagna L, Ponzoni M, et al. Human herpesvirus 8 (HHV-8/KSHV) and hematologic malignancies. Rev Clin Exp Hematol 2003;7:375–405. [PubMed] [Google Scholar]
- 4.Rettig MB, Ma HJ, Vescio RA, et al. Kaposi's sarcoma-associated herpesvirus infection of bone marrow dendritic cells from multiple myeloma patients. Science 1997;276:1851–4. 10.1126/science.276.5320.1851 [DOI] [PubMed] [Google Scholar]
- 5.Sadeghian MH, Mohammadnia Avval M, Ayatollahi H, et al. Is there any relationship between human herpesvirus-8 and multiple myeloma Lymphoma 2013;2013:1–5. 10.1155/2013/123297 [DOI] [Google Scholar]
- 6.Sadeghian M, Rezaei Dezaki Z, Shams S, et al. Correlation between human herpes virus 8 (Hhv8-) and plasma cell myeloma: a systematic review. Reviews in Clinical Medicine 2018;5:95–100. 10.22038/rcm.2018.32149.1235 [DOI] [Google Scholar]
- 7.Chavarot N, Lebbe C, Thervet E, et al. Bortezomib does not prevent the occurrence of Kaposi’s sarcoma in patients with haematological malignancies: two case reports. Acta Derm Venereol 2017;97:1138–9. 10.2340/00015555-2727 [DOI] [PubMed] [Google Scholar]
- 8.Reid EG. Bortezomib-induced Epstein-Barr virus and Kaposi sarcoma Herpesvirus Lytic gene expression: oncolytic strategies. Curr Opin Oncol 2011;23:482–7. 10.1097/CCO.0b013e3283499c37 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.National Cancer Institute . Clinical trial: bortezomib in treating patients with Relapsed or refractory AIDS-related Kaposi sarcoma. Clinicaltrials.Gov Identifier: Nct01016730. n.d. Available: https://clinicaltrials.gov/ct2/show/NCT01016730
- 10.Fostier K, Caers J, Meuleman N, et al. Impact of lenalidomide maintenance on the immune environment of multiple myeloma patients with low tumor burden after autologous stem cell transplantation. Oncotarget 2018;9:20476–89. 10.18632/oncotarget.24944 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Krown SE, Metroka C, Wernz JC. Kaposi’s sarcoma in the acquired immune deficiency syndrome: a proposal for uniform evaluation, response, and staging criteria. AIDS Clinical Trials Group Oncology Committee. J Clin Oncol 1989;7:1201–7. 10.1200/JCO.1989.7.9.1201 [DOI] [PubMed] [Google Scholar]
- 12.Billon E, Stoppa A-M, Mescam L, et al. Reversible rituximab-induced rectal Kaposi's sarcoma misdiagnosed as ulcerative colitis in a patient with HIV-negative follicular lymphoma. Clin Sarcoma Res 2018;8:11. 10.1186/s13569-018-0097-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pellom ST, Dudimah DF, Thounaojam MC, et al. Modulatory effects of bortezomib on host immune cell functions. Immunotherapy 2015;7:1011–22. 10.2217/imt.15.66 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Guillaume T, Rubinstein DB, Symann M. Immune reconstitution and immunotherapy after autologous hematopoietic stem cell transplantation. Blood 1998;92:1471–90. 10.1182/blood.V92.5.1471 [DOI] [PubMed] [Google Scholar]
- 15.Vivancos P, Sarrá J, Palou J, et al. Kaposi's sarcoma after autologous bone marrow transplantation for multiple myeloma. Bone Marrow Transplant 1996;17:669–71. [PubMed] [Google Scholar]
- 16.Baykal C, Atci T, Buyukbabani N, et al. The spectrum of underlying causes of iatrogenic Kaposi's sarcoma in a large series: a retrospective study. Indian J Dermatol 2019;64:392–9. 10.4103/ijd.IJD_217_18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fernández-Sánchez M, Iglesias MC, Ablanedo-Terrazas Y, et al. Steroids are a risk factor for Kaposi's sarcoma-immune reconstitution inflammatory syndrome and mortality in HIV infection. AIDS 2016;30:909–14. 10.1097/QAD.0000000000000993 [DOI] [PMC free article] [PubMed] [Google Scholar]